Hydrochloric acid Fasudil liposome injection and new application thereof
A technology of fasudil hydrochloride and fasudil hydrochloride, which is applied in the field of liposome injection of fasudil hydrochloride and its preparation, can solve problems such as poor stability, achieve the improvement of curative effect, increase the encapsulation rate, The effect of reducing leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
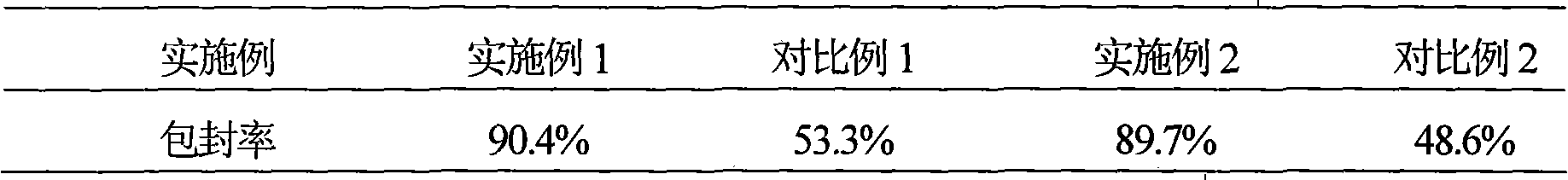
Examples
Embodiment 1
[0060] Example 1 Preparation of Fasudil Hydrochloride Liposomes
[0061] Prescription (1000 sticks):
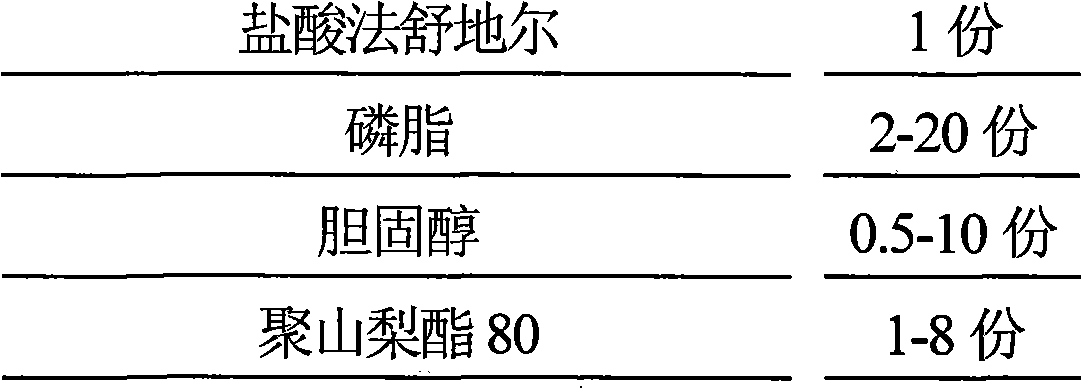
[0062] Fasudil Hydrochloride 15g
[0063] Dilauroyl Phosphatidylglycerol 180g
[0064] Cholesterol 90g
[0065] Polysorbate 80 30g
[0067] Citric acid-sodium citrate buffer solution with pH 4.5 300ml
[0068] Preparation Process
[0069] (1) Dissolve 180g of dilauroylphosphatidylglycerol, 90g of cholesterol and 30g of polysorbate 80 in 1000ml of hexane, slowly inject 1000ml of 0.05mol / L ammonium sulfate solution under stirring, heat and stir to remove n-hexane, place in an ice bath Ultrasound for 20 minutes to obtain blank liposomes;
[0070] (2) Blank liposome is placed in the dialysis bag, seals, and the dialysis bag is placed in 0.9% sodium chloride aqueous solution 975ml and dialyzes for 22 hours, removes the ammonium sulfate in the liposome external phase;
[0071] (3) Dissolve 15g of fasudil hydrochloride in 500ml of water, hea...
Embodiment 2
[0080] Example 2 Preparation of Fasudil Hydrochloride Liposomes
[0081] Prescription (1000 sticks):
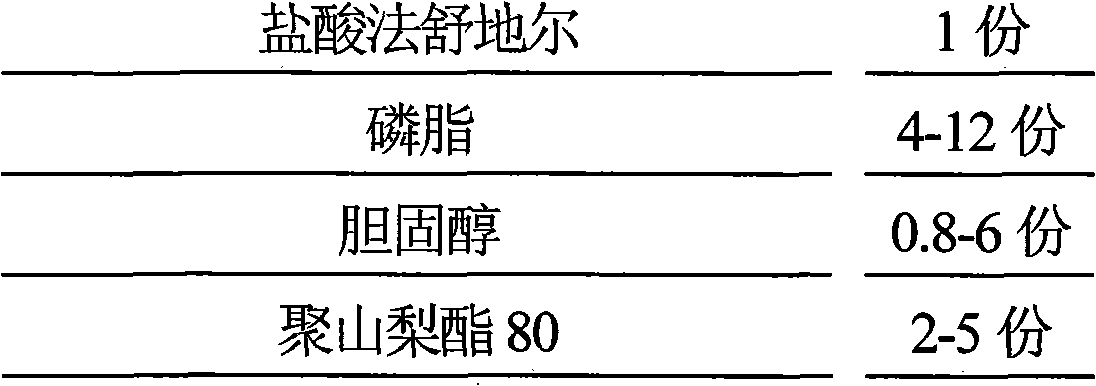
[0082] Fasudil Hydrochloride 30g
[0083] Dioleoylphosphatidylglycerol 120g
[0084] Cholesterol 24g
[0085] Polysorbate 80 150g
[0086] Glycerin 300g
[0087] Phosphoric acid-dipotassium hydrogen phosphate buffer solution at pH 5.2 500ml
[0088] Preparation Process
[0089] (1) Dissolve 120g of dioleoylphosphatidylglycerol, 24g of cholesterol and 150g of polysorbate 80 in 1200ml of methanol, slowly inject 1200ml of 0.02mol / L ammonium sulfate solution under stirring, heat and stir to remove the methanol, put it in an ice bath and sonicate 20min, get blank liposome;
[0090] (2) Blank liposome is placed in the dialysis bag, seals, and the dialysis bag is placed in 1125ml of 20% glycerol aqueous solution and dialyzes for 20 hours, removes the ammonium sulfate in the liposome external phase;
[0091] (3) Dissolve 30g of fasudil hydrochloride in 800ml of water, kee...
Embodiment 3
[0104] Example 3 Preparation of Fasudil Hydrochloride Liposomes
[0105] Prescription (1000 sticks):
[0106] Fasudil Hydrochloride 15g
[0107] Soy Phosphatidylinositol 120g
[0108] Cholesterol 60g
[0109] Polysorbate 80 55g
[0110] Glucose 65g
[0111] Phosphoric acid-disodium hydrogen phosphate buffer solution with a pH value of 5.8 300ml
[0112] Preparation Process
[0113] (1) Dissolve 120g of soybean phosphatidylinositol, 60g of cholesterol and 55g of polysorbate 80 in 1000ml of acetone, slowly inject 1000ml of 0.1mol / L ammonium sulfate solution under stirring, heat and stir to evaporate the acetone, and put it in an ice bath for 10 minutes of ultrasound , to obtain blank liposomes;
[0114] (2) Place the blank liposome in the dialysis bag, seal it, place the dialysis bag in 975ml of 5% glucose aqueous solution and dialyze for 22 hours, remove the ammonium sulfate in the liposome external phase;
[0115] (3) Dissolve 15g of fasudil hydrochloride in 500ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com