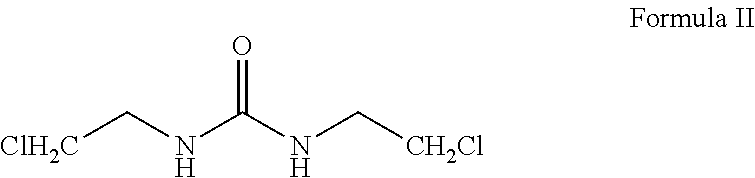
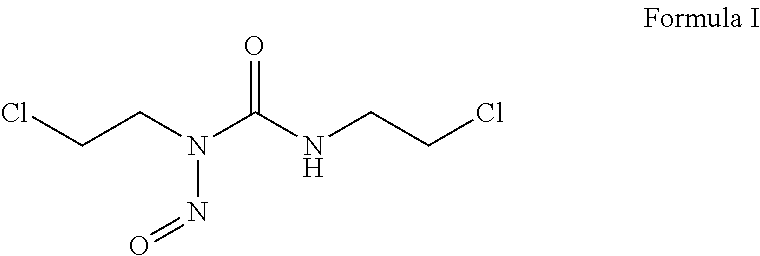
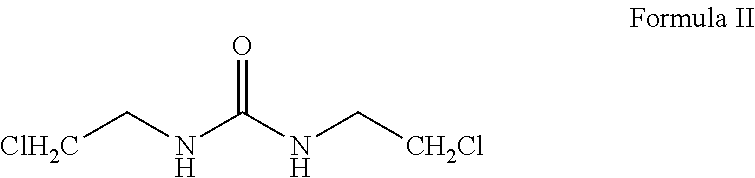
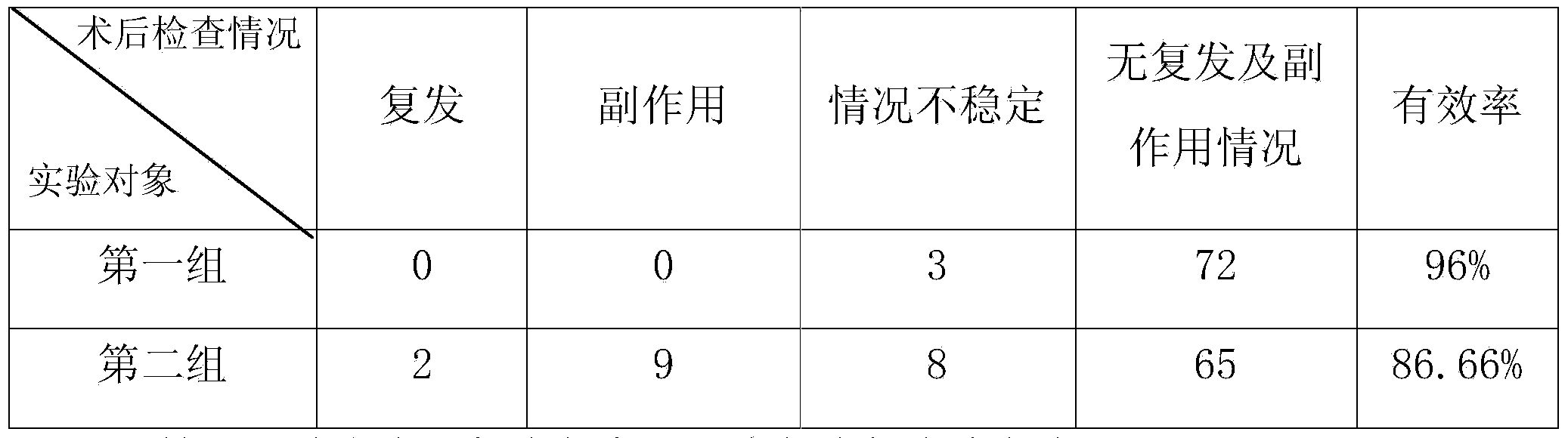
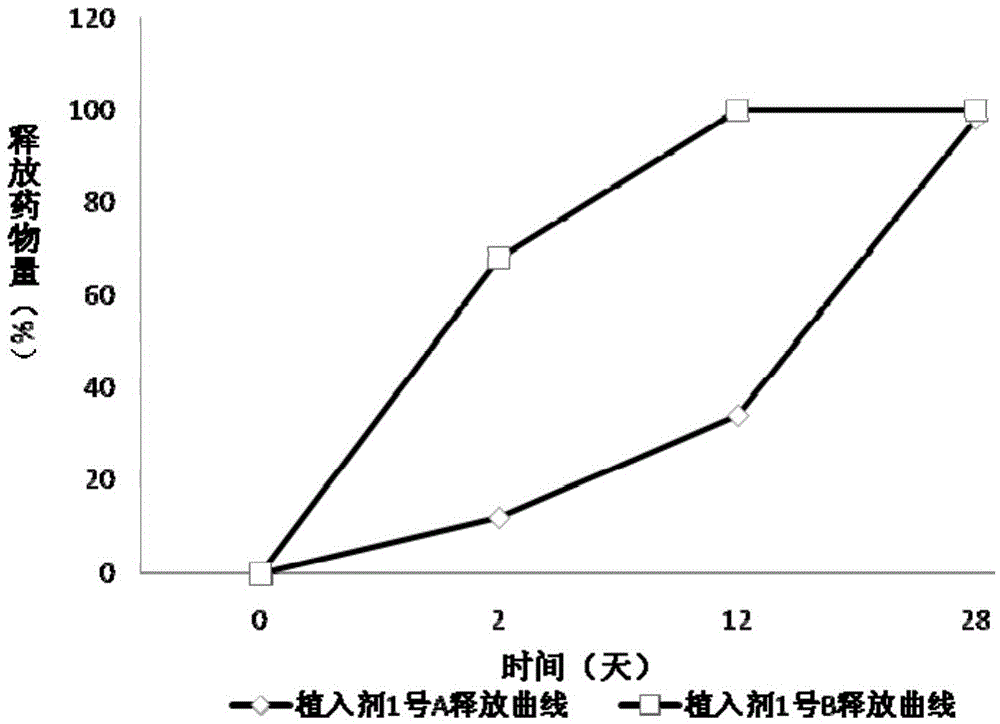
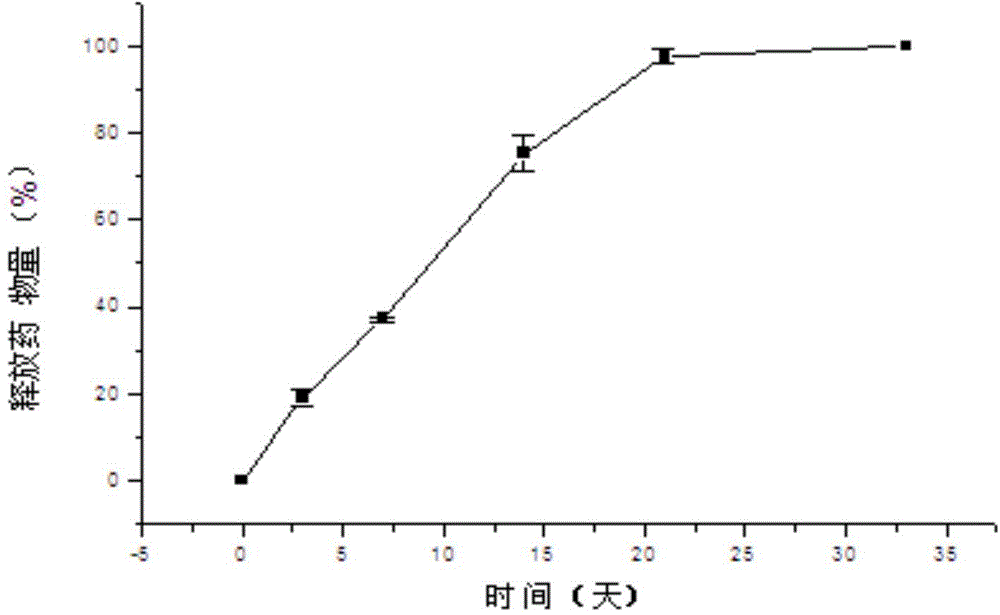
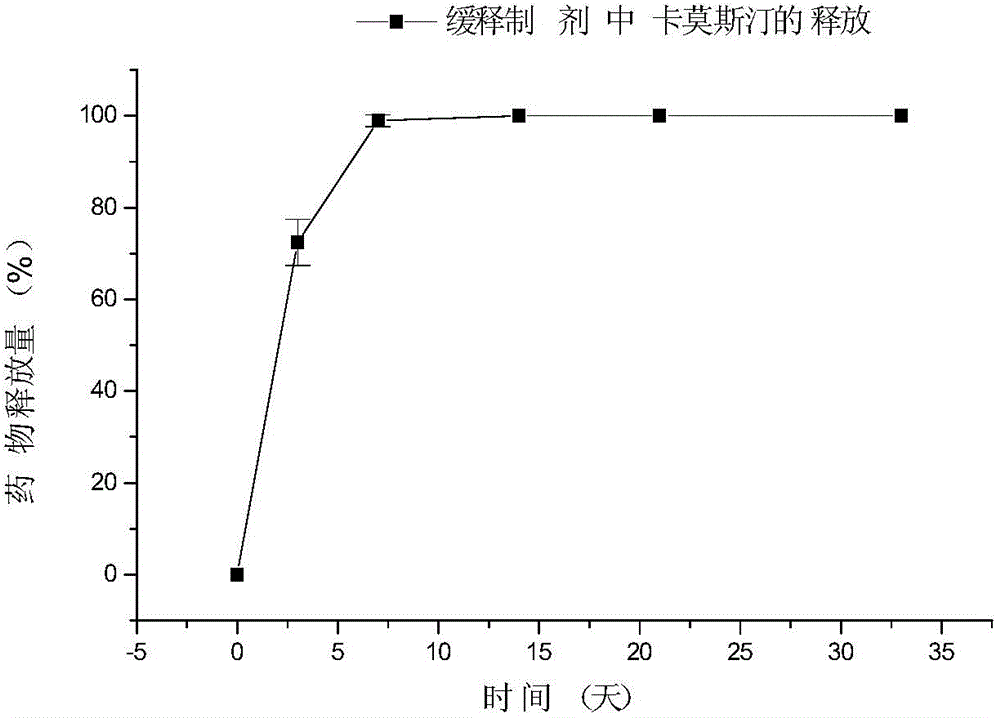
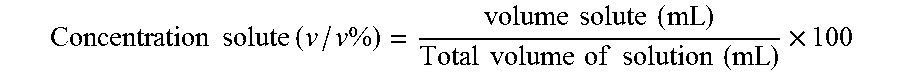Patents
Literature
76 results about "Carmustine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat certain types of cancer (including multiple myeloma, brain tumor, Hodgkin's disease, non-Hodgkin's lymphoma).
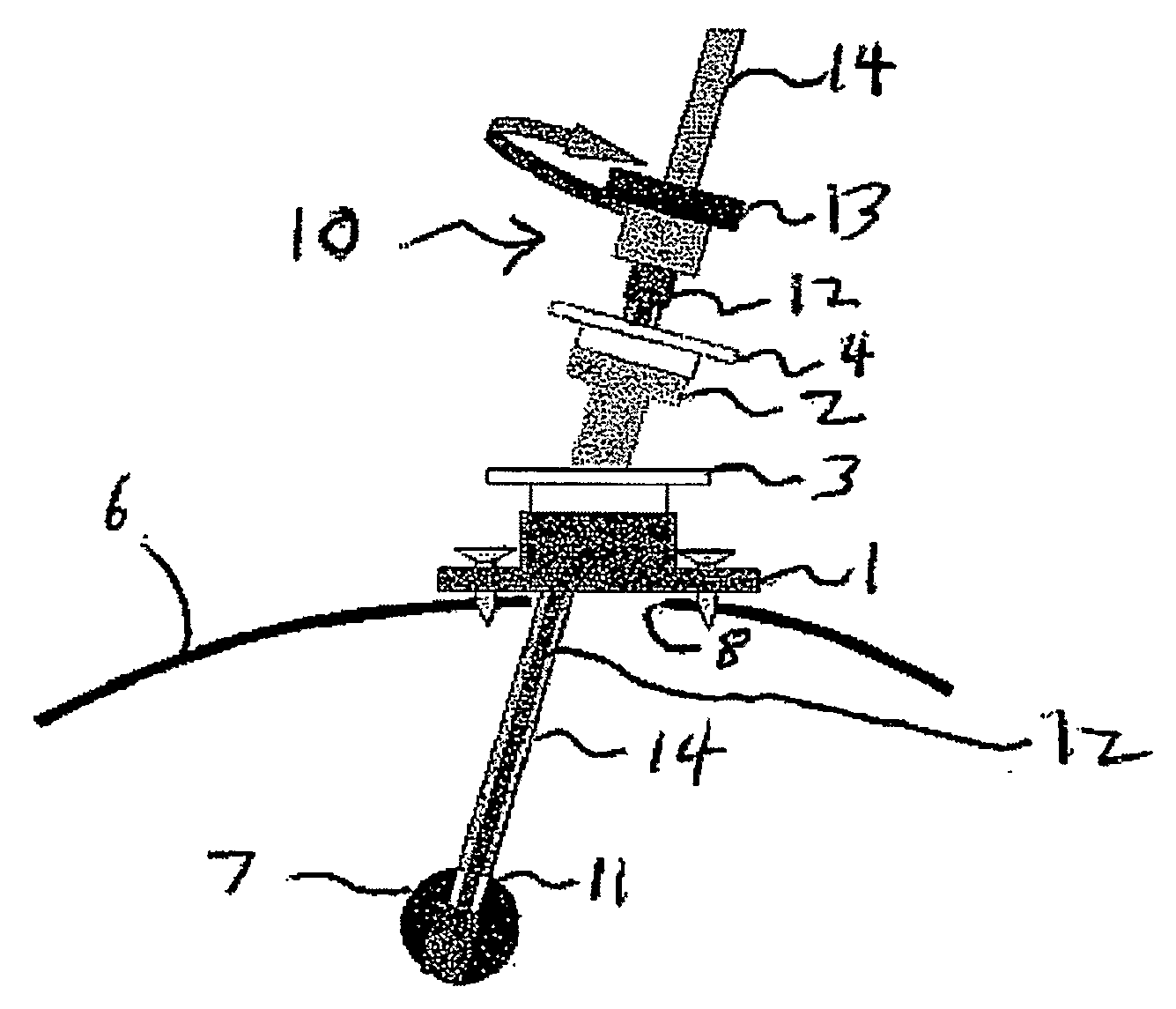
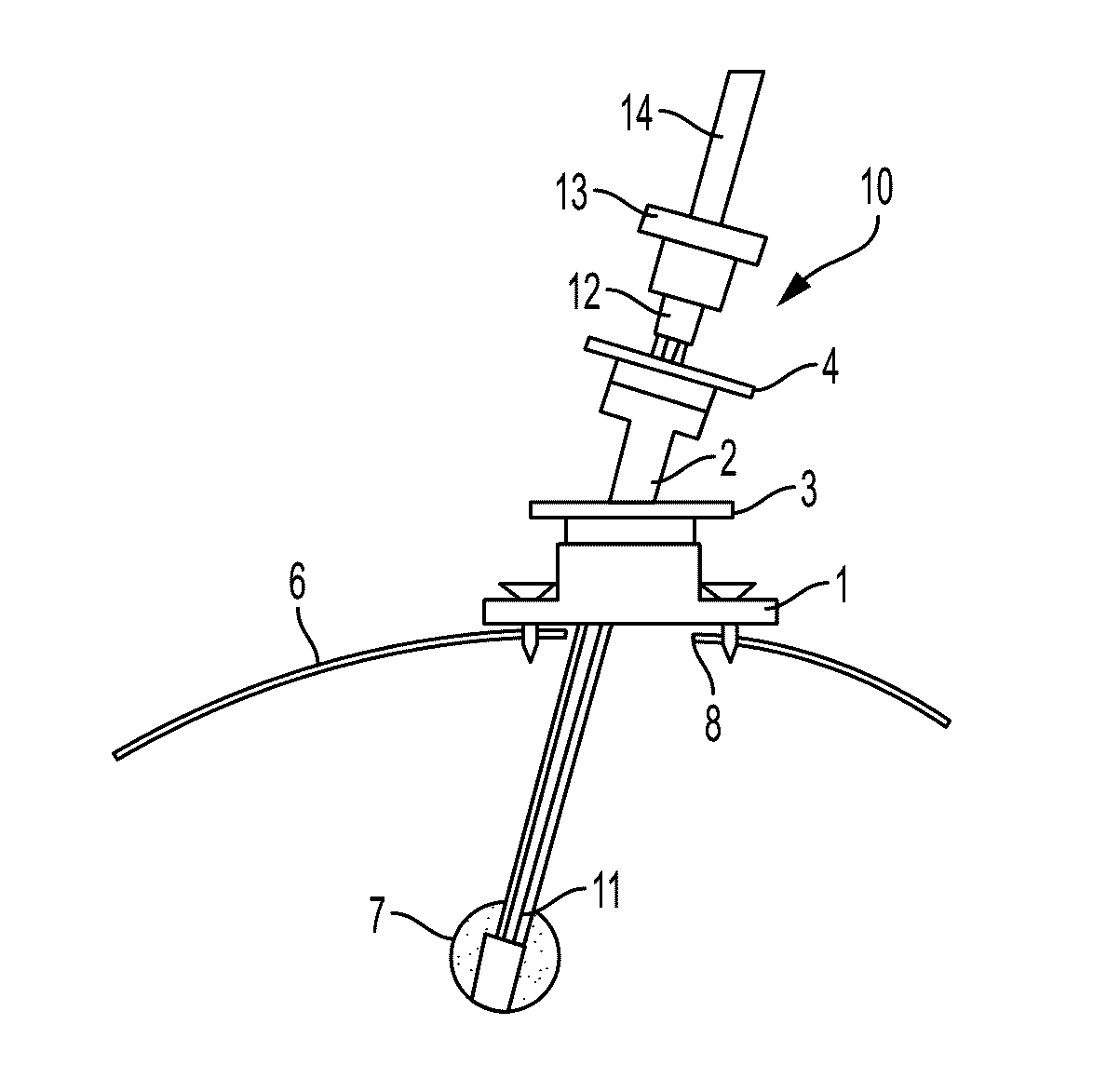
System and Method for Intracranial Implantation of Therapeutic or Diagnostic Agents
ActiveUS20090192487A1Precise positioningSimple and intuitive operationMedical devicesIntravenous devicesDiagnostic agentBrain tumor
A system and related method for delivering the anti-tumoral agent carmustine or other types of diagnostic or therapeutic agents into the brain of a patient with a brain tumor includes an insertion device, a skull mount, and a reformulated geometry of the carmustine compound (or other material) optimized for use in the insertion device and for maximized biodegradation time. The insertion device may be front loaded with the carmustine material (or other material) and inserted through the mount on a skull, to the location of the brain tumor, where the carmustine (or other material) is then released. It should be appreciated that the diagnostic and / or therapeutic system and related method thereof are not necessarily limited to the brain of a subject. It may also be used in the organ structures or tubular structures, as well as portions and locations thereof.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
Medical combination of Carmustine, the preparing method and use thereof
ActiveCN101032475AHydrolysis effectImprove stabilityPowder deliveryPharmaceutical non-active ingredientsSolubilityFreeze-drying
The present invention discloses one kind of carmustine medicine composition, which includes carmustine in effective treating amount, surfactant of Tween, and cosolvent of glycerin and / or polyglycol. The carmustine medicine composition may be injection or freeze dried powder for injection. The addition of Tween and cosolvent can raise the solubility of carmustine. The present invention also discloses the preparation process of the carmustine medicine composition.
Owner:MUDANJIANG HENGYUAN PHARMA CO LTD
Process for preparing medicine-carrying particle containing surface transferrin for glioma target chemical therapy
InactiveCN1683016AGrowth inhibitionImprove bioavailabilityPowder deliveryMacromolecular non-active ingredientsMicroparticlePolycaprolactone
The present invention discloses preparation process of medicine-carrying particle containing surface transferrin for glioma targeting chemotherapy, and belongs to the field of preparing technology of medicine-carrying targeting particle. Biodegradable polymer polylactic acid, polyglycolic acid, polycaprolactone or copolymer of lactic acid and glycolic acid and chemotherapeutical medicine carmustine, Adriamycin or taxol are dissolved in acetone, acetonitrile or dimethyl sulfoxide; and the solution is emulsified in solution of transferrin or combined with transferrin chemically after co-dialysis with cholesterol modified glucosan dialdehyde to prepare the medicine-carrying polymer particle containing surface transferrin. This kind of particle may be injected into tumor cavity to make medicine released crossing blood brain barrier and targeting glioma to reach the high inside inhibition of tumor. The present invention has simple preparation process and obvious curative effect.
Owner:TIANJIN UNIV
Carmustine precursor liposome powder injection formulation and process for preparing
InactiveCN1520808AAvoid slow breakdownAvoid side effectsAmide active ingredientsAntineoplastic agentsSide effectSterol
The precursor liposome powder for injection preparation has Carmustine in 3-12 wt% as main material and other components including phospholipid 19-25 wt%, sterol 3.5-6.5 wt% and support agent 54-73 wt%. Carmustine, phospholipid and sterol are compounded into solution; the solution is added into the support agent powder gradually and the mixture is vacuum dried to form Carmustine powder for injection preparation. Before use, the Carmustine powder for injection preparation is mixed with water for injection to form Carmustine liposome injection preparation. The injection preparation has Carmustine coated in double-layer liposome film of phospholipid and sterol and thus less toxic side effect of Carmustine to human body, so that it may be used continuously for raised cancer treating effect.
Owner:谷松兰 +4
Method of preparing superfie fiber formulation for carmustine
InactiveCN1687494ASlow release rateSmooth release behaviorFilament/thread formingMonocomponent polyesters artificial filamentSide effectHalf-life
The present invention relates to a method of preparing BCNU which has the biodegradation high polymer ultra-thin fiber form. Dissolve the BCNU into the solution of high polymer which can be biodegraded, electrospinning, so non-woven fabrics or felt of ultra-thin fiber wrapped with BCNU. The preparation is simple and cheap, the diameter of drug loading fiber can be controlled at the dimension of 0.2 - 2 ª–m, drug loading can be controlled at the range of 0.1 - 100%. The release of drug is study. It has high speed of drug releasing and can also release BCNU steadily for a long time. The fiber itself can biodegrades completely so it has little poisonous side effect. On the other hand, because BCNU is wrapped in the fiber and released slowly, so it conquers the disadvantage of short half-life, and also reduces its poisonous side effect. This trademark is suitable for part chemotherapy after the operation of tumour.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Anti-cancer drugs slow release agent comprising anticancer antibiotics and booster thereof
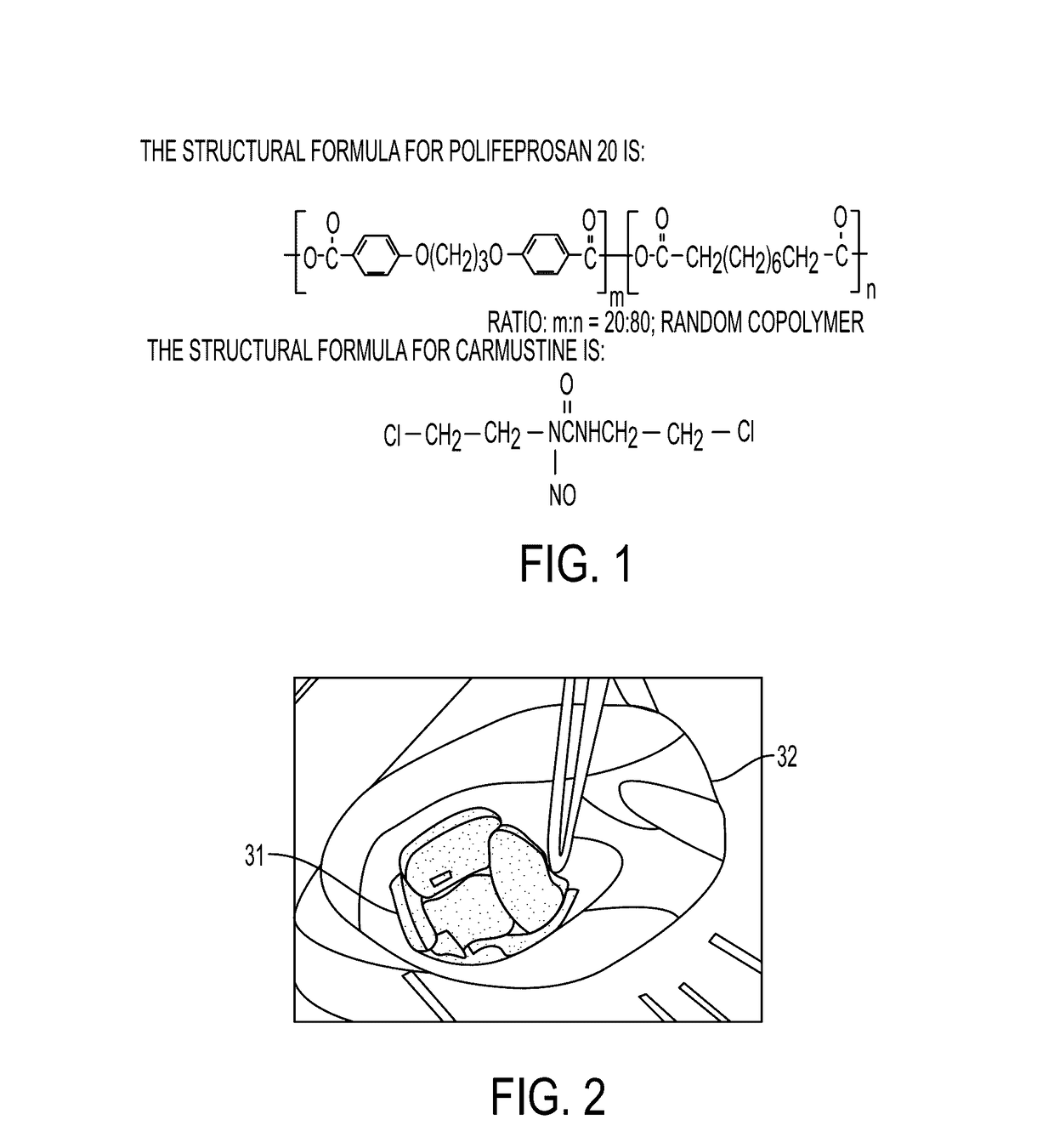
Disclosed is an anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer antibiotics are selected from Idarubicin, Valtaxin, Pirarubicin and Mitoxantrone, The anti-metabolite drugs are selected from Pemetrexed, Carmustine, Tegafur, Zalcitabine, Emtritabine, Galocitabine, Ibacitabine, Ancitabine, Decitabine, Flurocitabine, Enocitabine, Imidazoletabine, Capecittabine, Gemcitabine, Fludrarbine, Raltitrexed, Dexrazoxane, Cladribine, Nolatrexed and folic acid, The slow release auxiliary materials are selected from EVAc, Polifeprosan, sebacylic acid copolymer, lactic acid, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The slow release microspheres can also be prepared into slow release implanting agent for injection or placement in or around tumor.
Owner:SHANDONG LANJIN PHARMA
System and method for intracranial implantation of therapeutic or diagnostic agents
ActiveUS9669198B2Precise positioningSimple and intuitive operationMedical devicesDiagnostic recording/measuringDiagnostic agentBrain tumor
A system and related method for delivering the anti-tumoral agent carmustine or other types of diagnostic or therapeutic agents into the brain of a patient with a brain tumor includes an insertion device, a skull mount, and a reformulated geometry of the carmustine compound (or other material) optimized for use in the insertion device and for maximized biodegradation time. The insertion device may be front loaded with the carmustine material (or other material) and inserted through the mount on a skull, to the location of the brain tumor, where the carmustine (or other material) is then released. It should be appreciated that the diagnostic and / or therapeutic system and related method thereof are not necessarily limited to the brain of a subject. It may also be used in the organ structures or tubular structures, as well as portions and locations thereof.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +1
Anticancer sustained-release gel injection containing stines medicine
The invention relates to an anticancer sustained release gel injection with stine drugs, comprising sustained release microspheres with stine drugs, a amphiphilic block copolymer, solvent and releasing moderator; wherein, the mixture of the amphiphilic block copolymer and the solvent possesses sensitive gelation property; after in vivo injection, the injection can be transformed into nonflowing, biodegradable gel insoluble in water; the insoluble gel can release the contained drugs in local tumor for weeks, even months. Intra-tumor injection or local injection can be used to treat different tumors and unresectable tumors, control late complications and the postoperative residual tumor cell recurrent, reinforce the effect of radiotherapy and chemotherapy and the effect of radiotherapy particles; nimustine and carmustine and other stines; the amphiphilic block copolymer is a PLGA-PEG-PLGA copolymer with the molecular weight of PEG 1200-1600 accounting for 20% of the amphiphilic block copolymer weight; in the poly lactide coglycolide copolymer, the molar ratio of glycolide and lactide is 6:1.
Owner:济南基福医药科技有限公司
Carmustine intravenous emulsion and its preparation method
InactiveCN101143130AReduce releaseSmall toxicityAmide active ingredientsEmulsion deliveryMedicineCarmustine
The invention relates to a carmustine combination, which is used for the vein transfusion and is in a stable oil-in-water emulsion. The invention also relates to the method of mixing the carmustine into the oil to form the stable oil-in-water emulsion. The combination includes the carmustine, the oil, the water and a surfactant.
Owner:大道隆达(北京)医药科技发展有限公司
Fluorouracil containing anti-cancer sustained-release injection
InactiveCN101234084AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolSuspending Agents
The invention relates to anticancer sustained release injection which comprises sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise anticancer active components and sustained release auxiliary material; the menstruum is special menstruum that contains suspending agent. The anticancer active components are fotemustine, nimustine, carmustine or combination of bendamustine and mitozolomide, docetaxel, etoposide, teniposide, vinblastine, anastrozole, tamoxifen, fluorouracil or mitomycin C; the sustained release auxiliary material is polylactic acid and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer of polyethylene glycol, terminal carboxyl group polylactic acid copolymer, EVAc, fatty acid and decanedioic acid copolymer, etc.; viscosity of the suspending agent is 100cp-3,000cp (at 25 DEG C-30 DEG C), and the suspending agent is selected from sodium carboxymethylcellulose, etc. The sustained release microspheres can also be made into sustained release implant; the injection or implant is injected or placed in or around tumor so as to reduce general reaction of the drug and selectively improve and keep local concentration for about 30-50 days. The anticancer sustained release injection can be used solely and can also promote anti-tumor effects of non-operative treatments, such as chemotherapy and / or radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Implant agent treating for solid tumor
InactiveCN101204365ABoron compound active ingredientsPharmaceutical delivery mechanismNervous systemProstate cancer
The invention relates to a sustained-release implant for treating a solid tumor, which is characterized in that: the sustained-release implant contains an effective anticancer amount of bortezomib and sustained-release excipients. The solid tumor includes brain tumor, liver cancer, lung cancer, oesophagus cancer, gastric cancer, breast cancer, pancreatic cancer, thyroid cancer, nasopharyngeal cancer, ovarian cancer, endometrial cancer, cervical cancer, renal cancer, prostate cancer, bladder cancer, colon cancer, rectal cancer, skin cancer, head and neck cancer and primary or secondary cancer, caruncle or carcinosarcoma rooted at a peripheral nervous system, mucosa, glands, blood vessels, bone tissues and lymph nodes. The sustained-release excipients are mainly a biological polymer which is dissoluble and can be degraded and absorbed, in the degradation and absorption process of which carmustine is sustainedly released to part of the tumor, thus the entire toxicity of the carmustine is significantly reduced while an effective medicine consistency is maintained on part of the tumor. That the sustained-release implant is implanted inside part of the tumor can not only reduce the entire toxicity of the carmustine but also enhance the medicine consistency on part of the tumor, thereby increasing the curing effect of non-operative therapeutics such as chemotherapeutic drugs and radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
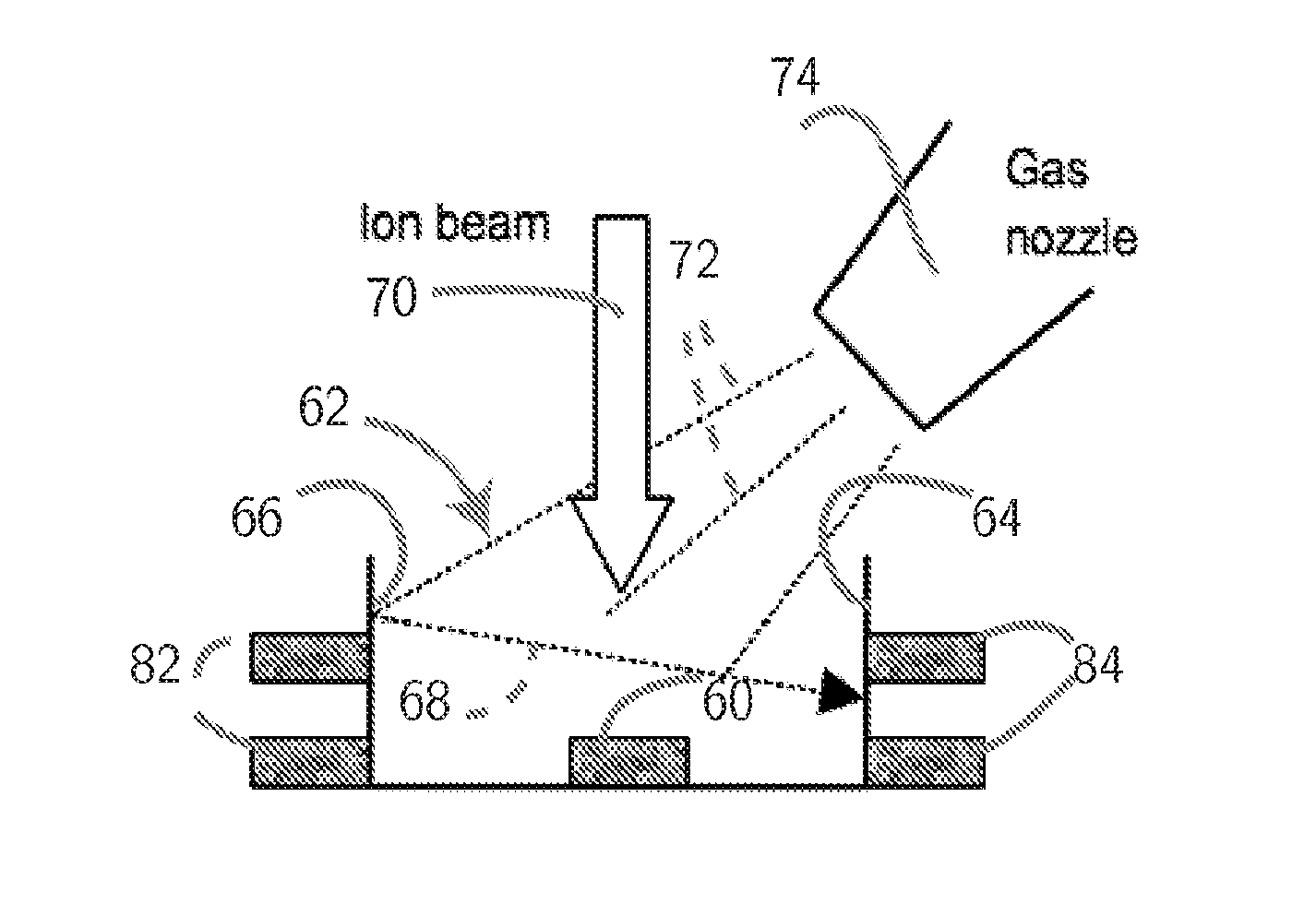
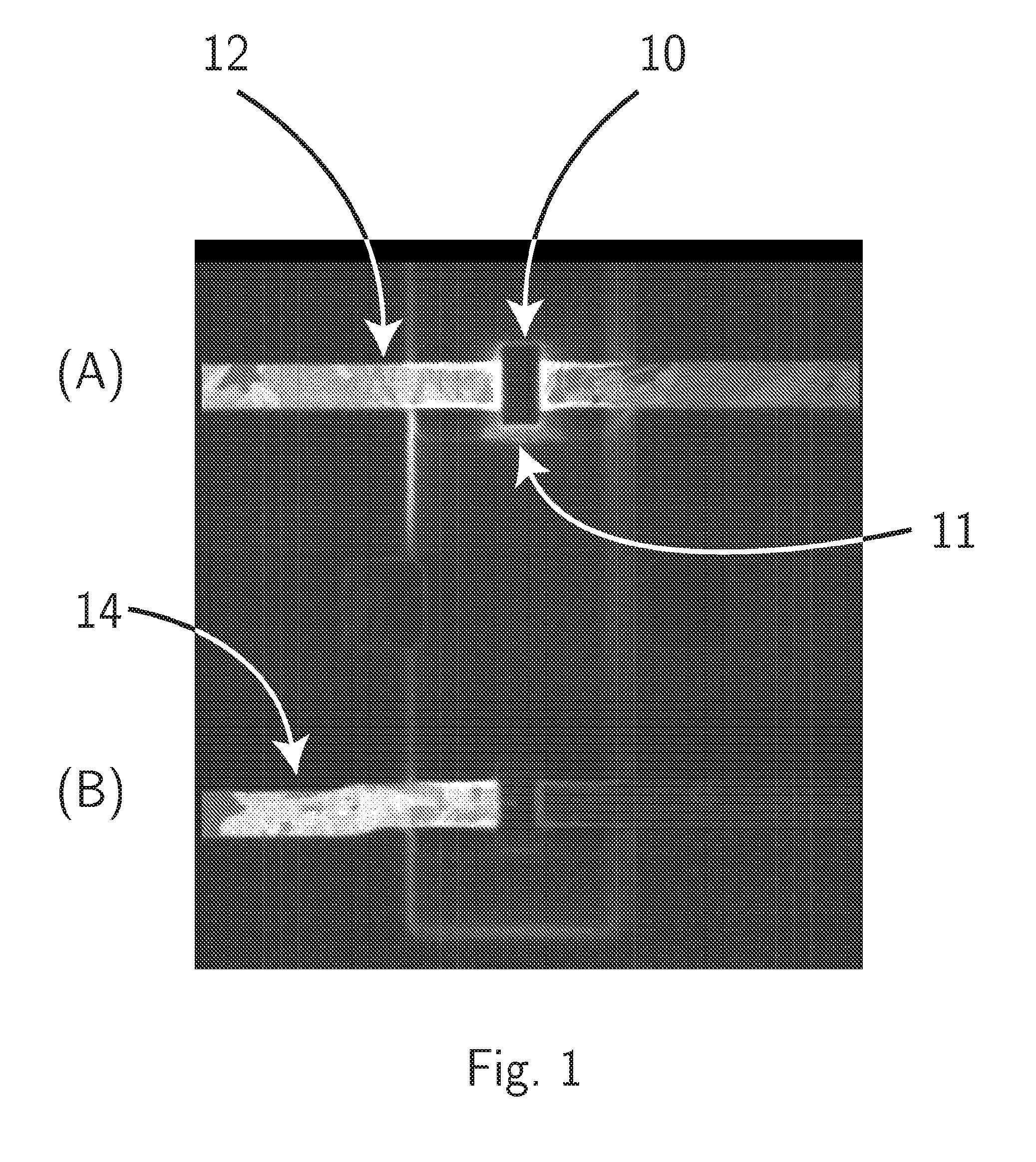
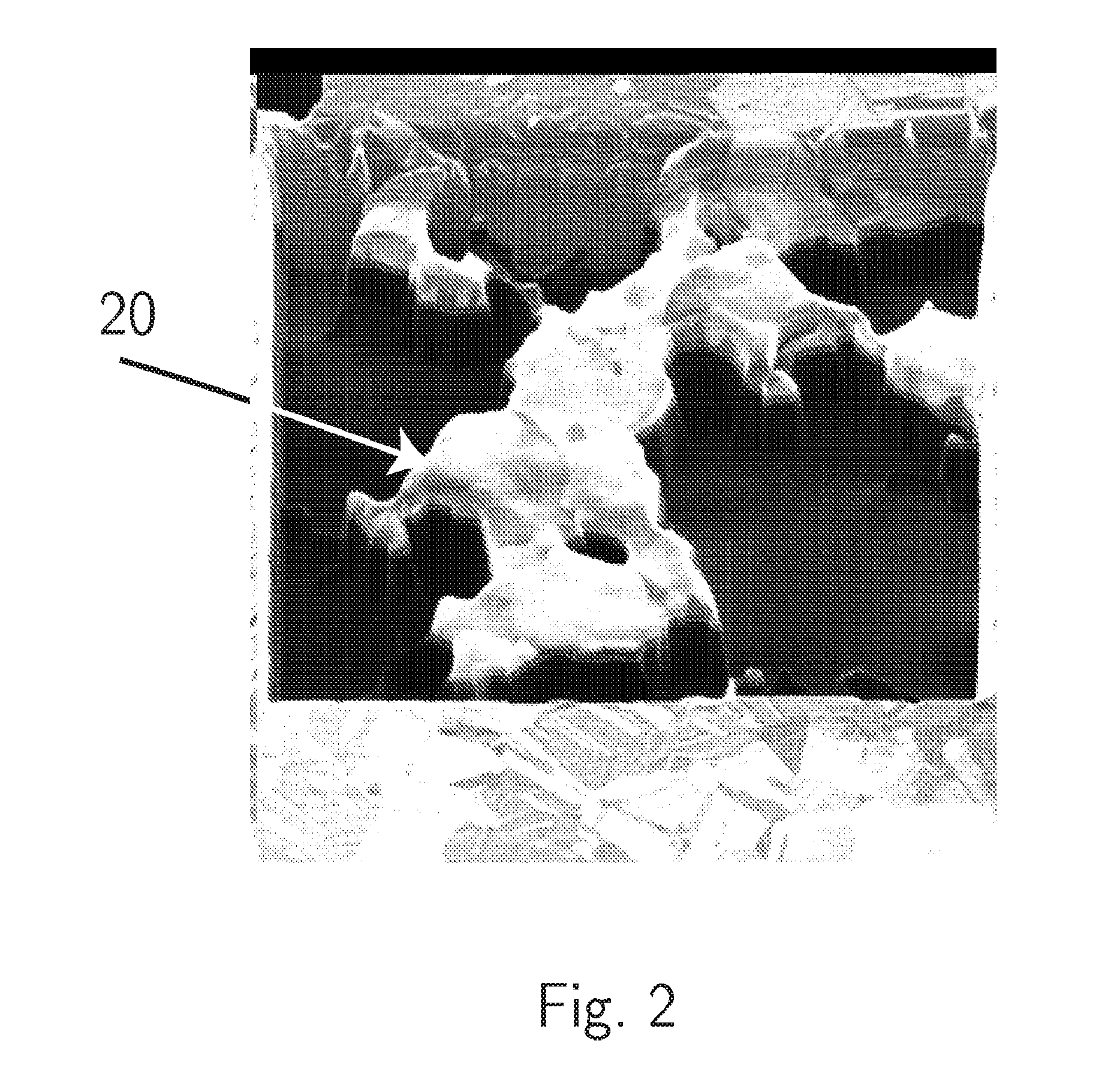
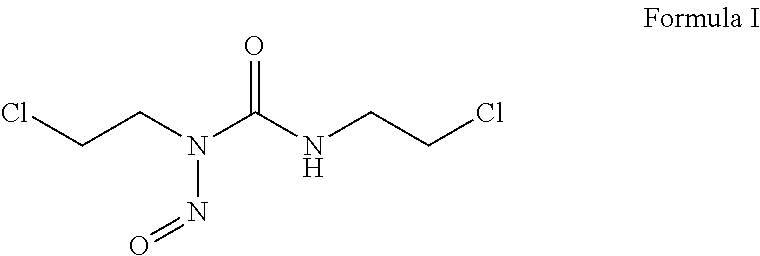
FIB Process for Selective and Clean Etching of Copper
InactiveUS20120211356A1Reduce volatilityHigh stickinessVacuum evaporation coatingSputtering coatingDielectricEtching
Etch assisting agents for focused ion beam (FIB) etching of copper for circuit editing of integrated circuits both prevent loss of adjacent dielectric due to sputtering by the ion beam, and render sputtered re-deposited copper on adjacent surfaces non-conductive to avoid electrical short circuits. The agents are characterized by having an N—N (N being Nitrogen) bonding in their molecules and boiling points between about 70° C. and about 220° C., and include hydrazine and water solutions, hydrazine derivatives, NitrosAmine derivatives saturated with two hydrocarbon groups selected from Methyl, Ethyl, Propyl and Butyl, NitrosAmine related compounds, and Nitrogen Tetroxide. Preferred agents are Hydrazine monohydrate (HMH), HydroxyEthylHydrazine (HEH), CEH, BocMH, BocMEH, NDMA, NDEA, NMEA, NMPA, NEPA, NDPA, NMBA, NEBA, NPYR, NPIP, NMOR and Carmustine, alone or in combination with Nitrogen Tetroxide. The agents are effective for etching copper in high aspect ratio (deep) holes.
Owner:TIZA LAB
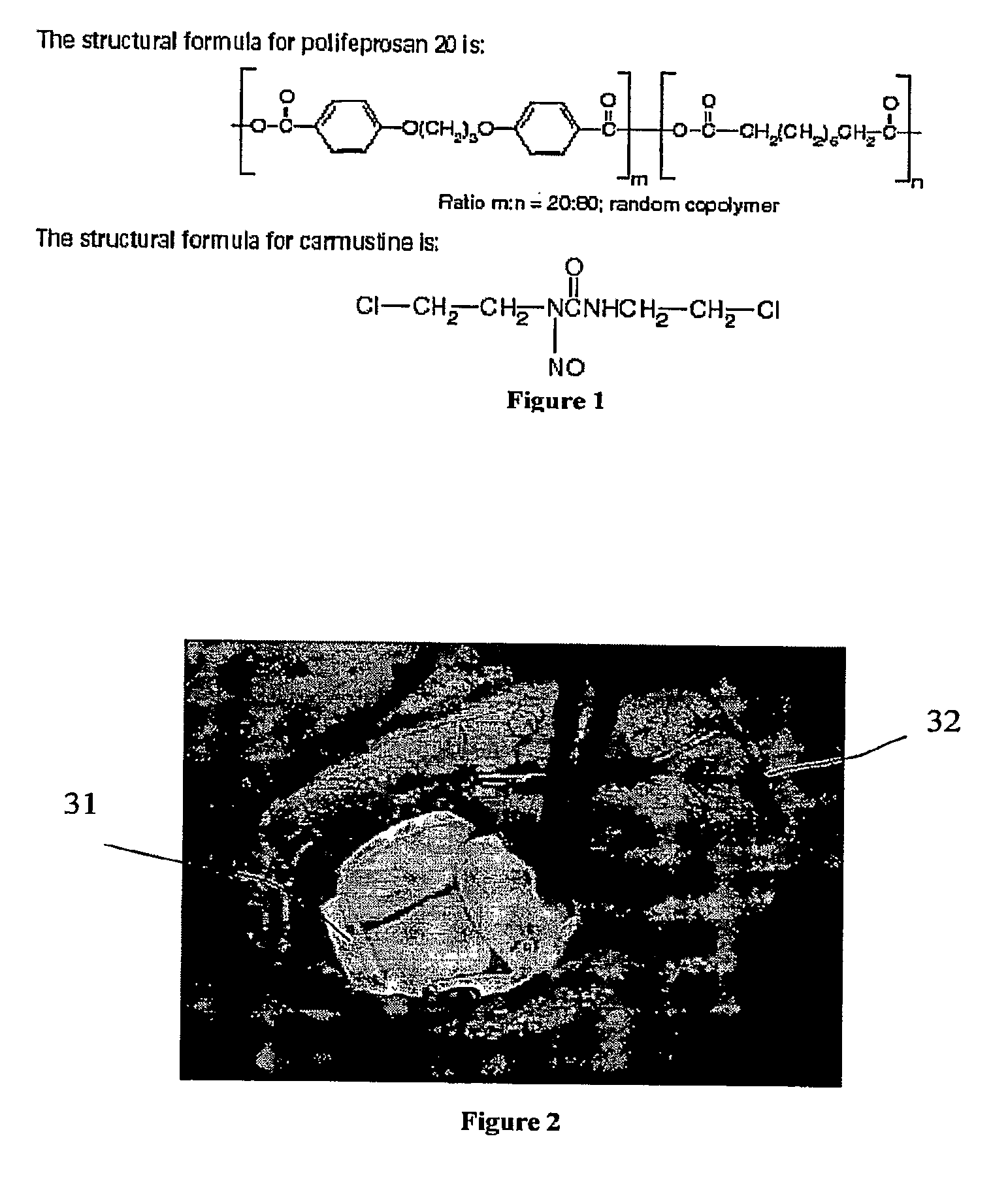
Carmustine Pharmaceutical Composition
The present invention provides pharmaceutical formulations of lyophilized carmustine suitable for pharmaceutical use. The present invention further provides methods of producing lyophilized carmustine. The pharmaceutical formulations can be used for any disease that is sensitive to treatment with carmustine, such as neoplastic diseases.
Owner:NAVINTA III INC
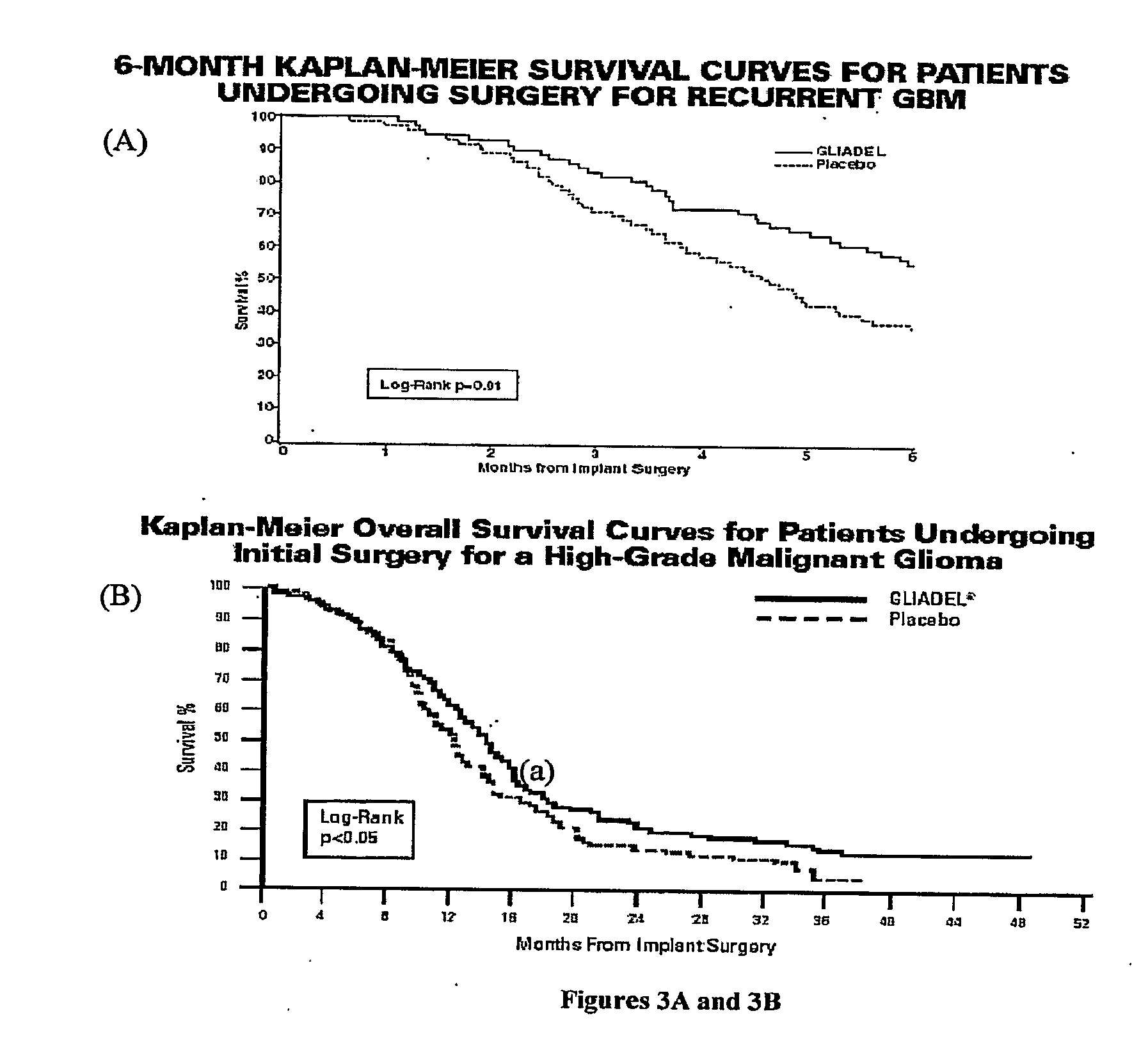
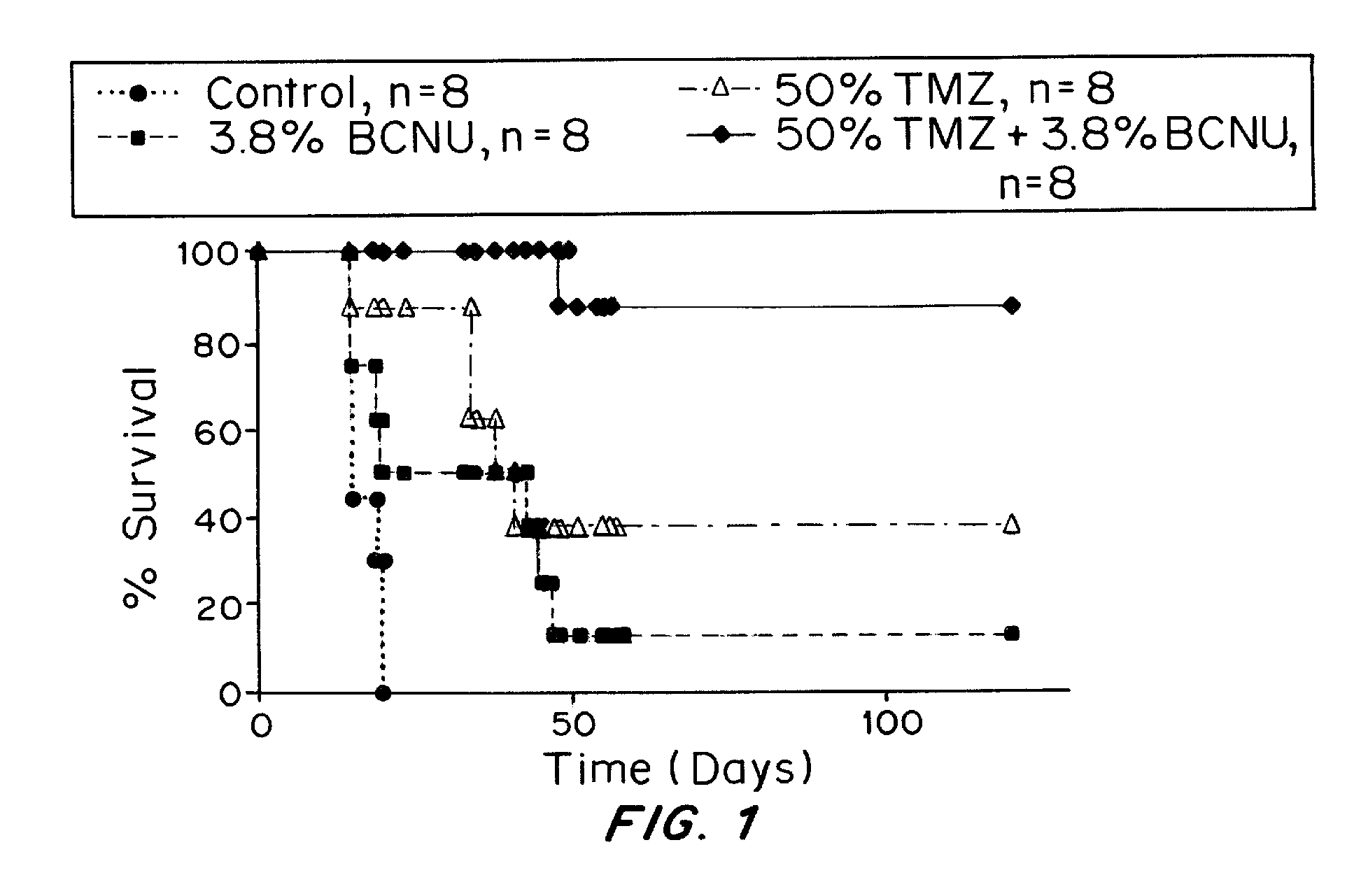
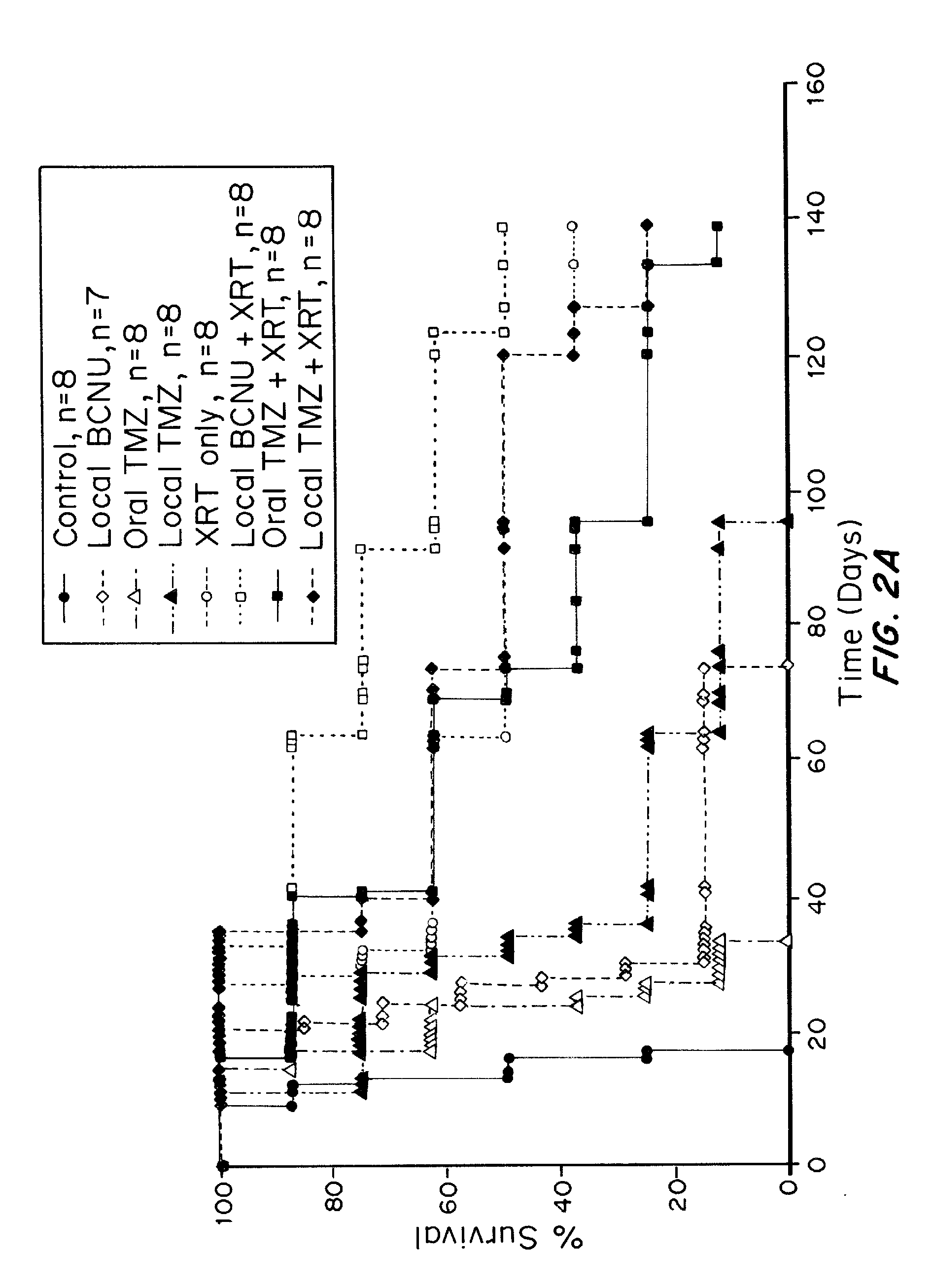
Combination of local temozolomide with local bcnu
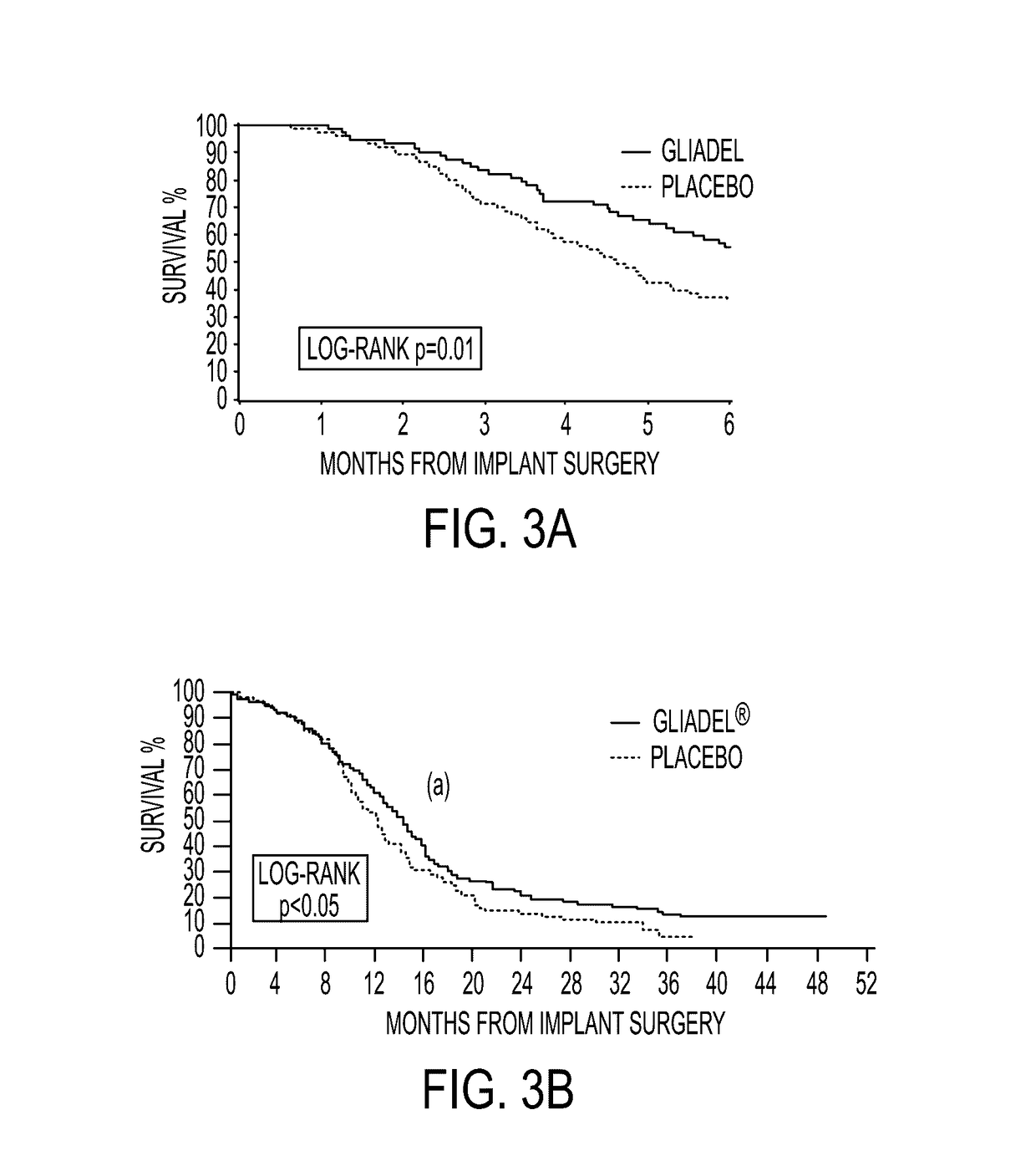
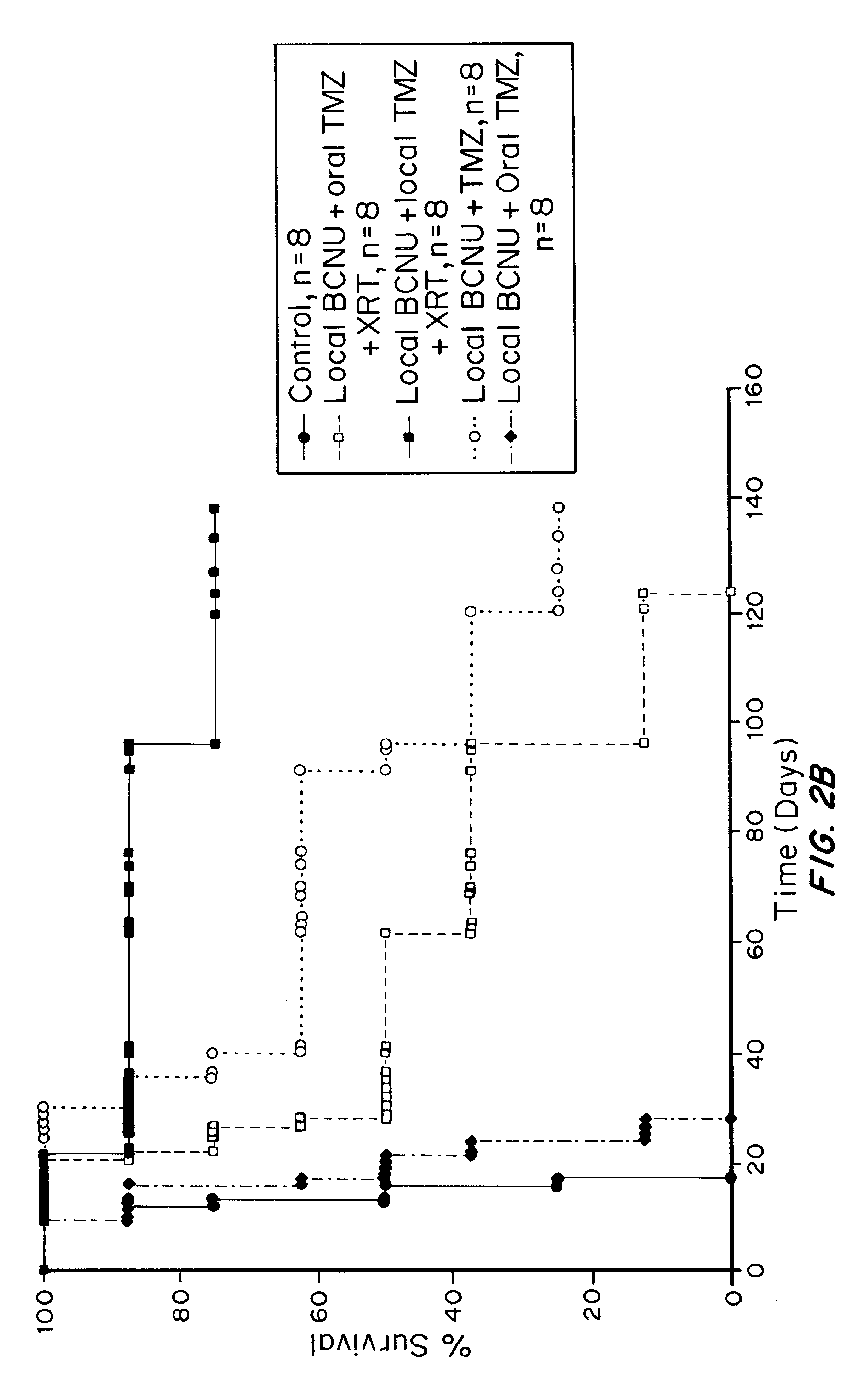
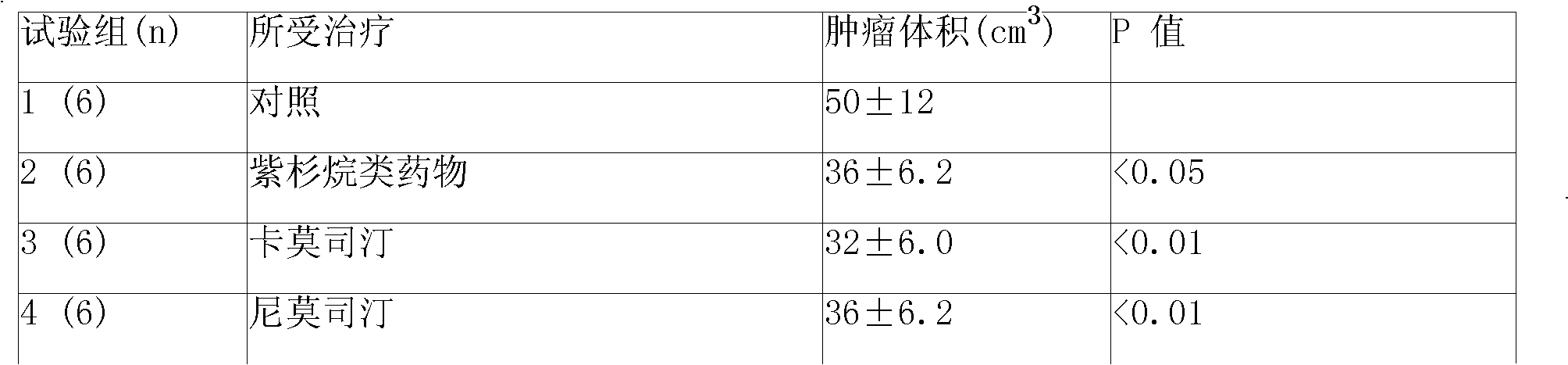
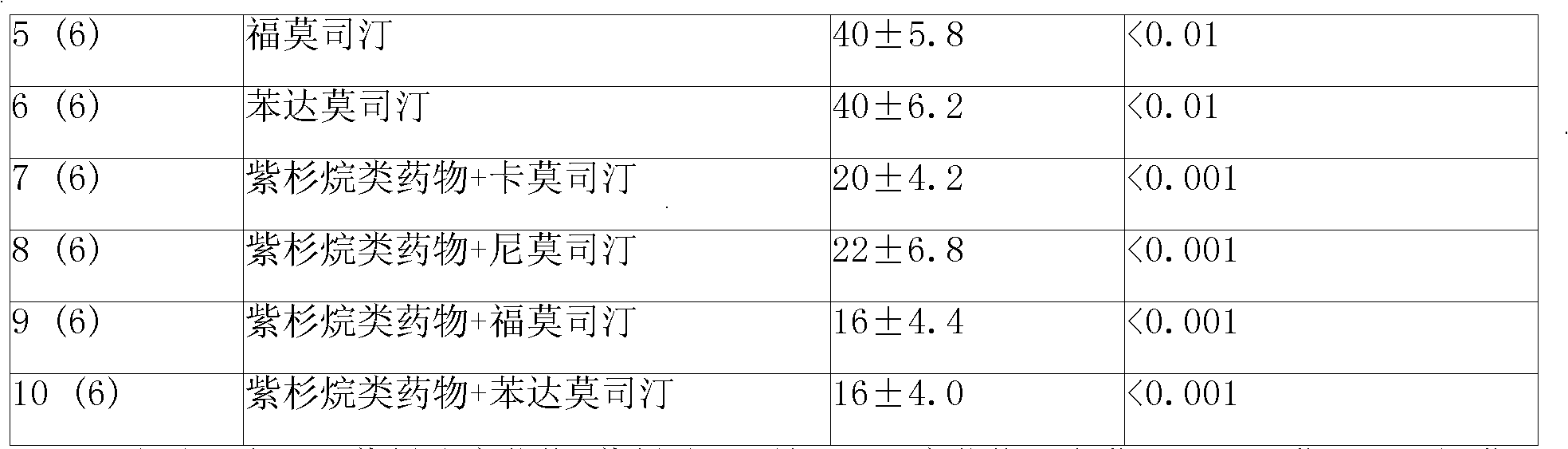
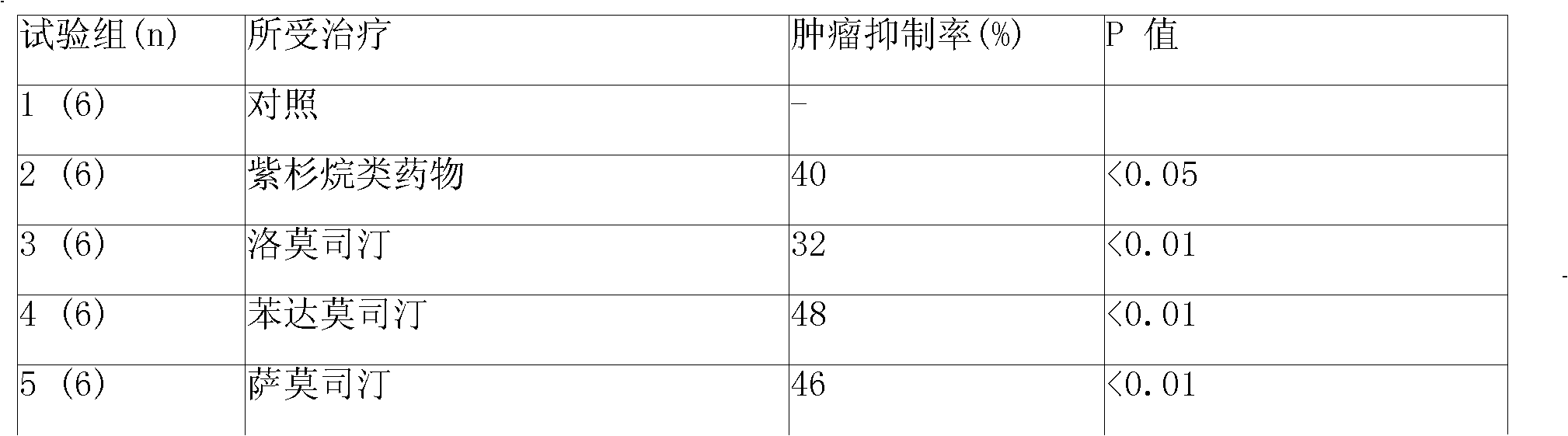
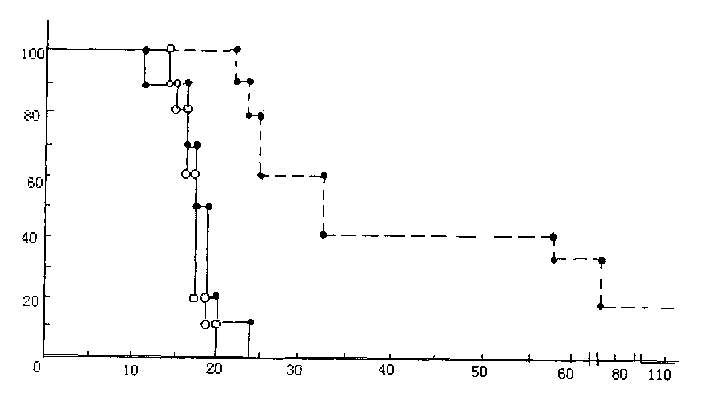
ActiveUS20110313010A1Improve survivalEfficient deliveryBiocideOrganic active ingredientsWhole bodyCarmustine
The additive effect of combined intracranial carmustine (“BCNU”) with intracranial temozolomide (“TMZ”), and particularly in combination with radiation (“XRT”), in the treatment of two rat intracranial glioma models, the 9L gliosarcoma and the F98 glioma, demonstrates that local delivery of both drugs, especially in combination with radiation, is far more effective than delivery of either drug alone or one systemically and one locally, either with or without radiation. The triple therapy showed a significant improvement in survival when compared to controls (p=0.0004), local BCNU (p=0.0043), oral TMZ (p=0.0026), local TMZ (p=0.0105), and the combinations of either BCNU and XRT (p=0.0378) or oral TMZ and local BCNU (p=0.0154).
Owner:ACCELERATING COMBINATION THERAPIES
Anticancer sustained-released gel injection and preparation method thereof
InactiveCN101336891APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTumor vesselSolvent
The invention relates to an anticancer slow-release gel injection and a preparation method thereof. The anticancer slow-release gel injection contains a taxane-like drug such as paclitaxel, docetaxel, hydroxypaclitaxel, epi-paclitaxel or deacetylpaclitaxel, a stine-like drug, an amphiphilic block polymer and a solvent. The composition exhibits the property of temperature-sensitive gelation, is a flowable liquid at the room temperature and turns into a stagnant and biodegradable water-insoluble gel in vivo in warm-blooded animals. The stine-like drug is selected from atrimustine, ambnmustine, nimustine, bendamustine, carmustine, elmustine, galamustine, fotemustine, estramustine, hesmustine, neptamustine, lomustine, semustine, ranimustine, semustine, tauromustine, tallimustine and spiromustine. The anticancer slow-release gel injection can release the drug slowly and locally around the tumor and retain the effective blood concentration for a plurality of weeks to a plurality of months, can not only kill tumor cells but also effectively inhibit tumor vessels, can reduce the general drug toxicity and can enhance the curative effect of chemotherapy particularly radioactive seed implantation.
Owner:济南基福医药科技有限公司
Application of Honokiol to preparing drugs for preventing or treating intracranial space occupying lesion and intracranial tissues and organs inflammation
ActiveCN102178666AGood anti-inflammatory effectGood effectNervous disorderHydroxy compound active ingredientsHonokiolCarmustine
The invention relates to the technical field of medicines and aims at solving the technical problem about providing a new effective choice for preventing or treating intracranial space occupying lesion and intracranial tissues and organs inflammation. The scheme for solving the technical problem provides an application of Honokiol to preparing drugs for preventing or treating intracranial space occupying lesion and intracranial tissues and organs inflammation. The invention further provides the application of Honokiol combined with carmustine (BCNU) to preventing or treating primary or metastatic tumors of brain, and the application prospect is bright.
Owner:CHENGDU JINRUI FOUND BIOTECH CO LTD
Sustained-release injection containing nitrosourea drugs
The invention provides a sustained-release injection containing nitrosourea drug (galamustine), which contains sustained-release microspheres and solvents. The sustained-release microspheres each comprise an anticancer-active component selected from nitrosourea drugs (such as nimustine and carmustine) and / or topoisomerase inhibitors, and a sustained-release agent. The solvents are common solvents or special solvents containing suspending agent. The viscosity of the suspending agent ranges from 100cp to 3000cp (at a temperature ranging from 20 DEG C to 30 DEG C). The suspending agent is selected from sodium carboxymethylcellulose and the like. The sustained-release agent is selected from p(LAEG-EOP) or p(DAPG-EOP) or other polyphosphate ester copolymers, or copolymer or blend of polyphosphate ester and PLA, polifeprosan, PLGA or poly(erucidic acid dipolymer-sebacic acid). The topoisomerase inhibitor is selected from camptothecin, hydroxycamptothecine, topotecan, lartotecan, irinotecan, etoposide or teniposide. The anticancer composition is also available in the dosage form of sustained-release implant, can retain the effective drug concentration for more than 60 days after intratumoral or local injection or implantation, can obviously reduce the systemic reaction to the drug, and can selectively enhance the curative effect of non-operative treatments such as radiotherapy and chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Carmustine sustained-release implantation agent for curing entity tumour
InactiveCN101176707APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTreatment effectWhole body
The invention relates to a slow-release implant agent of carmustine for tumor treatment, which is characterized in comprising effective amounts of carmustine, slow-release excipients and releasing regulatory agents in the slow-release implant agent. The excipients comprise macromolecules which are biologically compatible and degradable, mainly p (LAEG-EOP) and p (DAPG-EOP). The releasing regulatory agents are selected from one or more items from mannitol, sorbitol, xylitol, oligosaccharide, chitin, potassium salt, sodium salt, hyaluronic acid, collagen, chondroitin, gelatin and albumin. The slow-release implant agent slowly releases carmustine on the local tumor in the process of degradation and absorption, so the argent can evidently reduce the systemic toxicity and simultaneously apply to the effective drug concentration control on the local tumor. Therefore, the argent can be applied separately or combined with the non-surgical treatments such as chemotherapy drug and radiotherapy, which can be also widely used for tumor treatment of different phases, not only selectively improving the drug concentration on local tumor but also reinforcing the therapeutic effect of non-surgical treatments such as chemotherapy drug and radiotherapy.
Owner:SHANDONG LANJIN PHARMA
Carmustine slow-release tablet and its preparation method
InactiveCN1524580AControllable Release BehaviorRelieve painPharmaceutical non-active ingredientsAmide active ingredientsCarmustineDrug release
The invention relates to a carmustine slow release diaphragm and method for making same, which is a medical arrangement characterized by macromolecular compound carrying agent or antitumor agent, wherein carmustine and degradable high molecular polymer are used to form films to be implanted into tumor position through operation, the polymer is degraded, thus the medicament releases slowly at a finite speed to achieving the goal of localized treatment.
Owner:天津市第一中心医院 +1
Carmustine sustained-release implantation agent for curing entity tumour
InactiveCN101176713AIncreased sensitivityHigh clinical application valuePharmaceutical delivery mechanismPharmaceutical non-active ingredientsWhole bodyPotassium
The invention relates to a slow-release implant agent of carmustine, which is characterized by effective amounts of carmustine, slow-release excipients and releasing regulatory agents in the slow-release implant agent. The excipients comprise macromolecules which are biologically compatible and degradable, mainly PLA and PLGA. The releasing regulatory agents are selected from one or more items from mannitol, sorbitol, xylitol, oligosaccharide, chitin, potassium salt, sodium salt, hyaluronic acid, collagen, chondroitin, gelatin and albumin. The slow-release implant agent slowly releases carmustine on the local tumor in the process of degradation and absorption, so the argent can evidently reduce the systemic toxicity and simultaneously apply to the effective drug concentration control on the local tumor. Therefore, the argent can be applied separately or combined with the non-surgical treatments such as chemotherapy drug and radiotherapy, which can be also widely used for tumor treatment of different phases, not only selectively improving the drug concentration on local tumor but also reinforcing the therapeutic effect of non-surgical treatments such as chemotherapy drug and radiotherapy.
Owner:SHANDONG LANJIN PHARMA
Slow-release anticarcinogen contg. carmustine and fluorouracil
InactiveCN100998558AEasy injectionIncreased sensitivitySolution deliveryPharmaceutical non-active ingredientsAnticarcinogenCisplatin
A slow-release anticancer medicine in the form of injection or implant contains carmustine and its synergist chosen from 5-FU, cisplatin and 06-benzylguanine. For said slow-release injection, it is composed of the slow-release microballs containing the active anticancer component and slow-releasing auxiliary and the special solvent containing suspending aid.
Owner:SHANDONG LANJIN PHARMA +1
Carmustine powder injection and preparation technology thereof
ActiveCN102198100AThe tumor shrinks significantlyGood curative effectPowder deliveryPharmaceutical non-active ingredientsSide effectCholesterol
The invention relates to a pro-liposome carmustine powder injection and a preparation process thereof. The carmustine powder injection mainly comprises, by weight, 1.5 to 3 % of carmustine, 15 to 20% of granulesten, 4 to 6% of cholesterol and 71.1 to 79.5% of mannitol crystalline powders, wherein the granulesten comprises, by weight, 65 to 70% of phosphatidylcholine and 14 to 20% of phosphatidylethanolamine. The carmustine powder injection prepared by the preparation process is easy to disperse in water and convenient in storage, and has an entrapment rate of 100%, a small particle size, improved stability, small toxic and side effects and enhanced safety.
Owner:天津易诺利和科技有限公司
Carmustine pharmaceutical composition
Owner:NAVINTA III INC
Meningeoma nursing medicine and preparation method thereof
InactiveCN103721189ANot easy to relapseLittle side effectsNervous disorderInorganic active ingredientsMonkshoodsCarboplatin
The invention discloses a meningeoma nursing medicine and a preparation method thereof. The nursing medicine is a formula formed by combining traditional Chinese medicine and western medicine, wherein the western medicine comprises the following components: dichloro diethylamine, cyclophosphamide, ifosfamide, melphalan, chlorambucil, thiotepa, mitomycin, busulfan, lomustine, carmustine, temozolomide and carboplatin. The traditional Chinese medicine comprises the following components: Oldenlandia diffusa, dangshen, honey-fried licorice root, fructus forsythiae, realgar, edible tulip, Schisandra chinensis, ginseng, soroseris, beautyberry leaf, radix curcumae, hawthorn, pheretima, gastrodia elata, Minoru thorns, Rehmannia Glutinosa, Ligusticum wallichii, Angelica sinensis, Polygonum cuspidatum, bamboo leaf, radix semiaquilegiae, red flower, white paeony root, pubescent holly root, fried monkshood, herba eupatorii, ambergris, fingered citron, Radix Aucklandiae, radix scutellariae, radix bupleuri and serrate rabdosia herb; the meningeoma nursing medicine has the advantages of obvious treatment effect, low disease relapsing rate and low side effect.
Owner:刘玉含
Melphalan sustained-release implant treating for solid tumor
InactiveCN101204366AOrganic active ingredientsPharmaceutical delivery mechanismTherapeutic effectOligosaccharide
The invention relates to a sustained-release implant for treating a solid tumor, which is characterized in that: the sustained-release implant contains an effective anticancer amount of Melphalan and sustained-release regulator. The sustained-release excipient is the soluble degradable absorbable macromolecular polymer of organism, and the sustained-release excipient is mainly PLA and PLGA. The release regulating agent is chosen from a combination or a plurality of combinations of mannitol, sorbic alcohol, xylitol, oligosaccharide, chitin chitosan, potassium salt, sodium salt, hyaluronic acid, collagen, chondroitin, gelatin and albumin. The sustained-release implant can release carmustine to the part of the tumor in the process of the degradation and absorption; the effect medicine concentration can be maintained in the part of the tumor while the systemic toxic reaction is decreased obviously, therefore the sustained-release implant can be used independently or used with the other non-operative treatment such as the chemotherapeutics and the radiation therapy, etc., and the invention can be used for treatment of solid tumor in each period. The medicine concentration in the part of the tumor can be selectively improved, and effect of non-operative treatment such as the chemotherapeutics and the radiation therapy can be strengthened.
Owner:JINAN SHUAIHUA PHARMA TECH
Carmustine sustained-release implant for treating solid tumor and preparation method thereof
InactiveCN104523566APlay a clinical therapeutic effectSufficient concentrationOrganic active ingredientsPharmaceutical delivery mechanismLactideBrain metastatic tumors
The invention relates to a carmustine sustained-release implant for treating solid tumor and a preparation method thereof. The implant is characterized in that the sustained-release implant comprises a sustained-release auxiliary material and an effective anti-tumor component carmustine, wherein the sustained-release auxiliary material is a lactide-glycolide copolymer, the viscosity range of the copolymer is 0.3-0.6, the weight percentage of carmustine is 7.5-30% and the mass proportion of lactide to glycolide is 50: 50. The invention further relates to a method for preparing the sustained-release implant and an application of the implant for treating solid tumors such as pancreatic cancer, lung cancer, liver cancer, breast cancer, brain tumor, esophagus cancer, renal carcinoma, colorectal cancer and the like, liver and brain metastatic tumors. The sustained-release period of the implant is 3-4 weeks, wherein the accumulated release in previous two days is 5-12% of the total drug loading capacity and the accumulated release in previous 12 days is greater than 40% of the total drug loading capacity but less than 60% of the total drug loading capacity.
Owner:SHANDONG LANJIN PHARMA
Pharmaceutical composition of carmustine and its application in biomedicine
PendingCN106256834ANovel structureHas hypoglycemic effectOrganic active ingredientsMetabolism disorderDiabetes mellitusNatural product
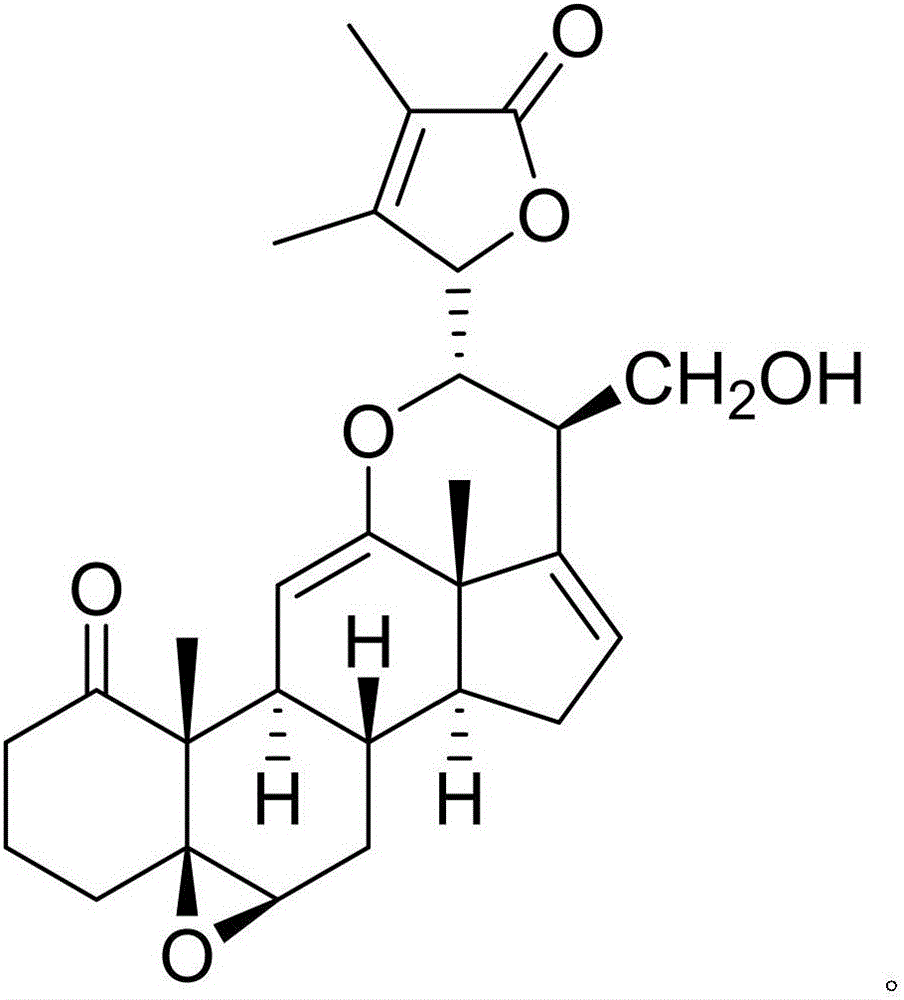
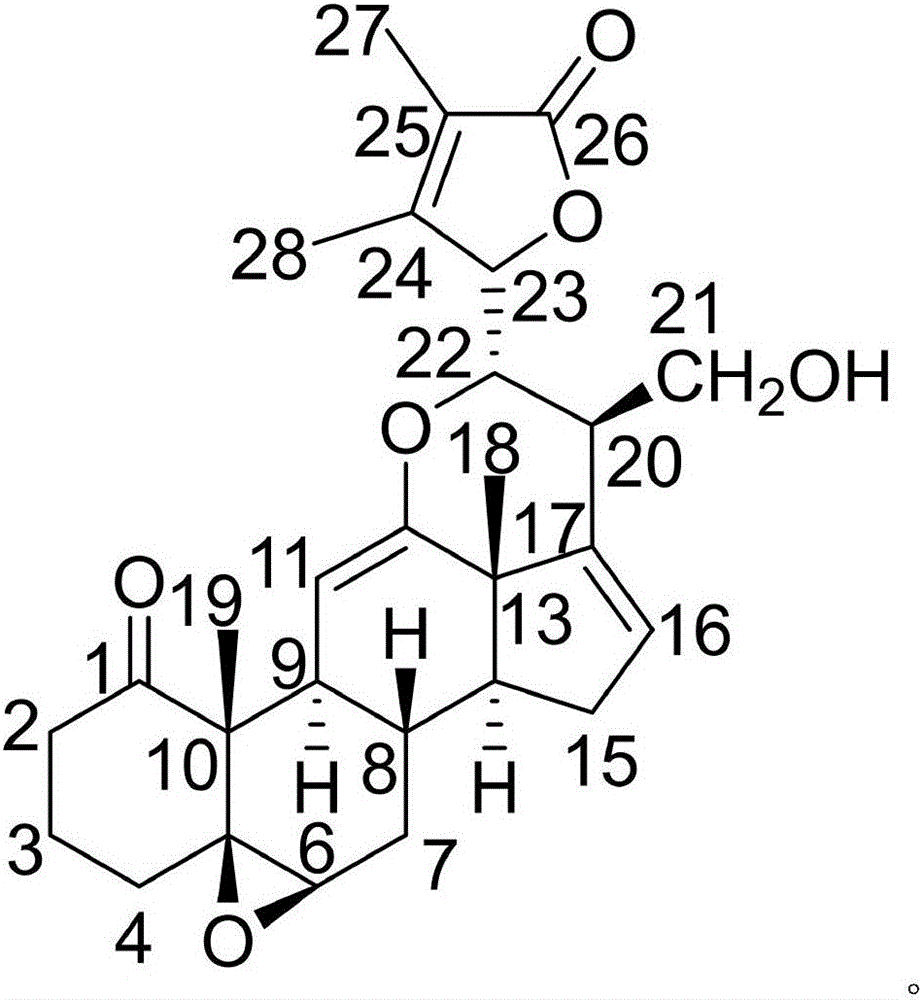
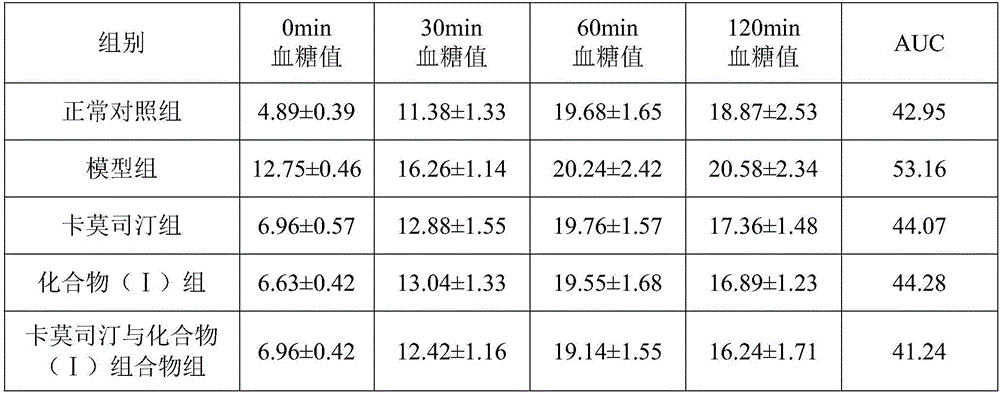
The invention discloses a pharmaceutical composition of carmustine and its application in biomedicine. The pharmaceutical composition of carmustine provided by the invention contains carmustine and a novel natural product compound (I ), the combination of carmustine and compound (I) can significantly improve the glucose tolerance of diabetic model mice, inhibit the activity of α-glucosidase, and improve the anti-oxidative damage ability of diabetic model mice. The activity of the combination of carmustine and compound (I) is stronger than the activity of carmustine or compound (I) alone, and can be developed into a drug for treating diabetes. Compared with the prior art, it has outstanding substantive characteristics and significant improvement.
Owner:黄亦琼
Slow release injection containing platinum compound and alkylating agent
InactiveCN101011345APharmaceutical delivery mechanismPharmaceutical non-active ingredientsCarboplatinAdjuvant
Disclosed is a slow release injection containing platinum-group compounds and / or alkylating agents, which comprises slow release microspheres and dissolvent, the slow release microspheres include platinum-group compounds selected from Tegafur, Capecittabine, Pemetrexed, Carboplatin or Gemcitabine, and / or alkylating agent anticancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with PLA, Polifeprosan, poly(dodecanedioic acid-tetradecanedioic acid) or poly(fumaric acid-sebacylic acid). The alkylating agent is selected from Carmustine, Nimustine, Fotemustine, Lomustine or bendamustine. The anticancer composition can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Docetaxel-containing anti-cancer sustained-release injection
InactiveCN101234085AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolSuspending Agents
The invention relates to anticancer sustained release injection which comprises sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise anticancer active components and sustained release auxiliary material; the menstruum is special menstruum that contains suspending agent. The anticancer active components are fotemustine, nimustine, carmustine or combination of bendamustine and mitozolomide, docetaxel, etoposide, teniposide, vinblastine, anastrozole, tamoxifen, fluorouracil or mitomycin C; the sustained release auxiliary material is polylactic acid and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer of polyethylene glycol, terminal carboxyl group polylactic acid copolymer, EVAc, fatty acid and decanedioic acid copolymer, etc.; viscosity of the suspending agent is 100cp-3,000cp (at 25 DEG C-30 DEG C), and the suspending agent is selected from sodium carboxymethylcellulose, etc. The sustained release microspheres can also be made into sustained release implant; the injection or implant is injected or placed in or around tumor so as to reduce general reaction of the drug and selectively improve and keep local concentration for about 30-50 days. The anticancer sustained release injection can be used solely and can also promote anti-tumor effects of non-operative treatments, such as chemotherapy and / or radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Anti-cancer composition loading both mtrosourea medicament and synergist
InactiveCN101011346APharmaceutical delivery mechanismPharmaceutical non-active ingredientsMitozolomideTreatment effect
Disclosed is a slow release injection agent of anticancer composition containing nitrosourea drugs and synergistic agent, which comprises slow release microspheres and dissolvent, wherein the slow release microspheres comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the nitrosourea drugs are selected from Carmustine, Nimustine or Fotemustine, the synergistic agent can be selected from tetrazine drugs such as Mitozolomide or temozolomide and / or anticancer antibiotics such as Adriamycin, Aclarubicin, Epirubicin, mitomycin or pidorubicin, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with polylactic acid, Polifeprosan, sebacylic acid and PLGA. The anticancer composition can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com