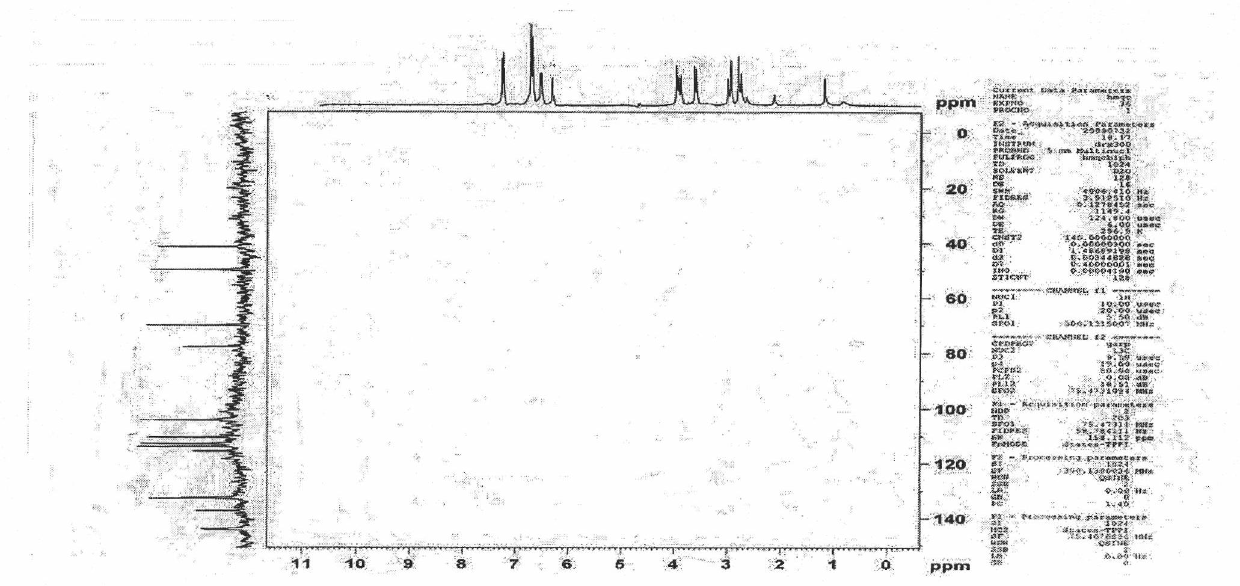
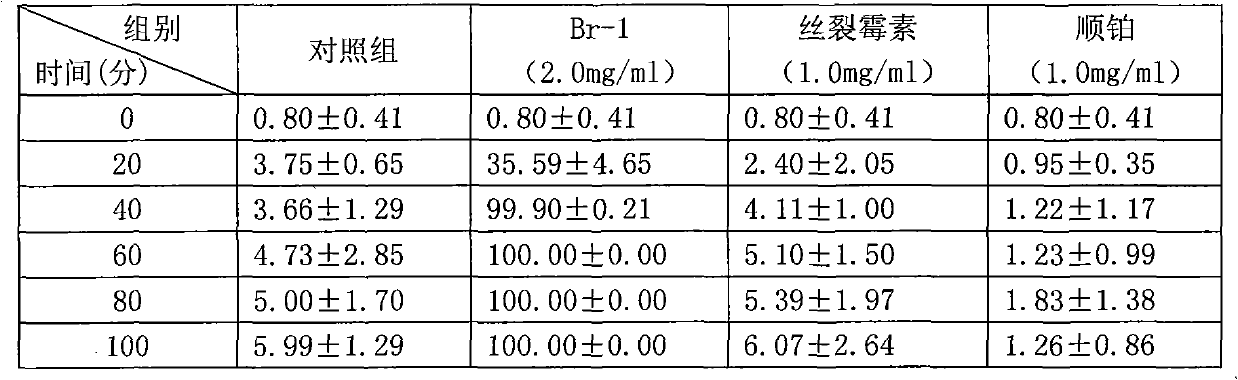
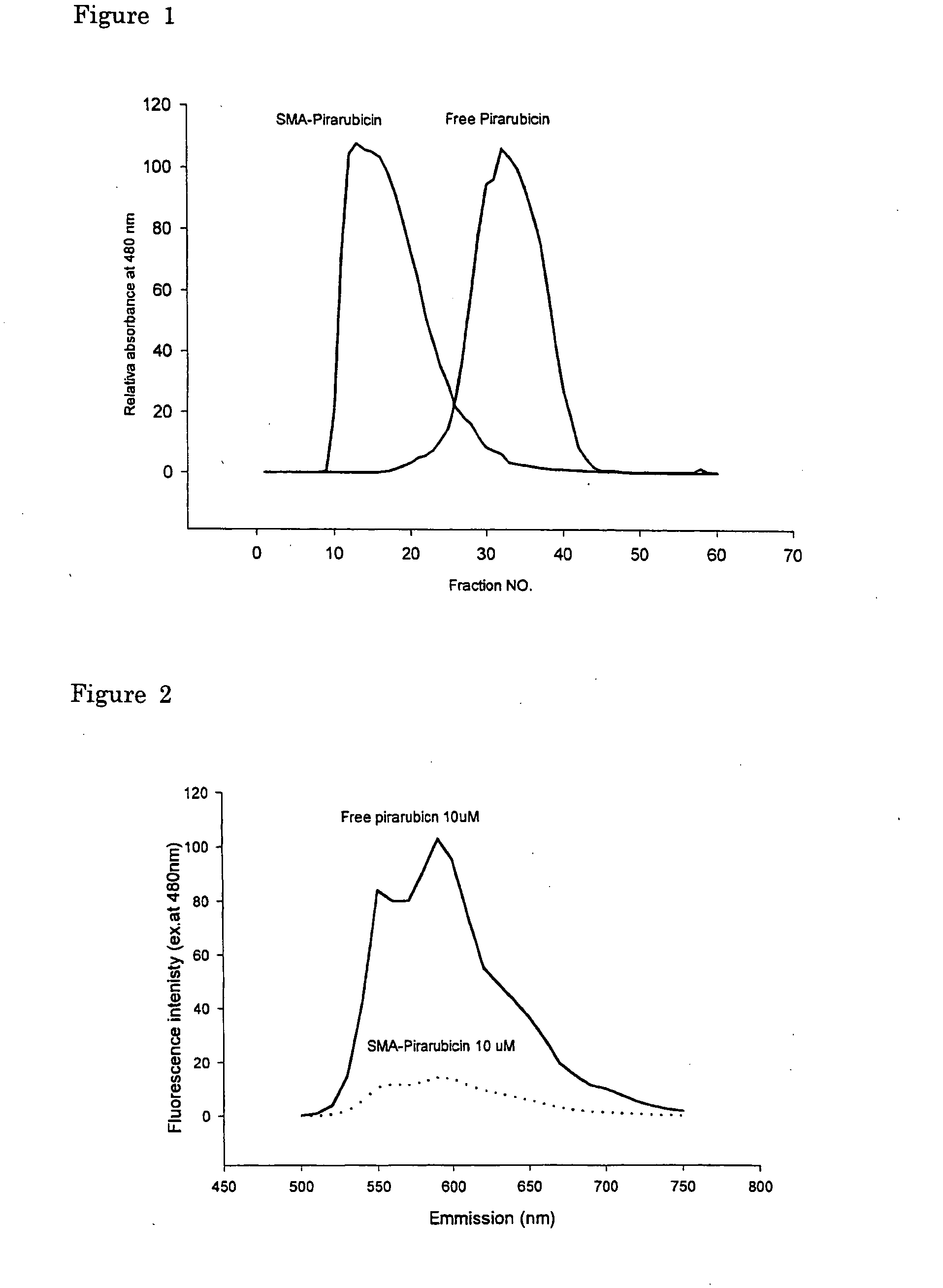
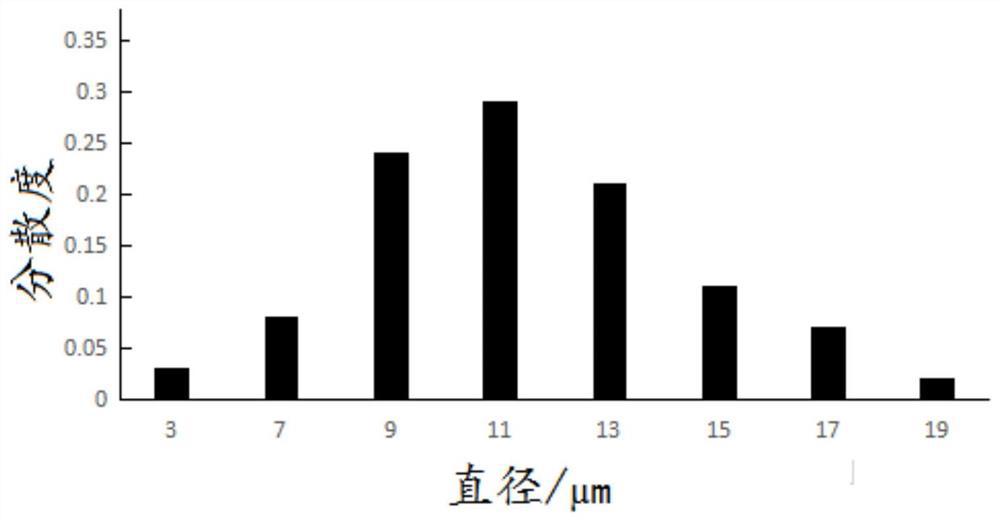
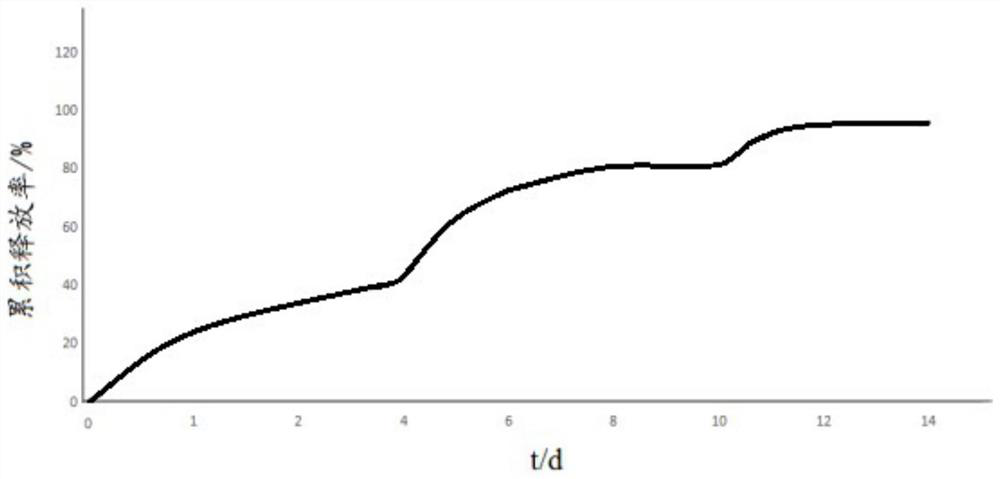
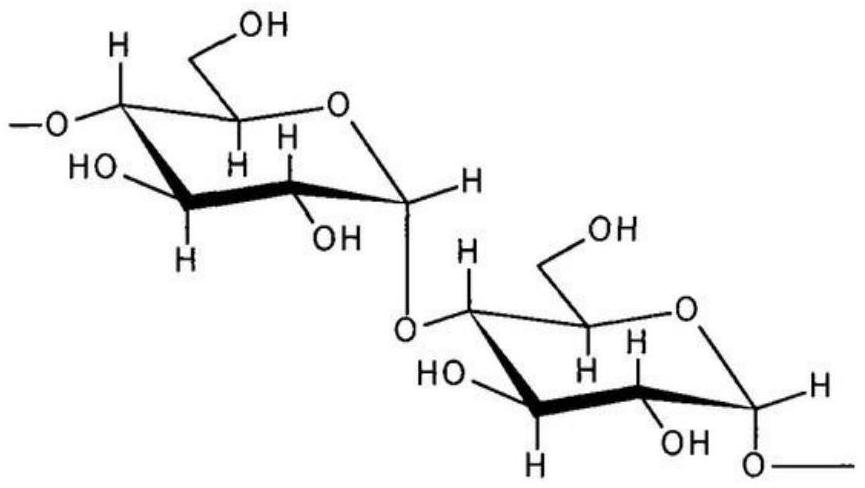
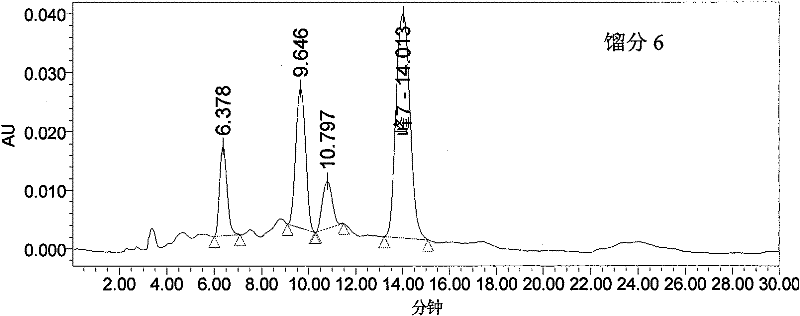
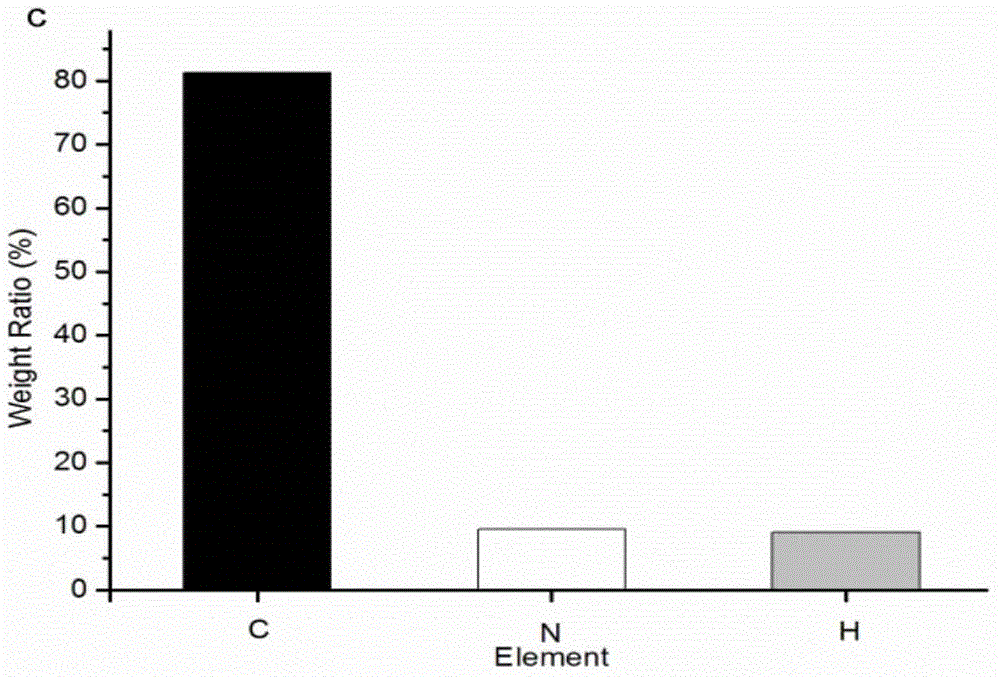
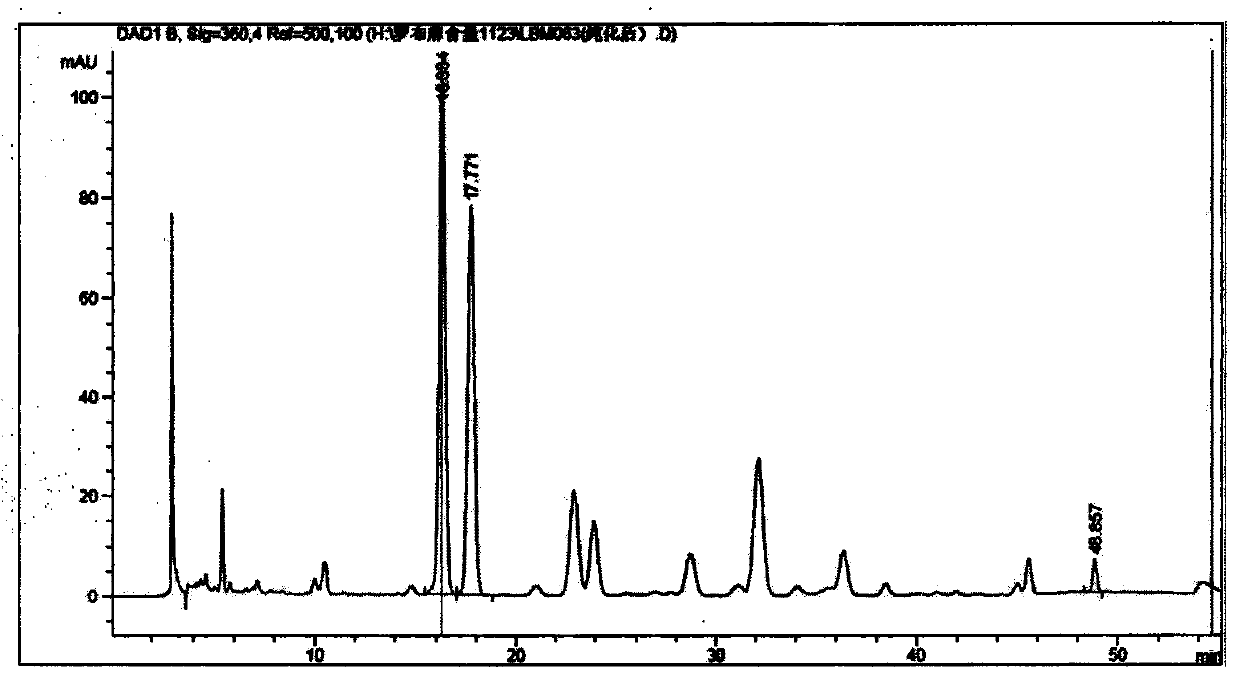
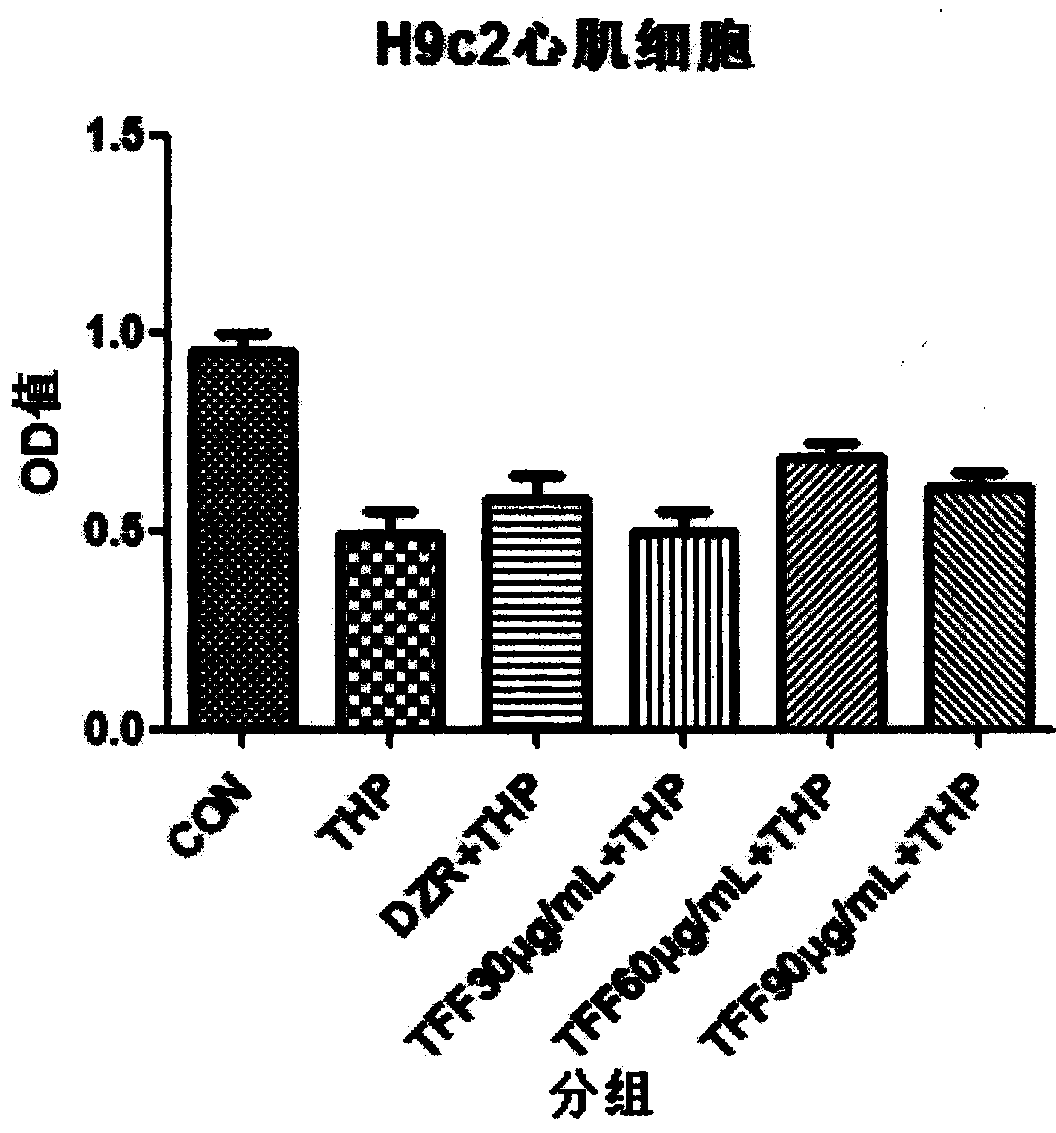
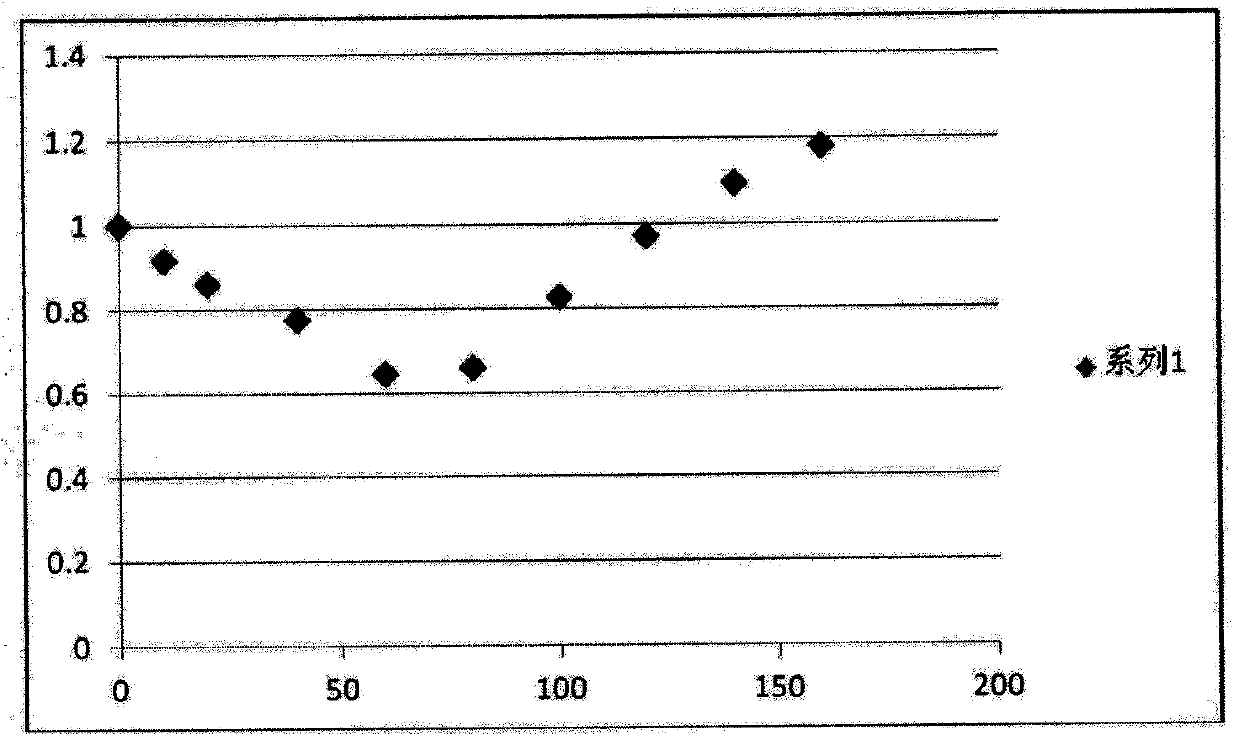
Patents
Literature
36 results about "Pirarubicin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
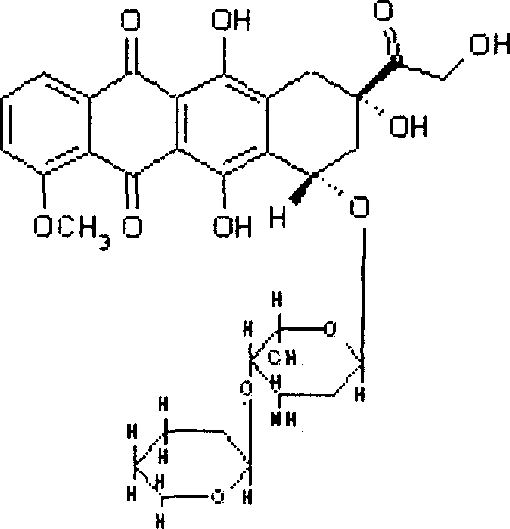
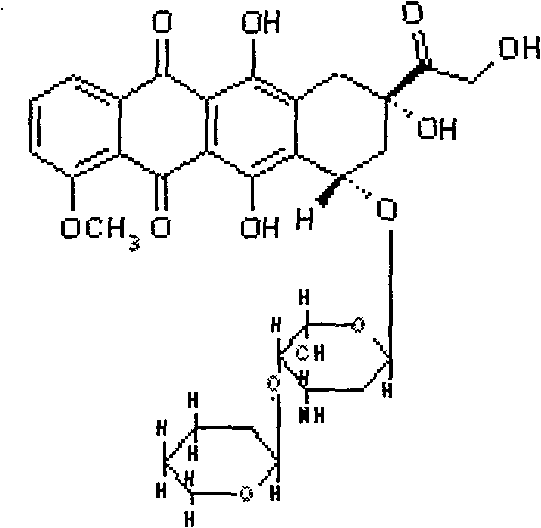
Pirarubicin (INN) is an anthracycline drug. An analogue of the anthracycline antineoplastic antibiotic doxorubicin. Pirarubicin intercalates into DNA and interacts with topoisomerase II, thereby inhibiting DNA replication and repair and RNA and protein synthesis. This agent is less cardiotoxic than doxorubicin and exhibits activity against some doxorubicin-resistant cell lines.
Preparation process for Sappan Wood extract perfusate and application thereof in treating bladder cancer
InactiveCN101904892ANo adverse reactionSignificant clinical effectAntineoplastic agentsPlant ingredientsFreeze-dryingEthyl acetate
The invention relates to a preparation method for a Sappan Wood extract, in particular to a preparation process for Sappan Wood extract perfusate and application thereof in treating bladder cancer, which solves the problem of the application of the Sappan Wood extract in treating bladder cancer tumors. The preparation process comprises the following steps: taking and smashing Sappan Wood, carrying out water extracting for three times, combining the extracting solutions obtained by three-time water extracting, and carrying out decompression concentrating; standing overnight, and removing sediments; adding petroleum ether, extracting, and retaining aqueous phase; adding ethyl acetate, extracting, and removing the aqueous phase; carrying out decompression volatilizing to remove all the ethyl acetate; weighing dry substances, adding purified water to enable the dry substance content to be 2%, heating for dissolving, standing overnight at room temperature, and filtering out sediments; and freeze-drying, and carrying out split charging. The experiment research result shows that the extract has obvious killing effect on bladder neoplasm cells; and compared with mitomycin, epirubicin, Pirarubicin and other anticancer drugs, which are clinically and frequently used, the Sappan Wood extract perfusate has the advantages of high anticancer activity, less toxicity, safety, effectiveness and the like.
Owner:山西华尚汇商贸有限公司
Antitumor agent and process for producing the same
InactiveUS20050208136A1Improving selective solid targeting capacityEliminate side effectsHeavy metal active ingredientsBiocideCancer cellPolyamine
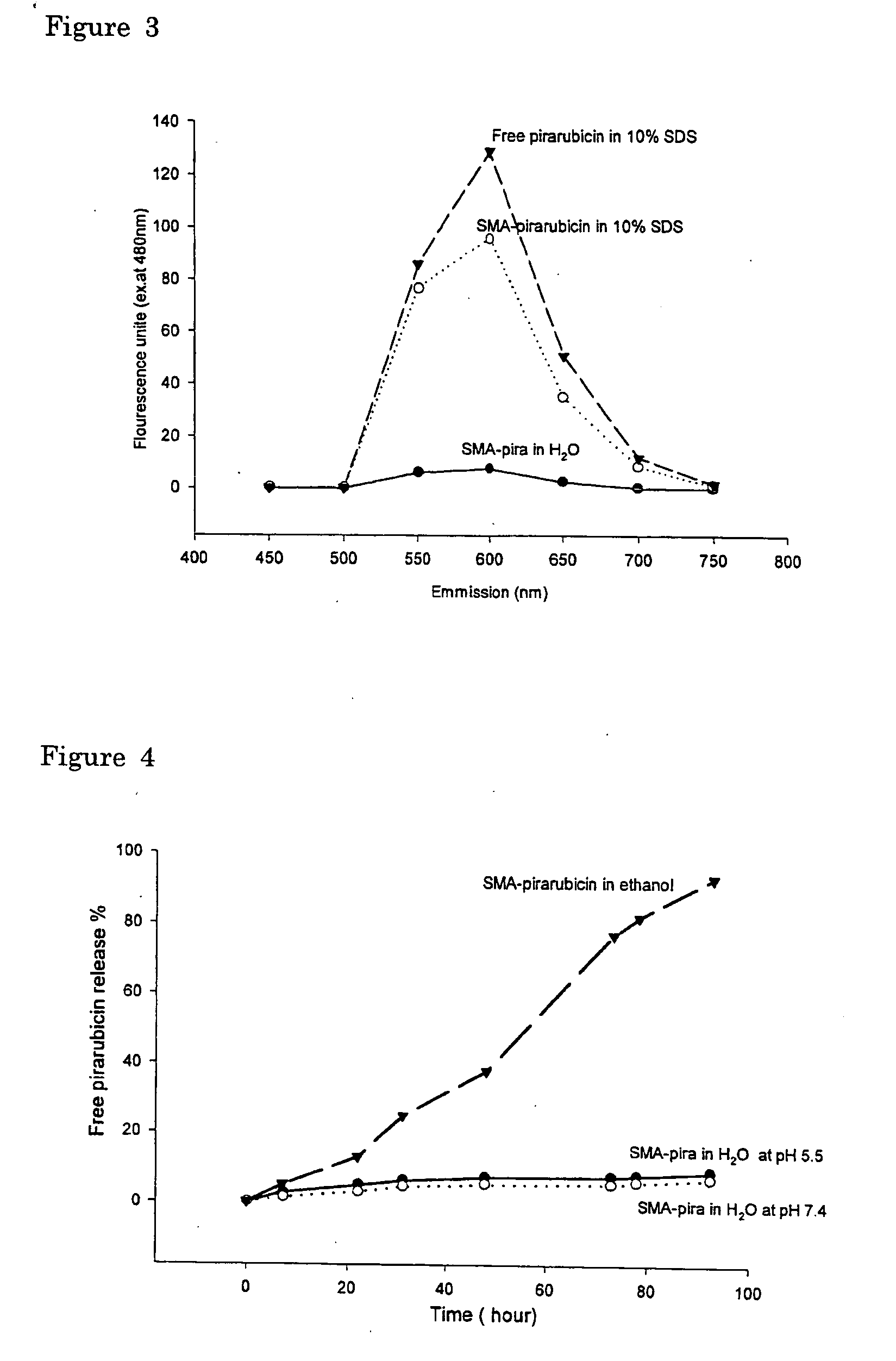
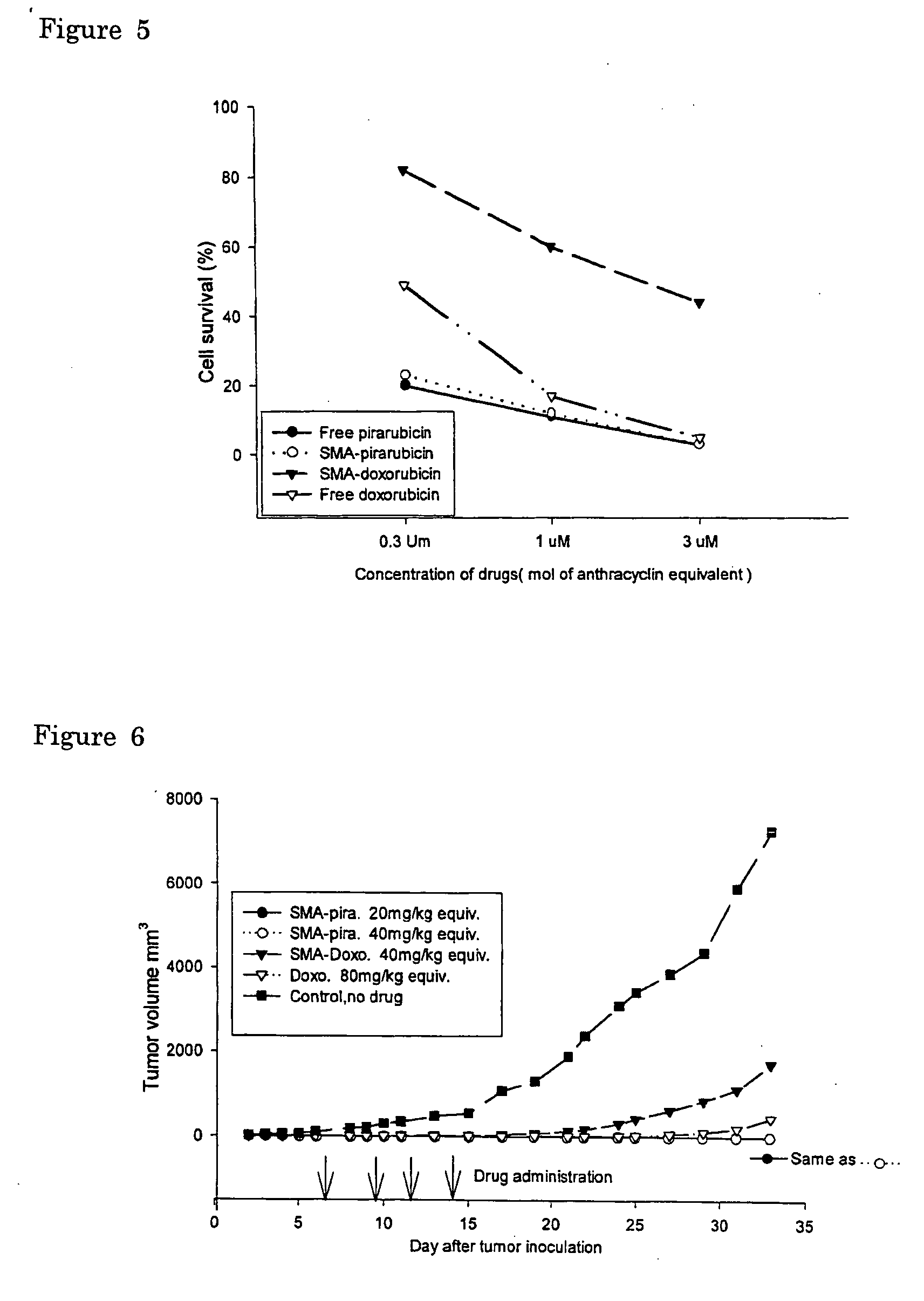
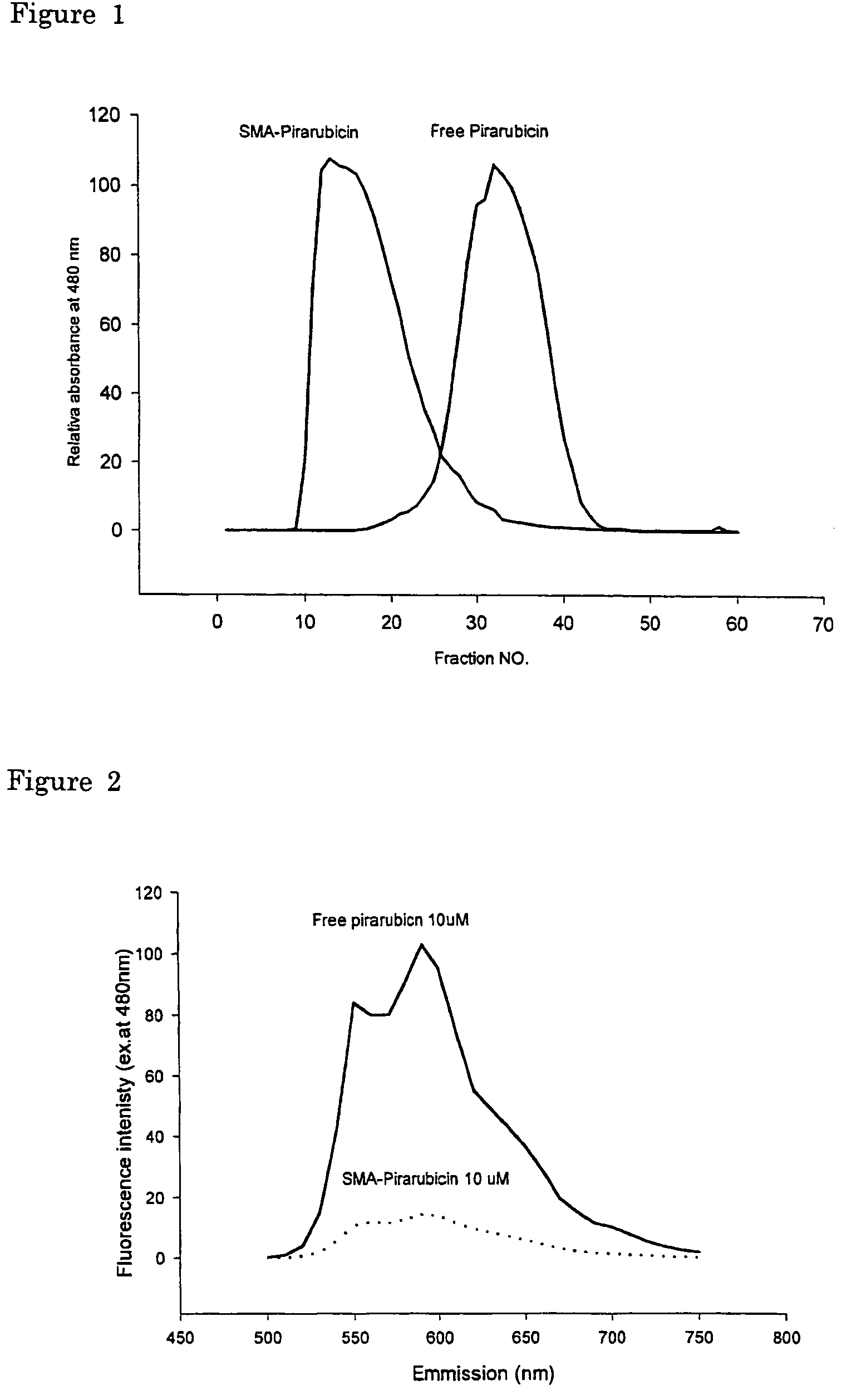
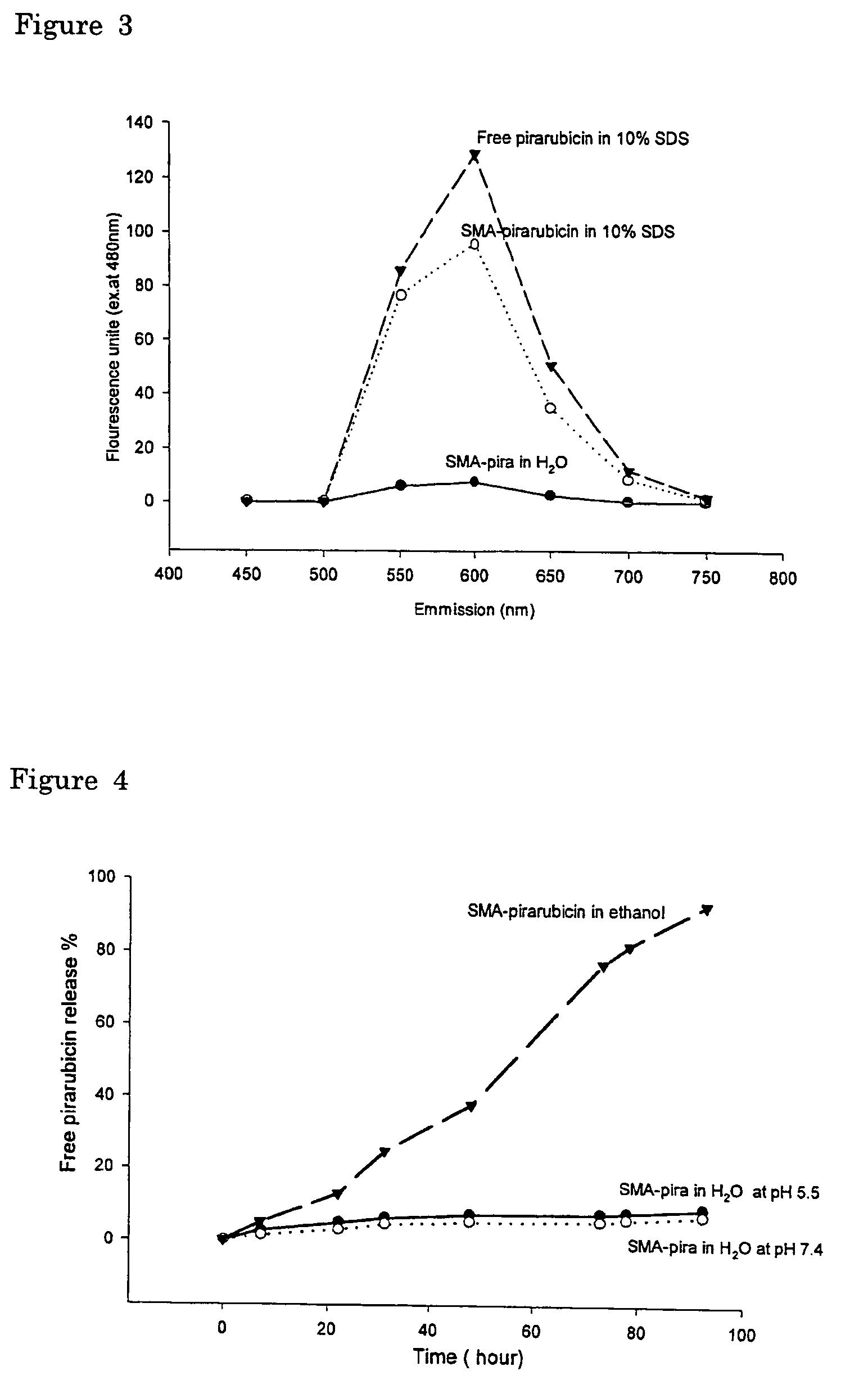
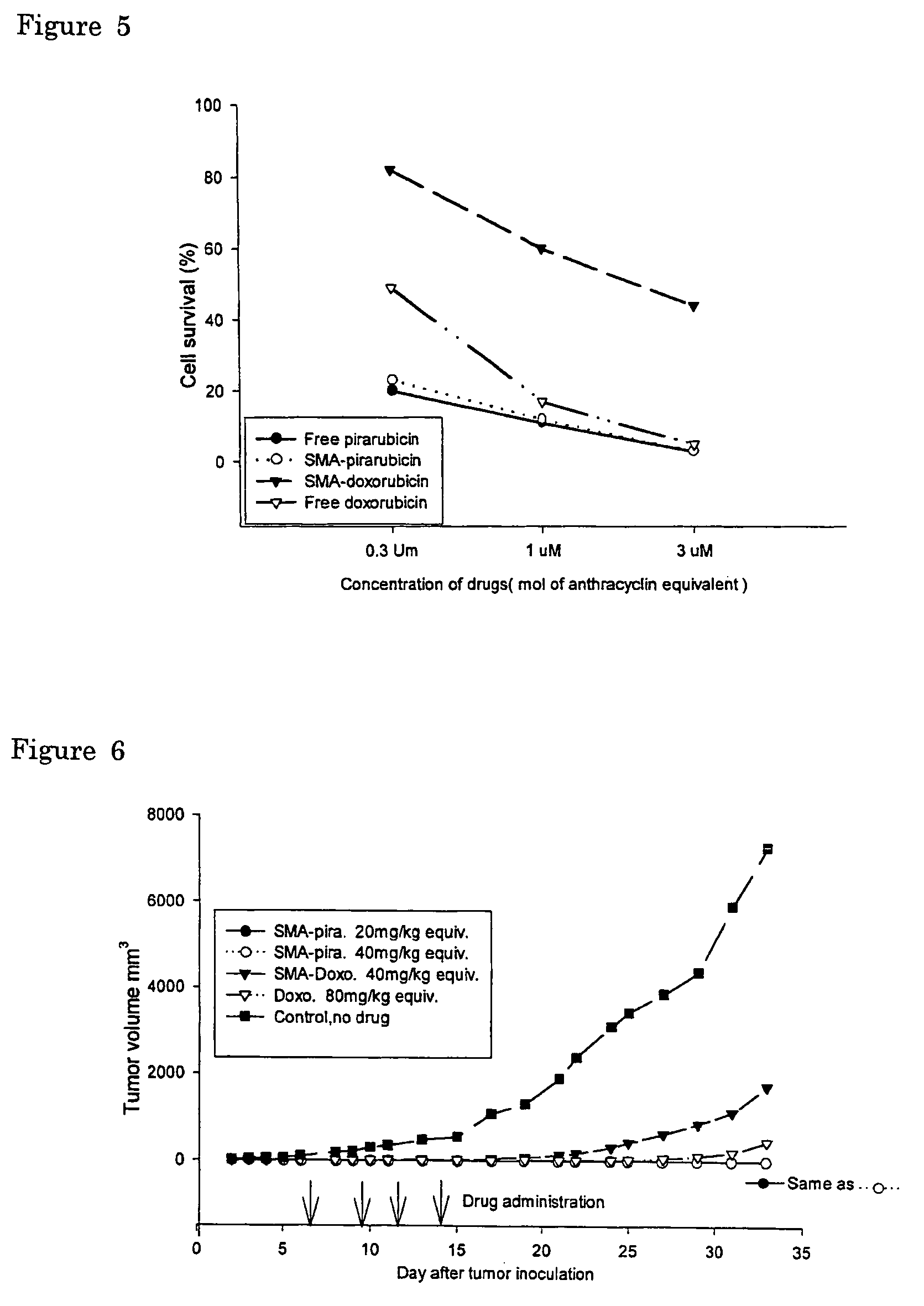
The present invention relates to polymeric antitumor agent which is formed in polymeric micelle complex by intermolecular bonding or mutual interaction between styrene maleic acid copolymer (SMA) and low molecule antitumor agent which is anthracyclins drug such as pirarubicin, doxorubicin, epirbicin, daunorbicin, acralbicin, or alkaloid antitumor agent such as cis-platinum, and taxol These polymeric antitumor agents may improve specificity to cancer cells so that it improves antitumor effect, while it may not be concentrated at normal organ or tissue, so that adverse effect may be diminished. These polymeric antitumor agents may be prepared by dissolving SMA and low molecule antitumor agent in aqueous solution, then in the presence of aqueous soluble carbodiimide, amino acids, or polyamine, adjusting pH to form micelle complex and recovering polymer fraction.
Owner:MAEDA HIROSHI
Anti-cancer drugs slow release agent comprising anticancer antibiotics and booster thereof
Disclosed is an anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer antibiotics are selected from Idarubicin, Valtaxin, Pirarubicin and Mitoxantrone, The anti-metabolite drugs are selected from Pemetrexed, Carmustine, Tegafur, Zalcitabine, Emtritabine, Galocitabine, Ibacitabine, Ancitabine, Decitabine, Flurocitabine, Enocitabine, Imidazoletabine, Capecittabine, Gemcitabine, Fludrarbine, Raltitrexed, Dexrazoxane, Cladribine, Nolatrexed and folic acid, The slow release auxiliary materials are selected from EVAc, Polifeprosan, sebacylic acid copolymer, lactic acid, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The slow release microspheres can also be prepared into slow release implanting agent for injection or placement in or around tumor.
Owner:SHANDONG LANJIN PHARMA
Slow released anticancer medicine preparation with both amrubicin and its synergist
The slow released anticancer medicine injection containing both amrubicin and its synergist consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent carboxymethyl cellulose, etc. and with viscosity of 100-3000 cp at 25 deg.c. The effective anticancer component is amrubicin, idarubicin, etc and / or antimetabolite composition selected from carmofur, tegafur, zalcitabine, etc. The slow releasing supplementary material is selected from EVAc, sebacic acid copolymer, lactic acid polymer, etc. The slow released microsphere may be also prepared into slow released implanting agent set around or inside the tumor to strengthen the chemotherapy or radiotherapy effect.
Owner:JINAN KANGQUAN PHARMA TECH
Compound recipe anti-cancer drugs slow release agent comprising anticancer antibiotics and booster thereof
Disclosed is a compound anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer effective ingredients include Aclarubicin, Idarubicin, Doxorubicin, Epirubicin, Valtaxin, Pirarubicin, Losaxantrone, Losoxantrone and / or anticancer antibiotic synergistic agents selected from phosphoinositide-3-kinase inhibitor, pyrimidine analogues and / or DNA restoration enzyme inhibitor, the slow release auxiliary materials are selected from polylactic acid copolymer EVAc, or sebacic acid copolymer, the viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C). The slow release microspheres can also be prepared into slow release implanting agent for lowering down the whole body toxicity reaction of the medicament when locally dispensing on the tumor, and for selectively increasing the tumor local medicinal concentration.
Owner:SHANDONG LANJIN PHARMA
Method for synthesizing polyvinyl alcohol embolization microspheres capable of loading chemotherapeutic drug pirarubicin
InactiveCN108236737AHigh drug loadingSimple methodOrganic active ingredientsSurgical adhesivesSulfonateTransarterial embolization
The invention relates to a method for synthesizing polyvinyl alcohol embolization microspheres capable of loading a chemotherapeutic drug pirarubicin. According to the present invention, based on theproblem of the loading of the chemotherapeutic drug pirarubicin by the existing polyvinyl alcohol embolization microspheres, a purpose of the present invention is to solve the disadvantage of the lowpirarubicin loading of the polyvinyl alcohol embolization microspheres; the technical scheme comprises that a ratio of a functionalized macromolecular hydrogel (Calli-B) to a drug-loading group 2-acrylamide-2-methyl propyl sodium sulfonate is 1.00:0.12, an axial flow type stirring paddle is selected, the stirring speed is controlled at 450-500 rpm, and the temperature of the reaction system is controlled at 48-60 DEG C when a polymer monomer solution is added; and by researching the use amount ratio of the drug-loading group 2-acrylamide-2-methyl propyl sodium sulfonate and the process, the problem of the loading of the pirarubicin in the polyvinyl alcohol embolization microspheres is solved, the microspheres can achieve a certain drug loading amount according to the requirements, and thedrug-loading microspheres can continuously release pirarubicin to the targeting tumor area at the tumor site, and are used for the drug-eluting bead transarterial chemoembolization on liver cancer.
Owner:SUZHOU HENGRUI CALLISYN BIOLOGICAL MEDICINE TECH CO LTD
Temperature controlled sustained-release injection containing anti-cancer medicine
InactiveCN101273965APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectVinorelbine
The invention relates to a temperature-controlled sustained-release injection containing an anti-cancer drug, which consists of the anti-cancer drug and an amphiphilic block copolymer hydrogel and has the temperature-sensitive gelatinization feature, the temperature-controlled sustained-release injection is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, thus allowing the drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the temperature-controlled sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be vincristine, vinorelbine, navelbine, vindesine, vinleurosine, vinrosidine, cephalotaxine, bleomycin, daunomycin, aclarubicin, epirubicin, idarubicin, pirarubicin, valrubicin, mitomycin C, actinomycin D, losoxantrone, mitoxantrone, mitozolomide, temozolomide and so on.
Owner:SHANDONG LANJIN PHARMA +1
Antitumor agent and process for producing the same
InactiveUS7682630B2Improving selective solid targeting capacityEliminate side effectsBiocideHeavy metal active ingredientsCancer cellDaunorubicin
The present invention relates to polymeric antitumor agent which is formed in polymeric micelle complex by intermolecular bonding or mutual interaction between styrene maleic acid copolymer (SMA) and low molecule antitumor agent which is anthracyclins drug such as pirarubicin, doxorubicm, epirbicin, daunorbicin, acralbicin, or alkaloid antitumor agent such as cis-platinum, and taxol These polymeric antitumor agents may improve specificity to cancer cells so that it improves antitumor effect, while it may not be concentrated at normal organ or tissue, so that adverse effect may be diminished. These polymeric antitumor agents may be prepared by dissolving SMA and low molecule antitumor agent in aqueous solution, then in the presence of aqueous soluble carbodiimide, amino acids, or polyamine, adjusting pH to form micelle complex and recovering polymer fraction.
Owner:MAEDA HIROSHI
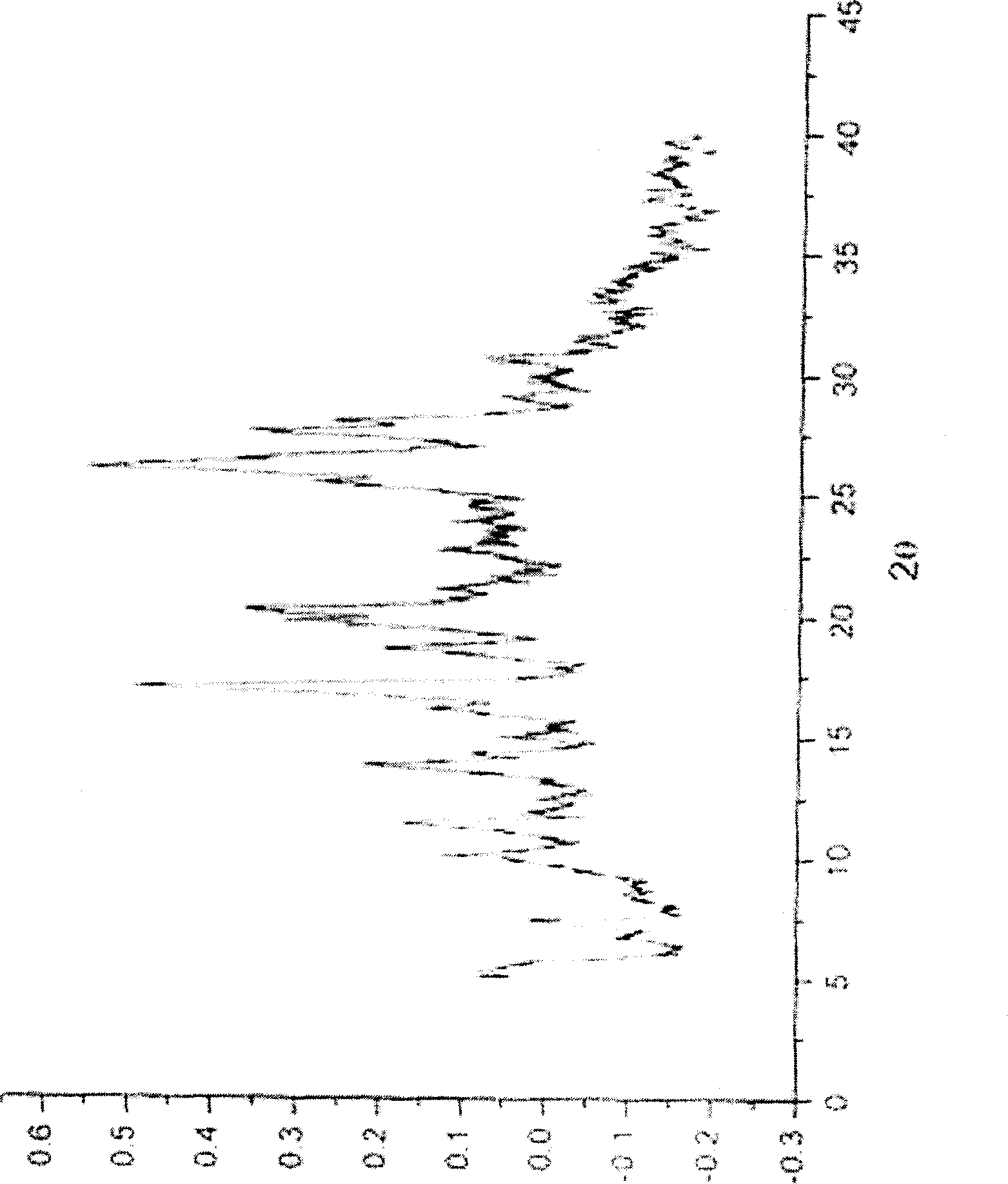
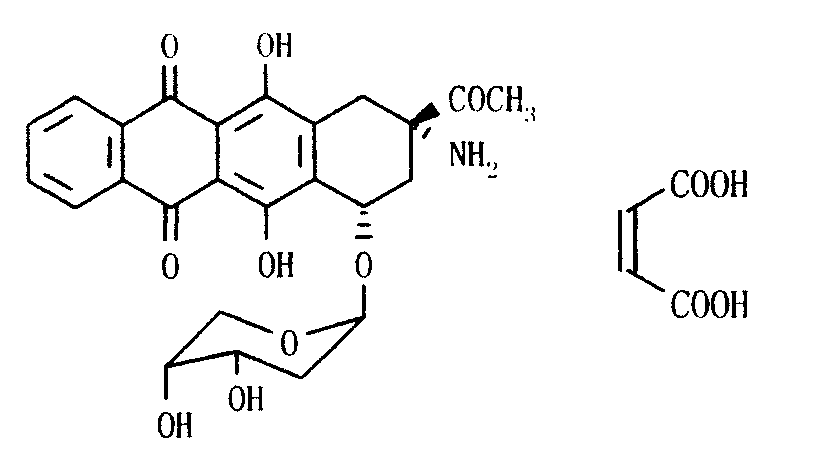
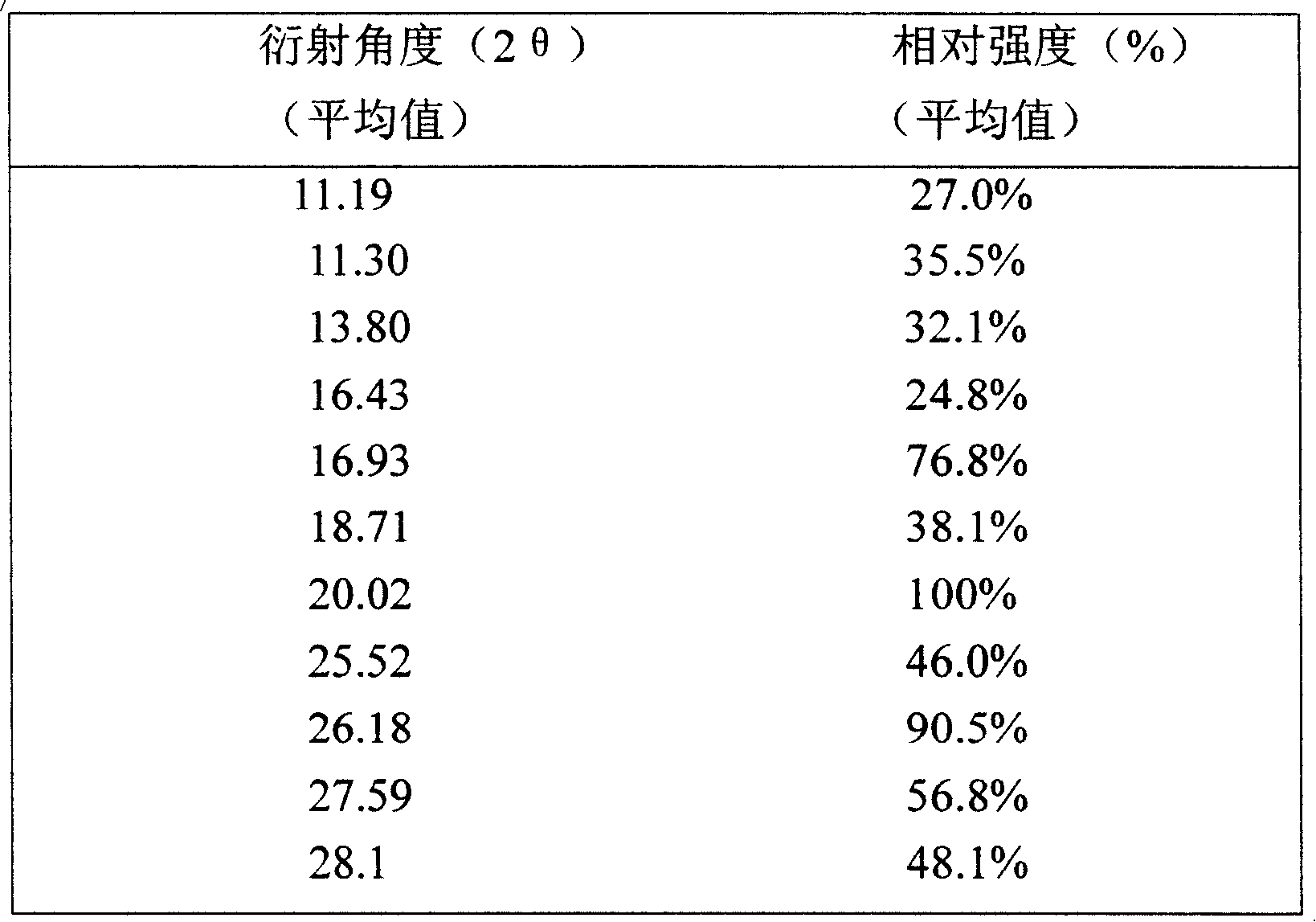
Ammonia maleate rubicin salt, and its preparing method and use
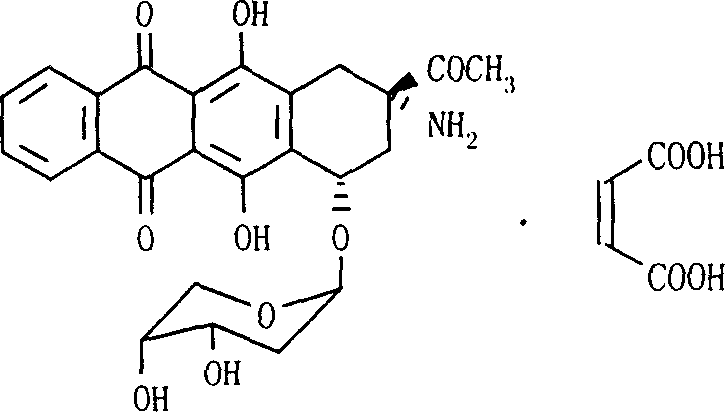
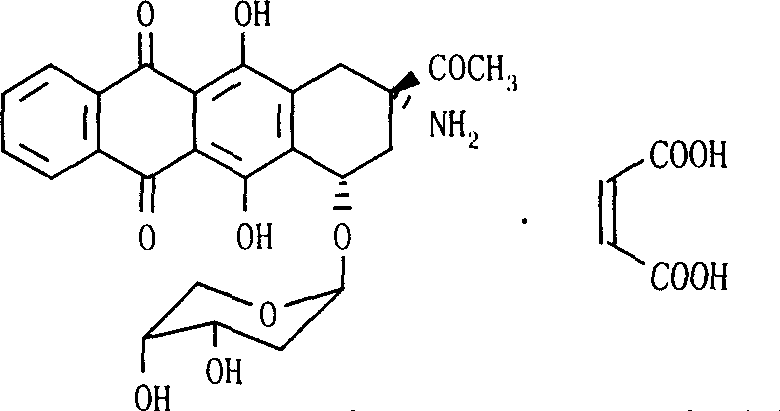
The invention relates to maleic acid ammonia pirarubicin salt and its preparation method, and its application in antineoplastics and pharmaceutical preparation. The preparation method includes reaction in solvent, freeze drying, or crystallizing.
Owner:JIANGSU HANSOH PHARMA CO LTD
Preparation and application of molecular site-directed targeted and activated short peptide adriamycin
ActiveCN106344930ABroad-spectrum inhibitionReduced chemotherapy toxicityOrganic active ingredientsPharmaceutical non-active ingredientsThreonineTert-leucine
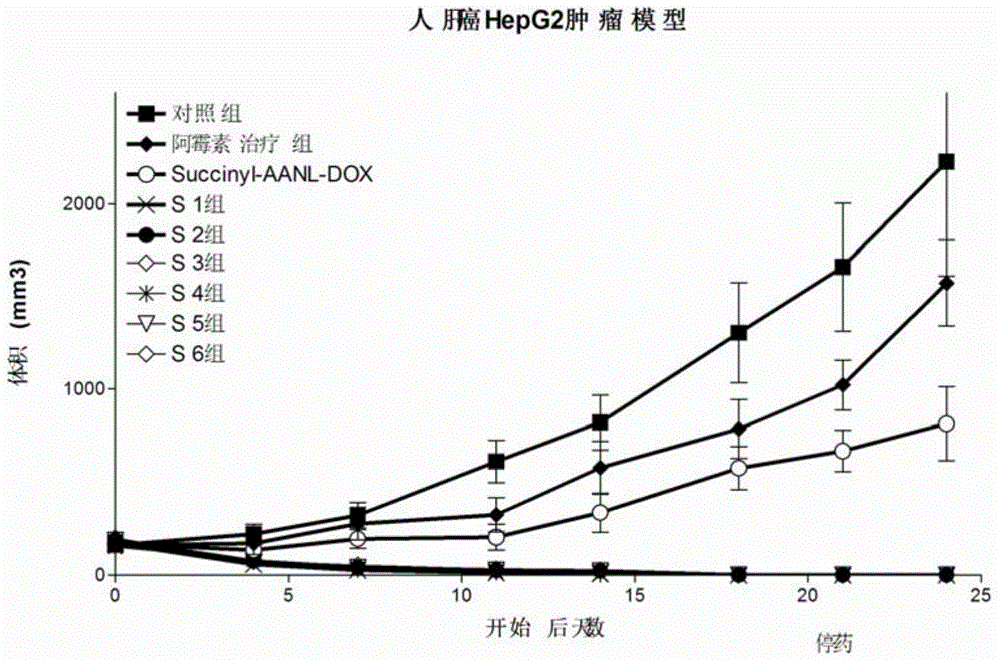
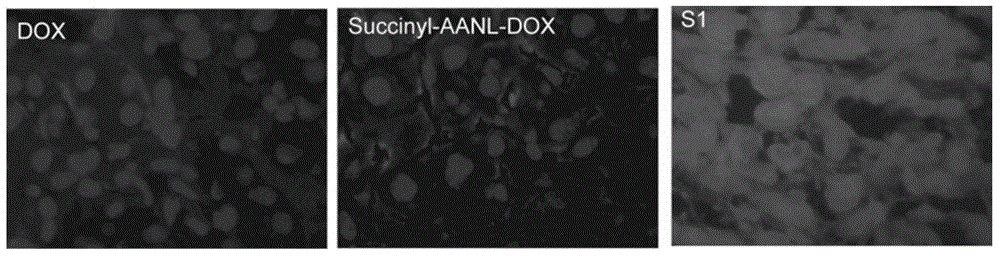
The invention relates to preparation and application of molecular site-directed targeted and activated short peptide adriamycin, in particular to a compound of the following formula I or pharmaceutically acceptable salt of the compound, a medicine composition of the compound and application of the compound in preparation of a medicine for treating or preventing cancers or cancer transferring. In the formula I, X is polar and nonpolar amino acids without charges, such as glycine, alanine, valine, leucine, isoleucine, serine, cysteine, methionine, asparaginate, glutamine and threonine; Z is adriamycin, pharmorubicin or pirarubicin; Z is connected with the lactose-XANL in the formula I through the amino of Z.
Owner:YAFEI (SHANGHAI) BIOLOG MEDICINE SCI & TECH CO LTD
Instant pyrobixi freeze-drying powdery injection and its production
An instant freeze-dried powder injection of pirarubicin is prepared from pirarubicin, excipient and cosolvent through proportionally dissolving them in the water for injection, aseptic filtering, loading in containers, and freeze-drying.
Owner:深圳万乐药业有限公司
Pirarubicin sustained-release implant treating for solid tumor
InactiveCN101204368AOrganic active ingredientsPharmaceutical delivery mechanismTreatment effectWhole body
The invention relates to a Pirarubicin sustained-release implant for treating a solid tumor, which is characterized in that: the sustained-release implant contains an effective anticancer amount of Pirarubicin and sustained-release excipients and a certain amount of sustained-release regulator. The solid tumors include the esophageal canner, the gastric canner, the breast canner, the ovarian canner, the prostate canner, the bladder canner, the liver canner, the lung canner, and the rectum canner. The sustained-release excipients are mainly a copolymer of glycollic acid and hydroxyacetic acid and one of the two materials, polifeprosan and poly-(L-lactide-co-ethyl phosphonate), or a combination of the two materials, in the process of degradation and absorption of which the Pirarubicin is sustainedly released to part of the tumor, thus the entire toxicity of the Pirarubicin is significantly reduced while an effective medicine consistency is maintained on part of the tumor. That the sustained-release implant is implanted inside part of the tumor can not only reduce the entire toxicity of the Pirarubicin but also enhance the medicine consistency on part of the tumor, thereby increasing the curing effect of non-operative therapeutics such as chemotherapeutic drugs and radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
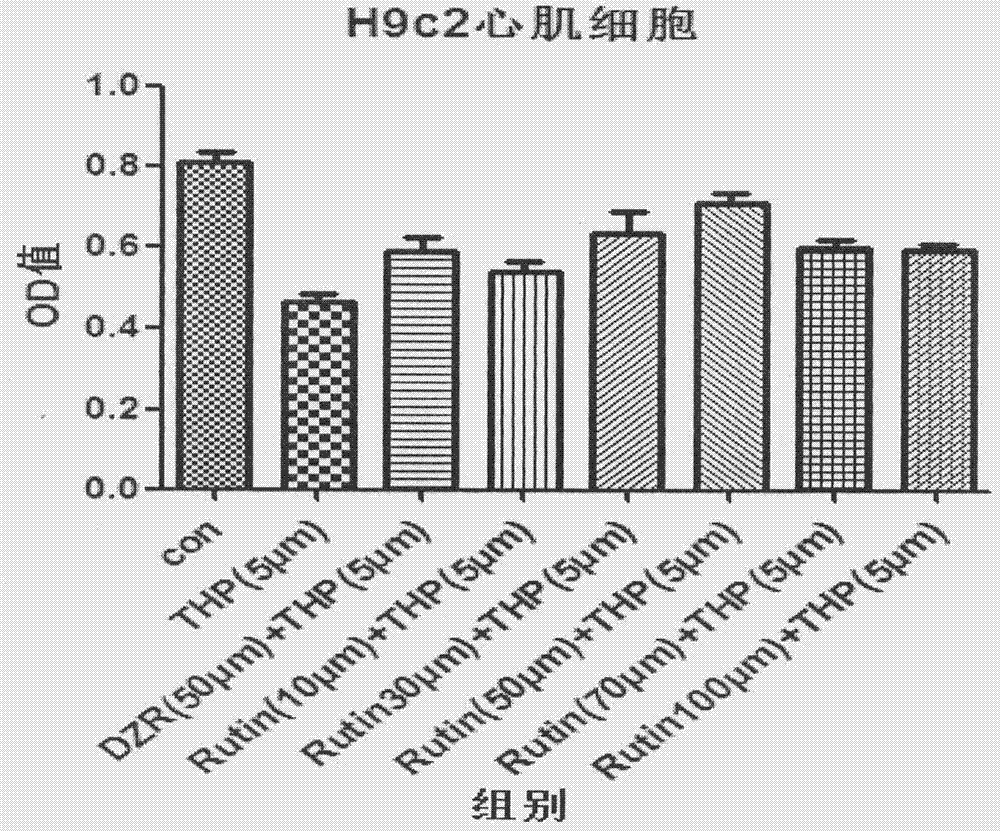
Anti-tumor pharmaceutical composition containing rutin drug
InactiveCN107115350AImprove anti-tumor activitySynergisticOrganic active ingredientsAntineoplastic agentsSide effectAnthracycline
Relating to the field of pharmacy, the invention in particular provides an anti-tumor pharmaceutical composition containing a rutin drug. The pharmaceutical composition is composed of a rutin drug and an anthracycline drug, wherein the rutin drug is rutin or troxerutin, and the anthracycline drug is epirubicin or pirarubicin. The pharmaceutical composition provided by the invention has better antitumor effect than anthracycline drugs, also effectively alleviates or avoids the cardiac toxicity of patients, and reduces the toxic and side effect of drugs.
Owner:任立群
Magnetic pirarubicin nano-drug composite
InactiveCN103536626AGood dispersionSmall toxicityHeavy metal active ingredientsOrganic active ingredientsDispersitySide effect
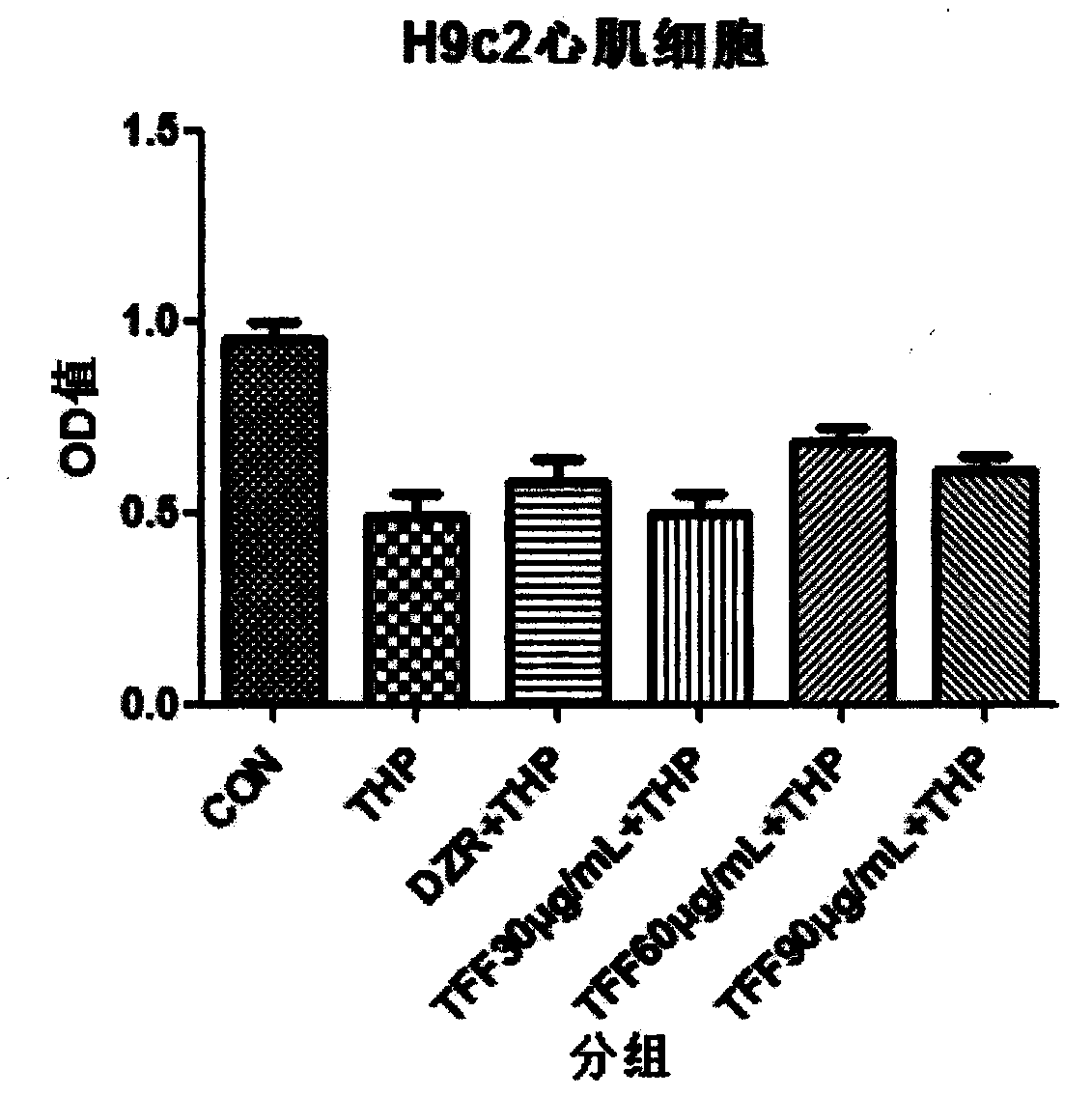
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a magnetic pirarubicin nano-drug composite. The magnetic pirarubicin nano-drug composite comprises pirarubicin, an Fe3O4 nanoparticle and 3-aminopropyl triethoxysilane, wherein the particle diameter of the Fe3O4 nanoparticle is 10-100 nanometers. The magnetic pirarubicin nano-drug composite disclosed by the invention has good stability and dispersity and property of being positioned to a tumour part in a targeted way through an external magnetic field and reduces the toxic side effect on a normal structure by restricting the dual inhibiting effect of THP and Fe3O4 on a cell on a specific tumour part.
Owner:代宏 +1
Method for purifying pirarubicin
InactiveCN111675738ALow impurity contentReduce corrosion damageSugar derivativesSugar derivatives preparationDivinylbenzenePhysical chemistry
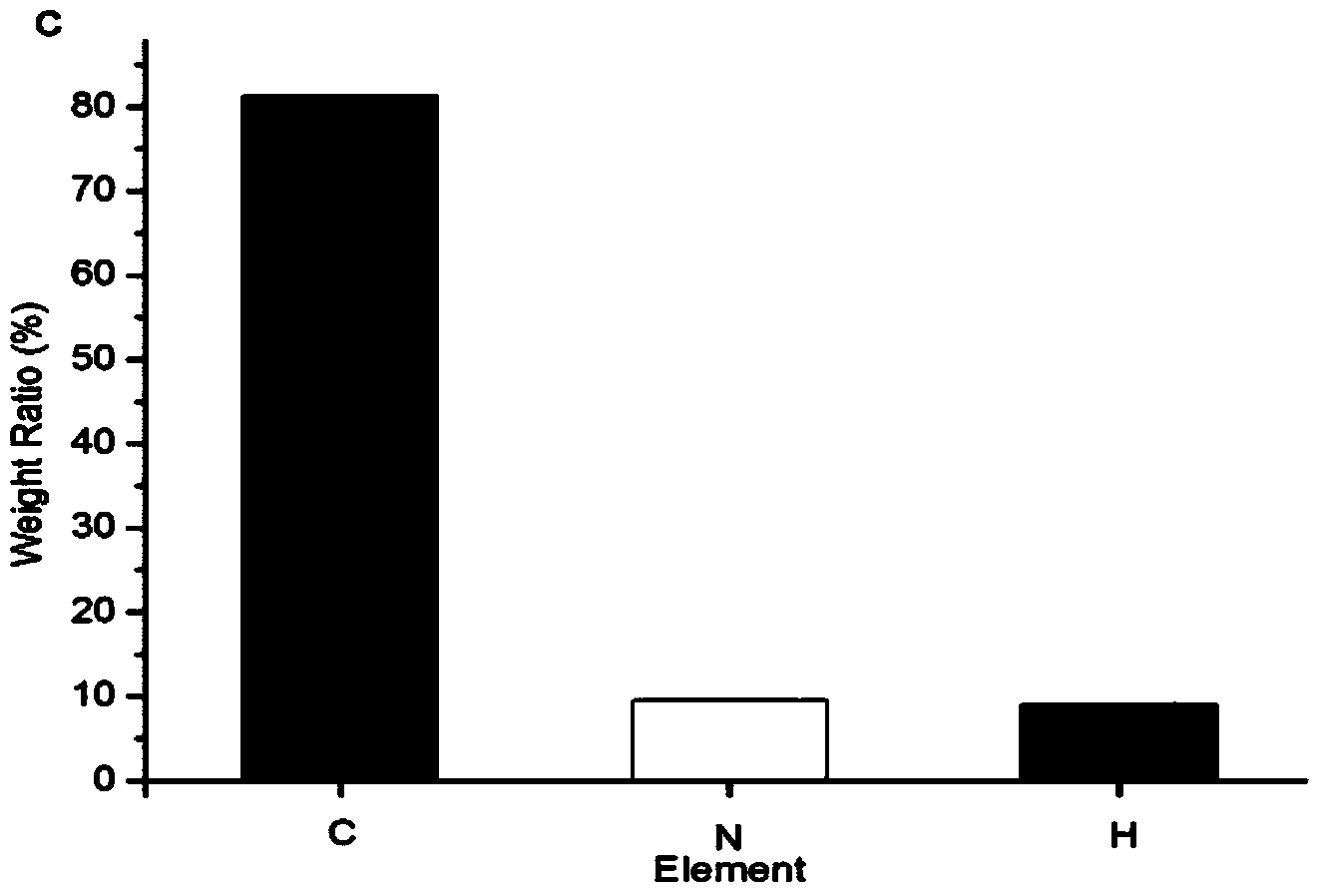
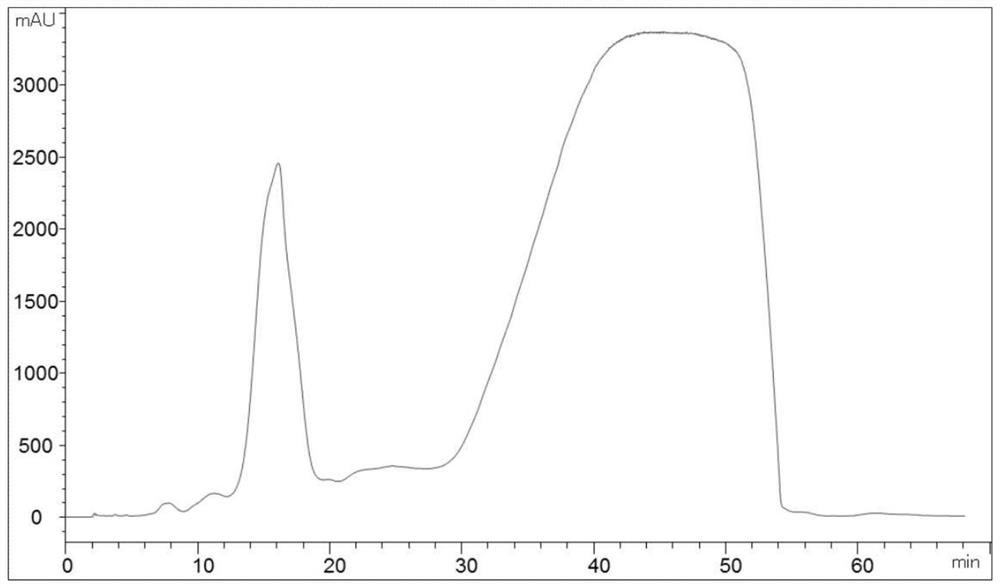
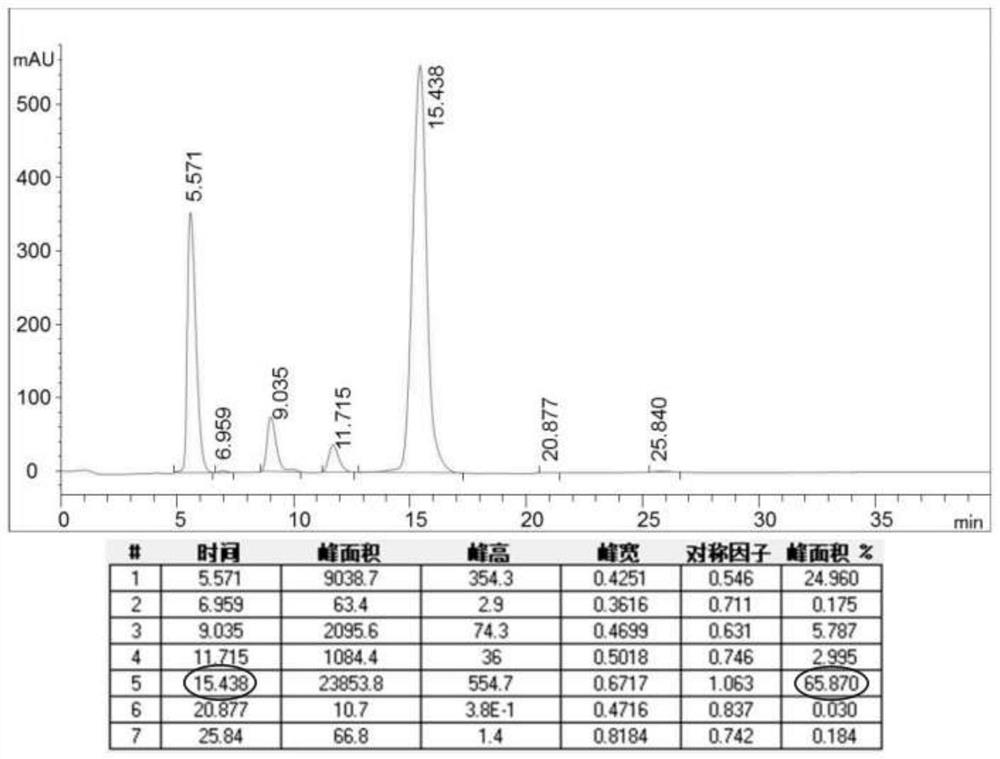
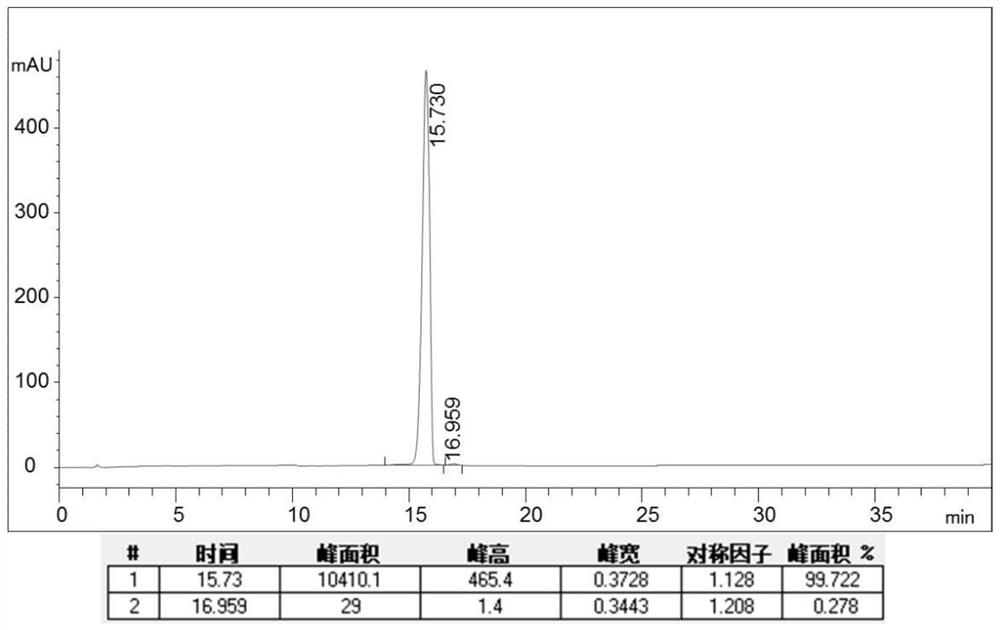
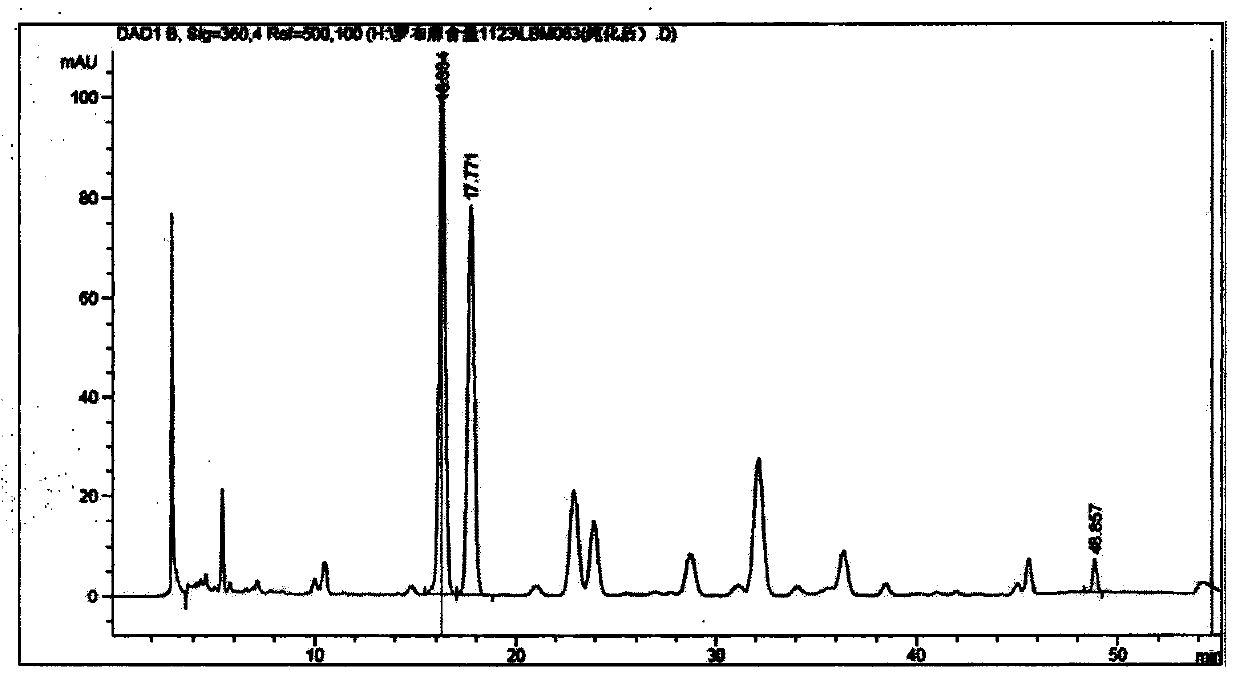
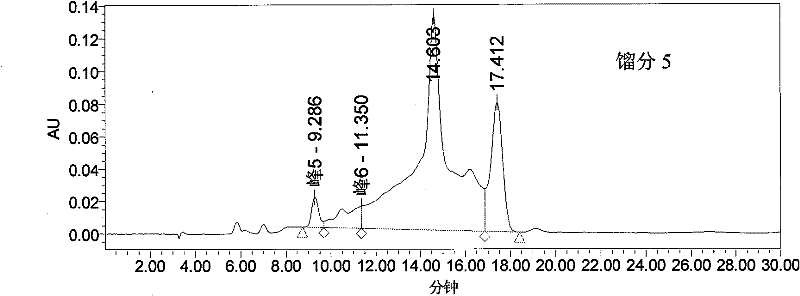
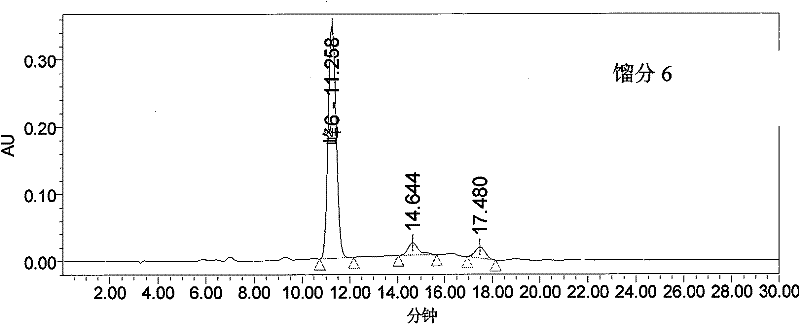
The invention provides a method for purifying pirarubicin. The method includes the steps: S1, preparing crude pirarubicin into a sample liquid; S2, injecting the sample liquid into a preparation column; and S3, performing elution for purification and separation so as to obtain a purified pirarubicin pure product, wherein solvents and eluents for purification and separation comprise acid, water andorganic phases, the packing of the preparation column is a silica gel matrix, and the silica gel matrix is bonded with octadecylsilane and divinylbenzene. According to the purification method, the silica gel matrix is adopted as the packing, the packing is special and characteristic filler with silica gel which is bonded with octadecylsilane and divinylbenzene, and pure pirarubicin is obtained after separation and purification are performed, wherein the purity of the pure pirarubicin can reach 99.5% or above, the content of one individual impurity is less than 0.1%, and the recovery rate is 76% or above; the method has convenient operation, time and effort can be saved, little pollution to the environment is caused; and the characteristic filler of the method has long life, and can be utilized repeatedly, and the purification method has been verified by scale-up production, and is suitable for industrial scale-up production.
Owner:SEPAX TECH
Thermostatic sustained release injection containing topoisomerase inhibitor and preparing method thereof
InactiveCN101229118APharmaceutical delivery mechanismPharmaceutical non-active ingredientsPolyesterPolyethylene glycol
A temperature control sustained release injection comprising topoisomerase inhibitor is composed of the topoisomerase inhibitor with an effective anti-cancer amount, an amphiphilic block copolymer, menstruum, and medicine release regulator with a fixed amount; wherein, the amphiphilic block copolymer consists of polyethylene glycol and polyester and an aqueous liquid of the amphiphilic block copolymer under a room temperature can change into a semi-solid or solid gel which can be absorbed by biodegradation and insoluble in water in a body of a hot blood animal; thereby being able to slowly release the Polyphosphate 3-Kinase inhibitor comprised in partial knub for a plurality of weeks to a plurality of months; an anti-cancer sustained release injection, being injected in the knub or in the surroundings of the knub or being arranged in a knub cavity after operation, can remarkably reduce the general reaction of the medicine, selectively enhance the curing effects of non operative treatments like chemotherapy and radiotherapy, and is used for curing the knub of different stages. The topoisomerase inhibitor is selected from self-hydroxy camptothecin, lartotecan, topotecan, irinotecan, etoposide, teniposid, podophyllotoxin, amrubicin, doxorubicin, esorubicin, pirarubicin and valrubicin.
Owner:SHANDONG LANJIN PHARMA +1
Anti-tumor pharmaceutical composition containing total flavonoids of apocynum venetum leaves
ActiveCN107115372AImprove anti-tumor activityProtectiveOrganic active ingredientsAntineoplastic agentsPharmacySide effect
Relating to the field of pharmacy, the invention in particular provides an anti-tumor pharmaceutical composition containing total flavonoids of apocynum venetum leaves. The pharmaceutical composition is composed of total flavonoids of apocynum venetum leaves and an anthracycline drug, wherein the anthracycline drug is pirarubicin or epirubicin. The pharmaceutical composition provided by the invention has better antitumor effect than anthracycline drugs, also effectively alleviates or avoids the cardiac toxicity of patients, and reduces the toxic and side effects.
Owner:任立群
Human polypeptide for constructing tumor pH response micro-robot and application thereof
ActiveCN110655559AGood biocompatibilityNon-immunogenicPowder deliveryPeptide-nucleic acidsLocal HyperthermiaNutrition
The invention relates to a human polypeptide for constructing tumor pH response micro-robot and an application thereof. The amino acid sequence is as shown in SEQ ID NO.1. The micro-robot loads pirarubicin and indocyanine green through self-assembled human peptide nanoparticles, and combines chemotherapy, photothermal therapy and hunger therapy as well as real-time diagnosis of tumors. The micro-robot is triggered in the microenvironment of tumor acidic pH, releases pirarubicin and insoluble deposits and reverses their fluorescence emission intensity. The released pirarubicin selectively enters tumor nuclei and kills them, while insoluble deposits can accumulate in tumor blood capillaries, block oxygen and nutrition supply and realize cancer hunger treatment. Under the irradiation of nearinfrared laser, indocyanine green effectively accumulated in tumor can induce local hyperthermia. The reverse reversal of ph response of fluorescence emission intensity of micro-robot can be used forreal-time diagnosis of tumors in vivo.
Owner:SOUTHEAST UNIV
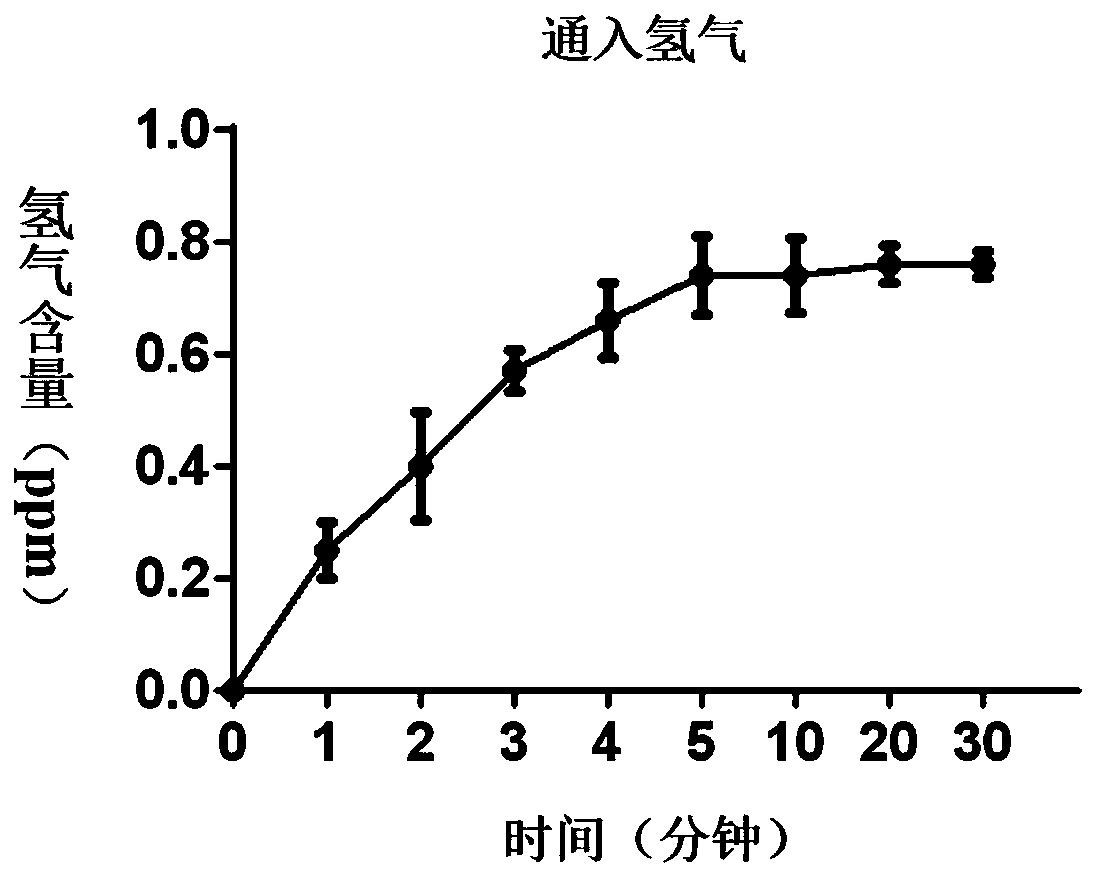
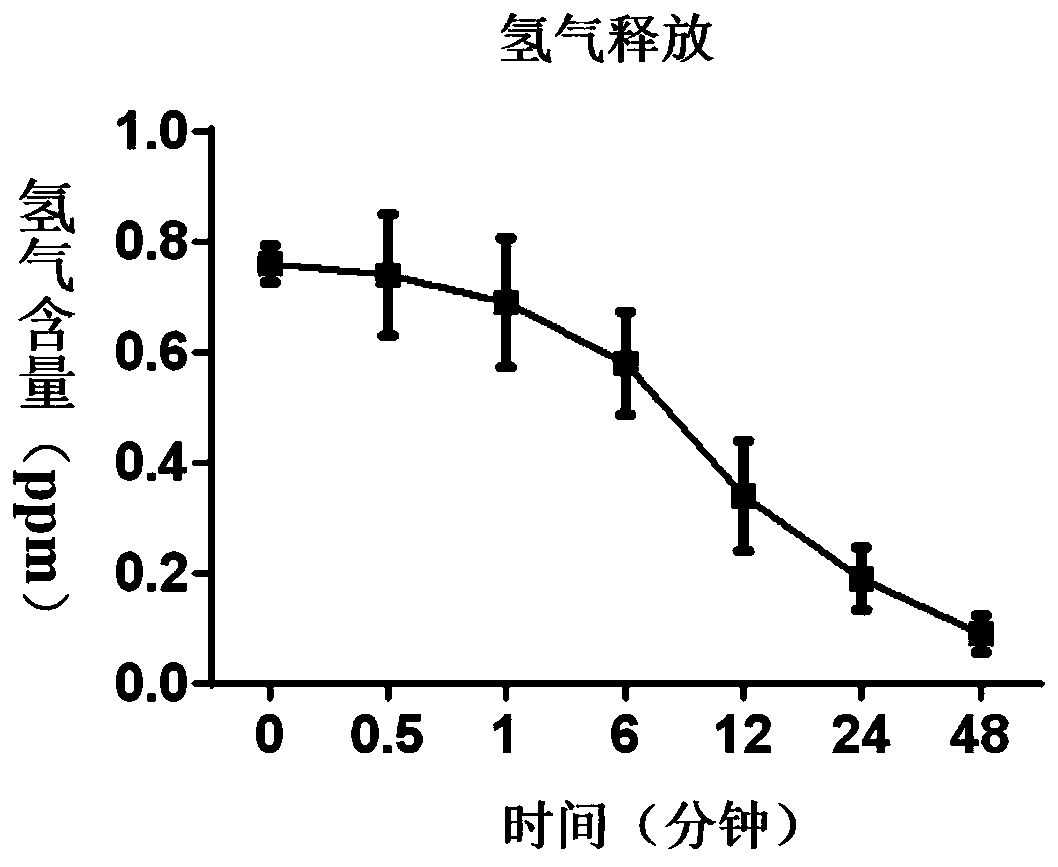
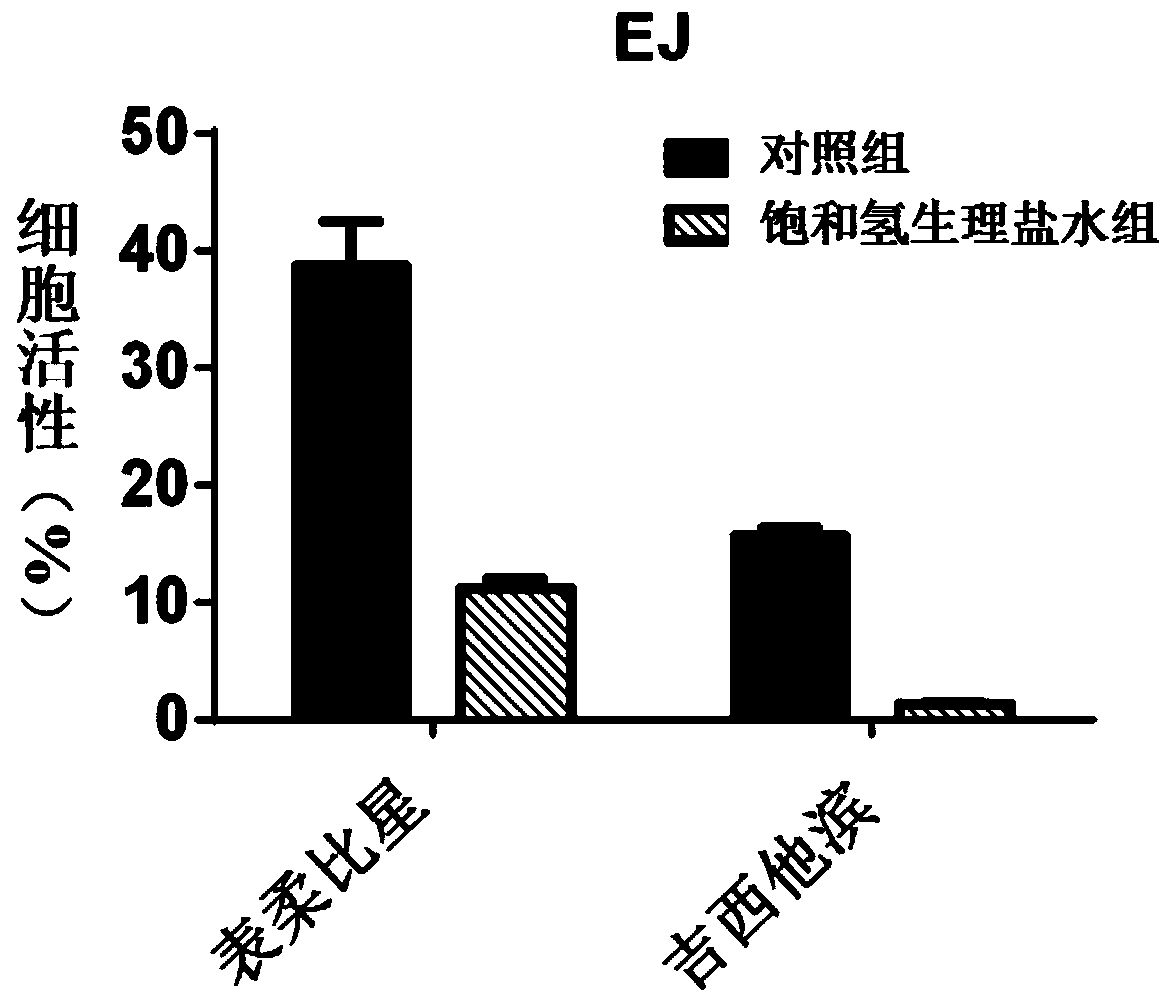
Composition for treating the bladder cancer and application of hydrogen physiological saline
InactiveCN110464736AGrowth inhibitionPromote apoptosisOrganic active ingredientsHeavy metal active ingredientsSide effectPhysiology
The invention discloses a composition for treating the bladder cancer and application of hydrogen physiological saline. The composition includes hydrogen physiological saline and chemotherapeutic drugs, wherein the chemotherapeutic drugs are one or more of cisplatin, adriamycin, hydroxycamptothecin, pirarubicin, epirubicin and gemcitabine. Through the hydrogen physiological saline, the growth of in-vitro bladder cancer cells can be inhibited significantly, apoptosis of tumor cells is promoted; and in addition, exogenous hydrogen taken through respiration or drinking or injection has good histocompatibility, safety, and few side effects, even if the taken exogenous hydrogen is excessive, the taken exogenous hydrogen can be discharged through respiration without residues.
Owner:SHENZHEN LUOHU PEOPLELS HOSPITAL
Gel injection of sustained-released topology enzyme inhibitor and preparation method thereof
InactiveCN101336892APharmaceutical delivery mechanismPharmaceutical non-active ingredientsPolyesterPolyethylene glycol
The invention relates to a slow-release topoismerase inhibitor gel injection which contains an anticancer-effective amount of topoismerase inhibitor microspheres (or microsphere and topoismerase inhibitor micropowder), an amphiphilic block polymer composed of polyethylene glycol and polyester, a solvent and a certain amount of slow-release regulator. The amphiphilic block polymer aqueous solution is liquid at the room temperature and turns into a semisolid or solid biodegradable water-insoluble gel in vivo in warm-blooded animals, so that the topoismerase inhibitor encapsulated therein is sustainedly and locally released around the tumor and the microspheres can further control the slow release for a plurality of weeks to a plurality of months. After the intratumoral injection or peritumoral injection or post-operative intracavitary injection or arterial embolization, the slow-release topoismerase inhibitor gel injection can significantly reduce the general drug reaction and selectively enhance the curative effect of non-operative treatment such as chemotherapy and radiotherapy, and is used for the treatment of tumors of different stages. The topoismerase inhibitor is selected from camptothecin, topoteean, etoposide, teniposide, esorubicin, pirarubicin and valrubicin.
Owner:济南基福医药科技有限公司
Combination therapies for treating cancer
ActiveUS11241421B2Prevent proliferationOrganic active ingredientsOrganic chemistryPharmaceutical medicineCombination therapy
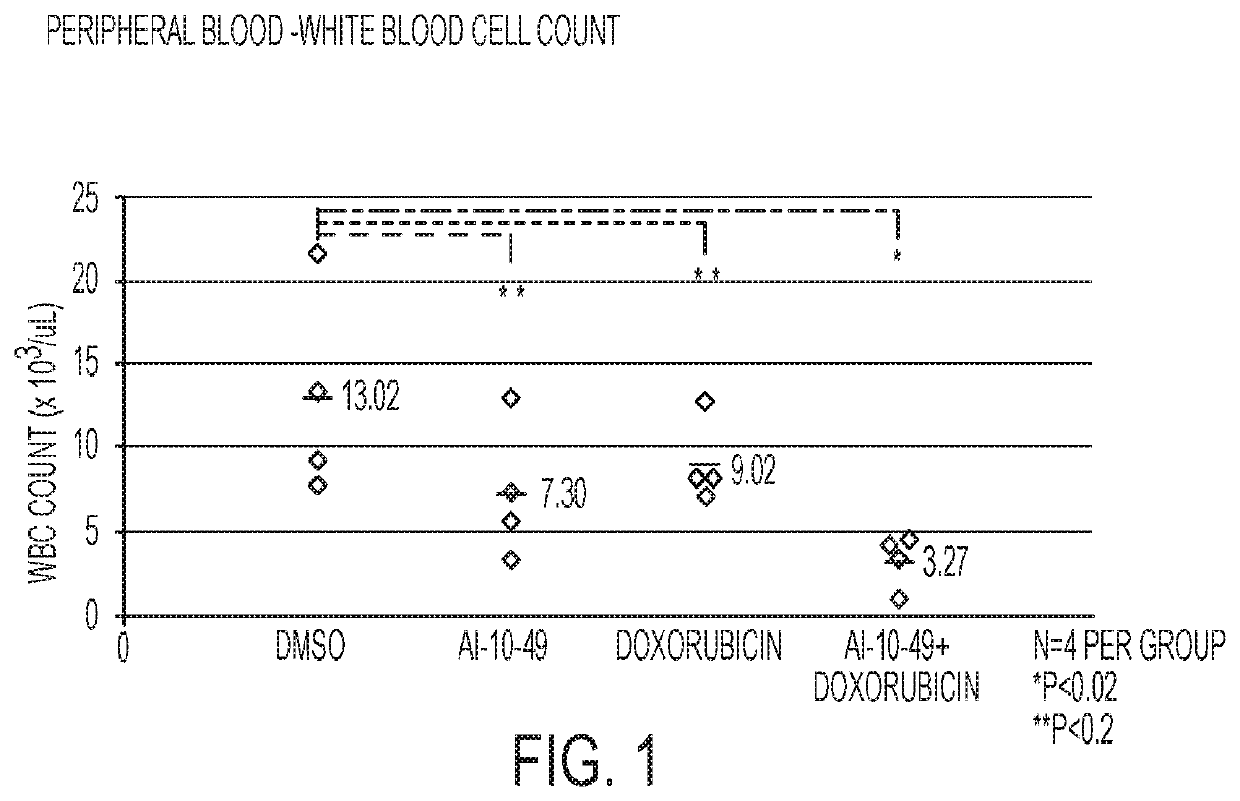
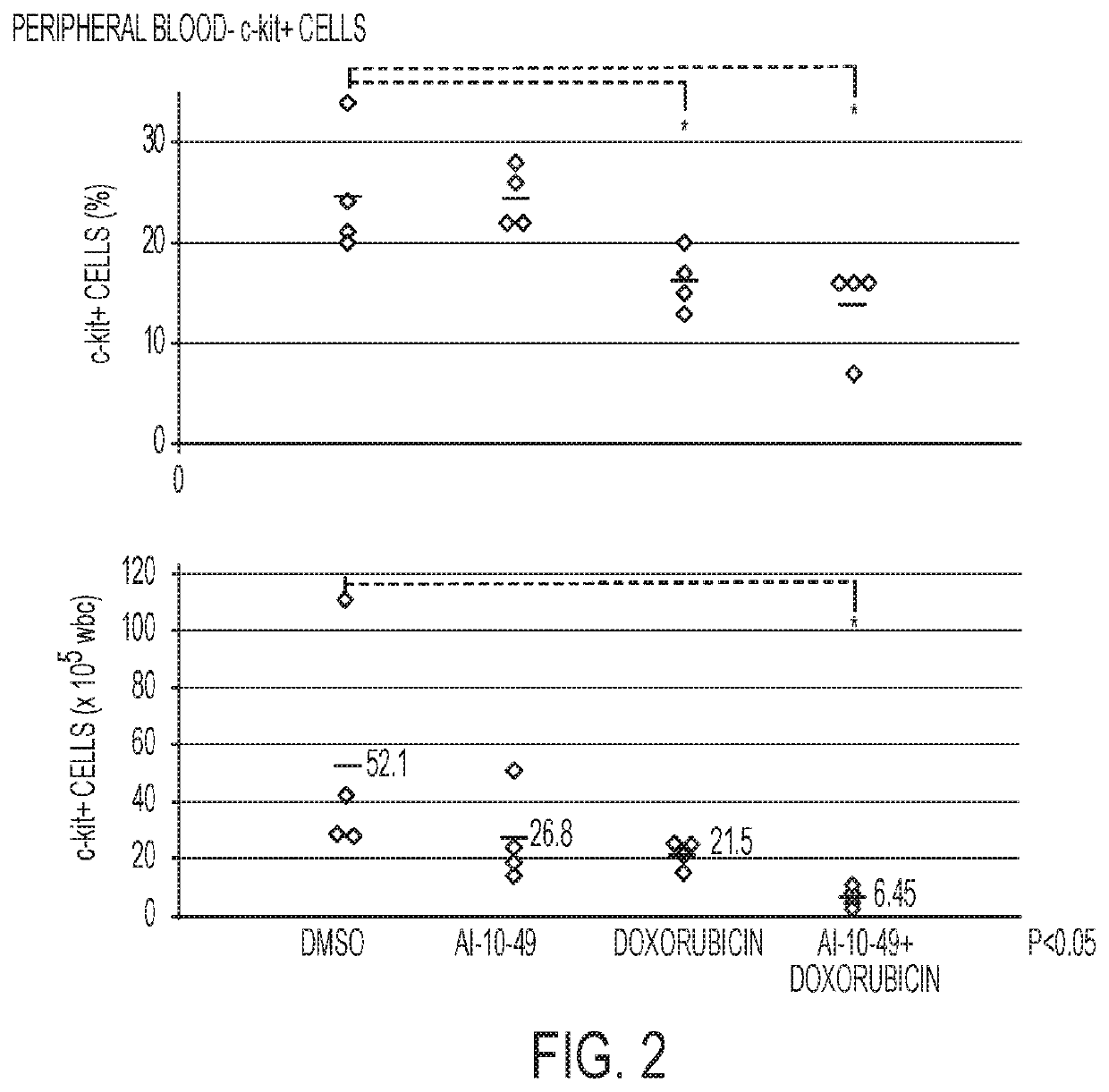
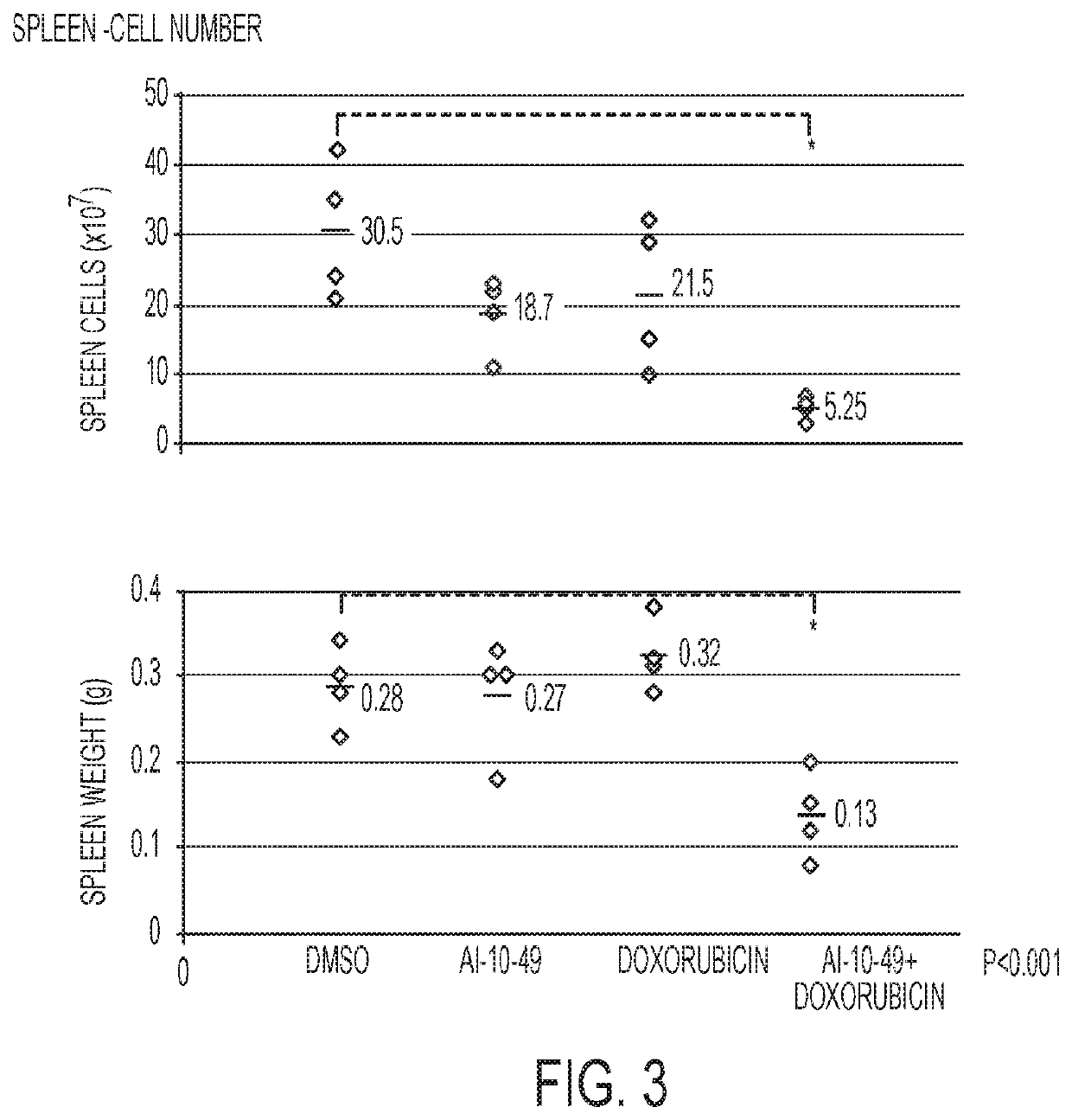
This invention relates to methods and compositions for treatment of inv(16) leukemia and particularly to treatment of acute myeloid leukemia. Disclosed is a method of treating inv(16) leukemia comprising the step of administering to a subject in need thereof a therapeutically effective combination of a) a compound of the formula (1) and b) a chemotherapeutic agent selected from the group consisting of pirarubicin, aclarubicin, mitoxantrone, doxorubicin, daunorubicin, idarubicin, epirubicin, cytarabine, pharmaceutically acceptable salts and mixtures thereof. The therapeutically effective combination synergistically inhibits proliferation of inv(16) leukemia cells. This invention also relates to pharmaceutical compositions comprising a therapeutically effective combination of the compound of formula (1) and the chemotherapeutic agent and a pharmaceutically acceptable excipient.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Drug containing pirarubicin, preparation method for drug, pharmaceutical composition and application of drug
ActiveCN110960542AStable intercalationStable deliveryPowder deliveryOrganic active ingredientsNucleic acid structureNanoparticle
The application provides a drug containing pirarubicin, a preparation method for the drug, a pharmaceutical composition and application of the drug. The drug comprises a nucleic acid nanoparticle andthe pirarubicin, and the pirarubicin is deposited on the nucleic acid nanoparticle; the nucleic acid nanoparticle includes a nucleic acid domain; the nucleic acid domain includes an a sequence, a b sequence and a c sequence; the a sequence includes an a1 sequence or a sequence of insertion, deletion or substitution of at least one base of the a1 sequence; the b sequence includes a b1 sequence or asequence of insertion, deletion or substitution of at least one base of the b1 sequence; and the c sequence includes a c1 sequence or a sequence of insertion, deletion or substitution of at least onebase of the c1 sequence. After the nucleic acid domain is modified by a target head, the drug containing the pirarubicin has a relatively good targeting property, can stably deliver the pirarubicin,and has very high reliability.
Owner:BAI YAO ZHI DA BEIJING NANOBIO TECH CO LTD
Anti-cancer drugs slow release agent comprising anticancer antibiotics and synergist thereof
Disclosed is an anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow releaseauxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer antibiotics are selected from Idarubicin, Valtaxin, Pirarubicin and Mitoxantrone, The anti-metabolite drugs are selected from Pemetrexed, Carmustine, Tegafur, Zalcitabine, Emtritabine, Galocitabine, Ibacitabine, Ancitabine, Decitabine, Flurocitabine, Enocitabine, Imidazoletabine, Capecittabine, Gemcitabine, Fludrarbine, Raltitrexed, Dexrazoxane, Cladribine, Nolatrexed and folic acid, The slow release auxiliary materials are selected from EVAc, Polifeprosan, sebacylic acid copolymer, lactic acid, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The slow release microspheres can also be prepared into slow release implanting agent for injection or placement in or around tumor.
Owner:SHANDONG LANJIN PHARMA
A kind of pirarubicin drug-loaded gelatin submicroemulsion material and its preparation method and application
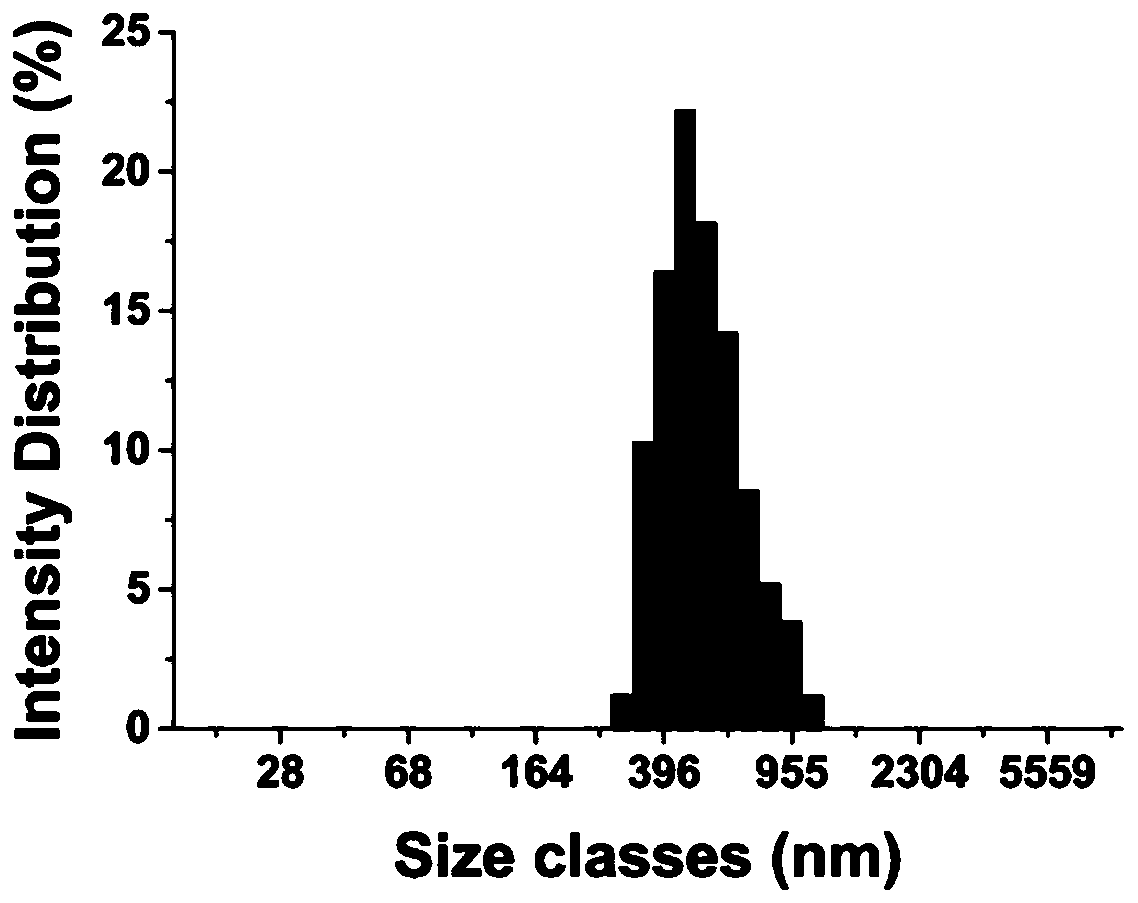
ActiveCN112754993BStable structureGood biocompatibilityOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical drugDrugs preparations
The present invention relates to the technical field of slow-release medicine preparation, specifically a pirarubicin drug-loaded gelatin submicroemulsion material and its preparation method and application. The present invention firstly prepares the gelatin water phase material A, and then mixes it with the oil phase Material B was blended to prepare W / O type blank gelatin material C, and pirarubicin was coated by using W / O type blank gelatin material C; pirarubicin was combined with W / O type blank gelatin material The compatibility of C is poor, so before coating, pirarubicin is dissolved in a glucose solution with a volume fraction of 5%, and under the action of glucose solution, pirarubicin is coated with W / O type Blank gelatin material C, and then obtain the structurally stable pirarubicin gelatin drug-loaded submicron emulsion material. The invention not only prepares a pirarubicin gelatin drug-loaded submicron emulsion material, but also the pirarubicin gelatin drug-loaded submicron emulsion material can be applied to the preparation of slow-release antitumor drugs.
Owner:PINGDINGSHAN UNIVERSITY
Compound recipe anti-cancer drugs slow release agent comprising anticancer antibiotics and synergist thereof
Owner:SHANDONG LANJIN PHARMA
Preparation process for Sappan Wood extract perfusate and application thereof in treating bladder cancer
Owner:山西华尚汇商贸有限公司
Magnetic pirarubicin nano-drug composite
InactiveCN103536626BGood dispersionSmall toxicityOrganic active ingredientsHeavy metal active ingredientsSide effectTriethoxysilane
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a magnetic pirarubicin nano-drug composite. The magnetic pirarubicin nano-drug composite comprises pirarubicin, an Fe3O4 nanoparticle and 3-aminopropyl triethoxysilane, wherein the particle diameter of the Fe3O4 nanoparticle is 10-100 nanometers. The magnetic pirarubicin nano-drug composite disclosed by the invention has good stability and dispersity and property of being positioned to a tumour part in a targeted way through an external magnetic field and reduces the toxic side effect on a normal structure by restricting the dual inhibiting effect of THP and Fe3O4 on a cell on a specific tumour part.
Owner:代宏 +1
A kind of antitumor pharmaceutical composition containing total flavonoids of apocynum leaf
ActiveCN107115372BImprove anti-tumor activityProtectiveOrganic active ingredientsAntineoplastic agentsSide effectApocynum venetum
Relating to the field of pharmacy, the invention in particular provides an anti-tumor pharmaceutical composition containing total flavonoids of apocynum venetum leaves. The pharmaceutical composition is composed of total flavonoids of apocynum venetum leaves and an anthracycline drug, wherein the anthracycline drug is pirarubicin or epirubicin. The pharmaceutical composition provided by the invention has better antitumor effect than anthracycline drugs, also effectively alleviates or avoids the cardiac toxicity of patients, and reduces the toxic and side effects.
Owner:任立群
Ammonia maleate rubicin salt, and its preparing method and use
The invention relates to maleic acid ammonia pirarubicin salt and its preparation method, and its application in antineoplastics and pharmaceutical preparation. The preparation method includes reaction in solvent, freeze drying, or crystallizing.
Owner:JIANGSU HANSOH PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com