Instant pyrobixi freeze-drying powdery injection and its production
A technology for pirarubicin and injection, which is applied in the field of instant pirarubicin for injection and its preparation, and can solve the problems of dissolution, slow speed, inconvenient medication and the like
Active Publication Date: 2007-09-05
深圳万乐药业有限公司
View PDF1 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0002] The currently marketed pirarubicin hydrochloride freeze-dried powder for injection is generally dissolved with water for injection or 5% glucose solution, and cannot be dissolved with normal saline, because when pirarubicin is dissolved with normal saline, the speed is very slow, in It is inconvenient to use medicine clinically
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 4
[0025] prescription
[0026] The above-mentioned preparation process is adopted.
[0027] Prescription 1
[0028] It can be seen from the test results that when lactose is used as an excipient, the stability of the prescription is much better than that of mannitol, so lactose is better when choosing an excipient.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
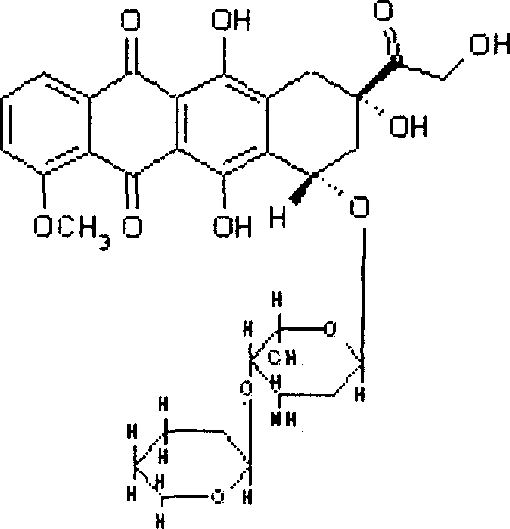
An instant freeze-dried powder injection of pirarubicin is prepared from pirarubicin, excipient and cosolvent through proportionally dissolving them in the water for injection, aseptic filtering, loading in containers, and freeze-drying.
Description
technical field [0001] The invention relates to an instant pirarubicin for injection and a preparation method thereof. The freeze-dried powder can improve the solubility of pirarubicin in normal saline. Background technique [0002] The currently marketed pirarubicin hydrochloride freeze-dried powder for injection is generally dissolved with water for injection or 5% glucose solution, and cannot be dissolved with normal saline, because when pirarubicin is dissolved with normal saline, the speed is very slow, and in It is very inconvenient to use medicine clinically. [0003] In the Chinese invention patent CN85107562 "Method for Improving the Solubility of an Anthracycline Glycoside Compound in an Injection Solution", it is proposed that for every 10 parts by weight of anthracycline glycoside, add 0.1 to 10 parts by weight of methyl p-hydroxybenzoate Esters can solve the solubility problem of the mentioned anthracycline glycosides in saline, such as doxorubicin, epirubicin...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/704A61K9/19A61K47/26A61K47/14A61K47/16A61K47/20A61P35/00
Inventor 欧阳德方卓秋绮王长平张汉利宝玉荣
Owner 深圳万乐药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com