A kind of pirarubicin drug-loaded gelatin submicroemulsion material and its preparation method and application
A technology of pirarubicin gelatin and pirarubicin, which is applied in the direction of pharmaceutical formulations, drug combinations, antineoplastic drugs, etc., to achieve the effects of increasing drug absorption, improving bioavailability, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of pirarubicin gelatin submicroemulsion material, comprising the steps of:
[0038] (1) Preparation of W / O blank gelatin powder:
[0039] S1. Prepare the aqueous phase: adjust the pH of the acetic acid solution to 5, dissolve 15 mg of gelatin in 1 mL of the acetic acid solution with a volume fraction of 1%, and filter to obtain the aqueous phase material A;
[0040] S2. Preparation of oil phase material B: After mixing paraffin oil and tween80 with a volume ratio of 100:6 evenly, oil phase material B is obtained;
[0041] S3. Preparation of W / O type blank gelatin: add the water phase material A of S1 dropwise to the oil phase material B of S2 according to the volume ratio of 1:4.5, stir and mix for 2.5 hours, and solidify by dropping glutaraldehyde for 4 hours. The sequence of ether, isopropanol aqueous solution and distilled water was repeatedly washed three times and freeze-dried to obtain a W / O type blank gelatin material C;
[0042] Wherein, t...
Embodiment 2
[0048] A preparation method of pirarubicin gelatin submicroemulsion material, comprising the steps of:
[0049] (1) Preparation of W / O blank gelatin powder:
[0050] S1. Prepare the water phase: adjust the pH of the acetic acid solution to 5.5, dissolve 20 mg of gelatin in 1 mL of the acetic acid solution with a volume fraction of 1%, and filter to obtain the water phase material A;
[0051] S2. Preparation of oil phase material B: After uniformly mixing paraffin oil and tween80 with a volume ratio of 100:5, oil phase material B is obtained;
[0052]S3. Preparation of W / O blank gelatin: Add the water phase material A of S1 dropwise to the oil phase material B of S2 at a volume ratio of 1:5, stir and mix for 2 hours, and solidify by adding glutaraldehyde dropwise for 4.5 hours. The sequence of ether, isopropanol aqueous solution and distilled water was repeatedly washed 4 times and freeze-dried to obtain W / O blank gelatin material C;
[0053] Wherein, the ratio of the total v...
Embodiment 3
[0059] A preparation method of pirarubicin gelatin submicroemulsion material, comprising the steps of:
[0060] (1) Preparation of W / O blank gelatin powder:
[0061] S1. Prepare the water phase: adjust the pH of the acetic acid solution to 6, dissolve 25 mg of gelatin in 1 mL of the acetic acid solution with a volume fraction of 1%, and filter to obtain the water phase material A;
[0062] S2. Preparation of oil phase material B: After uniformly mixing paraffin oil and tween80 with a volume ratio of 100:4, oil phase material B is obtained;
[0063] S3. Preparation of W / O blank gelatin: add the water phase material A of S1 dropwise to the oil phase material B of S2 at a volume ratio of 1:5.5, stir and mix for 1.5 hours, and solidify by dropping glutaraldehyde for 5 hours. The sequence of ether, isopropanol aqueous solution and distilled water was repeatedly washed 5 times and freeze-dried to obtain W / O type blank gelatin material C;
[0064] Wherein, the ratio of the total vo...
PUM
| Property | Measurement | Unit |
|---|---|---|
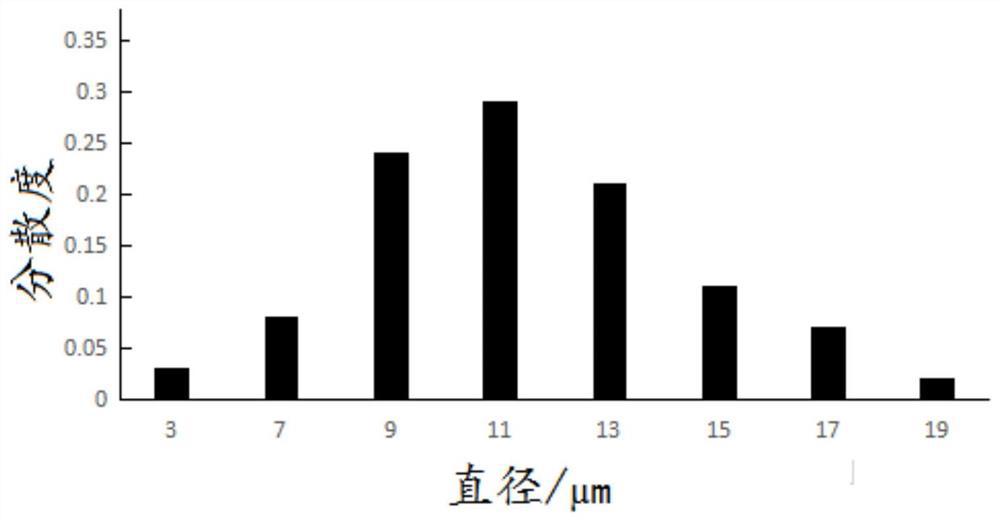
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com