Anti-tumor pharmaceutical composition containing rutin drug
An anti-tumor drug and composition technology, applied in the direction of drug combination, anti-tumor drug, medical preparation containing active ingredients, etc., can solve the problem of high risk of cardiovascular disease, lack of effective therapeutic drugs and intervention methods, and high risk question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
[0062] The compositions were prepared according to the ratios in Table 1 below.
[0063] For the preparation of the liquid compositions in Examples 1 to 32, the compositions of the present invention with corresponding concentrations can be prepared into the same solution, and then used directly when applied; they can also be prepared into solutions with corresponding concentrations, and then carried out They can be mixed and used directly at the time of administration; they can also be administered after being mixed at the time of use, or administered to the subject separately, and the sequence of their administration is not limited.
[0064] For the preparation of the solid compositions in Examples 1-32, the medicines of corresponding quality can be mixed directly and taken at the same time; they can also be made into unit preparations and taken to the subject at the same time when administered, or successively Take them, but the sequence of their use is not limited.
[0065...
experiment example 1
[0076] Experimental example 1 in vitro test
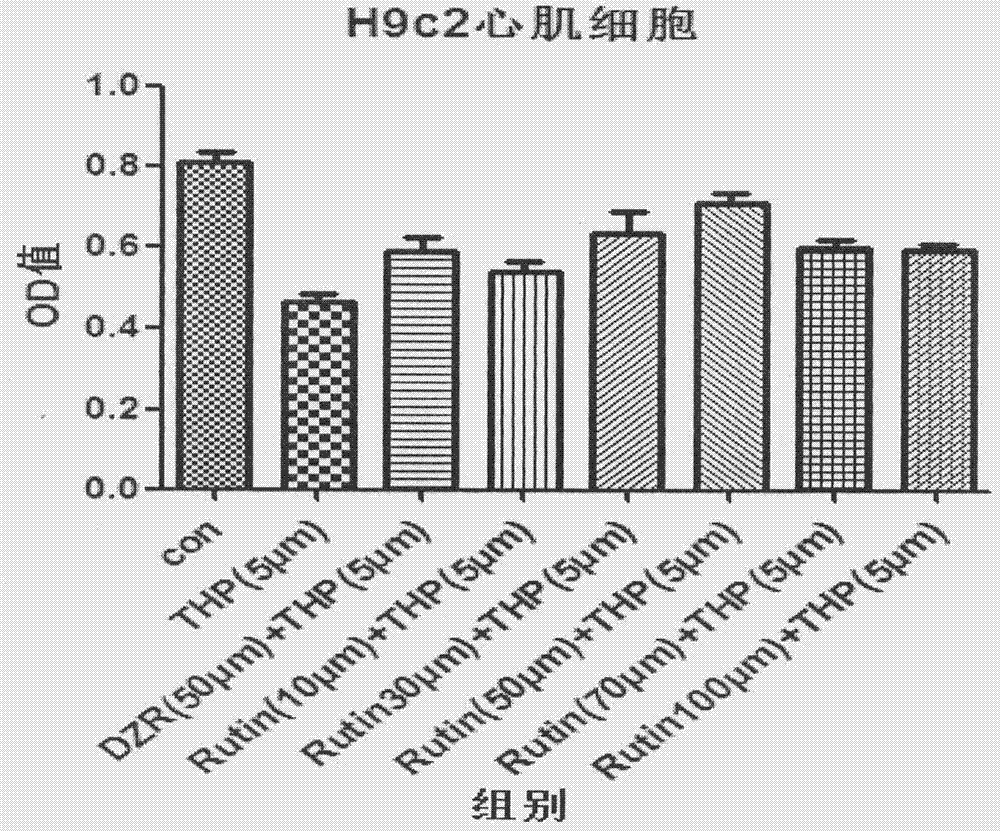
[0077] 1.1.1 Effect of different ratios of rutin and pirarubicin compositions on the inhibitory rate of H9c2 cells (MTT method)
[0078] experiment method:
[0079] H9c2 cardiomyocytes were seeded in 96-well culture plates. When the cells grew to about 80% of the culture well area, different treatments were carried out according to the needs of the experiment: (1) 10, 30, 50, 70 and 100 μmol / L different concentrations of Rutin (Rutin) was pretreated for 1 hour, discarded, washed twice with PBS, and cultured with 5 μmol / L pirarubicin for 24 hours; L-pirarubicin was cultured for 24 hours; 8 replicate wells were set up for each treatment factor. After terminating the culture, add 10 μL MTT to each well, shake gently, and incubate at 37°C. Then measure cell inhibition rate=(1-OD value of experimental group / OD value of control group)×100%. The measurement results are shown in Table 2 and figure 1 .
[0080] Data statistics The SPS...
experiment example 2
[0125] Experimental Example 2 In vivo test
[0126] Protective effects of different ratios of rutin and pirarubicin compositions on acute cardiotoxicity (injury) in Wistar rats
[0127] Preparation of anthracycline acute cardiotoxicity model: According to the replication method of animal model of human disease and the method in the article "The protective effect of schisandrin B and dexrazine on doxorubicin-induced cardiotoxicity".
[0128] Medicines, reagents and animals:
[0129] Drugs: Pirarubicin Hydrochloride (THP, Shenzhen Wanle Co., Ltd.), Dexpropylimine for Injection (DZR, Jiangsu Osaikang Pharmaceutical Co., Ltd.), Rutin (Rutin, Nanjing Jingzhu Pharmaceutical Technology Co., Ltd.) .
[0130] Reagents: Rat serum brain natriuretic peptide BNP (MEXN-R0119), serum cardiac troponin T (MEXN-R099) and creatine kinase isoenzyme MB (MEXN-R0995) kits were purchased from Shanghai Meixuan Biotechnology Co., Ltd. company. Malondialdehyde (A003-1), lactate dehydrogenase LDH (A0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com