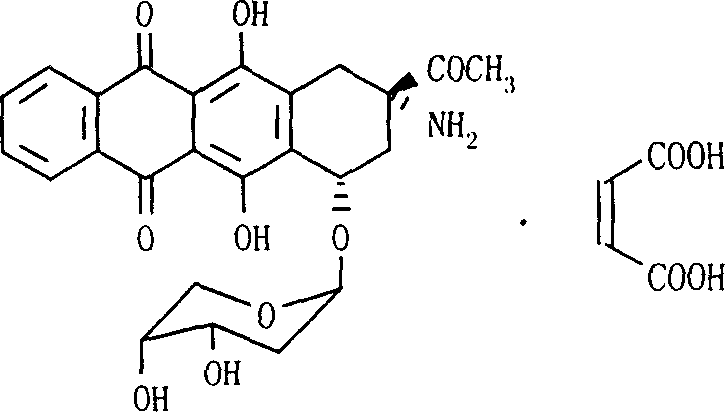
Ammonia maleate rubicin salt, and its preparing method and use
A kind of technology of amrubicin and amrubicin, which is applied in the field of amrubicin maleate and its preparation and use, and can solve the problems such as stability of amrubicin hydrochloride that is not disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of amrubicin maleate salt freeze-dried powder
[0025] In a 250ml reaction bottle, put 4.84g (10.0mmol) of amrubicin (10.0mmol) and 1.16g (10.0mmol) of maleic acid, add 320ml of deionized water, stir and dissolve, filter with a microporous membrane, freeze-dry, and obtain lyophilized Powder 5.89g, yield 98.2%.
Embodiment 2
[0026] Embodiment 2: the preparation of amrubicin maleate salt
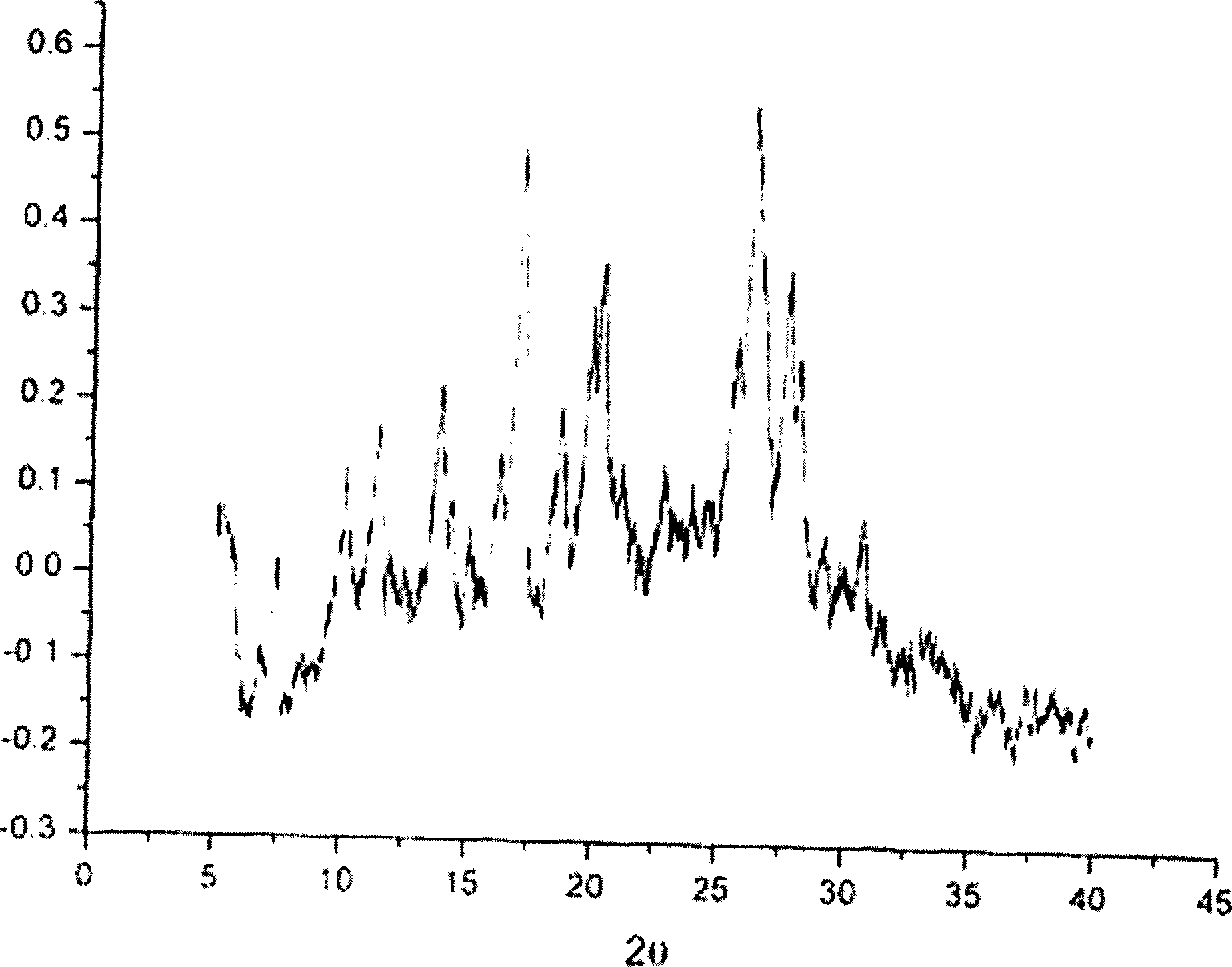
[0027] In a 2000ml reaction bottle, put 4.84g (10.0mmol) of amrubicin (10.0mmol) and 1.22g (10.5mmol) of maleic acid into it, add 420ml of methanol, stir and dissolve, filter, concentrate under reduced pressure at room temperature until turbid and crystals are precipitated, stir and drop After adding 240ml of isopropyl ether, a large number of orange-red crystals were gradually precipitated, then stirred at room temperature for 1 hour, filtered, washed with isopropyl ether, and vacuum-dried at room temperature to obtain 5.8 g of orange-red crystals, with a yield of 96.7%. Melting point: 146.9-147.8°C. X-diffraction pattern see figure 1 .
Embodiment 3
[0028] Embodiment 3: the preparation of amrubicin maleate salt
[0029] In a 2000ml reaction bottle, put 4.84g (10.0mmol) of amrubicin (10.0mmol) into it, 1.22g (10.5mmol) of maleic acid, add 420ml of methanol, stir and dissolve, concentrate under reduced pressure at room temperature until it becomes turbid and there are crystals, continue to concentrate until there is a large amount of Crystals were precipitated, then stirred at room temperature for 1 hour, filtered, washed with a small amount of methanol, and dried in vacuo at room temperature to obtain 4.48 g of orange crystals with a yield of 74.7%. Melting point: 145.7-146.8°C. The X-diffraction pattern is identical to the product of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com