Method for purifying pirarubicin
A technology of pirarubicin and purification method, applied in chemical instruments and methods, organic chemistry, preparation of sugar derivatives, etc., can solve the problems of cumbersome operation steps, little reference for industrial scale-up production, serious environmental pollution, etc. Small corrosion damage, saving maintenance costs, and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044]The packing of the preparation column in step S2 of the present invention is a silica gel substrate, and octadecylsilane and divinylbenzene are bonded on the silica gel substrate; wherein, the mass percent of octadecylsilane and divinylbenzene is (1 ~10):1, preferably (2~8):1, more preferably (4~6):1.
[0045] The eluent in steps S2 and S3 of the present invention is organic acid: water: organic solvent: ethyl acetate = 0.5~2: 100~400: 600~900: 20~80; wherein the organic acid is preferably formic acid, tris One or two of fluoroacetic acid; one or more of methanol, ethanol and acetonitrile as the organic solvent.
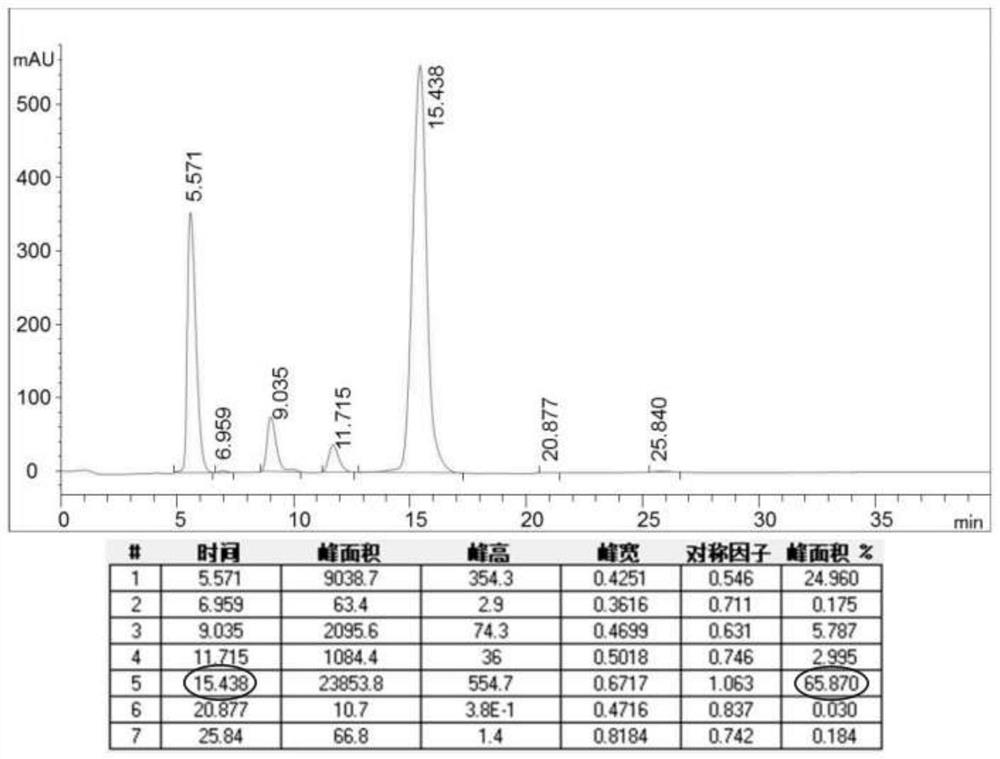
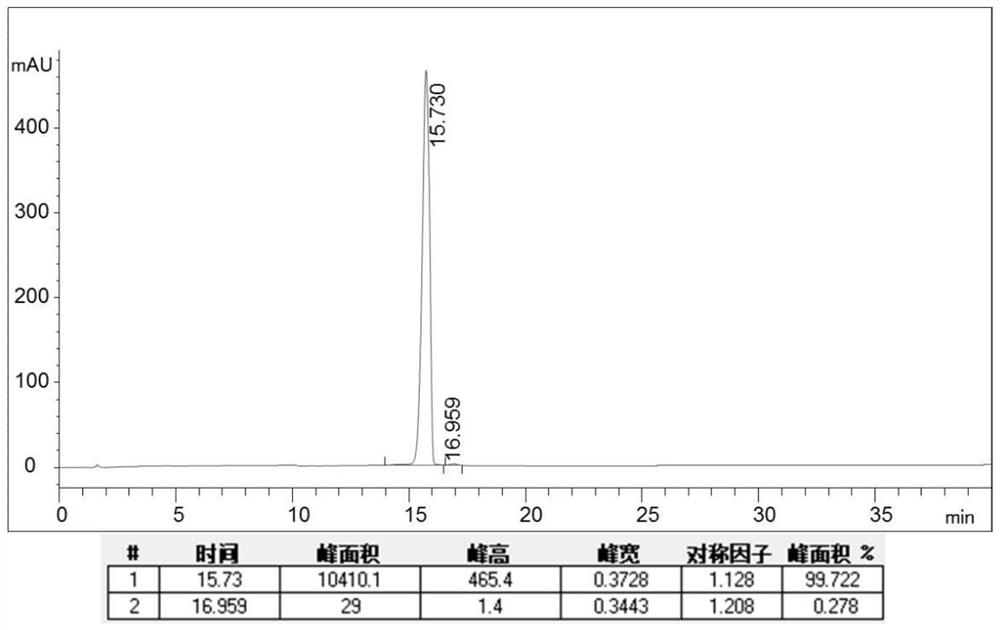
[0046] In step S3 of the present invention, the combined standard of pure pirarubicin is that the purity of the sample is higher than 99.5%, and the purity of the sample is less than 0.1%.
[0047] 1. Instruments and materials:
[0048] Crude pirarubicin, HPLC (ultraviolet detector), Sepax preparative liquid chromatograph LC6000, one analysis column Sepax-SAP...
Embodiment 1
[0064] Eluent volume ratio: formic acid: water: methanol: ethyl acetate = 1:300:700:50; flow rate: 400ml / min; injection volume: 400ml; column temperature: room temperature; sample: 100mg / ml; pressure: 1.1 MPa;
[0065] Instrument: Sepax preparative chromatograph LC6000; pirarubicin preparative column (100mm×250mm, containing about 0.98kg of filler);
[0066] Detection wavelength: UV@254nm; isocratic elution 70%C;
[0067] The specific method is as follows:
[0068] T1 solvent preparation: prepare two parts of solvent A and B in proportion, the solvent volume ratio is formic acid: water: methanol = 1:300:700, prepare 400ml, two parts; prepare one part of eluent C in proportion, the volume of eluent The ratio is formic acid: water: methanol: ethyl acetate = 1:300:700:50, prepare 30L, one part;
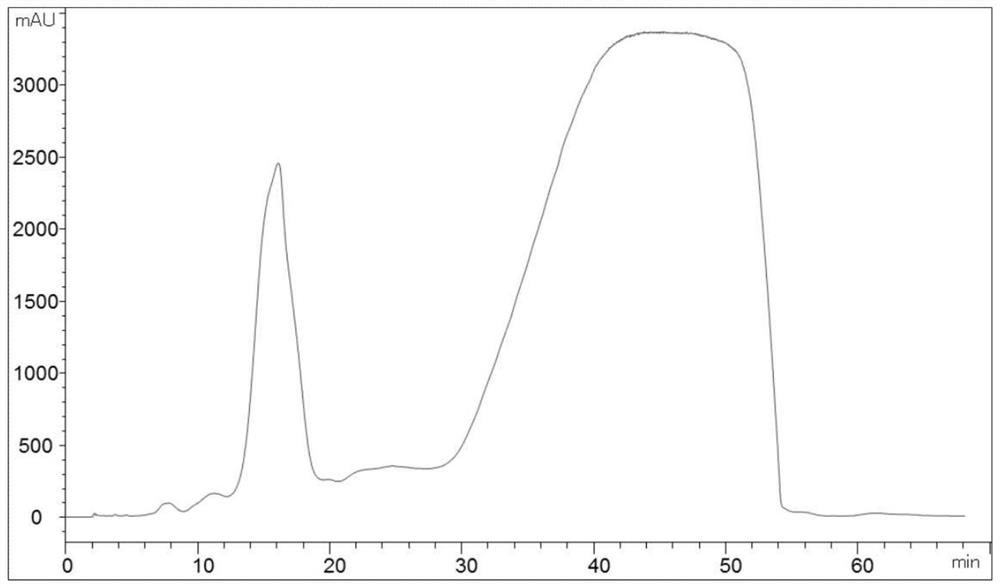
[0069] T2 sample is weighed: take by weighing pirarubicin crude product 40g (content 65.87%, see Table 1 or figure 2 ), and put into the Erlenmeyer flask;
[0070] T3 sample dissol...
Embodiment 2
[0094] Adopt the preparative chromatograph identical with embodiment 1, preparative column and elution method, its difference is:
[0095]Get pirarubicin crude product 65g (loading 6.6%), content 70.15%, with solvent (formic acid: water: acetonitrile=1:350:650, volume ratio) 400ml, magnetic stirring makes fully dissolve; : water: acetonitrile: ethyl acetate = 1:350:650:30) After elution and equilibration, the pirarubicin preparation column (100mm×250mm, containing about 0.98kg of filler mass), the flow rate of the sample solution was 200ml / min, and the Elution with eluent after sample, eluent flow rate 400ml / min, elution 60min altogether, collect pirarubicin purification sample and use HPLC to detect and collect pirarubicin component solution, merge qualified components, after lyophilization 49.68 g of pure pirarubicin was obtained, the purity detected by HPLC was 99.71%, the single impurity was less than 0.1%, and the recovery rate was 76.43%.
[0096] See Table 4 for the de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com