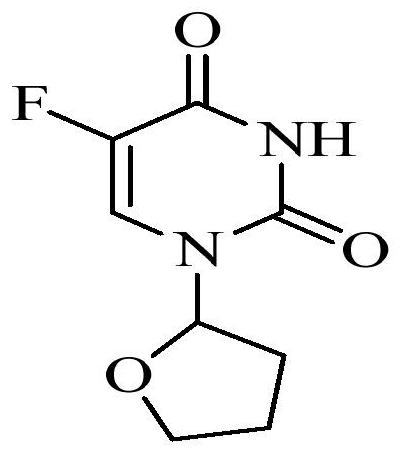
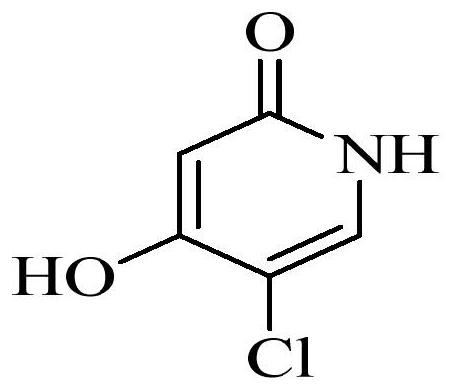
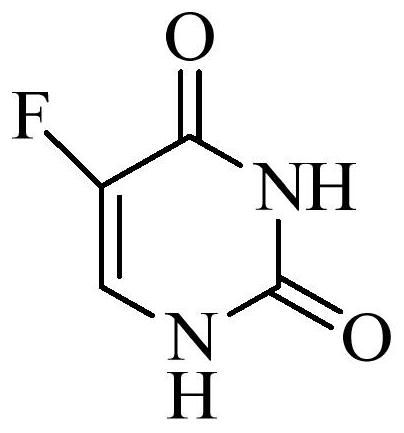
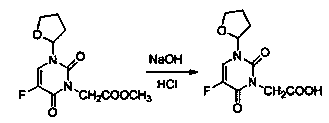Patents
Literature
72 results about "Tegafur" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
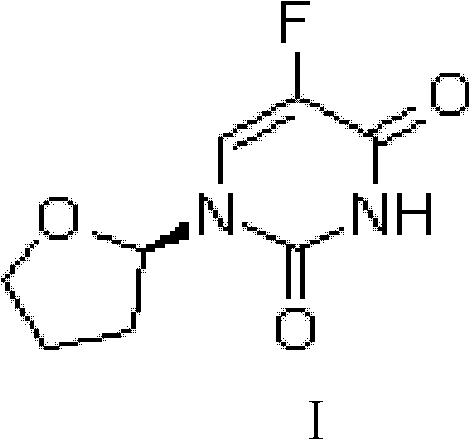
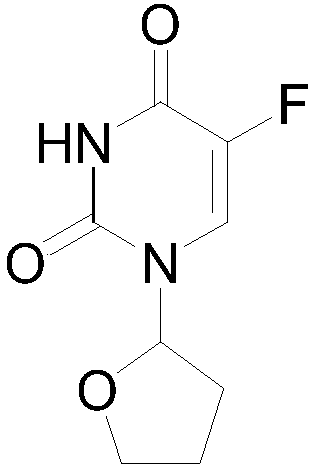
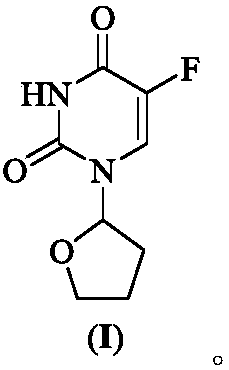
Tegafur is a chemotherapeutic prodrug of 5-fluorouracil (5-FU) used in the treatment of cancers. It is a component of the combination drug tegafur/uracil. When metabolised, it becomes 5-FU. It was patented in 1967 and approved for medical use in 1972.
Industrial tegafur synthesizing method
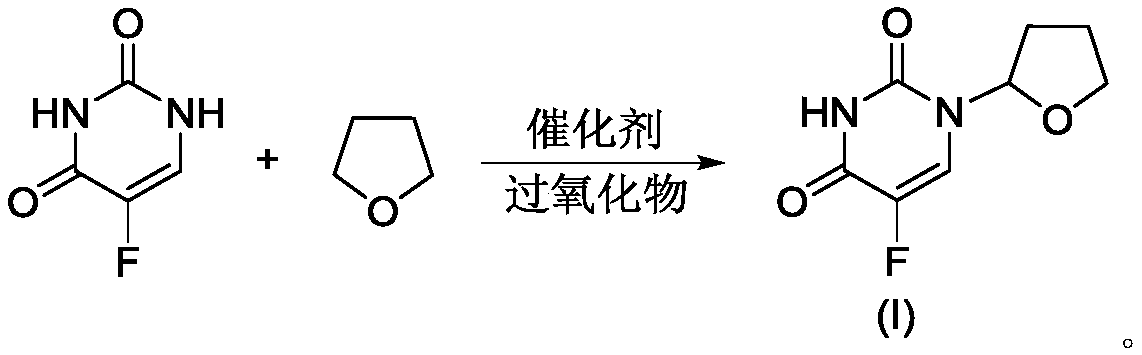
The invention belongs to the technical field of medicine, and specifically relates to an industrial tegafur synthesizing method comprising the steps that: under inert gas pressure control and the effects of a Lewis acid catalyst, 5-fluorouracil and 2,3-dihydrofuran are subjected to a substitution reaction in an aprotic polar solvent; and acidification and refining are carried out, such that tegafur is obtained. The method has the advantages of simple reaction process, high yield, less side reactions, mild reaction conditions, and the like. Pharmacopoeia standards can be satisfied with simple refining, and purity is higher than 99.7%.
Owner:SHANDONG NEWTIME PHARMA
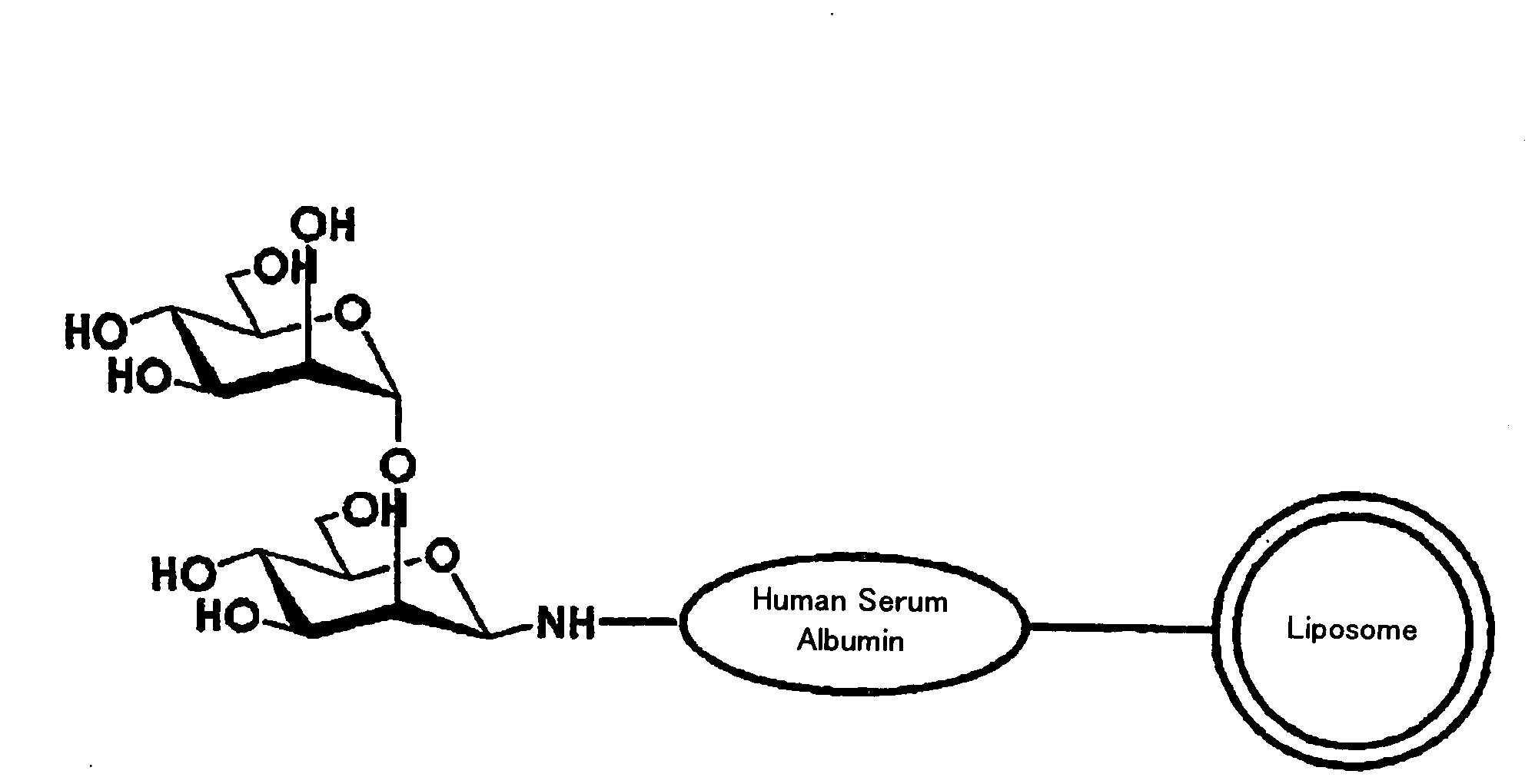
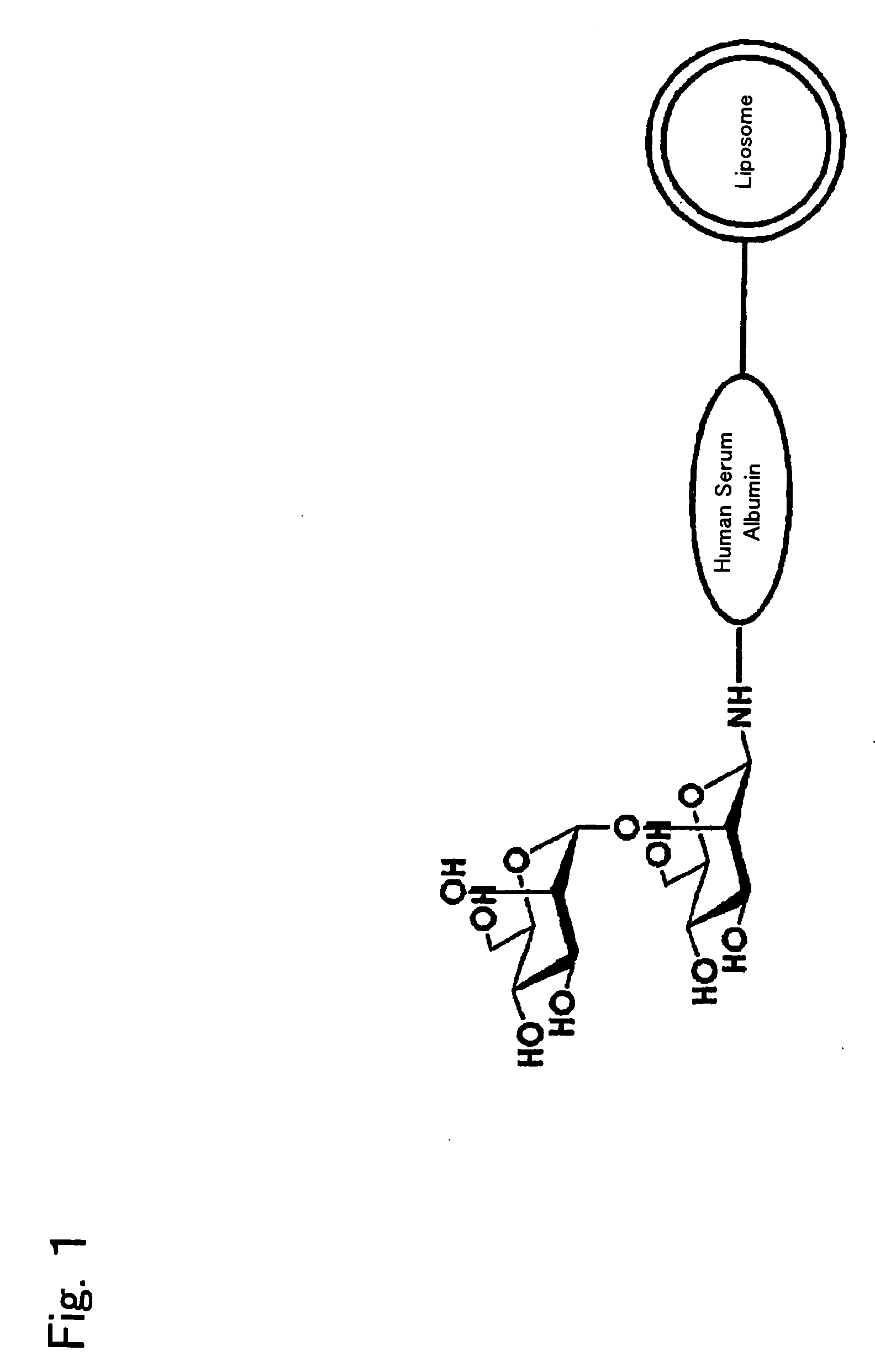
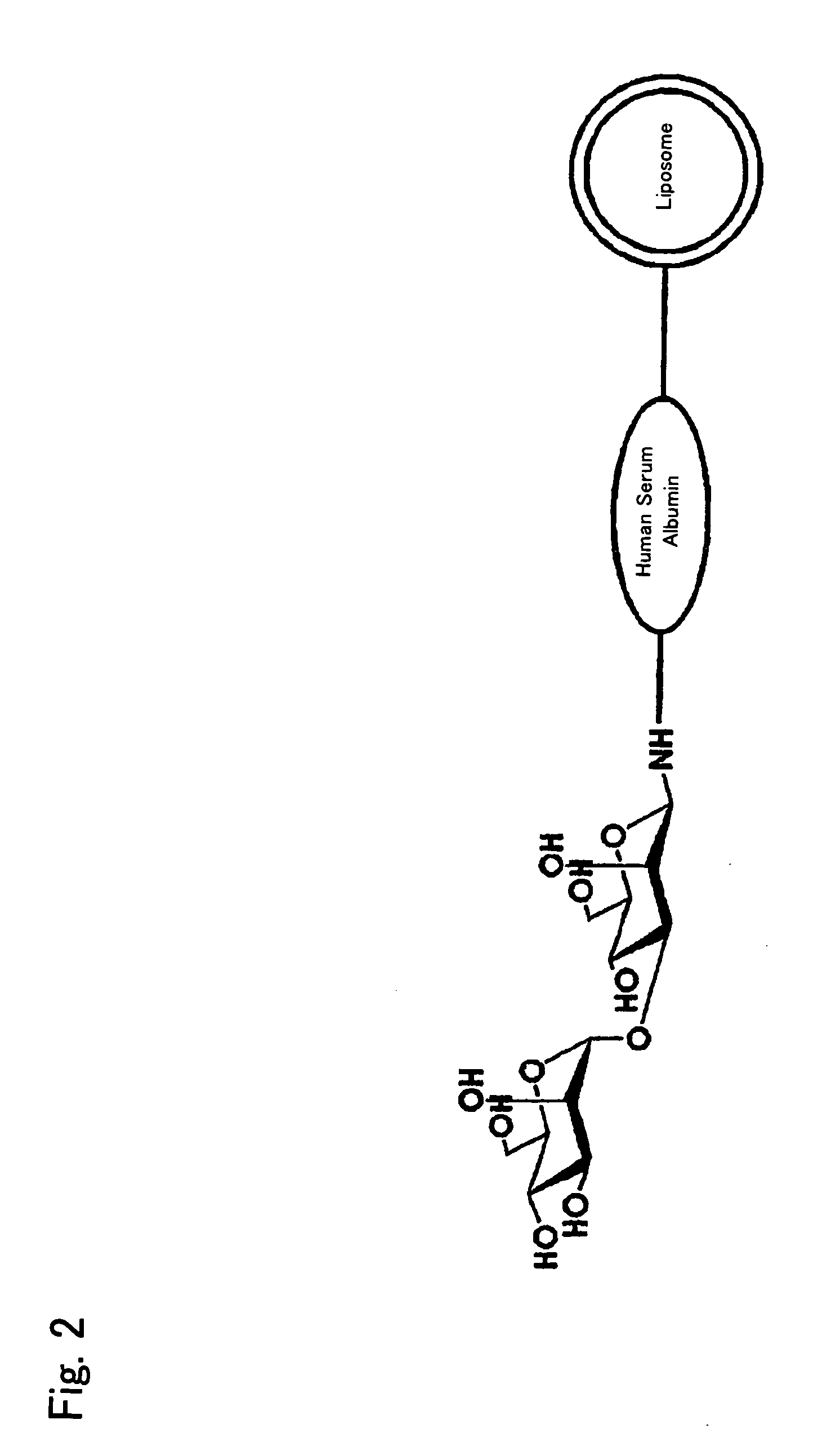
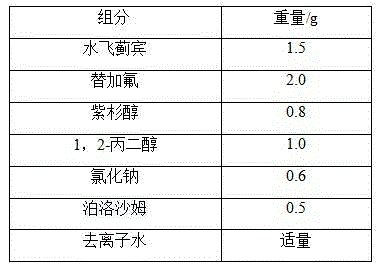
Liposome Preparation
InactiveUS20090169610A1Improve targetingReduce drug doseOrganic active ingredientsAntibody ingredientsGnRH AntagonistLiposome
The present invention provides cancer treatment preparations of excellent targetability. The sugar chain-modified liposomes of the present invention, which contain an aromatase inhibitor, anti-androgenic agent, lyase inhibitor, GnRH agonist, GnRH antagonist, anti-angiogenic agent, tyrosine kinase inhibitor, serine-threonine kinase inhibitor, antibody having an anticancer activity, ansamitocin, capecitabine, celmoleukin, docetaxel hydrate, gemcitabine hydrochloride, oxaliplatin, prednisolone, tegafur-uracil mixtures, zinostatin stimalamer or arsenic trioxide may be used as cancer treatment preparations having an excellent targetability.
Owner:SIEMENS AG +1
Tegafur/layered duplex metal hydroxide nanometer hybrid and preparation method thereof
InactiveCN101785860AHigh drug loadingGood slow releaseOrganic active ingredientsInorganic non-active ingredientsSide effectMedicine
The invention relates to a Tegafur / LDHs nanometer hybrid and a preparation method thereof, and aims to provide a method for preparing a Tegafur / LDHs nanometer hybrid for slow release of Tegafur to improve potency and reduce the toxic or side effect of medicaments by taking LDHs as a carrier. LDHs is taken as a main body, the Tegafur is taken as an intercalation object, and the Tegafur is assembled between LDHs layers by a coprecipitation method or a structure reconstruction method to prepare the Tegafur / LDHs nanometer hybrid. The Tegafur / LDHs nanometer hybrid and the preparation method have the advantages that: the nanometer hybrid prepared by the two methods is large in medicament-loading capacity and has good slow-release effect; the adopted preparation method is simple and the condition is moderate; the structure, compositions and the release rate of the Tegafur / LDHs can be controlled by adjusting a synthetic method or synthesis conditions or changing factors such as concentration, the pH, temperature, aging time and the like in the synthetic process of the medicaments.
Owner:QINGDAO UNIV OF SCI & TECH
Anti-cancer drugs slow release agent comprising anticancer antibiotics and booster thereof
Disclosed is an anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer antibiotics are selected from Idarubicin, Valtaxin, Pirarubicin and Mitoxantrone, The anti-metabolite drugs are selected from Pemetrexed, Carmustine, Tegafur, Zalcitabine, Emtritabine, Galocitabine, Ibacitabine, Ancitabine, Decitabine, Flurocitabine, Enocitabine, Imidazoletabine, Capecittabine, Gemcitabine, Fludrarbine, Raltitrexed, Dexrazoxane, Cladribine, Nolatrexed and folic acid, The slow release auxiliary materials are selected from EVAc, Polifeprosan, sebacylic acid copolymer, lactic acid, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The slow release microspheres can also be prepared into slow release implanting agent for injection or placement in or around tumor.
Owner:SHANDONG LANJIN PHARMA
A kind of method for preparing tegafur
InactiveCN102285972AImprove utilization efficiencyImprove timelinessOrganic chemistryFuranReaction rate
The invention relates to a method for preparing tegafur, which belongs to the field of drug synthesis. Follow the steps below: (1) Weigh 2,3-dihydrofuran and ethanol into the flask, add solvent tetrahydrofuran to it, then weigh 5-20% of the total mass of raw materials CuCl2, microwave irradiation 250W, react at 25°C for 0.6h. (2) Cool to room temperature, then add 5-fluorouracil with a molar ratio of 3:10 to 2,3-dihydrofuran, microwave irradiation 200-600W, reaction temperature 60-130°C, and react for 0.7h; After boiling the solvent, an oily substance was obtained; washed with ether to obtain a white solid, and the obtained solid was recrystallized with absolute ethanol to obtain the product. The process does not use toxic solvent chloroform, does not use high-boiling point solvents, and the one-step production cost is low. Microwave irradiation-assisted synthesis can speed up the reaction rate, and is widely used in organic substitution reactions.
Owner:JIANGSU UNIV
Slow released anticancer medicine preparation with both amrubicin and its synergist
The slow released anticancer medicine injection containing both amrubicin and its synergist consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent carboxymethyl cellulose, etc. and with viscosity of 100-3000 cp at 25 deg.c. The effective anticancer component is amrubicin, idarubicin, etc and / or antimetabolite composition selected from carmofur, tegafur, zalcitabine, etc. The slow releasing supplementary material is selected from EVAc, sebacic acid copolymer, lactic acid polymer, etc. The slow released microsphere may be also prepared into slow released implanting agent set around or inside the tumor to strengthen the chemotherapy or radiotherapy effect.
Owner:JINAN KANGQUAN PHARMA TECH
Tegafur/layered duplex metal hydroxide nanometer hybrid and preparation method thereof
InactiveCN101785860BHigh drug loadingGood slow releaseOrganic active ingredientsInorganic non-active ingredientsSide effectMedicine
Owner:QINGDAO UNIV OF SCI & TECH
Tegafur-layered double-metal hydroxide nanometer hybrid modified with polyethylene glycol derivative on surface and preparation of nanometer hybrid
InactiveCN103505741AGood dispersionImprove stabilityPowder deliveryOrganic active ingredientsDispersion stabilityNano hybrid
The invention discloses a tegafur-layered double-metal hydroxide nanometer hybrid modified with polyethylene glycol monomethyl ether sulfate on the surface (abbreviated to TF-LDH-MPS) and a preparation method of the nanometer hybrid. The invention aims to provide a method as follows: using the tegafur (TF) intercalated LDH (TF-LDH) nanometer hybrid as a model system, using the polyethylene glycol derivative (PEG) polyethylene glycol monomethyl ether sulfate (MPS) with pretending invisibility as a modifier to prepare the MPS modified TF-LDH nanometer hybrid. The nanometer hybrid can be used for improving the dispersion stability and the pretending invisibility of the tegafur-layered double-metal hydroxide, and improving targeted transport and controlled release effects of tegafur, so that the pharmaceutical effect is improved, and the toxic and side effects of the drug are reduced, and the like.
Owner:QINGDAO UNIV OF SCI & TECH
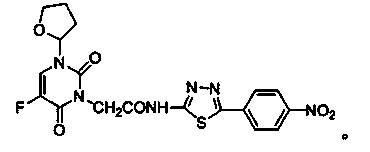
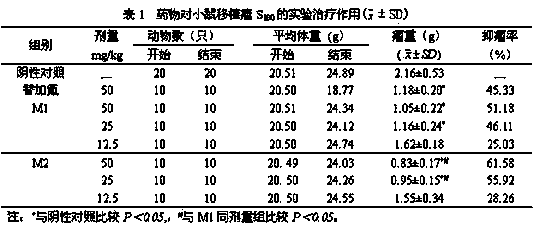
Tegafur derivative containing 1,3,4-thiadiazole heterocyclic ring and amide group
InactiveCN103864776AIncrease elimination timeLong duration of actionOrganic active ingredientsOrganic chemistryDitazoleStructural formula
The invention provides a tegafur derivative containing a 1,3,4-thiadiazole heterocyclic ring and an amide group. The structural formula of the tegafur derivative is as shown in the specification. A preparation method of the tegafur derivative comprises the steps of (1) dissolving a 3-(methoxycarbonylmethyl)tegafur in methanol, dropwise adding a sodium hydroxide solution, extracting by using ethyl acetate and distilled water and separating out an organic layer and a water layer, next, extracting by using ethyl acetate and blending the organic layers, and adding anhydrous sodium sulfate to obtain 1-(tetrahydro-2-furyl)-3-carbethoxy-5-fluoro-2,4-pyrimidinedione; (2) blending 1-(tetrahydro-2-furyl)-3-carbethoxy-5-fluoro-2,4-pyrimidinedione with dioxane, then adding sulfoxide chloride to obtain a 1-(tetrahydro-2-furyl)-3-chloracetyl-5-fluoro-2,4-pyrimidinedione solution, and then adding dioxane, evenly mixing and then sealing for later use; (3) taking 2-amino-5-p-nitrophenyl-1,3,4-thiadiazole, dioxane and triethylamine, and dropwise adding the 1-(tetrahydro-2-furyl)-3-chloracetyl-5-fluoro-2,4-pyrimidinedione solution obtained in the step (2) to obtain a pure product 1-(tetrahydro-2-furyl)-3-acetamido-[5-p-nitrophenyl-(1,3,4-thiadiazole-2-yl)]-5-fluoro-2,4-pyrimidinedione. The tegafur derivative has anti-tumor effect and no obvious toxicity, and is used for clinically treating malignant tumors.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
Synthetic method for antineoplastic medicine tegafur
InactiveCN104513230AHigh yieldHigh purityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsGeneration rateReaction temperature
The invention discloses a synthetic method for an antineoplastic medicine tegafur. The synthetic method comprises: adding a hydroxyapatite-fixedly supported magnesium chloride or magnesium trifluoromethanesulfonate catalyst, 5-fluorouracil and 2,3-dihydrofuran into dimethyl sulfoxide, adjusting the pH value of the solution to be 4-5, introducing inert gas and performing substitution reaction at 90-120 DEG C; and filtering and distilling the solvent to obtain an oily substance, repeatedly leaching with diethyl ether to obtain a white solid, and using anhydrous ethanol to recrystalize the obtained solid, so as to obtain the target product. The hydroxyapatite-fixedly supported magnesium chloride or magnesium trifluoromethanesulfonate catalyst is employed to replace a conventional Louis catalyst, the reaction conditions are optimized, and the pH value and the reaction temperature are adjusted, so that the generation rate of the group substitution reaction at the first site is obviously improved, the reaction selectivity is improved, and generation of side products is reduced. The reaction conditions are mild, operation is simple, the obtained compound is convenient for separation and purification, the high-yield high-purity tegafur product can be easily obtained after reaction, the yield is relatively substantially improved compared with that of a conventional technology, and the synthetic method is relatively suitable for industrial production.
Owner:丹阳恒安化学科技研究所有限公司
Preparation method of tegafur
ActiveCN110655506ALower activation energyLow reaction temperatureOrganic chemistryPtru catalystBiochemical engineering
The invention provides a preparation method of tegafur. The method mainly comprises the following steps: reacting 5-fluorouracil with tetrahydrofuran under the action of a catalyst, extracting, and recrystallizing to obtain a high-purity tegafur product. Compared with the prior art, the preparation method disclosed by the invention is simple to operate, mild in reaction condition, high in productyield, high in purity, less in pollution and suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
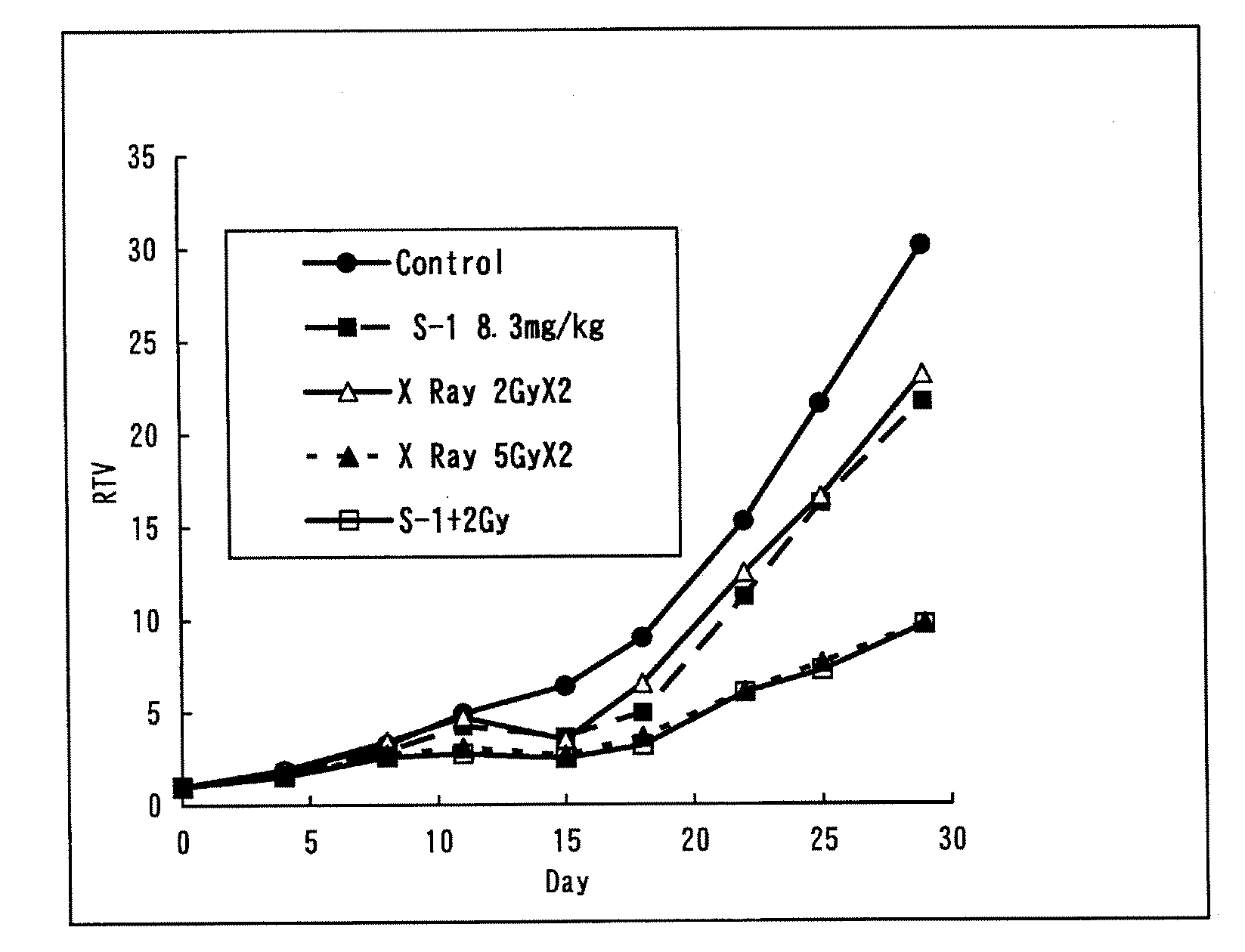
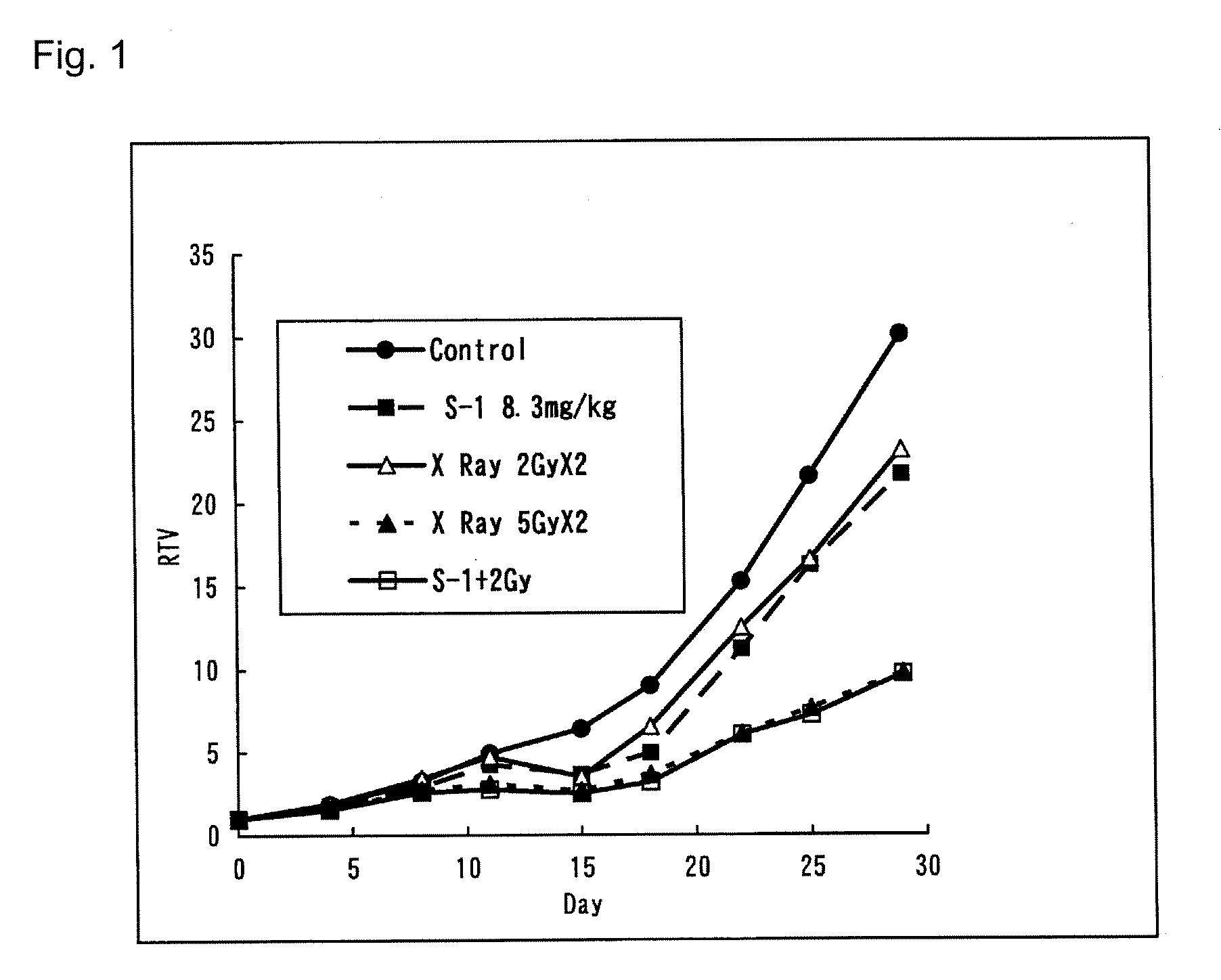
Radiotherapy Enhancer
InactiveUS20080275071A1Excellent cancer therapeutic effectReduce radiation doseBiocideUnknown materialsADR - Adverse drug reactionCancer Radiotherapy
Owner:TAIHO PHARMA CO LTD
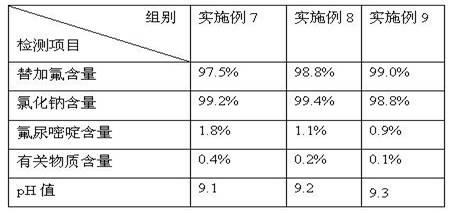
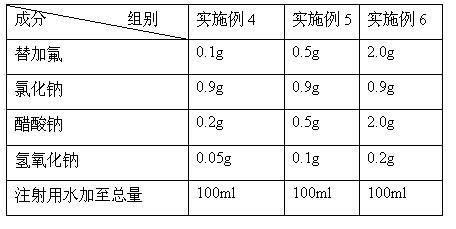
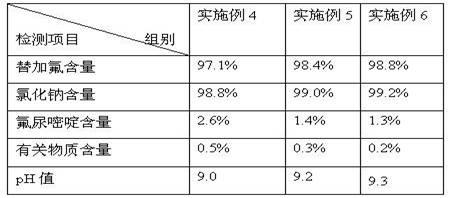
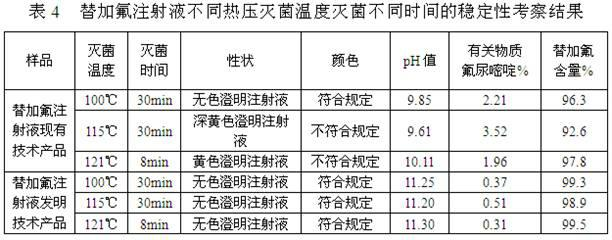
Preparation method of stable tegafur injection liquid
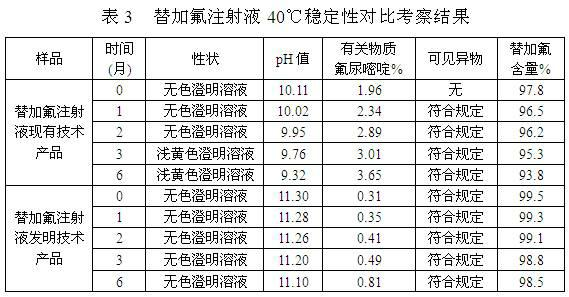
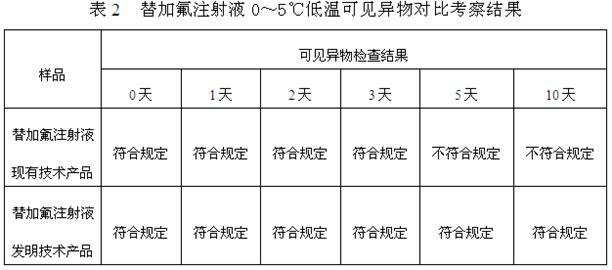
ActiveCN103989629AReduce contentGood for clinical useOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantColor changes
The invention discloses a stable tegafur Injection liquid and a preparation method thereof. The stable tegafur Injection liquid is mainly prepared from tegafur and injection water by using a sodium hydroxide solution and / or an acid solution to adjust the injection liquid pH value to be 11.1-12.0, adding an antioxidant and a pH value buffer, charging nitrogen for protection, and then sterilizing at high temperature. The preparation method can make the tegafur Injection liquid more stable, high temperature sterilization can be performed, compared with the prior art, related substances are greatly reduced, especially the problems that pH value is decreasing, the solution color changes into yellow, the visible foreign matter testing is unqualified and the related substance testing is unqualified in high temperature sterilization and storage processes of a tegafur Injection liquid prepared from products in the prior art can be solved, and the method can ensure the product meets the requirement of drug quality standards and facilitates clinical medicine use and promotion.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Method for stabilizing tegafur in aqueous solution
InactiveCN102100667AQuality improvementOrganic active ingredientsInorganic non-active ingredientsSodium acetateAnticarcinogen
The invention relates to a method for stabilizing tegafur in an aqueous solution, relating to the technical field of anticarcinogen tegafur injection application. The method comprises the following steps of: taking 0.7-1.4g of sodium chloride, and preparing a 5-30% sodium chloride solution by using water for injection; adding 0.1-3.0g of sodium carbonate, sodium phosphate or sodium acetate and 0.1-2.0g of tegafur to the sodium chloride solution, stirring and dissolving; and adding 0.01-0.3g of sodium hydroxide, regulating a pH value to be 8.5-10.5, adding the water for injection till the volume reaches 100ml, filling and sterilizing. The invention solve the technical problem that the stable injection with high addition amount can not be prepared due to the unstability of the tegafur in the aqueous solution; the prepared aqueous solution of tegafur completely meets the preparation requirement on injections with small capacity and large capacity, is safe, stable, and has reliable quality; in addition, the invention enlarges the application of the tegafur raw material and lays the foundation for the development of injection formulations, in particular injections with large capacity of more than 100ml.
Owner:山东华鲁制药有限公司
Novel pharmaceutical tegafur co-crystal and preparation method thereof
InactiveCN104496972AHigh activityInhibit synthesisCarboxylic acid amide separation/purificationAntineoplastic agentsKetoneSolvent
The invention belongs to the technical field of pharmaceutical co-crystals, particularly relates to a novel pharmaceutical tegafur co-crystal and a preparation method thereof, and more particularly relates to a novel co-crystal consisting of tegafur and an organic matter of 4-phenol benzamide. The co-crystal takes the tegafur as a pharmaceutical effective component (API); the selected matter for forming the co-crystal is the 4-phenol benzamide; the tegafur is connected with the 4-phenol benzamide by hydrogen bonds and a pi-pi accumulation effect; and the stoichiometric ratio (mole ratio) of both the tegafur and the 4-phenol benzamide is 1:1. The method for preparing the co-crystal disclosed by the invention is a temperature reduction type crystallization method which is simple and convenient to operate and easy to implement; used solvents are organic solvents which are low in boiling point and low in cost, such as acetonitrile and ketone; and convenience is provided for realizing industrial production.
Owner:ZHEJIANG UNIV
Preparation method of tegafur gimeracil oteracil impurity BCB
InactiveCN107089942AOrganic chemistryComponent separationChromatographic separationTegafur/gimeracil/oteracil
The invention discloses a preparation method of a tegafur gimeracil oteracil impurity BCB. The preparation method comprises the following steps: (1) carrying out reaction on gimeracil and tegafur in presence of a catalyst to obtain a mixture containing BCB and gimeracil; and (2) carrying out chromatographic separation to obtain a BCB impurity. The preparation method disclosed by the invention has the advantages that technology is simple, reaction conditions are mild, and a high-purity BCB product can be obtained.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
A kind of preparation method of tegafur
InactiveCN106397416BEasy to purifyMild reaction conditionsOrganic chemistryHigh pressureBiological activation
The invention provides a preparation method of tegafur. According to the preparation method, dimethylchlorosilane is adopted for activation, and tegafur is prepared via reaction of 5-fluorouracil with 2,3-dihydrofuran; reaction conditions are mild; no high temperature or high pressure is needed; no special equipment requirement is needed; operation is simple; compound purifying is convenient; tegafur products with purity of 99.9% or higher and yield of 80% or higher are obtained; and the preparation method is suitable for industrialized production.
Owner:深圳万乐药业有限公司
Anticancer composition loaded with anti-metabolism medicine fluorouracil and synergist thereof
InactiveCN101390829AOrganic active ingredientsPharmaceutical delivery mechanismAdditive ingredientSuspending Agents
Disclosed is an anticancer, which contains antimetabolics fluorouracil and the synergist thereof, and is a sustained-release injection. The anti-cancer combination is composed of sustained-release microspheres and solvent; wherein, the sustained-release microsphere contains effective anticancer ingredients and auxiliary material; the solvent is common solvent or special solvent which contains suspending agent. The viscosity of the suspending agent is 100cp-3000cp (20 DEG C-30 DEG C) and is selected from carboxymethyl cellulose; the antimetabolics is selected from methotrexate, fluorouracil, carmofur, tegafur, decitabine, capecitabine or gemcitabine; the synergist is tetrazine medicine or anticancer antibiotics; the auxiliary material is selected from p(LAEG-EOP), p(DAPG-EOP), or polyphosphate copolymer, or copolymer or mixture of polyphosphate, polylactic acid, polifeprosan, sebacic acid and PLGA; the anticancer combination also can be made into sustained-release implants which can be injected or implanted in tumor or the periphery of tumor with the effective medicine concentration maintained for more than 50 days; meanwhile, the systemic reaction of the medicine is obviously reduced; the treatment effects of the non-operative treatments such as chemotherapy or radiation treatment are selectively enhanced.
Owner:JINAN KANGQUAN PHARMA TECH
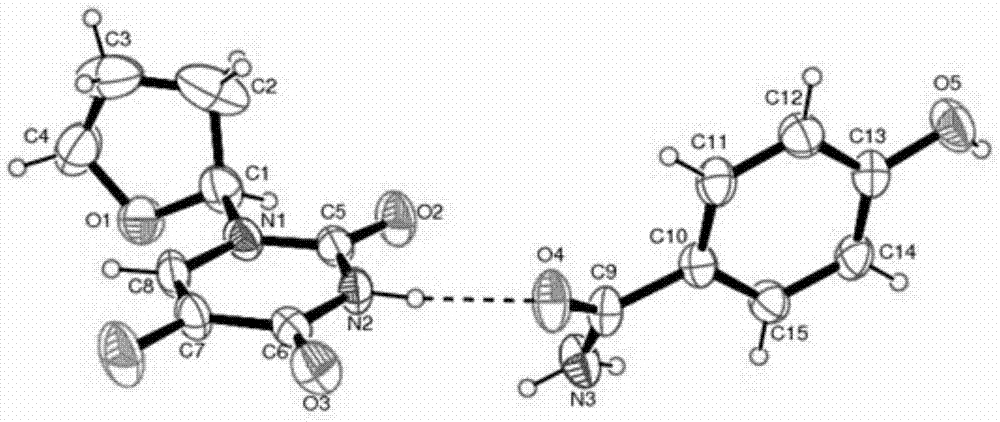
Tegafur-L-proline eutectic and preparation method thereof
ActiveCN111689947ASimple and fast operationGood chemical stabilityOrganic active ingredientsOrganic chemistry methodsPhysical chemistryPharmaceutical drug
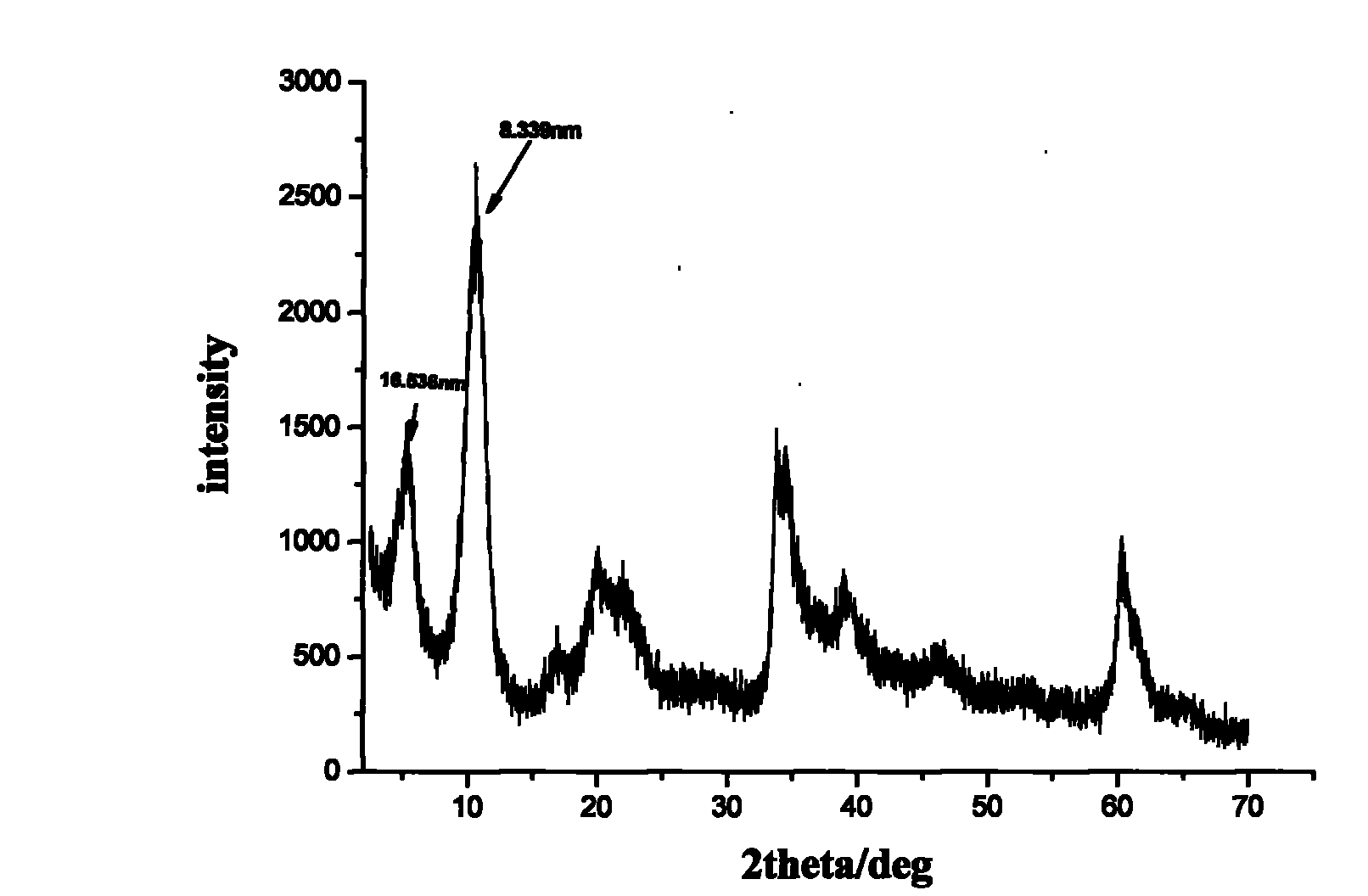
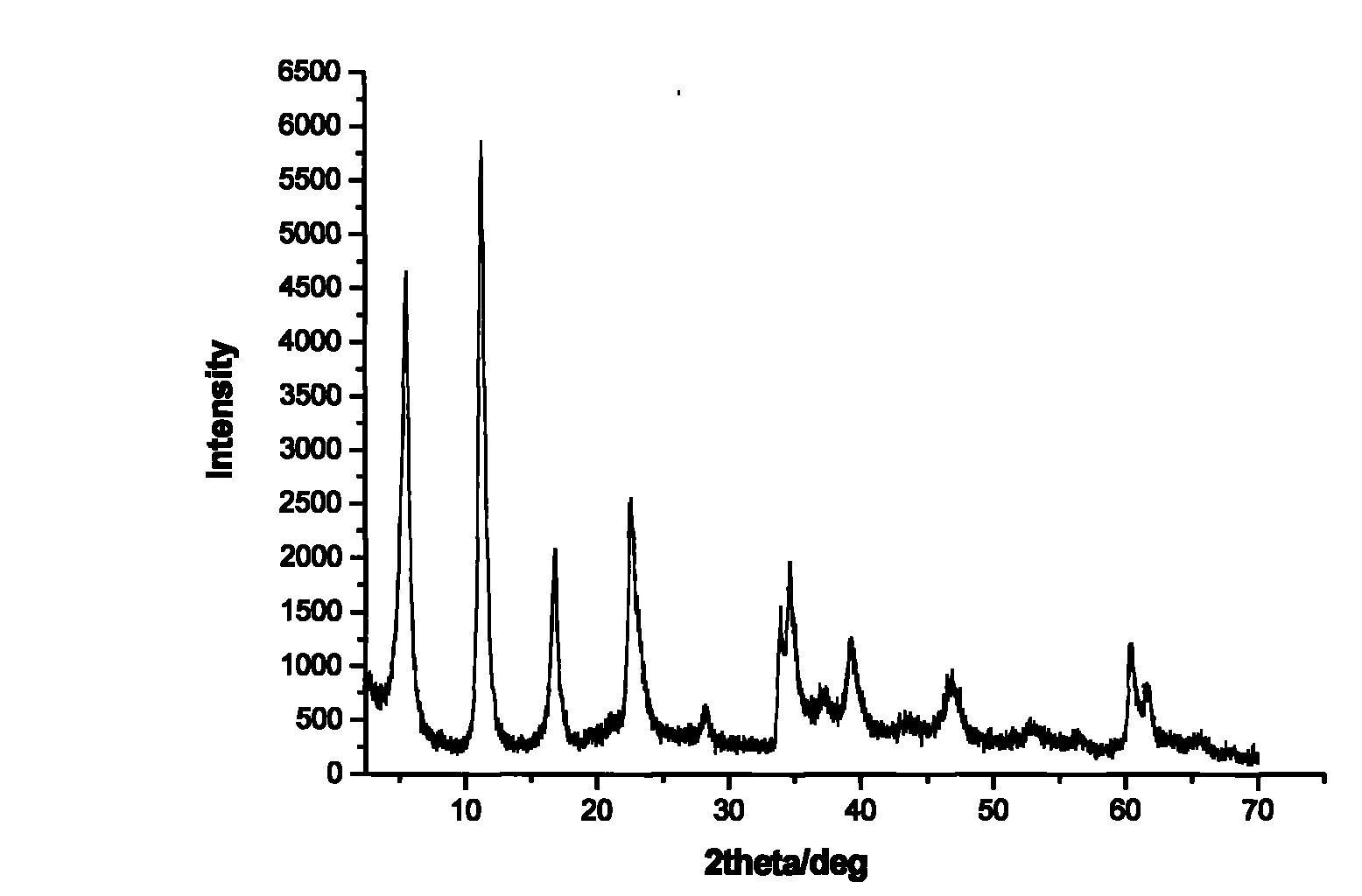
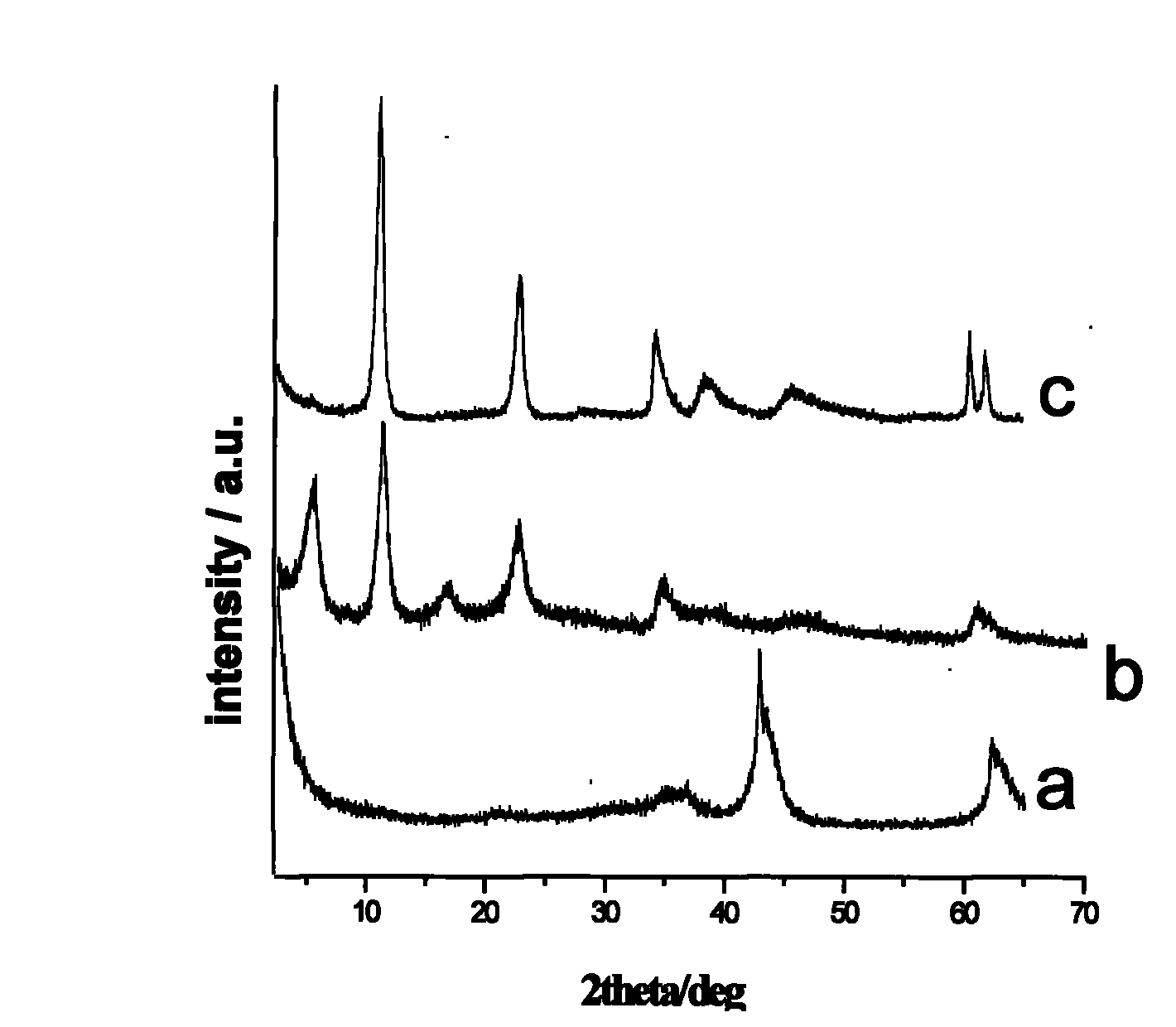
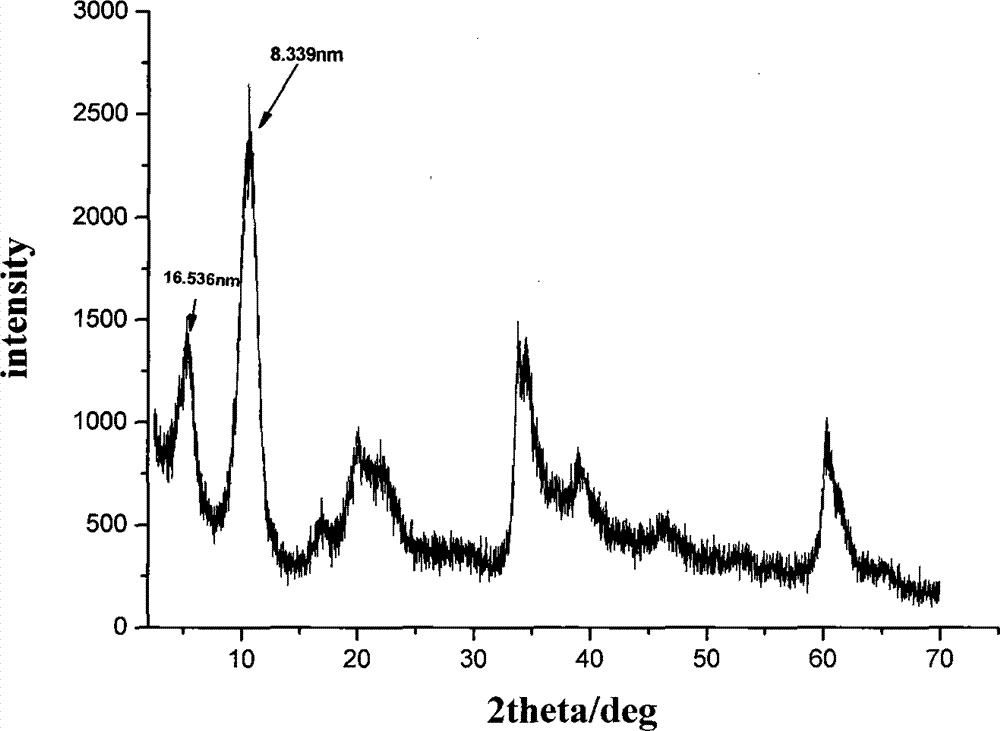
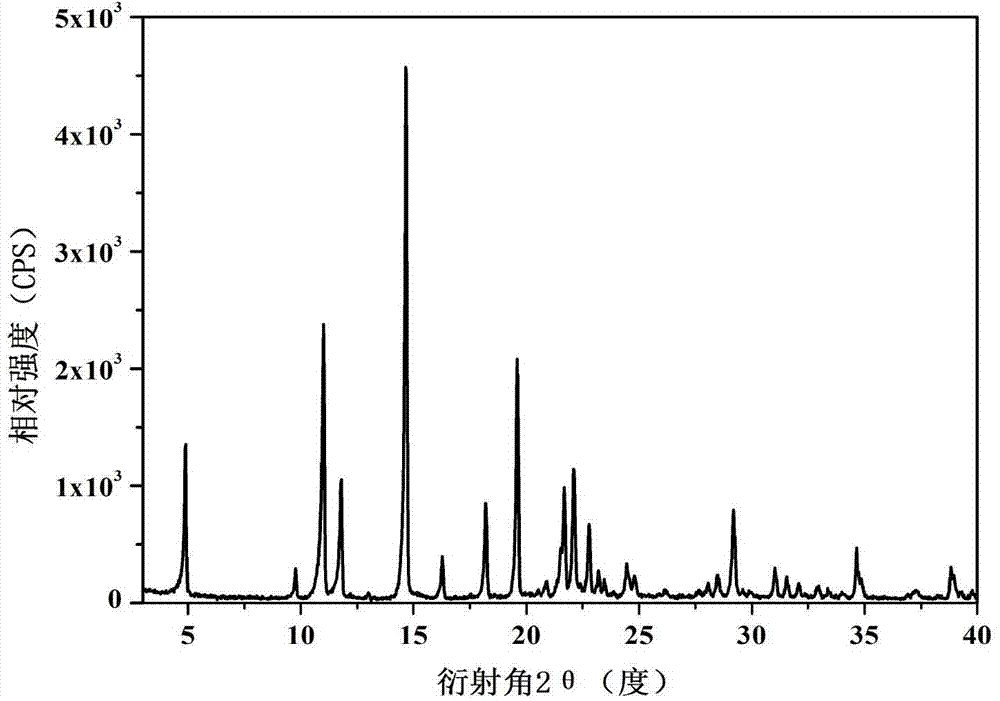
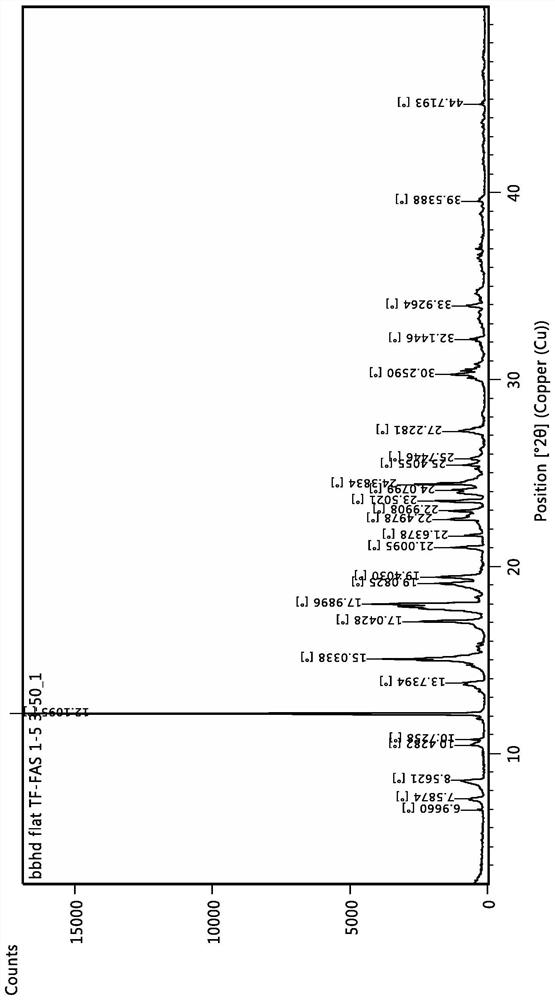
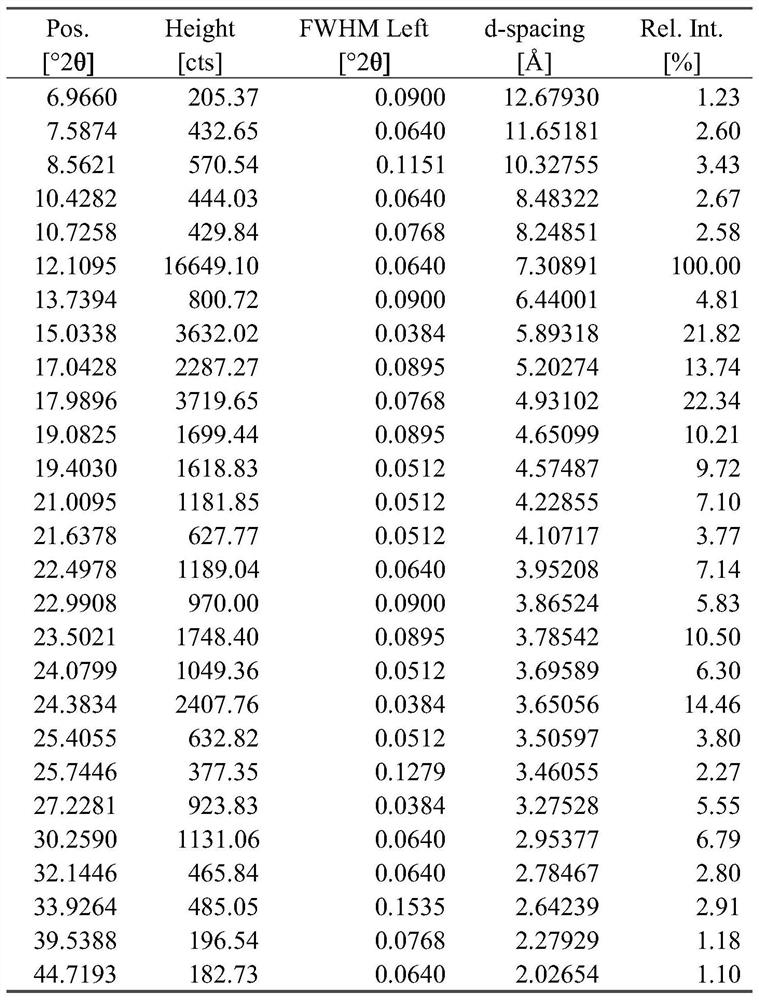
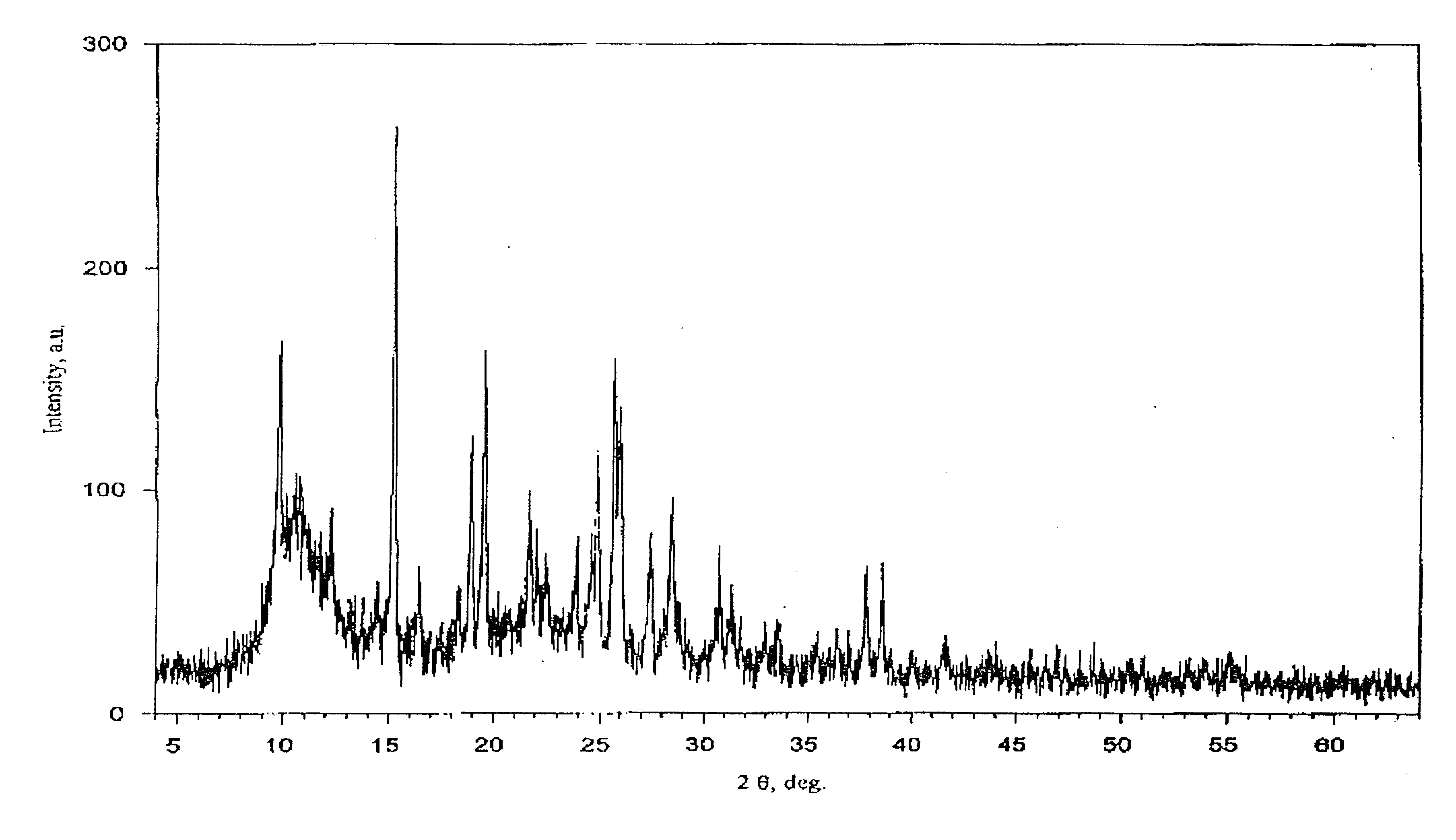
The invention discloses a tegafur-L-proline eutectic and a preparation method thereof, and belongs to the technical field of organic medicine eutectic. The molar ratio of tegafur to L-proline in the eutectic is 1:1. Under Cu-K[alpha] radiation, the an X-ray diffraction pattern has characteristic peaks, expressed by 2theta, at 7.0 + / - 0.2 degrees, 7.6 + / - 0.2 degrees, 8.6 + / - 0.2 degrees, 12.1 + / -0.2 degrees, 15.0 + / - 0.2 degrees and 18.0 + / - 0.2 degrees; wherein the eutectic is a monoclinic system, and the chiral space group is P21. The tegafur-L-proline eutectic crystal prepared by the preparation method disclosed by the invention is high in purity, relatively good in chemical stability and solubility and good in bioavailability.
Owner:LUNAN PHARMA GROUP CORPORATION
Sustained-released injection including platinum compound and the alkylate agent
The invention relates to an anti-cancer compound as a slow release injection which contains platinum compound and / or alkyl agent, formed by slow release micro ball and solvent. The slow release micro ball comprises the anti-cancer effective components and slow release findings, selected from platinum compound of kpeitabing, peimeiquse, caplatinum, or jxitabing and / or alkyl agent, the solvent is a common solvent or a special solvent with suspending agent, while the viscosity of suspending agent is 100cp-3000cp (at 20-30Deg. C), selected from carboxymethyl cellulose, the anti-cancer effective component is phosphoinositide 3-kinase restrainer and / or the phosphoinositide 3-kinase restrainer booster selected from self-anti-cancer antibiotics and / or tetrazine drug, the slow release finding is selected from phosphate polyester as p (LAEG-EOP) or p (DAPG-EOP), or the polyester or mixture of phosphate, PLA, polyphenyl, PLGA, poly (erucic acid dimmer-sebacic acid) or poly (fumaric acid-sebacic acid), and the alkyl agent is selected from ranimustine or the like. The anti-cancer compound can be made as slow release plant agent, to inject cancer or around cancer to hold the effective drug density for more than 60 days, while it can significantly reduce the general reaction of drug and selectively strengthen the effect of non-surgery treatments as chemotherapy or the like.
Owner:JINAN SHUAIHUA PHARMA TECH
Novel PD-1 tumor immunosuppressant and drug preparation method thereof
InactiveCN108498796AImprove antigen phagocytosisRapid responseHeavy metal active ingredientsOrganic active ingredientsApoptosisT cell
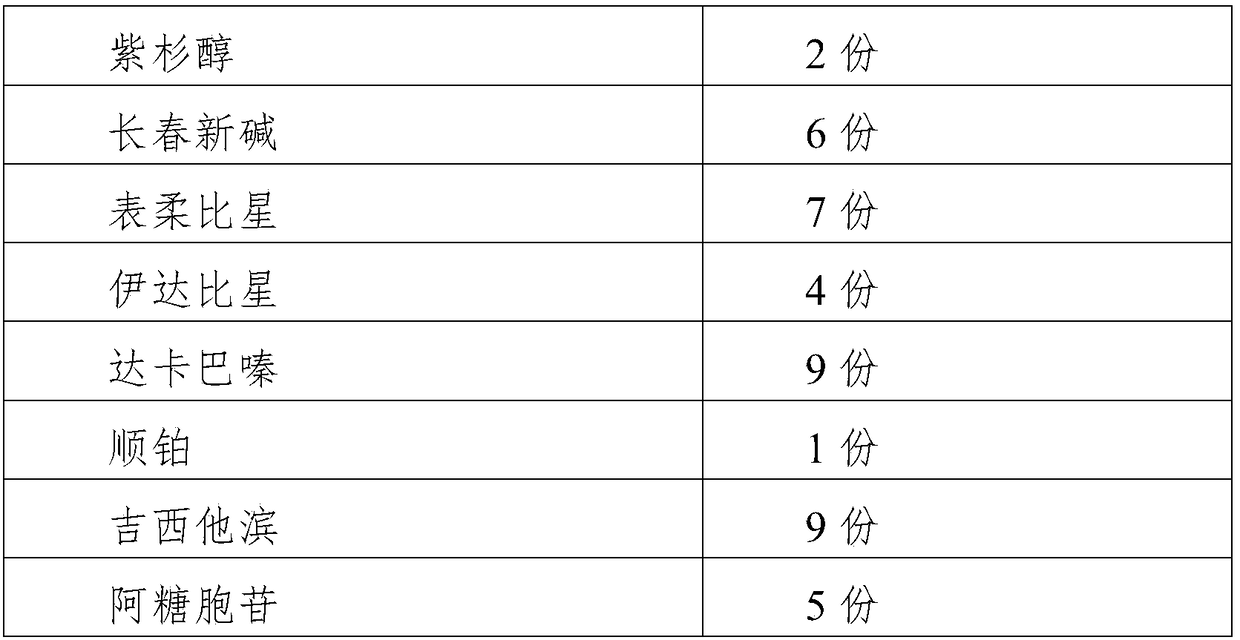
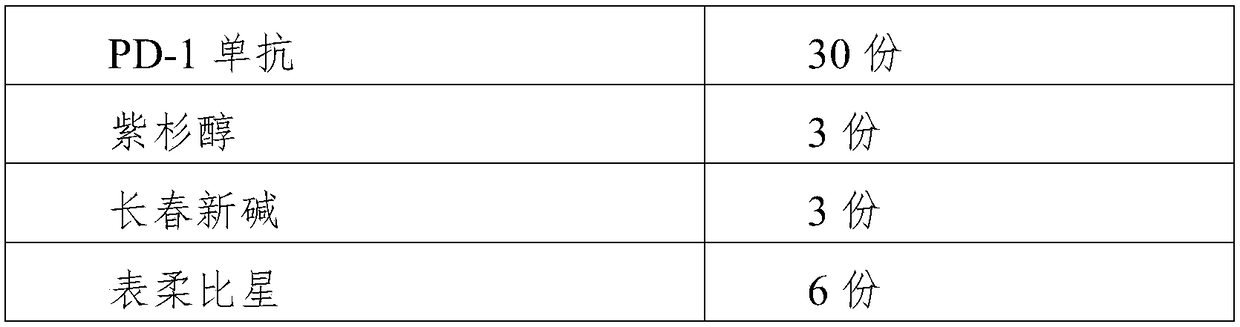
The invention relates to the technical field of drug inhibitors, in particular to a novel PD-1 tumor immunosuppressant and a drug preparation method thereof. The immunosuppressant is prepared from byweight, 30-45 parts of PD-1 monoclonal antibody, 2-6 parts of alkaloid, 4-7 parts of antibiotic, 3-9 parts of alkylating agent, 1-5 parts of platinum agent and 5-9 parts of metabolic antagonist; the alkaloid consists of one or more of paclitaxel, vincristine and docetaxel; the antibiotic consists of one or more of epirubicin, idarubicin and mitomycin; the alkylating agent is one or two of ifosfamide and dacarbazine; the platinum agent is one or two of cisplatin and oxaliplatin; the metabolic antagonist is one or more of gemcitabine, cytarabine and tegafur. The immunosuppressant can block the interaction between PD-L1 molecules expressed on tumor cells and receptors on activated T cells, inhibit the apoptosis of the activated T cells and improve the killing capability of the tumor cells.
Owner:HENAN TIANSHENG TAIFENG PHARM TECH CO LTD
Slow release injection containing platinum compound and alkylating agent
InactiveCN101011345APharmaceutical delivery mechanismPharmaceutical non-active ingredientsCarboplatinAdjuvant
Disclosed is a slow release injection containing platinum-group compounds and / or alkylating agents, which comprises slow release microspheres and dissolvent, the slow release microspheres include platinum-group compounds selected from Tegafur, Capecittabine, Pemetrexed, Carboplatin or Gemcitabine, and / or alkylating agent anticancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with PLA, Polifeprosan, poly(dodecanedioic acid-tetradecanedioic acid) or poly(fumaric acid-sebacylic acid). The alkylating agent is selected from Carmustine, Nimustine, Fotemustine, Lomustine or bendamustine. The anticancer composition can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Stable Tegafur injection and preparation method thereof
ActiveCN102178646AReduce contentGood for clinical useOrganic active ingredientsInorganic non-active ingredientsAntioxidantNitrogen
The invention discloses stable Tegafur injection and a preparation method thereof. The stable Tegafur injection is prepared mainly by the following steps of: mixing Tegafur and water for injection; adjusting the pH value to be 10.5 to 12.0 by using sodium hydroxide solution and / or acidic solution; adding an antioxidant and a pH value buffering agent and introducing nitrogen to perform protection; and performing high-temperature sterilization to prepare the injection. By the method, the Tegafur injection can be more stable; high-temperature sterilization can be performed; relative substances are greatly reduced compared with those in the prior art; particularly, the problems that the pH value is reduced, the color of the solution is changed to yellow, visible foreign matters are detected to be unqualified and relative substances are detected to be unqualified in the high-temperature sterilization and storage process of the Tegafur injection adopting the products in the prior art are solved; and the stable Tegafur injection can guarantee that the products meet the rule of the medicament quality standard and contributes to clinic administration and popularization.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Radiotherapy enhancer
InactiveUS20090281105A1Reduce doseReduce reduction reactionBiocideUnknown materialsRadical radiotherapyADR - Adverse drug reaction
Owner:TAIHO PHARMA CO LTD
Sustained-released injection including antimetabolite medicine and alkylate agent
The invention relates to a slow release injection which contains antimetabolite and / or alkyl agent, formed by slow release micro ball and solvent. The slow release micro ball comprises the anti-cancer effective components selected from platinum compound of kpeitabing, peimeiquse, caplatinum, or jxitabing and / or alkyl agent, the solvent is a common solvent or a special solvent with suspending agent, while the viscosity of suspending agent is 100cp-3000cp (at 20-30Deg. C), selected from carboxymethyl cellulose, the slow release finding is selected from phosphate polyester as p (LAEG-EOP) or p (DAPG-EOP), or the polyester or mixture of phosphate, PLA, polyphenyl, PLGA, poly (erucic acid dimmer-sebacic acid) or poly (fumaric acid-sebacic acid), and the alkyl agent is selected from ranimustine or the like. The anti-cancer compound can be made as slow release plant agent, to inject cancer or around cancer to hold the effective drug density for more than 50 days, while it can significantly reduce the general reaction of drug and selectively strengthen the effect of non-surgery treatments as chemotherapy or the like.
Owner:JINAN SHUAIHUA PHARMA TECH
Pharmaceutical composition for inhibiting proliferation of lung cancer cells and detection method
InactiveCN104873514AEnhanced inhibitory effectOrganic active ingredientsBiological testingCancer cellPharmaceutical drug
The invention provides a pharmaceutical composition for inhibiting proliferation of lung cancer cells, and belongs to the technical field of medicines. The pharmaceutical composition is prepared from silibinin, tegafur and taxane; the lung cancer cells are selected from non-small-cell lung cancer cell strains A549. The medicine composition has a good lung cancer cell inhibition effect and can obviously inhibit the proliferation of lung cancer cells.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
Slow release injection containing anti-metabolism medicament and alkylating agent
InactiveCN101011343APharmaceutical delivery mechanismPharmaceutical non-active ingredientsAdjuvantMicrosphere
Disclosed is a slow release injection containing antimetabolites and / or alkylating agents, which comprises slow release microspheres and dissolvent, the slow release microspheres include antimetabolites selected from Tegafur, Capecittabine, Pemetrexed, Carmofur or Gemcitabine, and / or alkylating agent anticancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with PLA, Polifeprosan, poly(dodecanedioic acid-tetradecanedioic acid) or poly(fumaric acid-sebacylic acid). The alkylating agent is selected from Carmustine, Nimustine, Fotemustine, Lomustine or bendamustine. The anticancer composition can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a period of effective concentration maintenance over 50 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN KANGQUAN PHARMA TECH
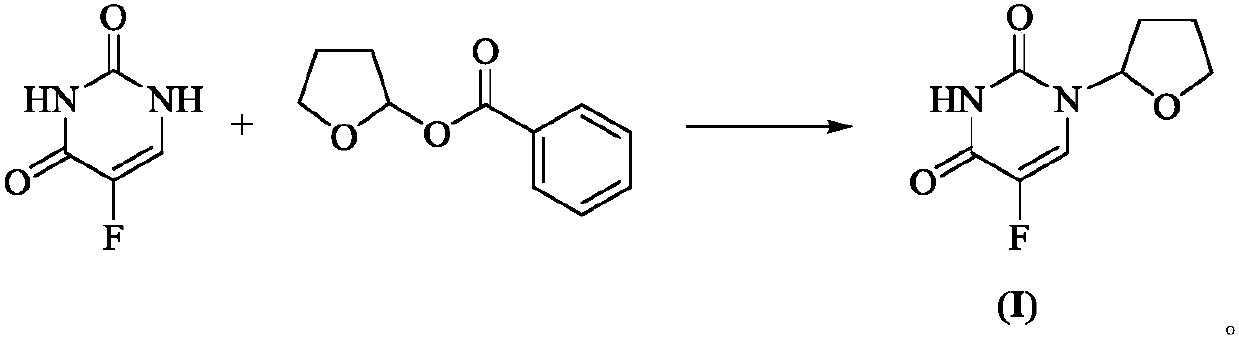
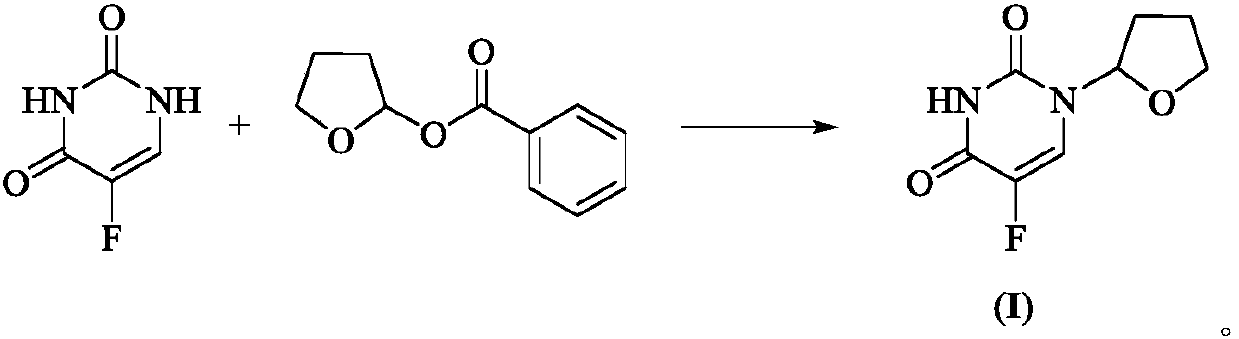
Preparation method of antitumor drug tegafur
ActiveCN110655507AEasy to operateSmooth reaction processOrganic chemistryChemical recyclingPtru catalystBiochemical engineering
The invention provides a preparation method of tegafur. The method mainly comprises the following steps: at room temperature, reacting 5-fluorouracil with 2-benzoyloxy tetrahydrofuran under the actionof an inorganic base and a phase transfer catalyst to obtain tegafur. Compared with the prior art, the method has the advantages of simple and easily available raw materials, simple operation, mild reaction conditions, stable quality, high yield, no pollution to the environment and suitability for industrial production, the purity of the obtained product is as high as 99.7%, and the maximum single impurity content is less than 0.1%.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for determining concentration of tegafur, gimeracil and 5-fluorouracil in plasma of tumor patient
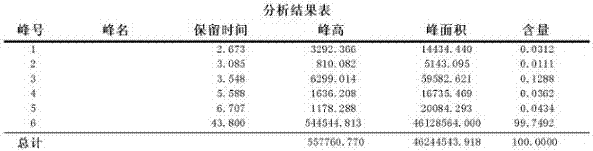
ActiveCN112834680AGood choiceSensitive highComponent separationESI mass spectrometryInternal standard
The invention relates to a method for determining the concentration of tegafur, gimeracil and 5-fluorouracil in plasma of a tumor patient. The tegafur, gimeracil and 5-fluorouracil in the plasma are analyzed through LC-MS / MS, a protein precipitation pretreatment method is adopted, a corresponding isotope label is used as an internal standard, an EclipseXDB-C18 column is adopted, gradient elution is carried out, and electrospray ionization (ESI) source tandem mass spectrometry detection is carried out. The method is high in specificity and selectivity, high in sensitivity and rapid in detection, and meets the requirement for large-batch sample analysis in clinical research.
Owner:苏州海科医药技术有限公司
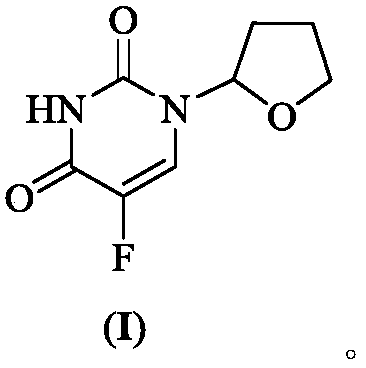
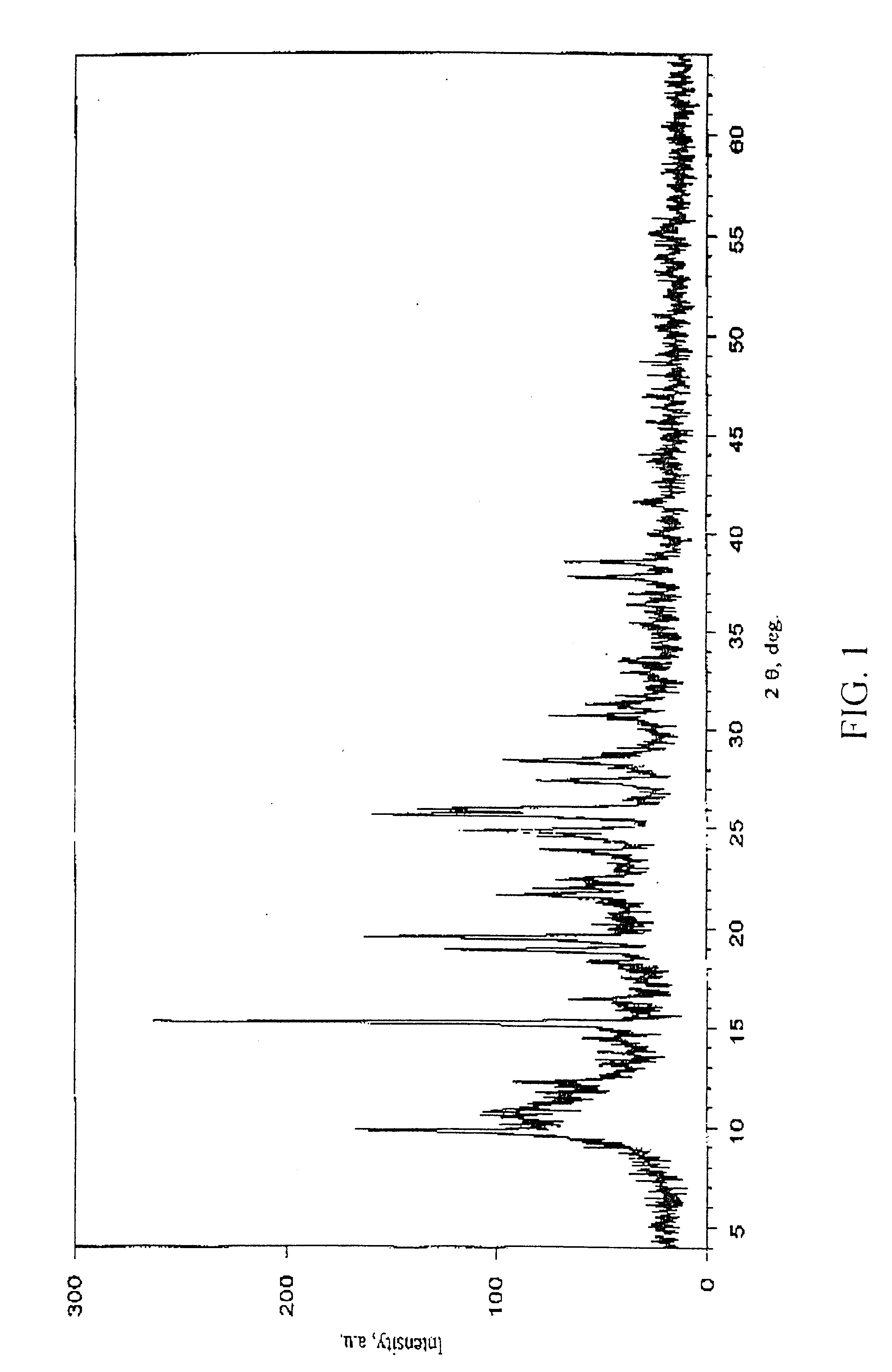
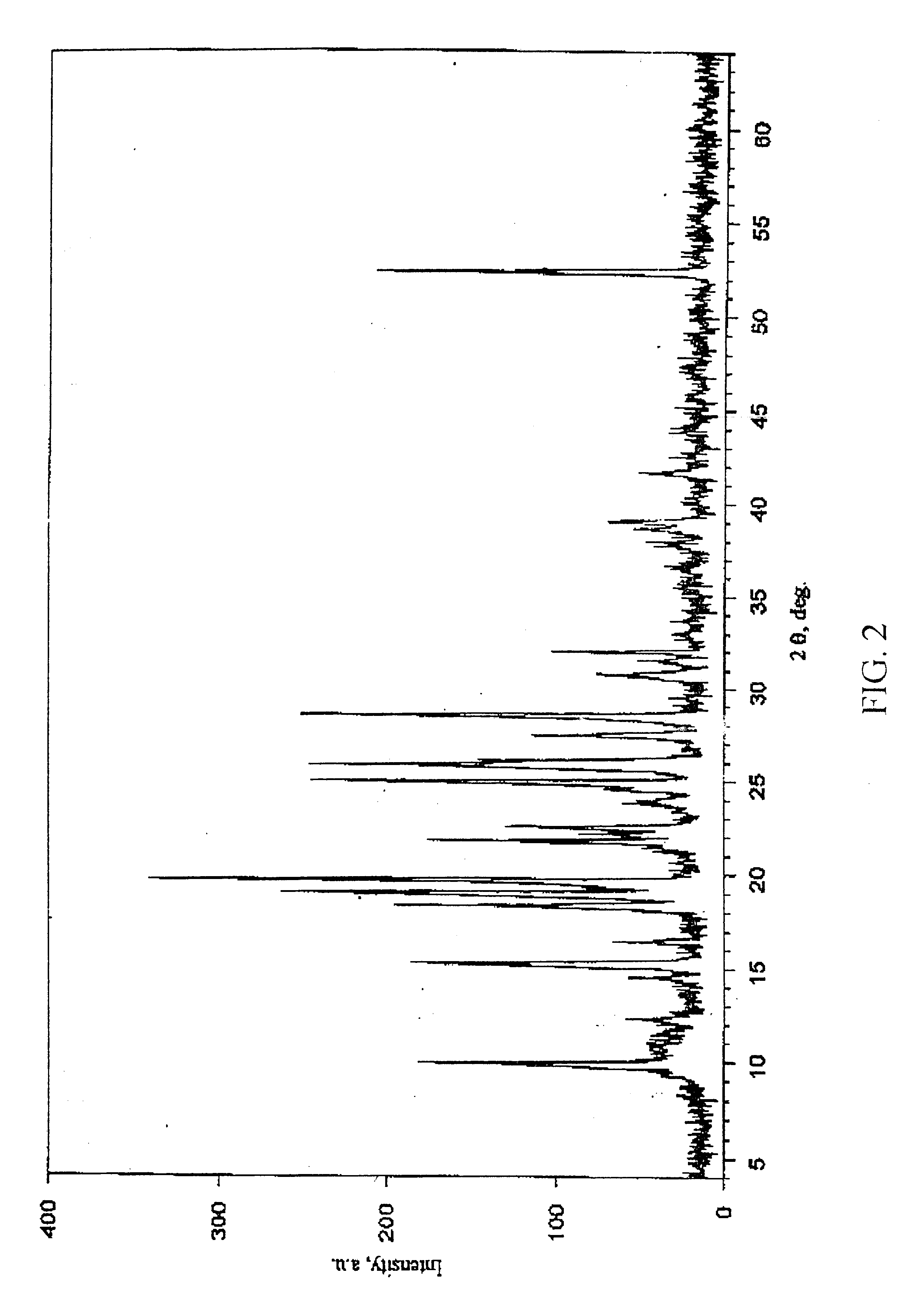
Crystalline modification of 5-fluoro-1-(tetrahydro-2-furyl) uracil and complex compounds based thereon, producing antineoplastic effect
The invention relates to a novel, heretofore unknown polymorphous modification of 5-fluoro-1-(tetrahydro-2-furyl)uracil (tegafur) having antineoplastic activity. This form is crystalline, and its characteristics it differs essentially from the modification known earlier. In particular, it has an enhanced specific activity. The new form is physically stable and may find application in medicine for treating oncological patients. New anticancer medicinal substances prepared in the form of stable molecular complexes on the basis of said new modification, in particular, crystalline complexes with 6-methyluracil, and amorphous complexes with the biologically active substances from licorice, are also described.
Owner:LEONIDOV NIKOLAI BORISOVICH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com