Stable Tegafur injection and preparation method thereof
A technology of tegafur and injection, which is applied in the field of medicine, can solve the problems of unqualified foreign body inspection, yellowing of solution color, drop of pH value, etc., and achieves the effect of being convenient for promotion and clinical medication.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
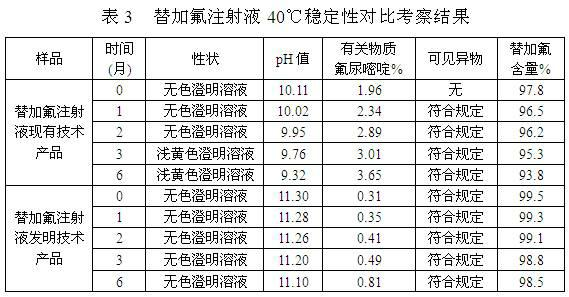
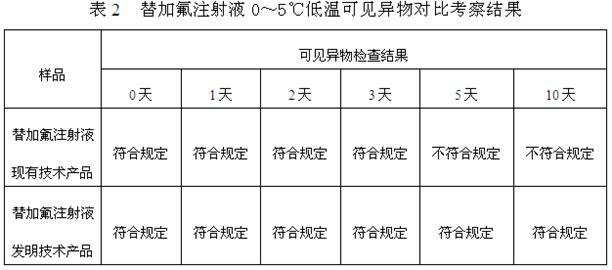
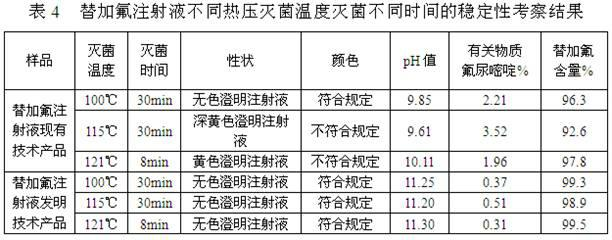
[0028] A stable tegafur injection and a preparation method thereof, comprising the steps of: (1) weighing 1 g to 100 g of tegafur, 10 mg to 50 g of an antioxidant, and 10 mg to 50 g of a pH buffer; (2) taking an acid solution and / or sodium hydroxide to be prepared into solutions of any concentration, and set aside; (3) Take 500ml of water for injection below 40°C, add antioxidants, pH buffers, and tegafur in sequence, stir, and use step (2) Adjust the pH value of the prepared acid solution and / or sodium hydroxide solution to 10.5-12.0, make up water for injection below 40°C to 1000ml; (4) add activated carbon to the solution obtained in step (3), and the amount of activated carbon is 0.005g- 0.5g / 100ml, stirred for 10-60 minutes, decarburized by filtration; (5) fine-filtered the injection solution obtained in step (4) until clarified, filled with nitrogen for protection, filled, and sterilized at high temperature.
[0029] The specific components and contents thereof of the pr...
Embodiment 2
[0036] The specific components and contents thereof of the present embodiment are as follows:
[0037] Tegafur 40.0g
[0038] L-cysteine 2.0g
[0039] Sodium citrate 5.0g
[0040] Citric acid and / or sodium hydroxide adjust the pH of the solution.
[0041]Citric acid and / or sodium hydroxide were prepared into 10% to 20% solutions respectively for later use; take 500ml of water for injection below 40°C, add L-cysteine, sodium citrate, and tegafur in sequence, and stir. Use the citric acid solution and / or sodium hydroxide solution prepared above to adjust the pH value to 10.5-12.0, make up water for injection below 40°C to 1000ml; add activated carbon to the obtained solution, the amount of activated carbon is 0.1g / 100ml, stir for 30 Minutes, decarburized by filtration; the resulting injection was fine-filtered until clarified, filled with nitrogen for protection, filled, and autoclaved at 115°C for 30 minutes to obtain the product.
Embodiment 3
[0043] The specific components and contents thereof of the present embodiment are as follows:
[0044] Tegafur 40.0g
[0045] L-cysteine 5.0g
[0047] Hydrochloric acid and / or sodium hydroxide to adjust the pH of the solution.
[0048] Hydrochloric acid and / or sodium hydroxide were prepared into 10% to 20% solutions respectively for later use; take 500ml of water for injection below 40°C, add L-cysteine, sodium carbonate, and tegafur in sequence, stir, and use the above Adjust the pH of the prepared hydrochloric acid solution and / or sodium hydroxide solution to 10.5-12.0, make up water for injection below 40°C to 1000ml; add activated carbon to the obtained solution, the amount of activated carbon is 0.1g / 100ml, stir for 30 minutes, and filter Decarburization; the resulting injection is finely filtered until clarified, filled with nitrogen for protection, filled, and sterilized by autoclaving at 100°C for 30 minutes.
[0049] When the pres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com