Method for stabilizing tegafur in aqueous solution
A technology of tegafur and aqueous solution, which is used in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., to achieve the effect of expanding the scope
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0021] (3) Add sodium hydroxide to adjust the pH to 8.5~10.5, add water for injection until the volume reaches 100ml, fill and sterilize.
[0022] The amounts of sodium chloride, sodium carbonate, tegafur and sodium hydroxide in Examples 1-3 are shown in Table 1 below.
[0023] Table 1 Statistical Table of Addition of Sodium Chloride, Sodium Carbonate, Tegafur and Sodium Hydroxide in Examples 1-3
[0024]
[0025] After the solutions in Examples 1-3 were sterilized, the components therein were tested, and the test results are shown in Table 2 below.
[0026] Table 2 The solution of embodiment 1-3 is through the composition detection result after sterilization
[0027]
[0028] Tegafur content=detected tegafur amount / added tegafur amount*100% in table 2,
[0029] Sodium chloride content = detected amount of sodium chloride / added amount of sodium chloride * 100%,
[0030] Fluorouracil content = the amount of detected fluorouracil / the amount after converting the ad...
Embodiment 4-6
[0036] (3) Add sodium hydroxide to adjust the pH to 8.5~10.5, add water for injection until the volume reaches 100ml, fill and sterilize.
[0037] The amounts of sodium chloride, sodium acetate, tegafur and sodium hydroxide in Examples 4-6 are shown in Table 3 below.
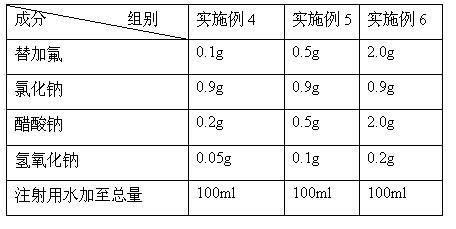
[0038] Table 3 The addition statistics of sodium chloride, sodium acetate, tegafur and sodium hydroxide in Examples 4-6
[0039]
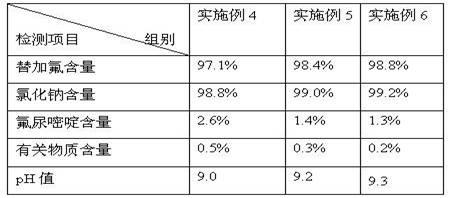
[0040] After the solutions in Examples 4-6 were sterilized, the components therein were tested, and the test results are shown in Table 4 below.
[0041] Table 4 The solution of embodiment 4-6 is through the composition detection result after sterilization
[0042]
[0043] Example 7-9
[0044] The steps of the method are as follows: (1) Take sodium chloride and prepare a 15% sodium chloride solution with water for injection;
[0045] (2) Add sodium phosphate and tegafur to the sodium chloride solution, stir to dissolve;
Embodiment 7-9
[0047] The amounts of sodium chloride, sodium phosphate, tegafur and sodium hydroxide in Examples 7-9 are shown in Table 5 below.
[0048] Table 5 The addition statistics of sodium chloride, sodium phosphate, tegafur and sodium hydroxide in Examples 7-9
[0049]
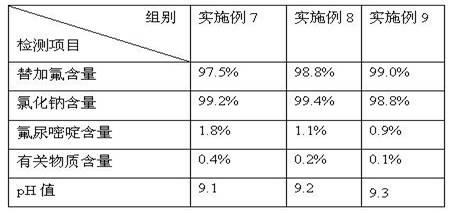
[0050] After the solutions in Examples 7-9 were sterilized, the components therein were tested, and the test results are shown in Table 6 below.
[0051] The solution of table 6 embodiment 7-9 is through the component test result after sterilization
[0052]
[0053] Comparative Examples 1-3
[0054] On the basis of embodiment 1-3, remove sodium carbonate, method step is identical with embodiment 1-3,
[0055] The amounts of sodium chloride, tegafur and sodium hydroxide in Comparative Examples 1-3 are shown in Table 7 below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com