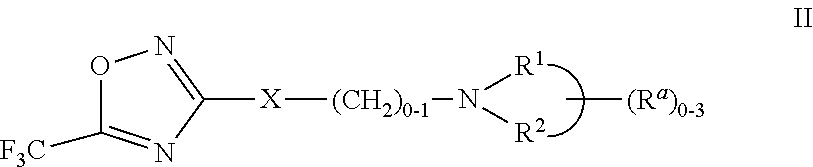Patents
Literature
171 results about "Ditazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ditazole is a non-steroidal anti-inflammatory agent with analgesic and antipyretic activity similar to phenylbutazone. It is also a platelet aggregation inhibitor which is marketed in Spain and Portugal under the trade name Ageroplas.
Gsk-3betainhibitor
InactiveUS20100069381A1Superior GSK-3 specific inhibitory activityImprove solubilityAntibacterial agentsBiocideDiseaseDitazole
For the purpose of providing a GSK-3β inhibitor containing an oxadiazole compound or a salt thereof or a prodrug thereof useful as an agent for the prophylaxis or treatment of a GSK-3β-related pathology or disease, the present invention provides a GSK-3β inhibitor containing a compound represented by the formula (I):wherein each symbol is as defined in the specification, or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
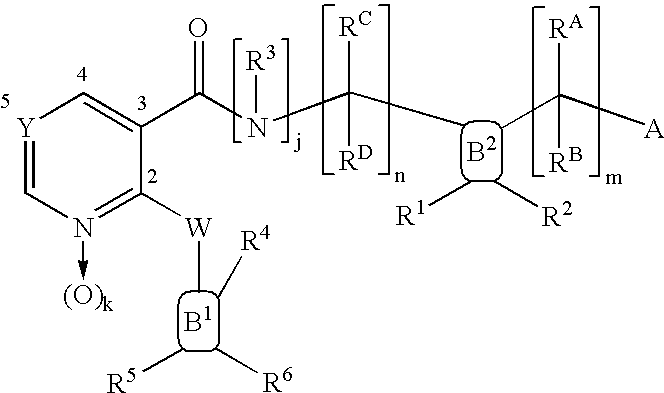
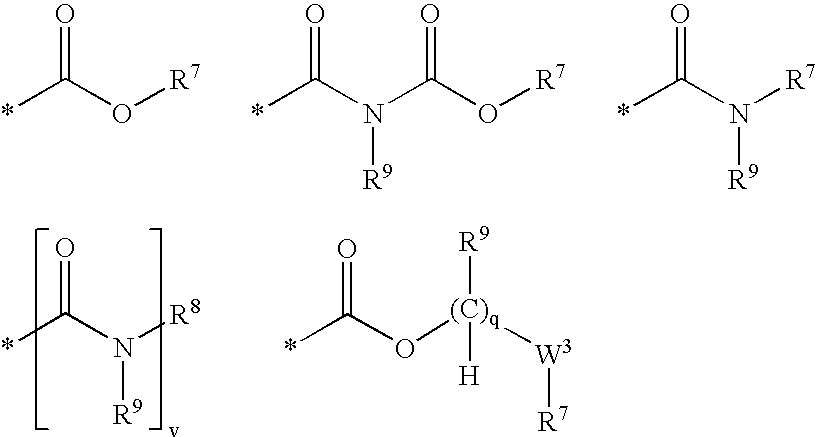
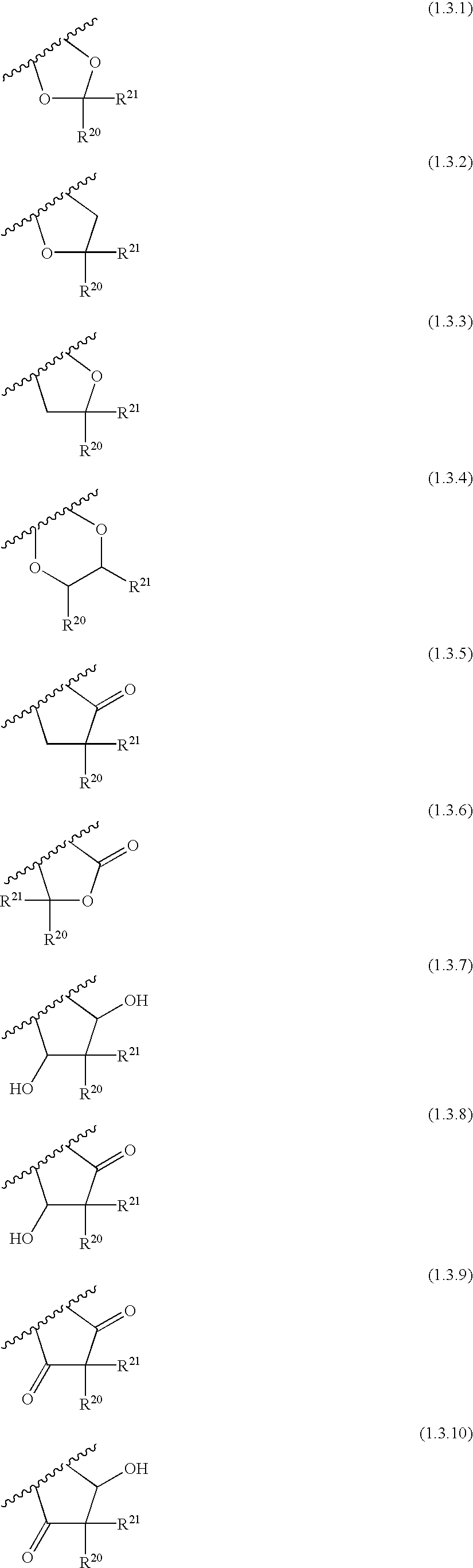
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Substituted-pent-4-ynoic acids
InactiveUS6037367AImprove the level ofSuppressing inappropriate activationBiocideOrganic chemistryMethyl groupCarbon atom
PCT No. PCT / US96 / 11613 Sec. 371 Date Sep. 14, 1998 Sec. 102(e) Date Sep. 14, 1998 PCT Filed Jul. 12, 1996 PCT Pub. No. WO97 / 03945 PCT Pub. Date Feb. 6, 1997Compounds of formula (I) wherein: R1 is -(CR4R5)nC(O)O(CR4R5)mR6, -(CR4R5)nC(O)NR4(CR4R5)mR6, (CR4R5)nO(CR4R5)mR6, or -(CR4R5)rR6: W is alkynyl or 2 carbon atoms; R3 is H or R7; Z is C(O)R13, (CH2)0-1C(O)OR13, (CH2)0-1C(O)NR10R13, (CH2)0-1C(R8R8)OR8, -NHC(O)R7, (CH2)0-1NR10R13, NH[C(O)C(O)OR8], CH2NH[C(O)CNR10R13], CH2S(O)qR7, CH[S(O)qR7]2, dithiolane, (tetrazol-5-yl), thiazol-2-yl, [1,2,4]thiadiazol-5-yl, [1,3,4]oxadiazol-2-yl, imidazol-2-yl, oxazol-2-yl, or (3- or 5-oxadiazolyl[1,2,4]; R7 is -(CR4R5)qR11 or C1-6 alkyl wherein the R11 or C1-6 alkyl group is unsubstituted or substituted one or more times by methyl or ethyl unsubstituted or substituted by 1-3 fluorines, -NR8R10, -CO2R8, -O(CH2qR8, -NR8C(O)R8 or R12; or the pharmaceutically acceptable salts thereof.
Owner:SMITHKLINE BECKMAN CORP
MEK inhibiting compounds
InactiveUS20050004186A1Inhibit phosphorylationAntibacterial agentsBiocidePercent Diameter StenosisImmunomodulations
This invention provides substituted Phenyl-(2-[1,3,4]thiadiazol-2-yl-phenyl)-amine and (2-[1,3,4]Oxadiazol-2-yl-phenyl)phenyl-amine compounds which act as inhibitors of MAPK / ERK Kinase (“MEK”) enzymes and pharmaceutical compositions and methods for their use in immunomodulation and in the treatment and alleviation of inflammation, and proliferative diseases such as cancer and restenosis.
Owner:PFIZER INC
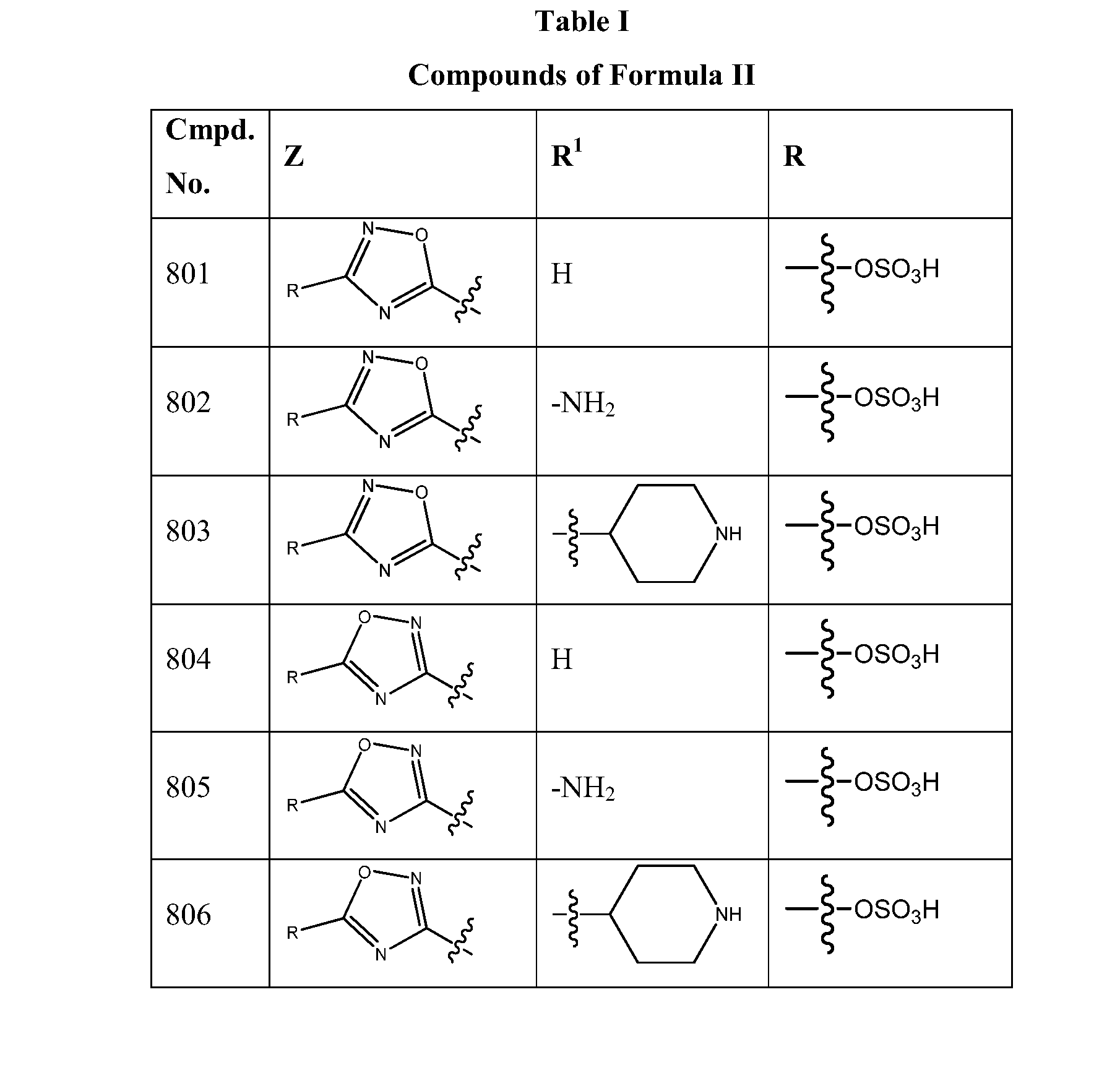
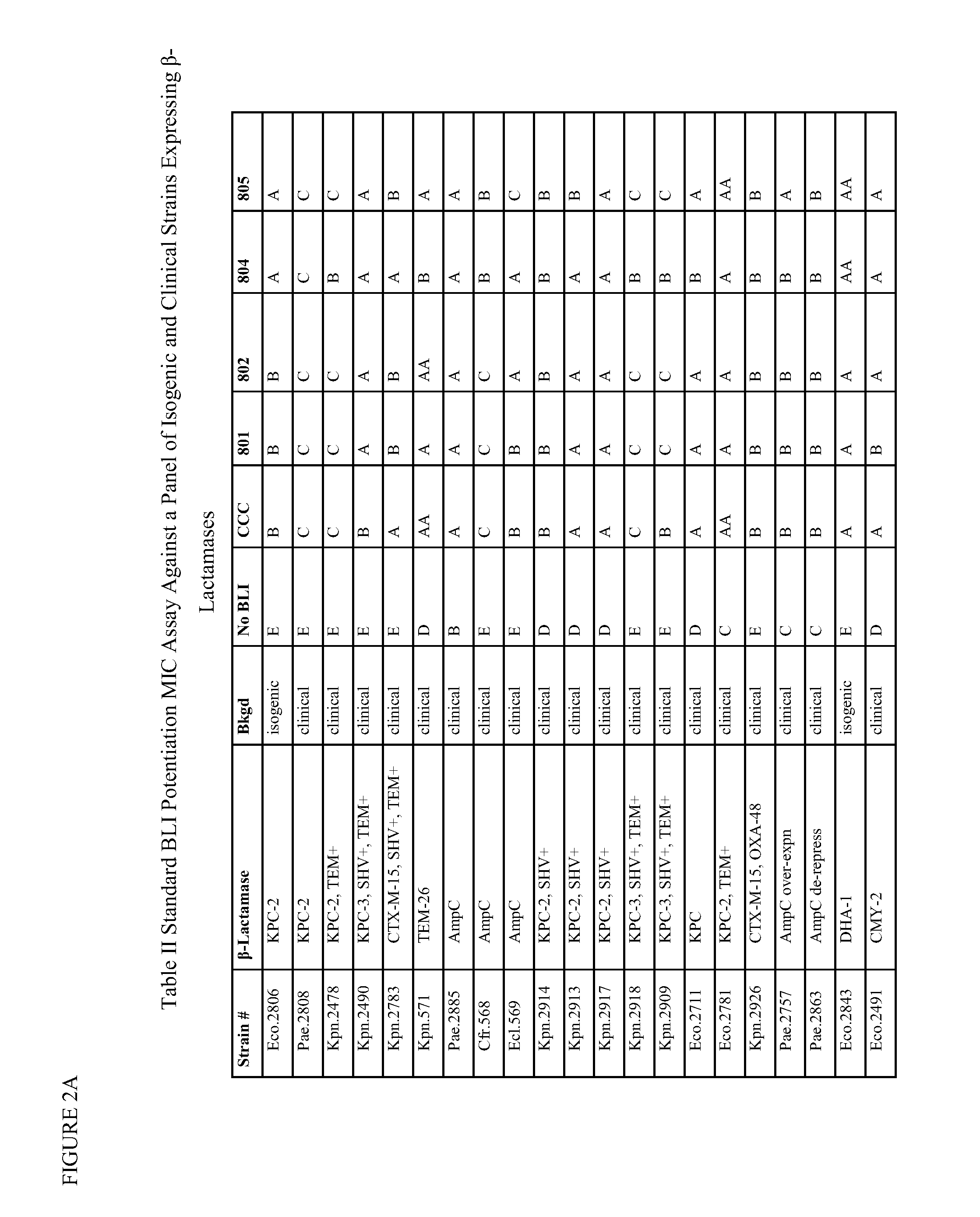
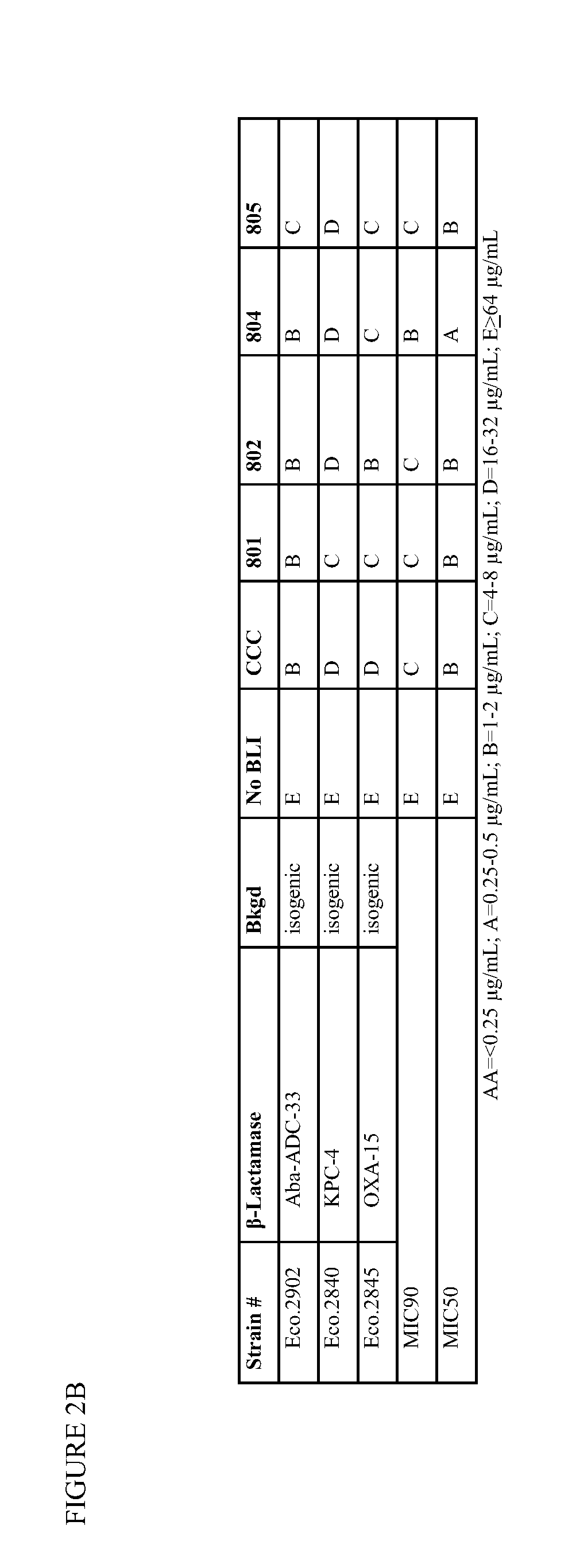
1,2,4-oxadiazole and 1,2,4-thiadiazole beta-lactamase inhibitors
β-Lactamase inhibitor compounds (BLIs) are disclosed, including compounds that have activity against class A, class C or class D β-lactamases. Methods of manufacturing the BLIs, and uses of the compounds in the preparation of pharmaceutical compositions and antibacterial applications are also disclosed.
Owner:MERCK SHARP & DOHME LLC
1,2,4-oxadiazole and thiadiazole compounds as immunomodulators
ActiveUS20180044303A1Suppress and inhibit programmed cell death (PD1) signaling pathwaySuppress and/or inhibit the programmed cell death 1 (PD1) signaling pathwayAntibacterial agentsOrganic active ingredientsPD-L1Thiadiazoles
The present invention relates to 1,2,4-oxadiazole compounds of formula (I) and their use to inhibit the programmed cell death 1 (PD-1) signaling pathway and / or for treatment of disorders by inhibiting an immunosuppressive signal induced by PD-1, PD-L1 or PD-L2.
Owner:AURIGENE ONCOLOGY LTD
1,3,4-oxadiazole and thiadiazole compounds as immunomodulators
InactiveUS20180044304A1Suppress and inhibit programmed cell death (PD-1) signaling pathwaySuppress and/or inhibit the programmed cell death 1 (PD-1) signaling pathwayOrganic active ingredientsOrganic chemistryPD-L1Thiadiazoles
The present invention relates to 1,3,4-oxadiazole and thiadiazole compounds of formula (I) and their use to inhibit the programmed cell death 1 (PD-1) signaling pathway and / or for treatment of disorders by inhibiting an immunosuppressive signal induced by PD-1, PD-L1 or PD-L2.
Owner:AURIGENE DISCOVERY TECH
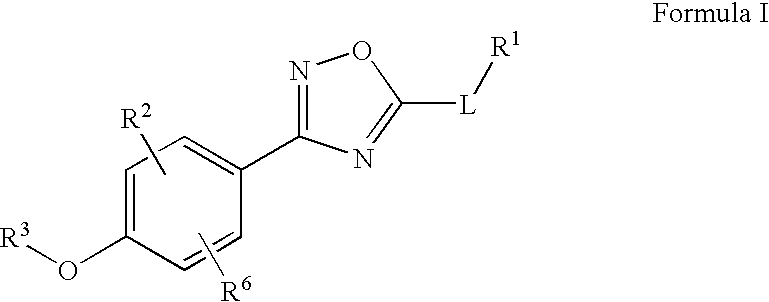
Oxadiazole compounds
ActiveUS7834039B2Reduce in quantityGood effectBiocideSenses disorderDiseaseG protein-coupled receptor
Novel oxadiazole compounds, pharmaceutical compositions containing such compounds and the use of those compounds or compositions as agonists or antagonists of the S1P family of G protein-coupled receptors for treating diseases associated with modulation of S1P family receptor activity, in particular by affording a beneficial immunosuppressive effect are disclosed.
Owner:ABBVIE INC
1,3,4-oxadiazole and 1,3,4-thiadiazole beta-lactamase inhibitors
β-Lactamase inhibitor compounds (BLIs) are disclosed, including compounds that have activity against class A, class C or class D β-lactamases. Methods of manufacturing the BLIs, and uses of the compounds in the preparation of pharmaceutical compositions and antibacterial applications are also disclosed.
Owner:MERCK SHARP & DOHME LLC
1,3,4-oxadiazole and 1,3,4-thiadiazole beta-lactamase inhibitors
β-Lactamase inhibitor compounds (BLIs) are disclosed, including compounds that have activity against class A, class C or class D β-lactamases. Methods of manufacturing the BLIs, and uses of the compounds in the preparation of pharmaceutical compositions and antibacterial applications are also disclosed.
Owner:MERCK SHARP & DOHME LLC
1,3,4-oxadiazole and 1,3,4-thiadiazole beta-lactamase inhibitors
β-Lactamase inhibitor compounds (BLIs) are disclosed, including compounds that have activity against class A, class C or class D β-lactamases. Methods of manufacturing the BLIs, and uses of the compounds in the preparation of pharmaceutical compositions and antibacterial applications are also disclosed.
Owner:MERCK SHARP & DOHME LLC
1,3,4-oxadiazole and 1,3,4-thiadiazole beta-lactamase inhibitors
β-Lactamase inhibitor compounds (BLIs) are disclosed, including compounds that have activity against class A, class C or class D β-lactamases. Methods of manufacturing the BLIs, and uses of the compounds in the preparation of pharmaceutical compositions and antibacterial applications are also disclosed.
Owner:MERCK SHARP & DOHME LLC
1,3,4-oxadiazole and 1,3,4-thiadiazole beta-lactamase inhibitors
Owner:MERCK SHARP & DOHME LLC
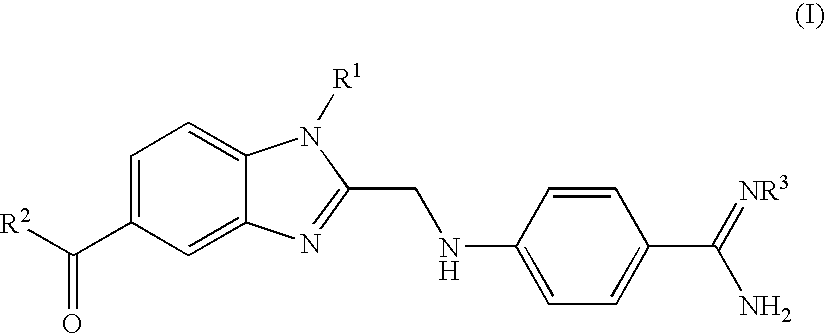
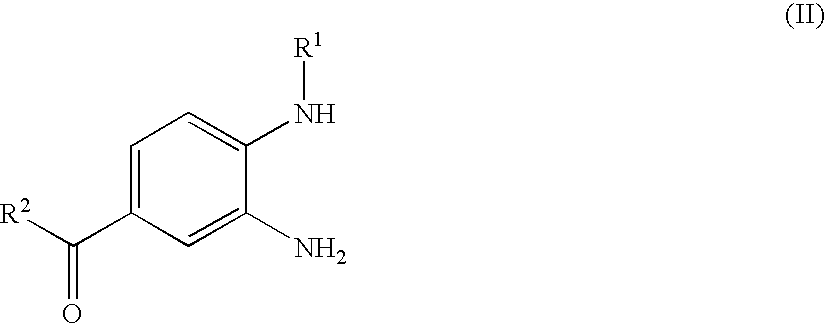
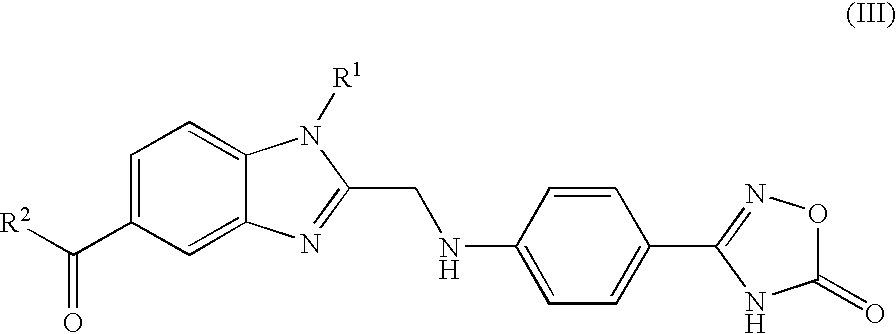
Process for the Preparation of the Salts of 4-(Benzimidazolylmethylamino)-Benzamides
The invention relates to a process for preparing a salt of an optionally substituted 4-benzimidazol-2-ylmethylamino)-benzamidine, characterised in that (a) an optionally correspondingly substituted diaminobenzene is condensed with 2-[4-(1,2,4-oxadiazol-5-on-3-yl)-phenylamino]-acetic acid, b) i) the product thus obtained is hydrogenated, ii) optionally the amidino group is carbonylated, without isolating the intermediate product of the hydrogenation beforehand and iii) without prior isolation of the intermediate product of the carbonylation the desired salt is isolated.
Owner:BOEHRINGER INGELHEIM INT GMBH
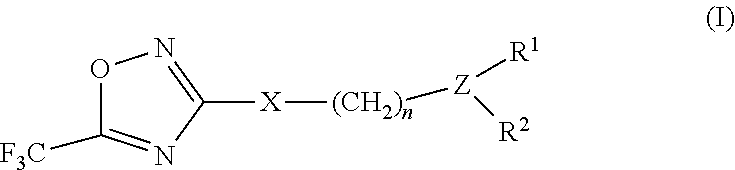
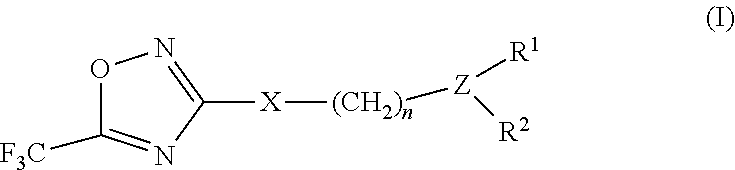
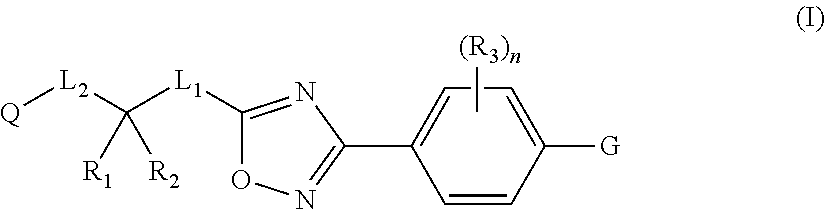
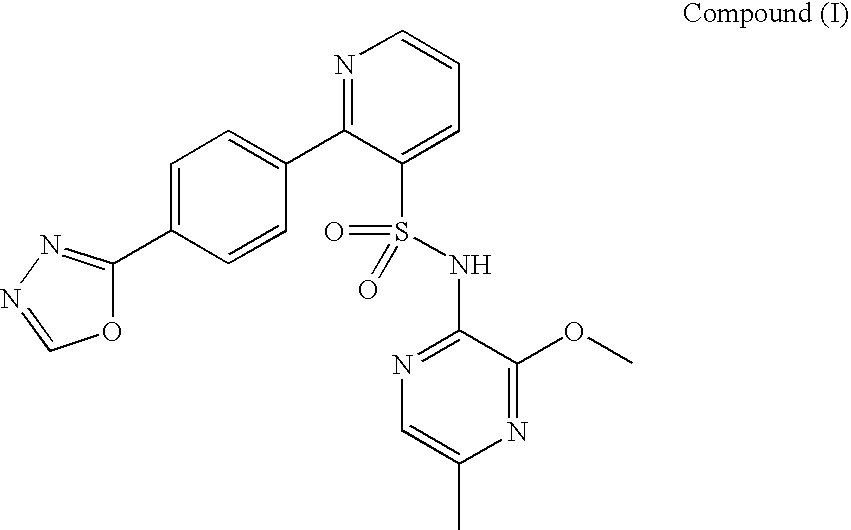
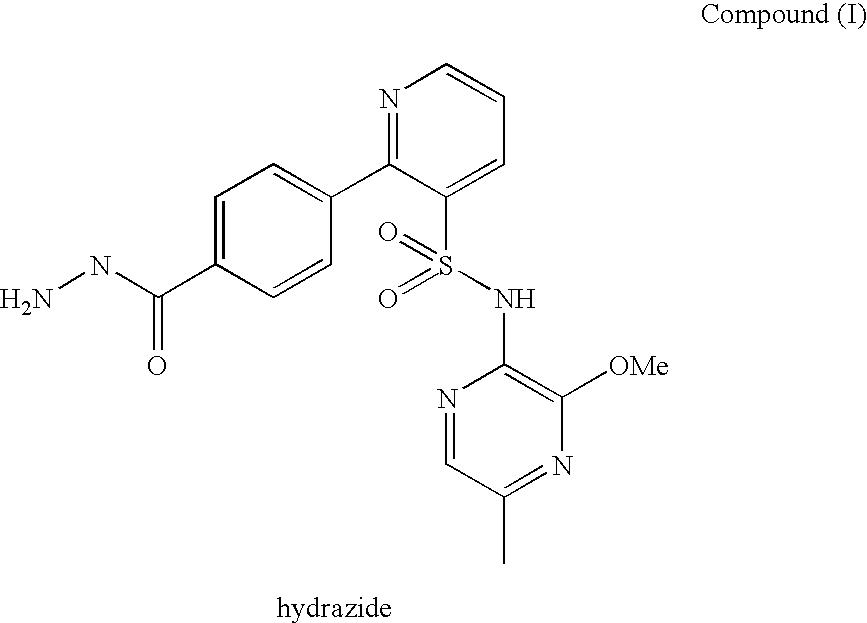
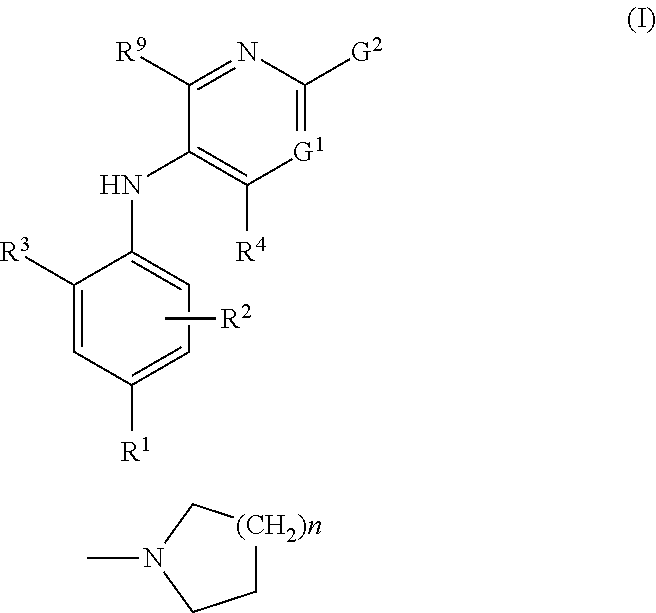
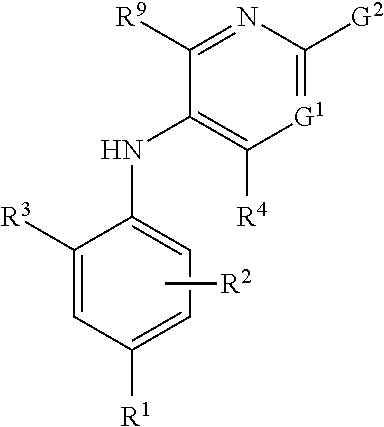
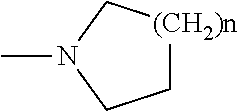
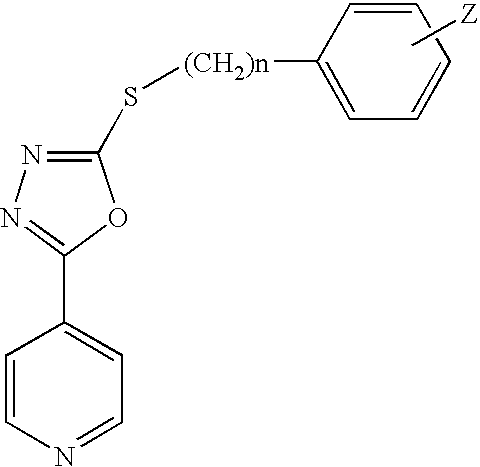
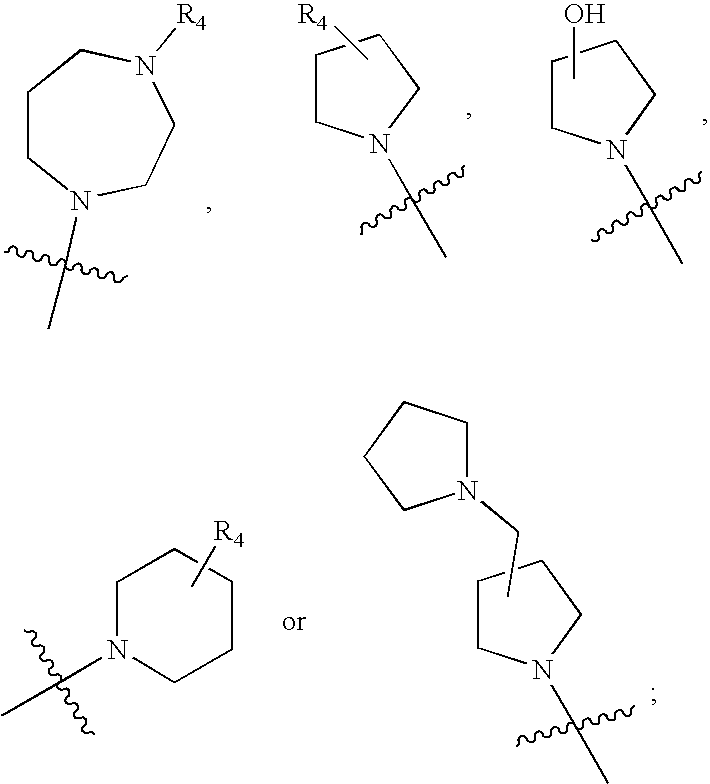
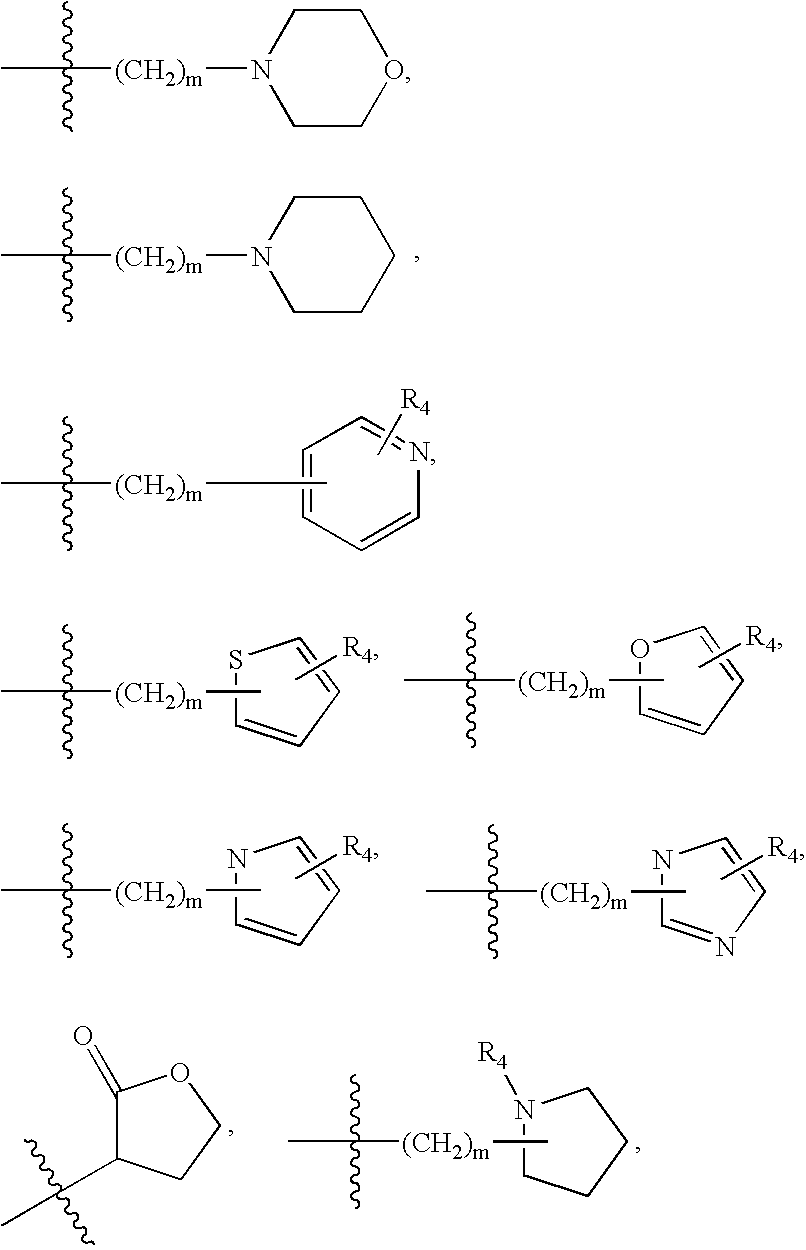
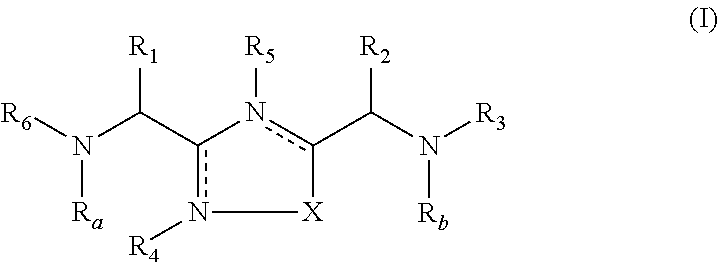
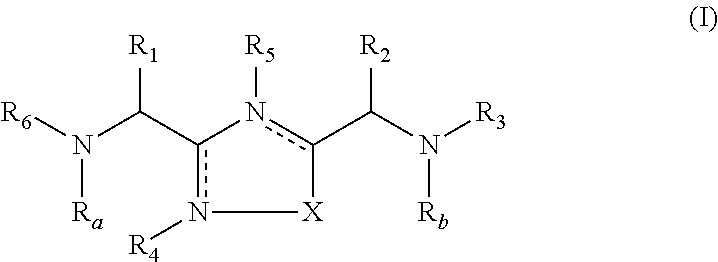
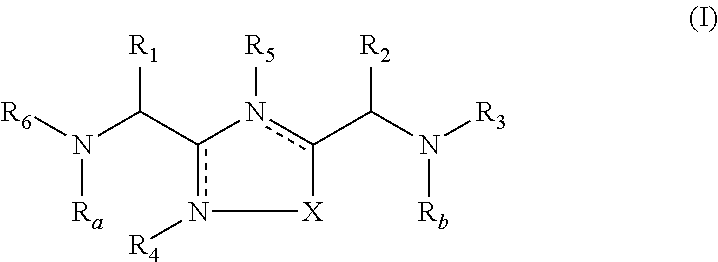
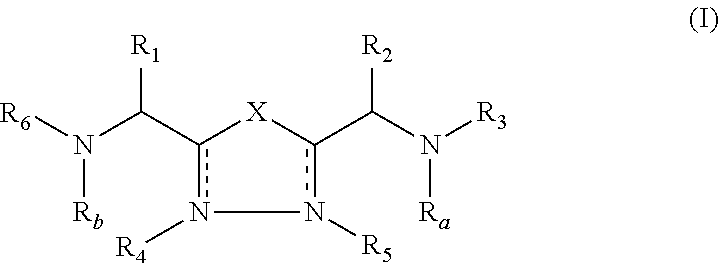
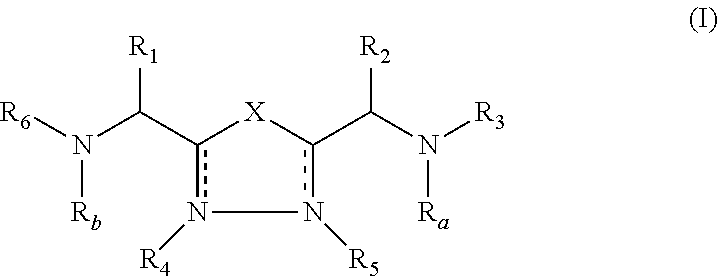
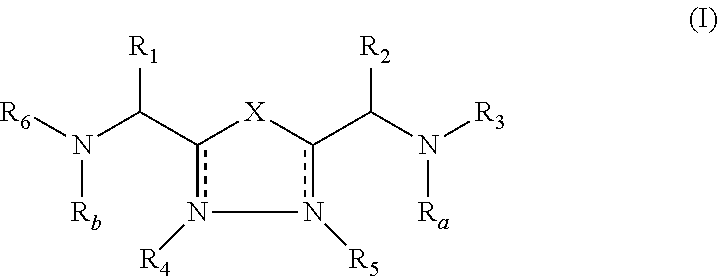
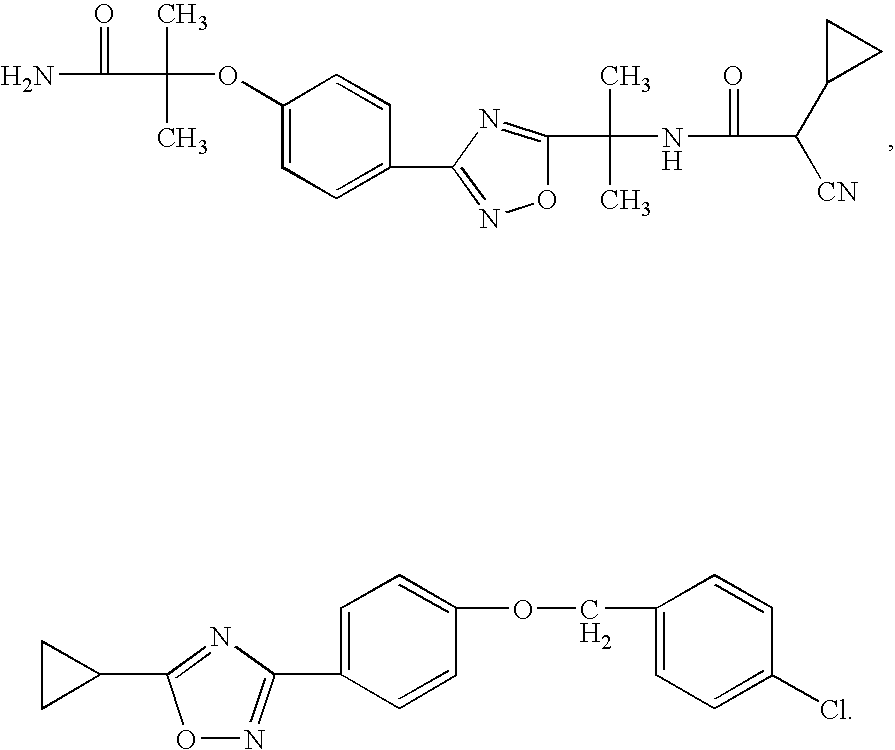
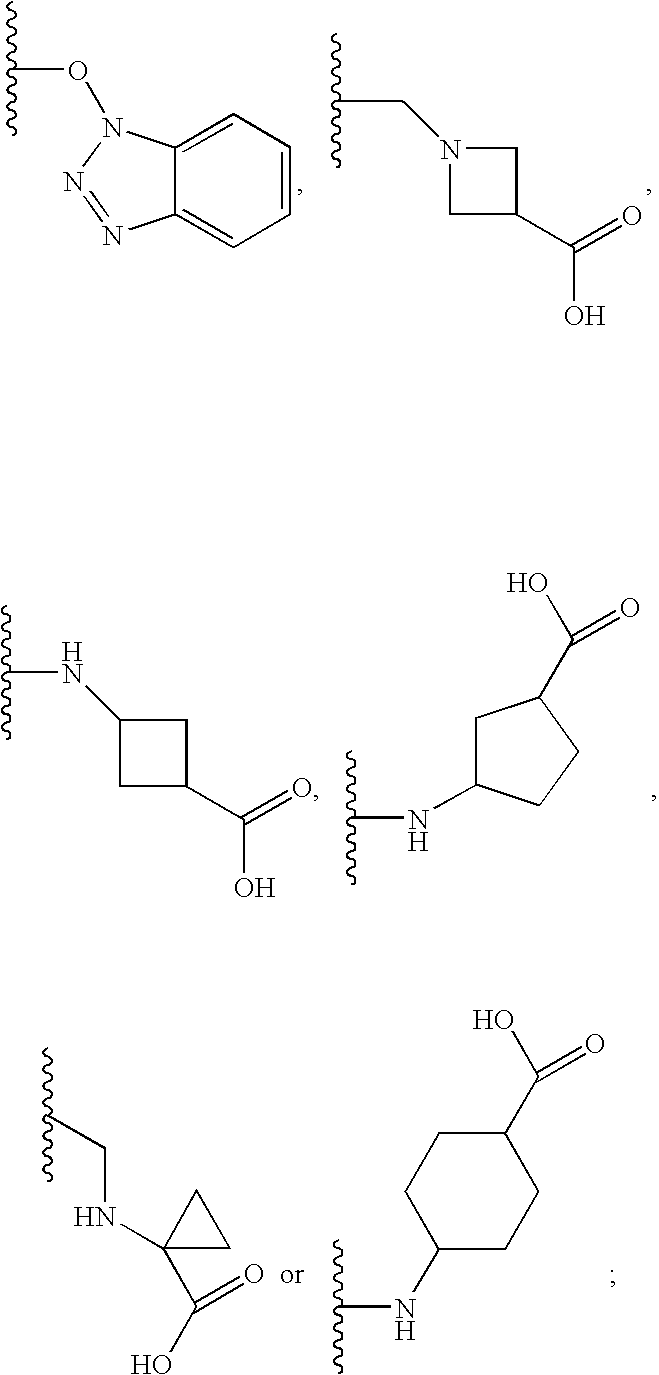
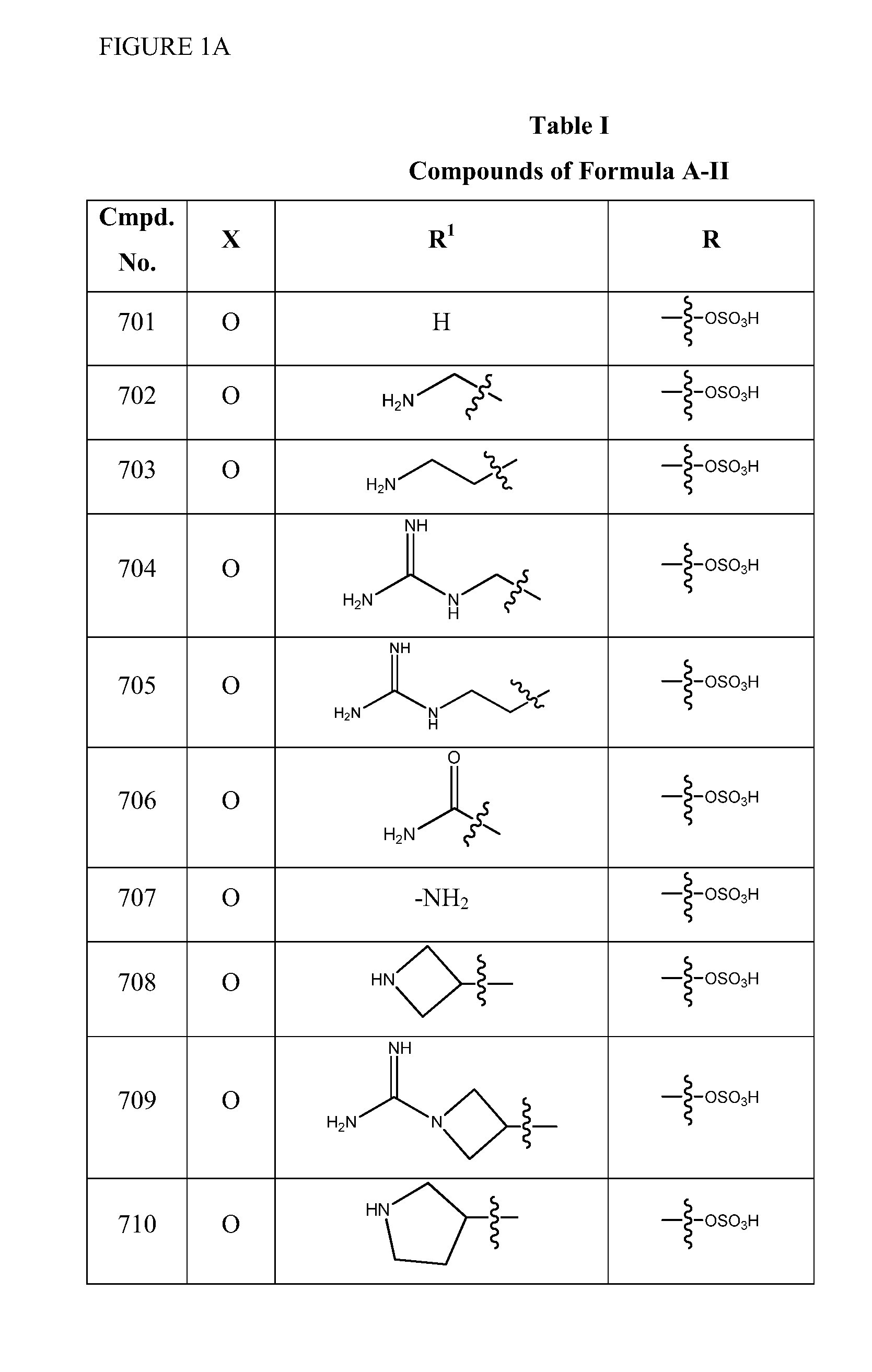
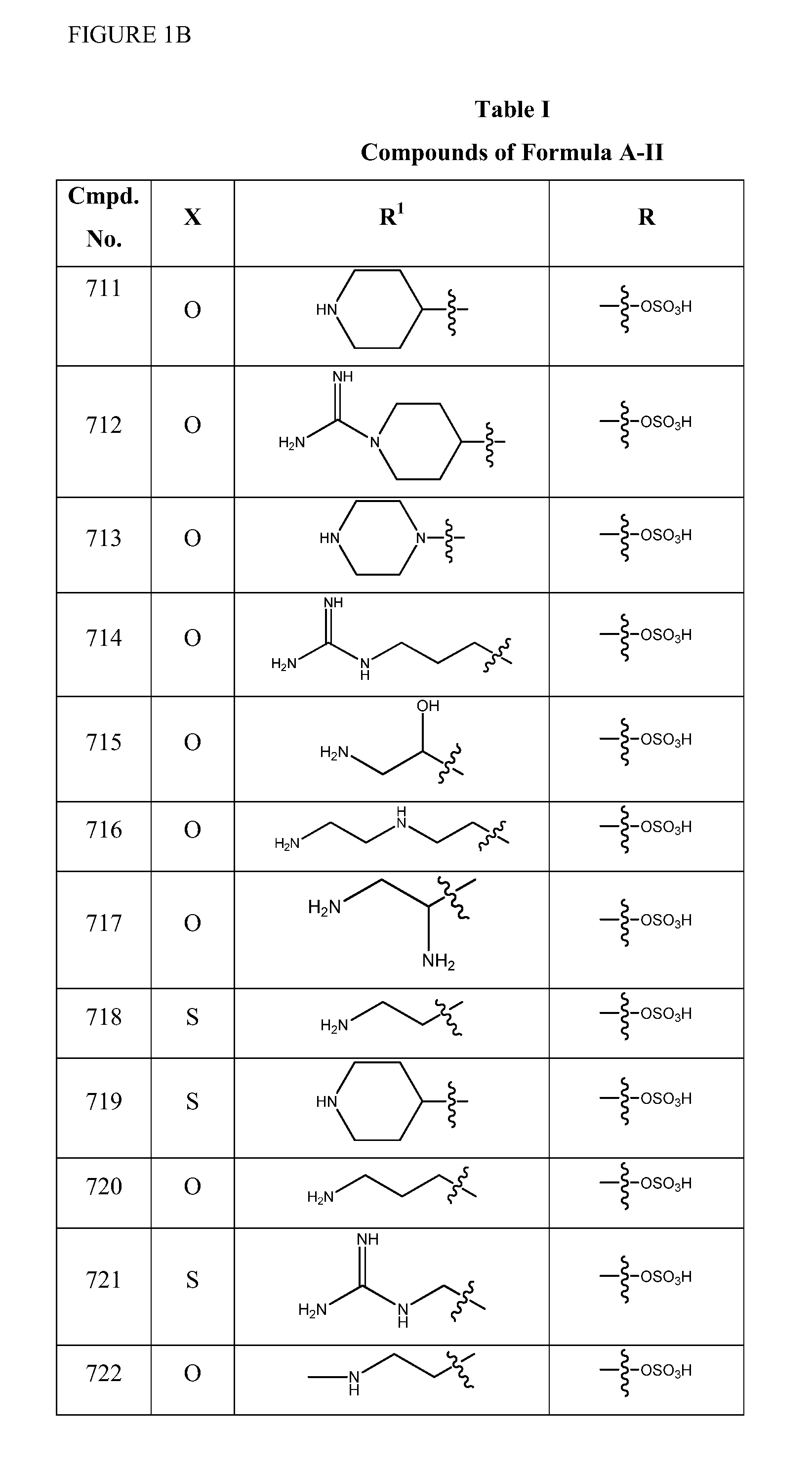
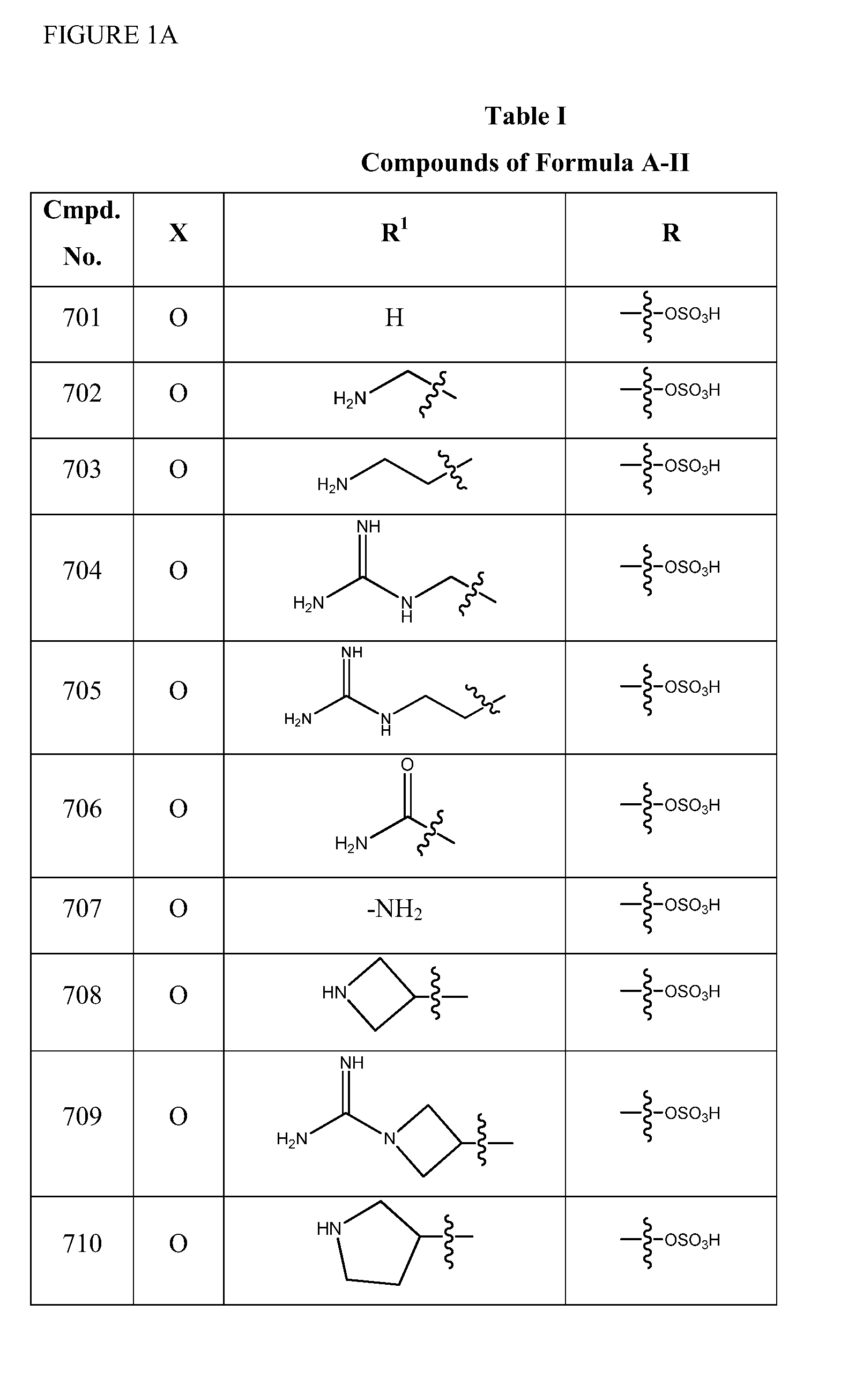
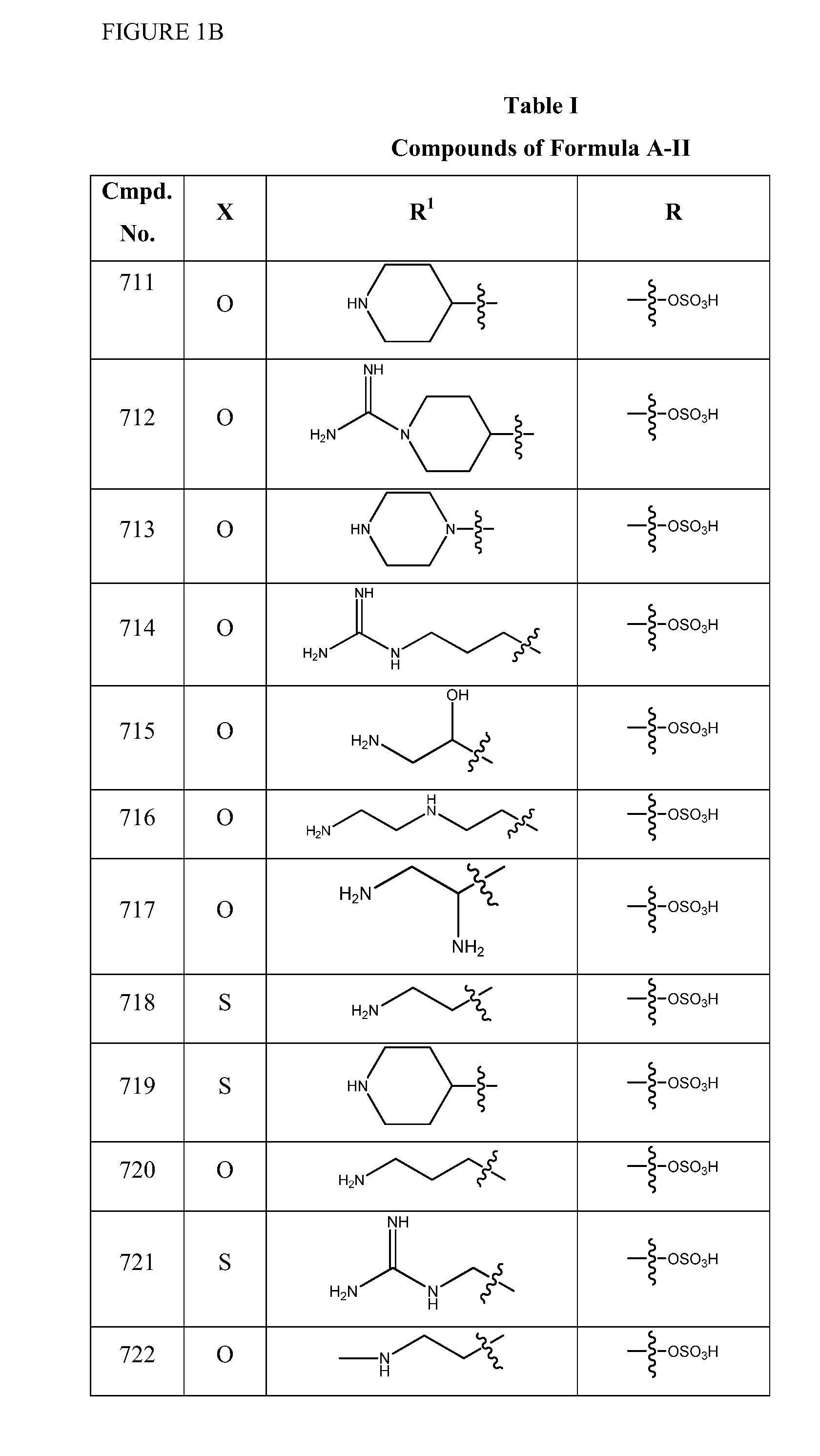
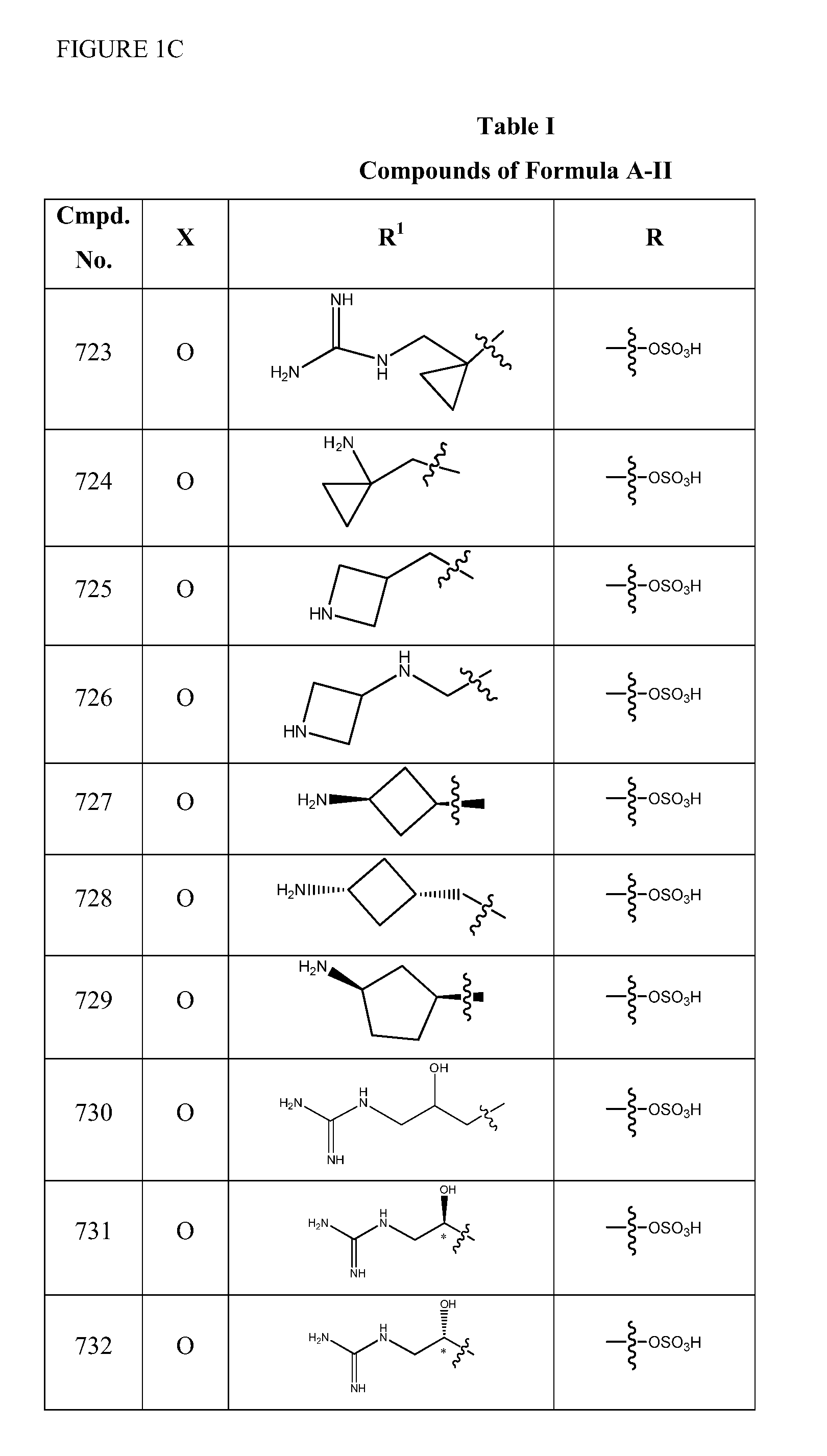
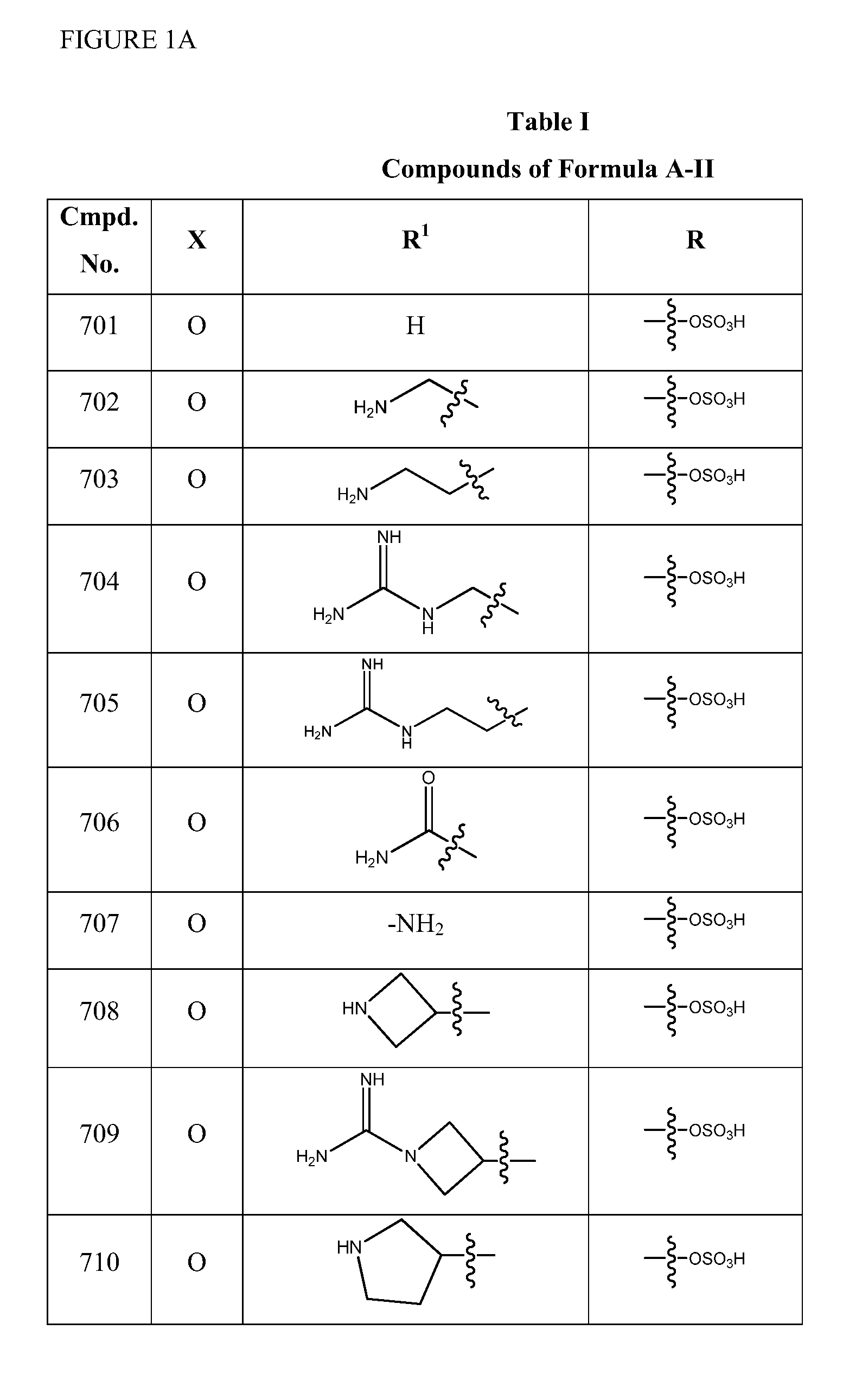
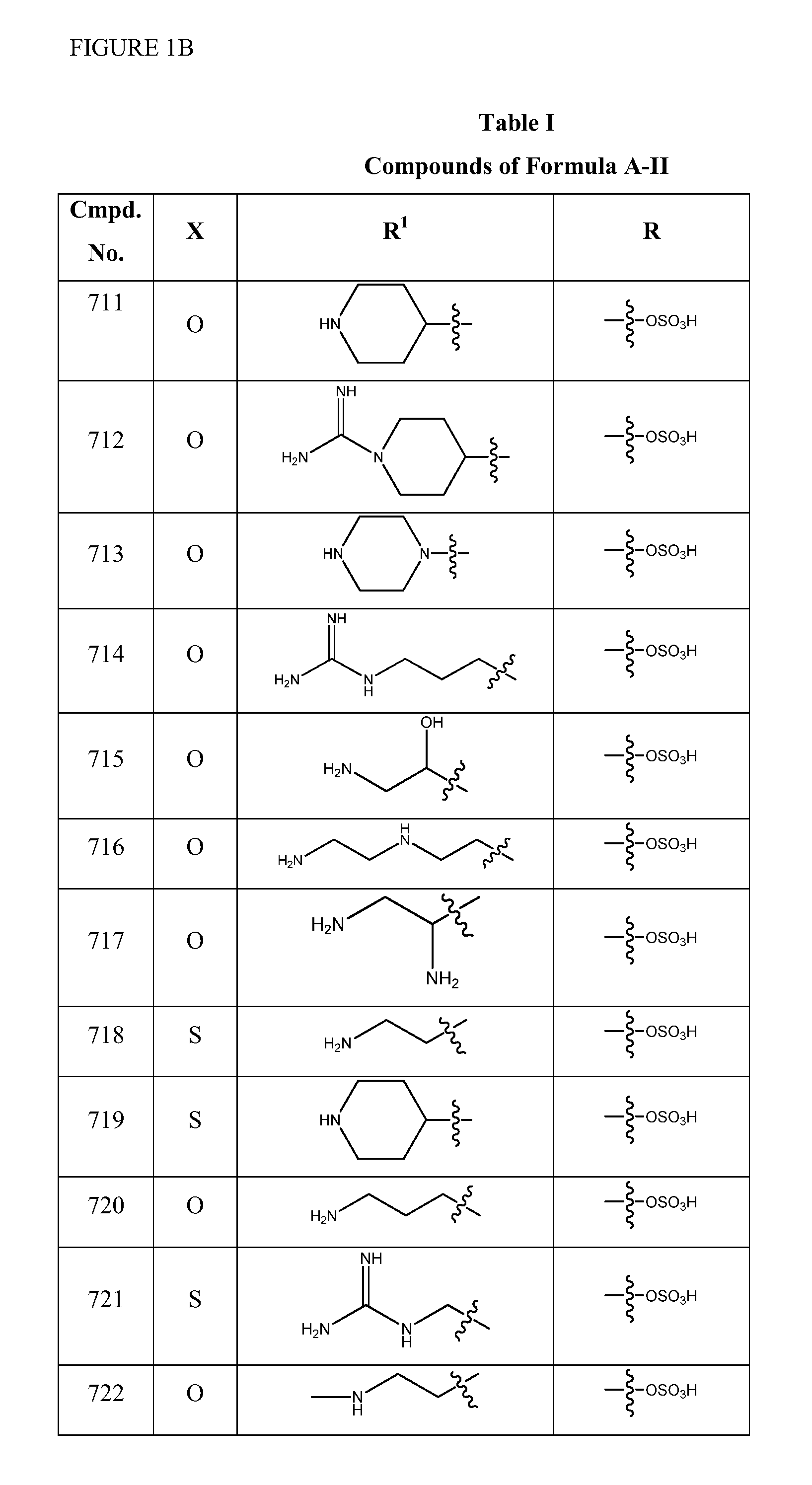
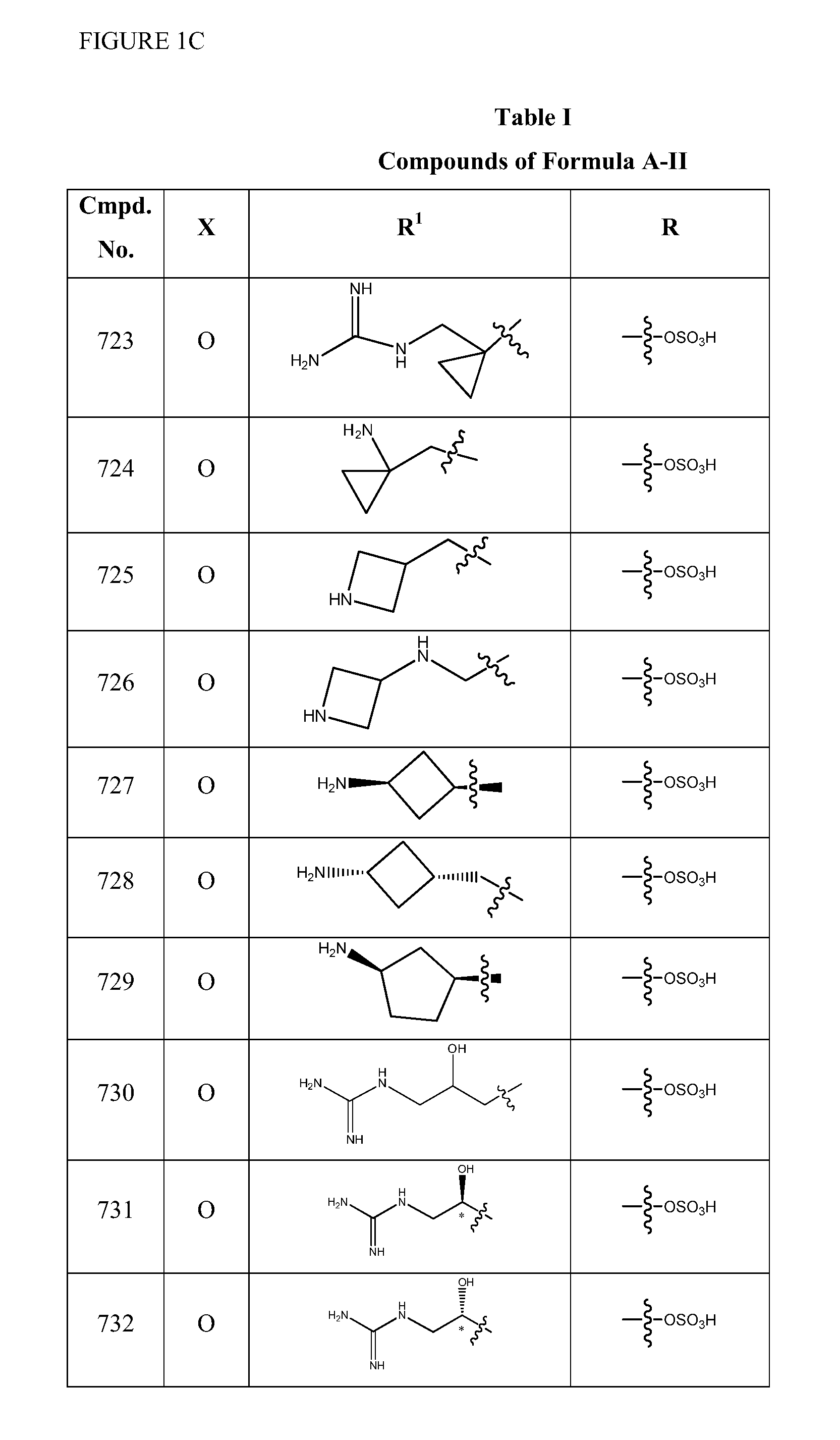
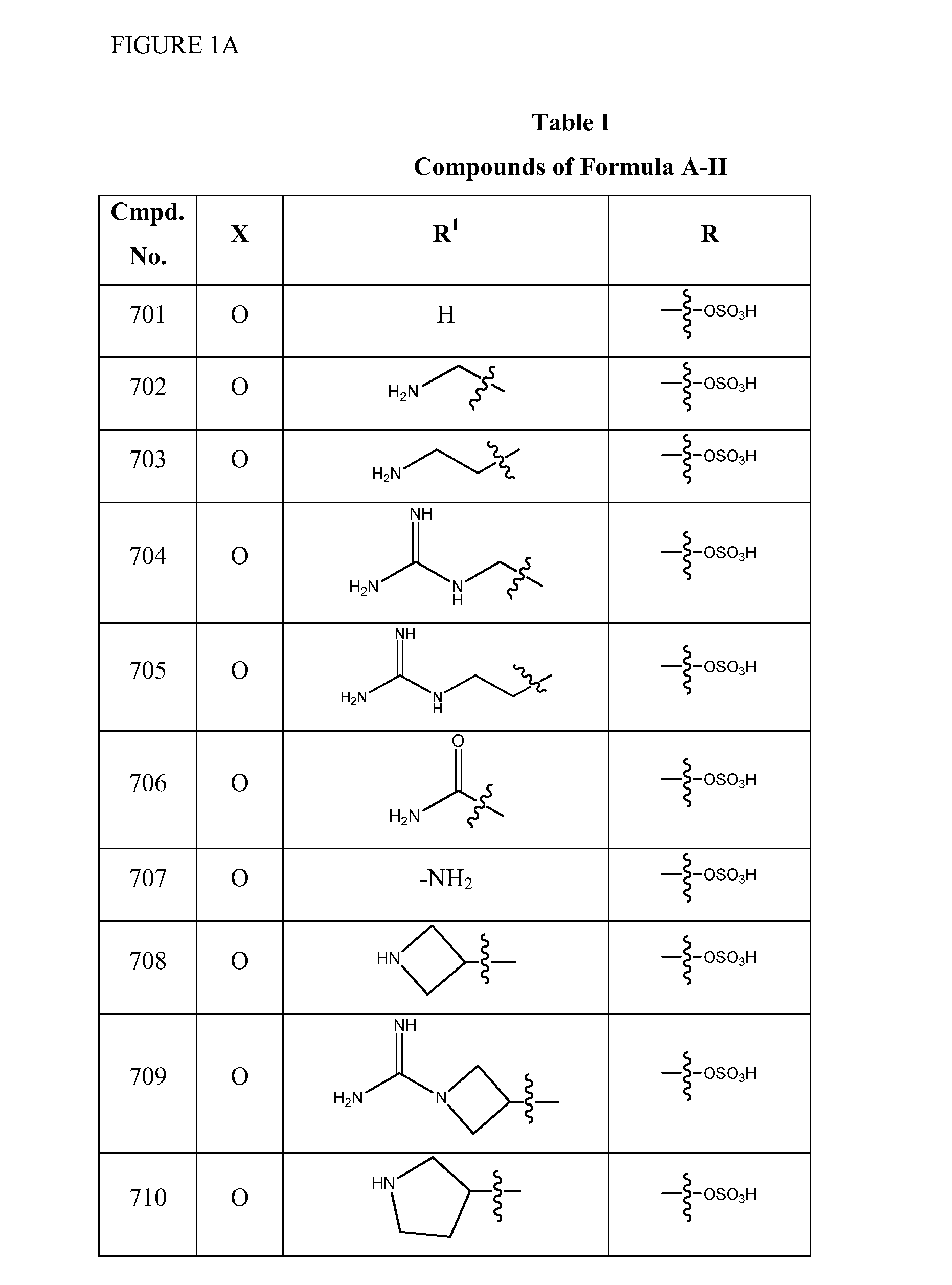
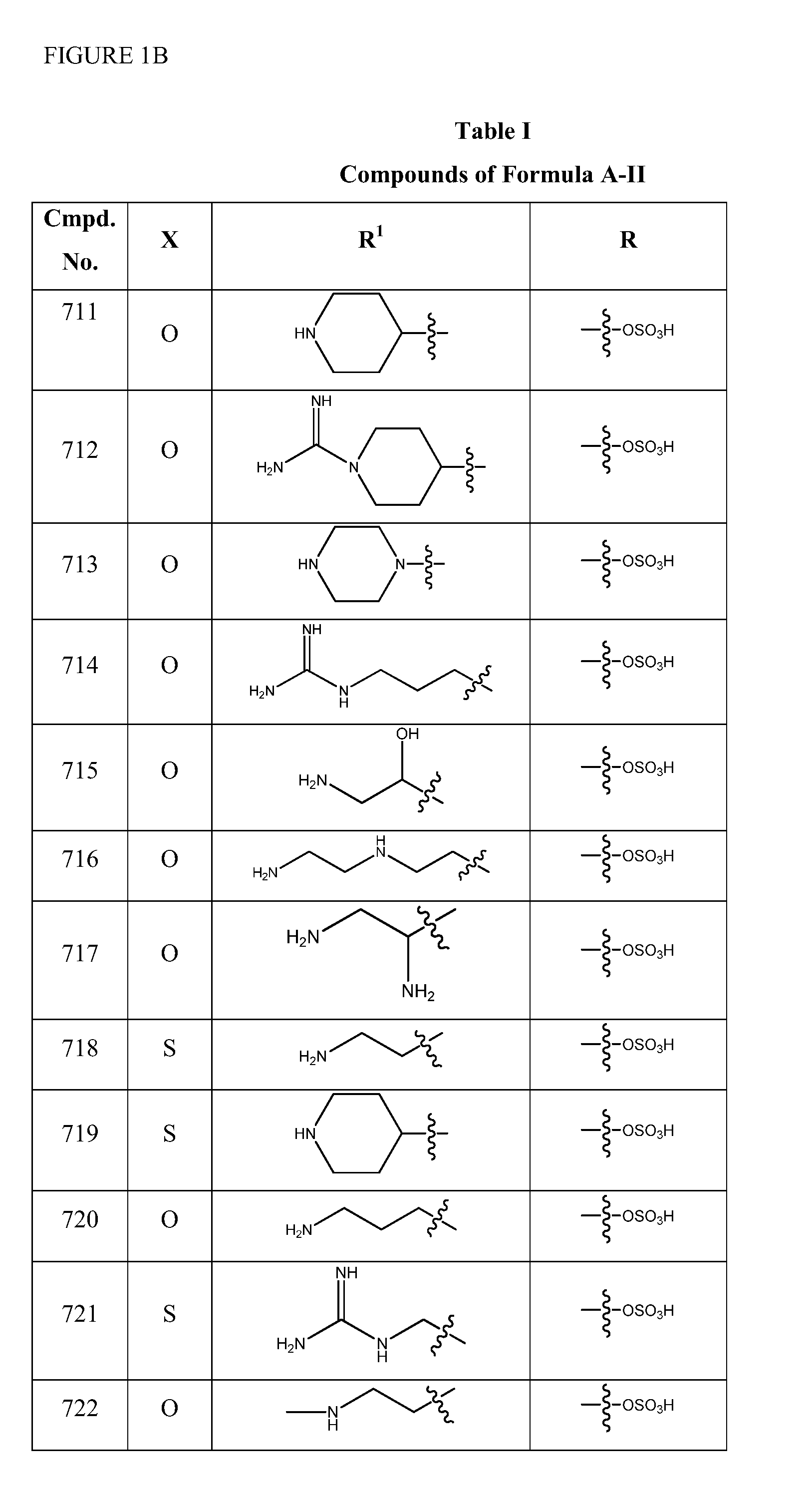
2-pyrrolidin-2-yl-[1,3,4]-oxadiazole compounds and their use as anti-depressants
Owner:GRUNENTHAL GMBH
3-aryl- heteroaryl substituted 5-trifluoromethyl oxadiazoles as histonedeacetylase 6 (HDAC6) inhibitors
The present invention is directed to substituted 5-trifluoromethyl oxadiazole compounds of generic formula (I) or a pharmaceutically acceptable salt thereof. In particular, the invention is directed to a class of aryl and heteroaryl substituted 5-trifluoromethyl oxadiazole compounds of formula I which may be useful as HDAC6 inhibitors for treating cellular proliferative diseases, including cancer, neurodegenerative diseases, such as schizophrenia and stroke, as well as other diseases.
Owner:MERCK SHARP & DOHME LLC
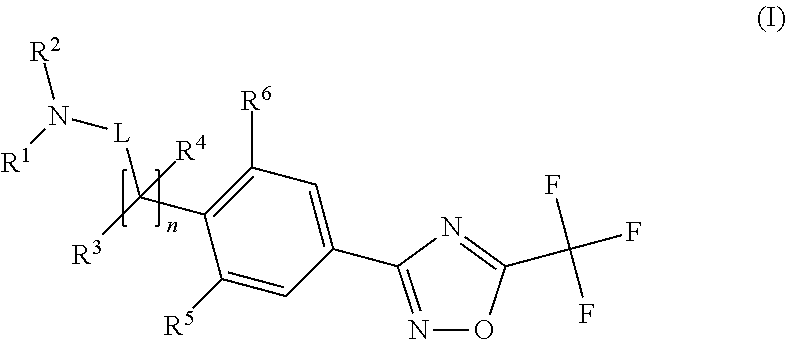
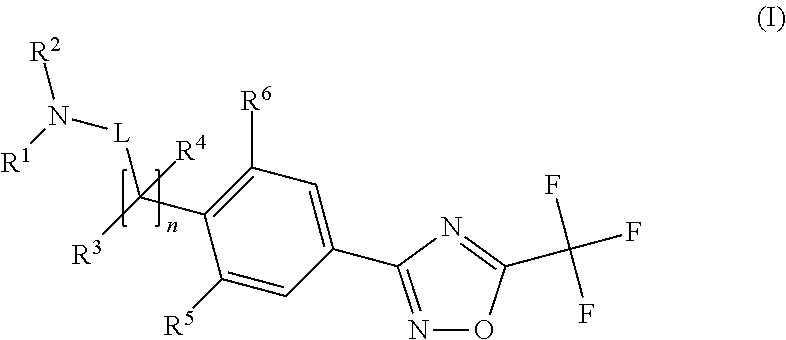
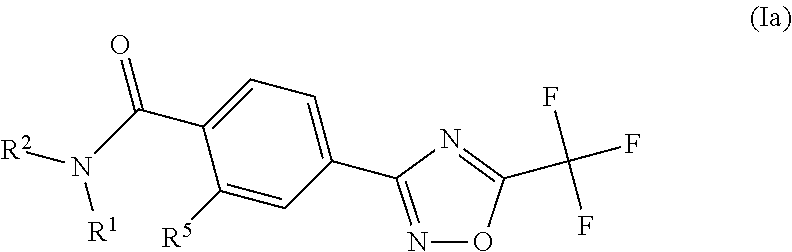
Substituted 3-phenyl-1,2,4-oxadiazole compounds
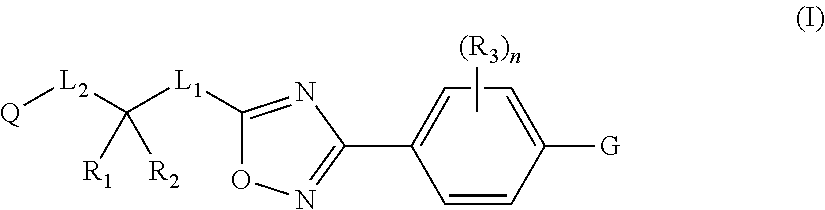
ActiveUS20130158001A1Reduce in quantityInhibit migrationBiocideOrganic chemistryTreatment fieldAutoimmune disease
Disclosed are compounds of Formula (I): (I) or stereoisomers, salts, or prodrugs thereof, wherein: (i) R1 and R2 are independently C1-C4 alkyl, or (ii) R1 and R2 together with the carbon atom to which they are attached, form a cyclic group; and Q is H, C1-6alkyl, phenyl or 5- to 6-membered heteroaryl substituted with zero to 3 substituents, and G is defined herein. Also disclosed are method of using such compounds as selective agonists for G protein-coupled receptor S1P1, and pharmaceutical compositions comprising such compounds. There compounds are useful in treating, preventing, or slowing the progression of diseases or disorders in a variety of therapeutic areas, such as autoimmune diseases and chronic inflammatory disease.
Owner:BRISTOL MYERS SQUIBB CO
Organometallic compound and organic electroluminescence device employing the same
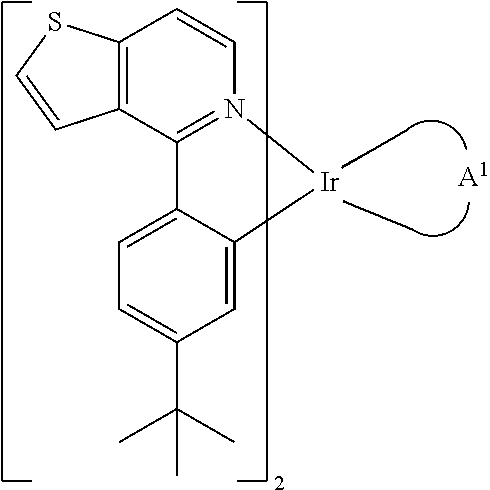
InactiveUS20130033171A1Indium organic compoundsDischarge tube luminescnet screensChemical structureCompound (substance)
Organometallic compounds and organic electroluminescence devices employing the same are provided. The organic compound has a chemical structure as represented below:wherein, A1 is diisopropyl carbodiimide ligand, 5-(2-pyridyl)-1,2,4-triazole ligand, acetylacetone with phenyl group ligand, 2-phenyl-1,3,4-oxadiazole ligand, or derivatives thereof. The organometallic compound of the disclosure can be applied in an organic electroluminescent device for enhancing the electroluminescent efficiency thereof.
Owner:IND TECH RES INST
Rhodamine derivative and preparation method and application thereof
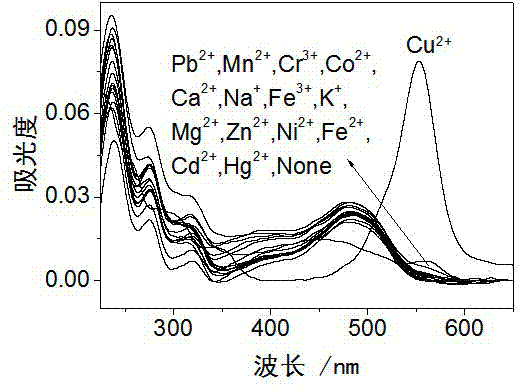
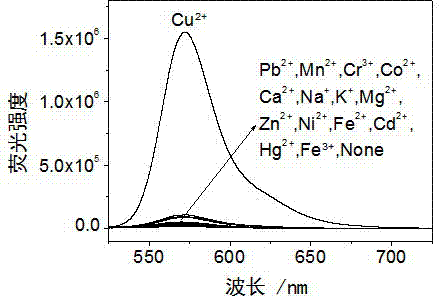
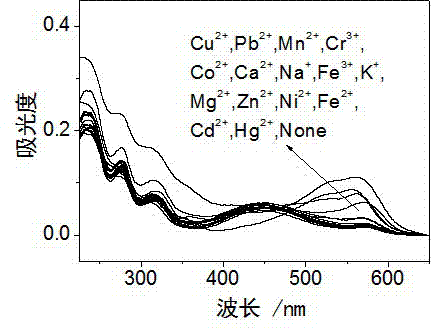
InactiveCN103113380AHigh selectivityHigh sensitivity detectionOrganic chemistryColor/spectral properties measurementsFluorescence spectrometryUv vis absorbance
The invention discloses a rhodamine derivative and a preparation method and application thereof. The rhodamine derivative is prepared by reacting rhodamine hydrazide and 4-chloro-7-nitro-2,1,3-benzoxaoxadiazole in the presence of an acid binding agent. The Cu<2+> and Fe<3+> can be respectively detected at high selectivity and high sensitivity. The designed and synthesized structure is short in synthetic route and mild in reaction conditions, the obtained derivative can be used for an aqueous phase system, and the ultraviolet-visible absorption spectrum and fluorescence spectrum are insensitive to the acid-base property of the system.
Owner:SUZHOU UNIV
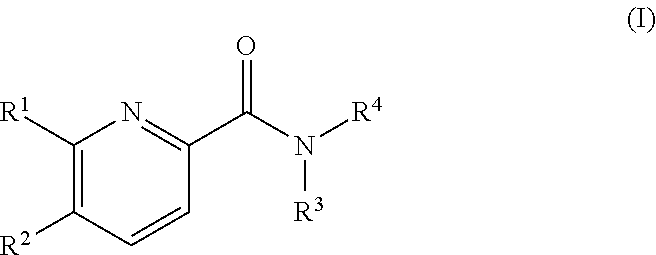
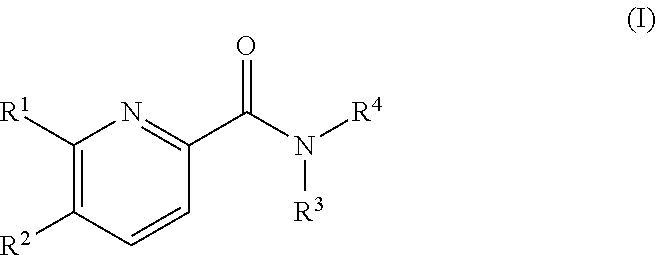
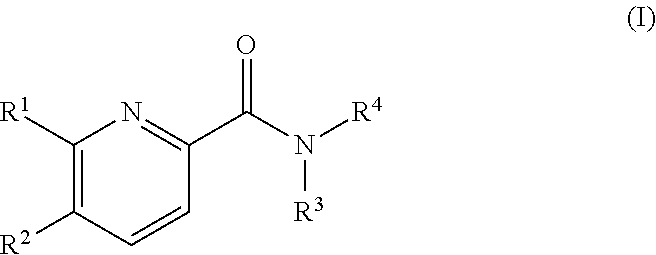
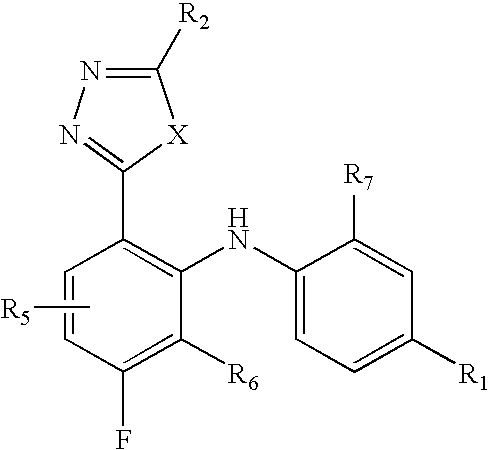
Pyridine derivatives as agonists of the CB2 receptor
The invention relates to a compound of formula (I)Wherein R1, R2, R3 and R4 are defined as in the description and in the claims. The said compounds of the invention are preferential agoniste of the Carsonabinocid Receptor 2 and thus useful as medicaments and may be used in treatment of chronic pain, atherosclerosis, ischemic / reperfusion injury and other related diseases.A representative compound of this invention is 6-cyclopropylmethoxy-5-(tetrahydro-pyradine-2-carboxglic acid [1-methyl-1-(5-methyl-(1,2,4]oxadiazol-3-yl)-ethyl)-amide.
Owner:F HOFFMANN LA ROCHE INC
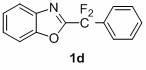
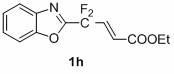
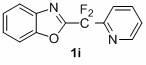
1,3-benzodiazole-containing compounds connected in series with gem-difluoromethylene groups and their synthesis methods
InactiveCN102260223AEfficient synthesisRaw materials are easy to getLiquid crystal compositionsOrganic chemistryDitazoleMeth-
The invention relates to a compound containing 1,3-benzodiazole connected in series with gem-difluoromethylene groups and a synthesis method thereof. The structural formula of this type of compound is: where R is: -COOEt-Ph, -COOCH3-Ph, -NO2-Ph, Ph, -OCH3-Ph, -CH3-Ph, -CH3-Ph, -NO2-Ph, Pyridine, (Z)-CH=CHCOOEt, (E)-CH=CHCOOEt or (E)-CH=CHPh; X is: O, S or NCH2CH2CH2CH3. The compounds containing 1,3-benzodiazoles in tandem with gem-difluoromethylene groups of the present invention exhibit unique properties in novel liquid crystal materials, so efficient synthesis of such compounds becomes very meaningful. The synthesis method has the characteristics of simple operation, short steps and convenient post-processing.
Owner:SHANGHAI UNIV
Trifluoromethyl-oxadiazole derivatives and their use in the treatment of disease
The invention relates to novel trifluoromethyl-oxadiazole derivatives of formula (I), and pharmaceutically acceptable salts thereof, (I) in which all of the variables are as defined in the specification, pharmaceutical compositions thereof, pharmaceutical combinations thereof, and their use as medicaments, particularly for the treatment of neurodegeneration, muscle atrophy or metabolic syndrome via inhibition of HDAC4.
Owner:NOVARTIS AG
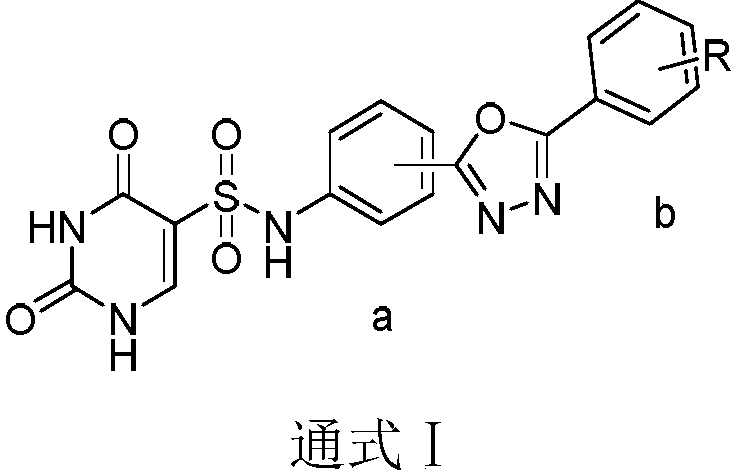
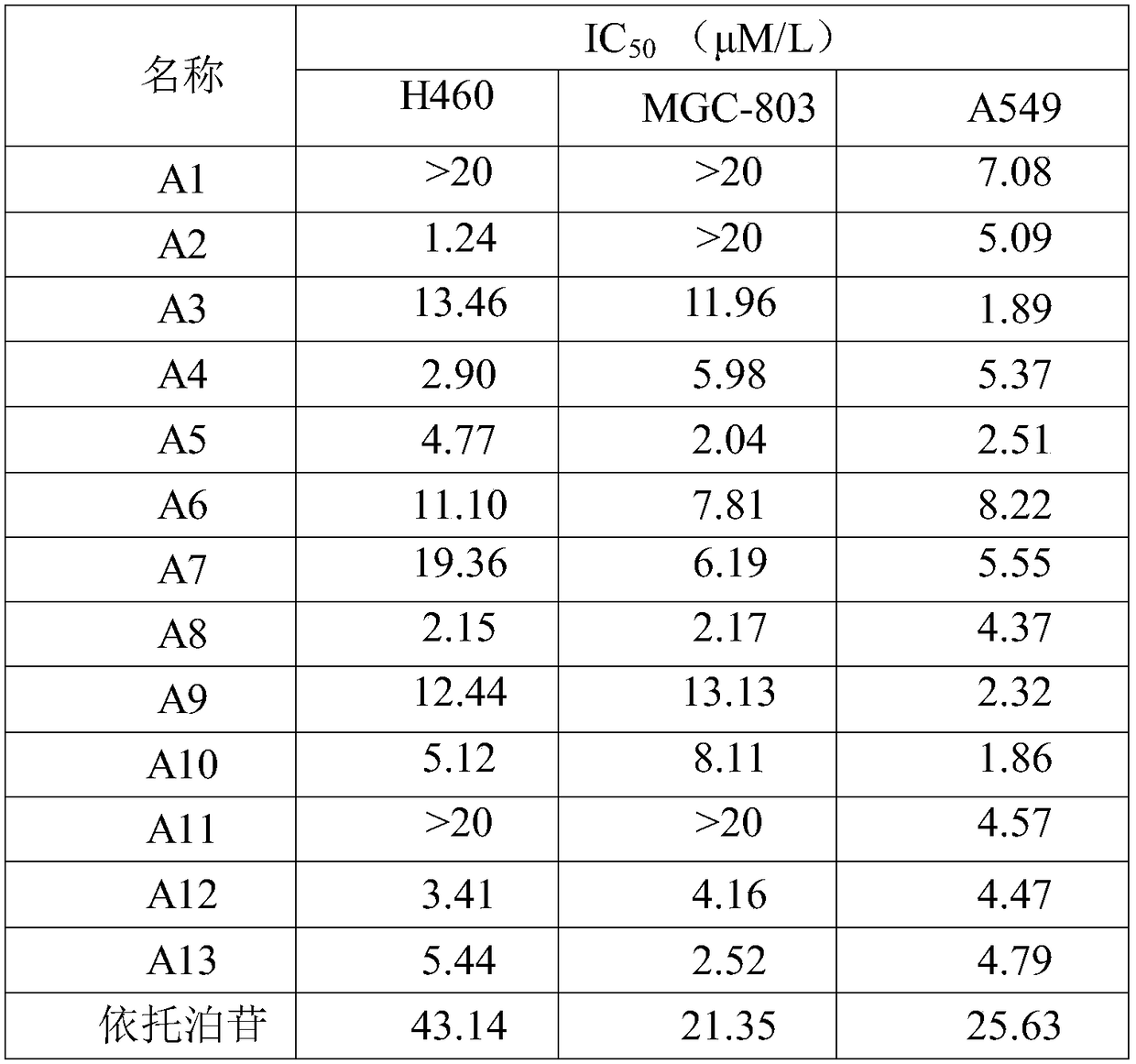
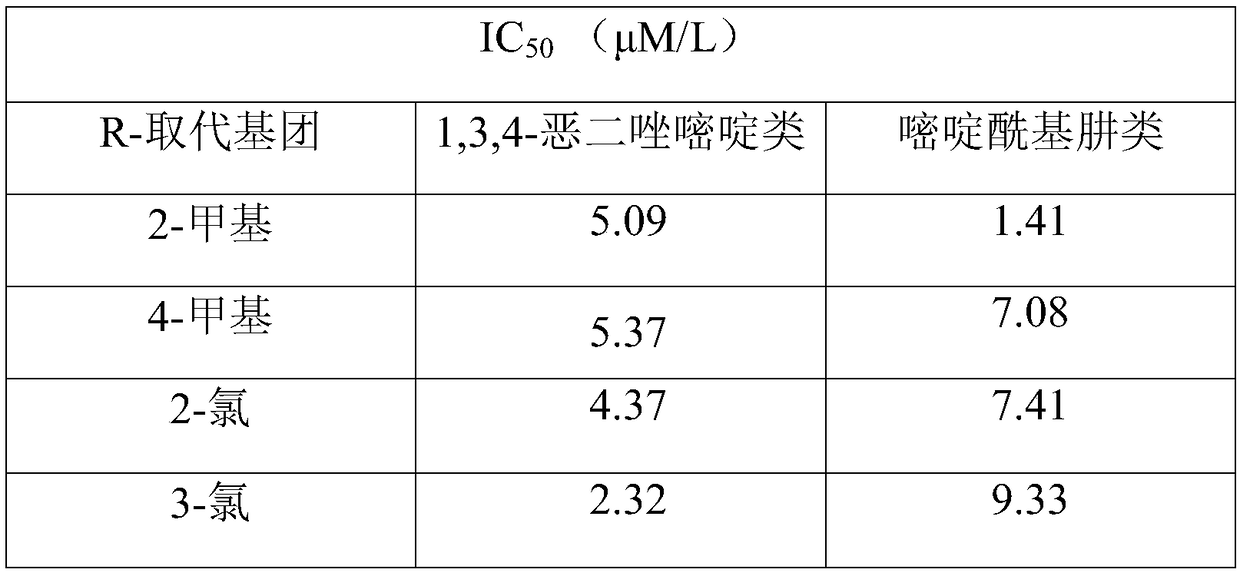
Pyrimidine type antitumor compounds having 1,3,4-oxadiazole structure and preparation method and application thereof
InactiveCN108774218AExcellent anti-proliferation abilityImprove anti-tumor effectOrganic active ingredientsOrganic chemistryChemical structureA549 cell
The invention belongs to the technical field of medicines, relates to compounds having antitumor activity and having specific chemical structures, and particularly relates to pyrimidine type antitumorcompounds having a 1,3,4-oxadiazole structure and a preparation method and application thereof. The general structure formula of the pyrimidine type antitumor compounds is shown as follows in the description. The novel compounds greatly improve antitumor effects, and have more excellent anti-proliferation capability for human lung cancer A549 cells when compared with pyrimidine acylhydrazine compounds. Synthetic steps are simplified in a synthesis process, thus making future industrial production possible.
Owner:中国医科大学
Therapeutic agent
Owner:PENTRAXIN THERAPEUTICS LTD
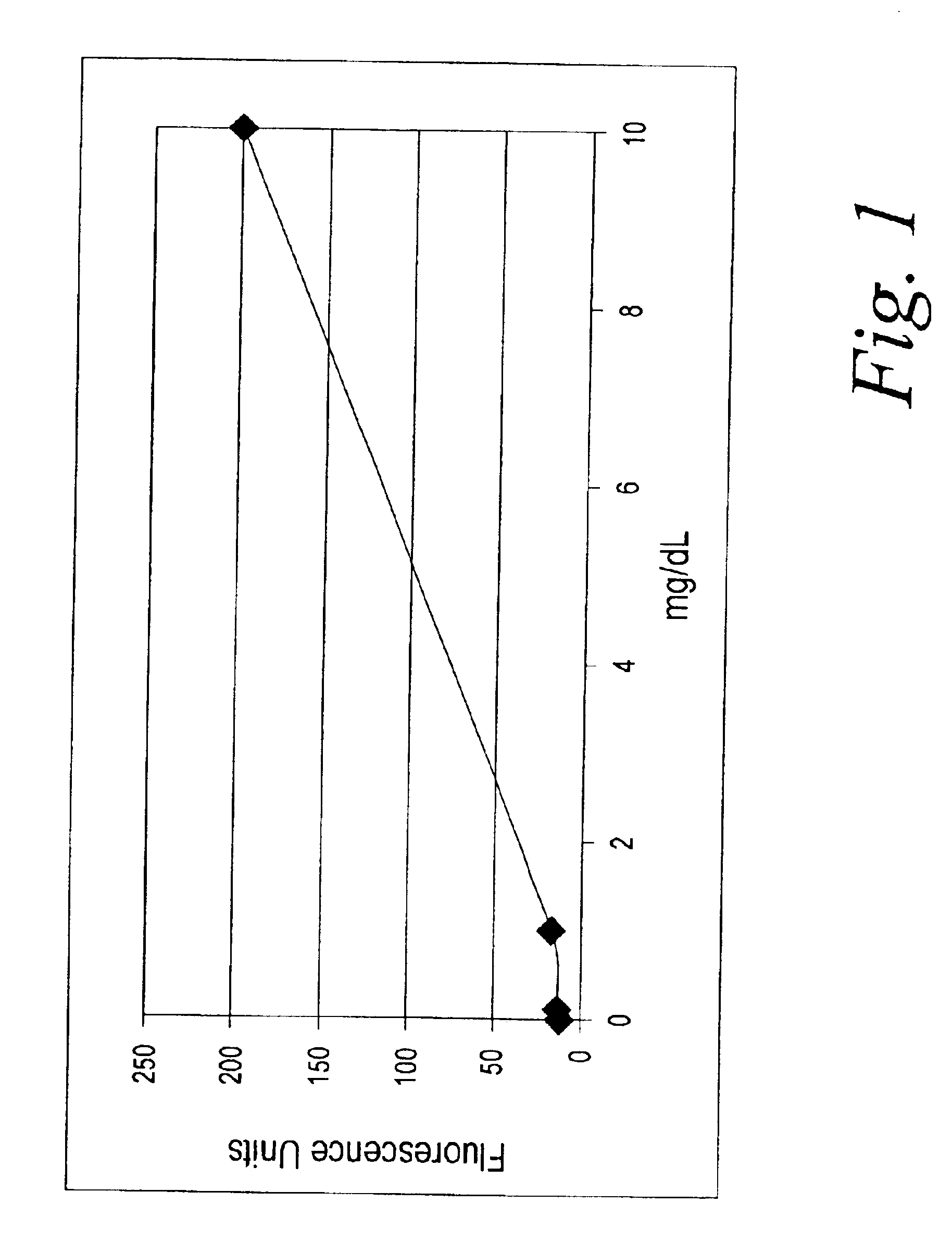
Fluorescent creatinine assay
InactiveUS6872573B2Analysis using chemical indicatorsMaterial analysis by observing effect on chemical indicatorCreatinine riseCopper
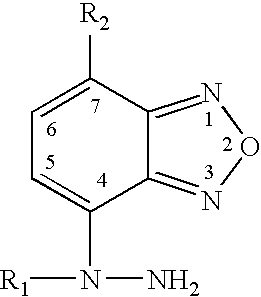
A method of detecting creatinine in body fluids using an indicator which produces a fluorescent response when oxidized in the presence of a copperII / creatinine complex. A preferred indicator is 4-(1-methylhydrazino)-7-nitro benzooxadiazole (MNBDH).
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
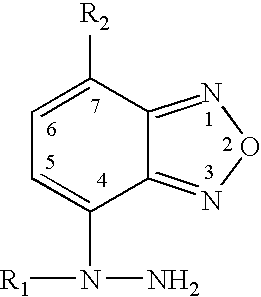
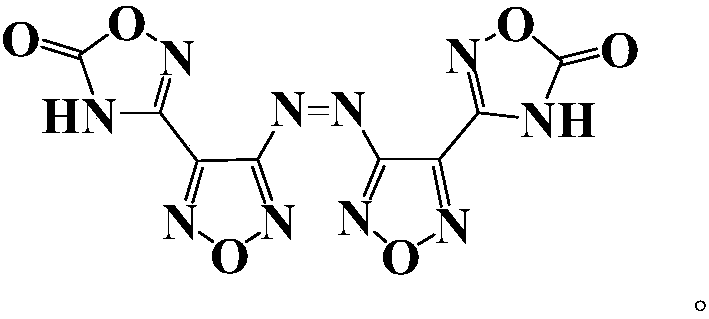
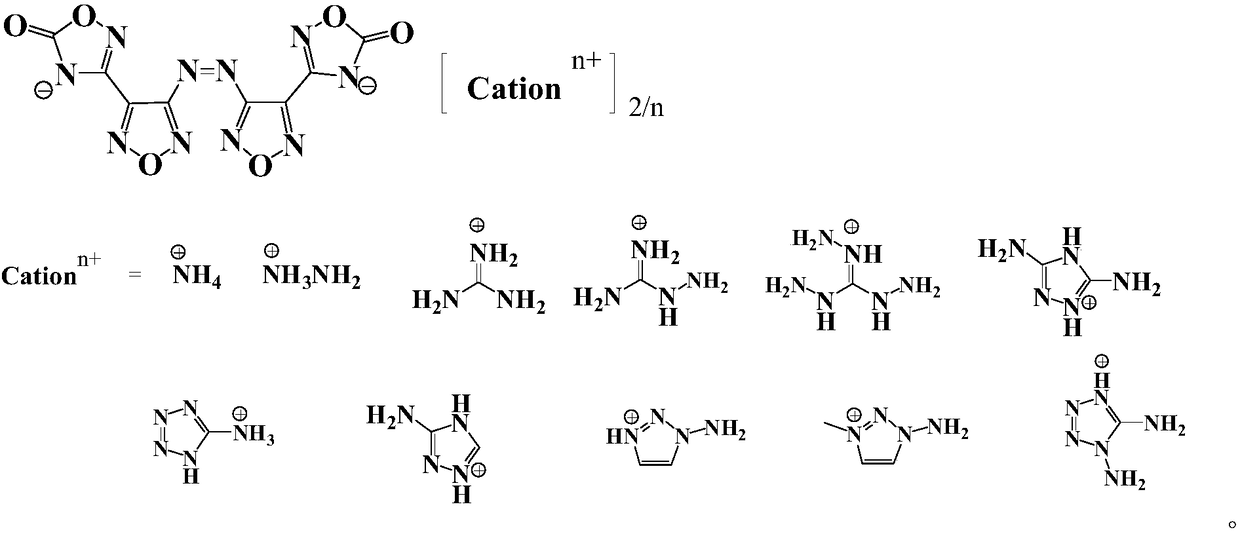
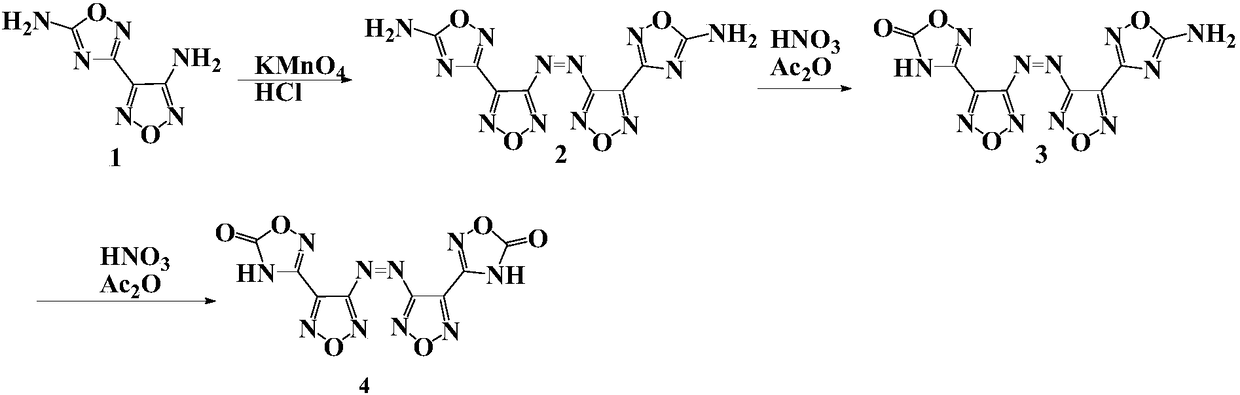
Compound and energetic ion salts thereof
ActiveCN108689959AImprove thermal stabilityThe synthesis method is simpleOrganic chemistryNitrated acyclic/alicyclic/heterocyclic amine explosive compositionsIonChemical compound
The present invention relates to 3,3'-azobis[1,2,4-oxadiazole-5-one-3-yl]-1,2,5-oxadiazole and energetic ion salts thereof, and belongs to the field of synthesis. 4-[5-amino-1,2,4-oxadiazole-3-yl-]-3-amino-1,2,5-oxadiazole is coupled by an aqueous solution of potassium permanganate to obtain 3,3'-azobis[5-amino-1,2,4-oxadiazole-3-yl-]-1,2,5-oxadiazole (compound 2), the compound 2 undergoes an oxidation reaction by a mixed solution of acetic anhydride and 100 wt% nitric acid (with a mass ratio of 2:1) to obtain 3,3'-azobis[1,2,4-oxadiazole-5-one-3-yl-]-1,2,5-oxadiazole, and the 3,3'-azobis[1,2,4-oxadiazole-5-one-3-yl-]-1,2,5-oxadiazole and an alkaline compound undergo a neutralization reaction to obtain the corresponding energetic ion salts. The above synthesis method has the advantages ofsafe and reasonable process, short reaction time, high yield, low production cost and basically no three wastes.
Owner:NANJING UNIV OF SCI & TECH
Composition 064
A pharmaceutical composition which comprises N-(3-methoxy-5-methylpyrazin-2-yl)-2-(4-[1,3,4-oxadiazol-2-yl]phenyl)pyridine-3-sulphonamide with mannitol and / or 5 microcrystalline cellulose is described.
Owner:ASTRAZENECA AB
Combinations comprising methotrexate and dhodh inhibitors
InactiveUS20110280831A1Inhibit inflammationGood curative effectBiocideNervous disorderDitazolePharmaceutical medicine
The present invention provides a combination which comprises (a) methotrexate and (b) a non-hepatotoxic DHODH inhibitor of formula (I): wherein: R1 is selected from the group consisting of hydrogen atoms, halogen atoms, C1-4 alkyl, C3-4 cycloalkyl, —CF3 and —OCF3, R2 is selected from the group consisting of hydrogen atoms, halogen atoms and C1-4 alkyl groups, R3 is selected from the group consisting of —COOR5, —CONHR5, tetrazolyl, —SO2NHR5 and —CONHSO2R5 groups, wherein R5 is selected from the group consisting of a hydrogen atom and linear or branched C1-4 alkyl groups, R4 is selected from the group consisting of a hydrogen atom and a C1-4 alkyl group, R9 is selected from the group consisting of a hydrogen atom and a phenyl group, G1 represents a group selected from N and CR6 wherein R6 is selected from the group consisting of hydrogen atoms, halogen atoms, C1-4 alkyl, C3-4 cycloalkyl, C1-4 alkoxy, —CF3, —OCF3, monocyclic N-containing C5-7 heteroaryl, monocyclic N— containing C3-7 heterocyclyl groups and C6-10 aryl groups which C6-10 aryl groups are optionally substituted with one or more substituents selected from halogen atoms and C1-4 alkyl groups, G1 represents a group selected from N and CR6 wherein R6 is selected from the group consisting of hydrogen atoms, halogen atoms, C1-4 alkyl, C3-4 cycloalkyl, C1-4 alkoxy, —CF3, —OCF3, mono-cyclic N-containing C5-7 heteroaryl, monocyclic N— containing C3-7 heterocyclyl groups and C6-10 aryl groups which C6-10 aryl groups are optionally substituted with one or more substituents selected from halogen atoms and C1-4 alkyl groups, G2 represents a group selected from: a hydrogen atom, a hydroxy group, a halogen atom, a C3-4 cycloalkyl group, a C1-4 alkoxy group and —NRaRb, wherein Ra represents a C1-4 alkyl group and Rb is selected from a group consisting of C1-4 alkyl group and C1-4alkoxy-C1-4 alkyl group, or Ra and Rb together with the nitrogen atom to which they are attached form a saturated 6 to 8 membered heterocyclic ring optionally containing one oxygen atom as an additional heteroatom, a monocyclic or bicyclic 5 to 10 membered heteroaromatic ring containing one or more nitrogen atoms which is optionally substituted by one or more substituents selected from halogen atoms, C1-4 alkyl, C1-4 alkoxy, C3-4 cycloalkyl, C3-4 cycloalkoxy, —CF3, —OCF3, and —CONR7R8, wherein R7 and R8 are independently selected from hydrogen atom, linear or branched C1-4 alkyl groups, C3-7 cycloalkyl groups, or R7 and R8 together with the nitrogen atom to which they are attached form a group of formula wherein n is an integer from 0 to 3, and a phenyl group which is optionally substituted by one or more substituents selected from halogen atoms, C1-4 alkyl, hydroxyl, C1-4 alkoxy, C3-4 cycloalkyl, C3-4 cycloalkoxy, cyano, —CF3, —OCF3, —CONR7R8, oxadiazolyl, triazolyl, pyrazolyl and imidazolyl groups, which oxadiazolyl, triazolyl, pyrazolyl and imidazolyl groups are optionally substituted by C1-4 alkyl or C3-7 cycloalkyl groups and wherein R7 and R8 are independently selected from hydrogen atom, linear or branched C1-4 alkyl groups, C3-7 cycloalkyl groups, or R7 and R8 together with the nitrogen atom to which they are attached form a group of formula wherein n is an integer from 0 to 3 or, when G′ represents CR6, G2 together with R6 forms a non-aromatic C5-10 carbocyclic group or a C6-10 aryl group, and the pharmaceutically acceptable salts and N-oxides thereof.
Owner:ALMIRALL
GSK-3β inhibitor
For the purpose of providing a GSK-3β inhibitor containing an oxadiazole compound or a salt thereof or a prodrug thereof useful as an agent for the prophylaxis or treatment of a GSK-3β-related pathology or disease, the present invention provides a GSK-3β inhibitor containing a compound represented by the formula (I):wherein each symbol is as defined in the specification, or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
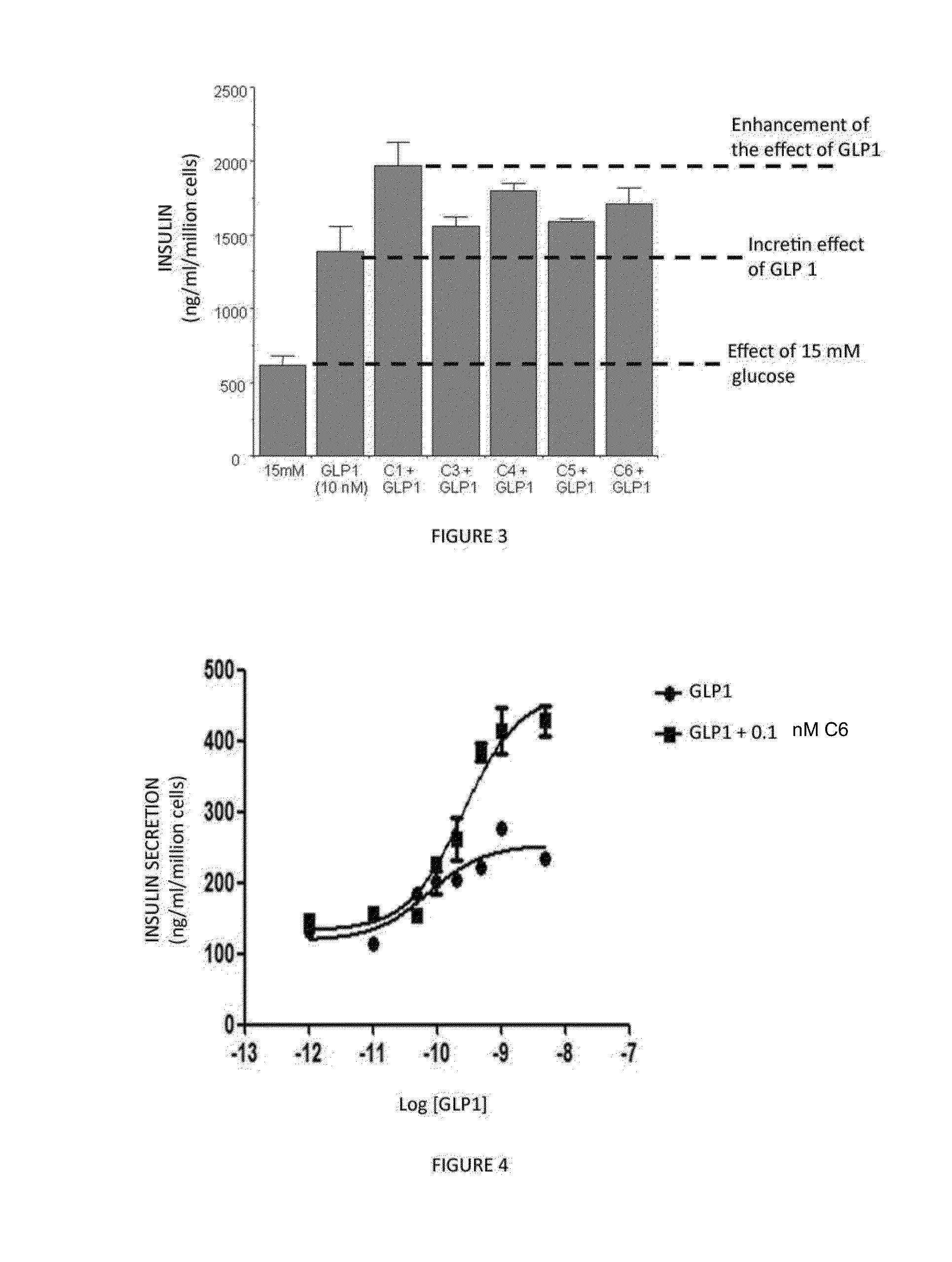
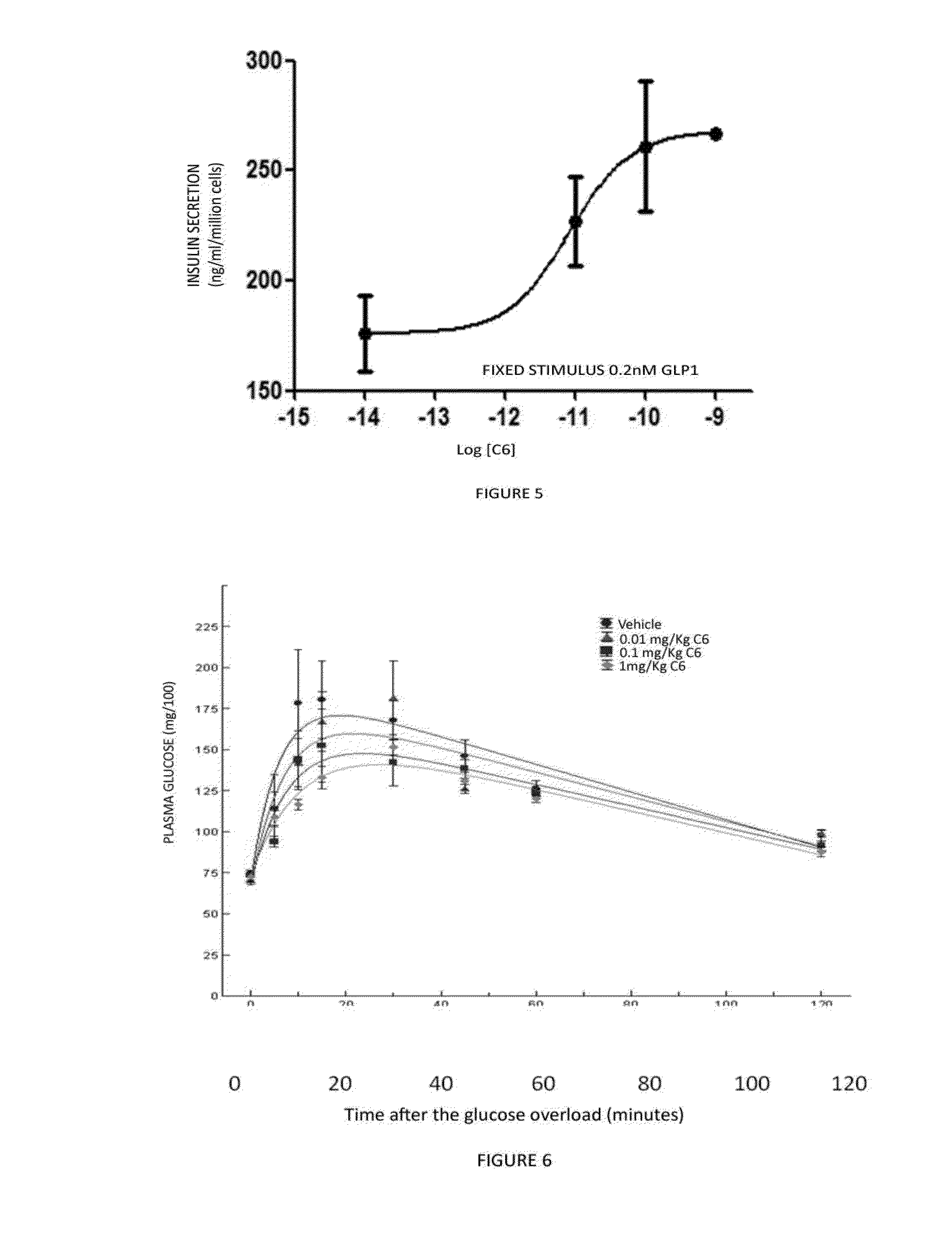
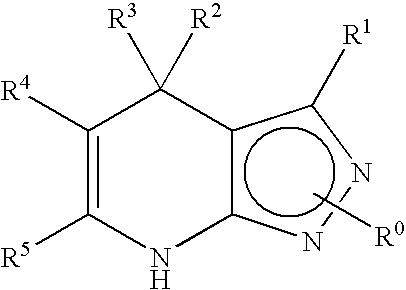
1,2,4-oxadiazole derivatives as drugs modulating the glp-1 peptide receptor
The present invention relates to the use of a compound of formula (I):or a pharmaceutically acceptable solvate or salt thereof in the preparation of a medicinal product for the prevention and / or the treatment of a disease in which the GLP-1 receptor participates or mediates, such as eating disorders or diseases, for example, obesity, anorexia, lipid dysfunction, diabetes, hyperinsulinism and cardiovascular diseases or metabolic syndrome.
Owner:VIVIA BIOTECH SL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![2-pyrrolidin-2-yl-[1,3,4]-oxadiazole compounds and their use as anti-depressants 2-pyrrolidin-2-yl-[1,3,4]-oxadiazole compounds and their use as anti-depressants](https://images-eureka.patsnap.com/patent_img/b4e6730d-4549-4e4b-99c5-bdb9382940fc/US07105538-20060912-C00001.png)
![2-pyrrolidin-2-yl-[1,3,4]-oxadiazole compounds and their use as anti-depressants 2-pyrrolidin-2-yl-[1,3,4]-oxadiazole compounds and their use as anti-depressants](https://images-eureka.patsnap.com/patent_img/b4e6730d-4549-4e4b-99c5-bdb9382940fc/US07105538-20060912-C00002.png)
![2-pyrrolidin-2-yl-[1,3,4]-oxadiazole compounds and their use as anti-depressants 2-pyrrolidin-2-yl-[1,3,4]-oxadiazole compounds and their use as anti-depressants](https://images-eureka.patsnap.com/patent_img/b4e6730d-4549-4e4b-99c5-bdb9382940fc/US07105538-20060912-C00003.png)