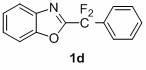
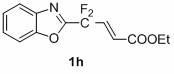
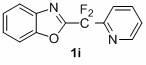
1,3-benzodiazole-containing compounds connected in series with gem-difluoromethylene groups and their synthesis methods
A technology of difluoromethylene and benzodiazole, applied in the field of 1,3-benzodiazole-containing compounds, can solve problems such as not yet reported, and achieve the effects of high research and application value and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Under nitrogen protection, 414 mg (1.5 mmol) ethyl 4-iodobenzoate and 248 mg (1 mmol) 2-bromodifluoromethane were added to a 25 ml two-neck bottle equipped with a magnet Base 1,3-benzoxazole, 2 ml dimethyl sulfoxide, 64 mg (1 mmol) copper powder, react at room temperature for 4 hours, stop the reaction, cool the reaction solution to room temperature, and directly use silica gel column chromatography to separate and purify , 32 mg of the product was obtained with a yield of 10%. The structure of this compound is:
[0025]
[0026] Chinese name: ethyl 4-(2-benzoxazolyl)difluoromethylbenzoate
[0027] English name: 4-(Benzooxazol-2-yl-difluoro-methyl)-benzoic acid ethyl
[0028] Molecular weight: 317.09
[0029] Appearance: white solid
[0030] Melting point: 59-61 degrees Celsius
[0031] H NMR spectrum (500 MHz, CDCl 3 ) chemical shift (in ppm): δ 8.17 (d, J = 8.5 Hz, 2H, ArH), 7.80 (d, J = 8.4 Hz, 3H, ArH), 7.59 (d, J = 8.1 Hz, 1H, ArH), 7.3...
Embodiment 2
[0036]Example 2: Under nitrogen protection, 414 mg (1.5 mmol) ethyl 4-iodobenzoate, 248 mg (1 mmol) 2-bromodifluoro Methyl 1,3-benzoxazole, 10 ml dimethylformamide, 64 mg (1 mmol) copper powder, stop the reaction after 4 hours at room temperature, cool the reaction solution to room temperature, and directly use silica gel column chromatography After separation and purification, 89 mg of the product was obtained with a yield of 28%.
Embodiment 3
[0037] Example 3: Under nitrogen protection, 1.1 g (4 mmol) ethyl 4-iodobenzoate, 2 g (8 mmol) 2-bromodifluoro Methyl 1,3-benzoxazole, 35 ml dimethylformamide, 1.2 g (18.4 mmol) copper powder, stop the reaction after 8 hours at room temperature, cool the reaction solution to room temperature, and directly use silica gel column chromatography After separation and purification, 0.98 g of the product was obtained with a yield of 77%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com