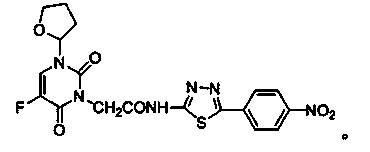
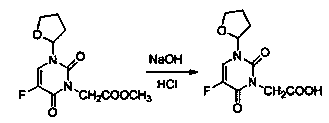Tegafur derivative containing 1,3,4-thiadiazole heterocyclic ring and amide group
A technology of amide groups and tegafur, which is applied in the field of tegafur derivatives, can solve the problems of high toxicity and achieve the effects of reducing toxicity, enhancing lipophilicity, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
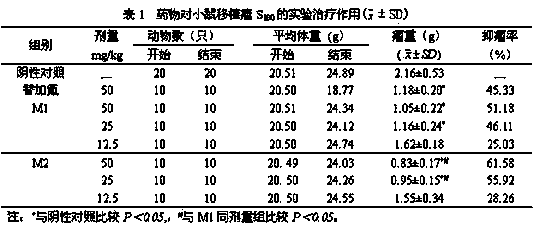
Image
Examples
Embodiment 1
[0019] Embodiment 1: A kind of preparation method of tegafur derivative containing 1,3,4-thiadiazole heterocycle and amide group, the steps are as follows:
[0020] ①Dissolve 3-(methoxycarbonylmethyl)tegafur in methanol, then add sodium hydroxide solution dropwise, mix sodium hydroxide and distilled water at a weight-to-volume ratio of 1:20-30 to make a solution, 45 React at ℃ for 2-4 hours, evaporate the solvent under reduced pressure, extract with ethyl acetate and distilled water to separate the organic layer and water layer, extract and combine the organic layers with water, adjust the pH value to 4, then extract with ethyl acetate, combine the organic layers, Add anhydrous sodium sulfate to dry, filter, and evaporate the solvent to obtain 1-(tetrahydro-2-furyl)-3-acetoxy-5-fluoro-2,4-pyrimidinedione;
[0021] ②Take 1-(tetrahydro-2-furyl)-3-acetoxy-5-fluoro-2,4-pyrimidinedione and dioxane, mix them in a ratio of 1:3-4 by weight and volume, and then add chlorine The volume...
Embodiment 2
[0023] Embodiment 2: A kind of preparation method of tegafur derivative containing 1,3,4-thiadiazole heterocycle and amide group, the steps are as follows:
[0024] ①Put 6.8g of 3-(methoxycarbonylmethyl)tegafur into a reaction flask, add 10ml of methanol, heat to dissolve, dissolve 1.4g of sodium hydroxide in 35ml of distilled water, add the sodium hydroxide solution dropwise, React at 45°C for 3 hours, evaporate the solvent under reduced pressure, add 50ml of distilled water and 50ml of ethyl acetate to extract and separate the organic layer and the water layer, extract the organic layer with 50 ml of water once, combine the water layers, and adjust the pH value with 10% hydrochloric acid to 4, then extracted twice with 50 ml of ethyl acetate, combined the organic layers, added 10 g of anhydrous sodium sulfate and dried for 30 minutes, filtered, and evaporated to remove the solvent to obtain 1-(tetrahydro-2-furyl)-3-acetate -5-fluoro-2,4-pyrimidinedione 4.7g, the yield is 73....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com