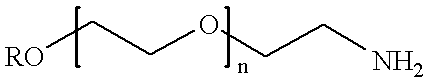Patents
Literature
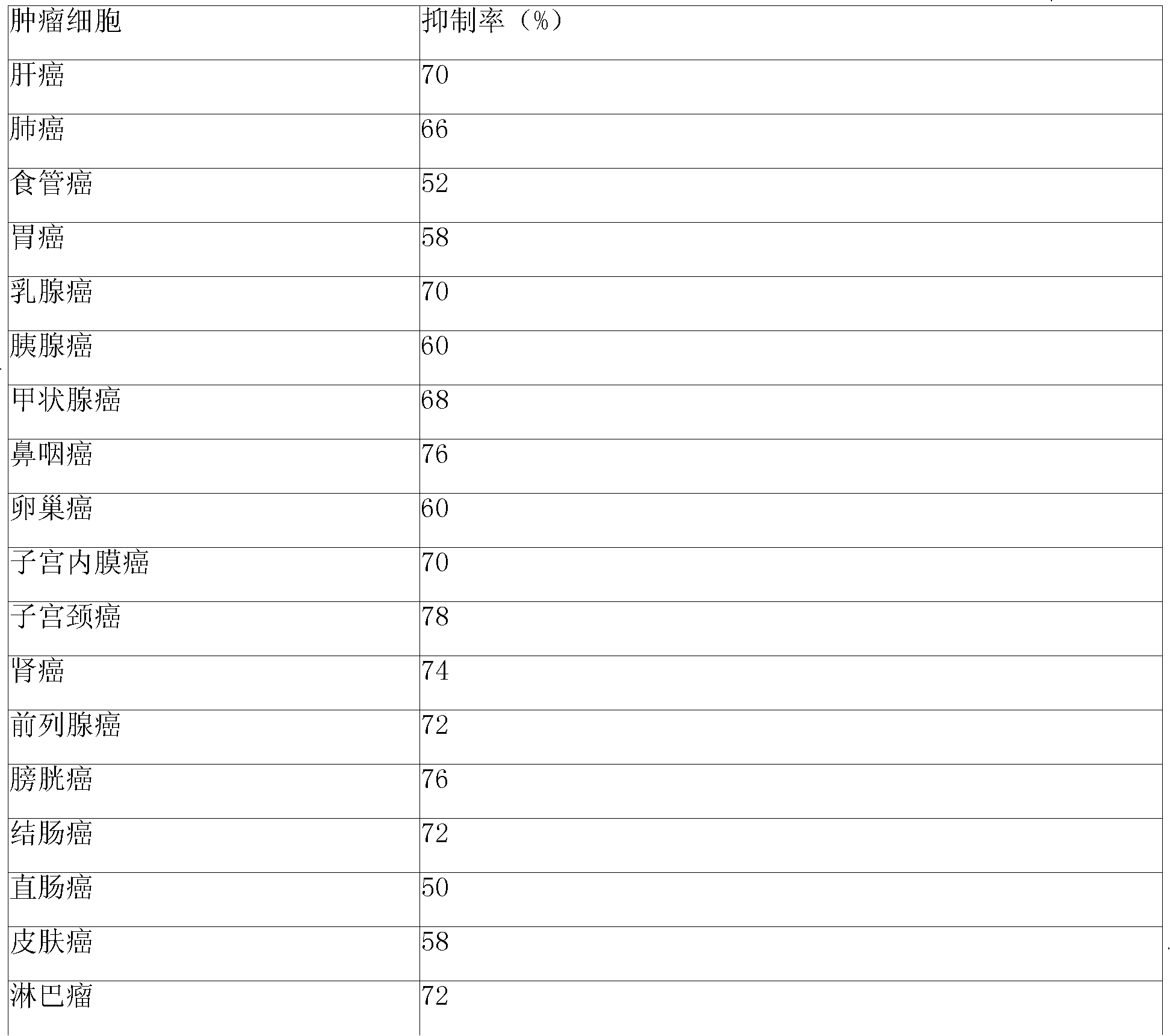
38 results about "Idarubicin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Idarubicin is used to treat a certain type of cancer (leukemia).
Antineoplastic conjugates of transferrin, albumin and polyethylene glycol
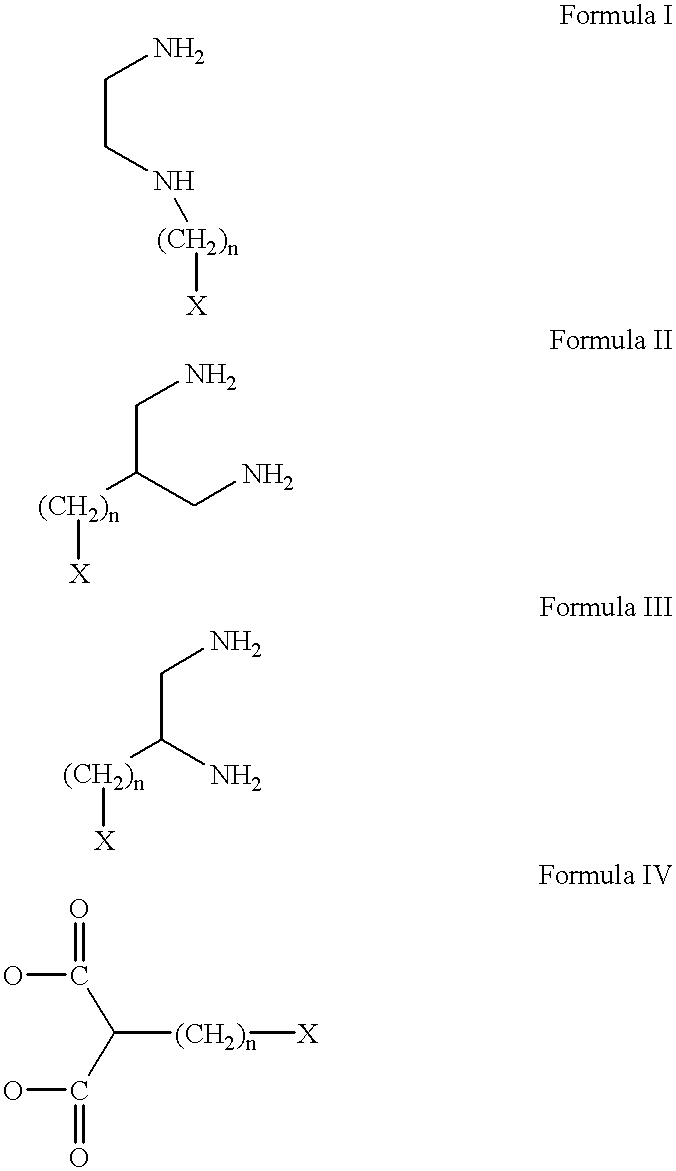
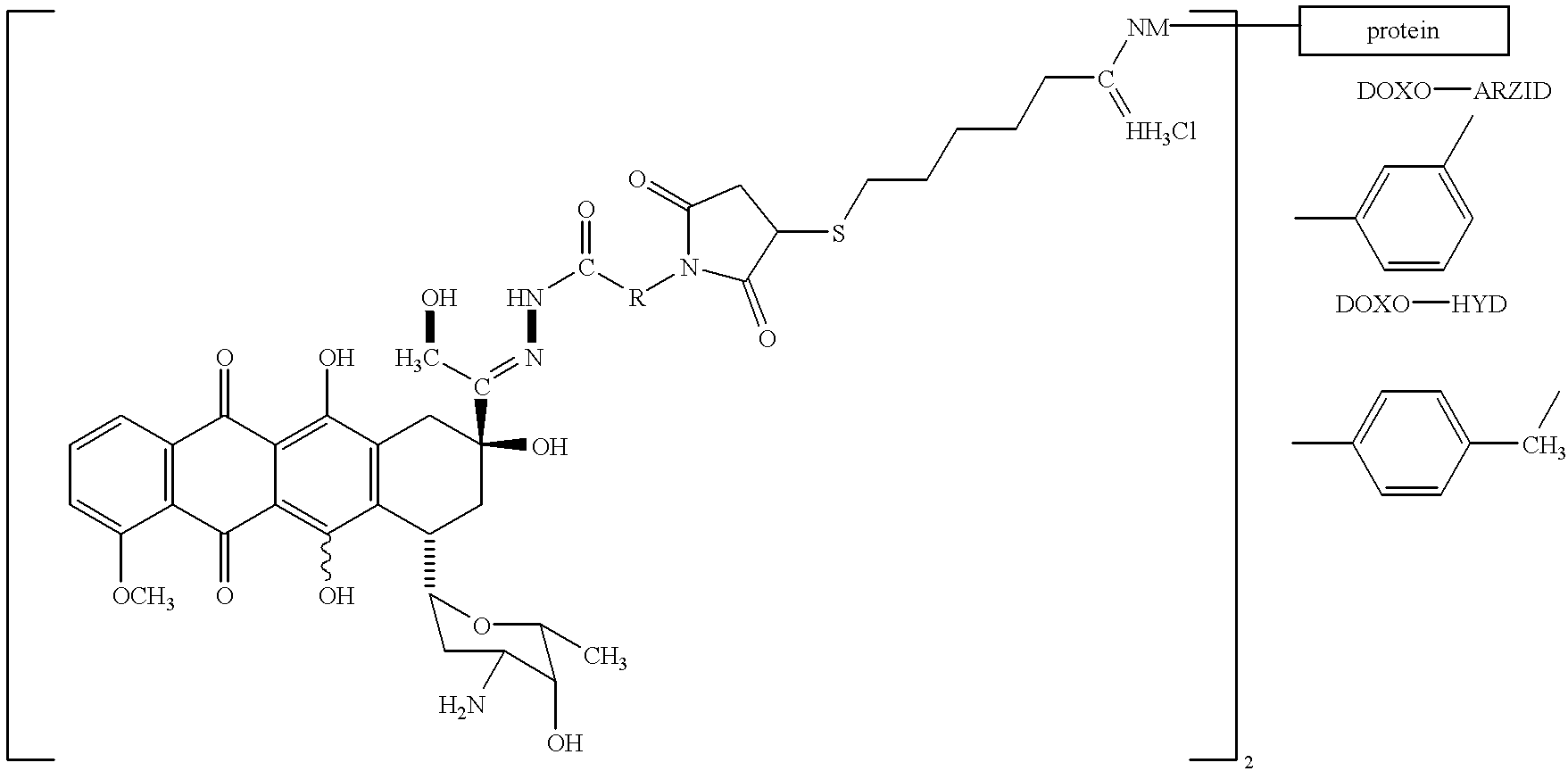
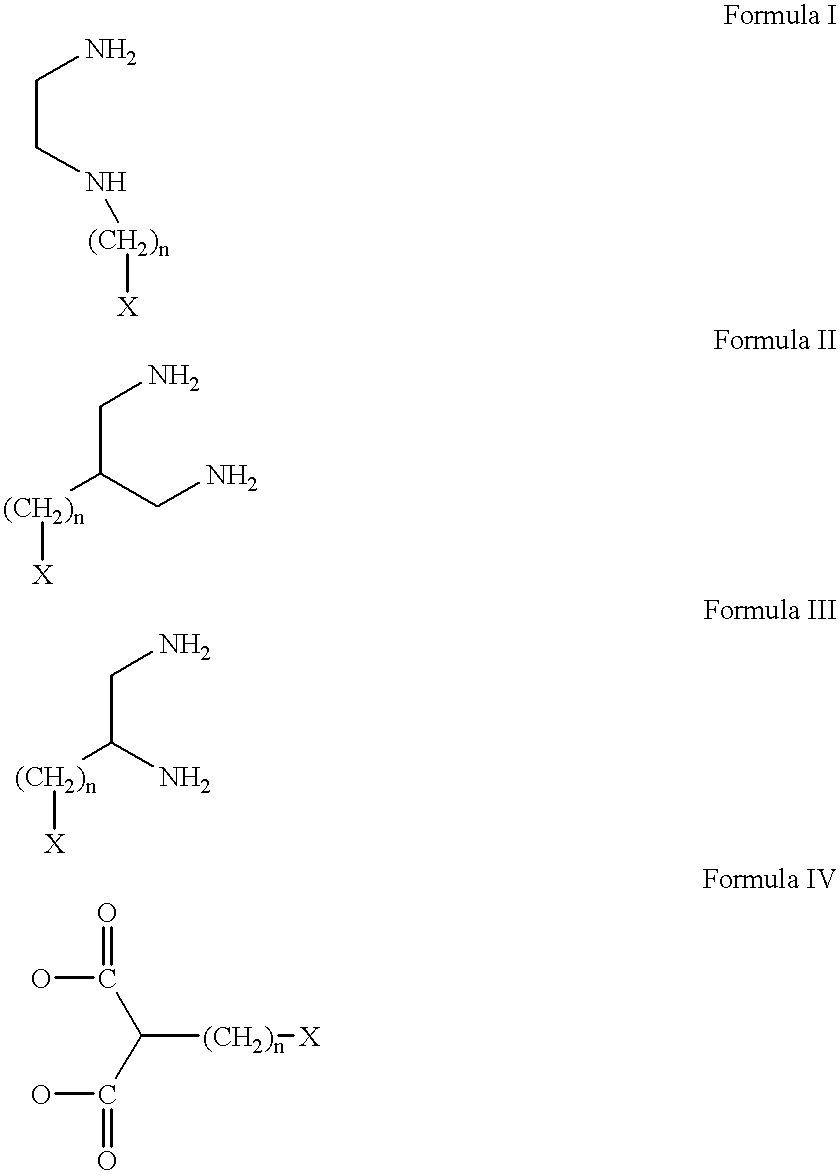
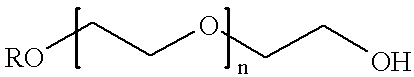
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R* H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
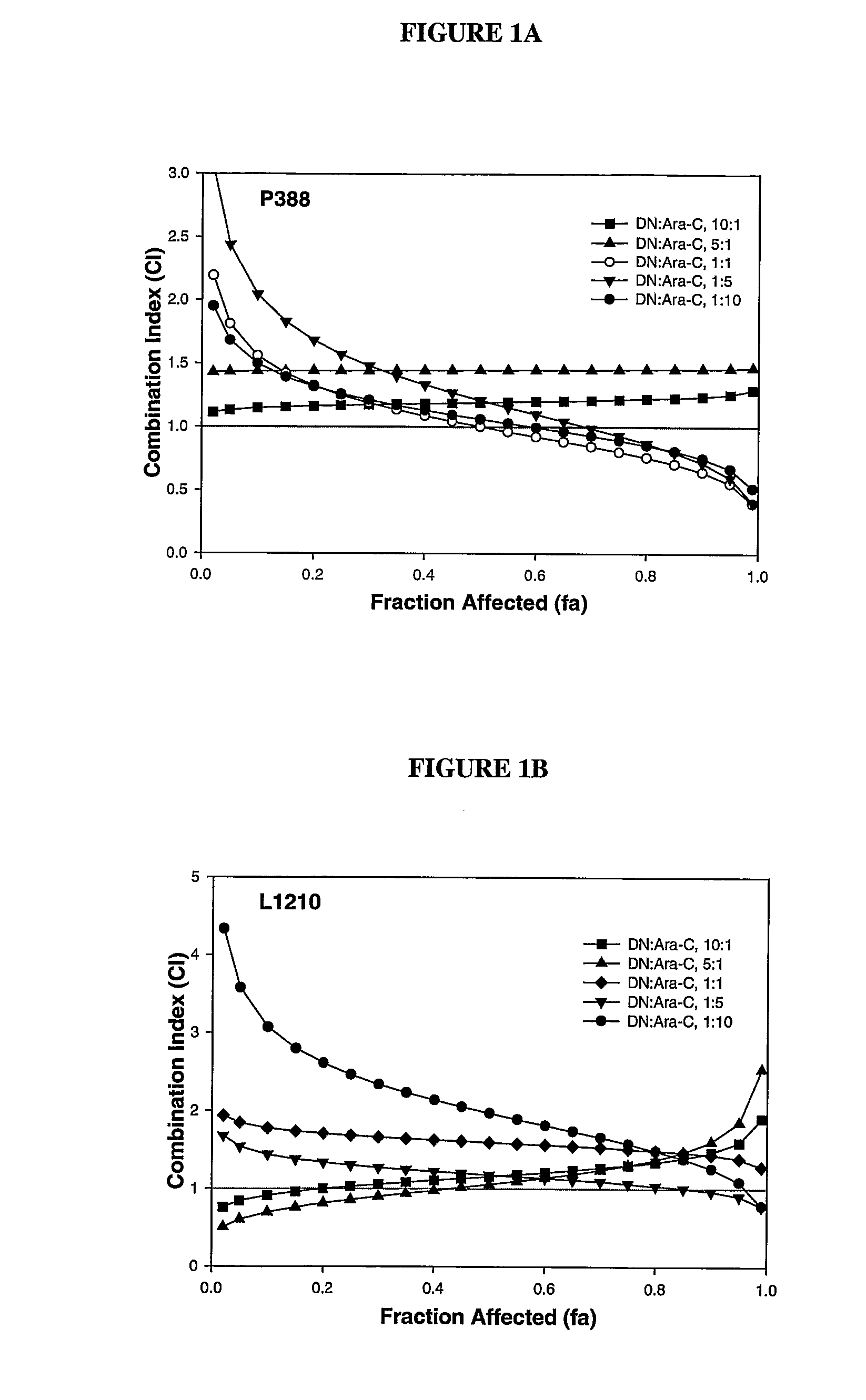
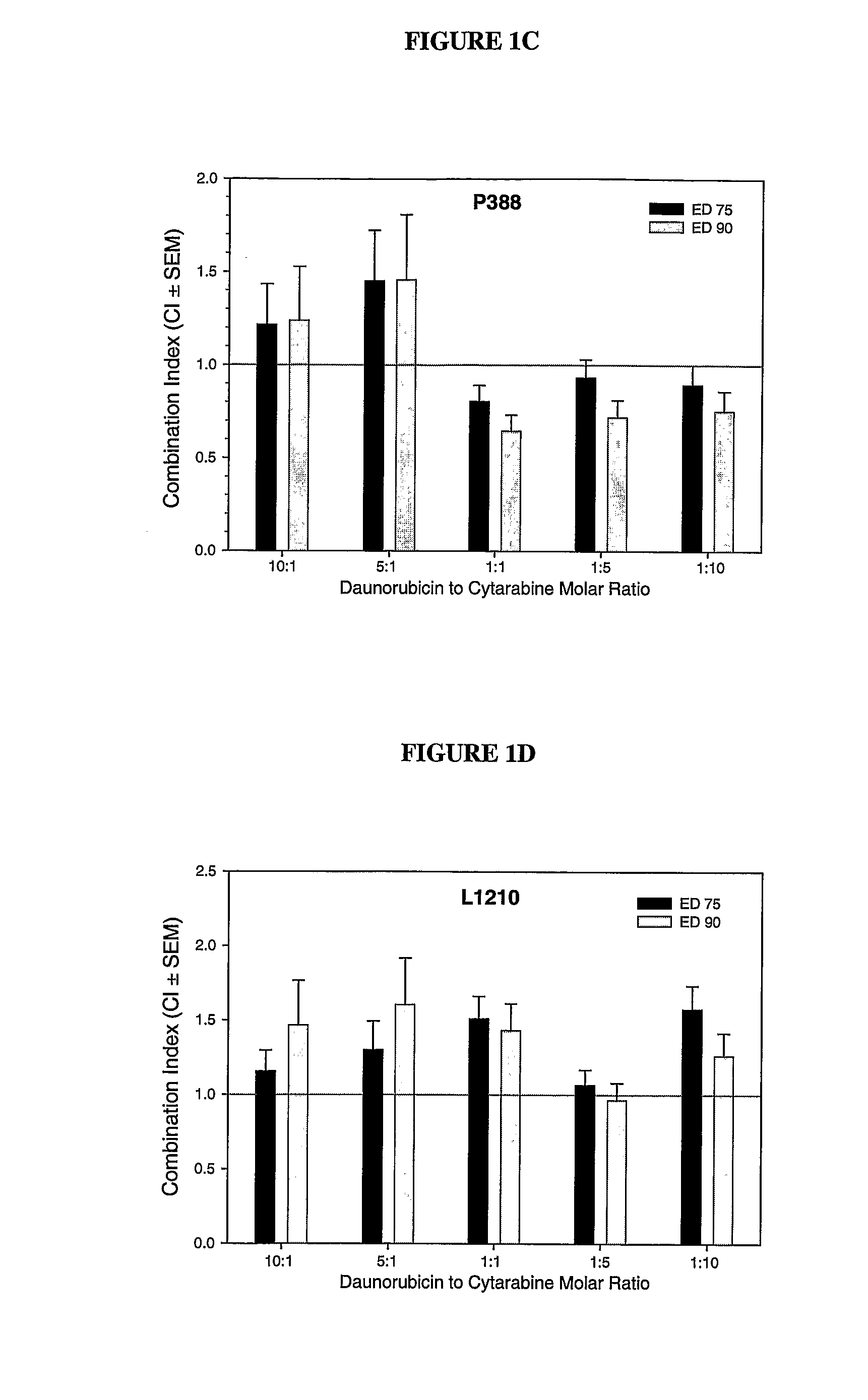
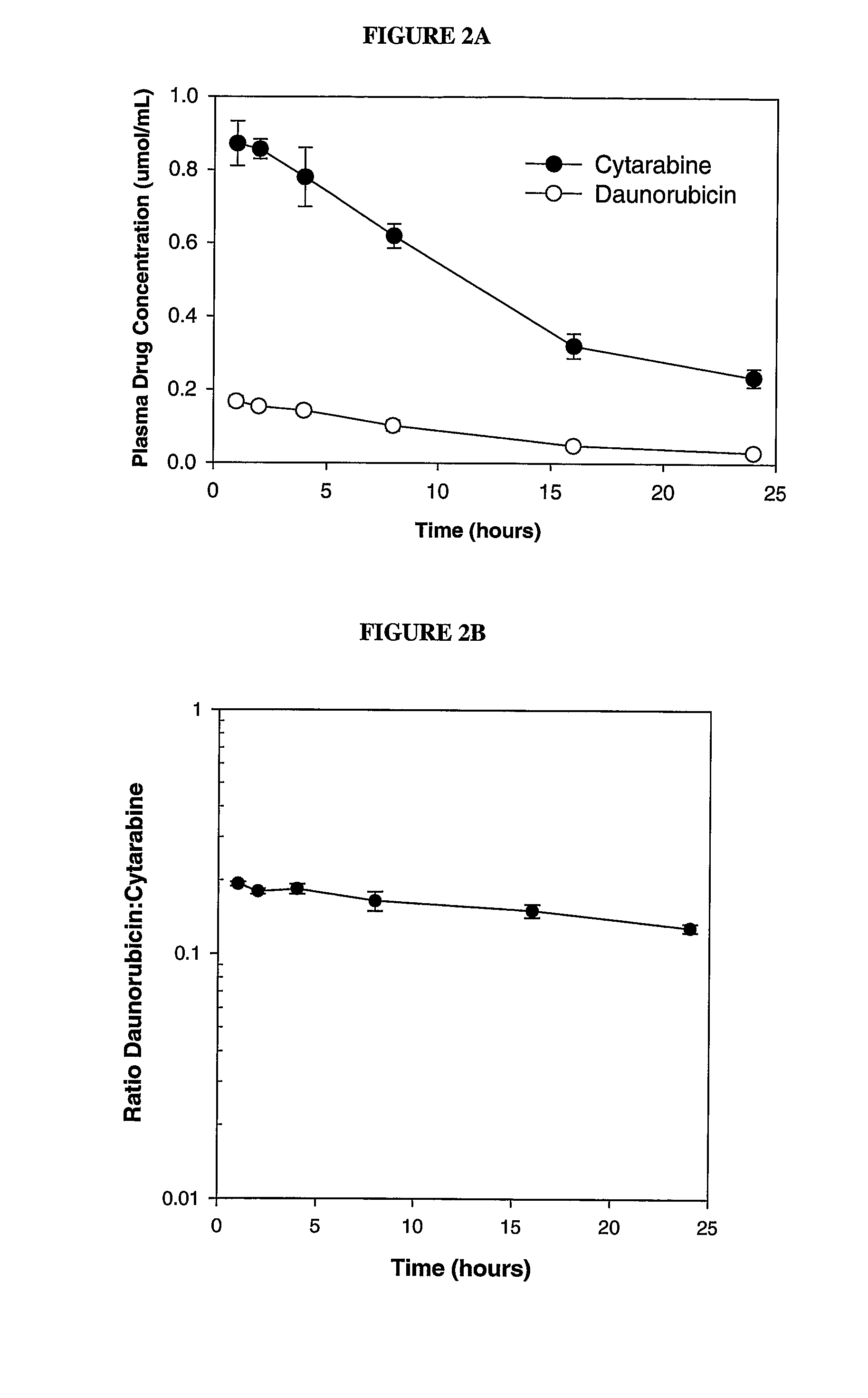
Liposomal formulations of anthracycline agents and cytidine analogs
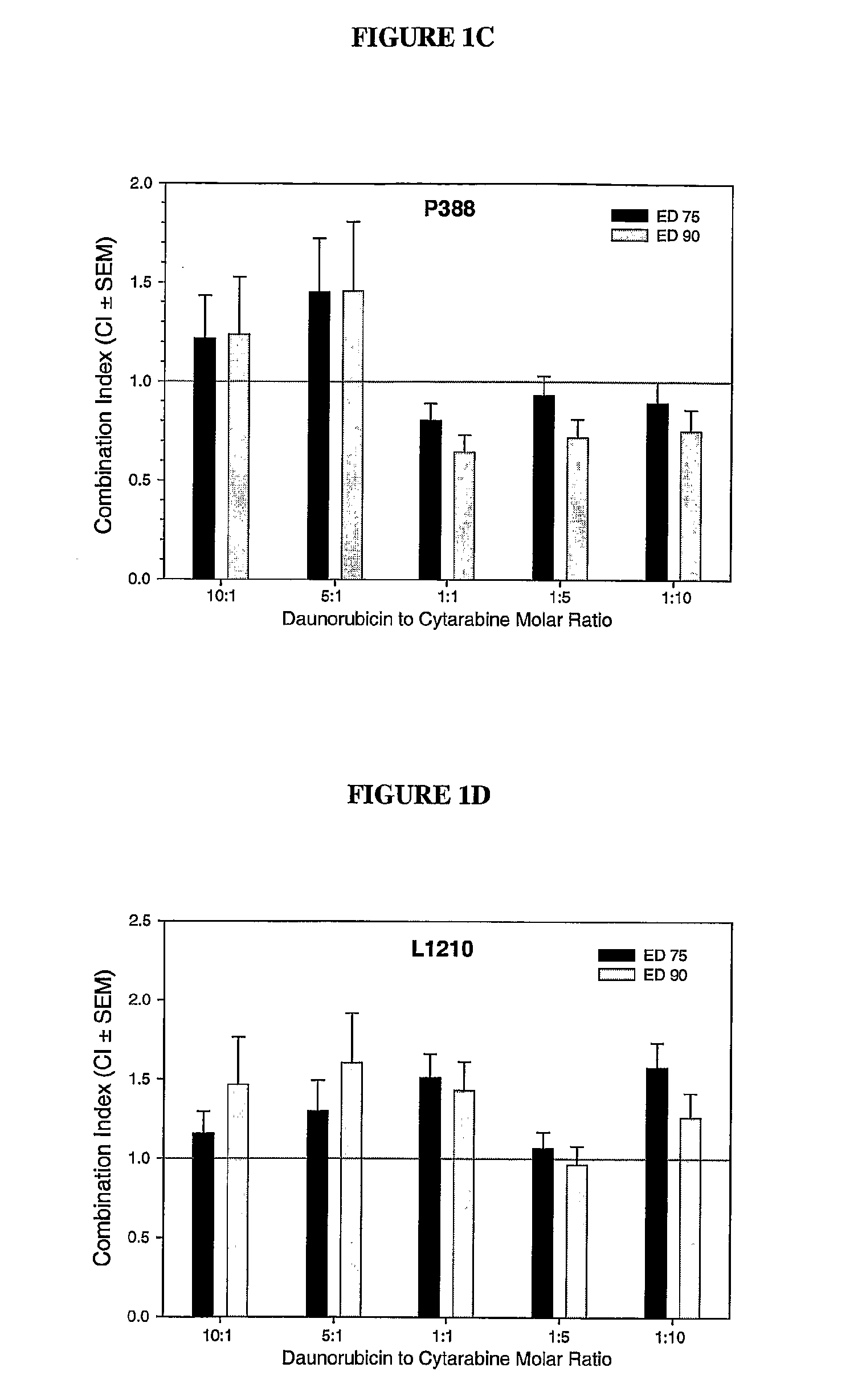
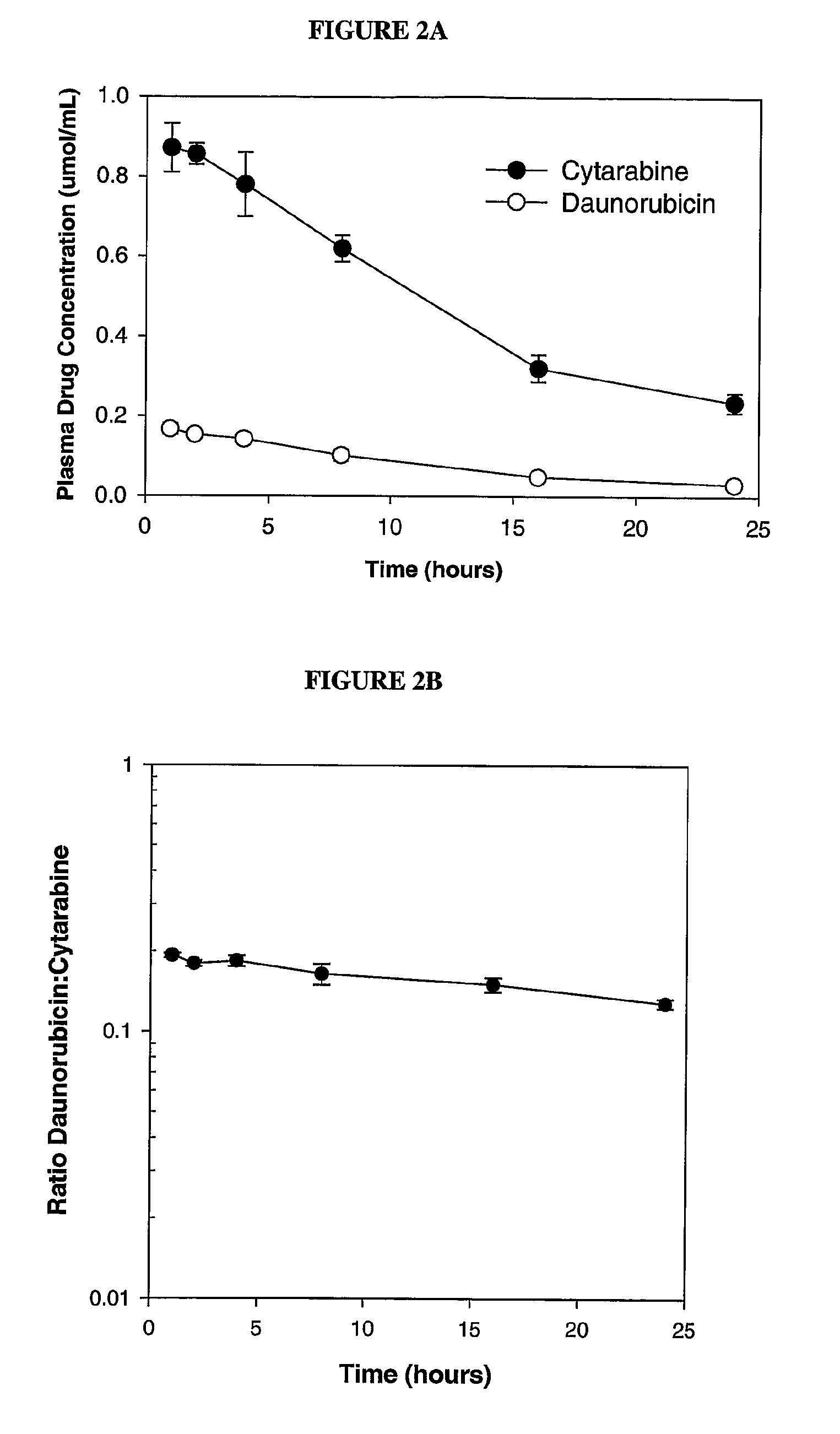
Compositions which comprise an anthracycline agent, and a cytidine analog are encapsulated in liposomal carriers. The preferred anthracycline agent is selected from the group of daunorubicin, doxorubicin, and idarubicin, while the preferred cytidine analog is selected from the group of cytarabine, gemcitabine, or 5-azacytidine. The combination of the anthracycline agent and cytidine analog encapsulated in said liposomal carriers are useful in achieving a drug retention and a sustained drug release for each therapeutic agent.
Owner:CELATOR PHARMA INC
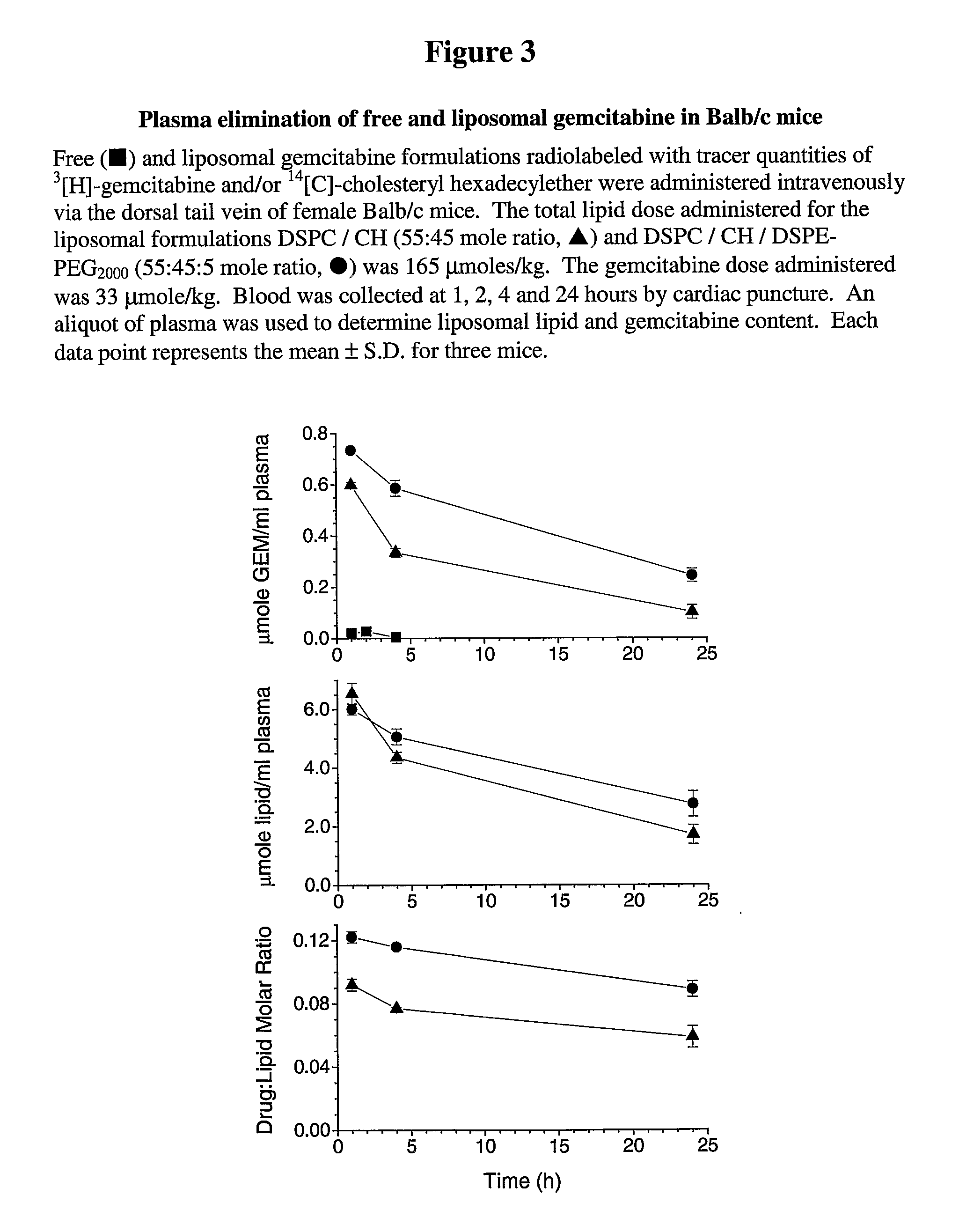
Free or Liposomal Gemcitabine Alone or in Combination with Free or Liposomal Idarubicin
InactiveUS20080213183A1Organic active ingredientsIn-vivo testing preparationsCisplatinMaximum tolerated dose
The use of the maximum tolerated dose (MTD) of individual drugs to determine appropriate administration ratios of drugs for combination therapy, wherein the ratios of drugs are fixed based on the same percentage of the MTD for each drug. Furthermore, antineoplastic compositions comprising liposomal encapsulated gemcitabine alone or in combination with free or liposomal encapsulated antineoplastic agents, such as idarubicin, irinotecan, etopside, cisplatin, cyclophosphamide, doxorubicin, or vincristine are diclosed.
Owner:BRITISH COLUMBIA CANCER AGENCY
Liposomal Formulations of Anthracycline Agents and Cytidine Analogs
Compositions which comprise an anthracycline agent, and a cytidine analog are encapsulated in liposomal carriers. The preferred anthracycline agent is selected from the group of daunorubicin, doxorubicin, and idarubicin, while the preferred cytidine analog is selected from the group of cytarabine, gemcitabine, or 5-azacytidine. The combination of the anthracycline agent and cytidine analog encapsulated in said liposomal carriers are useful in achieving a drug retention and a sustained drug release for each therapeutic agent.
Owner:CELATOR PHARMA INC
Anti-cancer drugs slow release agent comprising anticancer antibiotics and booster thereof
Disclosed is an anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer antibiotics are selected from Idarubicin, Valtaxin, Pirarubicin and Mitoxantrone, The anti-metabolite drugs are selected from Pemetrexed, Carmustine, Tegafur, Zalcitabine, Emtritabine, Galocitabine, Ibacitabine, Ancitabine, Decitabine, Flurocitabine, Enocitabine, Imidazoletabine, Capecittabine, Gemcitabine, Fludrarbine, Raltitrexed, Dexrazoxane, Cladribine, Nolatrexed and folic acid, The slow release auxiliary materials are selected from EVAc, Polifeprosan, sebacylic acid copolymer, lactic acid, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The slow release microspheres can also be prepared into slow release implanting agent for injection or placement in or around tumor.
Owner:SHANDONG LANJIN PHARMA
Slow released anticancer medicine preparation with both amrubicin and its synergist
The slow released anticancer medicine injection containing both amrubicin and its synergist consists of slow released microsphere and solvent. The slow released microsphere includes effective anticancer component and slow releasing supplementary material, and the solvent is special solvent containing suspending agent carboxymethyl cellulose, etc. and with viscosity of 100-3000 cp at 25 deg.c. The effective anticancer component is amrubicin, idarubicin, etc and / or antimetabolite composition selected from carmofur, tegafur, zalcitabine, etc. The slow releasing supplementary material is selected from EVAc, sebacic acid copolymer, lactic acid polymer, etc. The slow released microsphere may be also prepared into slow released implanting agent set around or inside the tumor to strengthen the chemotherapy or radiotherapy effect.
Owner:JINAN KANGQUAN PHARMA TECH
Compound recipe anti-cancer drugs slow release agent comprising anticancer antibiotics and booster thereof
Disclosed is a compound anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer effective ingredients include Aclarubicin, Idarubicin, Doxorubicin, Epirubicin, Valtaxin, Pirarubicin, Losaxantrone, Losoxantrone and / or anticancer antibiotic synergistic agents selected from phosphoinositide-3-kinase inhibitor, pyrimidine analogues and / or DNA restoration enzyme inhibitor, the slow release auxiliary materials are selected from polylactic acid copolymer EVAc, or sebacic acid copolymer, the viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C). The slow release microspheres can also be prepared into slow release implanting agent for lowering down the whole body toxicity reaction of the medicament when locally dispensing on the tumor, and for selectively increasing the tumor local medicinal concentration.
Owner:SHANDONG LANJIN PHARMA
High-stability combined drug delivery system for cancer treatment and construction method thereof
ActiveCN108478802ALittle side effectsStrong targetingOrganic active ingredientsPeptide/protein ingredientsChemical reactionSide effect
The invention relates to the technical field of nano medicines, in particular to a high-stability combined drug delivery system for cancer treatment and a construction method thereof. The combined drug delivery system takes graphene oxide as a carrier, apoptotic peptides are connected with the surface or the edge of the graphene oxide through a disulfide bond, adriamycin, doxorubicin hydrochloride, camptothecin or idarubicin and other anti-cancer drugs are connected under the Pi-Pi conjugate action and the electrostatic action, and bovine serum proteins coat the outsides. In the construction process, firstly, the surface of the graphene oxide is subjected to sulfydryl modification and bisulfide modification, then alkynyl is connected to the disulfide bond, the apoptotic peptides are connected through the disulfide bond under the chemical click between the alkynyl and an azide group, then the anti-cancer drugs are loaded, and the bovine serum proteins coat the outsides. Under the combined action of the apoptotic peptides and the anti-cancer drugs, a targeting effect on cancer cells is strong, and a side effect on normal cells is low; the stability, the dispersibility and the biocompatibility are good, and the stable dispersion can be maintained in water for 8 days or more.
Owner:ZHEJIANG UNIV OF TECH
Senolytic compounds
PendingCN110678187AHalogenated hydrocarbon active ingredientsCyclic peptide ingredientsDiseaseNitrofurazone
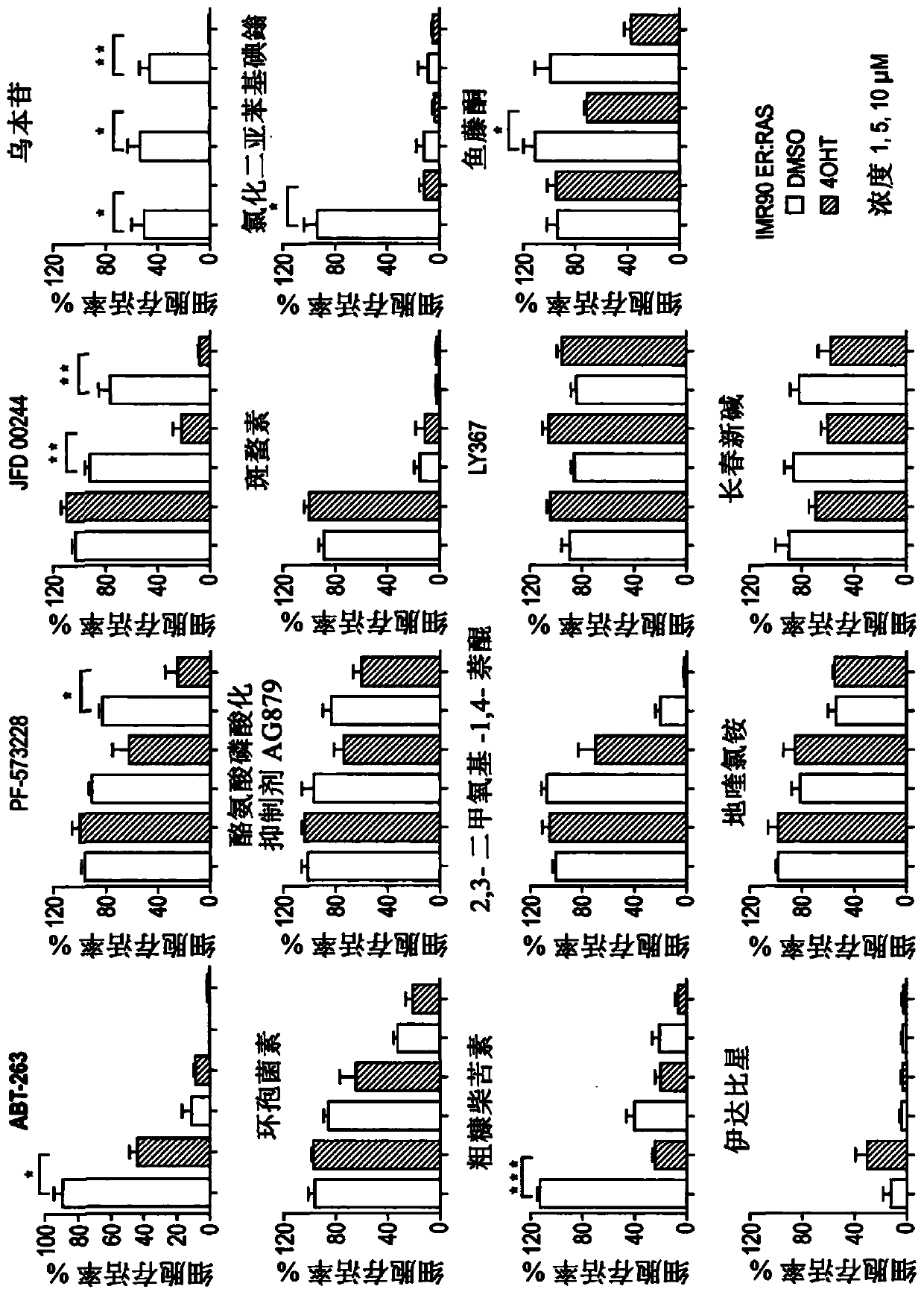
The present invention relates to an agent for use in selectively killing one or more senescent cells, wherein the agent is selected from the following: a cardiac glycoside or alglycone, a focal adhesion kinase (FAK) inhibitor, an HMG-CoA reductase inhibitor, JFD00244, Cyclosporine, Tyrphostin AG879, Cantharidin, Diphenyleneiodonium chloride, Rottlerin, 2,3-Dimethoxy-1,4-naphthoquinone, LY-367,265,Rotenone, Idarubicin, Dequalintum chloride, Vincristine, Nitazoxanide, Nitrofurazone, Temsirolimus, Eltrombopag, Adapalene, Azacyclonol, Enoxacin and Raltegravir, and pharmaceutically acceptable salts thereof. Another aspect relates to compounds for use in treating or preventing a senescence- associated disease or disorder, and methods relating thereto.
Owner:英国研究与创新公司
Temperature controlled sustained-release injection containing anti-cancer medicine
InactiveCN101273965APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectVinorelbine
The invention relates to a temperature-controlled sustained-release injection containing an anti-cancer drug, which consists of the anti-cancer drug and an amphiphilic block copolymer hydrogel and has the temperature-sensitive gelatinization feature, the temperature-controlled sustained-release injection is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, thus allowing the drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the temperature-controlled sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be vincristine, vinorelbine, navelbine, vindesine, vinleurosine, vinrosidine, cephalotaxine, bleomycin, daunomycin, aclarubicin, epirubicin, idarubicin, pirarubicin, valrubicin, mitomycin C, actinomycin D, losoxantrone, mitoxantrone, mitozolomide, temozolomide and so on.
Owner:SHANDONG LANJIN PHARMA +1
Antineoplastic conjugates of transferin, albumin and polyethylene glycol
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R*H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
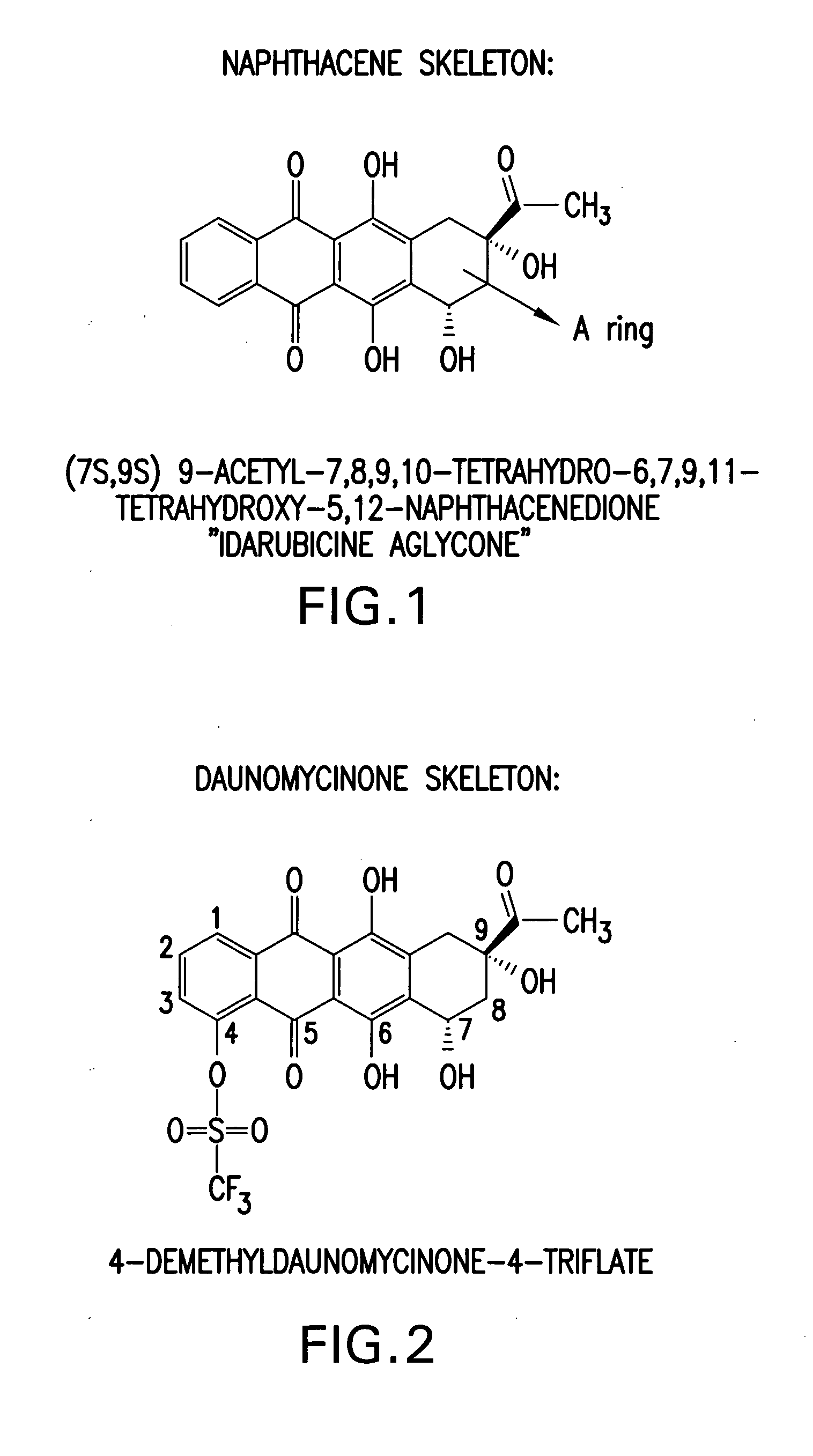
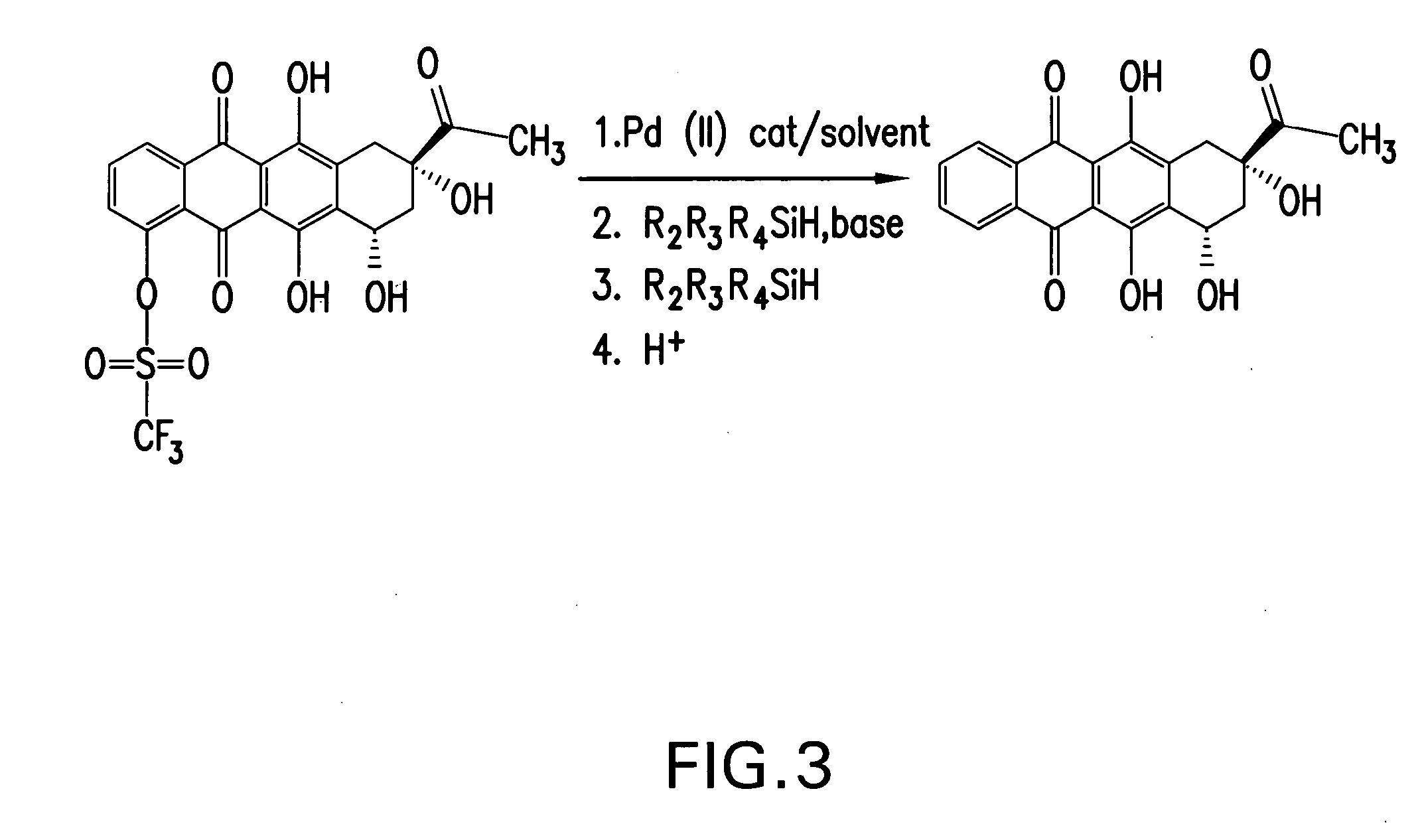
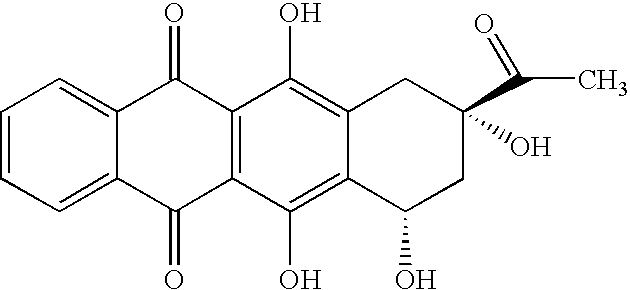
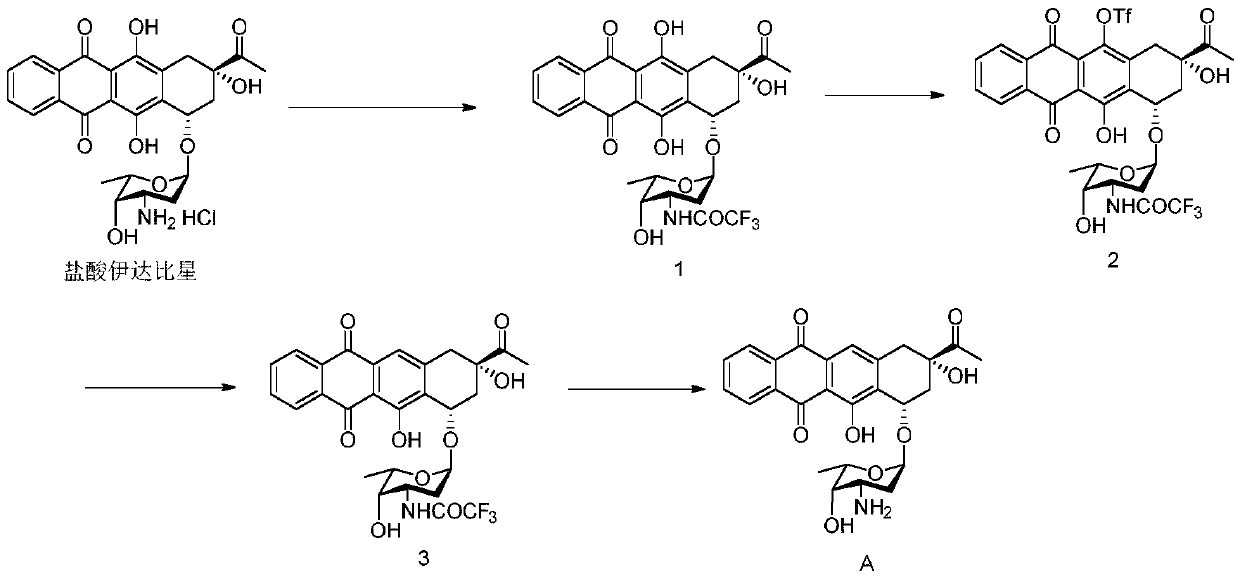
Synthesis of idarubicin aglycone
The present invention provides a new method of producing high quality idarubicin aglycone from 4-protected demethoxydaunomycinones such as 4-demethoxydaunomycinone-4-triflate.
Owner:SICOR SOC ITAL CORTICOSTEROIDI SPA
Pharmaceutical preparations and methods for inhibiting tumors
The invention provides pharmaceutical compositions and method for inhibiting growth of prostatic adenocarcinoma, stomach cancer, breast cancer, endometrial, ovarian or other cancers of epithelial secretion, or benign prostate hyperplasia (BPH). In one embodiment the pharmaceutical composition includes human rHuPSP94, antigenic portions thereof, and functionally equivalent polypeptides thereof. In another embodiment, the pharmaceutical composition includes a mixture of human rHuPSP94, antigenic portions thereof, and functionally equivalent polypeptides thereof and an anticancer drug which may be administered in an appropriate dosage form, dosage quantity and dosage regimen to a patient suffering from, for example of prostatic adenocarcinoma, stomach cancer, breast cancer, endometrial, ovarian or other cancers of epithelial secretion, benign prostate hyperplasia, or (BPH) gastrointestinal cancer. The anticancer drug of the latter mixture may be one selected from the group of drugs including mitomycin, idarubicin, cisplatin, 5-fluoro-uracil, methotrexate, adriamycin, daunomycin, taxol, taxol derivative, and mixtures thereof.
Owner:AENORASIS SA PHARMA & MEDICAL DEVICES
Idarubicin for the treatment of lymphoma in a dog
The present invention relates to a method of treating a lymphoma in a dog comprising administering to a dog in need of such treatment a therapeutically effective amount of idarubicin or a pharmaceutically acceptable salt thereof.
Owner:ZOETIS SERVICE LLC
Novel PD-1 tumor immunosuppressant and drug preparation method thereof
InactiveCN108498796AImprove antigen phagocytosisRapid responseHeavy metal active ingredientsOrganic active ingredientsApoptosisT cell
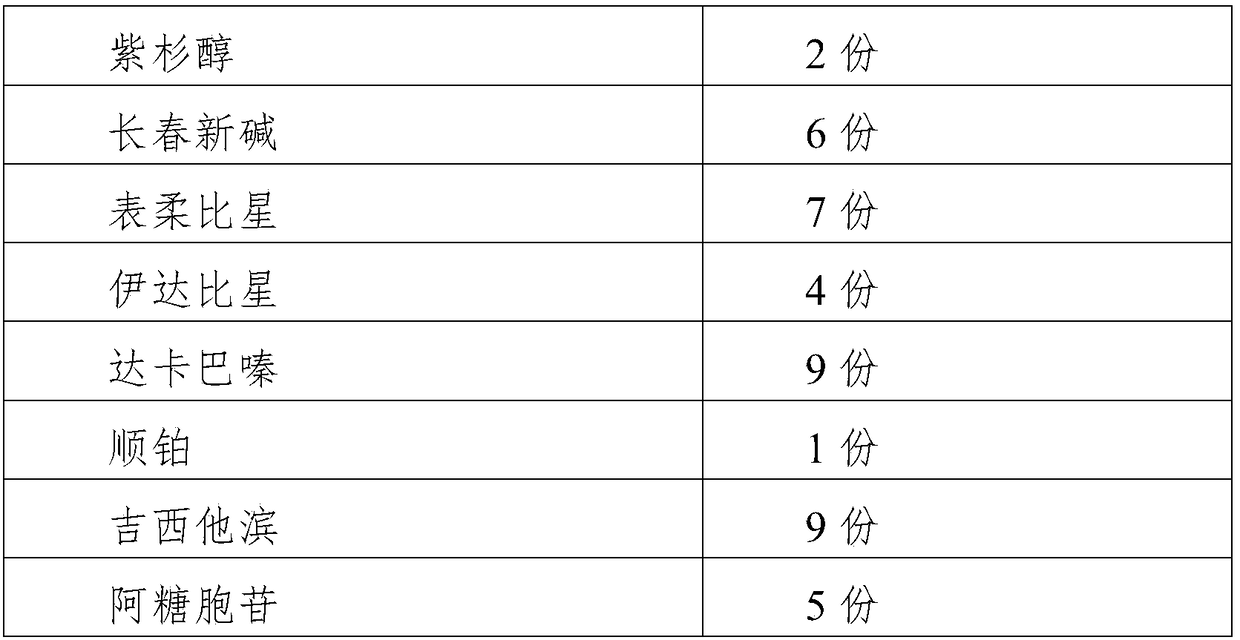
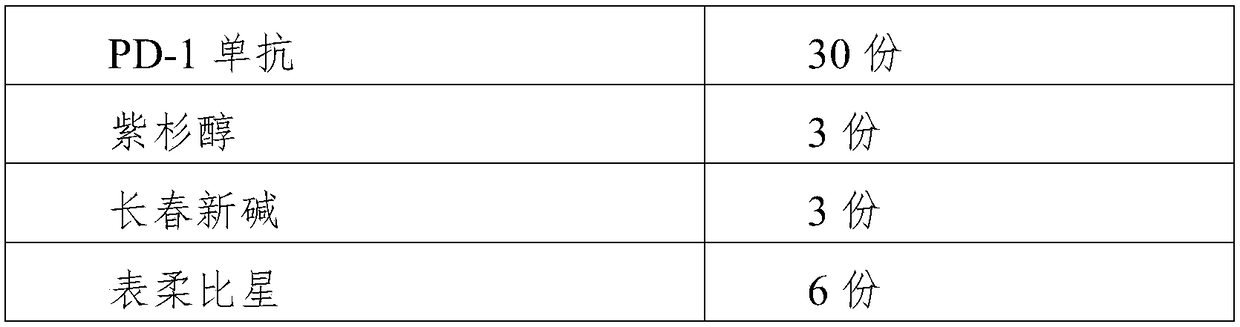
The invention relates to the technical field of drug inhibitors, in particular to a novel PD-1 tumor immunosuppressant and a drug preparation method thereof. The immunosuppressant is prepared from byweight, 30-45 parts of PD-1 monoclonal antibody, 2-6 parts of alkaloid, 4-7 parts of antibiotic, 3-9 parts of alkylating agent, 1-5 parts of platinum agent and 5-9 parts of metabolic antagonist; the alkaloid consists of one or more of paclitaxel, vincristine and docetaxel; the antibiotic consists of one or more of epirubicin, idarubicin and mitomycin; the alkylating agent is one or two of ifosfamide and dacarbazine; the platinum agent is one or two of cisplatin and oxaliplatin; the metabolic antagonist is one or more of gemcitabine, cytarabine and tegafur. The immunosuppressant can block the interaction between PD-L1 molecules expressed on tumor cells and receptors on activated T cells, inhibit the apoptosis of the activated T cells and improve the killing capability of the tumor cells.
Owner:HENAN TIANSHENG TAIFENG PHARM TECH CO LTD
Idarubicin liposome and preparation method thereof
InactiveCN104208024AImprove clinical efficacySimple processOrganic active ingredientsAntineoplastic agentsClinical efficacyCholesterol
The invention belongs to the technical field of medicine, and specifically discloses an idarubicin liposome and a preparation method thereof. The preparation method reduces the side and toxic effect of idarubicin, and improves the clinical effect. The idarubicin liposome is prepared by the following steps: mixing idarubicin, phosphatide, and cholesterol according to a certain ratio, and then making the mixture into idarubicin liposome through a film dispersion-ultrasonic extrusion combined method. The preparation method has the advantages of simple technology, high encapsulation rate, and suitability for massive production.
Owner:深圳市为泰医药科技有限公司
Lipiodol-based Anti-tumour emulsion for treating cancer
The present invention relates to a pharmaceutical composition including Lipiodol and a molecule with anti-tumour activity and secondarily a hydroxyethyl starch. The present invention also relates to the use of the compositions according to the invention for treating cancer. According to one embodiment, the pharmaceutical composition according to the invention has between 0.004% and 0.2% by weight of idarubicin, between 0.38% and 2.25% by weight of hydroxyethyl starch and secondarily between 60% and 68% by weight of Lipiodol, and water for injection (or even physiological saline) in a quantity sufficient (q.s.) for 100%.
Owner:GUERBET SA
Applications of anthracene ring type compounds in preparation of medicines treating AIDS
InactiveCN107041891AObvious anti-HIV effectOrganic active ingredientsAntiviralsT lymphocyteQuantification methods
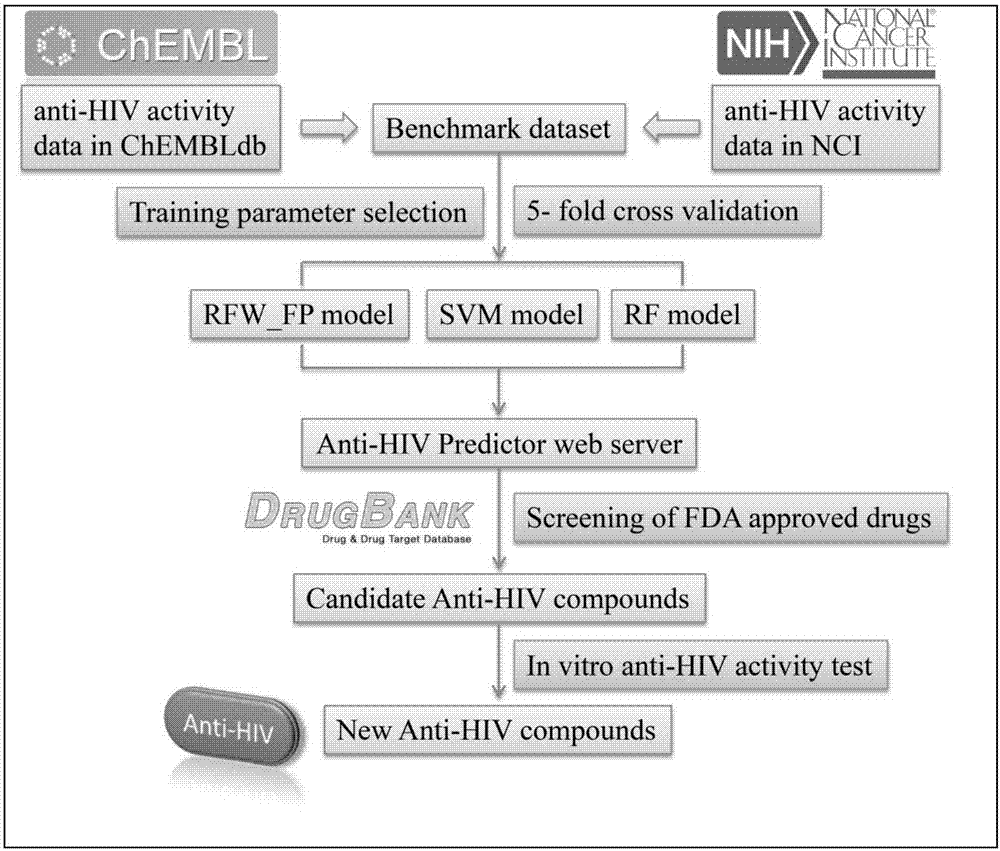
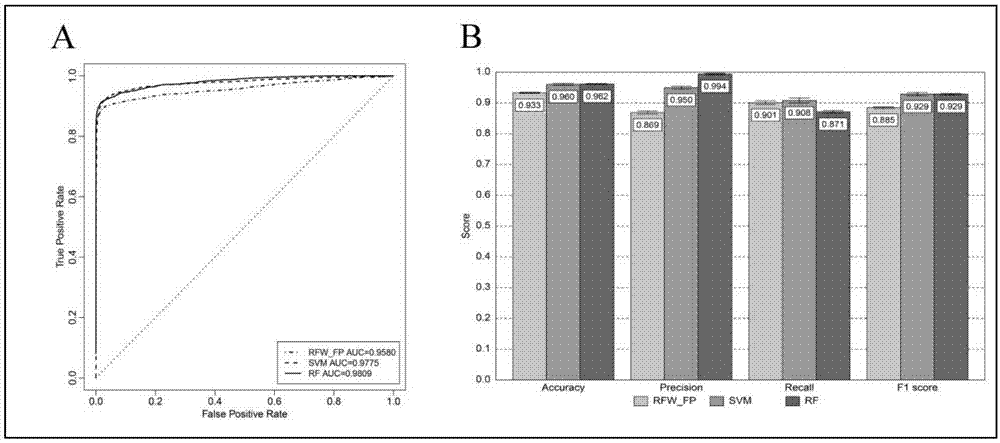
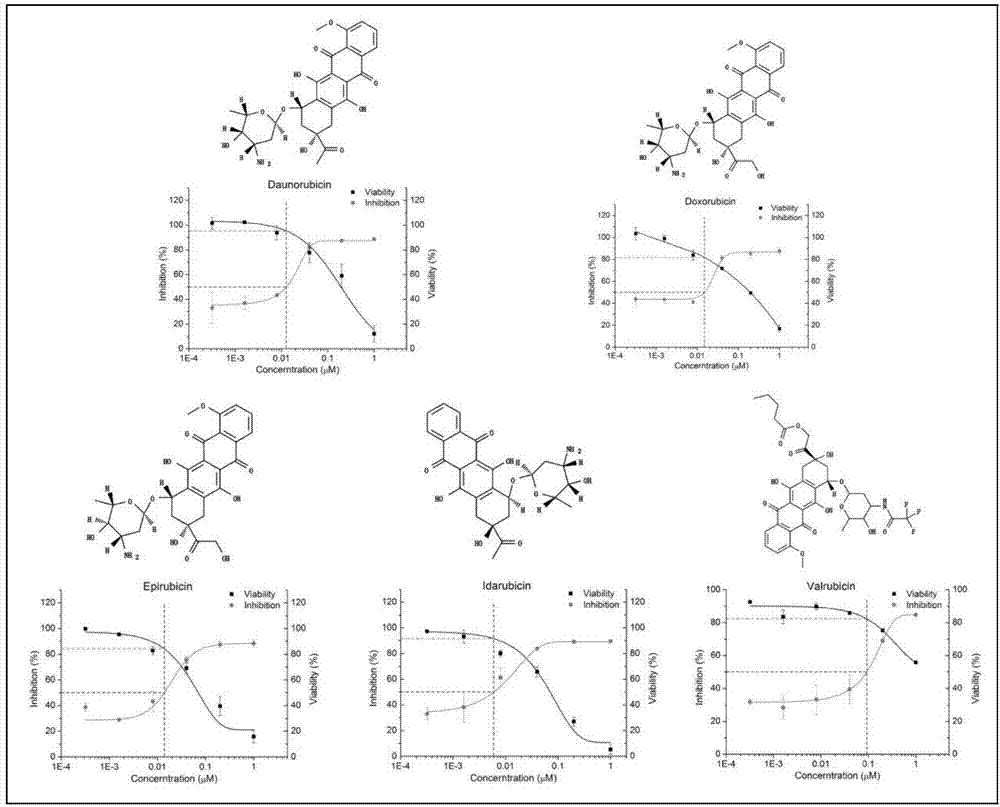
The invention provides applications of anthracene ring type compounds in preparation of medicines treating AIDS, especially applications of anthracene ring type compounds which are daunorubicin, doxorubicin, pharmorubicin, idarubicin and valrubicin in preparation of medicines treating AIDS. A human T lymphocyte line C8166 and an HIV-1 experimental strain that is HIV-1NL4-3 are selected to perform in-vitro cytotoxicity and anti-HIV activity experiments on the anthracene ring type compounds, wherein the cytotoxicity is detected by using an MTT colorimetric method, and the anti-HIV activity is detected by a symplasm inhibiting test method and an HIV-1p24 antigen quantification method. Through using different dosages of anthracene ring type medicines, results show that the anthracene ring type compounds have obvious anti-HIV effects so that the compounds can be applied for preparing medicines treating AIDS.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Liposomal formulations of anthracycline agents and cytidine analogs
Compositions which comprise an anthracycline agent, and a cytidine analog are encapsulated in liposomal carriers. The preferred anthracycline agent is selected from the group of daunorubicin, doxorubicin, and idarubicin, while the preferred cytidine analog is selected from the group of cytarabine, gemcitabine, or 5-azacytidine. The combination of the anthracycline agent and cytidine analog encapsulated in said liposomal carriers are useful in achieving a drug retention and a sustained drug release for each therapeutic agent.
Owner:CELATOR PHARMA INC
Methods and drug compositions for treating lyme disease
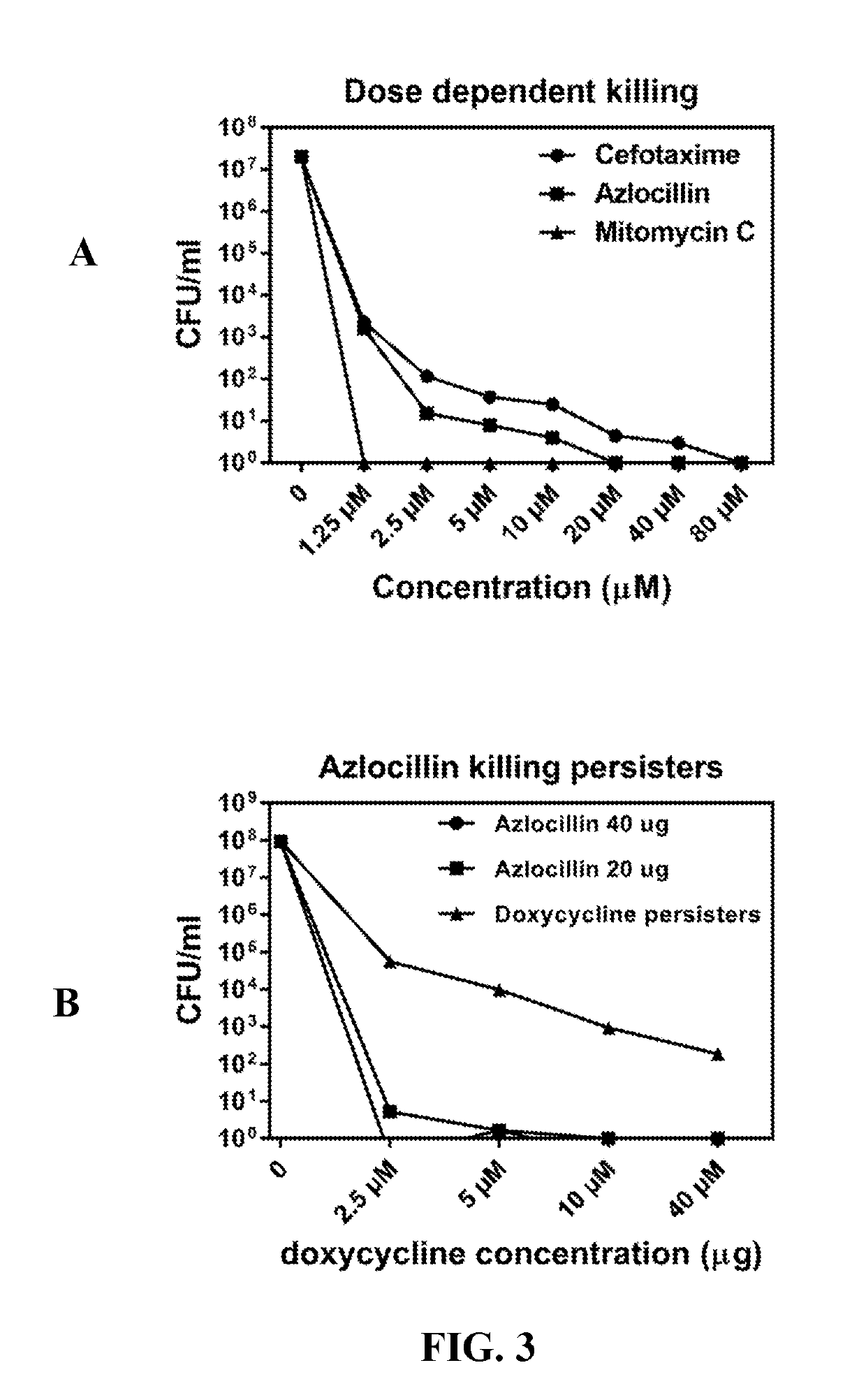
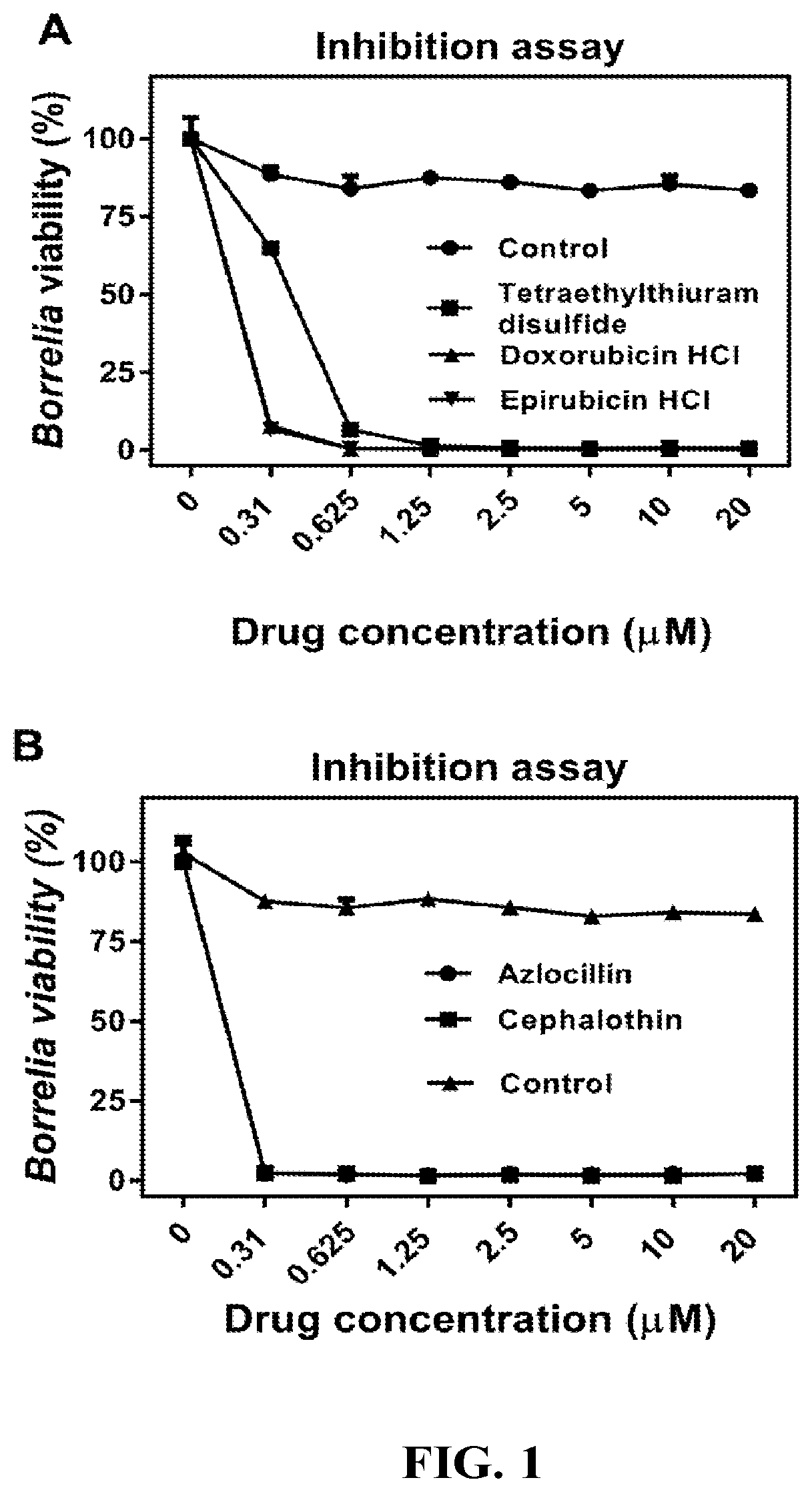
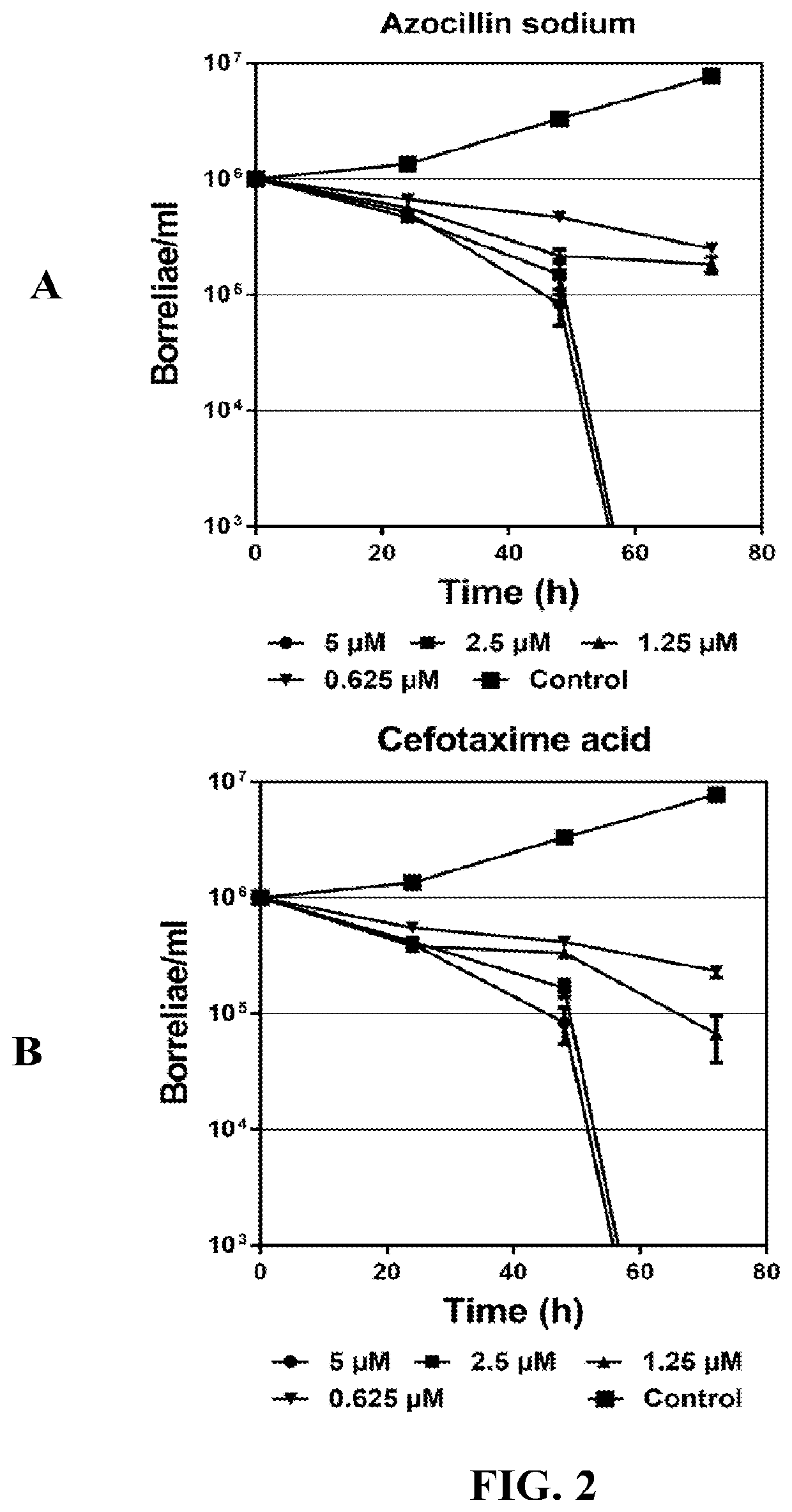
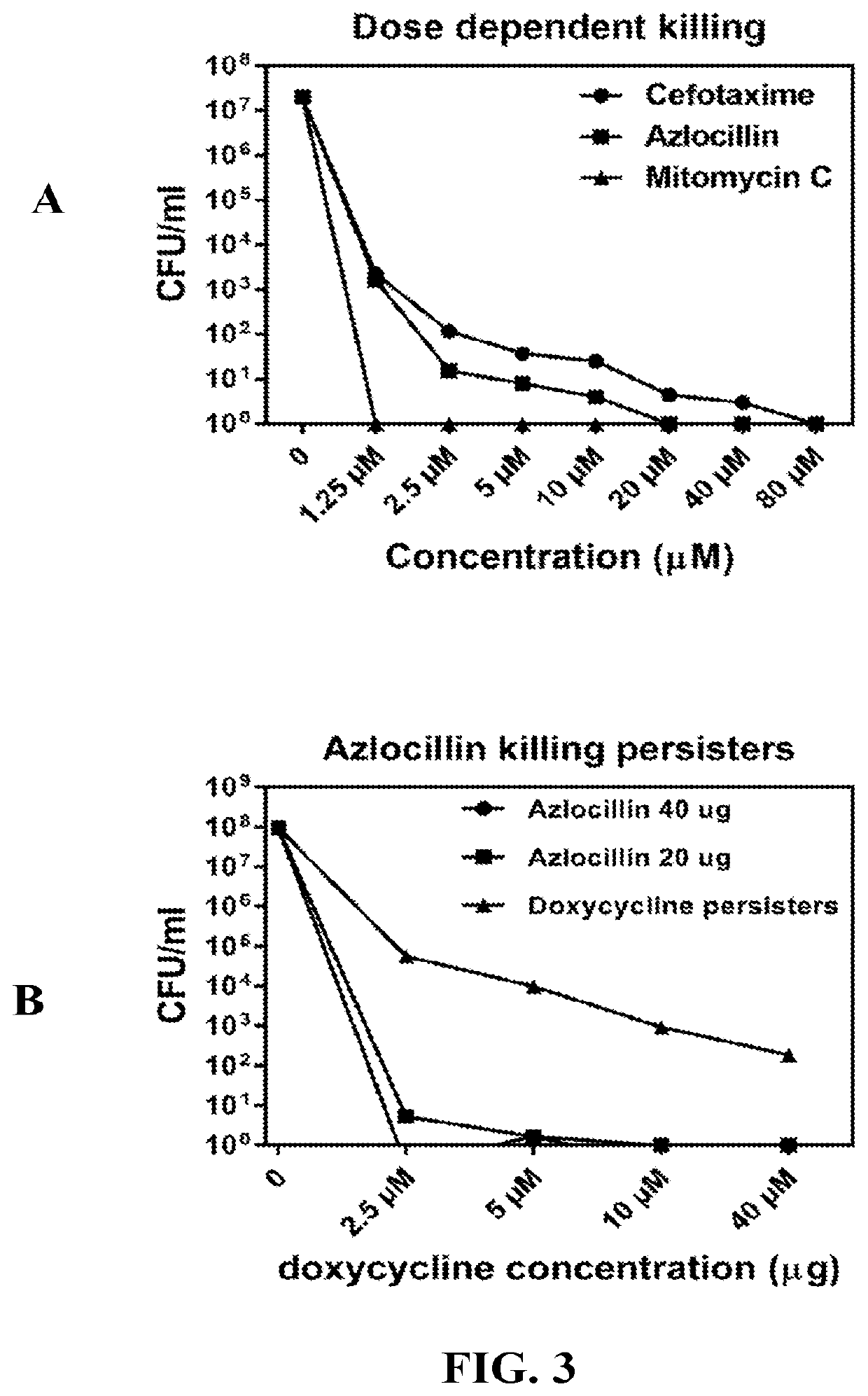
InactiveUS20190117630A1Antibacterial agentsHeterocyclic compound active ingredientsCefotaximeAzlocillin
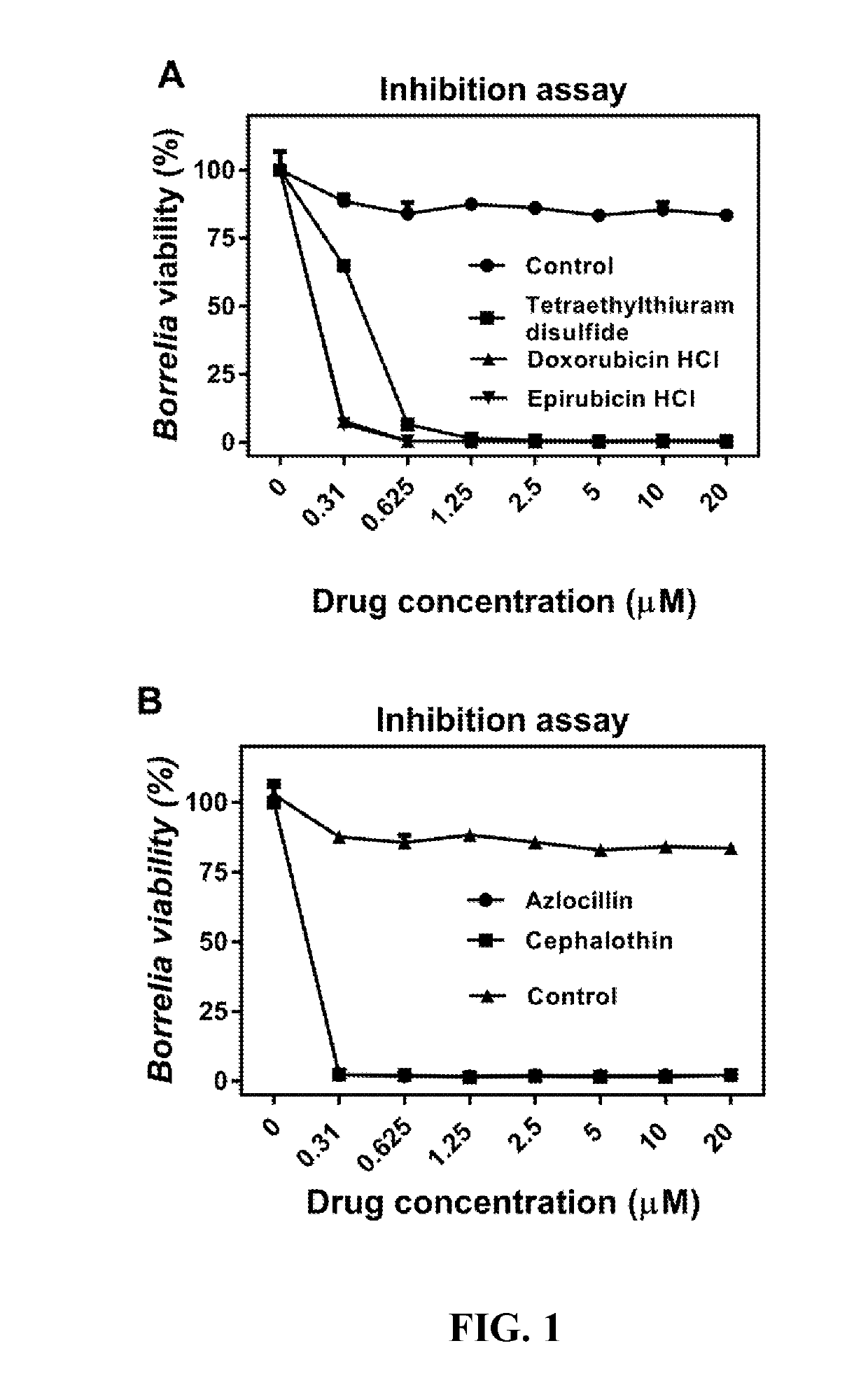
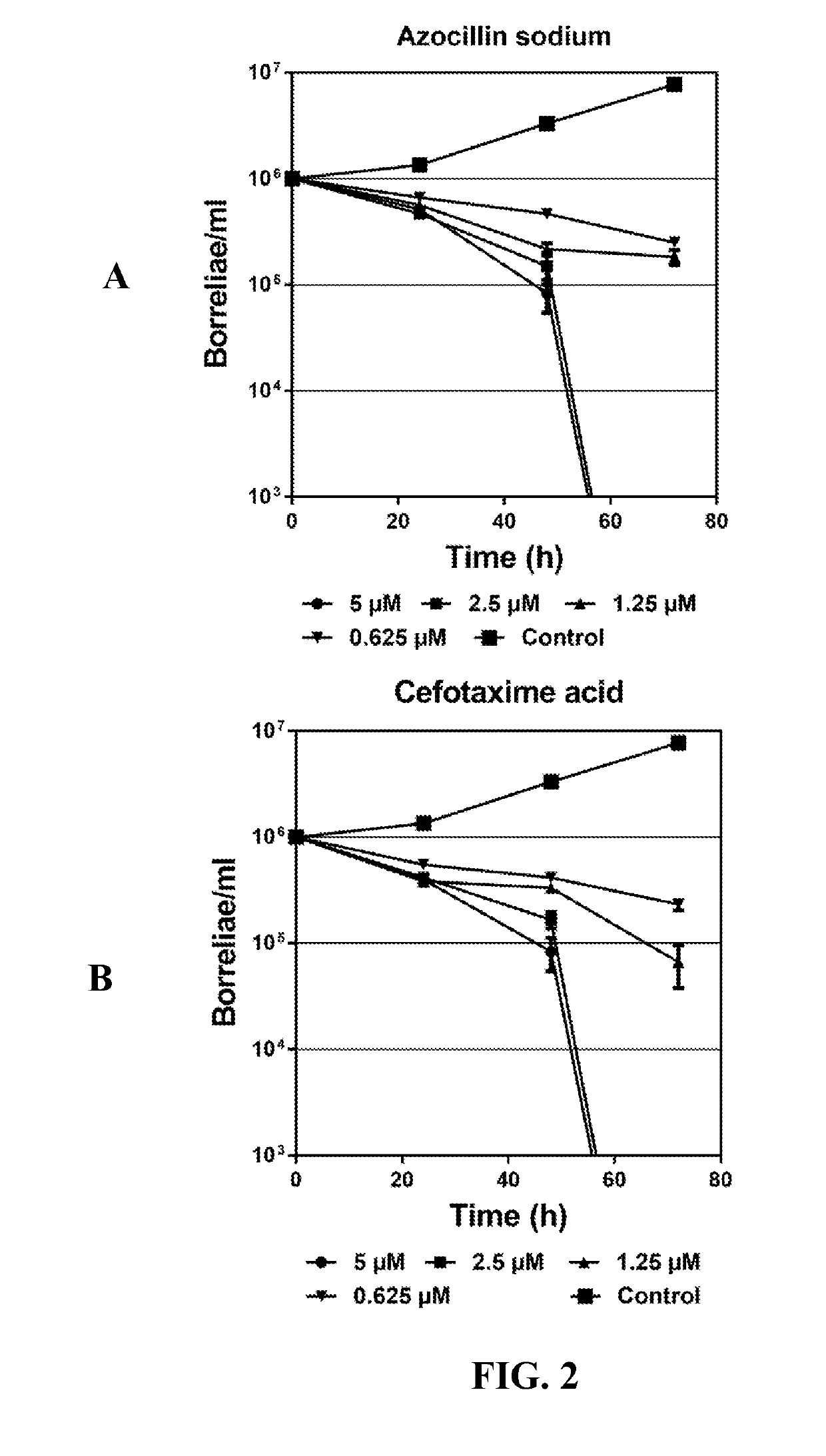
Disclosed herein are methods and drug compositions for treating Lyme disease and post-treatment Lyme disease syndrome (PTLDS) or chronic Lyme disease (CLD). In one embodiment, a method of treating a subject with Lyme disease involves administering an effective amount of a therapeutic agent selected from the group consisting of tetraethylthiuram disulfide, doxorubicin, epirubicin, azlocillin, cephalothin, josamycin, cefotaxime, cefazolin, erythromycin, calcimycin, gramicidin, cefdinir, gambogic acid, ceftazidime, ticarcillin, valinomycin, moxifloxacin, linezolid, idarubicin, tosufloxacin, loratadine, ceftriaxone, and combinations thereof, and pharmaceutical salts, hydrates, and solvates thereof.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Yitarbisin sustained-release implantation agent for curing entity tumour
InactiveCN101176711AOrganic active ingredientsPharmaceutical delivery mechanismPhosphateProstate cancer
The invention relates to an idarubicin sustained-release implant capability for curing solid tumors, such as lung cancer, esophageal cancer, gastric cancer, liver cancer, breast cancer, ovarian cancer, prostatic cancer, bladder cancer, colon cancer and rectum cancer. The invention is characterized in that: the sustained-release implant comprises idarubicin, sustained-release excipient and a certain amount of sustained-release regulator; the sustained-release excipient is mainly one or the combination of the copolymer of glycolic acid and hydroxyacetic acid, polifeprosan, poly (L-lactide-co-ethyl phosphate) and poly (L-lactide-co-propyl phosphate); the idarubicin can be released slowly into part of the tumor during the degradation and adsorption, significantly reducing the systemic toxicity and sustaining the effective medicine concentration simultaneously. The invention has the advantages that: the systemic toxicity of idarubicin can be significantly reduced; the effective medicine concentration can be improved selectively at part of the tumor; the invention can be applied to the prevention of tumor recurrence after operation and chemotherapy and combined therapy of various solid tumors and metastatic tumors that are not suitable for operation.
Owner:JINAN SHUAIHUA PHARMA TECH
Compositions and Methods for the Treatment of Acute Myeloid Leukemias and Myelodysplastic Syndromes
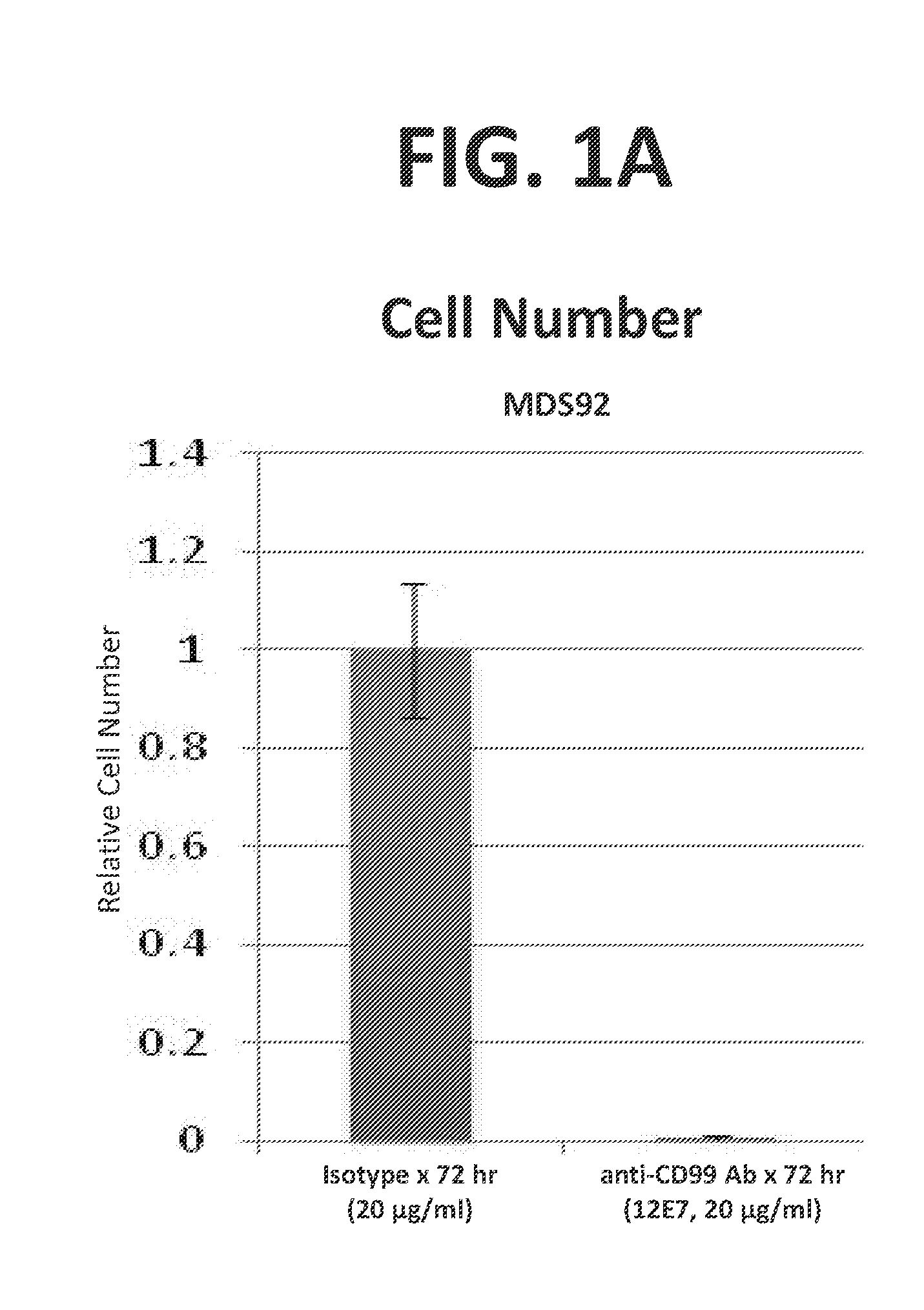
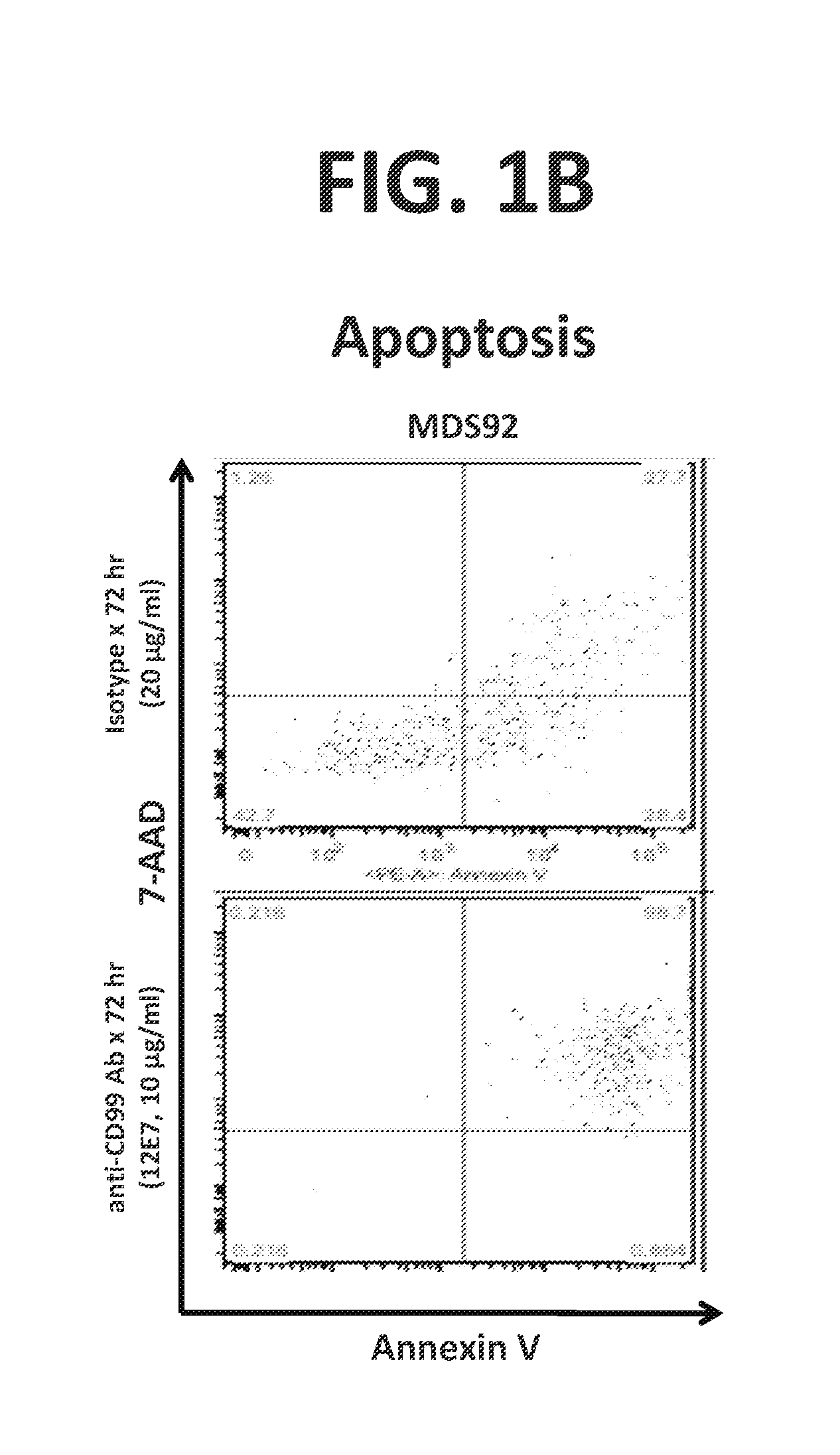
InactiveUS20160272720A1Promote aggregationInduce cell deathMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseCell Surface Proteins
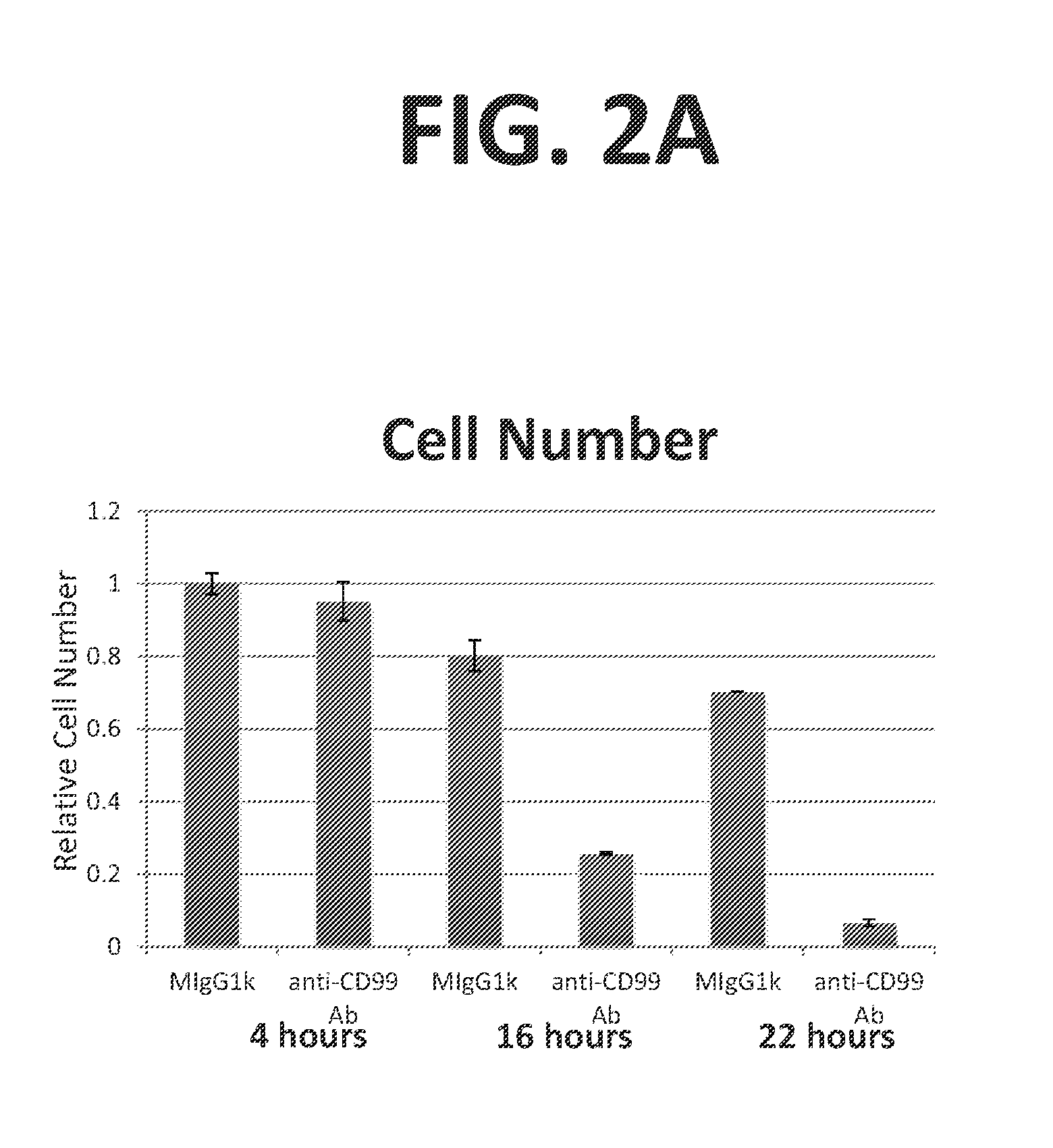
Provided are compositions and methods for the treatment of hematological conditions, in particular CD99+ acute myelogenous leukemias (AML) and myelodysplastic syndromes (MDS), which comprise one or more antibody that (a) binds to the extracellular domain of CD99, (b) ligates AML and / or MDS cell-surface expressed CD99, (c) promotes the capping / clustering / aggregation AML and / or MDS cell-surface expressed CD99, and (d) induces apoptosis in and consequent cytotoxicity of antibody-ligated CD99+ AML and / or MDS cells. Disclosed methods include methods for identifying AML and MDS patients that are susceptible to treatment with an anti-CD99 antibody by detecting the elevated expression of CD99 in a tissue sample or cell from an AML or MDS patient and for treating an AML and / or MDS patient exhibiting elevated CD99 gene and or cell-surface protein expression by administering a composition comprising an anti-CD99 antibody, either alone or in combination with one or more additional component such as a mobilizing agent, a transmigration blocking agent, and an AML and / or MDS chemotherapeutic agent, such as daunorubicin, idarubicin, cytarabine, 5-azacytidine, and decitabine.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Combination therapies for treating cancer
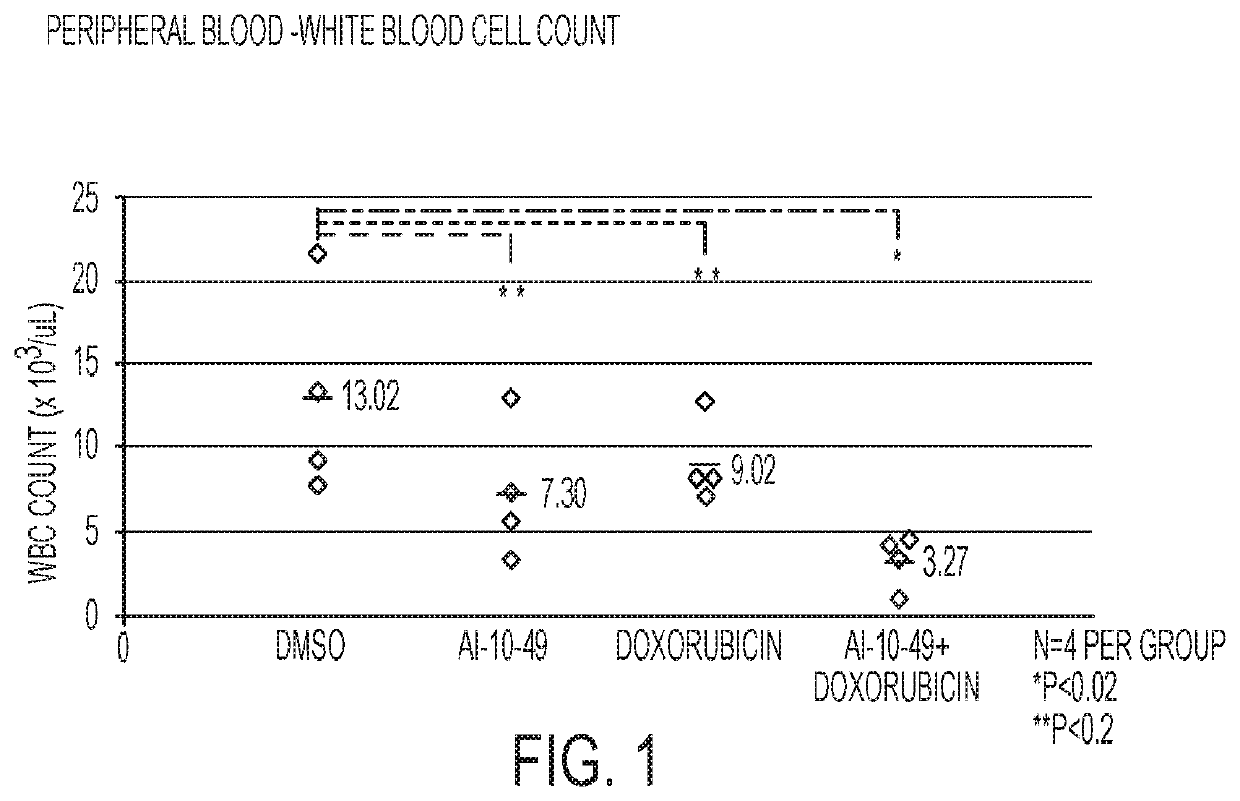
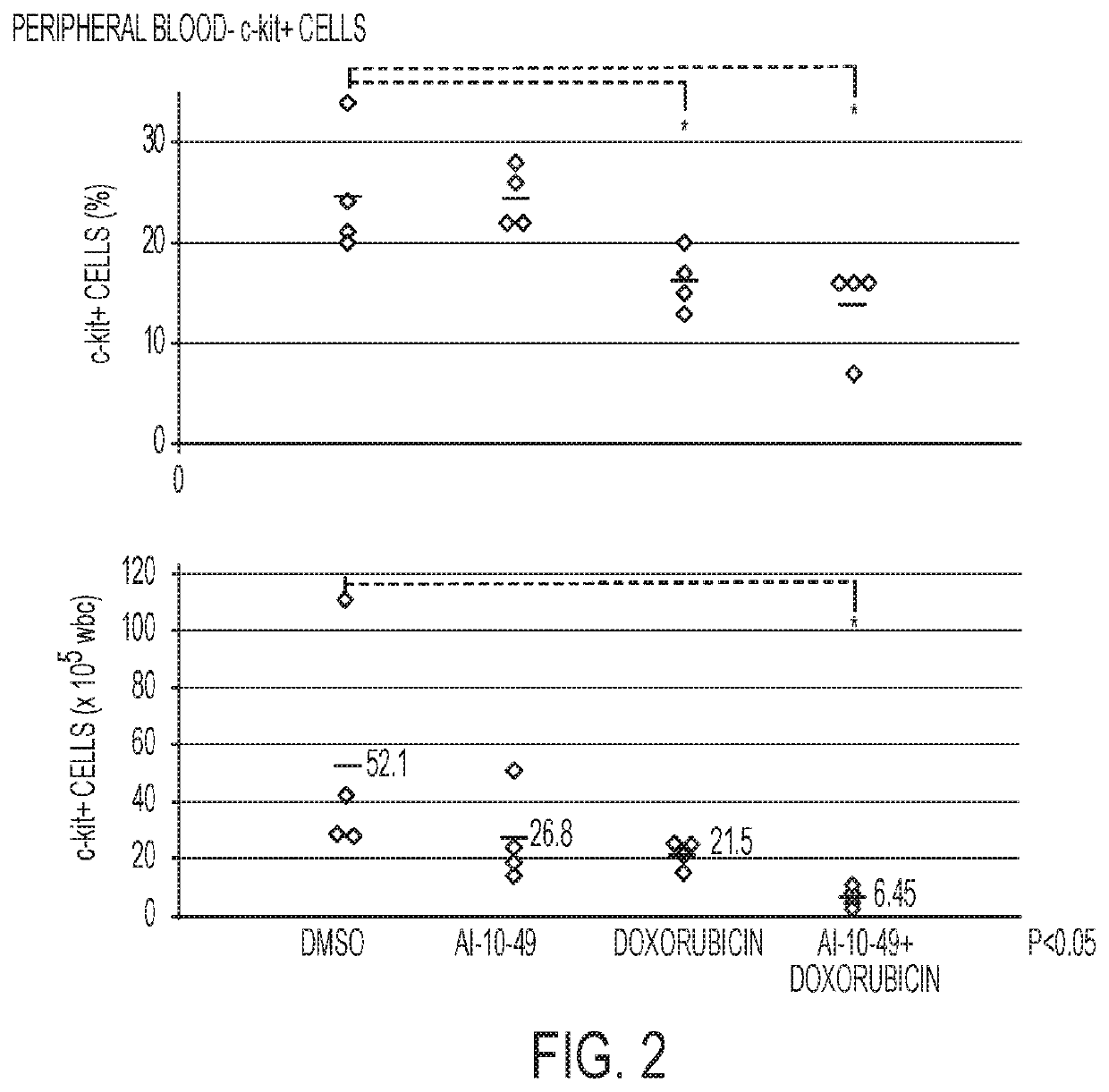
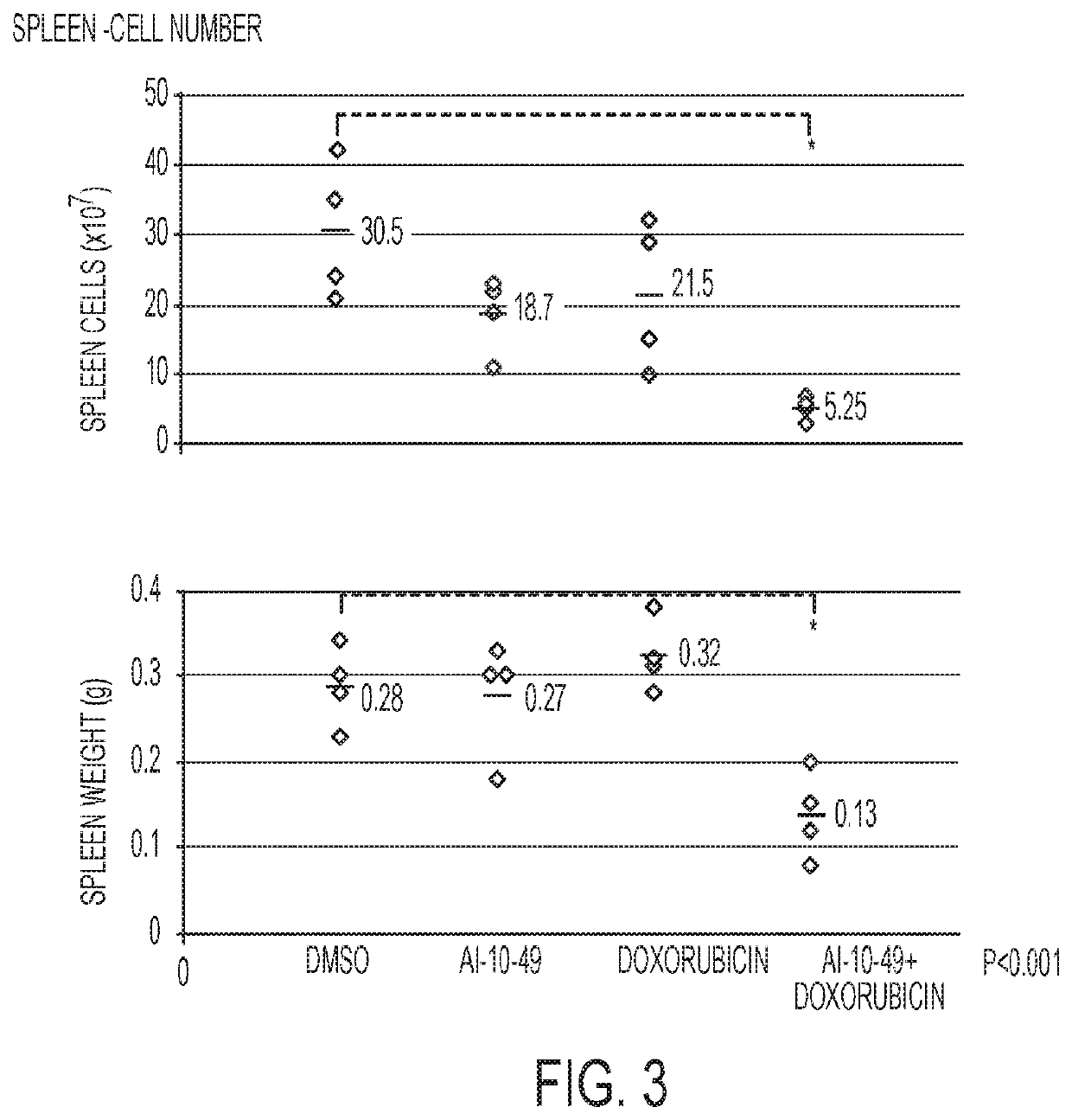
ActiveUS11241421B2Prevent proliferationOrganic active ingredientsOrganic chemistryPharmaceutical medicineCombination therapy
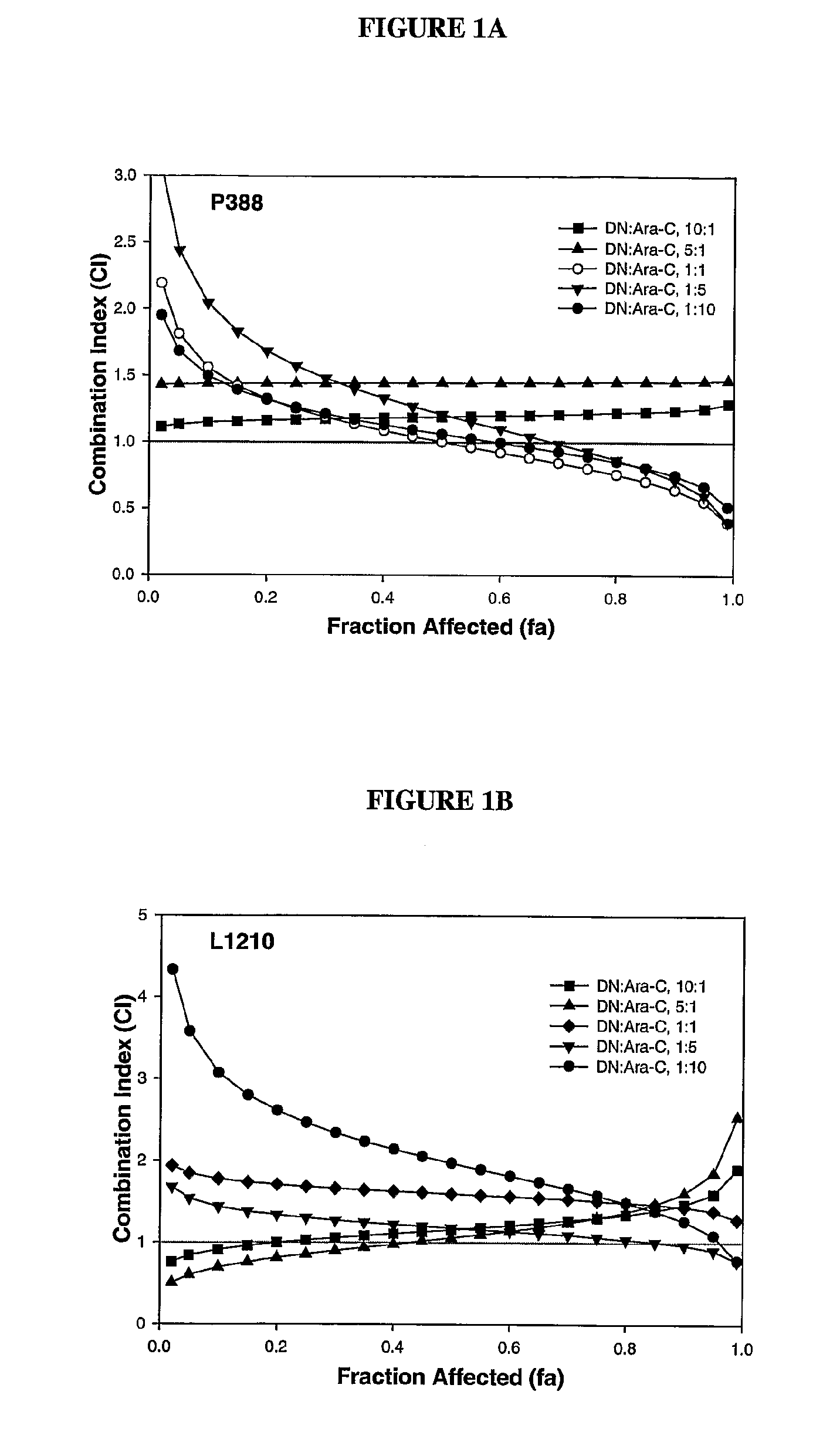
This invention relates to methods and compositions for treatment of inv(16) leukemia and particularly to treatment of acute myeloid leukemia. Disclosed is a method of treating inv(16) leukemia comprising the step of administering to a subject in need thereof a therapeutically effective combination of a) a compound of the formula (1) and b) a chemotherapeutic agent selected from the group consisting of pirarubicin, aclarubicin, mitoxantrone, doxorubicin, daunorubicin, idarubicin, epirubicin, cytarabine, pharmaceutically acceptable salts and mixtures thereof. The therapeutically effective combination synergistically inhibits proliferation of inv(16) leukemia cells. This invention also relates to pharmaceutical compositions comprising a therapeutically effective combination of the compound of formula (1) and the chemotherapeutic agent and a pharmaceutically acceptable excipient.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Anthracycline encapsulated with a polysaccharide for use in the treatment of tumours
InactiveUS20210052616A1Improve targeted drug deliveryHigher therapeutic indexPowder deliveryOrganic active ingredientsPharmaceutical drugDaunorubicin
The invention relates to a new form of a drug in the form of anthracycline encapsulated with a polysaccharide selected from epirubicin, daunorubicin, doxorubicin, idarubicin, especially encapsulated with dextran, for use in the treatment of specific tumours.
Owner:NANOVELOS
Anti-cancer drugs slow release agent comprising anticancer antibiotics and synergist thereof
Disclosed is an anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow releaseauxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer antibiotics are selected from Idarubicin, Valtaxin, Pirarubicin and Mitoxantrone, The anti-metabolite drugs are selected from Pemetrexed, Carmustine, Tegafur, Zalcitabine, Emtritabine, Galocitabine, Ibacitabine, Ancitabine, Decitabine, Flurocitabine, Enocitabine, Imidazoletabine, Capecittabine, Gemcitabine, Fludrarbine, Raltitrexed, Dexrazoxane, Cladribine, Nolatrexed and folic acid, The slow release auxiliary materials are selected from EVAc, Polifeprosan, sebacylic acid copolymer, lactic acid, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The slow release microspheres can also be prepared into slow release implanting agent for injection or placement in or around tumor.
Owner:SHANDONG LANJIN PHARMA
Antibacterial drug and application thereof
InactiveCN112076201ALow chance of drug resistanceLow cytotoxicityAntibacterial agentsOrganic active ingredientsStaphyloccocus aureusIsomerase
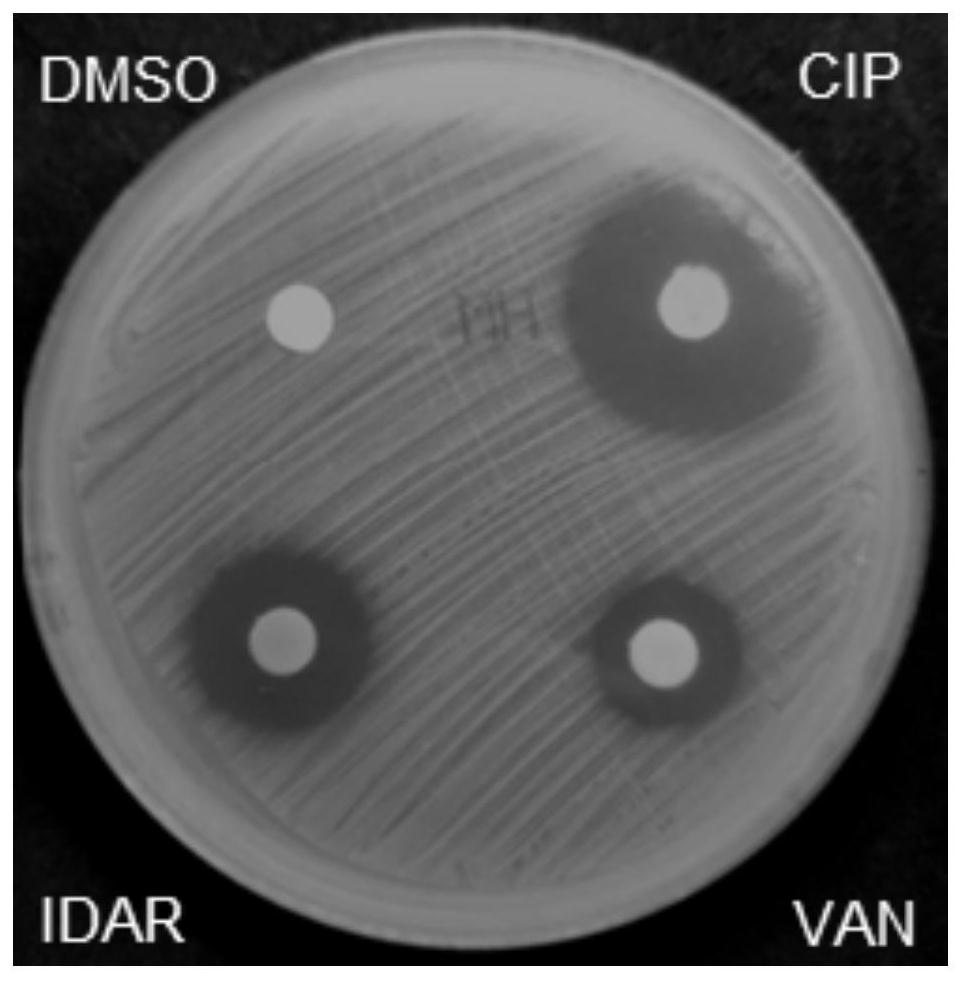
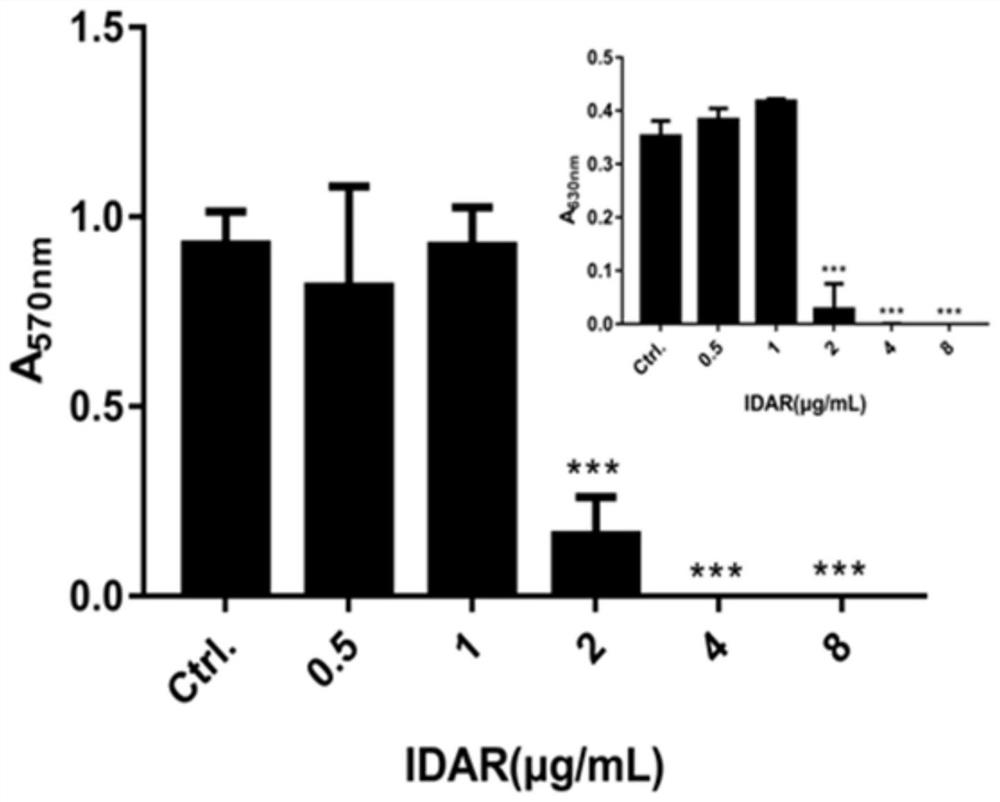
The invention discloses an antibacterial drug and application thereof. The antibacterial drug is idarubicin. The idarubicin achieves the effect of resisting staphylococcus aureus by blocking DNA topoisomerase IIA subunit, can be applied to preparation of drugs for resisting staphylococcus aureus infection, is low in drug resistance probability, is low in cytotoxicity and cardiotoxicity after beingcombined with traditional antibiotics, and has potential to become a new antibiotic.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
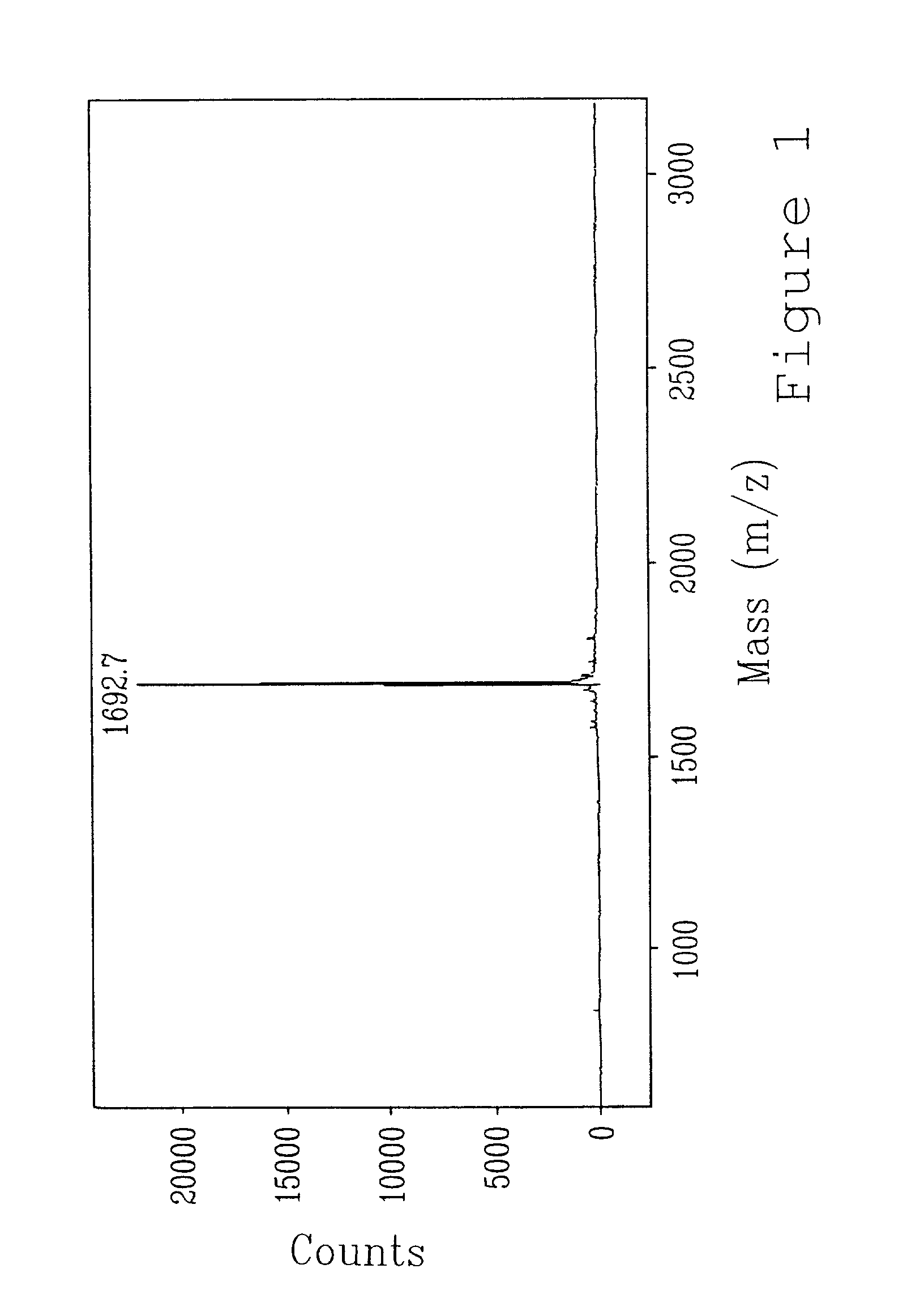
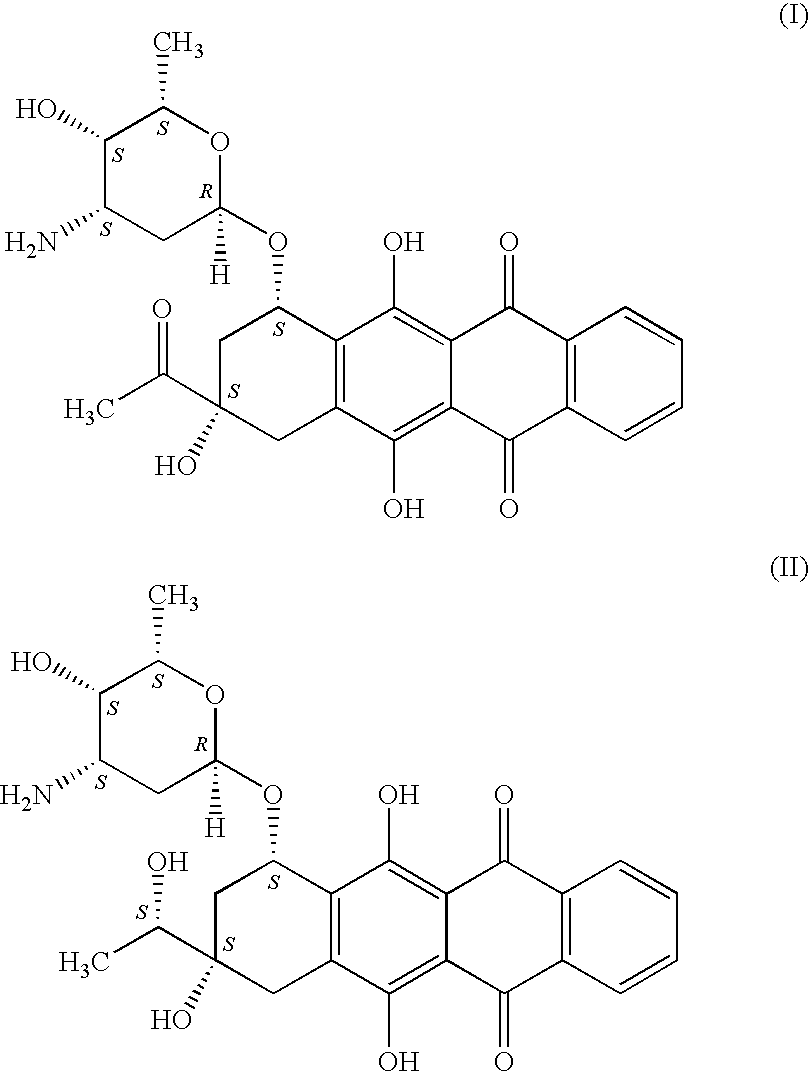
A stable and safe idarubicin pharmaceutical composition and preparation method thereof
ActiveCN107669693BImprove stabilityIncreased cardiotoxicityOrganic active ingredientsSugar derivativesReference samplePharmaceutical medicine
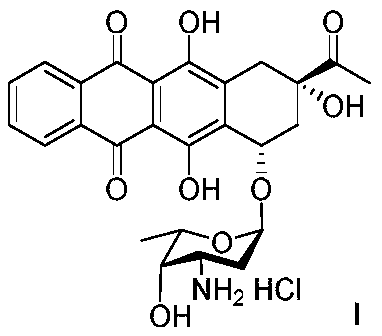
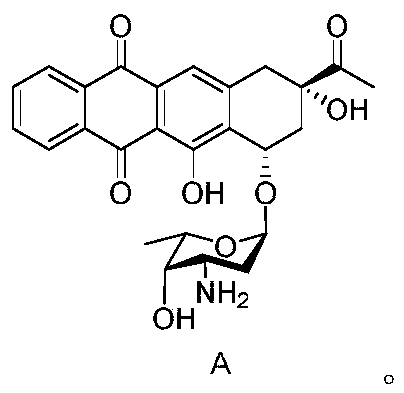
The invention discloses a stable and safe idarubicin pharmaceutical composition and a preparation method thereof. The pharmaceutical composition contains idarubicin or a pharmaceutically acceptable salt thereof and a compound of formula A or a pharmaceutically acceptable salt thereof in an amount of no more than 0.3%. According to the method, an impurity A in idarubicin is separated and confirmed.Prior to this, there is no report that the impurity A is present in the idarubicin. The impurity A is fully researched, it is found that the existence of the impurity A affects the physical and chemical stability of idarubicin and increases cardiac toxicity of idarubicin. The pharmaceutical composition enables idarubicin to maintain good stability. The invention further provides a method for preparing the impurity A. The high-purity impurity A can be acquired to serve as a reference sample or a standard sample for controlling the content of the impurity A in idarubicin and preparations thereof.
Owner:NANJING CHIA TAI TIANQING PHARMA
Medicine containing idarubicin, its preparation method, pharmaceutical composition and its application
ActiveCN111053919BStable intercalationStable deliveryPowder deliveryOrganic active ingredientsNucleic acid structureNanoparticle
The application provides a medicine containing idarubicin, its preparation method, pharmaceutical composition and application. The drug includes nucleic acid nanoparticles and idarubicin, and idarubicin is mounted on the nucleic acid nanoparticles; the nucleic acid nanoparticles include a nucleic acid domain, the nucleic acid domain includes a sequence, b sequence and c sequence, and the a sequence includes a1 The sequence or a1 sequence has at least one base insertion, deletion or substitution sequence, the b sequence contains the b1 sequence or the b1 sequence has at least one base insertion, deletion or substitution, and the c sequence contains the c1 sequence or the c1 sequence has at least one base substitution. Sequences of insertions, deletions or substitutions. The idarubicin-containing medicine provided by the present application, after the nucleic acid domain is modified by the target head, can have better targeting, can deliver idarubicin stably, and has high reliability.
Owner:BAI YAO ZHI DA BEIJING NANOBIO TECH CO LTD
Methods and drug compositions for treating lyme disease
InactiveUS20210059990A1Antibacterial agentsHeterocyclic compound active ingredientsCefotaximeAzlocillin
Disclosed herein are methods and drug compositions for treating Lyme disease and post-treatment Lyme disease syndrome (PTLDS) or chronic Lyme disease (CLD). In one embodiment, a method of treating a subject with Lyme disease involves administering an effective amount of a therapeutic agent selected from the group consisting of tetraethylthiuram disulfide, doxorubicin, epirubicin, azlocillin, cephalothin, josamycin, cefotaxime, cefazolin, erythromycin, calcimycin, gramicidin, cefdinir, gambogic acid, ceftazidime, ticarcillin, valinomycin, moxifloxacin, linezolid, idarubicin, tosufloxacin, loratadine, ceftriaxone, and combinations thereof, and pharmaceutical salts, hydrates, and solvates thereof.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Compound recipe anti-cancer drugs slow release agent comprising anticancer antibiotics and synergist thereof
Owner:SHANDONG LANJIN PHARMA
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com