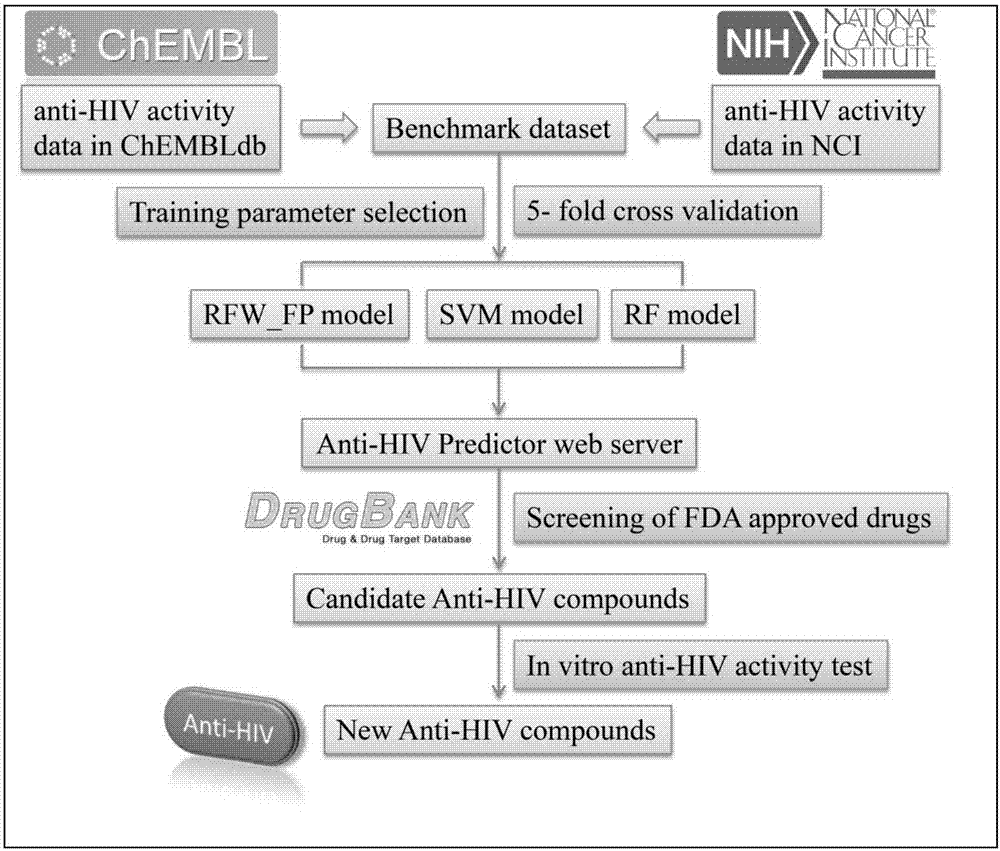
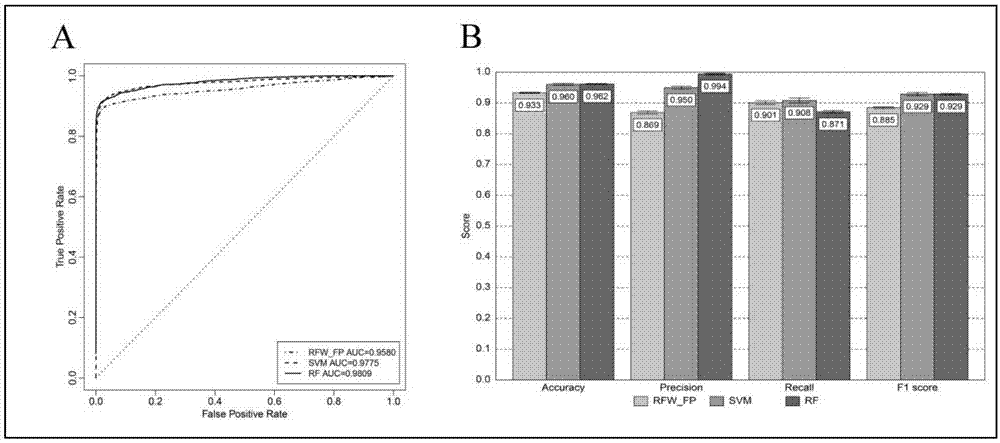
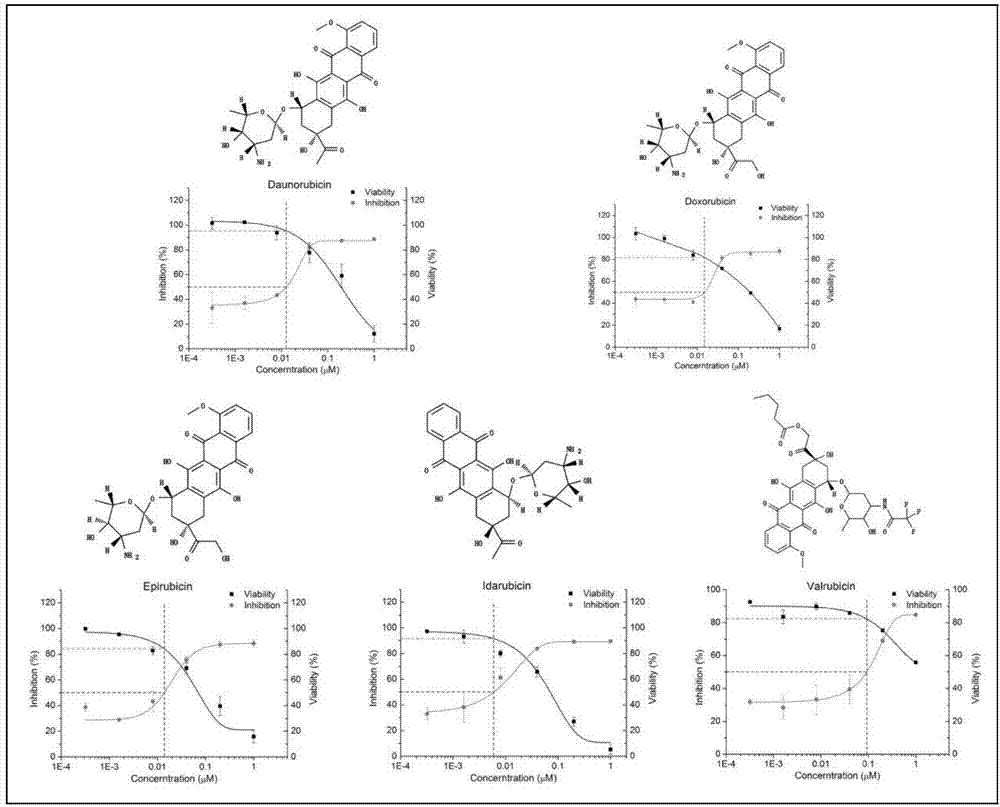
Applications of anthracene ring type compounds in preparation of medicines treating AIDS
An anthracycline compound, AIDS technology, applied in the application field of medicine, can solve problems such as no anti-HIV activity of anthracycline compound, no anti-HIV activity method, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Experimental purpose: To test the anti-HIV effect of anthracyclines.
[0024] 2. Experimental materials:
[0025] Measuring Drugs and Compounds:
[0026]The samples to be tested, Daunorubicin and Epirubicin, were purchased from Dalian Meilun Biotechnology Co., Ltd. The samples to be tested, Idarubicin and Valrubicin, were purchased from Beijing Lebo Biotechnology Co., Ltd. The sample to be tested, Doxorubicin (Doxorubicin), was purchased from Beijing Lamboride Trading Co., Ltd. The positive control compound azidothymidine (3'-Azido-3'-deoxythymidine, AZT) was purchased from Sigma. The sample to be tested is dissolved in RPMI-1640 complete medium or DMSO according to solubility, the sample stock solution concentration dissolved in DMSO is 50mM, and the sample stock solution concentration dissolved in RPMI-1640 complete medium is 10mM, 5mM or 2mM ( According to the solubility), the storage conditions are: -20°C; AZT is dissolved in RPMI-1640 complete medium, steril...
Embodiment 2
[0059] The anthracycline compound mentioned in Example 1 was dissolved in sterile water for injection to dissolve, and then filtered through a sterile funnel, finely filtered, potted, and sterilized to prepare an injection.
Embodiment 3
[0061] Take the anthracycline compound mentioned in Example 1, mix it uniformly with the excipient at a weight ratio of 1:8, granulate, and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com