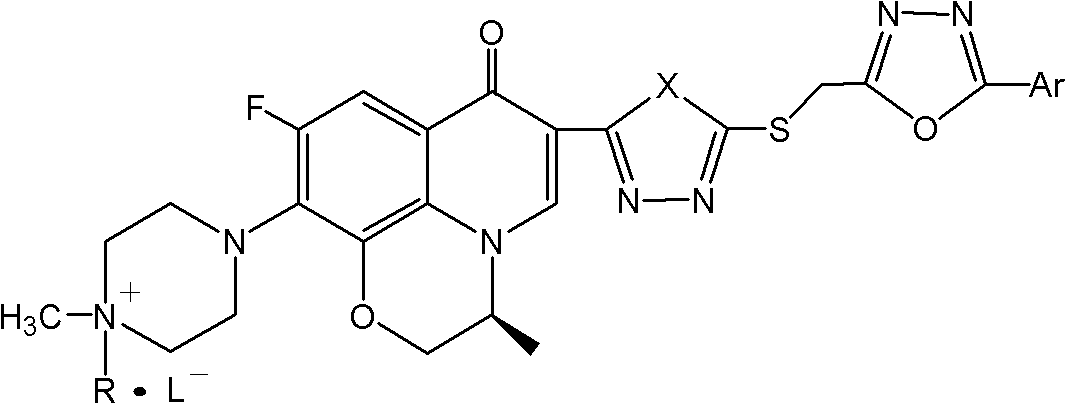
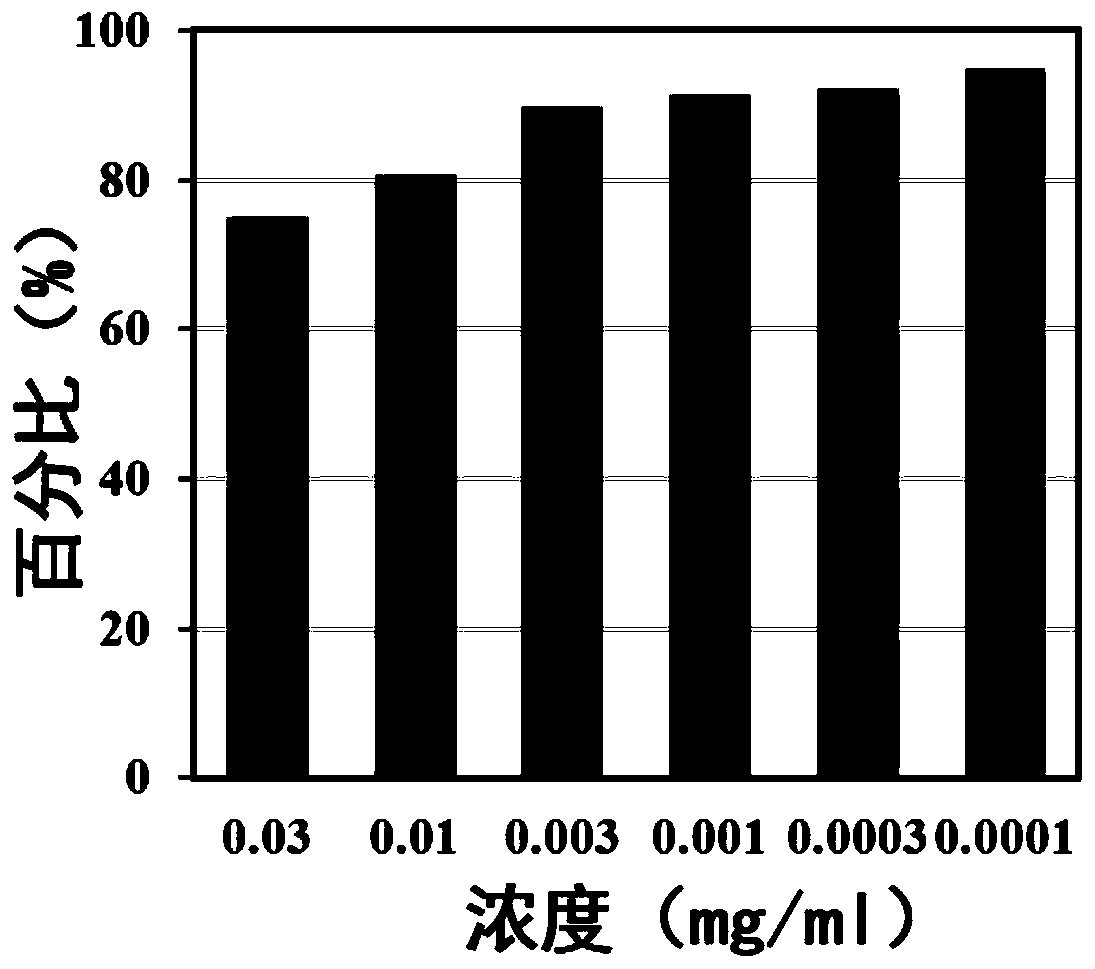
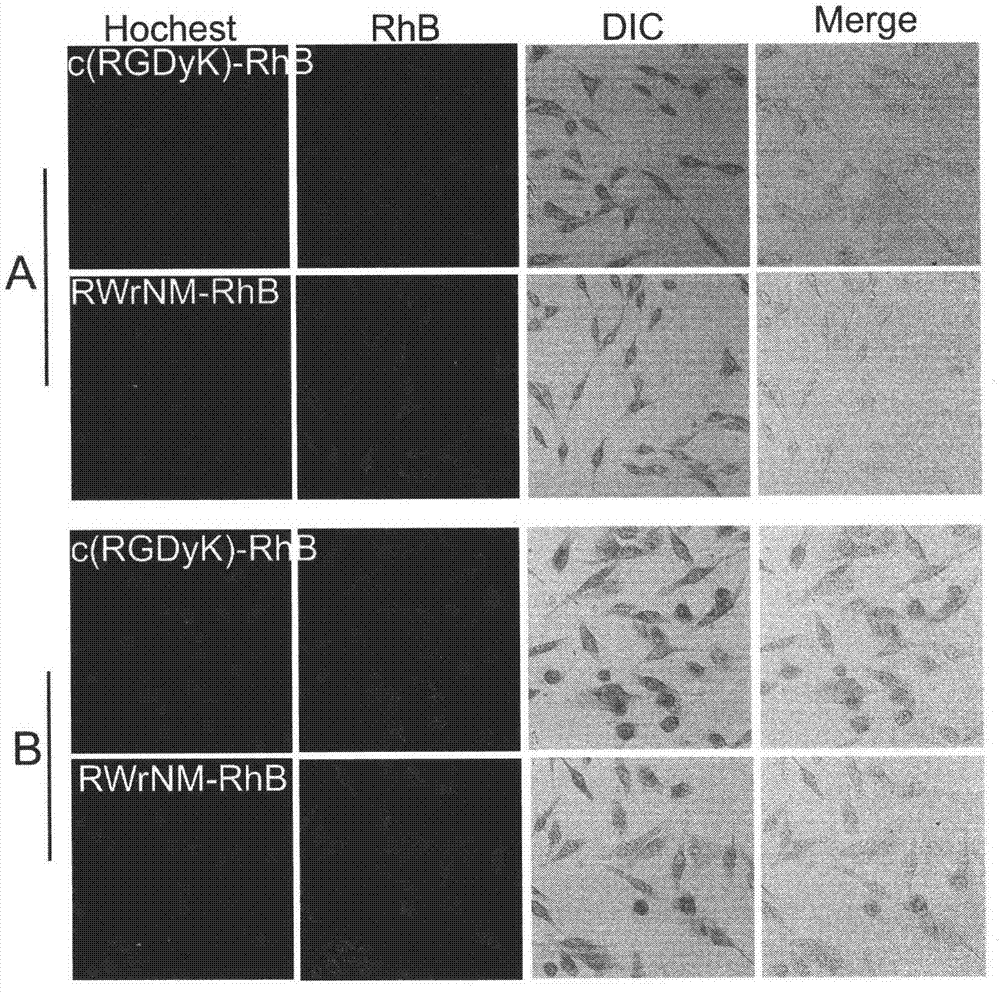
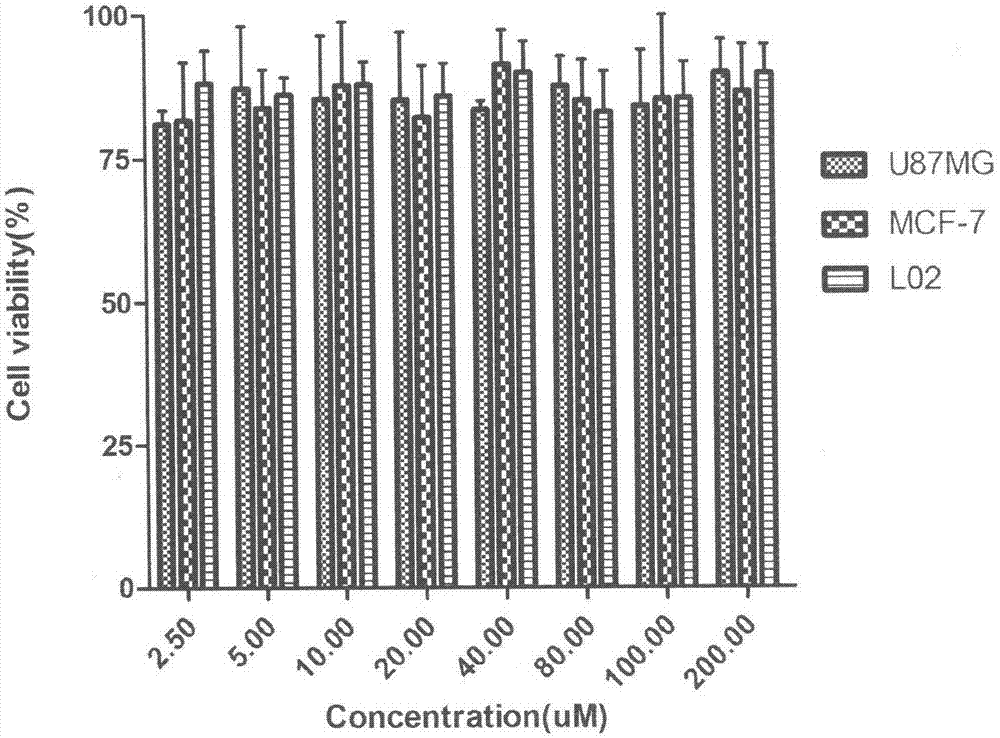
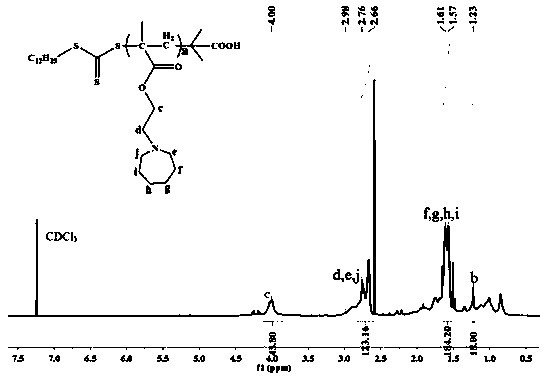
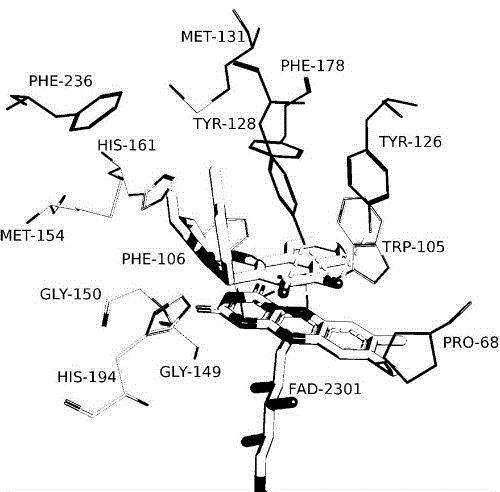
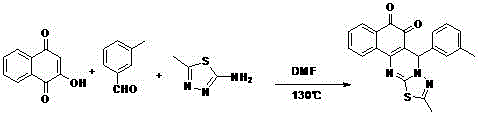
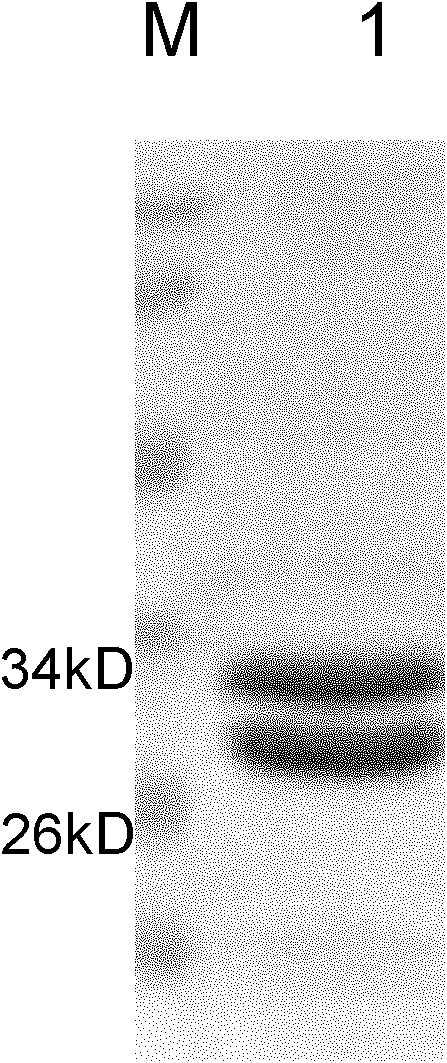Patents
Literature
127 results about "In vitro cytotoxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cytotoxicity is an in vitro test to determine whether the medical device will cause any cell death due to leaching of toxic substances or from direct contact.
Retrocyclins: antiviral and antimicrobial peptides
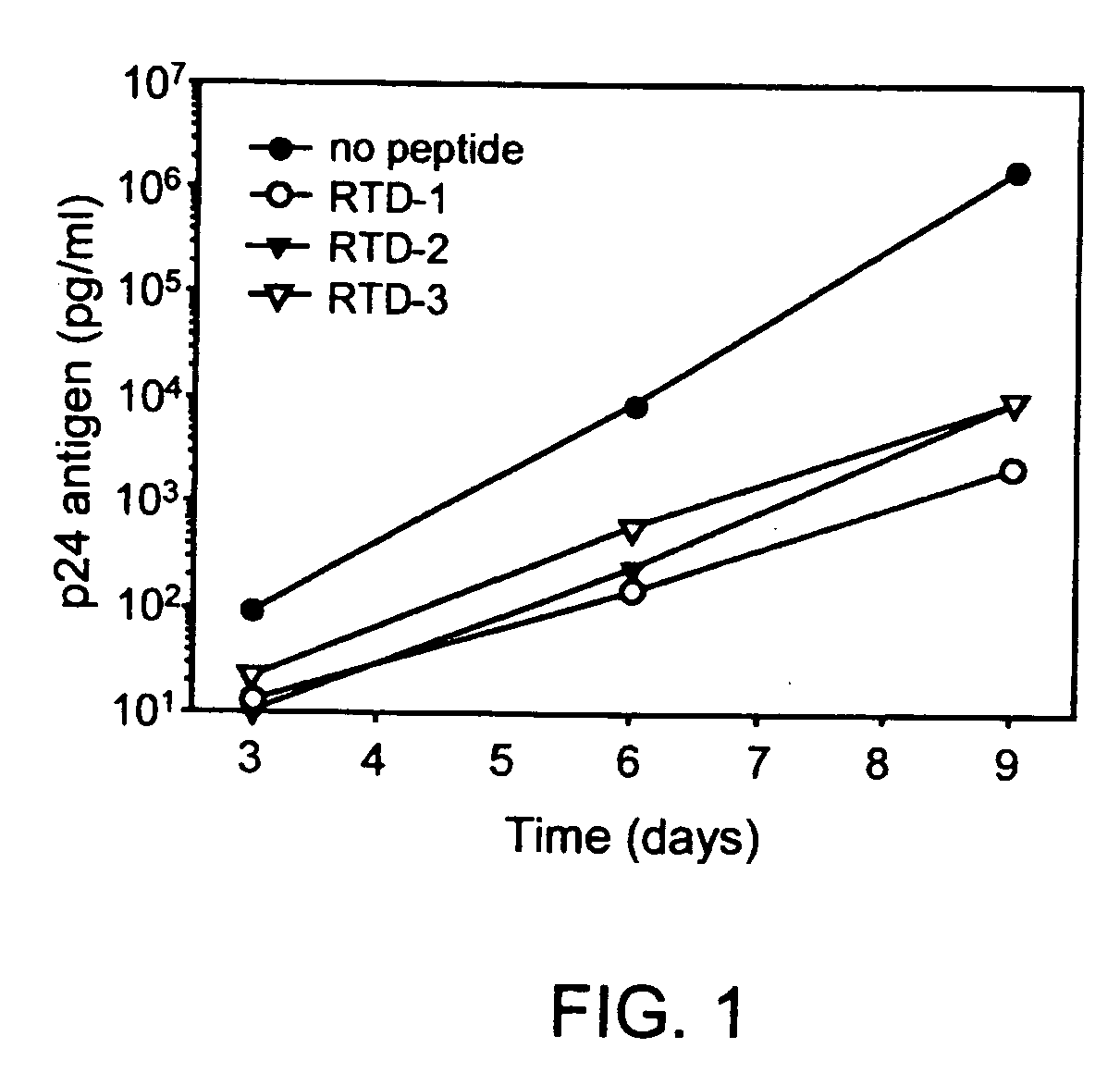
Retrocyclin peptides are small antimicrobial agents with potent activity against bacteria and viruses. The peptides are nonhemolytic, and exhibit minimal in vitro cytotoxicity. A pharmaceutical composition comprising retrocyclin as an active agent is administered therapeutically to a patient suffering from a bacterial and / or viral infection, or to an individual facing exposure to a bacterial and / or viral infection, especially one caused by the HIV-1 retrovirus or other sexually-transmitted pathogens.
Owner:RGT UNIV OF CALIFORNIA
Method for preparing docetaxel and sulforaphane loaded self-assembled nano-particle and application of nano-particle
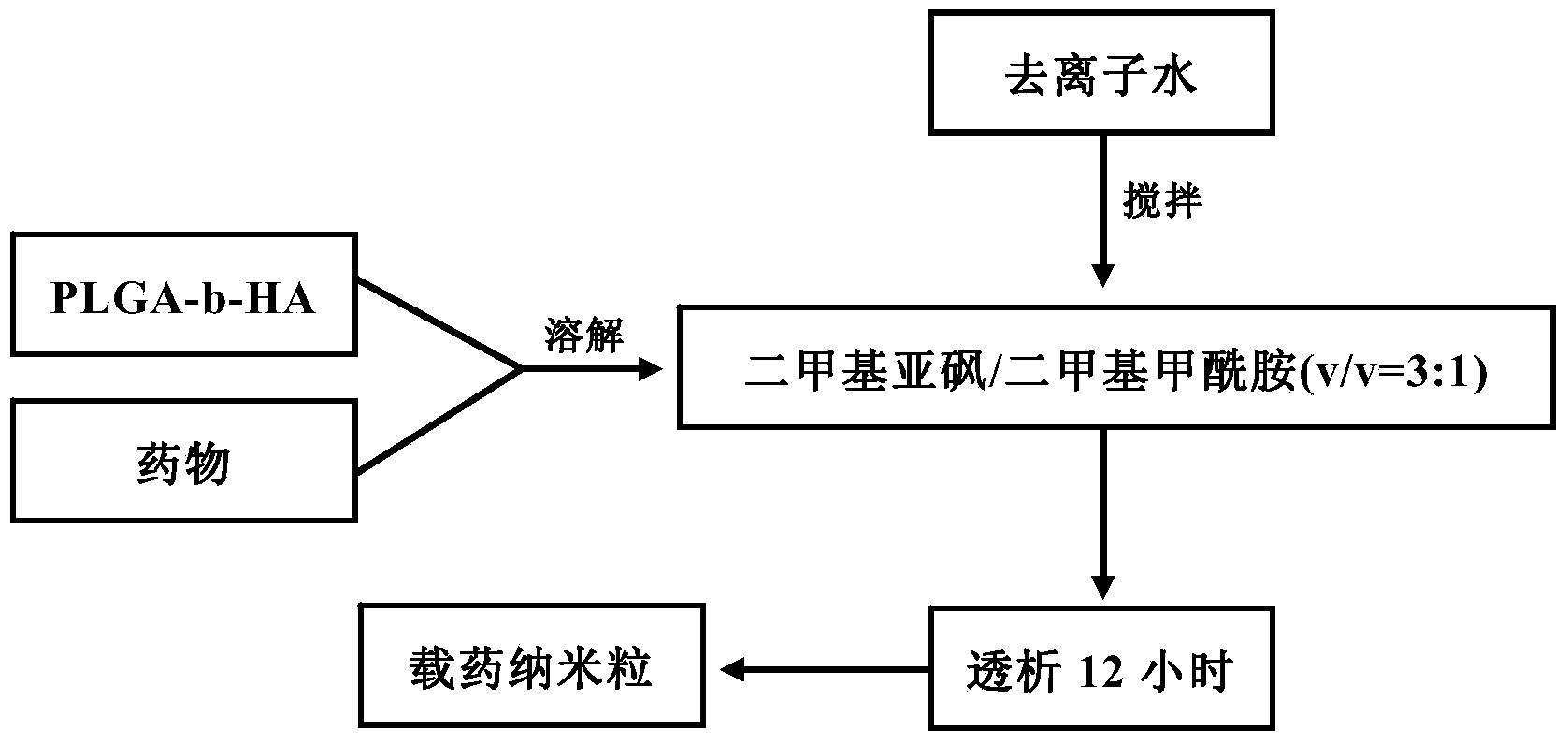
The invention relates to the technical field of medicines, and provides a docetaxel and sulforaphane loaded hyaluronic acid modified poly(lactic-co-glycolic acid) copolymer self-assembled nano-particle, a preparation method thereof and an application of the self-assembled nano-particle in preparing a medicine for treating breast cancer. The microscopic structure of the nano-particle comprises a hydrophobic PLGA (poly(lactic-co-glycolic acid)) core and a hydrophilic HA (hyaluronic acid) shell, wherein the PLGA core is used for wrapping a medicine DTX (docetaxel) or SFN (sulforaphane), and the hydrophilic shell HA can target a breast cancer stem cell highly expressing a CD44 receptor. In-vitro cytotoxicity tests indicate that the drug-loaded nano-particle can play a good role in resisting differentiation of mammary gland cells and breast cancer stem cells when compared with free medicines; in-vivo tumor inhibition experiments indicate that the drug-loaded medicine is more effective than free medicines and combined free medicines, and has a small systematic toxicity. The nano-particle can play a role in simultaneously performing target differentiating on mammary gland cells and breast cancer stem cells, and a new strategy is provided to treatment of breast cancer.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
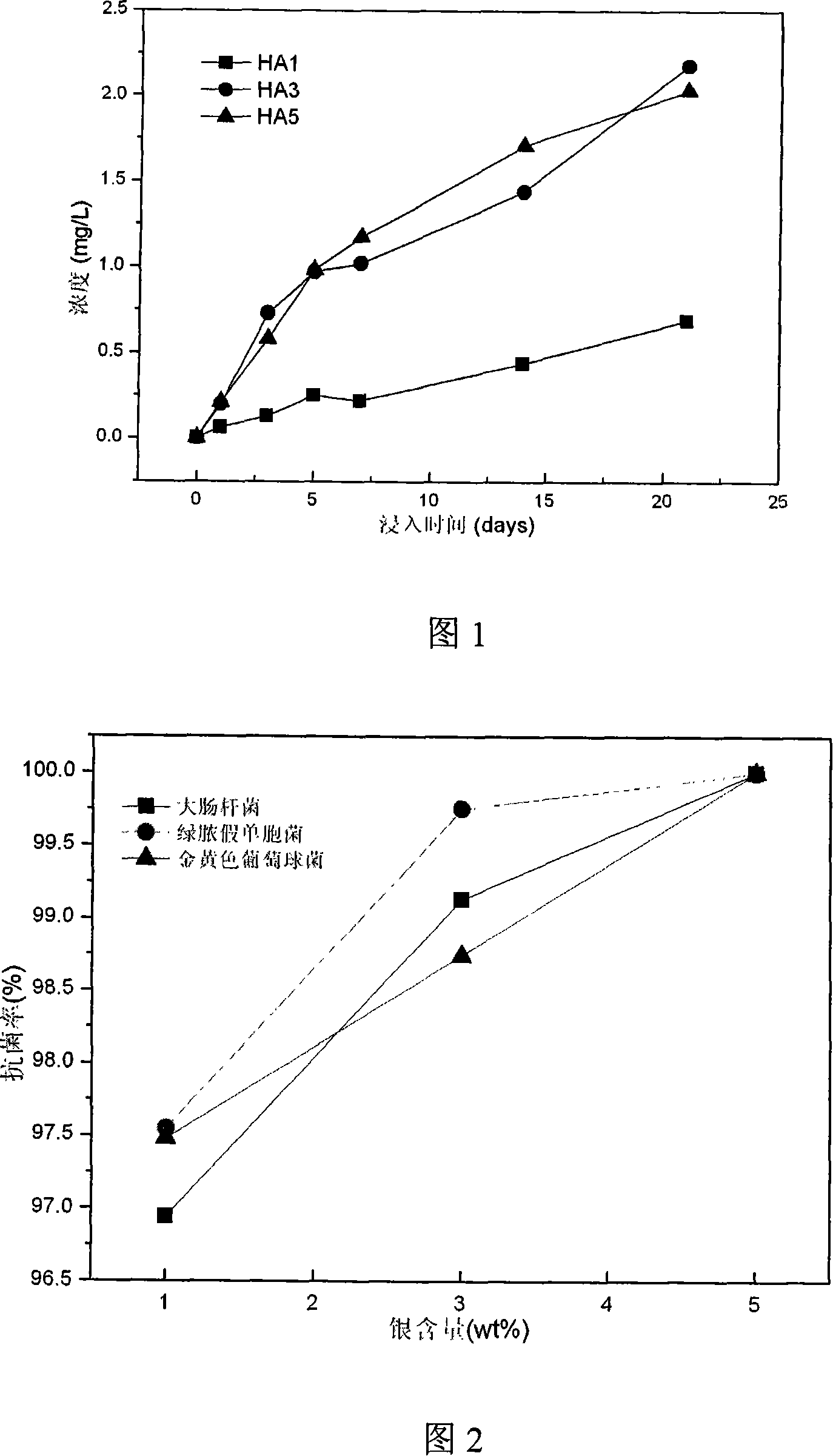
Anti-bacterial carboxy apatite composite coating, its preparing method and use
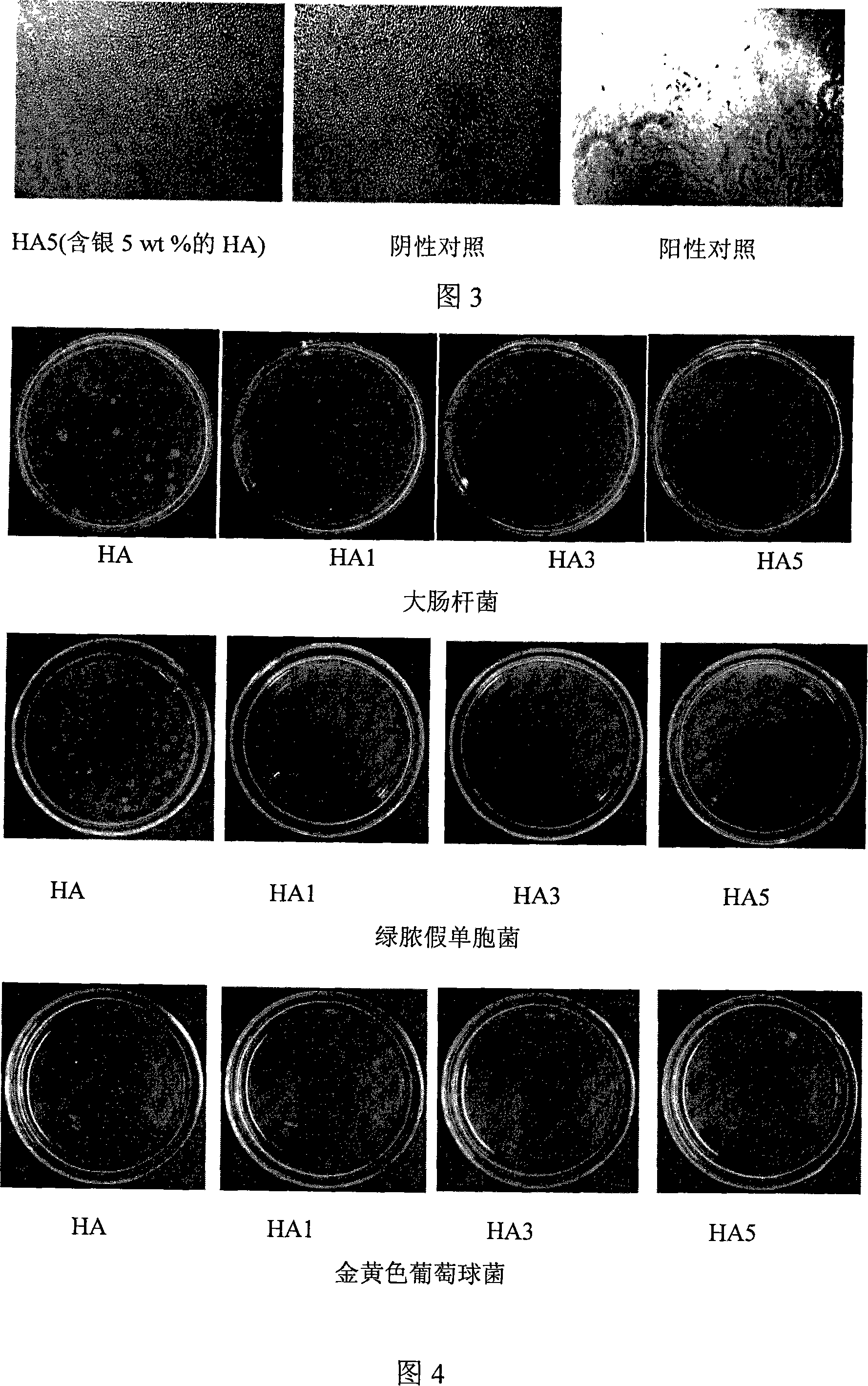
InactiveCN101070441AImprove biological activityImprove antibacterial propertiesAntifouling/underwater paintsPaints with biocidesApatiteAdditive ingredient
This invention relates to one kind of the antibacterial hydroxyl apatite compound coat, the preparation method and the application, its characteristic is the related compound coat is composed of the metal silver powder and the hydroxyl apatite powder, the metal silver powder is treated as the antibacterial increase ingredient, the silver powder' quality accounts for 1-5% of the compound coat, the particle size of the silver powder is 20-100mum, the particle size of the hydroxyl apatite powder is 10-100mum. This invention making the compound coat is realized by using the spray technics of the vacuum plasma. The provided compound coat has an above 95% excellent antibacterial effect on the coliform, the green pus pseudomonad and the golden yellow staphylococcus, and the cytotoxic rank in vitro of the compound coat is zero, has no cytotoxicity.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Submicron liposome suspensions obtained from preliposome lyophilizates
InactiveUS7238366B1Severe adverse effect on liposome integrityShorten hydration timePowder deliveryTetracycline active ingredientsLipid formationLiposome Vesicle
This invention provides an aqueous / t-butanol solvent-system, facile reconstitute, submicron-reconsitiute preliposome-lyophilaye and method of its preparation and use.In one embodiment this entails a modified method for the preparation of a submicron and stable liposome formulation of the non-cross-resistant anthracycline Annamycin is described. The optimal lipid composition was DMPC:DMPG at a 7:3 molar ratio and the optimal lipid:drug weight ratio 50:1. The selected formulation is a preliposome lyophilized powder that contains the phospholipids, Annamycin, and 1.7 mg Tween 20 per mg of Annamycin. The liposome suspension is obtained on the day of use by adding normal saline at 37° C. (1 ml per mg Annamycin) and hand-shaking for one minute. The presence of Tween 20 is essential in shortening the reconstitution step (from >2 hours to 1 minute), avoiding the early formation of free drug crystals, and reducing the median particle size (from 1.5 μm to 0.15-0.20 μm) without destruction of the liposome vesicles. The chemical stability of the preliposome powder at room temperature was >3 months and the chemical and physical stability of the liposome suspension at room temperature >24 hours. The in vitro cytotoxicity of the formulation was equivalent to that prepared by the standard evaporation method. The results of the study indicate that small amounts of surfactant may be used to enhance the reconstitution step and reduce the liposome size of lyophilized liposome formulations of lipophilic drugs.
Owner:BOARD OF REGENTS
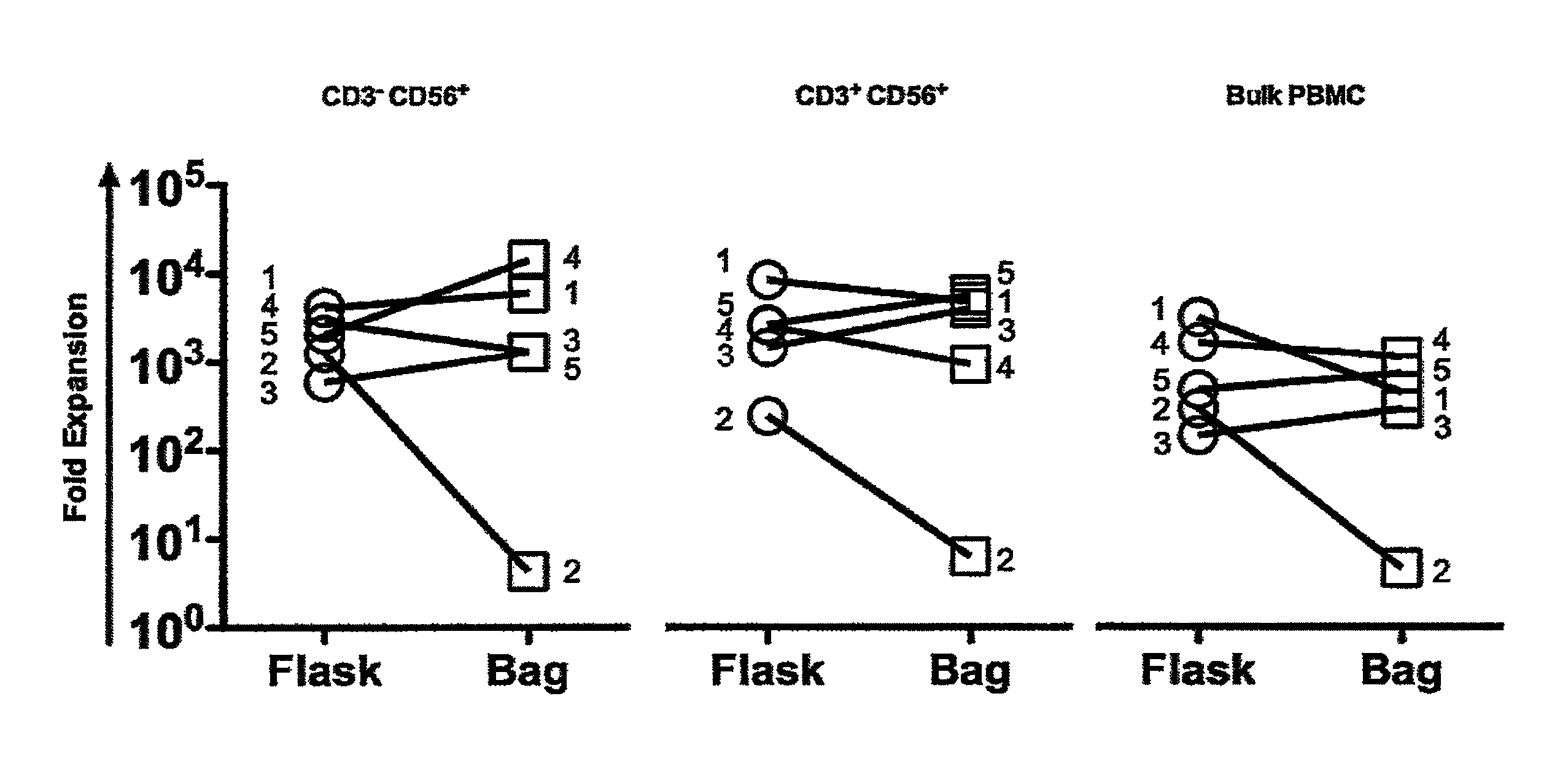
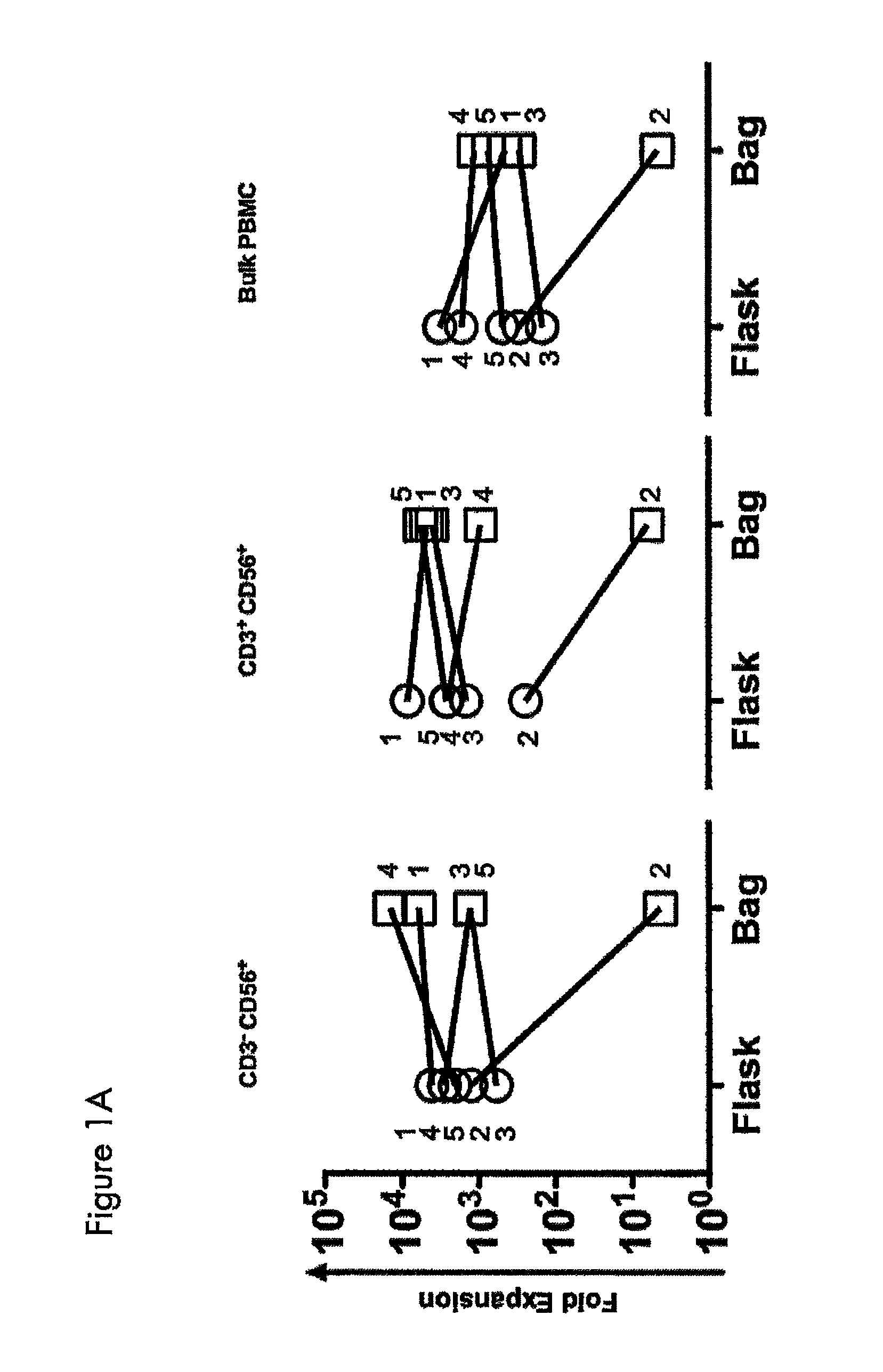
Expansion of NK cells
ActiveUS8877182B2Strong cytotoxicityBiocideMammal material medical ingredientsAbnormal tissue growthNatural Killer Cell Inhibitory Receptors
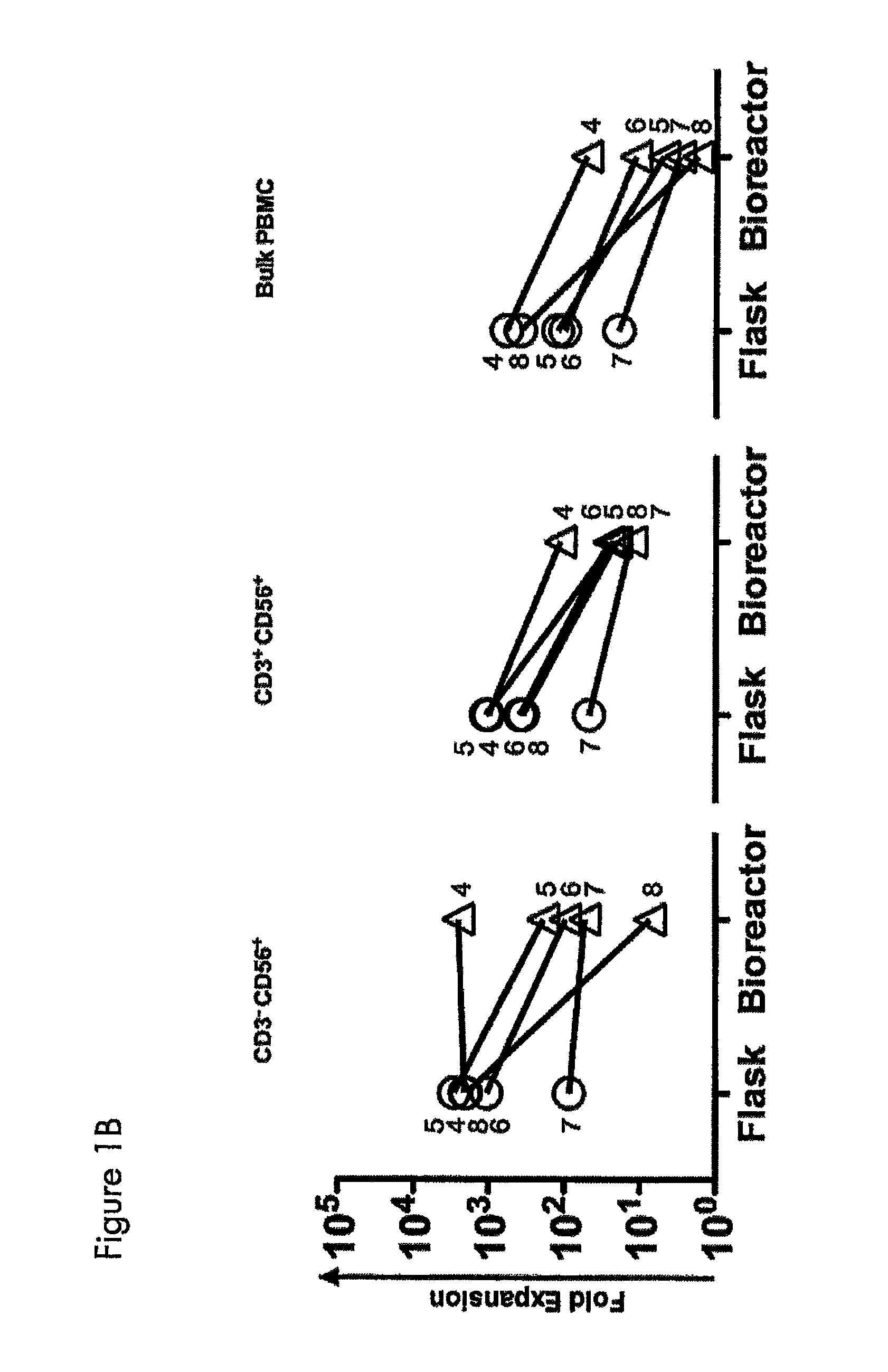
A method of obtaining expanded and activated natural killer (NK) cells with the phenotype CD3−CD56+ and NK-like T cells with the phenotype CD3+CD56+ comprises providing a cell sample of peripheral blood from a tumor bearing subject; isolating cells from the blood sample and re-suspending the cells in growth medium; adding the isolated cells to a closed cell culture bag bioreactor at a concentration of about 0.5×106 to about 2×106 / ml of growth medium; incubating and expanding the cells of step ii) with rocking motion agitation and heating until at least 50% of the expanded cell population comprises activated NK cells and NK-like T cells; and harvesting the expanded cell suspension of therapeutically active NK-cells and NK-like T cells from the bioreactor, wherein the cells exhibit an increased cytotoxicity compared to freshly isolated cells as determined by an in vitro cytotoxicity test.
Owner:CELLPROTECT NORDIC PHARMA
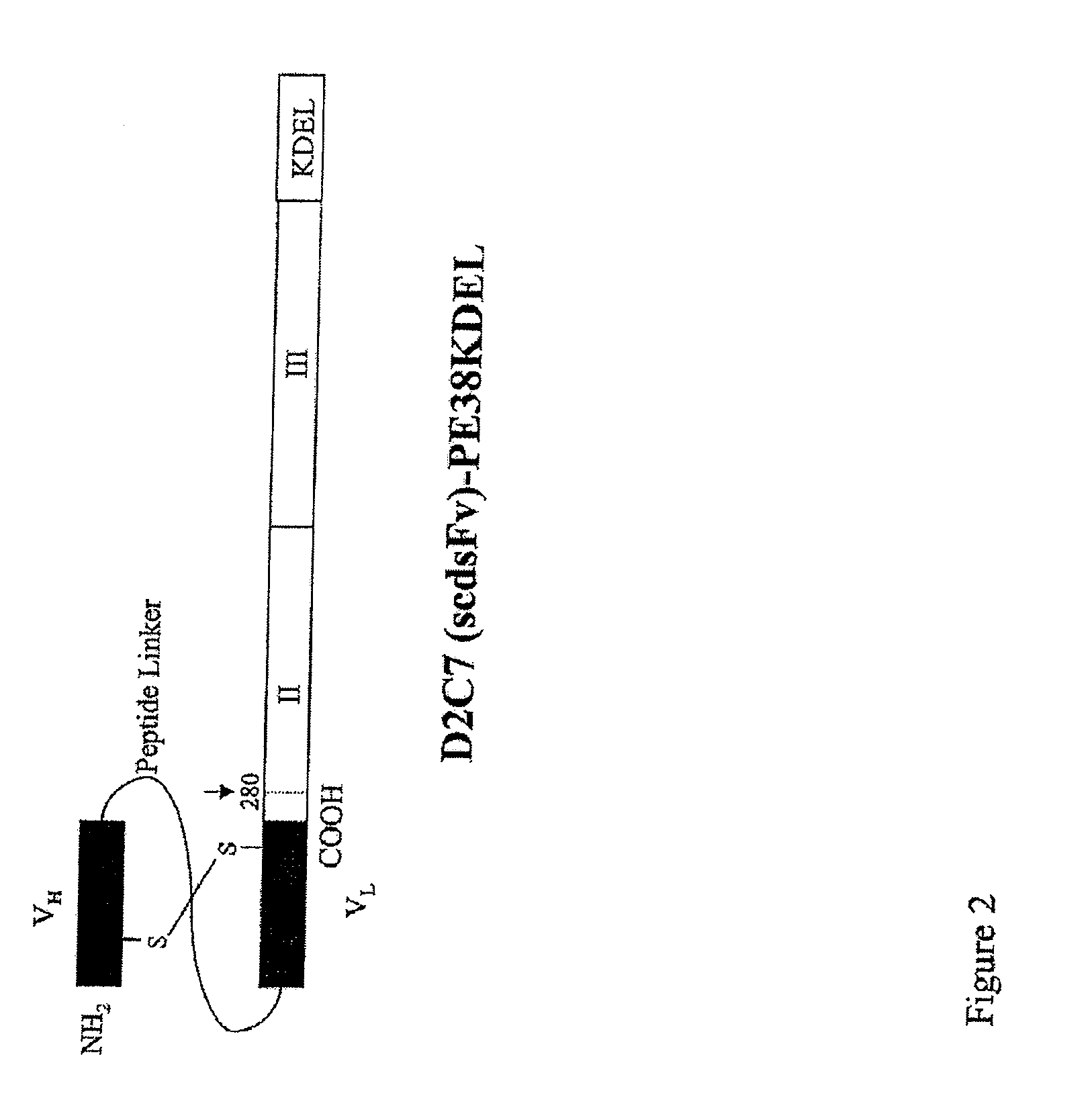
Dual Specific Immunotoxin for Brain Tumor Therapy
InactiveUS20130022598A1Polypeptide with localisation/targeting motifHybrid immunoglobulinsSingle-Chain AntibodiesAntiendomysial antibodies
We tested the in vitro and in vivo efficacy of a recombinant bispecific immunotoxin that recognizes both EGFRwt and tumor-specific EGFRvIII receptors. A single chain antibody was cloned from a hybridoma and fused to toxin, carrying a C-terminal peptide which increases retention within cells. The binding affinity and specificity of the recombinant bispecific immunotoxin for the EGFRwt and the EGFRvIII proteins was measured. In vitro cytotoxicity was measured. In vivo activity of the recombinant bispecific immunotoxin was evaluated in subcutaneous models and compared to that of an established monospecific immunotoxin. In our preclinical studies, the bispecific recombinant immunotoxin, exhibited significant potential for treating brain tumors.
Owner:DUKE UNIV
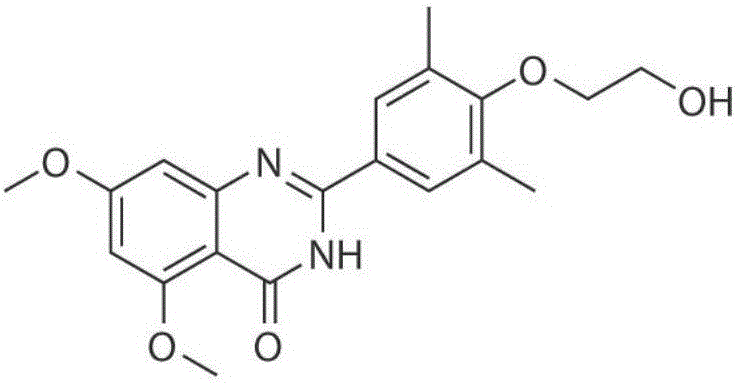
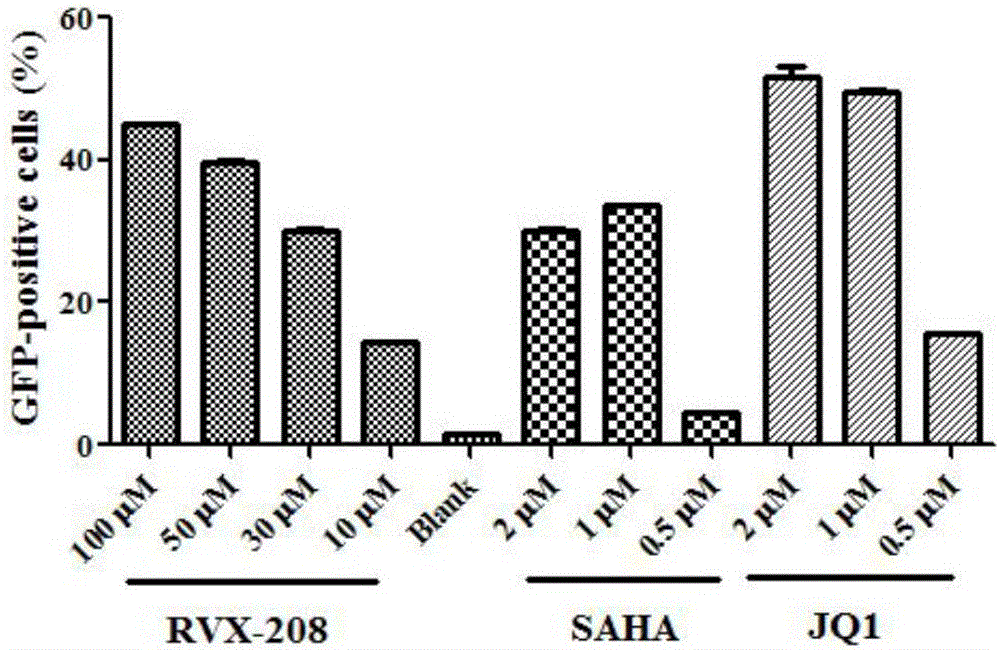
Application of RVX-208 used as HIV-1 latent infection reversal agent
InactiveCN106176753APromote activationImprove activation efficiencyPeptide/protein ingredientsAntiviralsProtein kinase C activationT cell
The invention discloses the application of RVX-208 as an HIV-1 latent infection reversing agent, belongs to the field of medicine, and relates to the application of RVX-208 (Apabetalone, RVX-000222) as an HIV-1 latent infection reversing agent. An important reason why HIV‑1 is difficult to be completely eliminated in the body is that HIV‑1 can lurk in the resting memory CD4 + in T cells. The RVX-208 of the present invention has good activity of activating HIV-1 latent cell pool in vitro, especially can efficiently activate the latent virus pool in HIV-infected patients, and can activate latent infection reversal agents such as protein kinase C with other mechanisms of action Drugs, histone deacetylase inhibitors and cytokines have a good synergistic effect. Compared with the known BET inhibitor JQ1, its in vitro cytotoxicity is greatly reduced, and the drug is safe and well tolerated. Therefore, RVX‑208 is expected to become a new and efficient HIV‑1 latent infection activator, so as to achieve the goal of completely eradicating the HIV‑1 latent infection pool and realizing the "functional" cure of HIV.
Owner:SOUTHERN MEDICAL UNIVERSITY
Levorotatory fluoroquinolone C3 diazole methyl sulfide quaternary ammonium salt, preparation method and application thereof
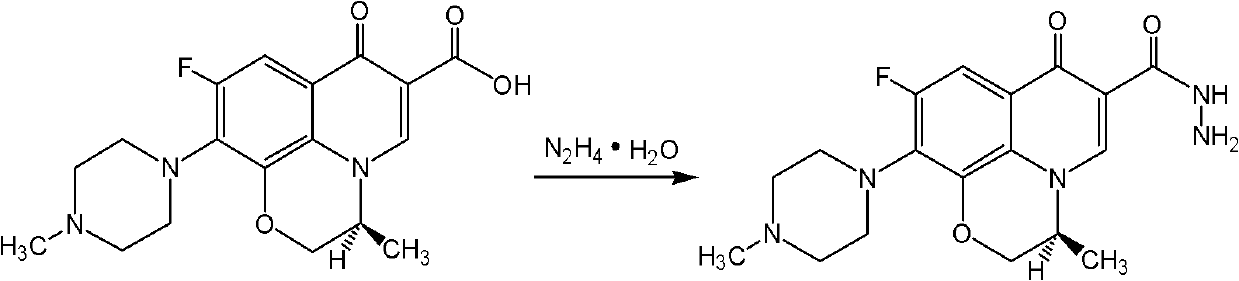
InactiveCN102443011AStrong in vitro cytotoxicityGood antitumor activityOrganic chemistryAntineoplastic agentsCancer cellMethyl sulfide
The invention relates to the technical field of medicines, in particular discloses a levorotatory fluoroquinolone C3 diazole methyl sulfide quaternary ammonium salt, and further discloses a preparation method and application of the levorotatory fluoroquinolone C3 diazole methyl sulfide quaternary ammonium salt in the pharmaceutical field simultaneously. The chemical structural formula of the levorotatory fluoroquinolone C3 diazole methyl sulfide quaternary ammonium salt is represented by a general formula I: the general formula I is described in the specification. The levorotatory fluoroquinolone C3 diazole methyl sulfide quaternary ammonium salt provided by the invention has stronger in-vitro cytotoxicity effect on experimental leukemia cancer cell strains and stronger anti-tumor activity, and can be mixedly prepared into anti-tumor medicines with human body acceptable acidic salts or pharmaceutical carriers.
Owner:HENAN UNIVERSITY
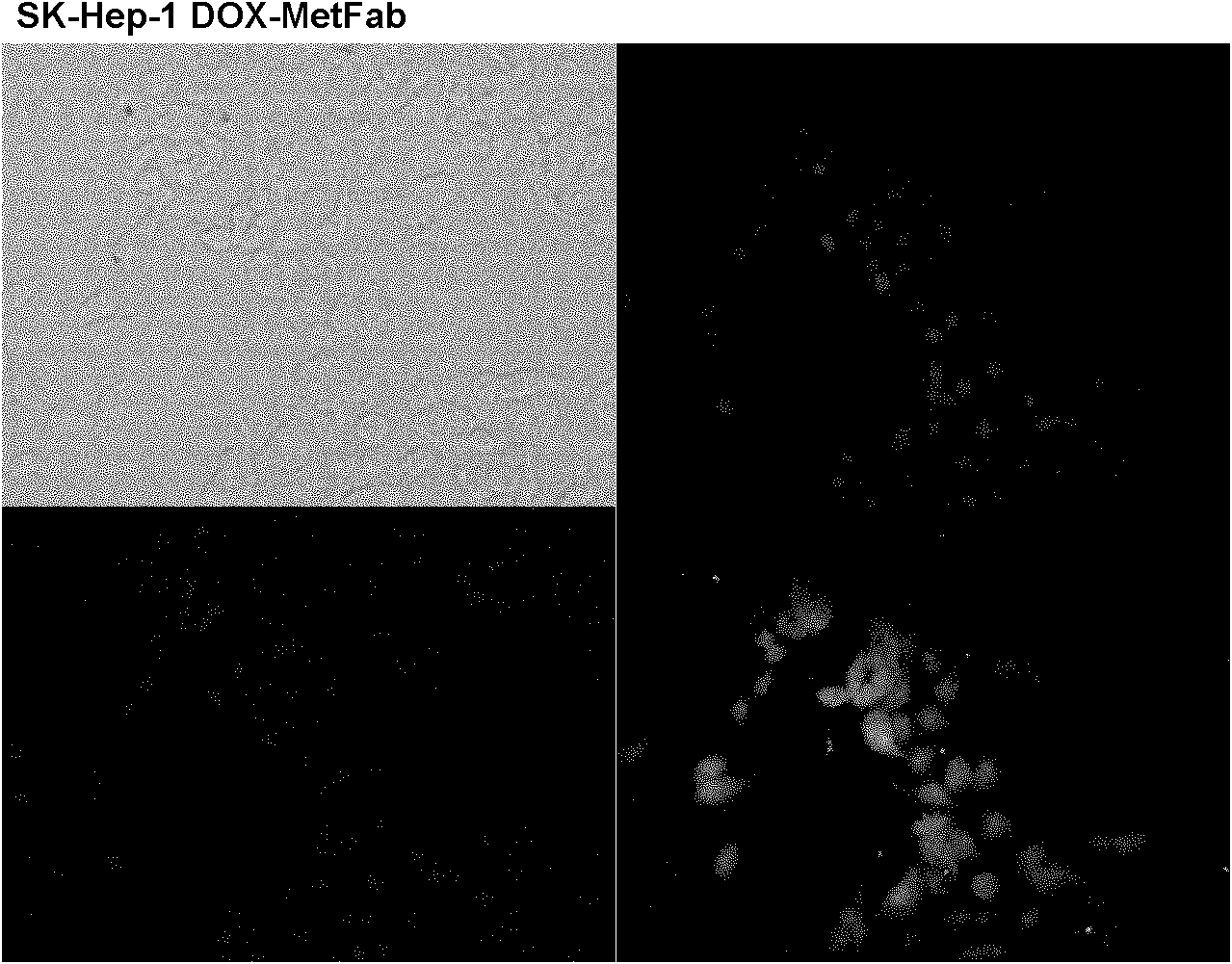
Anti-Met humanized Fab, anti-Met humanized Fab and doxorubicin conjugate and preparation method and application of anti-Met humanized Fab and doxorubicin conjugate
InactiveCN102174106AHigh affinityEffective internalizationOrganic active ingredientsDigestive systemSide effectIn vivo
The invention relates to anti-Met humanized Fab, an anti-Met humanized Fab and doxorubicin conjugate and a preparation method and application of the anti-Met humanized Fab and doxorubicin conjugate. The light-chain amino acid sequence is shown as the SEQ ID No.1, and the heavy-chain amino acid sequence is shown as the SEQ ID No.2. The light-chain nucleotide sequence is shown as the SEQ ID No.3, and the heavy-chain nucleotide sequence is shown as the SEQ ID No.4. The result of the in vitro cell toxicity test shows that the anti-Met humanized Fab and doxorubicin conjugate and free doxorubicin can effectively kill Met positive expression liver cancer cells, and the toxic effect of the anti-Met humanized Fab and doxorubicin conjugate on the Met positive expression cells is obviously less thanthat of the free doxorubicin. The result of the in vivo subcutaneous human liver cancer cell transplantation tumor treatment experiment on the nude mouse shows that the anti-Met humanized Fab and doxorubicin conjugate and the free doxorubicin can effectively inhibit the growth of subcutaneous human liver cancer cell transplantation tumor, and the anti-Met humanized Fab and doxorubicin conjugate can obviously alleviate side effects such as weight loss resulted from the chemotherapeutic medicament on the mouse.
Owner:SINOBIOWAY CELL THERAPY CO LTD
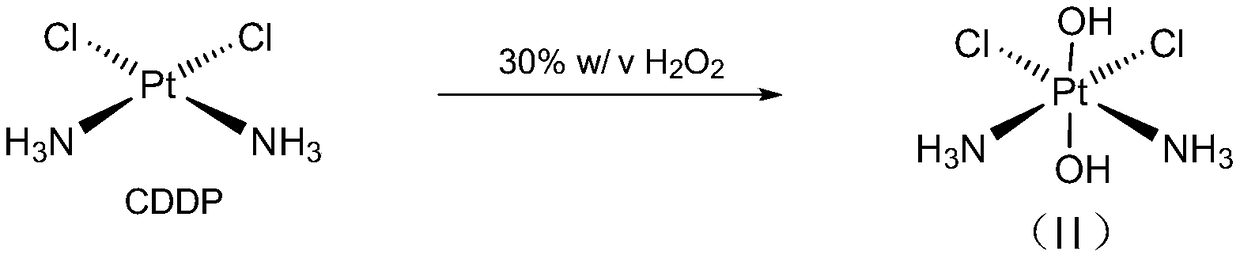
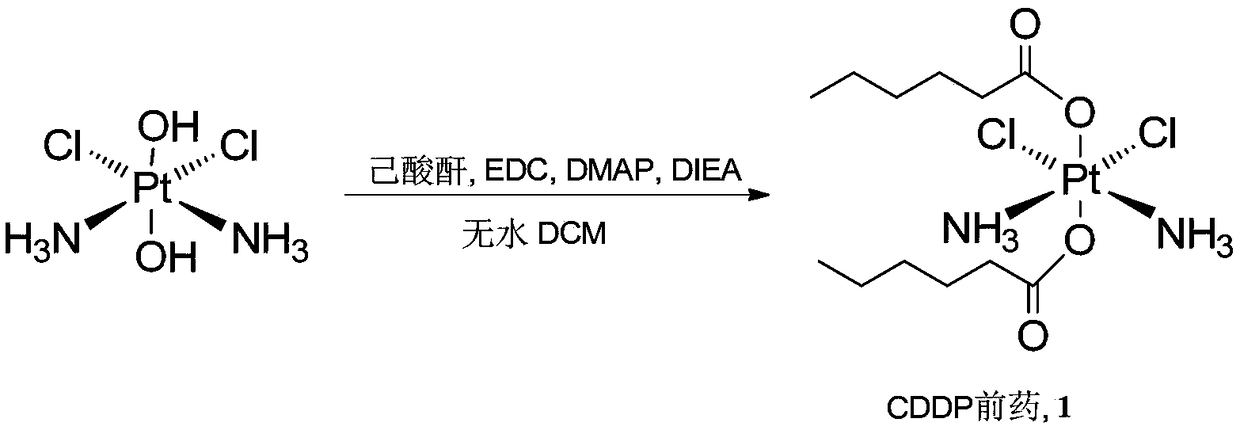
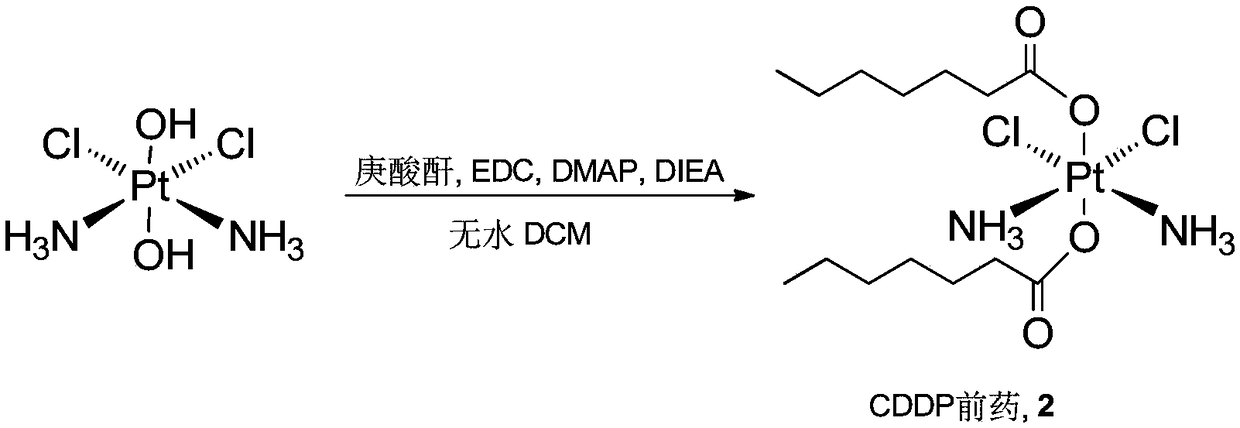
Cis-dammine dichloroplatinum prodrug, preparation method and application
InactiveCN109021026ABiologically activeExcellent ability to kill tumor cellsHeavy metal active ingredientsPlatinum organic compoundsStructural formulaWilms' tumor
The invention discloses a cis-dammine dichloroplatinum (CDDP) prodrug, a preparation method and application. The structural formula of the CDDP prodrug is shown as formula (I), and is generated by theesterification reaction of activated dihydroxy cisplatin with hydrophobic molecules. Characterization of the nano-preparation by dynamic light scattering and transmission electron microscopy indicates that the nanoparticles involved in the invention are uniformly distributed and are at about 30nm. In vitro cytotoxicity experiments show that the nano-drug can significantly inhibit the proliferation of tumor cells (A549 and LoVo). In vivo experiments show that compared with CDDP injections, on the basis of reducing the systemic toxicity, the nano-drug has the effect of inhibiting the non-smallcell lung cancer A549 subcutaneous tumor, and has good market prospects and clinical application value.
Owner:ZHEJIANG UNIV
System and method for high throughput screening of cancer cells
InactiveCN108027372ACompounds screening/testingAntipyreticHigh-Throughput Screening MethodsCancer cell
The present invention discloses a method for high throughput screening (HTS) for identifying an analyte with a measurable effect on cells. The aforementioned method comprises steps of: (a) providing an array comprising a plurality of cell samples; (b) providing at least one analyte to be tested; (c) contacting said cell samples with said analyte; and (d) detecting a signal indicative of said measurable effect on cells wherein alteration of said signal over time measured on said cell sample relative to a control sample is indicative of said measurable effect of said analyte on said cell sample.The current invention further discloses means and methods for identifying an analyte selected from the group consisting of: cannabis extract or a fraction thereof cannabinoid- type constitute non cannabinoid-type constitute and any combination thereof. The analyte is indicative of cytotoxic or anti proliferative or anti mitotic or cell growth inhibitory activity in vitro.
Owner:CANNABICS PHARMA
Retrocyclins: antiviral and antimicrobial peptides
Retrocyclin peptides are small antimicrobial agents with potent activity against bacteria and viruses. The peptides are nonhemolytic, and exhibit minimal in vitro cytotoxicity. A pharmaceutical composition comprising retrocyclin as an active agent is administered therapeutically to a patient suffering from a bacterial and / or viral infection, or to an individual facing exposure to a bacterial and / or viral infection, especially one caused by the HIV-1 retrovirus or other sexually-transmitted pathogens.
Owner:RGT UNIV OF CALIFORNIA
Recombinant expression vectors comprising a human codon-optimized marburg virus (MARV) angola glycoprotein gene insert and method of immunization employing said vector
Owner:UNITED STATES OF AMERICA
Viral vector driven mutant bacterial cytosine deaminase gene and uses thereof
InactiveUS20070225245A1Low efficiencyGreat fold substrate preferenceVectorsHydrolasesCytosine deaminaseHuman glioma
The instant invention has developed viral vectors encoding a mutant bacterial cytosine deaminase (bCD) gene, which have a higher affinity for cytosine than wild type bCD (bCDwt). The purpose of the present invention was to evaluate cytotoxicity in vitro and therapeutic efficacy in vivo of these vectors in combination with the prodrug 5-FC and ionizing radiation against human glioma. The present study demonstrates that infection with the viral vector expressing the mutant cytosine deaminase gene resulted in increased 5-FC-mediated cell killing, compared with vectors expressing the wild-type gene. Furthermore, a significant increase in cytotoxicity following infection with viral vector expressing the mutant cytosine deaminase gene and radiation treatment of glioma cells in vitro was demonstrated as compared to infection with viral vector expressing the wild-type gene. Animal studies showed significant inhibition of subcutaneous or intracranial tumor growth of D54MG glioma xenografts by the combination of AdbCD-D314A / 5-FC with ionizing radiation as compared with either agent alone, and with AdbCDwt / 5-FC plus radiation. These data indicate that combined treatment with this mutant enzyme / prodrug therapy and radiotherapy provides a promising approach for cancer therapy.
Owner:BUCHSBAUM DONALD J +3
Novel staurosporine analogue and preparation method and applications thereof
The invention relates to a novel staurosporine analogue and a preparation method and applications thereof, wherein two staurosporine analogue molecules with a novel structure is prepared from the Streptomyces Sp.FMA which is separated from the soil sample of the Sanya Mangrove in Hainan Island of China and has the strain preservation number of CCTCC M 2010021. The in vitro cytotoxicity test proves that the compound has cytotoxicity to leukemia cell HL-60 and lung cancer cell A549 and can be used as the cell antiblastic.
Owner:INST OF TROPICAL BIOSCI & BIOTECH CHINESE ACADEMY OF TROPICAL AGRI SCI +1
Double-drug albumin nanoparticles and preparation technology
InactiveCN109806241AGood effectSmall toxicityHydroxy compound active ingredientsMacromolecular non-active ingredientsSide effectMedicine
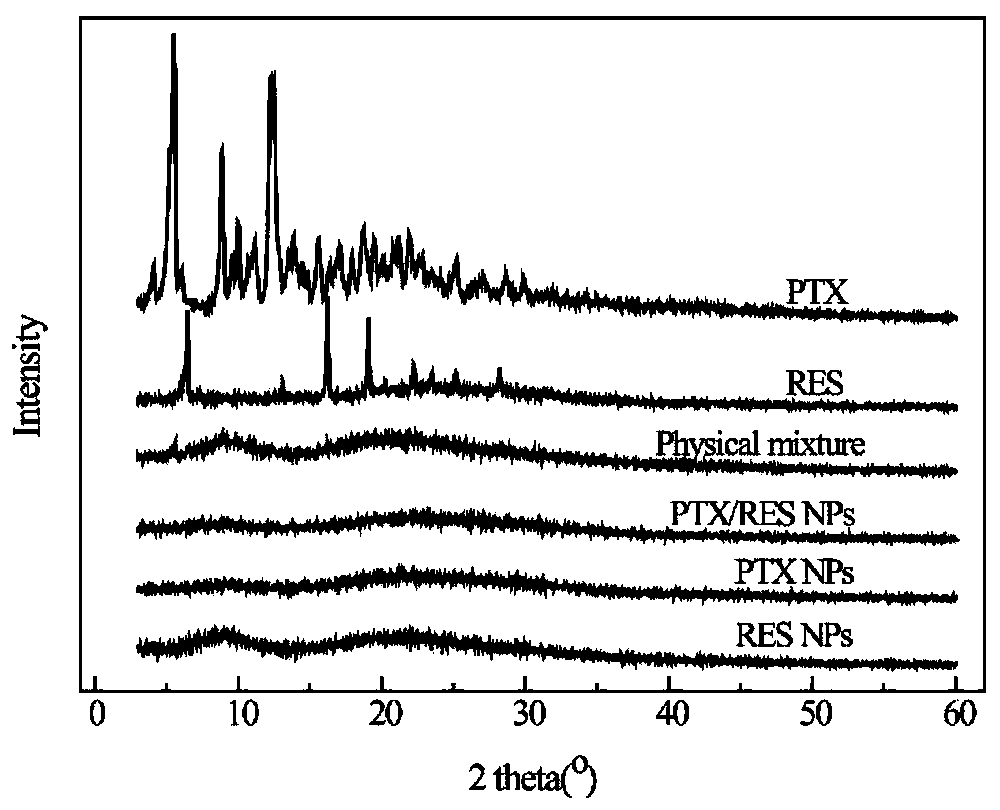
The invention provides paclitaxel / resveratrol albumin nanoparticles and a preparation technology. The preparation technology comprises the following steps: (1) preparing a paclitaxel and resveratrol storage solution; (2) preparing an albumin storage solution; (3) preparing double-drug albumin nanoparticles. According to the paclitaxel / resveratrol albumin nanoparticles, an anti-cancer drug paclitaxel and a bioactive substance resveratrol are combined to be administrated, so that the toxic side effect can be reduced and the anti-cancer effect is improved; the prepared double-drug albumin nanoparticles have the encapsulation efficiency of 99 percent and have good stability; the double-drug albumin nanoparticles provided by the invention can be released in sequence; the resveratrol is rapidlyreleased so that the effect of inhibiting P-gp is facilitated; a condition that the paclitaxel which is released subsequently is pumped out from tumor cells is avoided, so that the cytotoxicity on thedrug-resisting tumor cells is increased; an in-vitro cytotoxicity experiment of the double-drug albumin nanoparticles provided by the invention proves that the drug resistance of the tumor cells canbe reversed, and a cooperative anti-tumor effect of the paclitaxel and the resveratrol in the double-drug nanoparticles is realized.
Owner:LIAOCHENG UNIV
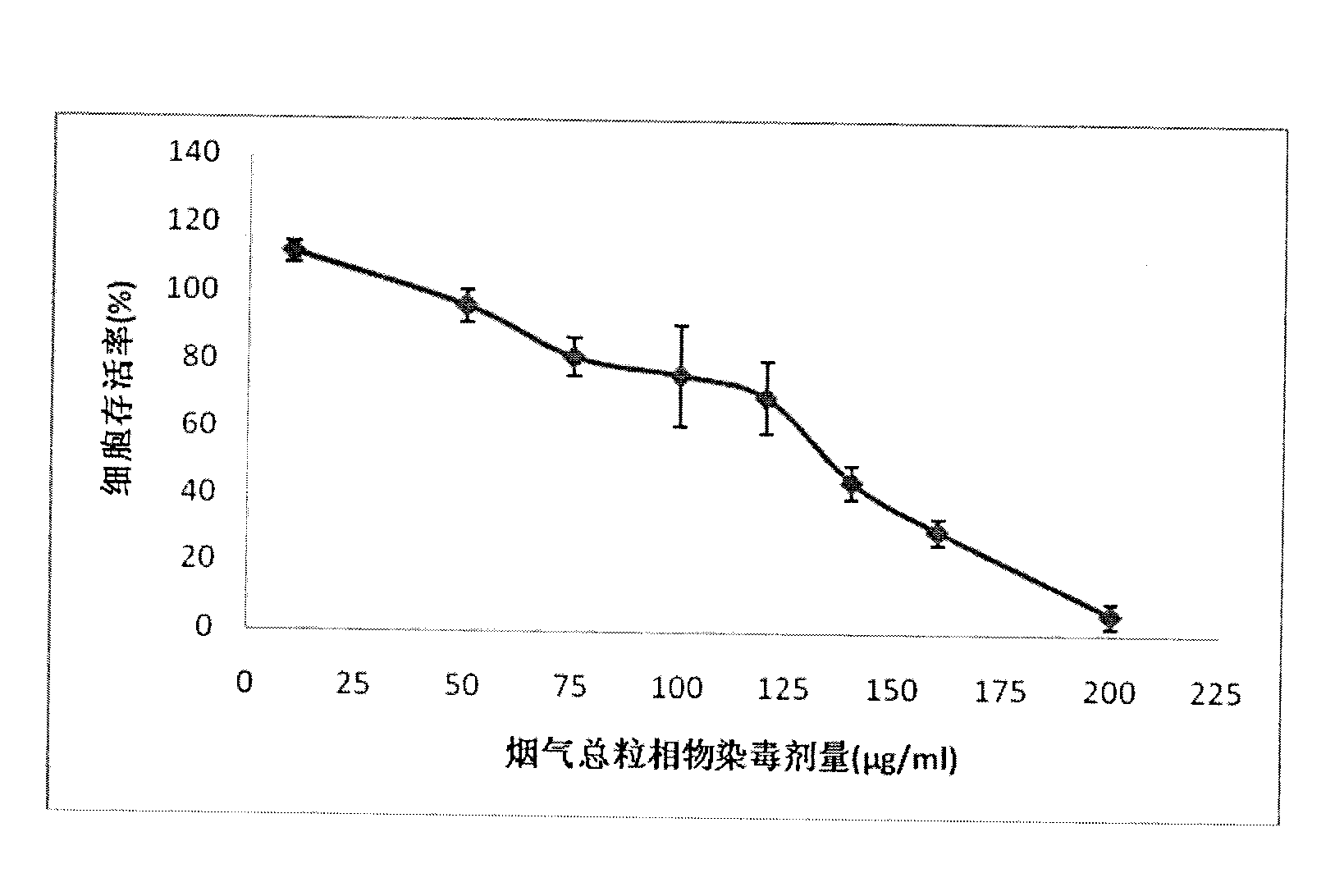
Method for testing in vitro cytotoxicity of cigarette smoke
ActiveCN102618619AWide linear rangeHigh sensitivityMicrobiological testing/measurementBiotechnologyStaining
The invention discloses a method for testing in vitro cytotoxicity of cigarette smoke. The method is characterized by comprising the following steps of: 1) preparing a laboratory reagent; 2) performing cell inoculation culture; 3) performing cigarette smoke contamination; 4) dyeing with water-soluble tetrazolium-1 (WST-1); and 5) obtaining a result and analyzing. Compared with the prior art, the method is characterized in that a test step for washing cells for multiple times is eliminated in the whole test process, so that the method is easy and convenient to operate; a step of replacing a nutrient solution is not required during dyeing with the WST-1, and a diluted nutrient solution can be directly added for reacting; formazan produced by the WST-1 is water-soluble, so that a subsequent dissolution step is eliminated; and absorbance detection can be finished in 2 hours after WST-1 dyeing, so that the test period is shortened, and the method is quick in comparison with the conventional testing method. The measuring method is quick and convenient to operate, and also has the advantages of high sensitivity and stable result; and the method can be applied to smoke cytotoxicity test of multiple cell lines.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
3D printing preparation method of skin tissue engineering scaffold and in vitro cell toxicity testing method of the same
InactiveCN107998451AIncrease added valueImprove mechanical propertiesAdditive manufacturing apparatusMicrobiological testing/measurementPorosityBiocompatibility Testing
The invention provides a 3D printing preparation method of a skin tissue engineering scaffold and an in vitro cell toxicity testing method of the scaffold. The 3D printing preparation method comprisesthe following steps: preparation of double aldehyde nano-crystalline cellulose used for a scaffold material, preparation of a gelatin solution, preparation of a double aldehyde nano-crystalline cellulose and gelatin compound hydrogel, and printing of the 3D tissue engineering scaffold. The problem that the tissue engineering scaffold needs the requirements of high porosity and high precision is solved by using a 3D biological printing technology. DAC is taken as a cross-linking agent, cross-linking occurs with GEL through a Schiff base reaction to form a network structure, so that the 3D printing tissue engineering scaffold has excellent mechanical property and is unlikely to crack, and simultaneously the additional value of plant fiber is also improved. The DAC / GEL hydrogel has good biocompatibility, is free of toxic and / or side effects or immunologic rejection, has biological activity besides degradation characteristic, and is extremely beneficial for the growth and differentiationof cells and the implementation of cell functions.
Owner:YANGZHOU UNIV
Method for preparing skin tissue engineering scaffold based on 3D bio-printing technology and in-vitro cytotoxicity testing method for scaffold
InactiveCN108114324AIncrease added valueHigh mechanical strengthAdditive manufacturing apparatusBiological testingCelluloseCross-link
The invention discloses a method for preparing a skin tissue engineering scaffold based on a 3D bio-printing technology and an in-vitro cytotoxicity testing method for the scaffold. The method comprises the following process steps: preparing high-strength cellulose nanofiber / gelatin complex hydrogel for a printing scaffold, printing a 3D tissue engineering scaffold and cross-linking the scaffold.According to the method disclosed by the invention, the requirements on high porosity and high precision of the tissue engineering scaffold are met by utilizing the 3D bio-printing technology. The CNFserves as a filling material of the GEL and has an effect of improving the mechanical strength of the GEL, and the printed scaffold is soaked in a genipin solution to be cross-linked. The skin tissueengineering scaffold prepared by the method has excellent mechanical property, and does not have any toxic or side effect or immunologic rejection. Meanwhile, the tissue engineering scaffold preparedby using the 3D printing technology has the advantages of being convenient, rapid and easy to control, and can be subjected to personalized customization according to depths, sizes and shapes of wounds of patients.
Owner:YANGZHOU UNIV
Application of pit-hole composite micro-nano-structure polysaccharide micro-sphere to preparation of haemostatic wound dressing
ActiveCN109364288AUnique surface "pit-hole" composite micro-nano structureUniform particle size distributionSurgical adhesivesPharmaceutical delivery mechanismMicro nanoHaemostatic function
The invention discloses an application of a pit-hole composite micro-nano-structure polysaccharide micro-sphere to preparation of haemostatic wound dressing. According to GB / T16886.5-2003 in-vitro cytotoxicity judgment standards, acquired pit-hole composite micro-nano-structure polysaccharide micro-sphere extracting solution has 0-grade cytotoxicity under the concentration of 10-40 micrograms / L and shows good biocompatibility, bleeding can be effectively stopped within 20s-50s, good physical and chemical property is achieved, hemolysis ratio is smaller than 5%, national standards are met, themicro-sphere has an instant haemostatic function for a liver injury wound surface (haemostatic time is shorter than 5 seconds), haemostatic materials are completely degraded 72 hours after, and any residue is omitted. The pit-hole composite micro-nano-structure polysaccharide micro-sphere can stop bleeding within 20 seconds when being used for excessive bleeding of a femoral artery, surgical woundsurface recovers normal one week after, and any haemostatic micro-sphere sample residues are omitted.
Owner:安徽中科迈德医疗科技有限公司
Spraying method for water-based two-component wood paint for teenage furniture
InactiveCN106670076AConvenient sourceNo emissionsPretreated surfacesSpecial surfacesWater basedUltraviolet lights
The invention discloses a spraying method for a water-based two-component wood paint for teenage furniture. The method belongs to the production field of furniture. The method comprises the following steps: taking TB-003 water-based two-component wood paint prime coating and TB-032 water-based two-component wood paint finishing coating as raw materials, and carrying out pre-treatment on the materials of the furniture, polishing for the surface of the furniture, primary roller coating for the prime coating, drying in an ultraviolet-light drying tunnel, polishing for the prime coating, secondary roller coating for the prime coating, high-finish-degree sanding and polishing for the prime coating, spraying for the finishing coating, and ultraviolet-light drying to prepare the finished product. The product is the furniture for teenagers, and has the following main advantages: 1. the product has an odour removal function, the surface of a paint film, the interior of a cabinet body and a store environment are free from the odour of the paint, and the odour generated by wood can be effectively sealed by the paint; 2. the paint film is high in hardness, resistant to chemicals, water, pollution and after-tack, high in transparency, and good in hand feeling; and 3. the coating layer of the paint film is high in resistance comprising resistance to allergy, irritative reaction to the skin, and in-vitro cytotoxicity.
Owner:徐州冠宁木业有限公司
Multifunctional specific DNA hybridization-gated mesoporous silica gene carrier and preparation method and application thereof
PendingCN110106204AAchieve specific releaseHave diversityInorganic non-active ingredientsOther foreign material introduction processesPolyetherimideTreatment effect
The invention relates to a multifunctional specific DNA hybridization-gated mesoporous silica gene carrier and a preparation method and application thereof. A mesoporous silica particle is adopted asa carrier body to produce particles with the particle size of 50-100 nm; the surface is modified with anchoring DNA, and a gated structure is formed on a DNA hybrid so that the nanoparticles can achieve the release of drugs on specific targets. At the same time, the surfaces of the particles are modified with polyetherimide loaded plasmid, so that a drug and gene combined treatment effect is achieved. A synthesis process of the carrier is simple, free of toxicant, fast and large in yield. In an in-vitro cytotoxicity test, the cell survival rate is 75.1-95%. The multifunctional specific DNA hybridization-gated mesoporous silica gene carrier has high biosafety.
Owner:TIANJIN UNIV
Integrin receptor alpha-v-beta-3 related 5-peptide
The invention belongs to the technical field of biomedicine production or the fields of protein polypeptide drugs and biomedical engineering, and in particular relates to a polypeptide which has high affinity with an integrin receptor and an application of the polypeptide like cancer diagnosis and treatment. The polypeptide is composed of five amino acids, and the sequence is arginine-tryptophan-arginine-asparagine-methionine (Arg-Trp-Arg-Asn-Met). On the basis of an in-vitro flow cytometry affinity determination experiment, the polypeptide disclosed by the invention has quite strong targeting on high-expression cells of the integrin receptor, and through an in-vitro laser confocal cell uptake experiment, the polypeptide can be targeted to high-expression tumor cells of the integrin receptor alpha-v-beta-3 and can basically avoid targeting on low-expression tumor cells and normal cells of the integrin receptor alpha-v-beta-3. An in-vitro cytotoxicity result shows that the 5-peptide disclosed by the invention is basically free from toxicity on cells, and the 5-peptide has a good application prospect in the diagnosis and the treatment of cancers.
Owner:CHINA PHARM UNIV
Novel withanolides compound, novel withanolides compound preparation method and medical application of novel withanolides compound
The invention belongs to the field of medicines, discloses a novel withanolides compound, a novel withanolides compound preparation method and a medical application of the novel withanolides compound and particularly relates to a novel withanolides compound obtained by separation from dried roots of attalanteae buxifoliae, a preparation method of the novel withanolides compound and a medical application of the novel withanolides compound. The novel withanolides compound which is reported for the first time can be obtained by separation and purification after extraction from the dried roots of attalanteae buxifoliae, and high purity is achieved. According to in-vitro tests, the novel withanolides compound with an effect of in-vitro resistance to breast cancers is capable of inhibiting proliferation of various breast cancer cells, shows high in-vitro cytotoxicity and can be further researched and developed for preparation of breast cancer treatment medicines.
Owner:林天样
pH responsive polymer nano-micelle, and preparation and application thereof
InactiveCN110664751AGood biocompatibilityPrevent proliferationOrganic active ingredientsPharmaceutical non-active ingredientsPolymer sciencePharmaceutical Substances
The invention discloses a preparation method for a pH responsive polymer nano-micelle. C7A is used as a base material, a hydrophobic macromolecule PC7A polymer is prepared by RAFT polymerization, thenPEG is used for chain extension of PC7A to obtain a PC7A-PEG polymer, then VI and PBA monomers are used for chain extension of PC7A-PEG to obtain a block compound PC7A-PEG-VI-PBA, and finally, the block compound PC7A-PEG-VI-PBA coordinates with a quantum dot CdSeTe QDs to obtain a CdSeTe@PC7A-PEG-VI-PBA polymer micelle. In vitro cytotoxicity studies show that the polymer nomo-micelle has good pHresponsiveness and biocompatibility, can be released in the weak acidic environment of tumor cells, and is expected to be a good drug delivery system.
Owner:NORTHWEST NORMAL UNIVERSITY
Ortho-naphthaquinone derivative, and preparation method and medicinal application thereof
The present invention relates to an ortho-naphthoquinone derivative, a preparation method and medical application thereof, and provides a compound with the structure shown as a formula I, a preparation method and application thereof in the manufacture of tumor medicaments. The compound of the invention has a hybrid structure of ortho -naphthoquinone with thiadiazole [3,2-a] pyrimidine, strong anti-cancer activity, metabolic stability and good selectivity. In vitro cytotoxicity and topoisomerase I inhibition tests show that the compound has strong inhibition on tested cancer cells and topoisomerase I; and NQO1 activity test shows that the compound is an effective substrate for NQO1, and mediated by NQO1, the compound cycles by a redox reaction, produces a large amount of reactive oxygen to induce oxidative stress, and selectively kills tumor cells. The compound can be used as an anti-cancer drug or a lead compound for further development. The method of the present invention has the characteristics of greenness, environmental protection, easily available raw materials, simple operation, and high yield.
Owner:XINXIANG MEDICAL UNIV
External preparation for treating eczematous dermatitis and preparation method thereof
InactiveCN109464621AHigh transdermal efficiencyDosage stableAntibacterial agentsHeavy metal active ingredientsRare-earth elementAllergic dermatitis
The invention relates to the technical field of preparations, in particular to an external preparation for treating eczematous dermatitis and a preparation method thereof. The external preparation adopts a rare earth element, glycyrrhizic acid, astaxanthin, beta-cyclodextrin and a cooling agent as main components. The components of the external preparation play a synergistic effect, so that the external preparation not only effectively exerts an effect of treating dermatitis of the rare earth element and glycyrrhizic acid and stably exerts an antioxidant effect of astaxanthin, but also has theadvantages of high transdermal efficiency, stable dosage form and the like. The external preparation provided by the invention has a plurality of prevention and treatment effects such as an antiallergic effect, an anti-itching effect and an anti-inflamed effect, has an obvious itching relieving effect, and can be used for treating dermatitis such as eczema, allergic dermatitis, contact dermatitisand psoriasis by inflammation diminishing, itching relieving, sterilization and the like. The preparation method of the external preparation provided by the invention has simple steps, no in vitro cytotoxicity, and is safe and effective, and controllable in quality.
Owner:GUANGXI XINYE BIOLOGICAL TECH
Method for testing in vitro cytotoxicity of cigarette smoke
ActiveCN102618619BWide linear rangeHigh sensitivityMicrobiological testing/measurementBiotechnologyStaining
The invention discloses a method for testing in vitro cytotoxicity of cigarette smoke. The method is characterized by comprising the following steps of: 1) preparing a laboratory reagent; 2) performing cell inoculation culture; 3) performing cigarette smoke contamination; 4) dyeing with water-soluble tetrazolium-1 (WST-1); and 5) obtaining a result and analyzing. Compared with the prior art, the method is characterized in that a test step for washing cells for multiple times is eliminated in the whole test process, so that the method is easy and convenient to operate; a step of replacing a nutrient solution is not required during dyeing with the WST-1, and a diluted nutrient solution can be directly added for reacting; formazan produced by the WST-1 is water-soluble, so that a subsequent dissolution step is eliminated; and absorbance detection can be finished in 2 hours after WST-1 dyeing, so that the test period is shortened, and the method is quick in comparison with the conventional testing method. The measuring method is quick and convenient to operate, and also has the advantages of high sensitivity and stable result; and the method can be applied to smoke cytotoxicity test of multiple cell lines.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
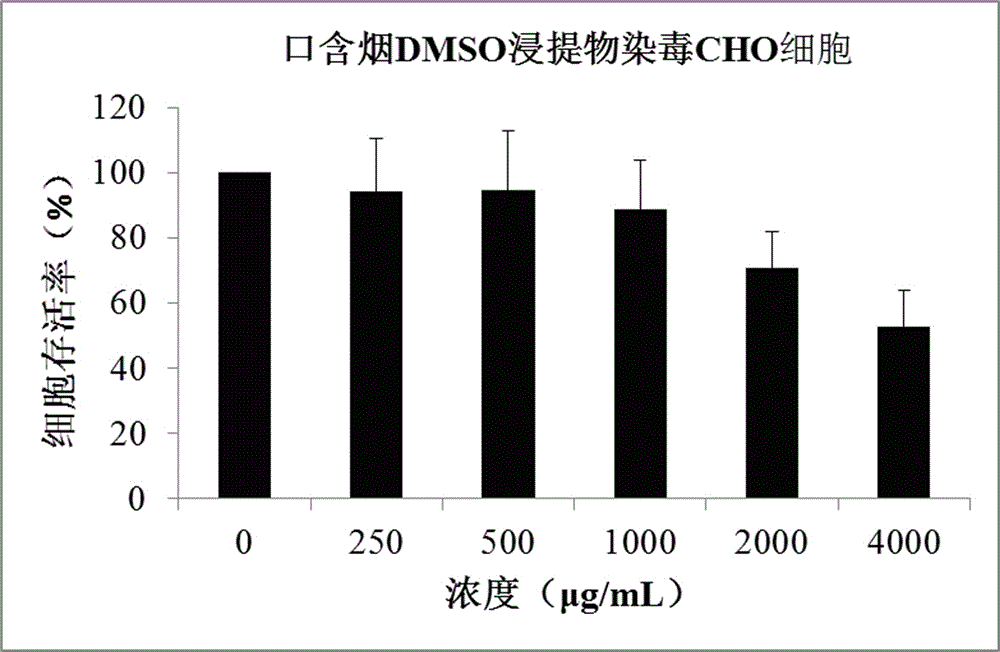
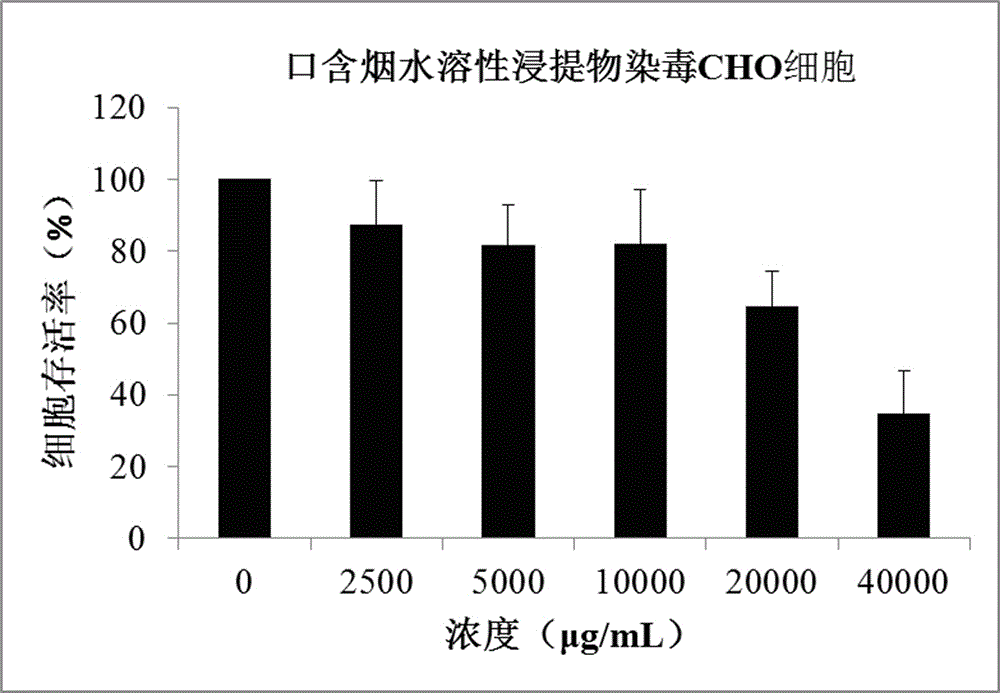
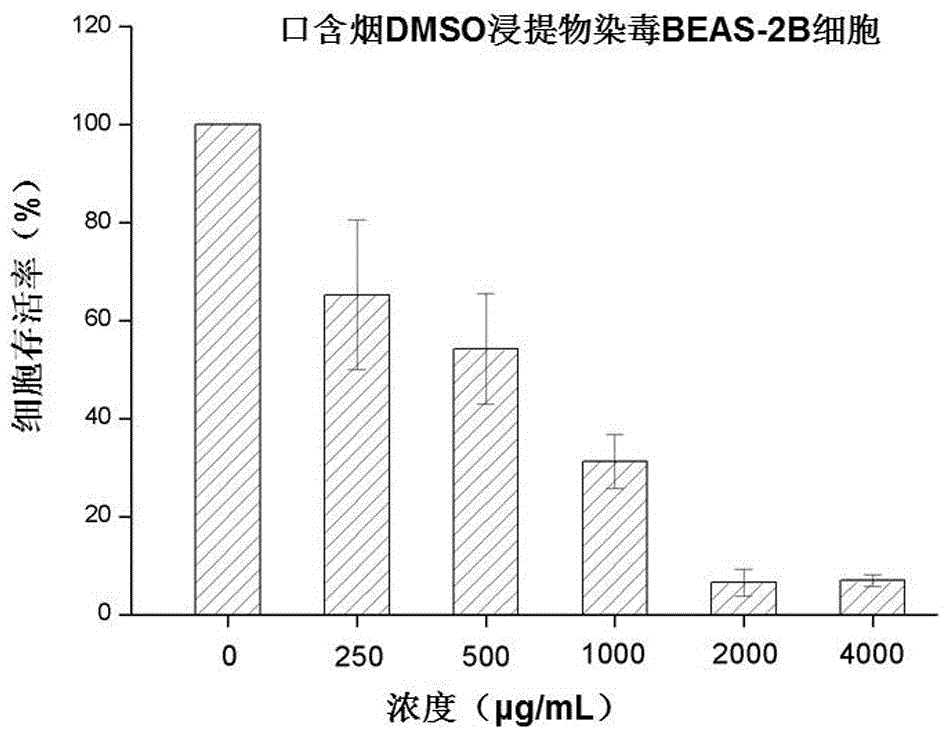
Method for testing in-vitro cytotoxicity of mouth cigarette extract
InactiveCN106191198ALow toxicityGood repeatabilityMicrobiological testing/measurementMaterial analysisContinuous lightCell culture media
A method for testing in-vitro cytotoxicity of mouth cigarette extract comprises the following steps that 1, the mouth cigarette extract is prepared; 2, cell inoculation culture is carried out; 3, the mouth cigarette extract is subjected to contamination; 4, a CCK-8 dyeing experiment is carried out; 5, a result is obtained, and analysis is carried out. The method is characterized in that in-vitro cytotoxicity testing of mouth cigarettes is established for the first time, when testing is carried out, the step of washing cells many times is omitted, and the experiment process is simpler and more convenient. A CCK-8 reagent can be directly added into a cell culture medium, no liquid replacement step is needed, the generated water-soluble formazan product can be directly subjected to light absorption value detection, a dissolving step before detection is omitted, and the experiment period and time are shortened; continuous light absorption value detection can be carried out many times, no additional experiment step needs to be added, and convenience is provided for looking for a proper detecting time point; the toxicity of the CCK-8 reagent on the cells is small, the influence of the background and experiment errors are avoided, and the testing result is good in repeatability and high in stability and sensitivity.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
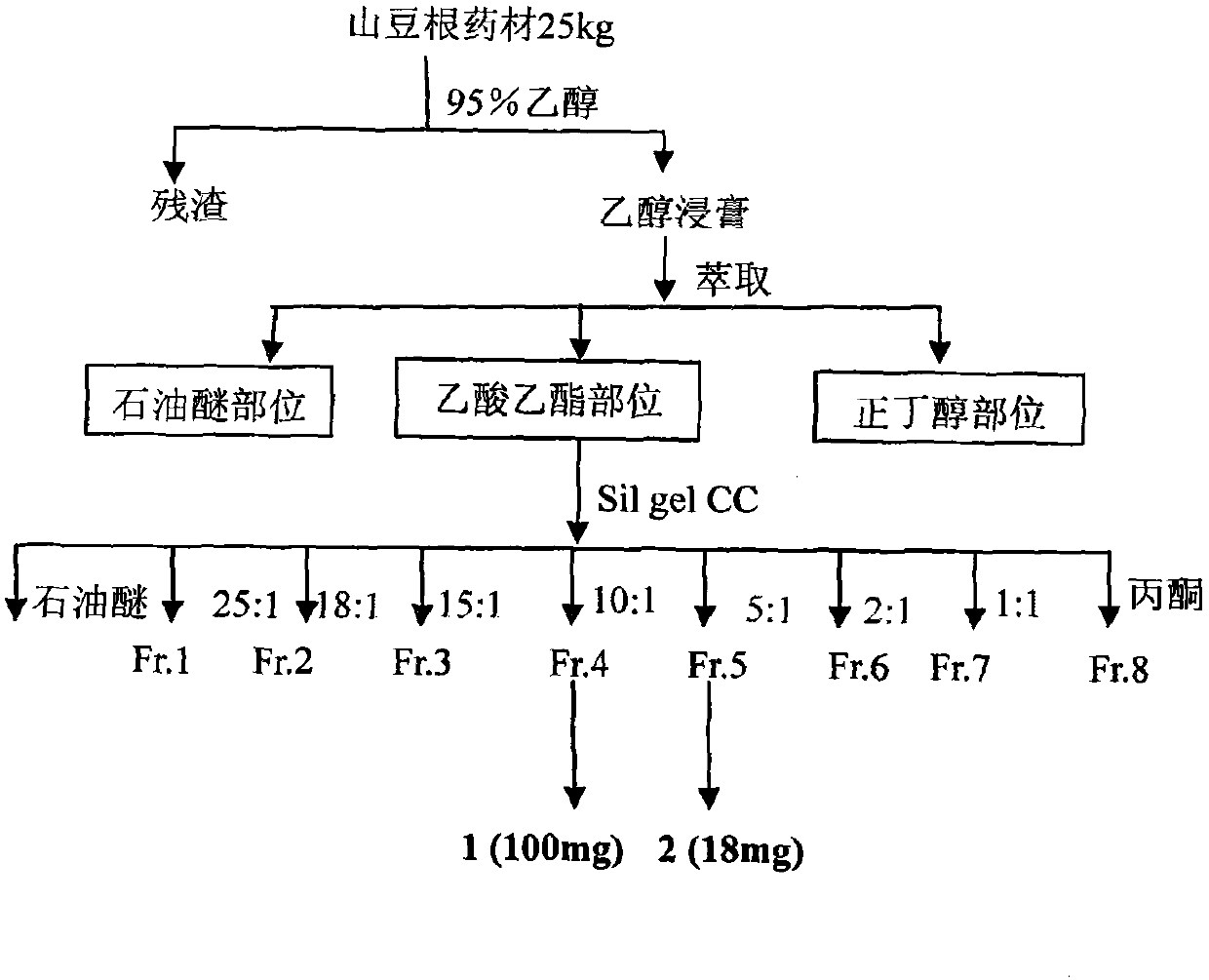
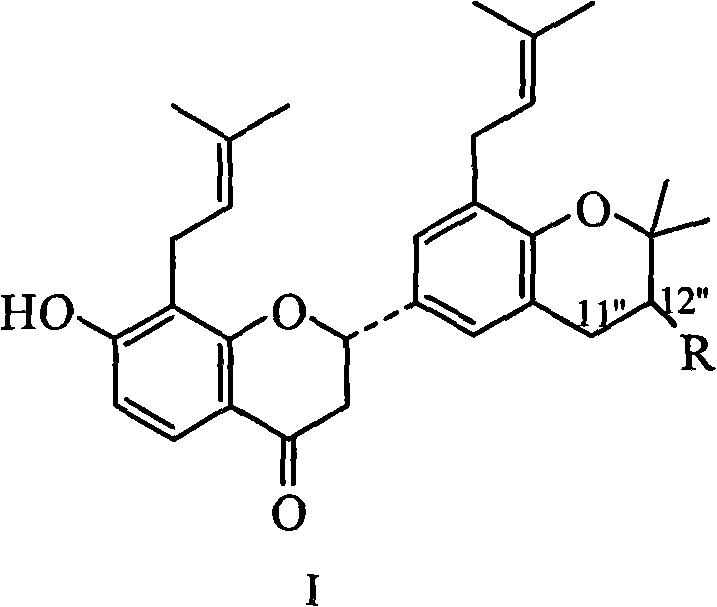
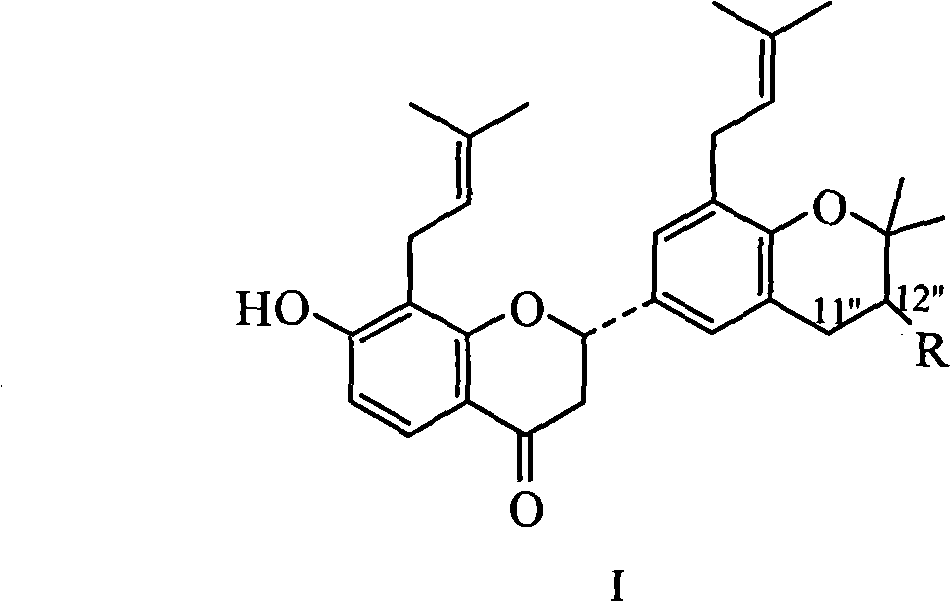
Prenylflavanone compound and use thereof in preparation of anti-tumor medicaments
InactiveCN102100692AOrganic active ingredientsAntineoplastic agentsProstate cancer cellSophora tonkinensis
The invention belongs to the pharmaceutical field of traditional Chinese medicines and relates to a prenylflavanone compound shown formula 1 and a new use thereof in preparation of anti-tumor medicaments. The flavonoid compound is extracted from a Sophora tonkinensis ethyl acetate part and proved to have stronger inhibition effect for tumor cell lines of lung cancer, prostatic cancer, nasopharyngeal cancer or colorectal cancer through an in vitro cytotoxicity screening test, wherein the compound comprises 5.41-7.19 mu g / mL of GI50 which has the inhibition effect on the cells of the nasopharyngeal cancer, 6.48-7.21 mu g / mL of the GI50 which has the inhibition effect on the cells of the lung cancer, 5.10-5.15 mu g / mL of the GI50 which has the inhibition effect on the cells of the prostatic cancer and 7.52-14.81 mu g / mL of the GI50 which has the inhibition effect on the cells of the colorectal cancer. The compound can be further used for preparing the anti-tumor medicaments for clinical treatment of the lung cancer, the prostatic cancer, the nasopharyngeal cancer or the colorectal cancer.
Owner:FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com