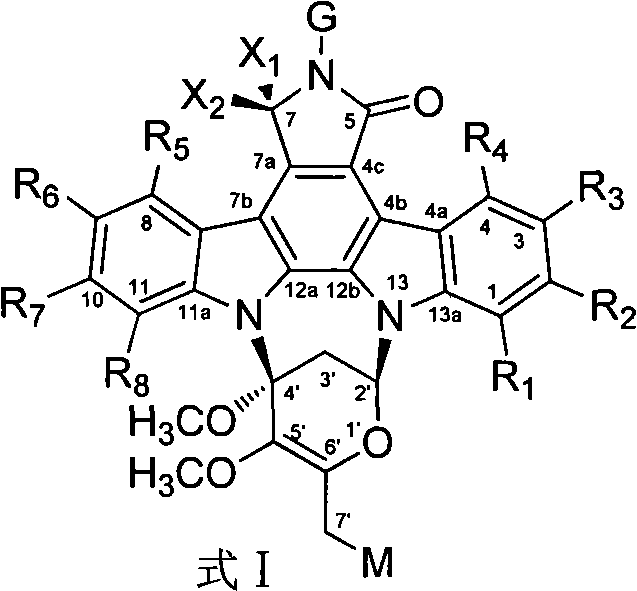
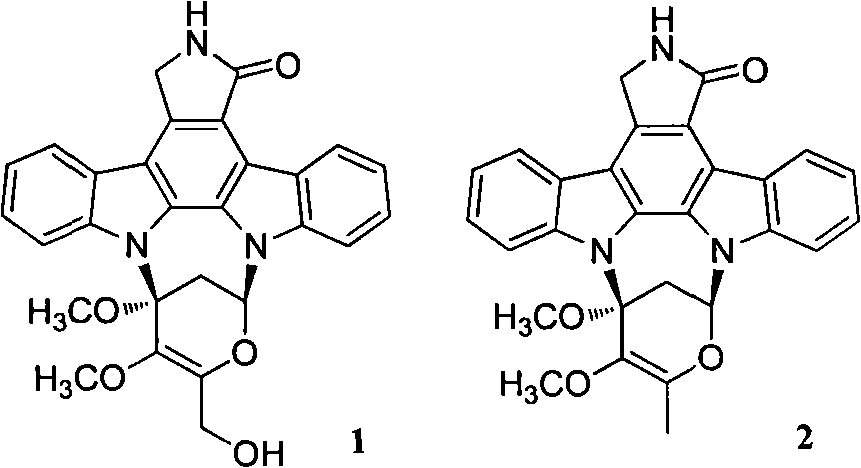
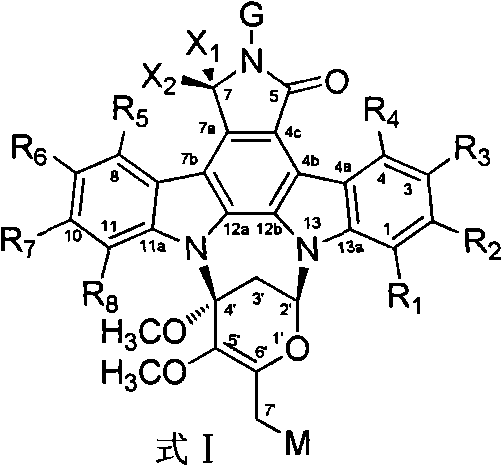
Novel staurosporine analogue and preparation method and applications thereof
A technology of staurosporine and analogues, which is applied in the field of a novel class of staurosporine analogues and their preparation and application, and can solve problems such as drugs that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] [Example 1] Fermentative production and separation and purification of compounds 1 and 2
[0052] 1 Fermentation production
[0053] According to the conventional method for cultivating microorganisms, take an appropriate amount of Streptomyces sp.FMA (CCTCC M 2010021), and inoculate it into 20 mL of seed medium (the medium consists of 0.2% soluble starch, 1.5% soybean powder, 0.5% yeast extract powder, CaCO 3 0.4%, peptone 0.2%, NaCl 0.4%, pH 7.2-7.4, prepared by artificial seawater) in a 30mm×200mm test tube, carry out seed culture on a rotary shaker (28°C, 200rpm, tilt 45°); after one week, take The seed culture was inoculated into a plurality of 1000 mL Erlenmeyer flasks containing 200 mL of fermentation medium (composition of the medium as above) at a 5% inoculum size, and fermented under the same conditions as the seed culture. The shaker was cultivated for 7 days, and a total of about 100 L of fermentation liquid was obtained.
[0054] 2 Obtaining the extract ...
Embodiment 2
[0063] [Example 2] Test of cell growth inhibitory activity
[0064] 1 test method
[0065] Preparation of the test sample solution: the test samples are the monomer compounds 1 and 2 synthesized in the above-mentioned Example 1. Accurately weigh an appropriate amount of sample and prepare a solution with the required concentration with DMSO for activity testing.
[0066] Cell lines and subculture of cells: HL-60 and A549 cell lines were used for activity testing. All kinds of cells were subcultured in RPMI-1640 medium containing 10% FBS in an incubator with 5% carbon dioxide at 37°C.
[0067] Cell Proliferation Inhibitory Activity Test Method
[0068] The invention uses the SRB method to test and evaluate the inhibitory activity of the tested samples on the proliferation of A549 and HL-60 cancer cells. SRB is a protein-binding dye that can bind to basic amino acids in biological macromolecules. The OD reading of the combined product at a wavelength of 515nm has a good line...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com