Cis-dammine dichloroplatinum prodrug, preparation method and application
A cisplatin and drug technology, applied in the field of medicinal chemistry and preparation, can solve the problems of poor water solubility of cisplatin, poor drug stability, low maximum tolerated dose, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 2
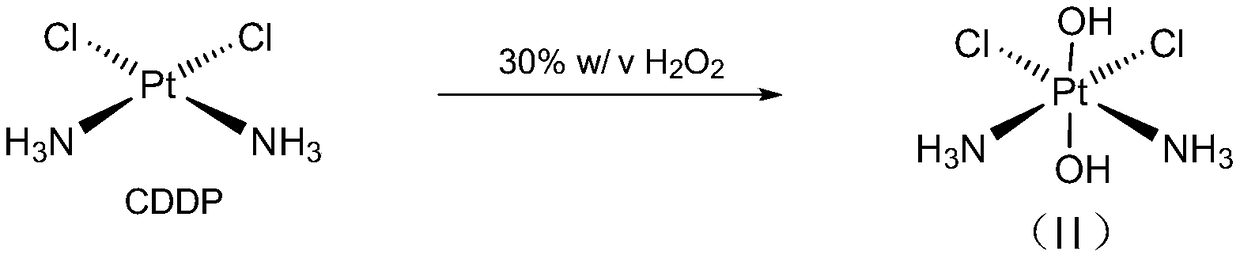
[0060] The synthesis of embodiment 1 dihydroxy diamine dichloroplatinum II, such as figure 1 Shown:
[0061] A 100 mL round bottom flask was charged with CDDP (1 g, 3.33 mmol) and 15 ml of 30% hydrogen peroxide (30%, w / v). Stir at 75°C in the dark for 5 hours, cool at 4°C for 2 hours, filter under reduced pressure to obtain light yellow crystals, wash with water, ethanol and ether, and dry to obtain product II (998 mg, yield 90%).
Embodiment 2
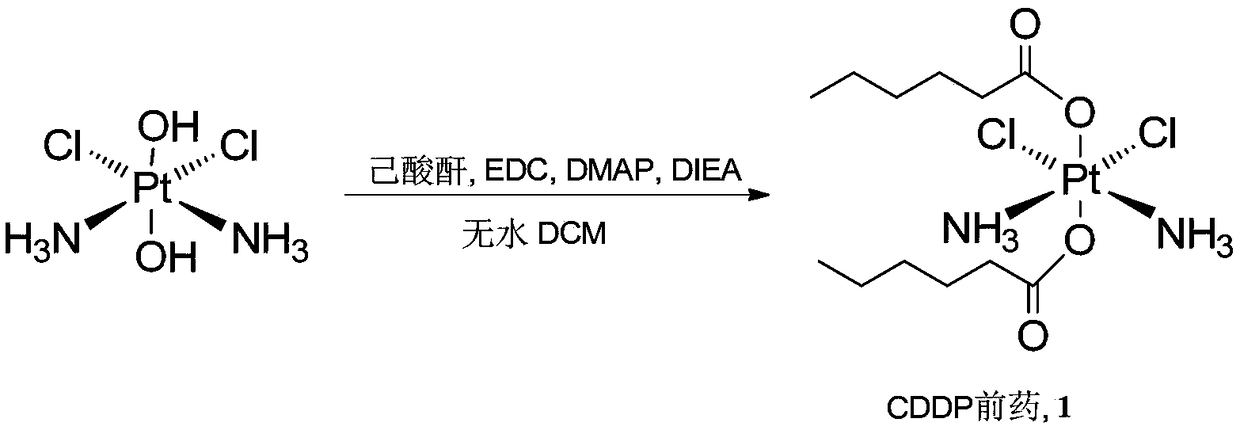
[0062] Example 2 Synthesis of CDDP prodrug 1 containing saturated alkane chain compound, such as figure 2 Shown:
[0063] Add II (100mg, 0.3mmol) and n-hexanoic anhydride (127mg, 0.6mmol) to a 100mL round bottom flask, dissolve in (DMF), and add N,N'-dicyclohexylcarbodiimide (0.3mmol). Stir at 70°C for 3 hours. After the reaction, add water to precipitate a light yellow solid; then wash with water and petroleum ether, and filter to obtain product 1 (95 mg, yield 60%).
[0064] product 1 1 The H NMR nuclear magnetic data is as follows:
[0065] 1 H NMR(400MHz,DMSO-d6):δ0.84-0.88(t,6H),1.23-1.27(m,8H),1.42-1.51(m,4H),2.16-2.22(m,4H),6.38- 6.63 (m, 6H).
Embodiment 3
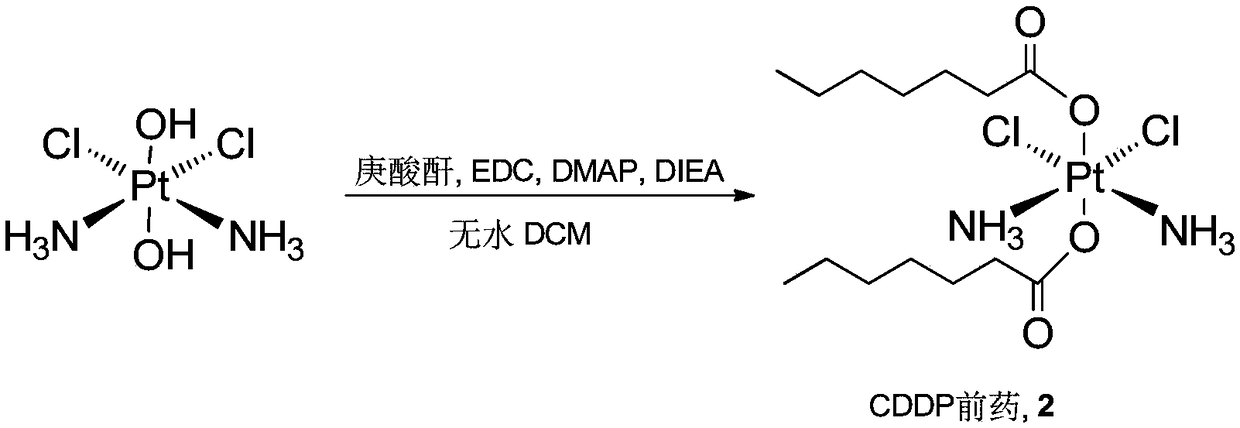
[0066] Example 3 Synthesis of CDDP prodrug 2 containing saturated alkane chain compound, such as image 3 Shown:
[0067] In a 100 mL round bottom flask, add II (100 mg, 0.3 mmol) and n-heptanoic anhydride (146 mg, 0.6 mmol), dissolve in 2 mL of anhydrous dimethylformamide (DMF), add 1-(3-dimethylaminopropyl )-3-Ethylcarbodiimide (0.3 mmol). Stir at 70°C for 3 hours, add water to precipitate after the reaction to obtain a yellowish solid; then wash with water and petroleum ether, and filter to obtain product 2 (109 mg, yield 65%).
[0068] product 2 1 The H NMR nuclear magnetic data is as follows:
[0069] 1 H NMR(400MHz,DMSO-d6):δ0.83-0.88(t,6H),1.21-1.25(m,12H),1.41-1.50(m,4H),2.17-2.22(m,4H),6.39- 6.63 (m, 6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com