Ortho-naphthaquinone derivative, and preparation method and medicinal application thereof
A technology of derivatives, o-naphthoquinone, applied in the field of medicinal chemistry, to achieve the effects of novel structure, easy availability of raw materials and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
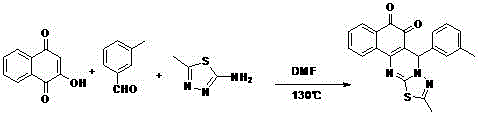
[0021] The synthesis of embodiment 1 compound
[0022] The reactants 1.74g 2-hydroxy-1,4-naphthoquinone, 1.20g 3-methylbenzaldehyde, 1.15g 5-methyl-2-amino-1,3,4-thiadiazole and 10mL dimethylformamide were added Into a 50mL round bottom flask, heat the reaction at 130°C for 5-8 hours. After the reaction was complete, the resulting mixture was cooled to room temperature, 50 mL of distilled water was added, precipitated and filtered, and purified by column chromatography to obtain the corresponding reddish-brown product 5-m-methylphenyl-2-methyl-5H-benzo[i] [1,3,4]Thiadiazolo[3,2-a]quinazoline-6,7-dione 1.16g, yield 31%.
[0023] 1 HNMR (400MHz, CDCl 3 )δ:8.37(d,1H,J=8.0Hz),8.10(d,1H,J=7.6Hz),7.72(t,1H,J=7.6Hz),7.56(t,1H,J=7.6Hz) ,7.29-7.10(m,4H),6.59(s,1H),2.54(s,3H),2.32(s,3H); 13 CNMR (100MHz, CDCl 3 )δ: 179.1, 175.9, 167.7, 154.2, 152.2, 140.2, 138.5, 134.6, 134.5, 131.1, 130.9, 129.7, 128.8, 128.7, 128.2, 126.7, 124.5, 111.8, 60.4, 21.5, 17.0 [M+Na] + :396.0773.
Embodiment 2
[0024] Embodiment 2 in vitro antitumor activity test
[0025] The antitumor activity of the target compounds was tested by MTT method. Human liver cancer cells HepG2 and colon cancer cells HCT116 were used as test cell lines, and adherent tumor cells in the logarithmic growth phase were selected, digested with trypsin, and formulated with RPMI1640 medium containing 10% calf serum to prepare 5000 cells / mL Cell suspension, seeded in 96-well culture plate, 200 μL per well, 37°C, 5% CO 2 Cultivate for 24h. Set up a negative control group, a positive control group and an administration group. The experimental group was replaced with new media containing different concentrations of tested samples, the control group was replaced with media containing an equal volume of solvent, and the positive control group was given the positive control drug doxorubicin (diluted with complete media to a concentration of 10 μmol L -1 ), set 3-5 parallel wells for each group, 37°C, 5% CO 2 Cultur...
Embodiment 3
[0026] Embodiment 3NQO1 activity test
[0027] 1mL reaction system contains 25mM Tris / HCl (pH7.4), 0.7mg / mL bovine serum albumin, 0.1% Tween-20, 200μM NADH, 77μM Cytochromec, 2μg recombinant human NQOl and 5-m-methylphenyl-2-methyl -5H-Benzo[i][1,3,4]thiadiazolo[3,2-a]quinazoline-6,7-dione (25 μΜ). Set the detection wavelength to 550nm, add NADH to start the reaction at room temperature, calculate the reduction rate from the initial linear part of the reaction curve, and convert the molar absorption coefficient of cytochrome c (21.1mM -1 cm -1 ), the results are expressed as μmol reduced cytochrome c / min / μgNQOl. The ability of the compound to generate active oxygen can be judged from the change of the amount of cytochrome c. The rate of the compound to generate active oxygen at the enzyme level is 1202±61 μmol reduced cytochrome c / min / μgNQOl.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com