A stable and safe idarubicin pharmaceutical composition and preparation method thereof
A technology of idarubicin and compounds, applied in the field of stable idarubicin pharmaceutical composition and its preparation, can solve problems affecting drug stability, drug efficacy or side effects, and achieve increased cardiotoxicity and good stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
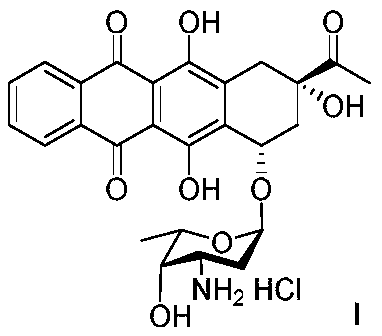
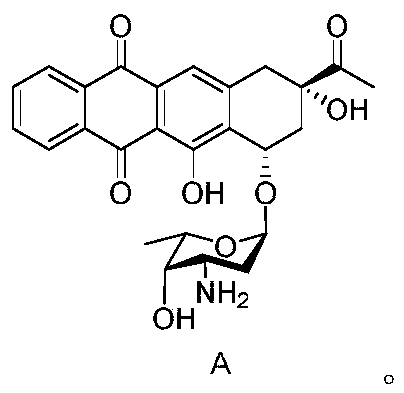
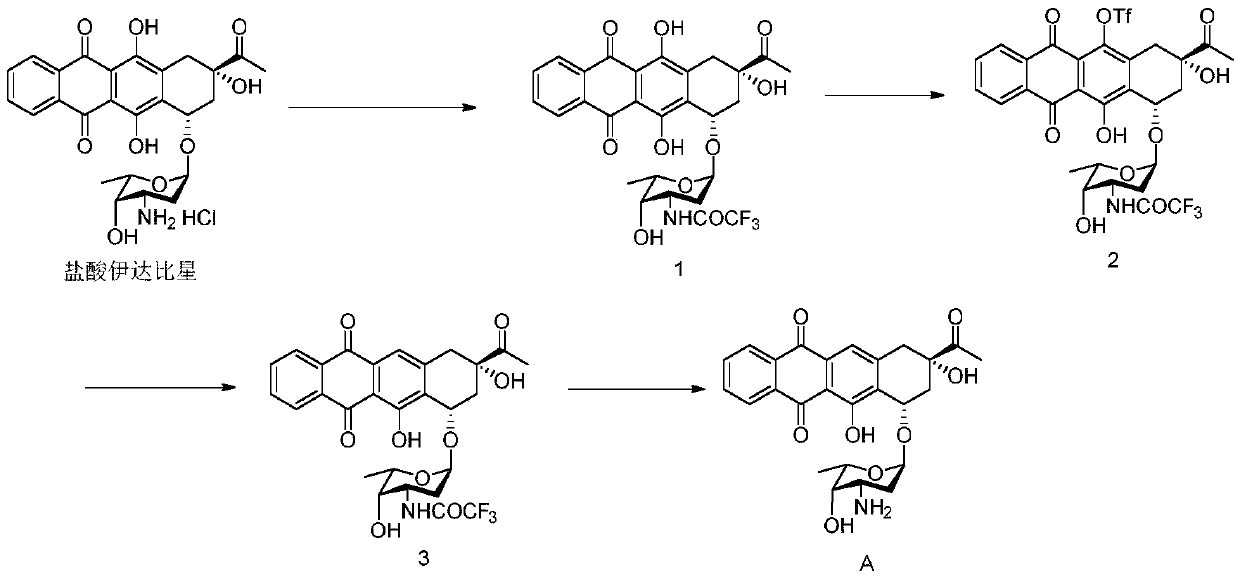
[0031] The preparation of embodiment 1 impurity A (hydrochloride)
[0032] (1) Preparation of compound 1
[0033] Dissolve 10 g of idarubicin hydrochloride in 100 ml of dichloromethane, add 10 g of triethylamine and 7.5 g of ethyl trifluoroacetate, and stir at room temperature for 4 h; add 100 ml of dichloromethane to dilute the reaction solution, wash twice with water, spin dry the organic phase, 10.4 g of solid was obtained (yield 94%, HPLC purity 97%).
[0034] (2) Preparation of Compound 2
[0035] Under anhydrous and oxygen-free conditions, 8g of compound 1 was dissolved in anhydrous dichloromethane (80ml), and trifluoromethanesulfonic anhydride (2eq) and triethylamine (2.2eq) were added under ice-cooling conditions, and reacted for 2 hours. Dilute the reaction solution with 80ml of methyl chloride, wash twice with water, and spin dry the organic phase to obtain 8.1g of solid (83% yield)
[0036] (3) Preparation of Compound 3
[0037] Dissolve 7g of compound 2 in diox...
Embodiment 2
[0040] Example 2 Toxicity studies of impurity A
[0041] Select 30 adult female Wistar rats, randomly divide them into blank group, positive drug group, and impurity A group, 10 rats in each group, administer once a week, and collect serum to detect brain natriuretic peptide (BNP) after 6 weeks of continuous administration and troponin I (cTnI) levels. Impurity A is prepared from Example 1. Idarubicin hydrochloride is self-made by the company, and its HPLC purity is 99.91%. The result is as follows:
[0042]
[0043] *P<0.05
[0044] It can be seen from the above table that compared with the blank group, the BNP and cTnl contents of the positive drug group and the impurity A group were significantly increased, and the value of the impurity A group was higher than that of the positive drug group, and the results were statistically different. This shows that both idarubicin and impurity A have certain cardiotoxicity, and the cardiotoxicity of impurity A is greater than th...
Embodiment 3
[0045] Example 3 Stability investigation
[0046] Composition samples with different contents of impurity A were subjected to accelerated test investigation under the conditions of temperature 40±2°C and relative humidity 75%±5%, left for 30 days, and samples were taken on day 0, day 10 and day 30, Investigate changes in product appearance and purity. The compositions whose mass ratios of idarubicin hydrochloride and impurity A are 1:0.2%, 1:0.3%, 1:0.4%, 1:0.5%, 1:0.6% are compositions 1, 2, 3, 4, 5. The purity of idarubicin hydrochloride is 99.9%, impurity A is prepared by embodiment 1. The results are shown in the table below:
[0047]
[0048]
[0049] The above results show that when the content of impurity A in the composition does not exceed 0.3%, idarubicin hydrochloride can maintain high stability, and when the content of impurity A reaches 0.4%, 0.5%, and 0.6%, the color of the product gradually becomes darker , the content of other impurities increases, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com