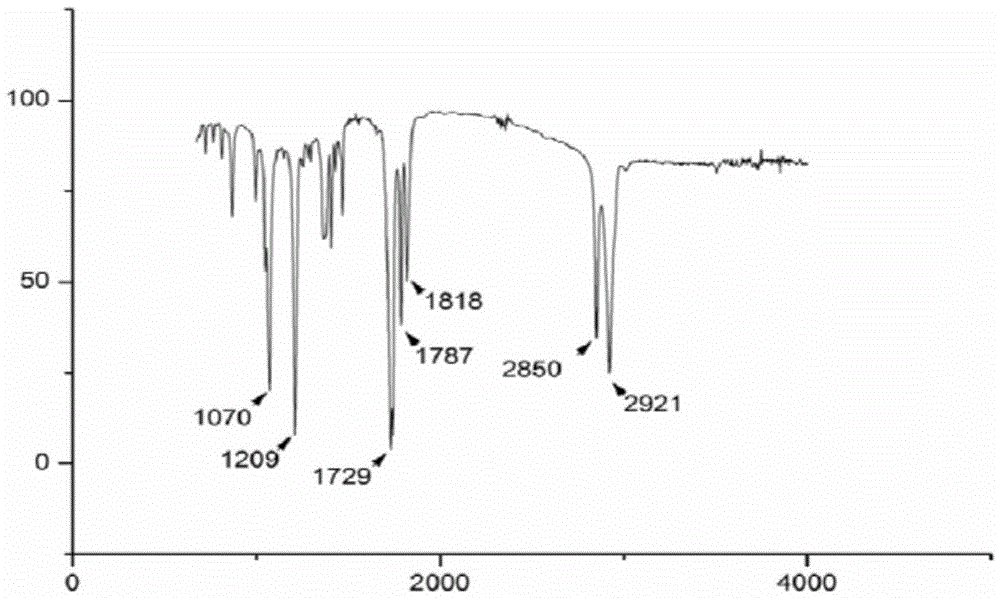
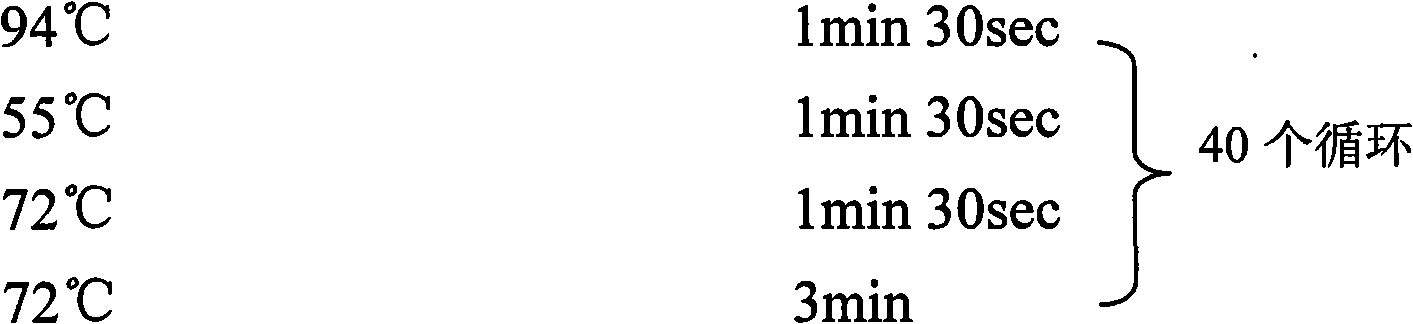Patents
Literature
52 results about "Lyme disease" patented technology
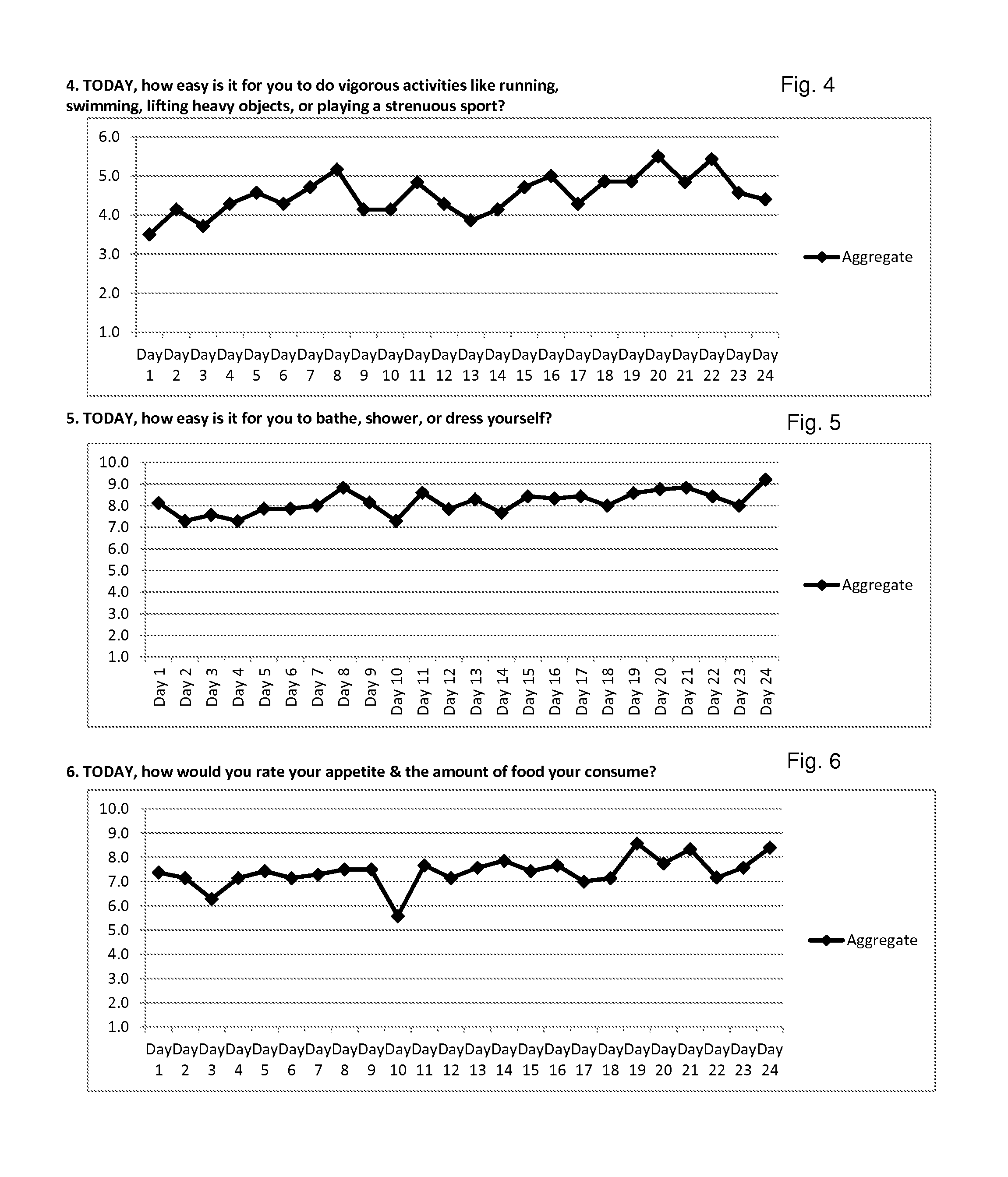
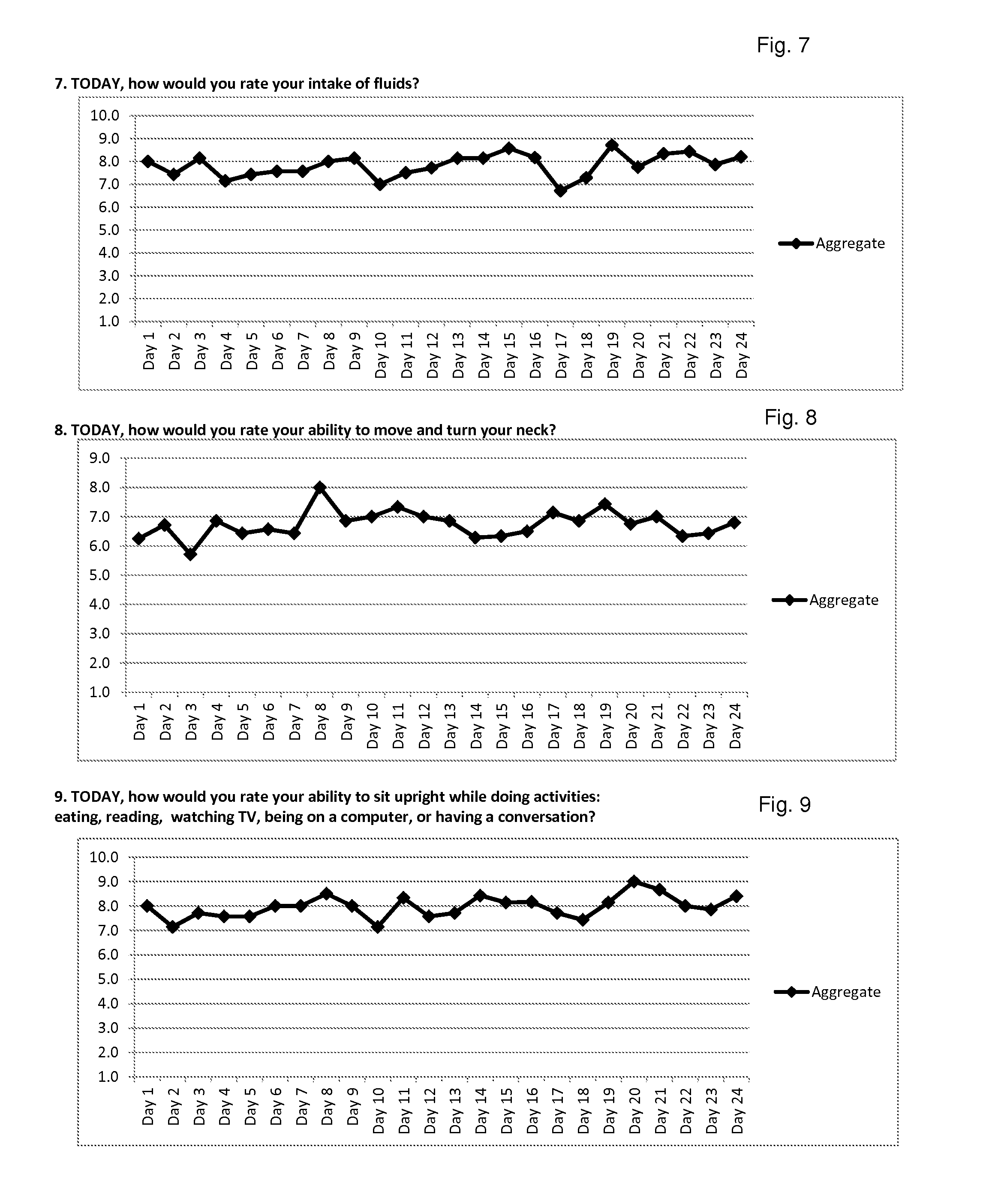
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A tick-borne disease caused by bacteria Borrelia burgdoferi.
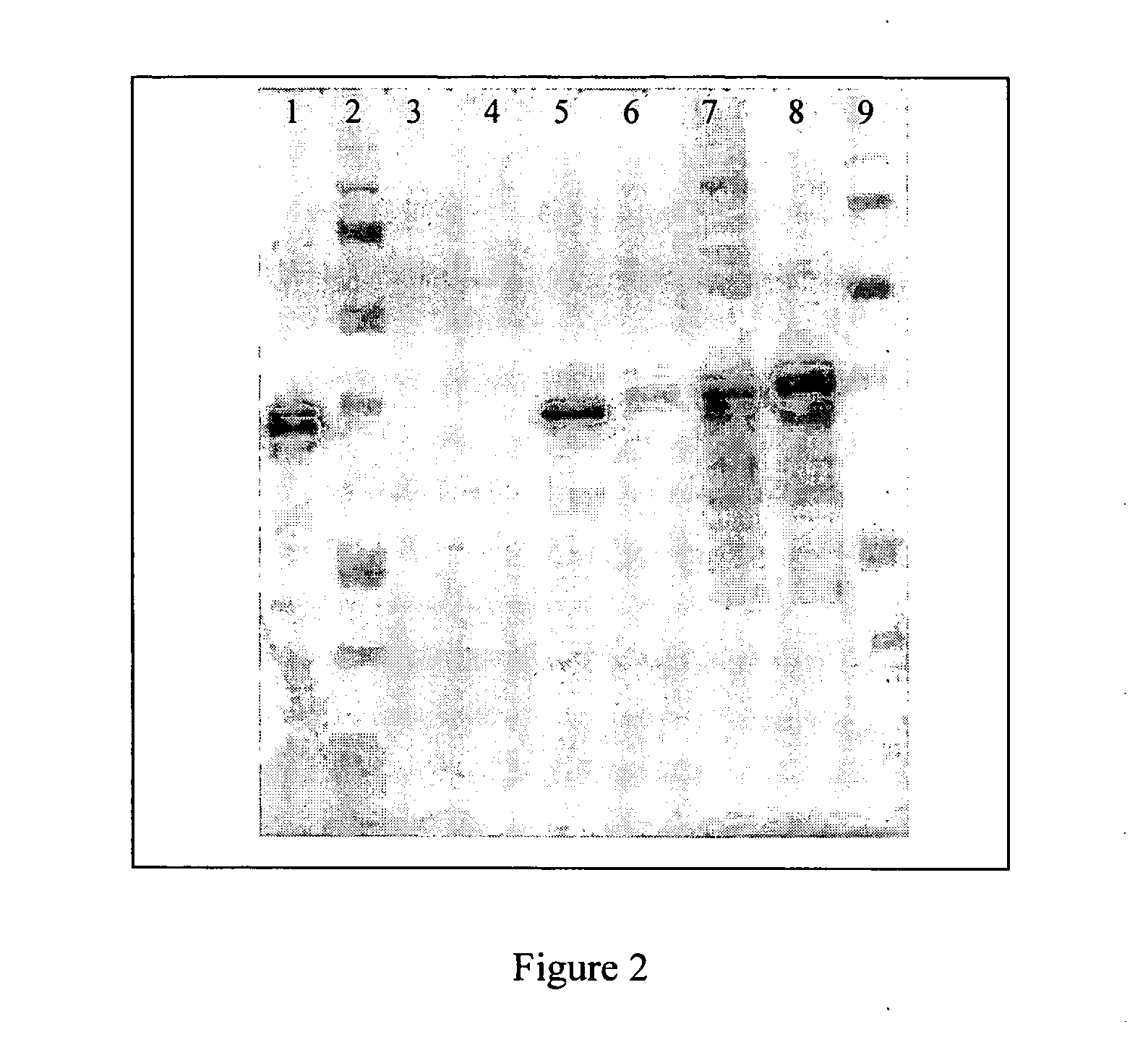
Lyme combination compositions and uses
InactiveUS6368603B1Safe and efficacious in dogNo exacerbation of diseaseAntibacterial agentsNanotechAntigenRabies
Disclosed and claimed are compositions containing a Borrelia burgdorferi antigen, and methods for making and using them. The antigen can be OspA. The compositions can contain at least one additional antigen from a pathogen other than Borrelia burgdorferi. The compositions are useful for eliciting an immunological response in a host mammal susceptible to Lyme Disease and to the mammalian pathogen other than Borrelia burgdorferi. Suitable host mammals include dogs, pups, horses, and, the additional antigen can be of a canine, equine or feline pathogen, such as rabies, canine distemper, adenovirus, coronavirus, parainfluenza and parvovirus. No significant efficacy interference is observed.
Owner:MERIAL LTD
TH2-specific gene
InactiveUS6190909B1Increase the number of cellsEffective in number of cellOrganic active ingredientsFungiContact dermatitisTransgene
The present invention relates to the discovery, identification and characterization of nucleic acids that encode a novel protein differentially expressed within the TH2 cell subpopulation (hereinafter referred to as STIF). The invention encompasses STIF nucleotides, host cell expression systems, STIF proteins, fusion proteins, polypeptides and peptides, antibodies to the STIF protein, transgenic animals that express a STIF transgene, or recombinant knock-out animals that do not express the STIF protein, and compounds that modulate STIF gene expression or STIF activity that can be used for diagnosis, drug screening, clinical trial monitoring, and / or used to treat STIF based disorders, such as proliferative disorders and T-lymphocyte-related disorders including, but not limited to, chronic inflammatory diseases and disorders, such as Crohn's disease, reactive arthritis, including Lyme disease, insulin-dependent diabetes, organ-specific autoimmunity, including multiple sclerosis, Hashimoto's thyroiditis and Grave's disease, contact dermatitis, psoriasis, graft rejection, graft versus host disease, sarcoidosis, atopic conditions, such as asthma and allergy, including allergic rhinitis, gastrointestinal allergies, including food allergies, eosinophilia, conjunctivitis, glomerular nephritis, certain pathogen susceptibilities such as helminthic (e.g., leishmaniasis) and certain viral infections, including HIV, and bacterial infections, including tuberculosis and lepromatous leprosy.
Owner:MILLENNIUM PHARMA INC
Diagnostic markers for neuropsychiatric disease
InactiveUS20130237454A1ConfidenceLibrary screeningDisease diagnosisNeuropsychiatric diseasePsychogenic disease
Biomarkers for the diagnosis of neuropsychiatric diseases are presented herein. In particular embodiments, biomarkers are identified that are useful for diagnosing multiple sclerosis, chronic fatigue syndrome, or Neurologic Lyme disease. Also encompassed is a method for diagnosing a patient with a neuropsychiatric disease, such as multiple sclerosis, chronic fatigue syndrome, or Neurologic Lyme disease, by analyzing biological samples isolated from the patient or the patient as a whole to assess levels of the biomarkers described herein.
Owner:SCHUTZER STEVEN E
Herbal remedy for treating Lyme disease
The present invention is directed to a composition for the treatment of Lyme disease, comprising: Uncaria tomentosa (Cat's Claw); Pau d'arco; Scutellaria baicalensis (Baikal Scullcap); Artemisinin; and Sambucus nigra (Elderberry).
Owner:BROWN PAUL R
Live bacterial vaccine
The present invention relates, e.g., to a Lactobacillus bacterium, which (1) expresses a recombinant polypeptide containing a lipoprotein signal sequence from the OspA protein of Borrelia burgdorferi, or an active variant of the leader sequence, operably linked to one or more heterologous polypeptide(s) of interest and / or (2) which comprises an expressible polynucleotide encoding a recombinant polypeptide, wherein the polynucleotide encodes a lipoprotein signal from the OspA protein of Borrelia burgdorferi, or an active variant thereof, which is operably linked to one or more heterologous polypeptide(s) of interest. In one embodiment, the heterologous polypeptide is from Yersinia pestis, the etiologic agent of plague. In another embodiment, the heterologous polypeptide is from Borrelia burgdorferi, the etiologic agent of Lyme disease. Also described are immunogenic compositions, such as live bacterial vaccines, comprising the bacterium; methods for eliciting an immune response against the polypeptide using the bacterium; and kits comprising the bacterium.
Owner:LACTRYS OCTROOI +1
Topical antibiotic composition for the prevention of Lyme disease
The invention relates to topical pharmaceutical compositions and methods related to Borrelia burgdorferi toxins, in particular, the present invention provides compositions and methods for the treatment of infections caused by Borrelia burgdorferi and in particular for the prevention of Lyme disease.
Owner:IXODES
Method for detecting Lyme disease pathogen in tick bodies and kit
ActiveCN101886113AIncreased sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesSpiroplasmaElectrophoresis
The invention relates to a loop-mediated isotheral amplification method (LAMP) for detecting Lyme disease pathogen in tick bodies, which is characterized by taking 16S rRNA gene as the target gene, using special software to design the LAMP for detecting the primers of the Lyme disease pathogen-Borrelia burgdorferi in the tick bodies, then selecting the primer of the specific fragment of the Lyme disease pathogen from the primers, extracting DNA after piercing the ticks to be detected, uniformly mixing the obtained DNA and the reaction buffer, the reaction mixture of the selected primer of the specific fragment and DNA polymerase to carry out amplification, adding loading buffer after activating the amplified product, then placing the mixture into agarose gel containing ethidium bromide to carry out electrophoresis detection and detecting whether the detected ticks carry the Lyme disease pathogen according to whether the specific band occurs after electrophoresis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Artemisinin with Berberine Compositions and Methods of Making
ActiveUS20130102625A1Effective treatmentBiocideSolid materialProgressive multifocal leukoencephalopathyYellow fever
An all-natural herbal composition and methods of preparing the same are provided. The novel Artemisinin Combination Therapy (ACT) consists of artemisinin and its derivatives and berberine, the two active substances mixed with various selected excipient compounds to form a single pill, tablet or capsule for treatment and prevention of malaria, dengue fever, yellow fever, dysentery, Lyme disease, babesiosis, progressive multifocal leukoencephalopathy, Helicobacter Pylori, and colitis, in adults and children. A tablet or pill for children is formulated to be chewable.
Owner:U S PHYTOTHERAPY
Protein chip for lyme disease flagellin antigen immunoserology diagnosis and preparation method and application of protein chip
InactiveCN104374921AHigh sensitivityStrong specificityBiological testingAgainst vector-borne diseasesSerodiagnosesADAMTS Proteins
The invention discloses a protein chip for lyme disease flagellin antigen immunoserology diagnosis and a preparation method and application of the protein chip. The protein chip is characterized in that borrelia burgdorferi recombination flagellin antigen probes are fixed on the surface of a solid phase carrier in a dot matrix mode; the solid phase carrier is a 16-amino-1-hexadecyl mercaptan modified gold foil chip which is combined with 4-(N-maleinimide methyl) cyclohexane-1-carboxylic acid succinimide ester and 4-(dimethylamino) pyridine. The protein chip disclosed by the invention can accurately detect an anti-flagellin antigen IgG antibody and an anti-flagellin antigen IgM antibody in serums of lyme disease patients, the operation is simple, and the detection result is stable.
Owner:ANHUI MEDICAL UNIV
Detection test paper for quickly diagnosing Lyme disease, and preparation method thereof
InactiveCN109596824AShort detection timeMeet the needs of on-site testingMaterial analysisAgainst vector-borne diseasesBiologyLyme disease
The invention discloses detection test paper for quickly diagnosing the Lyme disease, and a preparation method thereof. A method for detecting the Lyme disease by ELISA (Enzyme Linked Immunosorbent Assay) is long in detection time and is not suitable for substrate detection. The colloidal gold immunochromatography detection test paper for quickly diagnosing the Lyme disease comprises a bottom plate, a sample cushion, a Jinbiao cushion, a nitrocellulose membrane and a water adsorption cushion, wherein the sample cushion, the Jinbiao cushion, the nitrocellulose membrane and the water adsorptioncushion are arranged and connected in sequence and are all arranged on the bottom plate; the nitrocellulose membrane is provided with a first detection line, a second detection line and a quality control line, wherein the first detection line is provided with a mouse anti-human IgM (Immunoglobulin M) monoclonal antibody, the second detection line is provided with a mouse anti-human IgG (Immunoglobulin G) monoclonal antibody, and the quality control line is provided with a goat anti-mouse IgG polyclonal antibody; and the Jinbiao cushion is provided with Lyme disease recombinant antigen-colloidal gold conjugate. The detection test paper has the advantages of short detection time, high detection accuracy, high specificity and convenience in operation, and does not need the help of other equipment instruments.
Owner:HANGZHOU ALLTEST BIOTECH
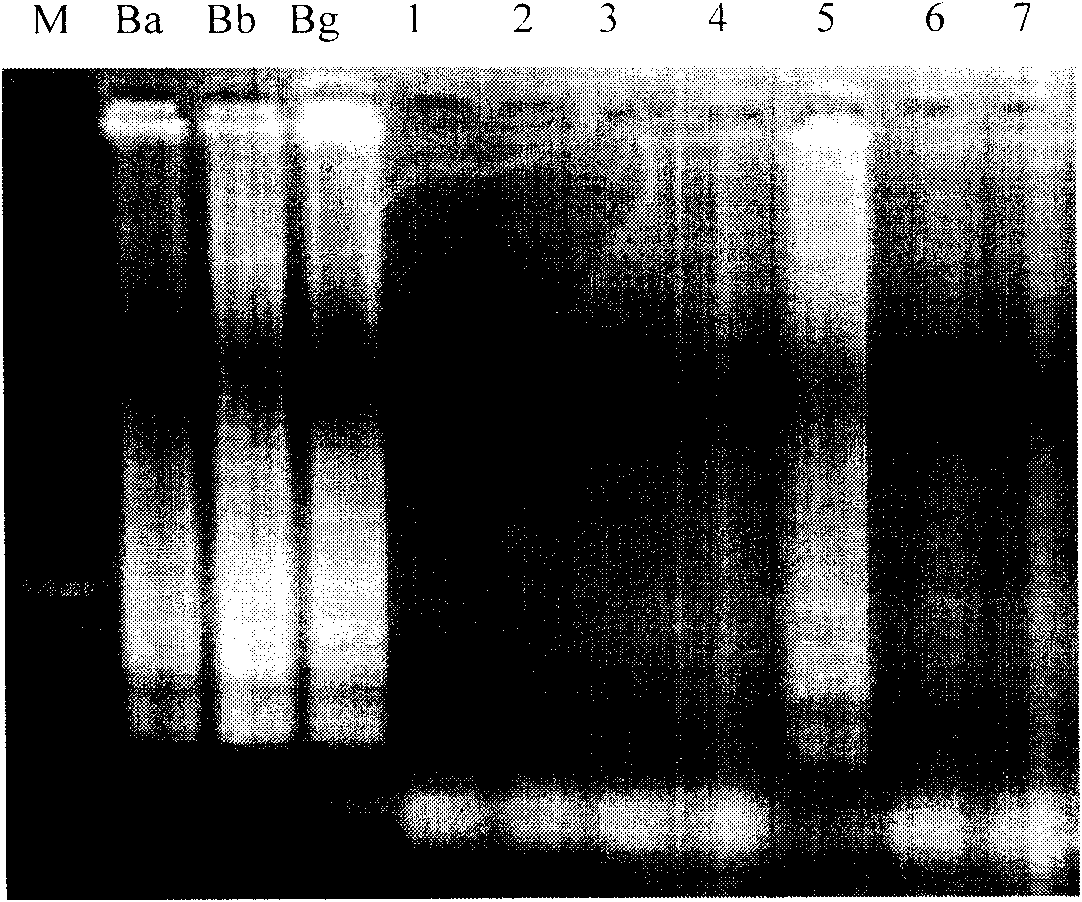
Oligonucleotides and methods for detecting Borrelia burgdorferi
InactiveUS20070172829A1Positive resistSugar derivativesMicrobiological testing/measurementBorrelia gariniiTest sample
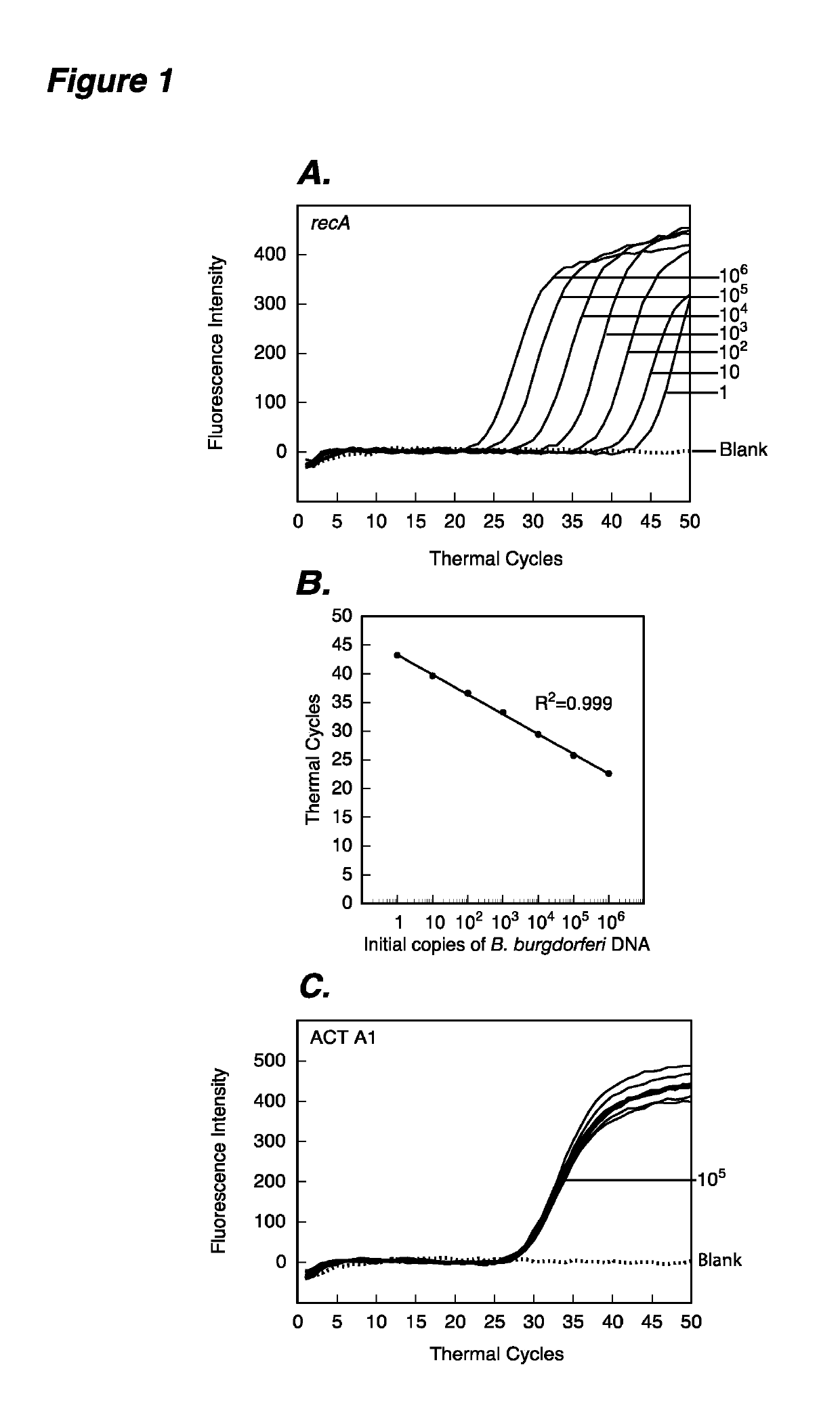
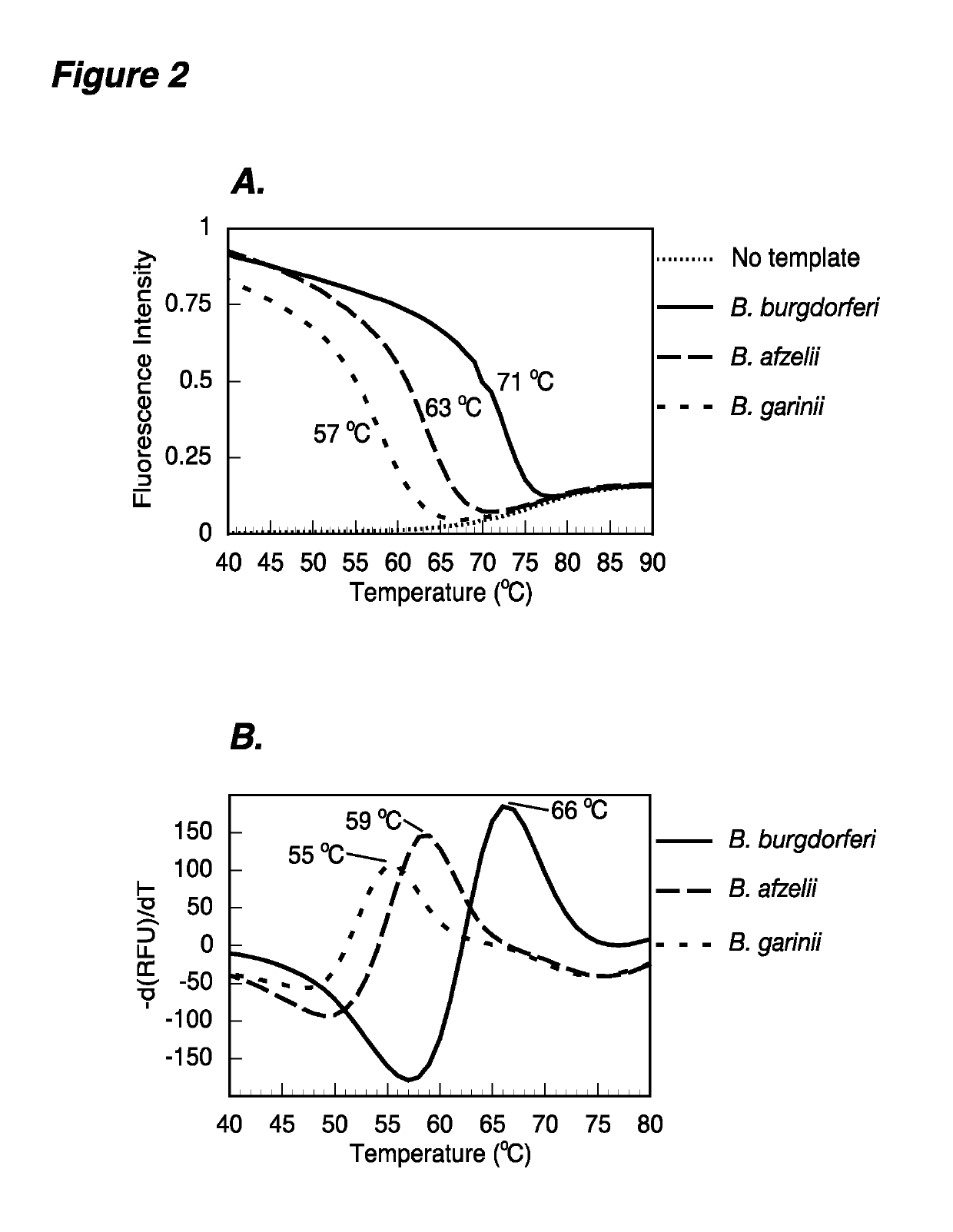
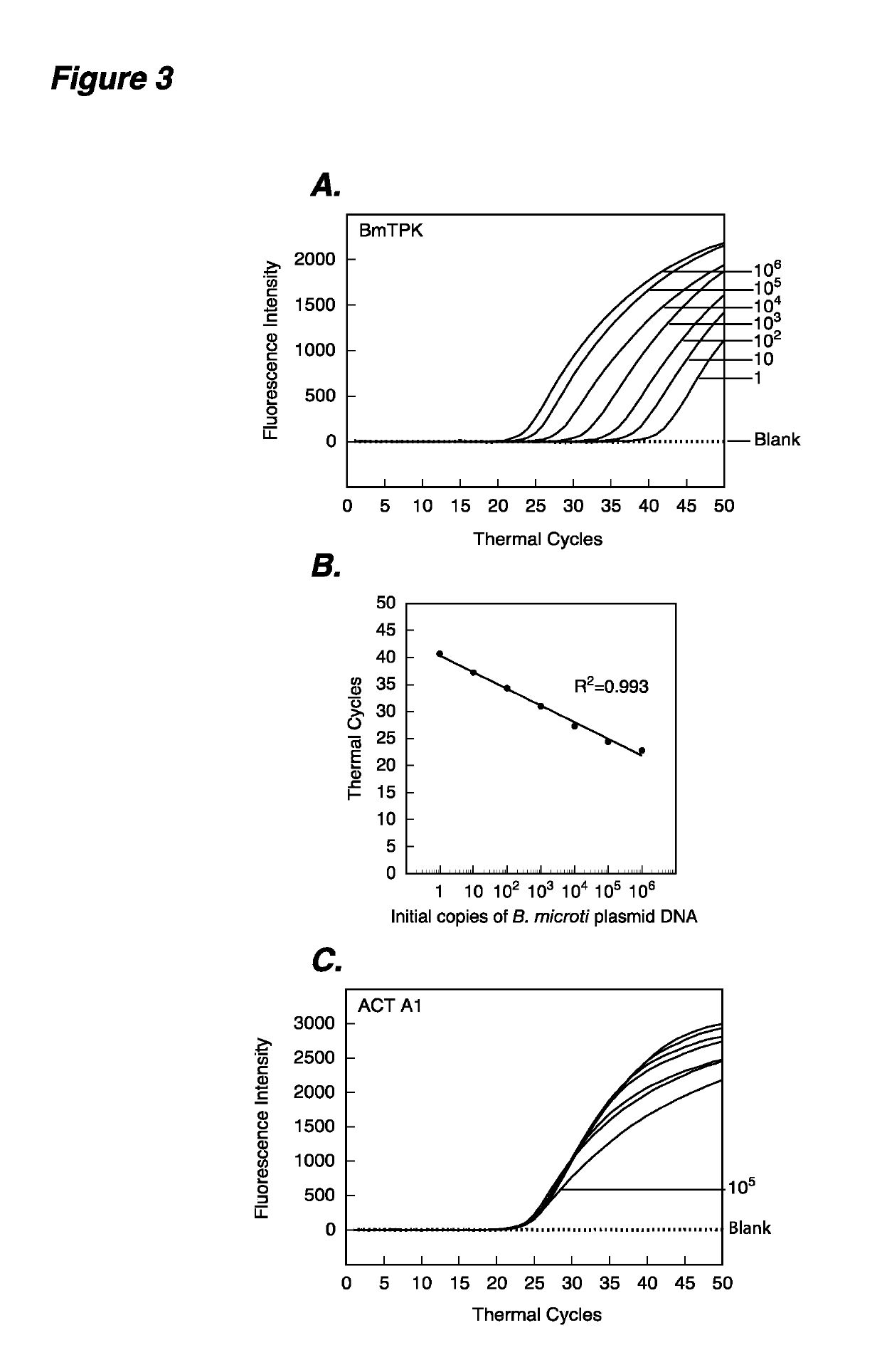
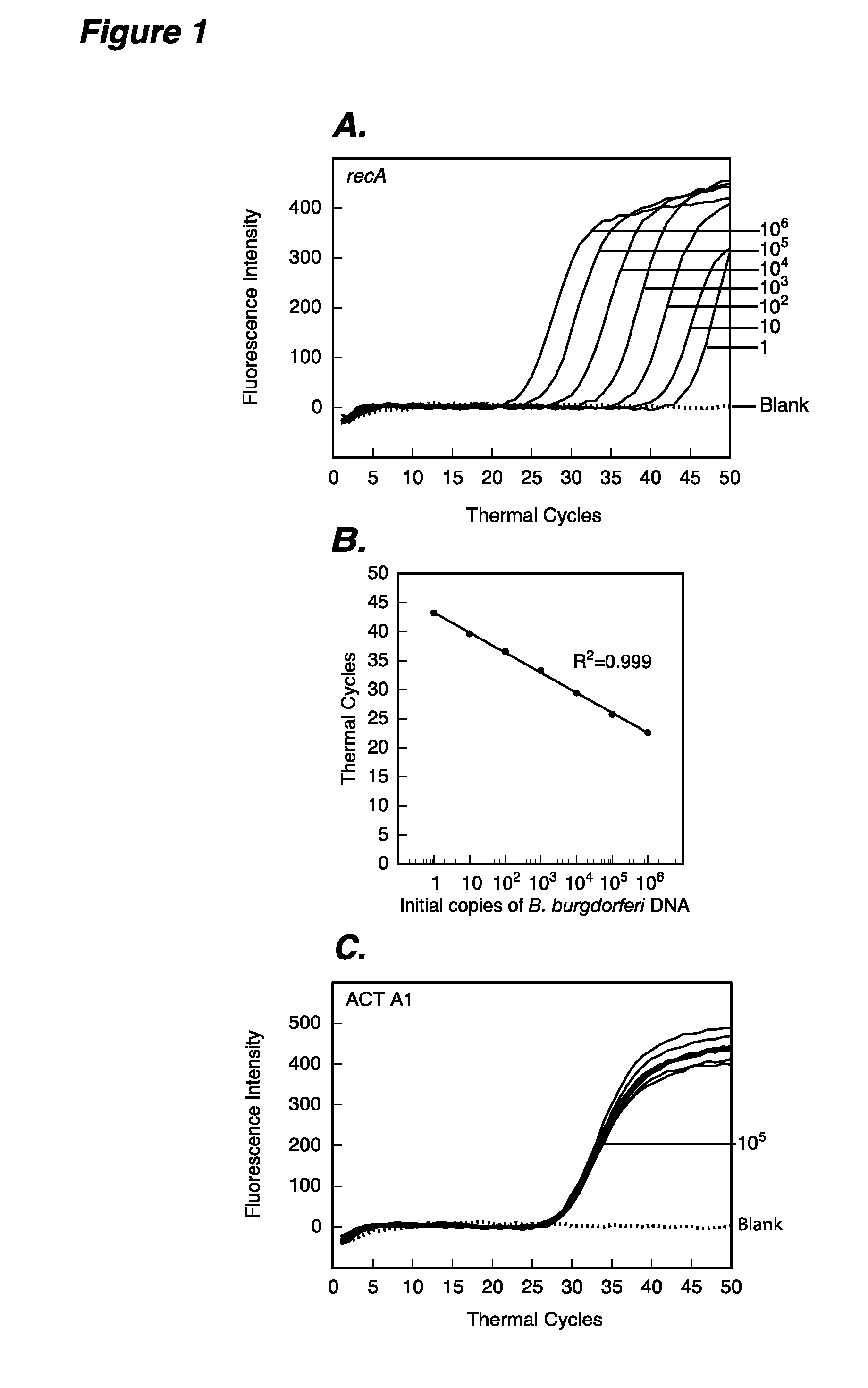
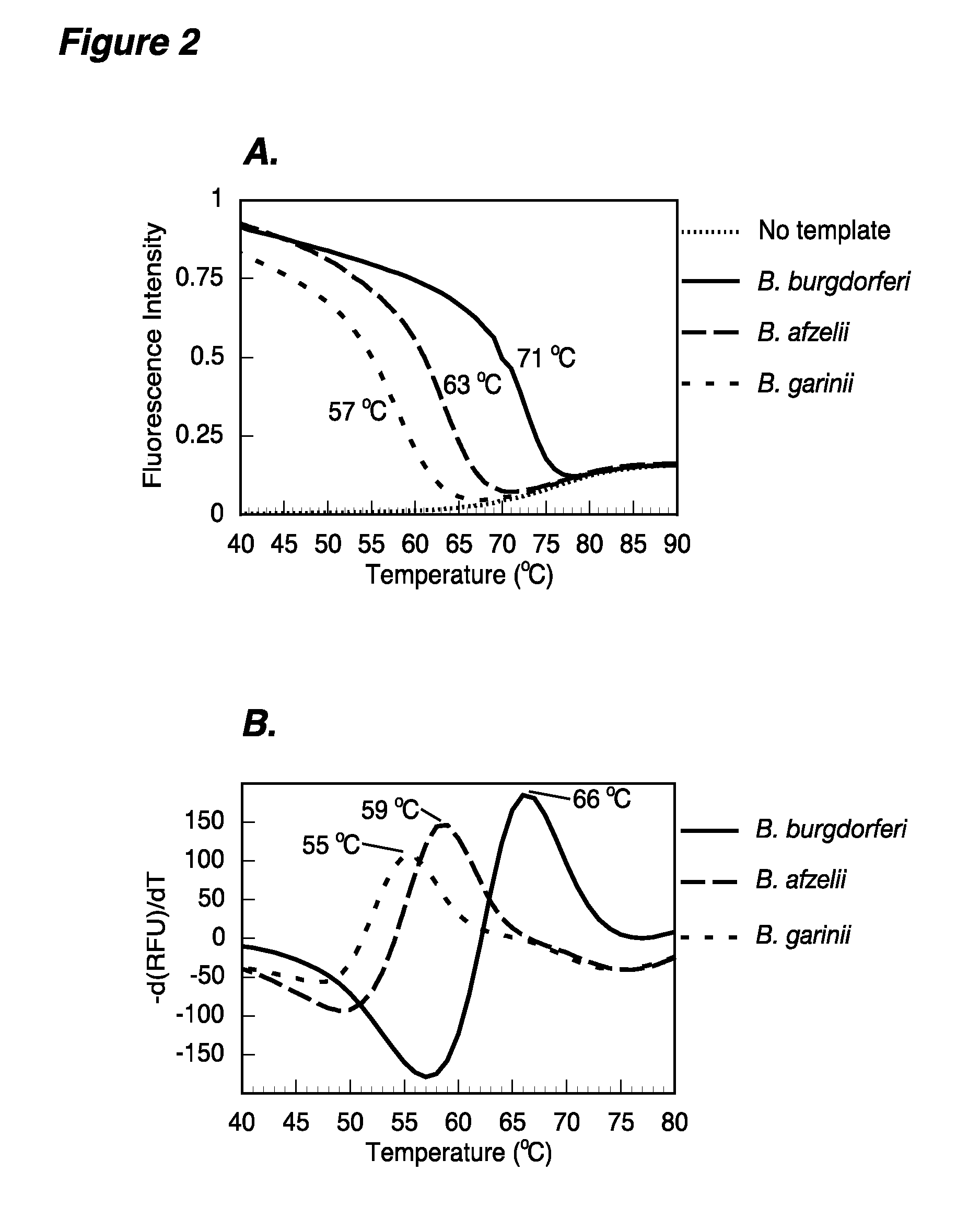
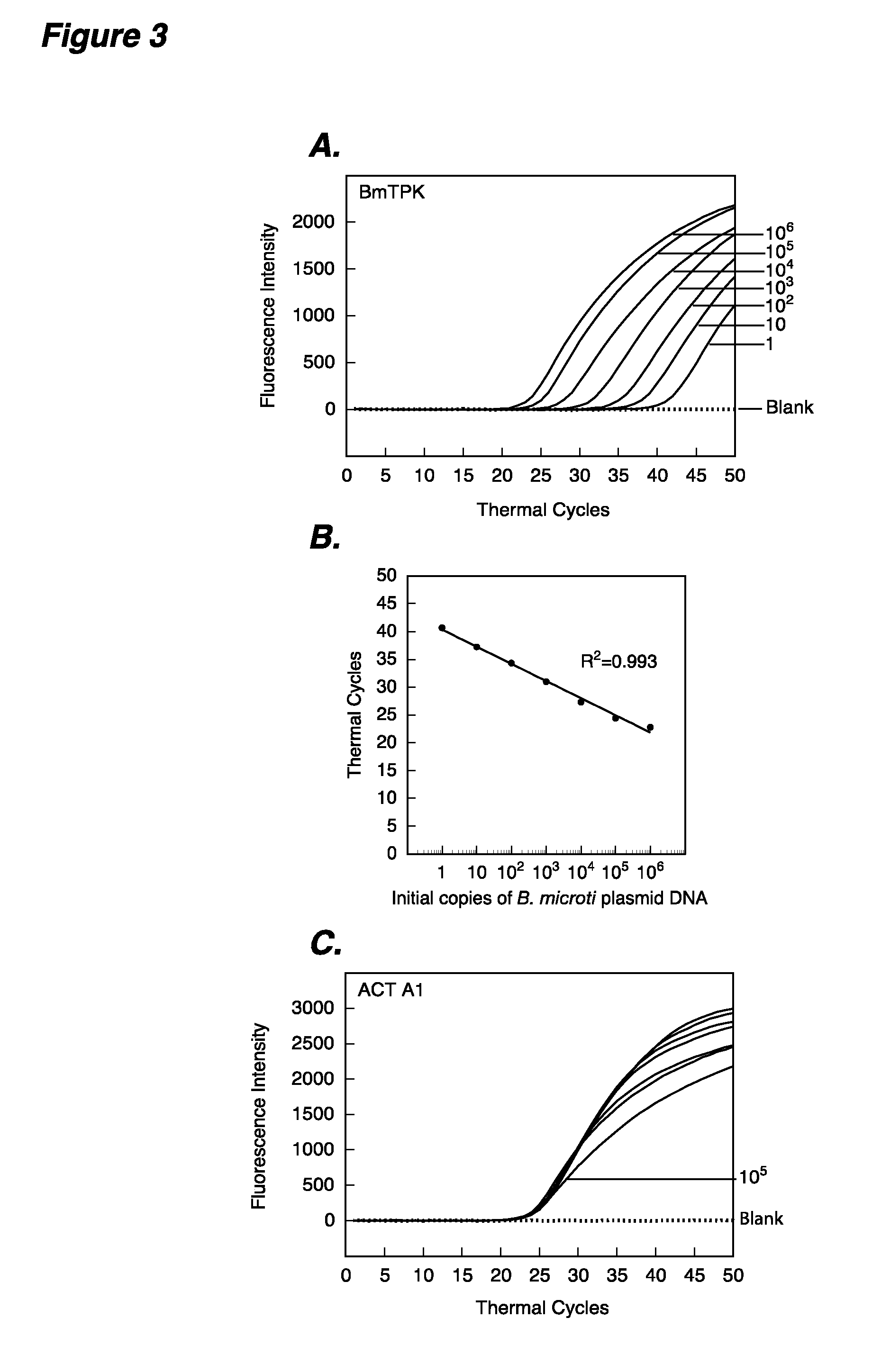
The present invention provides methods and compositions for determining the presence and / or amount of Borrelia burgdorferi nucleic acids in a test sample related to Lyme disease. In particular, substantially purified oligonucleotide primers and probes are described that can be used for qualitatively and quantitatively detecting Borrelia burgdorferi nucleic acid in a test sample by amplification methods. The present invention also provides primers and probes for generating and detecting control nucleic acid sequences that provide a convenient method for assessing internal quality control of the Borrelia burgdorferi assay.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
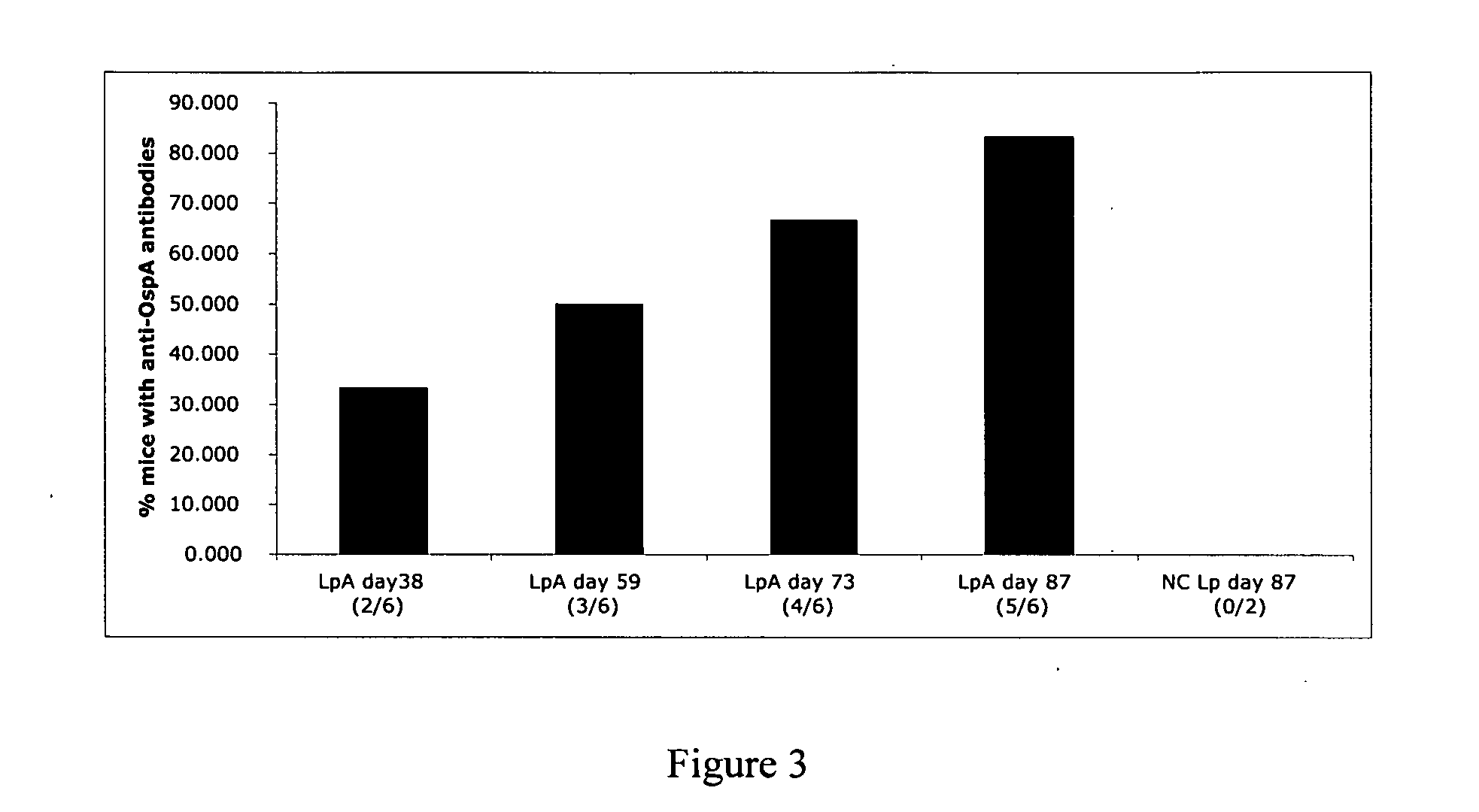
Methods for Diagnosing Lyme Disease
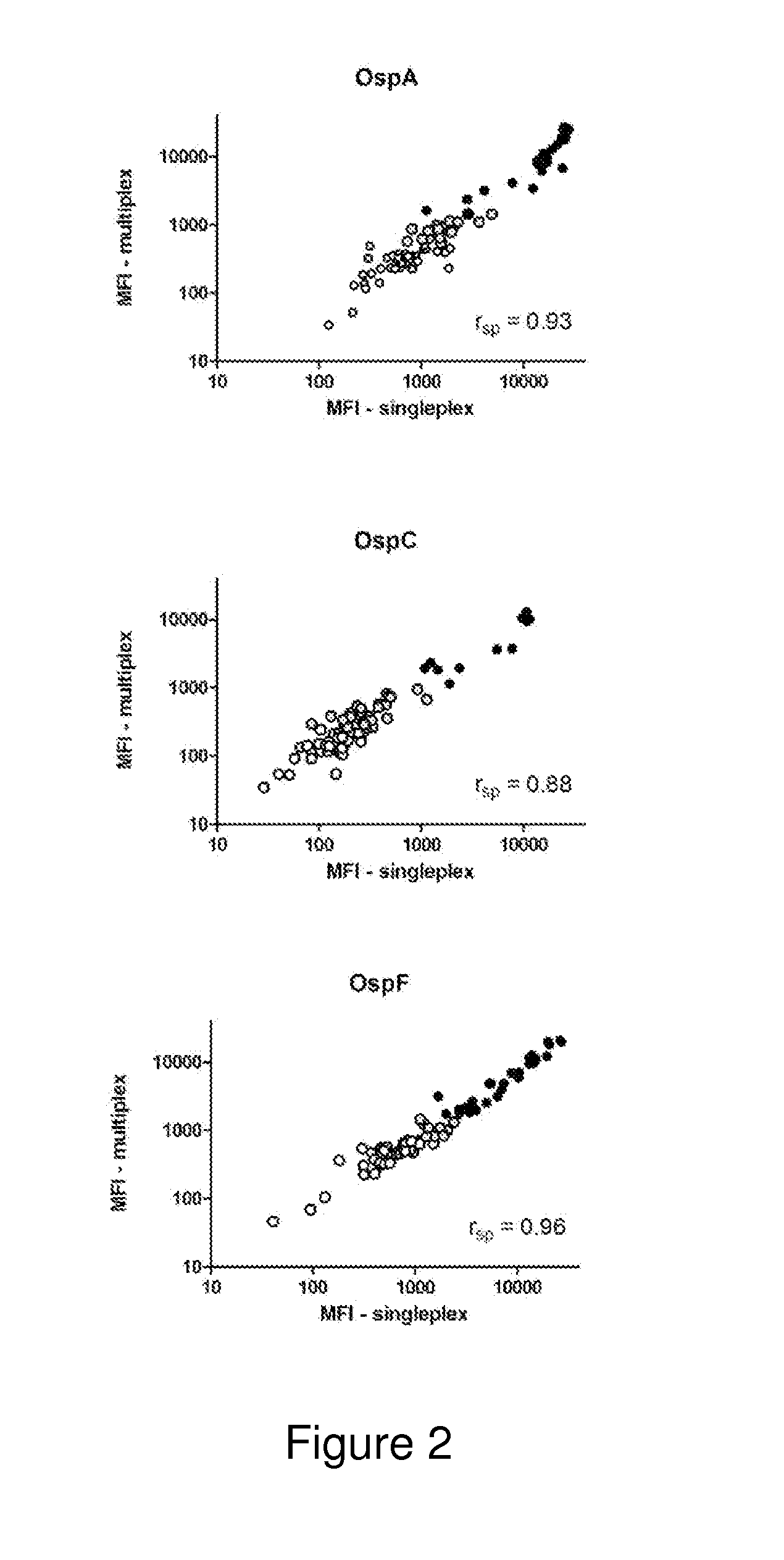
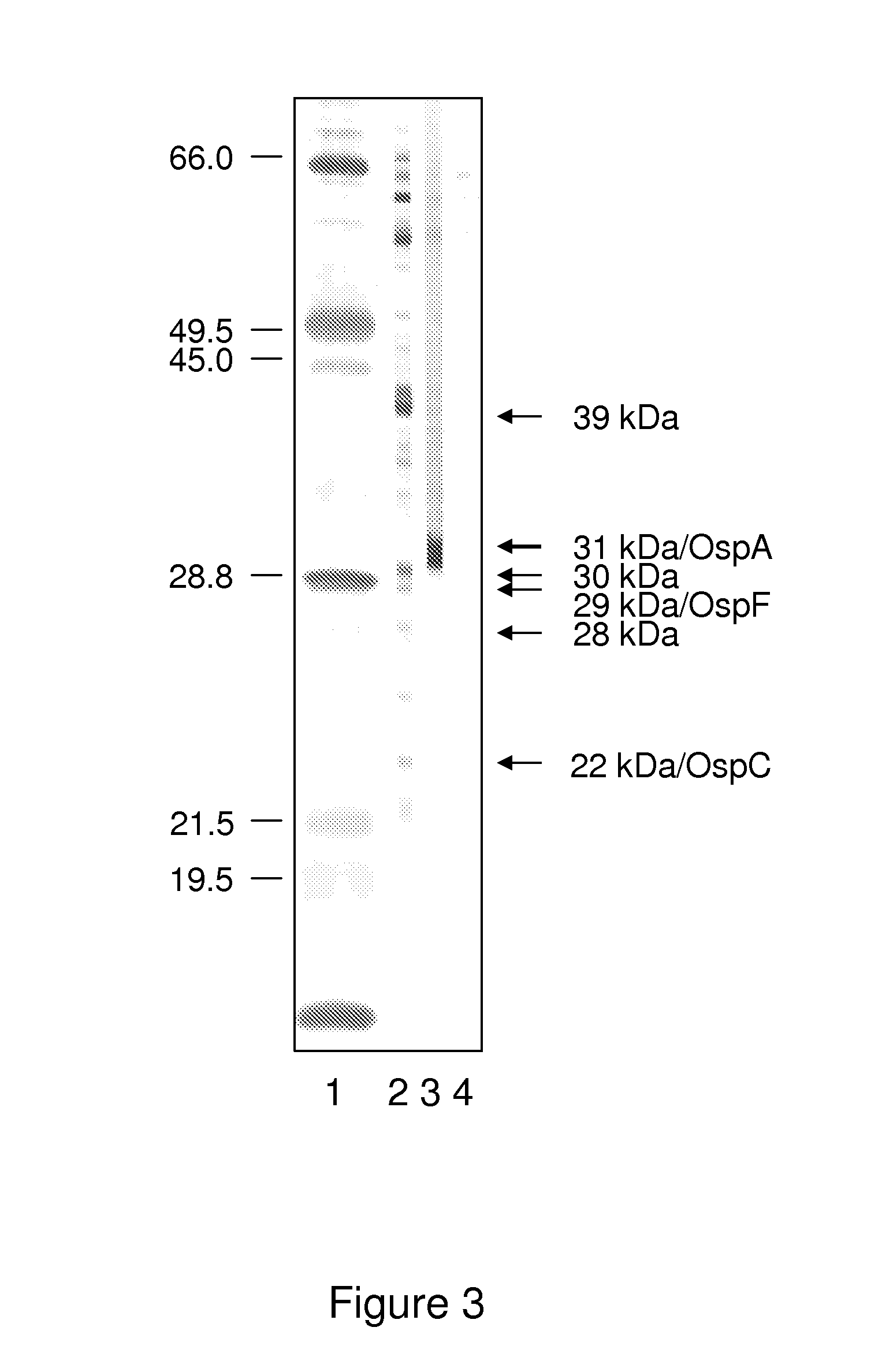
ActiveUS20130273572A1Biological material analysisPeptide preparation methodsOuter surface proteinBorrelia burgdorferi
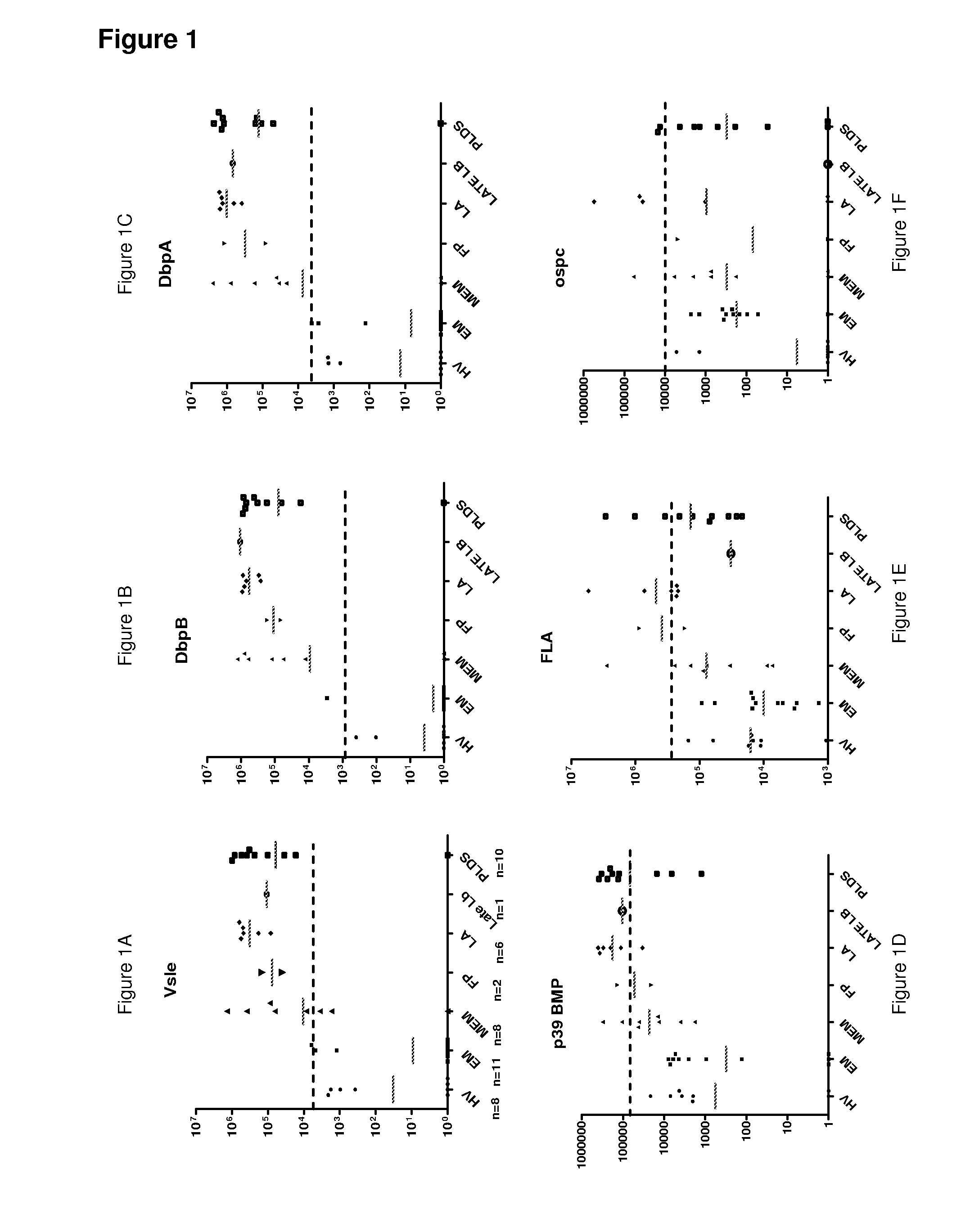
A method for diagnosing Lyme disease status in a mammal is provided. The method entails, in a biological sample obtained or derived from a mammal, determining antibodies to Borrelia burgdorferi (B. burgdorferi) outer surface proteins (Osp) OspA, OspC, and OspF. Based upon determining the OspA, OspC, and OspF antibodies, the mammal can be diagnosed as vaccinated, not vaccinated, infected or not infected with B. burgdorferi. Mammals that have early, intermediate or chronic B. burgdorferi infection can also be identified. The method is particularly suited for use with horses and dogs. Isolated or recombinant B. burgdorferi antigens and compositions that contain them are also provided.
Owner:CORNELL UNIVERSITY
Protein chip modified by succinyl-beta-cyclodextrin for detecting lyme disease and preparation and application of protein chip
ActiveCN105548577ALow biocompatibilityAvoid false positivesBiological material analysisBiological testingBeta-CyclodextrinsLyme disease
The invention belongs to the field of biomedicine detection, and particularly relates to a protein chip modified by succinyl-beta-cyclodextrin for detecting lyme disease and preparation and application of the protein chip. On the one hand, the invention provides a protein chip for detecting the lyme disease, comprising a solid phase carrier and a capture molecule fixed on the solid phase carrier, wherein the capture molecule contains a principal protein-like sequence expression E protein (VisE) of borrelia burgdorferi variable. In detection, each chip can detect multiple serum samples simultaneously, and is relatively high in flexibility and specificity, so that the occurrence probability of false positive detection and false negative detection among groups is reduced. Compared with a traditional method, the protein chip is applied to screening population at high-risk areas on a large scale; relatively simple, convenient and reliable detection methods can be provided; the screening efficiency as well as the positive detecting rate and accuracy are enhanced.
Owner:ANHUI MEDICAL UNIV
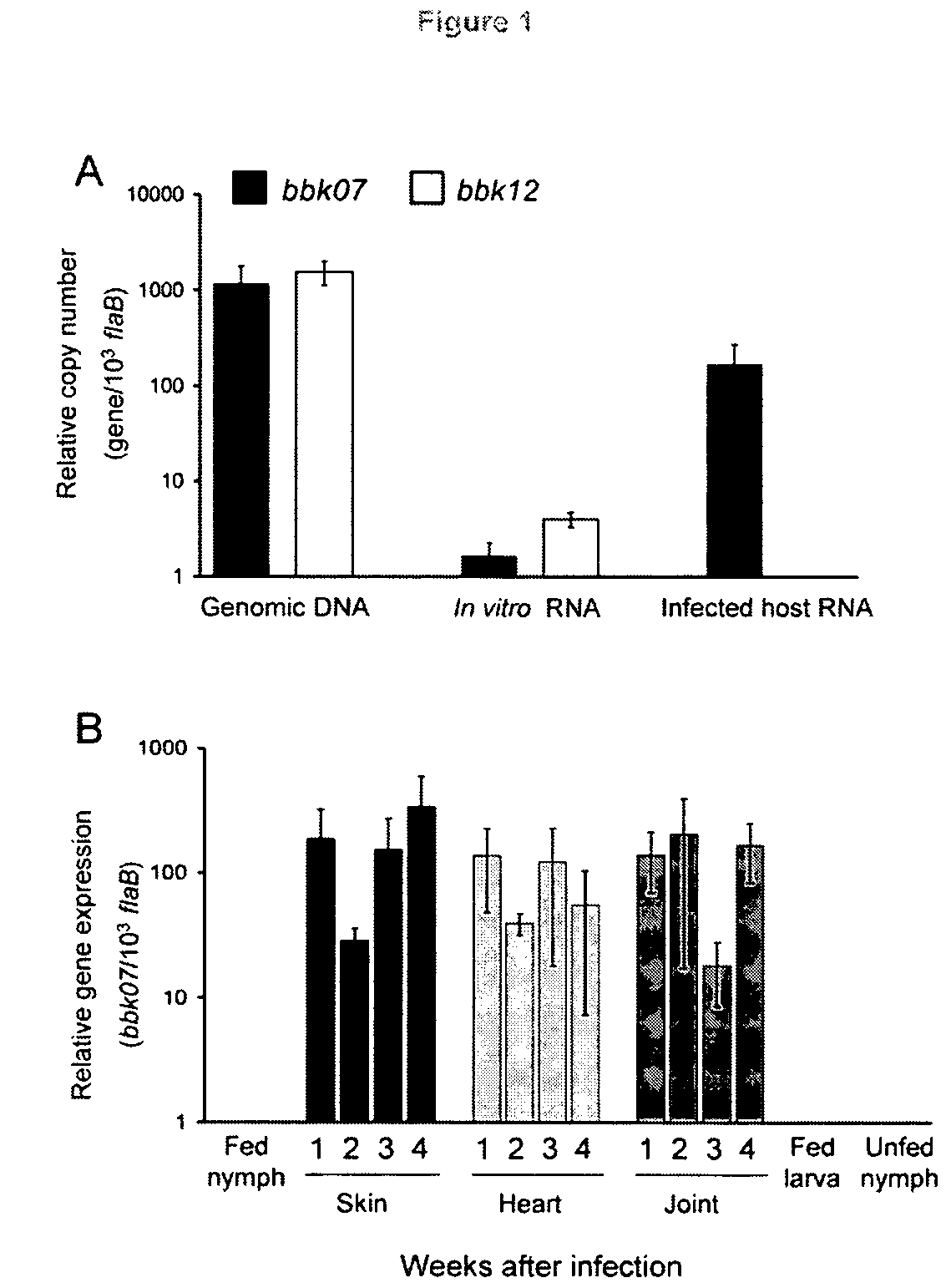
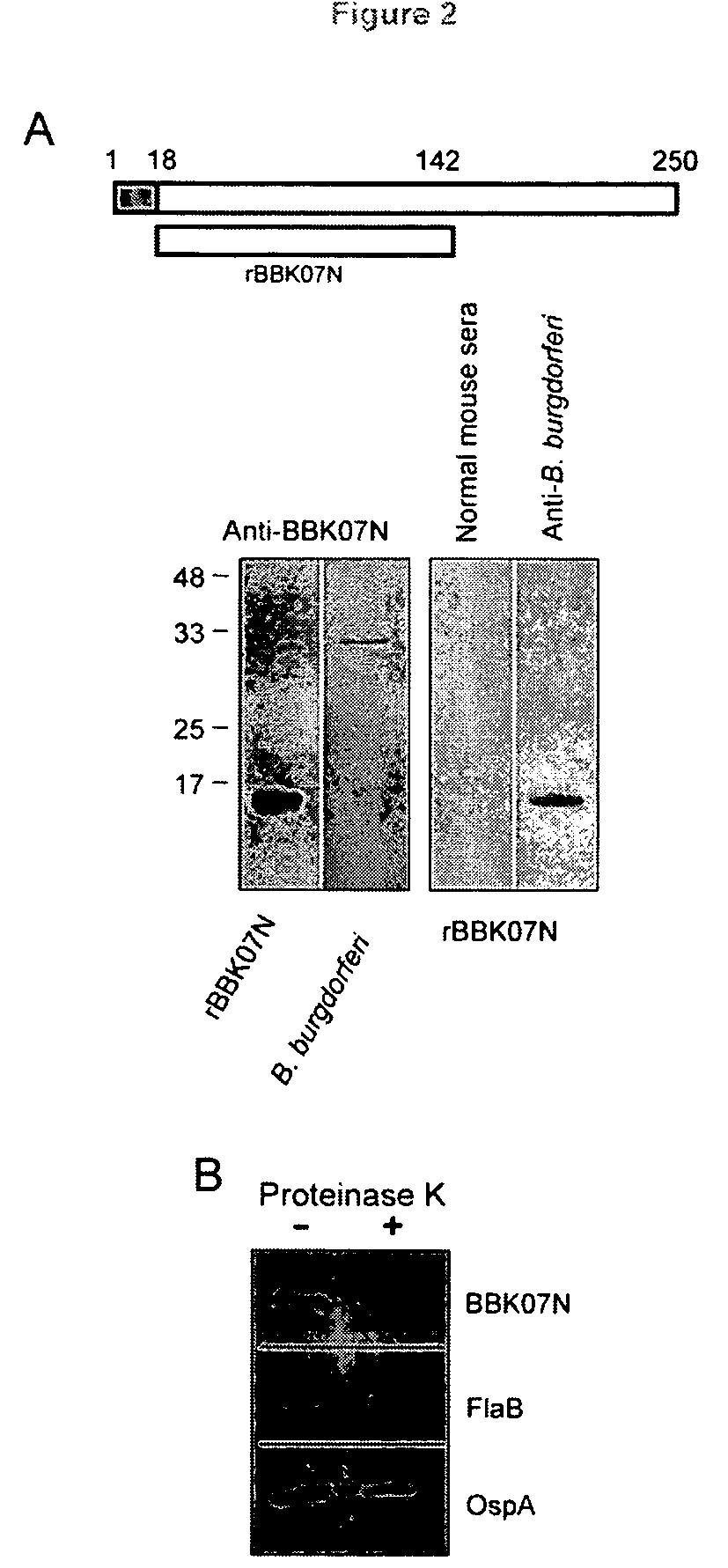
Characterization of BBK07 antigen of Borrelia burgdorferi and methods of use
InactiveUS8338566B2Low cross-reactivityHigh sensitivitySugar derivativesPeptide/protein ingredientsEpitopeNucleotide
In this application is described the characterization Borrelia burgdorferi lipoprotein BBK07, an in vivo expressed and surface-exposed immunogen. BBK07 expression in the infected hose can be detected at the RNA and protein level as early as the first week of infection. Therefore, described is the use of BBK07 antigen and immunogenic epitopes as well as bbk07 nucleotides in methods and kits for the diagnosis of Lyme disease.
Owner:UNIV OF MARYLAND
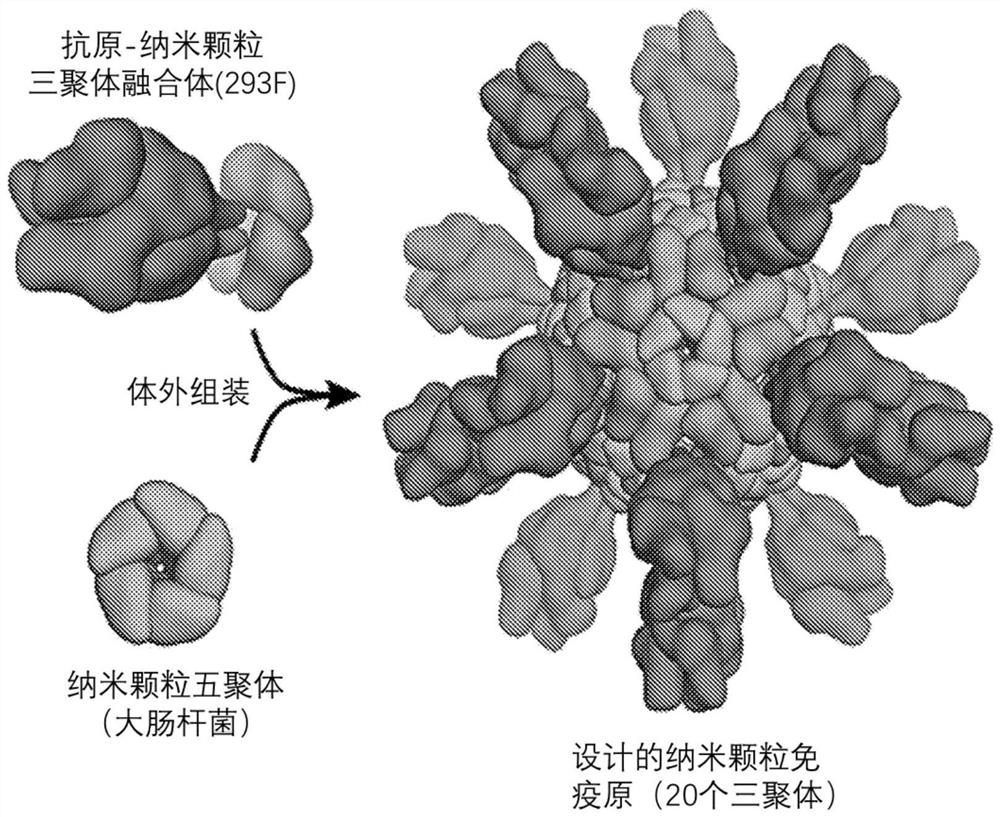
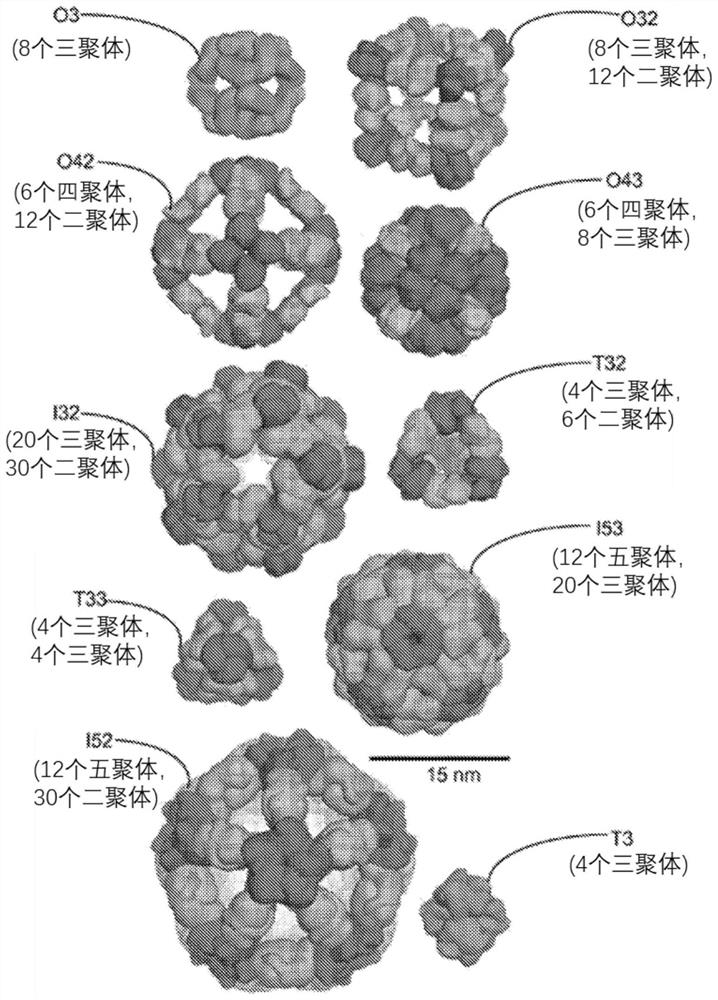
Self-asssembling nanostructure vaccines
The present disclosure provides nanostructures and nanostructure-based vaccines. Some nanostructures of the present disclosure display antigens capable of eliciting immune responses to infectious agents such as bacteria, viruses, and pathogens. Some vaccines of the present disclosure are useful for preventing or decreasing the severity of infection with an infectious agent, including, for exampleand without limitation, lyme disease, pertussis, herpes virus, orthomyxovirus, paramyxovirus, pneumovirus, filovirus, flavivirus, reovirus, retrovirus, meningococcus, or malaria. The antigens may be attached to the core of the nanostructure either non-covalently or covalently, including as a fusion protein or by other means disclosed herein. Multimeric antigens may optionally be displayed along asymmetry axis of the nanostructure. Also provided are proteins and nucleic acid molecules encoding such proteins, vaccine compositions, and methods of administration.
Owner:UNIV OF WASHINGTON
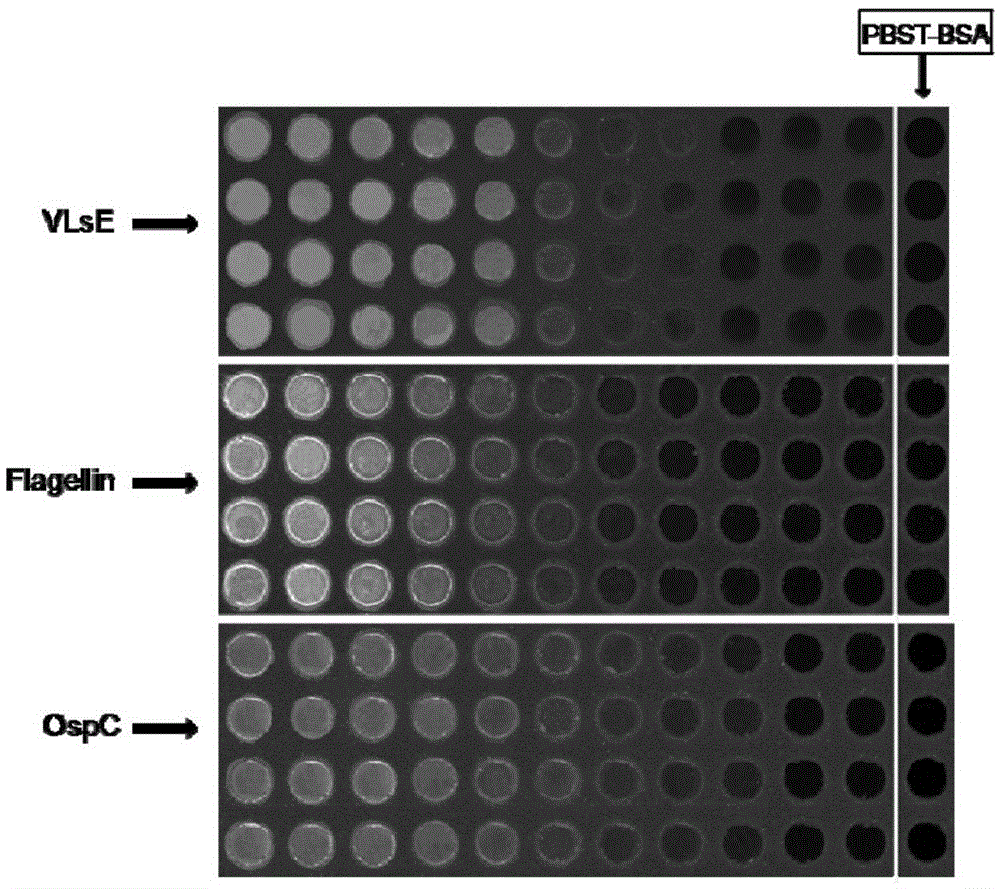
Protein combined chip for Lyme disease immunoserology diagnosis and preparation method and application thereof
InactiveCN105606821ALow biocompatibilityAvoid false positivesBiological material analysisBiological testingSerodiagnosesInfection types
The invention belongs to the field of biomedicine detection and particularly relates to a protein combined chip for Lyme disease immunoserology diagnosis and a preparation method and application thereof. The protein combined chip comprises a solid phase carrier and capture molecules fixed to the surface of the solid phase carrier, and the capture molecules comprise Borrelia burgdorferi flagellin, Borrelia burgdorferi outer membrane protein C (OspC) and Borrelia burgdorferi variable main protein sequence expression E protein (VlsE). The protein combined chip can further comprise capture molecules VlsE IR6 polypeptide. Compared with a traditional method, the protein combined chip can conveniently distinguish infection types on the basis of greatly increasing the positive rate.
Owner:ANHUI MEDICAL UNIV
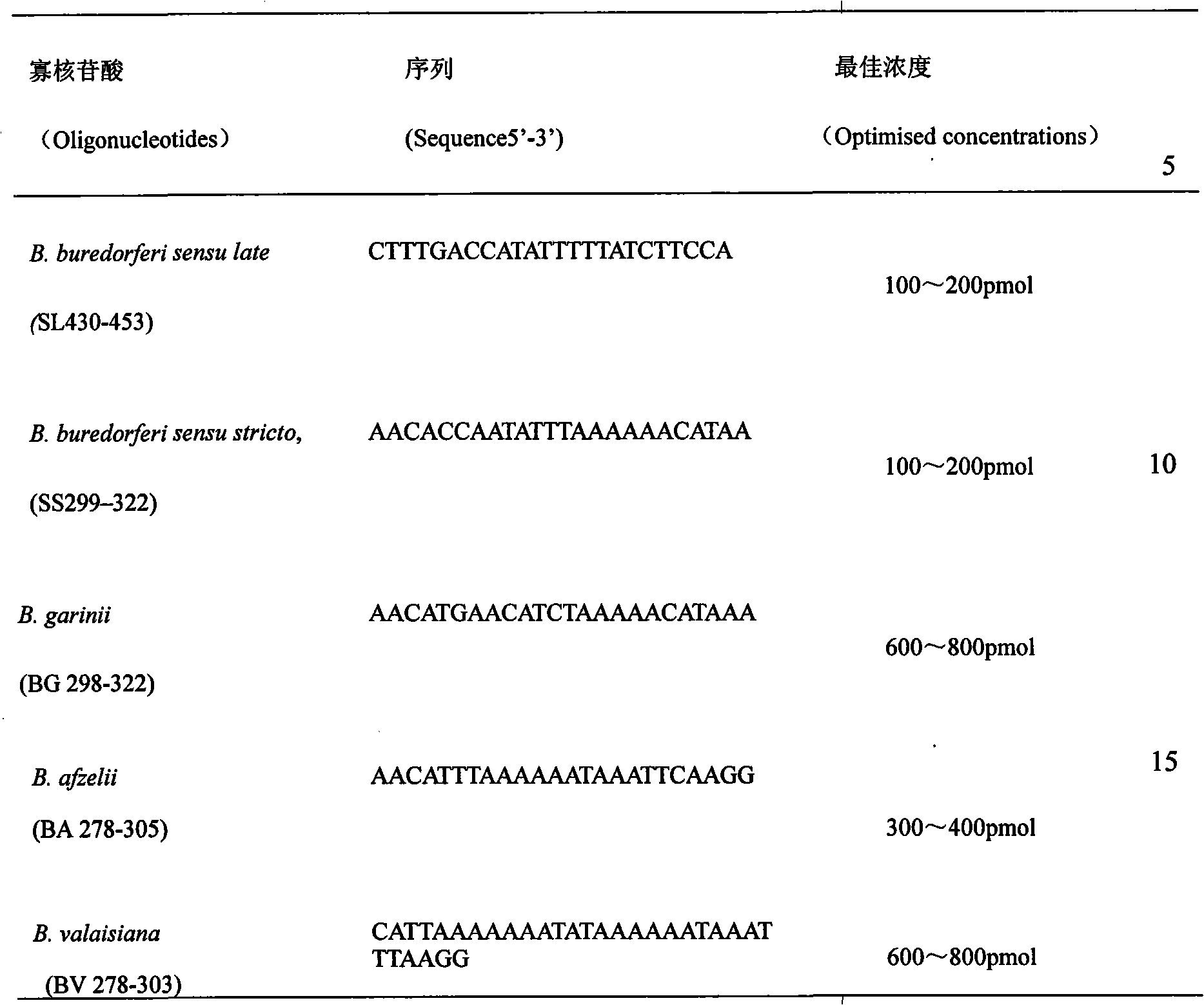
Kit for detecting Lyme bacteria in tick acarina and detection method thereof
InactiveCN101613757ADetailed diagnosisMicrobiological testing/measurementMicroorganism based processesSpiroplasmaBiotin
The invention relates to a kit for detecting Lyme bacteria in tick acarina and a detection method thereof. The kit for detecting the Lyme bacteria in the tick acarina at least comprises: Biodyne C membrane covalently bonded with universal probes of Lyme bacteria spirochetes and probes for specific oligonucleotide, B. burgdorferi sensu stricto, B. garinii, B. afzelii and B. valaisiana and a pair of primers which are designed on conservative regions at two ends of a gene sequence of spirochete 5S-23S rRNA and have 225 bp fragment that can be amplified, one primer of which is marked with biotin.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Topical antibiotic composition for the prevention of lyme disease
A topical pharmaceutical composition comprising an antibiotic is useful for the prevention of Lyme disease.
Owner:IXODES
Biomarker signatures for lyme disease and methods of use thereof
ActiveUS20170336409A1High success rateLow rate of successDisease diagnosisAgainst vector-borne diseasesDiseaseAnalyte
The present invention relates to methods for the diagnosing, prognosing, monitoring, differentiating, treating, and managing of Lyme disease in a subject. The methods according to the invention are characterized by the detection of a biomarker signature comprised of a combination of two or more analytes indicative of disease.
Owner:VERAMARX
Compositions and methods for screening for lyme disease
ActiveUS20130164759A1Inhibit bindingSugar derivativesPeptide/protein ingredientsLyme diseaseDisease agent
The invention provides compositions, methods, and kits for the diagnosis or detection of infection by a pathogen that causes Lyme disease in a subject.
Owner:UNITED STATES OF AMERICA
Vaccines and methods to treat lyme disease in dogs
InactiveCN104379164AAntibacterial agentsBacterial antigen ingredientsBorrelia gariniiBorrelia burgdorferi
The instant invention provides an immunogenic composition comprising an antigenic fragment of OspA protein of Borrelia burgdorferi and a chimeric protein containing antigenic fragments of different phylotypes of OspC protein of Borrelia burgdorferi. Vaccines incorporating the immunogenic composition of the invention, as well as methods of preventing Lyme disease in dogs and / or protecting dogs from Lyme disease using the vaccines are also provided.
Owner:ZOETIS SERVICE LLC +1
Compositions and methods for diagnosing and monitoring disease and treatment via antigen-specific molecules
InactiveUS20130089879A1Convenient treatmentSignificant valueMicrobiological testing/measurementPeptidesEnvironmental healthEnvironmental agent
This document provides methods and materials related to compositions and methods for diagnosing and monitoring treatment for sensitivity to an antigen. Compositions of substantially pure polypeptides, or antigenic fragments thereof, and methods of using such compositions for diagnosing Lyme disease, infections, exposure to toxic environmental agents, and food sensitivities, and monitoring a subject's response to treatment of the same are provided.
Owner:PHARMASAN LABS
Biomarker signatures for lyme disease and methods of use thereof
ActiveUS10274491B2Low rate of successBacterial antigen ingredientsSnake antigen ingredientsDiseaseAnalyte
Owner:VERAMARX
Sublingual Spray Formulation Comprising Dihydroartemesinin
InactiveUS20120157518A1Patient compliance is goodPrecise deliveryOrganic active ingredientsBiocideNasal cavityChemical compound
The invention provides pharmaceutical compositions for the treatment of neoplastic diseases, fluke infestations and Lyme disease, comprising compounds capable of providing dihydroartemesinin and a medium chain triglyceride formulated for transmucosal sublingual, buccal or nasal delivery, especially by a spray. Also provided are delivery devices containing the compositions.
Owner:LONDONPHARMA
Compositions and methods for screening for Lyme disease
The invention provides compositions, methods, and kits for the diagnosis or detection of infection by a pathogen that causes Lyme disease in a subject.
Owner:UNITED STATES OF AMERICA
Artemisinin with berberine compositions and methods of making
An all-natural herbal composition and methods of preparing the same are provided. The novel Artemisinin Combination Therapy (ACT) consists of artemisinin and its derivatives and berberine, the two active substances mixed with various selected excipient compounds to form a single pill, tablet or capsule for treatment and prevention of malaria, dengue fever, yellow fever, dysentery, Lyme disease, babesiosis, progressive multifocal leukoencephalopathy, Helicobacter Pylori, and colitis, in adults and children. A tablet or pill for children is formulated to be chewable.
Owner:ROSEN REECE D
Lyme disease reagent kit and its lyme disease detecting method
InactiveCN1469125ASimple and fast operationImproving immunogenicityBiological testingAgainst vector-borne diseasesProtein antigenEpizootic
The present invention is Lyme disease detecting reagent kit and its Lyme disease detecting method. The reagent kit includes P39 protein antigen coated carrier micropore plate, enzyme labeling antibody, substrate liquid, etc. It has the advantages of powerful and stable antigen immunogenicity and capability of mass production, and one set of reagent and several monoclonal mouse-to-human IgG antigens may be used to eliminate crossed display effect to result in clear background. The reagent kit is simple in operation, needs no special instrument and may be used in common hospital for wide epidemic disease survey and patient detection.
Owner:NO 261 HOSPITAL PLA
Multiplex diagnostic assays for Lyme disease and other tick-borne diseases
ActiveUS10480035B2Microbiological testing/measurementAgainst vector-borne diseasesDiseaseTick Borne Infections
The present invention provides novel methods of diagnosing and determining treatment strategies for Lyme disease and other tick-borne illnesses.
Owner:RUTGERS THE STATE UNIV
Multiplex diagnostic assays for lyme disease and other tick-borne diseases
ActiveUS20160289746A1Microbiological testing/measurementAgainst vector-borne diseasesTick Borne InfectionsTick-borne disease
The present invention provides novel methods of diagnosing and determining treatment strategies for Lyme disease and other tick-borne illnesses.
Owner:RUTGERS THE STATE UNIV
Bionanosensor detection device
ActiveUS20120178639A1Easy to useFast resultsMaterial nanotechnologyBioreactor/fermenter combinationsDiseaseNucleic acid detection
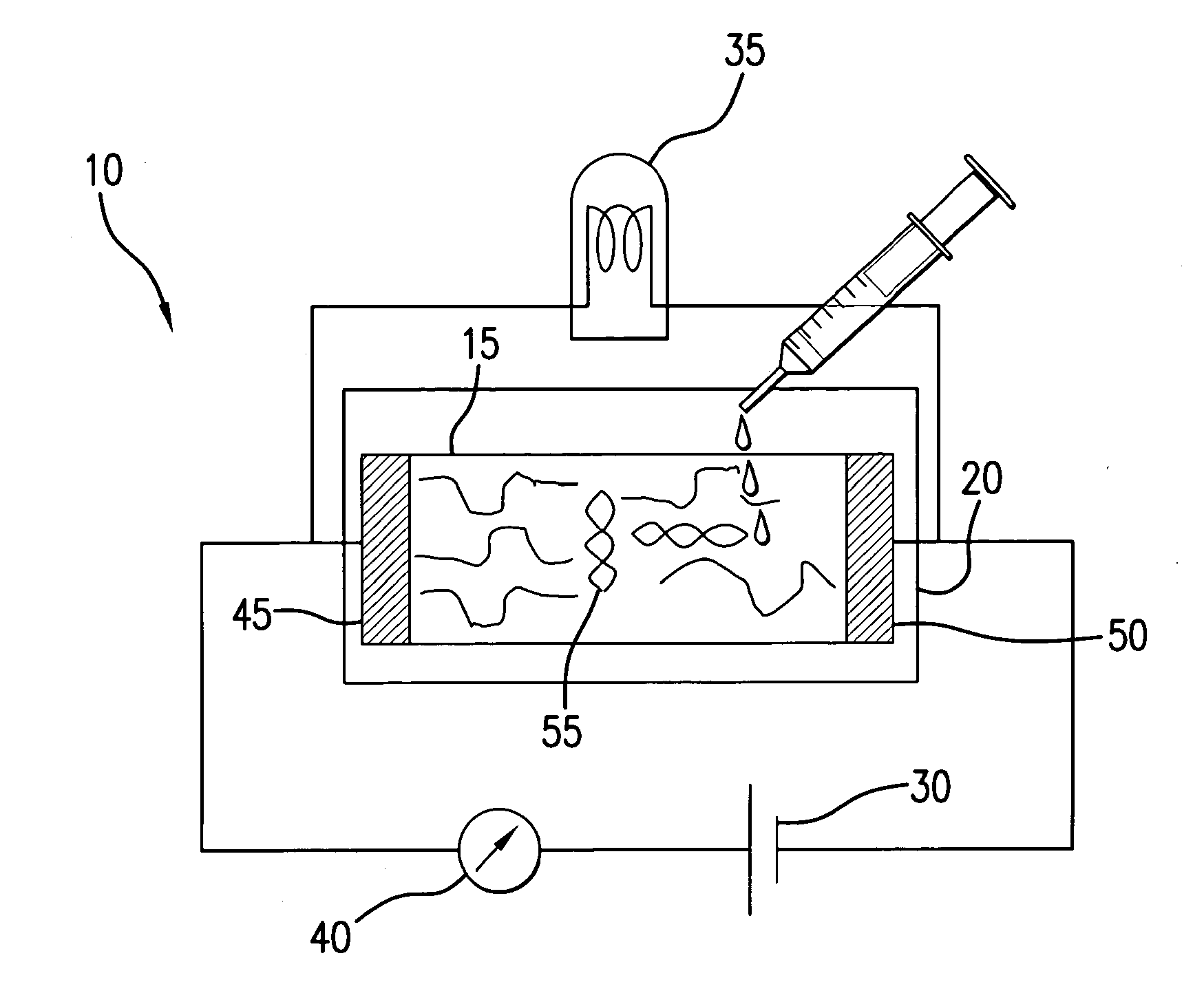
The present invention is directed to a nucleic acid detection device and method that incorporates bio-nanosensor technology to detect duplex DNA. The device is particularly applicable in detecting the presence or absence of duplex DNA and its correlation to the diagnosis of infectious diseases. In one embodiment, the infectious disease is Lyme disease or a bacterial or viral infection. The device comprises a bio-nanosensor element comprising ssDNA primed nanotubes, either single walled or multi-walled. The method comprises contacting the bio-nanosensor element with a test solution potentially containing DNA of interest. DNA of interest that hybridizes to the ssDNA results in a measurable change in the electrical properties of the bio-nanosensor. Correlations between the results provided by the device and the presence of disease states can result in rapid diagnosis of diseases such as Lyme disease or foodborne infections such as salmonellosis.
Owner:12-15 MOLECULAR DIAGNOSTICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com