Protein chip modified by succinyl-beta-cyclodextrin for detecting lyme disease and preparation and application of protein chip
A protein chip, Lyme disease technology, applied in the field of biomedical testing to achieve the effect of improving screening efficiency, positive detection rate and accuracy, reducing non-specificity, and reducing the incidence of false positives and false negatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The selection and surface chemical treatment of embodiment 1 carrier
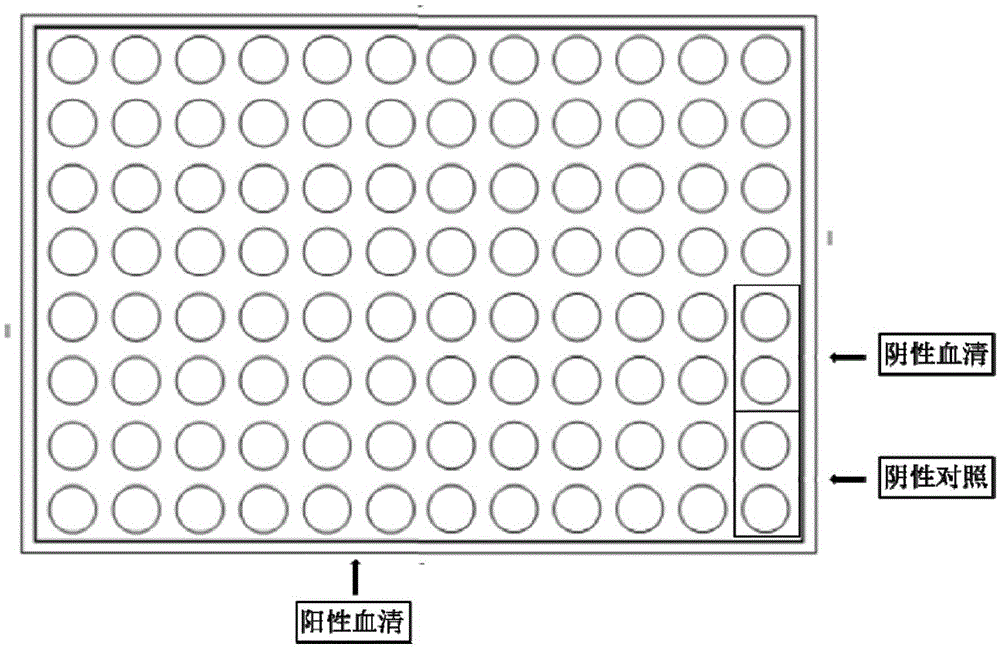
[0082] The present invention selects a gold foil chip from Interactiva Company (Ulm, Germany). Its substrate is a glass sheet covered with a layer of pure gold (purity 99.9%) with a thickness of 10nm. On the gold foil is a regionalized 50μm TEFLON Membrane array (96 holes*2, 8 rows*12 columns), the array aperture is 1.25mm, such as figure 1 shown.
[0083] Step 1. Cleaning of gold foil chips
[0084] Prepare TL1 solution (H 2 O:H 2 o 2 :NH3 ·H 2 O=5:1:1) into a stainless steel box, put the chip into the box, bathe in 82°C water for 5 minutes, rinse with deionized water 4 to 5 times, ethanol twice, each time for 3 minutes; air-dry with nitrogen, dry and store.
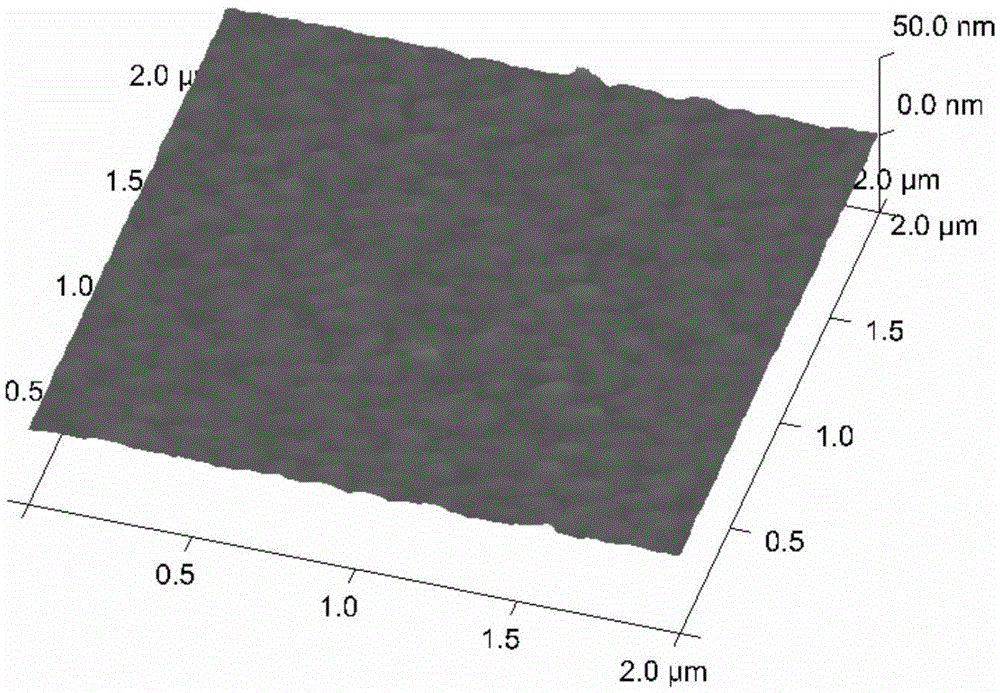
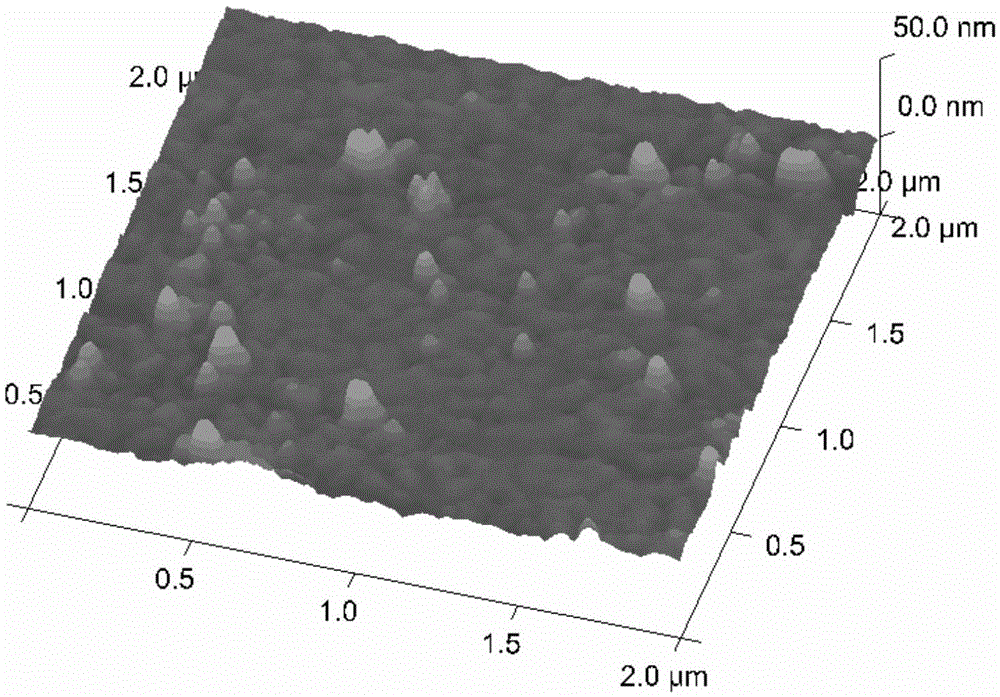
[0085] Step 2. Chemically modify the surface of the cleaned gold foil chip to obtain a solid phase carrier
[0086] After cleaning the gold foil chip, immerse it in the aforementioned modification solution 1, and incubate with shaking a...
Embodiment 2
[0089] Embodiment 2 quality control experiment
[0090] Preparation of incubation solution 1: Dissolve VlsE antigen in PBST-BSA solution, and prepare concentration gradients of 0.5μg / ml, 0.25μg / ml, 0.125μg / ml, 0.0625μg / ml, 0.0313μg / ml, 0.0156μg / ml , 0.0078μg / ml, 0.0039μg / ml, 0.0019μg / ml, 0.00095μg / ml, 0.000475μg / ml antigen solution.
[0091] Preparation of incubation solution 2: Dissolve human IgG in PBST-BSA solution, and configure the concentration gradient to be 0.5μg / ml, 0.25μg / ml, 0.125μg / ml, 0.0625μg / ml, 0.0313μg / ml, 0.0156μg / ml, 0.0078μg / ml, 0.0039μg / ml, 0.0019μg / ml, 0.00095μg / ml, 0.000475μg / ml solution;
[0092] Preparation of incubation solution 3: Dissolve Flagellin antigen in PBST-BSA solution, and prepare a concentration gradient of 0.5 μg / ml, 0.25 μg / ml, 0.125 μg / ml, 0.0625 μg / ml, 0.0313 μg / ml, 0.0156 μg / ml, 0.0078μg / ml, 0.0039μg / ml, 0.0019μg / ml, 0.00095μg / ml, 0.000475μg / ml solution;
[0093] Prepare incubation solution 4: dissolve the OspC antigen in PBST-BSA ...
Embodiment 3
[0118] Embodiment 3 repeatability experiment
[0119] Table 1 shows the repeatability experiment within a group. Each 16 wells is regarded as a group, and there are three groups in total. On the same day, the VlsE antigen with a concentration of 0.0156 μg / ml and the rabbit anti-VlsE antigen IgG antibody with a concentration of 50 μg / ml are incubated according to the steps and Cy3-labeled goat anti-rabbit IgG antibody at a concentration of 2.5 μg / ml.
[0120] Table 1
[0121]
[0122] Table 2 shows the repeatability experiment between groups. Each 24 wells is regarded as a group, and there are three groups in total. The VlsE antigen with a concentration of 0.0156 μg / ml and the rabbit anti-VlsE antigen IgG with a concentration of 50 μg / ml were incubated according to the steps within three days. Antibody and Cy3-labeled goat anti-rabbit IgG antibody at a concentration of 2.5 μg / ml.
[0123] Table 2
[0124]
[0125] In Tables 1 and 2, SD is the standard deviation, and CV...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com