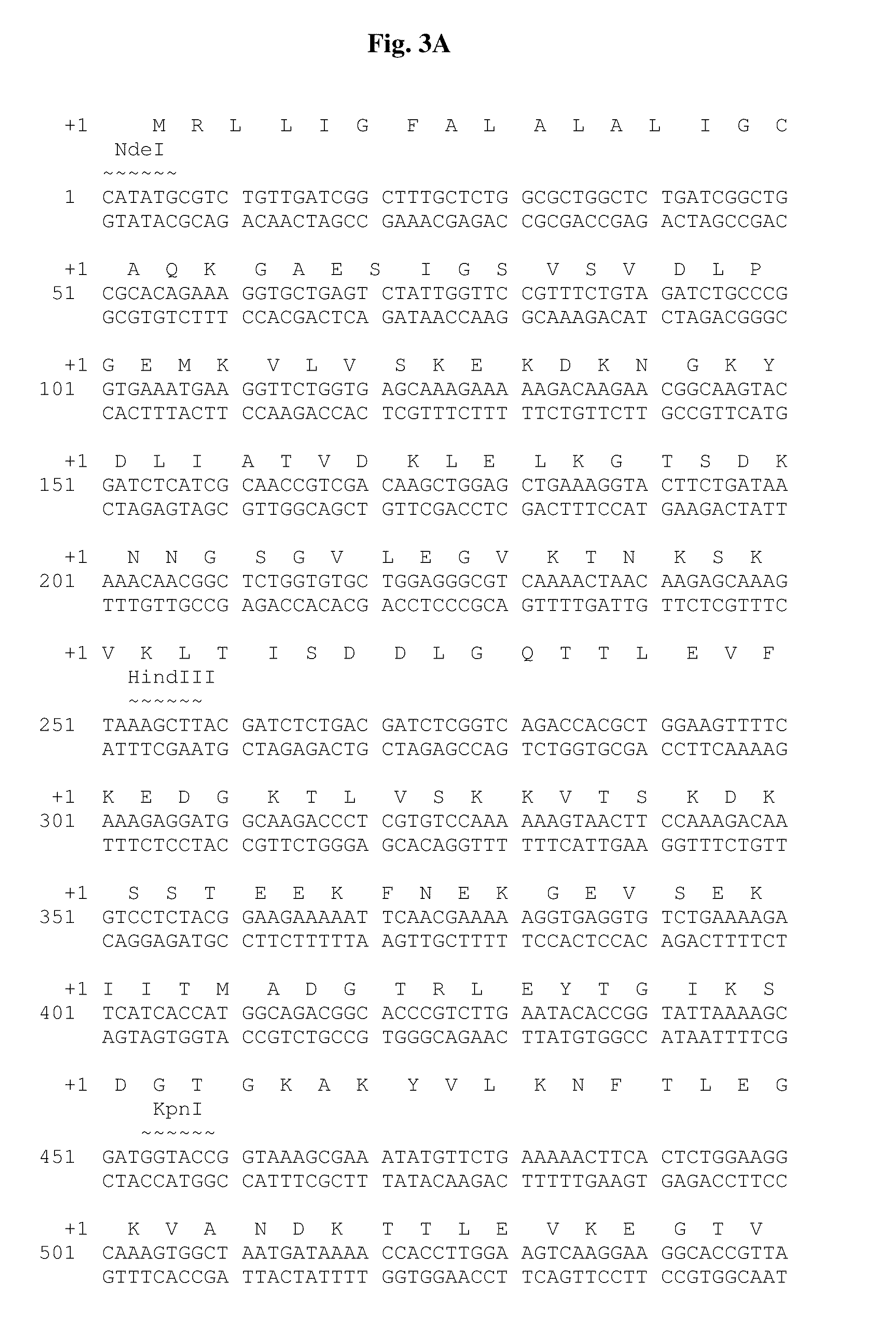Patents
Literature
34 results about "Borrelia afzelii" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Borrelia afzelii is a species of Borrelia a bacterium that can infect various species of vertebrates and invertebrates. Among 30 Borrelia known species, it is one of four which are likely to infect humans causing a variant of Lyme disease.
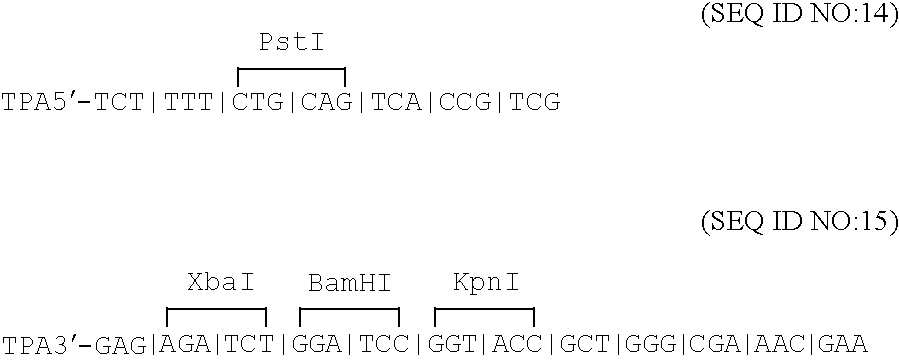
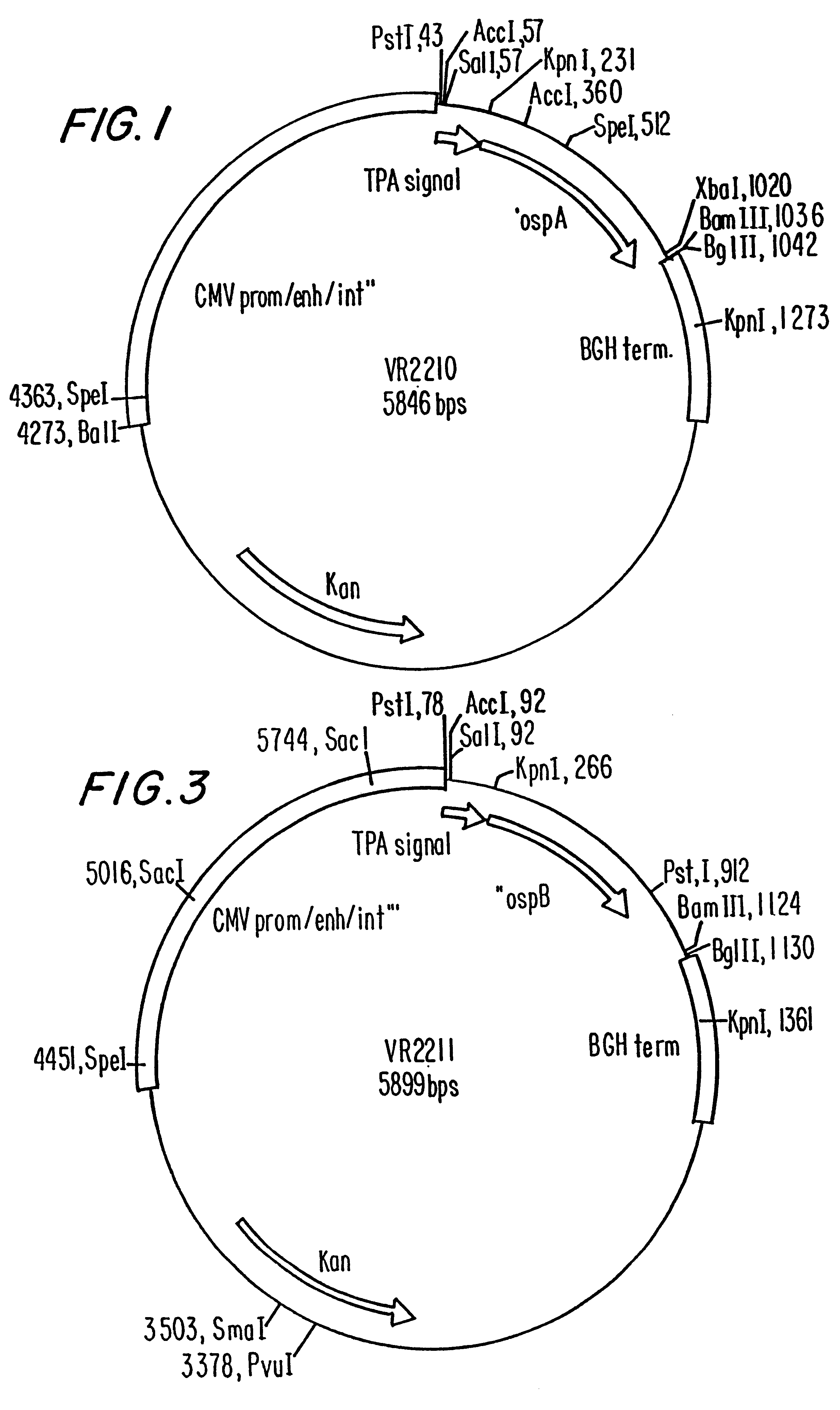
Compositions and methods for administering Borrelia DNA
Disclosed is a vaccine against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding Borrelia OspA or OspB, and a DNA encoding a terminator. Disclosed too is an immunogenic composition against Lyme Disease or its causative agent Borrelia burgdorferi (sensu stricto or sensu lato) containing a plasmid comprising a DNA encoding a promoter for driving expression in a mammalian cell, DNA encoding a leader peptide for facilitating secretion / release of a prokaryotic protein sequence from a mammalian cell, a DNA encoding a Borrelia OspC, and a DNA encoding a terminator. And, methods for making and using such vaccines and the immunogenic composition are also disclosed.
Owner:PASTEUR MERIEUX SERUMS & VACCINS SA
Lyme combination compositions and uses
InactiveUS6368603B1Safe and efficacious in dogNo exacerbation of diseaseAntibacterial agentsNanotechAntigenRabies
Disclosed and claimed are compositions containing a Borrelia burgdorferi antigen, and methods for making and using them. The antigen can be OspA. The compositions can contain at least one additional antigen from a pathogen other than Borrelia burgdorferi. The compositions are useful for eliciting an immunological response in a host mammal susceptible to Lyme Disease and to the mammalian pathogen other than Borrelia burgdorferi. Suitable host mammals include dogs, pups, horses, and, the additional antigen can be of a canine, equine or feline pathogen, such as rabies, canine distemper, adenovirus, coronavirus, parainfluenza and parvovirus. No significant efficacy interference is observed.
Owner:MERIAL LTD
Nucleic acid amplification oligonucleotides and probes to Lyme disease associated Borrelia
InactiveUS6074826AReduced thermal stabilityMaximize differenceSugar derivativesMicrobiological testing/measurementBorrelia gariniiHybridization probe
The present invention discloses hybridization assay probes, amplification primers, nucleic acid compositions and methods useful for detecting Borrelia nucleic acids. Hybridization assay probes and amplification primers that selectively detect Lyme disease-associated Borrelia and distinguish those Borrelia from Borrelia hermsii are disclosed. Other hybridization probes selectively detect Borrelia hermsii and not Lyme disease-associated Borrelia are also described.
Owner:GEN PROBE INC
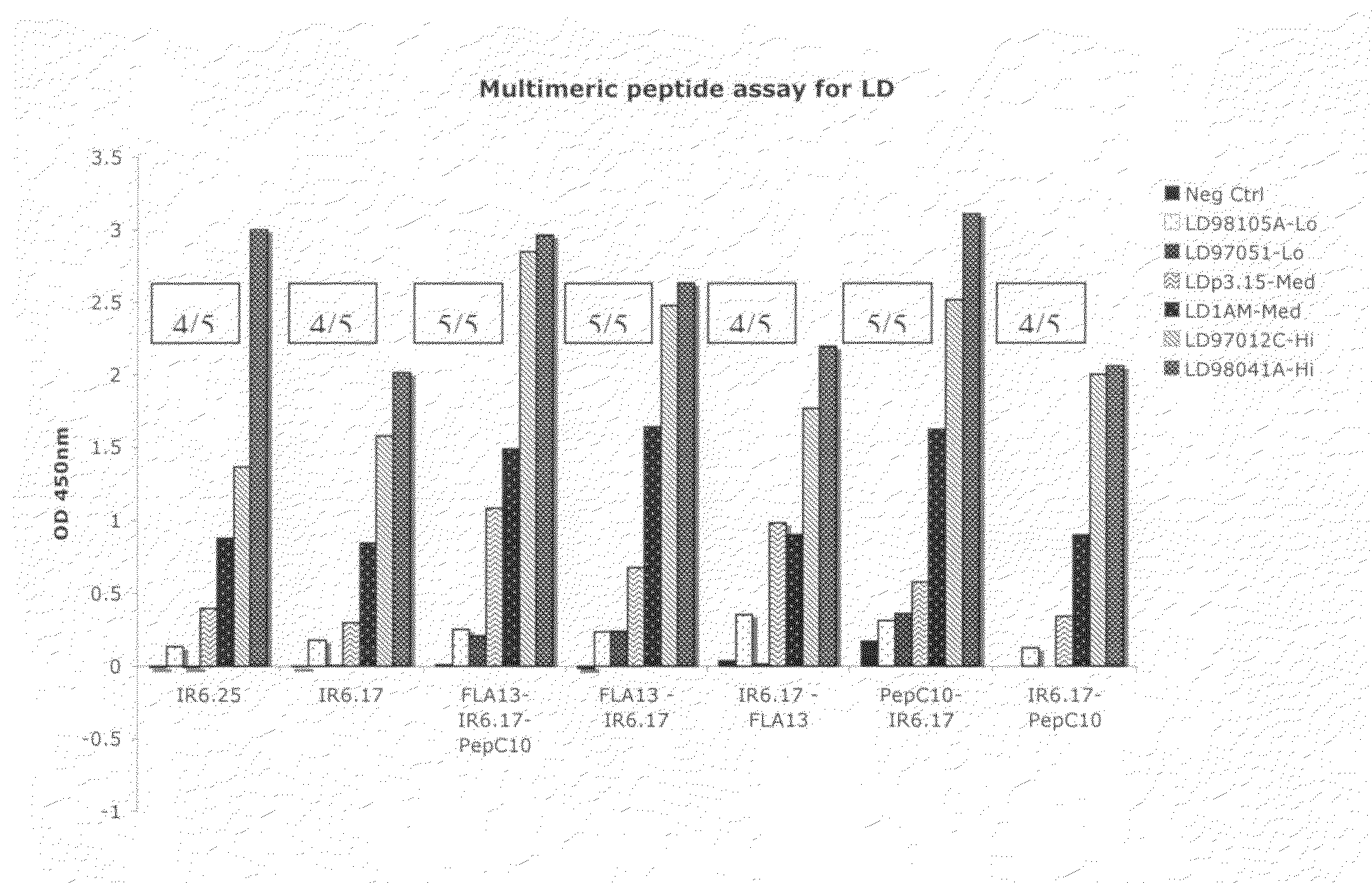
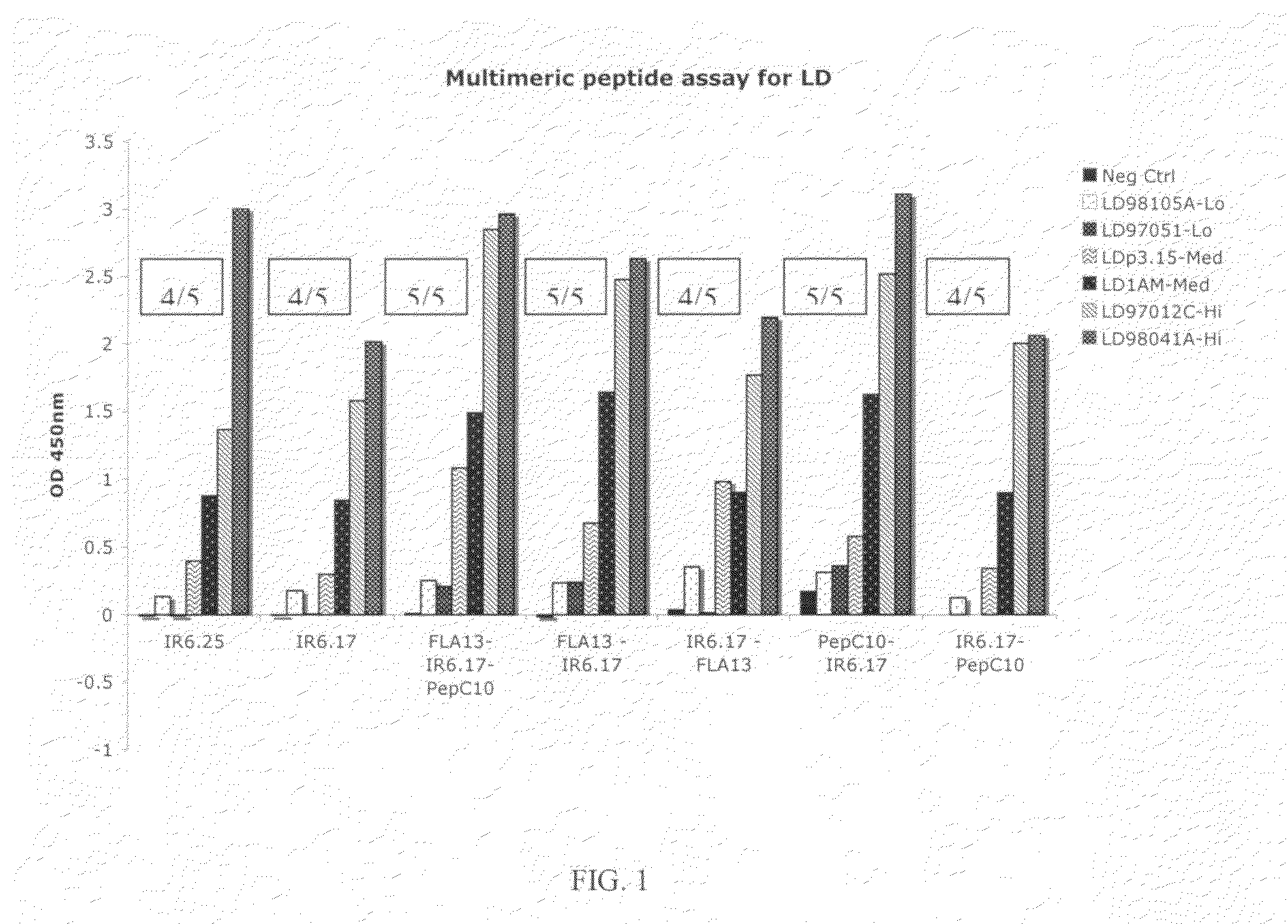
Peptide diagnostic agent for lyme disease
ActiveUS20090162875A1Bacterial antigen ingredientsPeptide/protein ingredientsEpitopeDiagnostic agent
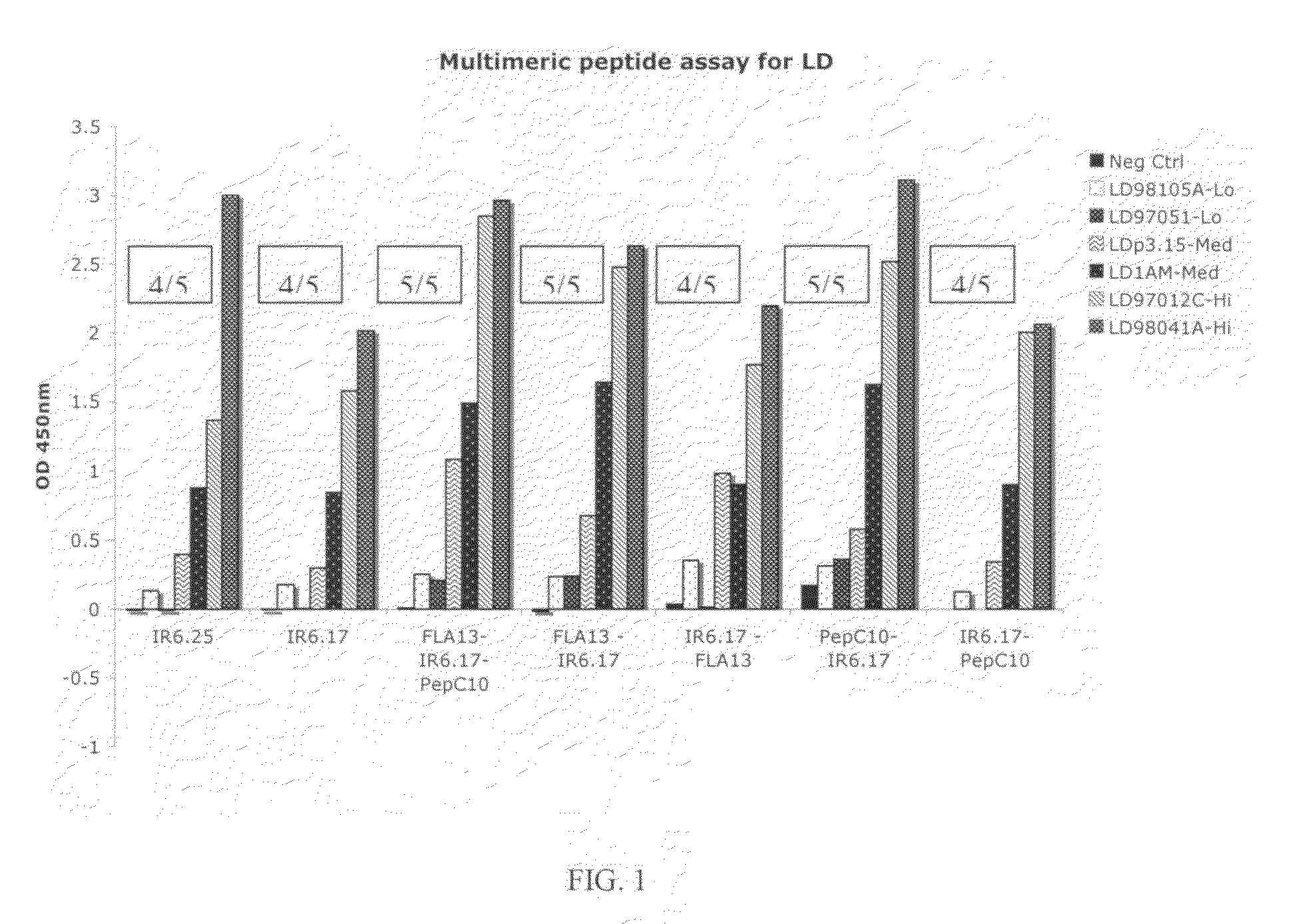
The present invention relates, e.g., to an isolated peptide consisting of the sequence MKKDDQIAAAIALRGMA (SEQ ID NO:1) or an active variant thereof, wherein the peptide or active variant can bind specifically to an antibody induced by a causative agent of Lyme disease (a pathogenic Borrelia), e.g. in a sample from a subject having Lyme disease. Also disclosed are linear multimeric peptides that contain the peptide represented by SEQ ID NO:1 as well as one or more additional peptide epitopes from other Borrelia proteins that can also bind specifically to an antibody as above. Compositions and diagnostic kits comprising a peptide of the invention are described, as are diagnostic assays using the peptide(s).
Owner:BIOPEPTIDES LLC
Peptide diagnostic agent for lyme disease
The present invention relates, e.g., to an isolated peptide consisting of the sequence MKKDDQIAAAIALRGMA (SEQ ID NO:1) or an active variant thereof, wherein the peptide or active variant can bind specifically to an antibody induced by a causative agent of Lyme disease (a pathogenic Borrelia), e.g. in a sample from a subject having Lyme disease. Also disclosed are linear multimeric peptides that contain the peptide represented by SEQ ID NO:1 as well as one or more additional peptide epitopes from other Borrelia proteins that can also bind specifically to an antibody as above. Compositions and diagnostic kits comprising a peptide of the invention are described, as are diagnostic assays using the peptide(s).
Owner:BIOPEPTIDES LLC
Oral vaccine for Borrelia
The present invention relates to vaccines for control of Borrelia infections in animal and human populations. In particular, the present invention provides compositions and methods comprising recombinant bacteria engineered to express one or more Borrelia burgdorferi antigens for use as Lyme disease vaccines. In some embodiments, the recombinant bacteria are freeze-dried.
Owner:US BIOLOGIC INC
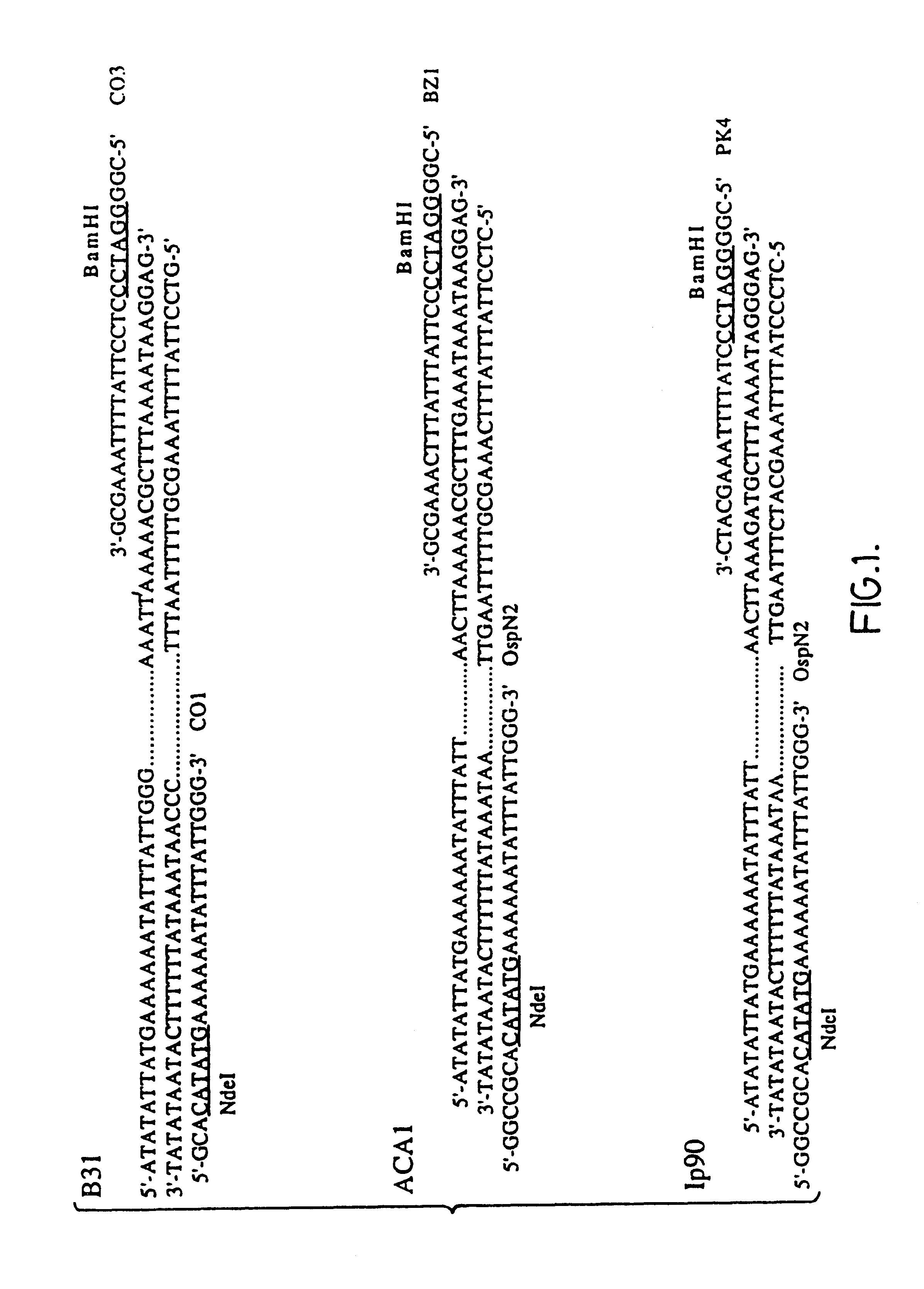
66 kDa antigen from Borrelia
InactiveUS6054296AReduce sensitivityHigh selectivityAntibacterial agentsAntibody mimetics/scaffoldsProtozoaAntigen
The present invention relates to nucleic acid molecules, polypeptides encoded by the same, antibodies directed thereto and a method of preparing such polypeptides including: (a) inserting an isolated DNA molecule coding for a polypeptide which is immunoreactive with a 66 kDa polypeptide derived from Borrelia garinii IP90 into an expression vector; (b) transforming a host organism or cell with the vector; (c) culturing the transformed host cell under suitable conditions; and (d) harvesting the polypeptide. The isolated DNA molecule is preferably at least 10 nucleotides in length, and the method may optionally include subjecting the polypeptide to post-translational modification. The host cell can be a bacterium, a yeast, a protozoan, or a cell derived from a multicellular organism such as a fungus, an insect cell, a plant cell, or a mammalian cell.
Owner:SYMBICOM
66 kDa antigen from Borrelia
InactiveUS6090586AReduce sensitivityHigh selectivityAntibacterial agentsAntibody mimetics/scaffoldsAntigenProtozoa
Owner:SYMBICOM
66 kDa antigen from Borrelia
InactiveUS6068842AReduce sensitivityHigh selectivityAntibacterial agentsAntibody mimetics/scaffoldsAntigenProtozoa
The present invention relates to nucleic acid molecules, polypeptides encoded by the same, antibodies directed thereto and a method of preparing such polypeptides including: (a) inserting an isolated DNA molecule coding for a polypeptide which is immunoreactive with a 66 kDa polypeptide derived from Borrelia garinii IP90 into an expression vector; (b) transforming a host organism or cell with the vector; (c) culturing the transformed host cell under suitable conditions; and (d) harvesting the polypeptide. The isolated DNA molecule is preferably at least 10 nucleotides in length, and the method may optionally include subjecting the polypeptide to post-translational modification. The host cell can be a bacterium, a yeast, a protozoan, or a cell derived from a multicellular organism such as a fungus, an insect cell, a plant cell, or a mammalian cell.
Owner:SYMBICOM
Diagnostic test for borrelia infection
InactiveUS6617441B1Inhibit digestionSugar derivativesAntibody mimetics/scaffoldsDiseaseLone star ticks
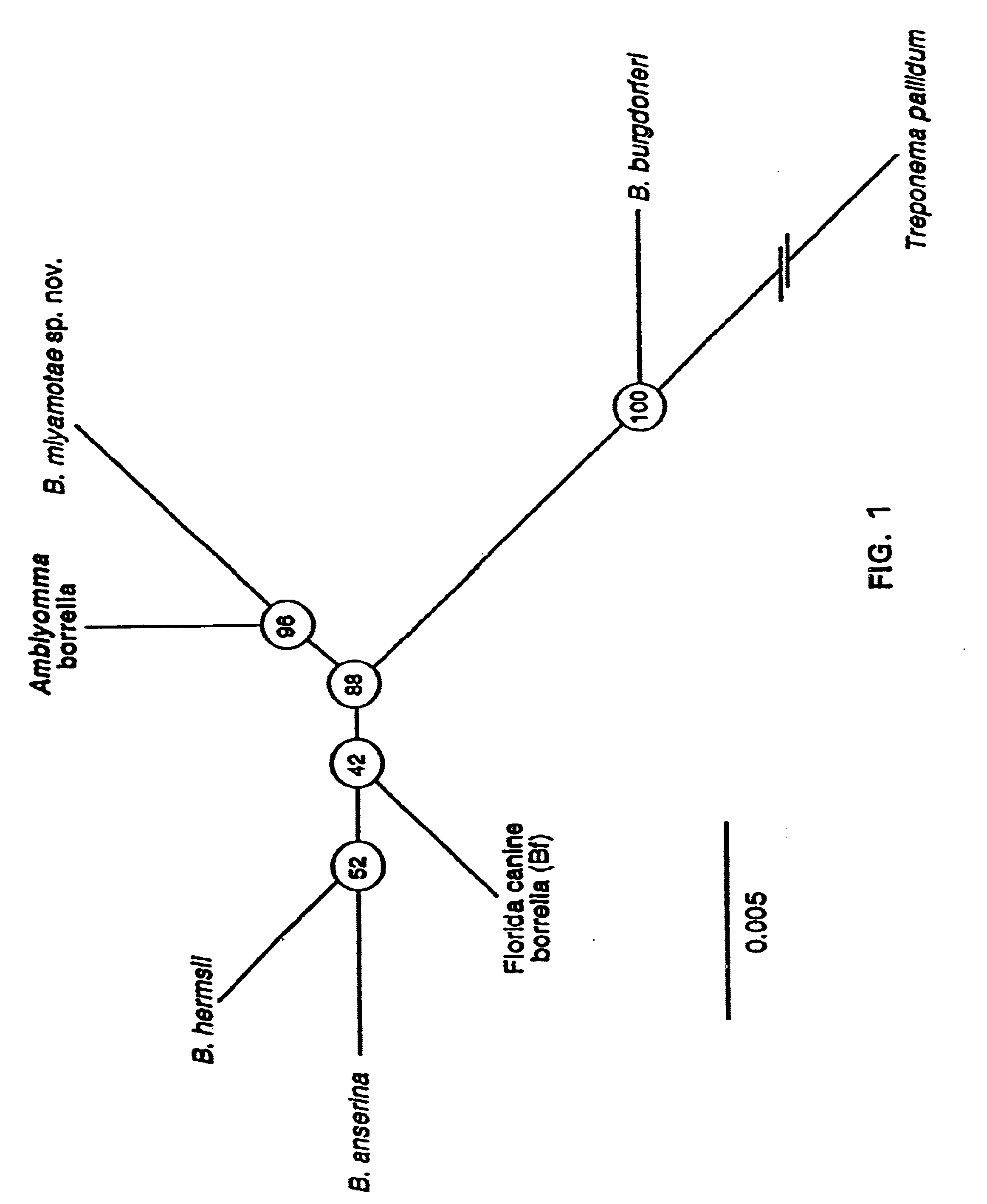
Bites from Amblyomma americanum, a hard tick, have been associated with a Lyme disease-like illness in the southeastern and south-central United States. Present in 2% of ticks collected in four states were uncultivable spirochetes. Through use of the polymerase chain reaction, partial sequences of the flagellin and 16s rRNA genes of microorganisms from Texas and New Jersey were obtained. The sequences showed that the spirochete was a Borrelia sp. but distinct from other known members of this genus, including B. burgdorferi, the agent of Lyme disease. Species-specific differences in the sequences of the flagellin protein, the flagellin gene and the 16s rRNA gene between the new Borrelia species and previously known species provide compositions and methods for assay for determining the presence of this new spirochete, or for providing evidence of past or present infection by this spirochete in animal reservoirs and humans.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
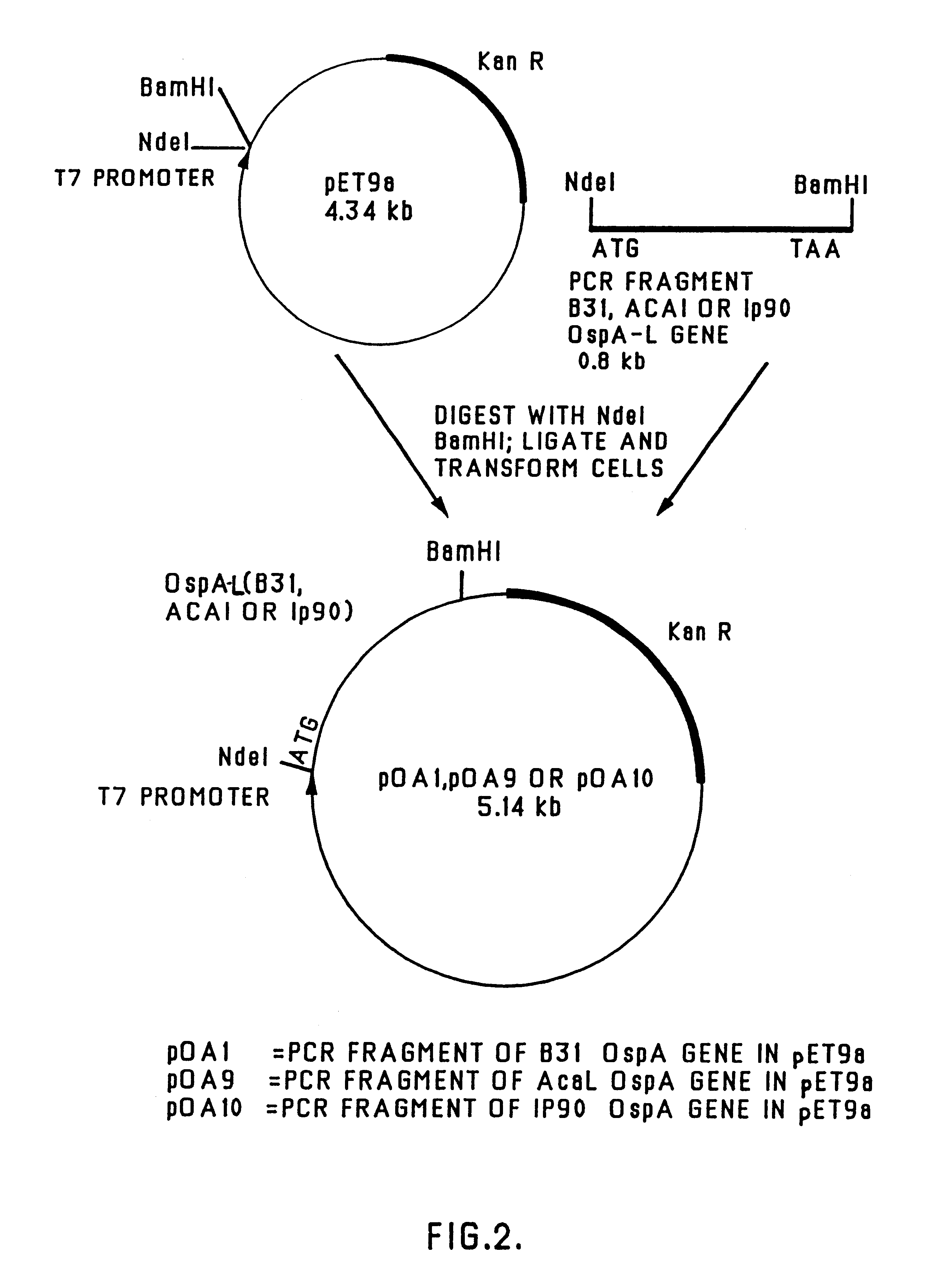
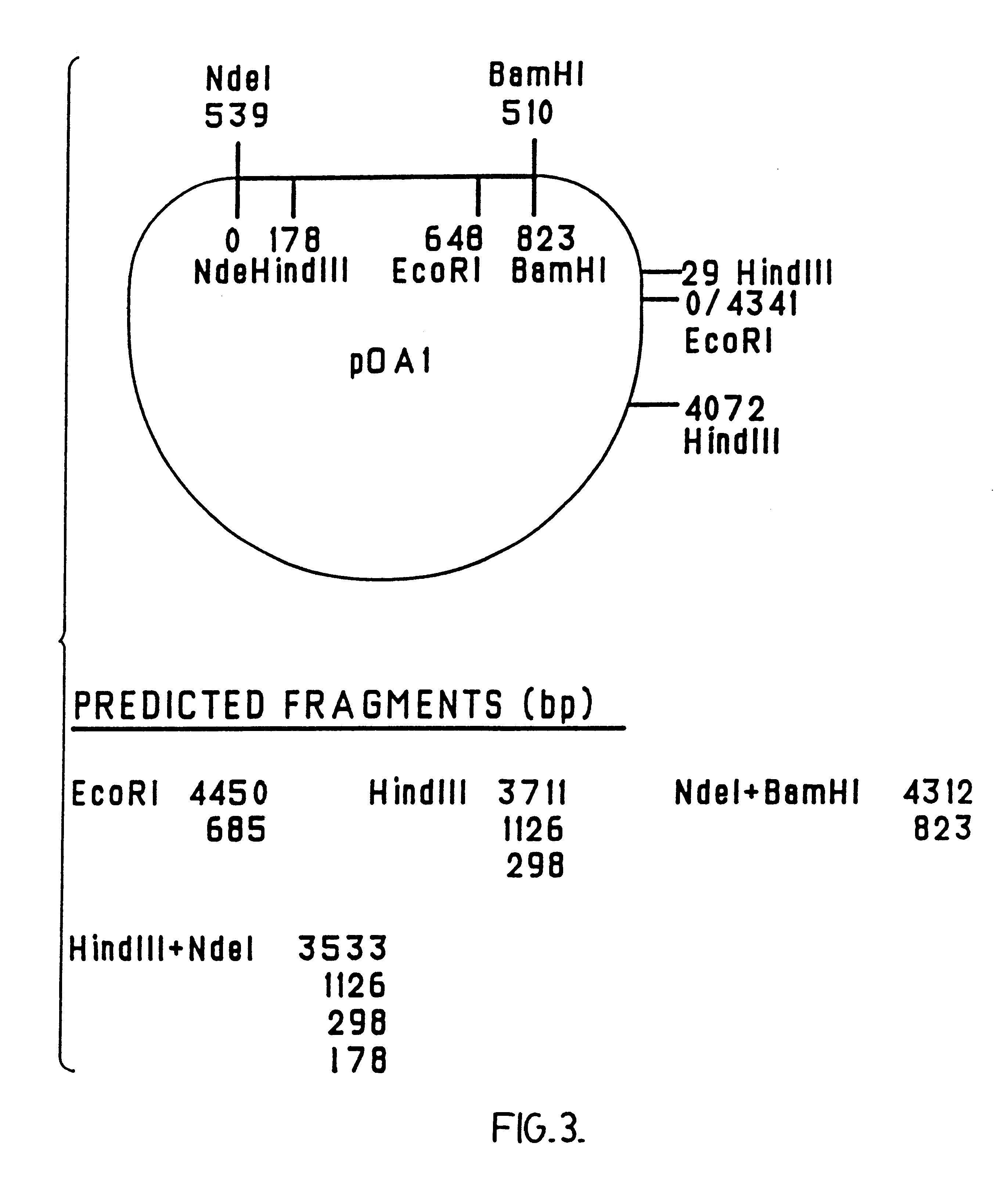
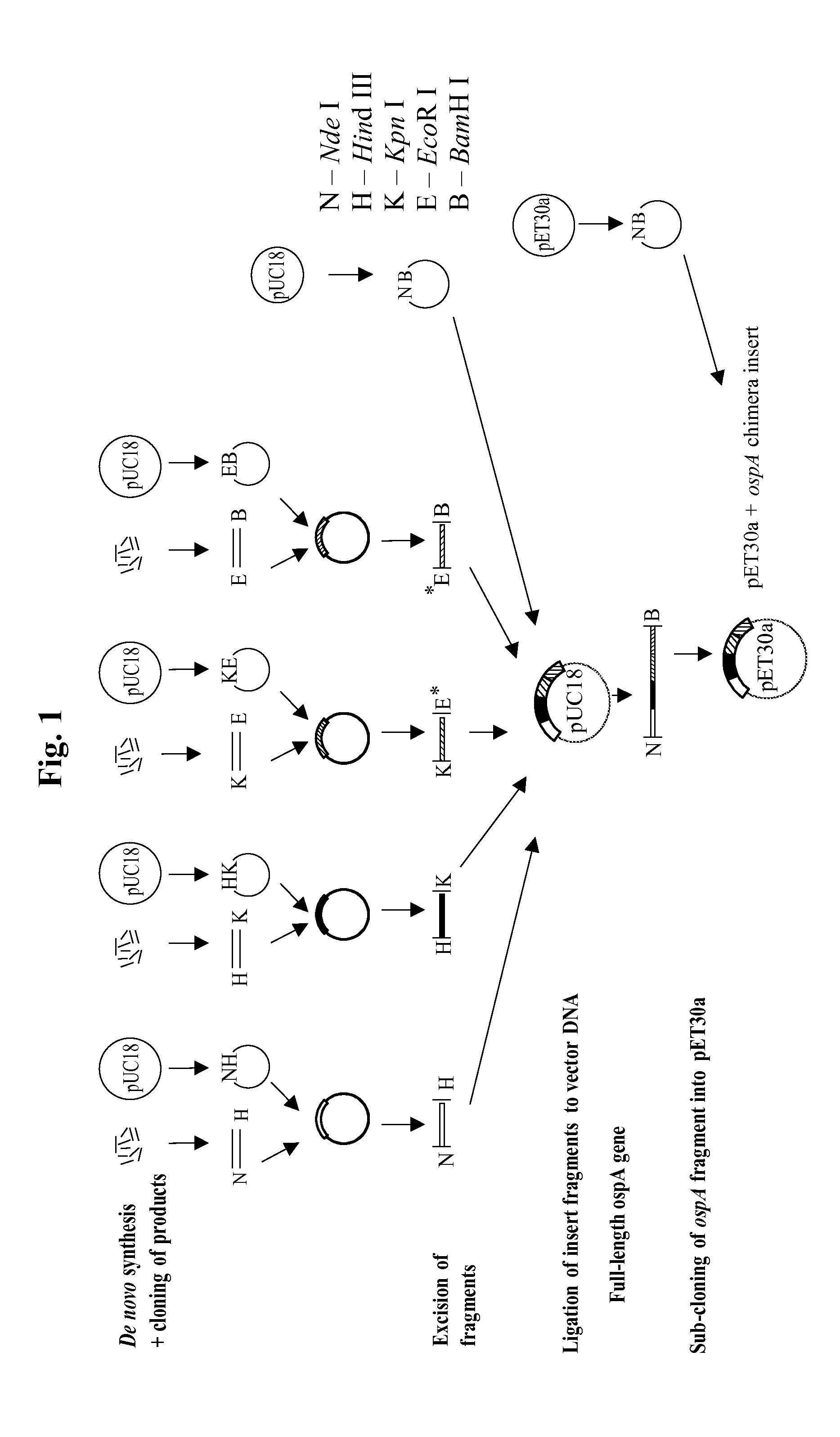
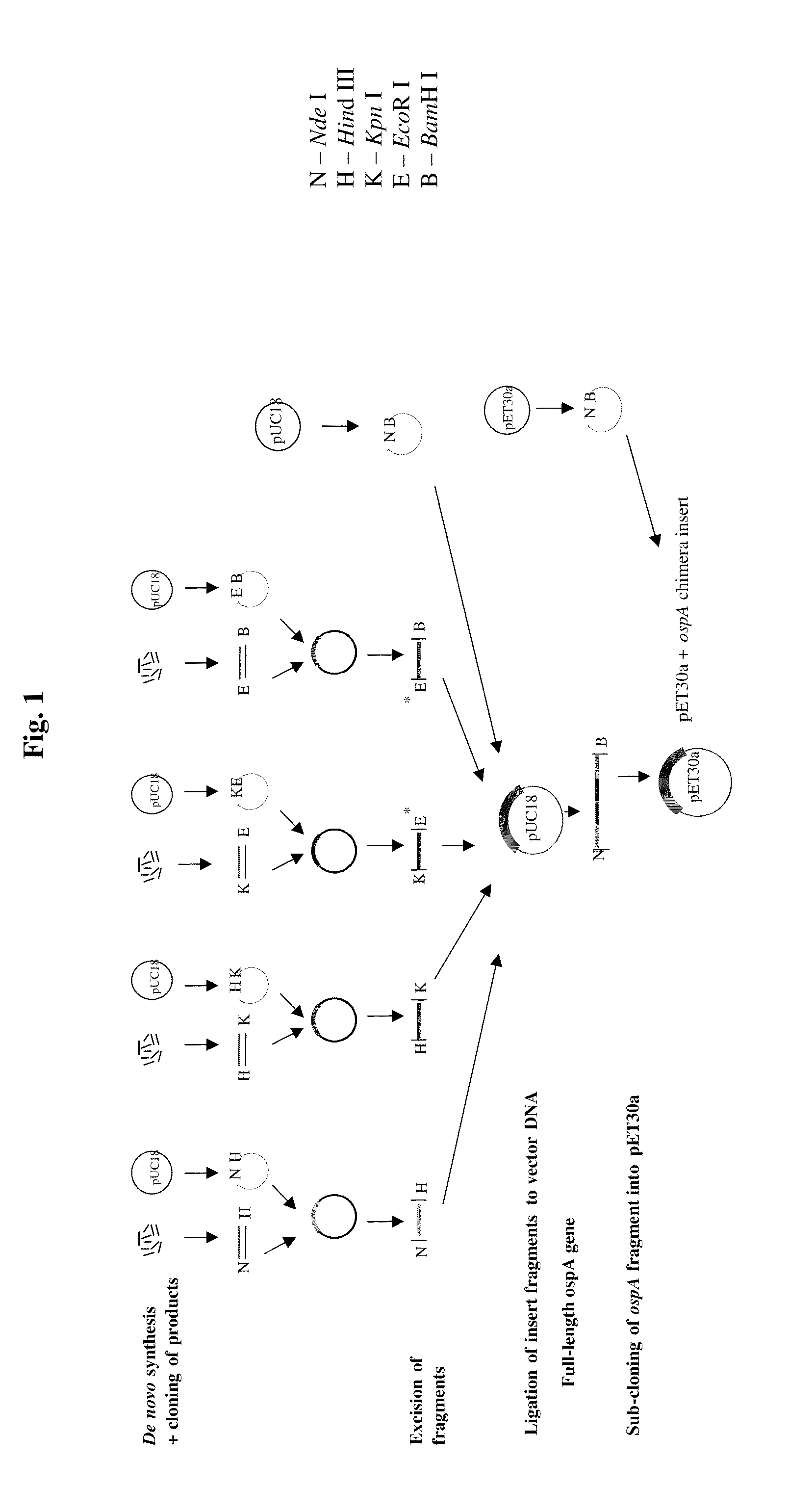
Chimeric ospa genes, proteins, and methods of use thereof
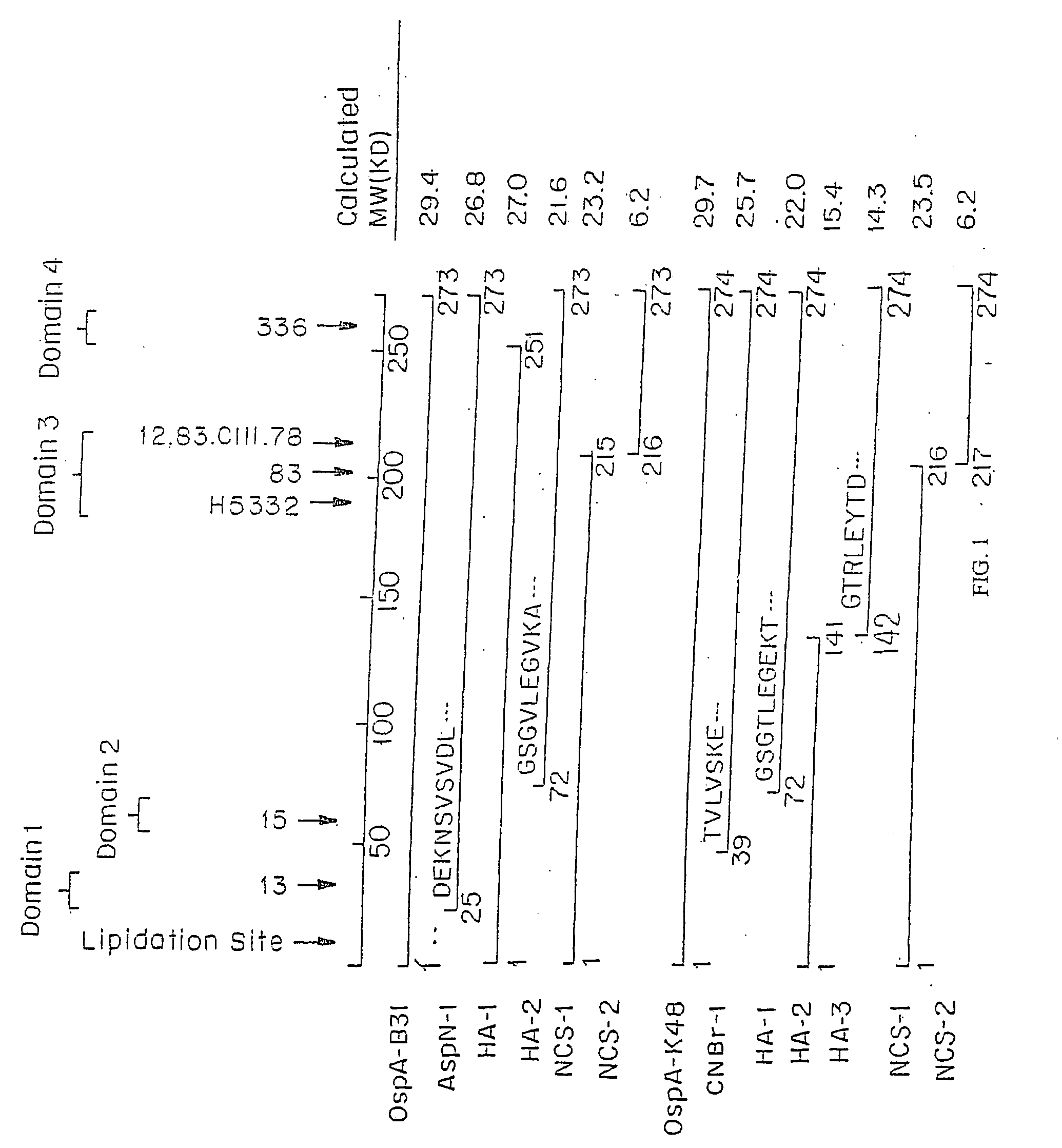
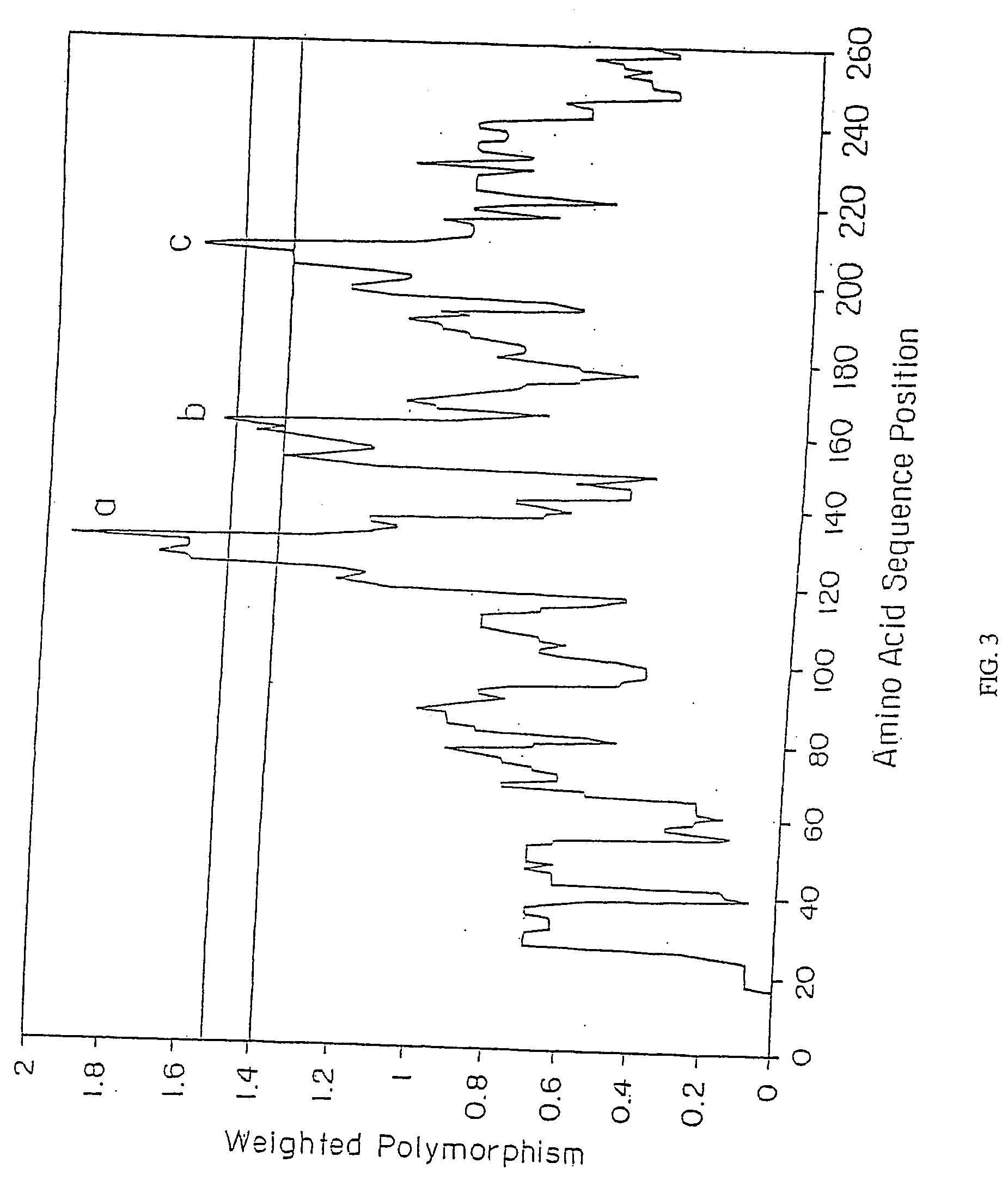
The invention relates to the development of chimeric OspA molecules for use in a new Lyme vaccine. More specifically, the chimeric OspA molecules comprise the proximal portion from one OspA serotype, together with the distal portion from another OspA serotype, while retaining antigenic properties of both of the parent polypeptides. The chimeric OspA molecules are delivered alone or in combination to provide protection against a variety of Borrelia genospecies. The invention also provides methods for administering the chimeric OspA molecules to a subject in the prevention and treatment of Lyme disease or borreliosis.
Owner:BAXALTA INC +2
Chimeric ospa genes, proteins, and methods of use thereof
The invention relates to the development of chimeric OspA molecules for use in a new Lyme vaccine. More specifically, the chimeric OspA molecules comprise the proximal portion from one OspA serotype, together with the distal portion from another OspA serotype, while retaining antigenic properties of both of the parent polypeptides. The chimeric OspA molecules are delivered alone or in combination to provide protection against a variety of Borrelia genospecies. The invention also provides methods for administering the chimeric OspA molecules to a subject in the prevention and treatment of Lyme disease or borreliosis.
Owner:BAXALTA GMBH
Live bacterial vaccine
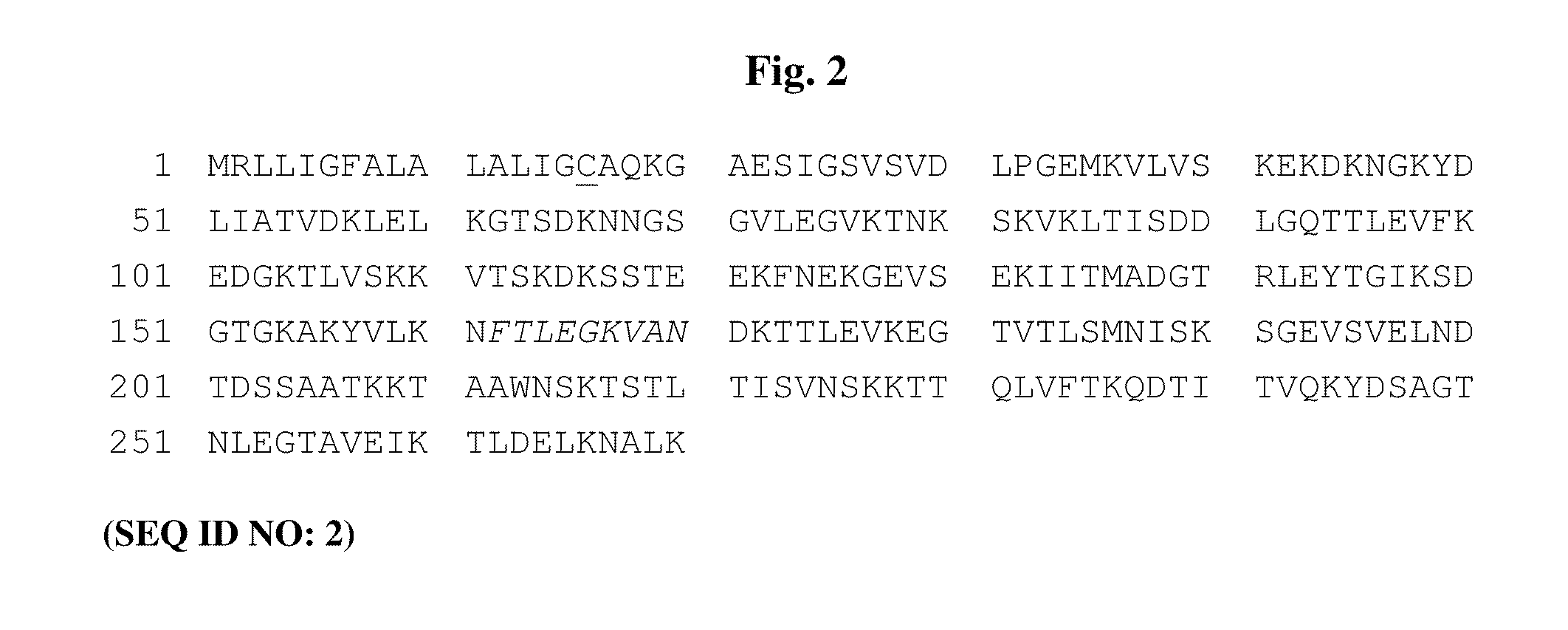
The present invention relates, e.g., to a Lactobacillus bacterium, which (1) expresses a recombinant polypeptide containing a lipoprotein signal sequence from the OspA protein of Borrelia burgdorferi, or an active variant of the leader sequence, operably linked to one or more heterologous polypeptide(s) of interest and / or (2) which comprises an expressible polynucleotide encoding a recombinant polypeptide, wherein the polynucleotide encodes a lipoprotein signal from the OspA protein of Borrelia burgdorferi, or an active variant thereof, which is operably linked to one or more heterologous polypeptide(s) of interest. In one embodiment, the heterologous polypeptide is from Yersinia pestis, the etiologic agent of plague. In another embodiment, the heterologous polypeptide is from Borrelia burgdorferi, the etiologic agent of Lyme disease. Also described are immunogenic compositions, such as live bacterial vaccines, comprising the bacterium; methods for eliciting an immune response against the polypeptide using the bacterium; and kits comprising the bacterium.
Owner:LACTRYS OCTROOI +1
Oral vaccine for Borrelia
The present invention relates to vaccines for control of Borrelia infections in animal and human populations. In particular, the present invention provides compositions and methods comprising recombinant bacteria engineered to express one or more Borrelia burgdorferi antigens for use as Lyme disease vaccines. In some embodiments, the recombinant bacteria are freeze-dried.
Owner:US BIOLOGIC INC
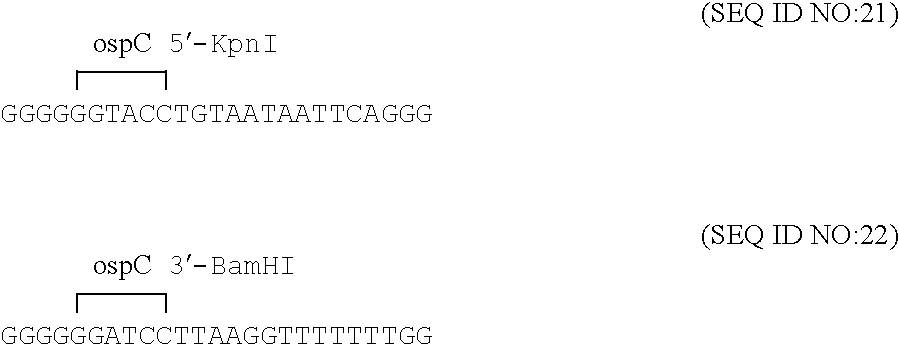
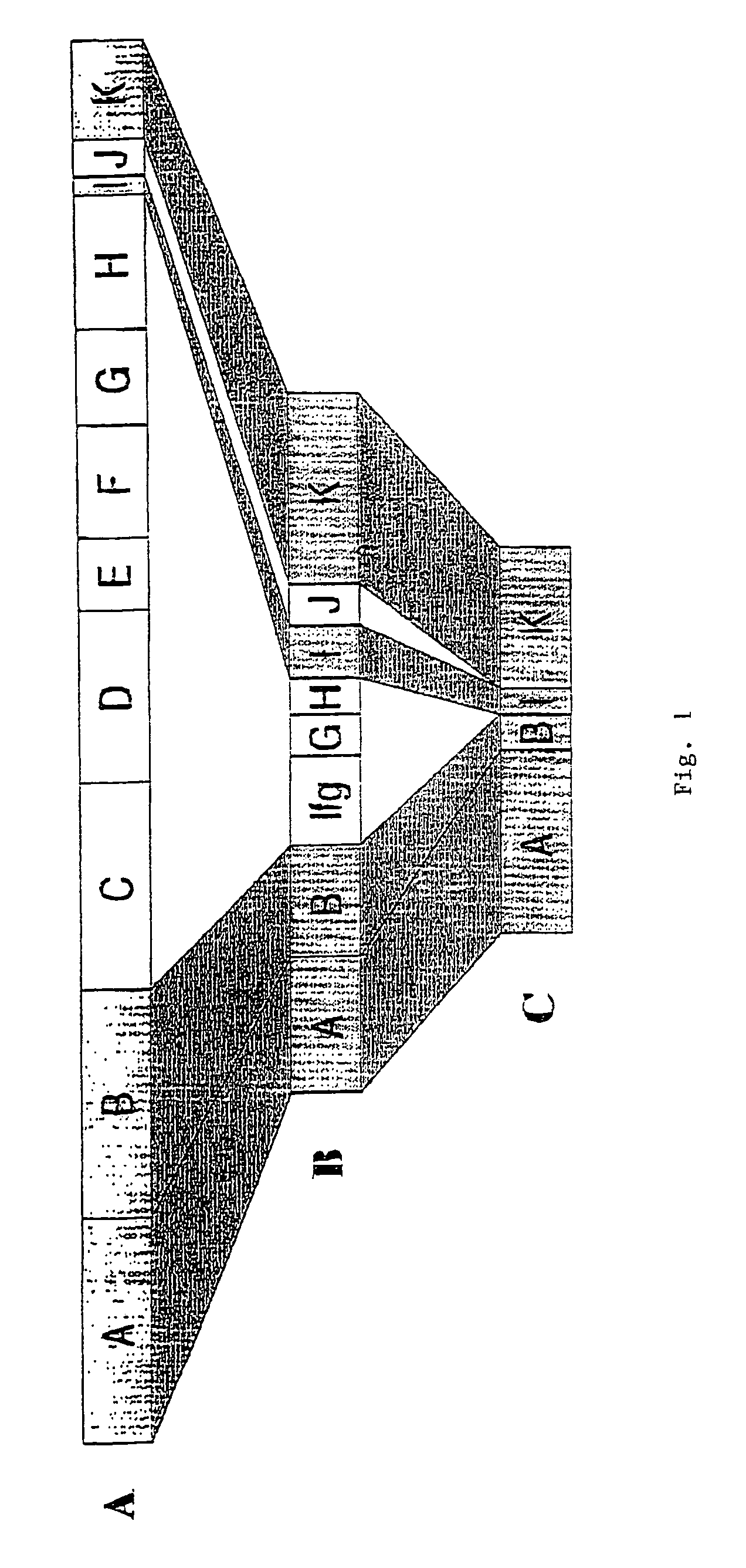
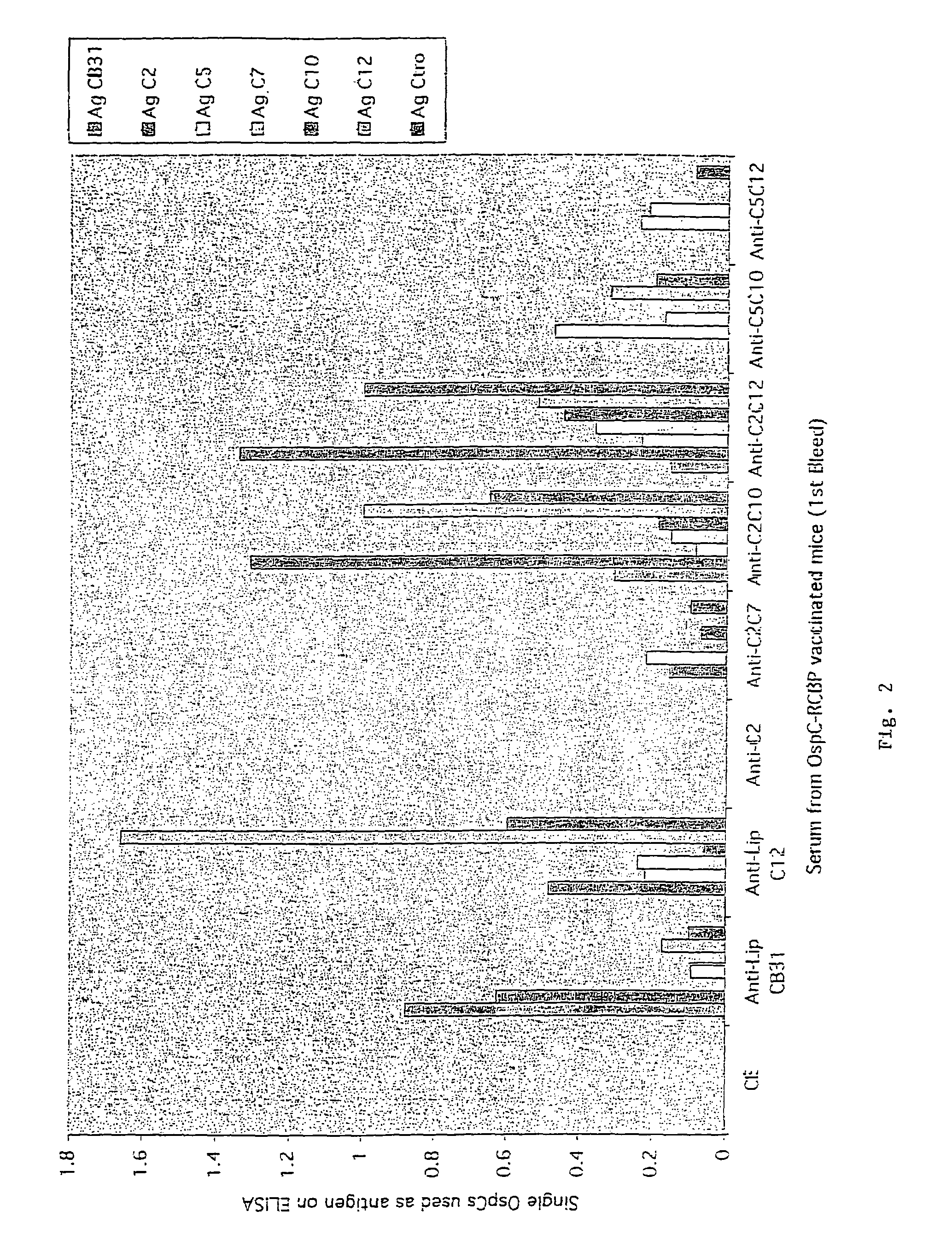
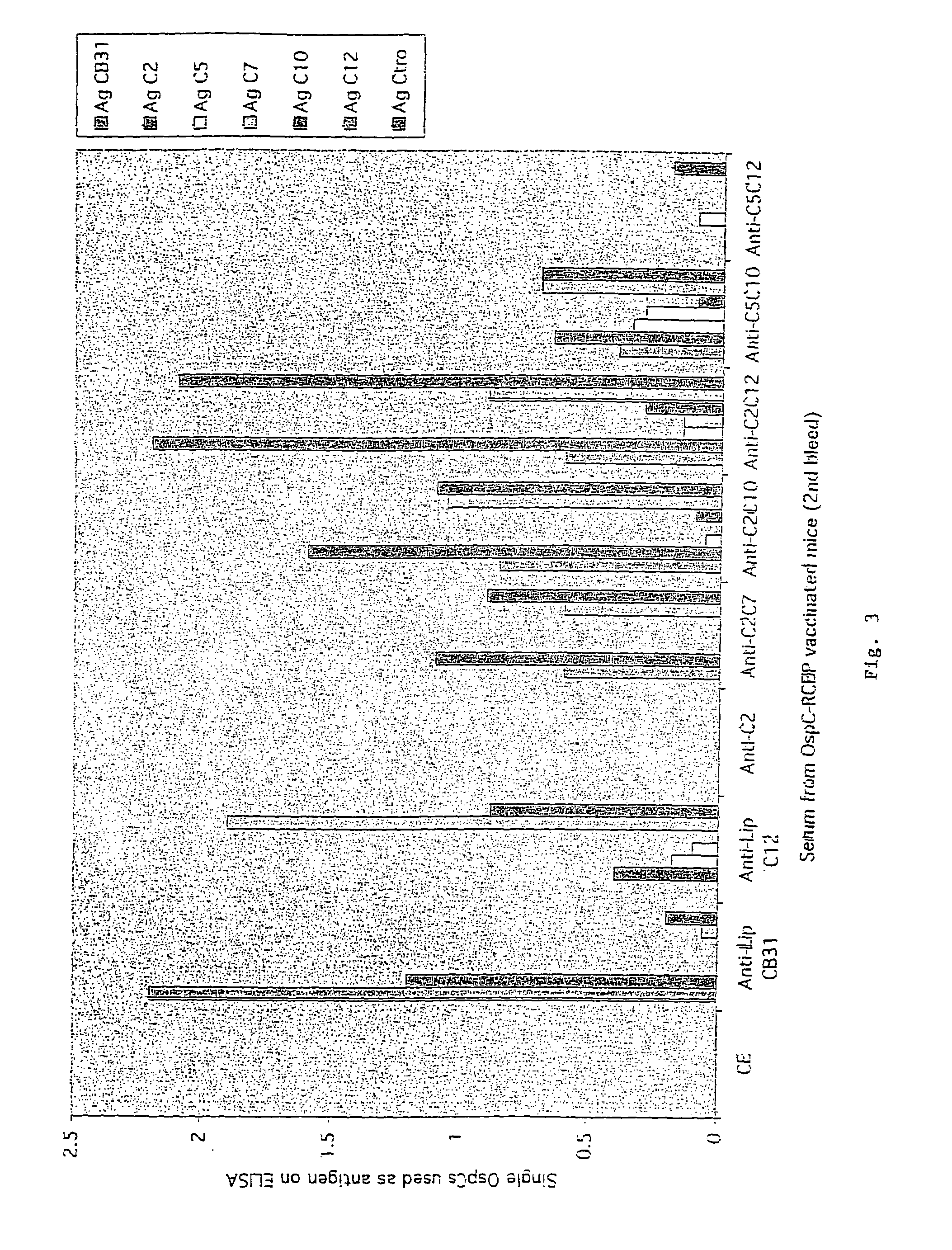
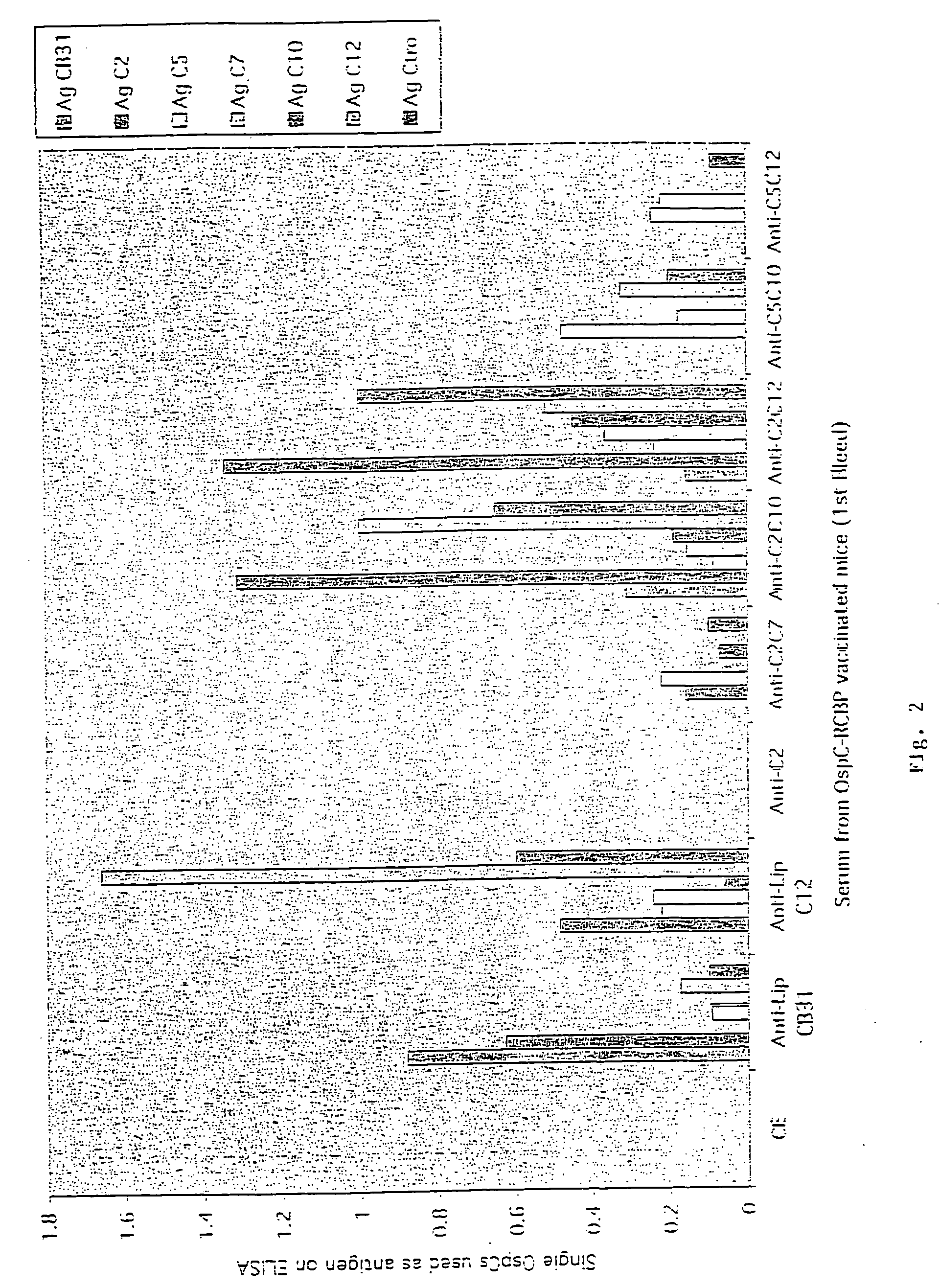
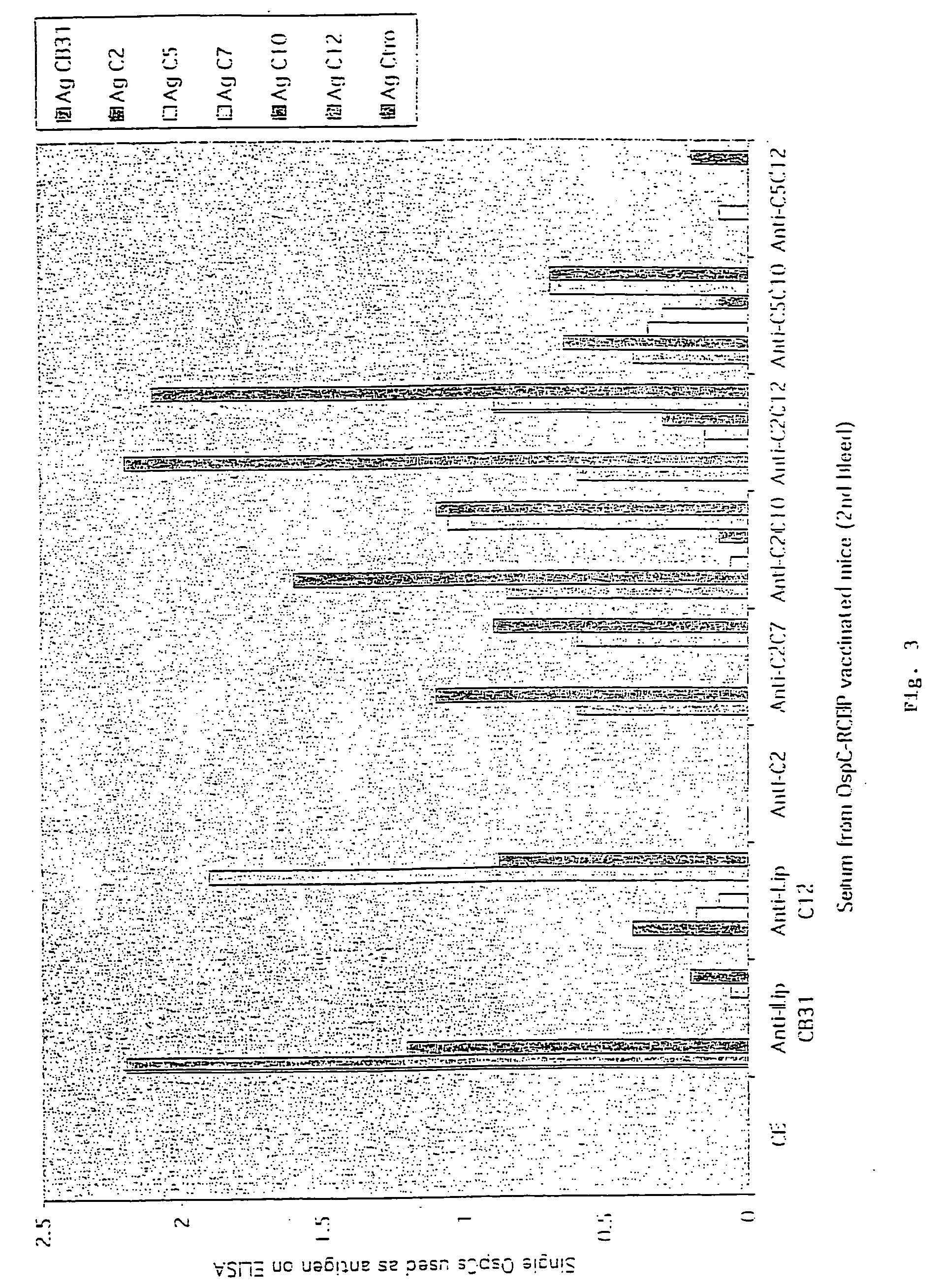
Groups of barrelia burgdorferi and borrelia afzelii that cause lyme disease in humans
InactiveUS7060281B1Simple methodIncrease productionPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseImmunogenicity
The present invention is drawn to an immunogenic composition comprising OspC polypeptides from Lyme Disease causing Borrelia. In one embodiment, the immunogenic composition of the present invention comprises at least one OspC polypeptide or immunogenic fragment thereof from each of Borrelia burgdorferi OspC families A, B, I and K. In another embodiment, the immunogenic composition of the present invention comprises at least one OspC polypeptide or immunogenic fragment thereof from each of Borrelia afzelii OspC families A and B.
Owner:BIOPEPTIDES LLC +2
Borrelia diagnostics and screening methods
InactiveUS20100278866A1Antibacterial agentsBacterial antigen ingredientsBorrelia gariniiScreening method
Compositions and methods of detecting Borrelia proteins, nucleic acid sequences encoding these proteins, and subject antibodies to these proteins in a sample are disclosed.
Owner:RGT UNIV OF CALIFORNIA
Topical antibiotic composition for the prevention of Lyme disease
The invention relates to topical pharmaceutical compositions and methods related to Borrelia burgdorferi toxins, in particular, the present invention provides compositions and methods for the treatment of infections caused by Borrelia burgdorferi and in particular for the prevention of Lyme disease.
Owner:IXODES
Decorin binding protein compositions
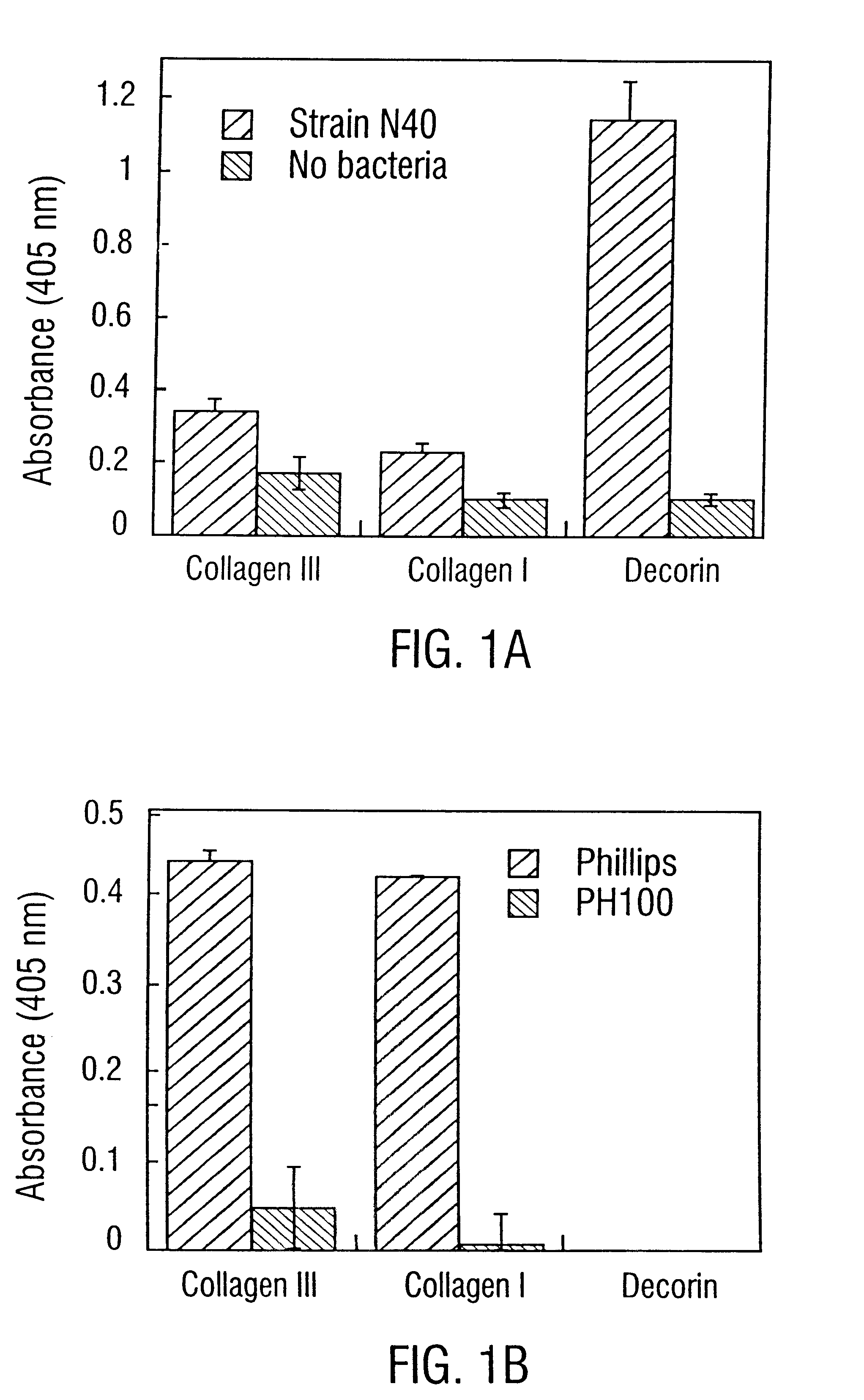
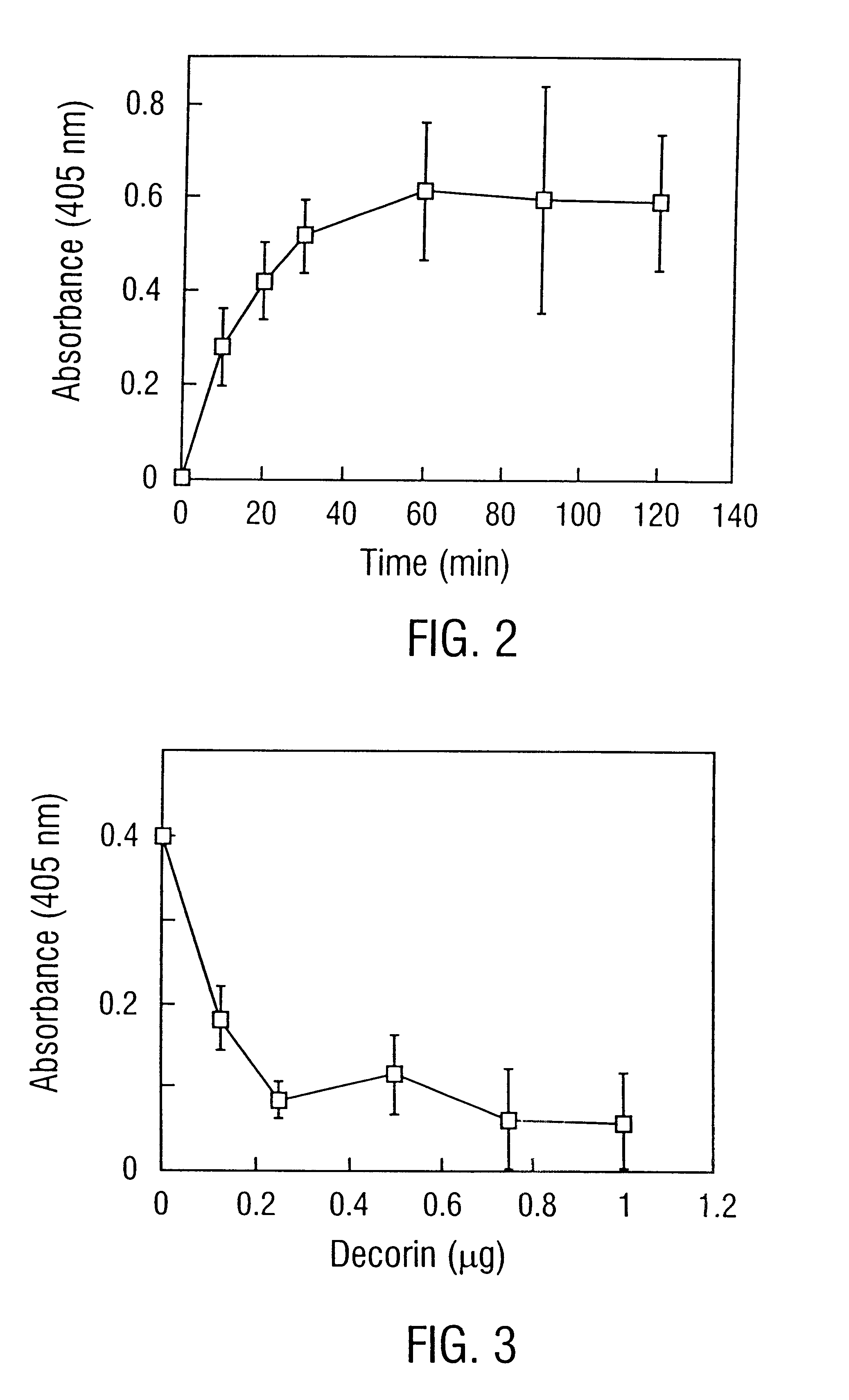
Disclosed are the dbp gene and dbp-derived nucleic acid segments from Borrelia burgdorferi, the etiological agent of Lyme disease, and DNA segments encoding dbp from related borrelias. Also disclosed are decorin binding protein compositions and methods of use. The DBP protein and antigenic epitopes derived therefrom are contemplated for use in the treatment of pathological Borrelia infections, and in particular, for use in the prevention of bacterial adhesion to decorin. DNA segments encoding these proteins and anti-(decorin binding protein) antibodies will also be of use in various screening, diagnostic and therapeutic applications including active and passive immunization and methods for the prevention of Borrelia colonization in an animal. These DNA segments and the peptides derived therefrom are contemplated for use in the preparation of vaccines and, also, for use as carrier proteins in vaccine formulations, and in the formulation of compositions for use in the prevention of Lyme disease.
Owner:TEXAS A&M UNIVERSITY
Borrelia Provocation Procedure Kit
ActiveUS20150353988A1Effective treatmentEffective and timely interventionBiocideMicrobiological testing/measurementBacteroidesSpiroplasma
The testing for the Lyme disease pathogen, (Borrelia bergdorferi (Bb) and all other Lyme Borrelia), is notoriously difficult. Bb is not by nature a blood loving bacteria, and prefers the climate of avascular tissues such as cartilage. The Borrelia Provocation technology disclosed herein proposes to uniquely lure the Lyme Borrelia spirochetes into the blood through a simple procedure of sub-dermal injection of benign tick saliva and co-factors. Lyme Borrelia can then be tested accurately after the provocation injection, utilizing any Lyme Borrelia detection test such as a specific PCR (polymerase chain reaction) test for the most accurate results, as there will be abundantly available DNA present in the blood if there is an existing infection. Treatment is more effective after provocation as well. The provocation protocol taught herein makes accurate, non-invasive, active direct Lyme infection testing possible, and creates a platform for an effective treatment as well.
Owner:TITAN COLLABORATIVE KITHE LLC
Recombinant constructs of Borrelia burgdorferi
InactiveUS20050271682A1Improve overall utilizationAvoid infectionBacteriaPeptide/protein ingredientsBorrelia gariniiBorreliella burgdorferi
Novel chimeric nucleic acids, encoding chimeric Borrelia proteins comprising OspC or an antigenic fragment thereof and OspA or an antigenic fragment thereof, are disclosed. Chimeric proteins encoded by the nucleic acid sequences are also disclosed. The chimeric proteins are useful as vaccine immunogens against Lyme borreliosis, as well as for immunodiagnostic reagents.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Vaccines and methods to treat lyme disease in dogs
InactiveCN104379164AAntibacterial agentsBacterial antigen ingredientsBorrelia gariniiBorrelia burgdorferi
The instant invention provides an immunogenic composition comprising an antigenic fragment of OspA protein of Borrelia burgdorferi and a chimeric protein containing antigenic fragments of different phylotypes of OspC protein of Borrelia burgdorferi. Vaccines incorporating the immunogenic composition of the invention, as well as methods of preventing Lyme disease in dogs and / or protecting dogs from Lyme disease using the vaccines are also provided.
Owner:ZOETIS SERVICE LLC +1
B.garinii polyclonal antibody and application
InactiveCN106432487AStrong specificityHigh potencySerum immunoglobulinsImmunoglobulins against bacteriaAdjuvantNew Zealand white rabbit
The invention discloses a B.garinii polyclonal antibody and an application. A BSK-II culture medium is inoculated with a B.garinii strain for enlargement culture, and a somatic antigen is obtained after inactivation; after the obtained somatic antigen and an adjuvant are fully emulsified, a New Zealand white rabbit is immunized by using multi-point intradermal injection; the immunized New Zealand white rabbit is anesthetized with chloral hydrate, whole blood is solidified at the room temperature after being collected, and serum containing the B.garinii polyclonal antibody is subjected to centrifugal separation. The B.garinii polyclonal antibody prepared with the method has good specificity and high titer, can effectively detect Lyme disease spirochete and has brighter application prospect.
Owner:杭州贝英福生物科技有限公司
Method for treating lyme disease
The present invention provides a method for treating Lyme disease and / or a Borrelia infection in animals comprising administering to an animal in need thereof a therapeutically effective amount of cefovecin.
Owner:ZOETIS SERVICE LLC
Obtaining mode and application of borrelia burgdorferi flagellin FlaB antibody
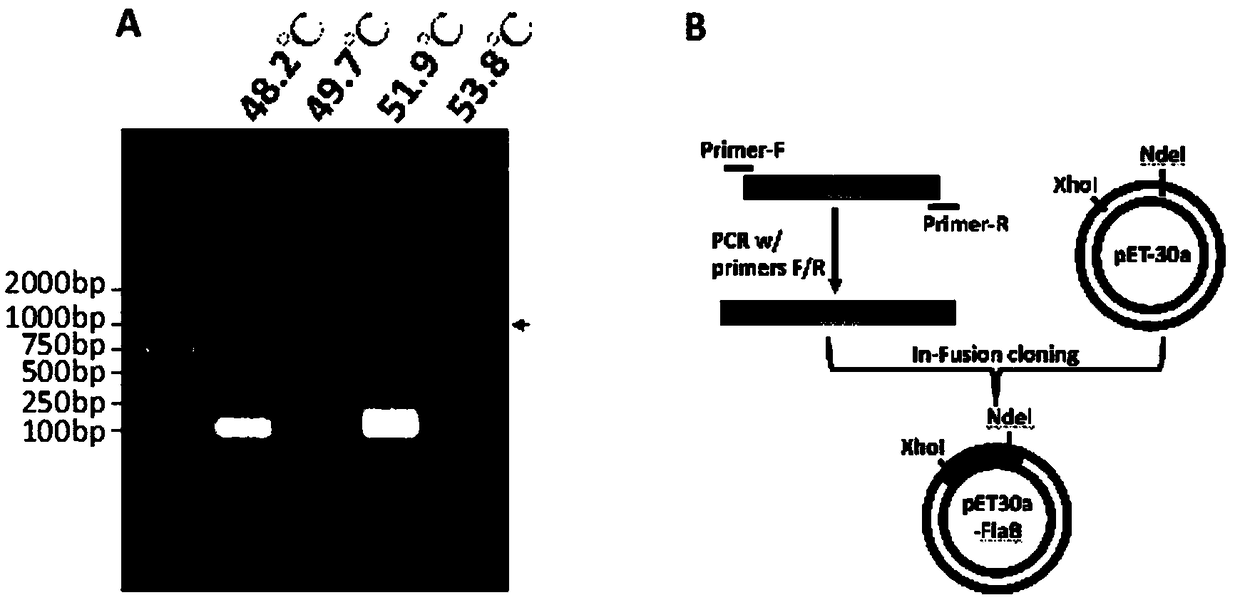
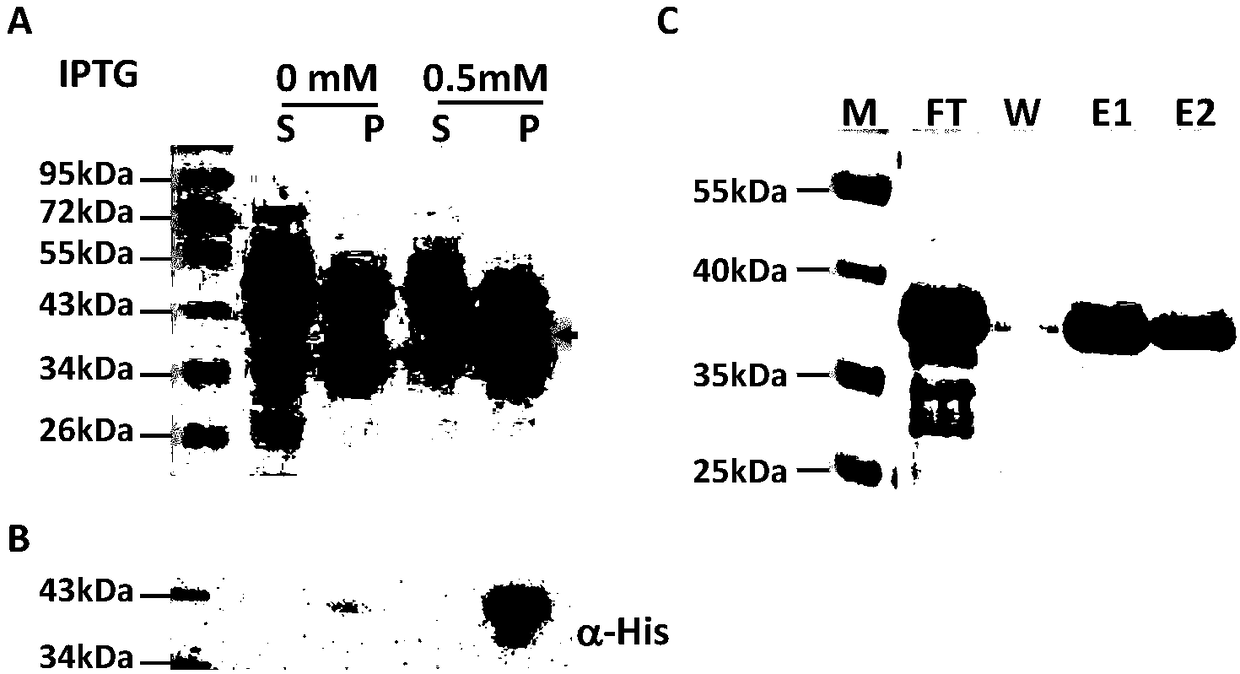
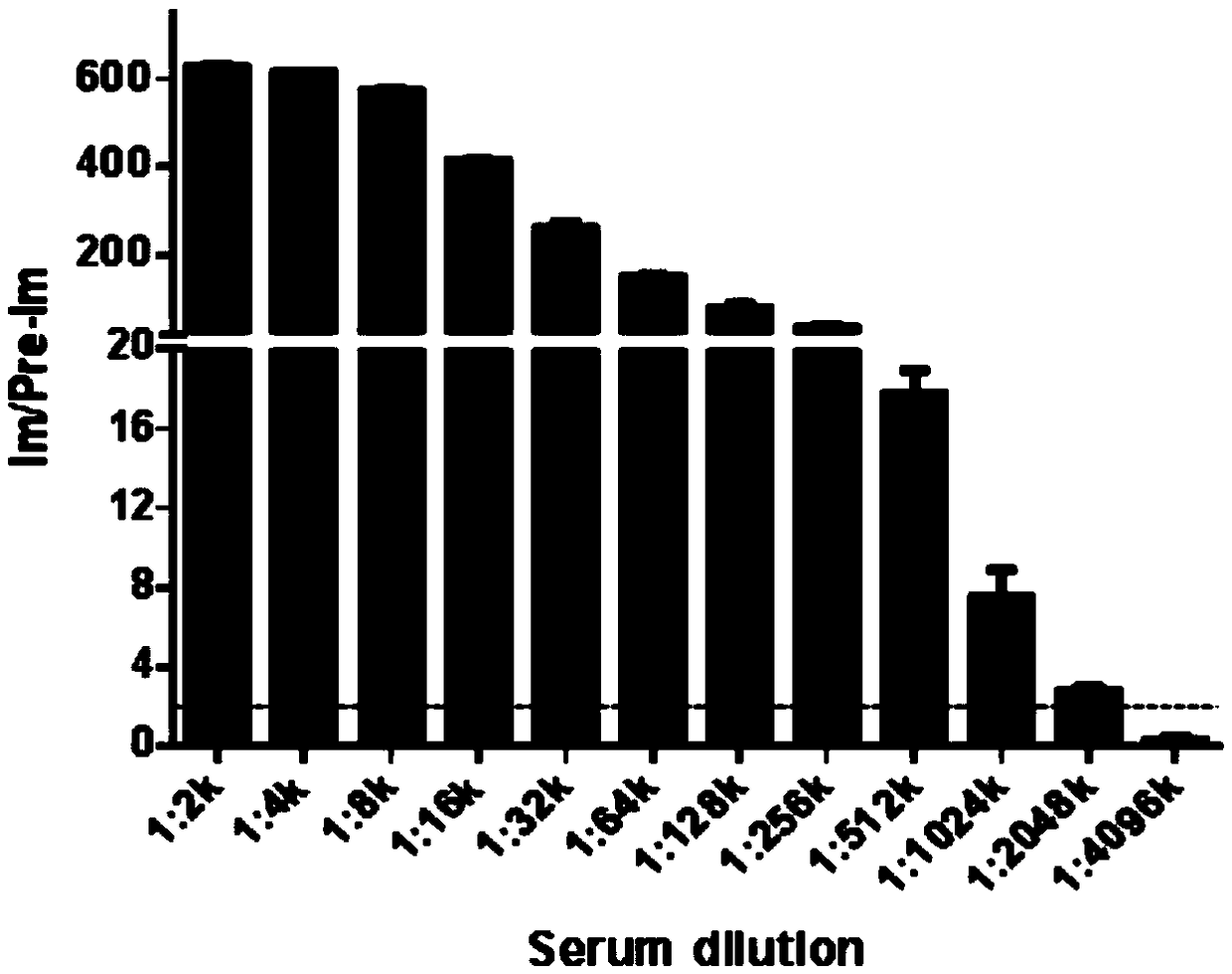
InactiveCN109134649AHigh purityHigh potencySerum immunoglobulinsBiological material analysisEscherichia coliSpiroplasma
The invention relates to preparation and application of a borrelia burgdorferi flagellin FlaB antibody. By means of a PCR method, a gene segment of FlaB is obtained from a borrelia burgdorferi genomethrough amplification, cloned into a prokaryotic expression vector pET30a containing a histidine tag and then transferred into escherichia coli BL21 competent cells. Induction expression is carried out through IPTG, affinity purification is performed to obtain FlaB protein, immunized mice obtain the borrelia burgdorferi flagellin FlaB antibody, and a solid foundation is laid for detection and deepresearch of borrelia burgdorferi.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Altered OSPA of Borrelia Burgdorferi
InactiveUS20140030285A1Improve stabilityLow cross-reactivityBacterial antigen ingredientsSugar derivativesBorrelia gariniiAntigenicity
Provided herein are OspA polypeptides from Lyme Disease-causing Borrelia having certain alteration(s). In one embodiment, the alteration(s) increase the conformational stability of the OspA polypeptide containing the alteration(s) while maintaining at least some of the antigenicity of the corresponding unaltered OspA polypeptide. In another embodiment, the altered OspA polypeptide has reduced cross-reactivity to hLFA-1, as compared to the corresponding unaltered OspA polypeptide.
Owner:BROOKHAVEN SCI ASSOCS +2
Method for treating Lyme disease
ActiveUS9265771B2Antibacterial agentsTetracycline active ingredientsTreated animalBorrelia Infections
The present invention provides a method for treating Lyme disease and / or a Borrelia infection in animals comprising administering to an animal in need thereof a therapeutically effective amount of cefovecin.
Owner:ZOETIS SERVICE LLC
Methods for diagnosing lyme disease
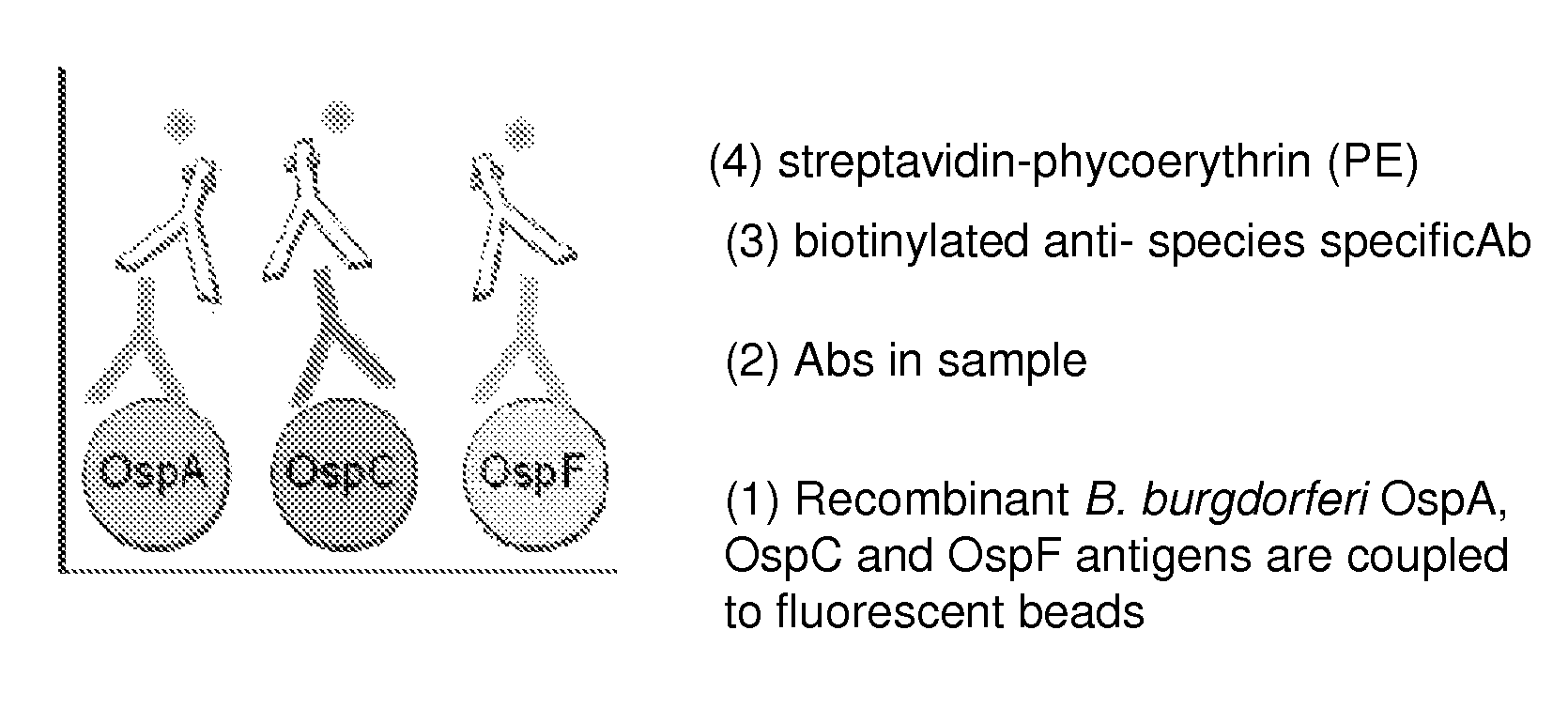
ActiveUS8946393B2Bacterial antigen ingredientsPeptide/protein ingredientsAntigenOuter surface protein
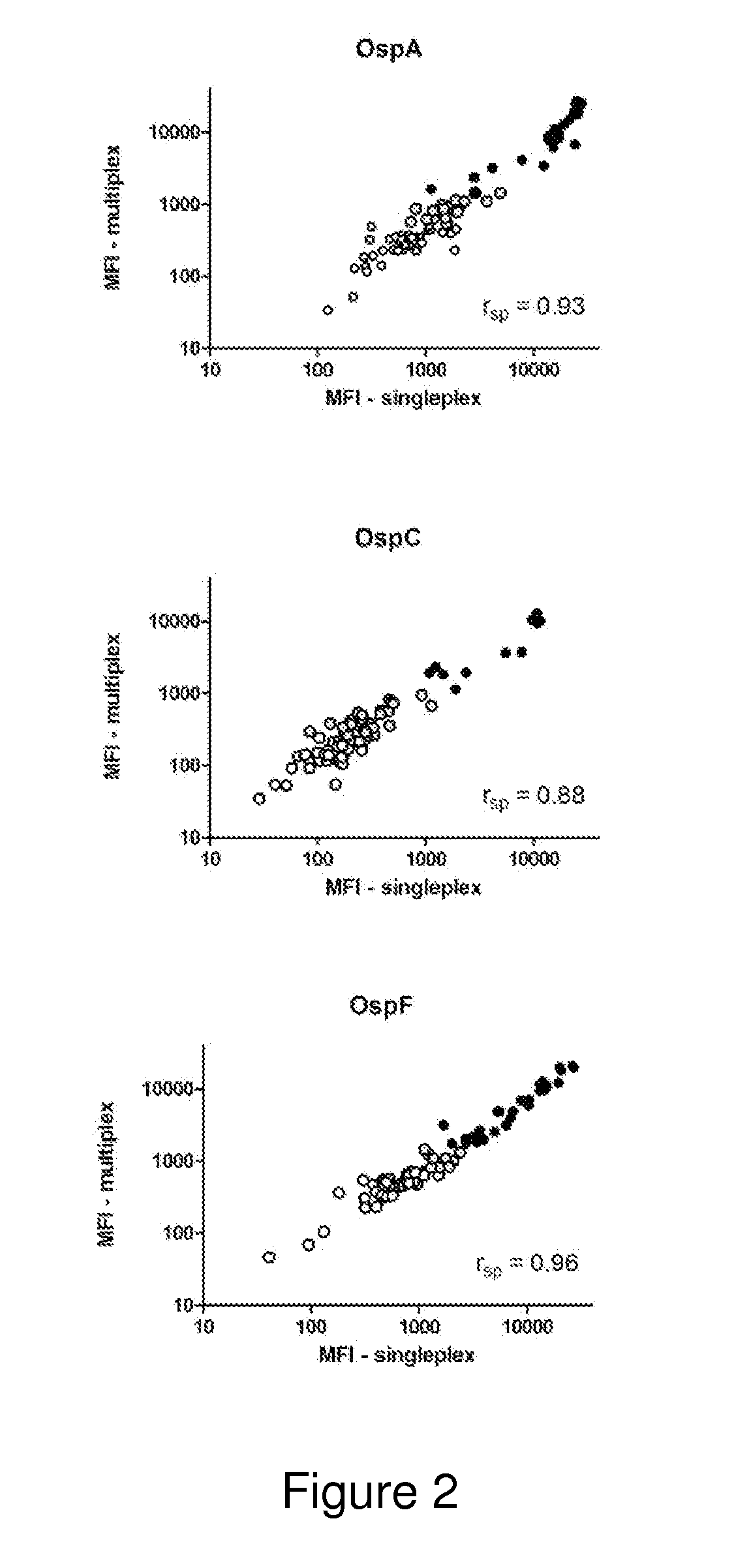
A method for diagnosing Lyme disease status in a mammal is provided. The method entails, in a biological sample obtained or derived from a mammal, determining antibodies to Borrelia burgdorferi (B. burgdorferi) outer surface proteins (Osp) OspA, OspC, and OspF. Based upon determining the OspA, OspC, and OspF antibodies, the mammal can be diagnosed as vaccinated, not vaccinated, infected or not infected with B. burgdorferi. Mammals that have early, intermediate or chronic B. burgdorferi infection can also be identified. The method is particularly suited for use with horses and dogs. Isolated or recombinant B. burgdorferi antigens and compositions that contain them are also provided.
Owner:CORNELL UNIVERSITY
Borrelia diagnostics and screening methods
InactiveUS20160195527A1Antibacterial agentsMicrobiological testing/measurementScreening methodNucleic acid sequencing
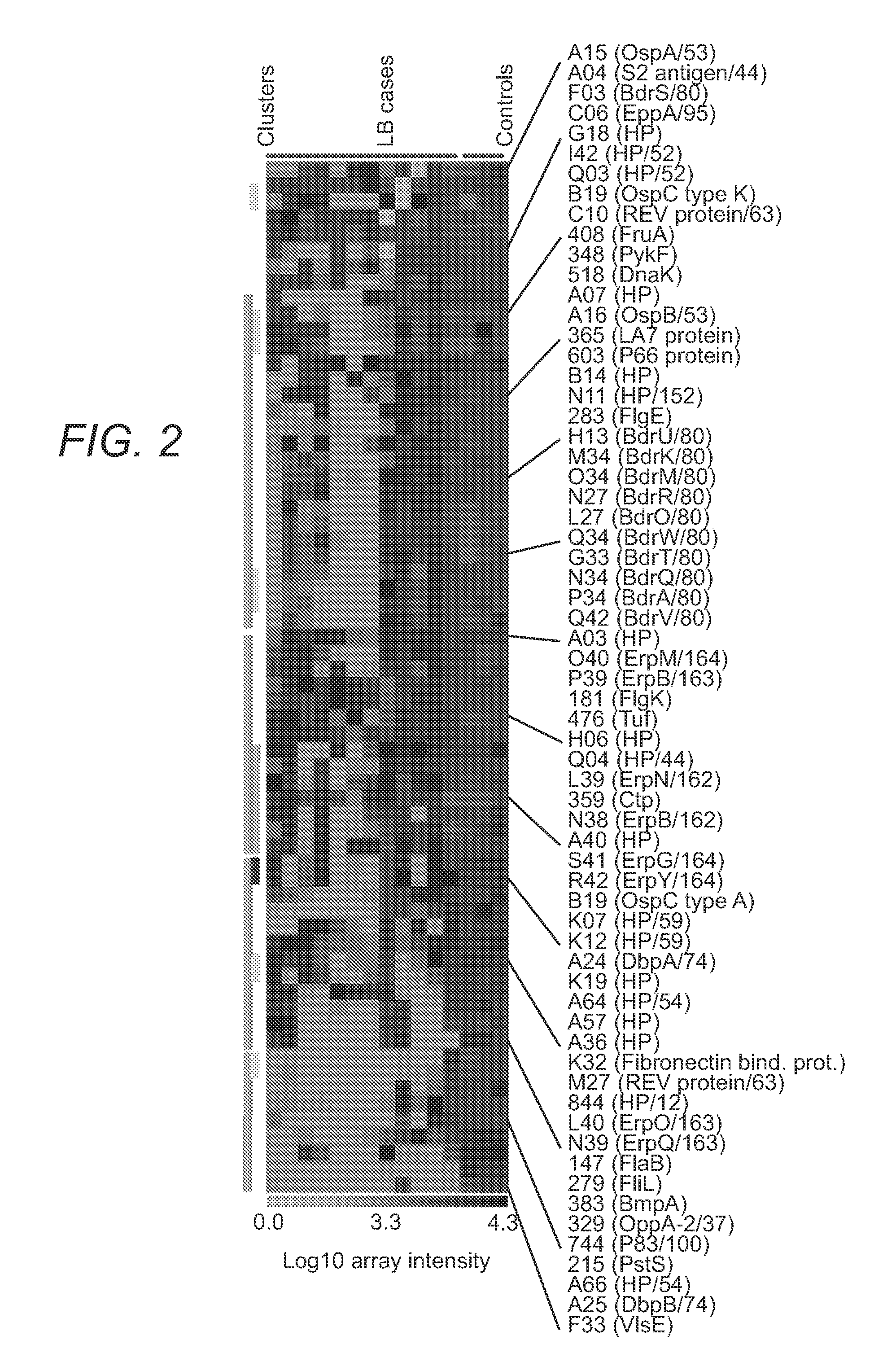
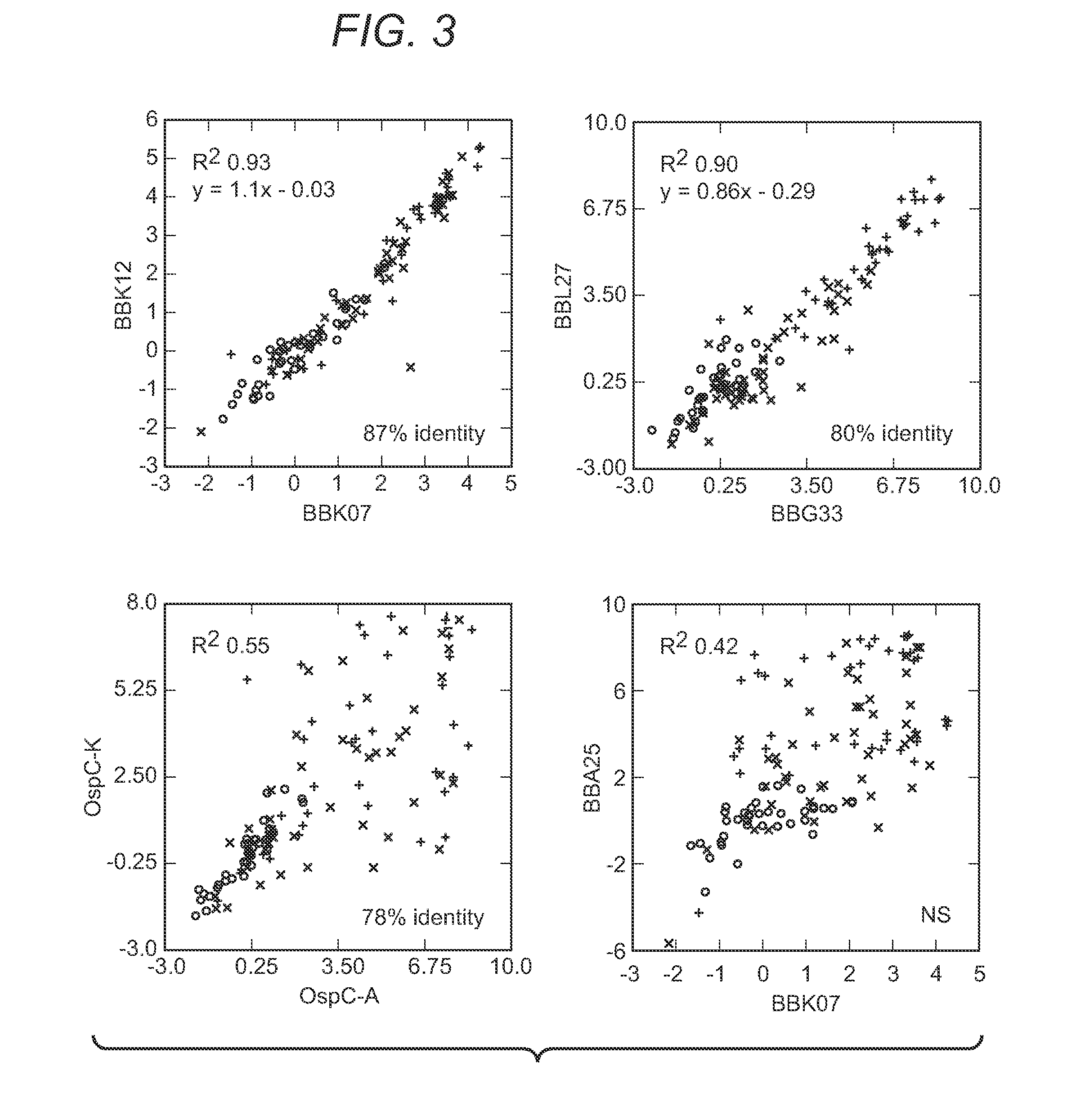
The present invention provides methods of detecting Borrelia species in a sample (e.g., a sample from a patient suspected of being infected). In particular, the present invention provides compositions and methods for detecting the presence of Borrelia proteins, nucleic acid sequences encoding these proteins, and subject antibodies to these proteins, where the proteins are selected from those listed in Table 3, including: BB0279 (FliL), BBK19, BBK07, BB0286 (FlbB), BBG33, BBL27, BBN34, BBP34, BBQ42, BBQ34, BBM34, BBN27, and BBH13.
Owner:RGT UNIV OF CALIFORNIA
Borrelia burgdorferi detection kit and application thereof
InactiveCN109097438AQuick checkObvious discolorationMicrobiological testing/measurementAgainst vector-borne diseasesSpiroplasmaRapid testing
The invention discloses a Borrelia burgdorferi detection kit. The Borrelia burgdorferi detection kit comprises a PCR (polymerase chain reaction) primer for detecting Borrelia burgdorferi, and a Borrelia burgdorferi detection system is provided; a bacteria solution is diluted 1 / 10<8> times in a PCR-immune colloidal gold test strip detection method, a detection band changes color obviously, a positive result can be judged clearly, and the detection kit is proved to be higher in detection sensitivity; by combining a high-sensitivity and high-specificity method of PCR in nucleic acid testing withan immune colloidal gold rapid testing technique in immunological detection and designing the unique primer, extracted target DNA is subjected to specific amplification, an amplification product in adeveloping solution binds with a gold labelled antibody fixed on the test strip, a stable and visible detection band and a quality control band are formed, and rapid detection of Borrelia burgdorferiis realized.
Owner:吉林出入境检验检疫局检验检疫技术中心
Groups of Borrelia burgdorferi and Borrelia afzelii that cause Lyme Disease in humans
InactiveUS20060182756A1Preventing of Lyme diseaseEffective preventionBacteriaPeptide/protein ingredientsDiseaseImmunogenicity
The present invention is drawn to an immunogenic composition comprising OspC polypeptides from Lyme Disease causing Borrelia. In one embodiment, the immunogenic composition of the present invention comprises at least one OspC polypeptide or immunogenic fragment thereof from each of Borrelia burgdorferi OspC families A, B, I and K. In another embodiment, the immunogenic composition of the present invention comprises at least one OspC polypeptide or immunogenic fragment thereof from each of Borrelia afzelii OspC families A and B.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com