Patents
Literature
102 results about "Intradermal injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Intradermal injection, often abbreviated ID, is a shallow or superficial injection of a substance into the dermis, which is located between the epidermis and the hypodermis. This route is relatively rare compared to injections into the subcutaneous tissue or muscle. ID injections are used only when certain therapies, such as tuberculin and allergy tests, require the specific benefits of this route of administration. Specific benefits are a higher immune responses for vaccinations, immunology and novel cancer treatments and faster drug uptake, since for certain small and well soluble proteins or molecules, ID route of administration is associated with fast uptake systemically compared to subcutaneous injections, applied in novel closed loop insuline infusion systems. Additionally, the body's reaction to substances is more easily visible since it is closer to the surface.
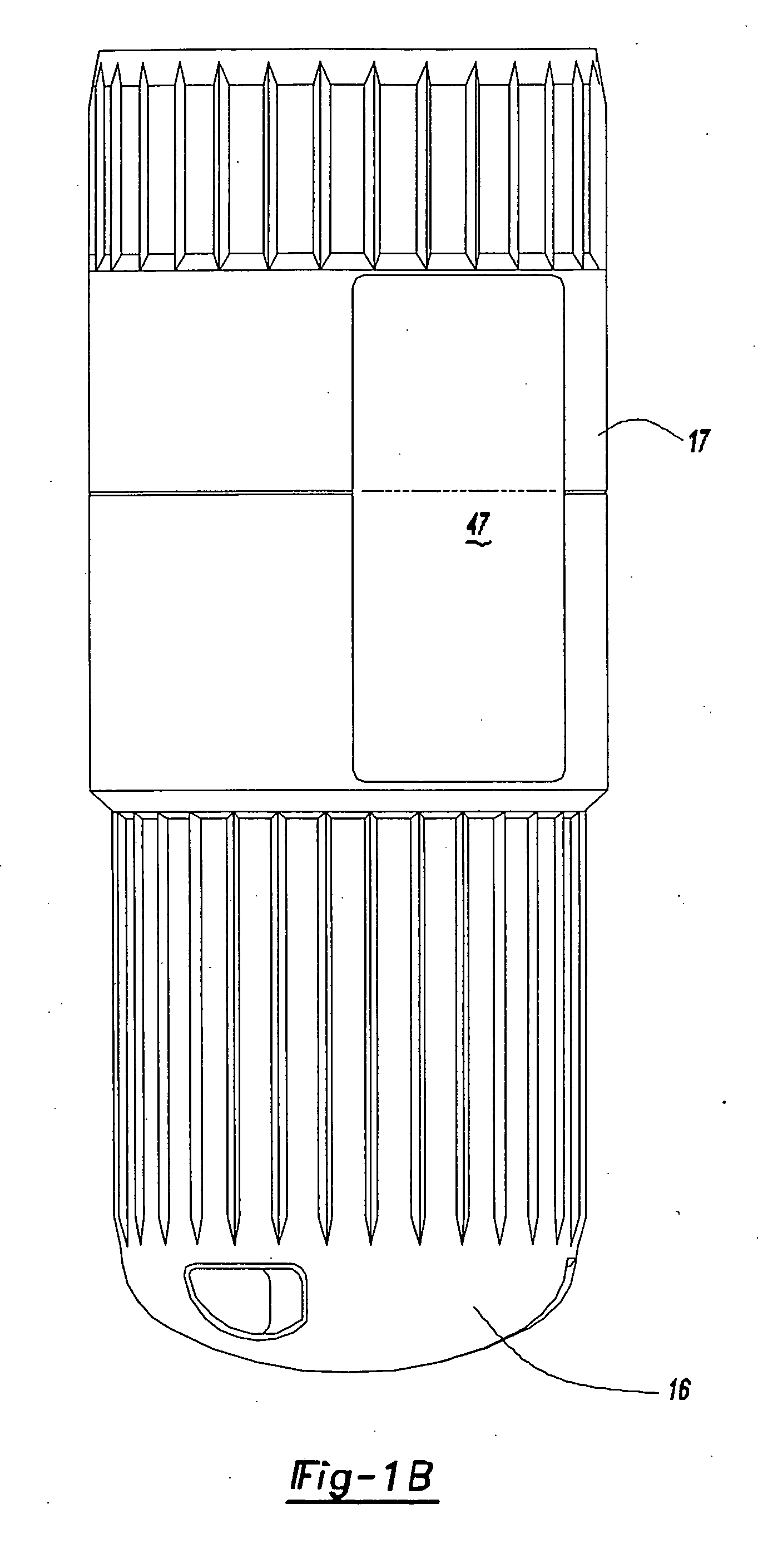
Intradermal injector
InactiveUS20050033234A1Avoid back pressureAvoid insufficient lengthAmpoule syringesAutomatic syringesInjection siteSkin contact
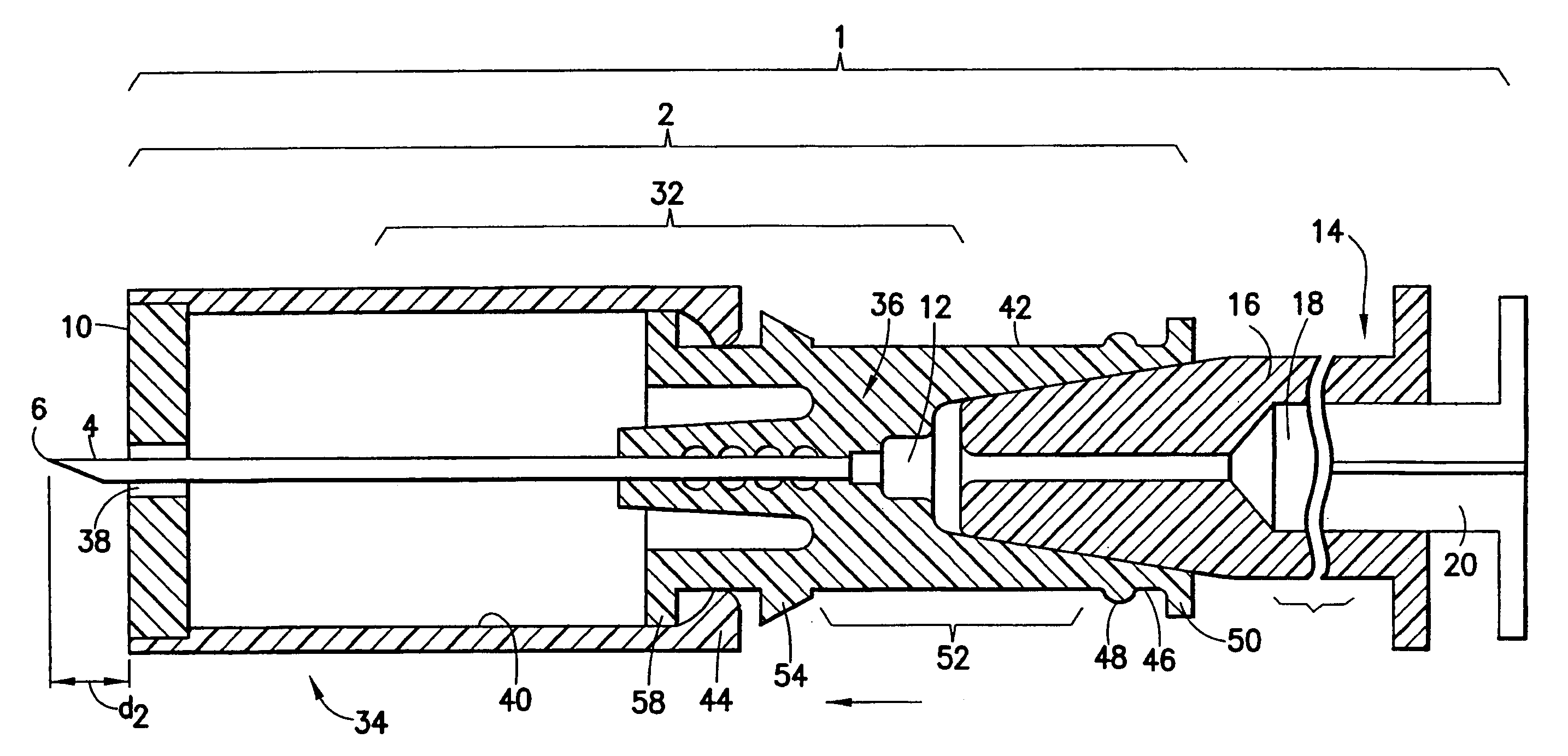
An injection device that comprises a chamber configured for containing a substance to be injected and a needle operatively associated with the chamber and having a length sufficient to deliver the substance to an intradermal injection site. A collar surrounds the needle, defining a collar cavity. The collar also has a peripheral forward skin-contacting surface that surrounds and is radially spaced from the needle and injection site by an area that is sufficiently large to allow a patient's skin to move into the collar cavity to properly position the needle for intradermal delivery of the substance to the injection site to allow spread of the injected substance under the skin while inhibiting or preventing backpressure within the skin from forcing the substance out through the injection site.
Owner:ANTARES PHARMA
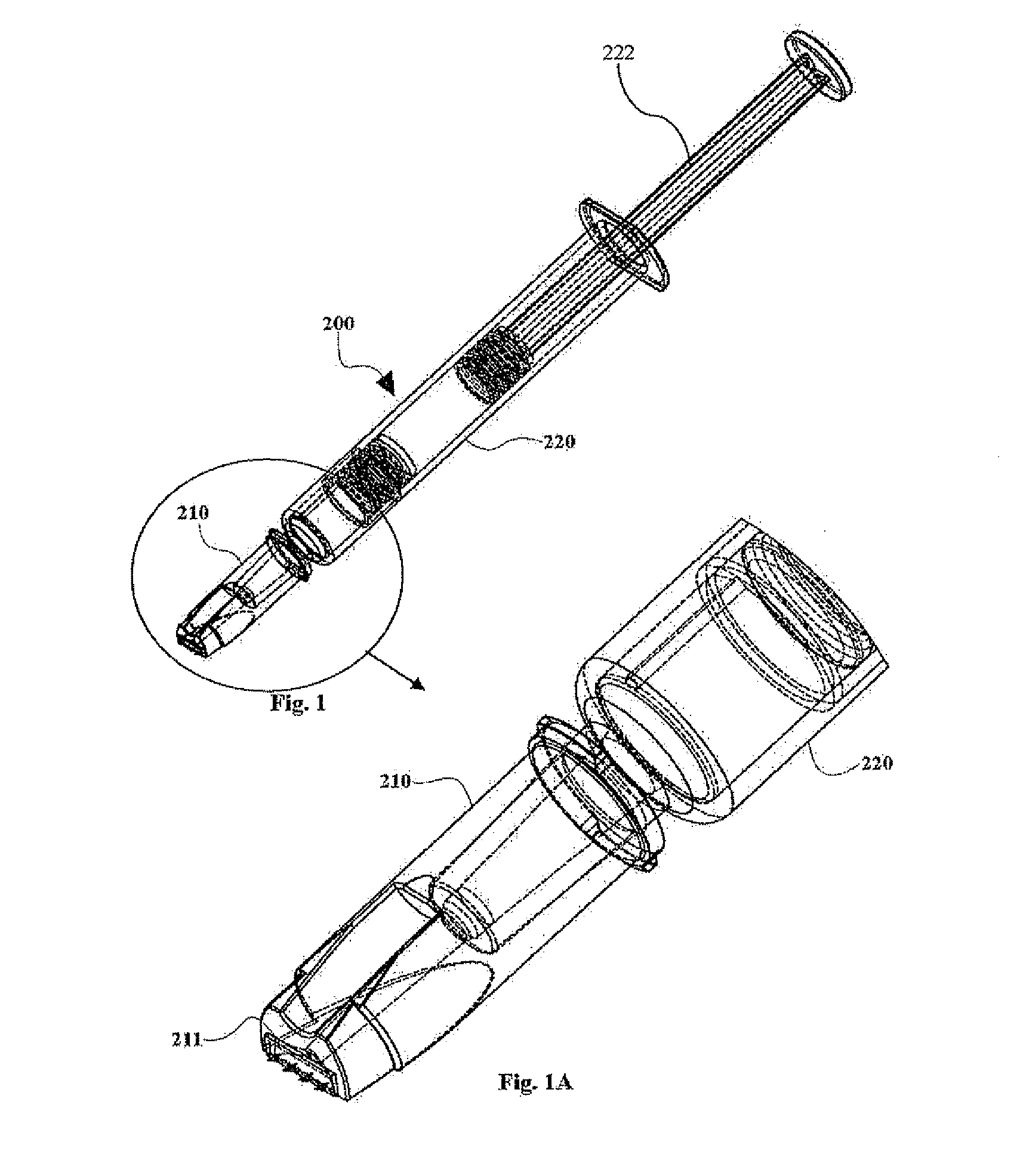
Prefillable intradermal delivery device
An intradermal delivery device for use in intradermally injecting substances into the skin of an animal includes a needle cannula supported by a hub portion that is attachable to a prefillable container. A limiter portion surrounds the needle cannula and extends away from the hub portion toward a forward tip of the needle cannula. The limiter portion includes a skin engaging surface extending in a plane generally perpendicular to an axis of the needle cannula. The skin engaging surface is received against skin of an animal to administer an intradermal injection. The forward tip extends beyond the skin engaging surface a distance that enables penetration of the needle cannula into the dermis layer of the skin of the animal enabling injection of the substance into the dermis layer of the animal. The device includes enclosure means that is moveable for concealing the needle cannula after the injection has been administered.
Owner:BECTON DICKINSON & CO
Methods and devices for intradermal injection
ActiveUS20100137831A1Minimal trainingMinimizes skillInfusion syringesMedical devicesBiomedical engineeringSkin surfaces
Devices and methods for intradermal (ID) administration of diagnostic and therapeutic agents, vaccines and other compounds into the dermal layer of the skin are disclosed. The devices and the methods simplify the ID injection process and increase the consistency of the placement of the needle tip in the dermal space close to the skin surface allowing for a shallow cannula placement in the dermis. Furthermore, the devices and methods broaden the number of sites suitable for ID injection and make the ID injection possible with limited training.
Owner:SID TECH LLC +1
Intradermal injector
InactiveUS8162886B2Avoid back pressureAvoid insufficient lengthAmpoule syringesAutomatic syringesInjection siteSkin contact
An injection device that comprises a chamber configured for containing a substance to be injected and a needle operatively associated with the chamber and having a length sufficient to deliver the substance to an intradermal injection site. A collar surrounds the needle, defining a collar cavity. The collar also has a peripheral forward skin-contacting surface that surrounds and is radially spaced from the needle and injection site by an area that is sufficiently large to allow a patient's skin to move into the collar cavity to properly position the needle for intradermal delivery of the substance to the injection site to allow spread of the injected substance under the skin while inhibiting or preventing backpressure within the skin from forcing the substance out through the injection site.
Owner:ANTARES PHARMA INC
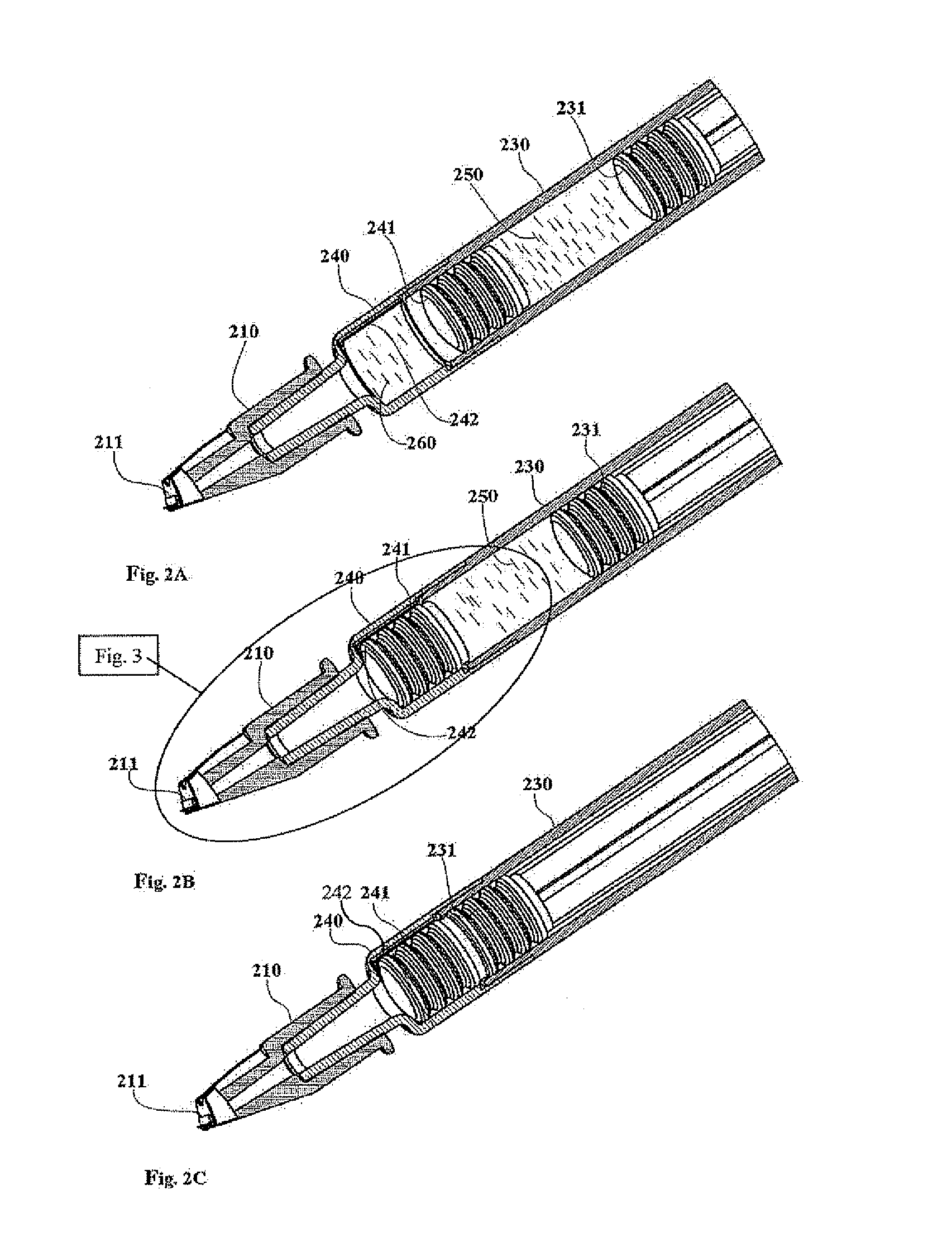
Intradermal syringe and needle assembly
A needle assembly for an intradermal injection device, and a drug delivery device, which includes a needle cannula and a limiter surrounding the needle cannula and includes a skin engaging surface on the limiter. The limiter is moveable from a first position in which an elongate portion of the needle cannula is exposed for access to a medication vial, to a locked second position in which the limiter is not movable from the second position to the first position. In the second position, the needle tip extends beyond the skin engaging surface a distance of about 3 mm or less.
Owner:BECTON DICKINSON & CO
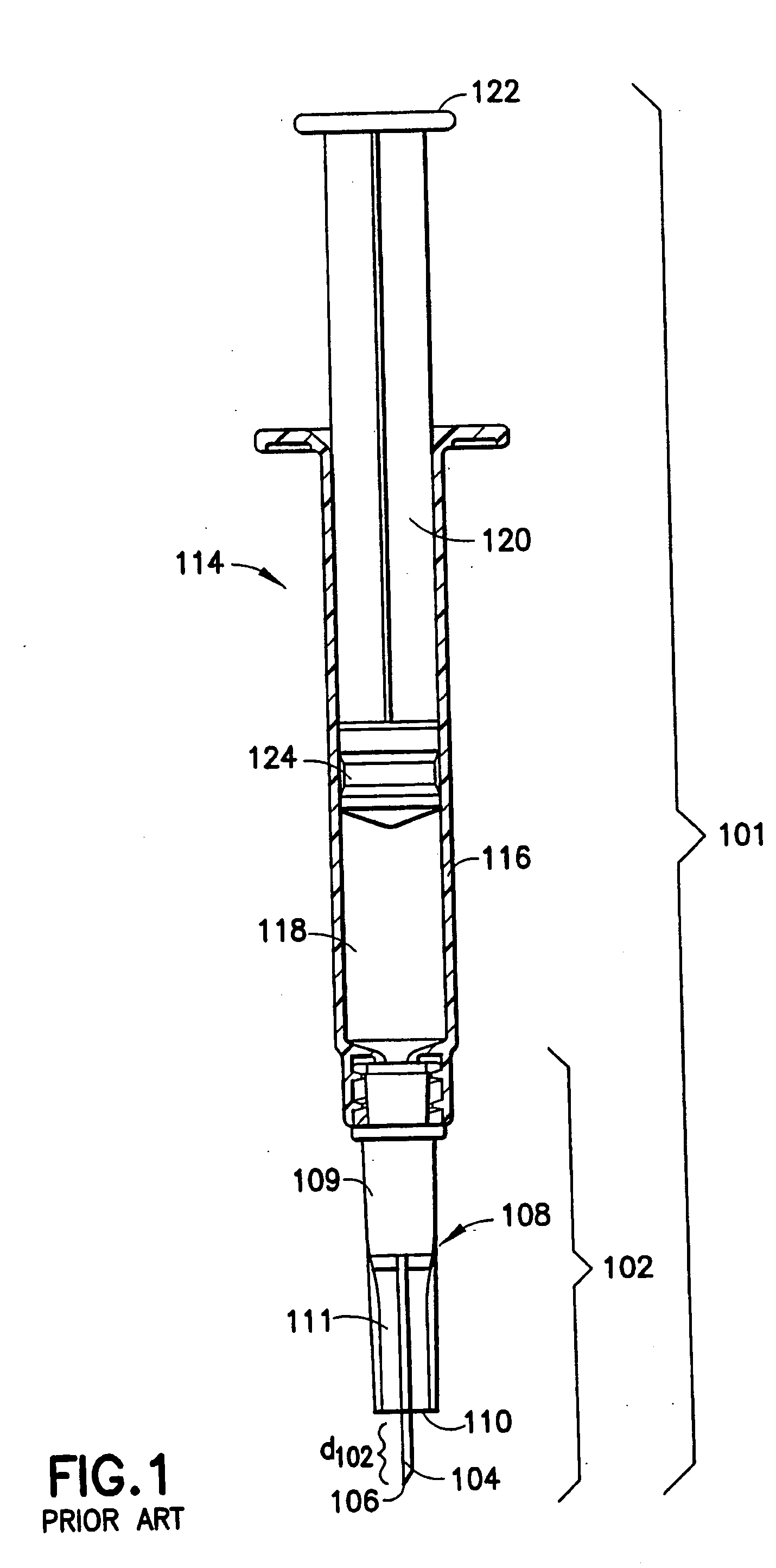
Intradermal needle
InactiveUS6843781B2Effectively and reliably deliver such substances intradermallyControl lengthAmpoule syringesMedical devicesMedicineInjected substance
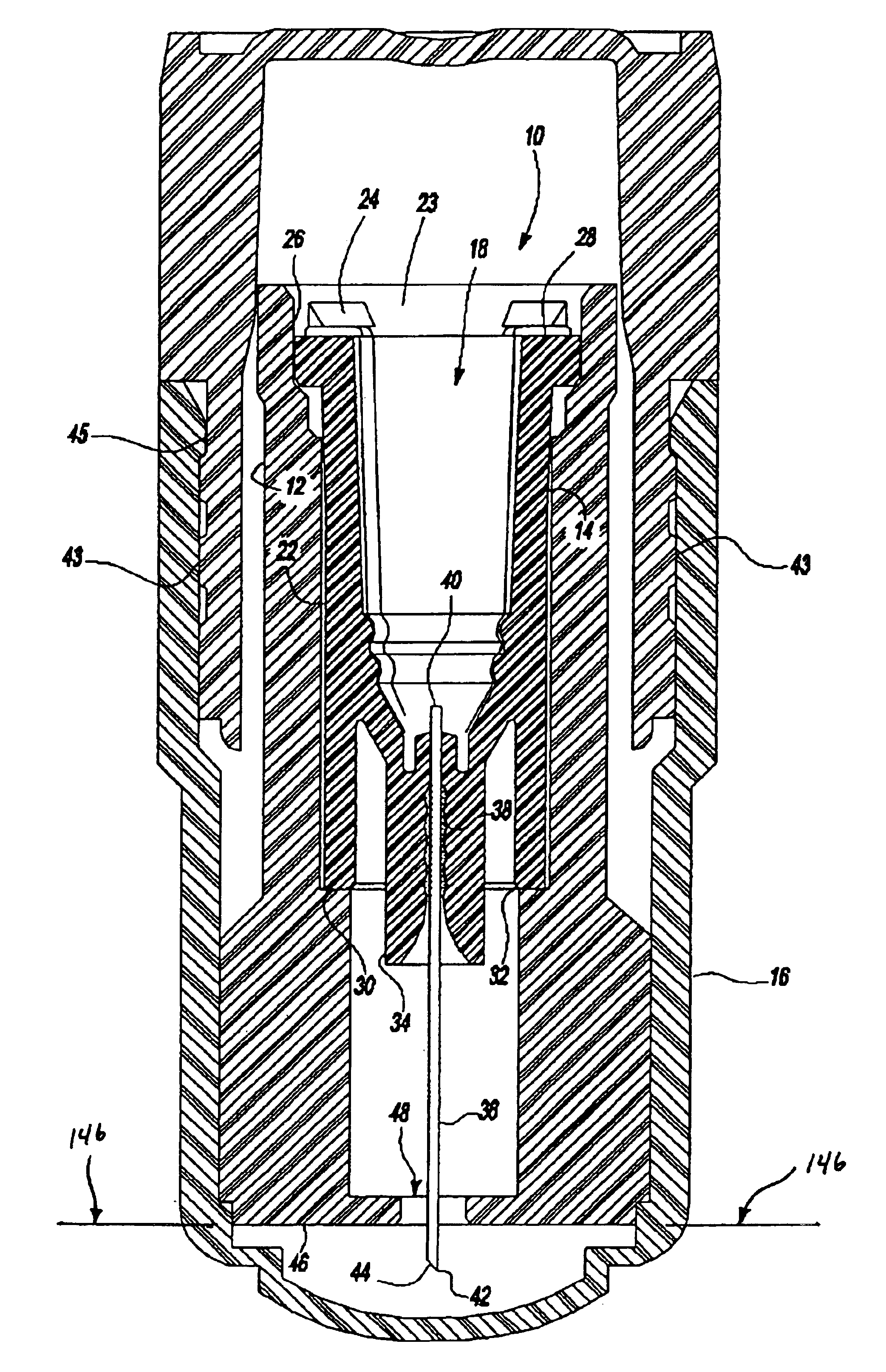
An intradermal needle assembly that is attachable to a prefillable container intended for intradermally injecting substances into an animal includes a needle cannula supported by a hub portion. The hub portion is adapted to receive the prefillable container just prior to administering the intradermal injection. A limiter portion surrounds the needle cannula and extends away from the hub portion toward a forward tip of the needle cannula, and includes a skin engaging surface with the needle cannula having a fixed angle of orientation, preferably generally perpendicular, relative to the plane of the skin engaging surface. The skin engaging surface is received against the skin of an animal to administer an intradermal injection. The forward tip extends beyond the skin engaging surface a distance enabling penetration of the needle cannula into the dermis layer of the skin of the animal enabling injection of the substance into the dermis layer.
Owner:BECTON DICKINSON & CO +1
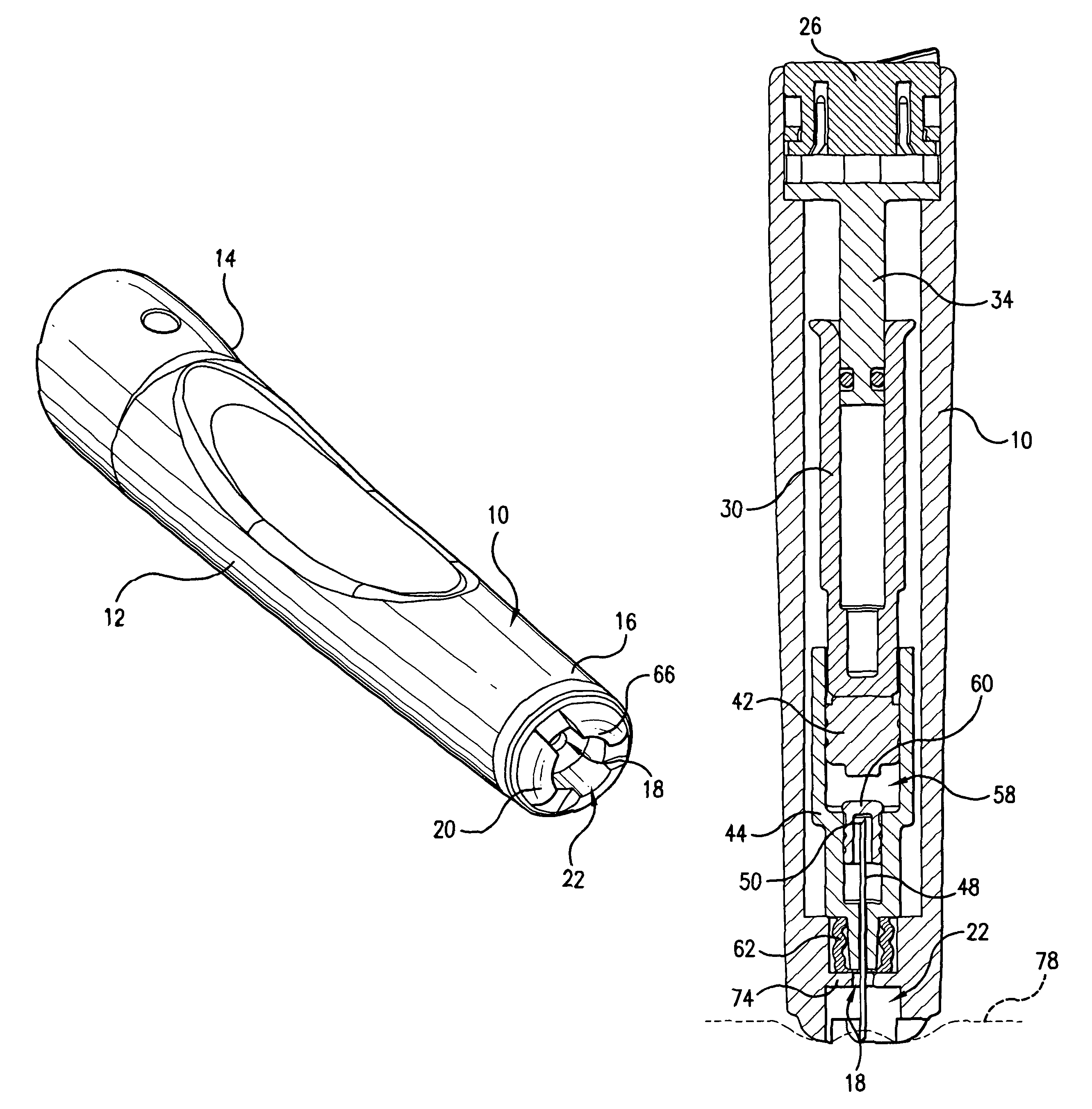
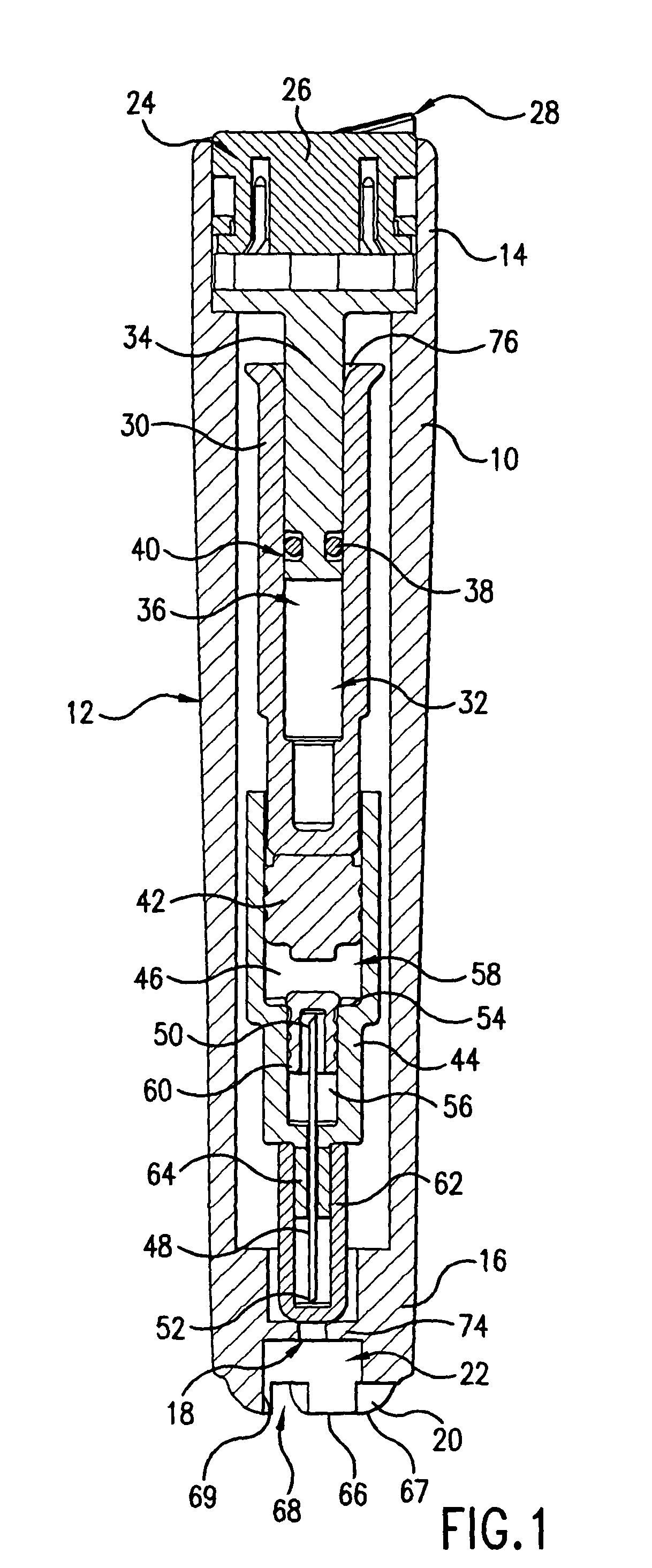
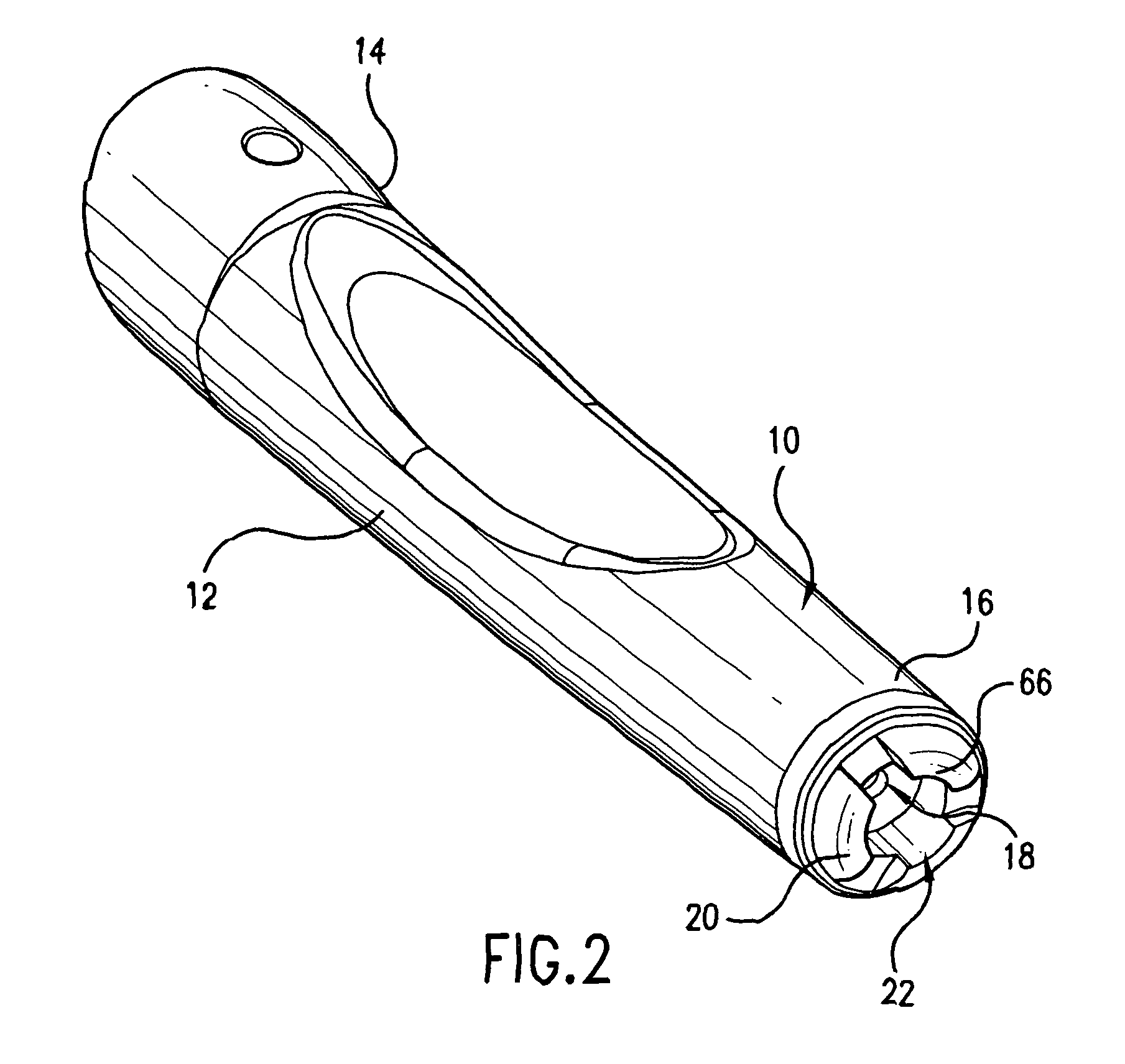
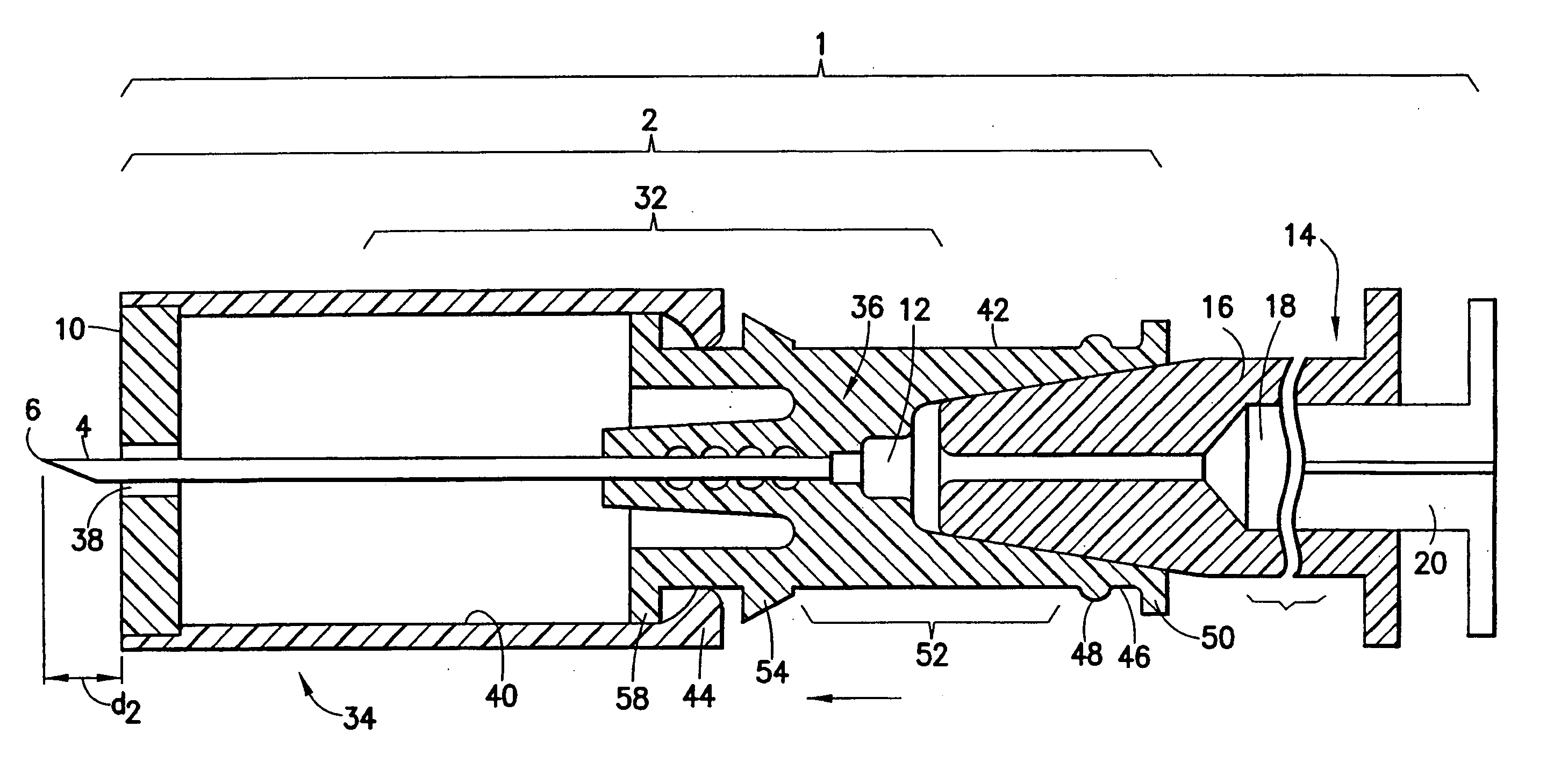
Prefillable intradermal delivery device with hidden needle and passive shielding
InactiveUS20050033230A1Simple methodReducing the potential for a biohazardInfusion needlesBiomedical engineeringIntradermal injection
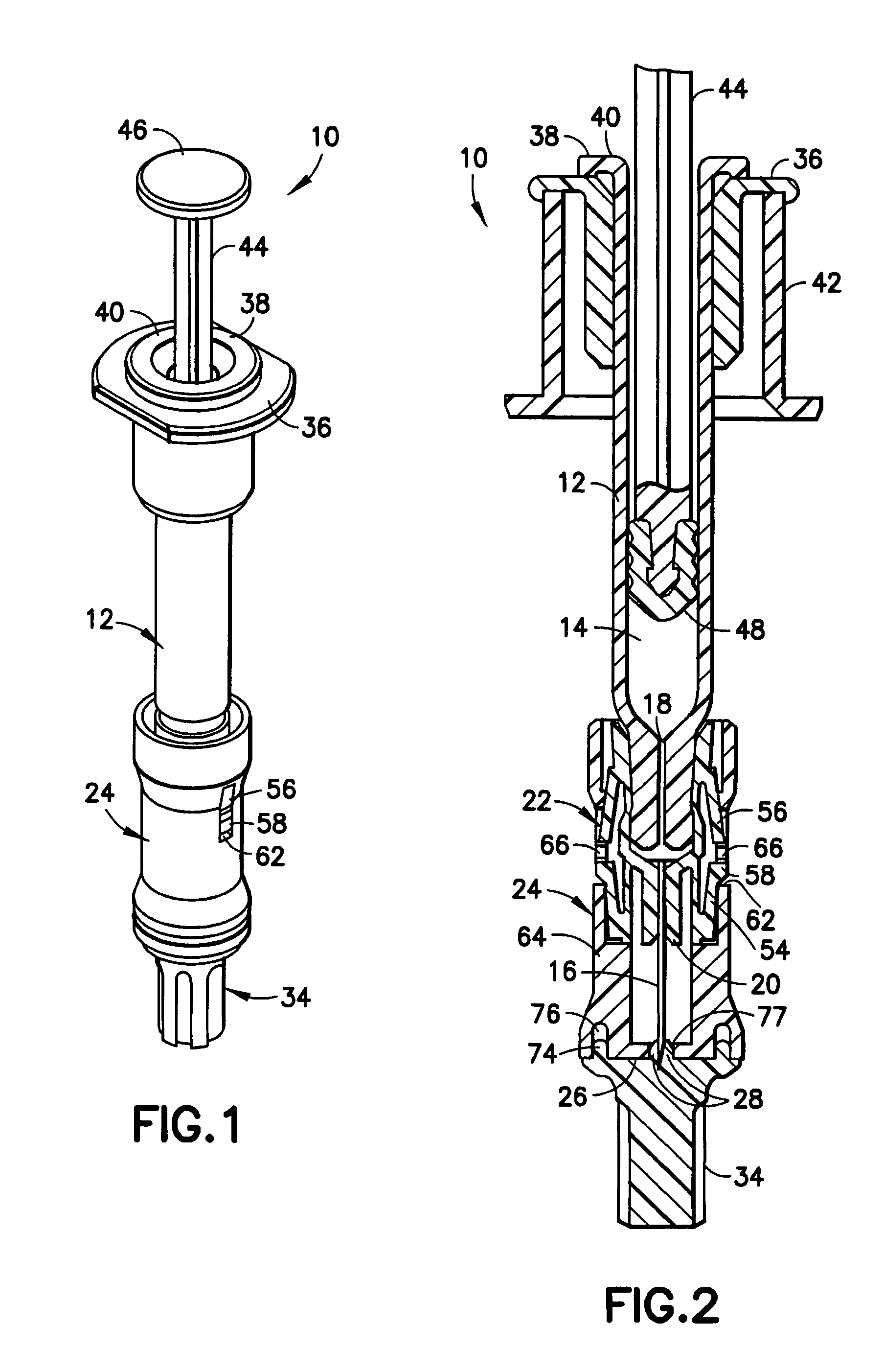
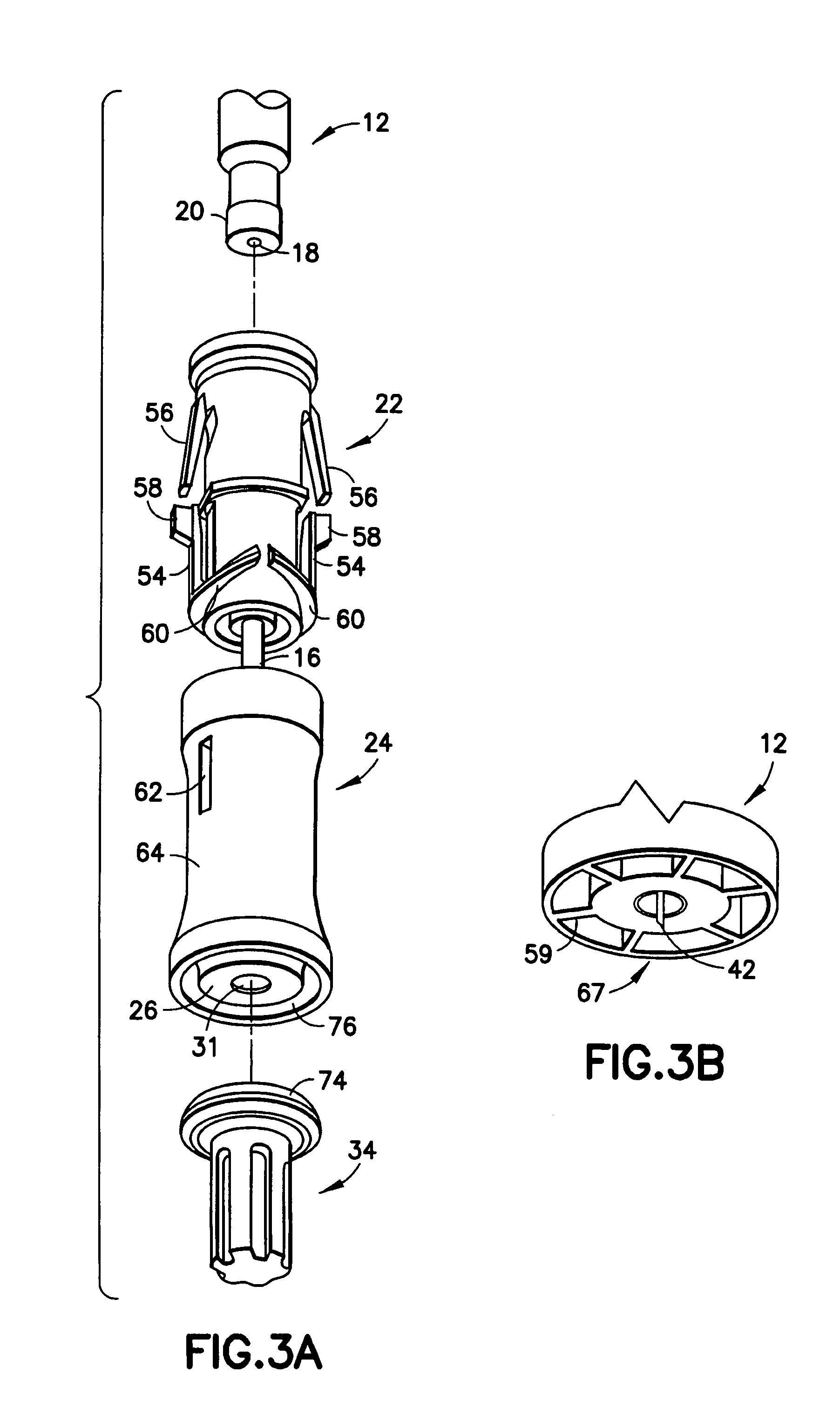
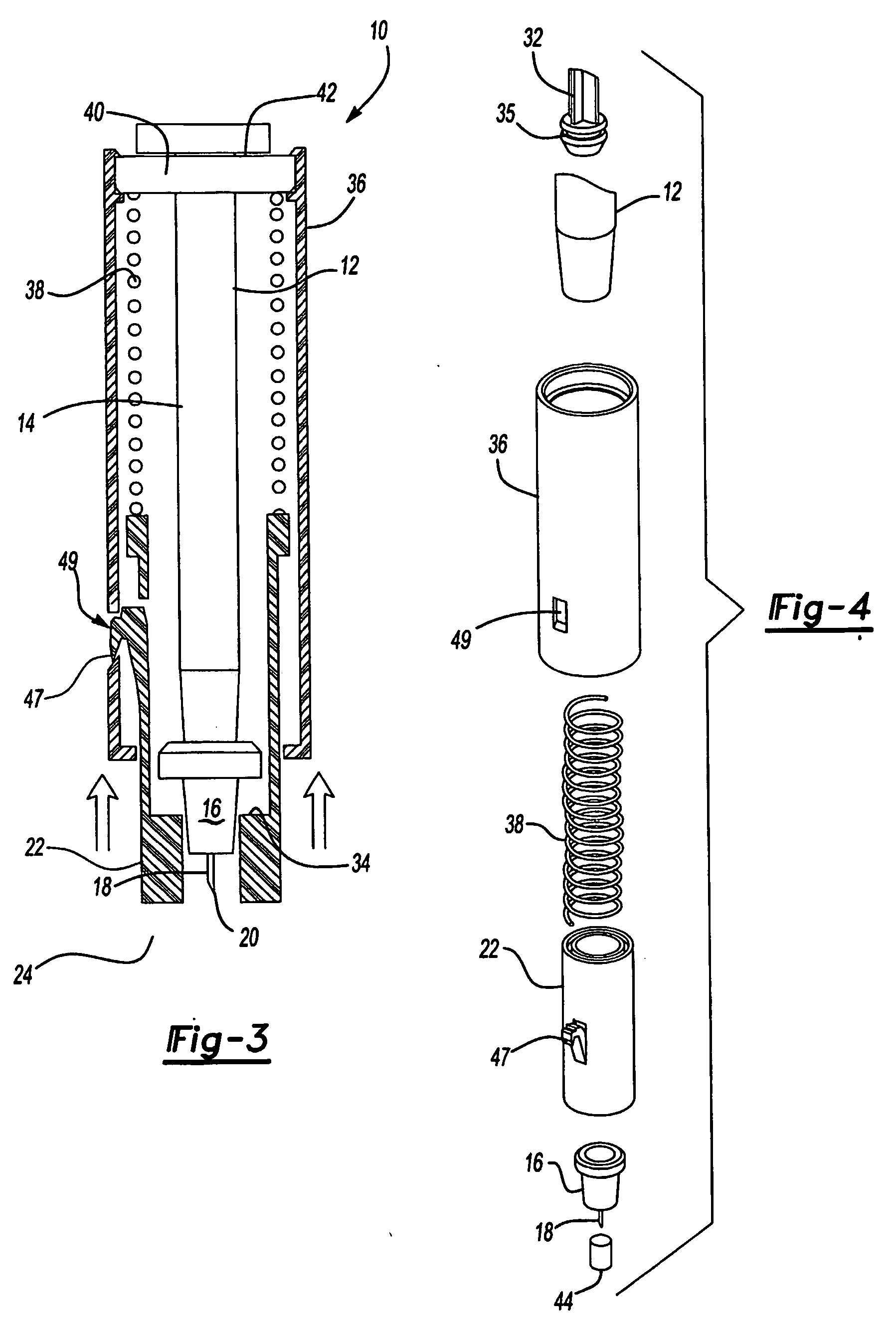
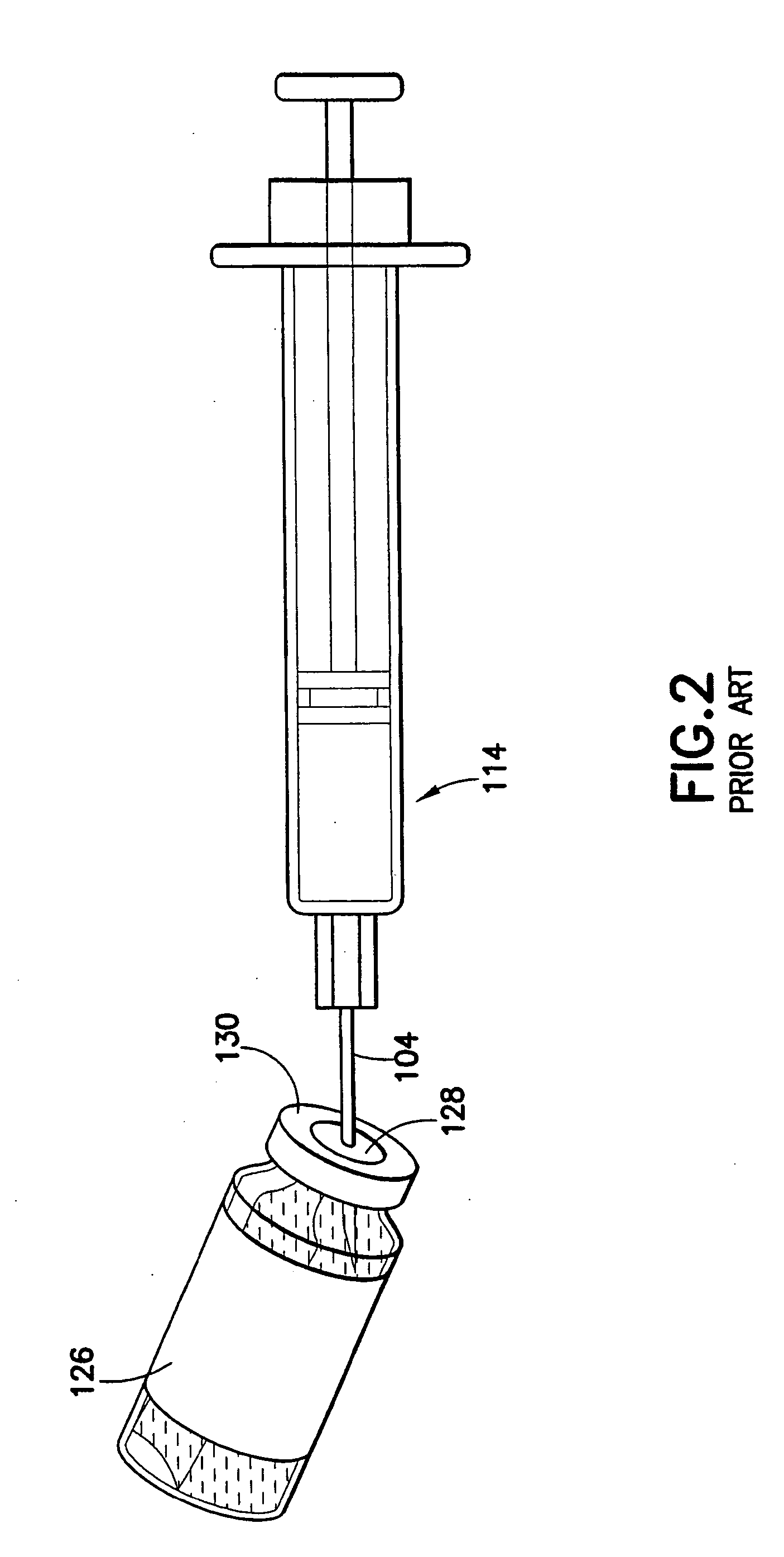
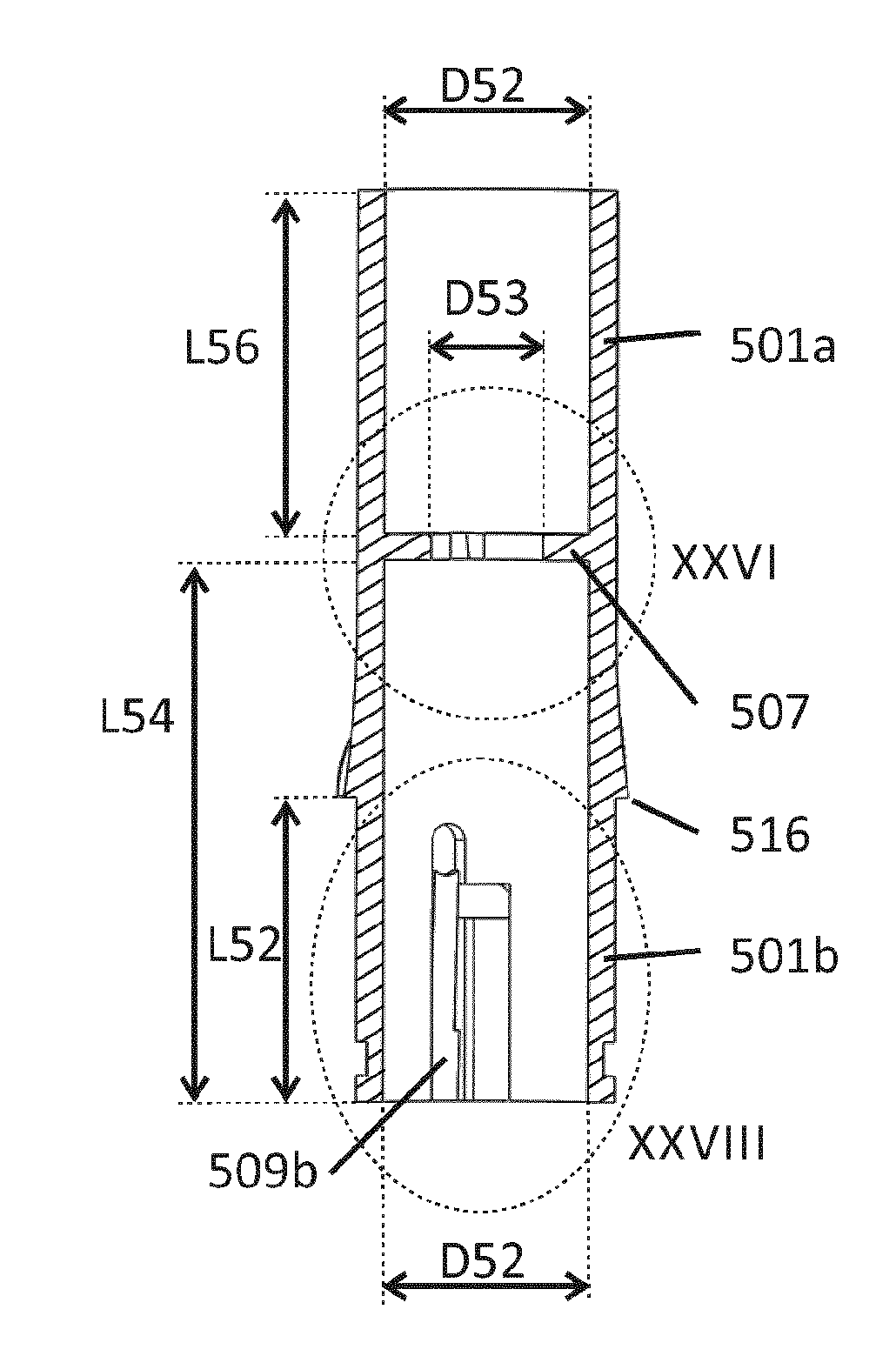
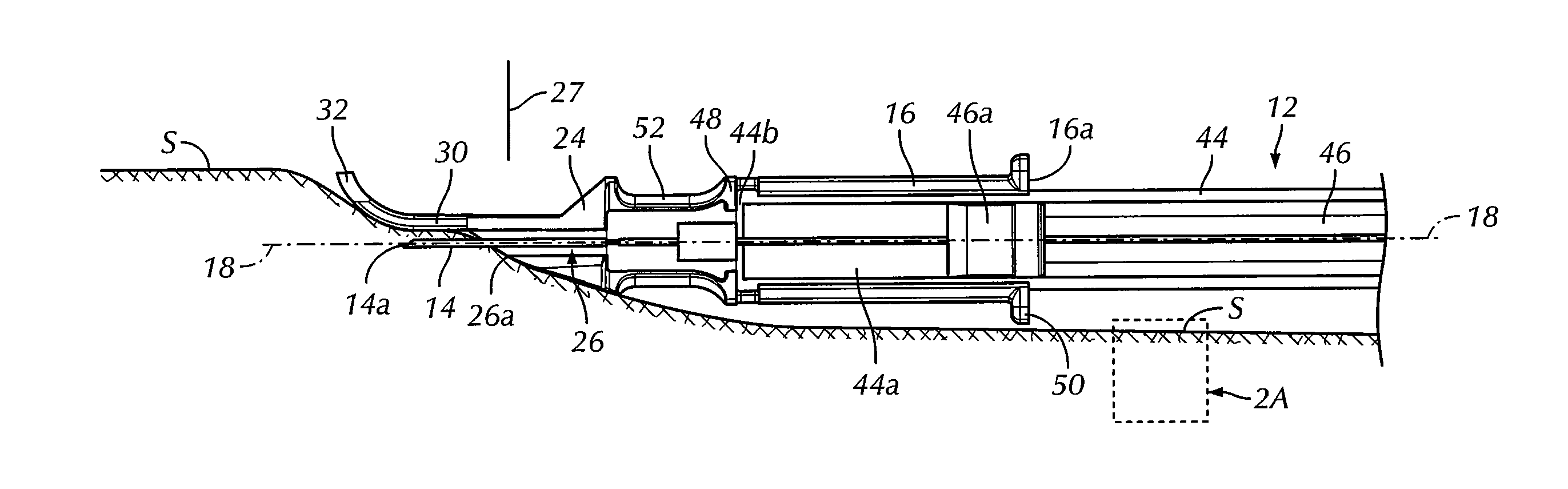
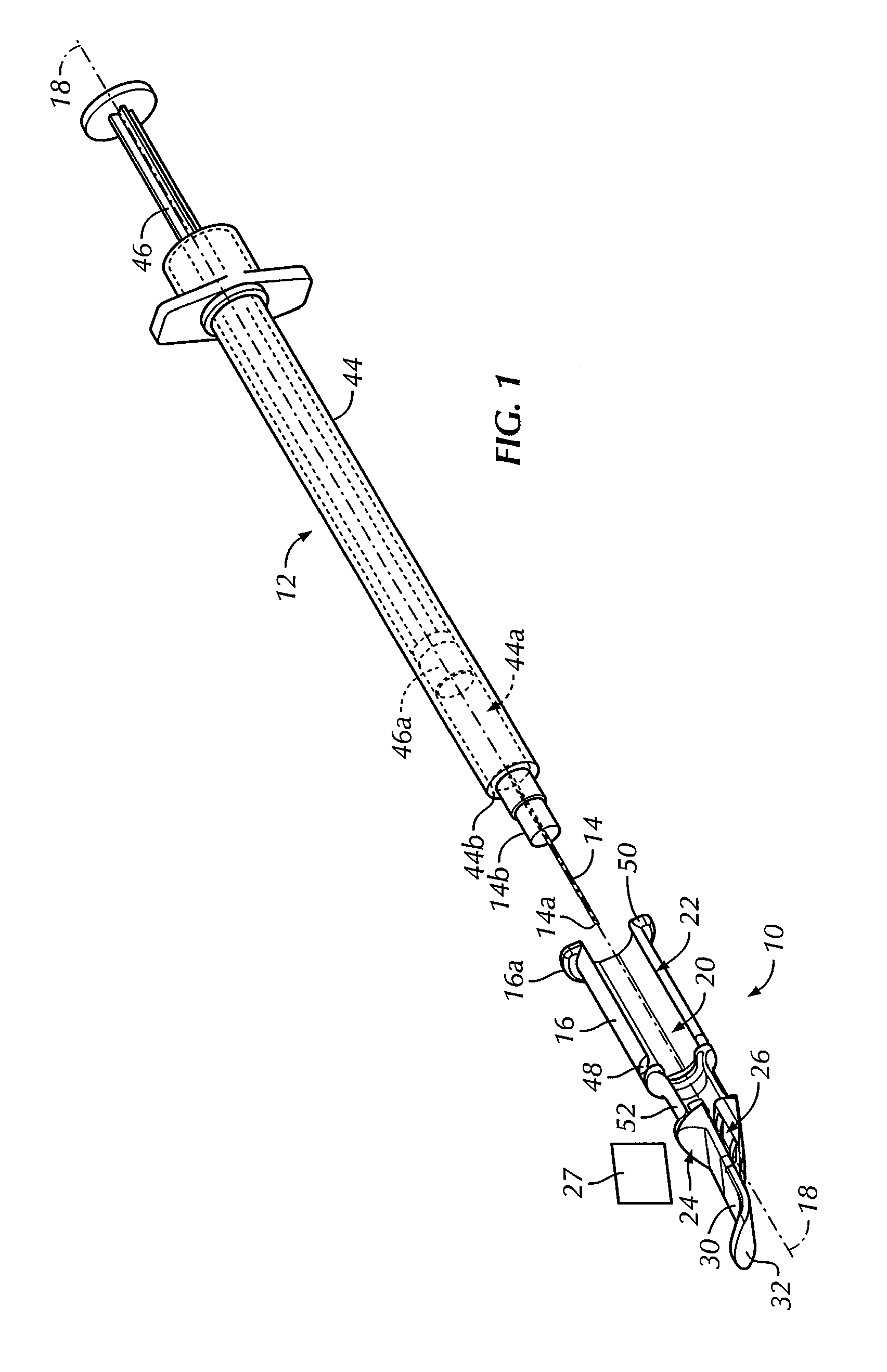
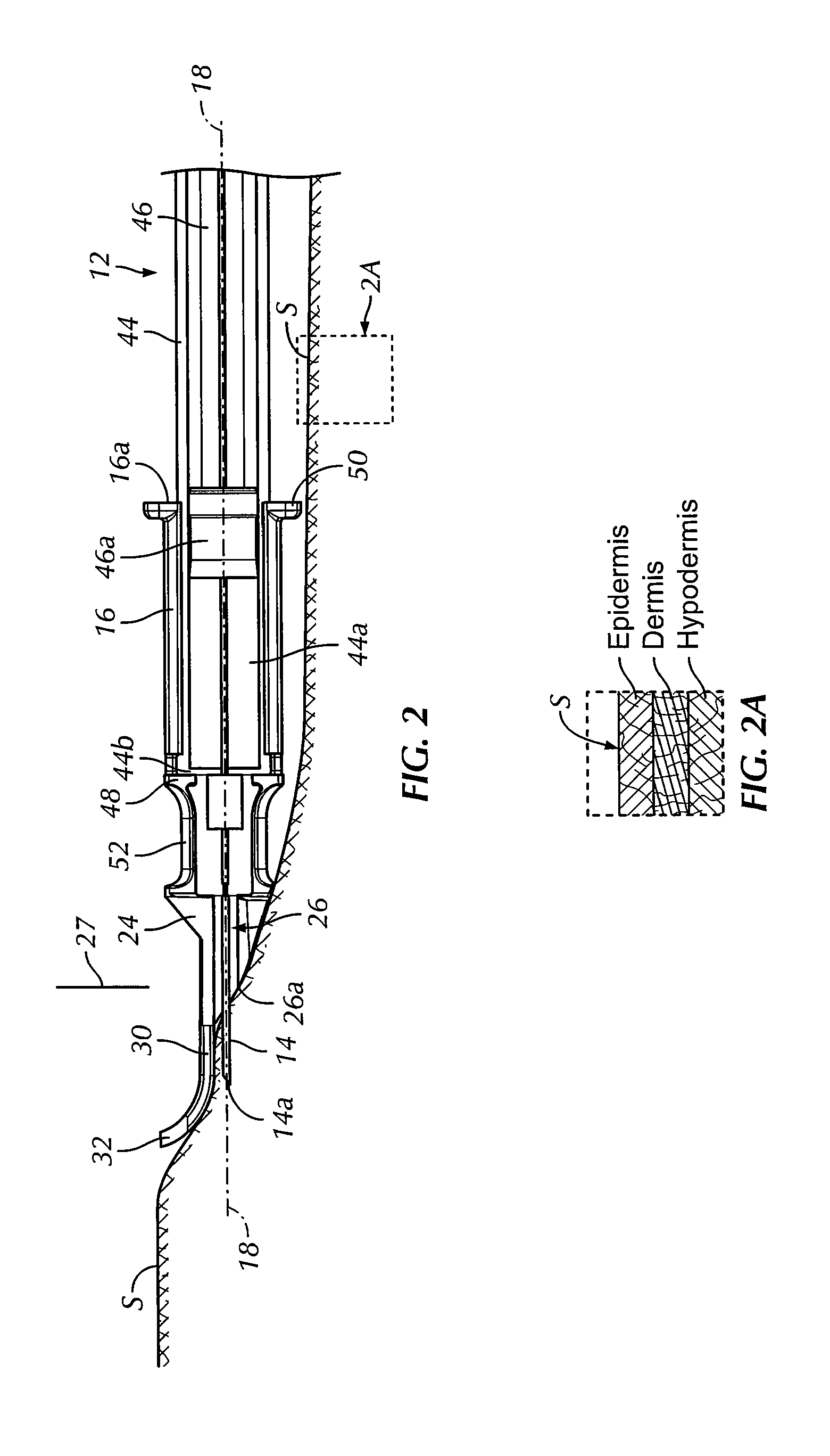
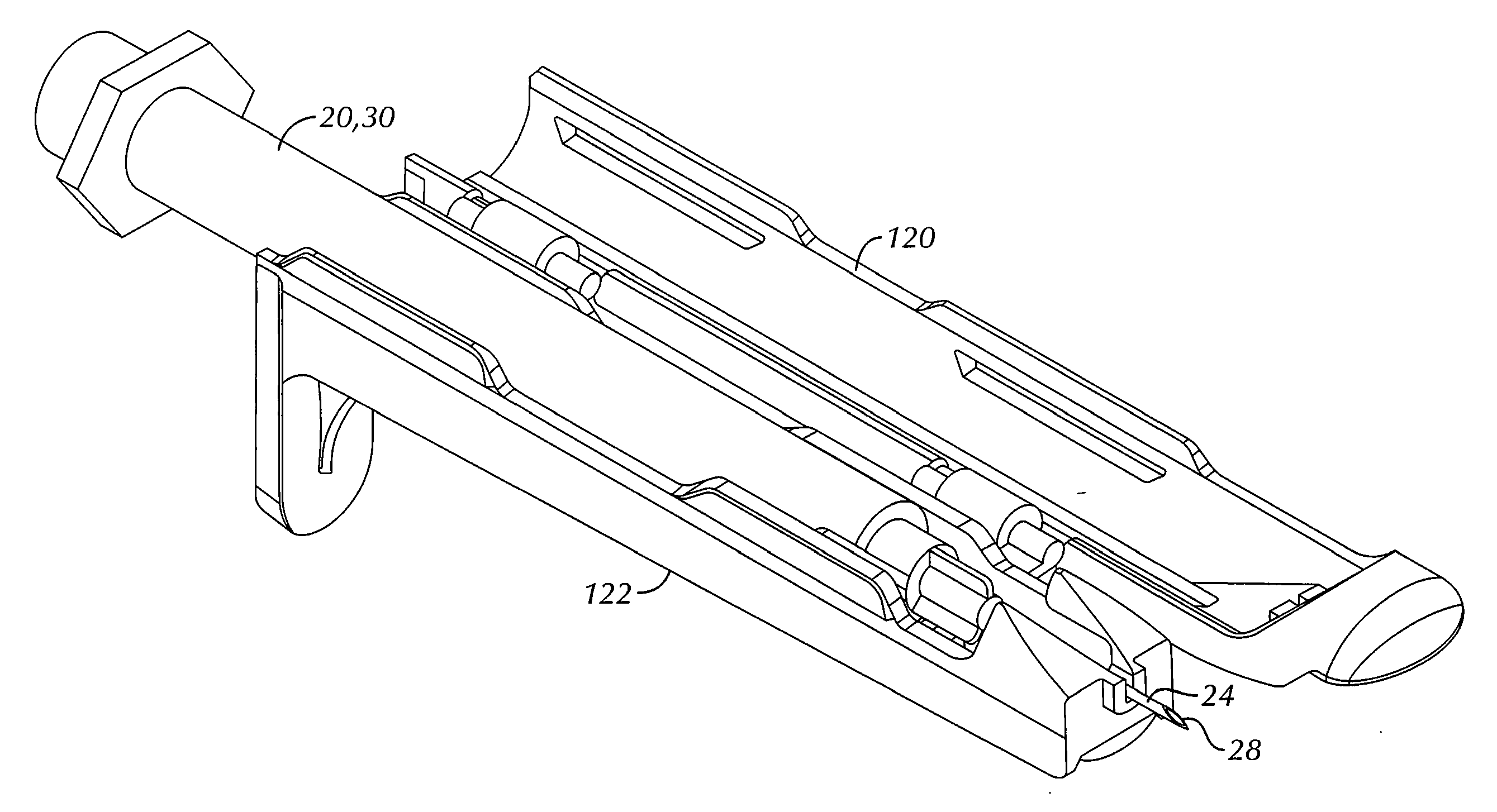
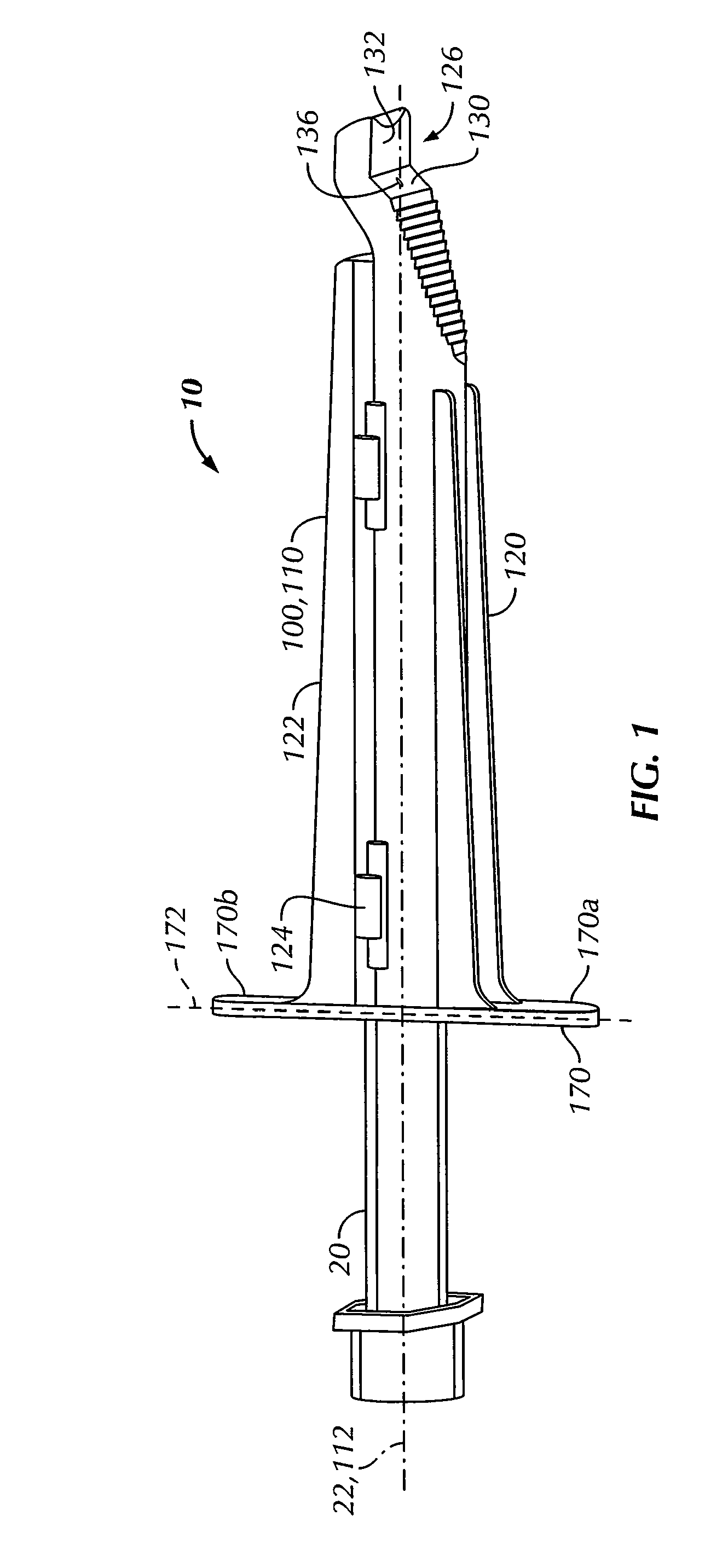
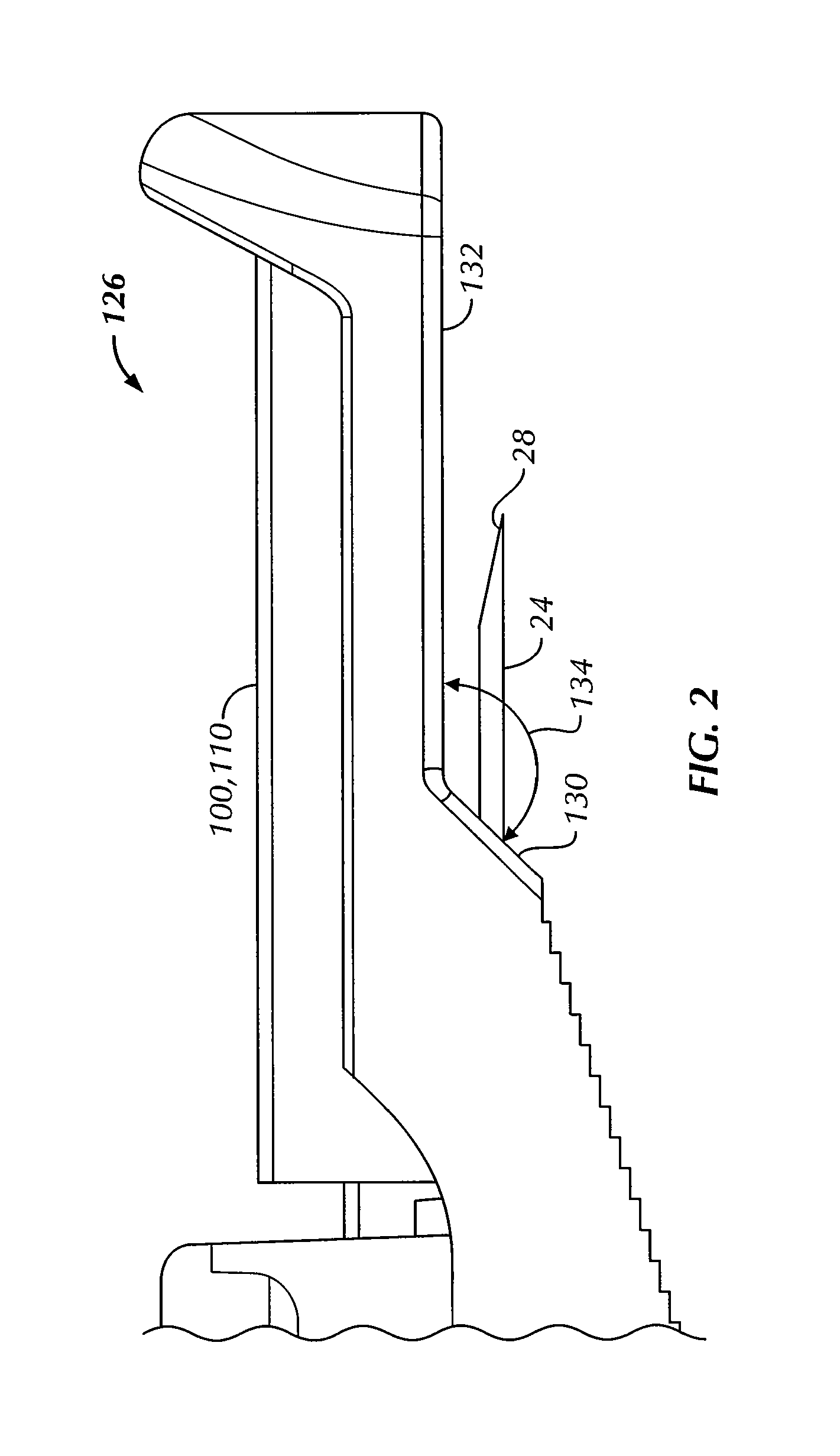
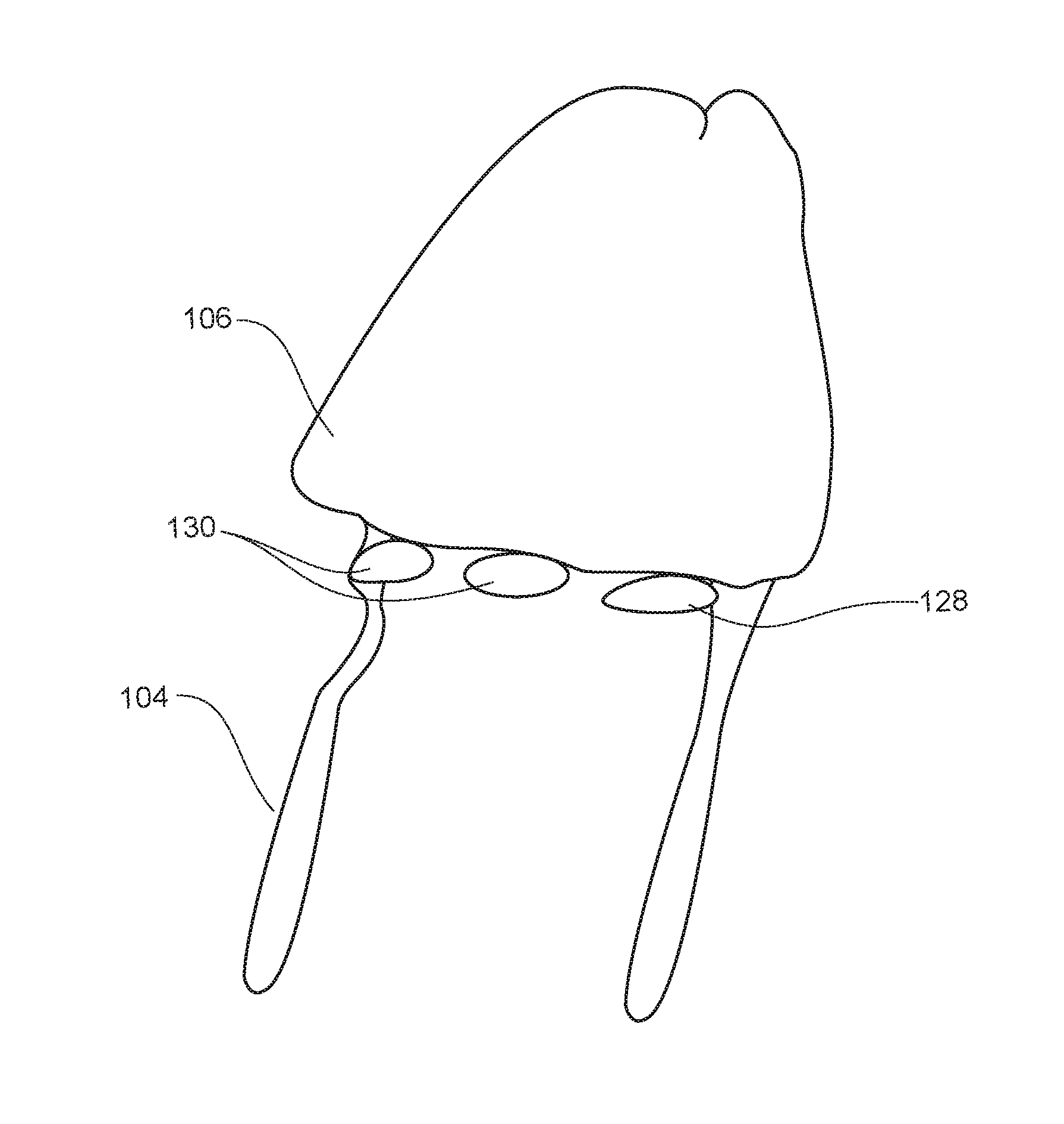
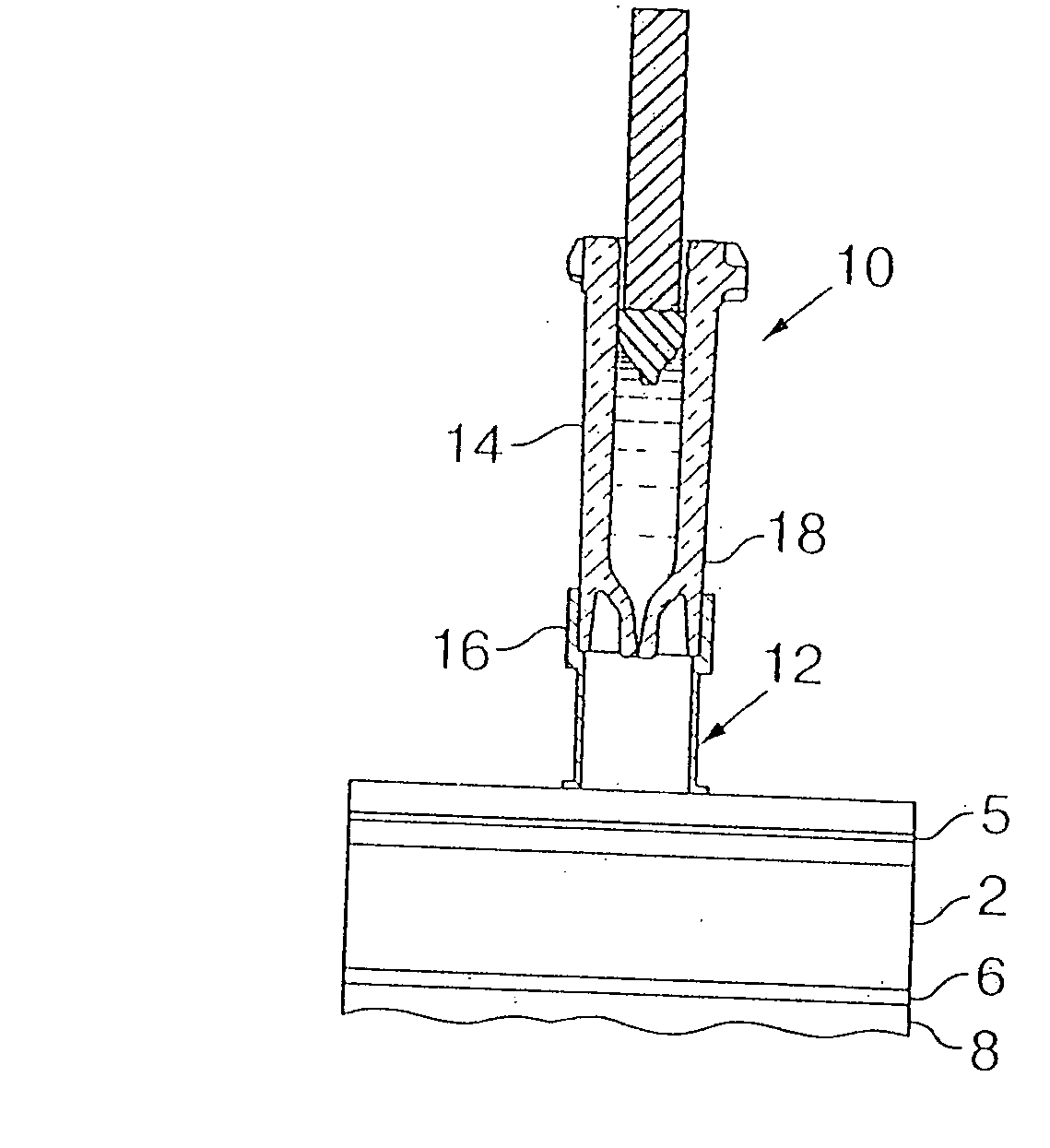
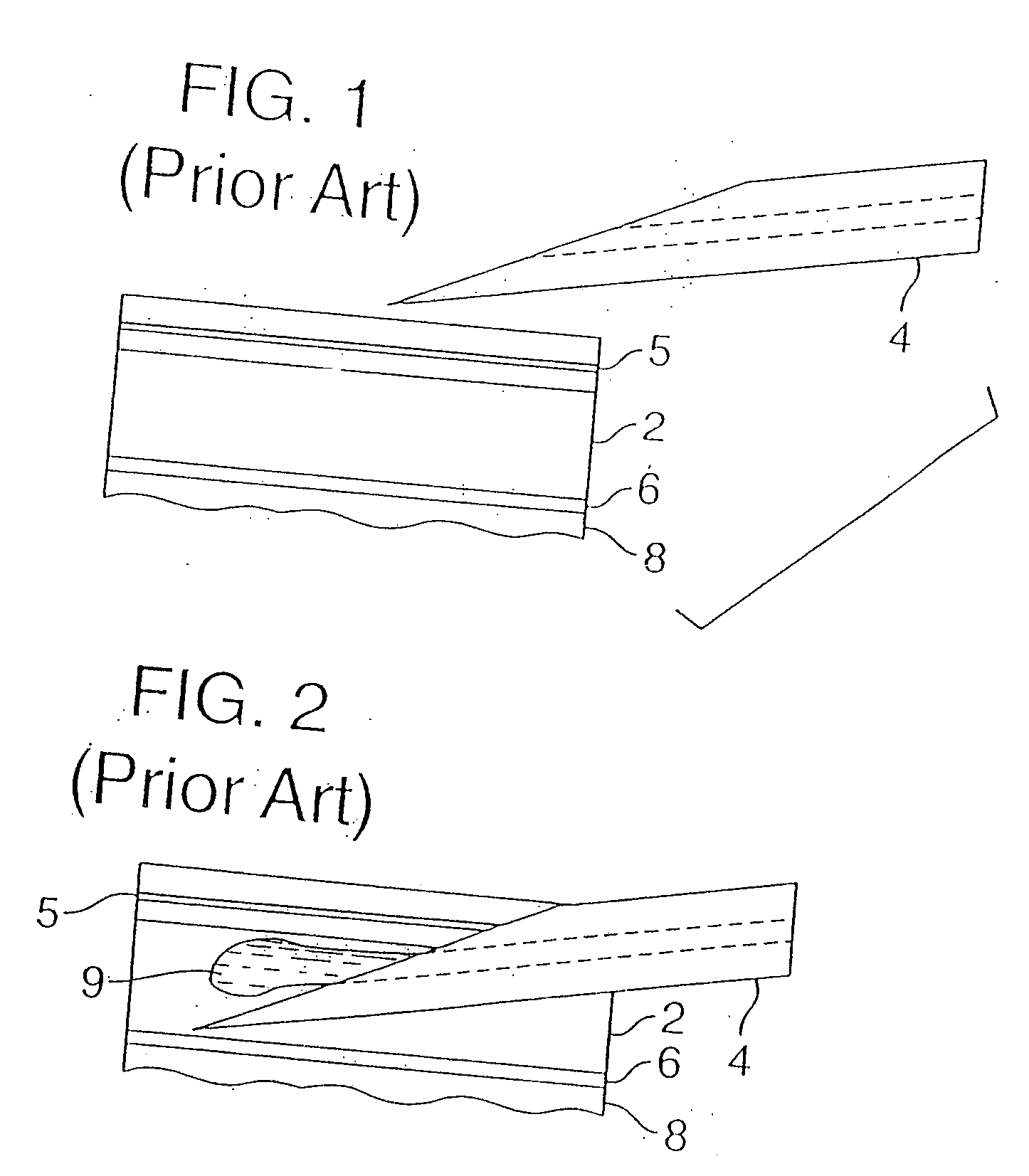
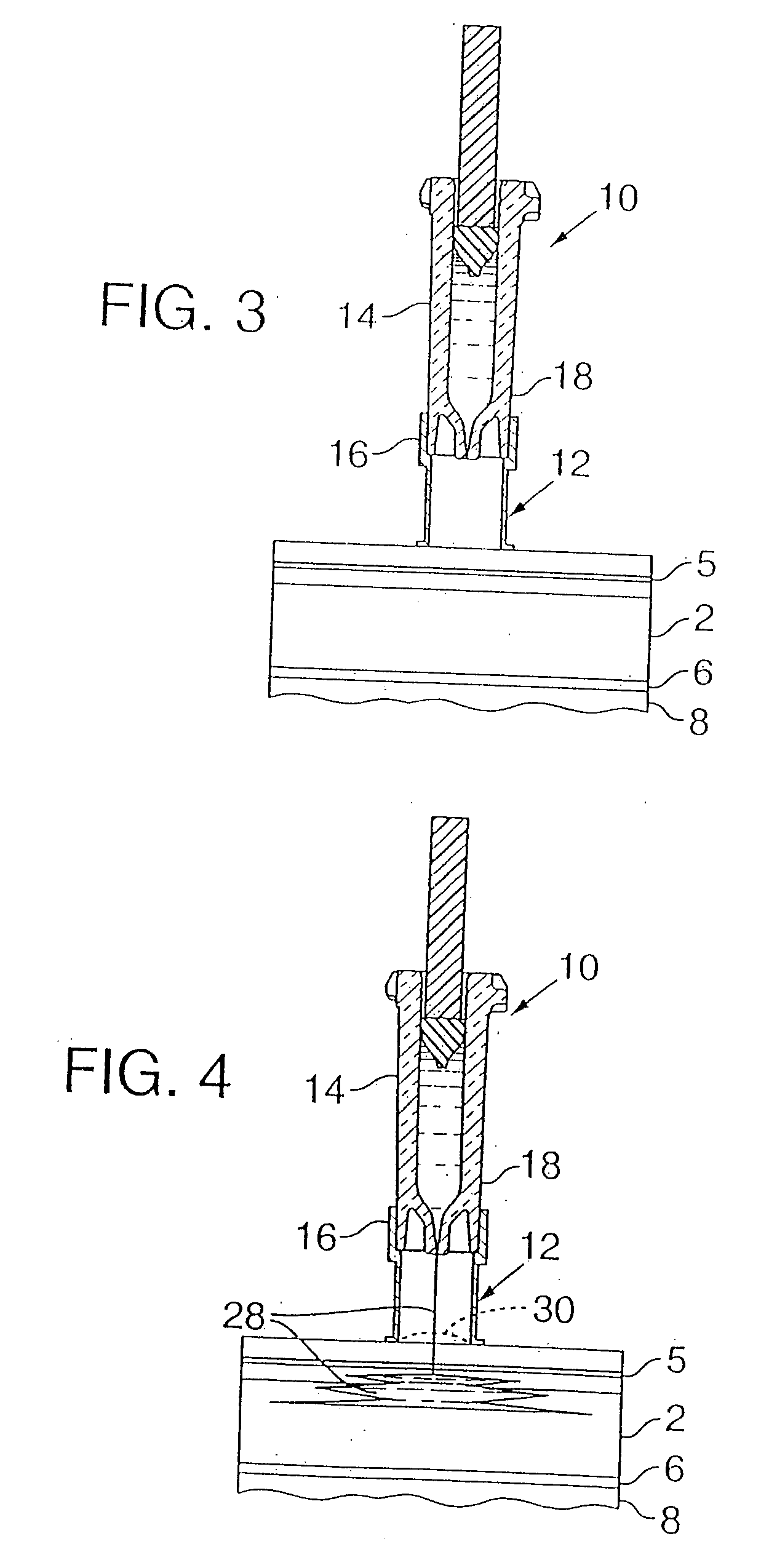
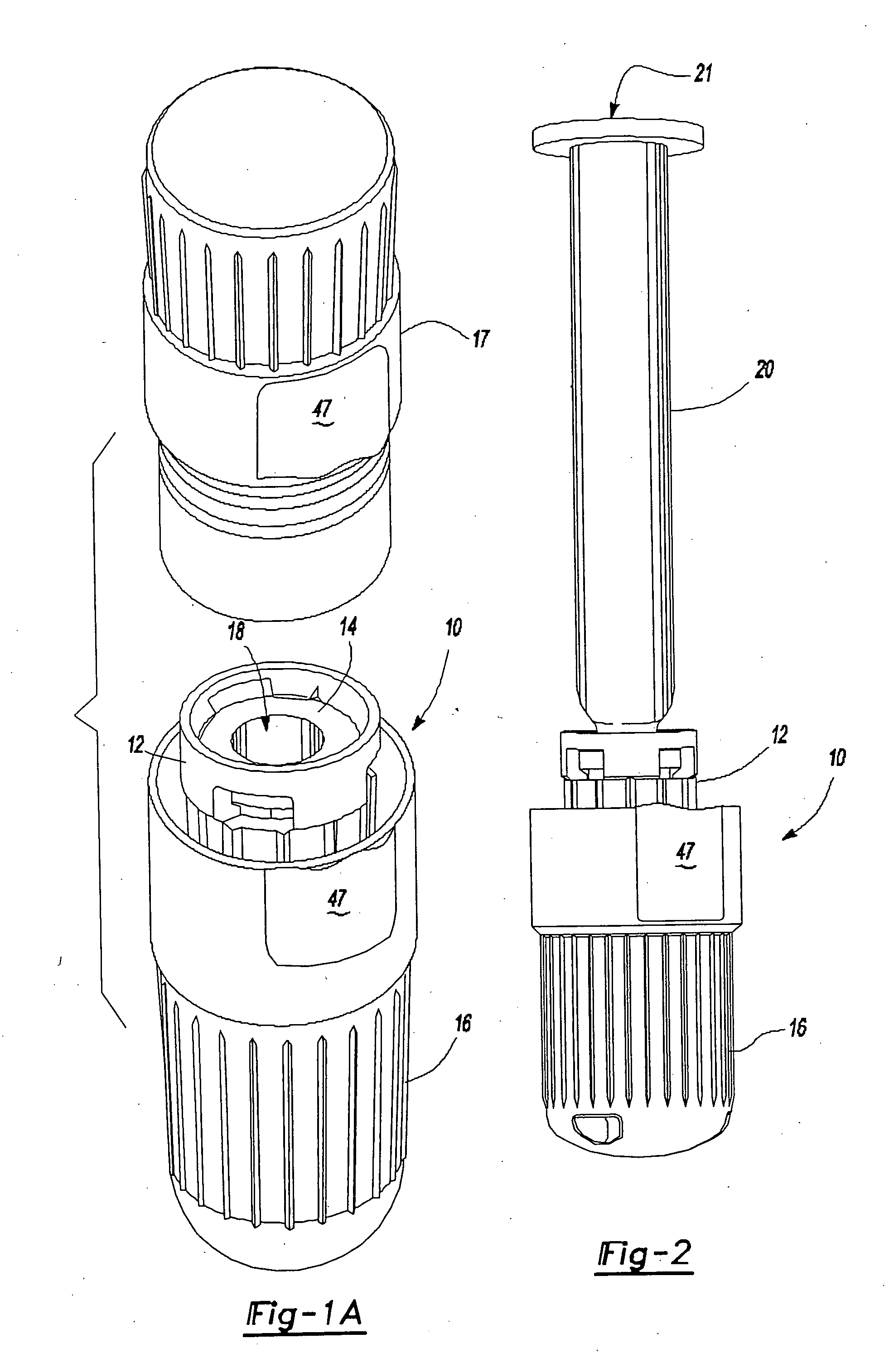
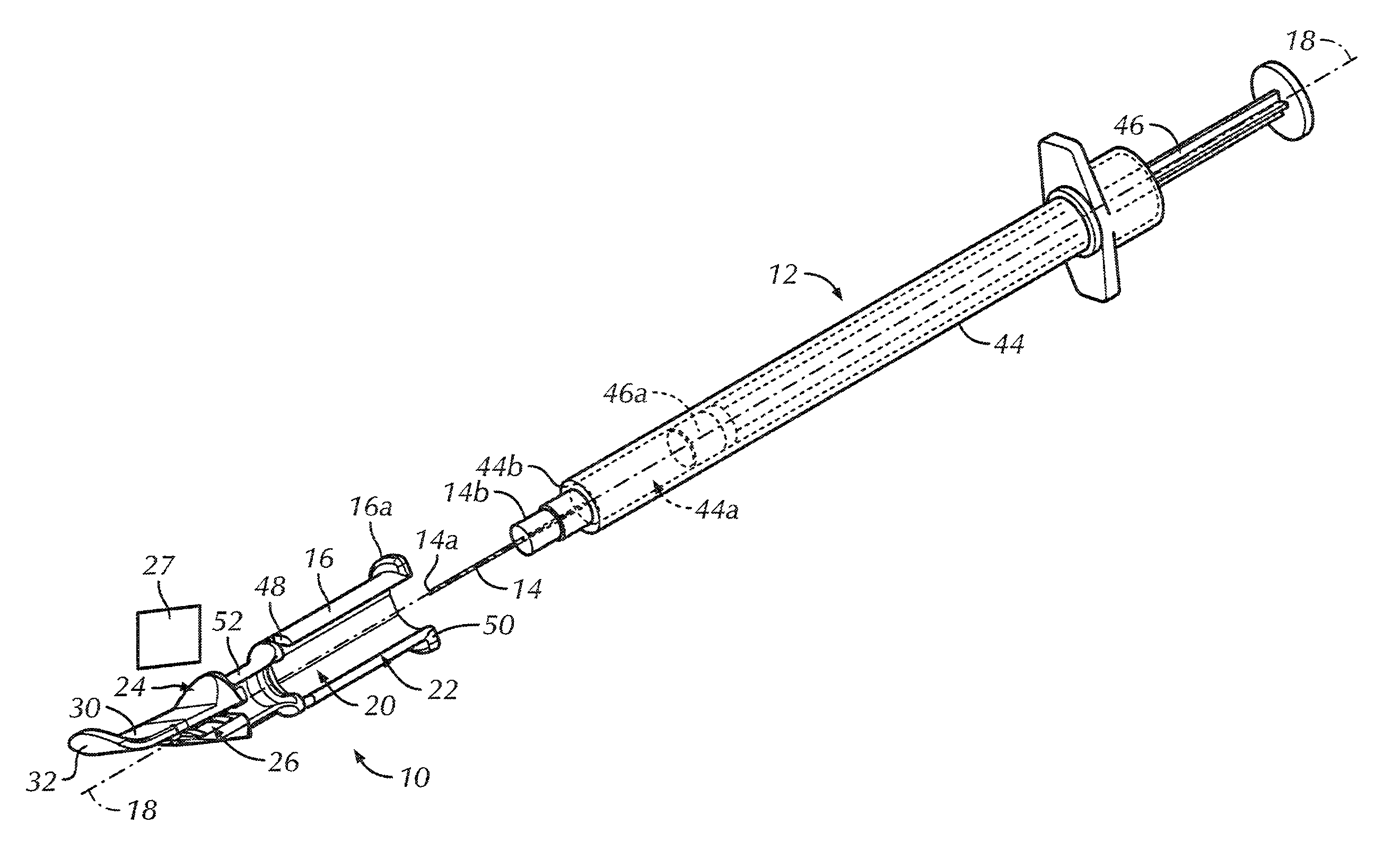
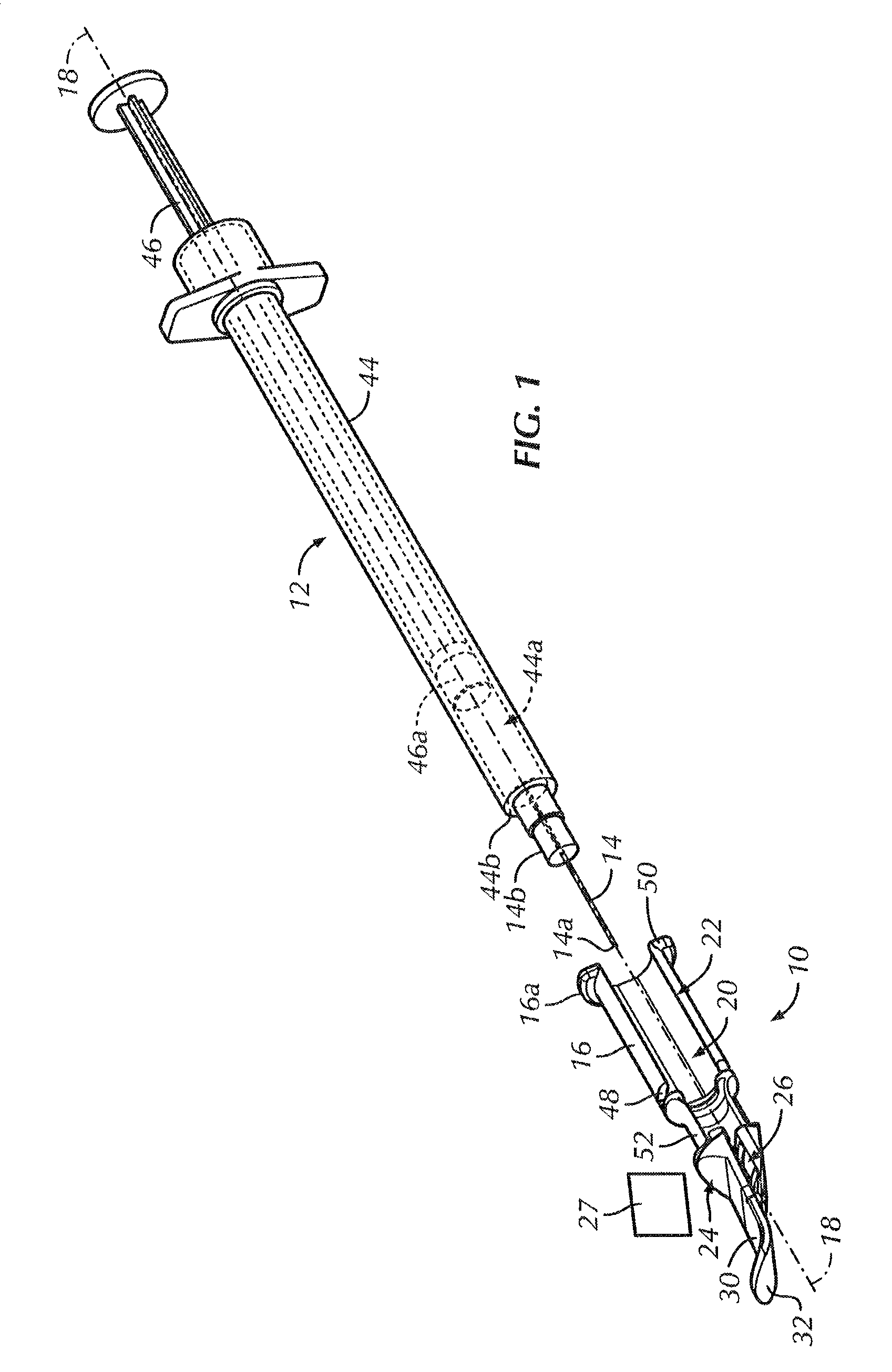
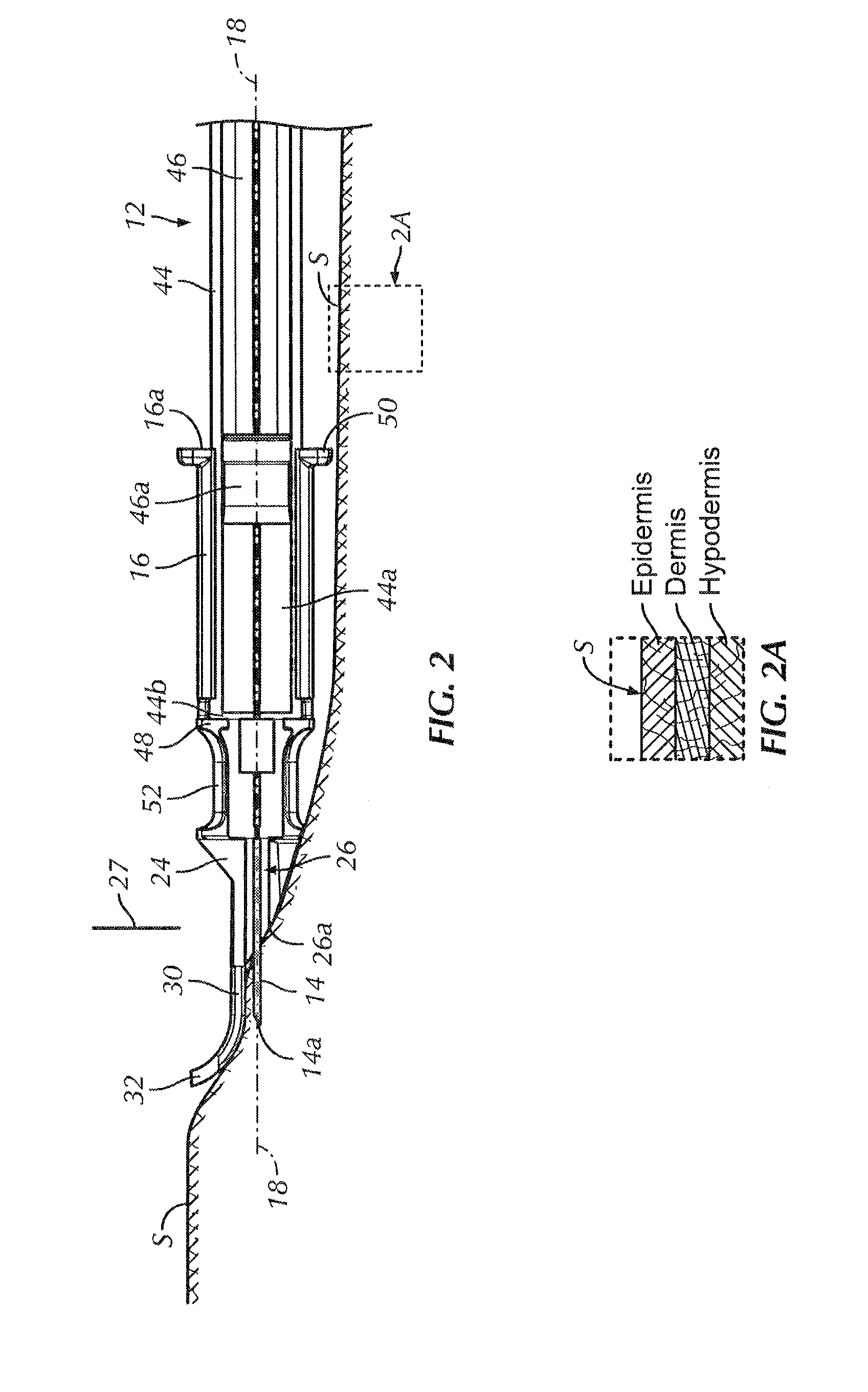
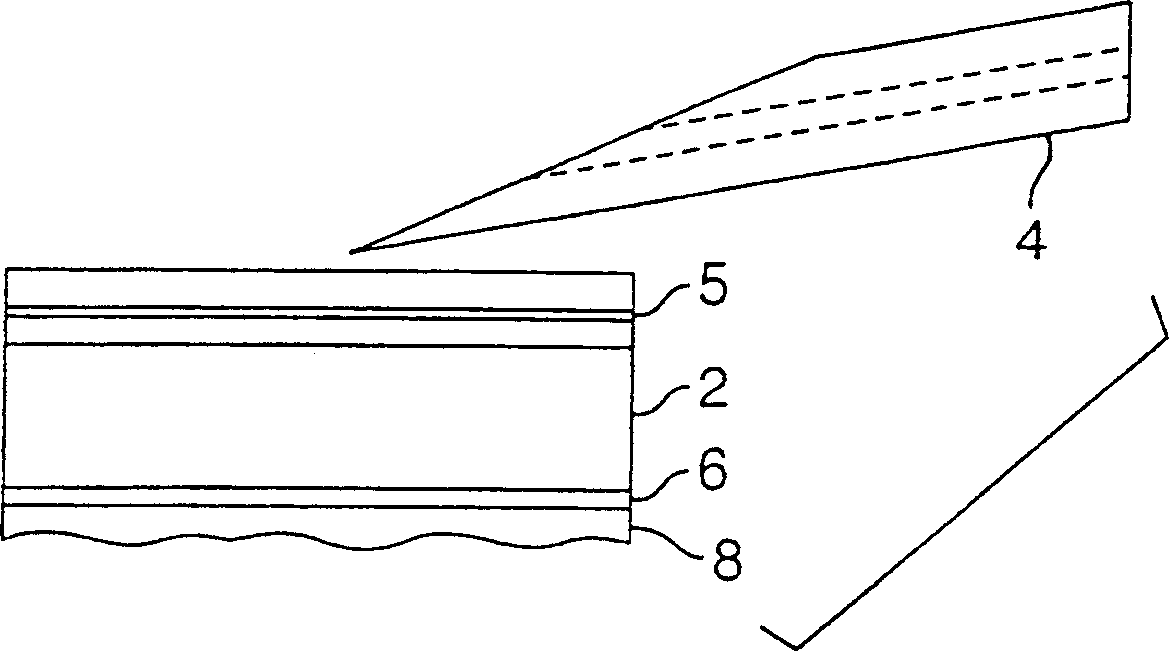
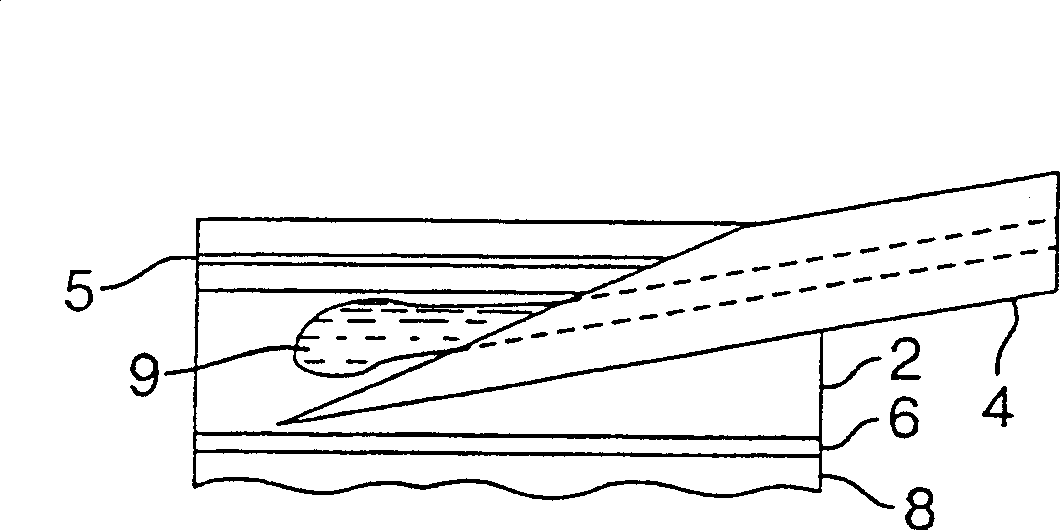
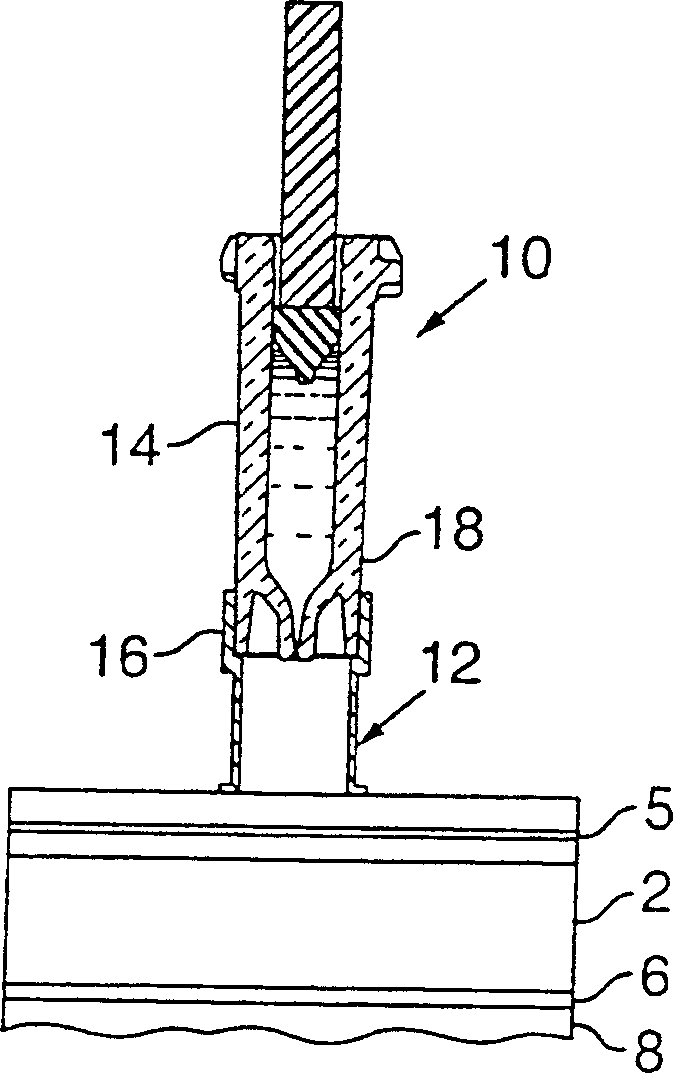
An intradermal needle assembly (10) for injecting a substance into the skin of an animal is disclosed. A hub (16) is attachable to a container (12) storing the substance. A needle (18) is supported by the hub (16) and has a forward tip (20) extending away from the hub (16). A limiter (22) surrounds the needle (18) and extends away from the hub (16) toward the forward tip (20) of the needle (18). The limiter (22) includes a generally flat skin engaging surface (24) extending in a plane generally perpendicular to an axis of the needle (18) and is adapted to be received against the skin (26) to administer an intradermal injection. The limiter (22) is movable between a first position (28), wherein the skin engaging surface (24) shields the needle (18), and a second position (30), wherein the forward tip (20) extends beyond the skin (26) upon depressing the skin engaging surface (24) against the skin (26).
Owner:BECTON DICKINSON & CO
Nanocomposite temperature-sensitive gel and preparation method and application thereof
InactiveCN102525882AEasy to prepareSuitable for mass productionGenetic material ingredientsEmulsion deliveryHypodermic injectionLung cancer
The invention relates to a nanocomposite temperature-sensitive gel and a preparation method and an application thereof. The preparation method comprises the following steps of: coating antitumor active substances with a high molecular polymer serving as a carrier material to obtain nanoparticles; and adding a temperature-sensitive high molecular material to obtain the nanocomposite temperature-sensitive gel. The preparation method disclosed by the invention is simple and convenient, is suitable for large-scale production, and is particularly suitable for preparing medicaments or diagnostic reagents having the characteristics of long cycle, biodegradability, slow release, passive targeting, active targeting, active substance conveying function and tumor resistance. An antitumor medicament prepared with the method disclosed by the invention is suitable for ways such as intravenous injection, intramuscular injection, hypodermic injection, intradermal injection, intratumor injection, tumor-side injection, oral administration or transdermal medicament delivery and the like, is applied to treatment and diagnosis of pancreatic cancer, liver cancer, lung cancer, gastric cancer, colorectal cancer, esophageal cancer, prostatic cancer, uterine cancer and ovarian cancer, and has a good application prospect.
Owner:SHANGHAI INST OF ONCOLOGY
Dual Chamber Injector Integrated With Micro-Needles
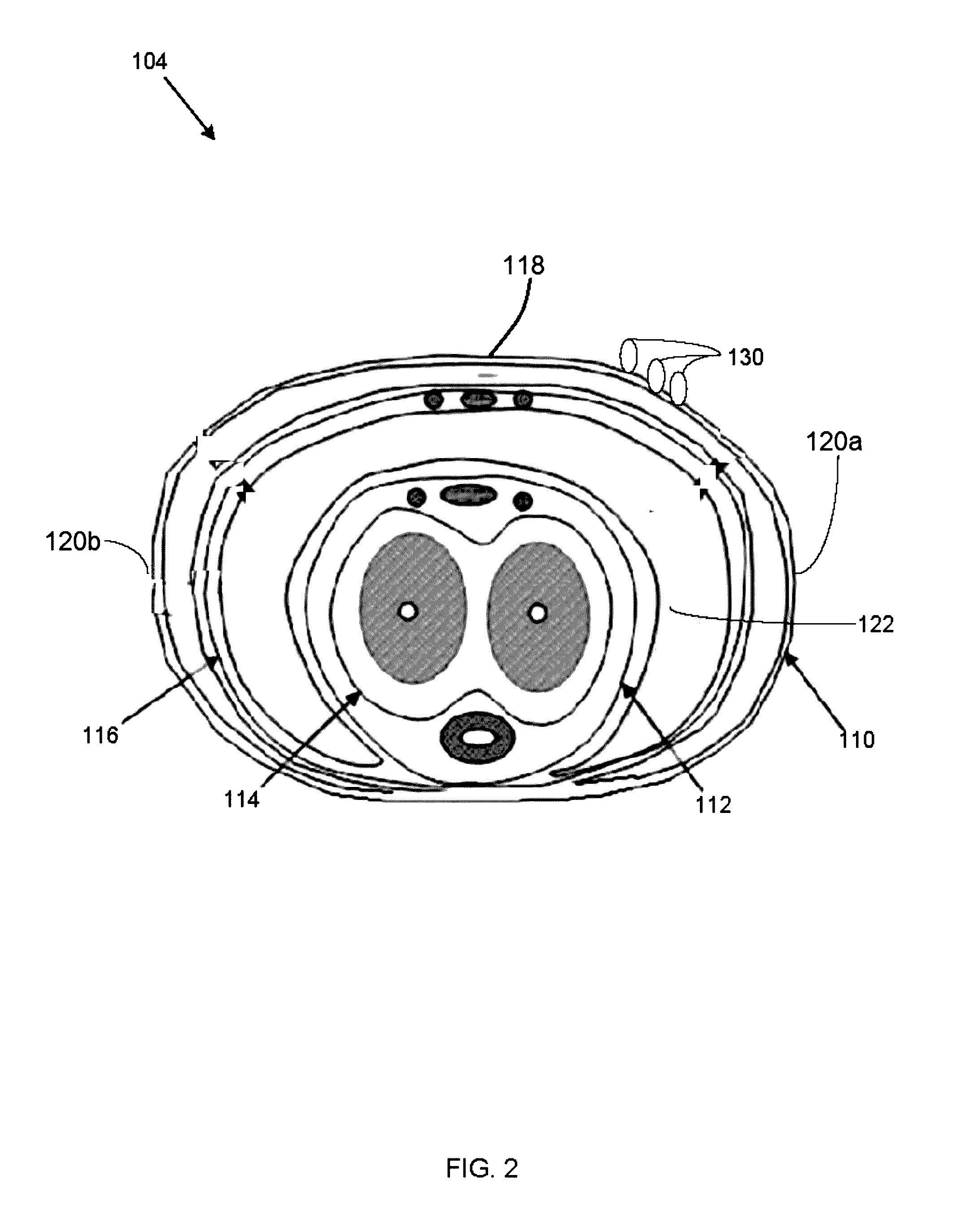
This invention relates to a medical injection system for the intradermal injection of two or more fluid substances via micro-needles, and most preferably via a linear array of micro-needles, although the array need not be linear. Injecting two fluid substances contained in two separate chambers of the delivery device can be done by various methods. These methods may be generally categorized as, a) sequential injection, b) simultaneous injection, c) parallel injection and d) closed-loop injection processes. The present invention includes devices configured to perform all three of these methods.
Owner:NANOPASS TECH LTD
Intradermal syringe and needle assembly
ActiveUS20050203459A1Prevent penetrationMedical devicesInfusion needlesTissue skinIntradermal injection
A needle assembly for an intradermal injection device, and a drug delivery device, having a needle cannula having a needle tip and a limiter surrounding the needle cannula and having a skin engaging surface, wherein the limiter is moveable from a first position in which an elongate portion of the needle cannula is exposed, to a locked second position in which the limiter is not movable from the second position to the first position and in which the needle tip extends beyond the skin engaging surface a distance of about 3 mm or less.
Owner:BECTON DICKINSON & CO
Methods and devices for intradermal injection
Owner:SID TECH LLC +1
Intradermal Injection Device
ActiveUS20150073344A1Reduce the amount requiredMaintain positionInfusion syringesInfusion needlesDermatomalBiomedical engineering
An intradermal injection device comprises a housing, a foot with an opening for passage of a needle; a reservoir movably mounted in the housing, and having a hollow space for holding a fluid; a hollow needle movably mounted in the housing and having a first end for penetrating the subject's skin and a second end for penetrating the reservoir, a plunger movably mounted in the housing for moving the needle through the opening for penetrating the subject's skin, and for pressing the fluid out of the reservoir. The reservoir is frictionally mounted inside the housing by first friction means, and the plunger is frictionally mounted to the reservoir by second friction means. The force to overcome the second friction is larger than the force to overcome the first friction.
Owner:NOVOSANIS NV
Intradermal injection device
InactiveUS20070185460A1Control leakageSimple interfaceAmpoule syringesInfusion needlesInjection deviceTissue skin
An intradermal injection device comprising a unitary body having an open distal end and a proximal end having a skin engaging surface defined thereon, a reservoir defined between the proximal and distal ends for accommodating a drug substance, and a channel defined at the proximal end of the unitary body and extending through, and distally from, the skin engaging surface to the reservoir. A needle cannula having a sharpened proximal end and a distal end may be provided in the channel. The needle cannula is secured in the channel with the distal end being in communication with the reservoir and the proximal end of the needle cannula extending from the skin engaging surface a distance in the range of about 0.5 mm to 3.0 mm such that the skin engaging surface limits penetration of the proximal end of the needle cannula to the dermis layer of the skin of a patient.
Owner:BECTON DICKINSON & CO
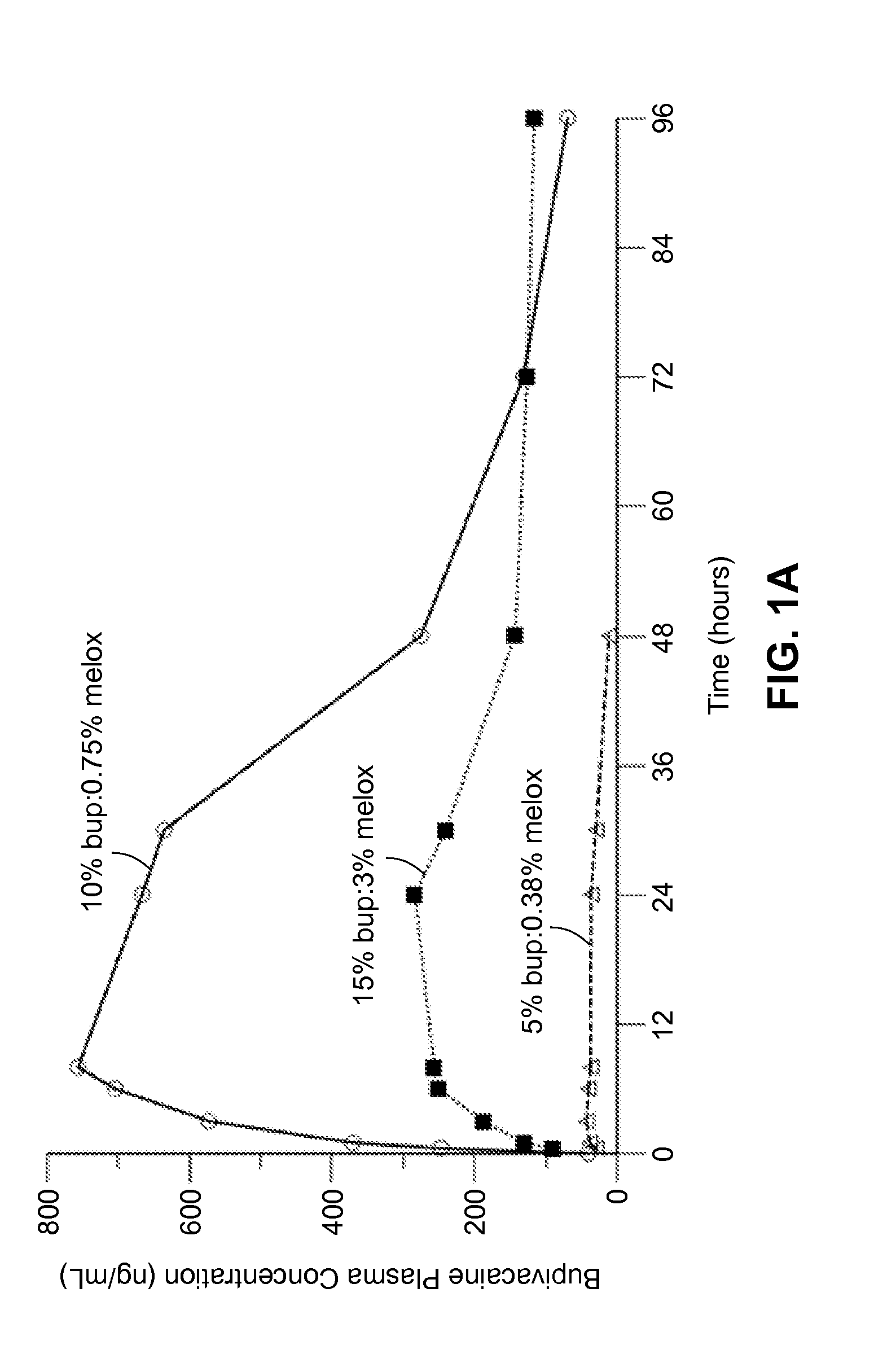
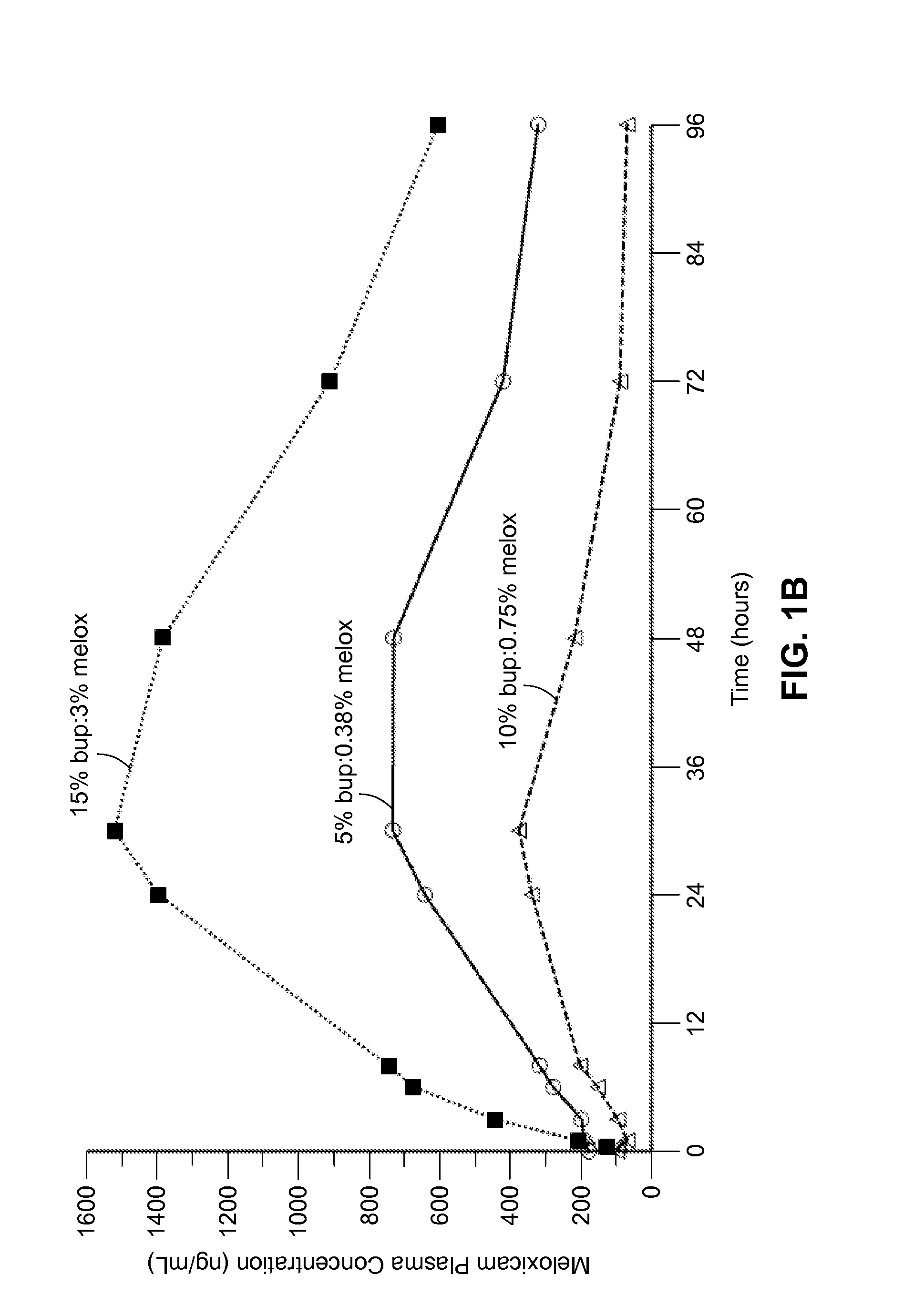
Long-acting polymeric delivery systems
ActiveUS20150297729A1Efficient releaseImprove the level ofPowder deliveryBiocideAnesthetic AgentActive agent
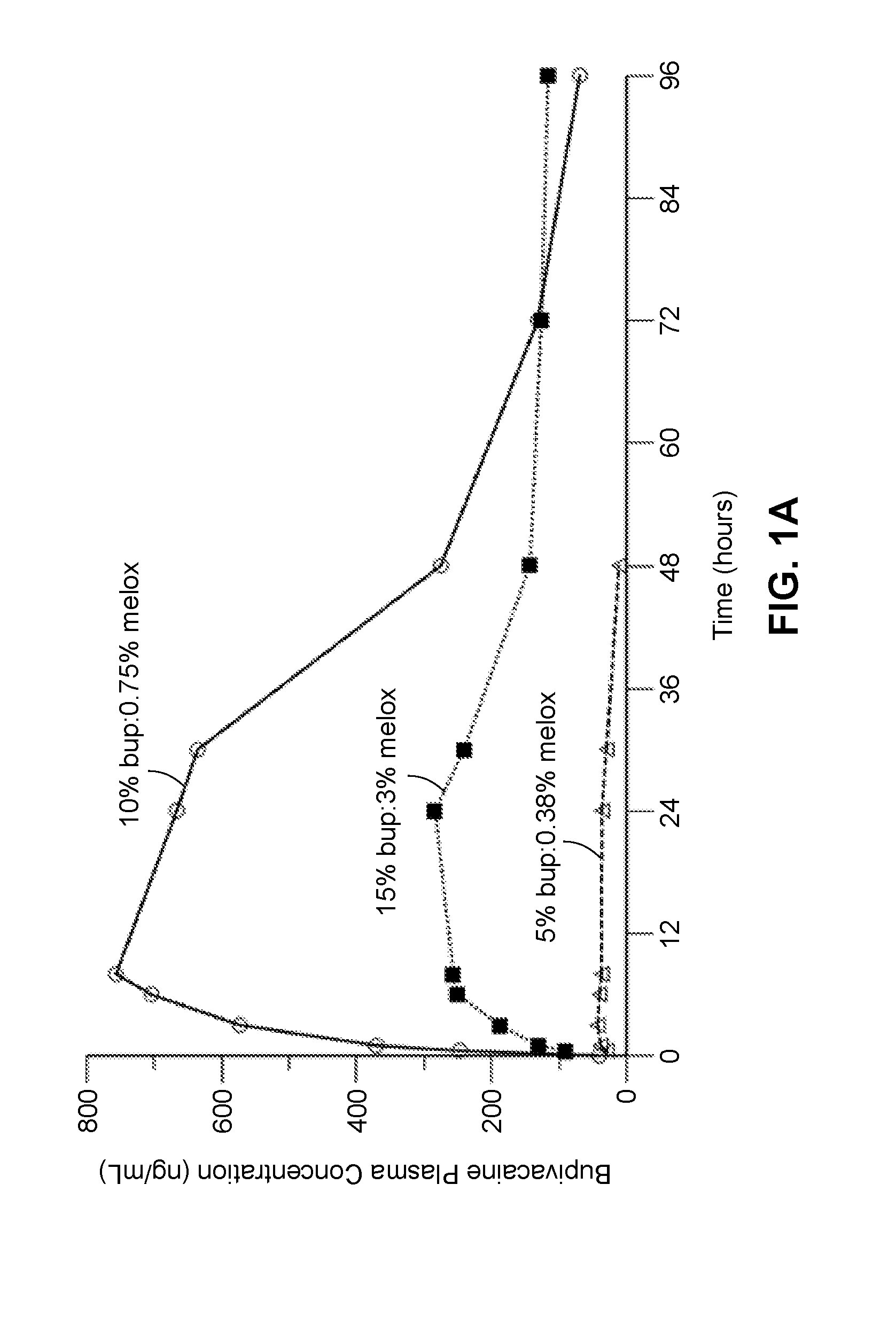
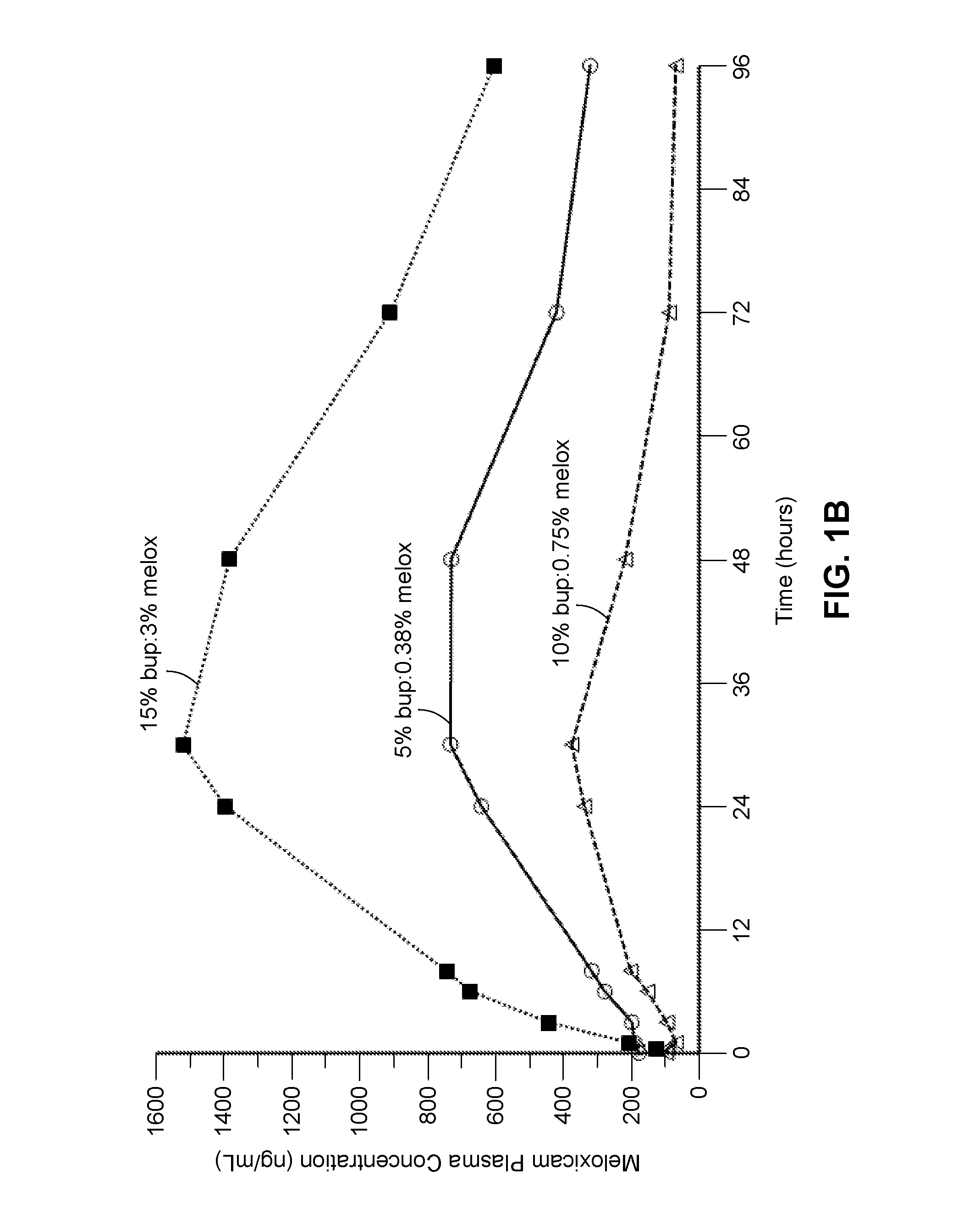
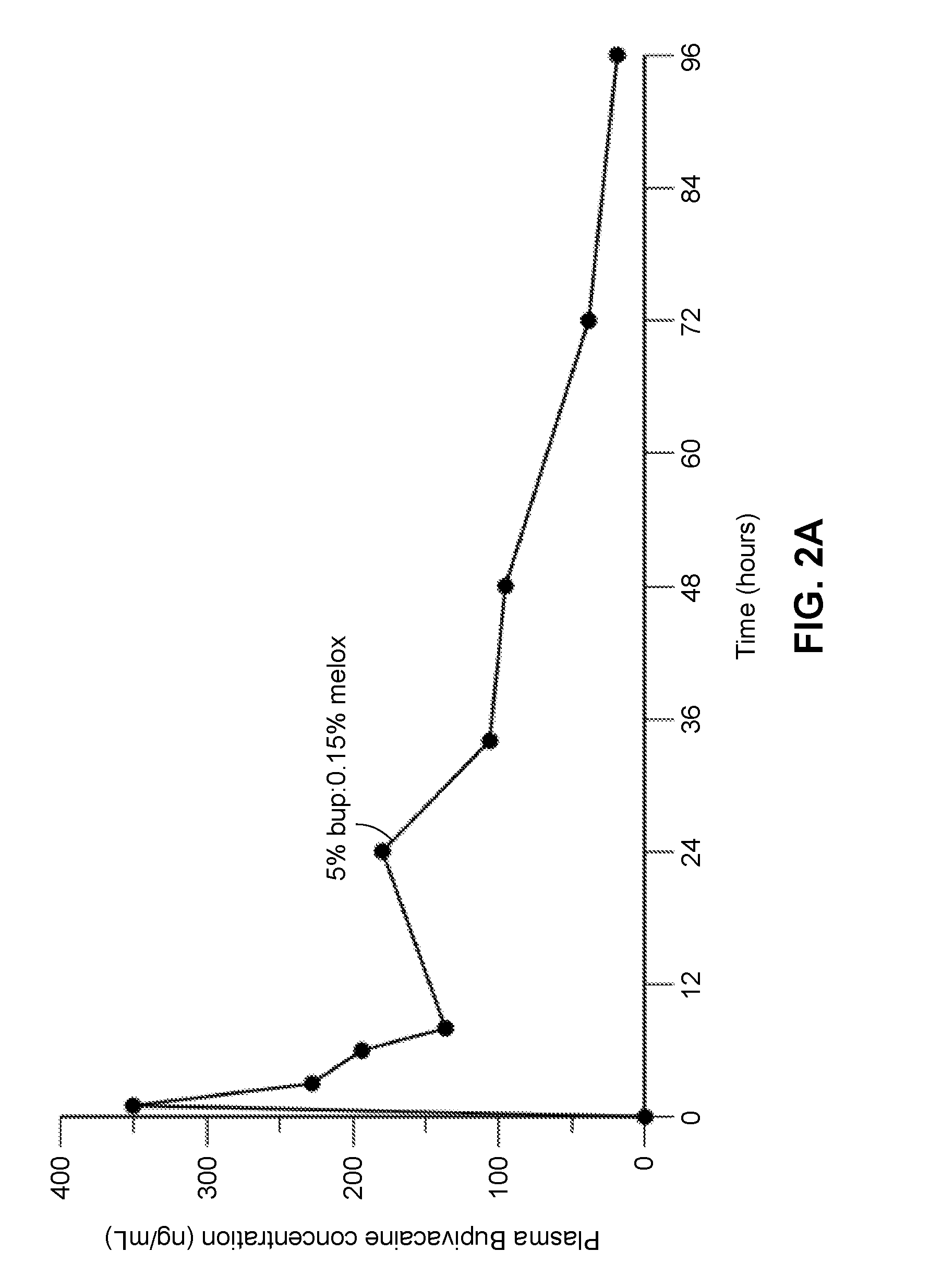
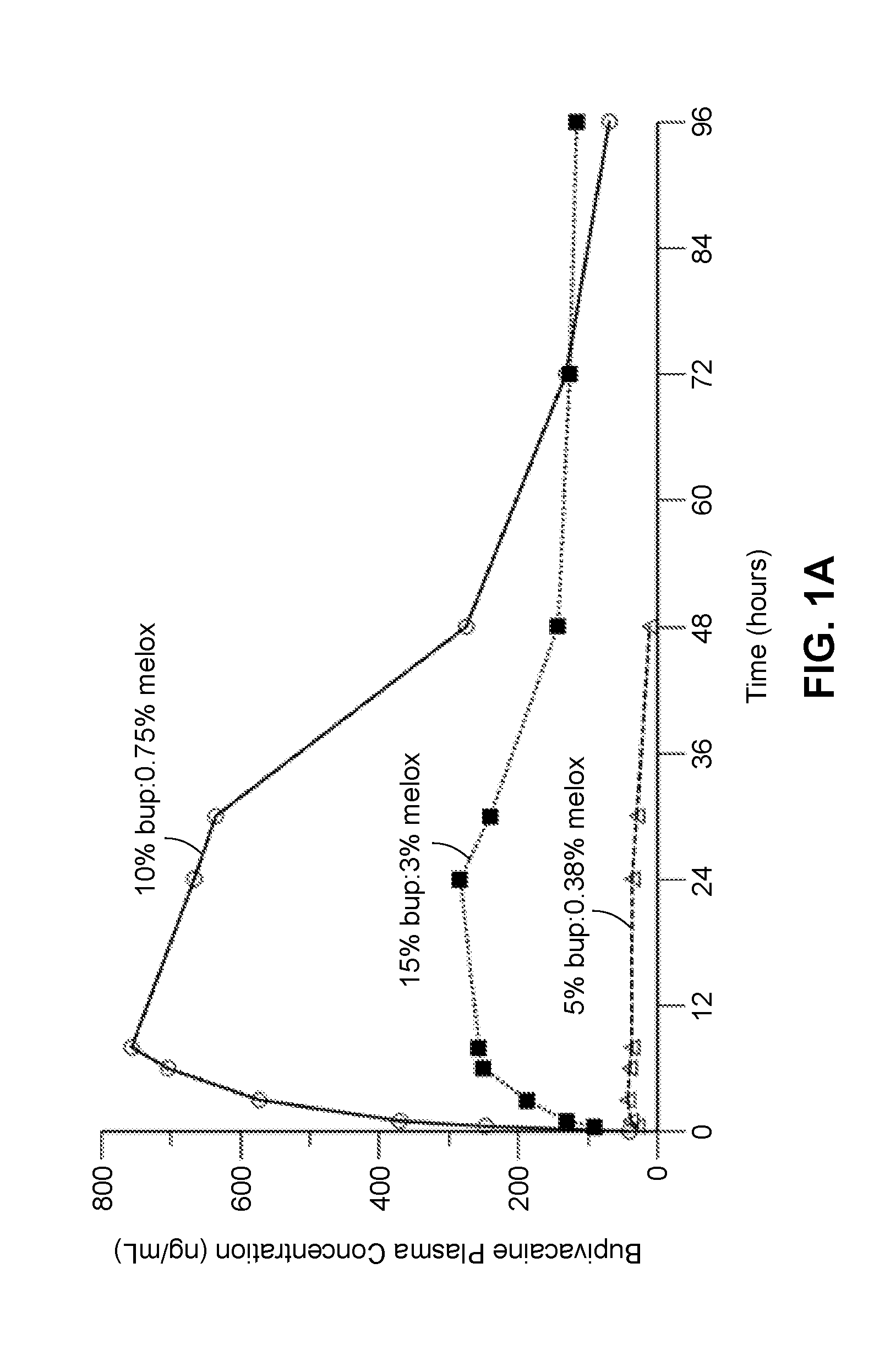
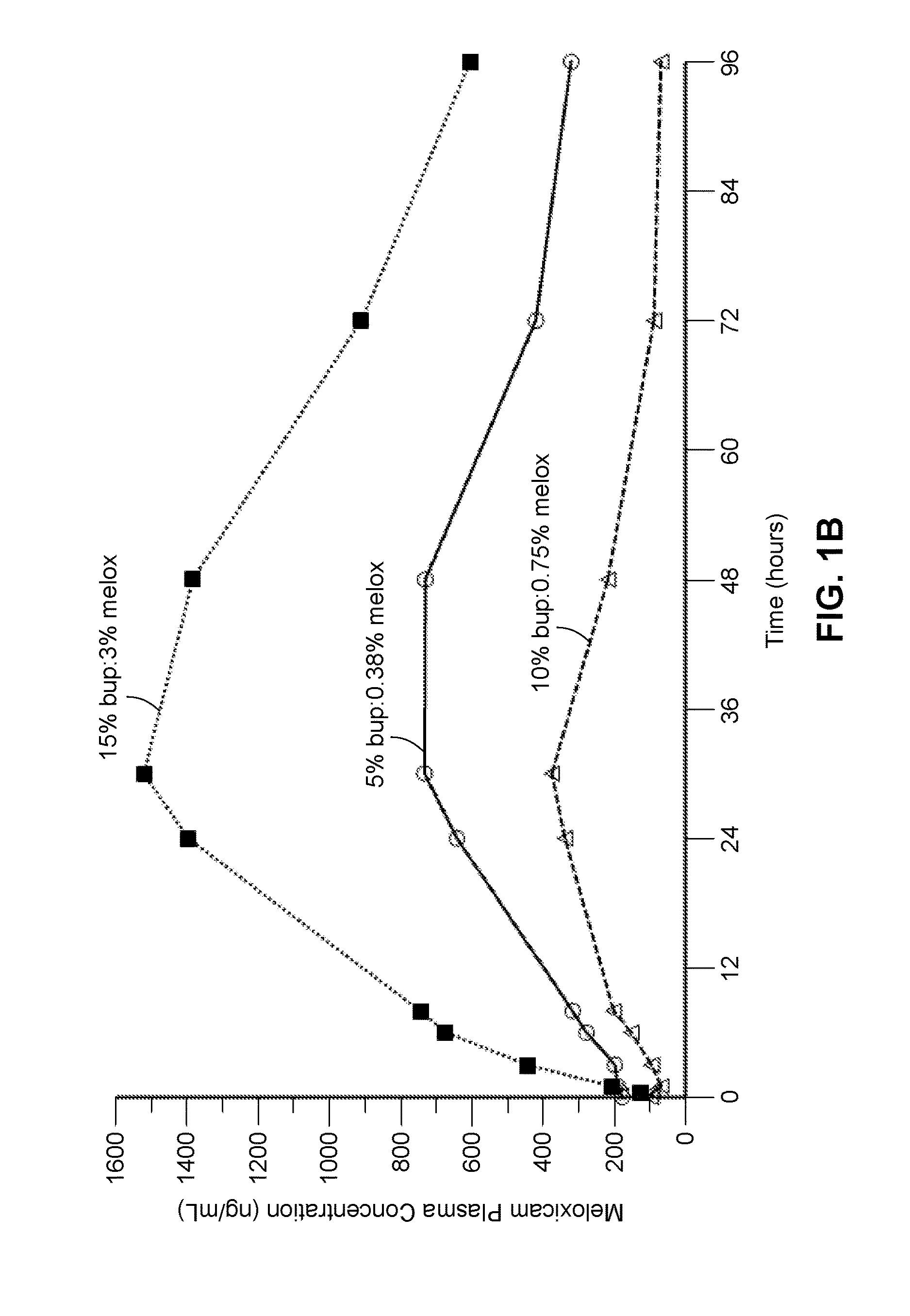
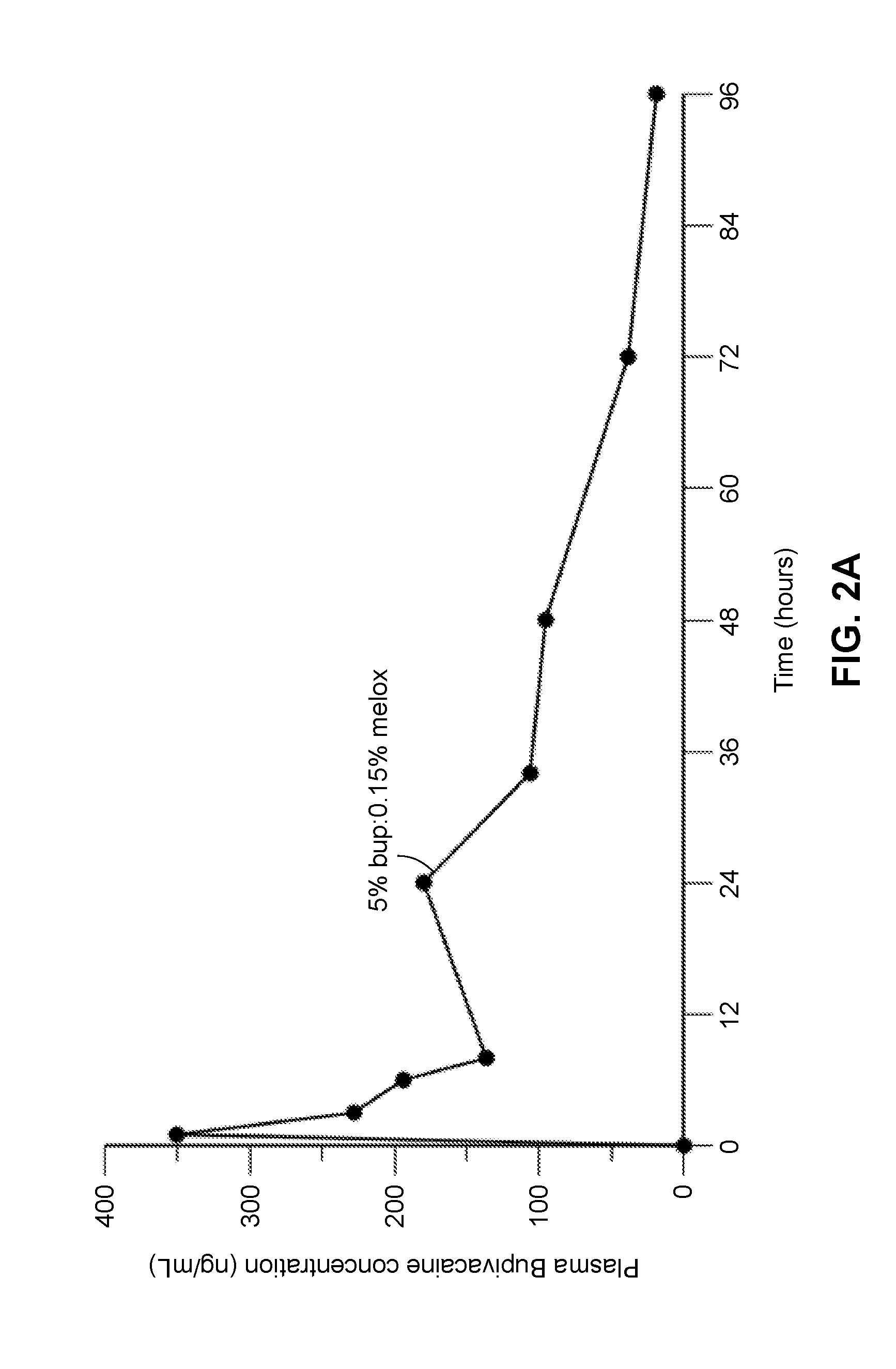
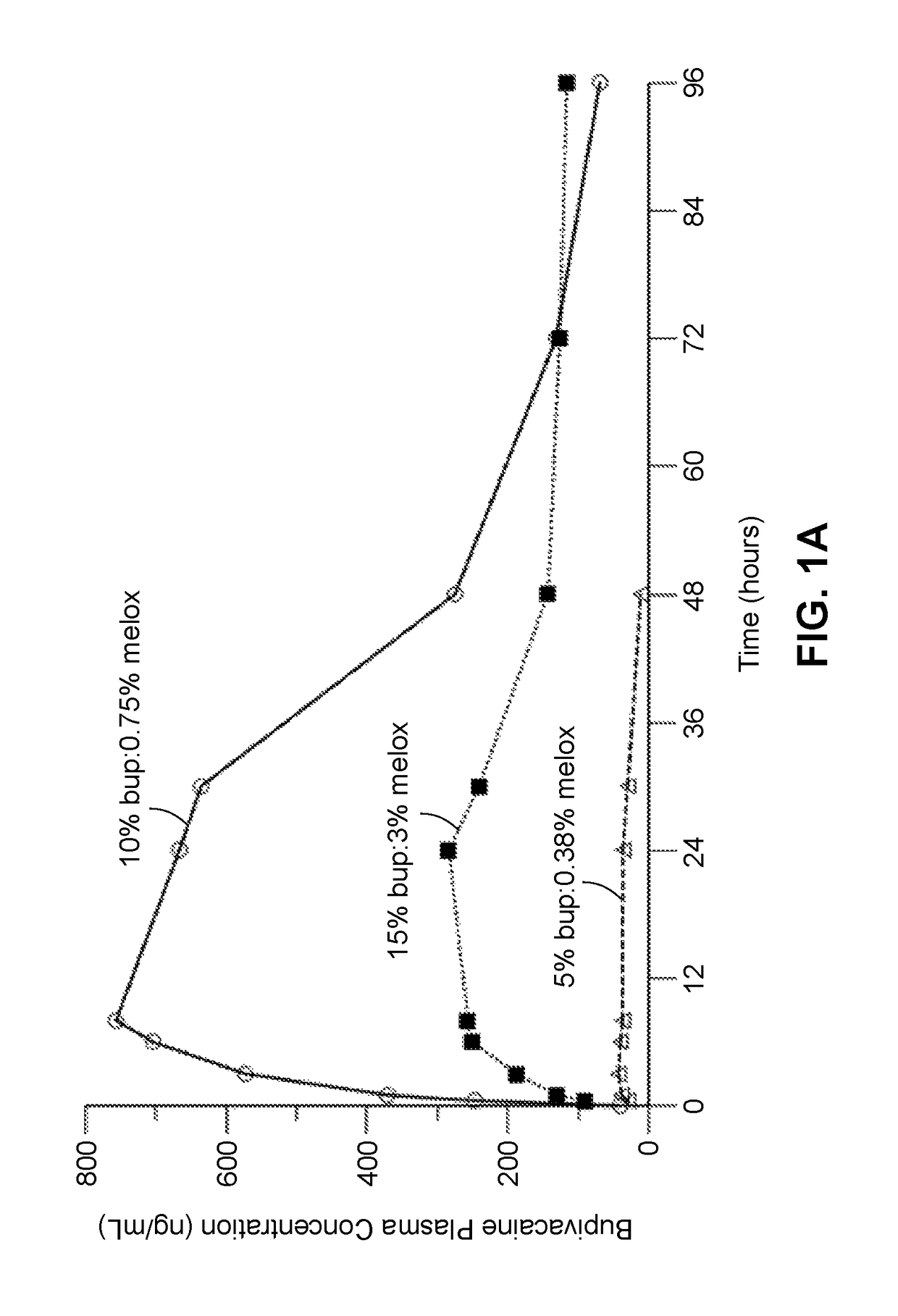
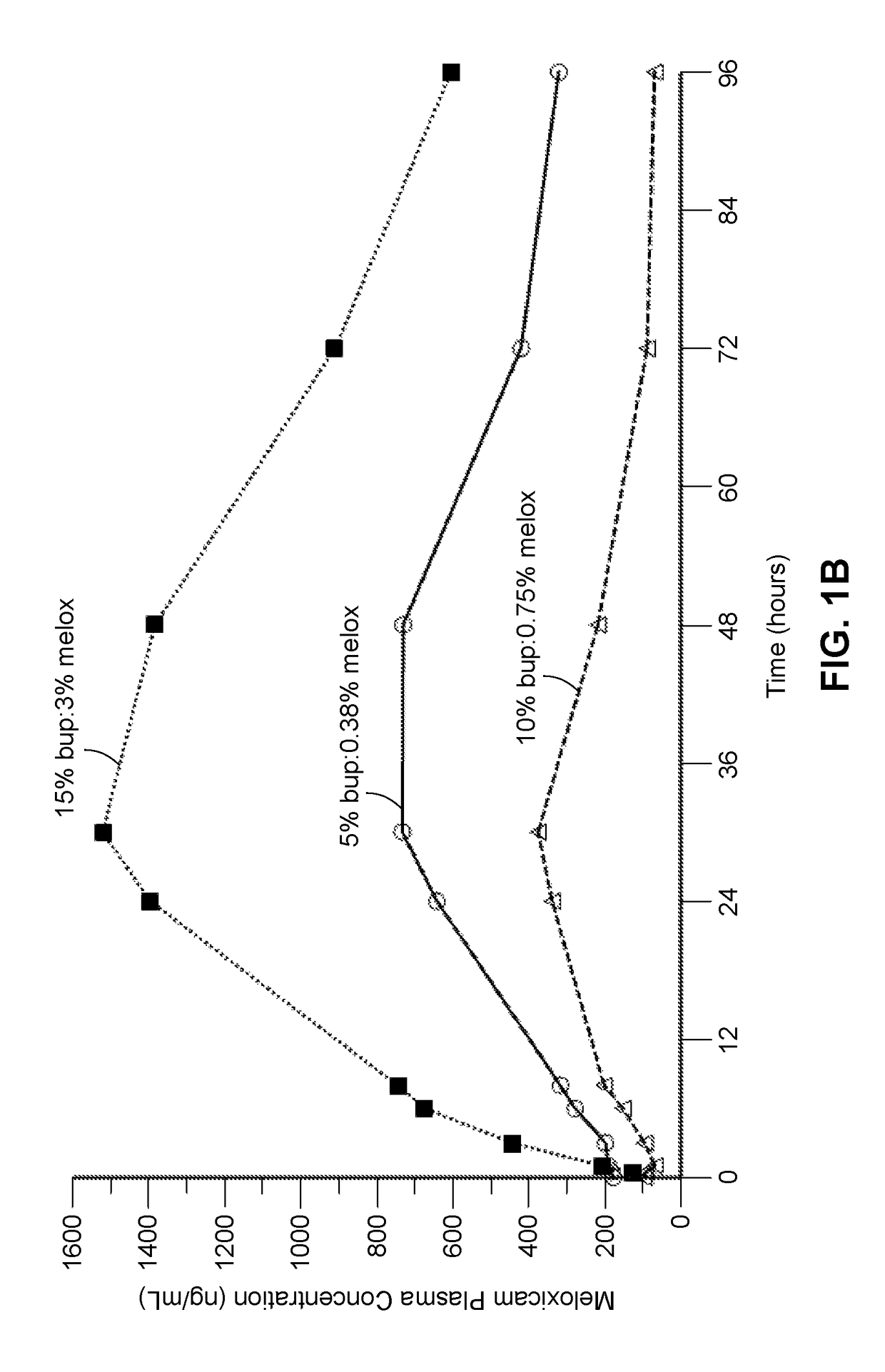
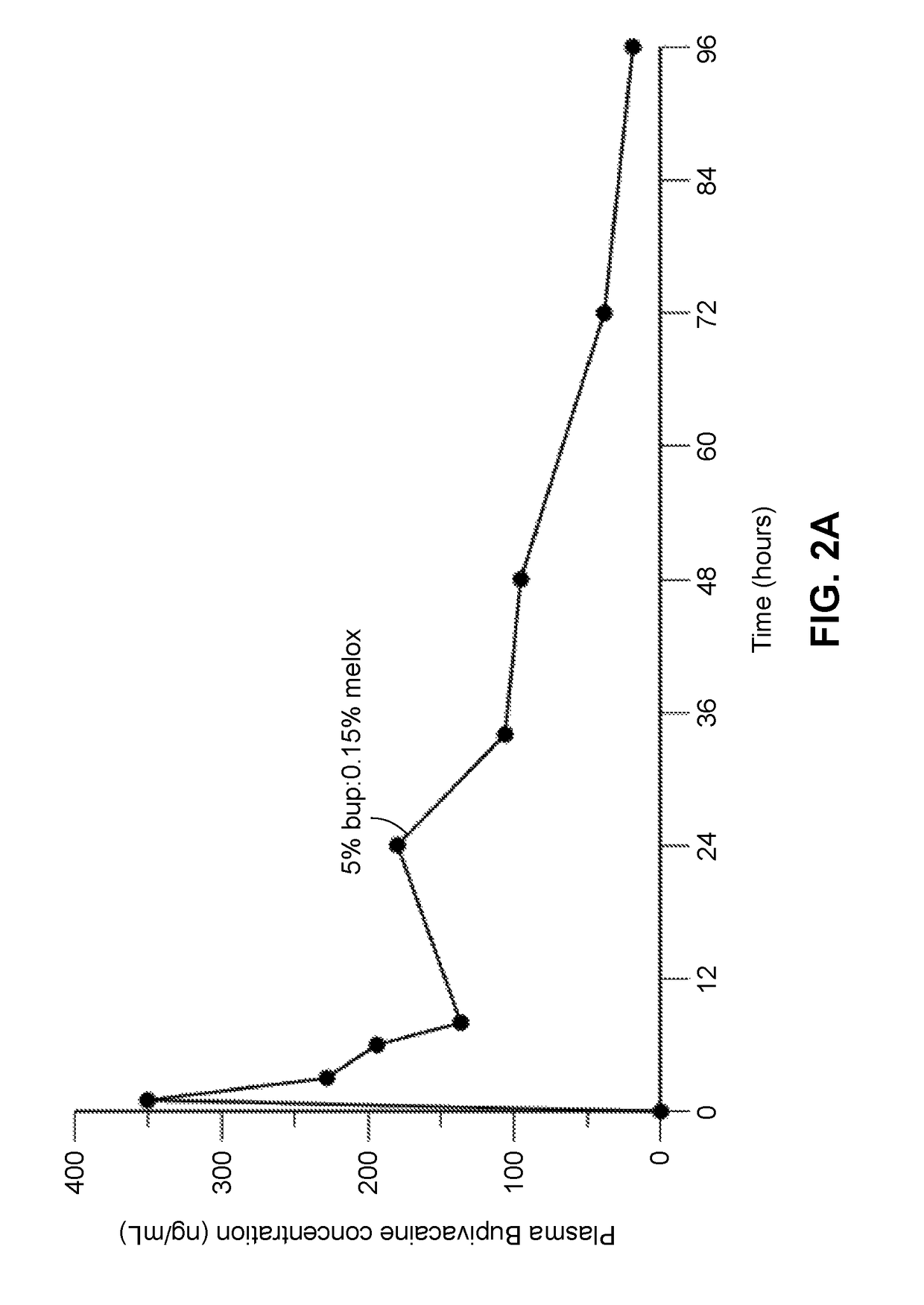
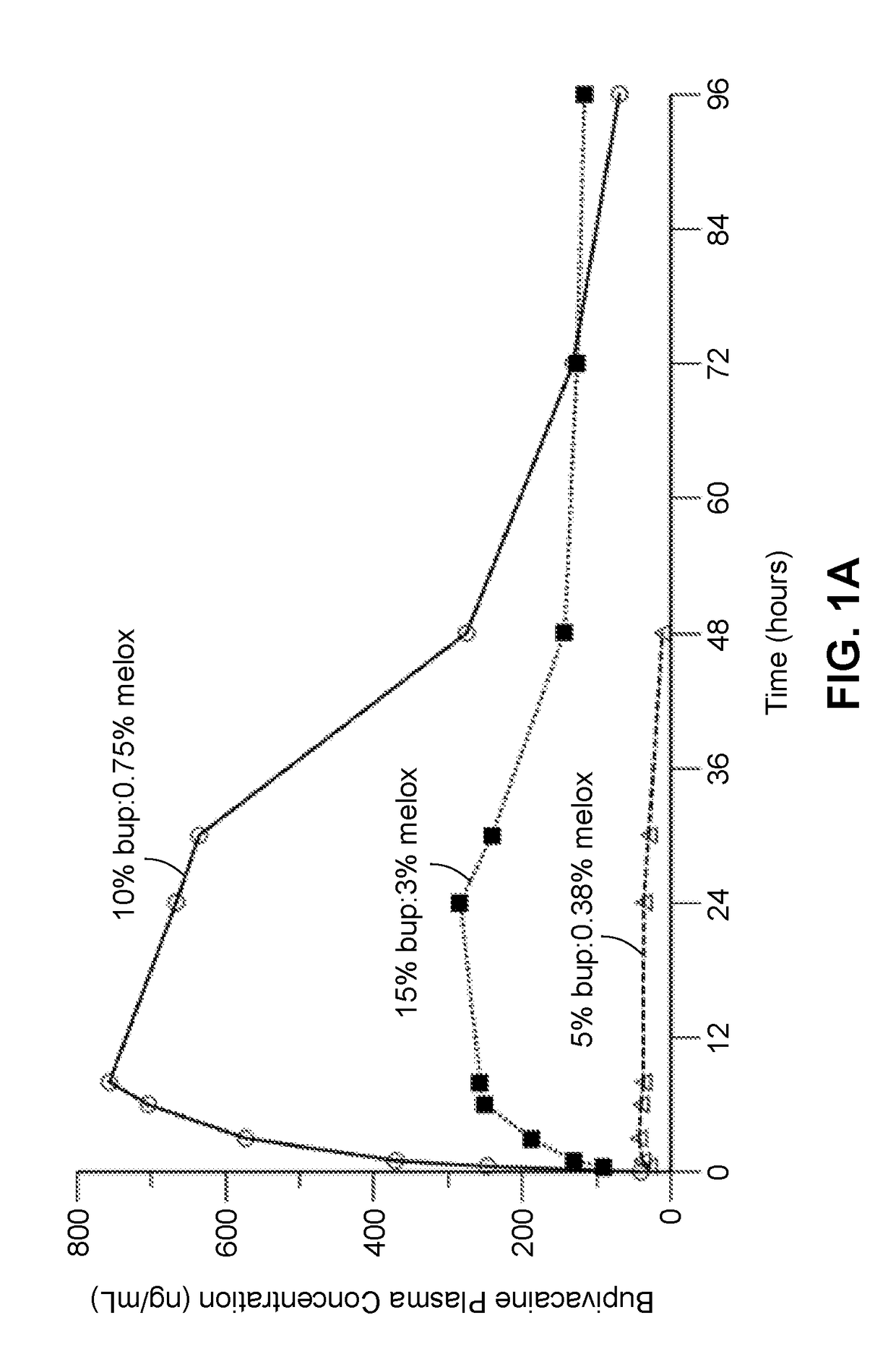
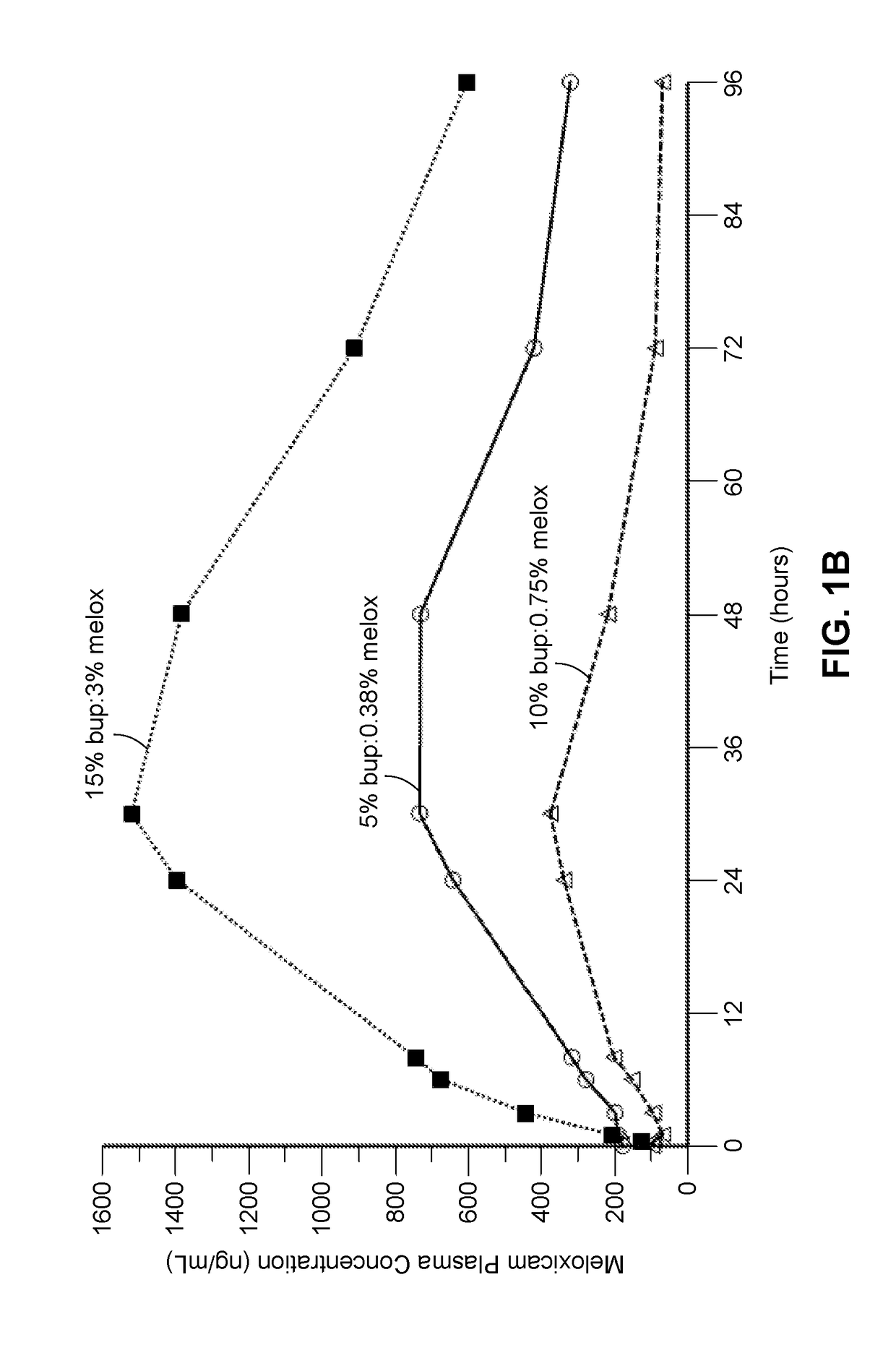
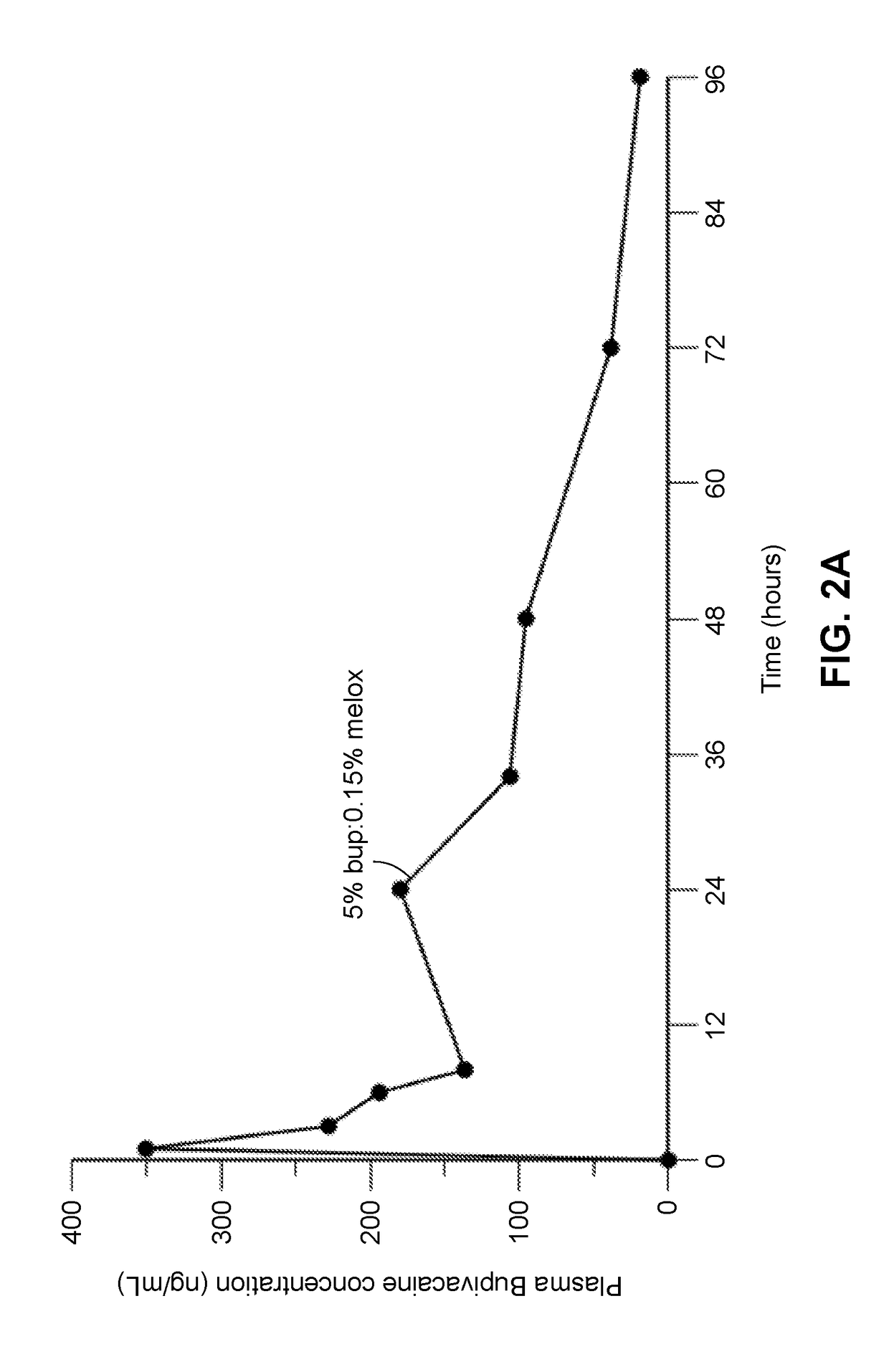
Compositions comprised of a delivery vehicle or delivery system and an active agent dispersed within the delivery vehicle or system, wherein the delivery vehicle or system contains a polyorthoester polymer and a polar aprotic solvent. Also disclosed are low viscosity delivery systems for administration of active agents. The low viscosity delivery systems have a polyorthoester polymer, a polar aprotic solvent and a solvent containing a triglyceride viscosity reducing agent. Compositions described include an amide- or anilide-type local anesthetic of the “caine” classification, and a non-steroidal anti-inflammatory drug (NSAID), along with related methods, e.g., for treatment of post-operative pain or for prophylactic treatment of pain. The compositions are suitable for delivery via, e.g., direct application and instillation, intradermal injection, subcutaneous injection, and nerve block (perineural).
Owner:HERON THERAPEUTICS
Mycobacterial immunotherapy for cancer treatment
InactiveUS20100247440A1Method securityEffective treatmentUltrasonic/sonic/infrasonic diagnosticsBacterial antigen ingredientsCancer therapyEffective treatment
Methods of Mycobacterial immunotherapy for the treatment of cancer are described. In certain cases, these methods concern administration of attenuated Mycobacteria by intradermal injection into non tumor tissues. Methods of the invention, provide safe and effective treatments for malignant tumors and the compositions for use in such treatments. Methods for determine the effectiveness of such immunotherapies are also described.
Owner:ONCOVAC
Intradermal injection adapter
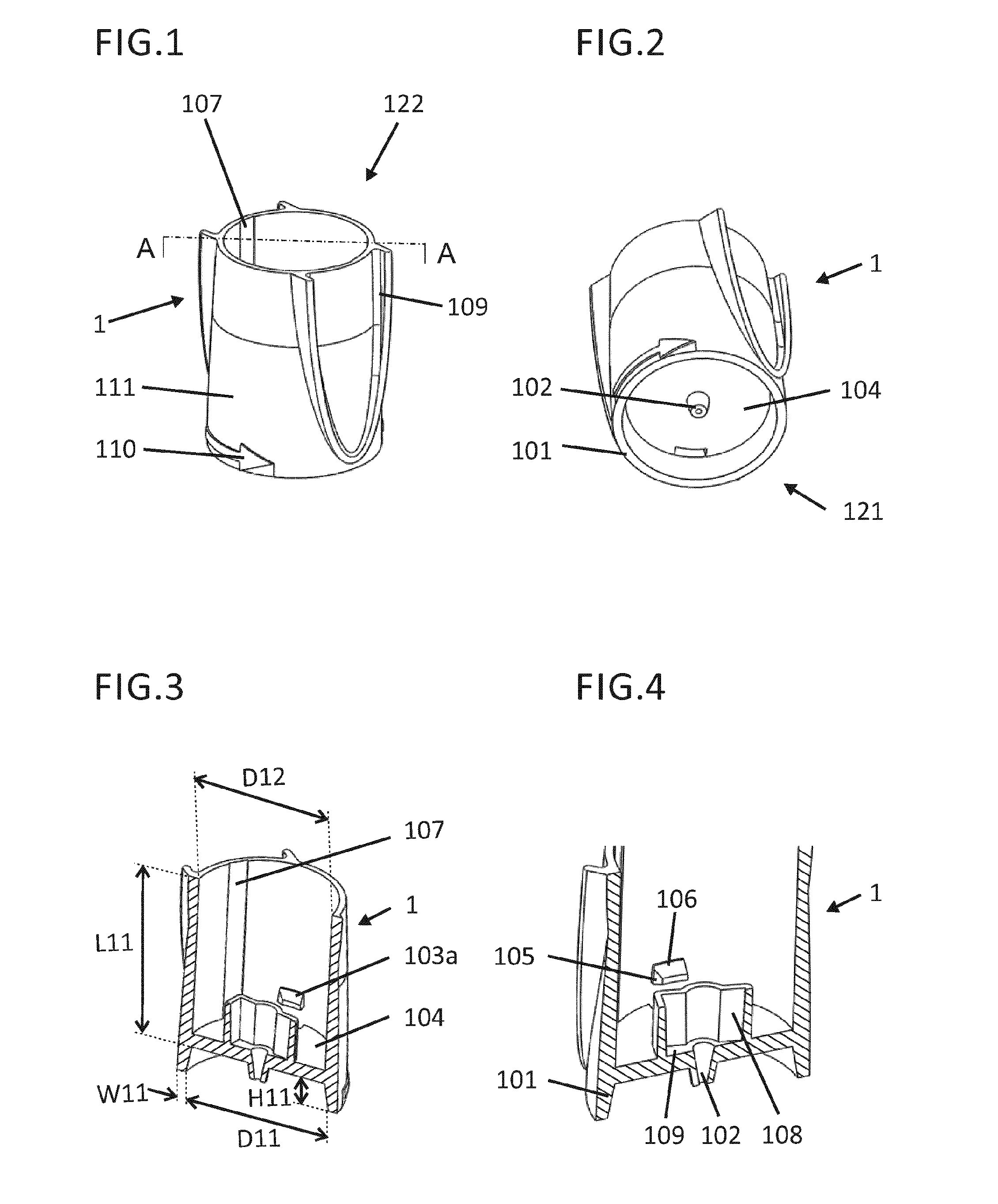
An intradermal injection assembly for injecting a medicament into skin could form an intradermal needle with an adapter, an intradermal syringe with an adapter or an adapter for a merger with a syringe with a cannula. The intradermal adapter in these devices has a body having a longitudinal axis, a central portion having a cannula channel and a distal protrusion extending generally parallel to the longitudinal axis. The distal protrusion of the adapter has a first skin contacting surface extending generally parallel to the longitudinal axis. At least a portion of the distal protrusion is generally transparent such that the portion of the cannula that extends distally relative to a demarcation plane can be viewed through the distal protrusion during insertion of the cannula into a patient's skin.
Owner:SID TECH LLC
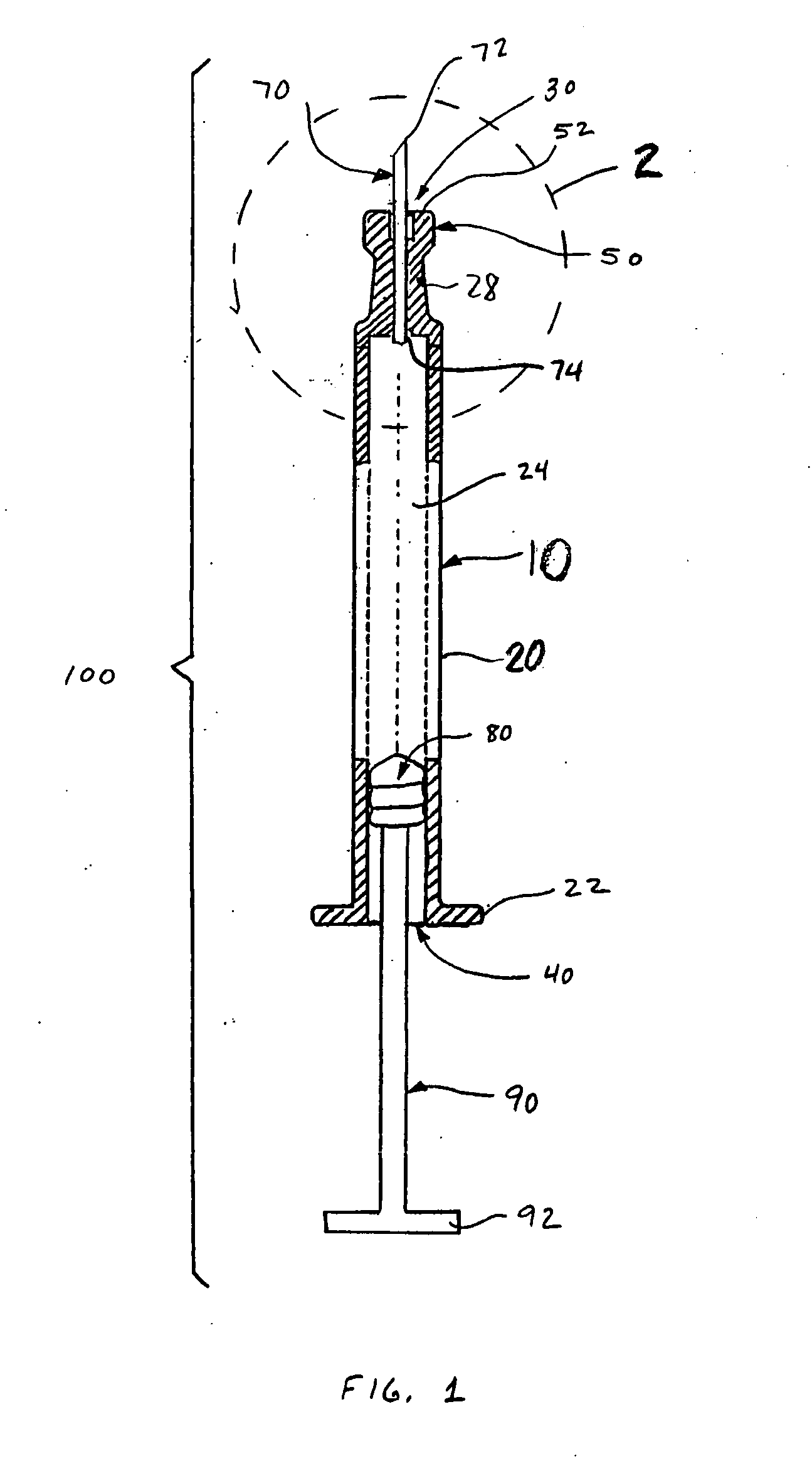
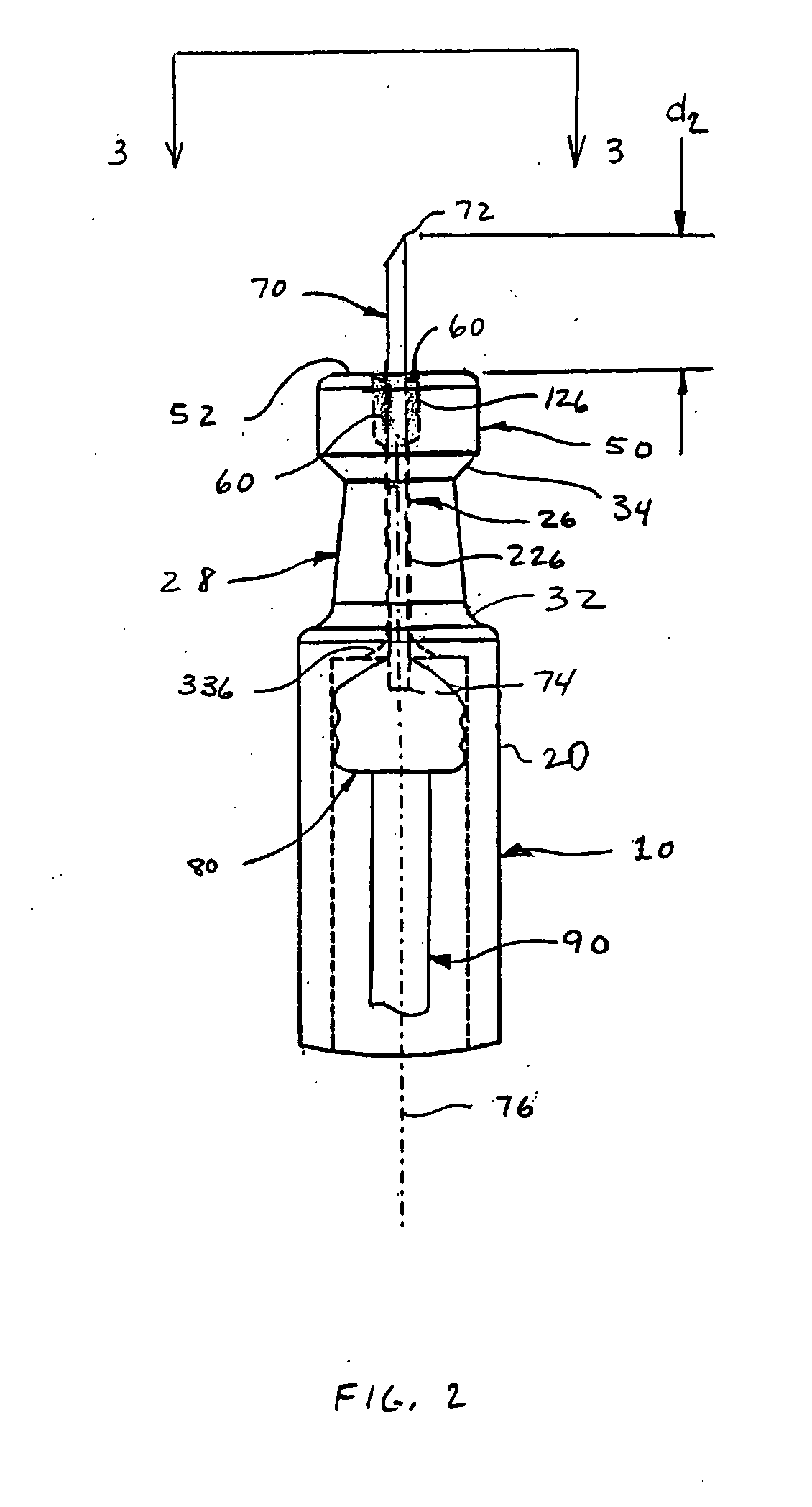
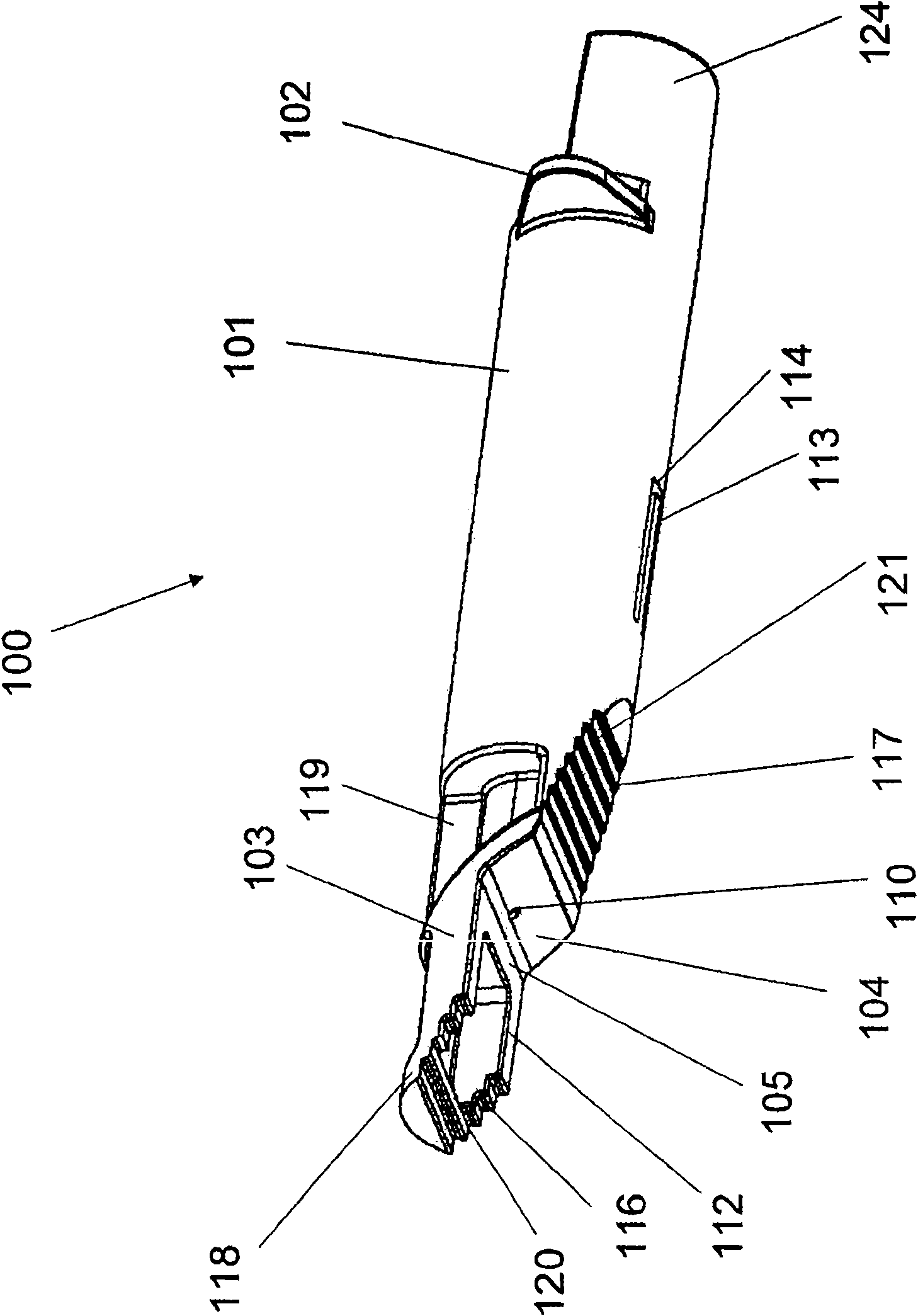
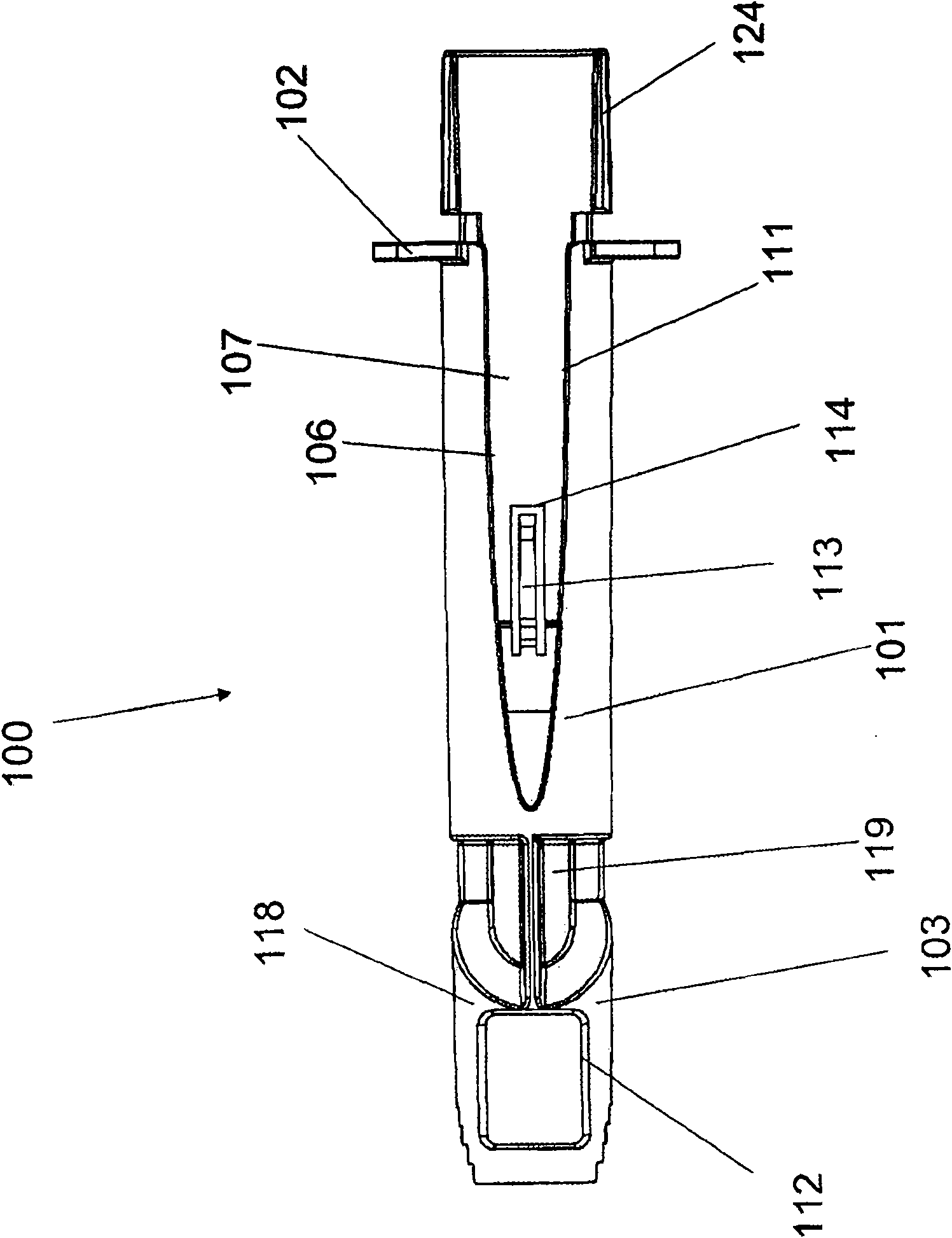
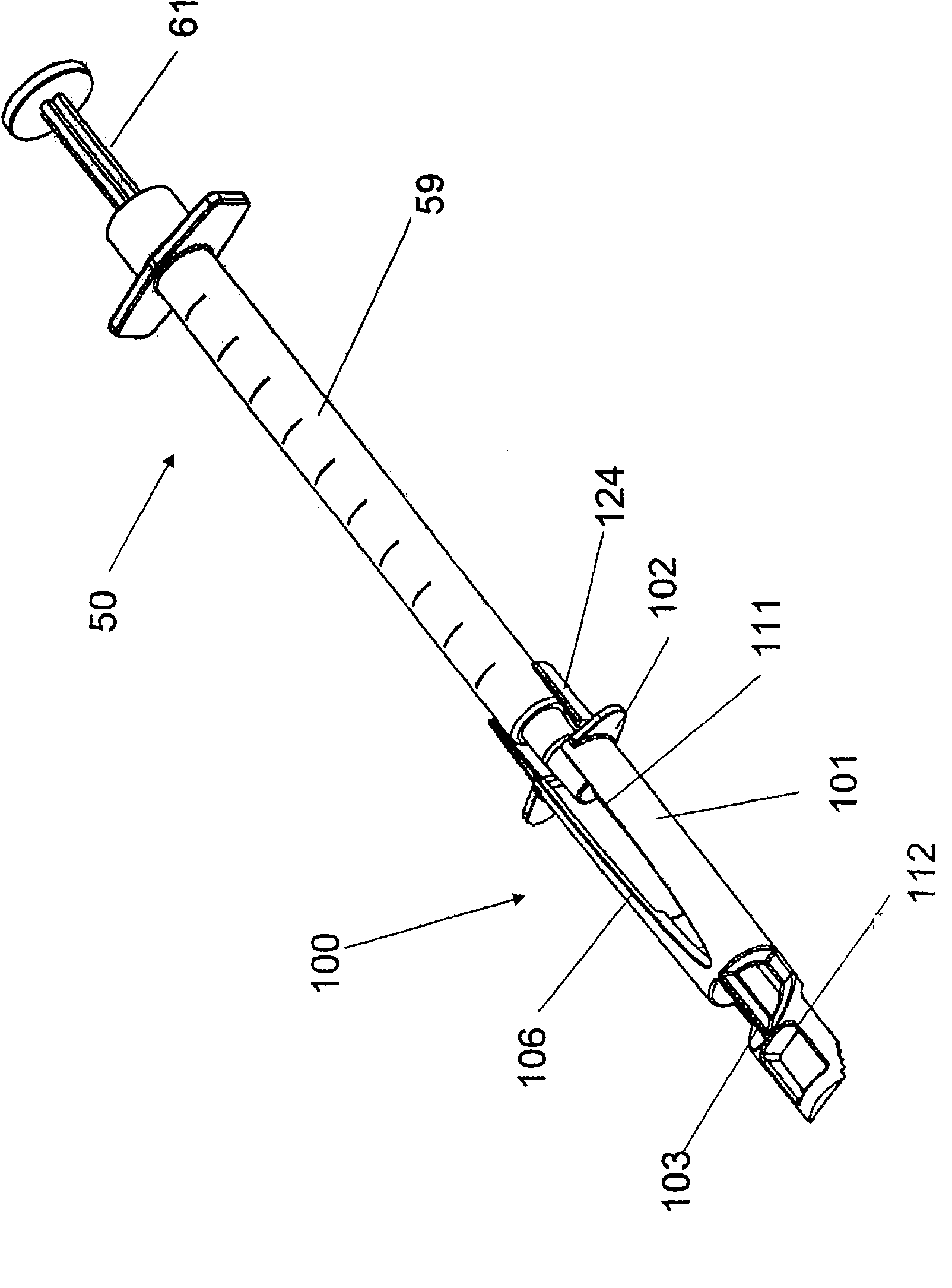
Alignment of a Needle in an Intradermal Injection Device
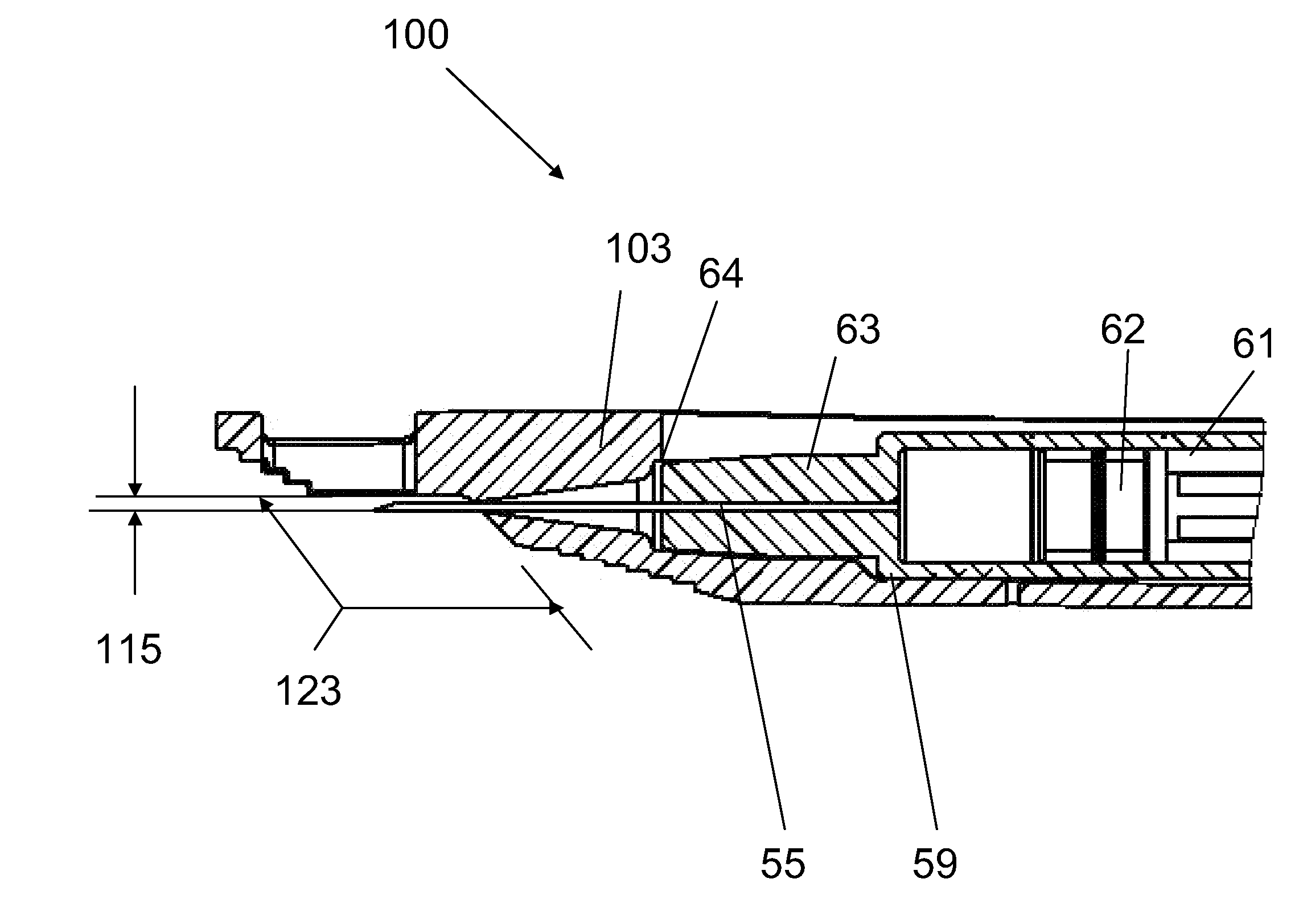
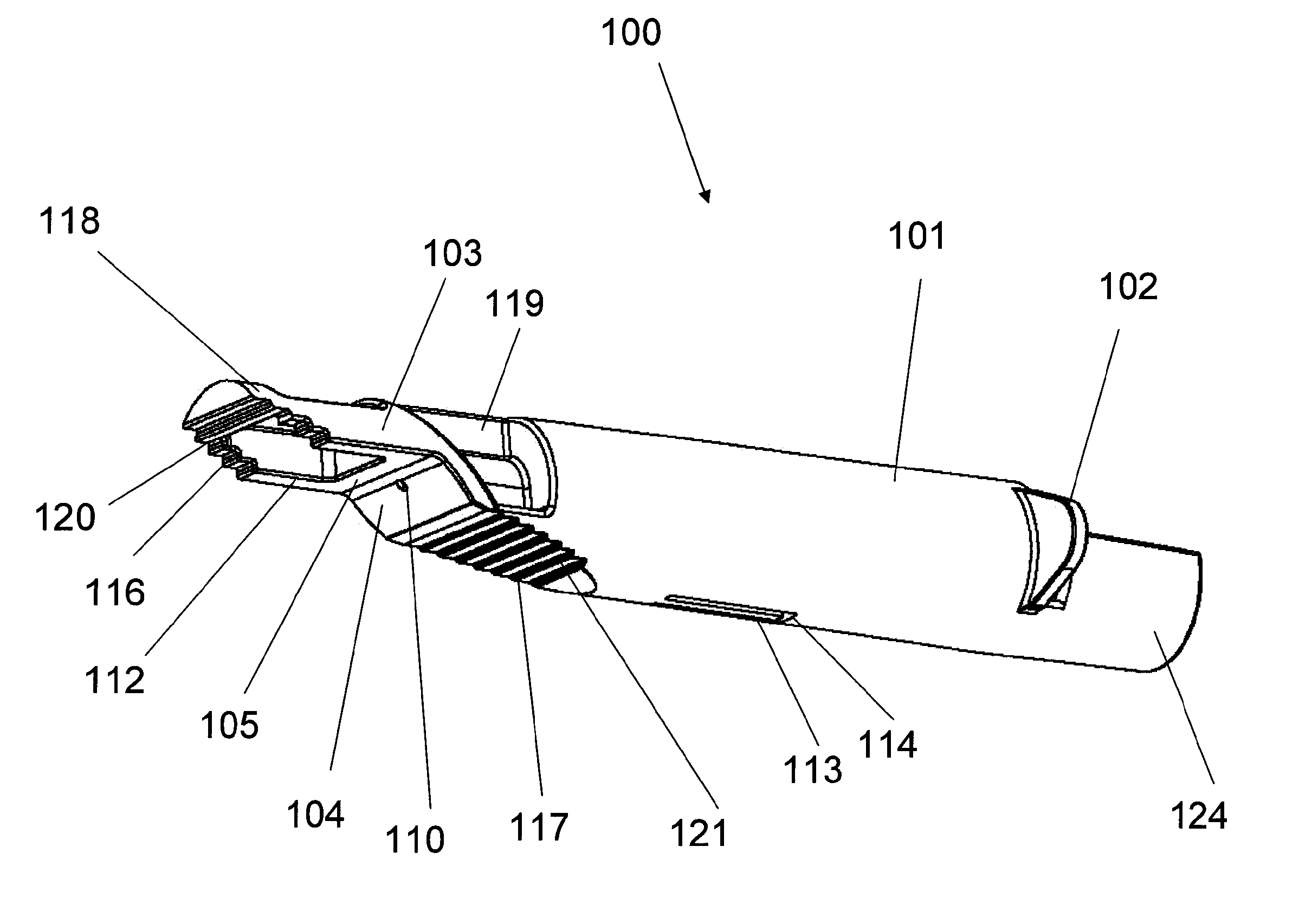
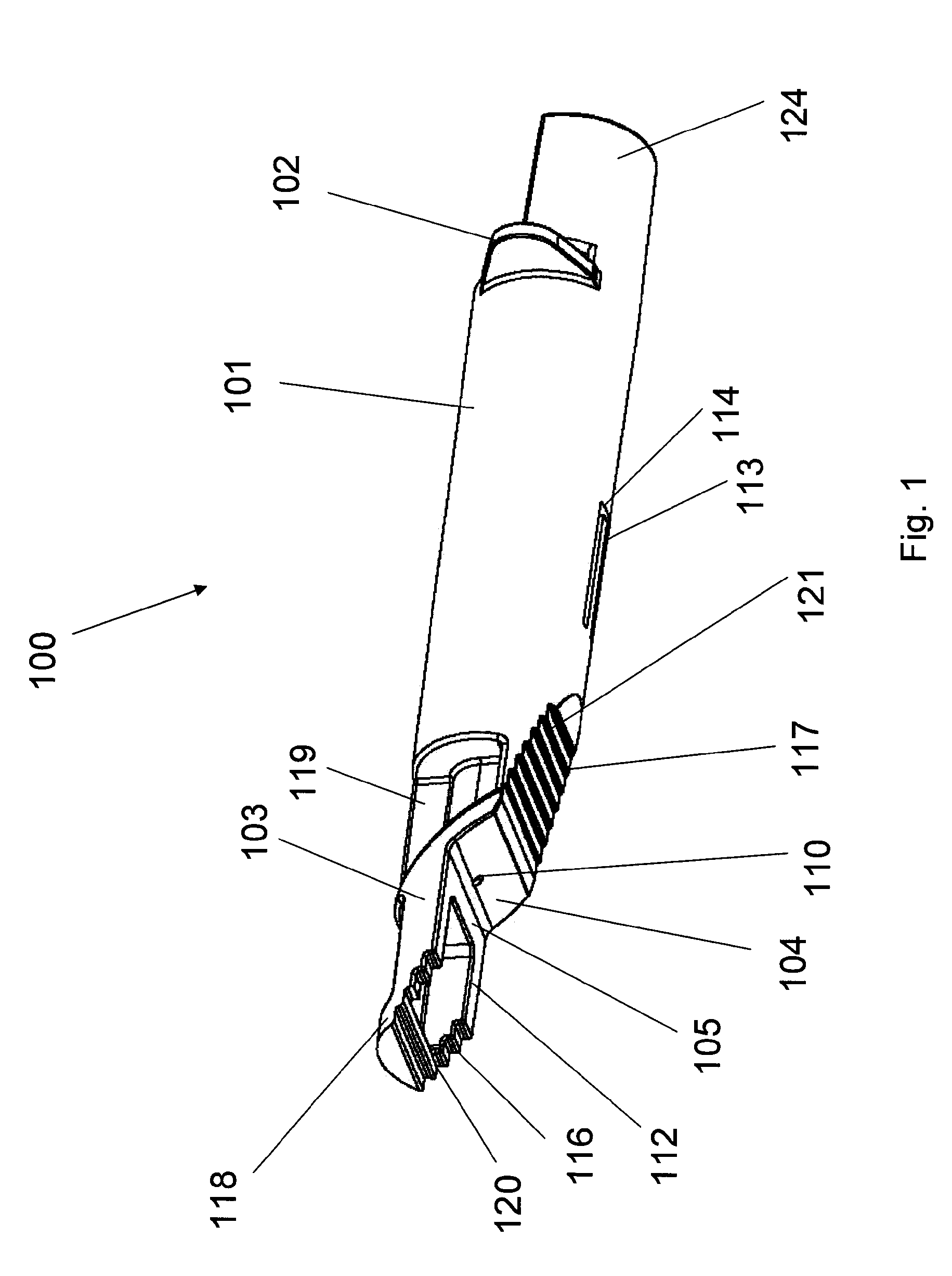
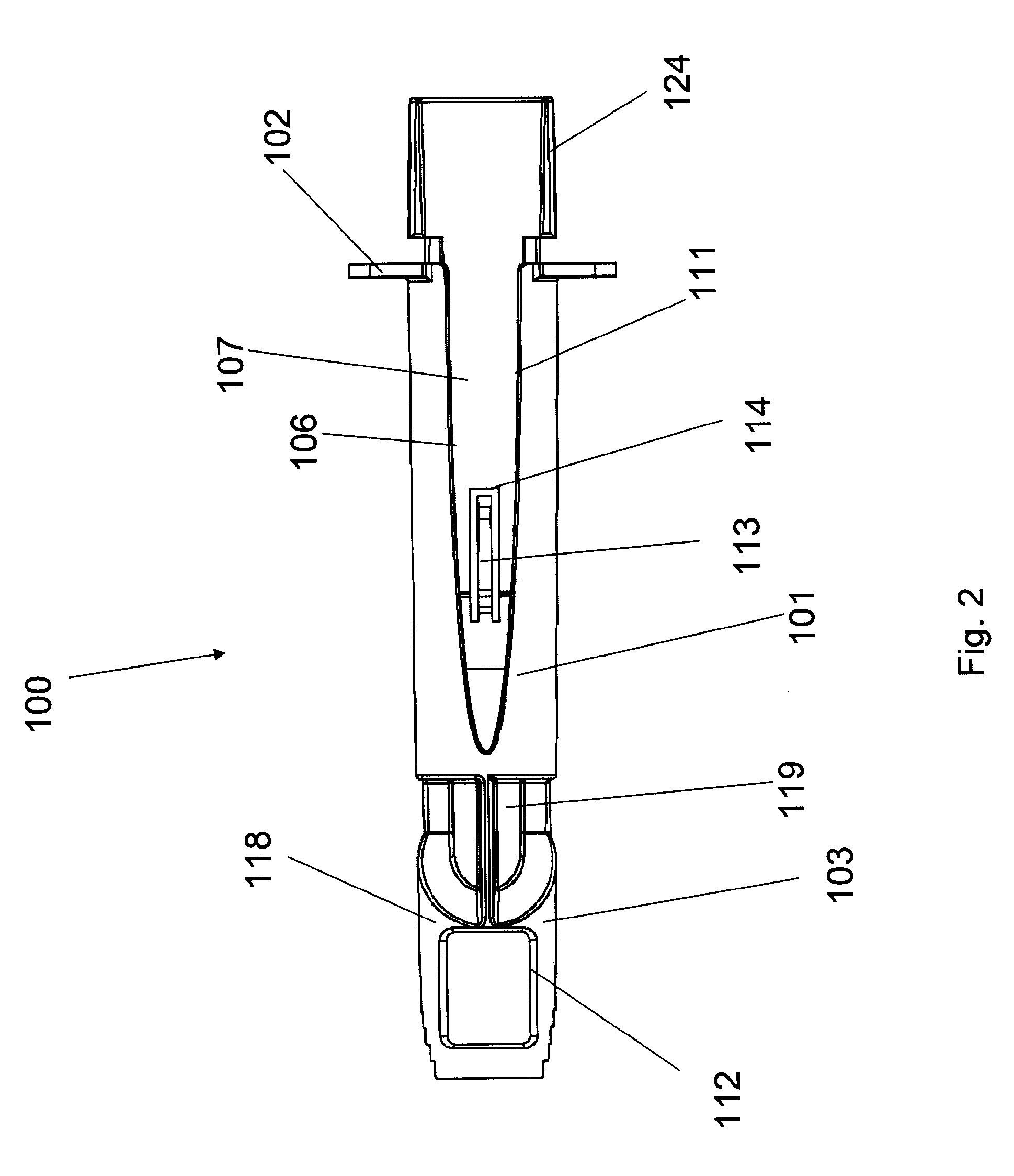
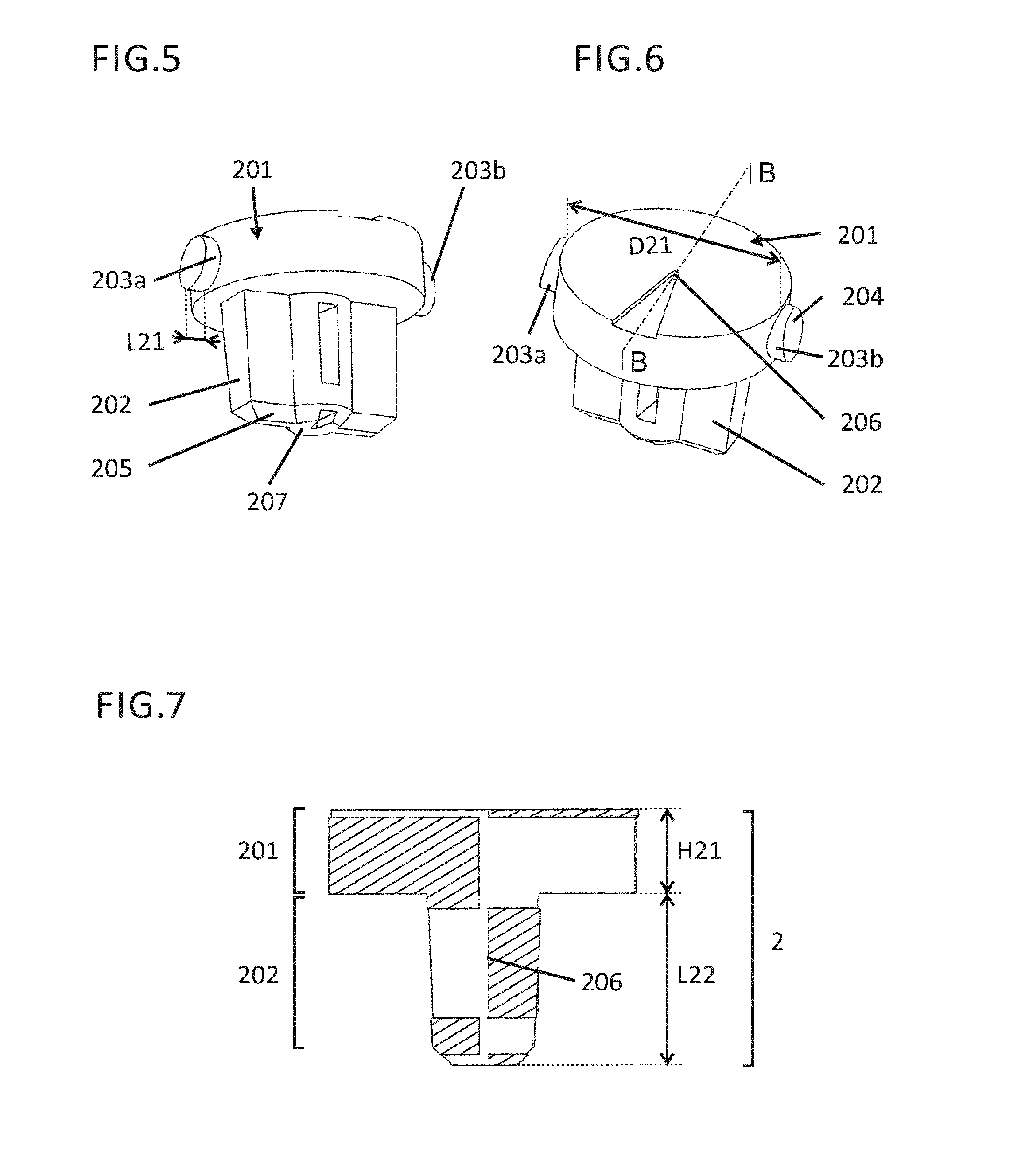
An adapter device (100, 200) for use in combination with a syringe (20) to form an assembly (10) for delivering an intradermal injection. The adapter device comprises a body (110, 210) which is connectable to the syringe. A second primary skin contacting surface (232) is positioned at a distal end (126, 226) of the body. At least one support element (140, 150, 240) is connected to the body. With the assembly in an assembled condition, the at least one support element supports a needle cannula (24) connected to the syringe. The needle cannula is supported by the at least one support element intermediate a base (26) and a tip (28) of the needle cannula. At least a terminal portion of the needle cannula extends axially in a direction substantially parallel to at least a planar portion of the second primary skin contacting surface.
Owner:SID TECH LLC +1
Long-acting polymeric delivery systems
ActiveUS20170035777A1Efficient releaseImprove the level ofAntipyreticAnalgesicsAnesthetic AgentDelivery vehicle
Compositions comprised of a delivery vehicle or delivery system and an active agent dispersed within the delivery vehicle or system, wherein the delivery vehicle or system contains a polyorthoester polymer and a polar aprotic solvent. Also disclosed are low viscosity delivery systems for administration of active agents. The low viscosity delivery systems have a polyorthoester polymer, a polar aprotic solvent and a solvent containing a triglyceride viscosity reducing agent. Compositions described include an amide- or anilide-type local anesthetic of the “caine” classification, and a non-steroidal anti-inflammatory drug (NSAID), along with related methods, e.g., for treatment of post-operative pain or for prophylactic treatment of pain. The compositions are suitable for delivery via, e.g., direct application and instillation, intradermal injection, subcutaneous injection, and nerve block (perineural).
Owner:HERON THERAPEUTICS
Long-acting polymeric delivery systems
ActiveUS20160375140A1Improved profileEffective pain reliefPowder deliveryNervous disorderAnesthetic AgentActive agent
Compositions comprised of a delivery vehicle or delivery system and an active agent dispersed within the delivery vehicle or system, wherein the delivery vehicle or system contains a polyorthoester polymer and a polar aprotic solvent. Also disclosed are low viscosity delivery systems for administration of active agents. The low viscosity delivery systems have a polyorthoester polymer, a polar aprotic solvent and a solvent containing a triglyceride viscosity reducing agent. Compositions described include an amide- or anilide-type local anesthetic of the “caine” classification, and a non-steroidal anti-inflammatory drug (NSAID), along with related methods, e.g., for treatment of post-operative pain or for prophylactic treatment of pain. The compositions are suitable for delivery via, e.g., direct application and instillation, intradermal injection, subcutaneous injection, and nerve block (perineural).
Owner:HERON THERAPEUTICS
Minimally Invasive Method of Augmenting a Glans Penis with Intradermal Filler Material
InactiveUS20160354516A1Increase girthMaintaining sensationSurgical needlesMedical devicesFilling materialsInjection point
A method for augmenting the glans penis provides a minimally invasive introduction of injectable biocompatible material, including hyaluronic acid, to increase the girth of the glans penis while maintaining sensation in the penis. The method initially performs preoperative penile inspection of the girth of a penis. A biocompatible material is injected at a 12 o'clock, a 3 o'clock and 9 o'clock injection point relative to the glans penis. Where damage to the dorsal pedicle may occur, the injection points is at a 10'oclock and a 2 o'clock injection point. The biocompatible material is nontoxic and nonimmunogenic. The biocompatible can also be injected below the corona of the glans penis to create a mushroom effect causing nodules and pearls and intradermal injection causes blebs. Then, postoperative compressive dressing is applied to the penis. Then the penis is measured for postoperative changes in penile girth, and postoperative sexual satisfaction is recorded.
Owner:CHUANG GARY
Intradermal injection system for injecting DNA-based injectables into humans
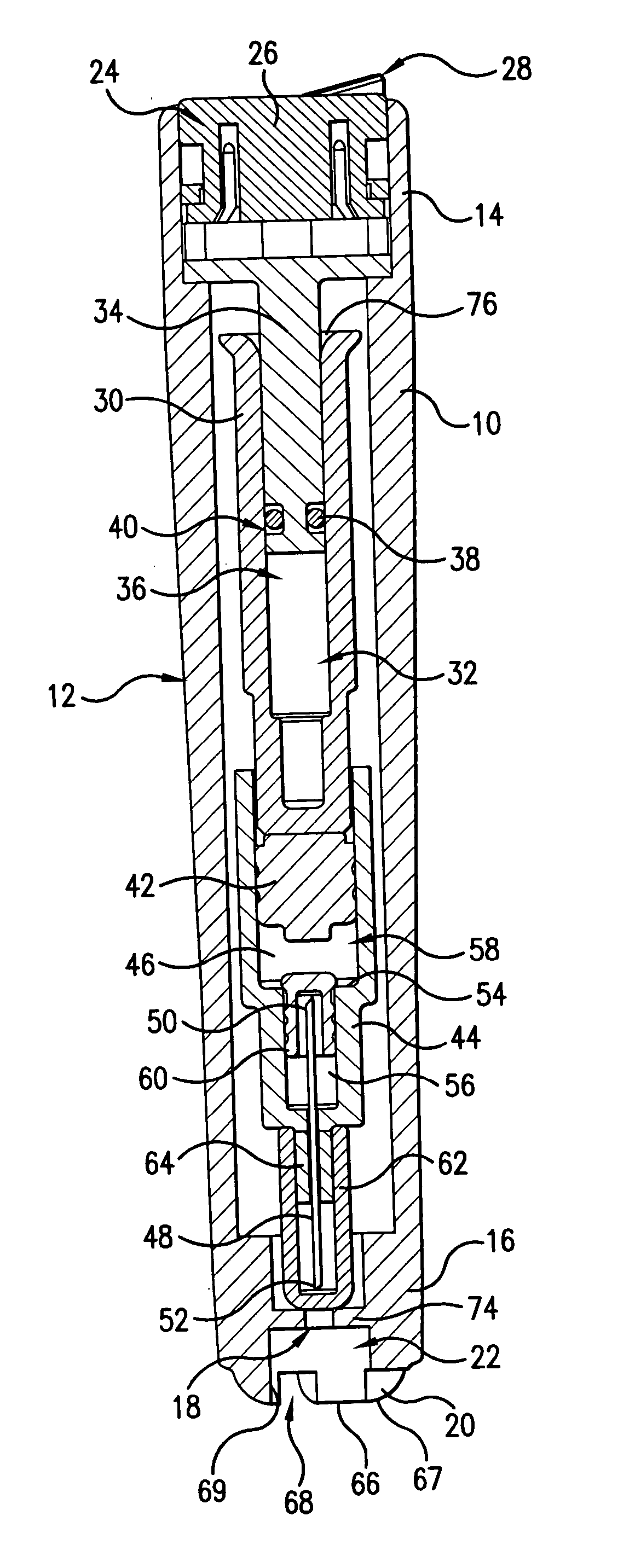
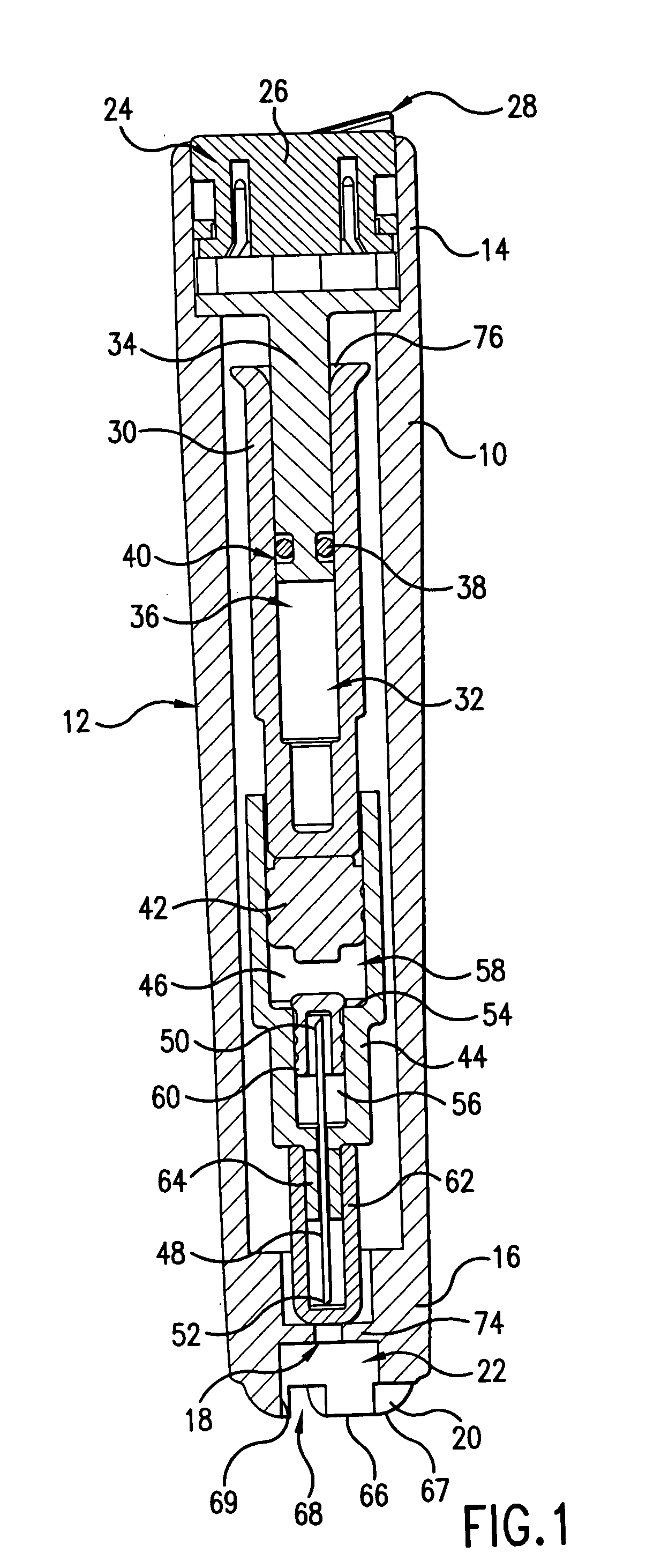
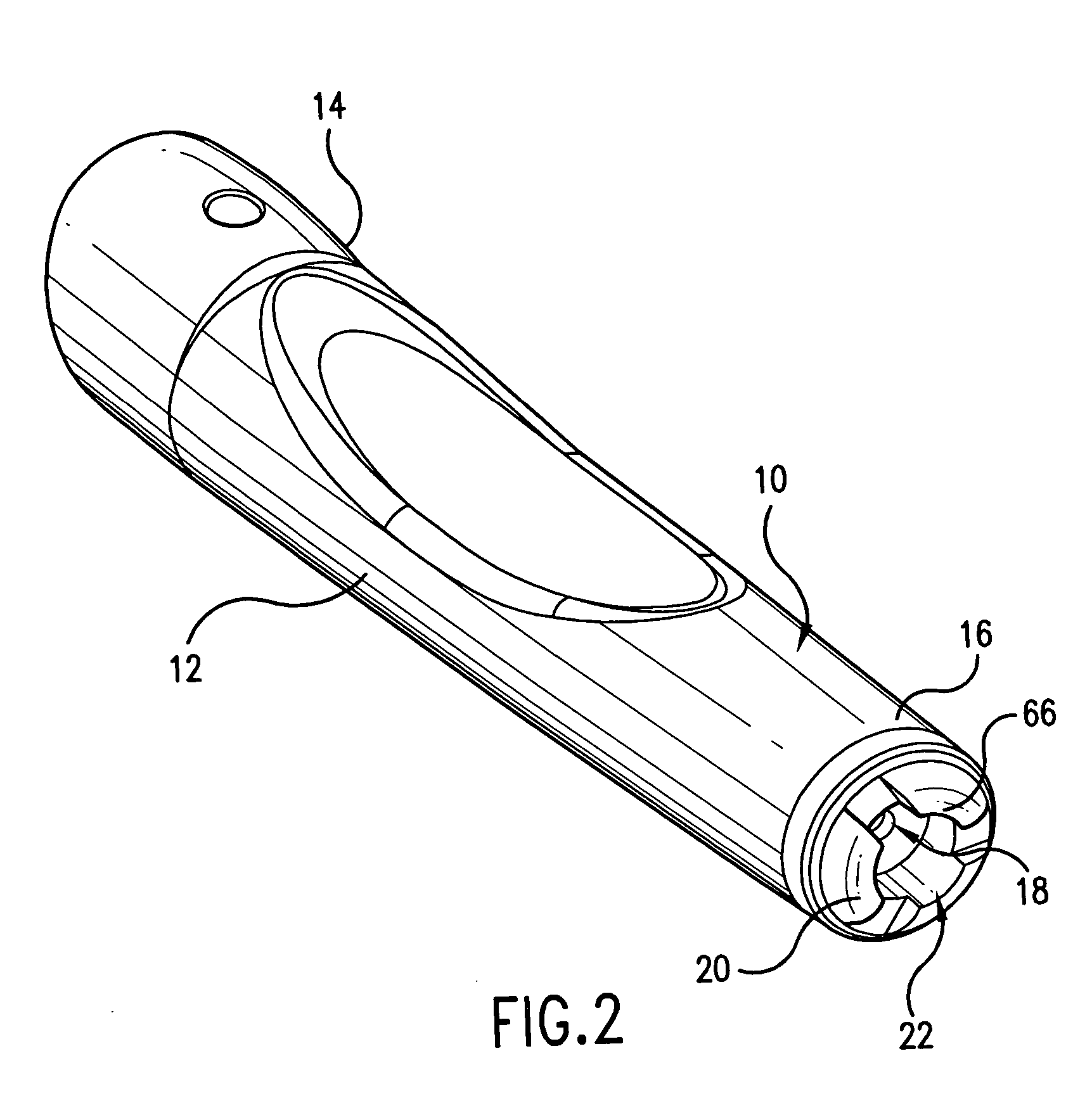
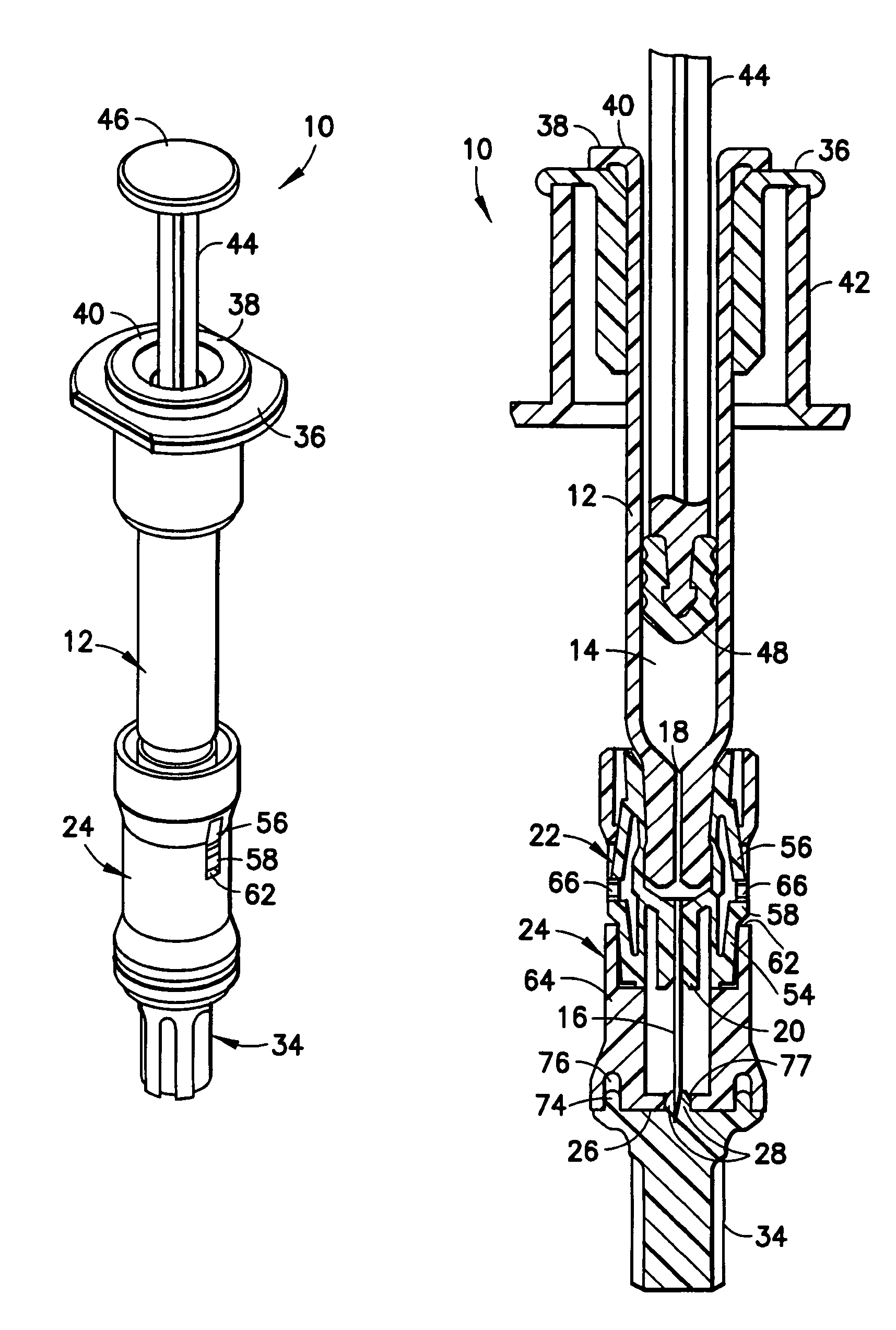
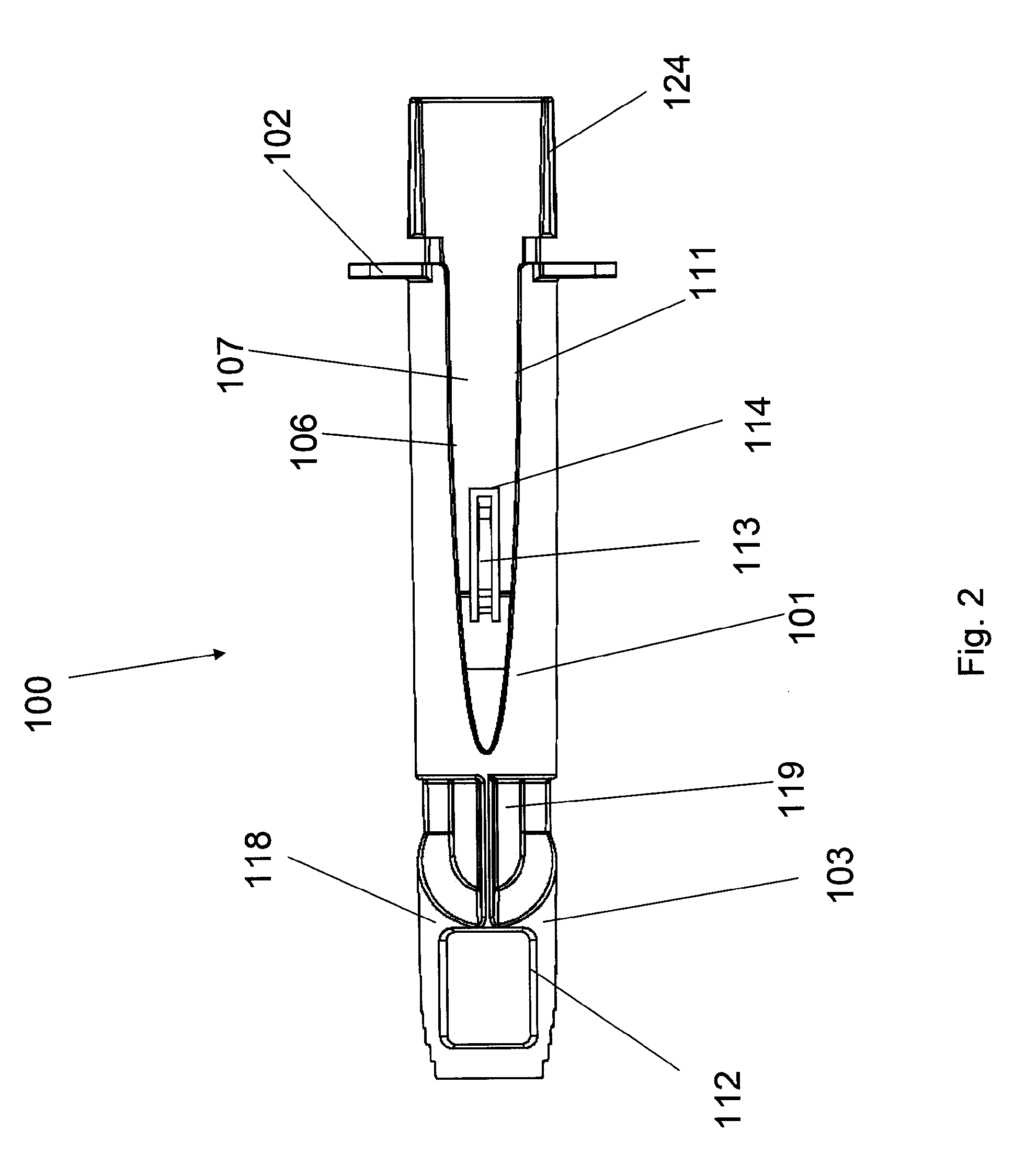
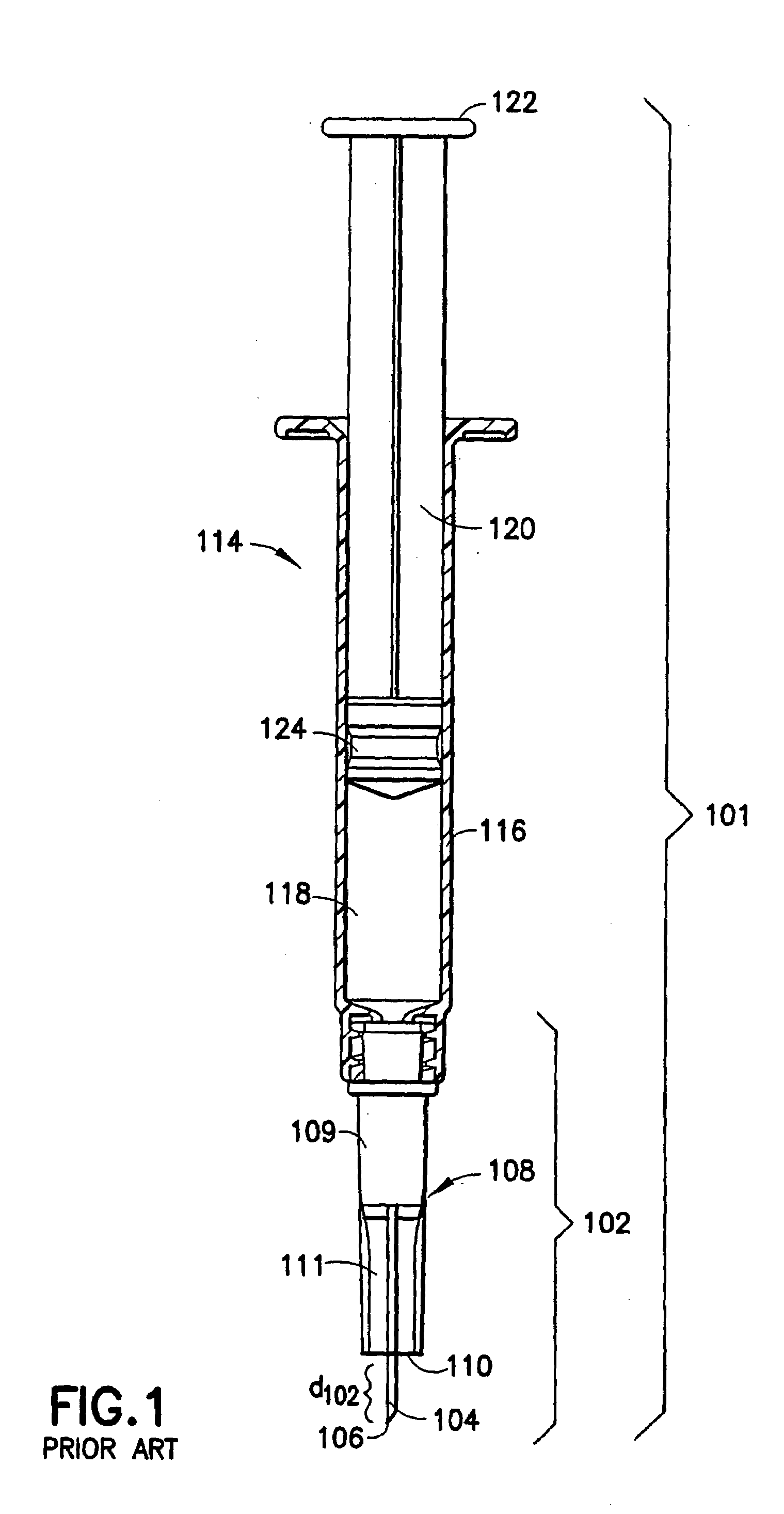
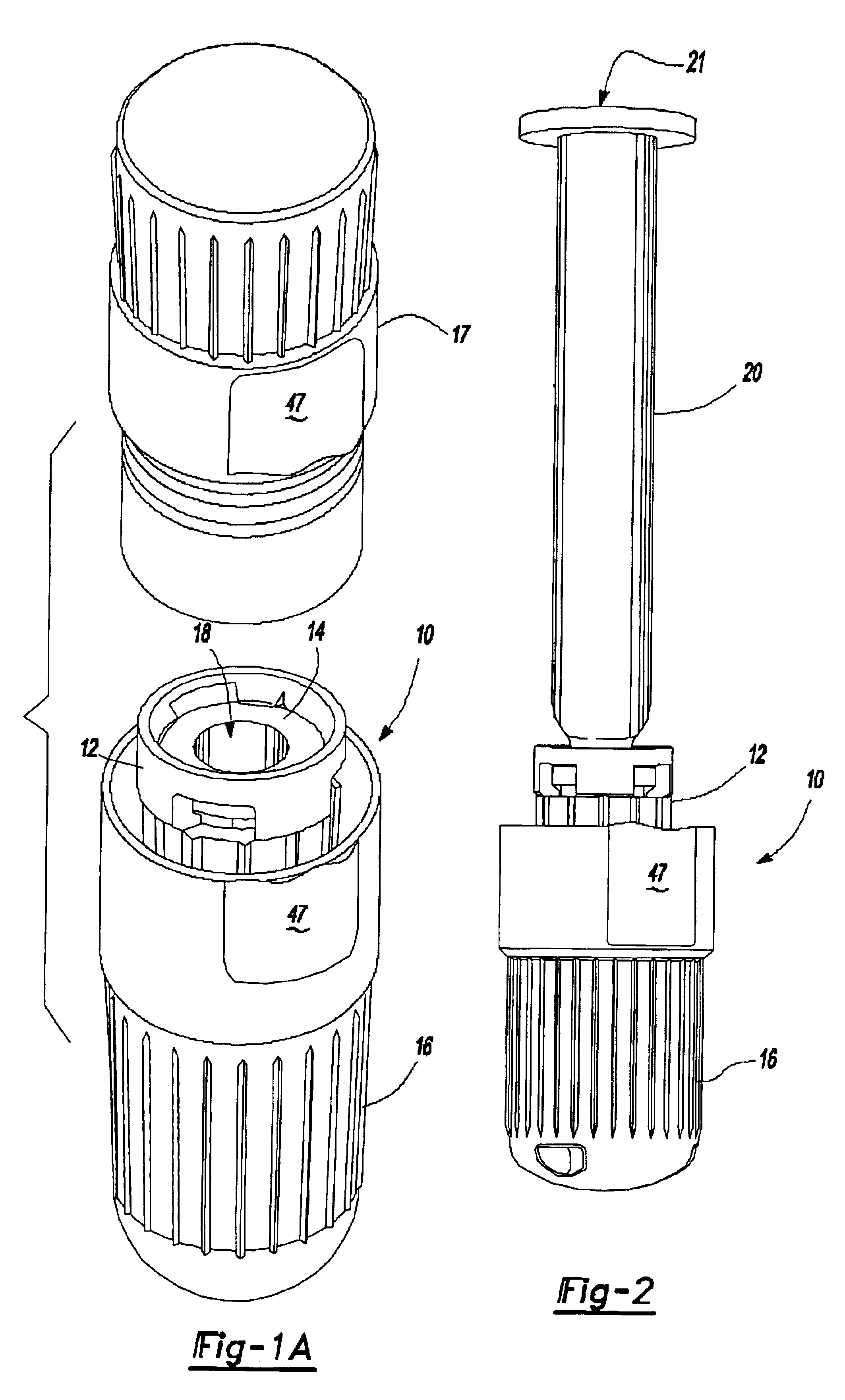
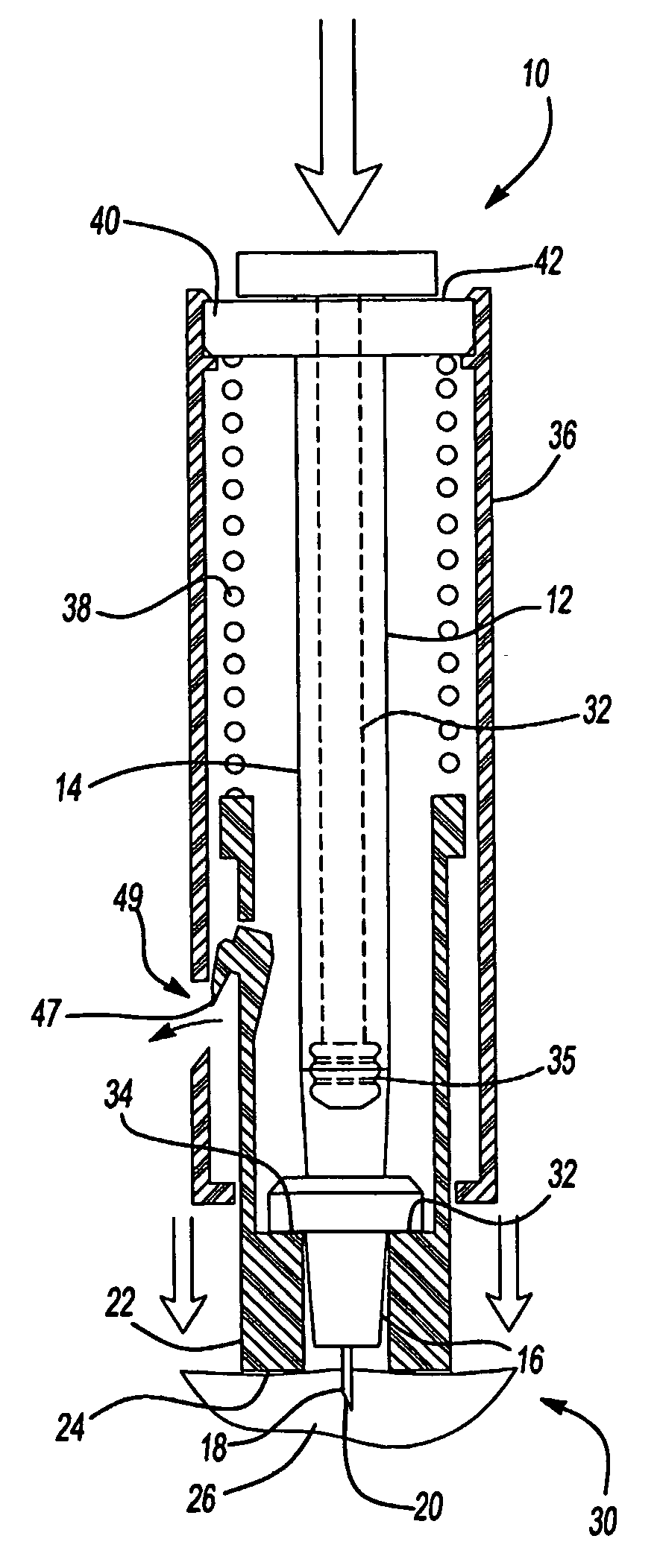
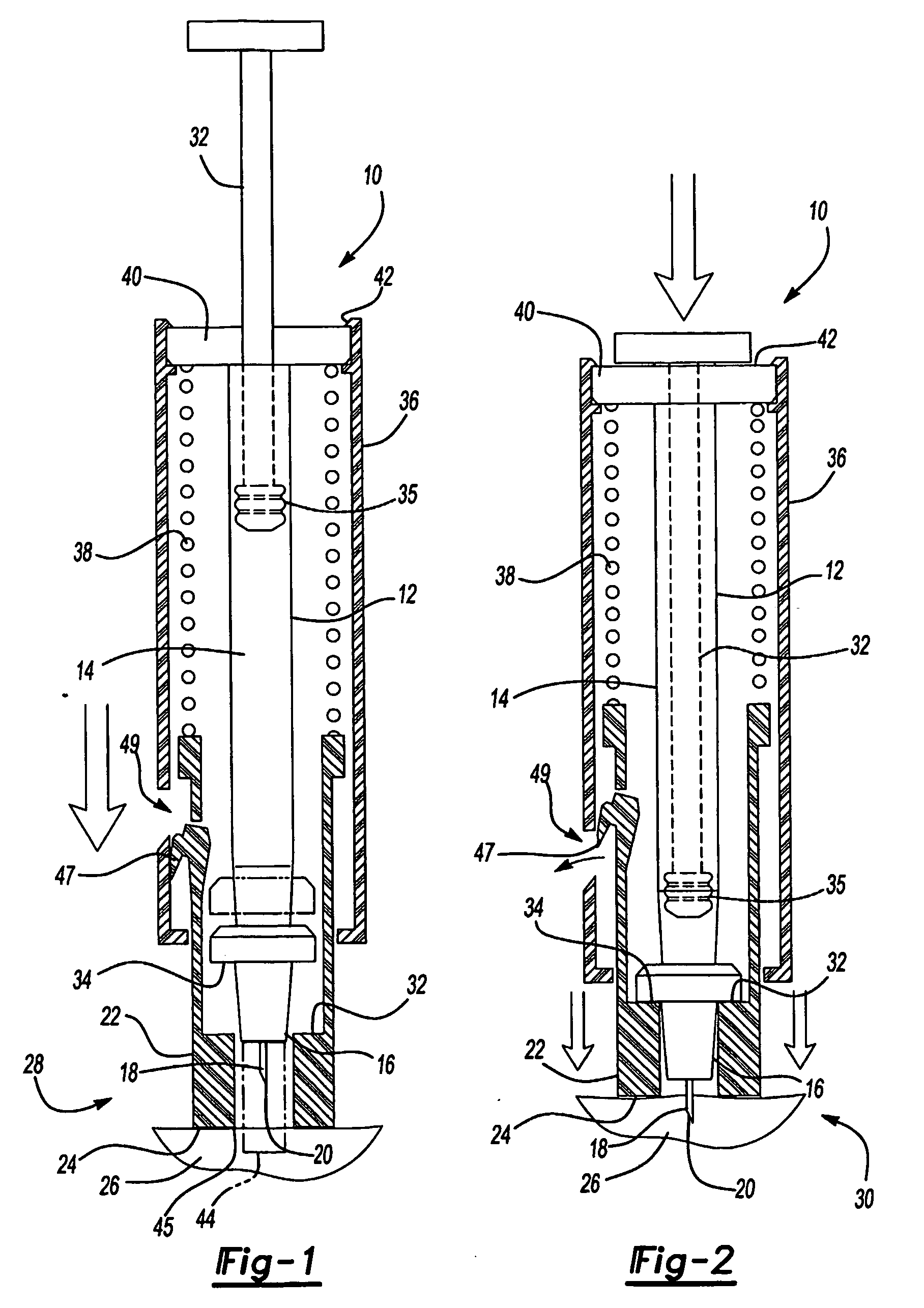
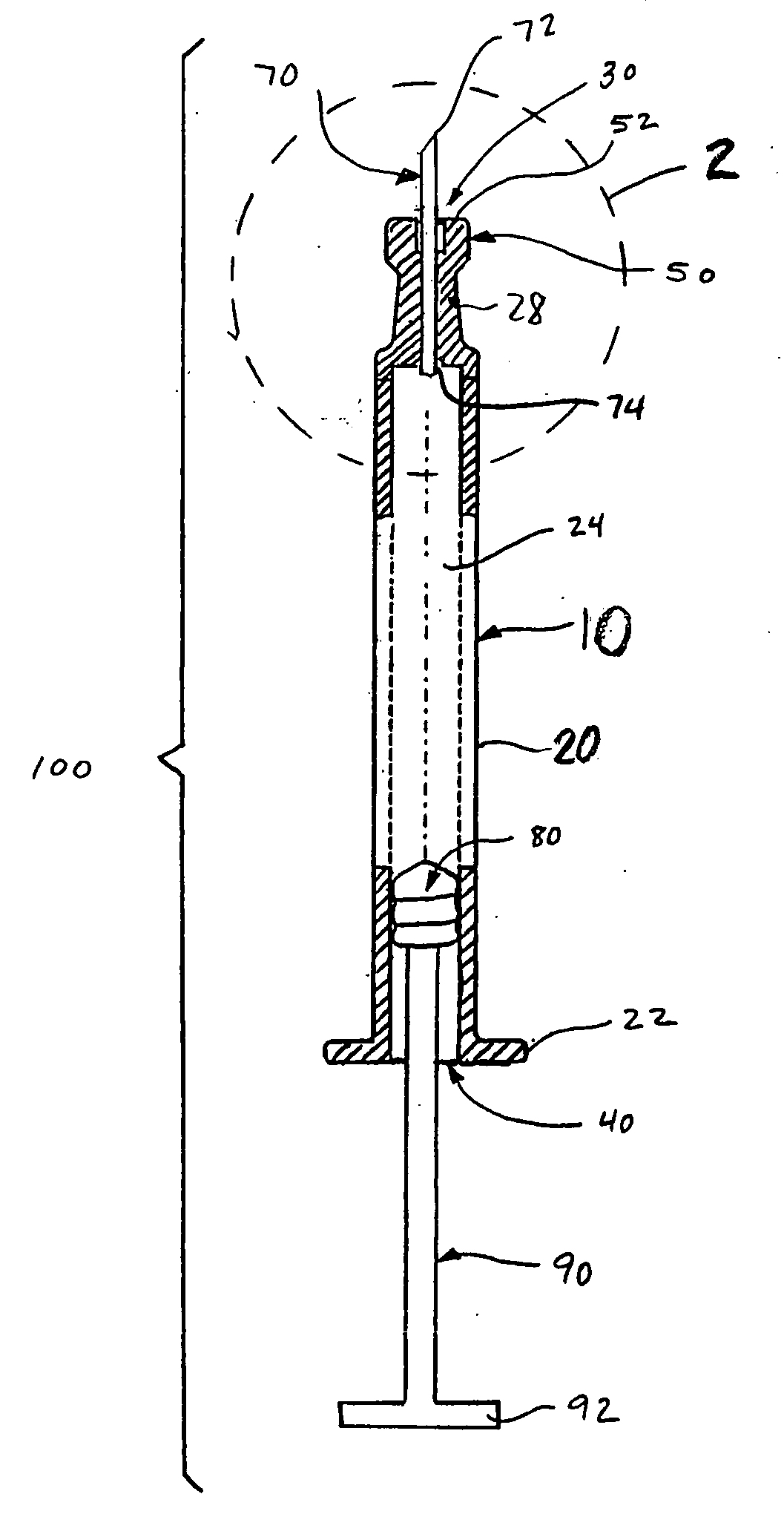
A system for injecting DNA-based medications into humans is provided by the present invention. The system includes a needle-free injector with an injection orifice of approximately 0.004 inches for supplying DNA-based medication at an initial pressure of from 3900 to 4300 psi, and then immediately declining to a level of about 2800 to 3800 psi, and then immediately cutting off pressure to terminate the injection. The injector includes an annular adapter for spacing the injection orifice from the skin of the patient. The adapter includes an abutment against which the injector is disposed so that the orifice is spaced approximately 0.76-1.0 inch from the skin of the patient, the adapter having an inner diameter at the distal end of approximately 0.50-0.70 inches.
Owner:STOUT RICHARD R +2
Intradermal needle
InactiveUS20050113753A1Effectively and reliably deliver such substances intradermallyControl lengthAmpoule syringesMedical devicesInjected substanceTissue skin
An intradermal needle assembly that is attachable to a prefillable container intended for intradermally injecting substances into an animal includes a needle cannula supported by a hub portion. The hub portion is adapted to receive the prefillable container just prior to administering the intradermal injection. A limiter portion surrounds the needle cannula and extends away from the hub portion toward a forward tip of the needle cannula, and includes a skin engaging surface with the needle cannula having a fixed angle of orientation, preferably generally perpendicular, relative to the plane of the skin engaging surface. The skin engaging surface is received against the skin of an animal to administer an intradermal injection. The forward tip extends beyond the skin engaging surface a distance enabling penetration of the needle cannula into the dermis layer of the skin of the animal enabling injection of the substance into the dermis layer.
Owner:ALCHAS PAUL G +3
Treatment of autism using botulinum toxins
A method of treating ASD (autism) in a patient in need thereof comprises administering a botulinum toxin to the patient. The botulinum toxin may be administered by subcutaneous / intradermal injection. The subcutaneous / intradermal injection may be administered to and / or around the vicinity of a trigeminal nerve, a cervical nerve, a thoracic nerve, a lumbar nerve, and a sacral nerve of the patient. In infants or toddlers—from about 1 to 5 year olds, it is used to prevent or minimize damage to the developing brain; in older children and adult Autism Spectrum Disorder (ASD) patients, it will be used to reduce or eliminate their symptoms.
Owner:PENLAND FOUND
Methods and devices for intradermal injection
Devices and methods for intradermal (ID) administration of diagnostic and therapeutic agents, vaccines and other compounds into the dermal layer of the skin are disclosed. The devices and the methods simplify the ID injection process and increase the consistency of the placement of the needle tip in the dermal space close to the skin surface allowing for a shallow cannula placement in the dermis.Furthermore, the devices and methods broaden the number of sites suitable for ID injection and make the ID injection possible with limited training.
Owner:SID TECH LLC +1
Long-acting polymeric delivery systems
ActiveUS9801945B2Enhance the imageEffective pain reliefPowder deliverySurgeryDelivery vehicleActive agent
Compositions comprised of a delivery vehicle or delivery system and an active agent dispersed within the delivery vehicle or system, wherein the delivery vehicle or system contains a polyorthoester polymer and a polar aprotic solvent. Also disclosed are low viscosity delivery systems for administration of active agents. The low viscosity delivery systems have a polyorthoester polymer, a polar aprotic solvent and a solvent containing a triglyceride viscosity reducing agent. Compositions described include an amide- or anilide-type local anesthetic of the “caine” classification, and a non-steroidal anti-inflammatory drug (NSAID), along with related methods, e.g., for treatment of post-operative pain or for prophylactic treatment of pain. The compositions are suitable for delivery via, e.g., direct application and instillation, intradermal injection, subcutaneous injection, and nerve block (perineural).
Owner:HERON THERAPEUTICS
Intradermal pen adapter
An intradermal injection adapter includes a body having a longitudinal axis and a central portion located distally relative to the body along the longitudinal axis. The central portion has a cannula channel therethrough extending generally parallel to the longitudinal axis. A cannula within the cannula channel has a sharpened tip for injection into a patient and a sharpened opposite proximal end for injection into a pen injector. A distal protrusion has a first skin contacting surface extending generally parallel to the longitudinal axis. The first skin contacting surface is spaced from the cannula such that a distal portion of the cannula extends generally parallel to the first skin contacting surface.
Owner:SID TECH LLC
Long-acting polymeric delivery systems
ActiveUS20170304455A1Enhance the imageEffective pain reliefPowder deliveryPharmaceutical non-active ingredientsAnesthetic AgentActive agent
Compositions comprised of a delivery vehicle or delivery system and an active agent dispersed within the delivery vehicle or system, wherein the delivery vehicle or system contains a polyorthoester polymer and a polar aprotic solvent. Also disclosed are low viscosity delivery systems for administration of active agents. The low viscosity delivery systems have a polyorthoester polymer, a polar aprotic solvent and a solvent containing a triglyceride viscosity reducing agent. Compositions described include an amide- or anilide-type local anesthetic of the “caine” classification, and a non-steroidal anti-inflammatory drug (NSAID), along with related methods, e.g., for treatment of post-operative pain or for prophylactic treatment of pain. The compositions are suitable for delivery via, e.g., direct application and instillation, intradermal injection, subcutaneous injection, and nerve block (perineural).
Owner:HERON THERAPEUTICS
Gel compound of monoclonal antibody drugs
InactiveCN104784105AReduce frequency of useExtended stayPeptide/protein ingredientsAerosol deliveryTreatment effectCell-Extracellular Matrix
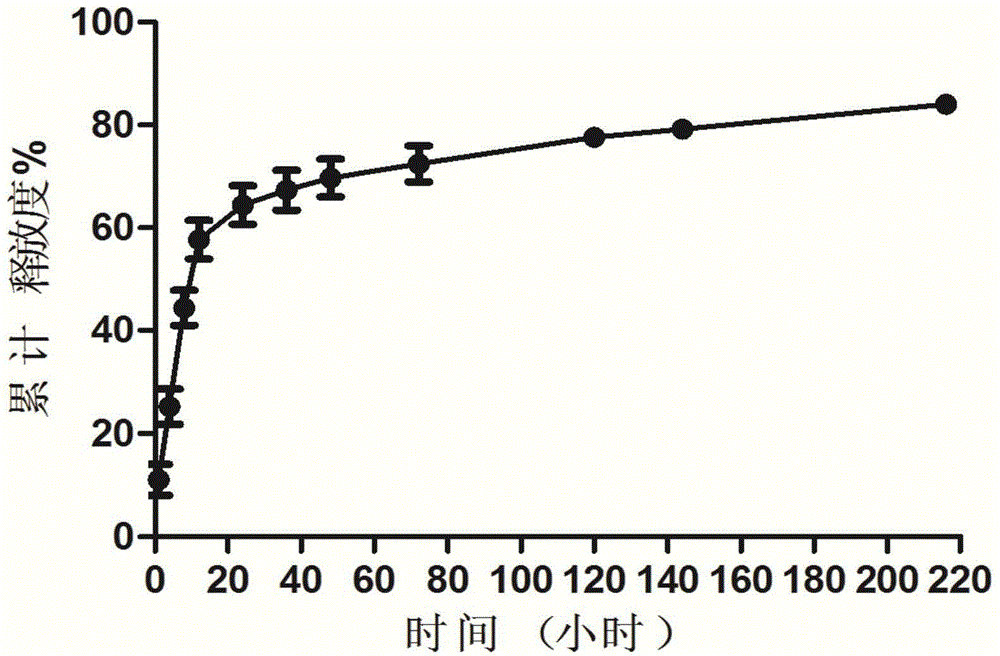
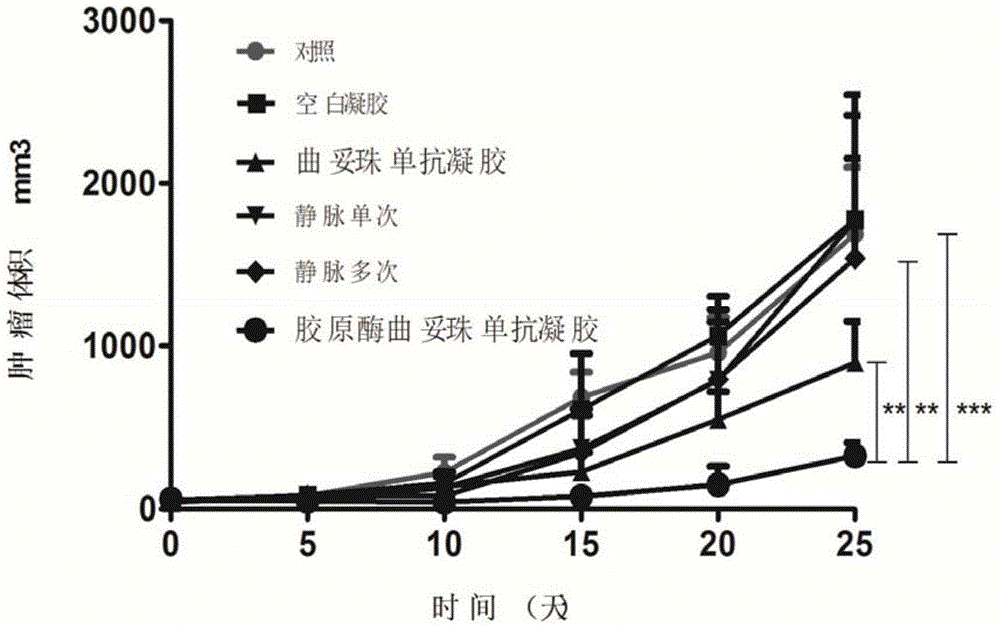
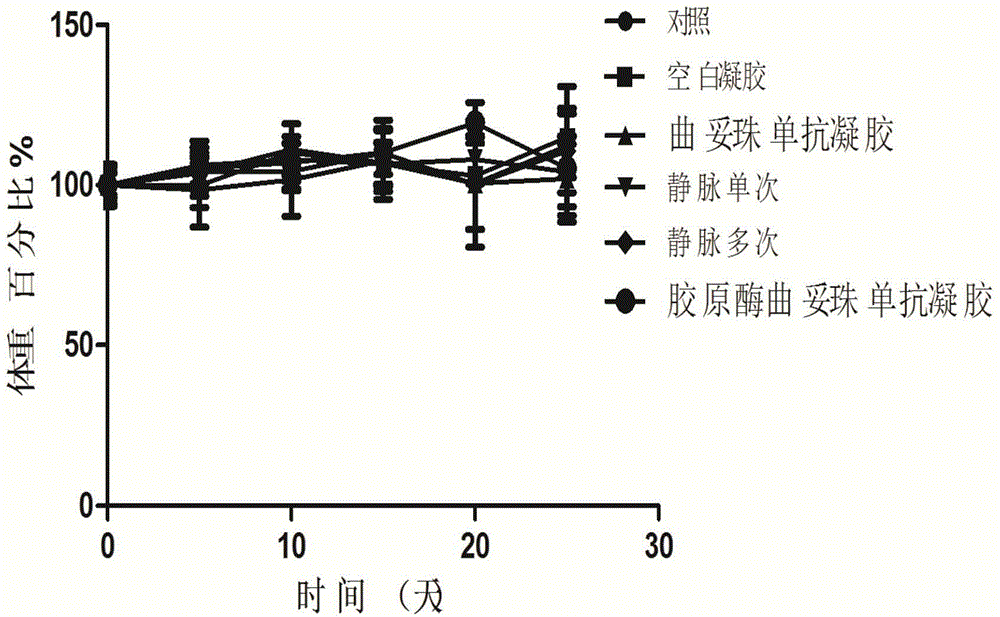
The invention relates to a gel compound of monoclonal antibody drugs, which can be provided for patients to perform intradermal injection, surrounding-tumor injection or intratumor injection on clinic, and belongs to the technical field of medicinal preparation. The gel compound of the monoclonal antibody drugs comprises monoclonal antibodies, at least one kind of extracellular matrix degrading enzymes, such as collagenase or hyaluronidase, a temperature-sensitive gel material, menstruum, a buffering agent, a stabilizing agent, a nonionic surfactant and an osmotic regulating agent. The invention further provides a method and uses of the gel compound of the monoclonal antibody drugs. The gel compound of the monoclonal antibody drugs, which is disclosed by the invention, has the effects of prolonging the release of the monoclonal antibodies, and promoting the tissue osmosis of monoclonal antibodies or the osmosis of the monoclonal antibodies into tumors; the gel compound of the monoclonal antibody drugs has the advantage of reducing drug administration times and enhancing treatment effects.
Owner:PEKING UNIV
Smearing type intradermal injection capable of directly reaching dermis layer and preparation method thereof
ActiveCN105616187AKeep healthyRepair damageCosmetic preparationsToilet preparationsMedicineUltraviolet
The invention discloses smearing type intradermal injection capable of directly reaching the dermis layer and a preparation method thereof. The smearing type intradermal injection comprises, by weight, 10-30 parts of hyaluronic acid and 10-30 parts of collagen. The smearing type intradermal injection has the advantages that the smearing type intradermal injection is high in activity, appropriate in component and reasonable in combination, the smearing type intradermal injection can permeate the epidermis to reach the dermis layer when the smearing type intradermal injection is applied to the face or smeared to the epidermis of the skin, the hyaluronic acid and collagen lost due to aging are injected to allow the skin to absorb and store moisture with the weight being 1000 times of the weight of the skin, the regenerative function of cells is awakened, the withered and fractured cells of the skin are repaired again, the compact, smooth and elastic skin can be achieved again in a short time, effects of removing yellowness and whitening and resisting wrinkles and tightening the skin are achieved, the skin can restore elasticity, be transparent, bright and compact and form a moisture shield, different mechanisms are coordinated to bring an anti-ultraviolet effect into play, ultraviolet permeation can be reduced, the skin damage caused by the ultraviolet can be repaired, dual-protection and a good effect are achieved, and the smearing type intradermal injection has a quite good anti-allergic function.
Owner:티안지우안
Intradermal injection system for injecting DNA-based injectables into humans
InactiveCN1399567AJet injection syringesTissue/virus culture apparatusNeedle Free InjectionNeedle free
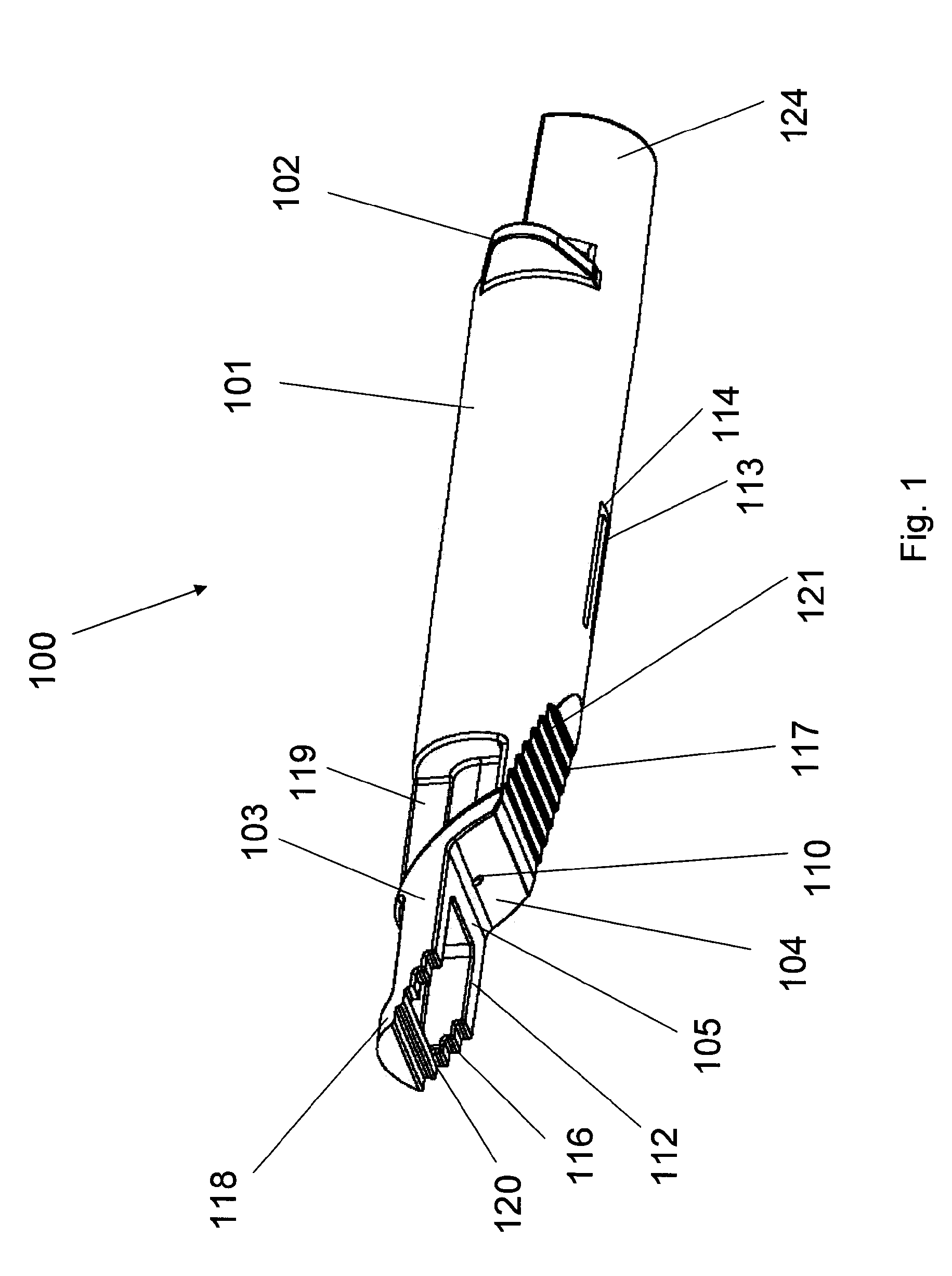
A system for injecting DNA-based medications into humans is provided by the present invention. The system includes a needle-free injector (10) with an injection orifice of approximately 0.004 inches for supplying DNA-based medication at an initial pressure of from 3900 to 4300 psi, and then immediately declining to a level of about 2800 to 3800 psi, and then immediately cutting off pressure to terminate the injection. The injector includes an annular adapter (12) for spacing the injection orifice from the skin of the patient. The adapter includes an abutment (16) against which the injector is disposed so that the orifice is spaced approximately 0.76-1.0 inch from the skin of the patient, the adapter having an inner diameter at the distal end of approximately 0.50-0.70 inches.
Owner:BIOJECT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com