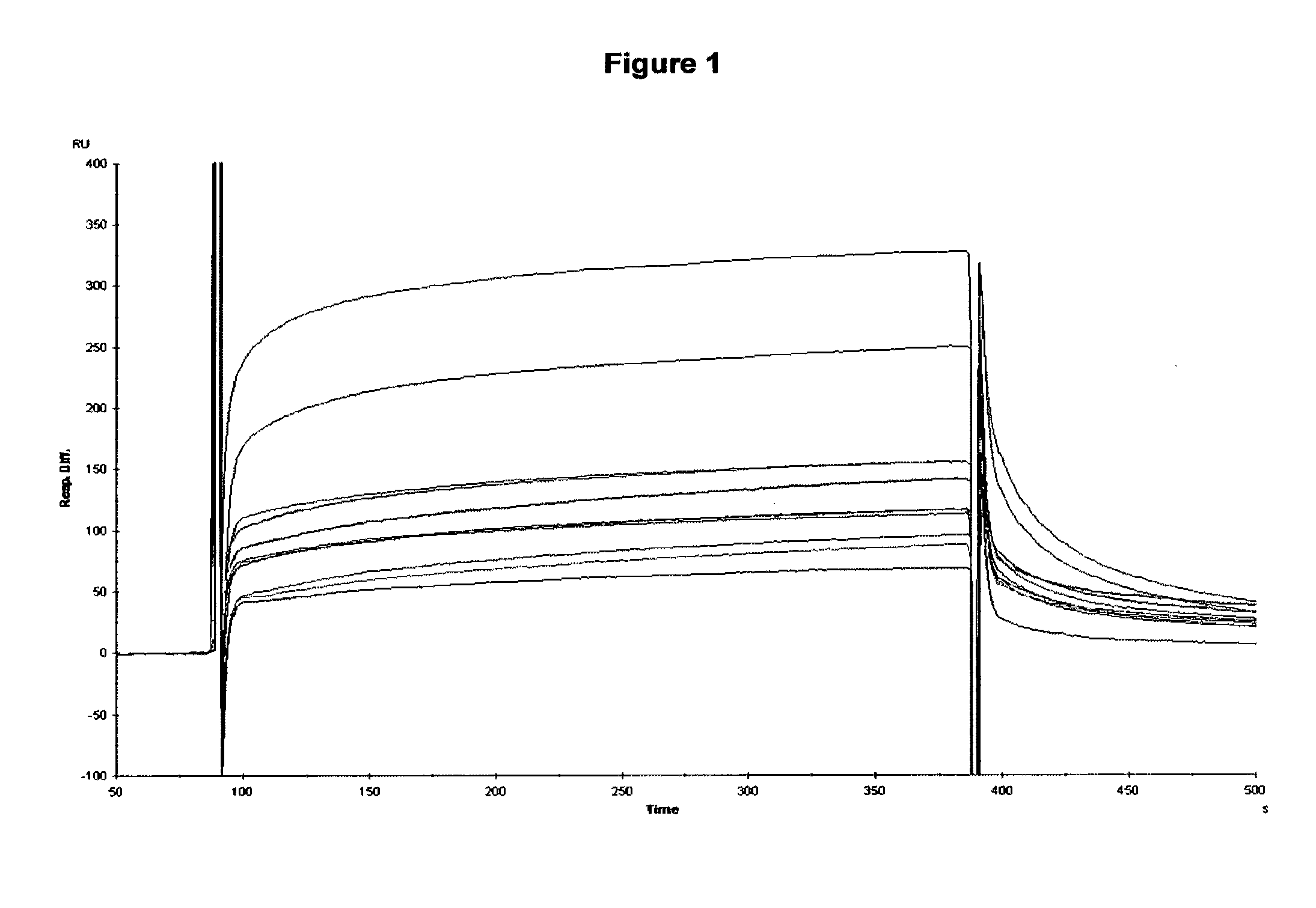
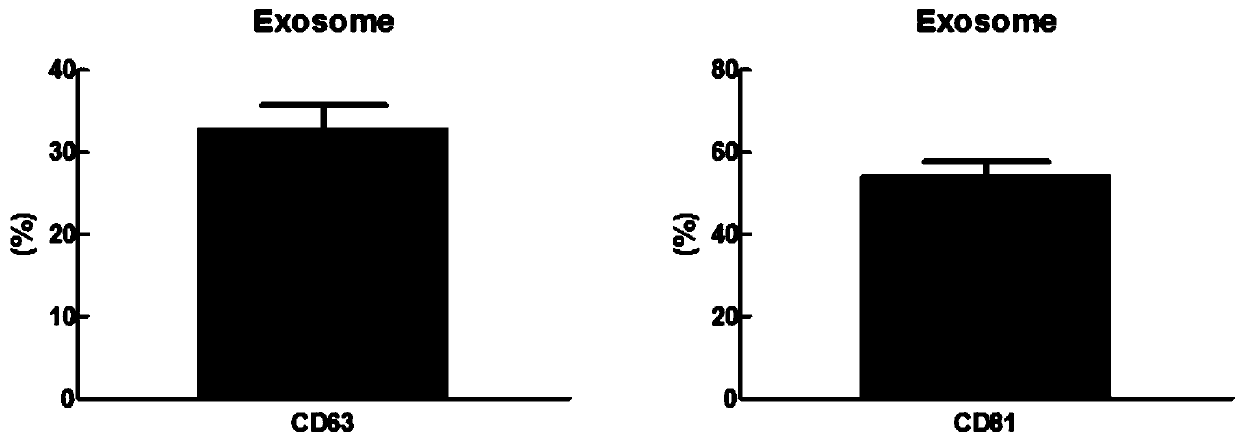
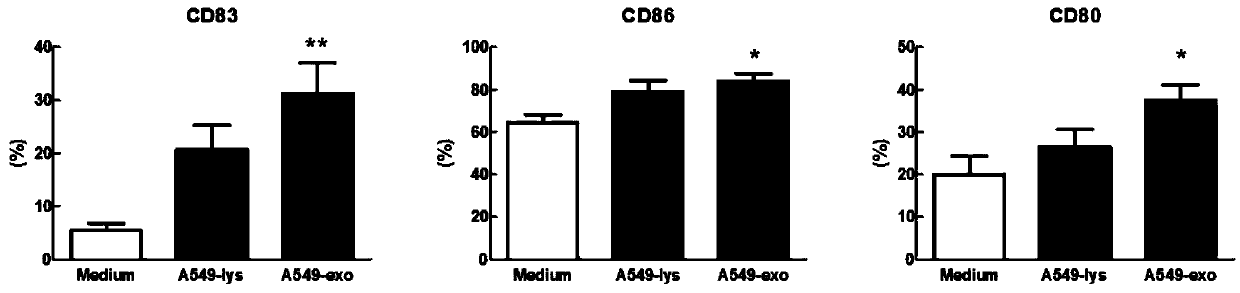
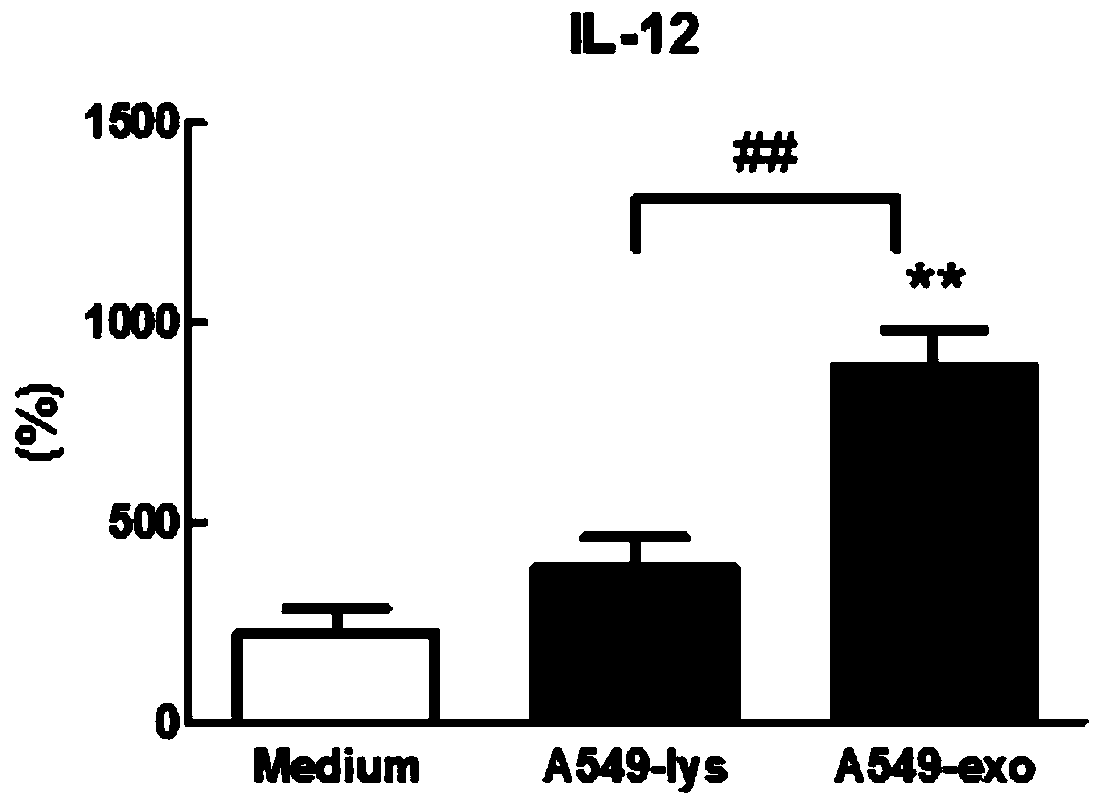
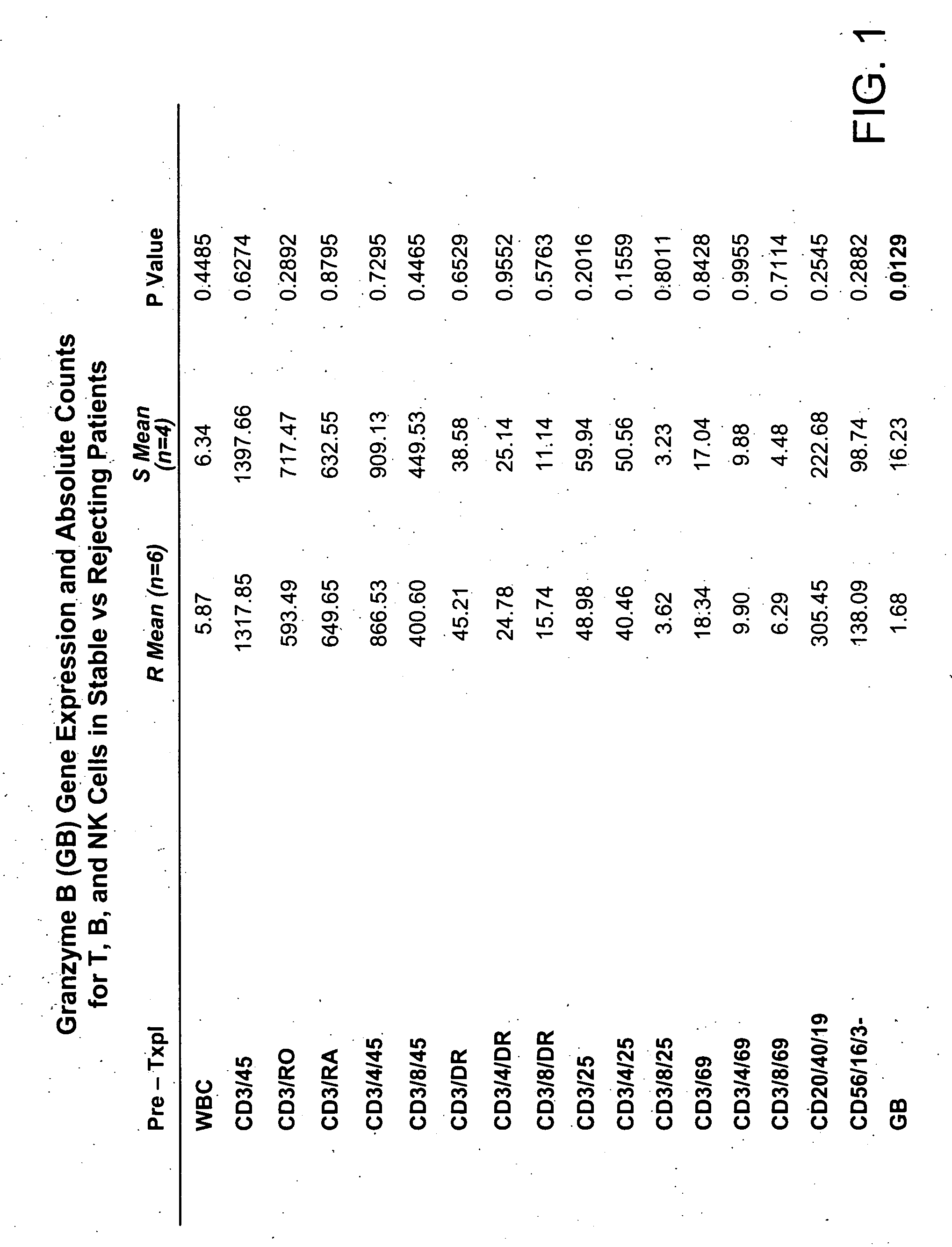
Patents
Literature
173 results about "Somatic antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A somatic antigen is an antigen located in the cell wall of a gram-positive or gram-negative bacterium.
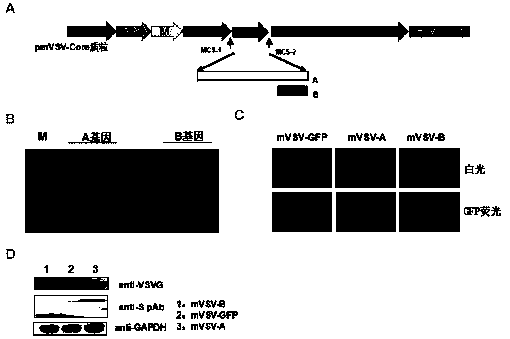
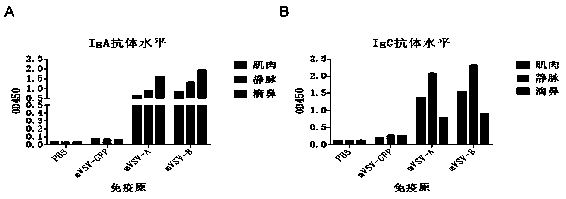
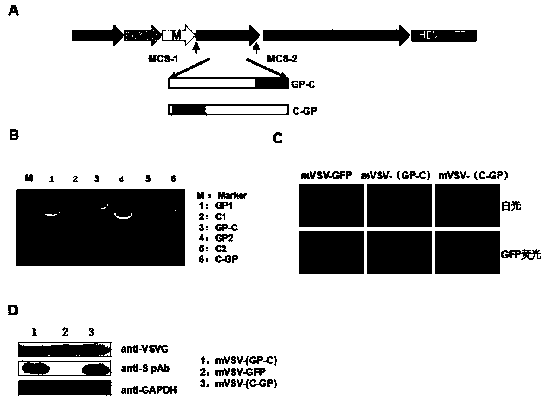
mVSV virus vector and virus vector vaccine, and COVID-19 vaccine based on mVSV mediation
ActiveCN111088283AEnhance immune responseStrong immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousReceptor
The invention provides an mVSV virus vector, i.e., attenuated mVSV obtained after multiple modification mutations occur to an M protein amino acid site of a wild Indiana strain VSV, and an optimized heterologous antigen gene is preferentially integrated to a double cloning site area of an mVSV packaging core plasmid pmVSV-Core at the same time. The mVSV virus vector vaccine comprises a heterologous antigen gene which fuses or embeds a target virus between G and L genes of an mVSV vector envelope, wherein the antigen gene comprises an enveloped and embedded antigen gene encoding the target virus, an embedded combination antigen gene or a fused antigen gene; the mVSV virus vector is embedded or fused with a dominant antigen of spike protein S of an SARS-CoV-2 pathogen; the dominant antigen is preferably selected from a receptor binding domain of spike protein S, namely RBD; and a COVID-19 vaccine based on mVSV mediation is formed. The vaccine has good prevention or treatment effect on COVID-19 infected people.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
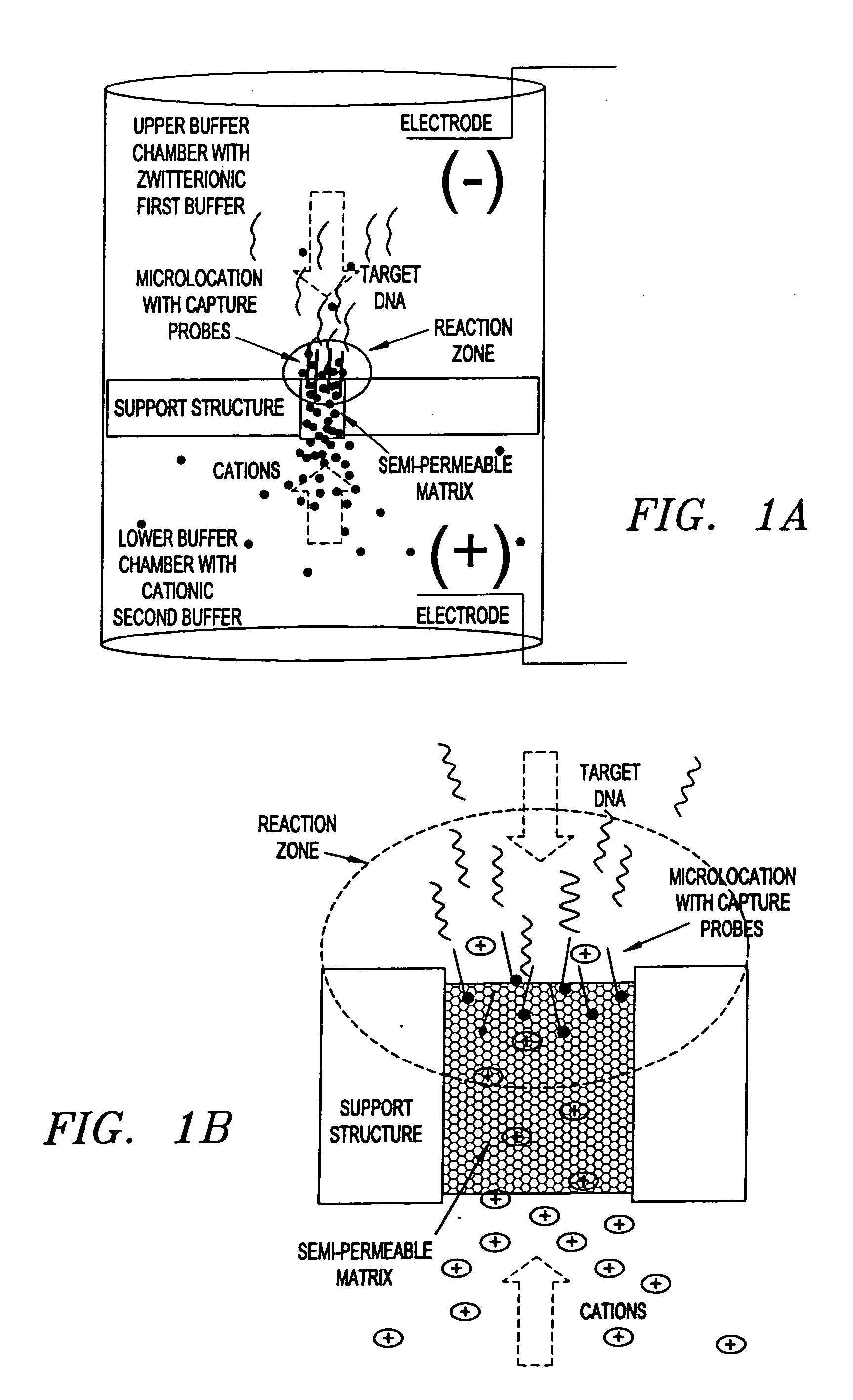
Electronic systems and component devices for macroscopic and microscopic molecular biological reaction, analyses, and diagnostics
InactiveUS20050026202A1Favorable zoneImprove responseBioreactor/fermenter combinationsMaterial nanotechnologyMultiplexBiopolymer
This invention pertains to the design, fabrication, and uses of an electronic system which can actively carry out and control multi-step and multiplex reactions in macroscopic or microscopic formats. In particular, these reactions include molecular biological reactions, such as nucleic acid hybridizations, nucleic acid amplification, sample preparation, antibody / antigen reactions, clinical diagnostics, combinatorial chemistry and selection, drug screening, oligonucleotide and nucleic acid synthesis, peptide synthesis, biopolymer synthesis, and catalytic reactions. A key feature of the present invention is the ability to control the localized concentration of two or more reaction-dependant molecules and their reaction environment in order to greatly enhance the rate and specificity of the molecular biological reaction
Owner:ADOR DIAGNOSTICS SRL
Methods for detecting proteins
The invention provides methods for detecting antigens comprising forming an antibody / antigen complex in which the antibody is coupled to a polynucleotide having a known sequence. The sequence of the polynucleotide is identified in order to identify the antibody, thereby detecting the antigen.
Owner:HELICOS BIOSCIENCES CORPORATION
Virus like particle comprising pd-1 antigen or pd-1 ligand antigen
ActiveUS20150017194A1SsRNA viruses positive-senseAntibody mimetics/scaffoldsVirus Structural ProteinsStructural protein
The present invention provides a virus like particle comprising a virus structural protein and an antigen derived from PD-1 or a ligand of PD-1, and a composition or kit comprising thereof, its use in immune response etc.
Owner:VLP THERAPEUTICS LLC
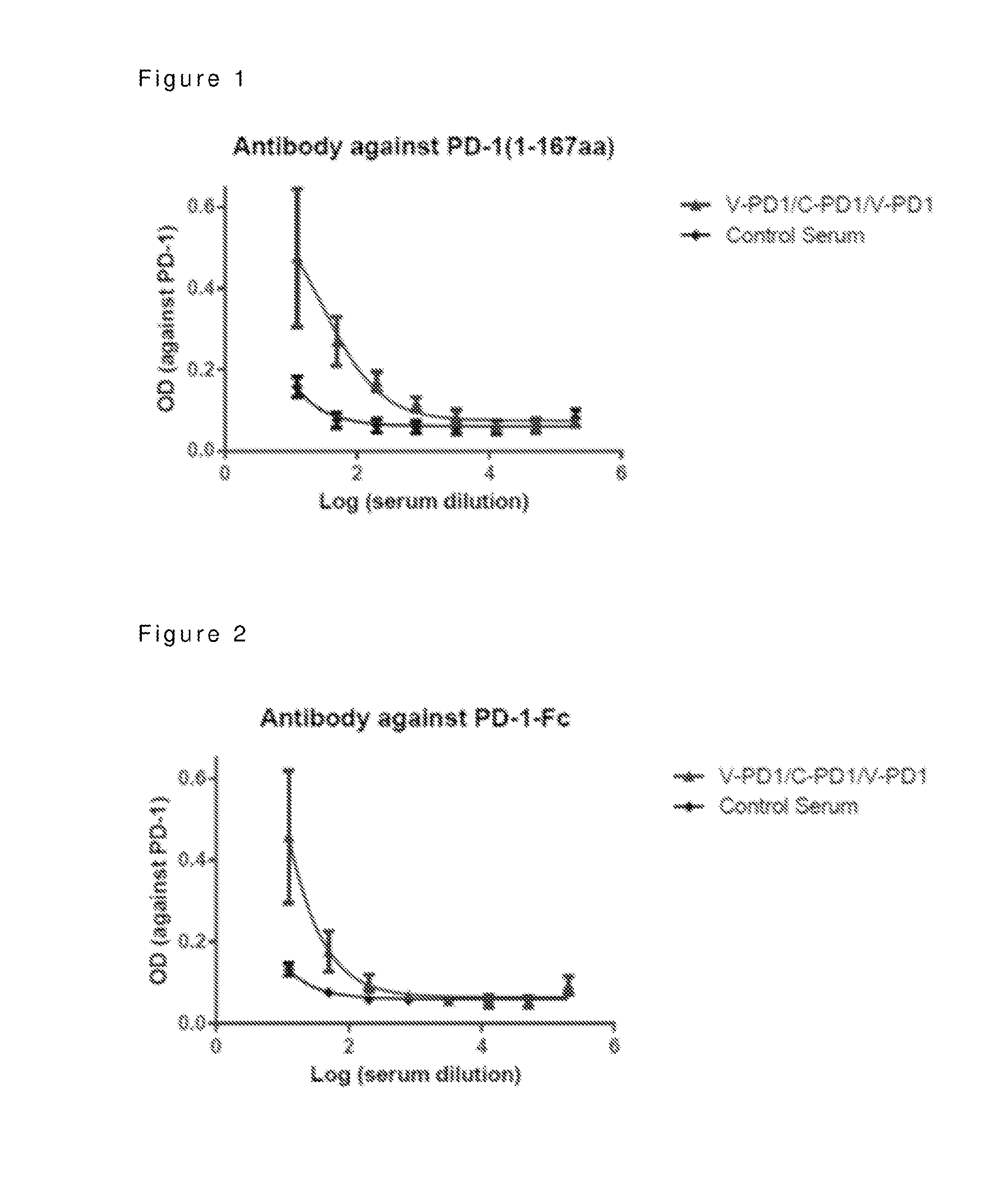
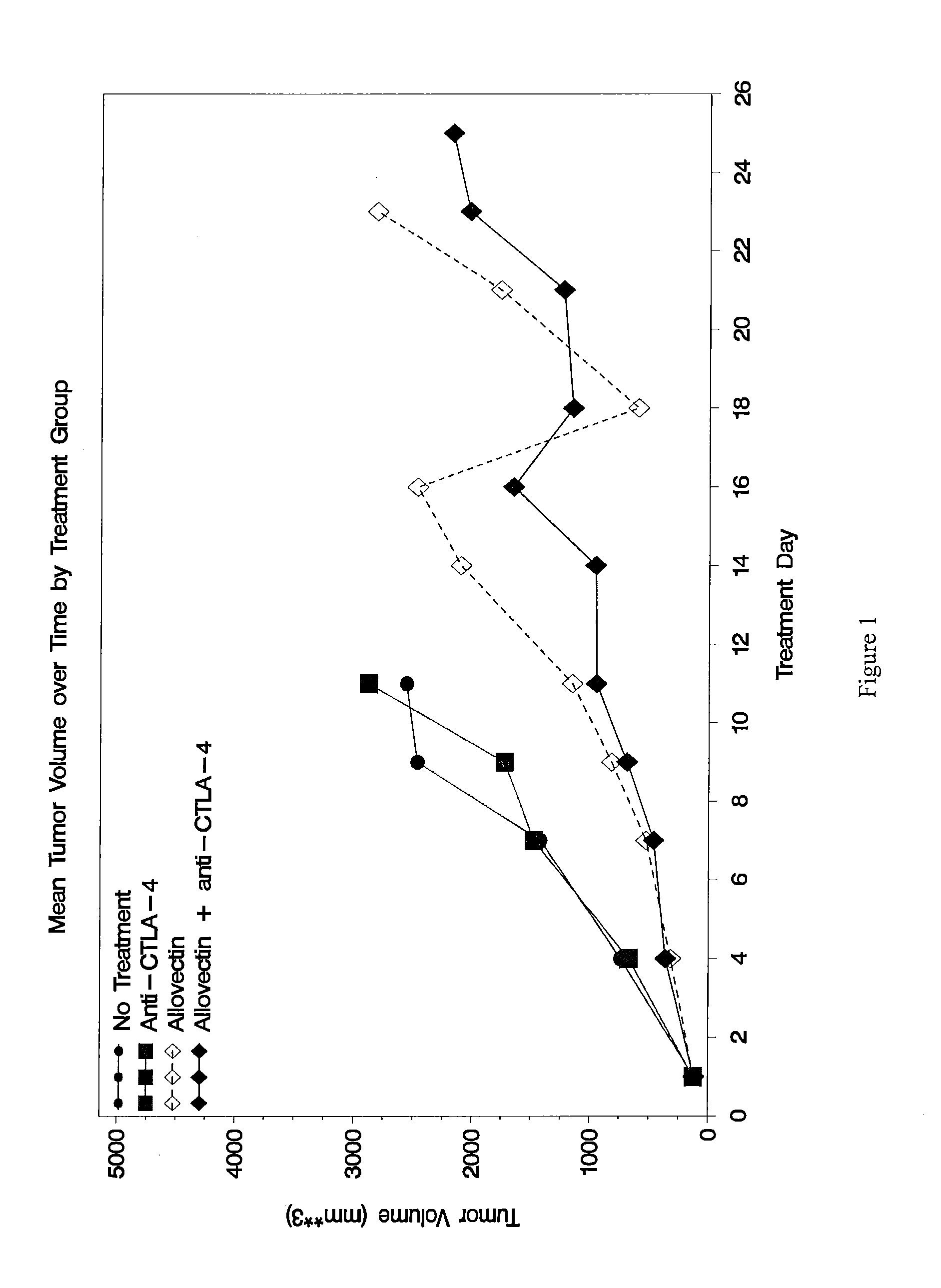
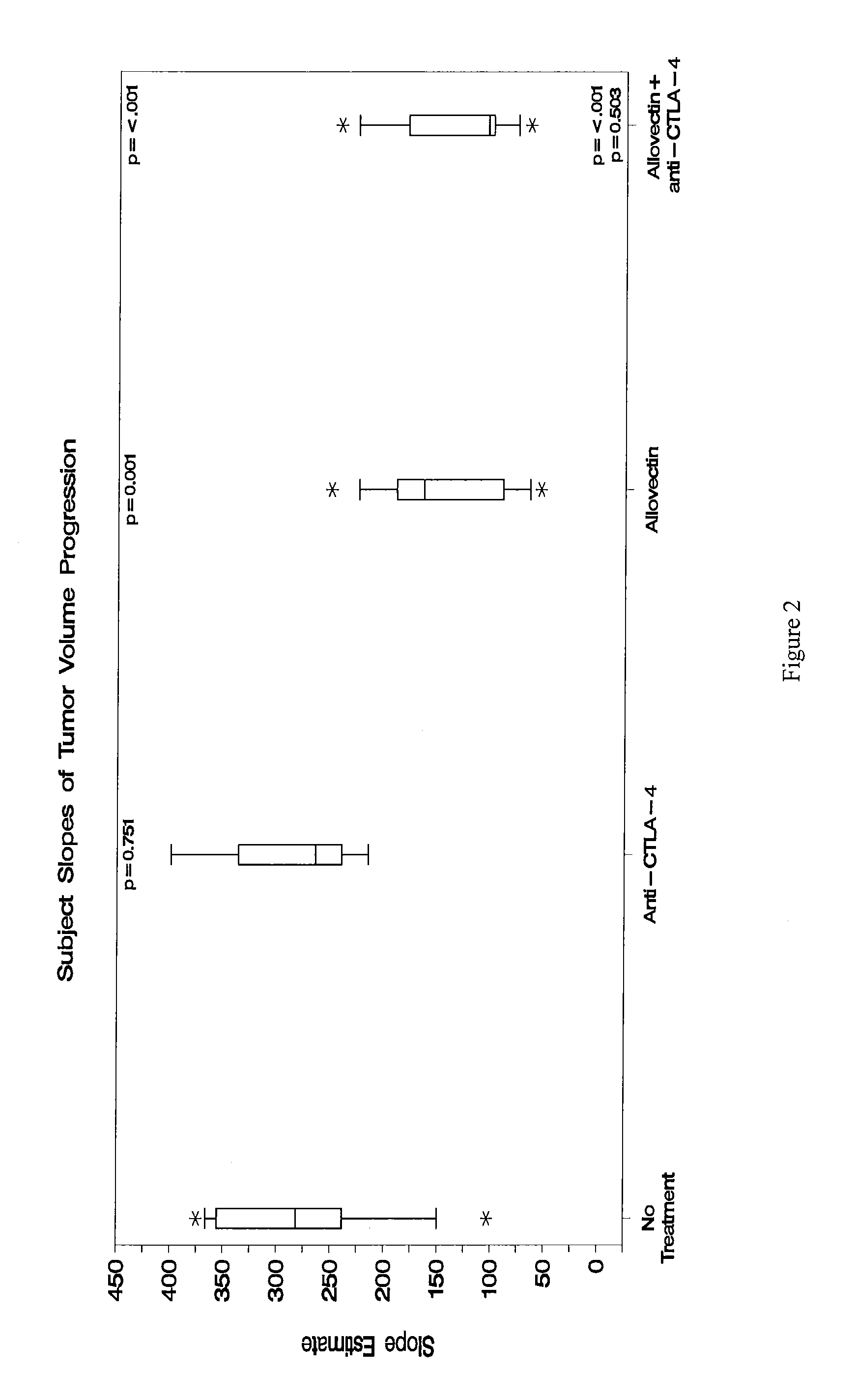
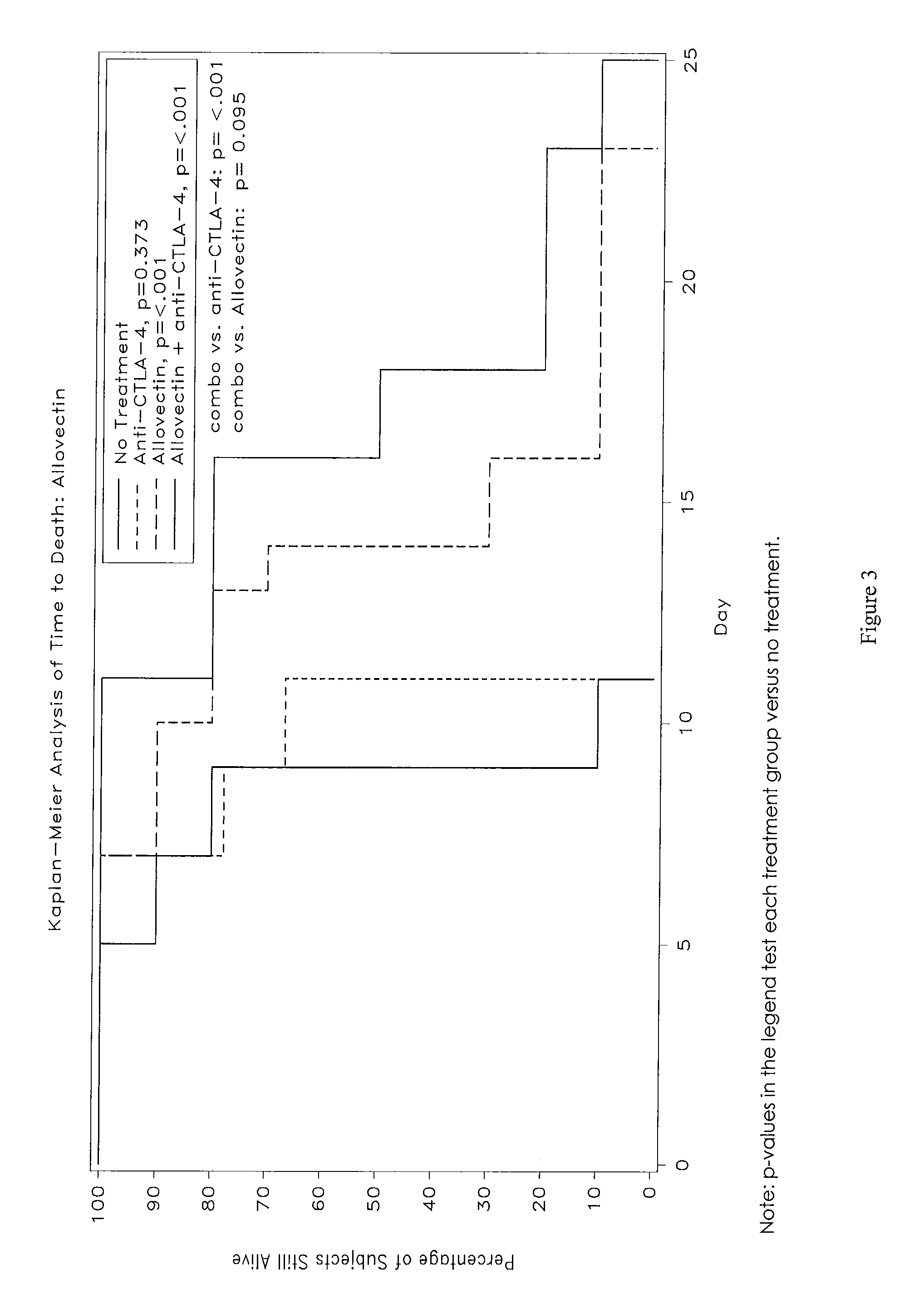
Synergistic Anti-tumor efficacy using alloantigen combination immunotherapy
InactiveUS20130280265A1Increased activationOrganic active ingredientsAntibody ingredientsImmunotherapeutic agentEfficacy
The present disclosure provides combinations of immunotherapeutics and methods for treating medical conditions that are characterized by the lack of an effective immune response, for example as would result following a down-regulation of MHC class I, such as in cancer. The immunotherapeutic compositions of the invention, which can be used to treat the medical conditions, include one or more immunostimulatory antibodies or molecules having specificity for CTLA-4, PD-1, PD-L1, PD-L2, CD40, OX40, CD137, GITR, ILT2, or ILT3, or ligands for these molecules (e.g., an isolated fully-human monoclonal antibody) in association with one or more alloantigens, such as, vector(s) capable of expressing protein(s) or peptide(s) that stimulate T-cell immunity against tissues or cells, formulated in a pharmaceutically acceptable carrier. The proteins or peptides may comprise class I major histocompatibility complex (MHC) antigens, β2-microglobulins, or cytokines. The MHC antigen may be foreign to the subject. The MHC antigen may be HLA-B7.
Owner:VICAL INC
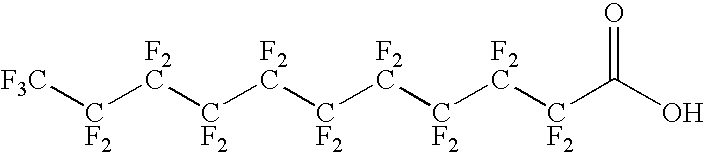
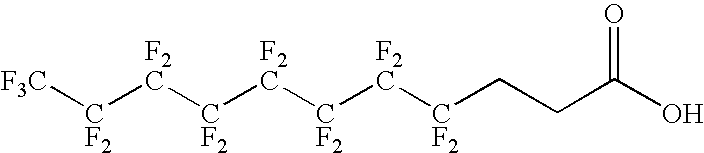
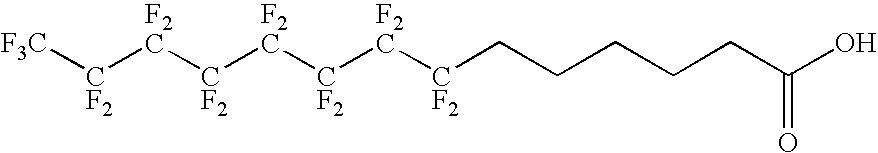
Antigen delivery vectors and constructs
ActiveUS20060013820A1Improving immunogenicityOptimise steric presentation of antigenAntibacterial agentsOrganic active ingredientsVirologyAntigen delivery
The present invention relates to fluorocarbon vectors for the delivery of antigens to immunoresponsive target cells. It further relates to fluorocarbon vector-antigen constructs and the use of such vectors associated with antigens as vaccines and immunotherapeutics in animals.
Owner:ALTIMMUNE UK LTD
Suppression of transplant rejection
InactiveUS20070166307A1Vertebrate cellsImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune conditionRegulatory T cell
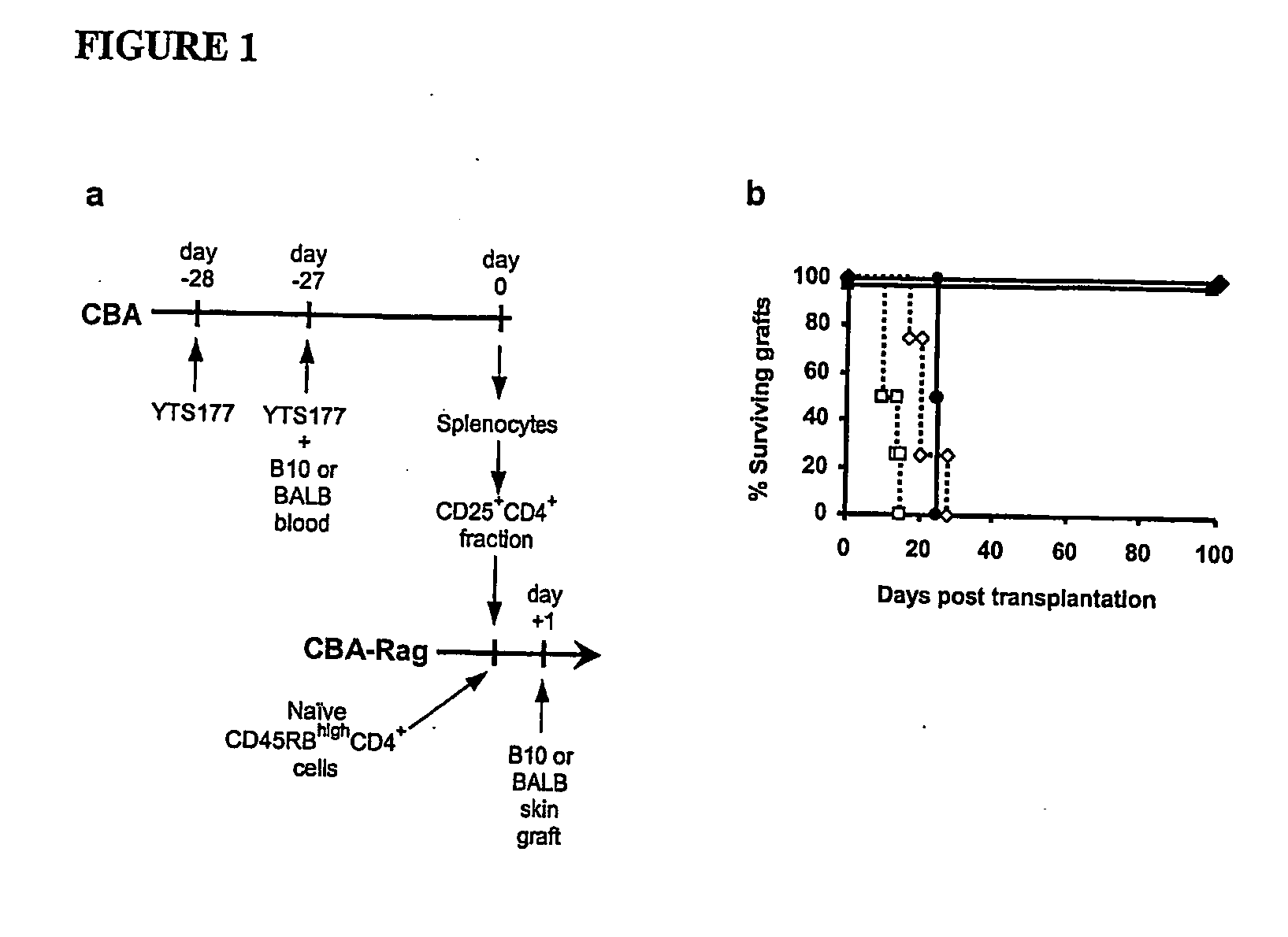
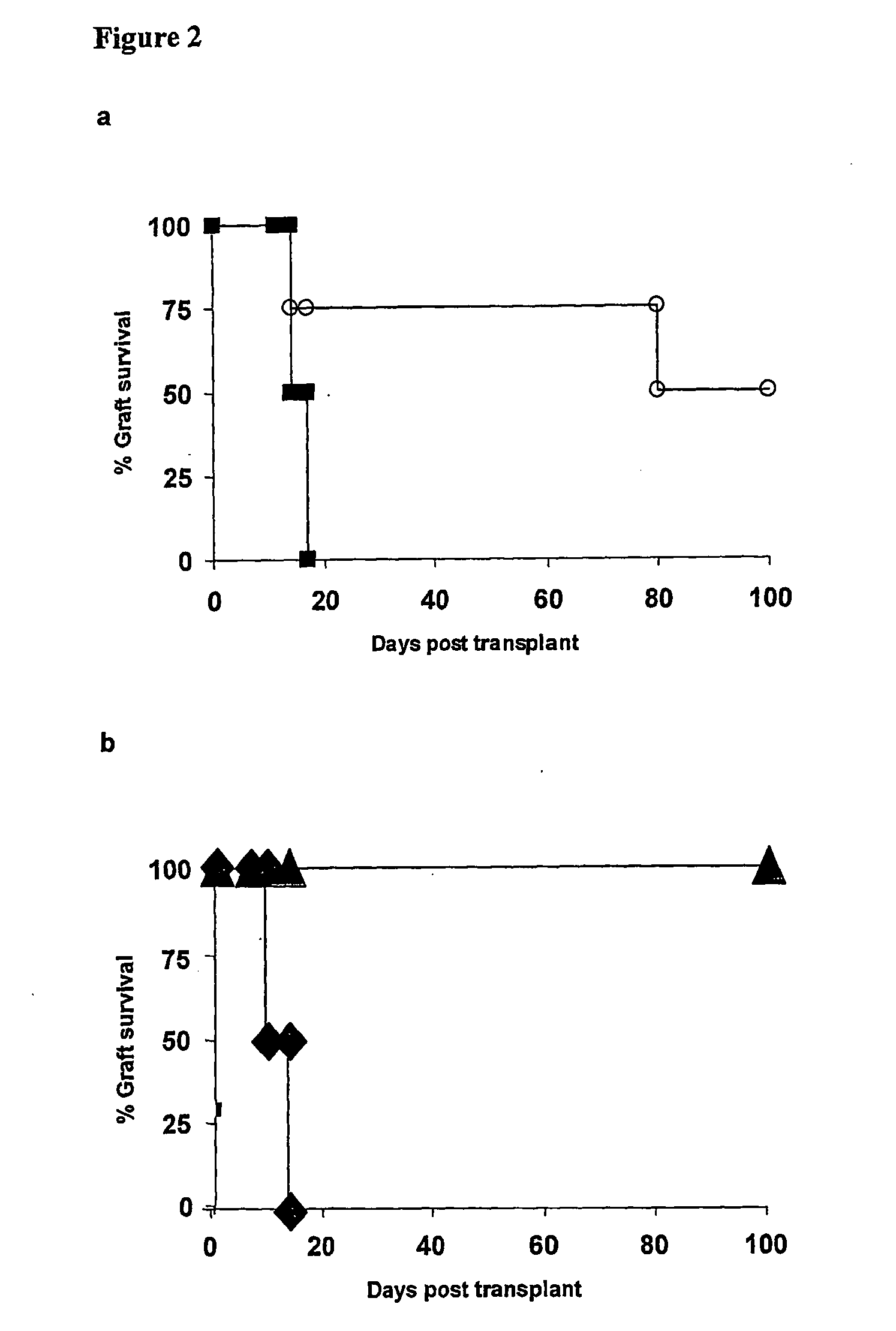
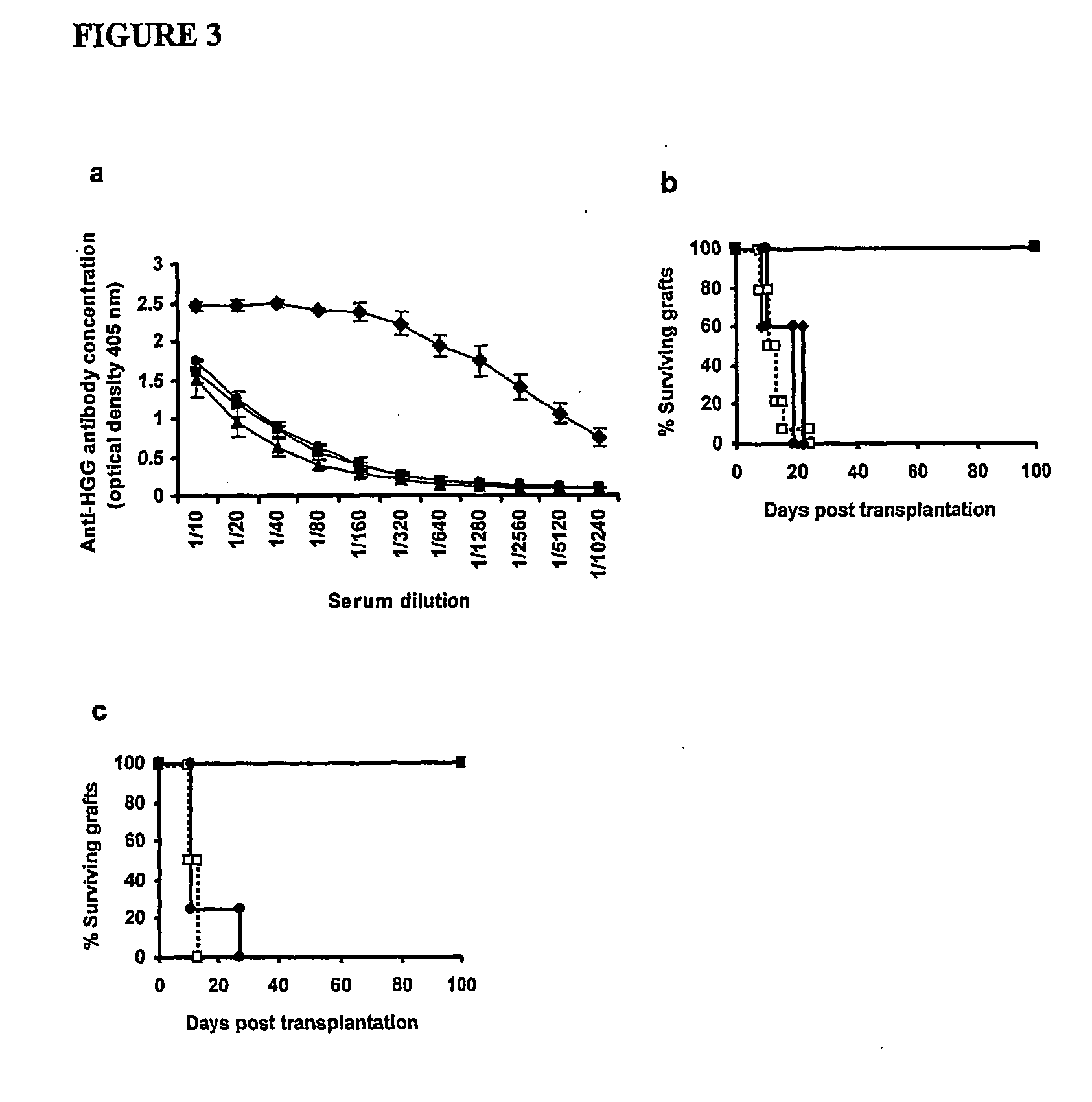
The present invention relates to a transplant rejection in an animal suppressed by administration of an antibody directed at a cell surface antigen selected from the group consisting of CD4, CD8, CD154, LFA-1, CD80, CD86 and ICAM-1, preferably an anti-CD4 antibody, together with a non-cellular protein antigen to generate in the animal a population of regulatory T-lymphocytes; reactivating said population of regulatory T-lymphocytes by further administration to the animal of the non-cellular protein antigen; and transplanting said organ or tissue whilst said population of regulatory T-lymphocytes is activated. Regulatory T cells can be generated ex vivo by culturing T cells with an antibody directed at a cell surface antigen selected from the group consisting of CD4, CD8, CD154, LFA-1, CD80, CD86 and ICAM-1, in the presence of cells that present either alloantigen or a non-cellular protein antigen. Ex vivo generated T-lymphocytes can be used as an alternative method of overcoming transplant rejection or in combination with the in vivo method. A similar approach can be adopted for the treatment of autoimmune conditions.
Owner:ISIS INNOVATION LTD
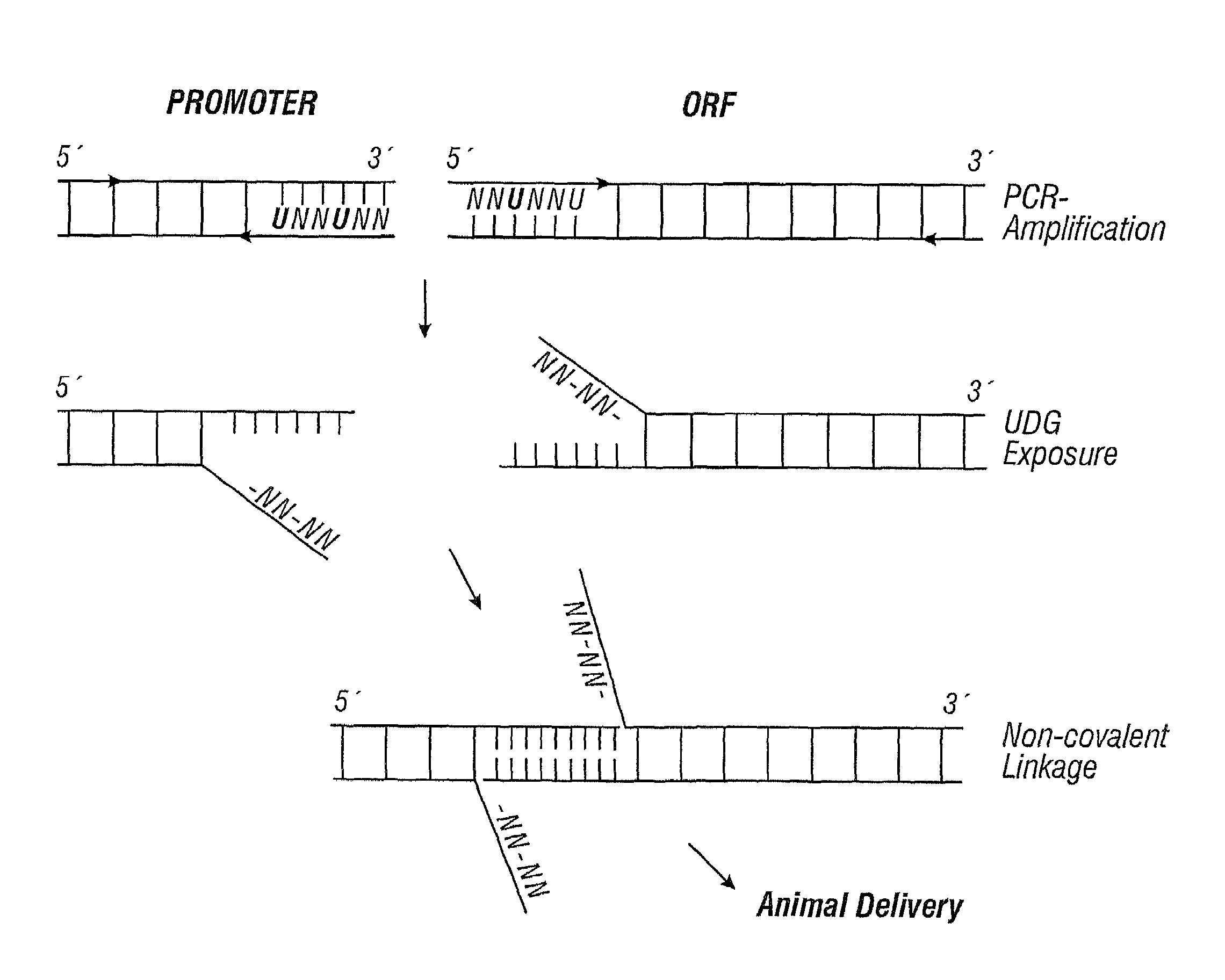
Linear and circular expression elements
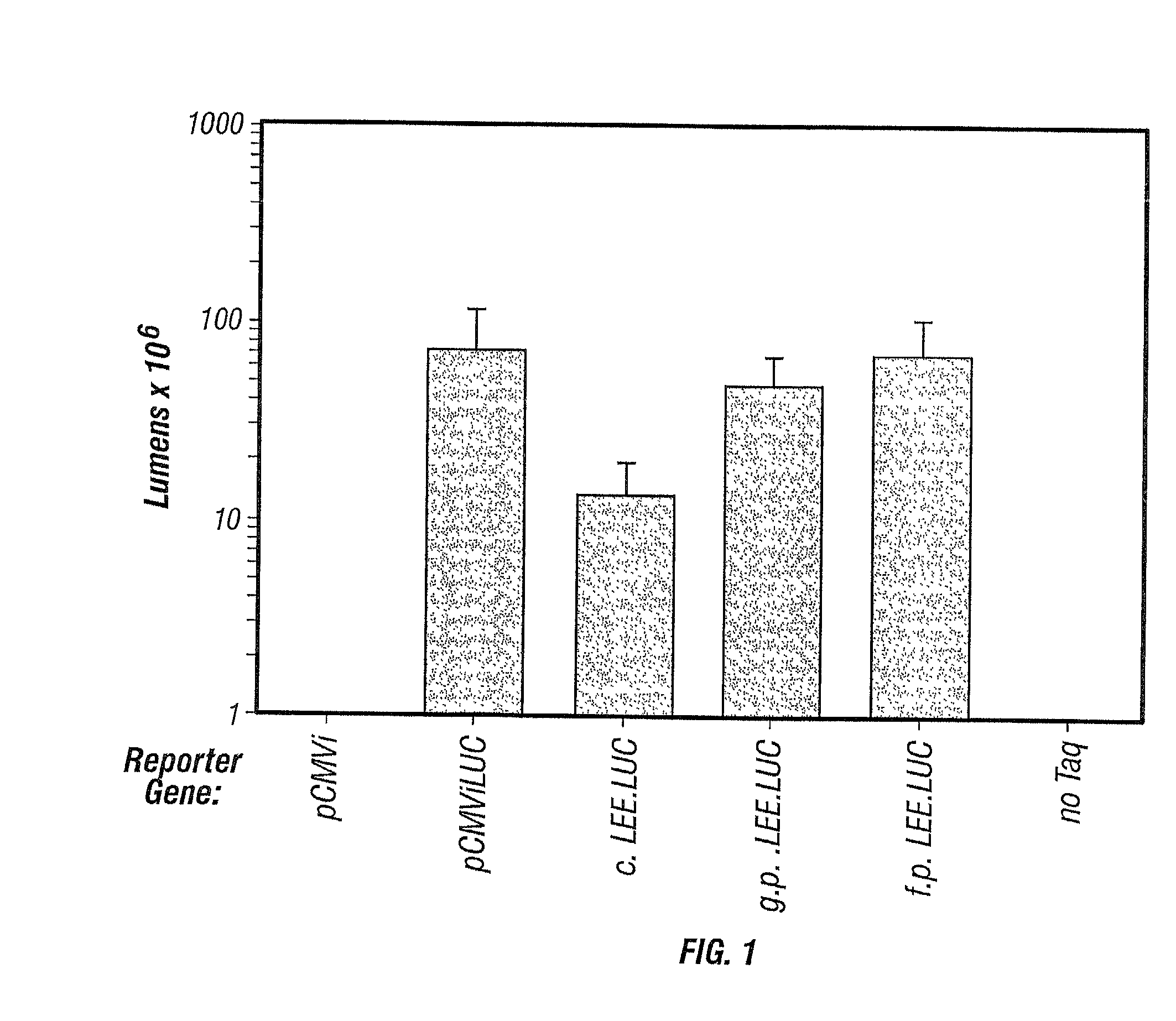
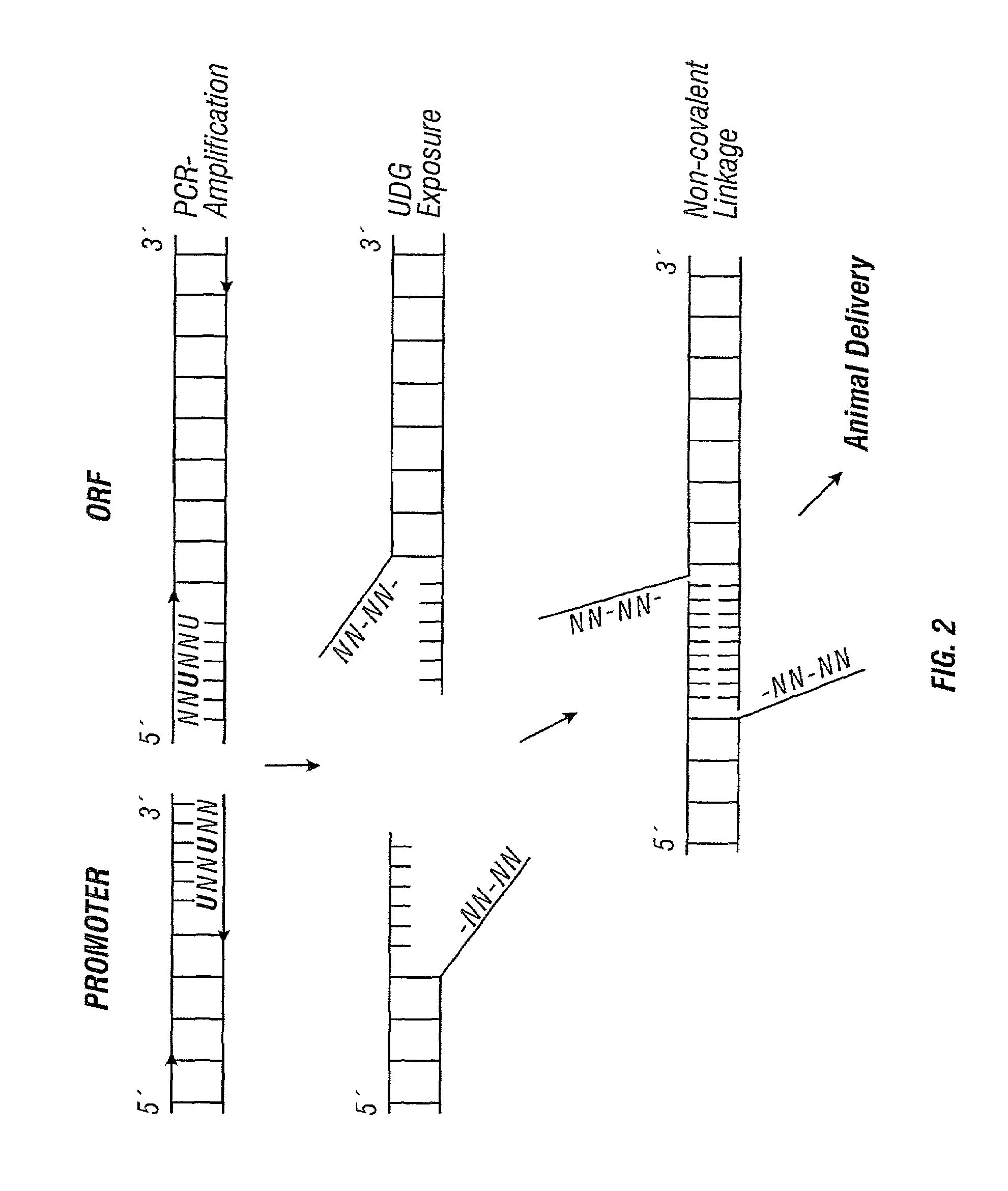
The present invention relates to linear expression elements (LEEs) and circular expression elements (CEEs), which are useful in a variety of molecular biology protocols. Specifically, the invention relates to the use of LEEs and CEEs to screen for gene function, biological effects of gene function, antigens, and promoter function. The invention also provides methods of producing proteins, antibodies, antigens, and vaccines. Also, the invention relates to methods of making LEEs and CEEs, and LEEs and CEEs produced by such methods.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Molecule detection signal amplification technique
InactiveCN102492772AAchieving High Sensitivity DetectionRealize visual detectionMicrobiological testing/measurementBiological testingBiotin-streptavidin complexAntigen
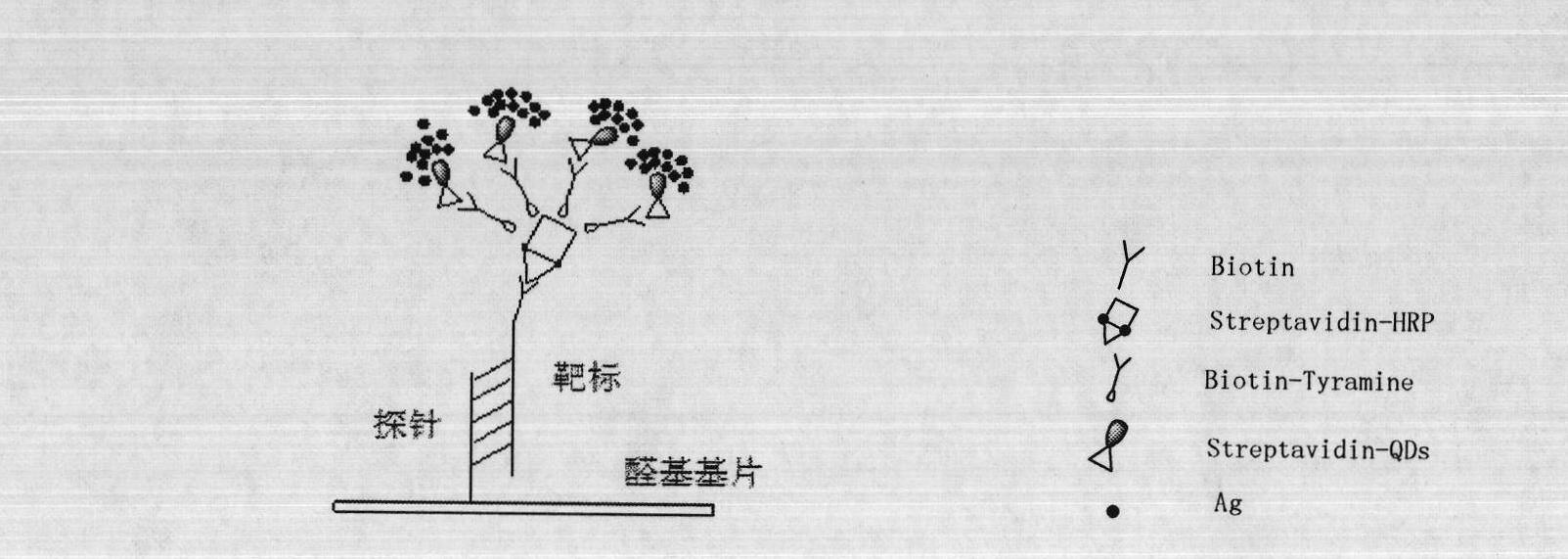
The invention relates to novel molecule detection signal amplification technique, which is characterized in that the molecule detection signal amplification technique is a biomolecule detection method with high sensitivity which is formed by streptavidin, an antigen, an antibody, polypeptide and the like which are marked by quantum dots through tyramine signal amplification and silver strengthening dyeing signal amplification. The detection method comprises the following steps: the biomolecule to be detected is combined with nucleic acid, the antibody, the antigen, the polypeptide and the like which are fixed on a solid phase material, the quantum dots are introduced to molecule to be detected through biomolecule specificity combination, silver strengthening dyeing is carried out on the quantum dots by using silver dyeing reagents, and signals are amplified. Obtained signals can be scanned through common optical scanners, analyzed through common imaging instruments or observed through naked eyes. The detection method achieves high sensitivity detection of the biomolecule on one hand, and reduces application cost of biomolecule detection on the other hand.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Method for obtaining tumor specific T cell receptor
InactiveCN109485721AHigh killing efficiencyImmunoglobulin superfamilyGenetically modified cellsT-Cell Receptor GeneWilms' tumor
The invention discloses a method for obtaining a tumor specific T cell receptor. The method comprises the following steps: firstly, obtaining a T cell receptor gene sequence in an antigen peptide specific T cell which comes from an immunoreactive positive and / or tumor symptom relieved tumor patient after antigen peptide treatment; then, preparing an antigen specific T cell receptor according to the antigen specific T cell receptor gene sequence; introducing the antigen peptide specific T cell receptor gene into the T cell, and expressing the antigen peptide specific T cell receptor gene to obtain the specific TCR-T cell. The specific TCR-T cell can be used for successfully killing gene mutation corresponding to antigen peptide and HLA typed tumor cell, and has high killing efficiency and agood application prospect.
Owner:杜学明
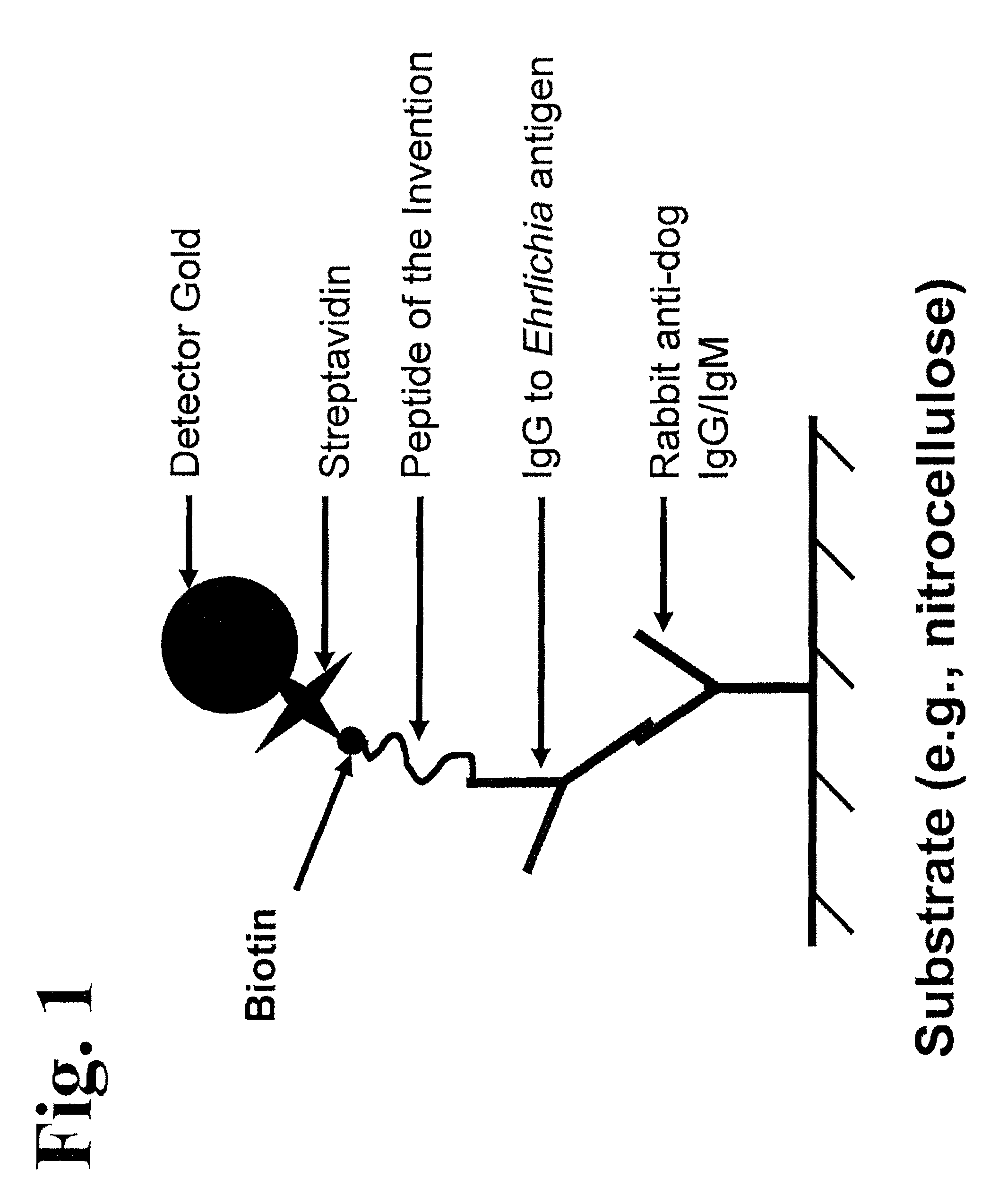
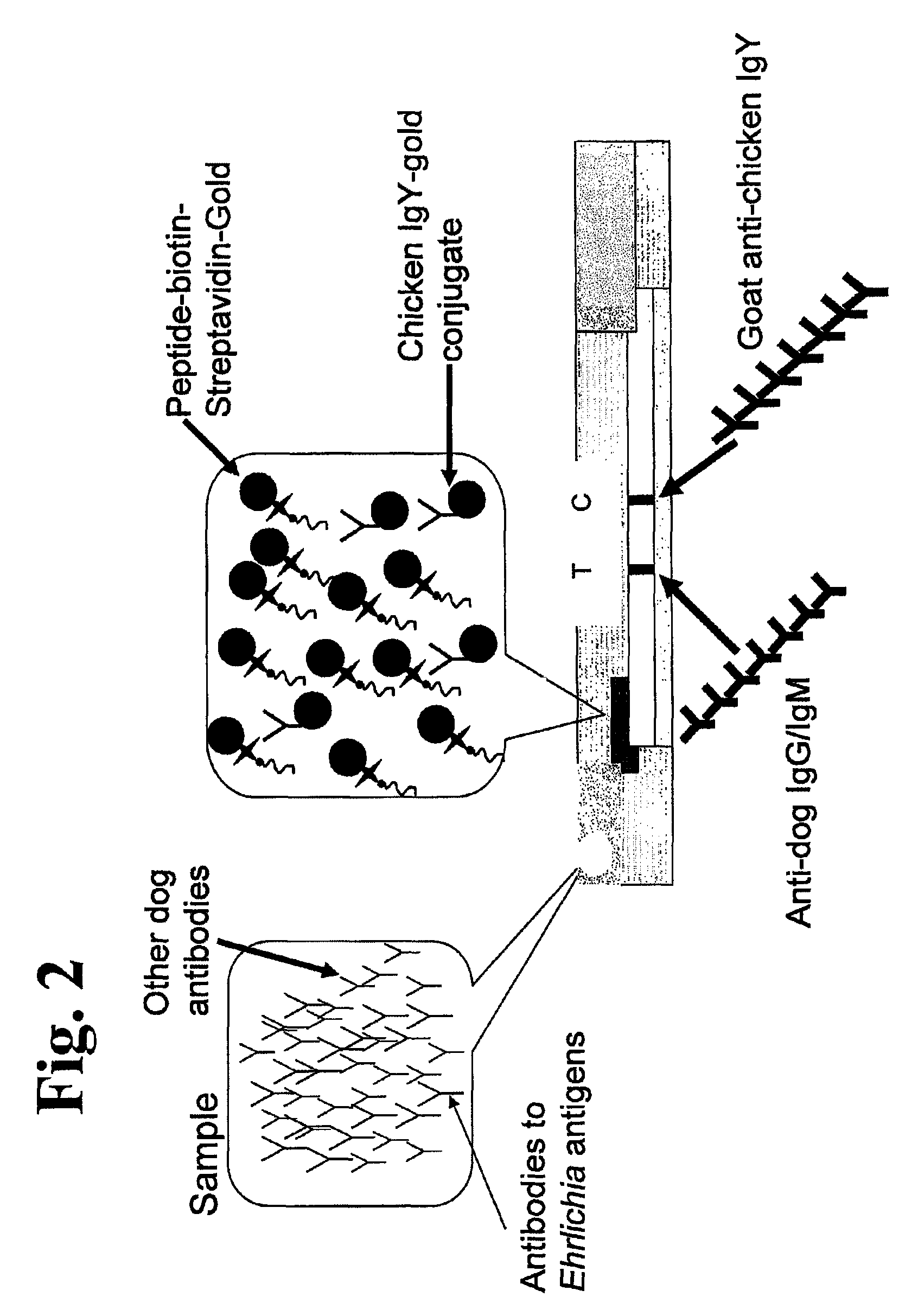
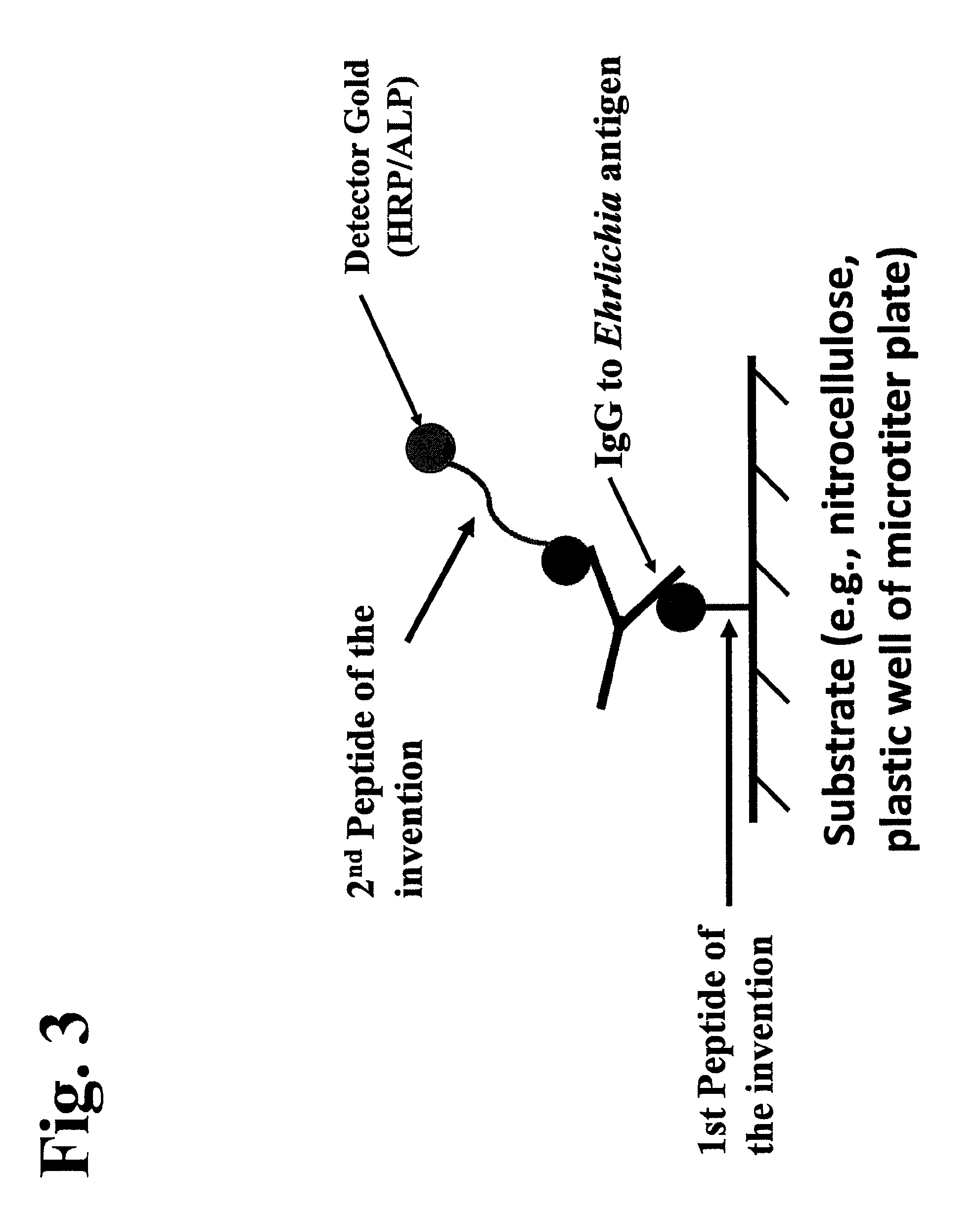
Peptides, devices, and methods for the detection of ehrlichia antibodies
ActiveUS8828675B2Simple and inexpensive and rapid and sensitive and accurate detectionAvoid serologic cross-reactivityPeptide/protein ingredientsBiological material analysisCell Membrane ProteinsMonocyte
Owner:ZOETIS SERVICE LLC
Rapid detection nanosensors for biological pathogens
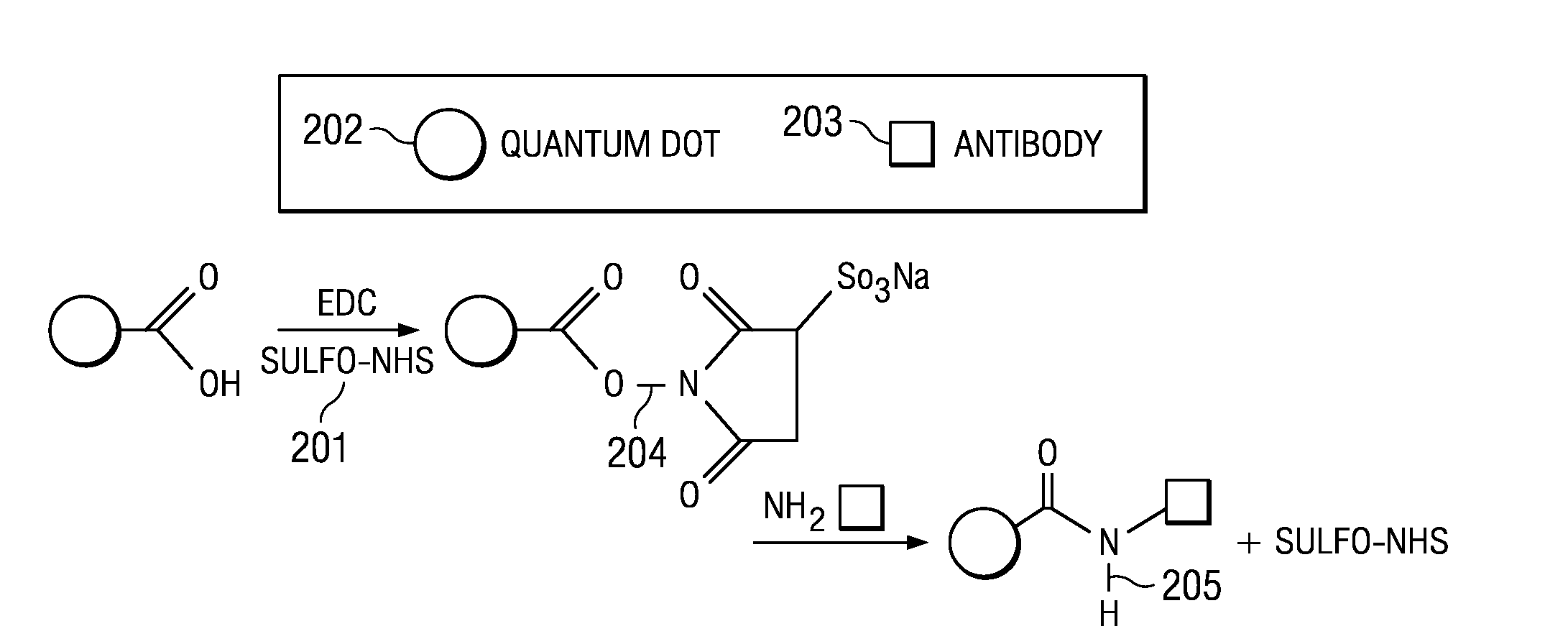
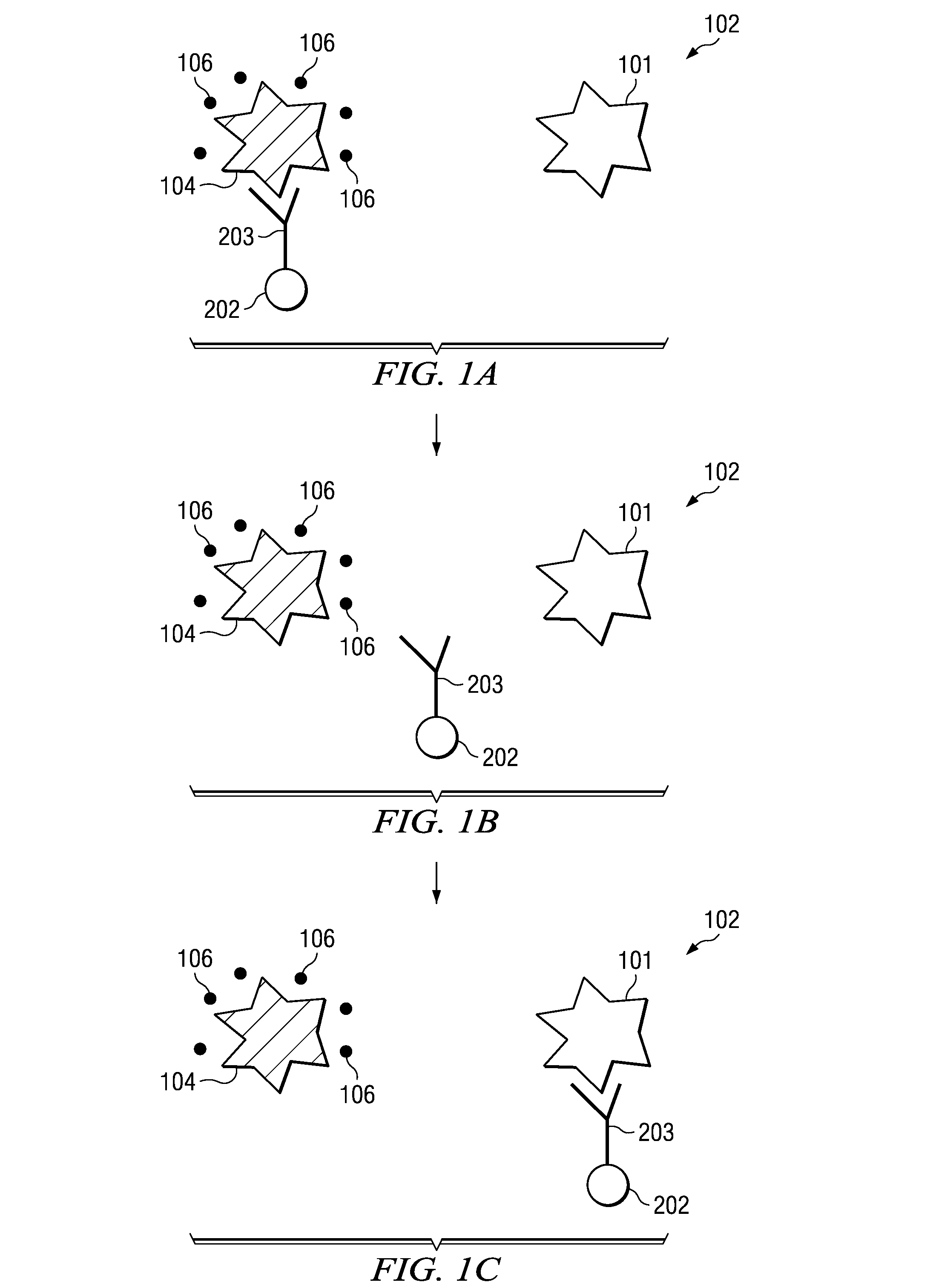
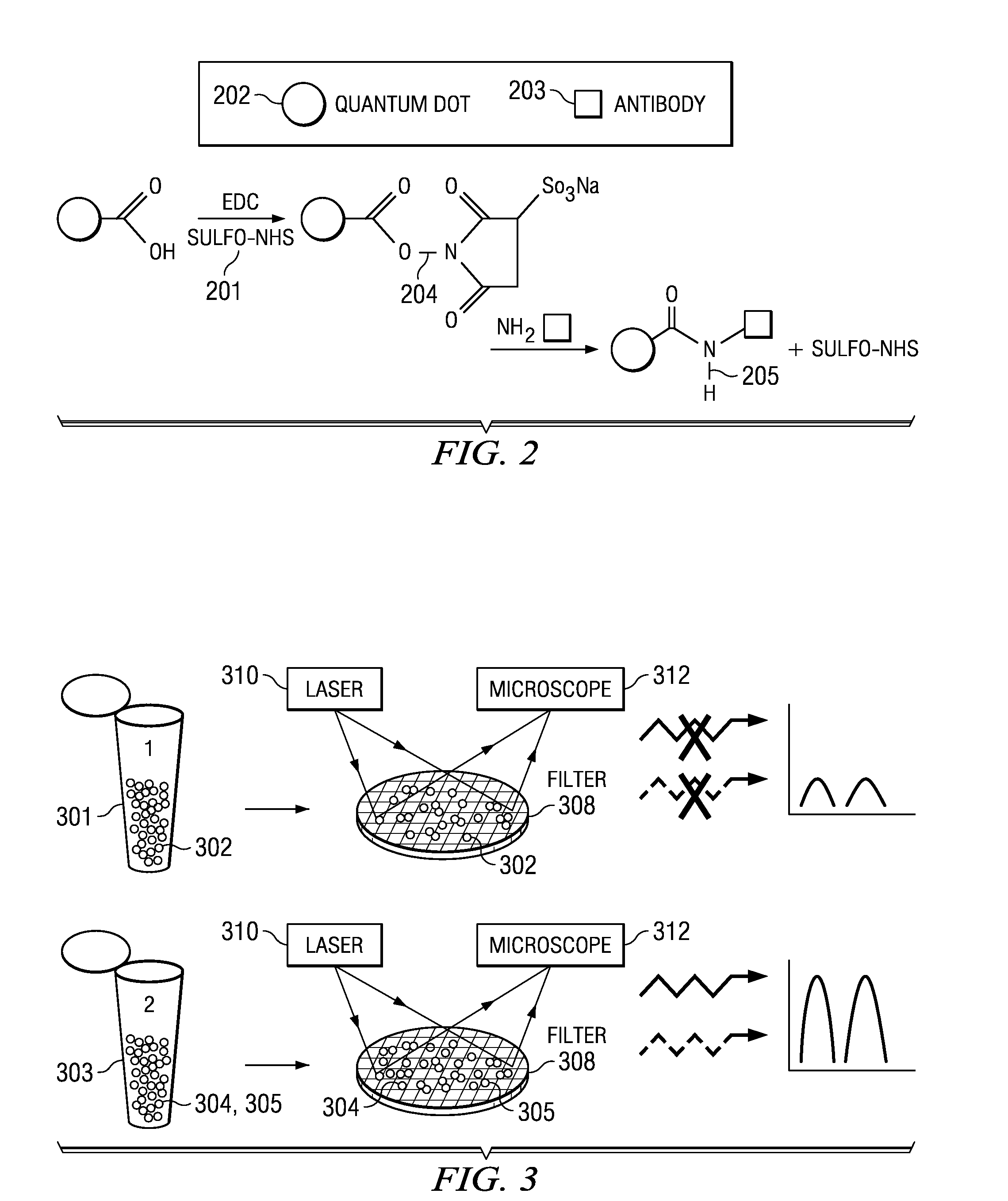
InactiveUS20100105082A1High sensitivityIncrease signal strengthBioreactor/fermenter combinationsBiological substance pretreatmentsPhotodetectorFluorescence
An assay test solution, a method for using, and an apparatus for the rapid detection of multiple pathogens using a FRET-based phenomenon. A volume of fluid, possibly containing pathogens, is passed through an intake and combined with an assay solution of quantum dot / antibody-antigen / quencher complexes that dissociate and recombine with the pathogens into quantum dot / antibody-pathogen complexes. The quantum dot / antibody-antigen / quencher and quantum dot / antibody-pathogen complexes are captured on a detection filter which is illuminated by a light source. The quantum dot / antibody-pathogen complexes, but not the quantum dot / antibody-antigen / quencher complexes, fluoresce when excited by the light from the light source and the fluorescence is picked up by a photodetector, indicating the presence of the pathogens.
Owner:EPIR TECH INC
Anti P2X7 receptor antibodies and fragments thereof
ActiveUS9127059B2Sugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsBinding siteReceptor for activated C kinase 1
The invention relates to an antigen binding site for binding to a P2X7 receptor, the antigen binding site being defined by general formula 1:FR1-CDR1-FR2-CDR2-FR3-CDR3-FR4
Owner:BIOSCEPTRE PTY LTD
Anti p2x7 receptor antibodies and fragments thereof
ActiveUS20120282278A1High affinitySugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen binding siteP2x7 receptor
Owner:BIOSCEPTRE PTY LTD
Single-index microfluidic chip, and production method and application method thereof
ActiveCN109569754AQuality is not affectedEnsure fluid dosingLaboratory glasswaresBiological testingEngineeringAntibody antigen
The invention discloses a single-index microfluidic chip, and a production method and an application method thereof. The single-index microfluidic chip comprises a chip body. A sampling cavity, a quantitative reaction cavity and a waste liquid cavity are formed in the chip body; a first backflow prevention device and a second backflow prevention device are arranged at the front end and the rear end of the quantitative reaction cavity correspondingly; at least one of the first backflow prevention device and the second backflow prevention device is a rubber plug anti-backflow device that comprises a rubber plug, a fluid input pipe capable of raising the fluid delivery height and a fluid output pipe capable of lowering the liquid delivery height. The front end and the rear end of the quantitative reaction cavity are provided with the backflow prevention devices, and accordingly, quantification of fluids in the quantitative reaction cavity can be guaranteed; during antibody / antigen coating, antibody / antigen coating solvents can be injected into the fluid input pipe / fluid output pipe of the rubber plug anti-backflow device, and then are subjected to incubation, washing, packaging, and vacuumizing by a vacuum drying oven before being provided with a rubber plug, so that the single-index microfluidic chip is suitable for mass production. Basically, the quality of coated antibodies / antigens is not affected by the packaging time and the packaging process.
Owner:NANJING LANSION BIOTECH CO LTD
Suspension chip detection method for simultaneously detecting hepatitis B virus, hepatitis C virus, treponema pallidum and human immunodeficiency virus pathogens
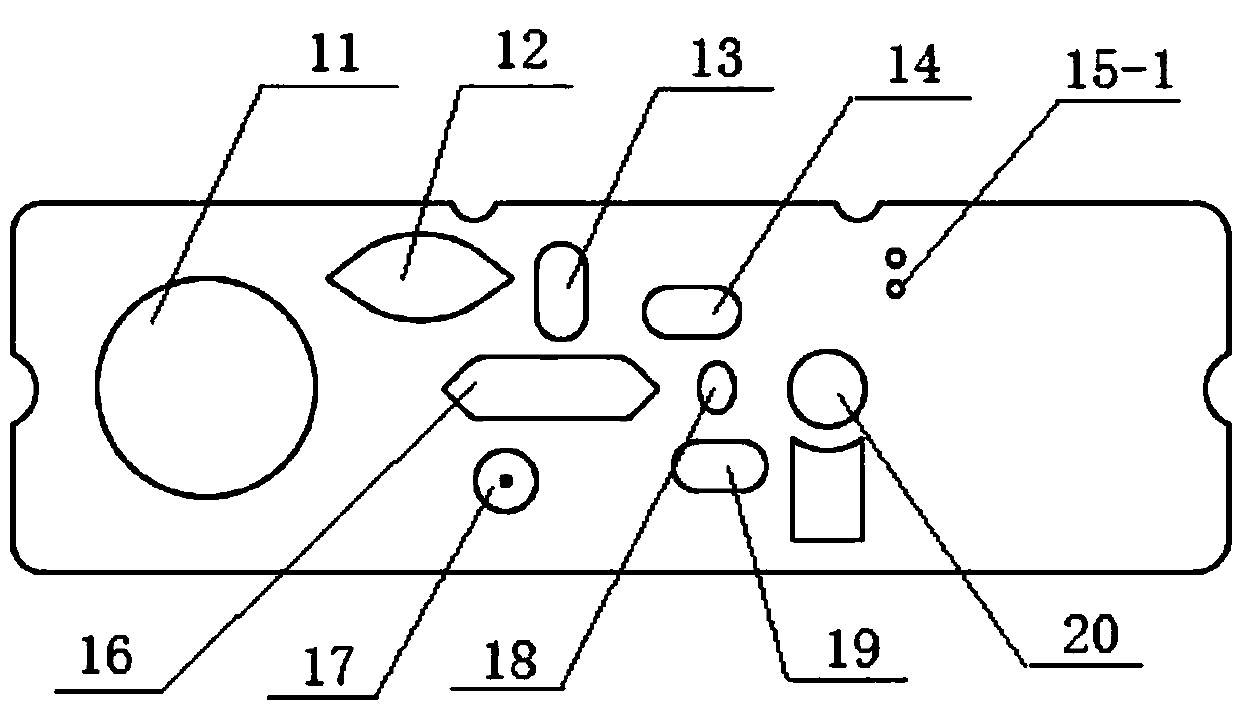
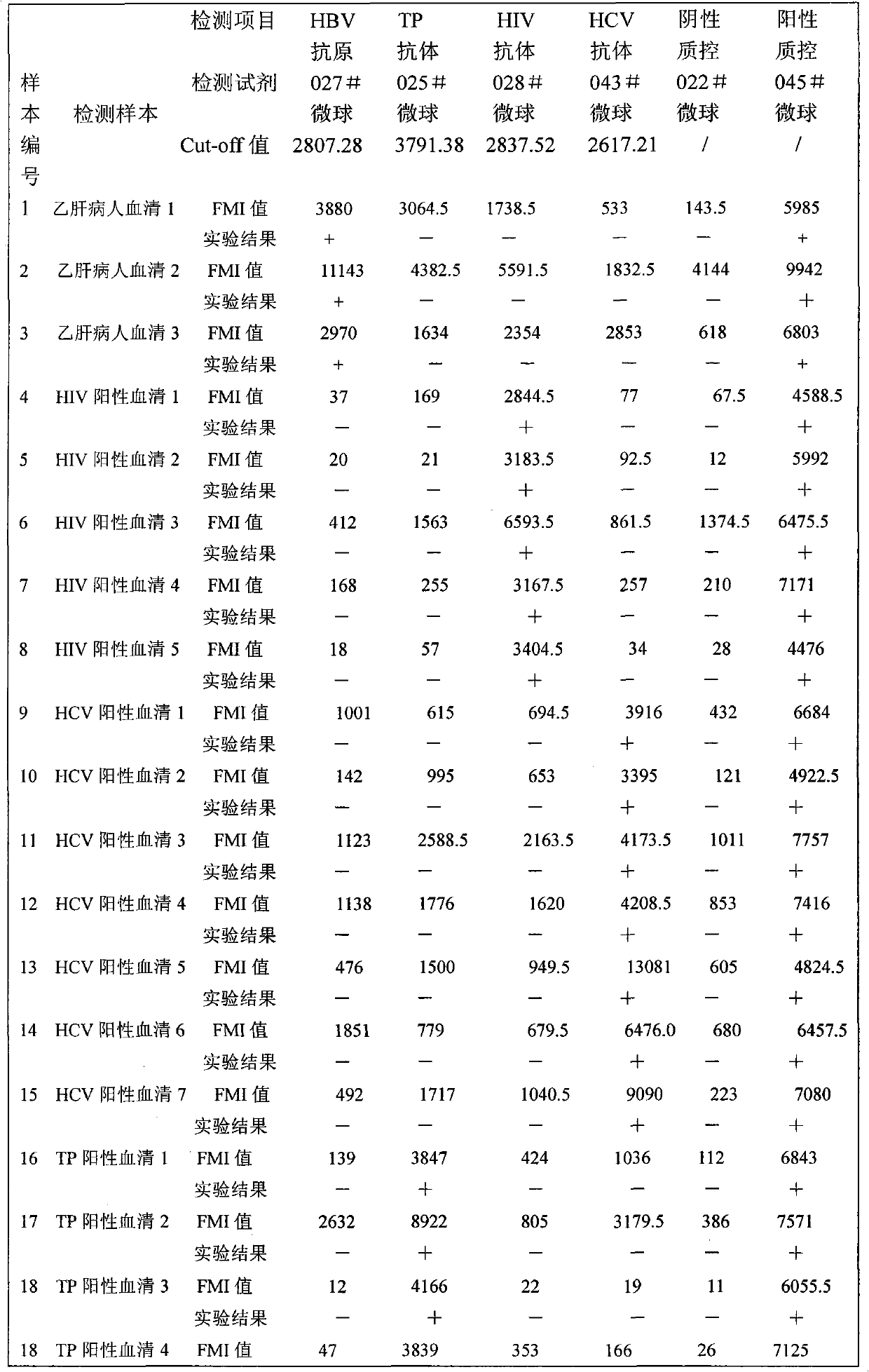
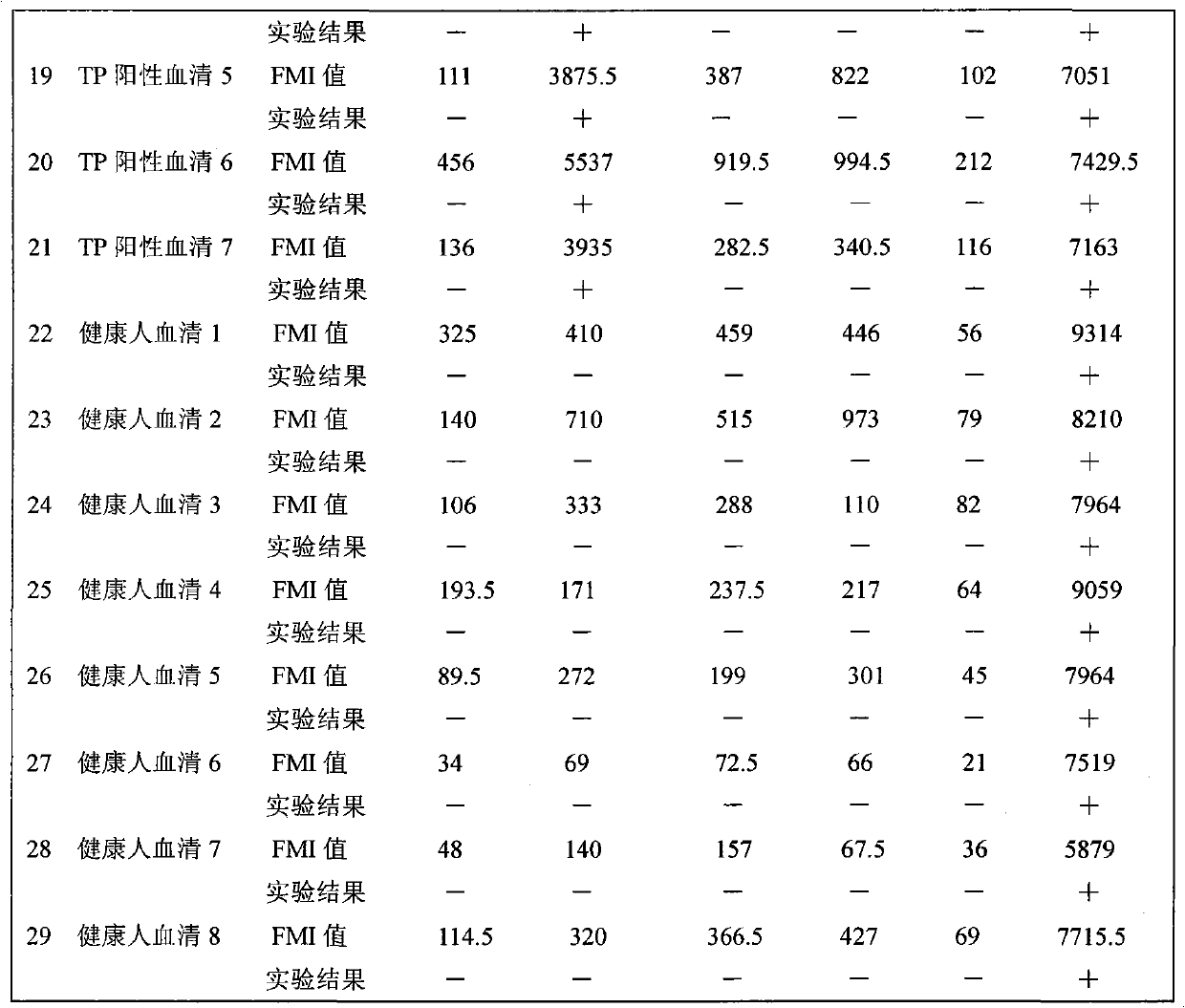
The invention relates to a suspension chip detection method for simultaneously detecting hepatitis B virus, hepatitis C virus, treponema pallidum and human immunodeficiency virus pathogens. Plastic color coded microspheres with diameter of between 5.5 and 6.5 mu m are taken as vectors for a suspension chip; and the carriers are coated with monoclonal hepatitis B virus surface antibodies, HIV1, gP41 antigens, HCV NS3 antigens or treponema pallidum TP15 antigens. Through the suspension chip, the hepatitis B virus, hepatitis C virus, treponema pallidum and human immunodeficiency virus pathogens in a sample to be detected can be very conveniently and quickly detected at the same time; and the method has the advantages of accurate and reliable detection result, high specificity, small using amount of samples and high-throughput detection.
Owner:中华人民共和国陕西出入境检验检疫局
Monoclonal antibodies against duck Tembusu virus, antigen detection kit and application
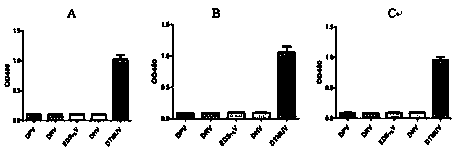
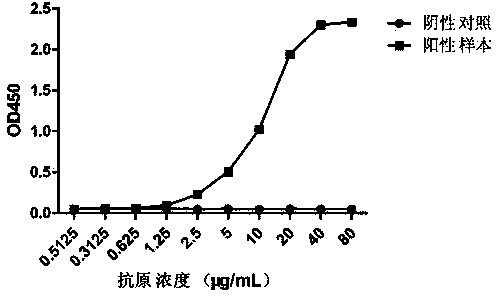
ActiveCN104109206AQuick checkFast detection methodImmunoglobulins against virusesMicroorganism based processesTembusu virusCell strain
The invention provides monoclonal antibodies against the duck Tembusu virus, an antigen detection kit and application. According to the invention, the DTMUV-JXSP cell strain of the duck Tembusu virus is used as immunogen for preparation of the monoclonal antibodies which include monoclonal antibodies respectively secreted by hybridoma cells with respective accession numbers of CGMCC No. 8104, CGMCC No. 8107 and CGMCC No. 8106; the monoclonal antibody 3B4 secreted by the hybridoma cell with the accession number of CGMCC No. 8106 is used as a primary antibody coated ELISA plate, the monoclonal antibody 3F2 secreted by the hybridoma cell with the accession number of CGMCC No. 8107 is subjected to enzyme labeling and used as an ELISA secondary antibody, so the double-antibody sandwiched detection kit is established and the kit has good sensitivity and specificity. According to the invention, effective means are provided for large-scale clinical detection of the duck Tembusu virus.
Owner:北京市动物疫病预防控制中心
Antibody drug conjugates
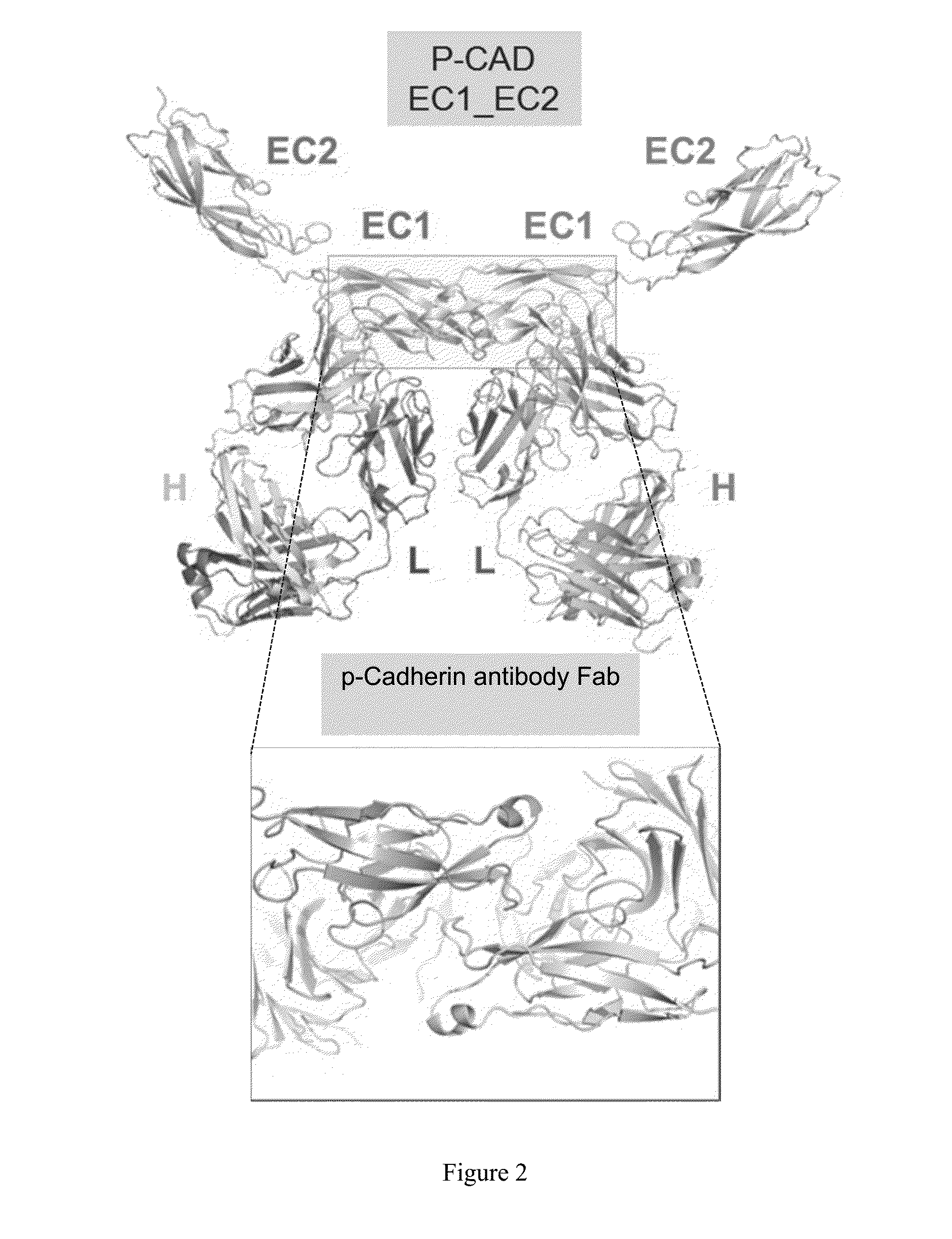
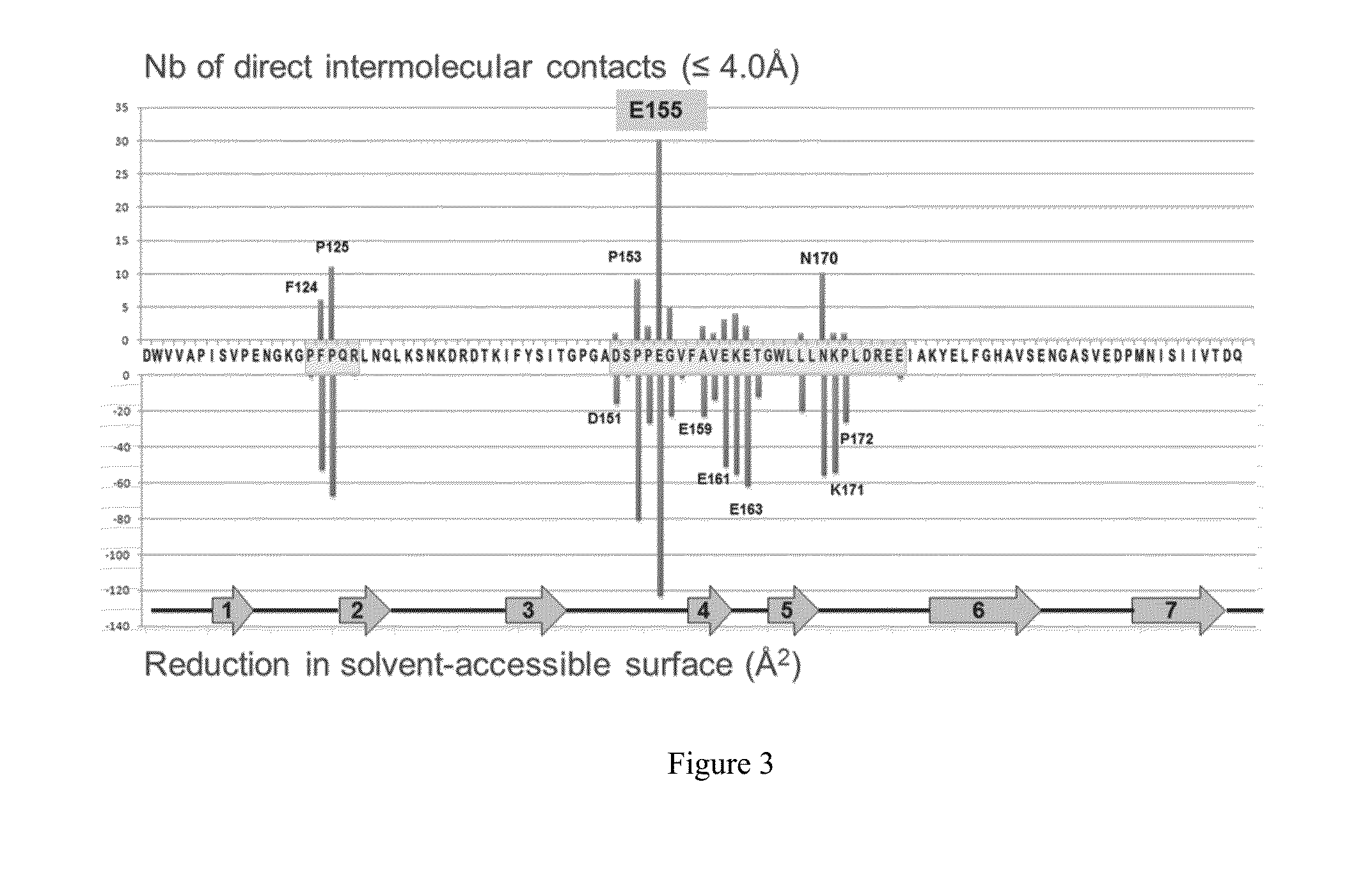
This application discloses anti-P-cadherin antibodies, antigen binding fragments thereof, and antibody drug conjugates of said antibodies or antigen binding fragments. The invention also relates to methods of treating cancer using the antibodies, antigen binding fragments, and antibody drug conjugates. Also disclosed herein are methods of making the antibodies, antigen binding fragments, and antibody drug conjugates, and methods of using the antibodies and antigen binding fragments as diagnostic reagents.
Owner:NOVARTIS AG
Anti-CD40 antibodies and methods of treating cancer
ActiveUS9676862B2Good effectHybrid immunoglobulinsIn-vivo radioactive preparationsDiseaseDendritic cell
The present invention relates to antibodies (and fragments, variants, fusions and derivatives thereof) with multivalent binding specificity for CD40, which have a potency for dendritic cell activation which is higher than, or is equal to, the potency for B cell activation and wherein the antibody, antigen-binding fragment, or fusion, variant or derivative thereof has an affinity (KD) for CD40 of less than 1×10−10 M, which have utility in the treatment of diseases such as cancer. The invention also relates to pharmaceutical compositions, uses, methods and kits comprising such antibodies.
Owner:ALLIGATOR BIOSCI
Calibration product stabilizer, detection kit for determining C peptide and detection method
ActiveCN109917134ALow costImprove responseChemiluminescene/bioluminescenceBiological testingC-peptideReaction curve
The invention belongs to the technical field of biomedical examination, and particularly relates to a calibration product stabilizer, a C peptide determination detection kit and a detection method. The kit comprises a calibration product, a reagent 1, a reagent 2, a reagent R3 and a reagent R4. According to the kit, an acridinium ester-labeled anti-C peptide antibody, an antigen and a horse radishperoxidase-labeled anti-C peptide antibody are utilized to form an antibody-antigen-antibody compound. A triggering agent is added into the compound without a washing process, the compound is continuously detected for a period of time, the peak area is calculated every 0.02-0.05 S, and a dosage-reaction curve is made by using C peptide with known concentration and the calculated peak area; and the content of the C peptide in the sample to be detected is calculated according to the curve. The kit for detecting the C peptide by adopting the spatial proximity chemiluminiscence method provided bythe invention has the advantages of strong calibration product stability, strong reagent anti-interference capability, high accuracy, good specificity and wide linear range, and is suitable for beingused by medical and research institutions at all levels in combination with instrument measurement.
Owner:GUANGZHOU JINDE BIOTECH
Liver cirrhosis detection kit and detection method thereof
InactiveCN104865385AEasy to operateThe test result is accurateDisease diagnosisBiological testingSolid phasesChemistry
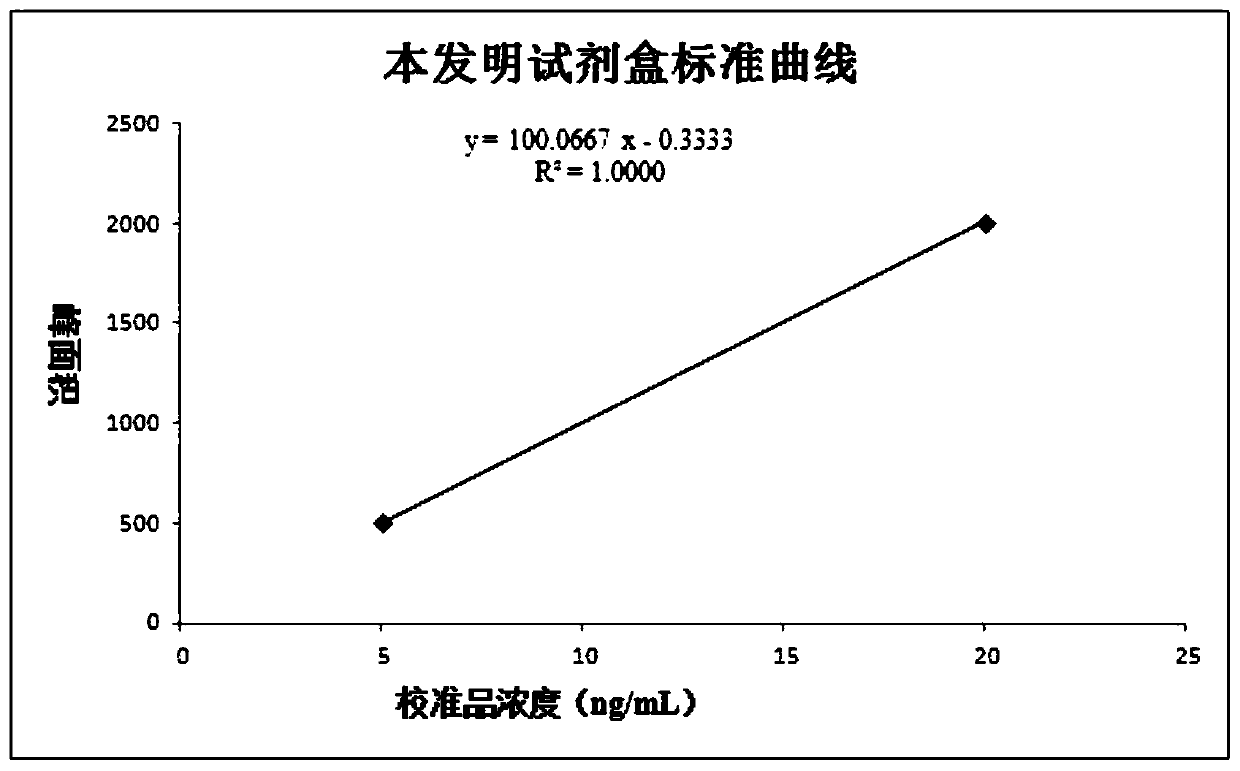
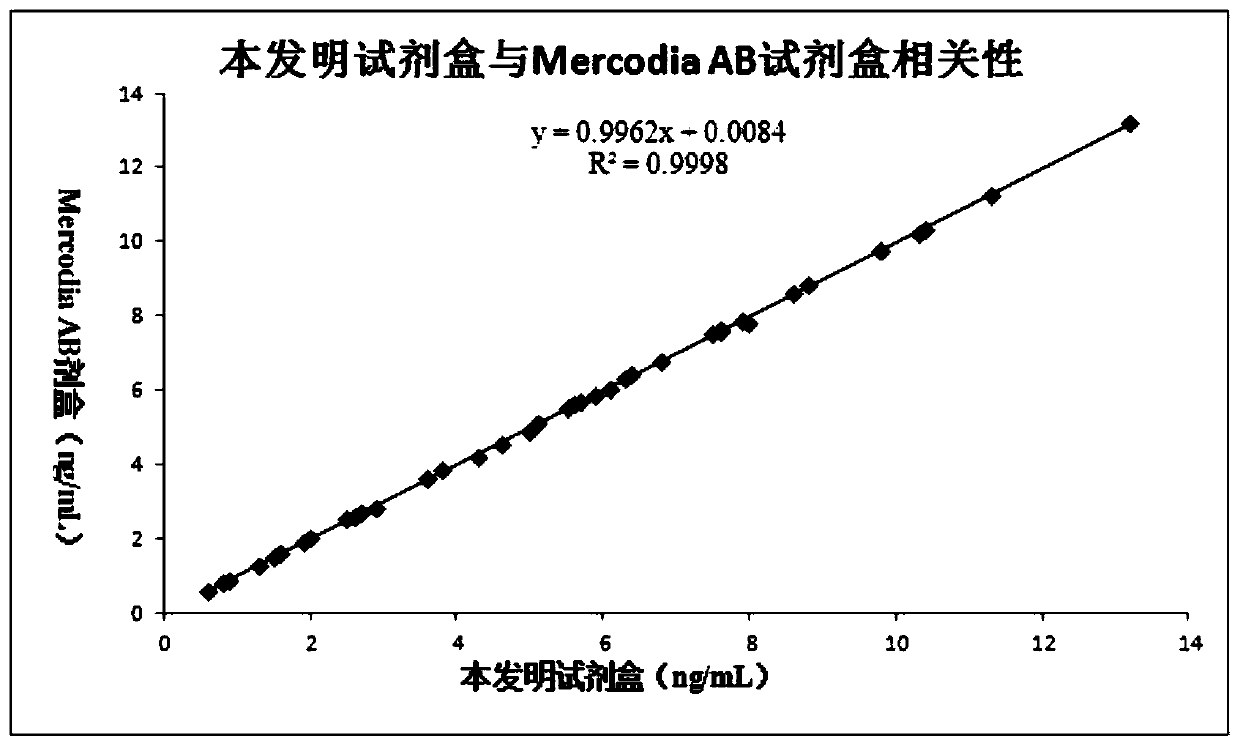
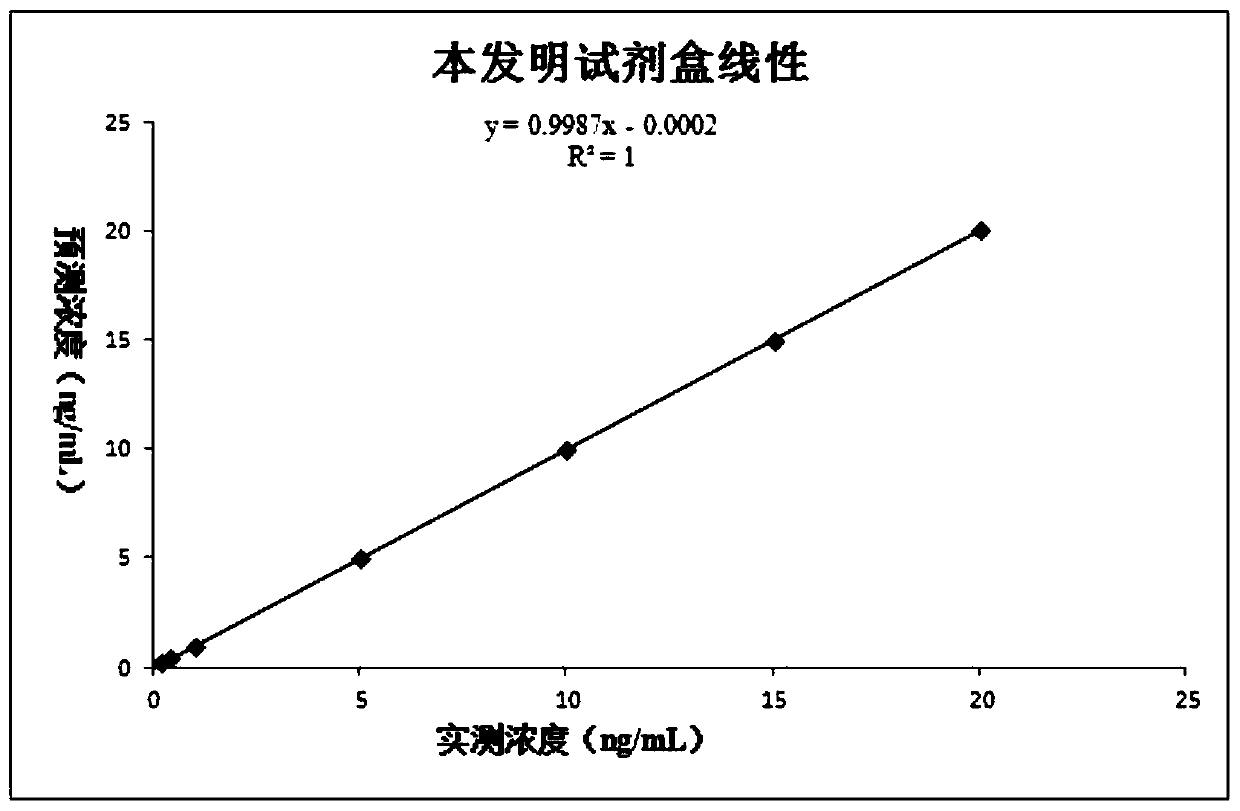
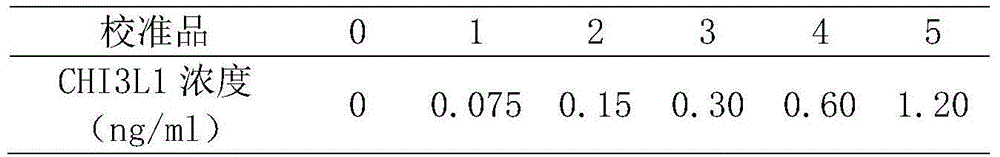
This invention provides a liver cirrhosis detection kit and detection method, and belongs to the biotechnology field. According to the detection method, anti-human chitinase 3 protein 1 (CHI3L1) antibody coats a microporous plate for preparation of a solid phase antibody, when in use, a to-be-tested sample is added into the microporous plate coated with the antibody, if the to-be-tested sample contains a tested matter, the to-be-tested sample can be combined on the solid phase antibody to form an antigen-antibody complex; then a biotin-labeled anti-human CHI3L1 antibody is added to form an antibody-antigen-biotin-labeled antibody complex; horse radish peroxidase (HRP)-labeled avidin is added to form an antibody-antigen-biotin-labeled antibody-enzyme labeled avidin complex; and finally the complex is added into 3, 3 ', 5, 5 '-tetramethyl benzidine substrate system for chromogenic reaction. After the chromogenic reaction is completed, an enzyme standard instrument is used or testing. The liver cirrhosis detection kit has the advantages of fast, simple and accurate determination of test results.
Owner:TIANYIKANG TIANJIN HOSPITAL MANAGEMENT CO LTD
Oncostatin m receptor antigen binding proteins
ActiveUS20160137739A1Amenable to commercial productionAmenable to useNervous disorderAntipyreticDiseaseAntigen binding
The invention provides anti-oncostatin M receptor-β (OSMR) antigen binding proteins. e.g., antibodies and functional fragments, derivatives, muteins, and variants thereof. OSMR antigen binding proteins interfere with binding of OSM and / or IL-31 to OSMR. In some embodiments, anti-OSMR antigen binding proteins are useful tools in studying diseases and disorders associated with OSMR and are particularly useful in methods of treating diseases and disorders associated with OSMR and binding of OSM and / or IL-31 to OSMR.
Owner:KINIKSA PHARMA LTD
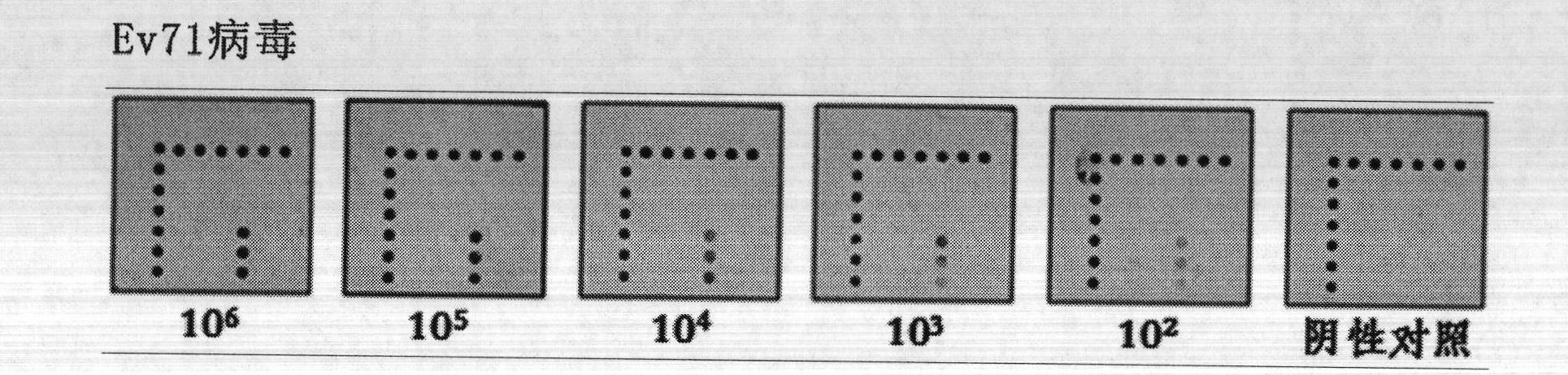
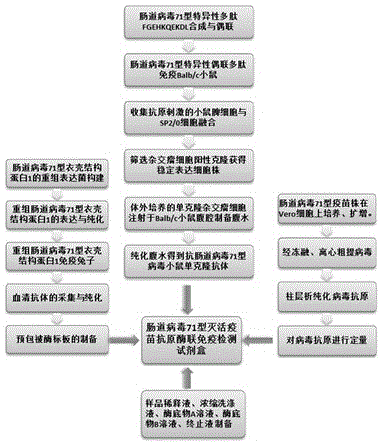
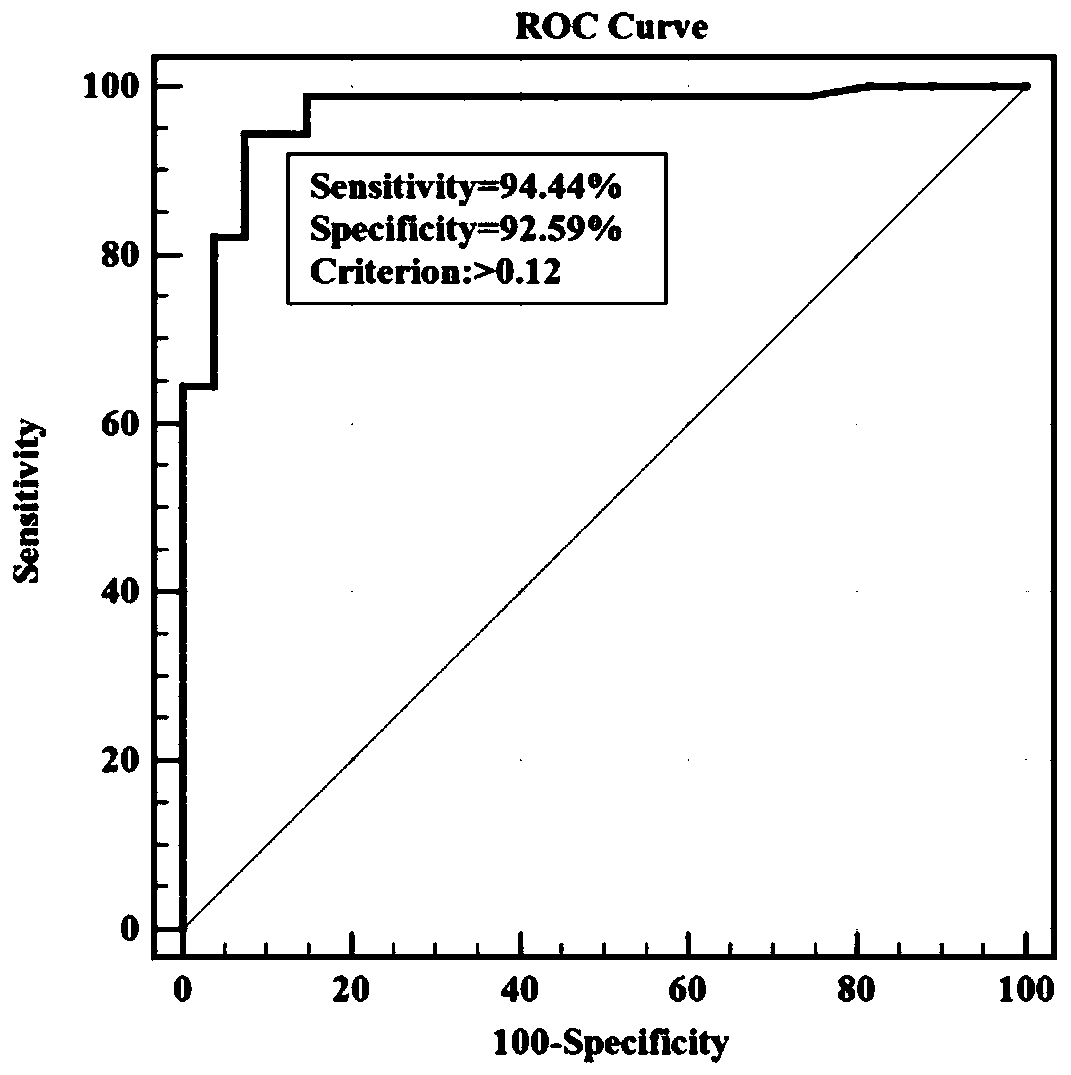
ELISA (enzyme-linked immunosorbent assay) kit for EV (enterovirus) 71 inactivated vaccine antigen
The invention relates to an ELISA (enzyme-linked immunosorbent assay) kit for an EV (enterovirus) 71 inactivated vaccine antigen. The detection kit comprises an EV71 pre-coated polyclonal antibody ELISA plate, a sample diluent, a second antibody, an enzyme-labeled antibody, a concentrated cleaning solution, an enzyme substrate solution and a stop buffer, wherein the ELISA plate is pre-coated with a polyclonal antibody prepared by taking recombinant EV71 structural protein 1 as an immune source; the second antibody adopts a monoclonal antibody prepared by taking keyhole limpet hemocyanin coupled polypeptide sequence FGEHKQEKDL as the immune source; the enzyme-labeled antibody adopts a horse radish peroxidase labelled goat anti-mouse immunoglobulin antibody; and an antibody standard is placed in the kit. The ELISA kit has better sensitivity when measuring the titer of the EV71 inactivated vaccine antigen, and has better linearity in the range of 5.9-750 ng / ml, and the linearly dependent coefficient r2 is larger than 0.99. The kit for measuring the titer of the EV71 inactivated vaccine antigen simply and conveniently has good specificity, accurate quantification, high sensitivity and good repeatability.
Owner:ZHEJIANG PUKANG BIOTECH
Indirect ELISA detection method for detecting porcine epidemic diarrhea virus antibody and kit thereof
PendingCN111381032APurification production process is simple and easyHigh expressionSsRNA viruses positive-senseVirus peptidesInclusion bodiesVirus Protein
The invention discloses an indirect ELISA detection method for detecting a porcine epidemic diarrhea virus antibody and a kit thereof. The recombinant porcine epidemic diarrhea virus COE protein is successfully expressed by cloning a gene for encoding the COE protein into a eukaryotic expression vector pPIC9K and then transforming the eukaryotic expression vector pPIC9K into a pichia pastoris GS115 competent cell for inducible expression. Compared with a prokaryotic expression inclusion body antigen, the purification production process of the COE protein is simple, convenient and feasible, andthe COE protein is high in expression quantity and purity and is closer to a virus protein natural structure; the COE protein is used as a coating antigen; the indirect ELISA detection method for detecting the porcine epidemic diarrhea virus antibody and the kit thereof are successfully constructed, the detection results of the method and the kit thereof are high in accuracy, strong in specificity, high in sensitivity and good in repeatability, and the kit needs a small amount of samples, is simple, convenient and rapid to operate and is low in cost; therefore, the method and the kit thereofhave a wide application prospect in detection of the porcine epidemic diarrhea virus antibody.
Owner:SOUTH CHINA AGRI UNIV
Vaccine composition as well as preparation method and application thereof
ActiveCN104258389AAntibacterial agentsBacterial antigen ingredientsMycoplasma synoviae antigenPathogen
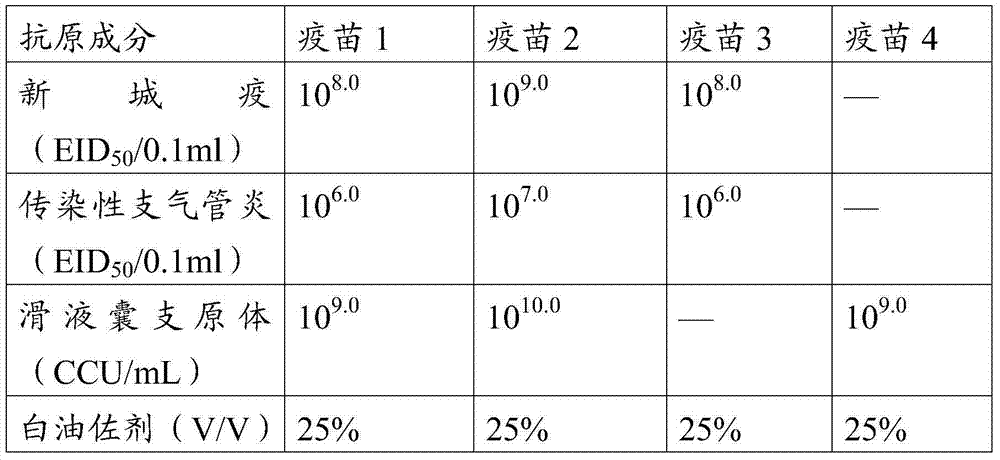
The invention provides a vaccine composition. The vaccine composition comprises an immune amount of an avian newcastle disease virus antigen, an avian infectious bronchitis virus antigen and an avian mycoplasma synoviae antigen and a carrier. The invention further provides a preparation method and application of the vaccine composition. The immune effect of the trigeminal vaccine is superior to the immune effect of combining newcastle disease-infectious bronchitis inactivated vaccines with mycoplasma synoviae inactivated single vaccine. The invention further provides a vaccine composition which contains a mycoplasma gallisepticum antigen in addition to the three antigens, and can be used for effectively resisting attack of four pathogens.
Owner:PU LIKE BIO ENG
Application method of tumor cell-derived exosome antigen in DC vaccine
PendingCN110604813AEfficient removalImprove the activation efficiency of source DCCancer antigen ingredientsAntineoplastic agentsAntigenMedicine
The invention discloses an application method of a tumor cell-derived exosome antigen in a DC vaccine. The invention discloses a preparation method of a tumor vaccine, which comprises the following step: sensitizing DC cells by using a tumor cell exosome to obtain the tumor vaccine. The tumor cell supernatant exosome provided by the invention has good stability, low toxicity and good cell compatibility, and is convenient to operate and apply and popularize in clinical tumor immunotherapy, and the method is a simple, feasible and practical application technology and method. The problems that inexisting DC vaccine construction, antigen sources are lacked, compatibility is poor, instability is caused, and an external DC antigen carrier has biotoxicity and is difficult to operate are solved.
Owner:SHENZHEN PEOPLES HOSPITAL
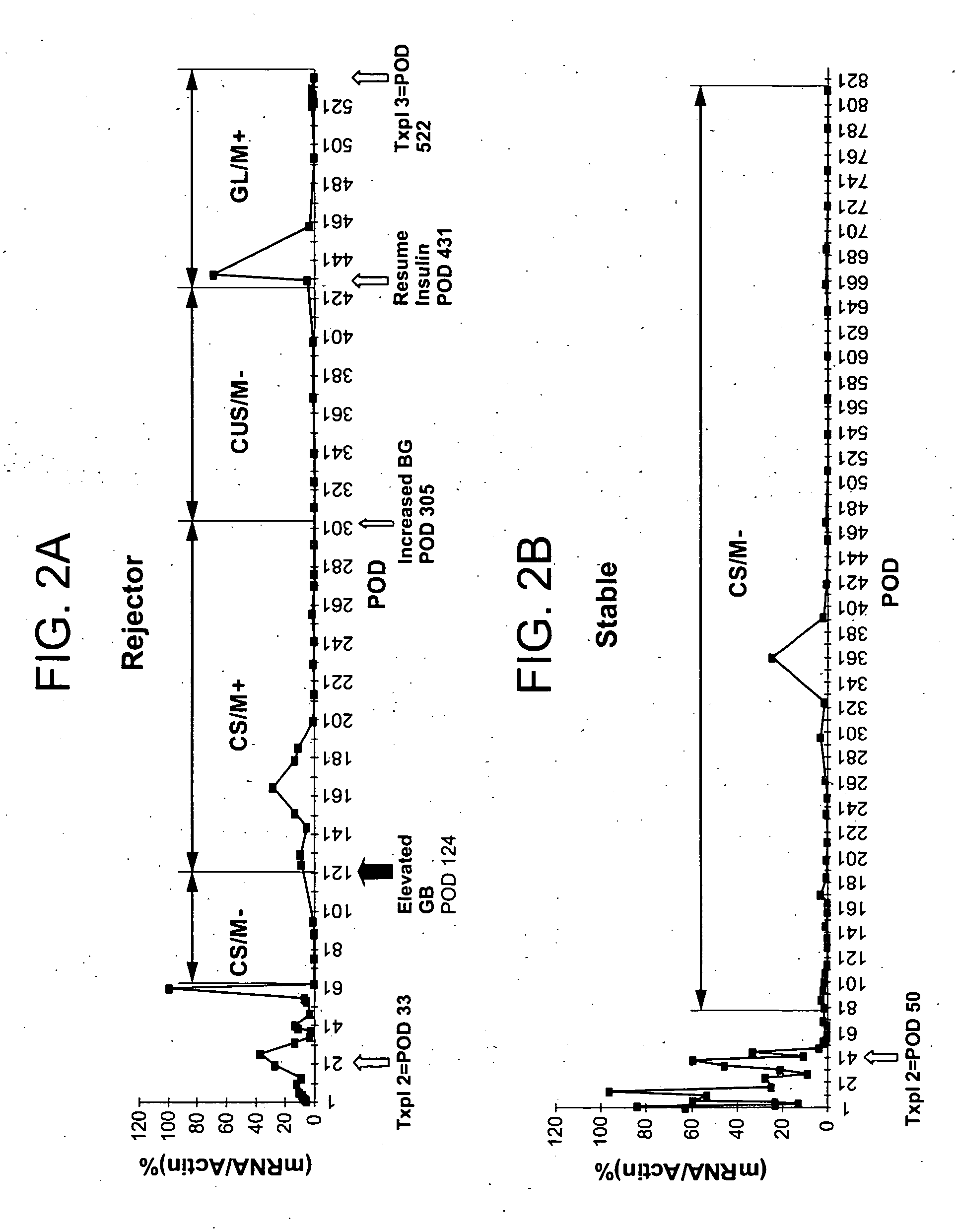
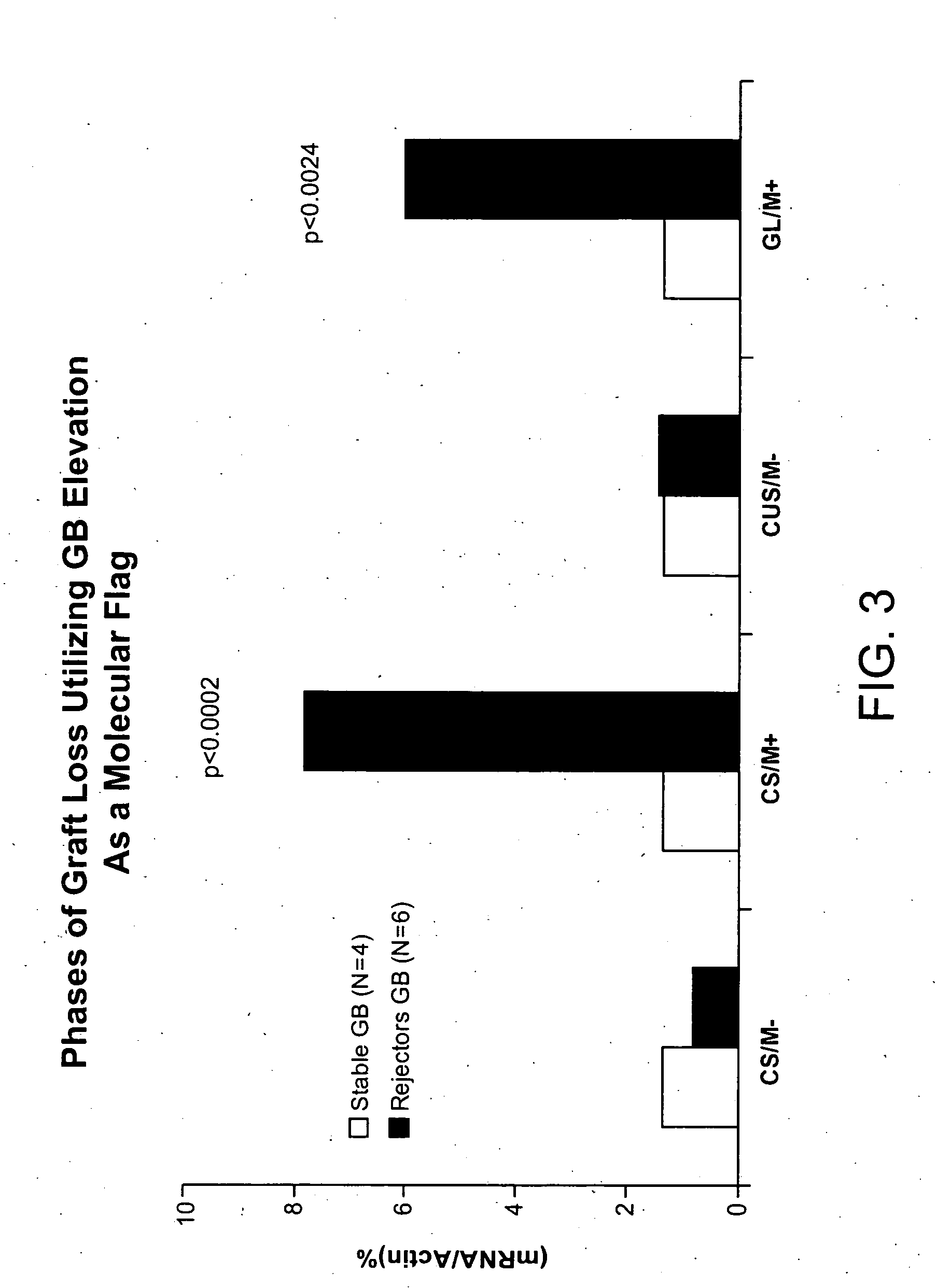
Molecular dissection of cellular responses to alloantigen or autoantigen in graft rejection and autoimmune disease
InactiveUS20060263343A1High expressionBiocideMicrobiological testing/measurementAutoimmune conditionAuto antigen
An antigen-specific T-cell response to alloantigen, tissue-specific antigen (e.g., islet antigen or other autoantigens involved in autoimmune disease), or self (or host) antigen is detected at an early stage of graft rejection or recurrent autoimmunity. An increase in cytotoxic lymphocyte gene (CLG) expression in peripheral blood is a risk factor for development of deleterious immune responses, which may be confirmed by functional assays. For example, the distinction between production of regulatory or inflammatory cytokines by T cells may dissect the type of immune response which is being induced: the survival of transplanted islet cells used to treat type 1 diabetes may be monitored, loss of the transplant by graft rejection (i.e., an alloantigen target) may be distinguished from autoimmune disease (i.e., a self or host antigen target).
Owner:BECKMAN COULTER INC +1
Kit of Mycobacterium tuberculosis and detection method thereof
InactiveCN102081091AEasy to synthesizeHigh OD valueMicrobiological testing/measurementColor/spectral properties measurementsBiotin-streptavidin complexSingle strand dna
The invention discloses a kit of Mycobacterium tuberculosis and a detection method thereof. The kit contains an enzyme-linked immunosorbent assay (ELISA) plate, a biotin-labeled single-stranded DNA adaptor, horseradish peroxidase-labeled streptavidin and a chromogenic substrate. In the detection method provided by the invention, the biotin-labeled single-stranded DNA adaptor of Mycobacterium tuberculosis H37Rv is used as a probe and a method which is similar to the indirect enzyme-linked immunosorbent assay (ELISA) method is adopted to detect Mycobacterium tuberculosis; and Mycobacterium tuberculosis or sputum to be detected or a body fluid sample, and a control sample are added in the ELISA plate for incubation, Mycobacterium tuberculosis combined with the plate is trapped by the biotin-labeled single-stranded DNA adaptor and then washed, the horseradish peroxidase-labeled streptavidin is added, the substrate is added for developing, and the optical density (OD) value is detected by a microplate reader after developing when the absorption wavelengh is 450nm. When the kit provided by the invention detects Mycobacterium tuberculosis or the Mycobacterium tuberculosis or somatic antigen in sputum, blood and body fluid, the detection speed is high, the detection is convenient and safe, the detection time can be saved, the efficiency is high, the cost is low, the detection sensitivity and accuracy are high, and the application prospect is wide.
Owner:WUHAN UNIV
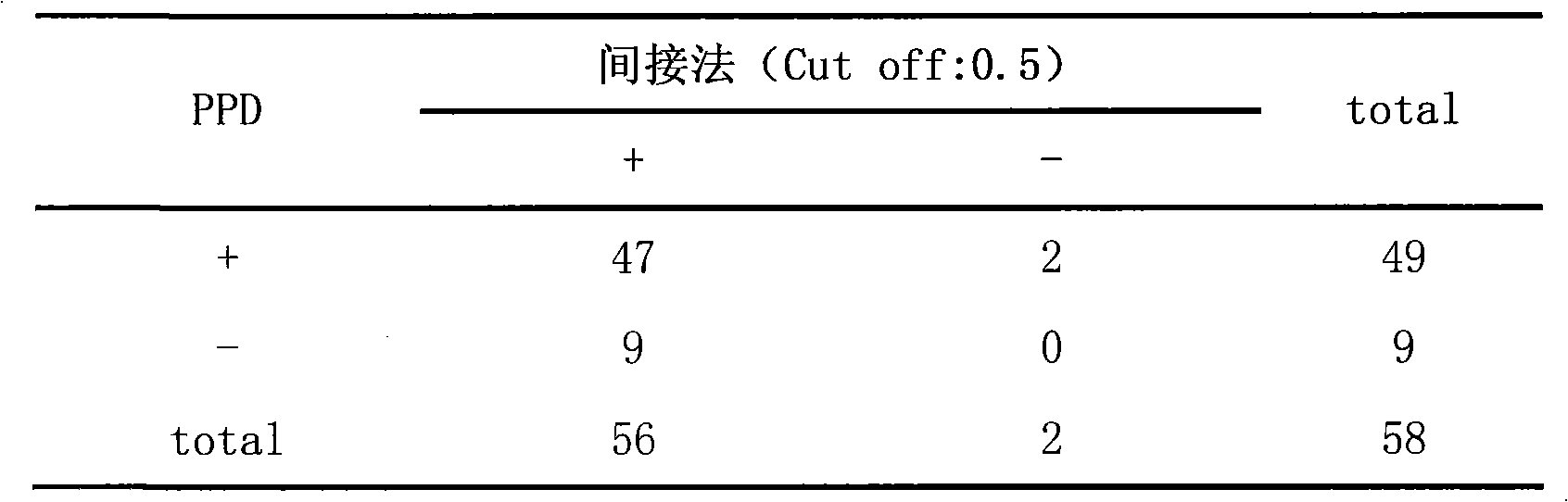
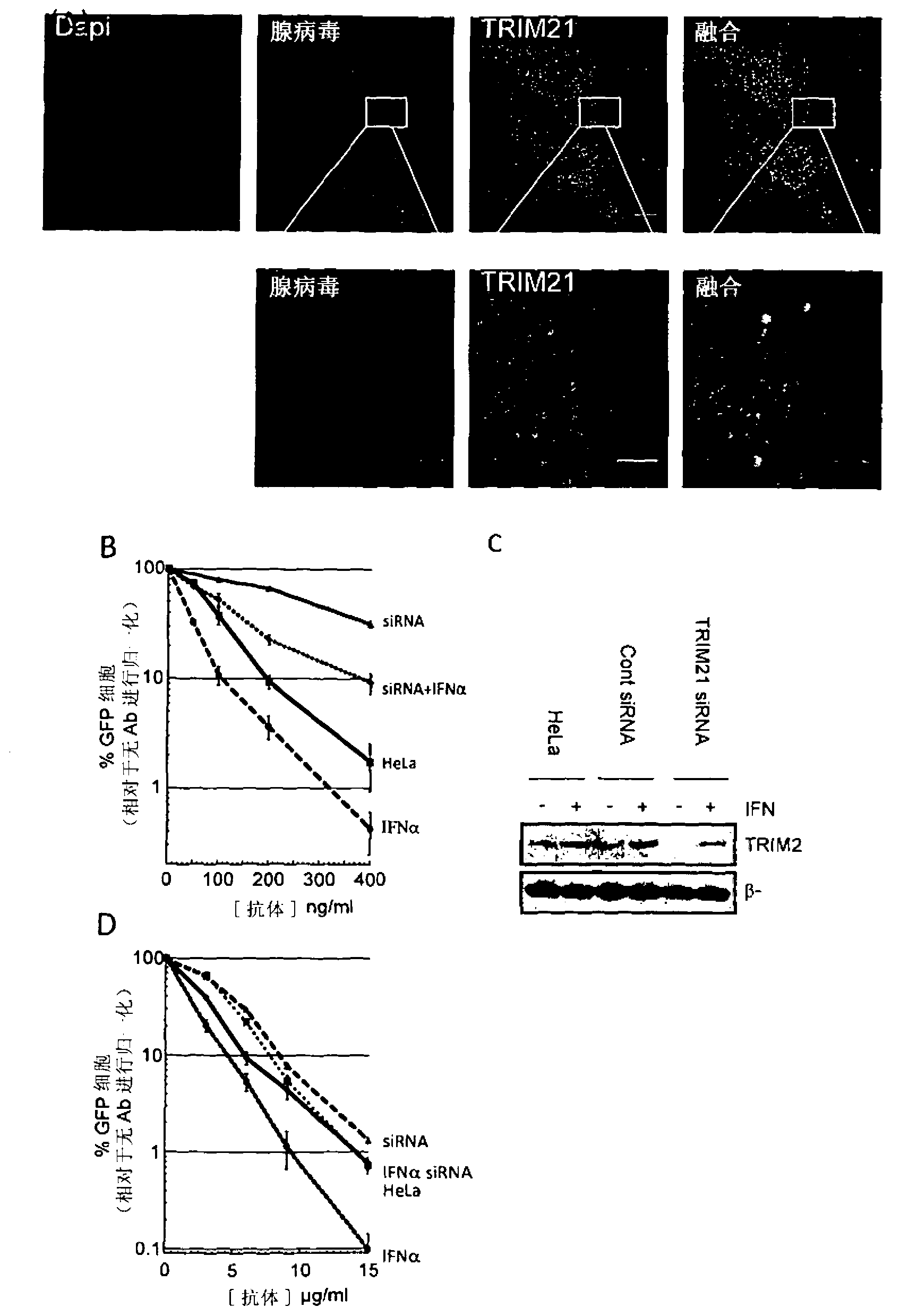
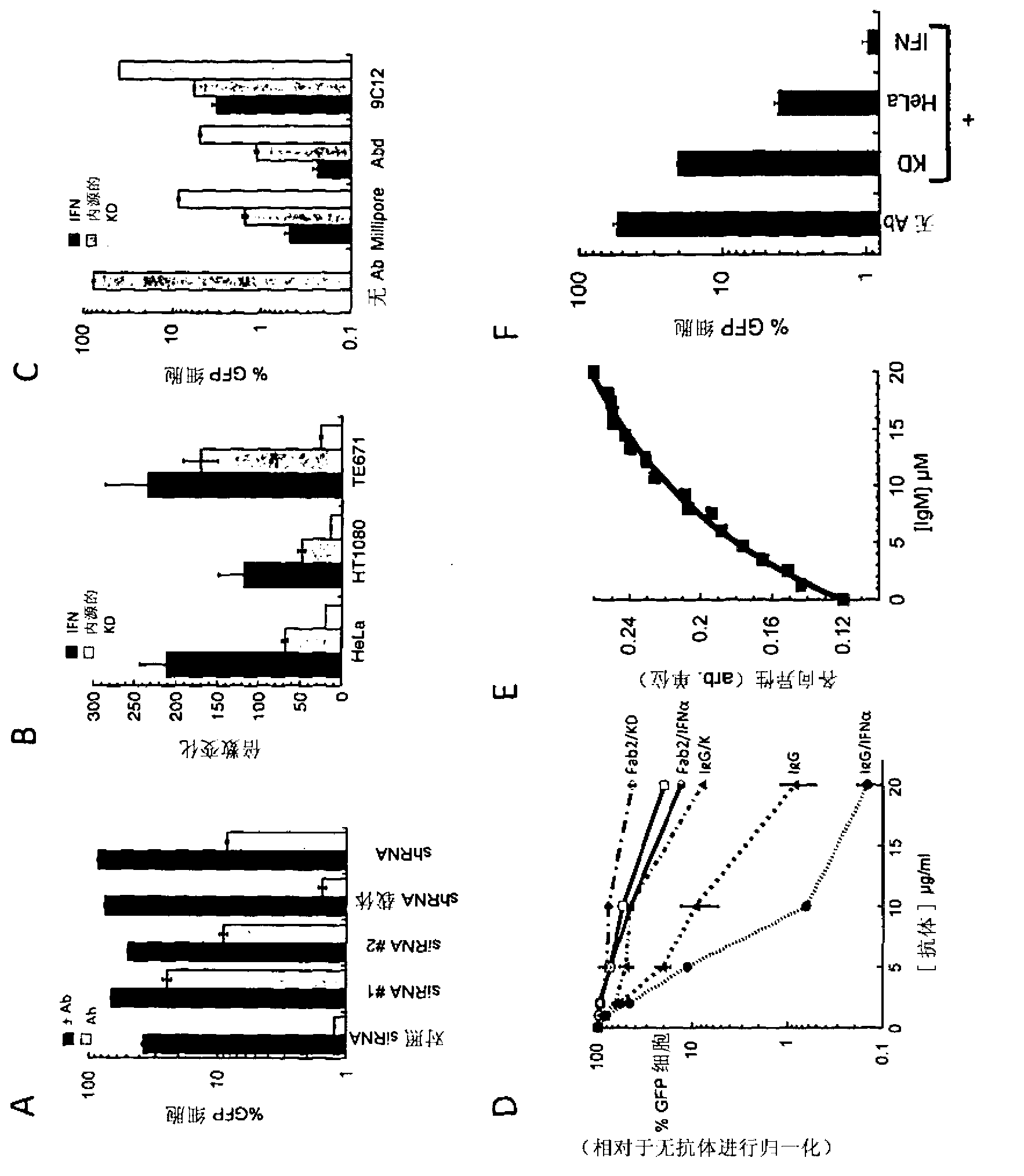
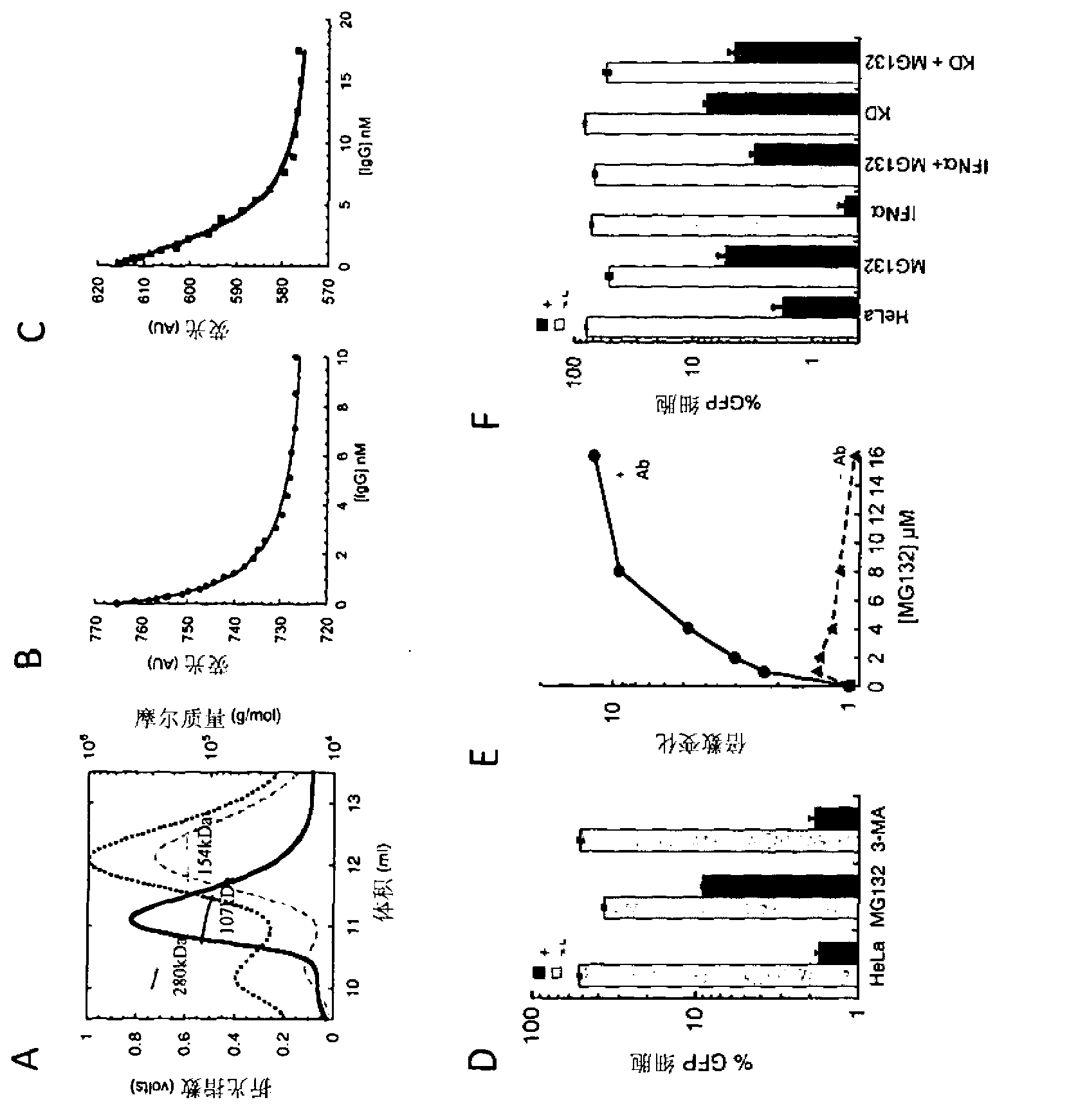
Intracellular immunity
Owner:MEDICAL RESEARCH COUNCIL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com