Anti P2X7 receptor antibodies and fragments thereof
a technology of p2x7 receptor and antibody, applied in the field of purinergic receptors, can solve the problem of difficult to obtain serological reagents that bind to non-functional p2xsub>7, and achieve the effect of increasing the affinity for p2x7 receptor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identifying Dab Leads for Binding to Non Functional Receptors on Live Cells
Objective:
[0260]The experiments described here have been to find antigen binding sites that bind the E200 peptide.
Background:
[0261]Antisera that bind P2X7 have low affinity for P2X7 as expressed on live cancer cells since the conformation of the epitope target on live cancer cells differs. To identify dAb leads for high affinity binders, we first needed to identify a suitable target, knowing that good sequence diversity of binders is required in order to widen the screening of conformational space to encompass suitable lead compounds. We selected the E200 peptide as a suitable target to identify dAb leads.
Materials and Methods:
[0262]The E200 peptide was made by solid phase synthesis at Chiron Mimotopes. A range of conjugates were synthesized to identify those most likely to be useful for screening purposes. These included protein conjugates BSA, DT and ovalbumin linked to the C-terminal Cys reside on E200 pep...
example 2
Determining Activity of dAb Leads in dAb-Fc Format
Objective:
[0273]The experiments described here have been to improve affinity of antigen binding sites that bind the E200 peptide through formatting lead dabs as dAb-Fc.
Background:
[0274]Co-operative binding of the lead dabs was achieved by producing standard format dAb-Fc with human type IgG1 Fc subtype. These formats enabled more considered screening of the lead dAb clones by enabling the elimination of high affinity lead dabs for which formatting as dAb-Fc provided little benefit due to solubility issues. Favourable conformational solutions would then be selected for additional rounds of screening.
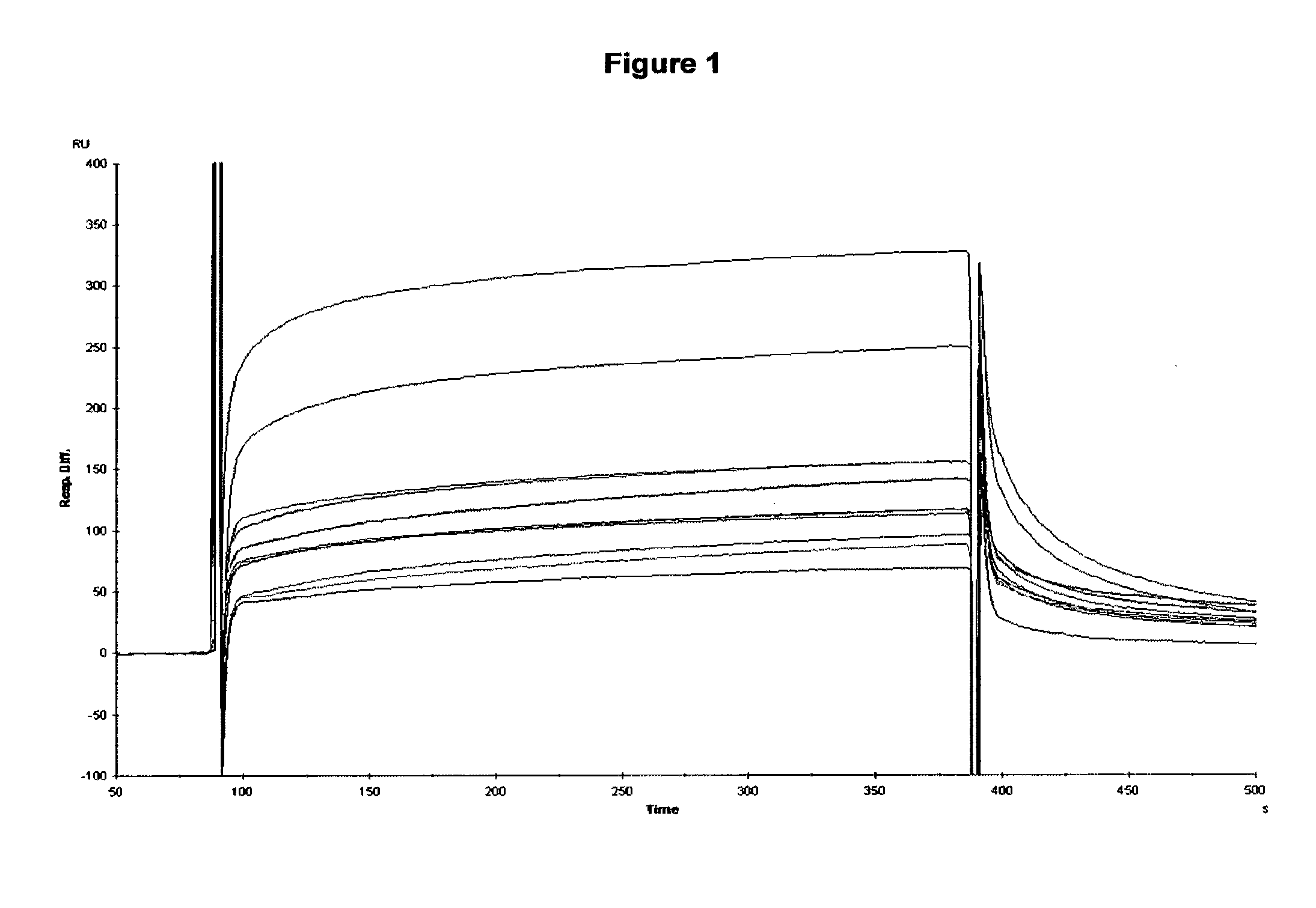
[0275]Results: The first formatted dabs PEP2-4 and PEP2-5 that had been chosen as high affinity leads from Example 1 showed little additional binding to the E200 peptide whereas PEP2-2 and others (2-47, 2-42) benefited with a typical improvement in KD of 100-1000 times. Formatting of the various leads resulted in good expression as reveale...
example 3
Determination of a Conformational Epitope for Screening dAb Leads Against
Objective:
[0277]The experiments described here have been to determine an appropriate conformational epitope for finding dAbs that bind the E200 peptide and also bind a conformational epitope.
Background:
[0278]The high affinity binders are to bind to a non functional P2X7 receptor extra cellular domain. The sequence of P2X7 is shown in SEQ ID NO:1. There are a number of possible constructs that could be developed but we had to determine which of these would model the conformational epitopes as observed on a live cancer cell. We particularly needed a target that could be bound to a solid phase for later affinity maturation experiments.
[0279]We started with ECD1 that has the structure 47-332 because this constitutes all the amino acids forming the extracellular domain between the transmembrane domains TM1 and TM2 including the putative intramembraneous segment at the C-terminus of the segment from 325-332. By inclu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com