Patents
Literature
90 results about "Auto antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Autoantigen [ô′tō·an′tijin] an endogenous body constituent that stimulates the production of autoantibodies and an autoimmune reaction.
Methods and compositions for expanding T regulatory cells
InactiveUS20070172947A1Peptide/protein ingredientsAntibody mimetics/scaffoldsType 1 diabetesStem cell
The present invention provides methods and compositions for expanding Treg cells ex vivo or in vivo using one or more conjugates comprising a costimulatory moiety that stimulates at least one of three signals involved in Treg cell development and / or using dendritic cells pulsed with antigens and modified to display TGF-β, or hematopoetic stem cells or bone marrow cells modified to display TGF-β. The methods and compositions are useful, for example, in the treatment and prevention of autoimmune disease, including Type 1 diabetes and in preventing foreign graft rejection, as well as to establish mixed chimerism, induce tolerance to autoantigens, alloantigens or xenoantigens, beta cell regeneration, prevention of foreign graft rejection, and treatment of a genetically inherited hematopoietic disorder.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Human immune therapies using a cd27 agonist alone or in combination with other immune modulators
InactiveUS20130336976A1Promotes strong expression of 4-1BBImprove responseAntibacterial agentsAntimycoticsIMMUNE STIMULANTSCD8
Methods of inducing T cell proliferation and expansion in vivo for treating conditions wherein antigen-specific T cell immune response are therapeutically desirable such as cancer, infection, inflammation, allergy and autoimmunity and for enhancing the efficacy of vaccines are provided. These methods comprise the administration of at least one CD27 agonist, preferably an agonistic CD27 antibody, alone or in association with another moiety such as immune stimulant or immune modulator such as an anti-CD40, OX-40, 4-1BB, or CTLA-4 antibody or an agent that depletes regulatory cells, or a cytokine. These mono and combination therapies may also optionally include the administration of a desired antigen such as a tumor antigen, an allergen, an autoantigen, or an antigen specific to an infectious agent or pathogen against which a T cell response (often CD8+) is desirably elicited.
Owner:UNIV OF SOUTHAMPTON
Immunogenic peptides and their use in immune disorders
ActiveUS20100303866A1High expressionNervous disorderPeptide/protein ingredientsAuto antigenAutoimmune disease
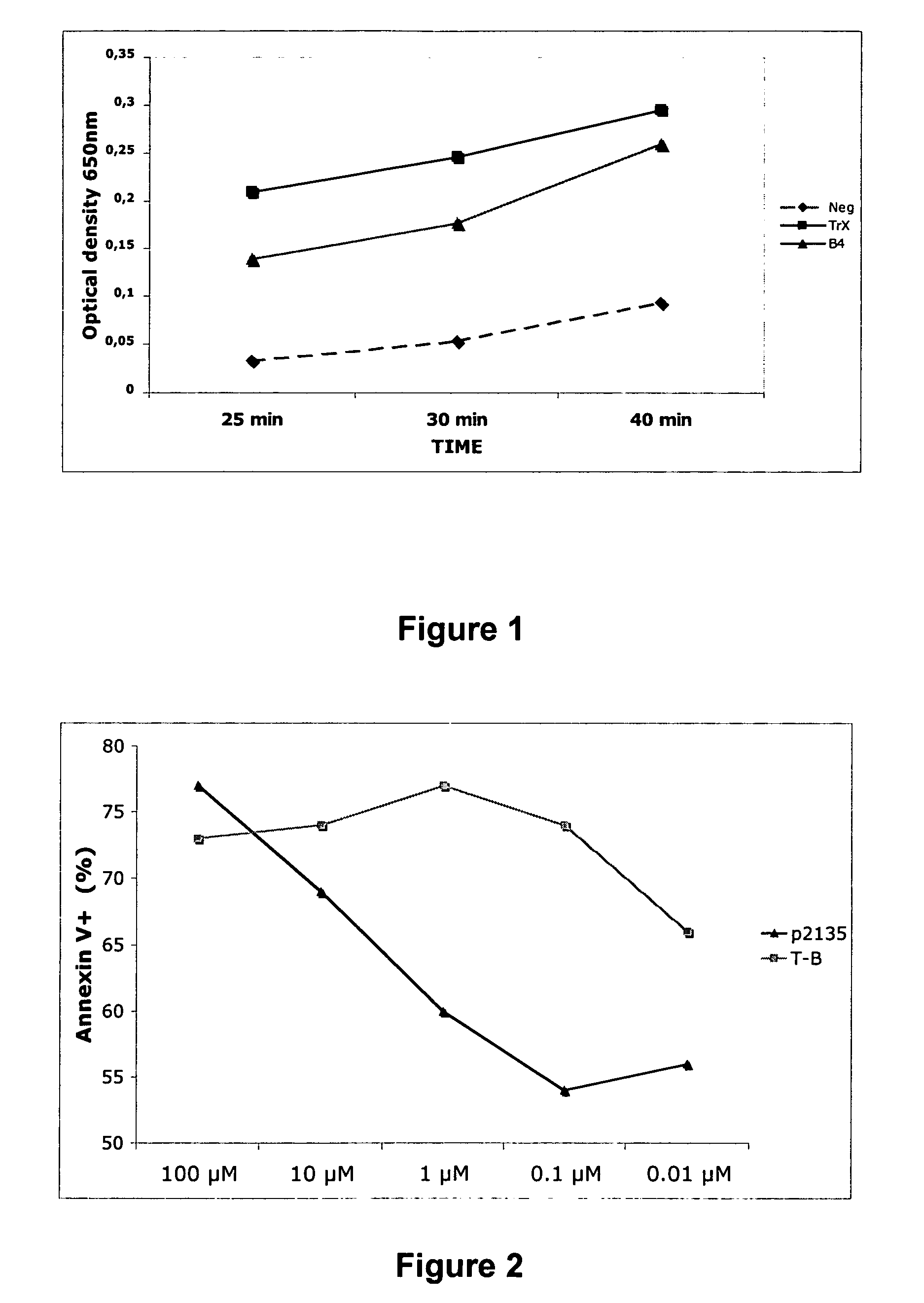
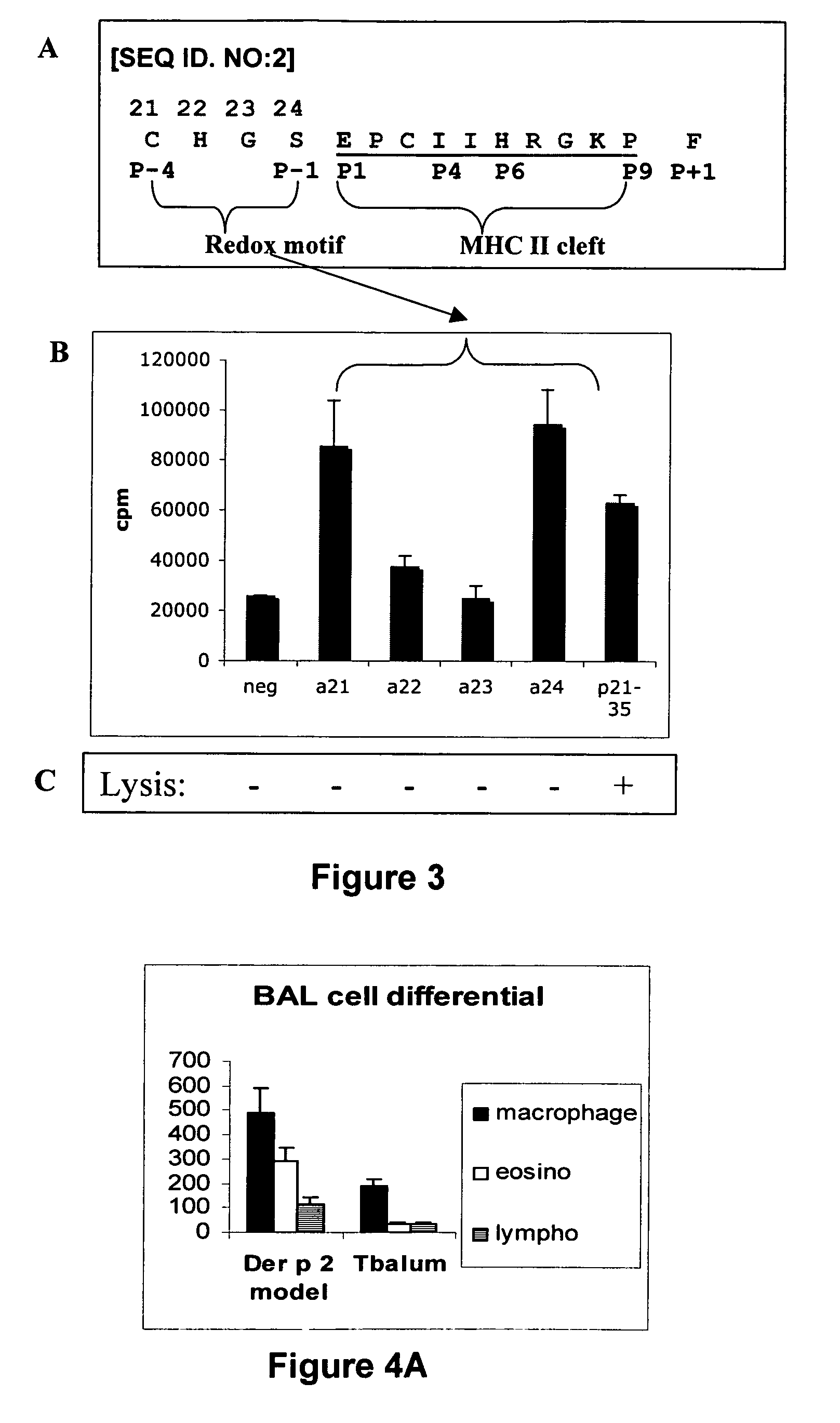
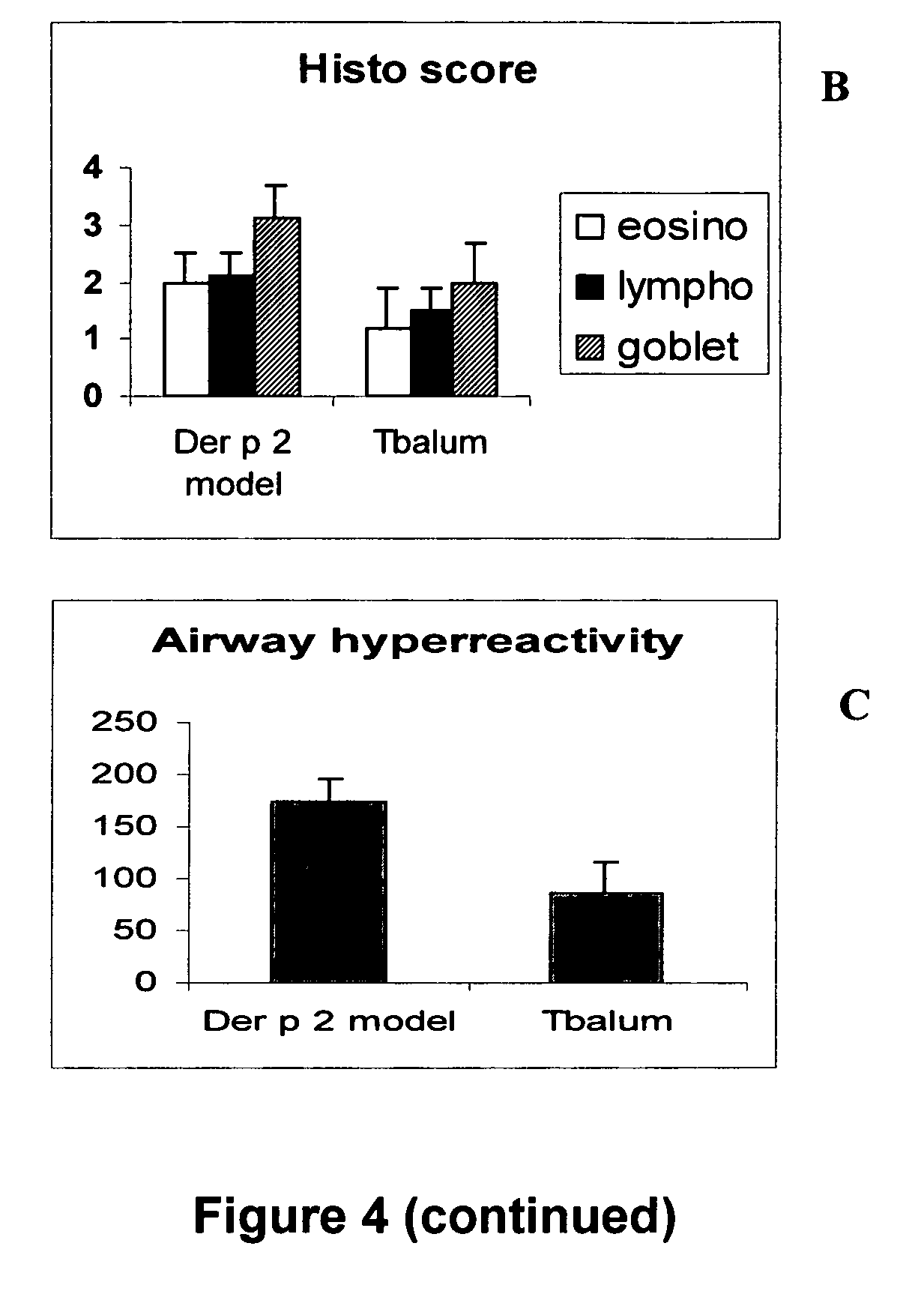
The present invention provides novel peptides and homologues thereof. The peptides of the invention comprise (i) a T-cell epitope of an antigen (self or non-self) with a potential to trigger an immune reaction presented by a class II major histocompatibility complex (MHC) determinant and recognised by CD4+ T cell more specifically of an allergen or auto-antigen, coupled, optionally through the use of a linker to (ii) an amino acid sequence having a reducing activity, such as a thioreductase sequence. The peptides of the invention have been shown to be useful a medicine, more in particular for the prevention or treatment of immune disorders, more specifically of allergic disorders or autoimmune diseases. The present invention thus provides for the use of said peptides for the manufacture of a medicament for the prevention or treatment of an immune disorder and further provides for methods of treatment or preventing immune disorders by using said peptides. The present invention also provides for compositions comprising said peptides.
Owner:IMCYSE
Viral display vehicles for treating multiple sclerosis
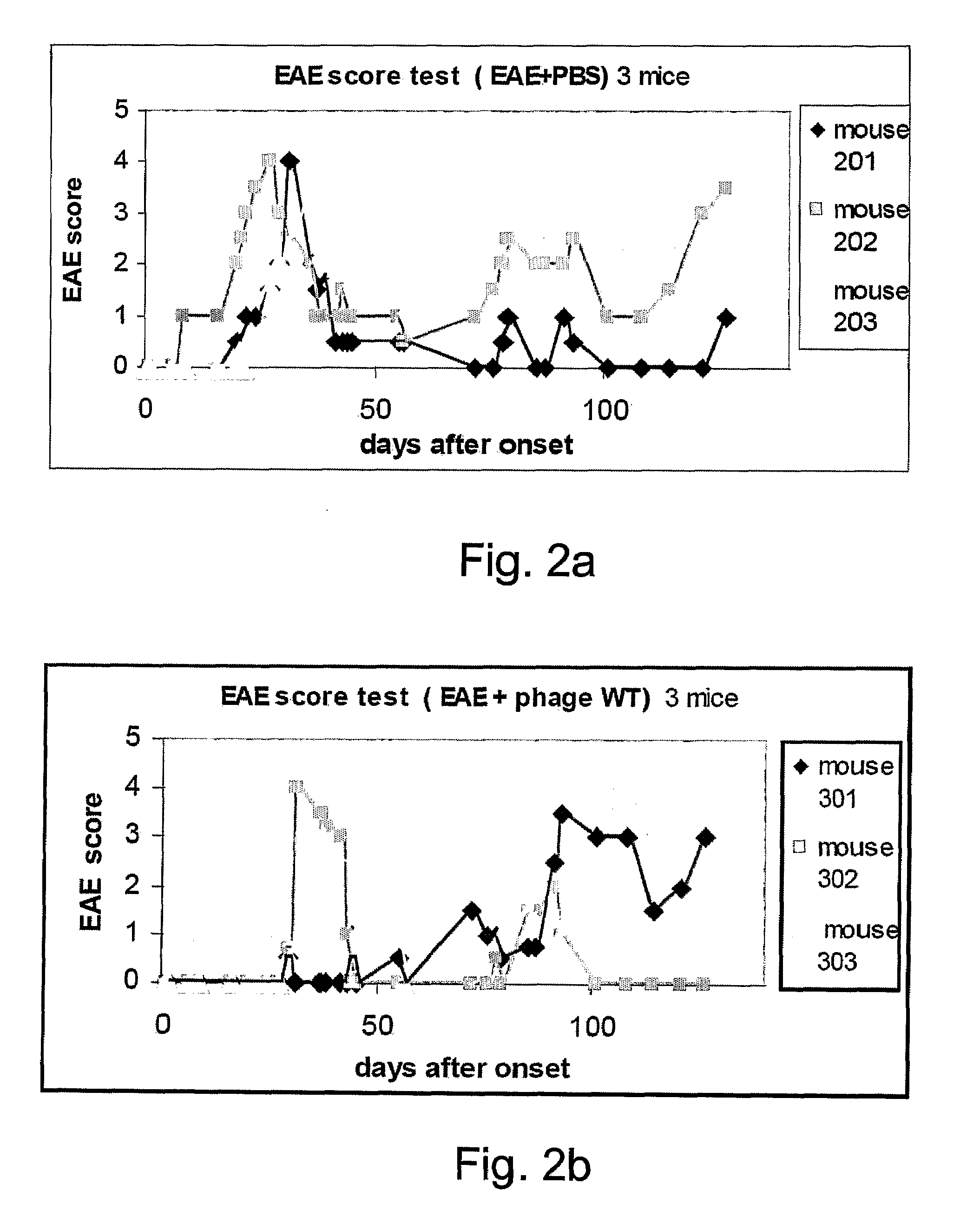
Provided are viral display vehicles which display multiple sclerosis associated antigens on the surface thereof for induction of immune tolerance to autoantigens such as MOG. Also provided are methods and pharmaceutical compositions for treating multiple sclerosis using the viral display vehicles of the present invention.
Owner:RAMOT AT TEL AVIV UNIV LTD
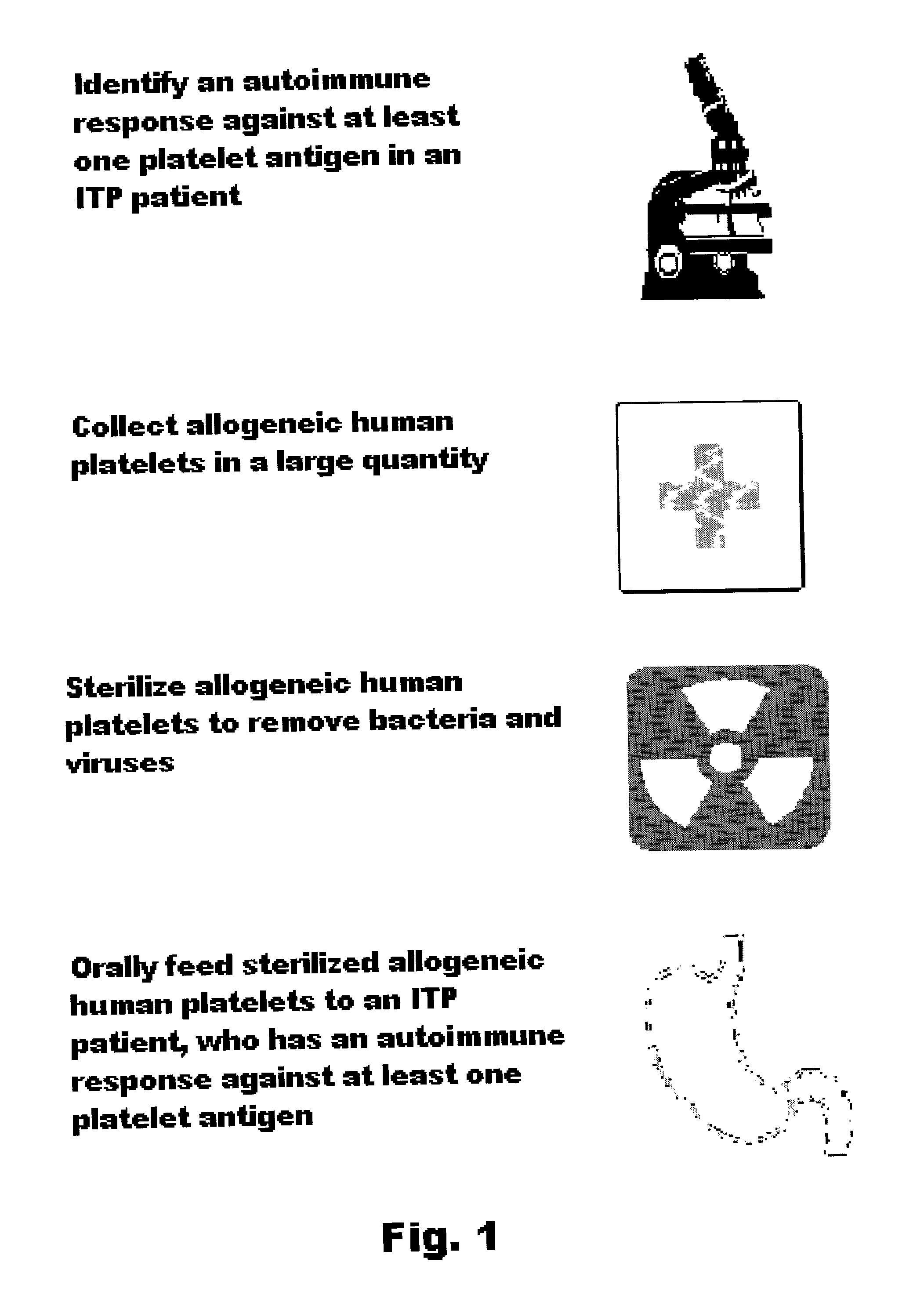
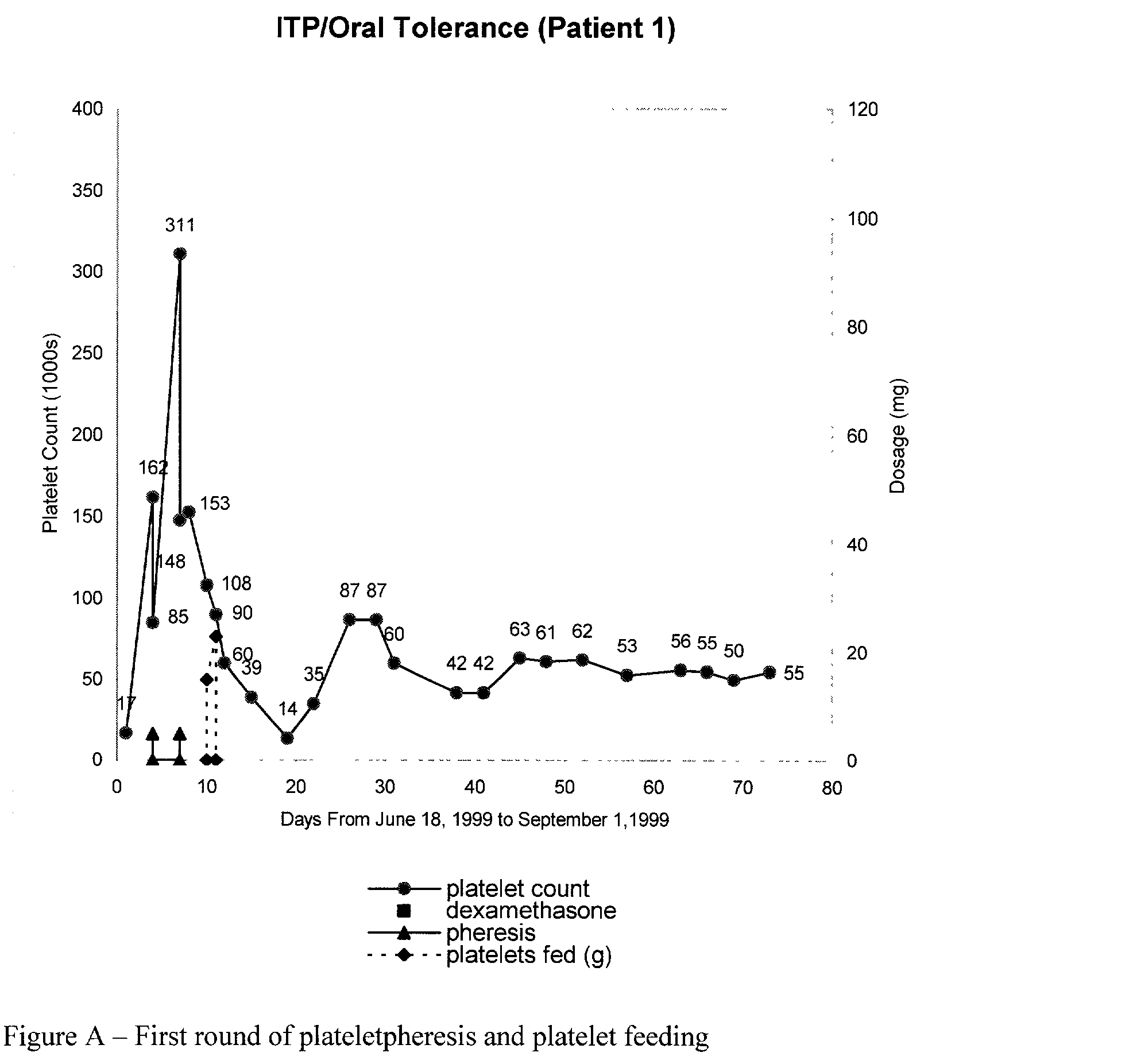
Oral tolerance using allogeneic platelets in ITP
ITP may be treated using a method that involves identifying the autoimmune response, collecting allogeneic platelets, sterilizing allogeneic platelets and feeding them orally. Auto-antigens contained on the surface of allogeneic platelets administered to the intestines deactivate or delete lymphocytes responsible for auto-antibody production. This treatment can be used for ITP, specifically targets the cause of the disease and provides the possibility of a sustained response without further medication.
Owner:KASHA JOHN R JR
Specific Inhibition of Autoimmunity and Diseases Associated With Autoantigens
InactiveUS20090053249A1Enhance cell viabilityProlong survival timeOrganic active ingredientsNervous disorderAntigenCellular component
Owner:ISOGENIS INC
Autoantigenic fragments, methods and assays
InactiveUS6855515B1Reduce the impactRelieve symptomsDisease diagnosisAntibody medical ingredientsAntigenAssay
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
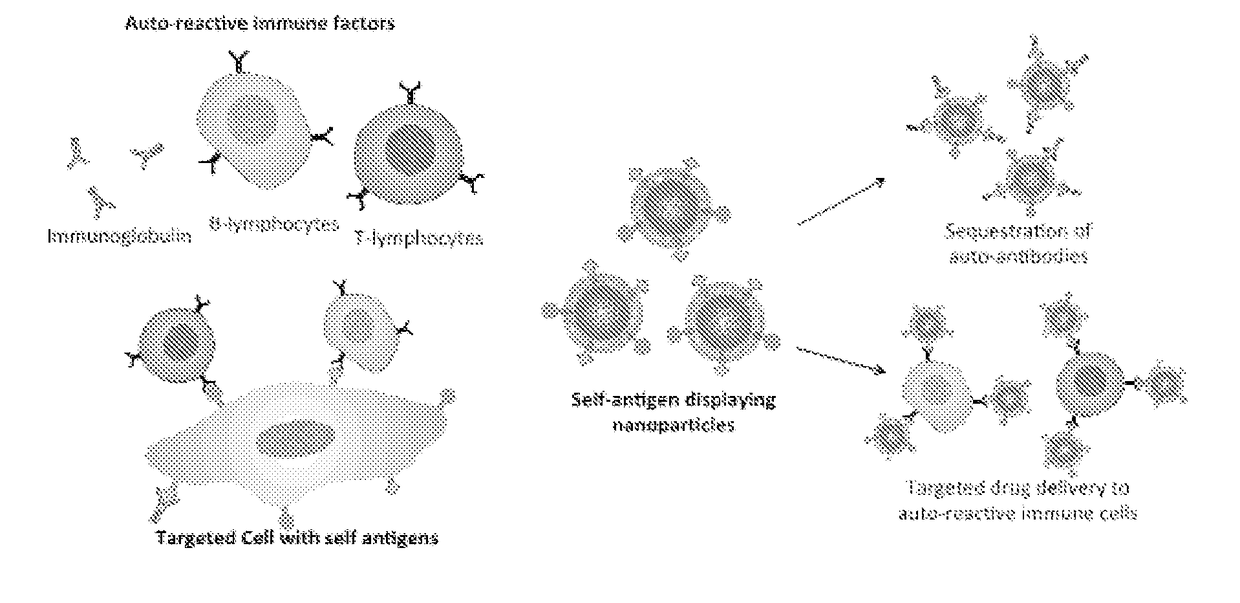
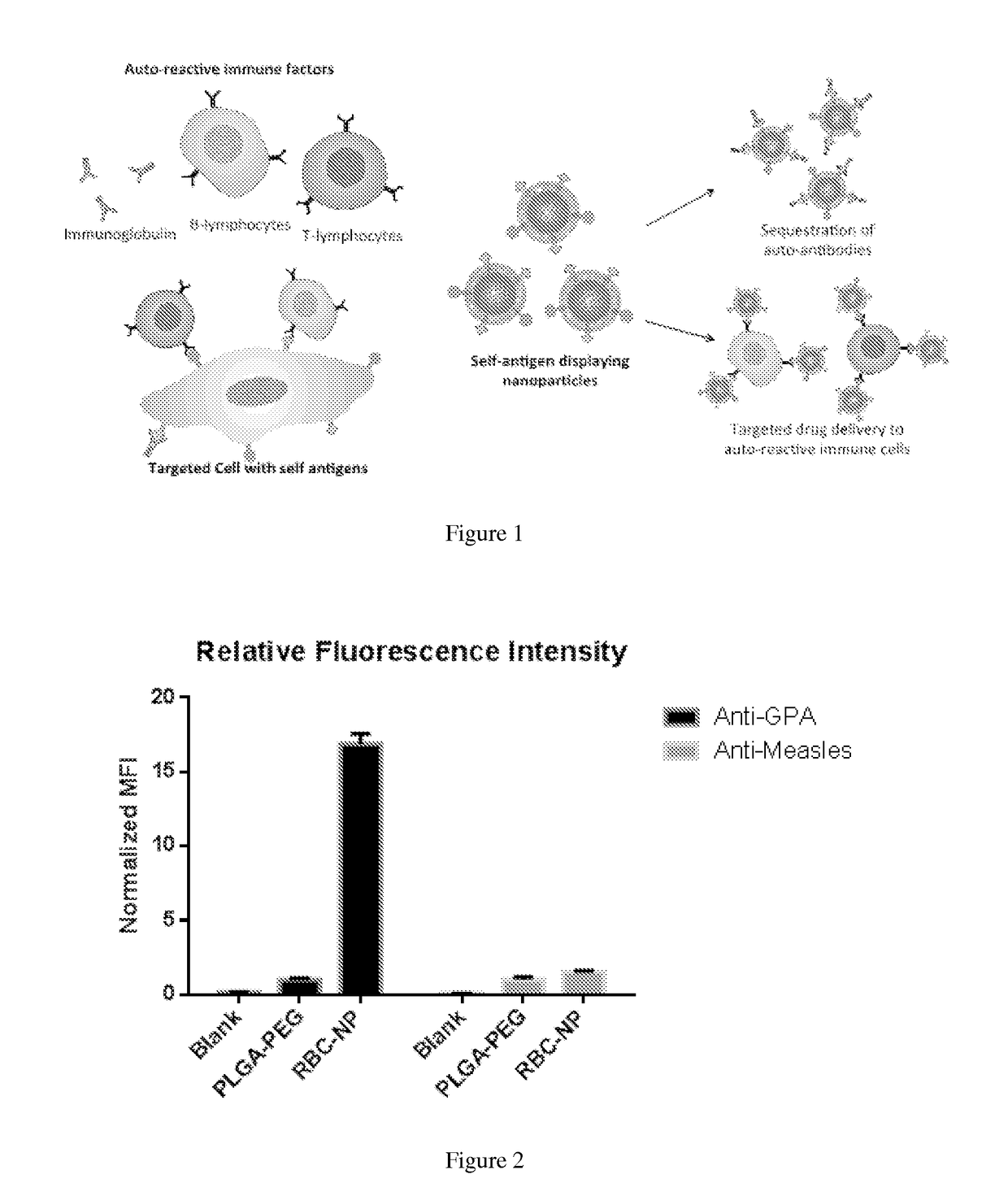
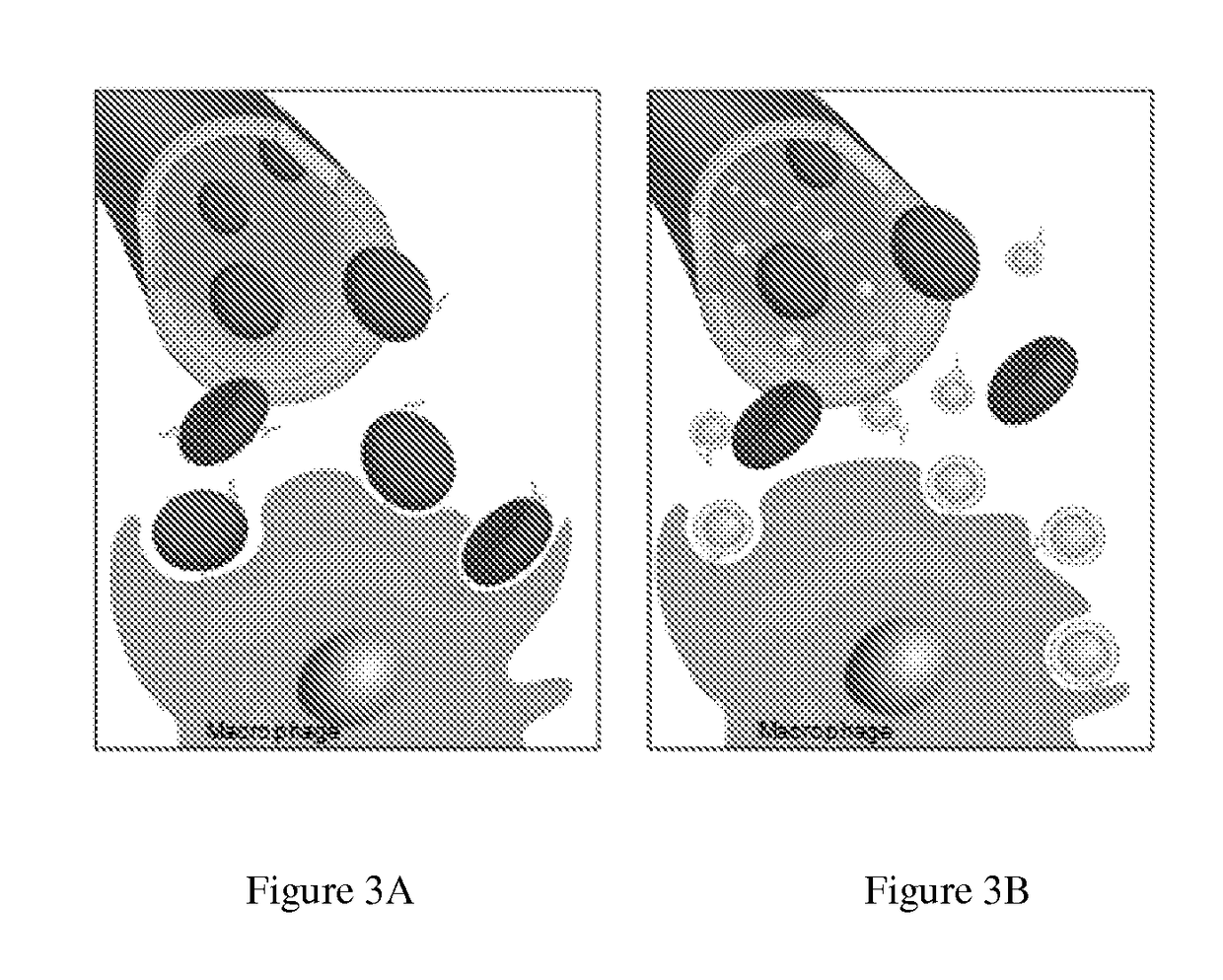
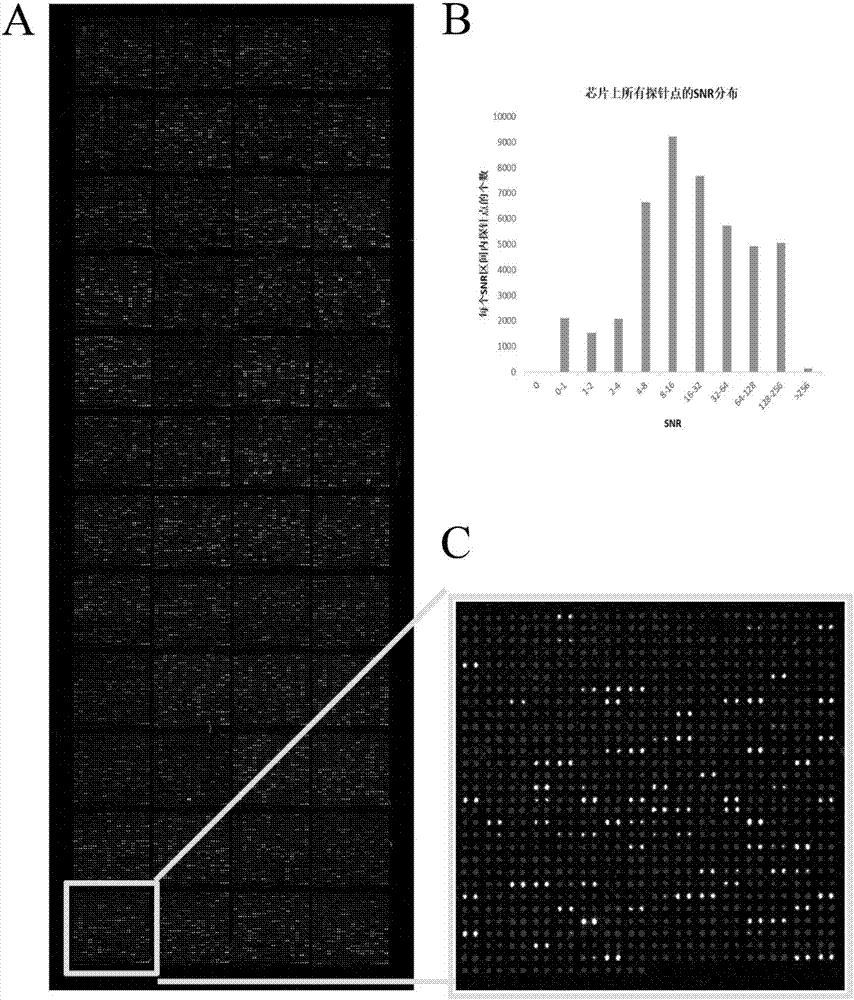
Self-Antigen Displaying Nanoparticles Targeting Auto-Reactive Immune Factors and Uses Thereof
PendingUS20170274059A1Normalize the immune regulationRelieve symptomsMammal material medical ingredientsCarrier-bound antigen/hapten ingredientsNanoparticleCytotoxic drug
The invention provides a composition, and method of use thereof, comprising self-antigen displaying nanoparticles to target auto-reactive immune components for treating and / or preventing the autoimmune diseases associated therewith. The nanoparticles can also be loaded with cytotoxic drugs for targeted cell killing or with immune-tolerizing compounds to normalize the immune regulation.
Owner:RGT UNIV OF CALIFORNIA
ACPA-negative RA diagnosis marker and application thereof
ActiveCN106950365AHigh sensitivityImprove featuresDisease diagnosisBiological testingDiseaseAuto antigen
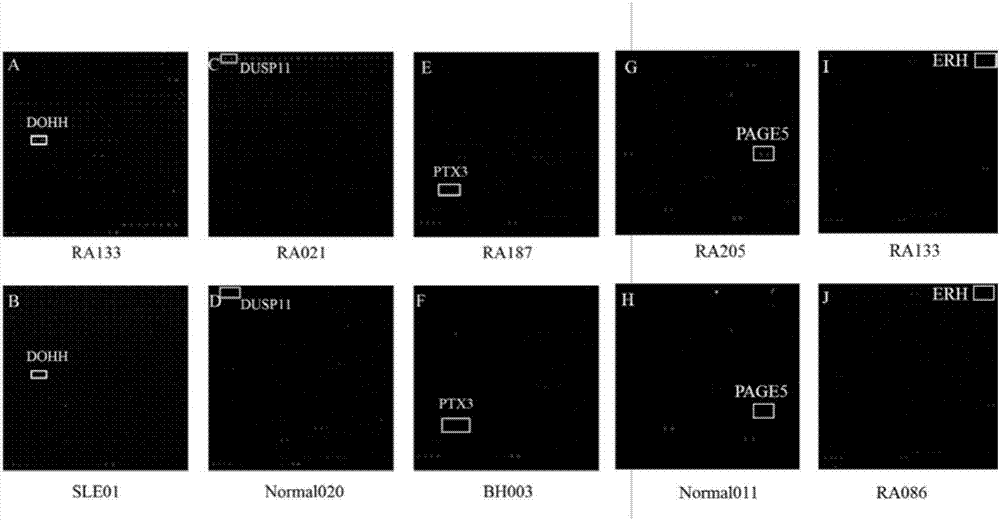
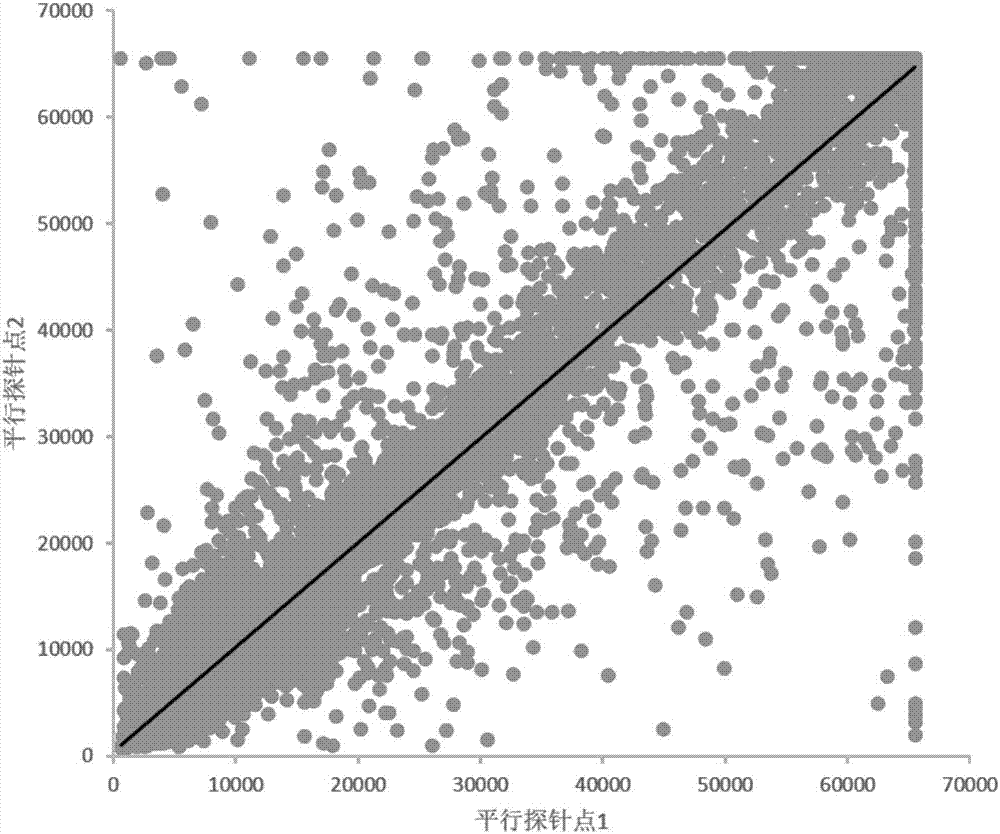
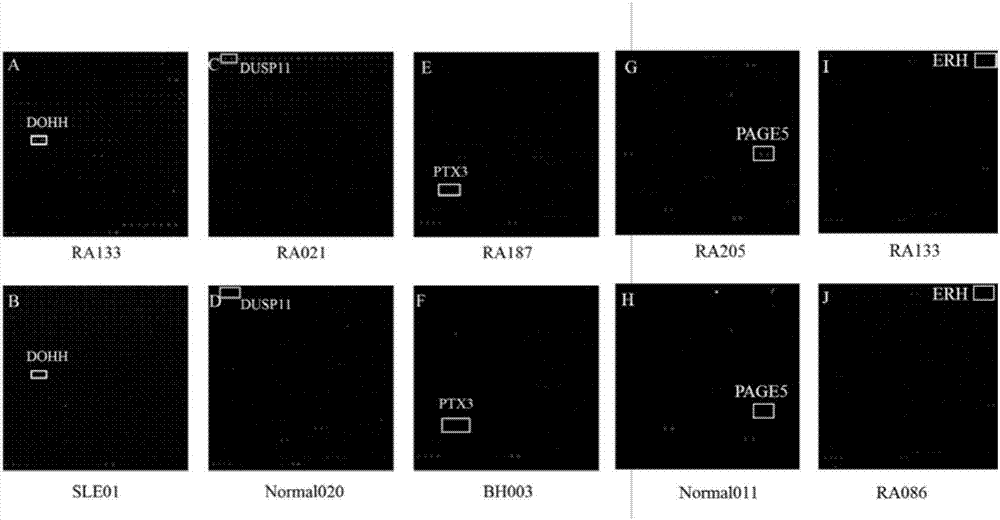
The invention provides applications of deoxyhypusine dioxygenase, namely, DOHH, or a fragment thereof in preparation of a reagent for diagnosis of anti-citrullinated protein antibody negative (ACPA-negative) rheumatoid arthritis (RA). In the method, a high-density protein chip is hybridized with RA serum to screen 35 proteins, which are candidate auto-antigens of the ACPA-negative RA. It is identified that four protein antigens, DOHH, DUSP11, PTX3 and PAGE5, have high sensitivity and specificity in ACPA-negative serum of the RA, wherein the four protein antigens are all novel auto-antigens. The method shows that the DOHH, DUSP11, PTX3 and PAGE5 can be used as diagnosis markers for the ACPA-negative RA.
Owner:北京埃克帕生物医学科技有限公司
Protein chip, protein chip diagnostic kit, preparation method and using method
ActiveCN105527435AFacilitate early diagnosisConvenient census screeningMaterial analysisProtein markersHigh risk populations
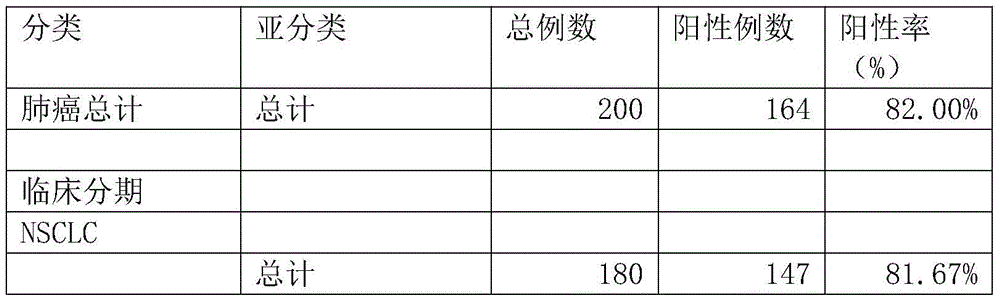
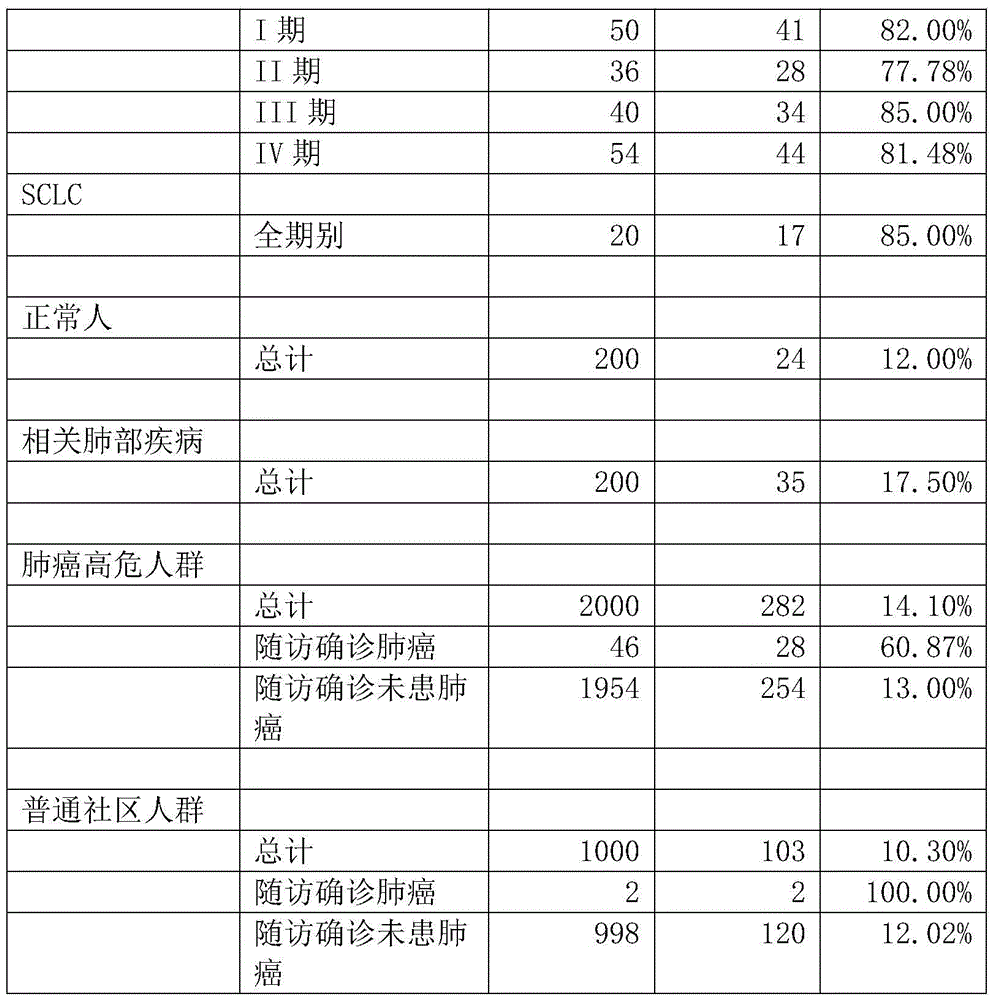
The invention relates to a protein chip, a protein chip diagnostic kit, a preparation method and a using method. The protein chip contains the following protein markers of autologous antigen protein: a P53 antigen fragment, an SOX2 antigen fragment, a COPB1 antigen fragment, an EFHD2 antigen fragment, an EIF4G3 antigen fragment and a PCNA antigen fragment, wherein the characteristic amino acid sequence of the P53 antigen fragment is as shown in ID.1sequence; the characteristic amino acid sequence of the SOX2 antigen fragment is as shown in ID.2sequence; the characteristic amino acid sequence of the COPB1 antigen fragment is as shown in ID.3sequence; the characteristic amino acid sequence of the EFHD2 fragment is as shown in picture 4seeqeunce; the characteristic amino acid sequence of the EIF4G3 antigen fragment is as shown in ID.5seqeunce; the characteristic amino acid sequence of the PCNA antigen fragment is as shown in ID.6sequence. Early diagnosis or general investigation screening for suspected patients of lung cancer or high-risk population of lung cancer can be carried out extremely conveniently, quickly, noninvasively and efficiently.
Owner:GUANGZHOU BIO BLUE TECH CO LTD
Diagnosis marker for ACPA-negative RA and application thereof
ActiveCN106950366AHigh sensitivityImprove featuresDisease diagnosisBiological testingAuto antigenAnti–citrullinated protein antibody
The invention provides applications of Pentaxin related protein 3, namely, PTX3 or a fragment thereof, in preparation of a reagent for diagnosis of anti-citrullinated protein antibody negative (ACPA-negative) rheumatoid arthritis (RA). In the method, a high-density protein chip is hybridized with RA serum to screen 35 proteins, which are candidate autoantigens of the ACPA-negative RA. It is identified that four protein antigens, DOHH, DUSP11, PTX3 and PAGE5, have high sensitivity and specificity in ACPA-negative serum of the RA, wherein the four protein antigens are all novel auto-antigens. The method shows that the DOHH, DUSP11, PTX3 and PAGE5 can be used as diagnosis markers for the ACPA-negative RA.
Owner:北京埃克帕生物医学科技有限公司
Human monoclonal antibodies and methods for producing the same
ActiveUS20110318758A1Immunoglobulins against bacteriaImmunoglobulins against virusesViral antigensTumour-associated antigen
The present invention provides for methods of producing human monoclonal antibodies against a wide variety of antigens including bacterial and viral antigens, as well as tumor antigens, and various autoantigens. Also provided are the antibodies themselves, nucleic acids encoding such antibodies, cells producing such antibodies, and methods of using such antibodies for diagnostic assays and passive immunity against disease states such as infection and cancer.
Owner:MUSC FOUND FOR RES DEV
Auto-antigen biomarkers for lupus
InactiveUS20130331283A1Easy diagnosisImproved prognosisPeptide librariesLibrary screeningAntigenAutoantibody
Owner:SENSE PROTEOMIC LTD
Methods and compositions for the treatment of autoimmune disease
InactiveUS20120128646A1Relieve symptomsEasy to produceOrganic active ingredientsBiocideChromogranin AAntigen
The present invention is related to the development and treatment of autoimmune disease. Autoimmune diseases can result from tissue damage caused by the activation of autoreactive T cells by autoantigens. For example, peptide fragments of naturally occurring proteins (i.e., for example, chromogranin A) may activate autoreactive T cells that result in the destruction of pancreatic β islet cells, possibly by the release of inflammatory cytokines (i.e., for example, interferon-γ). One naturally occurring biologically active chromogranin A peptide fragment, WE14, may comprise a diabetogenic autoantigen. Truncation and extension analysis of WE14 indicates that the stimulating binding register of WE14 occupies only half of the mouse IAg7 peptide binding groove, leaving positions p1 to p4 empty. Inhibition of autoantigen-autoreactive T cell binding may provide therapeutic as well a prophylactic treatments for autoimmune diseases
Owner:NAT JEWISH HEALTH +2
Vaccine
The present invention relates to an isolated polypeptide useful for immunisation against self-antigens. In particular the invention relates to a self-protein that is capable of raising auto-antibodies when administered in vivo. The invention particularly relates to rendering human cytokines immunogenic in humans. The invention further relates to pharmaceutical compositions comprising such compounds and their use in medicine and to methods for their production.
Owner:GLAXO GRP LTD
Auto-antigen biomarkers for lupus
InactiveUS20150204866A1Easy diagnosisImproved prognosisPeptide librariesLibrary screeningAuto antigenAutoantibody
Owner:SENSE PROTEOMIC LTD
RNA for treatment of autoimmune diseases
ActiveUS20200061166A1Improve stabilityExtend your lifeNervous disorderWhole-cell/virus/DNA/RNA ingredientsImmunologic disordersAutoimmune condition
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITATSM EDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GEMEINNUTZIGE GMBH +2
Medicaments and methods to treat autoimmune disease and cancer
InactiveCN101687019ANervous disorderPeptide/protein ingredientsImmunologic disordersAutoimmune condition
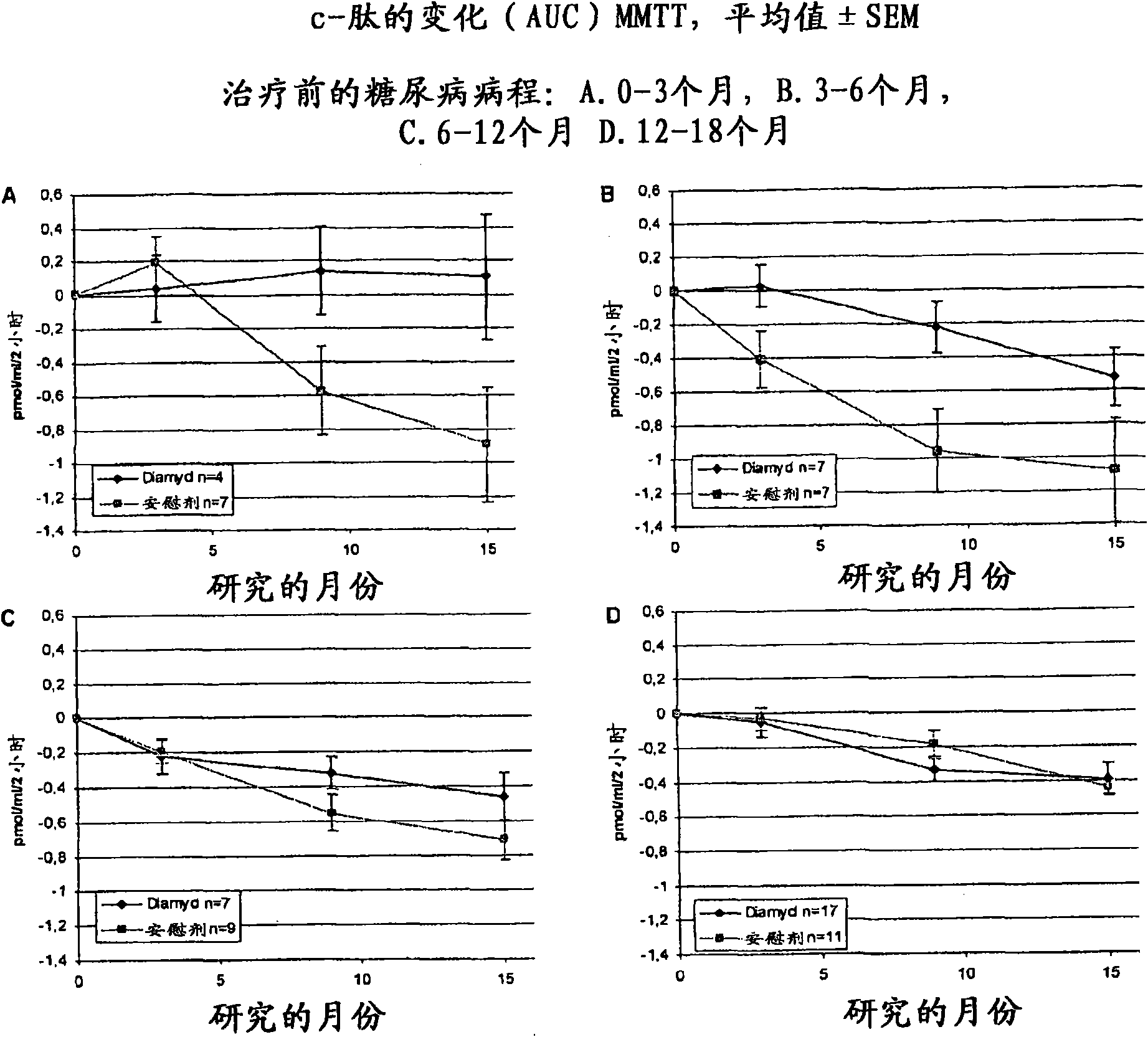
The present invention relates to methods and formulations for GAD- vaccination to evoke a systemic effect rather that a GAD-specific effect. The present invention may therefore be used in the treatment of disease in humans not bearing on a GAD-specific effect. The invention includes a method to treat an autoimmune disease or disorder by administering at least one sequestered autoantigen in a primeand boost regimen for sensitization purposes followed by a boost for treatment purposes. This may be done upon diagnosis. Examples of sequestered autoantigens include: GAD65, GAD67, Pro-Insulin, Basic Myelin Protein, MOG and Chondrotoin II. The present invention relates to medications and methods for treatment of autoimmune disease within six months from diagnosis.
Owner:DIAMYD THERAPEUTICS
Saliva immunoassay for detection of antibodies for cardiovascular disease
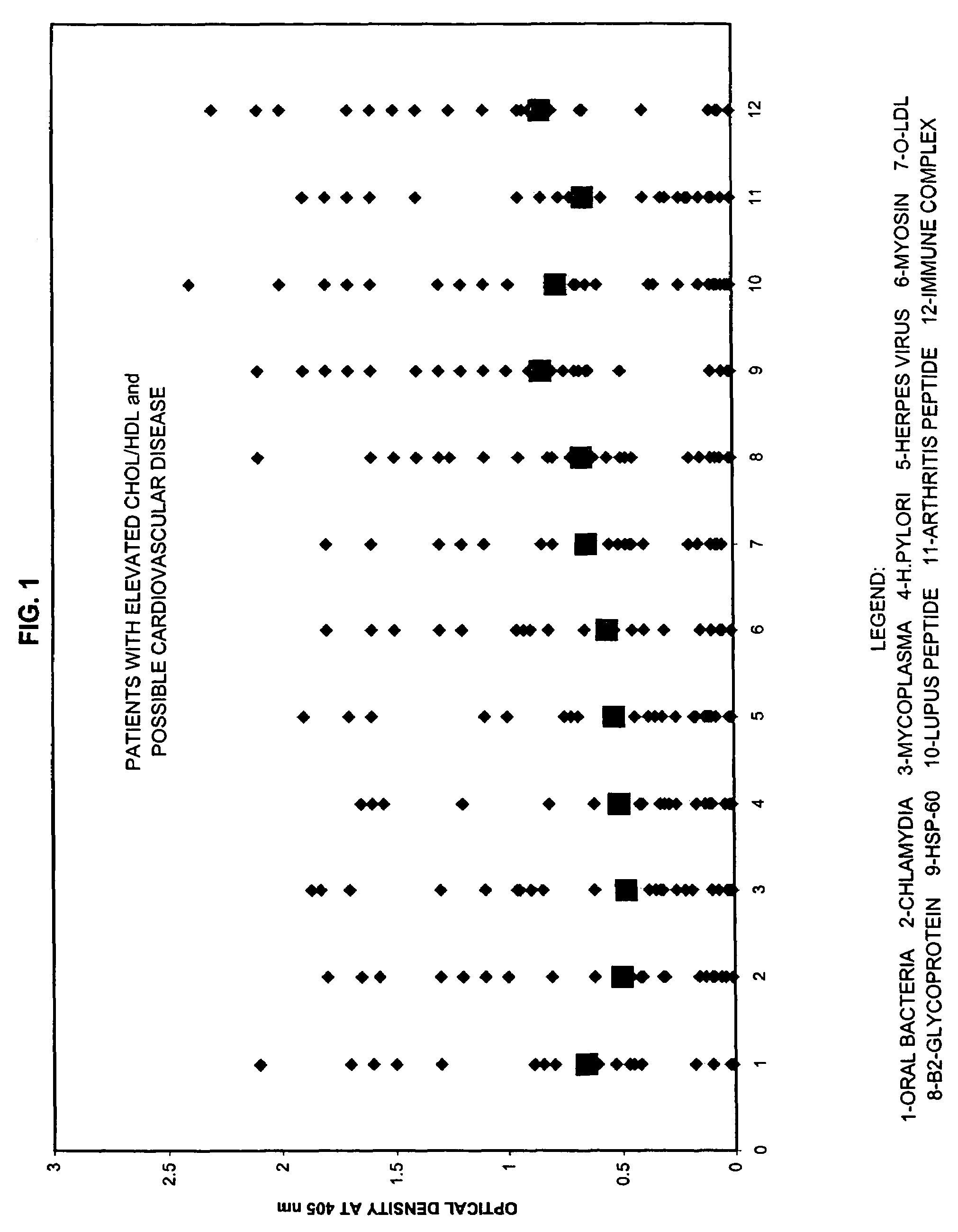
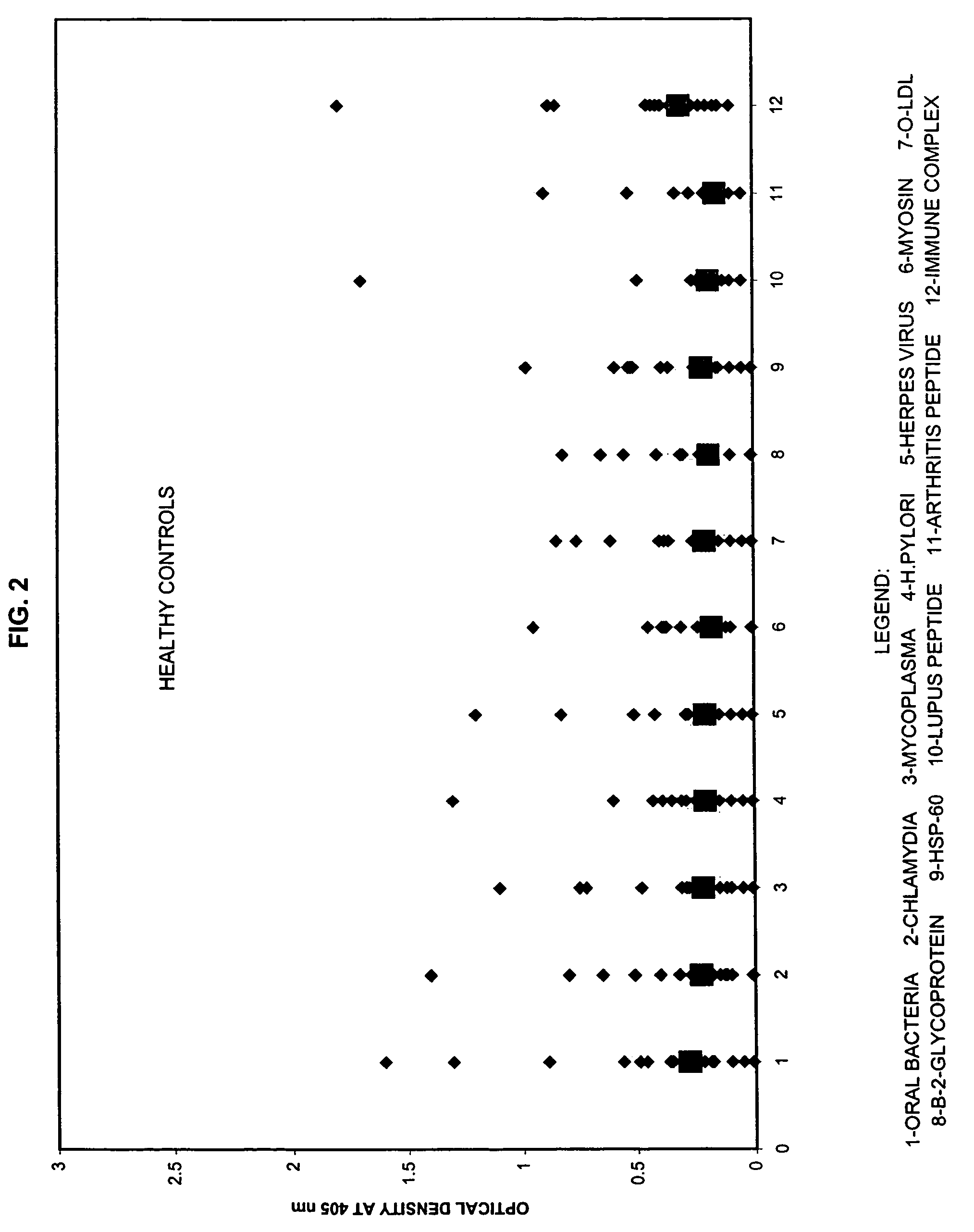
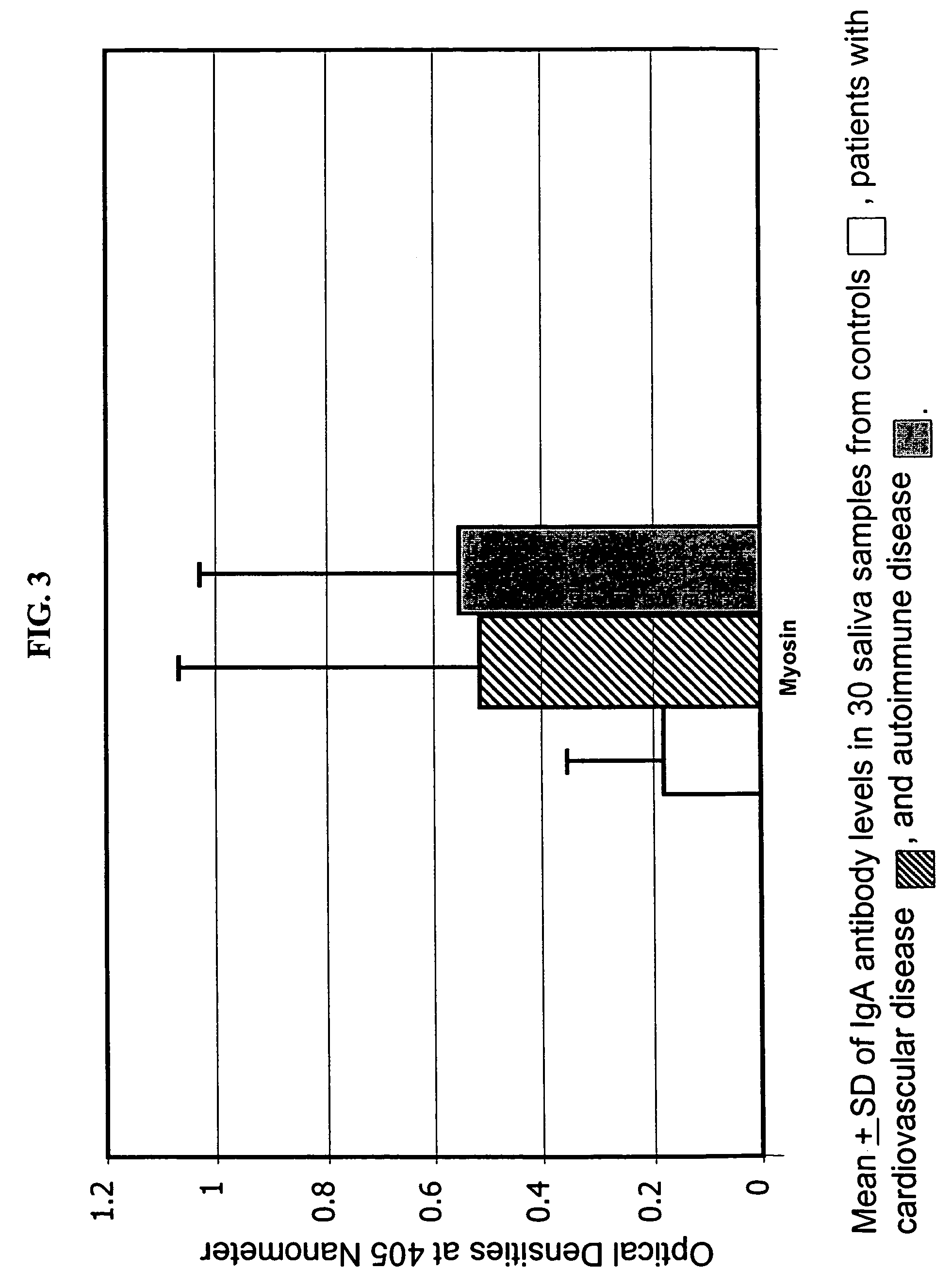
A method for diagnosing the likelihood and severity of cardiovascular disease in a patient is disclosed. The method determines the levels of antibodies against autoantigens, including myosin, oxidized LDL, β-2-glycoprotein, heat shock protein-60, platelet glycoprotein, and immune complexes. It then compares the results to normal levels to determine the likelihood and severity of cardiovascular disease.
Owner:IMMUNOSCI LAB
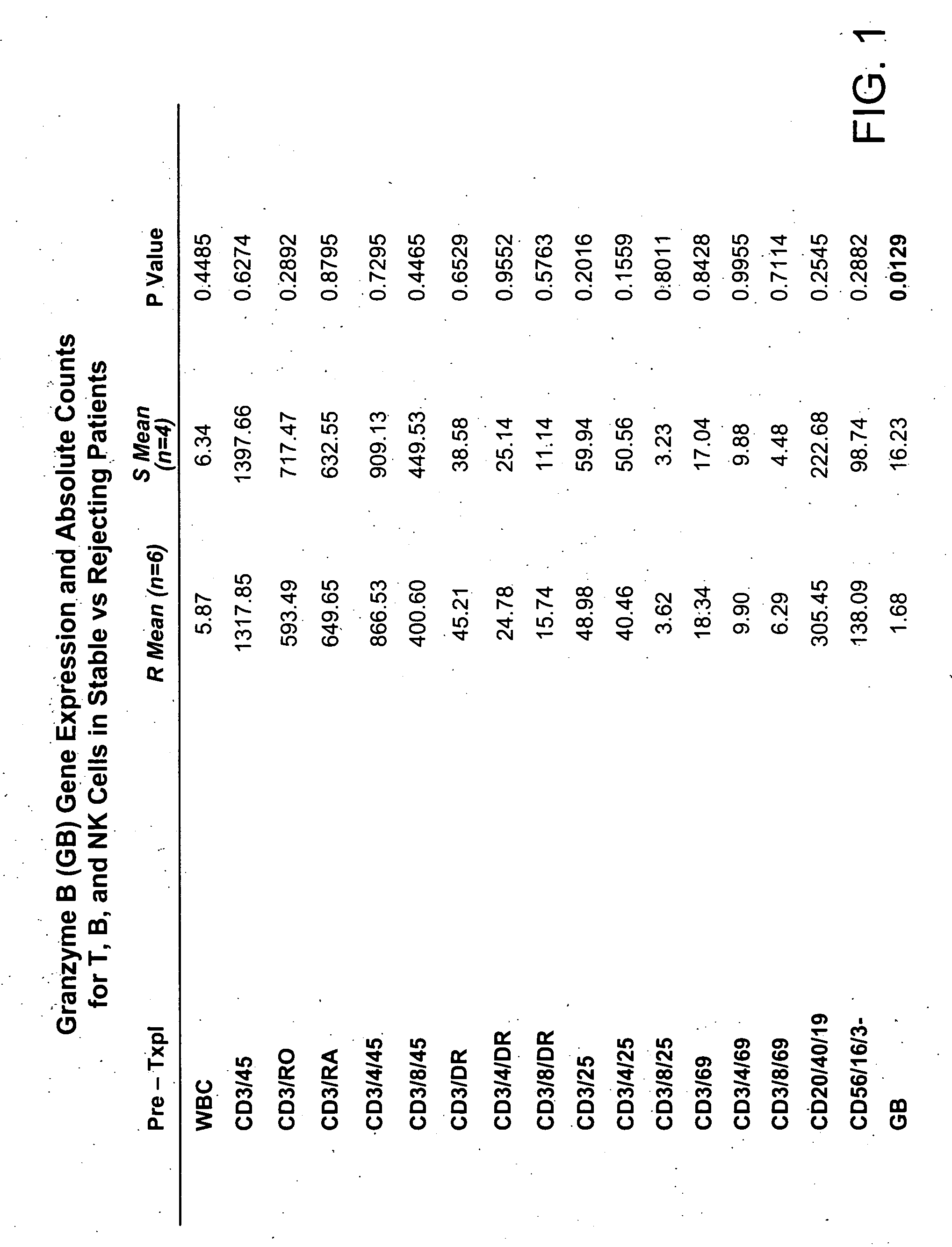
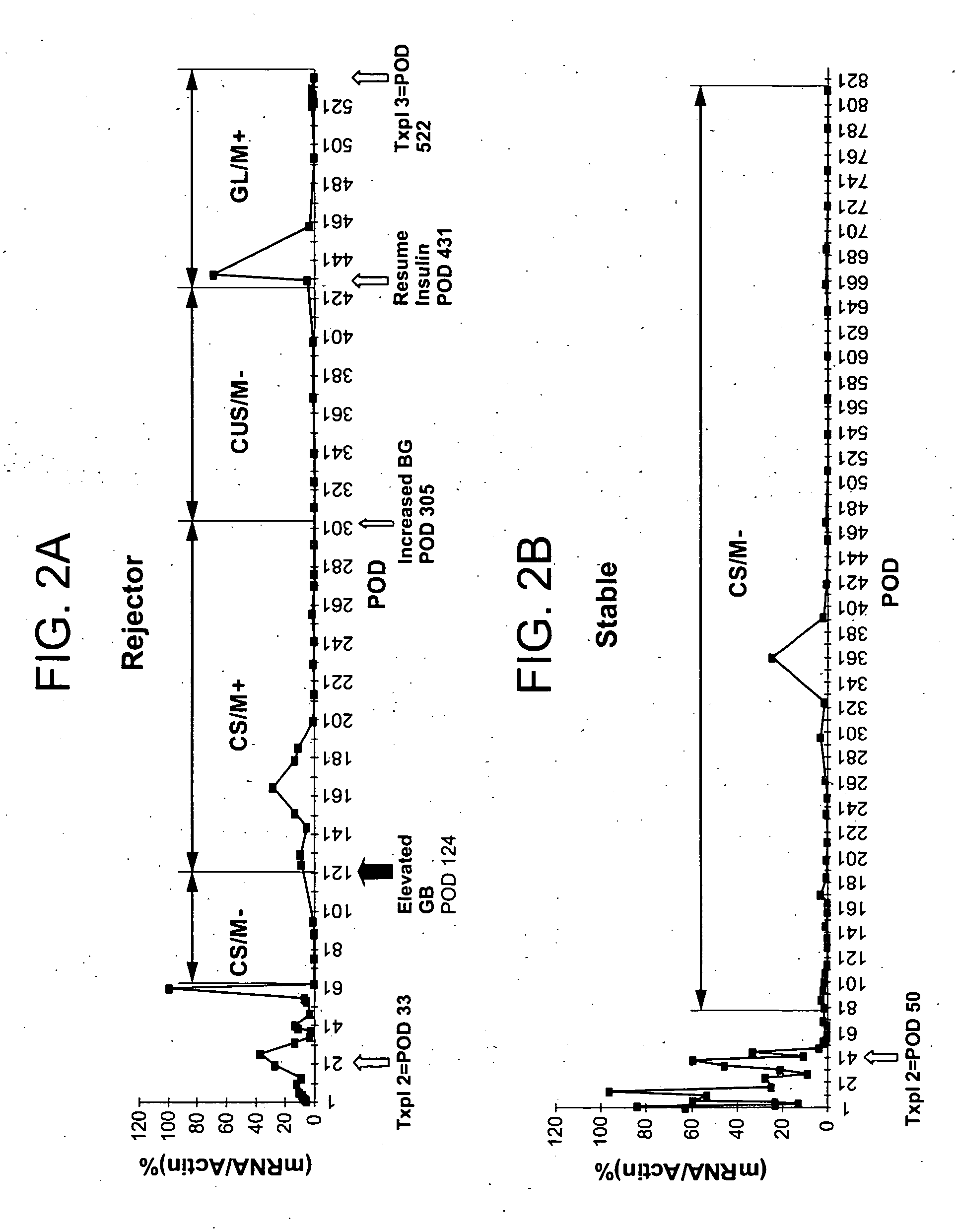
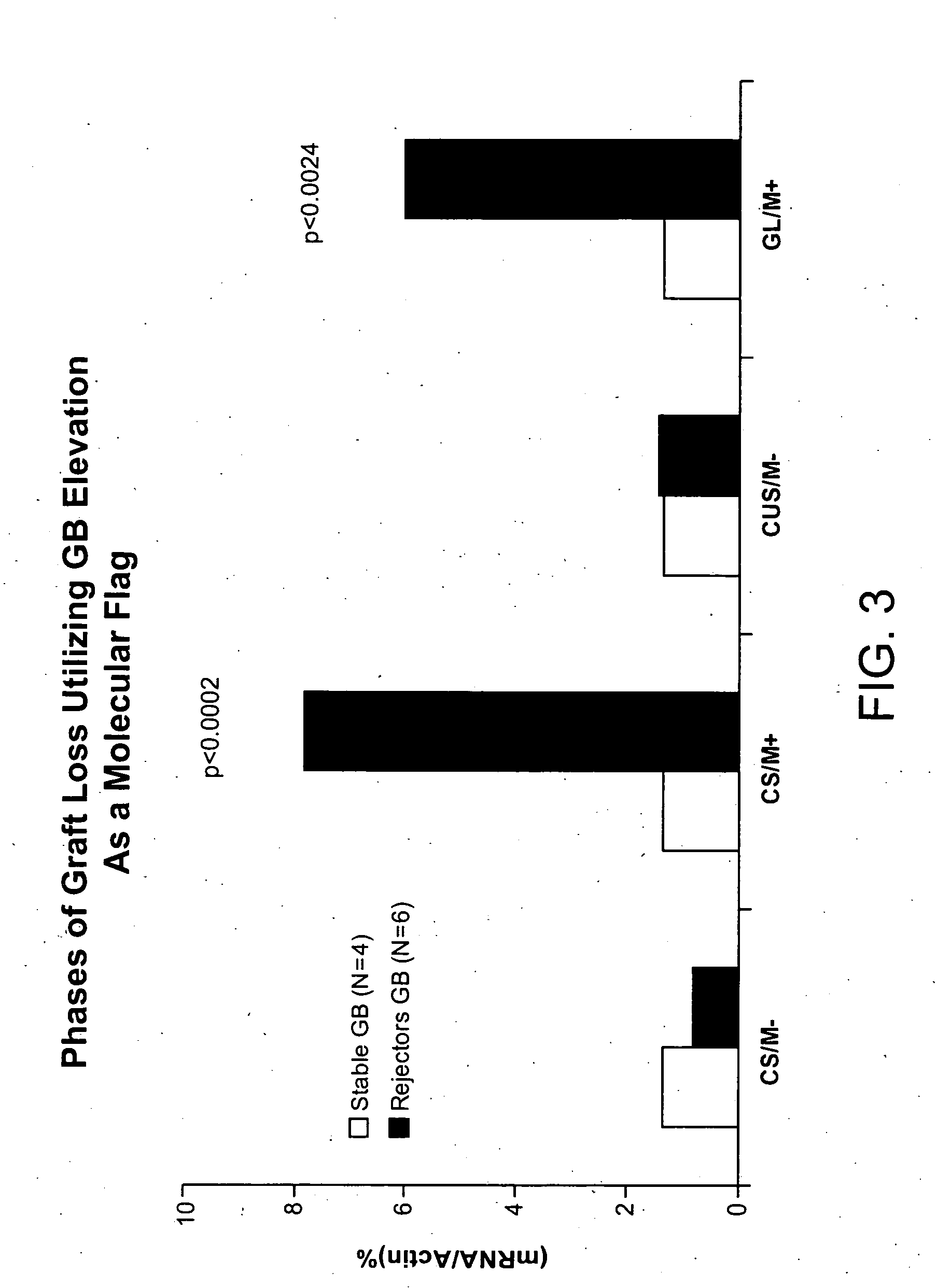
Molecular dissection of cellular responses to alloantigen or autoantigen in graft rejection and autoimmune disease
InactiveUS20060263343A1High expressionBiocideMicrobiological testing/measurementAutoimmune conditionAuto antigen
An antigen-specific T-cell response to alloantigen, tissue-specific antigen (e.g., islet antigen or other autoantigens involved in autoimmune disease), or self (or host) antigen is detected at an early stage of graft rejection or recurrent autoimmunity. An increase in cytotoxic lymphocyte gene (CLG) expression in peripheral blood is a risk factor for development of deleterious immune responses, which may be confirmed by functional assays. For example, the distinction between production of regulatory or inflammatory cytokines by T cells may dissect the type of immune response which is being induced: the survival of transplanted islet cells used to treat type 1 diabetes may be monitored, loss of the transplant by graft rejection (i.e., an alloantigen target) may be distinguished from autoimmune disease (i.e., a self or host antigen target).
Owner:BECKMAN COULTER INC +1
Human monoclonal antibodies and methods for producing the same
The present invention provides for methods of producing human monoclonal antibodies against a wide variety of antigens including bacterial and viral antigens, as well as tumor antigens, and various autoantigens. Also provided are the antibodies themselves, nucleic acids encoding such antibodies, cells producing such antibodies, and methods of using such antibodies for diagnostic assays and passive immunity against disease states such as infection and cancer.
Owner:MUSC FOUND FOR RES DEV
BP180 single chain antibody of humanized anti-bullous pemphigoid antigen
InactiveCN101333255ABlock inflammatory responseImmunoglobulins against animals/humansAntibody ingredientsAntigenSingle-Chain Antibodies
The invention discloses a human-derived anti-bullous pemphigoid antigen BP180 ScFv; the gene sequence of the BP180 ScFv structurally belongs to the single-chain antibody (scFv) and is composed of a light chain variable region gene and a heavy chain variable region gene. The antibody preparation method mainly comprises the following steps: a, separating the peripheral blood mononuclear cells of bullous pemphigoid patients, extracting the RNA and constructing a phage antibody library through RT-PCR amplification of the antibody light and heavy chain variable region genes; b, selecting the BP180 phage antibodies from the phage antibody library; c, obtaining the human-derived anti-BP180 ScFv of soluble expression through genetic engineering means; d, identifying the binding activity, specificity, affinity and other immunological characteristics of the obtained antibody. The antibody of the invention can block the binding activity between the bullous pemphigoid autoantibody and the corresponding self-antigen and can block the activation of the complement-mediated inflammatory response as a result of the combination of the autoantibodies, and can be used in bullous pemphigoid treatment.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Synthetic human genes and polypeptides and their use in the treatment of autoimmune diseases
InactiveUS8088389B1Peptide/protein ingredientsAntibody mimetics/scaffoldsAutoimmune diseaseSelf-Antigens
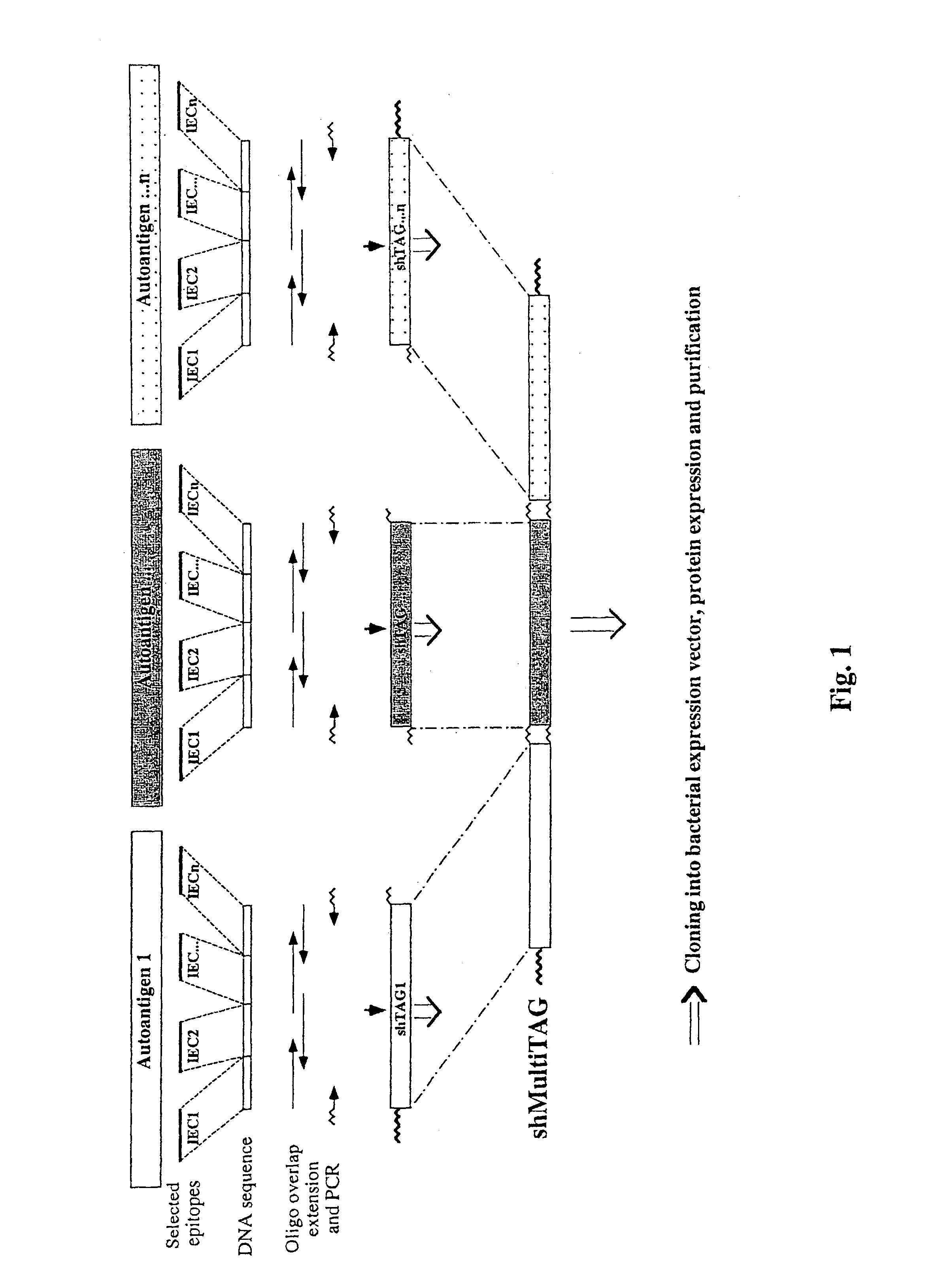
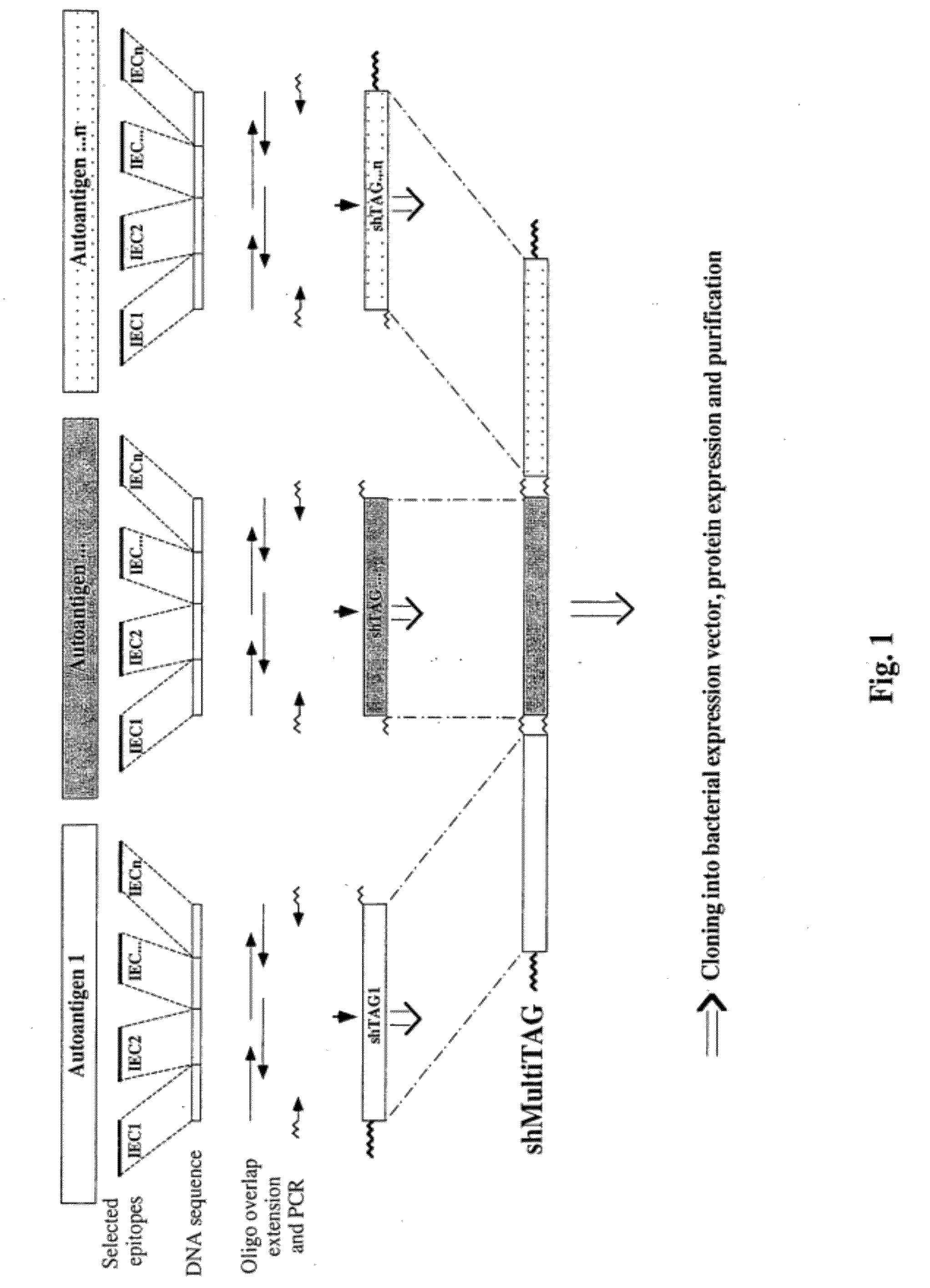
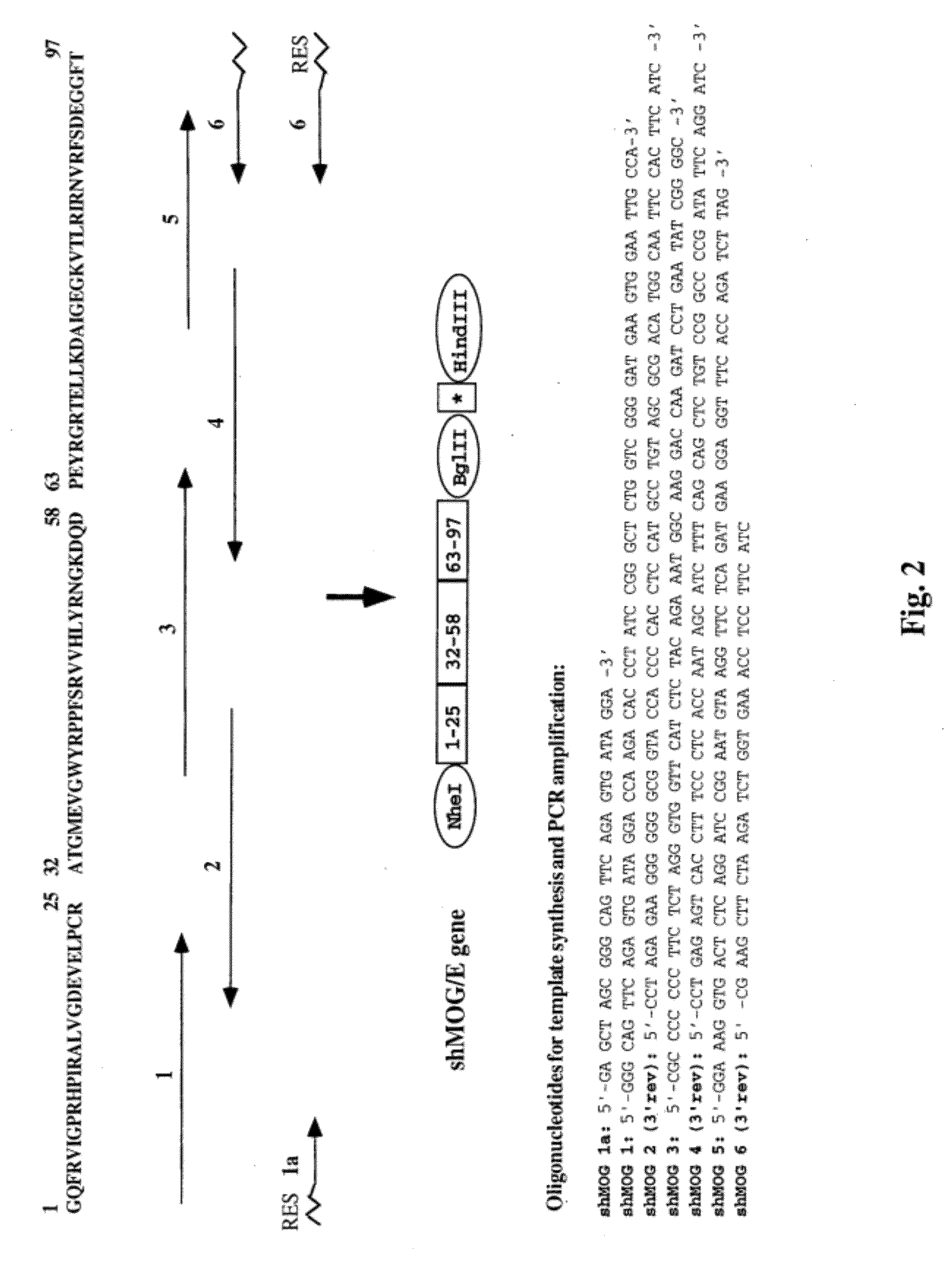
Synthetic human target autoantigen genes comprising sequences coding for at least two immunogenic epitopic clusters (hereinafter IEC) of autoantigen(s) related to a specific autoimmune disease, wherein said at least two IECs may be derived from a sole autoantigen or from at least two different autoantigens related to said autoimmune disease, and polypeptides encoded thereby, can be used for the treatment and the diagnosis of autoimmune diseases such as multiple sclerosis (MS), insulin-dependent diabetes mellitus (IDDM), rheumatoid arthritis (RA), myasthenia gravis (MG) and uveitis.
Owner:YEDA RES & DEV CO LTD
Method for characterizing autoimmune disorders
The invention relates to methods of characterizing autoimmune diseases by detecting and measuring at least one analyte using multiplexed assay systems. According to one embodiment, a target is detected and measured by different bead sets having different reactants. According to another approach the ratio of self-antigen to autoantibody is measured by exposing a sample suspected of containing self-antigen and autoantibody to a bead set associated with monoclonal antibody specific for the self-antigen and a bead set associated with the self-antigen.
Owner:LUMINEX
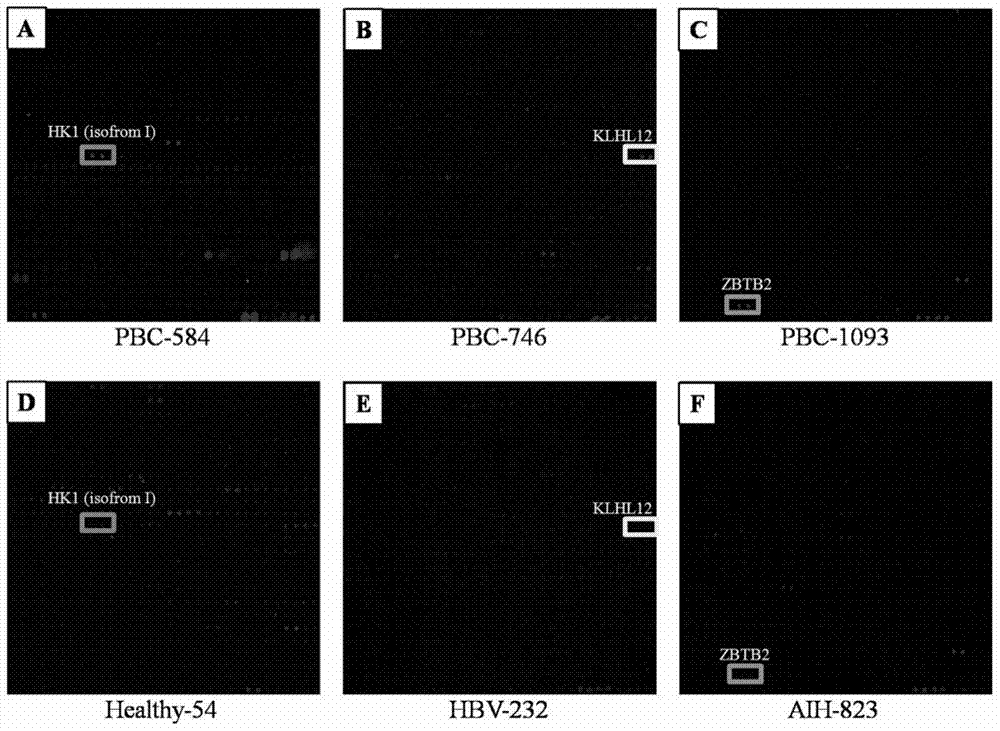
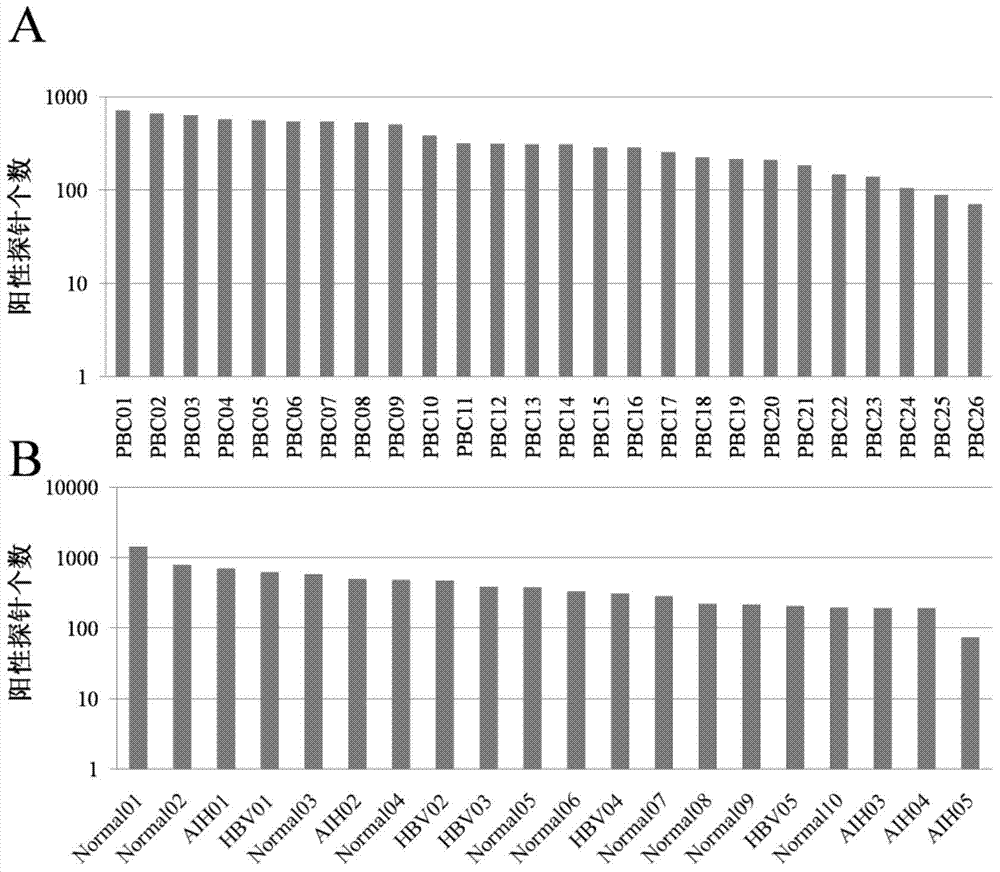
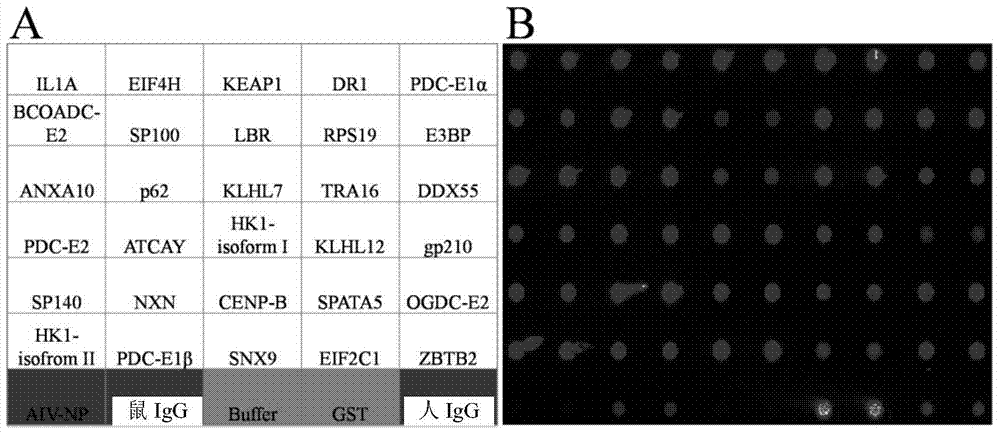
Specific autoantigen of primary biliary cirrhosis (PBC) and application thereof
InactiveCN104292322AHigh sensitivityImprove featuresDisease diagnosisBiological testingDiseasePrimary biliary cirrhosis
The invention discloses a specific autoantigen EIF2C1 of primary biliary cirrhosis (PBC). According to the specific autoantigen of PBC and the application thereof, six novel autoantigens of PBC are obtained through screening; during the detection on the blood serum of a PBC patient, the positive rate of each autoantigen reaches over 15%, so that the new-found autoantigens are potential biomarkers which are used for accurately diagnosing PBC through distinguishing other autoimmune diseases.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI +1
Use of effector-function-deficient antibodies for treatment of auto-immune diseases
ActiveUS20090317382A1Prevention of pathogenic effect of anti-AChRInhibition effectMetabolism disorderAntipyreticDiseaseAuto antigen
The invention relates to the treatment of autoimmune diseases and disorders, in particular myasthenia gravis, by administration of effector-function-deficient antibodies, wherein said effector-function-deficient antibodies are capable of competing with one or more of the auto-antibodies involved in mediating the antibody-mediated auto-immune disease or disorder for binding to a target auto-antigen.
Owner:GENMAB AS
Targeted Drug-Formaldehyde Conjugates And Methods Of Making And Using The Same
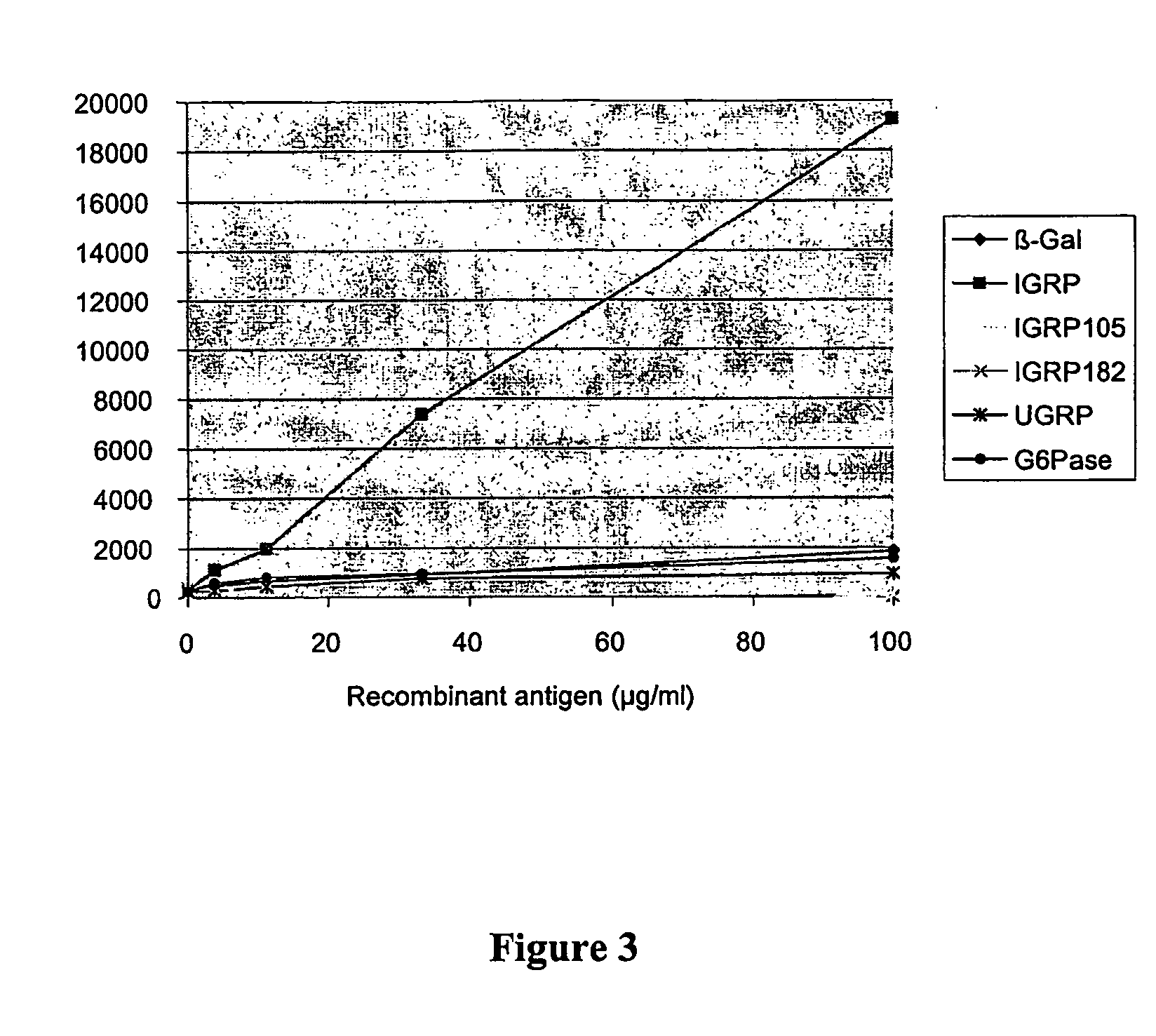
A method and compositions for detecting autoimmunity to islet glucose-6 phosphatase related protein (IGRP). Detection of IGRP autoantibodies alone, and in combination with other molecules such as the 65-kDa form of glutamate decarboxylase (GAD65), insulin and insulin granule membrane proteins ICA512 (IA-2) and phogrin (IA-2) auto-antigens, provides an effective and reliable chemical assay for the diagnosis of autoimmune (type 1) diabetes. Additionally, this invention provides therapeutic regimens based on IGRP and related molecules for the amelioration of the diabetic clinical condition. Therefore, IGRP alone or in combination with other autoimmune diabetes associated antigens such as insulin, IA-2 and GAD65, is useful in the prediction (diagnosis), treatment (therapy), and prevention (prophylaxis) of diabetes.
Owner:HUTTON JOHN C +3
Synthetic human genes and polypeptides and their use in the treatment of autoimmune diseases
Synthetic human target autoantigen genes comprising sequences coding for at least two immunogenic epitopic clusters (hereinafter IEC) of autoantigen(s) related to a specific autoimmune disease, wherein said at least two IECs may be derived from a sole autoantigen or from at least two different autoantigens related to said autoimmune disease, and polypeptides encoded thereby, can be used for the treatment and the diagnosis of autoimmune diseases such as multiple sclerosis (MS), insulin-dependent diabetes mellitus (IDDM), rheumatoid arthritis (RA), myasthenia gravis (MG) and uveitis.
Owner:BEN NUN AVRAHAM +2
Autoantigenic fragments, methods and assays
InactiveUS20050112137A1Reduce impactRelieve symptomsGenetic material ingredientsDisease diagnosisAuto antigenAssay
Owner:ROSEN ANTONY +5
Matrix-embedded tolerance-promoting adjuvants for subcutaneous immunotherapy
InactiveUS20180085452A1Easy to prepareGood biocompatibilityOrganic active ingredientsPeptide/protein ingredientsAdjuvantAuto antigen
The present invention relates to compositions and methods for the application of tolerance-promoting adjuvants embedded in alum-, microcrystalline tyrosine-, or hydrogel-based formulations for allergen- or autoantigen-specific immunotherapy.
Owner:TOLEROGENICS S A R L +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com