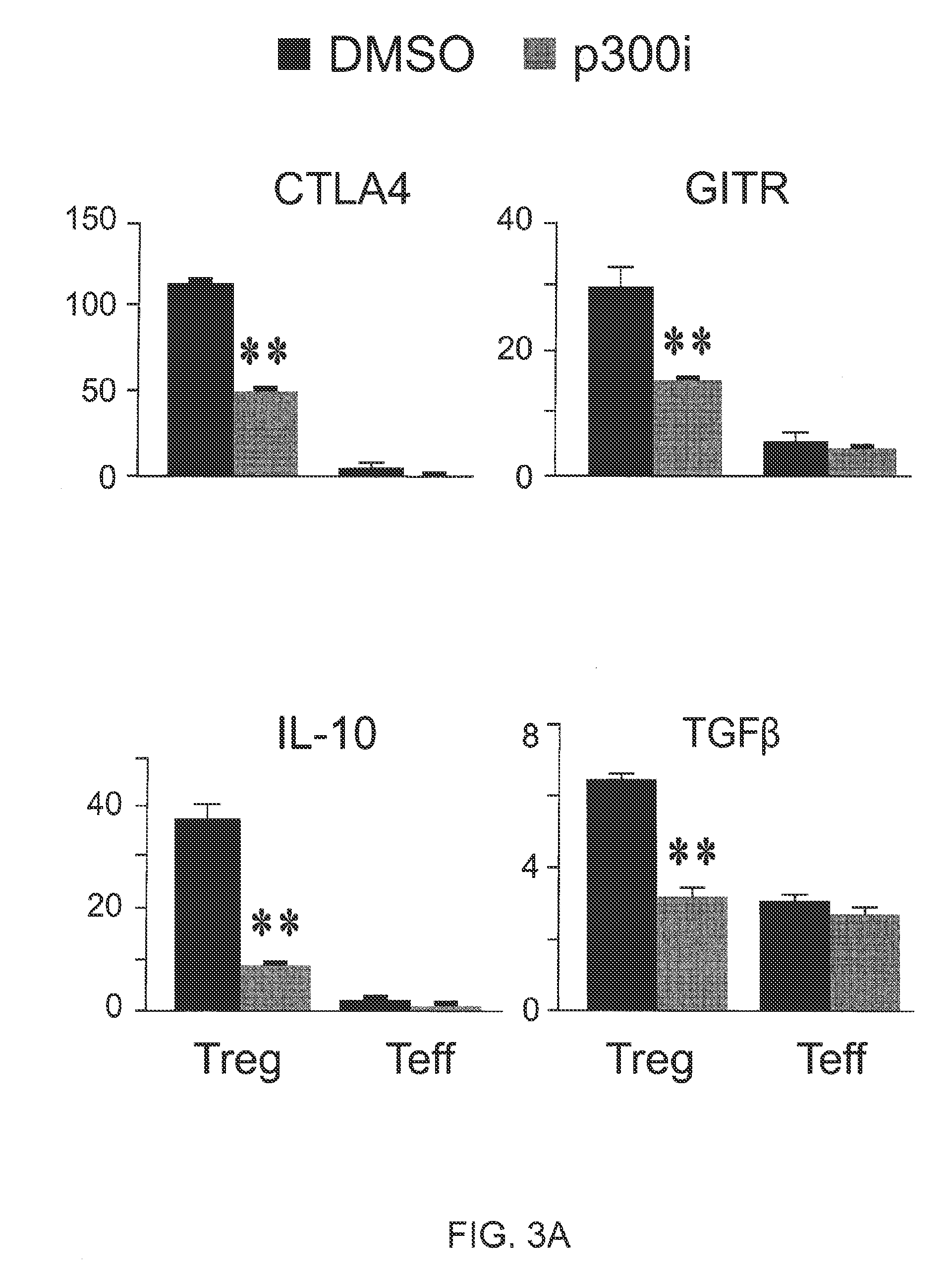
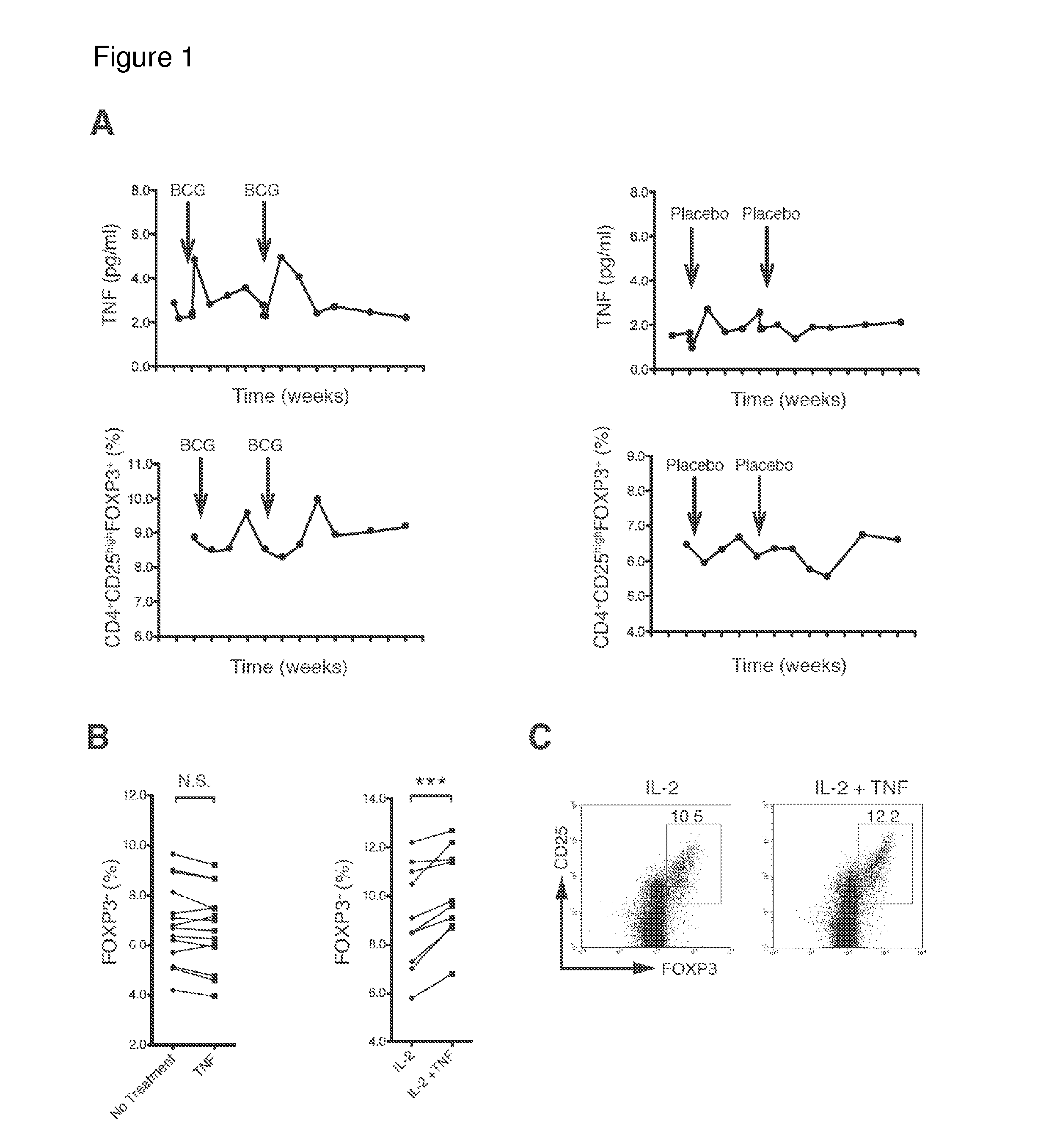
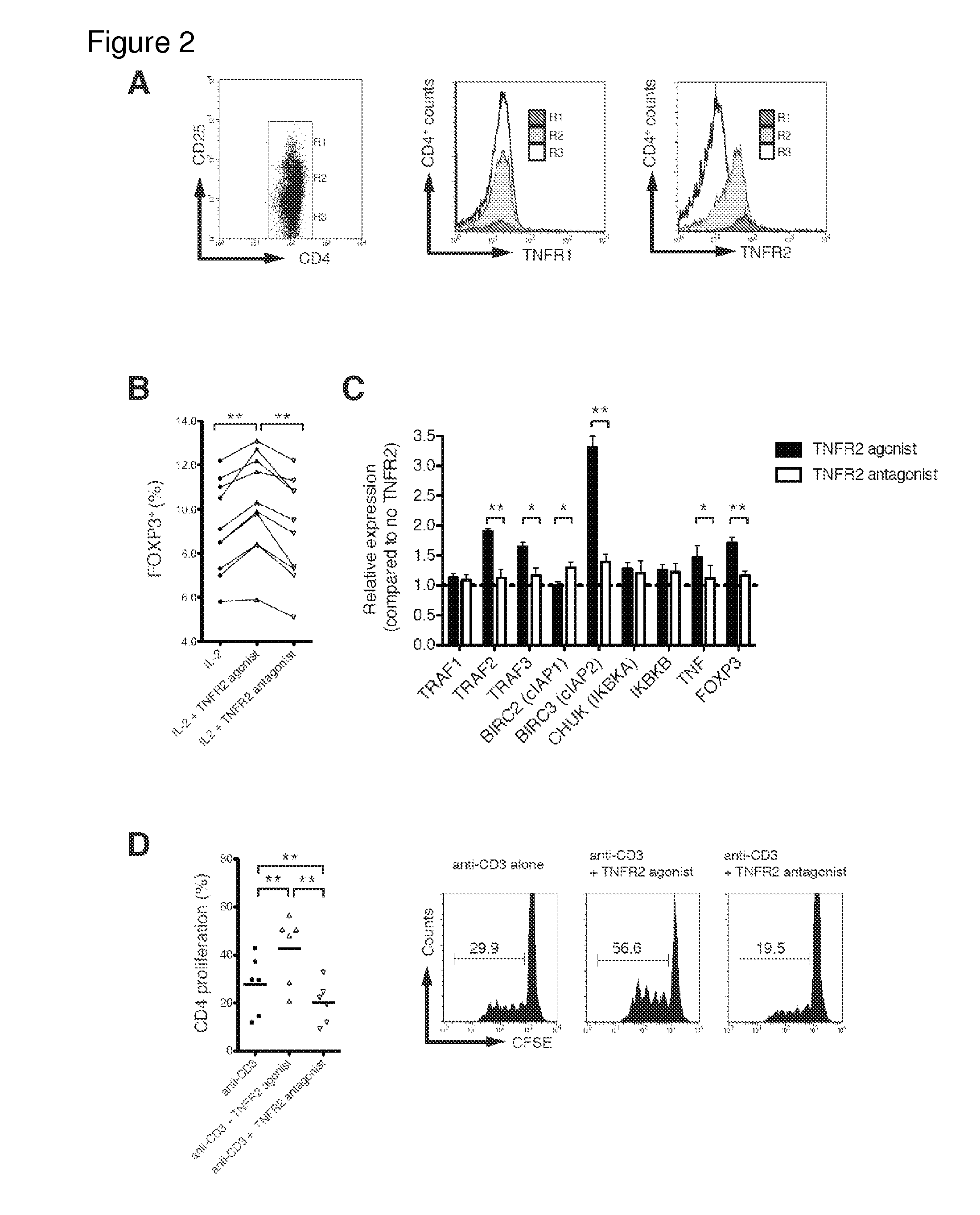
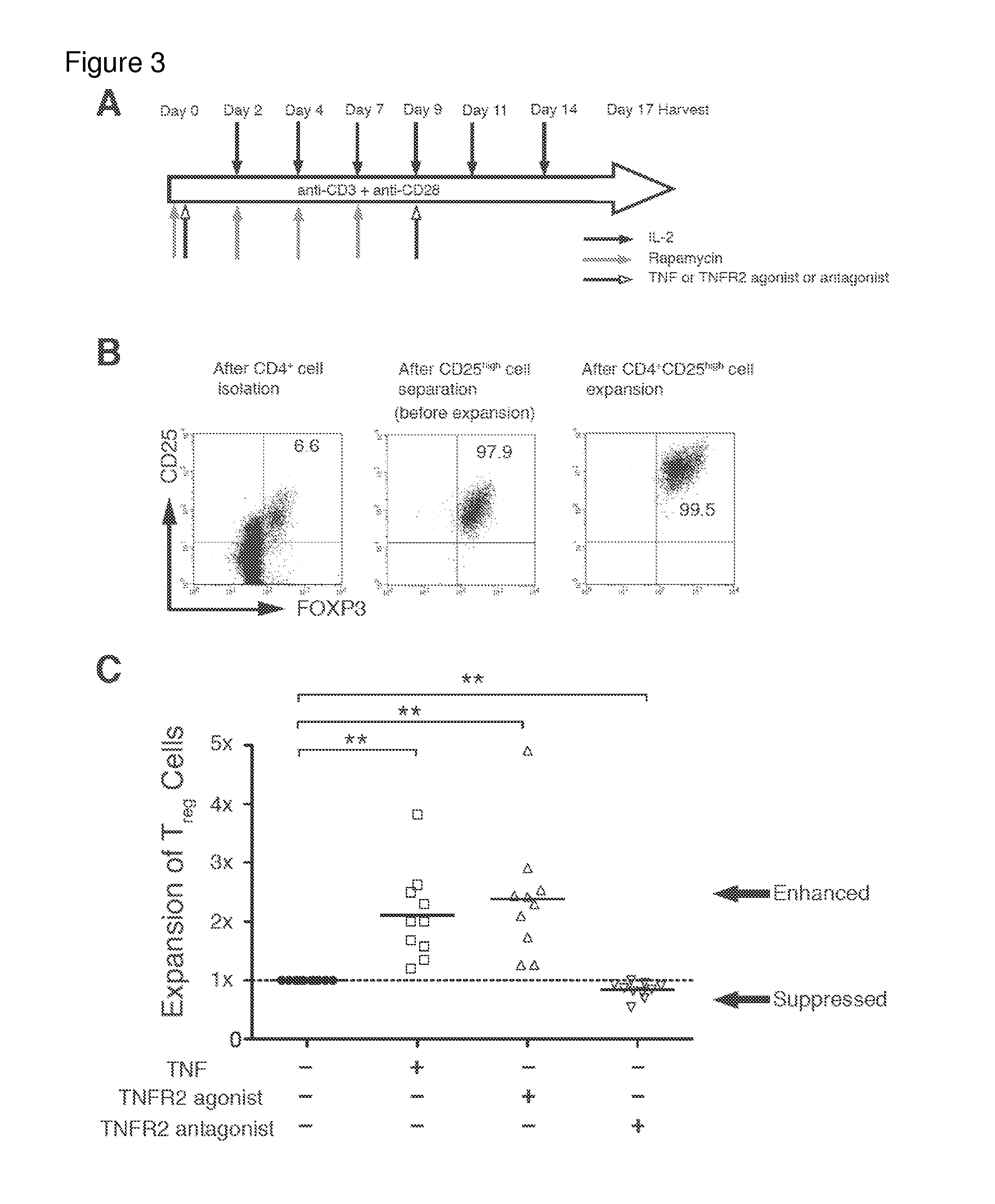
Patents
Literature
51 results about "T-regulatory cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stem cell mediated treg activation/expansion for therapeutic immune modulation
ActiveUS20080159998A1High activityInduce upregulationBiocideGenetic material ingredientsAntigenT cell
Disclosed are cells, methods of modulating cells, and therapeutic uses of the cells for the immune modulation of mammals in need thereof. Immune modulation including alteration of cytokine profile, cytotoxic activity, antibody production and inflammatory states is achieved through the administration of various cell types that have been unmanipulated or manipulated in order to endow specific biological activity. Cellular subsets and administration of the subsets in combination with various agents are also provided. One embodiment teaches the previously unknown finding that adipose tissue derived mononuclear cells contain T cells with immune regulatory properties that alone or synergistically with various stem cells induce immune modulation upon administration. Another embodiment is the finding that stimulation of stem cell activation results in stem cell secondary activation of immune modulatory cells, one type which is T regulatory cells (Tregs). One specific embodiment involves extraction of a heterogenous stem cell pool, which contains T regulatory cells, treatment in culture of the population with agents known to stimulate stem cell activation, then subsequent extraction and administration of the purified Tregs. Other embodiments include expansion of Tregs in the presence of antigen in order to generate anti-specific Tregs.
Owner:XON CELLS
Redirected, genetically-engineered t regulatory cells and their use in suppression of autoimmune and inflammatory disease
InactiveUS20100135974A1Effective quantityOvercome scarcityBiocideAntipyreticIntracellular signallingInflammatory Bowel Diseases
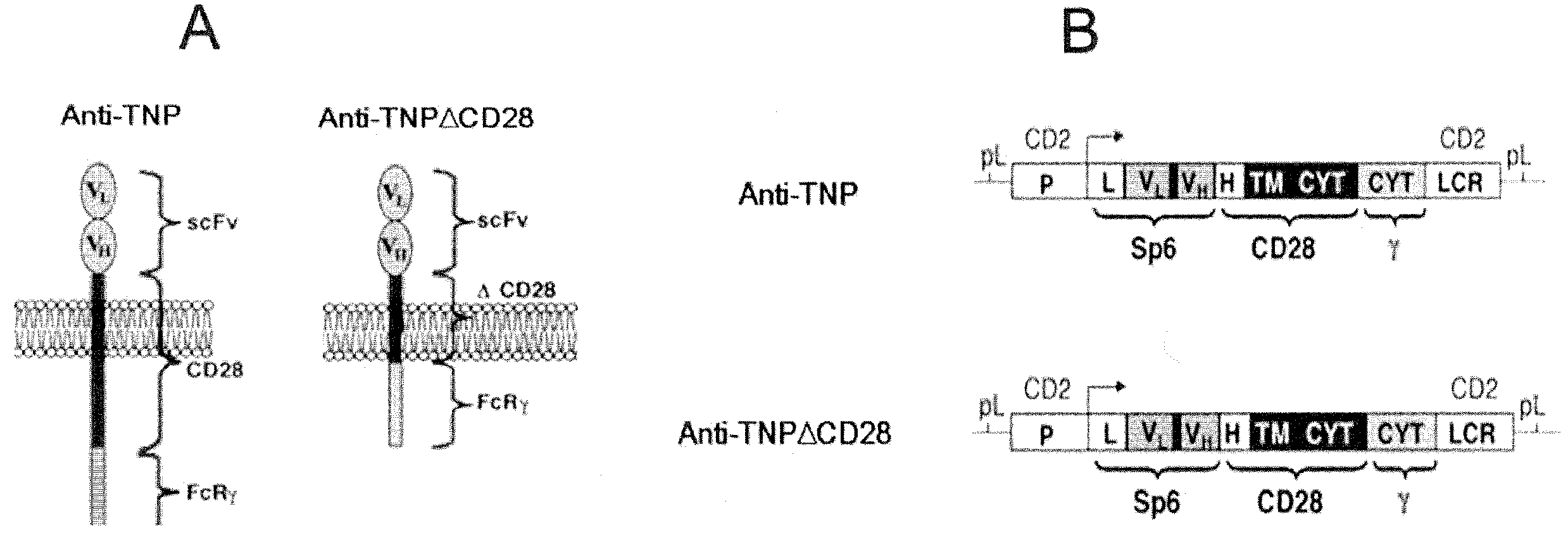
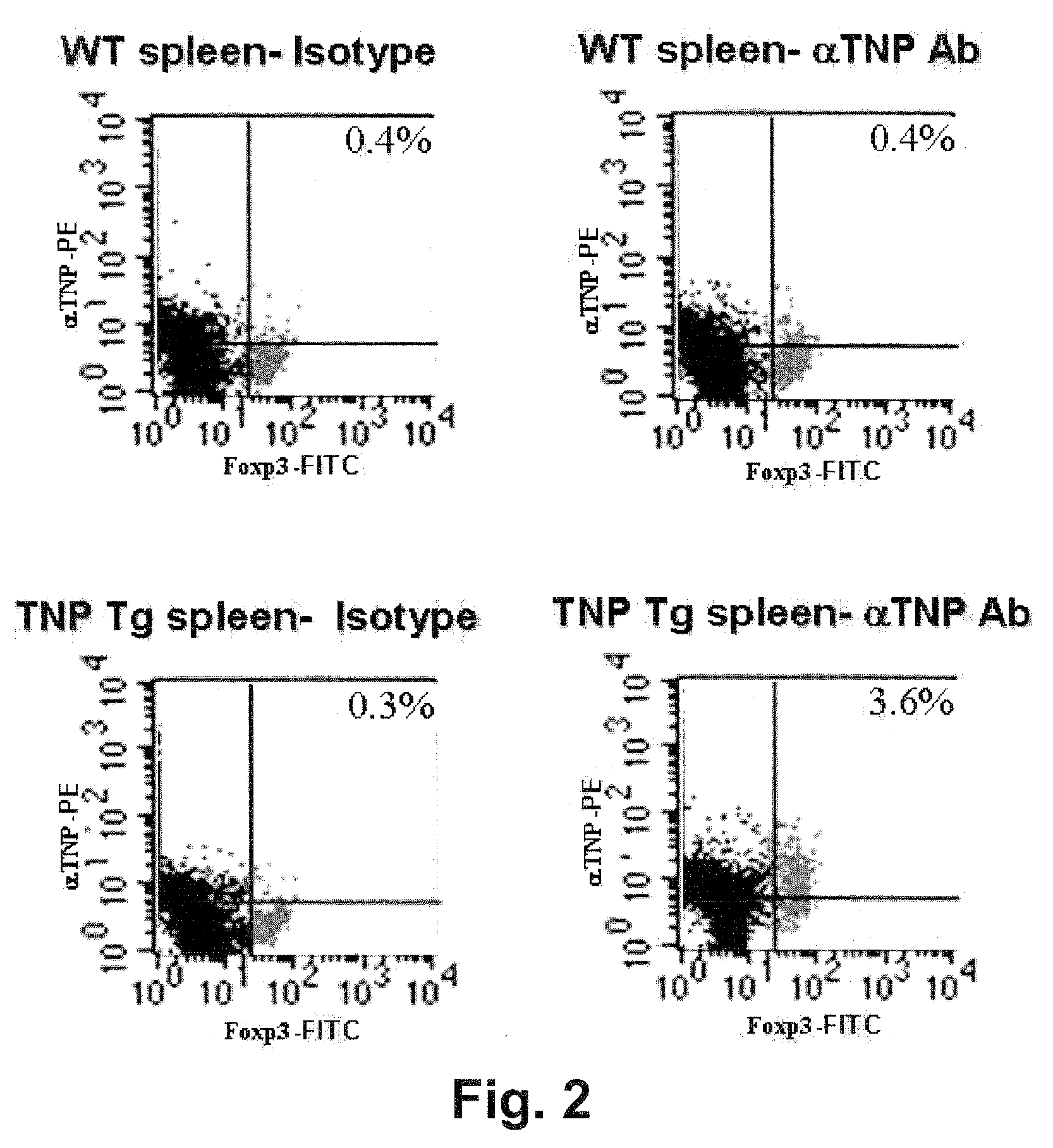
A redirected Treg cell is endowed with specificity toward a selected target antigen or ligand. The cell contains a chimeric receptor polypeptide that is expressed in a single, continuous chain, with an extracellular recognition region displayed on the surface of the cell, a transmembrane region and an intracellular signaling region. The extracellular recognition region is specific for the selected target antigen or ligand. The intracellular signaling region includes a combination of T-cell signaling polypeptide moieties, which combination, upon binding of the extracellular recognition region to the selected target antigen or ligand, triggers activation of the redirected Treg cells to cause suppression of T-cell mediated immunity. Such redirected Treg cells may be used to suppress undesired activity of T effector cells thereby mediating an immune or inflammatory response. They are particularly useful in treating T effector cell-mediated diseases, such as inflammatory bowel disease, transplant rejection and GVH disease.
Owner:YEDA RES & DEV CO LTD
Treatment methods for autoimmune disorders
ActiveUS20100260781A1Promote regulatory cell-mediated suppressionAvoid seizuresBiocideSenses disorderDiseaseT-regulatory cell
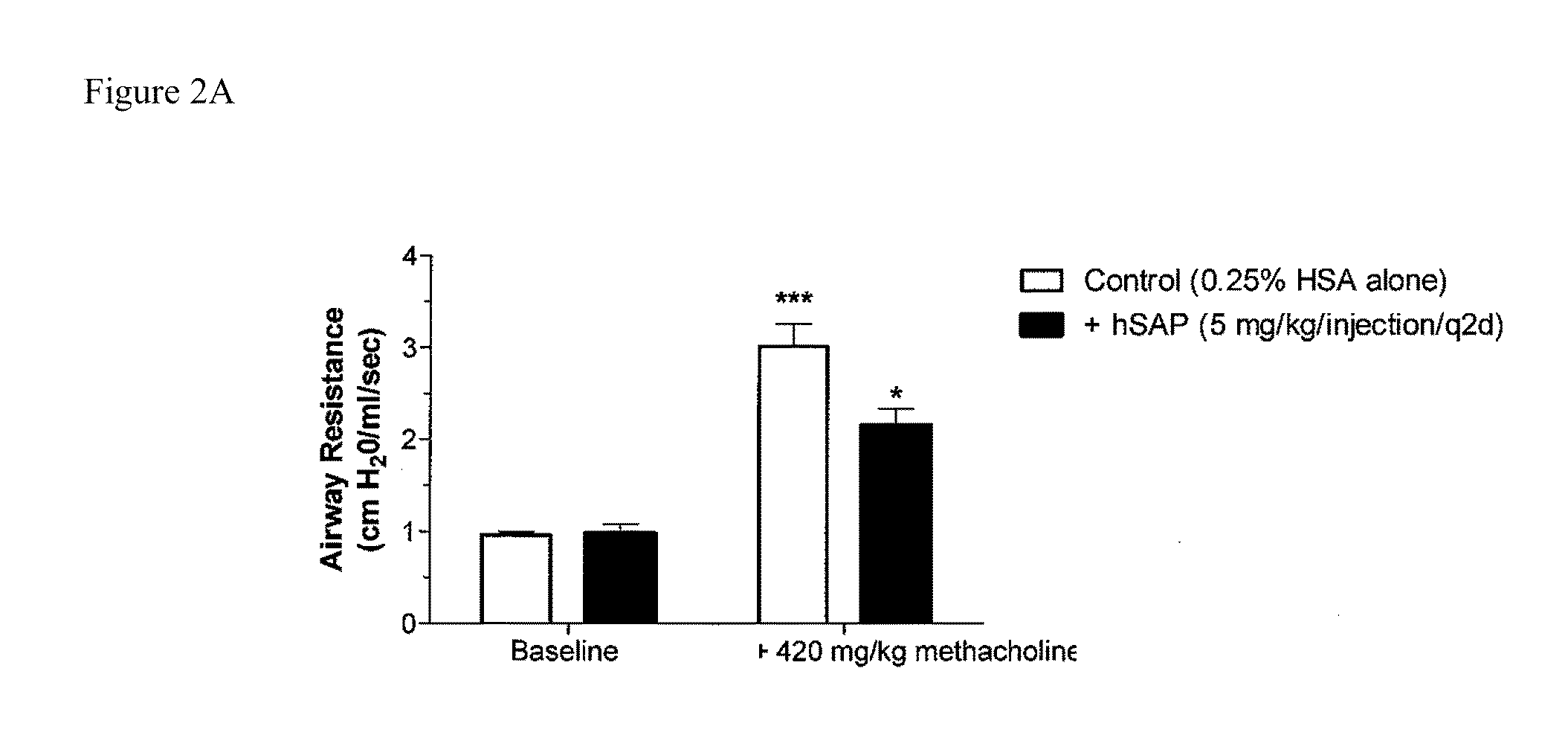
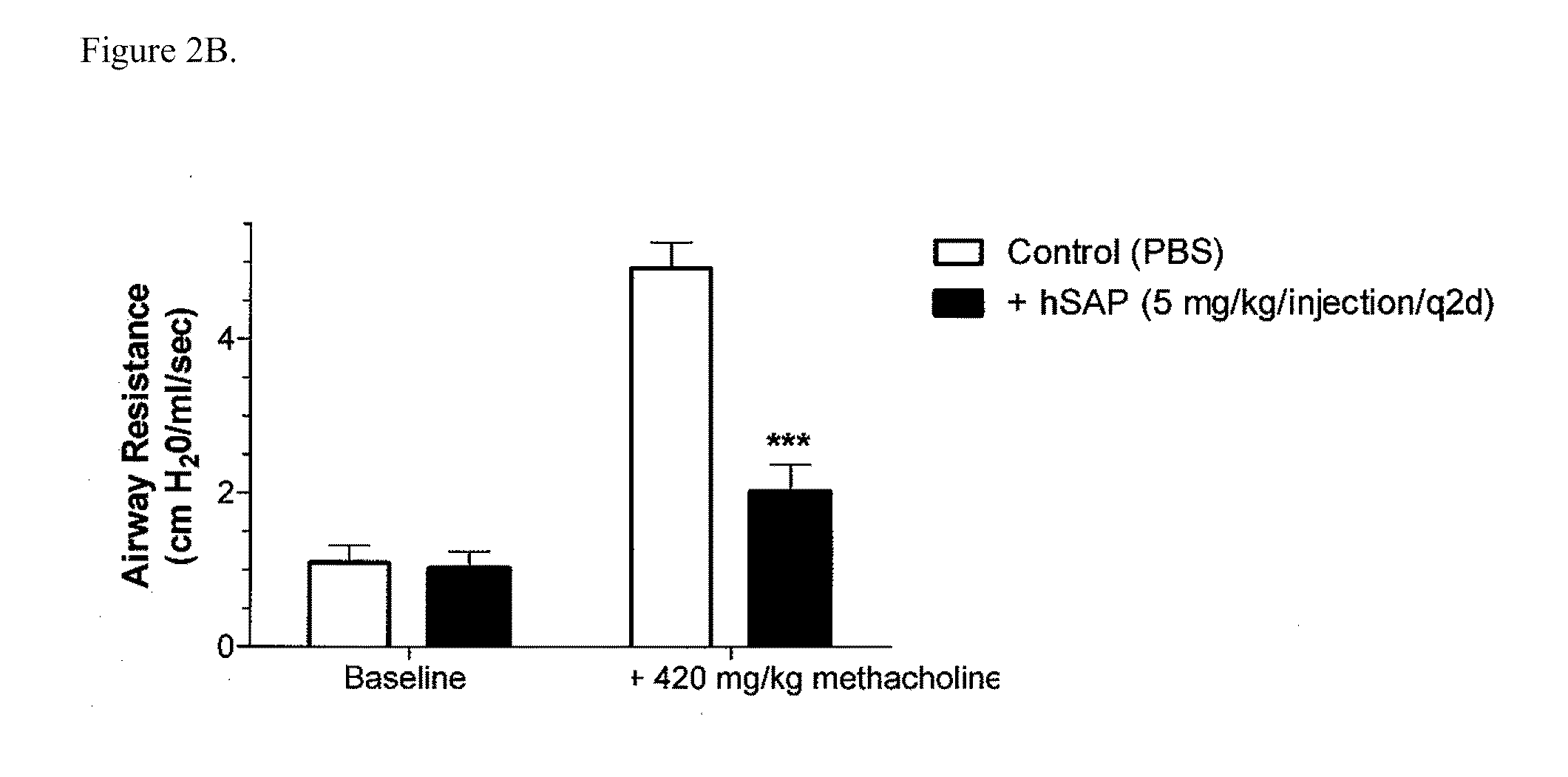
The present invention provides methods and compositions for expanding T regulatory cells ex vivo or in vivo using one or more SAP agonists. The methods and compositions are useful in the treatment of autoimmune diseases and in preventing foreign graft rejection.
Owner:PROMEDIOR
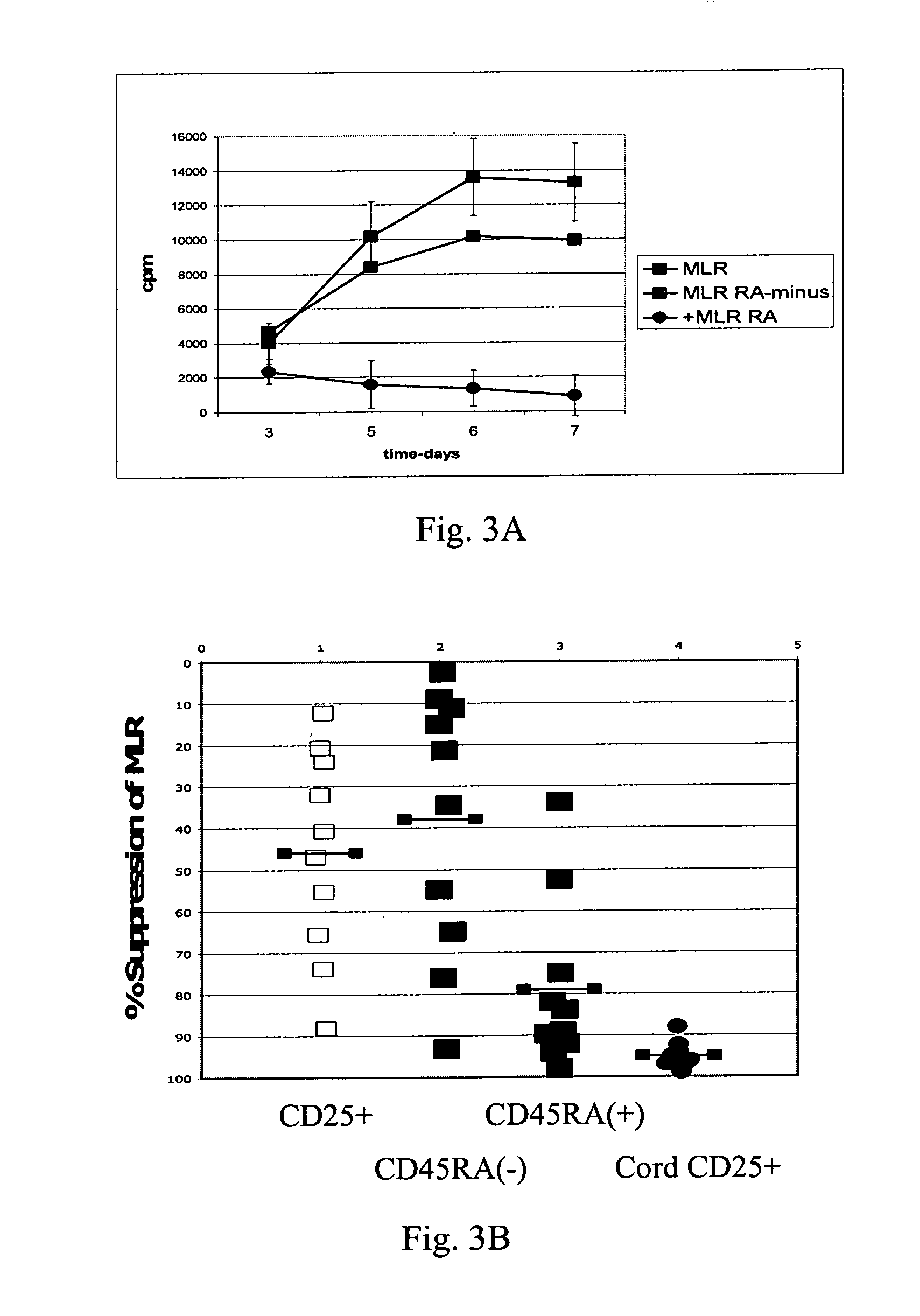
Methods for the isolation and expansion of cord blood derived T regulatory cells
ActiveUS20060062763A1Prevent proliferationBiocideArtificial cell constructsCord blood stem cellT-regulatory cell
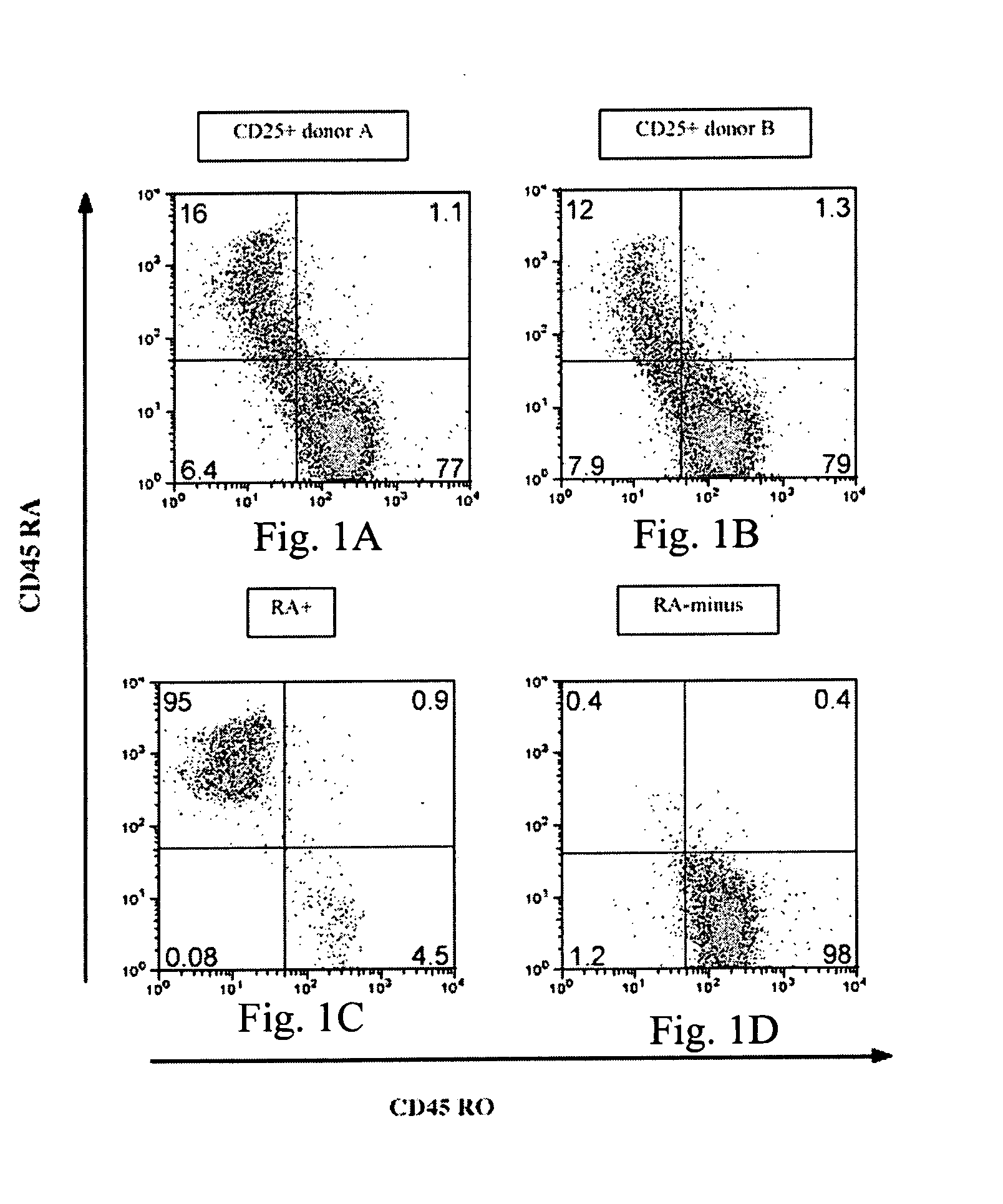
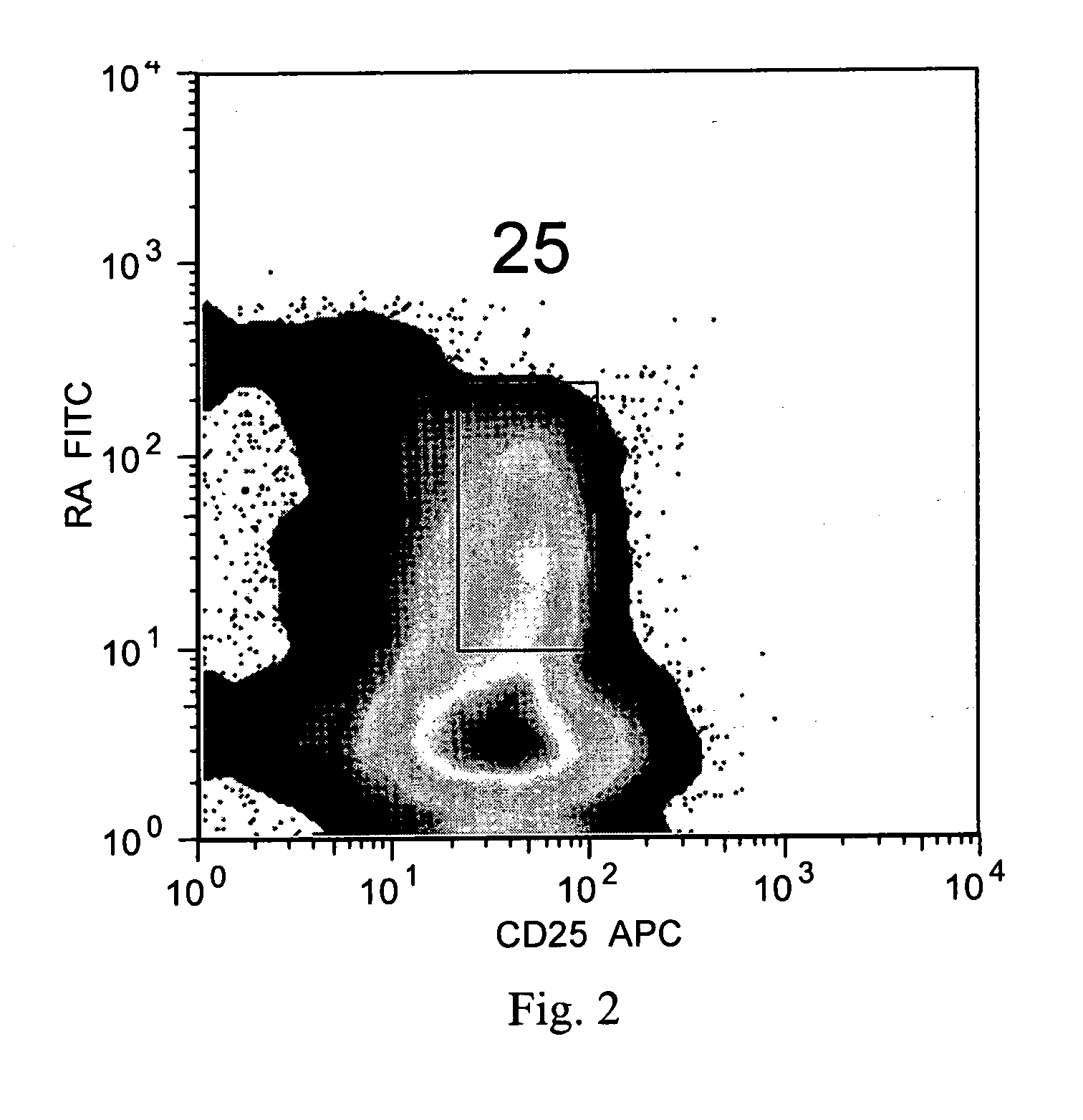
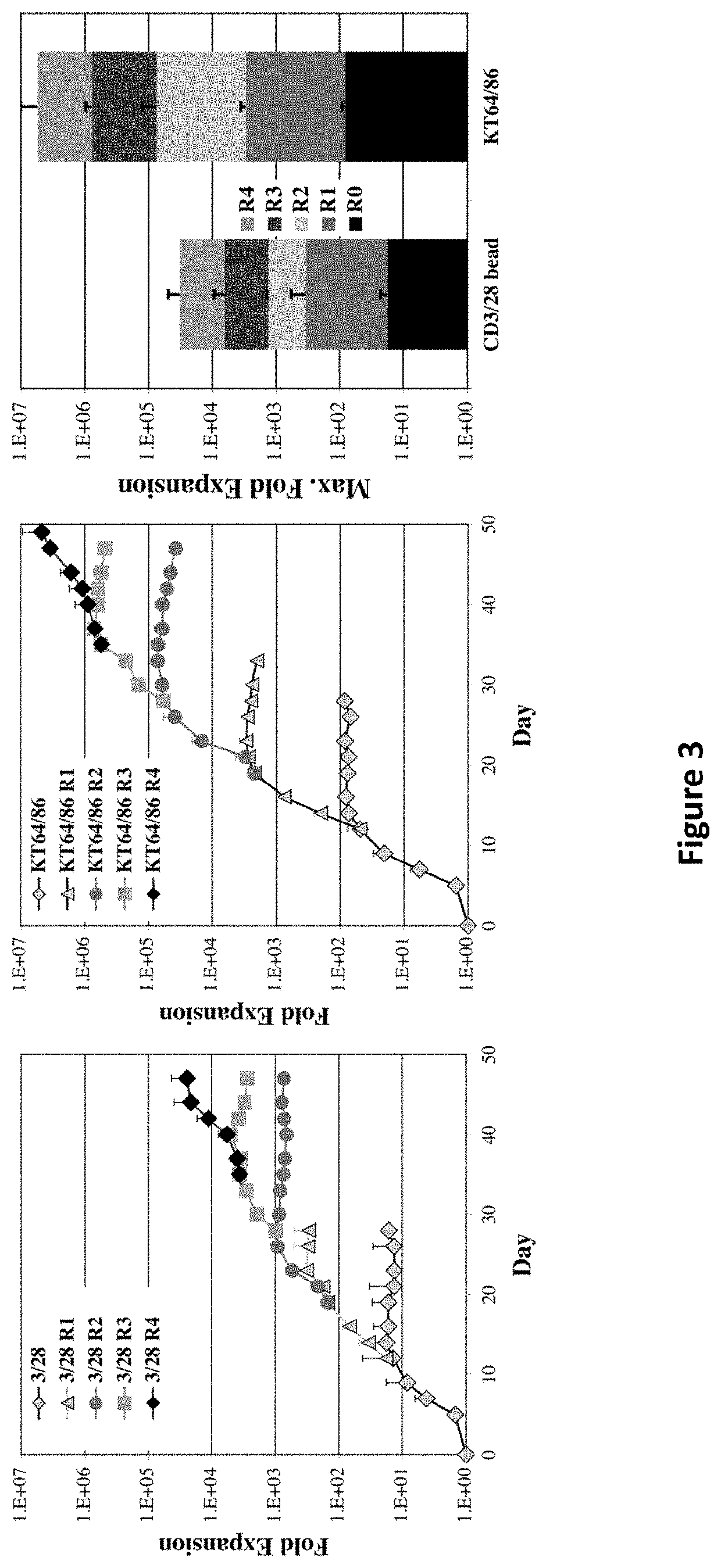
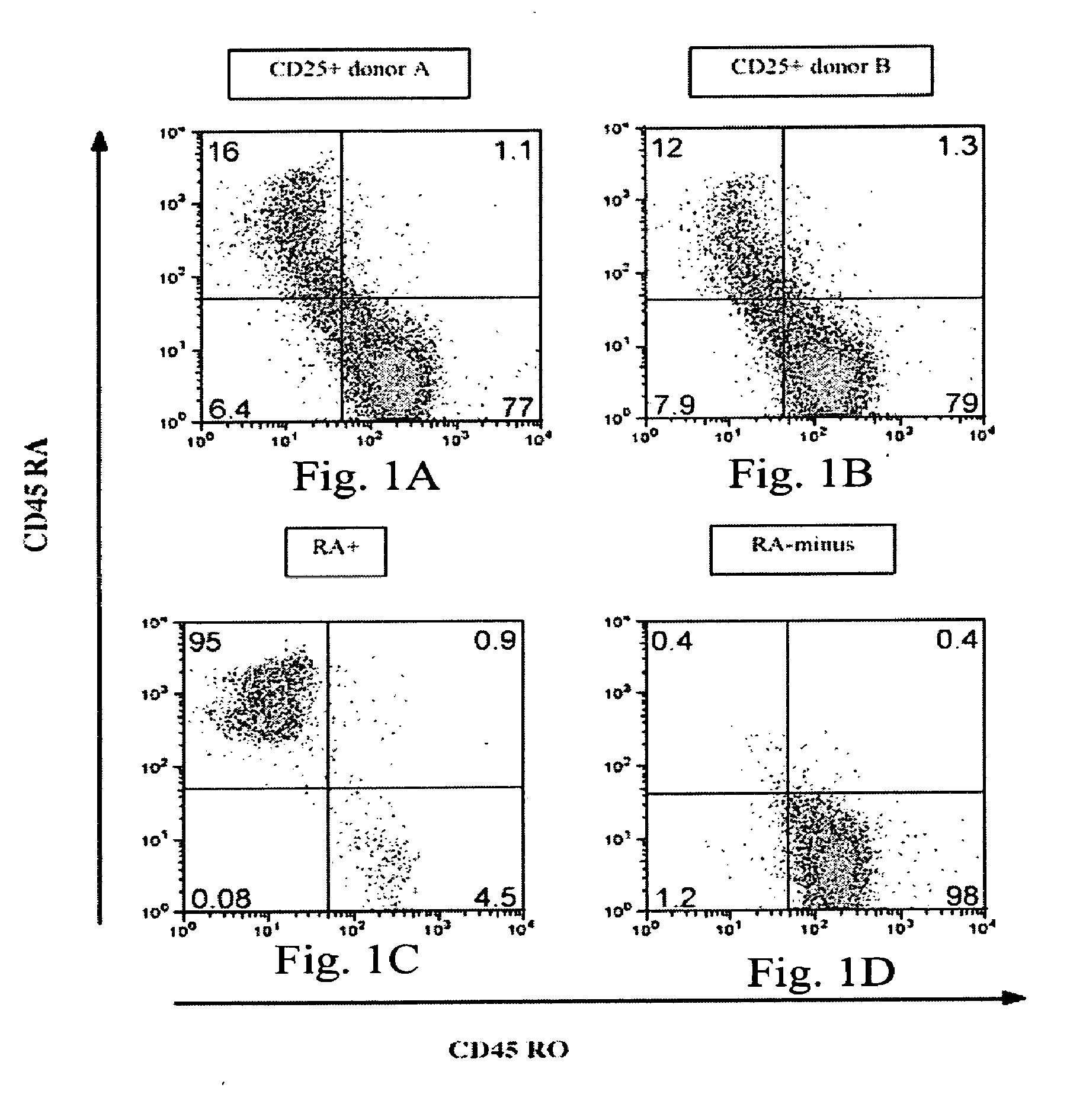
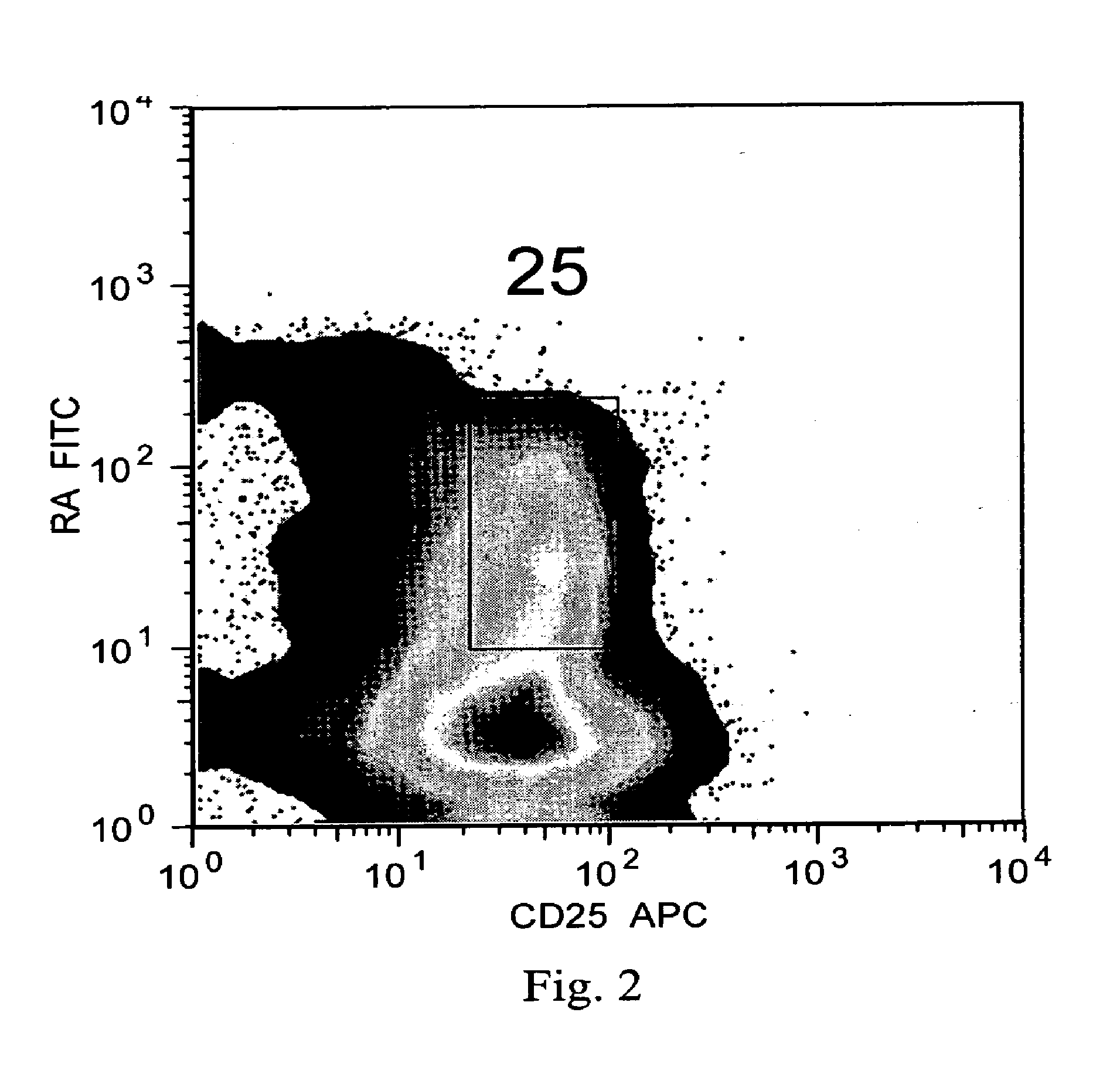
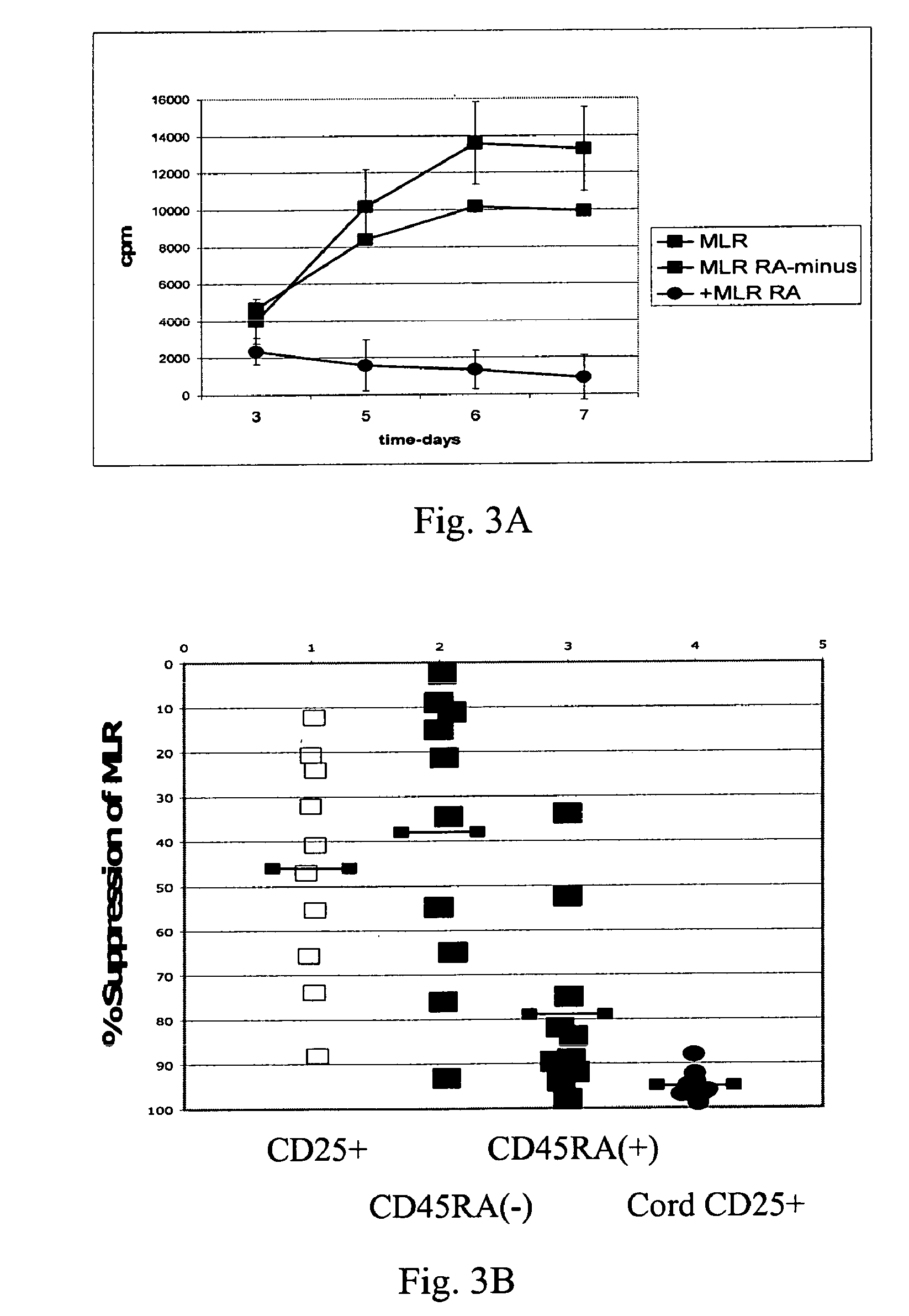
The present invention encompasses methods, and kits for the isolation and expansion of T regulatory cells having the CD45RA+ phenotype, including such cells from human umbilical cord blood.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Control of vagal stimulation
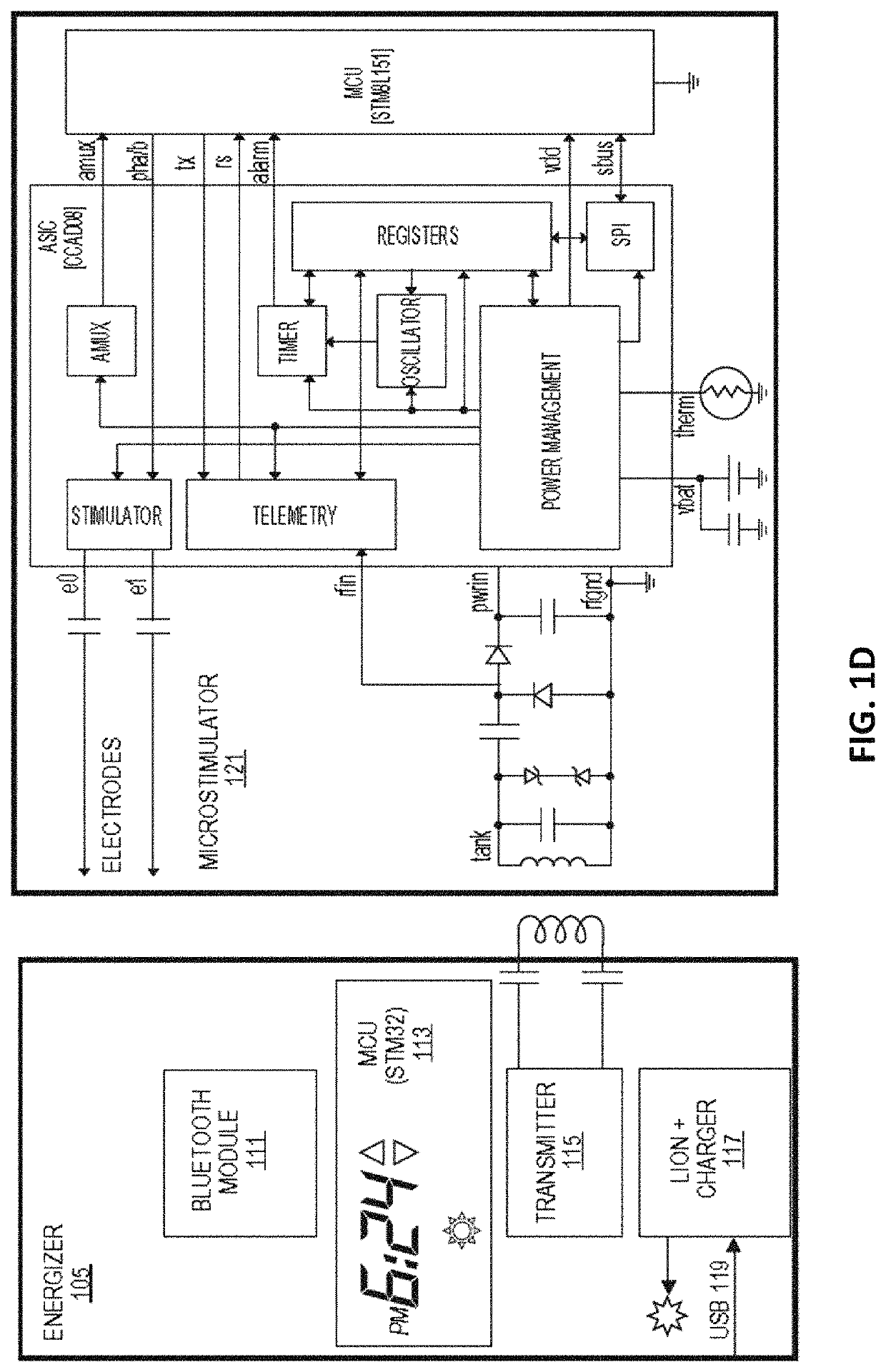
ActiveUS20170203103A1Increase amplitudeLower Level RequirementsSpinal electrodesImplantable neurostimulatorsRR intervalT-regulatory cell
Methods and apparatuses for stimulation of the vagus nerve to treat inflammation including adjusting the stimulation based on one or more metric sensitive to patient response. The one or more metrics may include heart rate variability, level of T regulatory cells, particularly memory T regulatory cells, temperature, etc. Stimulation may be provided through an implantable microstimulator.
Owner:SETPOINT MEDICAL CORP
Myeloid Suppressor Cells, Methods For Preparing Them, and Methods For Using Them For Treating Autoimmunity
The present invention relates to novel myeloid suppressor cells (MSCs) and to methods of isolating these MSCs are also included. The MSCs of the present invention can be used to treat or prevent autoimmune diseases or alloimmune responses. The MSCs of the present invention may also be used to reduce a T cell response, induce T regulatory cells, and produce T cell tolerance.
Owner:MT SINAI SCHOOL OF MEDICINE
Method for culturing autologous peripheral blood lymphocyte
InactiveCN104371974AInhibitionEnhance killing activityBlood/immune system cellsBiological activationCytokine
The invention relates to a method for culturing an autologous peripheral blood lymphocyte. The method comprises the following steps: (1) separating a mononuclear cell from peripheral blood, resuspending in an X-VIVO15 serum-free culture medium to obtain cell concentration of 1*10<6> / mL, and culturing for 3 days; (2) supplementing the X-VIVO15 serum-free culture medium to 100 mL, adding IL-21*10<3> U / mL, and culturing for 1 day; (3) supplementing the X-VIVO15 serum-free culture medium to 200-240 mL, adding IL-21*10<3> U / mL, and culturing for 3 days; (4) supplementing the X-VIVO15 serum-free culture medium to 1000 mL, adding IL-21*10<3> U / mL, CTLA-4mAb100n g / mL and PD-1mAb100n g / mL; and (5) culturing for 5-7 days to prepare the autologous peripheral blood lymphocyte. The method disclosed by the invention can be used for improving the activation efficiency and amplification efficiency of an effector cell group by adding multiple monoclonal antibodies and cell factors to the X-VIVO15 serum-free culture medium, and can be used for effectively reducing the content of T regulatory cells by covering CTLA-4 and PD-1 molecules of the surfaces of all CIK cells by loading CTLA-4 and PD-1 antibodies in vitro especially, thus further enhancing the killing effect of the CIK cells on tumors.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Compositions and methods for treating foxp3+ treg related diseases
InactiveUS20130323283A1Increase productionSlow tumor growthBiocidePeptide/protein ingredientsDiseaseTest sample
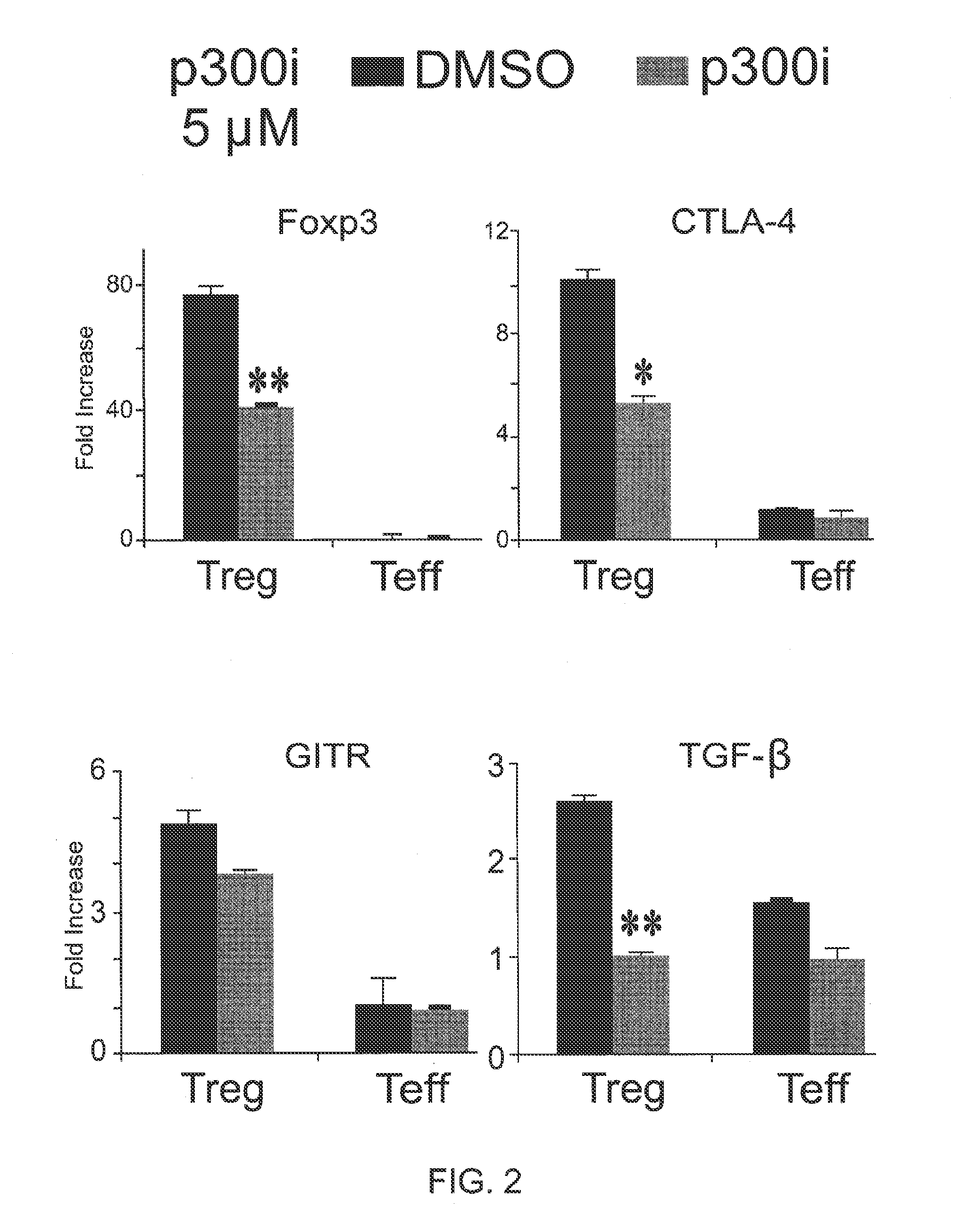
Methods for treating or preventing a Foxp3+ T regulatory cell (Treg) related disease in a subject in need thereof comprise administering to the subject an effective amount of a pharmaceutical composition comprising an inhibitor of a histone / protein acetyltransferase (HAT). Methods for identifying an agent useful for treating or preventing a Foxp3+ T regulatory cell (Treg) related disease comprise (a) contacting a candidate agent with a test sample comprising Foxp3+ T regulatory cells (Tregs), and (b) comparing a function of the Foxp3+ Tregs in the test sample with that in a control sample, wherein inhibition of the function of the Foxp3+ Tregs in the test sample when compared with the control sample indicates that the candidate agent is an agent useful for treating or preventing a Foxp3+ Treg related disease.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods for expansion or depletion of t-regulatory cells
Owner:THE GENERAL HOSPITAL CORP
Methods to Expand a T Regulatory Cell Master Cell Bank
InactiveUS20130101567A1Reduce probabilityExpand the populationBiocideCulture processT-regulatory cellCell therapy
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Methods for expansion or depletion of T-regulatory cells
Owner:THE GENERAL HOSPITAL CORP
Stem cell mediated treg activation/expansion for therapeutic immune modulation
ActiveUS8241621B2High activityIncrease the number ofBiocideGenetic material ingredientsT cellBiological activation
Disclosed are cells, methods of modulating cells, and therapeutic uses of the cells for the immune modulation of mammals in need thereof. Immune modulation including alteration of cytokine profile, cytotoxic activity, antibody production and inflammatory states is achieved through the administration of various cell types that have been unmanipulated or manipulated in order to endow specific biological activity. Cellular subsets and administration of the subsets in combination with various agents are also provided. One embodiment teaches the previously unknown finding that adipose tissue derived mononuclear cells contain T cells with immune regulatory properties that alone or synergistically with various stem cells induce immune modulation upon administration. Another embodiment is the finding that stimulation of stem cell activation results in stem cell secondary activation of immune modulatory cells, one type which is T regulatory cells (Tregs). One specific embodiment involves extraction of a heterogenous stem cell pool, which contains T regulatory cells, treatment in culture of the population with agents known to stimulate stem cell activation, then subsequent extraction and administration of the purified Tregs. Other embodiments include expansion of Tregs in the presence of antigen in order to generate anti-specific Tregs.
Owner:XON CELLS
Methods and compositions for expanding T regulatory cells
InactiveCN101378783AHigh affinityEfficient signal transductionCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsDendritic cellAutoimmune disease
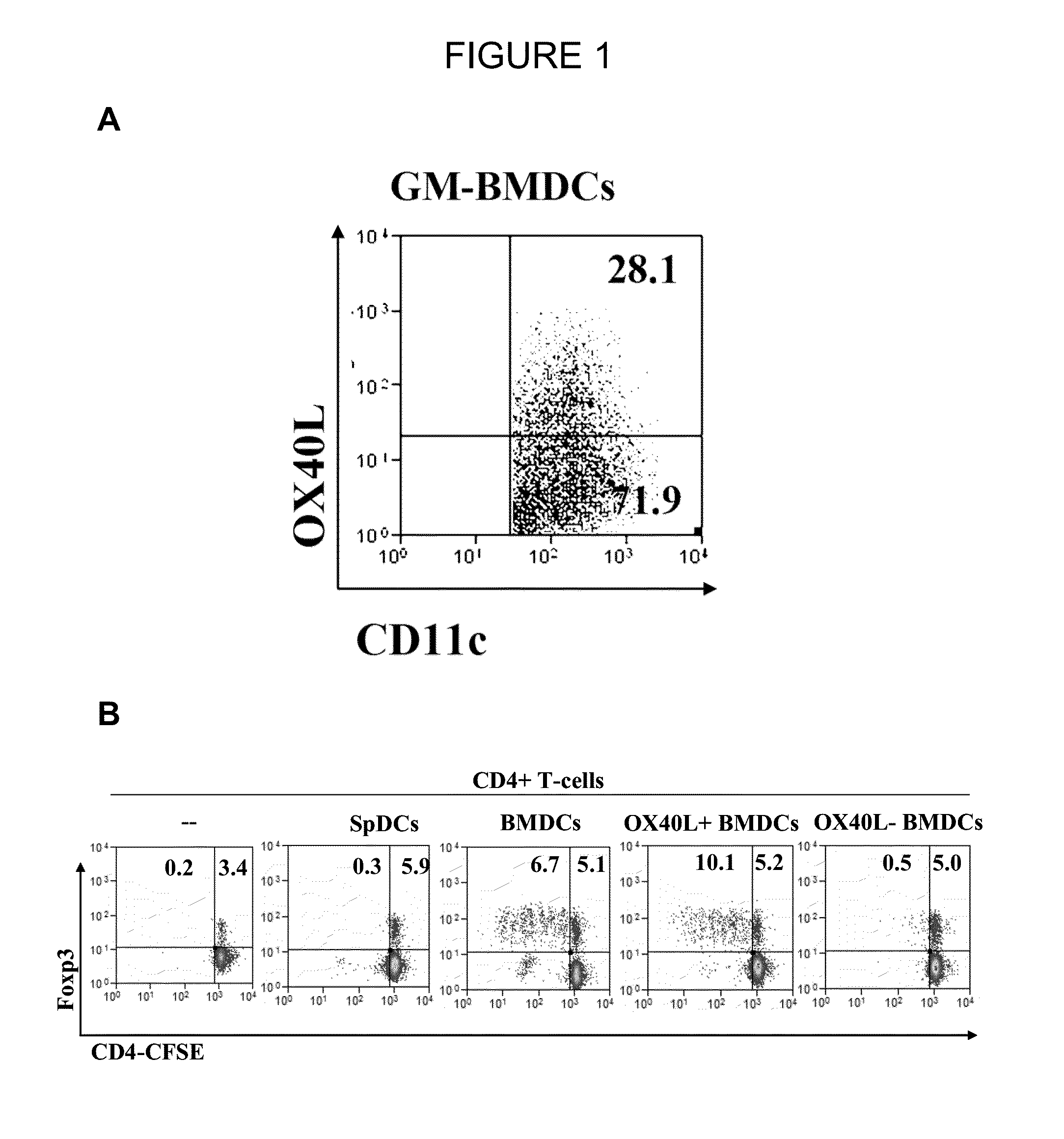
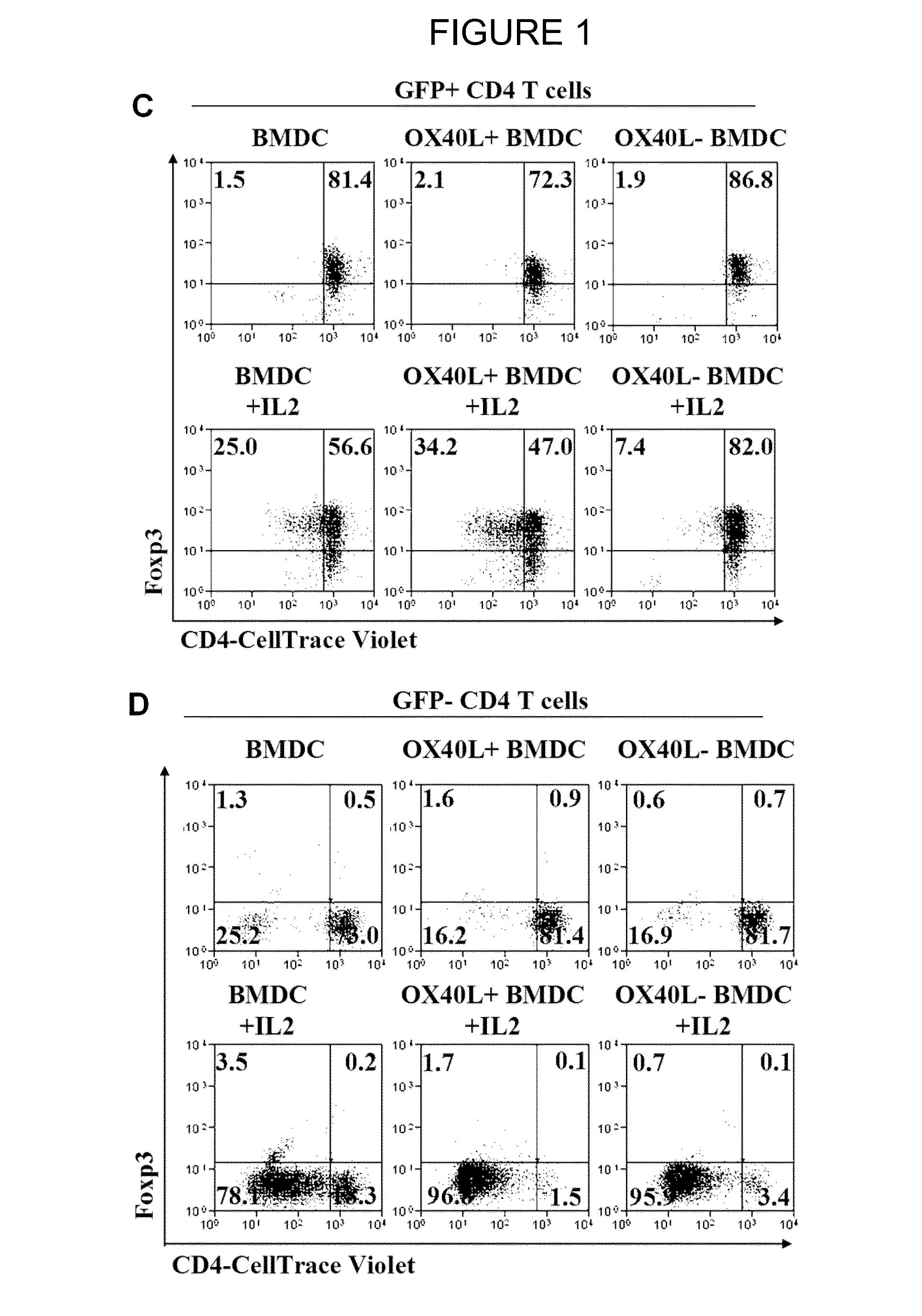
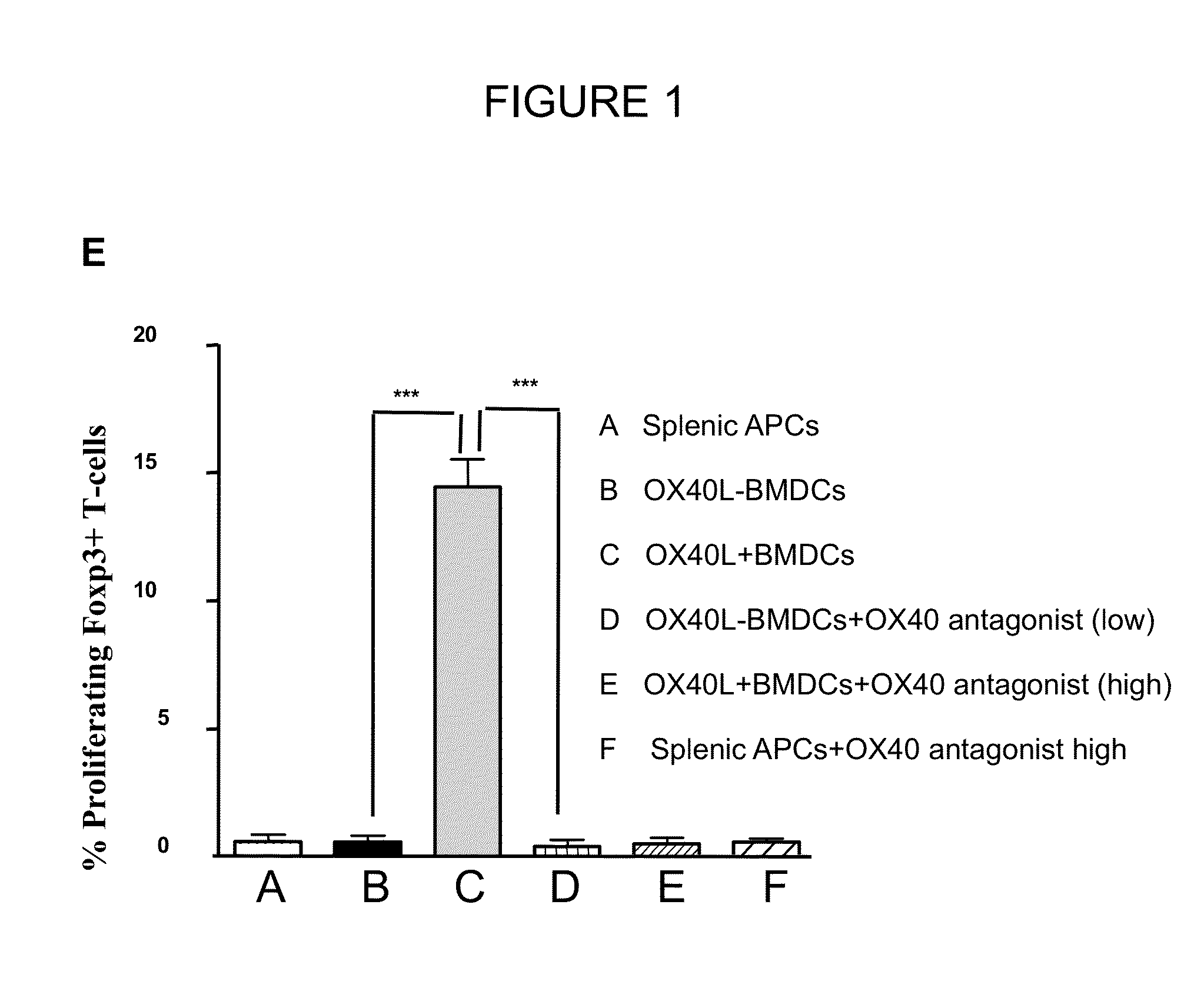
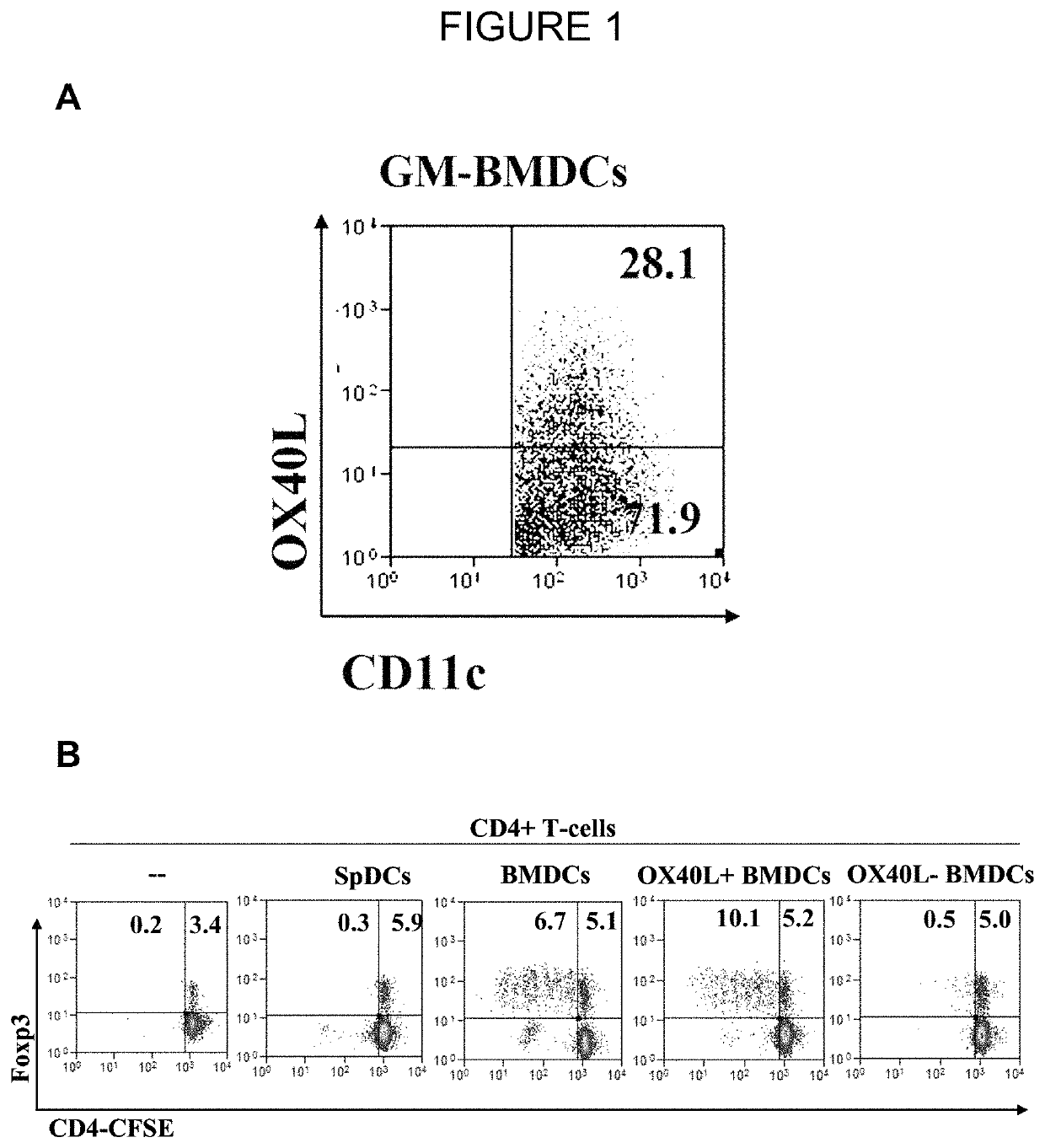
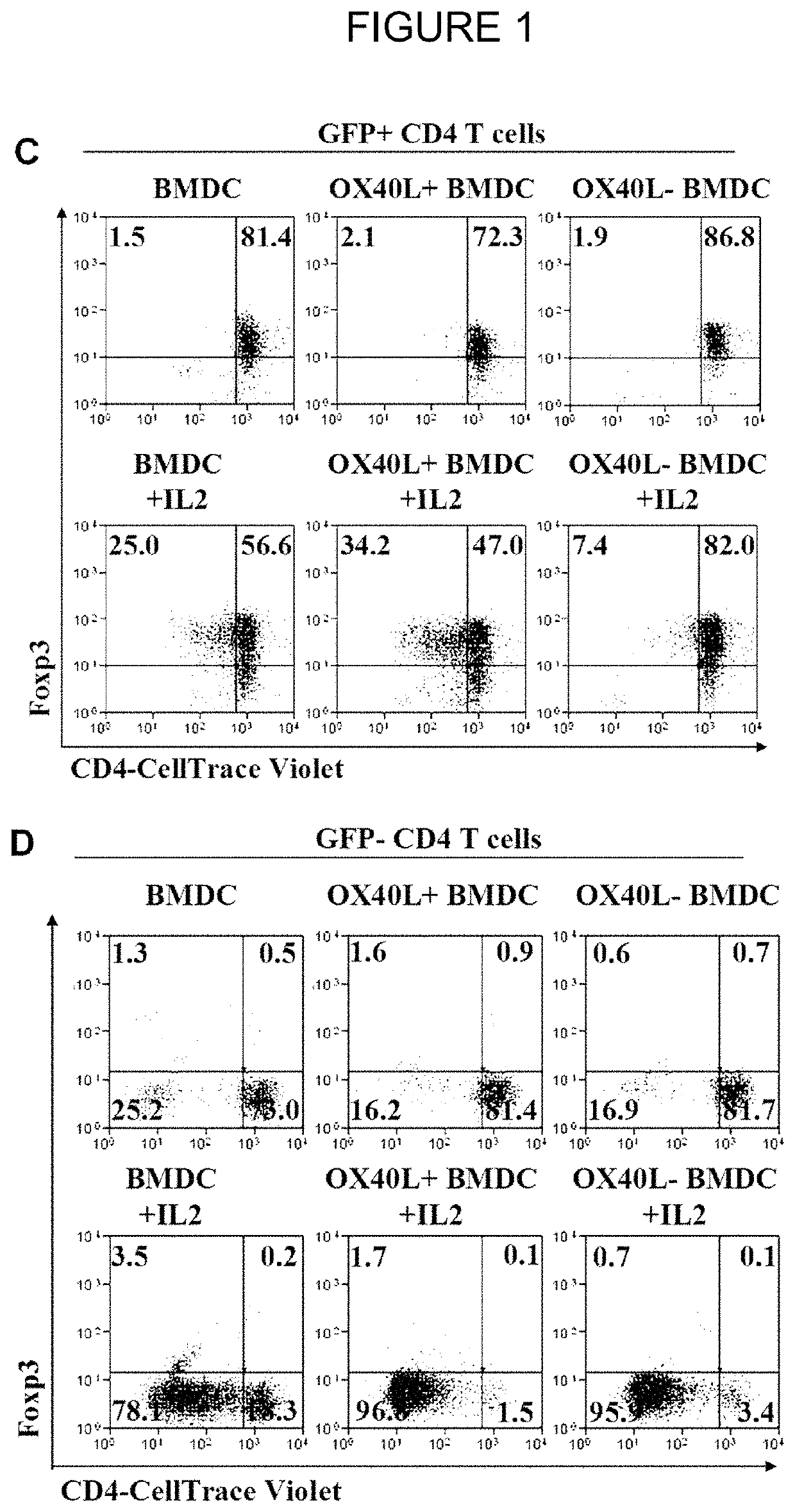
The present invention provides methods and compositions for expanding Treg cells ex vivo or in vivo using one or more conjugates comprising a costimulatory moiety that stimulates at least one of three signals involved in Treg cell development and / or using dendritic cells pulsed with antigens and modified to display TGF-beta, or hematopoetic stem cells or bone marrow cells modified to display TGF-beta. The methods and compositions are useful, for example, in the treatment and prevention of autoimmune disease, including Type 1 diabetes and in preventing foreign graft rejection, as well as to establish mixed chimerism, induce tolerance to autoantigens, alloantigens or xenoantigens, beta cell regeneration, prevention of foreign graft rejection, and treatment of a genetically inherited hematopoietic disorder.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Methods and compositions for expanding T regulatory cells
InactiveUS7745215B2Peptide/protein ingredientsAntibody mimetics/scaffoldsAutoimmune conditionDendritic cell
The present invention provides methods and compositions for expanding Treg cells ex vivo or in vivo using one or more conjugates comprising a costimulatory moiety that stimulates at least one of three signals involved in Treg cell development and / or using dendritic cells pulsed with antigens and modified to display TGF-β, or hematopoetic stem cells or bone marrow cells modified to display TGF-β. The methods and compositions are useful, for example, in the treatment and prevention of autoimmune disease, including Type 1 diabetes and in preventing foreign graft rejection, as well as to establish mixed chimerism, induce tolerance to autoantigens, alloantigens or xenoantigens, beta cell regeneration, prevention of foreign graft rejection, and treatment of a genetically inherited hematopoietic disorder.
Owner:UNIV OF LOUISVILLE RES FOUND INC
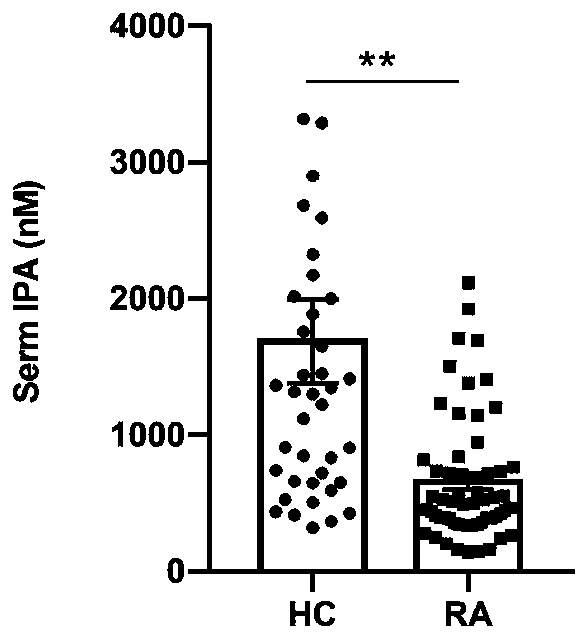
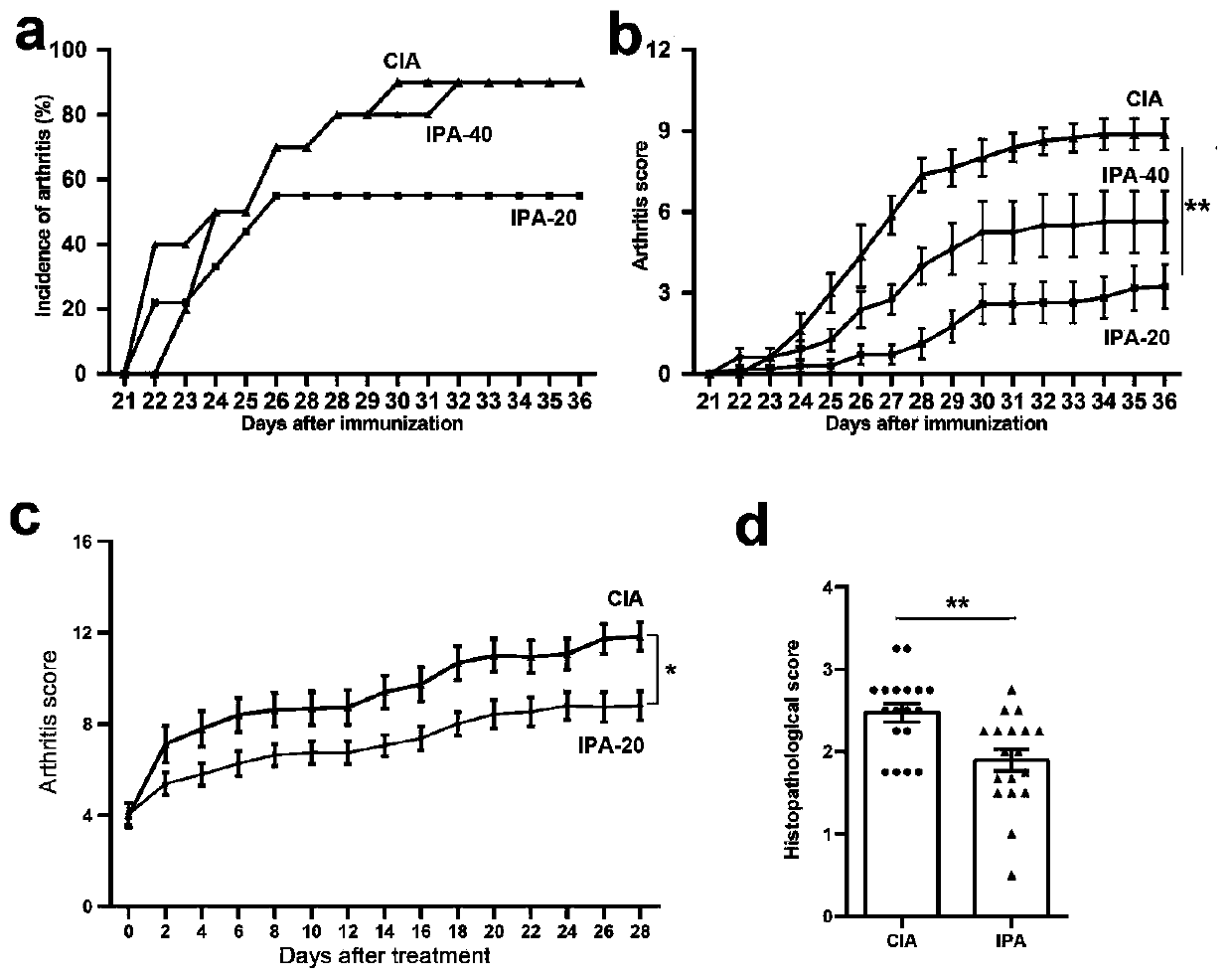
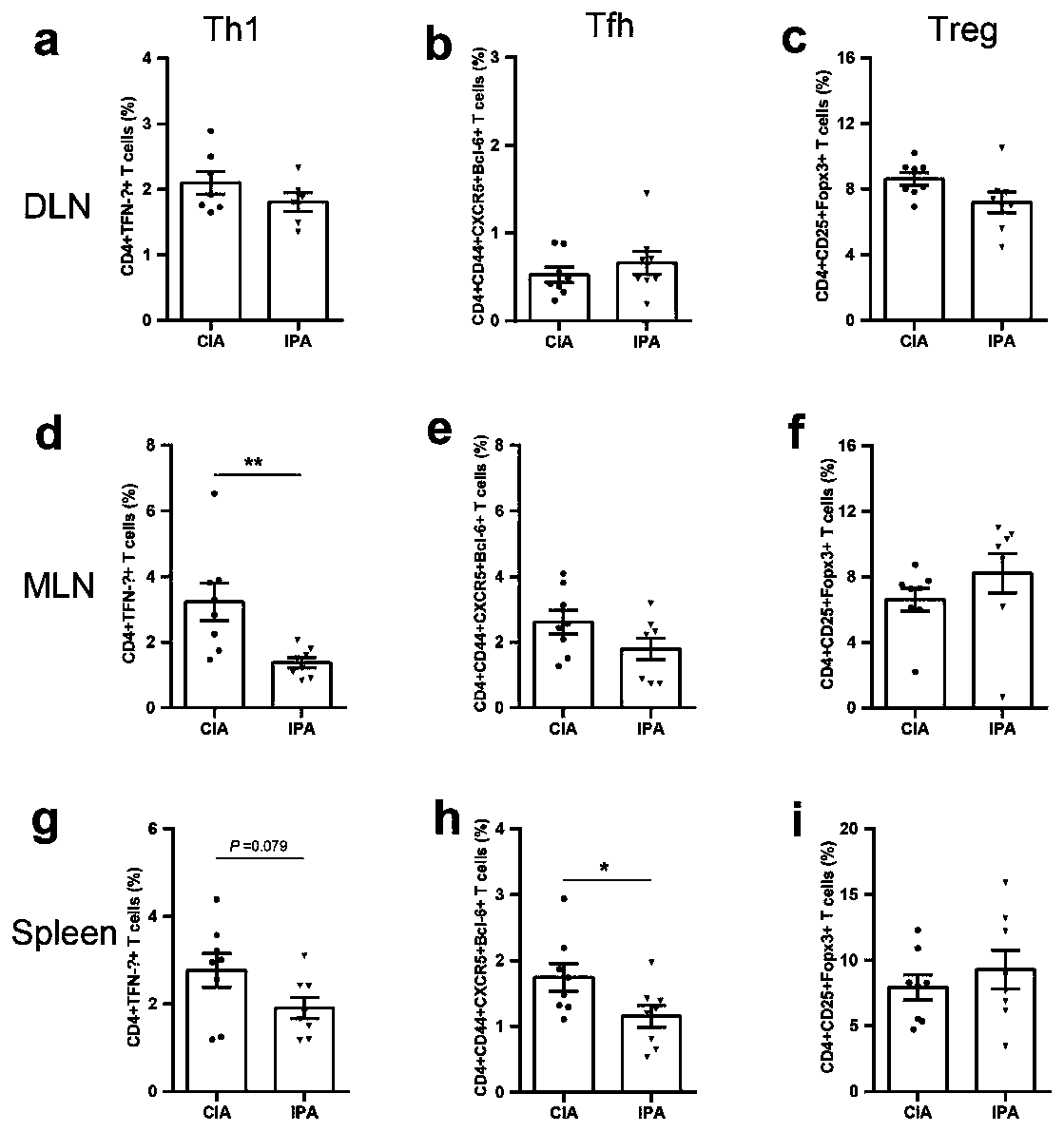
Application of indole-propioponic acid to preparing medicine for controlling rheumatoid arthritis
The invention provides application of indole-propioponic acid to preparing medicine for controlling rheumatoid arthritis, and relates to the technical field of rheumatoid arthritis disease treatment.Through the indole-propioponic acid, occurrence and development of experimental arthritis can be remarkably inhibited, and joint swelling symptoms are effectively relieved. The indole-propioponic acidcan inhibit a proinflammatory cell Th1 and a follicular helper T cell (Tfh) and increase the proportion of an anti-inflammation T regulatory cell (T regulatory, Treg). The indole-propioponic acid canalso reduce expression of proinflammatory factors IL-1beta, IL-6 and IL-17A in serum and anti-CII (anti-type II collage antibody), and can increase secretion of beta-endorphin with the analgesic effect. The indole-propioponic acid has anti-inflammation and immunosuppression effects, the unbalance state of immune cells in organisms can be corrected, and the aim of controlling rheumatoid arthritisis thus achieved.
Owner:PEOPLES HOSPITAL PEKING UNIV
T-REG Cell Expansion
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Methods To Protect Transplanted Tissue From Rejection
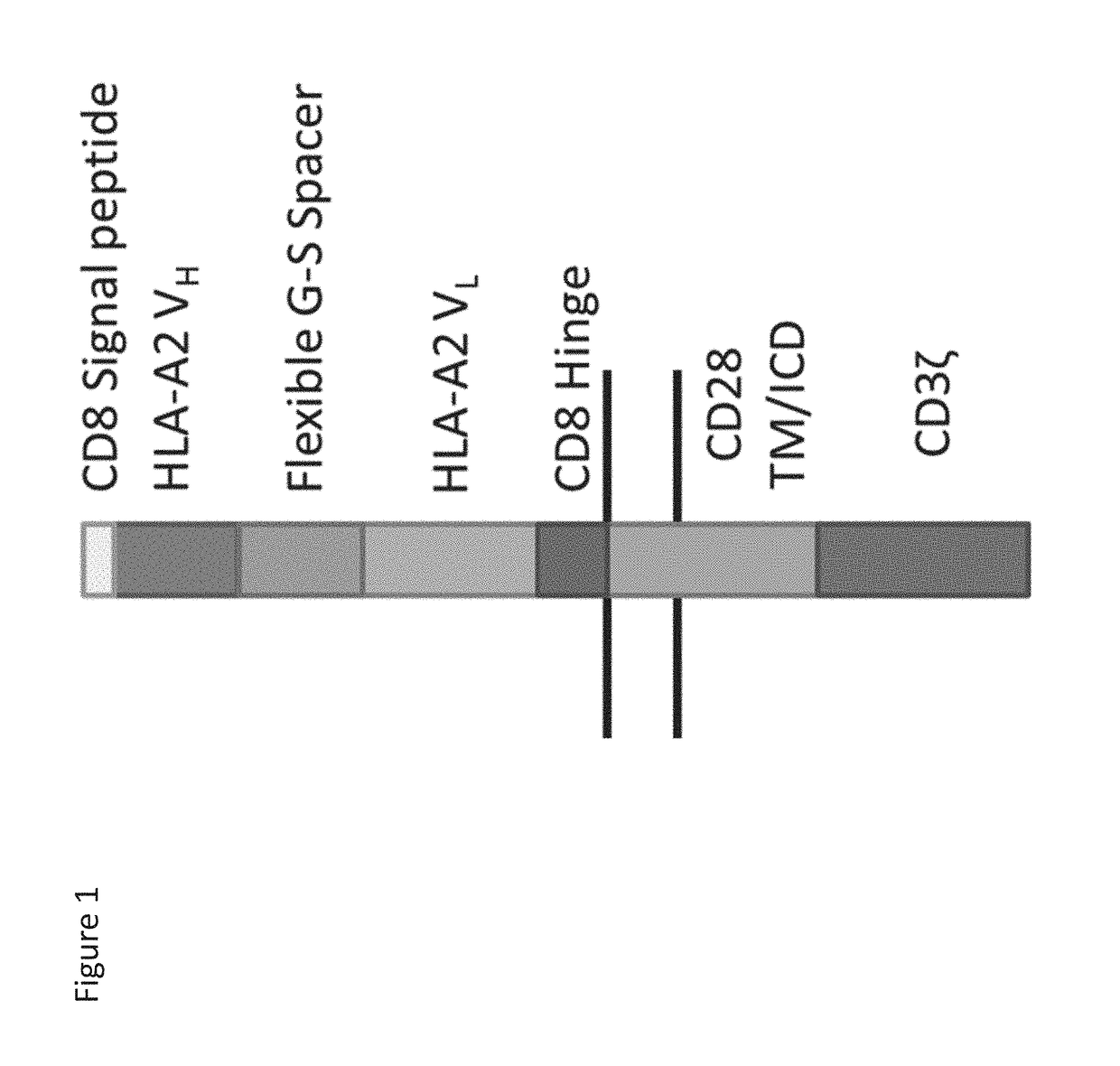
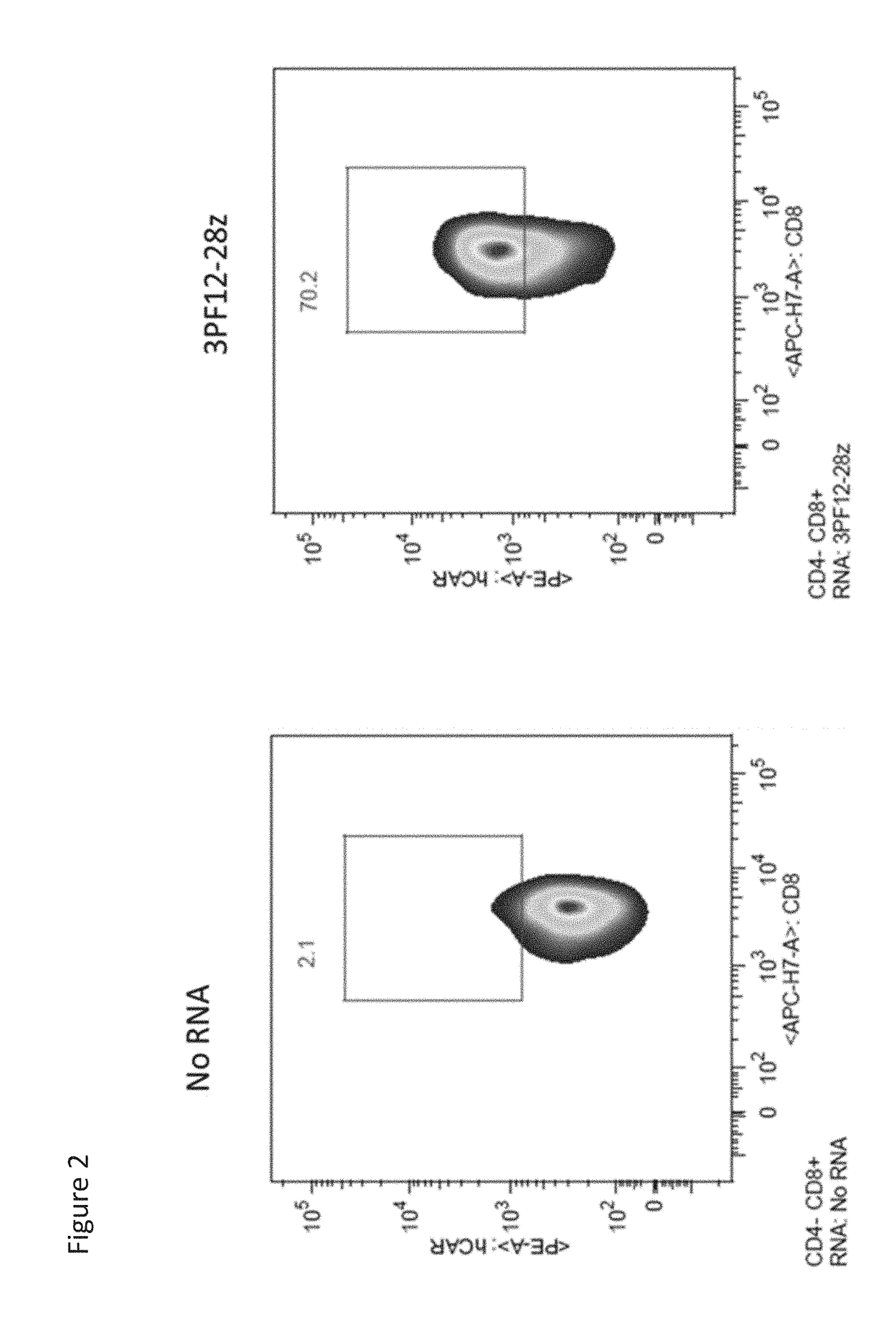
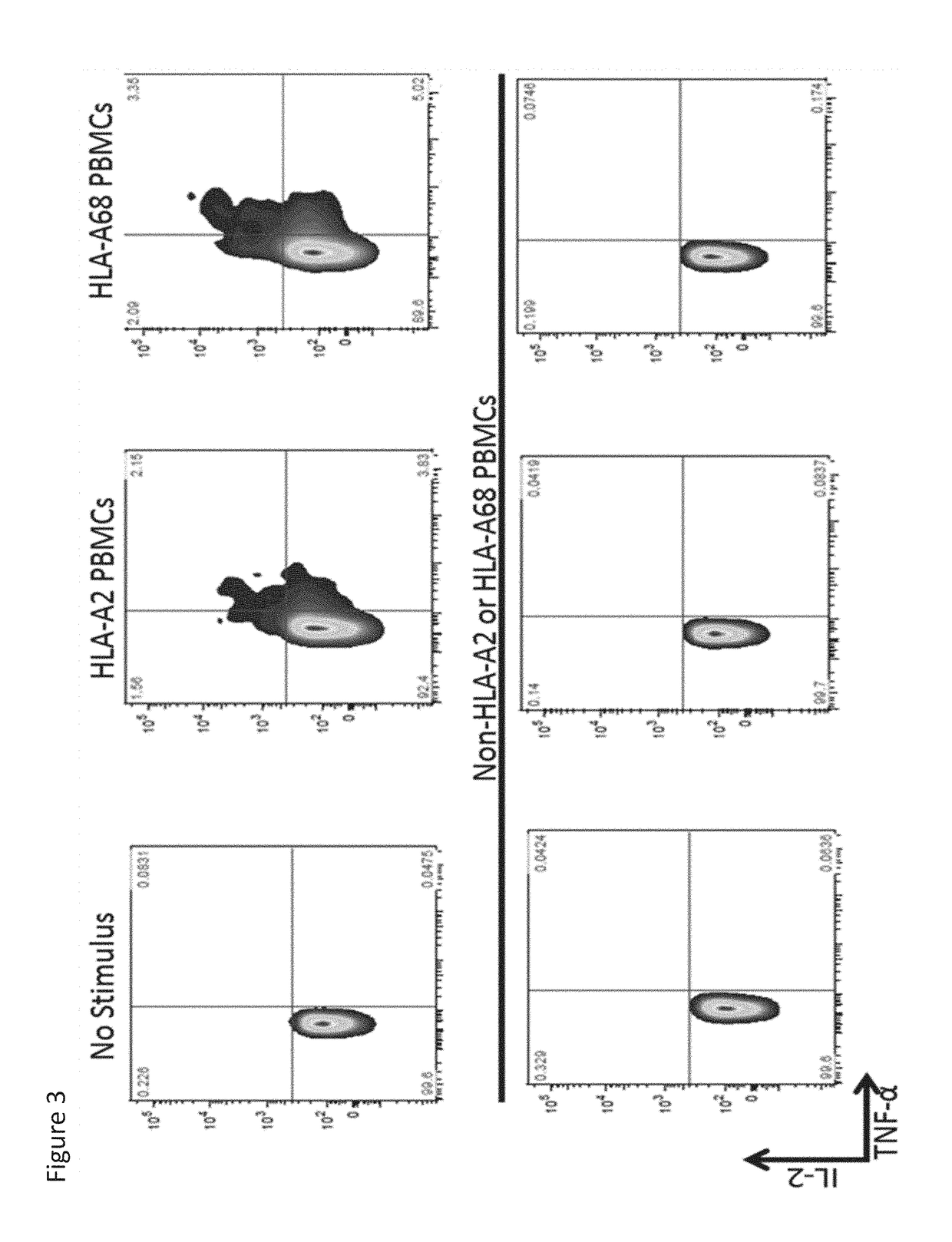
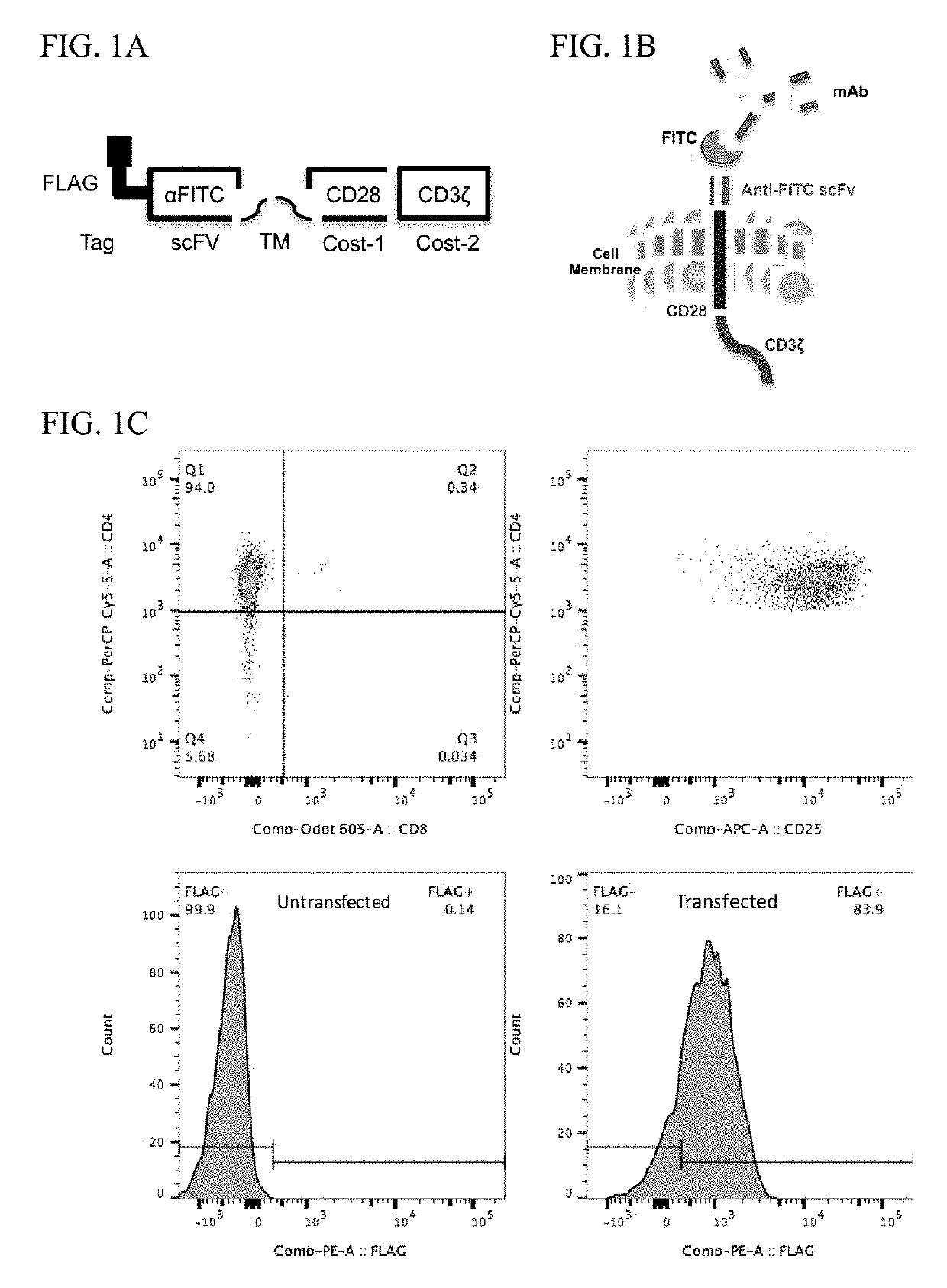
PendingUS20180282416A1Potent immunosuppressive effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyT-regulatory cellChimeric antigen receptor
The present invention includes compositions and methods for an HLA-A2 specific chimeric antigen receptor (CAR). In certain embodiments the HLA-A2 specific CAR is expressed on a T regulatory cell. In certain embodiments, the HLA-A2 specific CAR protects transplanted tissue from rejection.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
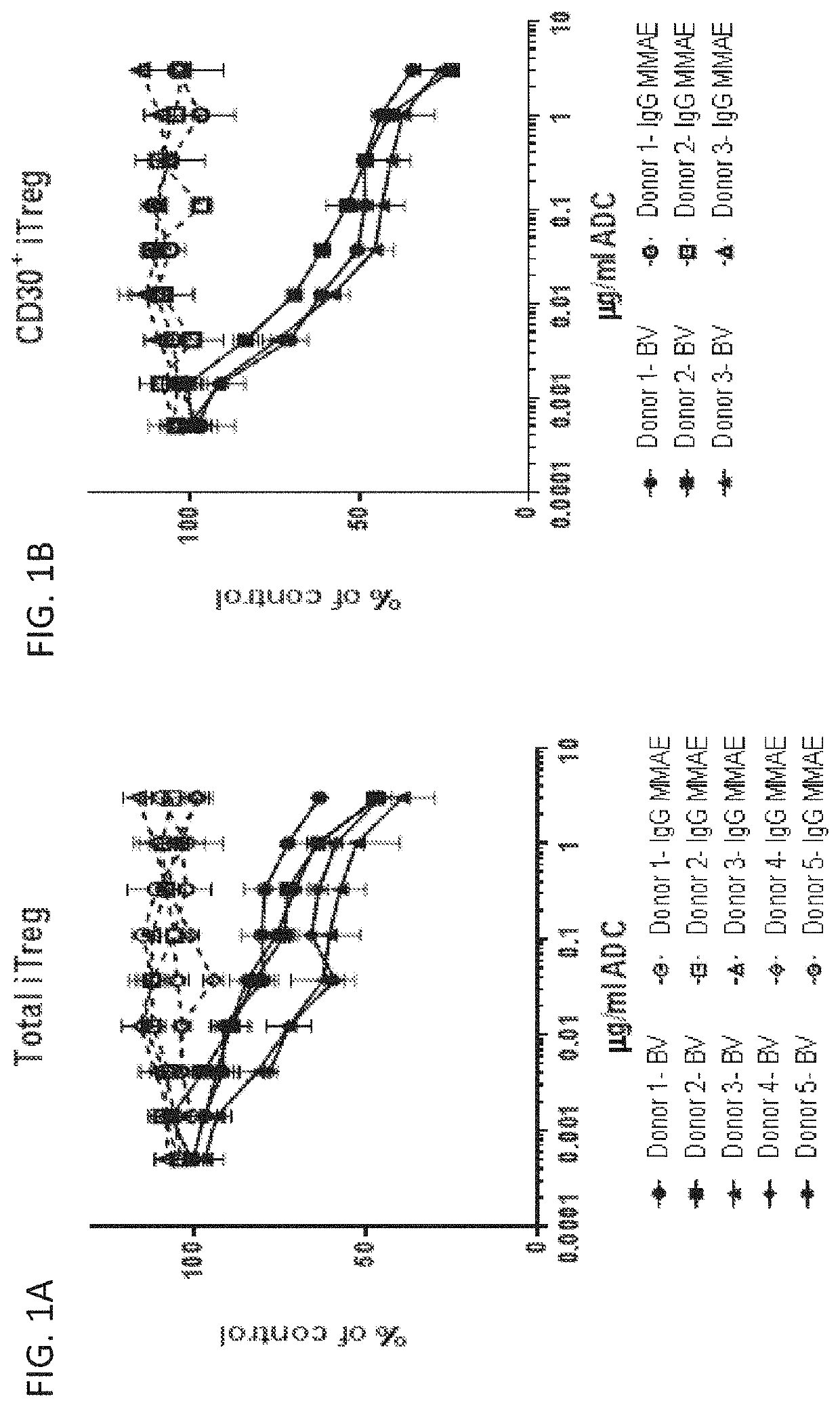
Modulating the immune response using antibody-drug conjugates
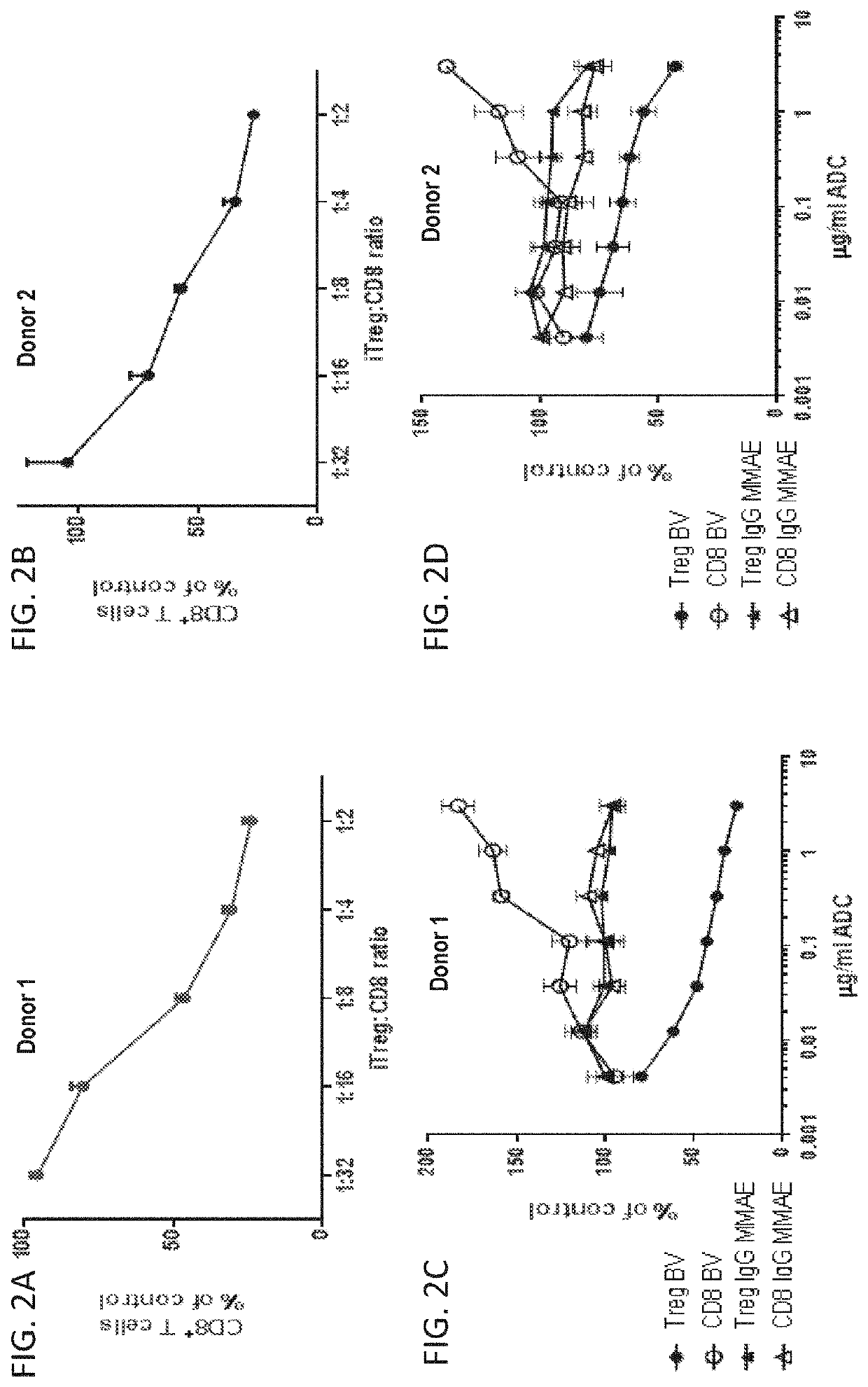
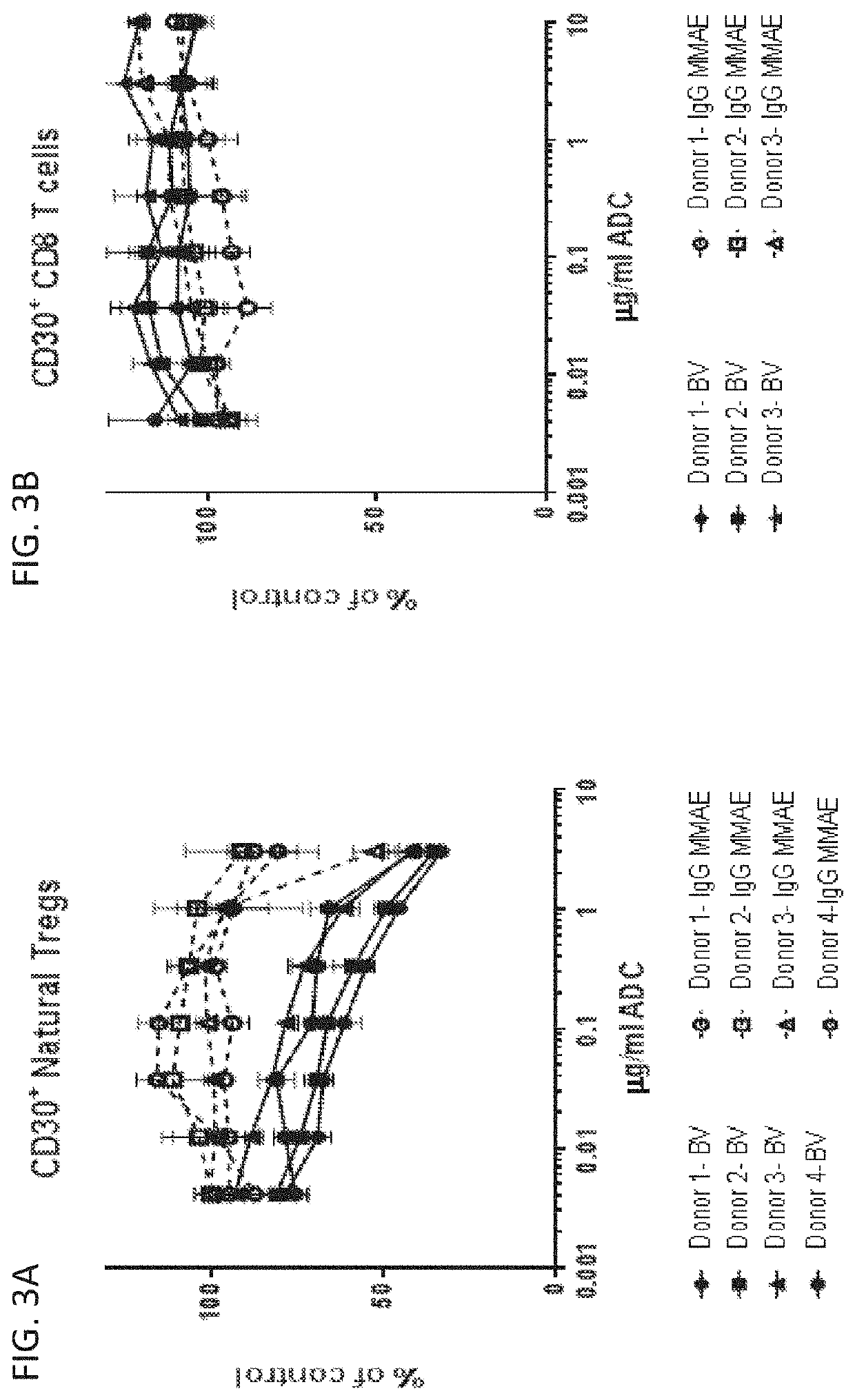
InactiveUS20200239585A1Reduced activityReduce in quantityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesT-regulatory cell
The invention provides methods and compositions for modulating the immune response in a subject, such as decreasing the activity of CD30+ T regulatory cells and increasing the ratio of CD8+ T cells to CD30+ T regulatory cells, through administration of antibody drug-conjugates that bind to CD30. The invention also provides articles of manufacture or kits comprising said antibody drug-conjugates that bind to CD30 for modulating the immune response.
Owner:SEAGEN INC
Methods to Expand a T Regulatory Cell Master Cell Bank
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Methods for the Isolation and Expansion of Cord Blood Derived T Regulatory Cells
ActiveUS20120282694A1Artificial cell constructsBlood/immune system cellsCord blood stem cellT-regulatory cell
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Regulatory t cells targeted with chimeric antigen receptors
InactiveUS20190307795A1Easy to transplantIncrease chimerismPeptide/protein ingredientsAntipyreticAntigenRegulatory T cell
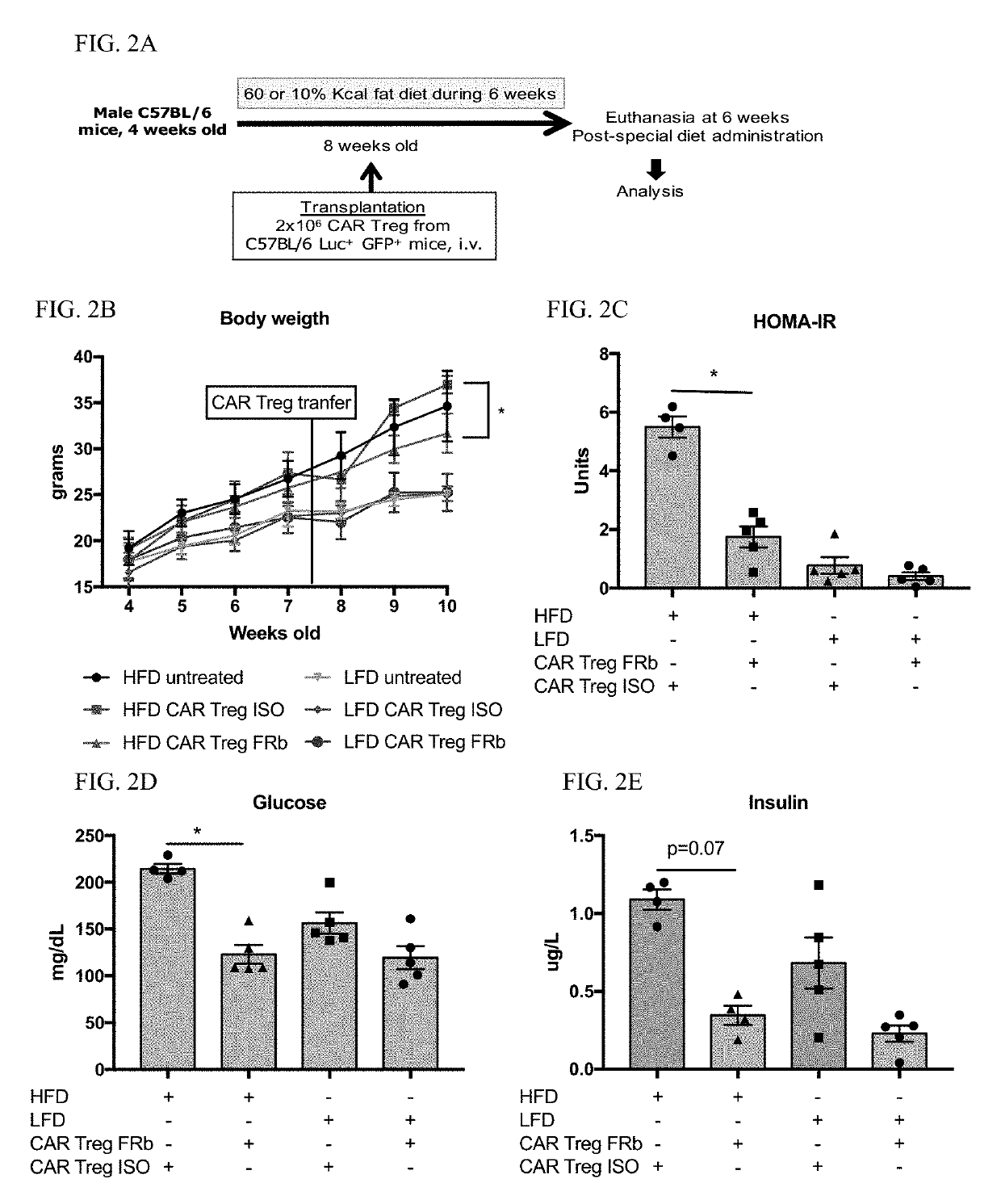
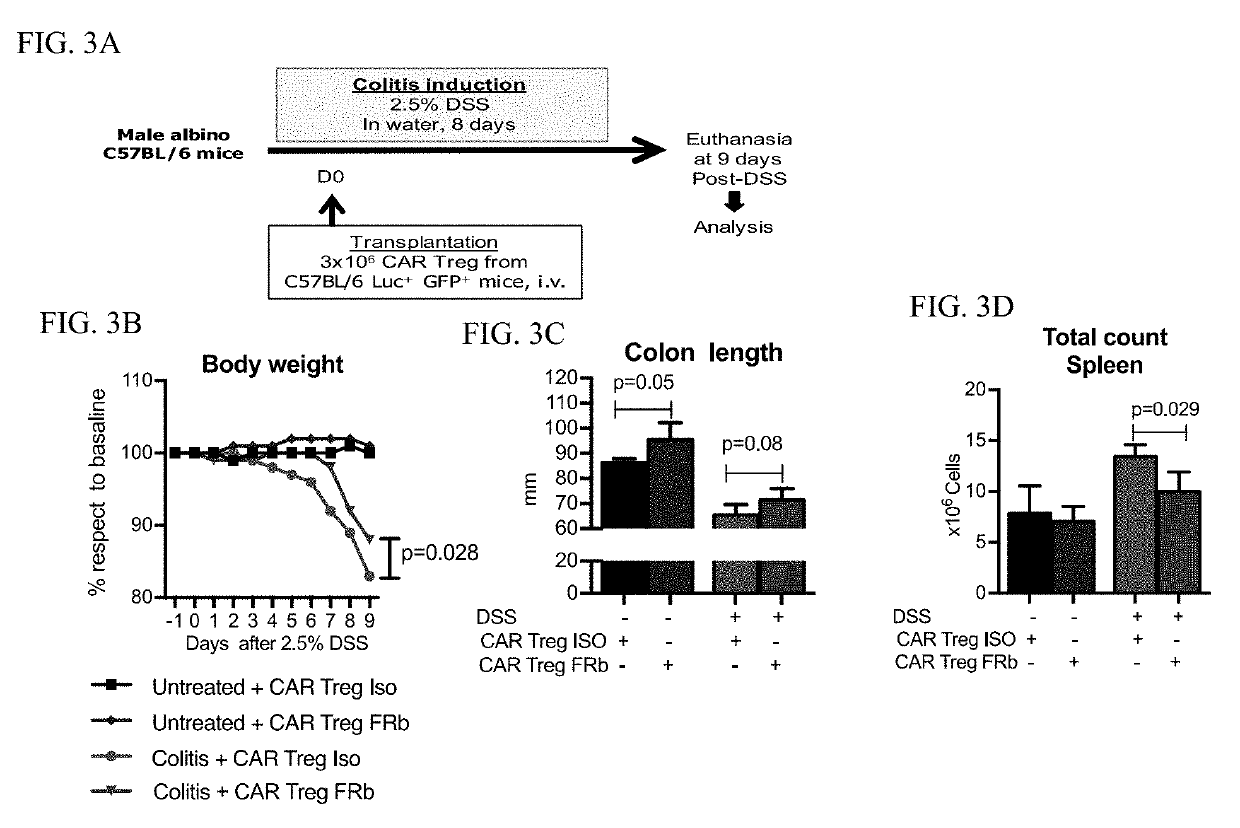
Regulatory T cells (Treg) are engineered to express a chimeric antigen receptor (CAR), that specifically binds folate receptor beta; and are administered to an individual for treatment of inflammation at sites characterized by the presence of activated myeloid cells. Also provided are methods for utilized engineered T regulatory cells to enhance hematopoietic cell transplantation.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Approach for modulating regulatory t cells and inhibiting tumor growth
PendingCN112703039APeptide/protein ingredientsImmunoglobulins against growth factorsRegulatory T cellAutoimmune responses
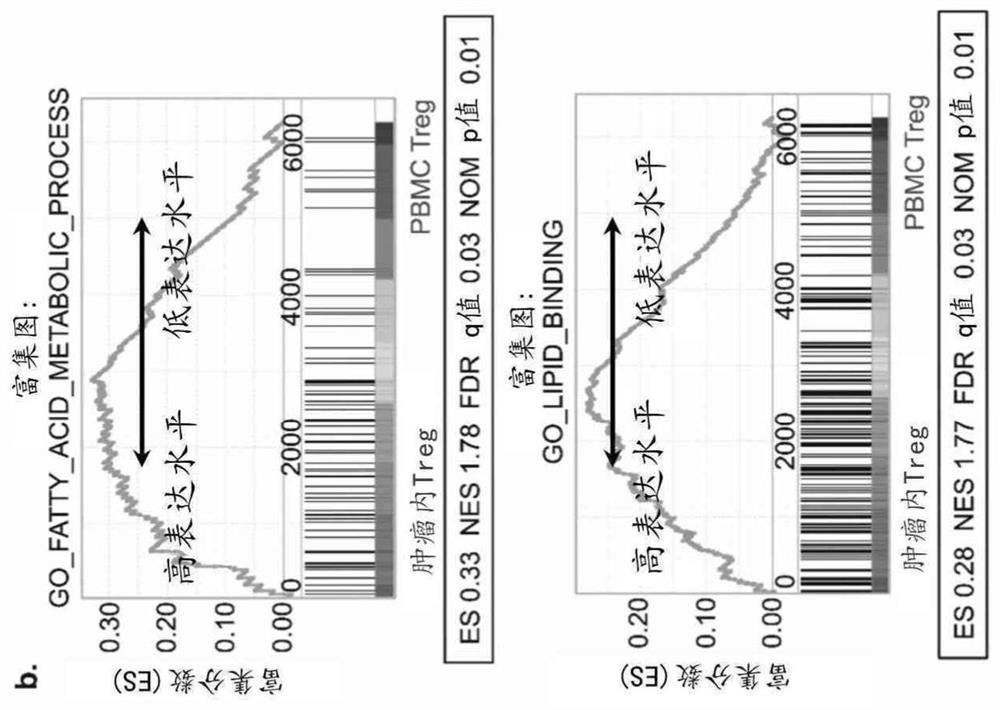
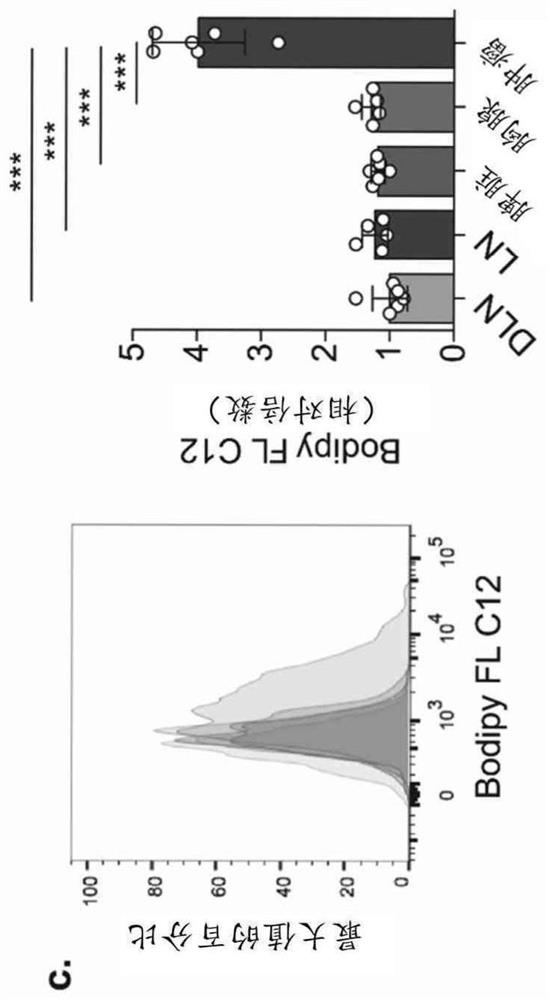
Provided herein is approach that specifically modulates the activity and / or the number of intratumoral regulatory T (Treg) cells in a subject. Such an approach can be used to reduce the number of intratumoral T regulatory cells in a subject as well as to inhibit tumor growth in a subject having a cancer without eliciting autoimmune responses. The approach relies on the inhibition of CD36 or of PPARbeta.
Owner:UNIVERSITY OF LAUSANNE
T-REG cell expansion
ActiveUS10696946B2Peptide/protein ingredientsNervous system cellsImmunologic disordersAutoimmune condition
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Treatment methods for autoimmune disorders
ActiveUS10702583B2Promote regulatory cell-mediated suppressionAvoid seizuresBiocideSenses disorderImmunologic disordersAutoimmune condition
The present invention provides methods and compositions for expanding T regulatory cells ex vivo or in vivo using one or more SAP agonists. The methods and compositions are useful in the treatment of autoimmune diseases and in preventing foreign graft rejection.
Owner:PROMEDIOR
Methods for enhancing proliferation of t regulatory cells
PendingUS20190358258A1Improve the level ofIncrease capacityImmunoglobulins against animals/humansMammal material medical ingredientsPlant Germ CellsKaryotype
The invention is generally directed to a method of enhancing proliferation of T regulatory cells (Tregs) in vitro, comprising contacting Tregs with cells (I), or conditioned medium from the cells, in the presence of one or more Treg stimulation agents. The Treg stimulation agent(s) is present in an amount and for a time effective to stimulate proliferation of the Tregs. The cells (I) are present in an amount and for a time effective to enhance proliferation of the Tregs. The cells (I) are non-embryonic stem, non-germ cells characterized by one or more of the following: extended replication in culture and express markers of extended replication, express markers of pluripotentiality, and have broad differentiation potential, are not tumorigenic or transformed, and have a normal karyotype. The invention is also directed to methods for immune modulation using the proliferated Tregs, cell banks, drug discovery methods, populations, and compositions of the proliferated Tregs.
Owner:KINGS COLLEGE LONDON +1
T regulatory cells and uses thereof
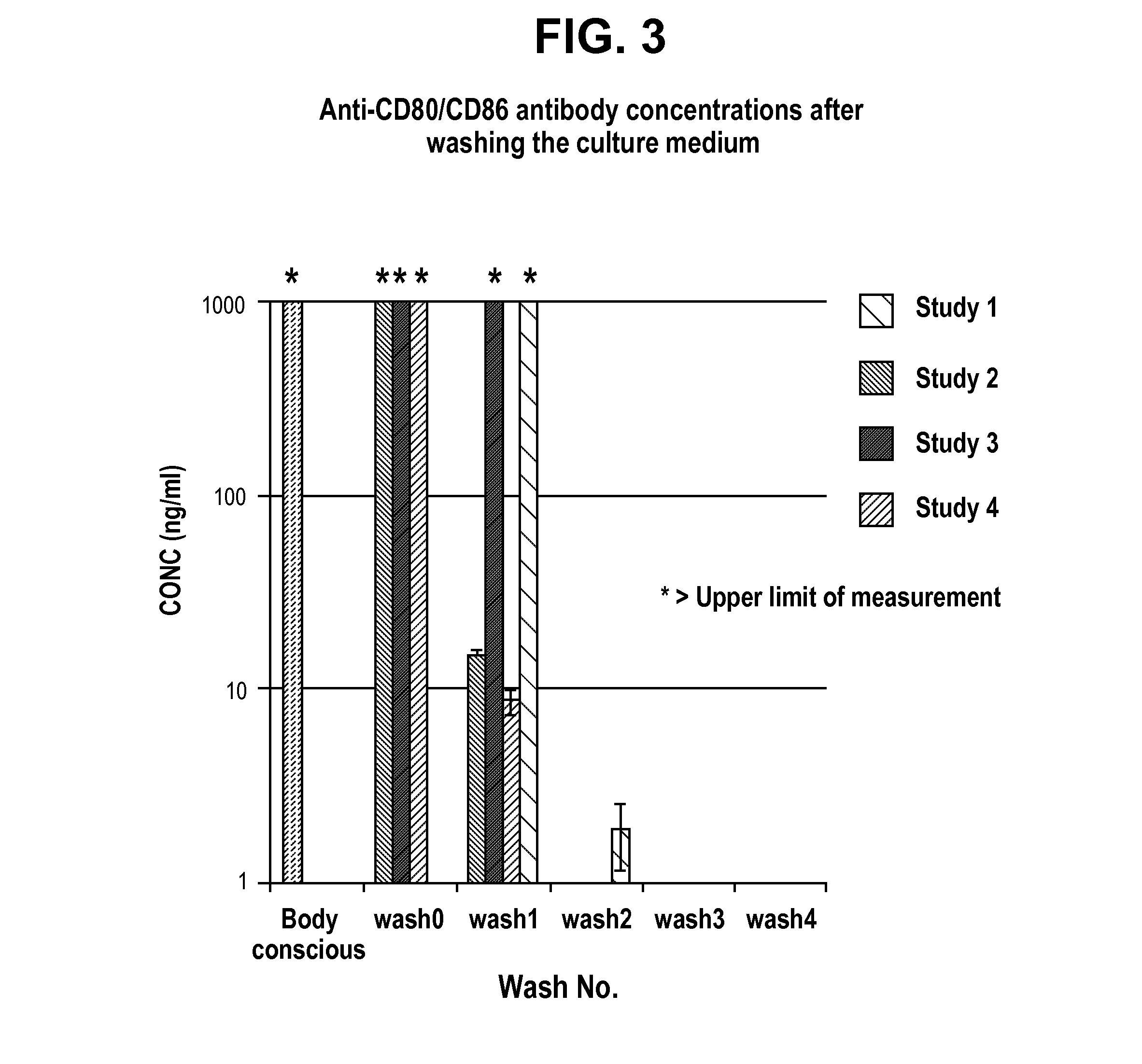
InactiveUS20160046715A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsRegulatory T cellCD80
The present application relates to generation of regulatory T cells, particularly those generated in the presence of anti-CD80 and anti-CD86 antibodies. The present application also relates to uses of the regulatory T cells in treating subjects undergoing organ transplantation. The present application also relates to uses of the regulatory T cells in treating subjects undergoing tissue grafts. Regulatory T cells may be administered to a subject along with one or more antibodies.
Owner:JUNTEN BIO CO LTD
Control of vagal stimulation
ActiveUS10695569B2Lower Level RequirementsIncrease amplitudeSpinal electrodesElectrocardiographyPhysical medicine and rehabilitationRR interval
Methods and apparatuses for stimulation of the vagus nerve to treat inflammation including adjusting the stimulation based on one or more metric sensitive to patient response. The one or more metrics may include heart rate variability, level of T regulatory cells, particularly memory T regulatory cells, temperature, etc. Stimulation may be provided through an implantable microstimulator.
Owner:SETPOINT MEDICAL CORP
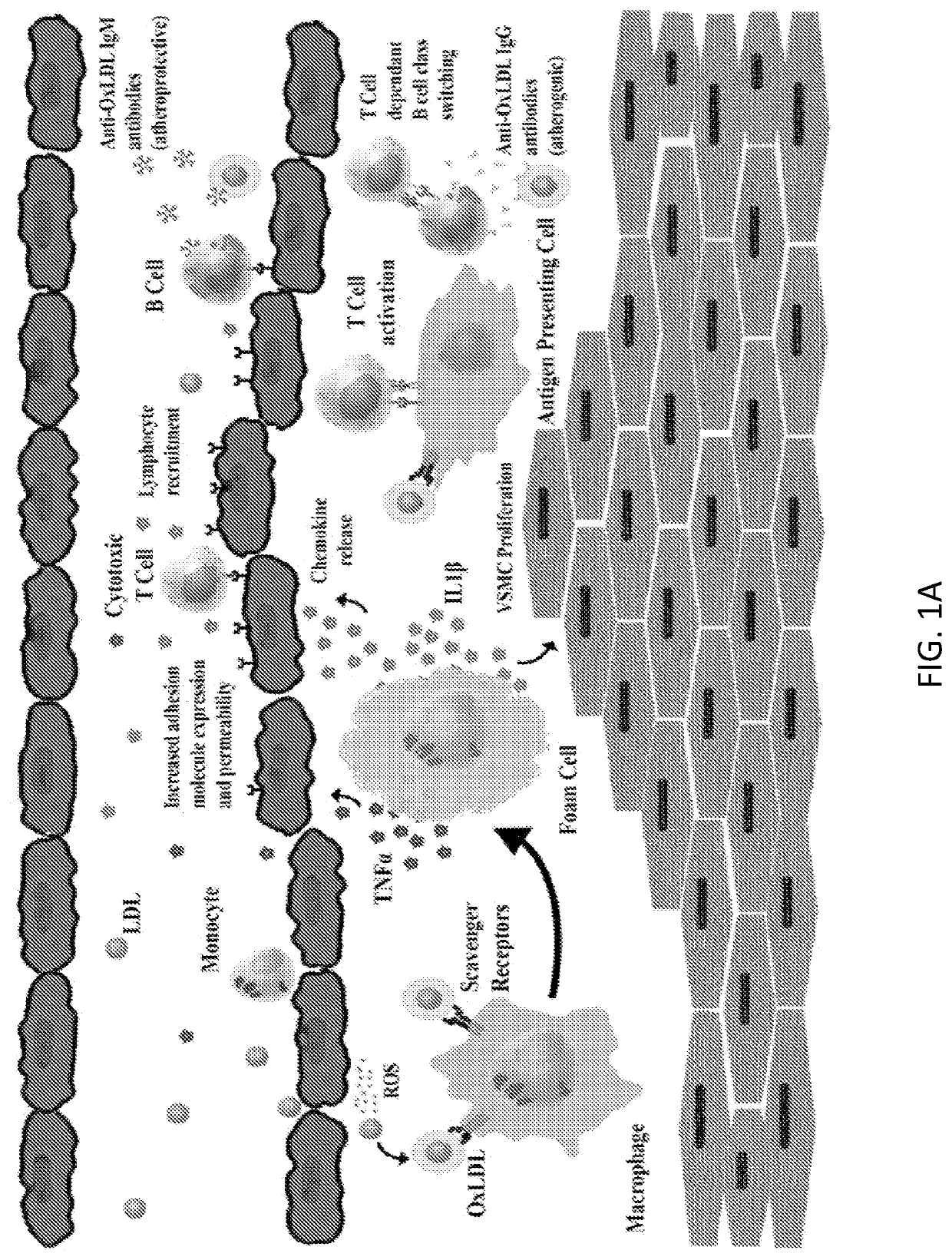
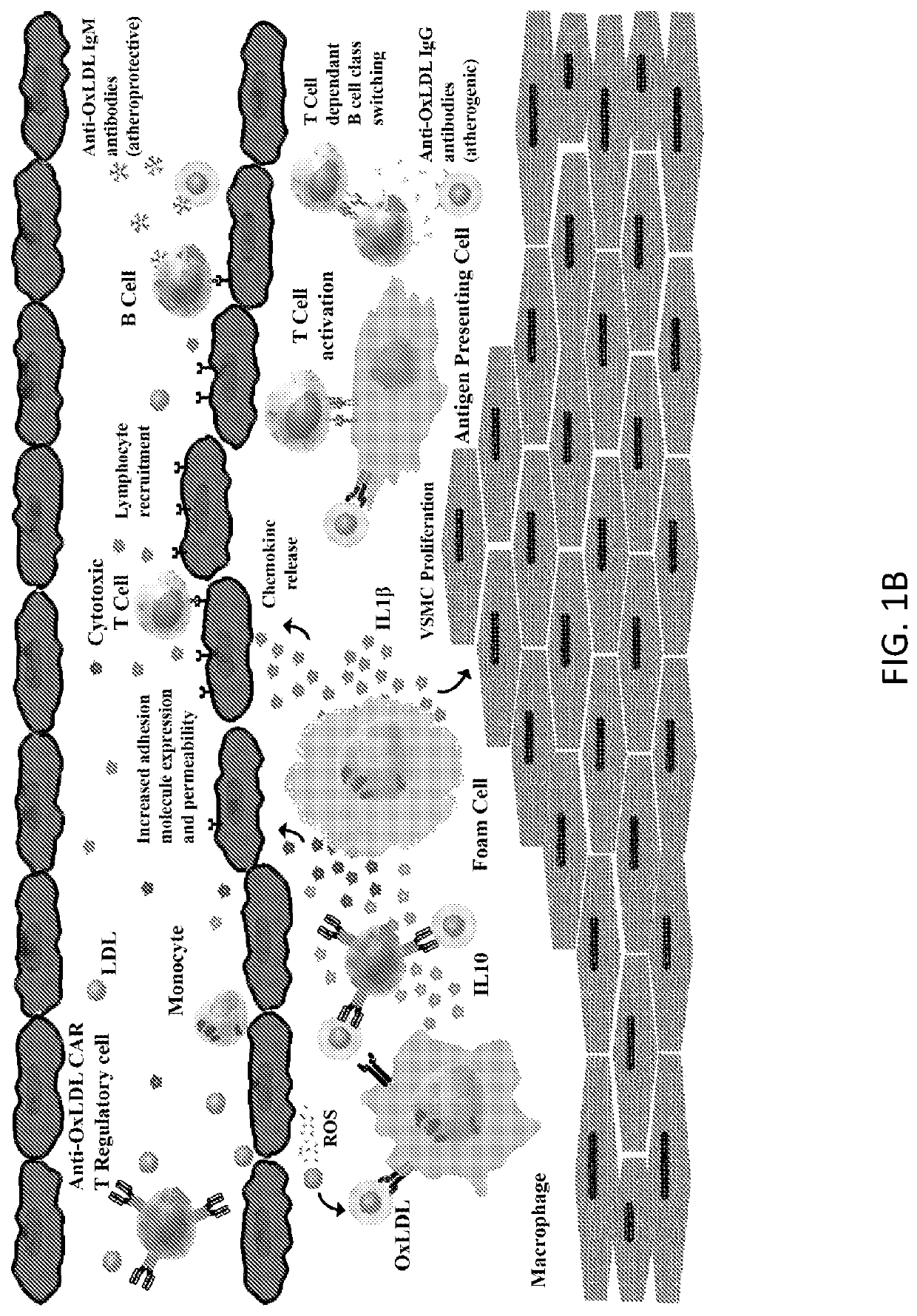
Chimeric Antigen Receptor T Regulatory Cells for the Treatment of Atherosclerosis
PendingUS20210060071A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyA lipoproteinLow-density lipoprotein
The present invention includes compositions and methods for treating atherosclerosis. In certain embodiments, atherosclerosis is treated using a chimeric antigen receptor (CAR) T cell specific for modified low-density lipoprotein.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
T-REG Cell Expansion
ActiveUS20180119100A1Nervous system cellsMammal material medical ingredientsImmunologic disordersDisease
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com