Medicaments and methods to treat autoimmune disease and cancer
A technology for autoimmunity and diseases, applied in diseases, allergic diseases, metabolic diseases, etc., can solve problems such as weak effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0057] [054] With the foregoing summary in mind, the following presents a detailed description of the preferred embodiment (presently believed to be the best mode) of the invention.
[0058] [055] The invention is further understood from the following patient studies.
[0059] method
[0060] [056] This study was approved by the Research Ethics Committee of Linkoping University in Sweden and the Swedish regulatory authorities.
[0061] Research design
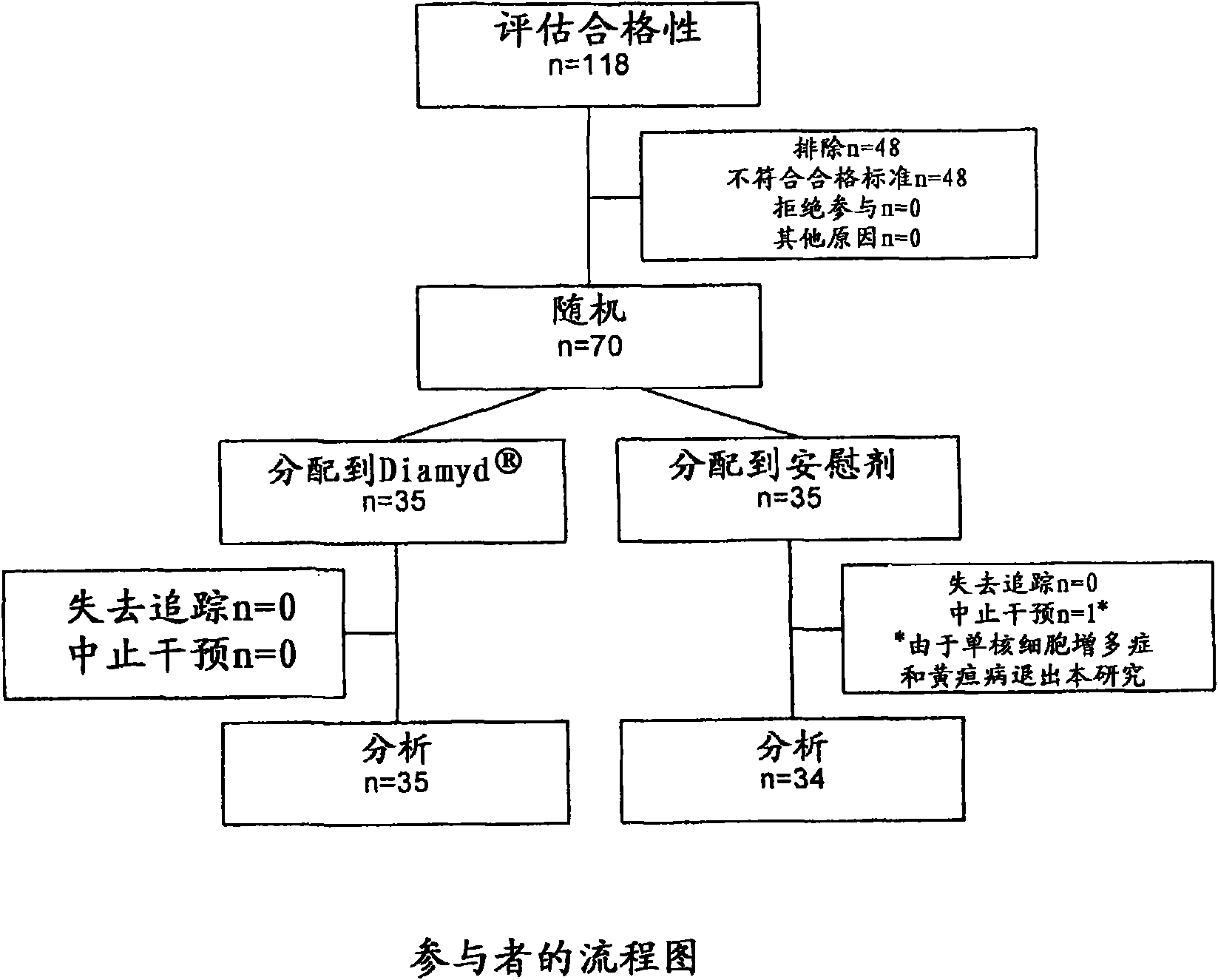
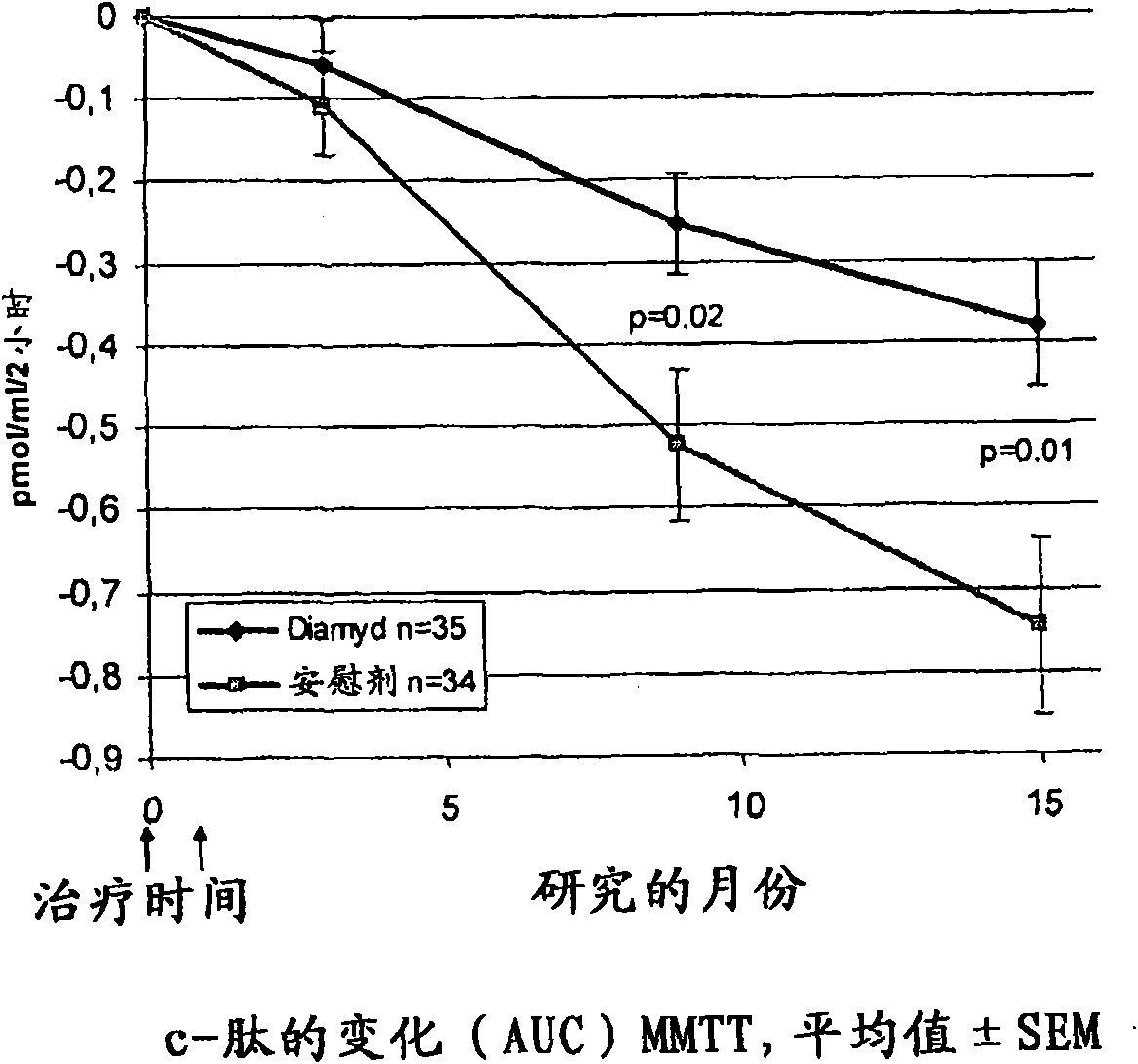
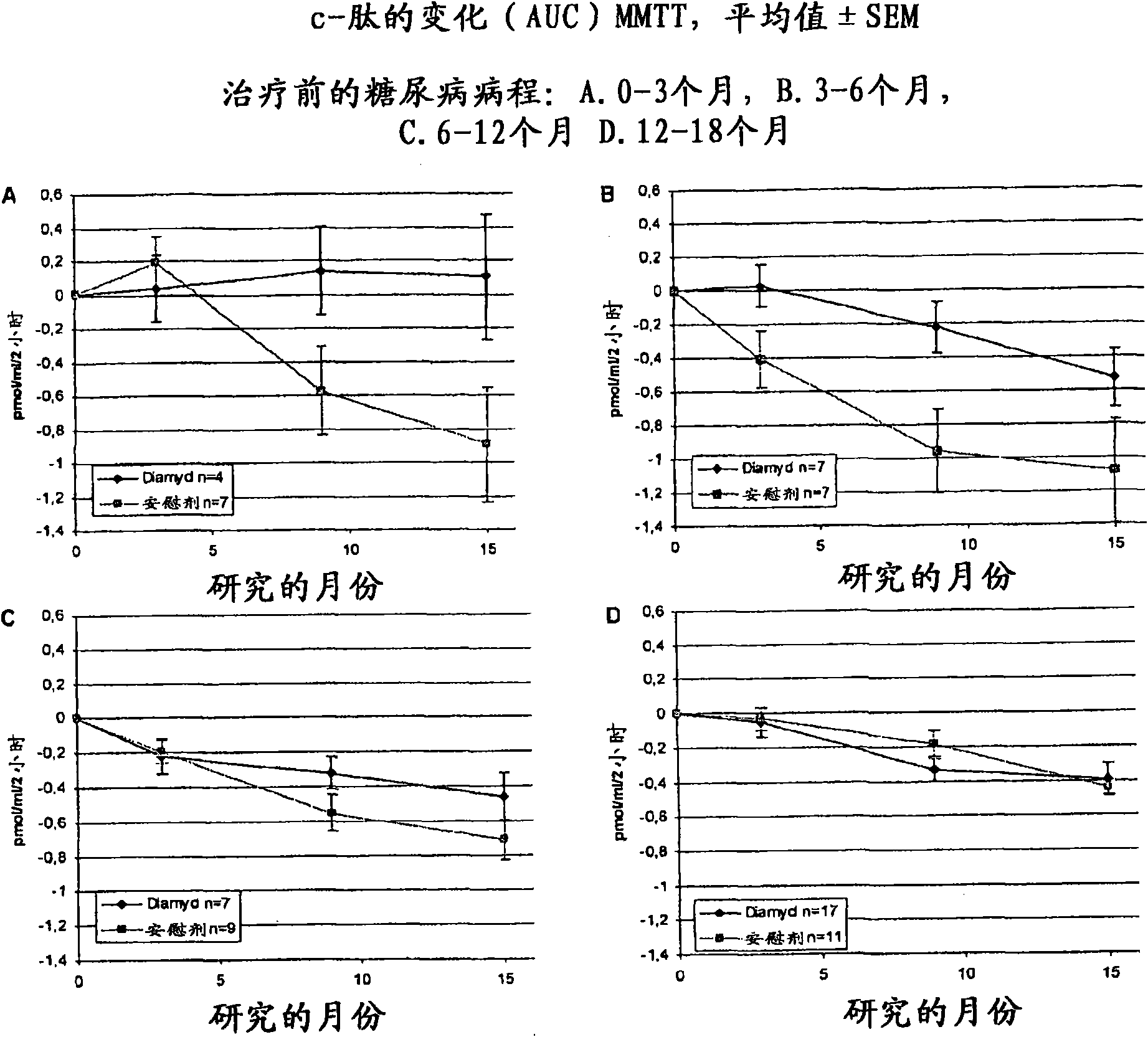
[0062] [057] In 8 pediatric clinics in Sweden, the presence of GAD65 autoantibodies (GADA) and fasting C-peptide levels greater than 0.1 pmol / ml were associated with 10-18 year olds who had shown disease within the preceding 18 months. T1D patients were screened. A total of 70 patients were eligible and were randomized to receive 20 μg of recombinant human GAD65 formulated in alum ( Double-blind treatment of Diamyd Medical, Stockholm, Sweden, 35 patients) or placebo (same formulation without rhGAD65; 35 patients).
[0063...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com