Targeted Drug-Formaldehyde Conjugates And Methods Of Making And Using The Same
a technology of formaldehyde and conjugates, which is applied in the field of targeted drugformaldehyde conjugates and methods of making and using the same, can solve the problems of large socioeconomic toll, blindness, kidney disease and premature death, and places a heavy burden on healthcare facilities worldwid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Test for T-cell Proliferation in Response to IGRP Protein and IGRP Protein Fragments
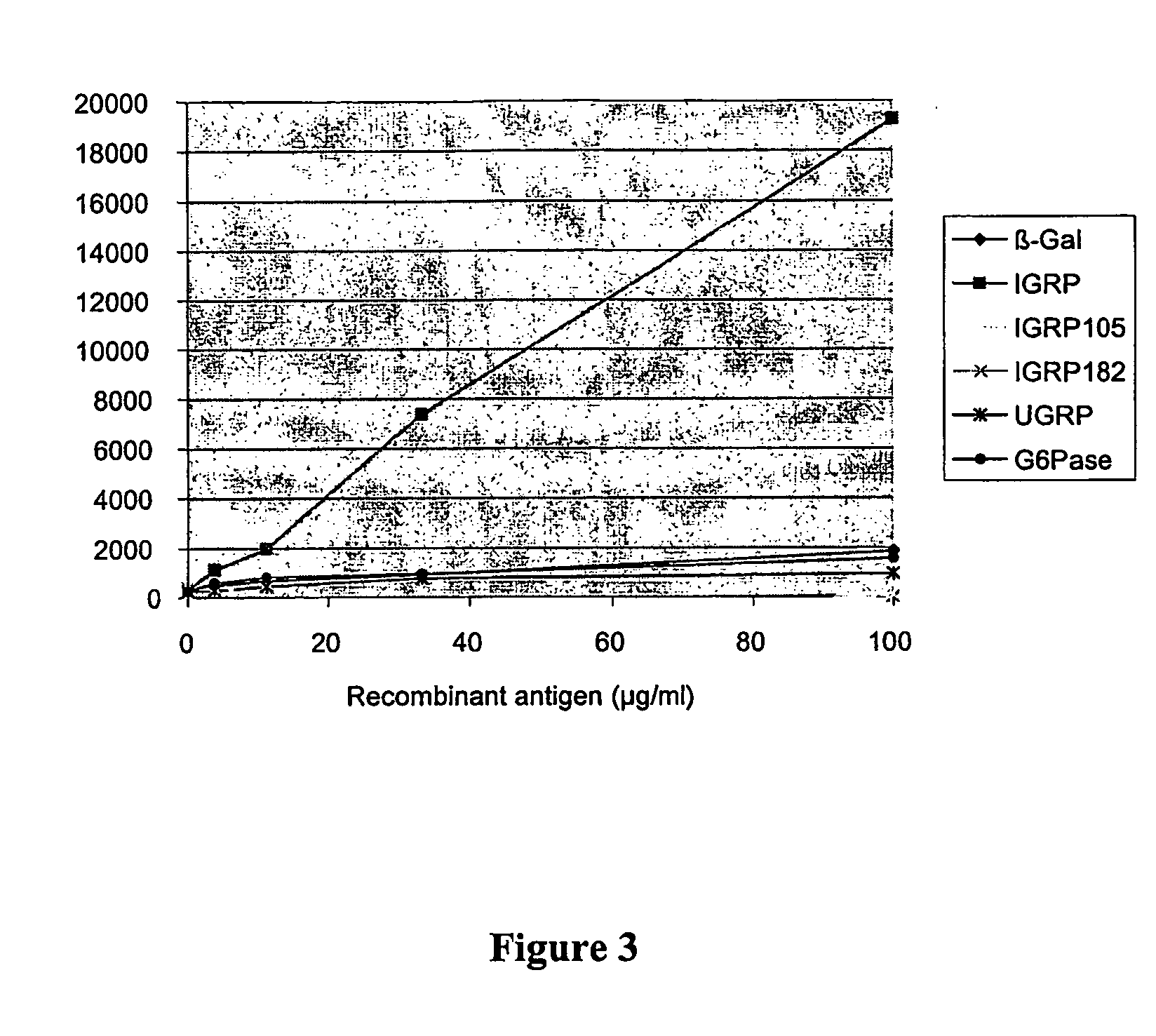
[0092] T-cell proliferative responses to recombinant IGRP (3-100 μg / ml) were observed from mixed lymphocyte preparations of pre-diabetic 12 week old female NOD mice (SImax=10). The fusion partner, β-galactosidase itself produced a minimal response in the assay as did equivalent constructs with G6Pase and UGRP. Constructs that were truncated C-terminally by 183 and 250 amino acids did not evoke a response suggesting that epitopes were confined to the C-terminal half of the molecule.
example 2
Test for antibodies to IGRP
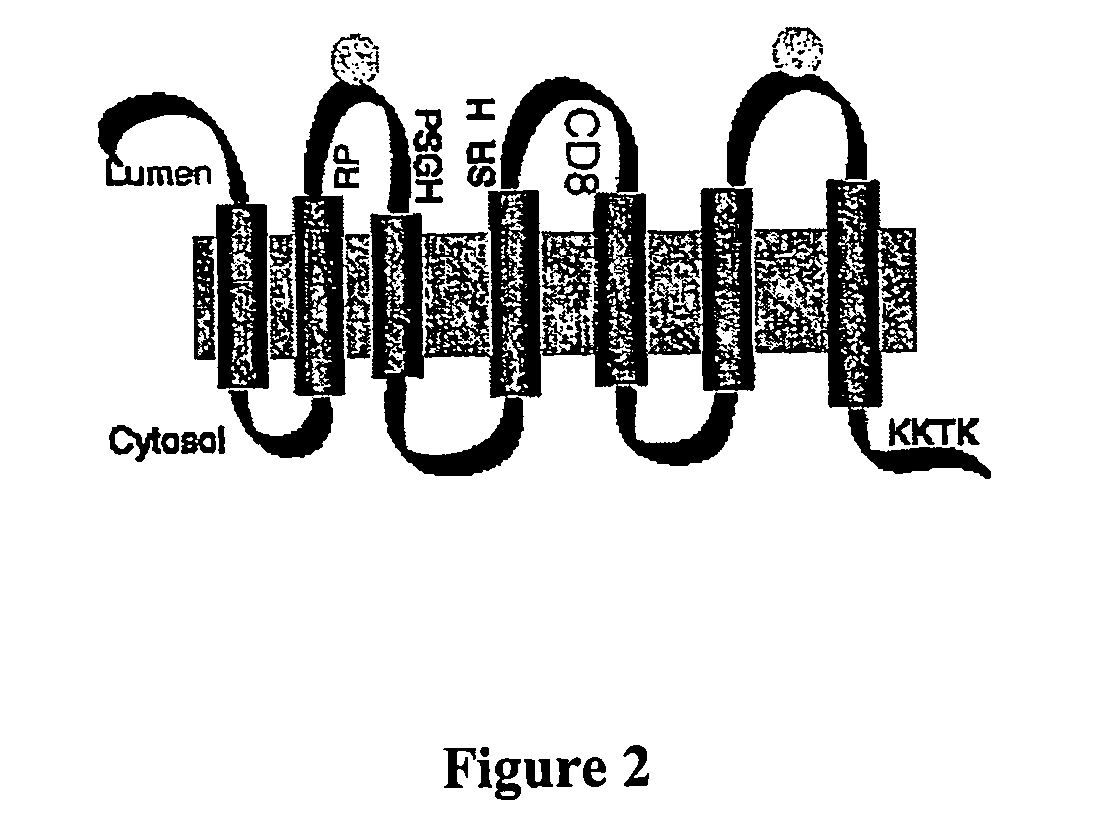
[0093] IGRP was investigated as a humoral autoantigen in diabetic human subjects and NOD mice using a series of assays based either upon immunoprecipitation of 35S-labelled in vitro translated protein generated from reticulocyte lysates, or ELISAs based on the binding of antibodies to recombinant protein immobilized on microtiter plates or PVDF membranes. The assays easily detected antibodies from rabbits immunized with an IGRP COOH-terminal peptide or recombinant antigen (antibody dilution 1:50 to 1:8000) but failed to demonstrate the presence of autoantibodies in spontaneous diabetic or prediabetic samples, a high proportion of which were positive for one or more other autoantigens (insulin, GAD65 and ICA512). Other assays in which IGRP was translated in vitro with dog pancreatic microsomes to mimic its insertion into membranes and core glycosylation were similarly negative. Thus, any humoral autoimmune response remains to be characterized despite testi...
example 3
Identification of the Major IGRP Epitope
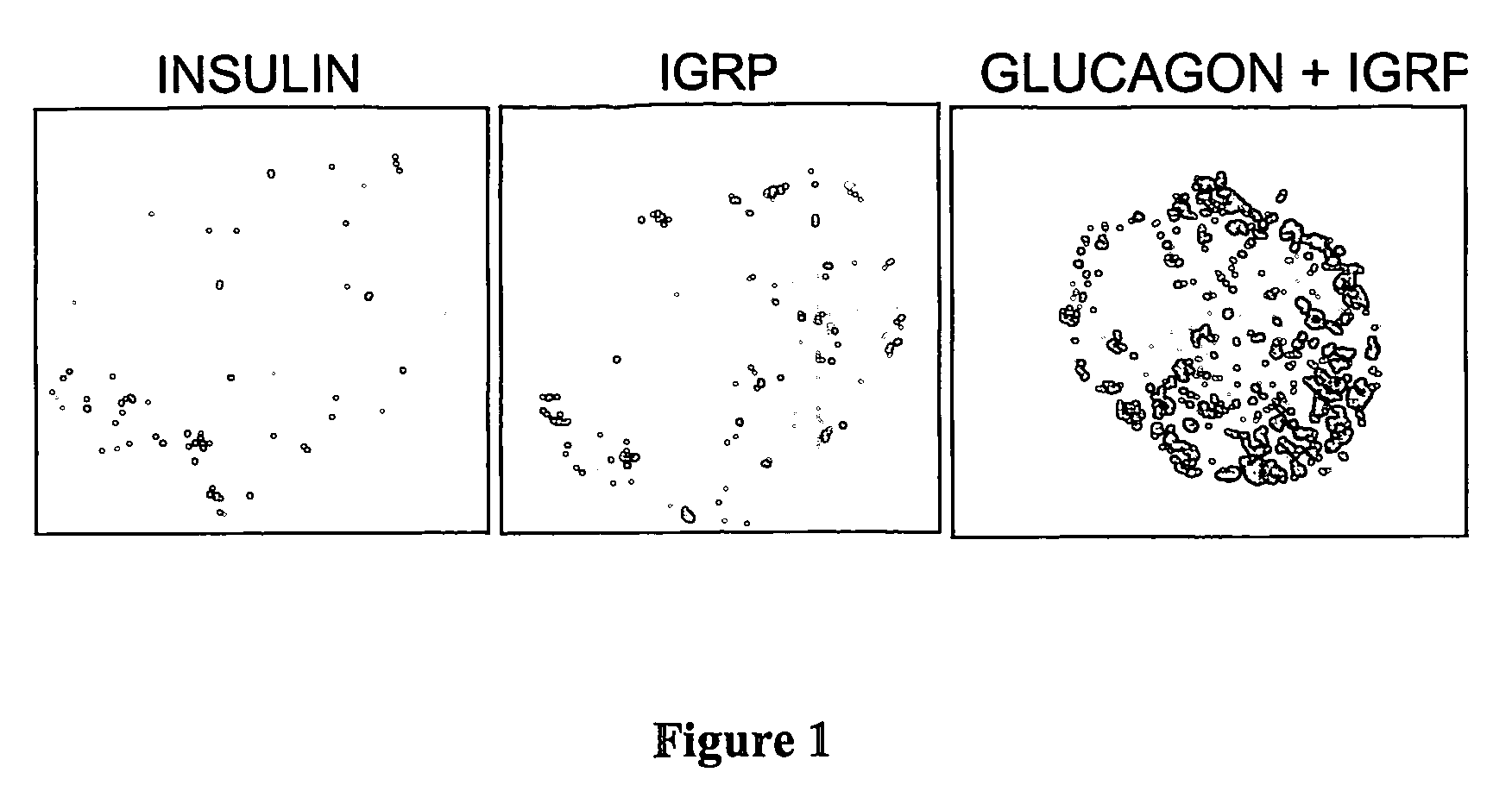
[0094] MHC class I tetramers were generated using the NIH tetramer facility. These were loaded with a series of peptides including the native mouse IGRP peptide (VYLKTNVFL (SEQ ID NO.: 6); peptide C, FIG. 4) corresponding to the NRP-V7 mimeotope (KYNKANVFL (SEQ ID NO.: 7)) that stimulates the NY8.3 CD8 T-cell clone. A procedure was developed for the isolation of mononuclear cells from the whole pancreas of NOD mice yielding approximately 0.5-1×106 CD45 positive cells (lymphocytes) per mouse (12-16wk females) for tetramer binding assays (FIG. 4). The lymphocyte population contained 10-15% CD8 positive cells of which 8% bound the native peptide tetramer. These assays confirm the remarkable finding that a major proportion of the CD8 cells in the NOD pancreas target one specific IGRP epitope. The procedure developed here represents a technical advance on previous tetramer studies of activated NRP reactive T-cell in NOD mice which required up to 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com