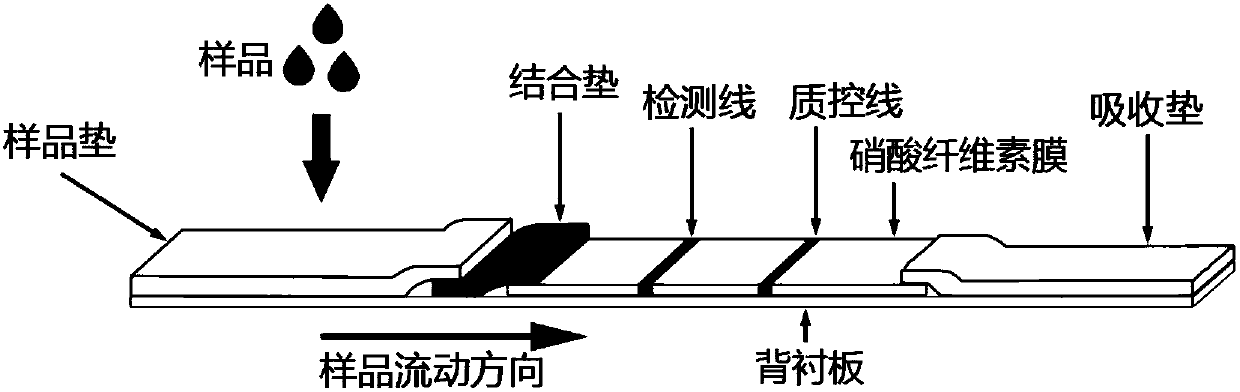
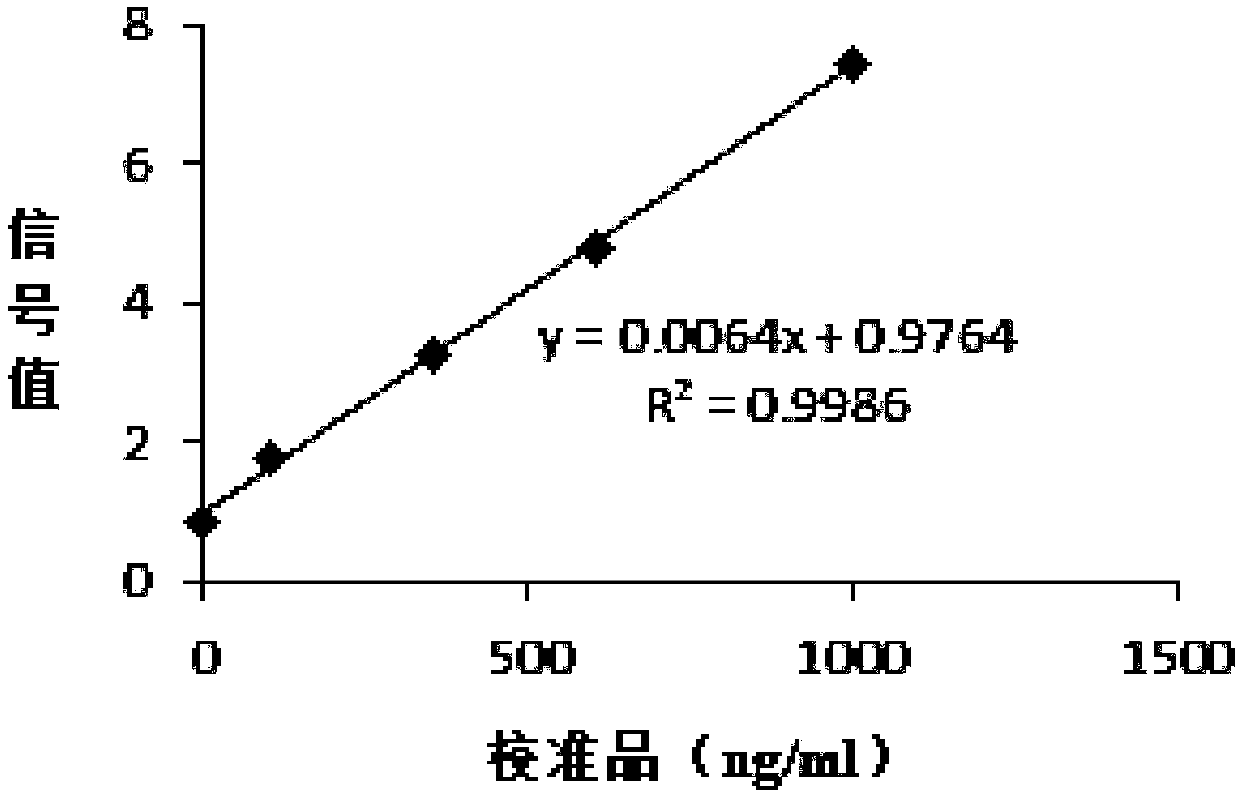
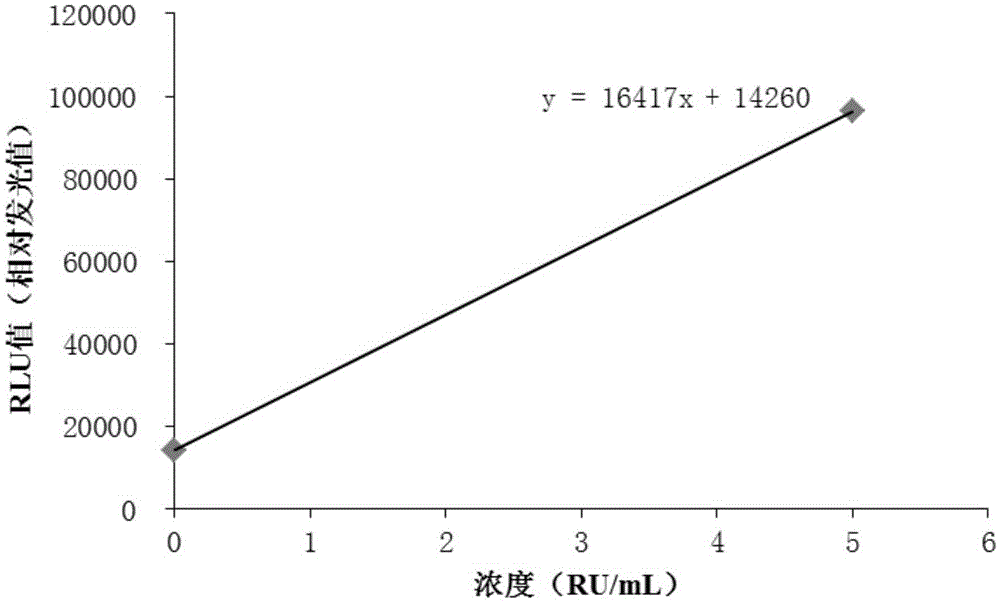
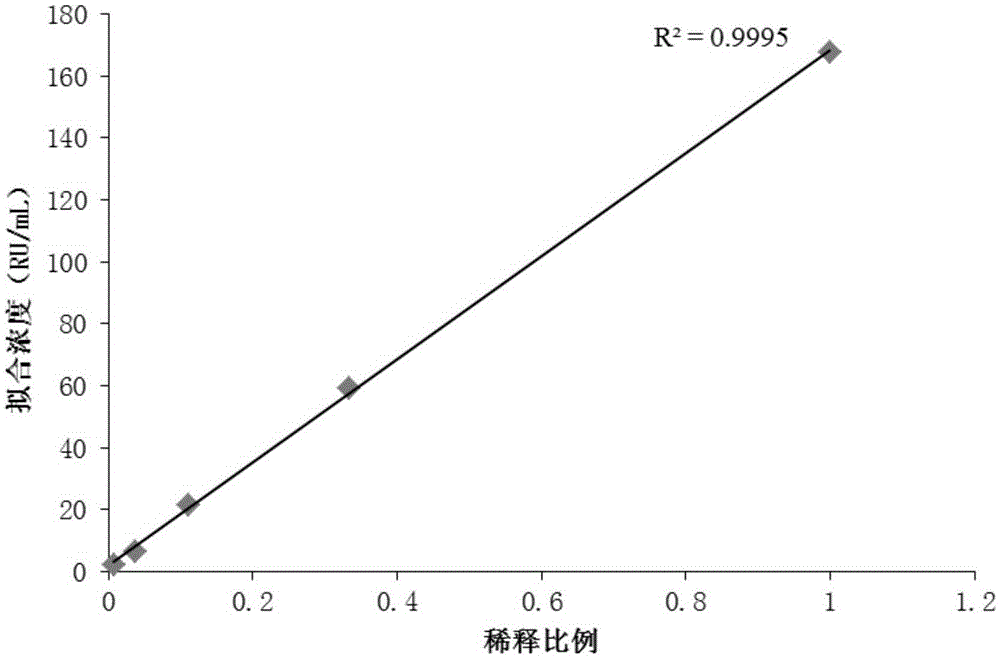
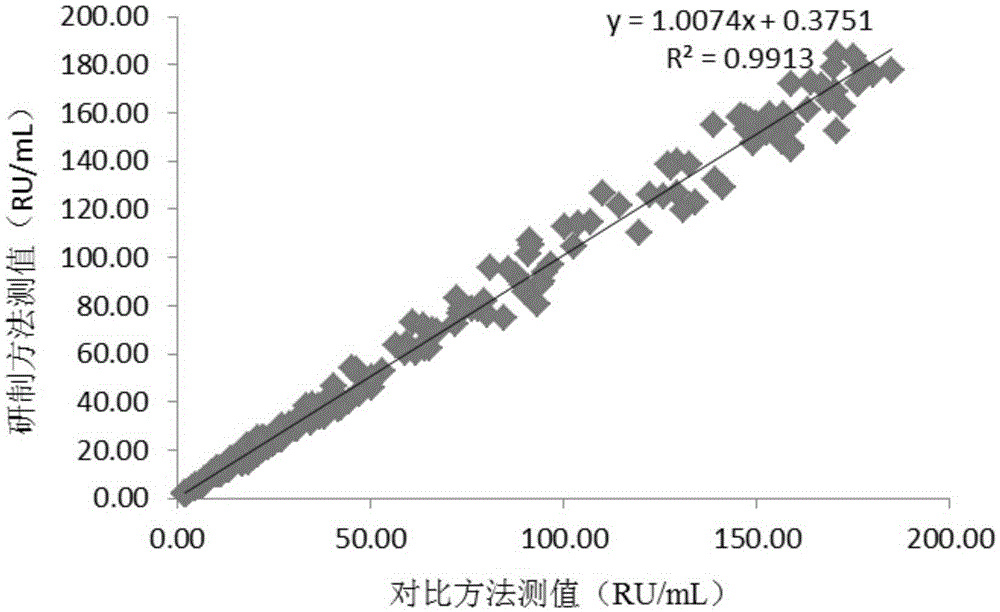
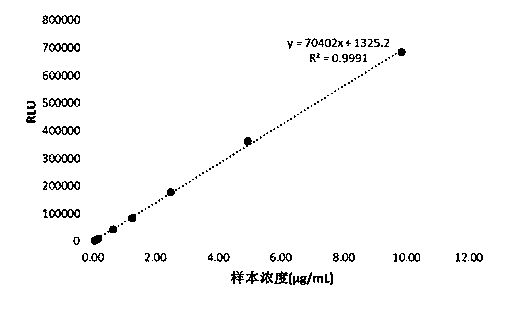
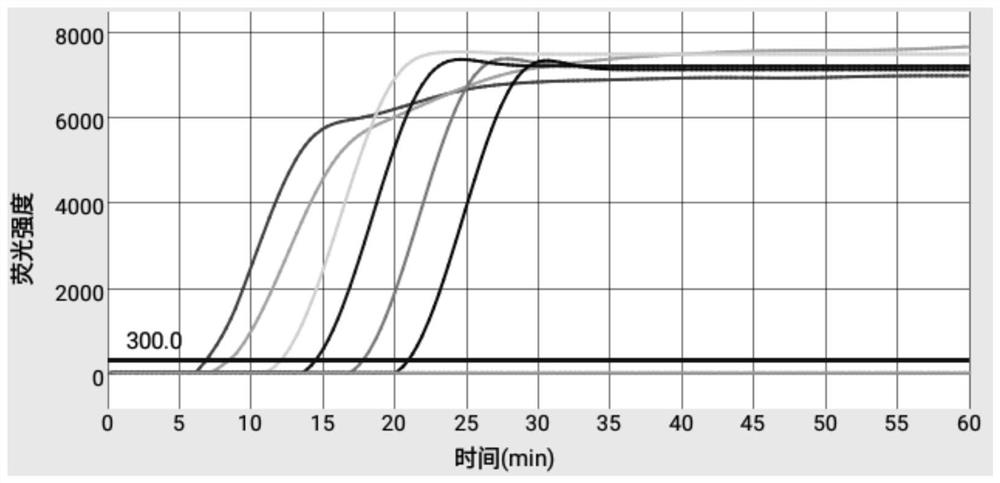
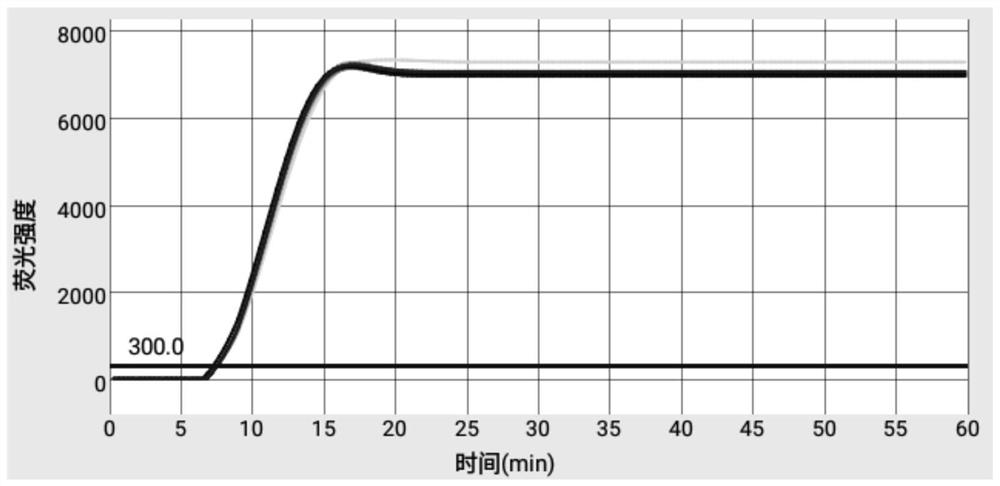
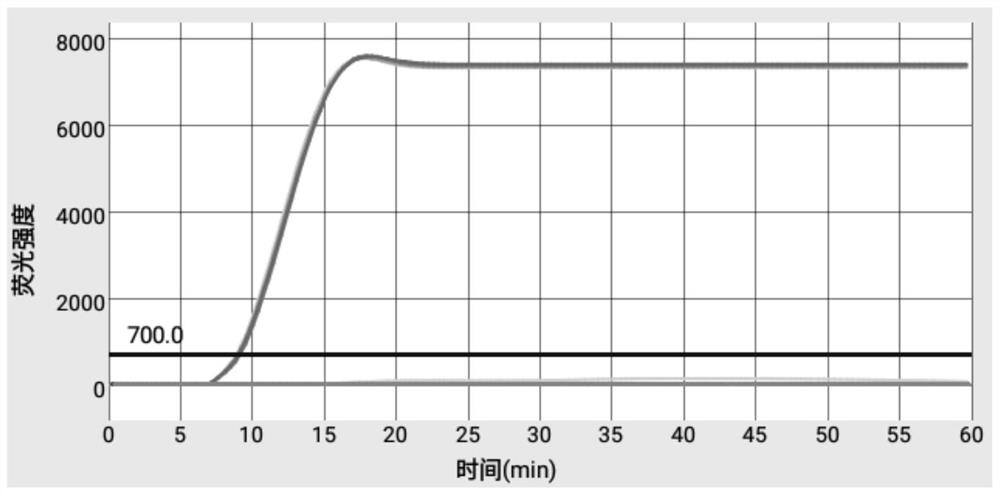
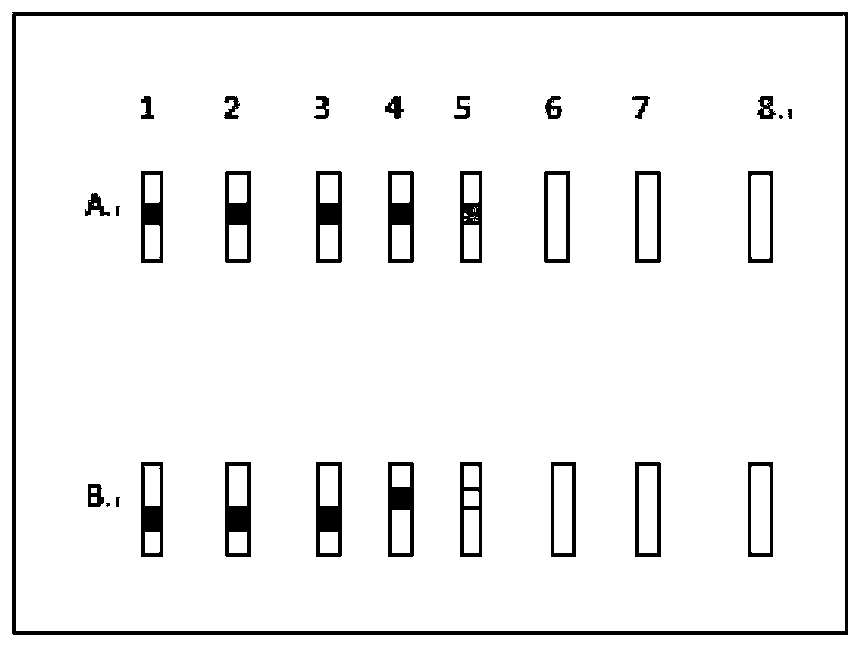
Patents
Literature
70results about How to "Avoid non-specific reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
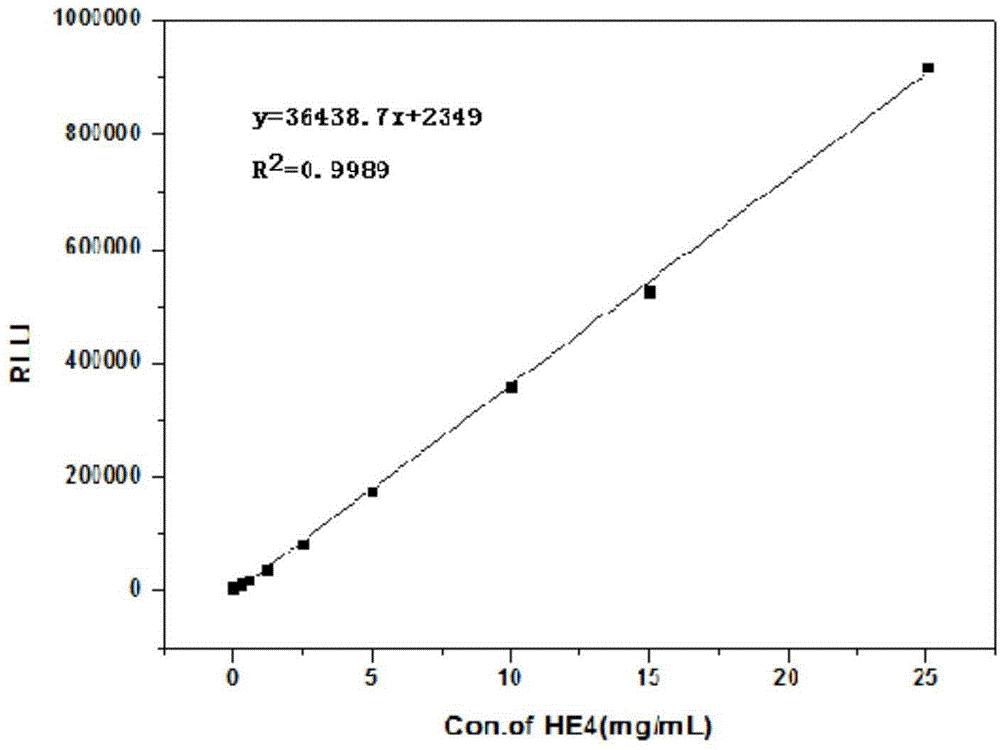
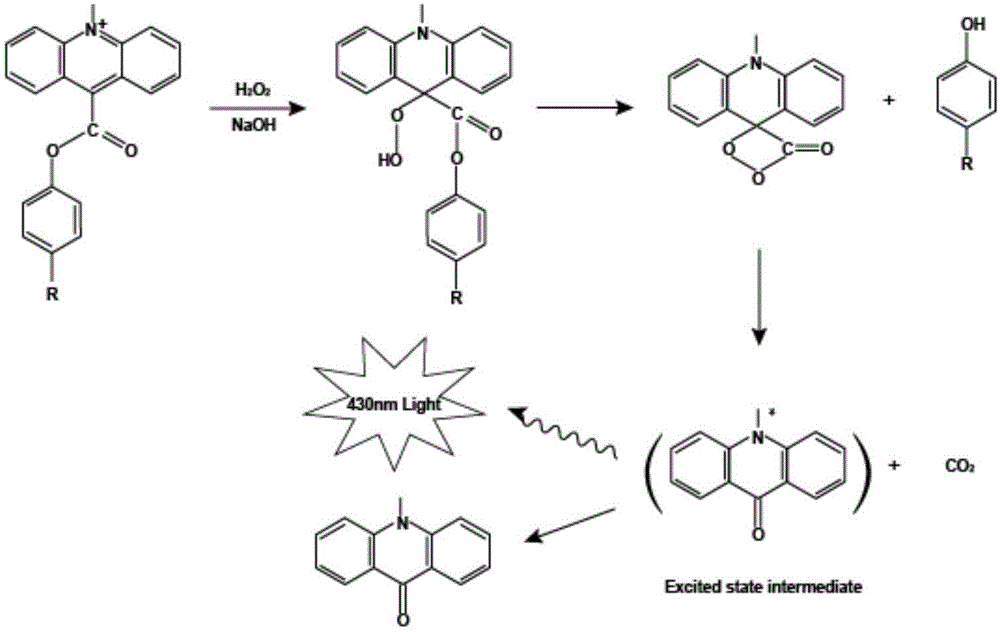
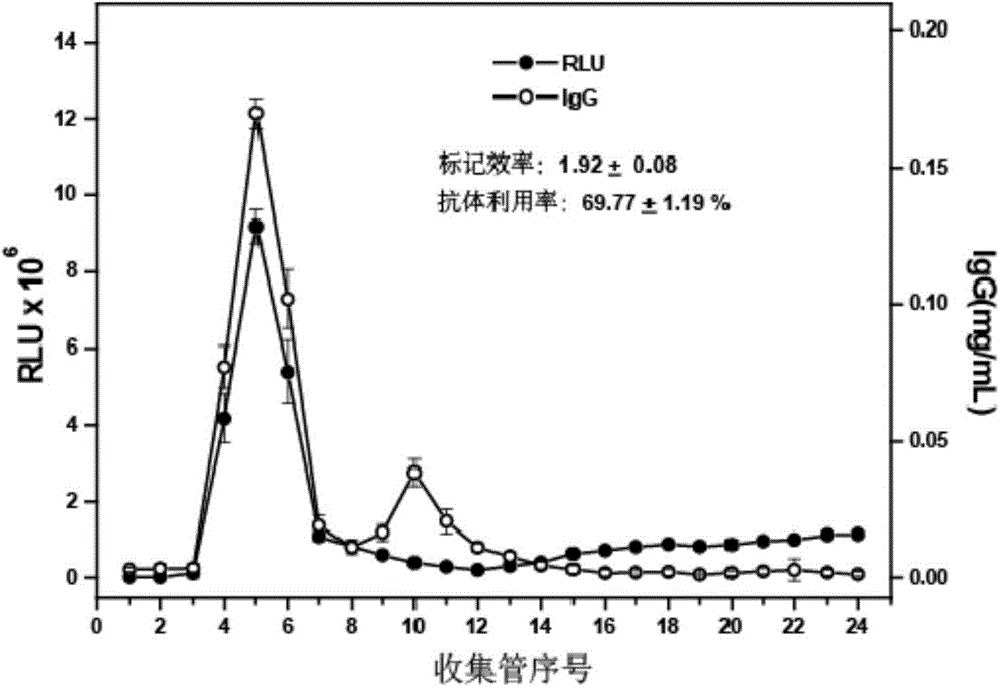
Goldmag particle-based acridinium ester chemiluminescence immunological detection method of HE4
ActiveCN104897901AGood response specificityAvoid cross reactionChemiluminescene/bioluminescenceBiological testingAntigenSolid phases
The invention provides a goldmag particle-based acridinium ester chemiluminescence immunological detection method of human epididymis secretory protein (HE4). The goldmag particle-based acridinium ester chemiluminescence immunological detection method mainly comprises following steps: (1) goldmag particle is taken as an immunoreaction and solid-phase separation carrier, and HE4 coated antibody is connected with the surface of the goldmag particle via coupling; (2) blank sites on the surface of the goldmag particle, which are not combined with the HE4 coated antibody, are blocked with a blocking solution; (3) acridinium ester (AE) is used for marking HE4 labelled antibody; (4) a sample to be detected, and the acridinium ester marked HE4 antibody are added into the blocked HE4 antibody coated goldmag particle for reaction so as to obtain a double-antibody sandwich compound, wherein the acridinium ester marked HE4 antibody is capable of realizing specific binding with HE4 antigen; (5) washing is carried out; and (6) chemiluminescence detection is carried out. The goldmag particle-based acridinium ester chemiluminescence immunological detection method is high in detection sensitivity, specificity, accuracy, and stability, and is simple and rapid; linearity range is wide; no radioactive contamination is caused; and operation is safe.
Owner:XIAN GOLDMAG NANOBIOTECH
Recombinant antibody of anti-human cardiac troponin I as well as construction method and application thereof
ActiveCN103694355AAvoid non-specific reactionsHybrid immunoglobulinsImmunoglobulins against animals/humansAntigenAntibody
The invention relates to a recombinant antibody of an anti-human cardiac troponin I. The recombinant antibody comprises a light chain consisting of a light chain variable region of murine antibodies of the anti-human cardiac troponin I and a light chain constant region of human IgG1 and a heavy chain consisting of a heavy chain variable region of murine antibodies of the anti-human cardiac troponin I and a heavy chain constant region of human IgG1. Compared with the murine antibodies, the recombinant antibody has the advantages that specificity and appetency of a parental antibody for identifying antigens are kept, and nonspecific reaction between the parental antibody and an anti-mouse antibody in human serum is avoided. Therefore, according to the clinical diagnosis application aspect, the recombinant antibody has higher application value compared with the murine antibodies. In addition, the invention also relates to a method for constructing the recombinant antibody of the anti-human cardiac troponin I as well as application of the recombinant antibody.
Owner:FAPON BIOTECH INC +1
Immunochromatographic test strip, and production method and application thereof
InactiveCN107907679AGuaranteed specificityIndicating validityMaterial analysisQuality control systemMonoclonal antibody
The invention discloses an immunochromatographic test strip, and a production method and an application thereof. The test strip is characterized in that a labeling compound carries an inert protein connected with a special label and used for quality control, and a quality control line contains a monoclonal antibody specifically binding to the special label carried by the inert protein. The test strip has an independent quality control system, so the obtained test result has a high specificity and an anti-interference property, non-specific reactions brought by the self-bearing antibodies in human blood samples are effectively avoided, and the effectiveness of the test strip is effectively indicated.
Owner:NANJING VAZYME MEDICAL TECH CO LTD +1
NT-ProBNP detection kit and using method thereof
ActiveCN107656071ADetection speedSimplify operation stepsDisease diagnosisBiological testingChemistryEnzyme
The invention discloses an NT-ProBNP detection kit. The NT-ProBNP detection kit comprises a calibrator, a cleaning solution, a substrate solution, a pretreatment solution, an enzyme conjugate workingsolution and a magnetic bead conjugate working solution; the pretreatment solution contains pyridine, the enzyme conjugate working solution contains NT-ProBNP antibody labeled by enzyme, and the magnetic bead conjugate working solution contains magnetic beads labeled by the NT-ProBNP antibody. The NT-ProBNP detection kit can accurately measure NT-ProBNP in a whole blood sample, the lowest limit detection of the kit is 20 pg / ml, the linearity range is 20-5,000 pg / ml, the detection sensitivity is high, the linearity range is wide, and the result is accurate. The invention further discloses a using method of the NT-ProBNP detection kit. The NT-ProBNP detection kit is simple in using step, the detection time of an NT-ProBNP is shortened, and quick and sensitive detection of the NT-ProBNP is achieved.
Owner:NANTONG EGENS BIOTECH
Specific B cell epitope polypeptide of NS1 protein of encephalitis B virus and use thereof
ActiveCN102206249AStrong specificityReduce false positive ratePeptidesGenetic engineeringMolecular ImmunologyScreening method
The invention discloses the sequence of a specific B cell epitope polypeptide of an NS1 protein of encephalitis B virus and the use of the epitope polypeptide in the diagnosis of encephalitis B virus and a screening method of specific B cell epitope polypeptide of NS1 protein of encephalitis B virus, and belongs to the field of molecular immunology. The amino sequence of the epitope polypeptide is represented by SEQ ID No.1 or SEQ ID No.2. The specific B cell epitope synthetic polypeptide of the JEV NS1 protein, the coupling antigen of the synthetic epitope polypeptide and the epitope fusion expression protein can be used for specifically detecting a JEV NS1 protein antibody generated in body which is immunized and infected. The epitope polypeptide can be used for diagnosis of JEV infection, evaluation on immunity effect and identification and diagnosis of immunity of inactivated vaccine and natural infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Single-index microfluidic chip, and production method and application method thereof
ActiveCN109569754AQuality is not affectedEnsure fluid dosingLaboratory glasswaresBiological testingEngineeringAntibody antigen
The invention discloses a single-index microfluidic chip, and a production method and an application method thereof. The single-index microfluidic chip comprises a chip body. A sampling cavity, a quantitative reaction cavity and a waste liquid cavity are formed in the chip body; a first backflow prevention device and a second backflow prevention device are arranged at the front end and the rear end of the quantitative reaction cavity correspondingly; at least one of the first backflow prevention device and the second backflow prevention device is a rubber plug anti-backflow device that comprises a rubber plug, a fluid input pipe capable of raising the fluid delivery height and a fluid output pipe capable of lowering the liquid delivery height. The front end and the rear end of the quantitative reaction cavity are provided with the backflow prevention devices, and accordingly, quantification of fluids in the quantitative reaction cavity can be guaranteed; during antibody / antigen coating, antibody / antigen coating solvents can be injected into the fluid input pipe / fluid output pipe of the rubber plug anti-backflow device, and then are subjected to incubation, washing, packaging, and vacuumizing by a vacuum drying oven before being provided with a rubber plug, so that the single-index microfluidic chip is suitable for mass production. Basically, the quality of coated antibodies / antigens is not affected by the packaging time and the packaging process.
Owner:NANJING LANSION BIOTECH CO LTD
Multi-index microfluidic chip and using method thereof
ActiveCN109738632AEasy to moveFlow boostLaboratory glasswaresBiological testingFiltrationEngineering
The invention discloses a multi-index microfluidic chip and a using method thereof. The multi-index microfluidic chip comprises a chip body, wherein the chip body is provided with a sample injection chamber, a plurality of quantitative reaction chambers and a waste liquid chamber; the sample injection chamber comprises a sample filter pool, a filter membrane and a sample injection part disposed atthe port of the sample filter pool; the filter sample pool is arranged in the shape of a palm-leaf fan, and the liquid outlet of the sample filter pool is arranged on the narrow side wall; the lowerend of the sample injection part has a sample injection hole penetrating the sample filter pool; the upper end of the sample injection part comprises two parts of a flow guiding surface and a ventingboss respectively; the flow guiding surface is a curved surface which is gradually tapered from the outside to the inside; the venting boss has a venting hole penetrating the sample filter pool; the venting boss is located close to the wide side wall of the sample filter pool, the flow guiding surface is disposed adjacent to the narrow side wall of the sample filter pool; and the bottom of the sample filter pool is provided with a plurality of rib protrusions along the flow direction of the fluid. Therefore, the invention can effectively improve the filtration efficiency of the microfluidic chip.
Owner:NANJING LANSION BIOTECH CO LTD
Detection method for specific antibodies IgM of mycoplasma pneumonia and influenza viruses based on micro-fluidic chip
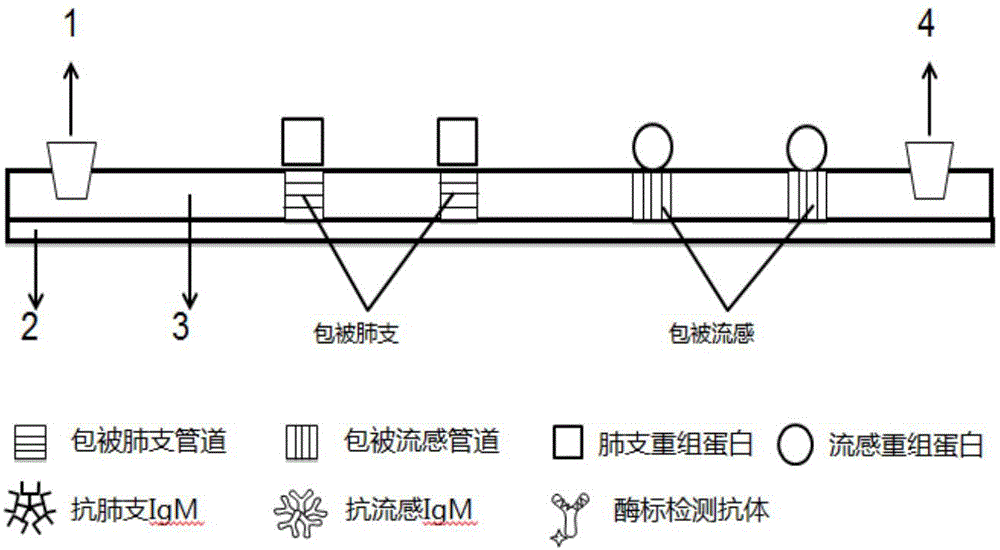
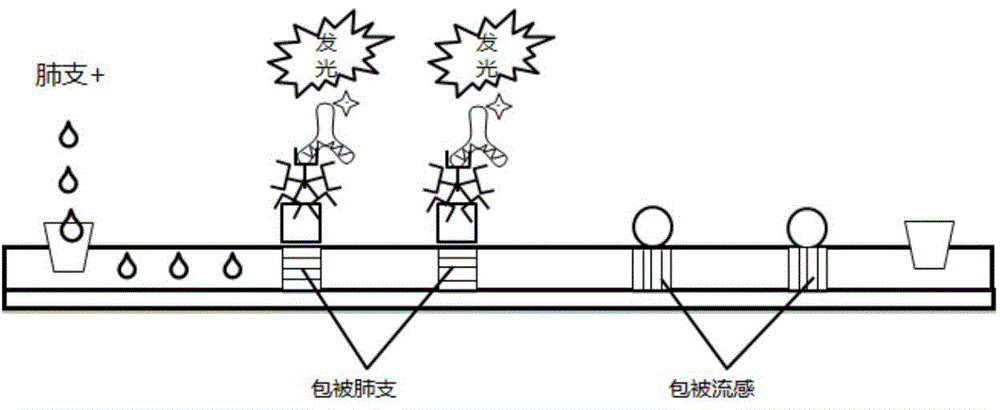
ActiveCN104360060AAvoid non-specific reactionsMeet testing needsChemiluminescene/bioluminescenceBiological material analysisSmall sampleSpecific antibody
The invention relates to a detection method for specific antibodies IgM of mycoplasma pneumonia and influenza viruses based on a micro-fluidic chip and application of the detection method. The method is characterized by taking the micro-fluidic chip with a plurality of pipelines as a reaction platform and detecting by using a chemical luminescent signal so as to independently or simultaneously detect the serum specific antibodies IgM of mycoplasma pneumonia and influenza viruses. As the micro-fluidic chip is combined with chemiluminiscence, the detection method integrates the advantages of rapidness, high efficiency and small sample amount of the micro-fluidic chip and high specificity of chemiluminiscence, also has the advantages of simplicity in operation, low cost, rapidness and the like, is a multi-target, real-time and high-sensitivity detection method and can be applied to rapid diagnosis of pneumonia and influenza.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Myoglobin detection reagent kit and method for applying same
ActiveCN107907691AAvoid swallowingMeet the needs of rapid diagnosisChemiluminescene/bioluminescenceBiological material analysisMagnetic beadWhole blood sample
The invention belongs to the field of in-vitro diagnostic reagent kits, and particularly relates to a myoglobin detection reagent kit and a method for applying the same. The myoglobin detection reagent kit comprises calibration products, reagents R1, enzyme conjugate working solution R2, magnetic bead conjugate working solution M, cleaning solution and chemiluminescent substrates. The reagents R1contain imidazole components, blood cells in whole blood can be quickly removed by the imidazole components, and accordingly the possibility that magnetic beads are swallowed by the blood cells can beeffectively prevented; the cleaning solution contains sodium lauryl sulfate components, accordingly, cleaning effects can be enhanced, and non-specific binding of the chemiluminescent substrates canbe prevented. The myoglobin detection reagent kit and the method have the advantages that finger peripheral whole blood or anticoagulant venous whole blood can be directly used as a to-be-detected sample for the myoglobin detection reagent kit, the whole blood sample can be directly detected without being pretreated, accordingly, the detection speed can be greatly increased, operation steps can besimplified, the application range of the myoglobin detection reagent kit can be expanded, and large-scale popularization and application can be facilitated.
Owner:NANTONG EGENS BIOTECH
Double-particle compounded C-reactive protein detection kit
The invention provides a double-particle compounded C-reactive protein detection kit and relates to the field of in vitro diagnosis of medical immunology. The kit consists of three parts, namely an R1 reagent, an R2 reagent and a calibrator, wherein the R1 reagent is a phosphate buffer system and comprises the following components in mass ratio: 30-80mmol / l of phosphate buffer with the pH value of 7.0-7.6, 60-120mm / l of polyethylene glycol 6000-10000 and 10-20 mmol / L of disodium edta; the R2 reagent is an antibody sensitized grain suspension and comprises compounded CRP (C-Reactive Protein) antibody particles, phosphate buffer with the pH value of 7.0-7.6, disodium edta and the like; and the calibrator is a bovine serum substrate and comprises 0.2-2.2% of sodium azide, 1-10% of tween-20 and 1-3% of bovine serum albumin. The kit provided by the invention adopts an antibody coated with compounded latex particles, is high in sensitivity and can be used for detecting 0.2mg / l of samples and improving the linear value.
Owner:上海睿康生物科技有限公司
cTnI detection kit and using method thereof
ActiveCN107918022AAccurate measurementStrong specificityDisease diagnosisBiological testingMagnetic beadWhole blood sample
The invention discloses a cTnI detection kit which comprises a calibration product, a cleanout fluid, a substrate solution, a pretreatment liquid, an enzyme conjugate working solution and a magnetic bead conjugate working solution, wherein the pretreatment liquid contains imidazole; the enzyme conjugate working solution contain an enzyme labeled cTnI antibody; and the magnetic bead conjugate working solution contains cTnI antibody labeled magnetic beads. The cTnI detection kit can realize accurate determination of cTnI in a whole blood sample, the detection steps are simplified, and the detection efficiency is improved. The lowest detection limit of the kit is 0.02ng / ml, the linear range is 0.02-50ng / ml, the detection sensitivity is high, the linear range is wide, and the detection resultis accurate. The invention further discloses a using method of the cTnI detection kit. The using method is simple in using steps, the detection time of the cTnI is effectively shortened, and rapid andsensitive detection of the cTnI is realized.
Owner:NANTONG EGENS BIOTECH CO LTD
CK-MB detection kit and use method thereof
The invention discloses a CK-MB detection kit which comprises calibrator, cleaning fluid, a substrate solution, a pretreatment solution, an enzyme conjugate working solution and magnetic bead conjugate working solution, wherein the pretreatment solution contains imidazole, the enzyme conjugate working solution contains an enzyme marked CK-MB antibody, and the magnetic bead conjugate working solution contains CK-MB antibody marked magnetic beads. By means of the CK-MB detection kit, accurate measurement of CK-MB in a whole blood sample can be achieved, detection steps are simplified, and a detection efficiency is improved. The lowest detection limit of the kit is 1ng / ml, a linearity range is 1 to 300ng / ml, detection sensitivity is high, the linearity range is wide, and detection results areaccurate. The invention further discloses a use method of the CK-MB detection kit. Use steps of the use method are simple, CK-MB detection time is effectively shortened, and quick and flexible detection of the CK-MB is benefit to achieve.
Owner:NANTONG EGENS BIOTECH CO LTD
Method and kit for detecting TORCH IgM antibodies and preparation method of kit
InactiveCN104155449AGenerally short-lived responseAvoid issues with poor sensitivityBiological material analysisLanthanideIgm antibody
The invention discloses a kit for detecting TORCH IgM antibodies. The kit comprises a solid carrier which is coated with an antibody combined with the TORCH IgM antibodies, a TORCH antigen which is marked with lanthanide, a TORCH IgM antibody calibrator, a sample diluent, an experiment buffering solution, a concentration cleaning solution and an enhancement solution. The kit can simultaneously detect multiple pathogene IgM antibodies in TORCH pathogene under the same detection condition and is high in sensitivity, high in specificity, good in precision, high in accuracy, little in blood consumption amount, rapid, convenient and favorable for early diagnosis on the infection of the TORCH pathogene. The method has the characteristic that the detection linear range is wide, the diluting times or diluting ratio of a high-value sample can be reduced, and the accuracy of a detection result can be improved.
Owner:GUANGZHOU FENGHUA BIOENG
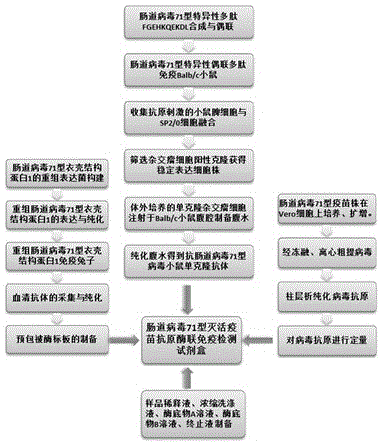
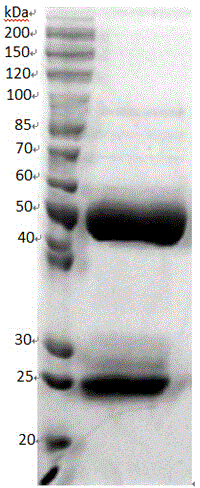
ELISA (enzyme-linked immunosorbent assay) kit for EV (enterovirus) 71 inactivated vaccine antigen
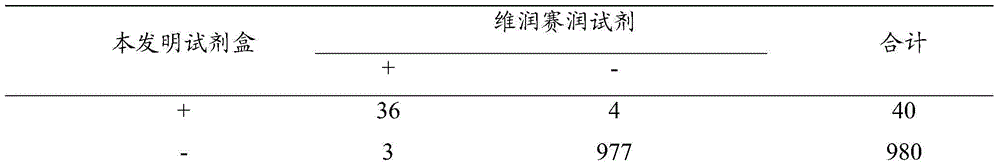
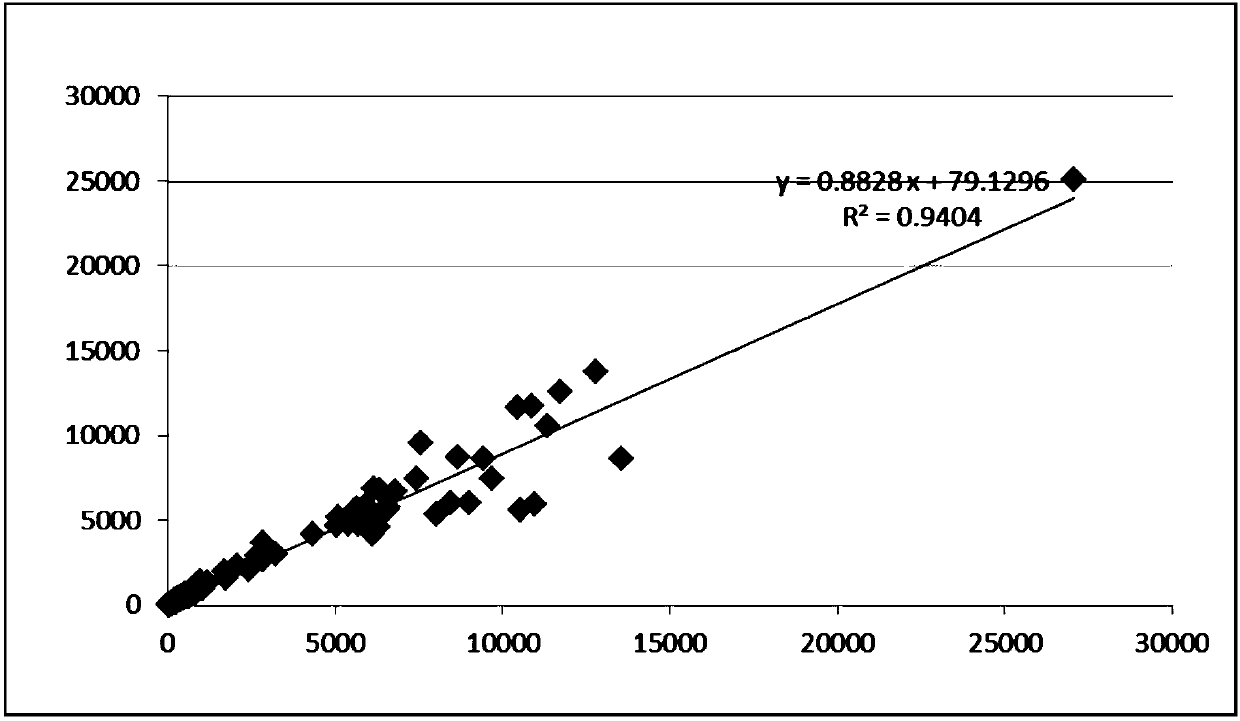
The invention relates to an ELISA (enzyme-linked immunosorbent assay) kit for an EV (enterovirus) 71 inactivated vaccine antigen. The detection kit comprises an EV71 pre-coated polyclonal antibody ELISA plate, a sample diluent, a second antibody, an enzyme-labeled antibody, a concentrated cleaning solution, an enzyme substrate solution and a stop buffer, wherein the ELISA plate is pre-coated with a polyclonal antibody prepared by taking recombinant EV71 structural protein 1 as an immune source; the second antibody adopts a monoclonal antibody prepared by taking keyhole limpet hemocyanin coupled polypeptide sequence FGEHKQEKDL as the immune source; the enzyme-labeled antibody adopts a horse radish peroxidase labelled goat anti-mouse immunoglobulin antibody; and an antibody standard is placed in the kit. The ELISA kit has better sensitivity when measuring the titer of the EV71 inactivated vaccine antigen, and has better linearity in the range of 5.9-750 ng / ml, and the linearly dependent coefficient r2 is larger than 0.99. The kit for measuring the titer of the EV71 inactivated vaccine antigen simply and conveniently has good specificity, accurate quantification, high sensitivity and good repeatability.
Owner:ZHEJIANG PUKANG BIOTECH
D-dimer detection kit and use method thereof
ActiveCN107942051ADetection speedSimplify operation stepsChemiluminescene/bioluminescencePre treatmentEnzyme
The invention belongs to the field of in vitro diagnosis kits, and concretely relates to a D-dimer detection kit and a use method thereof. The D-dimer detection kit comprises a calibration product, areagent R1, an enzyme conjugate working solution R2, a magnetic bead conjugate working solution M, a cleaning solution and a chemiluminescent substrate, and the reagent R1 contains an imidazole component, so blood cells in the whole blood can be quickly eliminated to effectively avoid the possibility of blood cells swallowing magnetic beads. The kit can directly use fingertip whole blood or anticoagulant venous whole blood as a sample to be tested, and can directly detect the whole blood sample without preprocessing the whole blood sample, so the detection speed is greatly improved, the operation steps are simplified, the application range of the kit is enlarged, and large-scale promotion and application are easy.
Owner:NANTONG EGENS BIOTECH
Array digital PCR chip and application method thereof
PendingCN107287112AImprove accuracyAvoid non-specific reactionsBioreactor/fermenter combinationsBiological substance pretreatmentsPcr chipEngineering
The invention specifically relates to an array digital PCR chip and an application method thereof, belonging to the technical field of experimental instruments used in biochemistry. The array digital PCR chip comprises a substrate and a cover plate, wherein the upper surface of the substrate is provided with a plurality of chutes; the central part of the bottom surface each chute is recessed to form a PCR reaction cell; and the lower surface of the cover plate is provided with a plurality of slide block bulges respectively in snapping connection with the chutes. When the cover plate covers the substrate, the slide block bulges are snapped with the corresponding chutes; and a liquid storage chamber is formed between each slide block bulge and its corresponding chute. According to the invention, through cooperation between the substrate and the cover plate, liquid in each liquid storage chamber simultaneously enters the PCR reaction cells and is mixed with primers and probes in the PCR reaction cells when the cover plate slides, so non-specific reaction is avoided, and the accuracy of subsequent detection data is improved.
Owner:兰州海关技术中心 +1
Immunoassay method and immunoassay kit to be used therein
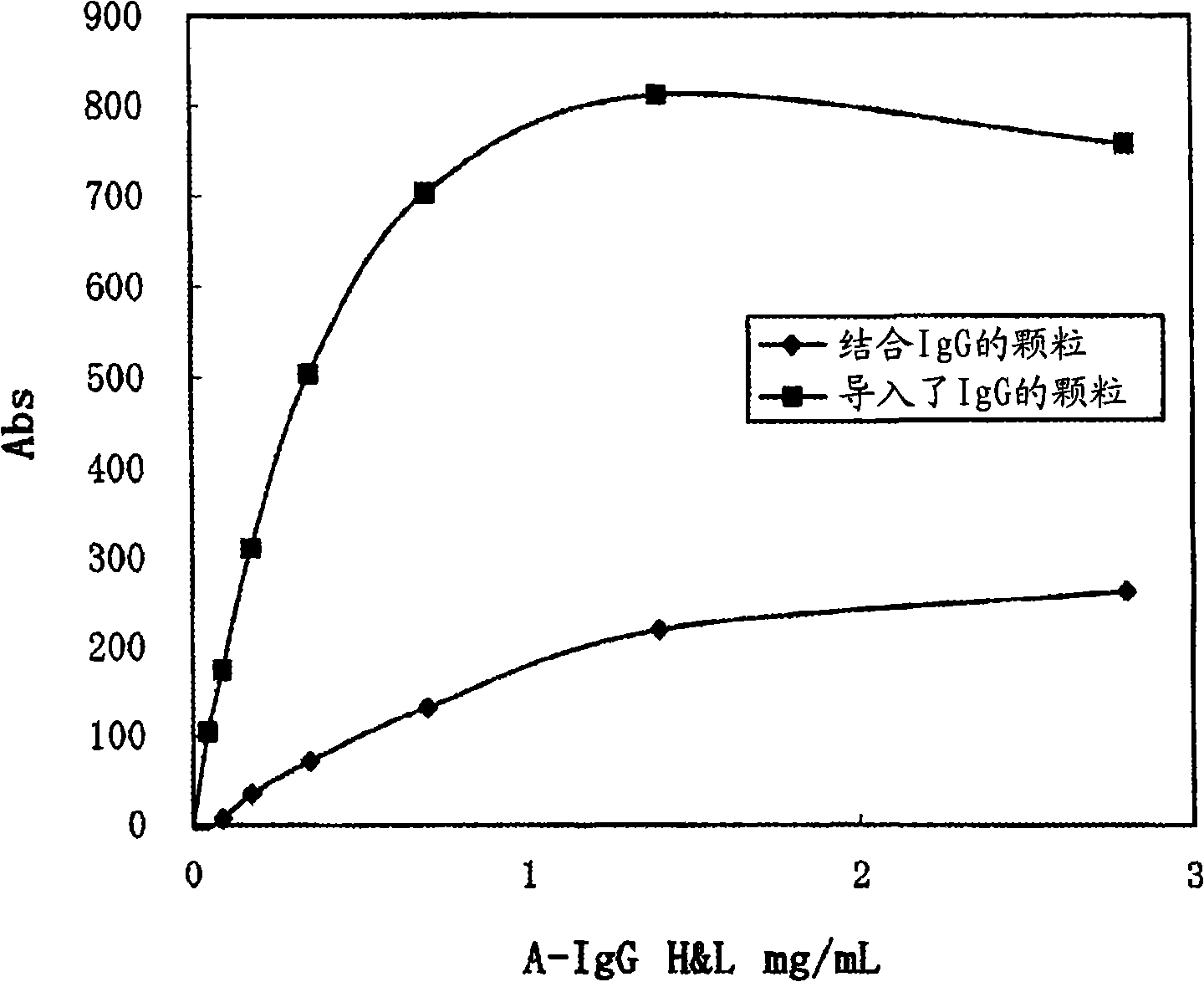
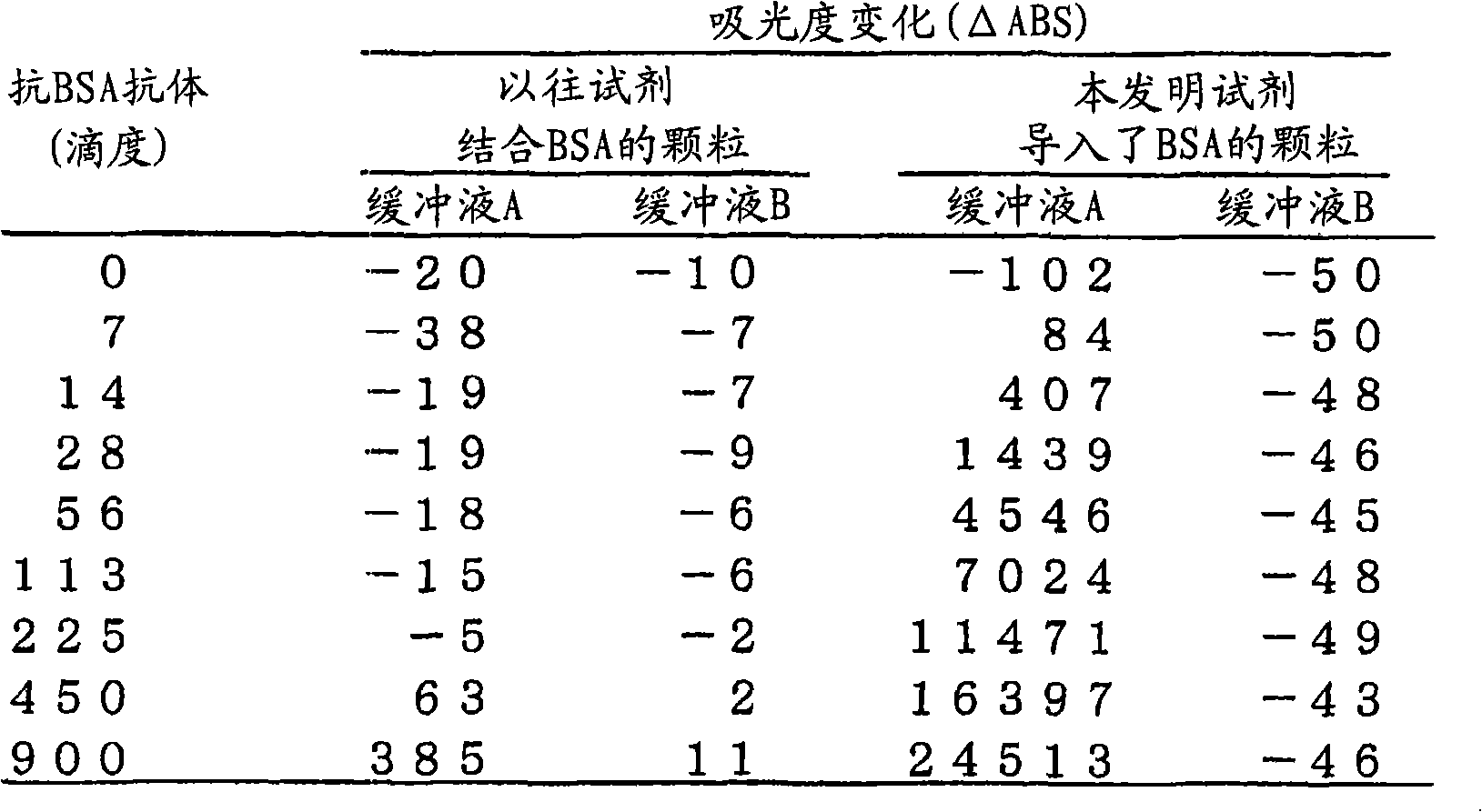
InactiveCN101147065AImprove detection accuracyHigh quantitative accuracyImmunoglobulinsMaterial analysisAntigen-antibody reactionsBiochemistry
In an immunoassay method with the use of antibody-sensitized particles, it is intended to prevent the occurrence of a false positive reaction. An immunoassay method which comprises mixing a specimen with antibody-sensitized particles having been sensitized with an antibody capable of reacting a subject to be assayed in the specimen, measuring the aggregation of the antibody-sensitized particles due to the antigen-antibody reaction and thus detecting or quantifying the subject to be assayed in the specimen, wherein the specimen is pretreated with a cation exchanger before the mixing. As the cation exchanger as described above, use can be made of, for example, a cation exchanger having sulfonate or carboxyl group as a functional group. It is not restricted in form. Namely, it may be a cation exchange filter, a cation exchange column or the like.
Owner:ARKRAY INC
Method of producing polymer microparticles
InactiveCN101542286AHigh detection sensitivityAvoid non-specific reactionsMaterial analysisAntigenMicroparticle
Owner:MITSUBISHI CHEM MEDIENCE +1
Primers and kit for rapidly detecting African swine fever virus
PendingCN110791592AThe test result is accurateImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationNanoparticleAfrican swine fever virus
The invention relates to primers and kit for rapidly detecting African swine fever virus. Four primers are provided, the kit comprises the primers for detecting nucleic acid of the African swine fevervirus and 18 [mu]L of a reaction solution, and the reaction solution comprises the following reagents: 1.8 [mu]L of 40 mM KCl, 1.8 [mu]L of 100 mM(NH4)2SO4, 1.8 [mu]L of 80 mM MgSO4, 1.8 [mu]L of 1%Tween-20, 0.9 [mu]L of28 mM dNTPs, 1.8 [mu]L of 8000 U / mL Bst enzyme, 0.18 [mu]L of 60 U specific endonuclease FEN1, 0.9 [mu]L of 1 mM SYBRGREEN fluorochrome, 1.8 [mu]L of 4.0*10<-5> mol / L gold nanoparticles and 0.22 [mu]L of ultrapure water. Compared with the prior art, the primers and kit for rapidly detecting the African swine fever virus have the following advantages: four different specific primers are provided, so that the accuracy of detection results for the African swine fever virus is higher, accurate detection results can be rapidly given in combination with an improved LAMP technology and a microfluidic chip technology, and the purpose of jointing detecting multiple different indexes of the same sample can be achieved.
Owner:宁波爱基因科技有限公司
Polypeptide-ELISA (enzyme-linked immunosorbent assay) kit for detecting specific antibody of N (nucleocapsid) protein of SFTSV (severe fever with thrombocytopenia syndrome virus)
ActiveCN105510580AReduce peptideReduce false positivesMaterial analysisPositive controlHorse radish peroxidase
The invention relates to detection kits in the technical field of biology, in particular to a polypeptide-ELISA (enzyme-linked immunosorbent assay) kit for detecting a specific antibody of an N (nucleocapsid) protein of an SFTSV (severe fever with thrombocytopenia syndrome virus). The kit comprises an ELISA plate pre-coated with synthetic SFTSV N protein linear B-cell antigenic polypeptide, a sample diluent, negative control serum, positive control serum, an HRP (horse radish peroxidase) marked antibody, a concentrated cleaning solution, an enzyme substrate solution and a stop buffer. The kit has the advantages of high specificity, good repeatability and simplicity, convenience and rapidness in operation, and can be widely used for evaluating SFTSV infection conditions.
Owner:ZHEJIANG PUKANG BIOTECH
Kit used for quantitative determination anti-nucleosome antibody Ig G via magnetic micro particle chemiluminiscence, and preparation method and detection method thereof
InactiveCN105301235AEasy to useGuaranteed detection effectMaterial analysisQuantitative determinationQuality control
The invention belongs to the technical field of immunological detection, and more specifically relates to a kit used for quantitative determination anti-nucleosome antibody Ig G via magnetic micro particle chemiluminiscence, and a preparation method and a detection method thereof. The kit comprises an anti-nucleosome antibody Ig G calibration material, an anti-nucleosome antibody Ig G reagent 1, an anti-nucleosome antibody Ig G reagent 2, an anti-nucleosome antibody Ig G magnetic separation reagent, an anti-nucleosome antibody Ig G quality control product, and a cleaning solution. The invention also discloses the preparation method and the detection method of the kit. The detection method is invented based on conventional membrane strip immunization and enzyme linked immunosorbent assay, sensitivity and linearity range are increased 10<3> to 10<5>, quantitative determination is realized, sensitivity, specificity, and accuracy are high, cost is low, operation is simple and convenient, result judgment is objective, fully automatic application is realized via combination with an automatic chemicaluminescence immunity analyzer, and application prospect is promising.
Owner:北京贝尔医疗设备有限公司
Pet D-dimer detecting kit for micro chemiluminescence immune assay system
PendingCN109444412AGuaranteed stabilityAvoid non-specific reactionsMaterial analysisSerum igeDisease
The inventio discloses a pet D-dimer detecting kit for a micro chemiluminescence immune assay system. The pet D-dimer detecting kit for the micro chemiluminescence immune assay system comprises D-dimer calibration products, enzyme conjugate working solution, D-dimer antibody-coated capillary tubes, cleaning solution and chemiluminescence substrates. The pet D-dimer detecting kit for the micro chemiluminescence immune assay system is high in sensitivity and specificity, wide in linear range, low in sample loading quantity, low in capacity of required plasma or serum samples and simple in operation method; only through simple operations such as manual adding of a small quantity of samples, users can achieve the aim of rapid and highly accurate pet disease diagnosis.
Owner:CHENGDU POLYTECH BIOLOGICAL TECH CO LTD
Primer and kit for efficiently detecting Erysipelothrix rhusiopathiae
PendingCN112029878ASimple and fast operationSuppresses non-specific reactionsMicrobiological testing/measurementDNA/RNA fragmentationSwine ErysipelasMedicine
The invention discloses a primer and kit for efficiently detecting Erysipelothrix rhusiopathiae. According to the primer and the kit, an improved LAMP technology serves as a gene amplification reaction principle, and gold nanoparticles are added into a reaction system to adsorb ssDNA and protease and inhibit nonspecific reactions during heating, thus, the aim of warm start is achieved, and the nonspecific reactions during heating are avoided; by combining the improved LAMP technology and a microfluidic chip technology, an accurate detection result can be presented rapidly, and the aim of jointdetection on the same sample with a plurality of different indexes is achieved; and meanwhile, reaction reagents are pre-buried into a microfluidic chip, a user only needs to add a sample, and operations are simple and convenient.
Owner:宁波爱基因科技有限公司
Technology for improving activity of enzyme-labeled working fluid anti-human IgA alkaline phosphatase
The invention provides a technology for improving activity of an enzyme-labeled working fluid anti-human IgA alkaline phosphatase. The technology comprises preparing an enzyme-labeled secondary antibody working solution according to the characteristics of anti-human IgA alkaline phosphatase self. The improvement comprises successively adding the following substances into the prepared enzyme-labeled secondary antibody: (1) adding a low-concentration high-molecular polymer, and dissolving the added high-molecular polymer in the prepared enzyme-labeled secondary antibody working solution; and (2) then adding anti-human IgA alkaline phosphatase into the enzyme-labeled secondary antibody working solution added with the high-molecular polymer, and stirring to fully dissolve the anti-human IgA alkaline phosphatase, so as to obtain an enzyme-labeled secondary antibody working solution with activity-improved anti-human IgA alkaline phosphatase. According the method, by adding the low-concentration high-molecular polymer, the effects of improving the reaction activity of anti-human IgA alkaline phosphatase and improving the detection sensitivity are realized.
Owner:BEIJING H&J NOVOMED
Method for preparing a nucleic acid with high precision used in detecting a nucleic acid with a nucleic acid polymerase
ActiveCN104350159AHigh sensitivityAvoid non-specific reactionsMicrobiological testing/measurementDNA preparationPolymerase LComputational biology
Owner:BIONEER
The testing method of adenosine deaminase
InactiveCN106282309ABest combination conditionsImprove consistencyMicrobiological testing/measurementXanthine oxidationPurine nucleoside phosphorylase
The invention discloses the testing method of adenosine deaminase. The testing reagent of adenosine deaminase is made up of the following two parts: Reagent 1 includes disodium hydrogen phosphate 8.5-12 mmol / L, aminoantipyrine 1.5-2.2 mmol / L, purine nucleoside phosphorylase 2500-2900 U / L, xanthine oxidase 500-700 U / L, peroxidase 510-710 U / L, ascorbinase 1800-2800 U / L, certain stability reagent and certain assistant factors. Reagent 2 includes adenosine 10-14 mmol / L and EHSPT 1.5-2.5 mmol / L. The testing reagent formula of adenosine dehydrogenase in this invention and the confirmation of the testing parameters and referential range are good for the consistency of the clinical testing results. it will bring a lot of benefits to the clinical diagnosis. The sensitivity of this method is very high and it is superior to the similar products sold on the market.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Primer and test kit for efficiently detecting porcine Japanese encephalitis virus
PendingCN111996293ASimple and fast operationSuppresses non-specific reactionsMicrobiological testing/measurementDNA/RNA fragmentationNanoparticleEncephalitis Viruses
The invention discloses a primer and a test kit for efficiently detecting porcine Japanese encephalitis virus; an improved LAMP technology is taken as a gene amplification reaction principle, gold nanoparticles are added into a reaction system, and ssDNA and protease are adsorbed, thereby inhibiting non-specific reaction in a heating process, achieving the purpose of hot start, and avoiding non-specific reaction in the heating process; the improved LAMP technology and a microfluidic chip technology are combined, an accurate detection result can be rapidly given, the purpose of joint inspectionof multiple different indexes of the same sample is achieved, and meanwhile, a reaction reagent is pre-buried in a microfluidic chip, a user only needs to add the sample, and operation is easy and convenient.
Owner:宁波爱基因科技有限公司
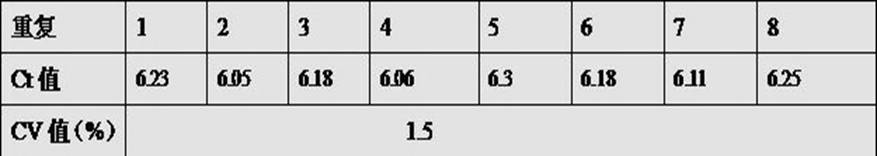
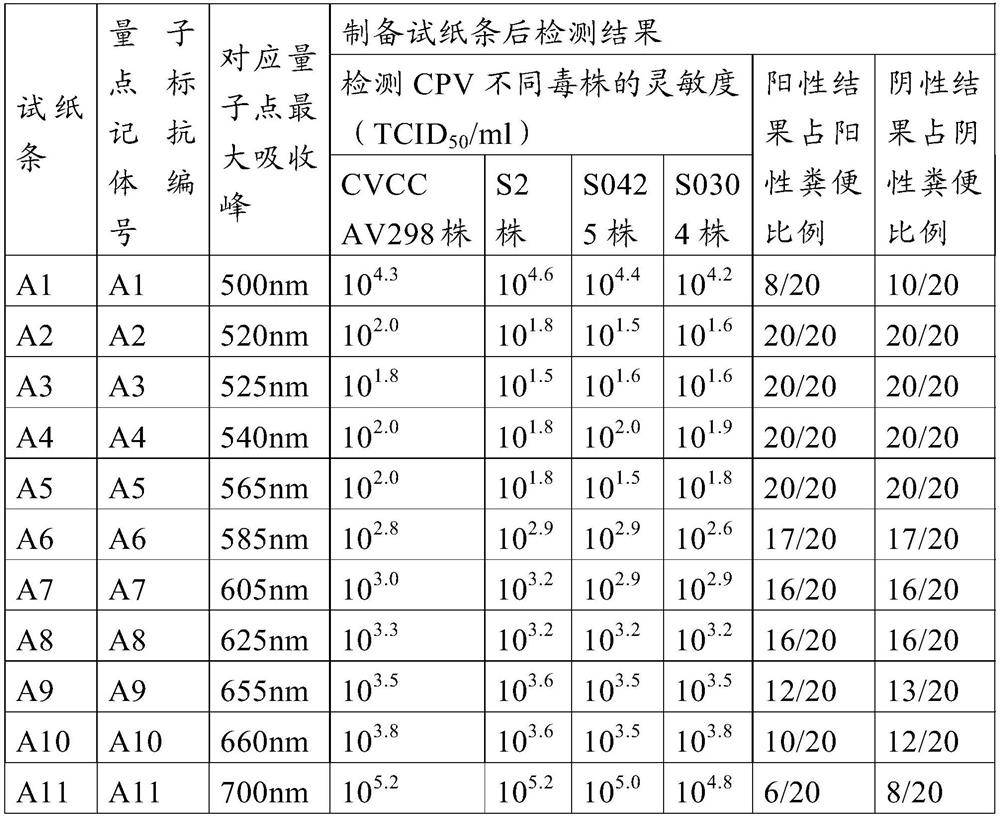
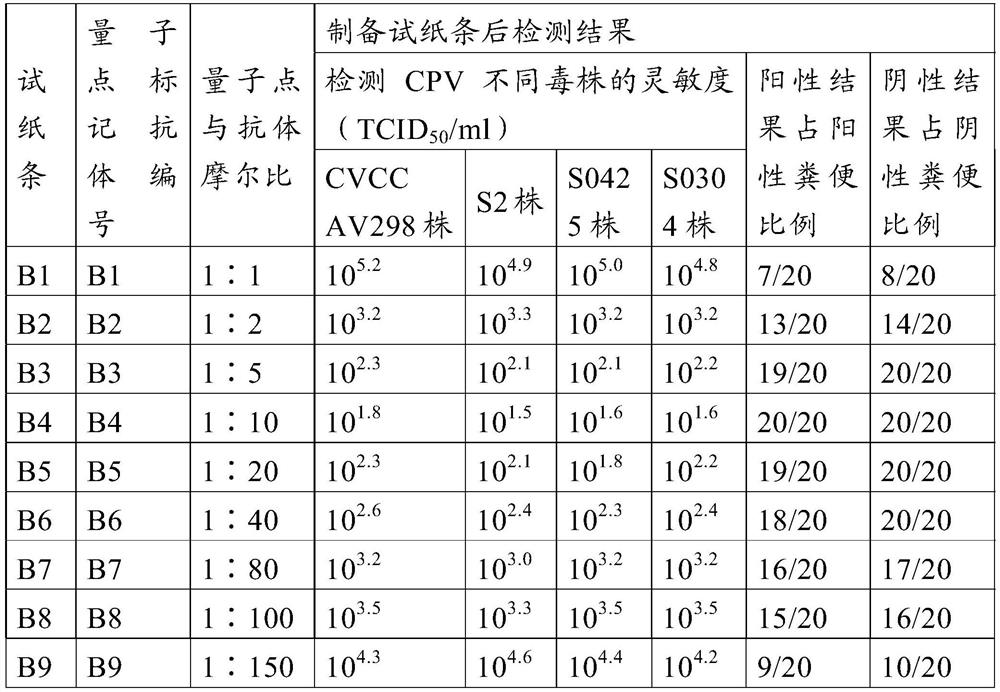
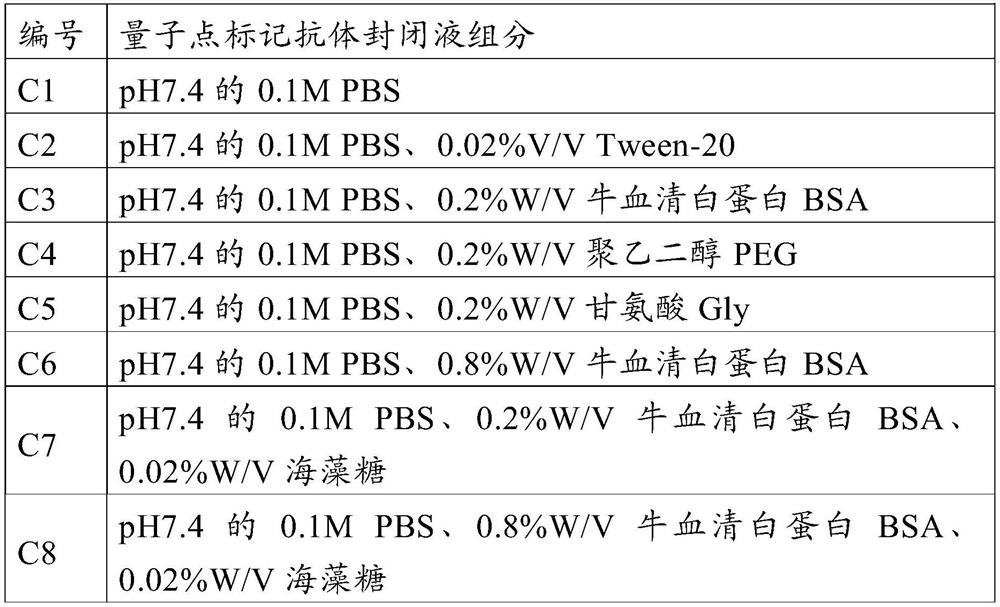
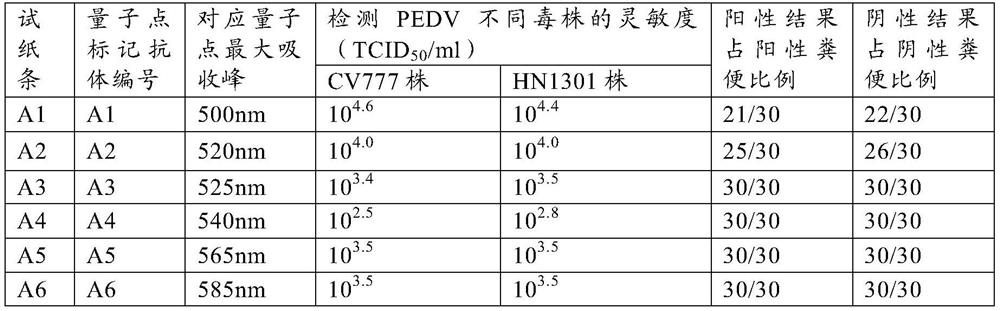
Method for preparing quantum dot labeled anti-canine virus antibody immunochromatographic test strip, prepared test strip and application
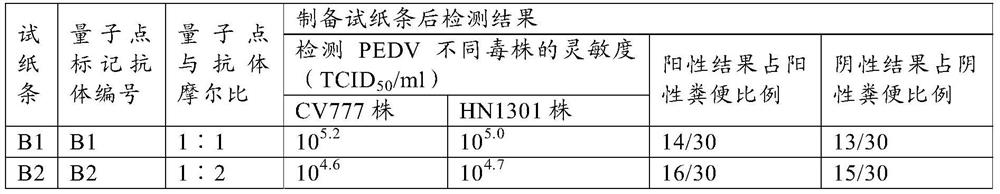
ActiveCN111665363AHigh sensitivityRapid real-time quantitative detectionBiological material analysisBiological testingImmobilized AntibodiesViral antibody
The invention provides a method for preparing a quantum dot labeled anti-canine virus antibody immunochromatographic test strip. According to the method, high-sensitivity detection of a virus antigenin a sample is realized by optimizing the ratio of quantum dots to a canine virus antibody, an antibody confining liquid, an antibody reconstitution fluid, a labeled antibody and a fixed antibody, andthe sensitivity of the method is 50 times higher than that of a colloidal gold immunochromatography test strip. The preparation method disclosed by the invention is universally used for various anti-canine virus antibody pairs, and the quantum dot labeled immunochromatographic test strip prepared from various canine virus antibody pairs can sensitively detect virus antigens; meanwhile, the storage life can be prolonged to 27 months. The invention also relates to the quantum dot labeled immunochromatographic test strip prepared by the method, and a kit containing the quantum dot labeled immunochromatographic test strip.
Owner:北京中科基因技术股份有限公司
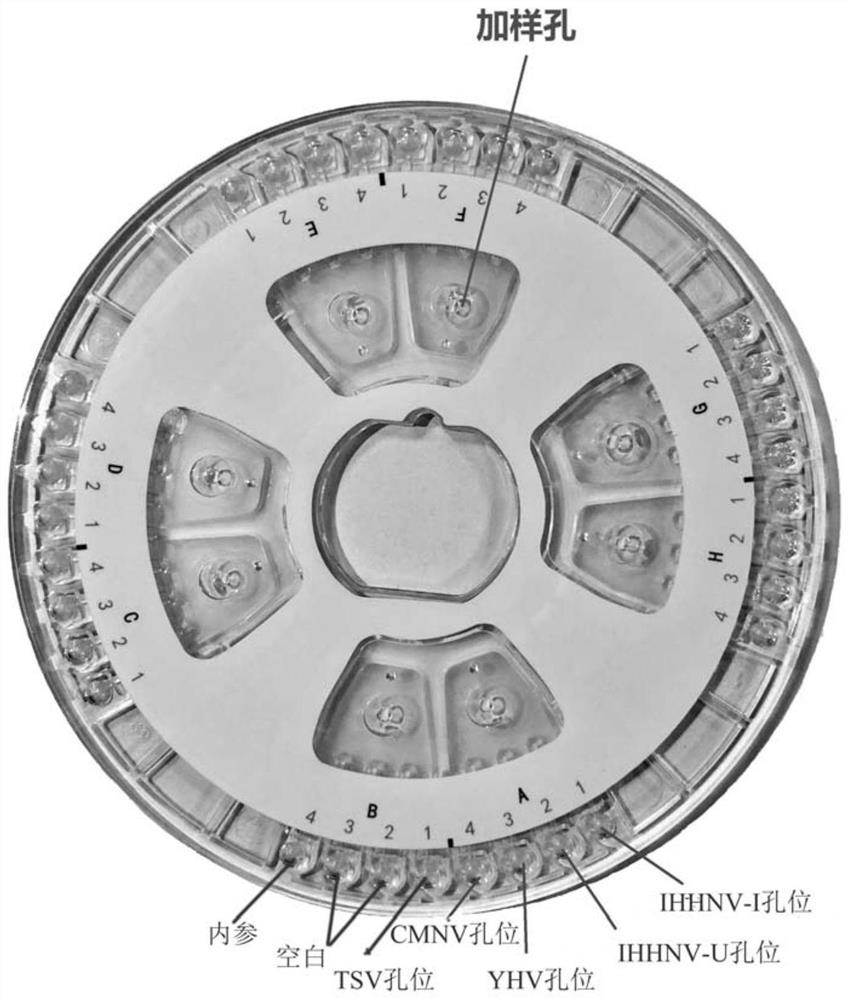
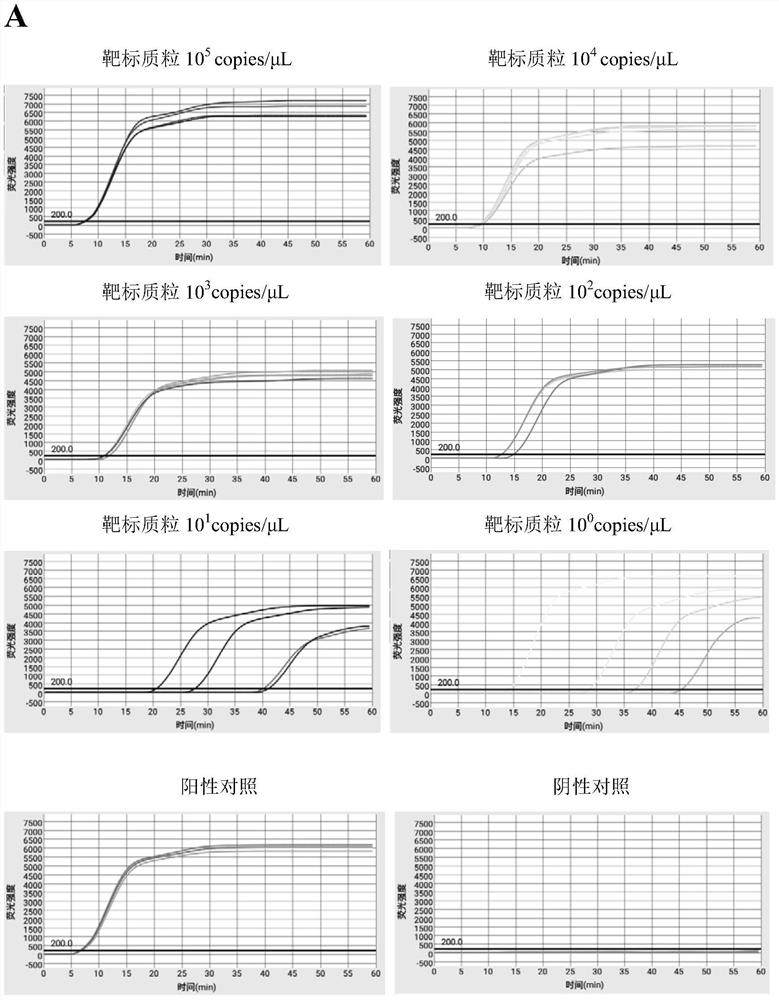
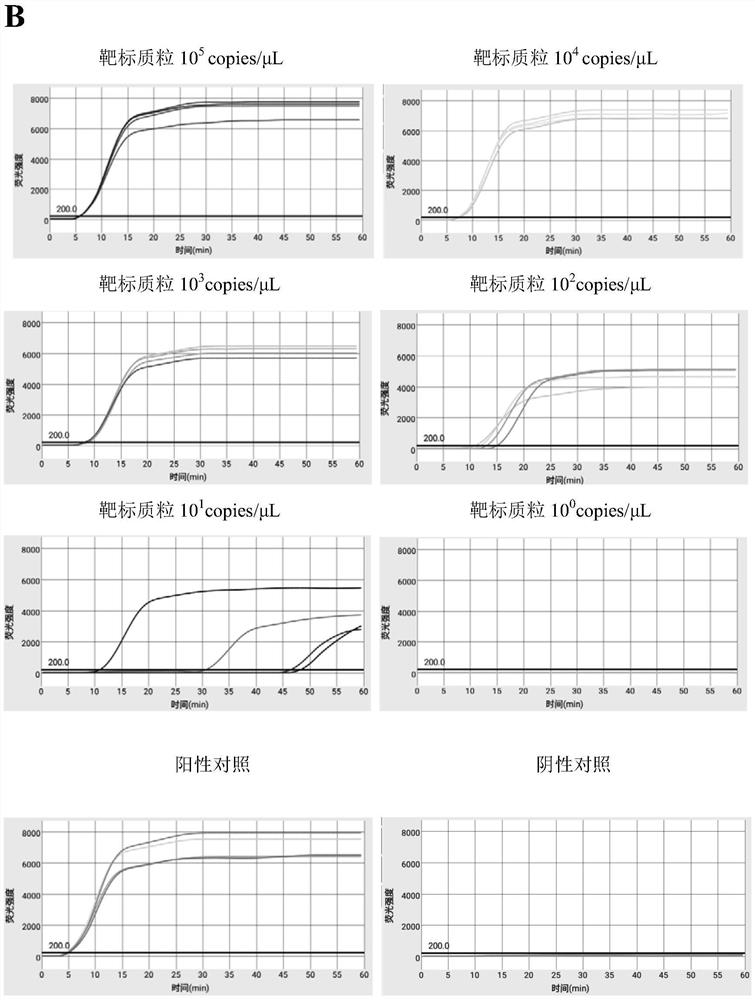
Efficient quintuplet primer for detecting shrimp diseases and kit
PendingCN112981006ASuppresses non-specific reactionsTo achieve the purpose of hot startMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyNanoparticle
The invention discloses an efficient quintuplet primer for detecting shrimp diseases and a kit. According to the primer and the kit, an improved LAMP technology serves as a gene amplification reaction principle, and gold nanoparticles are added into a reaction system to adsorb ssDNA and protease and inhibit nonspecific reactions during heating, thus, the aim of warm start is achieved, and nonspecific reactions during heating-up are avoided; and by combining the improved LAMP technology and a microfluidic chip technology, an accurate detection result can be presented rapidly, and the aim of joint detection on the same sample with a plurality of different indexes is achieved; meanwhile, reaction reagents are pre-buried into a microfluidic chip, five-index detection on one same is achieved, a user only needs to add a sample, and operations are simple and convenient; and the instrument is equipped with lithium batteries, so that on-site rapid detection is facilitated.
Owner:宁波爱基因科技有限公司 +1
Method for preparing quantum dot labeled anti-porcine virus antibody immunochromatographic test strip, prepared test strip and application
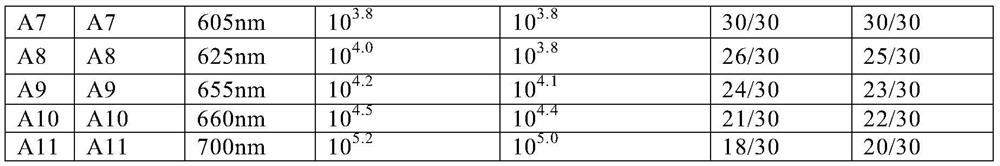
PendingCN111665362AAchieving High Sensitivity DetectionWide applicabilityBiological material analysisBiological testingImmobilized AntibodiesViral antibody
The invention provides a method for preparing a quantum dot labeled anti-porcine virus antibody immunochromatographic test strip. According to the method, by optimization of the ratio of the quantum dots to an anti-porcine virus antibody, an antibody confining liquid, an antibody reconstitution fluid, a labeled antibody and a fixed antibody, high-sensitivity detection of the virus antigen in a sample is realized, and the sensitivity of the method is 50 times higher than that of a colloidal gold immunochromatography test strip. The preparation method disclosed by the invention is universal forvarious anti-porcine virus antibody pairs, and the quantum dot labeled immunochromatographic test strip prepared from various anti-porcine virus antibody pairs can sensitively detect virus antigens; meanwhile, the storage life can be prolonged to 27 months. The invention also relates to the quantum dot labeled immunochromatographic test strip prepared by the method, and a kit containing the quantum dot labeled immunochromatographic test strip.
Owner:北京中科基因技术股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com