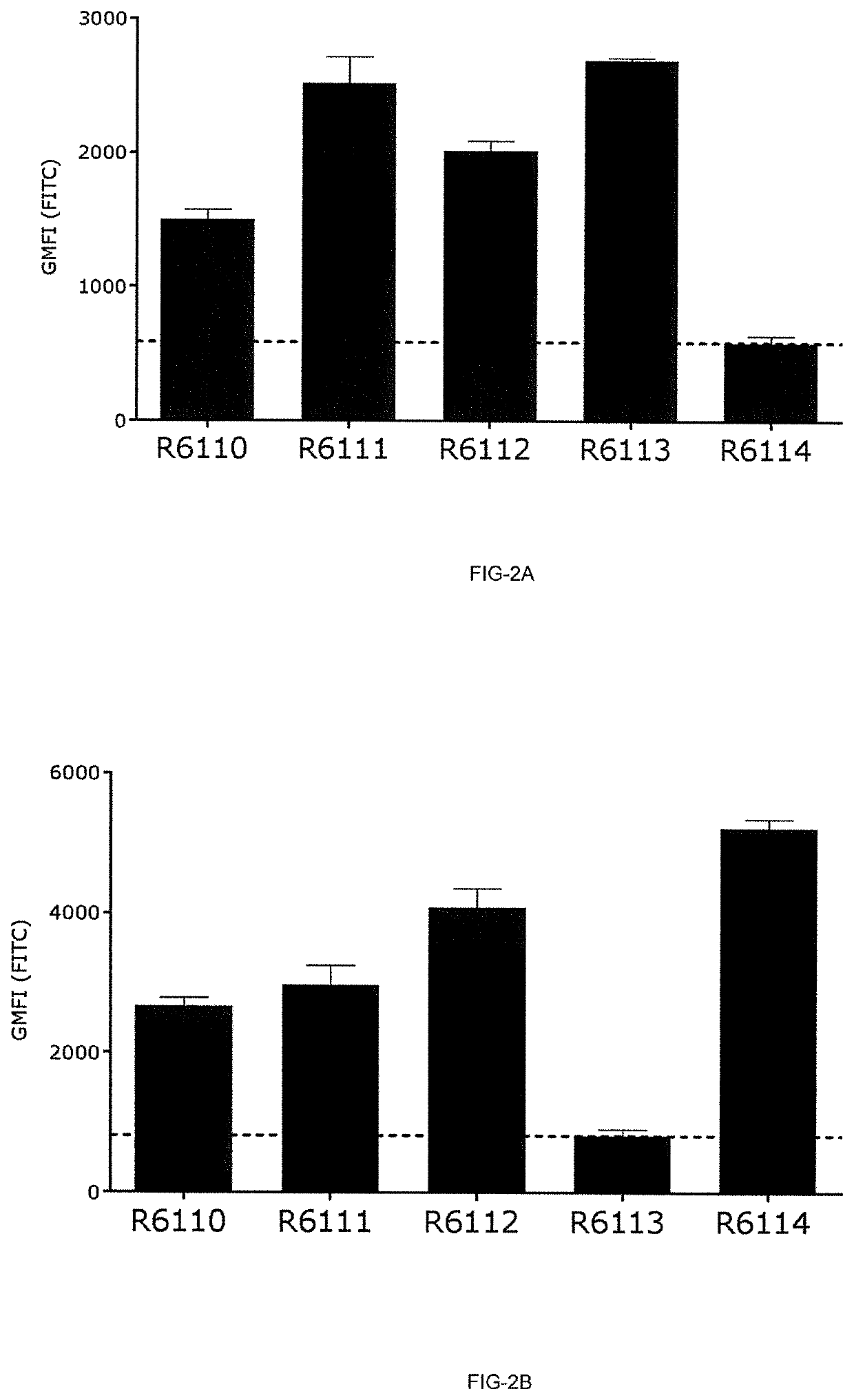
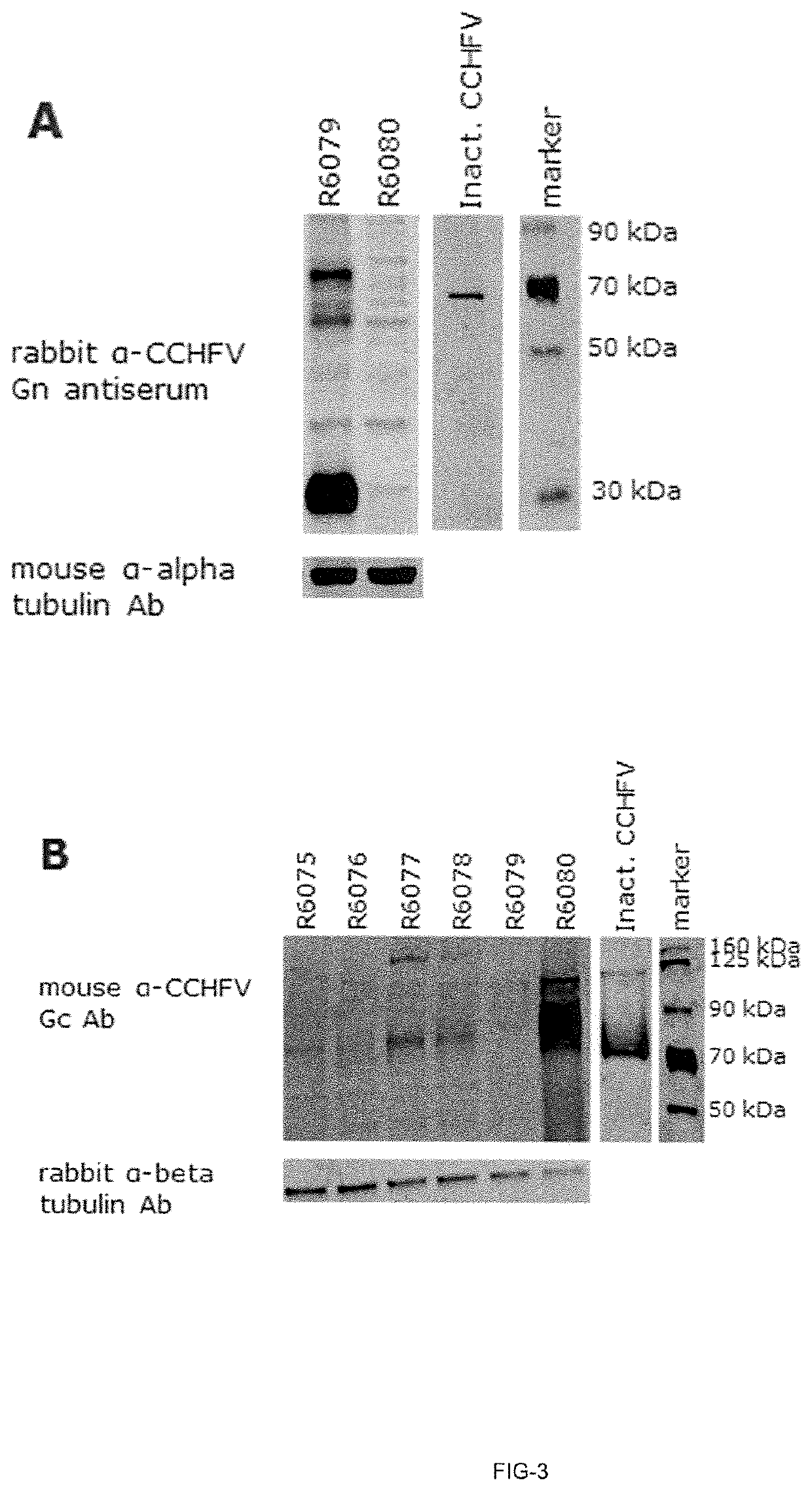
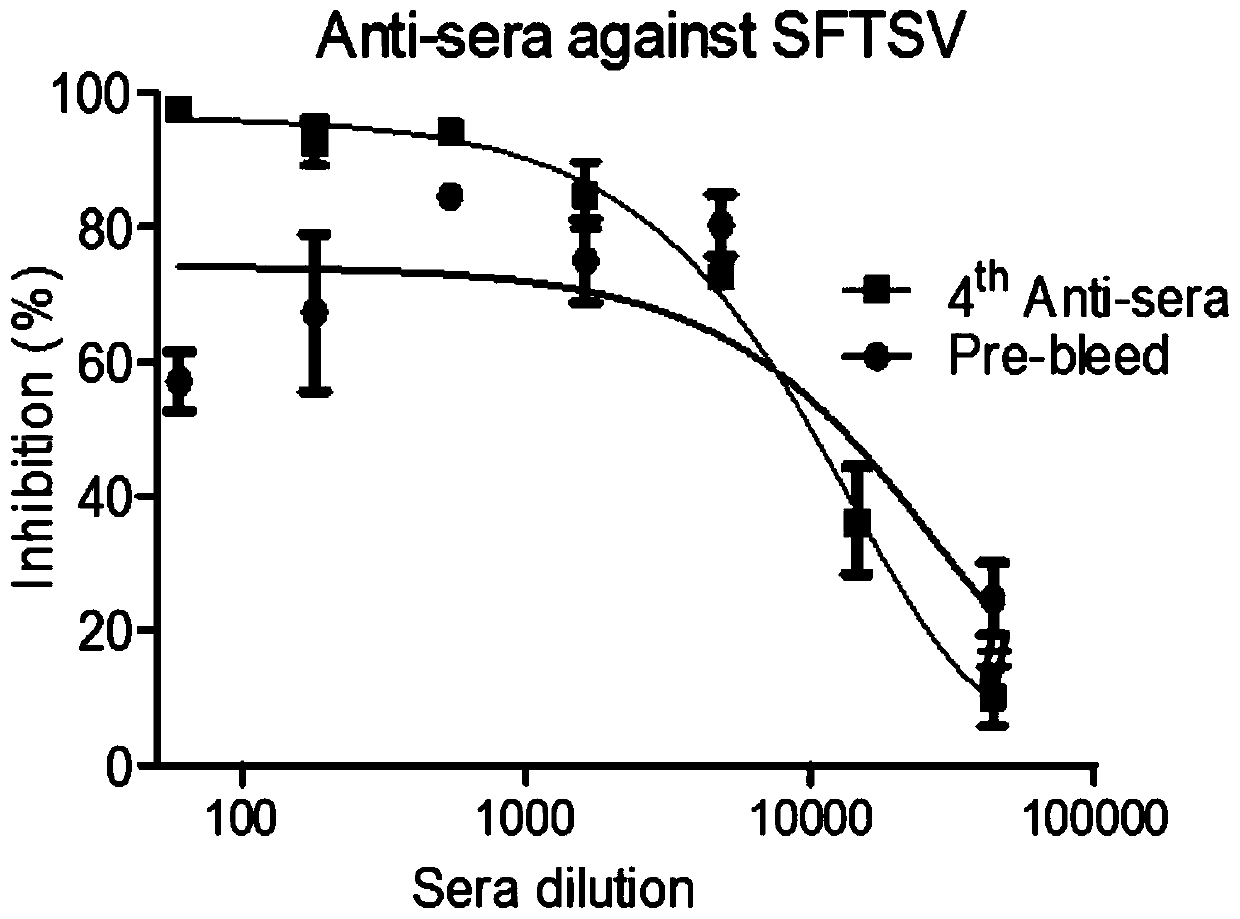
Patents
Literature
38 results about "Severe fever with thrombocytopenia syndrome virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Severe fever with thrombocytopenia syndrome (SFTS) is a newly emerging infectious disease.
Entire gene sequence of severe fever with thrombocytopenia syndrome virus (SFTSV) and application
ActiveCN102070704AStrong specificityPeptide/protein ingredientsGenetic material ingredientsPolymerase LStructural protein
The invention relates to a severe fever with thrombocytopenia syndrome virus (SFTSV), an entire gene sequence represented by Hubei isolate HB29, amino acid sequences of coding proteins and application. The entire gene sequence of the virus is subjected to homology analysis. The virus belongs to bunyaviridae and comprises three gene segments, namely, L, M and S which represent polymerase and glycoprotein (Gn and Gc), nucleoprotein (NP) and non-structural proteins (NSs) of the virus respectively, and the three segments are all positioned on the branch of phlebovirus but farther from other viruses of phlebovirus. The entire gene sequence and the coding proteins of the virus can be used for developing drugs, vaccines or diagnostic reagents for preventing and treating the epidemic diseases caused by the SFTSV.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Bunyavirales vaccine
ActiveUS20210030864A1Efficient expressionSsRNA viruses negative-senseViral antigen ingredientsDiseaseBunyavirales
The present invention is directed to an artificial nucleic acid, particularly to an artificial RNA, and to polypeptides suitable for use in treatment or prophylaxis of an infection with a virus of the order Bunyavirales, particularly Severe fever with thrombocytopenia syndrome virus (SFTSV), Rift Valley fever virus (RVFV), or Crimean-Congo hemorrhagic fever virus (CCHFV), or a disorder related to such an infection. The present invention further concerns a Bunyavirales vaccine, particularly a SFTSV, RVFV, or CCHFV vaccine. The present invention is directed to an artificial nucleic acid, polypeptides, compositions and vaccines comprising the artificial nucleic acid or the polypeptides. The invention further concerns a method of treating or preventing a disorder or a disease, first and second medical uses of the artificial nucleic acid, polypeptides, compositions and vaccines. Further, the invention is directed to a kit, particularly to a kit of parts, comprising the artificial nucleic acid, polypeptides, compositions and vaccines.
Owner:CUREVAC SE
Codon optimized severe fever with thrombocytopenia syndrome virus (SFTSV) glycoprotein Gn gene sequence carrying tPA signal peptide and nucleic acid vaccine thereof
InactiveCN106011155AEfficient removalEffective combinationSsRNA viruses negative-senseViral antigen ingredientsSequence signalWild type
The invention belongs to the technical field of biological medicines, and relates to a codon optimized severe fever with thrombocytopenia syndrome virus (SFTSV) glycoprotein Gn gene sequence carrying tPA signal peptide and a nucleic acid vaccine thereof. The SFTSV glycoprotein Gn nucleic acid vaccine is composed of the codon optimized SFTSV glycoprotein Gn gene sequence and a eukaryotic expression vector pJW4303, and the 5'end of the SFTSV glycoprotein Gn gene sequence is connected with a tPA signal peptide sequence. Compared with a nucleic acid vaccine in a wild type state, the nucleic acid vaccine can express objective protein more efficiently and can effectively secrete the objective protein out of cells in gene expression, and an immune system can be effectively stimulated to produce good humoral immune response after a mammal is inoculated with the nucleic acid vaccine.
Owner:李军 +6
Quantitative determination kit for neutralizing antibodies of virus and application thereof
The invention belongs to the field of a biotechnology and particularly relates to a quantitative determination kit for neutralizing antibodies of virus and application of the quantitative determination kit to detection of the neutralizing antibodies of virus of severe fever with thrombocytopenia syndrome. Virus NP proteins of the severe fever with thrombocytopenia syndrome are subjected to prokaryotic expression to prepare a rat source monoclonal antibody or polyclonal antibody aiming at NP proteins. The tissue cell half infection amount (TCIF50) of determined viruses is measured by detecting the NP proteins through an enzyme linked immunosorbent assay. A specimen to be detected and the equal amount of virus liquid are mixed and inoculated with cells. The monoclonal antibody or the polyclonal antibody of the NP proteins is monoclonal antibody; a specimen neutralizing antibody valence is detected by a double-antibody enzyme linked immunosorbent assay. The quantitative determination kit can be applied to the aspects of clinical immune effect evaluation of severe fever with thrombocytopenia syndrome vaccines, blood serum epidemiologic studies of the severe fever with thrombocytopenia syndrome of crowds or faunas, in-vitro valence determination and estimation of the manually-prepared virus-neutralizing antibodies of the severe fever with thrombocytopenia syndrome. The method provided by the invention has the characteristics of sensitivity, rapidness, specificity, high flux and the like.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Detecting kit for severe fever with thrombocytopenia syndrome viruses (SFTSV) and preparation method of detecting kit
InactiveCN105296674AEasy to operateMicrobiological testing/measurementMicroorganism based processesNucleic acid detectionFluorescence
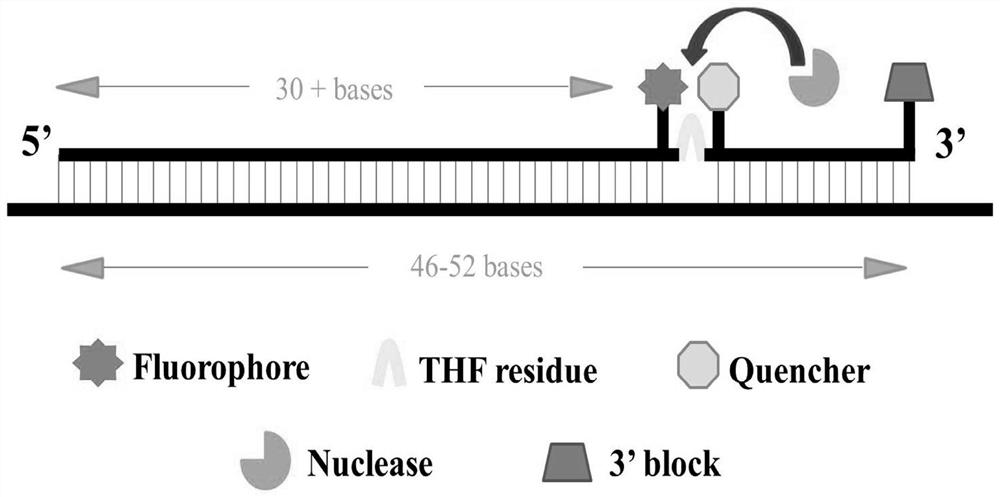
The invention belongs to the technical field of biology and particularly relates to a reverse transcription-recombinase polymerase amplification (RT-RPA) detecting kit for severe fever with thrombocytopenia syndrome viruses (SFTSV) and a preparation method of the detecting kit. The detecting kit is composed of an RT-RPA reaction system, an RNA enzyme inhibitor, an SFTS virus fragment L positive plasmid Puc-L, a negative quality control, a fragment L RPA primer and an exo probe. A rapid, sensitive and specific isothermal real-time fluorescent nucleic acid detection method for SFTSV is established by using the specific primer and the probe through real-time fluorescent detection realized by preparing the RT-RPA reaction system and placing a Twista real-time fluorescent detection instrument to carry out real-time fluorescent detection. The invention relates to application of the specific RPA primer and the exo probe to clinical differential diagnosis of SFTSV infection and identification of a virus isolate strain.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Antibody that binds to envelope glycoprotein of severe fever with thrombocytopenia syndrome virus and use for same
ActiveUS20190112360A1Effective Detection and DiagnosisSsRNA viruses negative-senseVirus peptidesSevere fever with thrombocytopenia syndrome virusEnv Glycoproteins
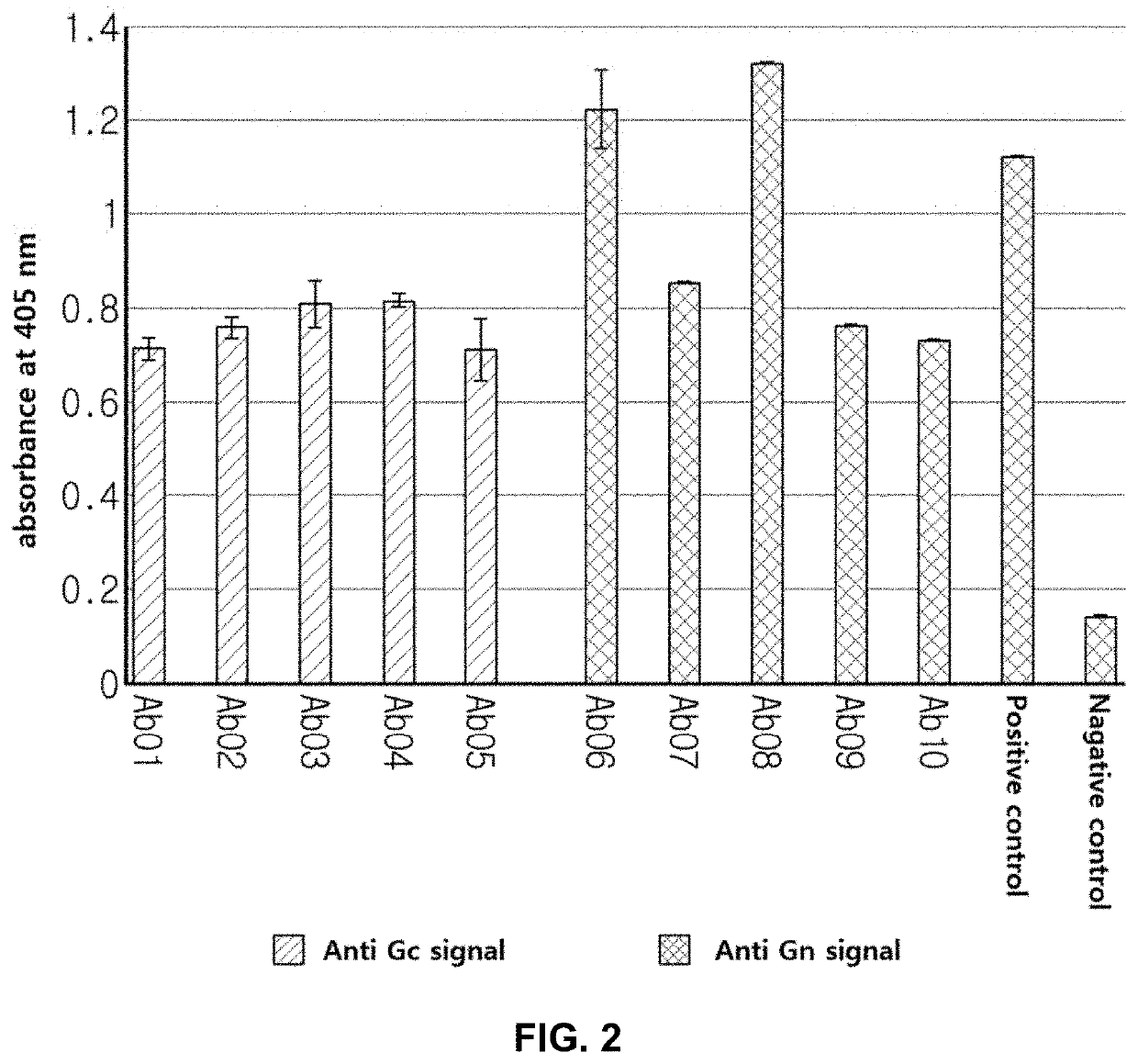
The present invention relates to an antibody which specifically binds to the envelope glycoprotein of severe fever with thrombocytopenia syndrome virus (SFTSV), the pathogen of severe fever with thrombocytopenia syndrome (SFTS), and is used in order to effectively detect or diagnosis SFTSV and treat SFTS.
Owner:SEOUL NAT UNIV R&DB FOUND +1
Recombinant virus vector and vaccine and preparation method and application thereof
ActiveCN108715866AEasy to trainHigh titerSsRNA viruses negative-senseViral antigen ingredientsVector vaccineViral vector
The invention belongs to the technical field of immune and discloses a recombinant virus vector and a vaccine against virus SFTS (severe fever with thrombocytopenia syndrome) and a preparation methodand application thereof. The recombinant virus vector is DNA molecules virus vector with glycoproteins Gn / Gc which can encode severe fever with thrombocytopenia syndrome virus. Furthermore, the recombinant virus and the virus transcription composite and the like transform host cells and collect viral particles with infective capacity which is a live virus vector vaccine. Compared with the prior technology, the vaccine has the advantages of easy cultivation, effective infection, easy utilization, capacity of stimulating host to produce strong immune reaction to cells, strong immune reaction tofluid and relatively strong immune reaction to mucosa, good safety, and the like.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Polypeptide-ELISA (enzyme-linked immunosorbent assay) kit for detecting specific antibody of N (nucleocapsid) protein of SFTSV (severe fever with thrombocytopenia syndrome virus)
ActiveCN105510580AReduce peptideReduce false positivesMaterial analysisPositive controlHorse radish peroxidase
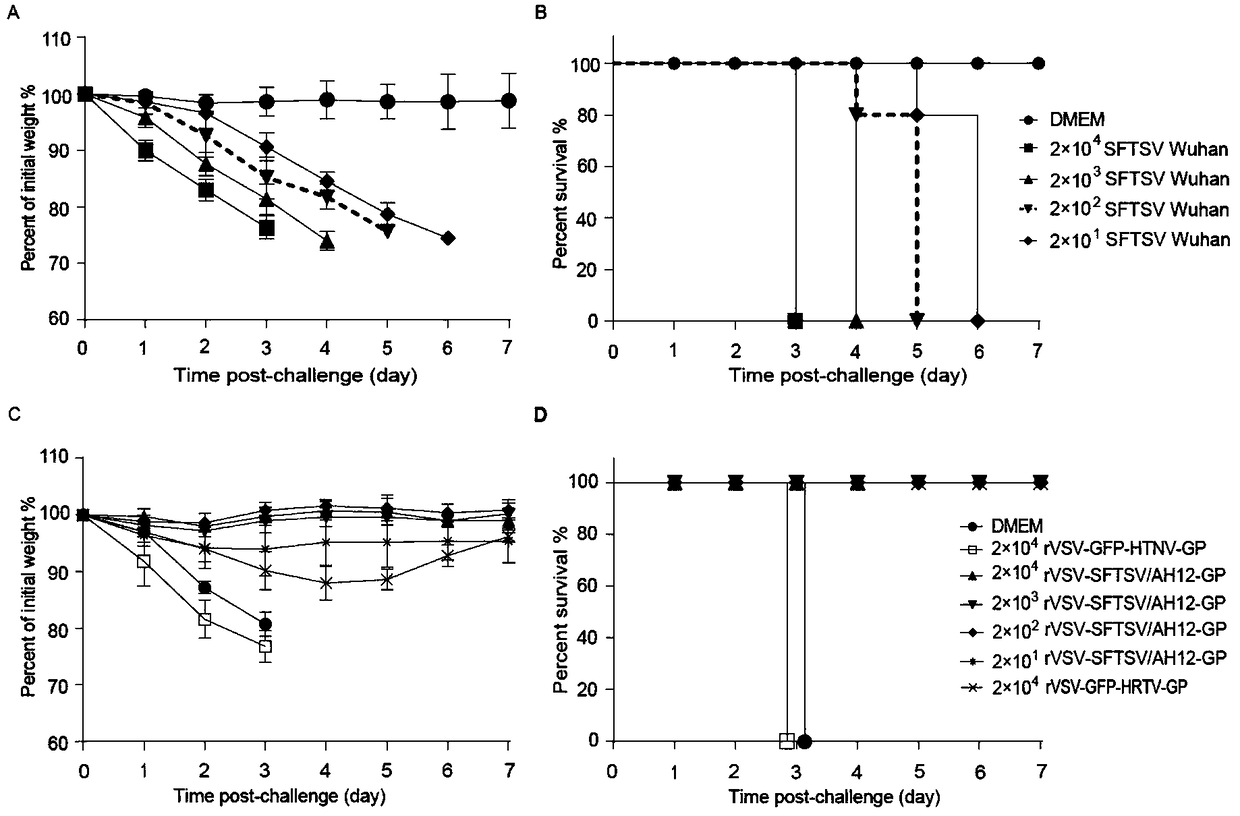
The invention relates to detection kits in the technical field of biology, in particular to a polypeptide-ELISA (enzyme-linked immunosorbent assay) kit for detecting a specific antibody of an N (nucleocapsid) protein of an SFTSV (severe fever with thrombocytopenia syndrome virus). The kit comprises an ELISA plate pre-coated with synthetic SFTSV N protein linear B-cell antigenic polypeptide, a sample diluent, negative control serum, positive control serum, an HRP (horse radish peroxidase) marked antibody, a concentrated cleaning solution, an enzyme substrate solution and a stop buffer. The kit has the advantages of high specificity, good repeatability and simplicity, convenience and rapidness in operation, and can be widely used for evaluating SFTSV infection conditions.
Owner:ZHEJIANG PUKANG BIOTECH
Enzyme linked immunosorbent assay kit for detecting sever fever with thrombocytopenia syndrome virus antigen
The invention discloses an enzyme linked immunosorbent assay kit for detecting sever fever with thrombocytopenia syndrome virus antigen. The enzyme linked immunosorbent assay kit comprises a reagent A, a reagent B, a reagent C, a reagent D, a reagent E, a reagent F, and a reagent H. The reagent A is an ELISA coated plate coated with a capture antibody; the reagent B is a PBS buffer solution; the reagent C is an antigen NP solution with the concentration of 15.6 ng / mL; the reagent D is an AuNP-Ab2-HRP probe concentrated solution with the gold nanoparticle concentration being 100 microgram / mL; for the reagent E, a 0.01 M PBS buffer solution containing 5% of BSA and having a pH value of 7.4 is used as a probe diluent; the reagent F is skim milk powder; the reagent G is 3, 3', 5, 5'-tetramethyl benzidine; and the reagent H is 0.1 M of HCL. The kit is high in sensitivity and specificity, and the aggregation concentration of enzymes on each immune sandwich structure is enhanced. The absorbance value under a specific wavelength is measured, and the sever fever with thrombocytopenia syndrome virus antigen NP can be qualitatively and quantitatively detected.
Owner:TIANJIN UNIV
Codon-optimized severe fever with thrombocytopenia syndrome virus nucleoprotein gene and its nucleic acid vaccine
InactiveCN104830874BHelps stimulate immune responseStimulate immune responseViral antigen ingredientsGenetic material ingredientsSequence signalFhit gene
The invention belongs to the technical field of biomedicine and relates to codon-optimized severe fever with thrombocytopenia syndrome virus nucleoprotein gene and nucleic acid vaccine thereof. The codon-optimized gene sequence takes into account the codon usage preference of mammalian cells, and the sequence is shown in SEQ ID NO.2. The involved severe fever with thrombocytopenia syndrome virus nucleoprotein nucleic acid vaccine is composed of codon-optimized severe fever with thrombocytopenia syndrome virus nucleoprotein gene sequence and eukaryotic expression vector pJW4303, whose 5' end is connected with tPA signal peptide sequence. The nucleic acid vaccine effectively stimulates the immune system after immunizing the mammal, and produces a better humoral immune response.
Owner:JIANGSU PROVINCE HOSPITAL
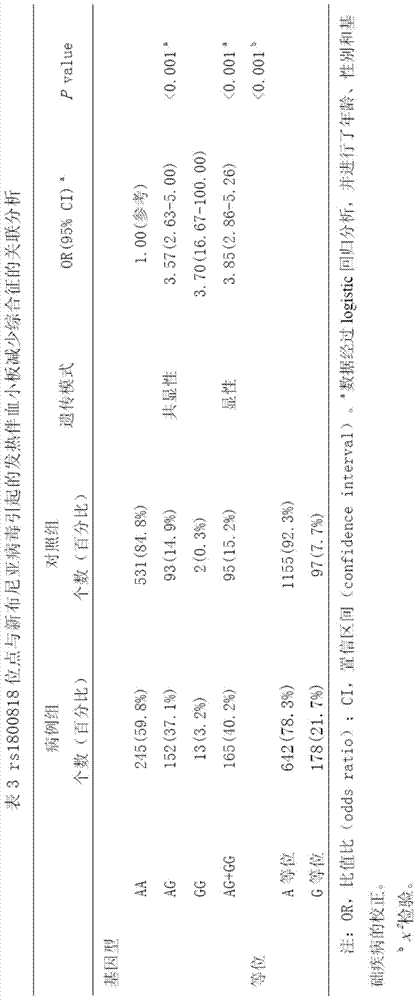
Application of rs1800818 to detection of severe fever with thrombocytopenia syndrome caused by severe fever with thrombocytopenia syndrome virus
ActiveCN104789673AMicrobiological testing/measurementDNA/RNA fragmentationNucleotideSevere fever with thrombocytopenia syndrome virus
The invention discloses application of rs1800818 to detection of severe fever with thrombocytopenia syndrome (FTLS) caused by severe fever with thrombocytopenia syndrome virus (FTLSV). The technical scheme to be protected is about application of substances for detecting the polymorphism or the genotype of rs1800818 in a personal genome to preparation of a product for screening FTLS and application of substances for detecting the polymorphism or the genotype of rs1800818 in a personal genome to preparation of a product for forecasting the condition of an FTLS patient. The substances for detecting the polymorphism or the genotype of rs1800818 can be combined with other substances (such as a substance for detecting other single nucleotide polymorphism or genotype related to FTLS) to prepare a product for screening FTLS patients or forecasting the conditions of FTLS patients.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Polypeptide-ELISA kit for detecting specific antibody against envelope glycoprotein of severe fever with thrombocytopenia syndrome virus
The invention relates to the field of biotechnology, especially to a polypeptide-ELISA kit for detecting a specific antibody against the envelope glycoprotein (Gn and Gc) of the severe fever with thrombocytopenia syndrome virus (SFTSV). The kit comprises an enzyme-labeled plate coated with SFTSV envelope glycoprotein dominant linear B cell antigen polypeptide, a sample diluents, negative control serum, positive control serum, a horseradish peroxidase (HRP) labeled antibody, a concentrated washing solution, an enzyme substrate solution and a stopping solution. The kit has the advantages of high specificity and good repeatability, can simply and conveniently detect the specific antibody against SFTSV, reduces false positive results, and is applicable to large-scale serological detection and epidemiological investigation and assessment the infection condition of SFTSV.
Owner:ZHEJIANG PUKANG BIOTECH
Antibody that binds to envelope glycoprotein of severe fever with thrombocytopenia syndrome virus and use for same
ActiveUS10947299B2Effective Detection and DiagnosisSsRNA viruses negative-senseVirus peptidesPlateletSevere fever with thrombocytopenia syndrome virus
The present invention relates to an antibody which specifically binds to the envelope glycoprotein of severe fever with thrombocytopenia syndrome virus (SFTSV), the pathogen of severe fever with thrombocytopenia syndrome (SFTS), and is used in order to effectively detect or diagnosis SFTSV and treat SFTS.
Owner:SEOUL NAT UNIV R&DB FOUND +1
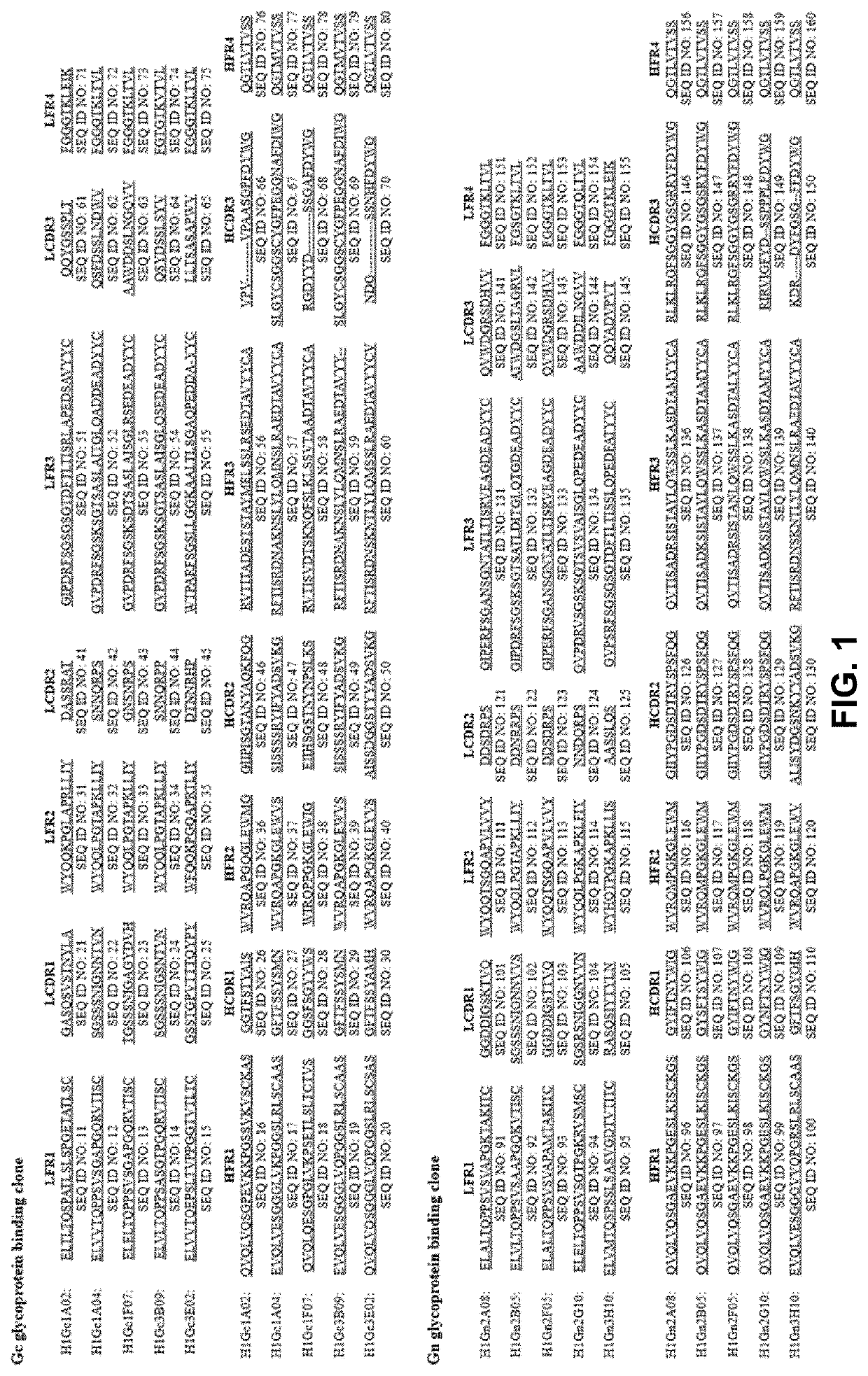
Severe fever with thrombocytopenia syndrome virus (SFTSV) inhibitor and application thereof
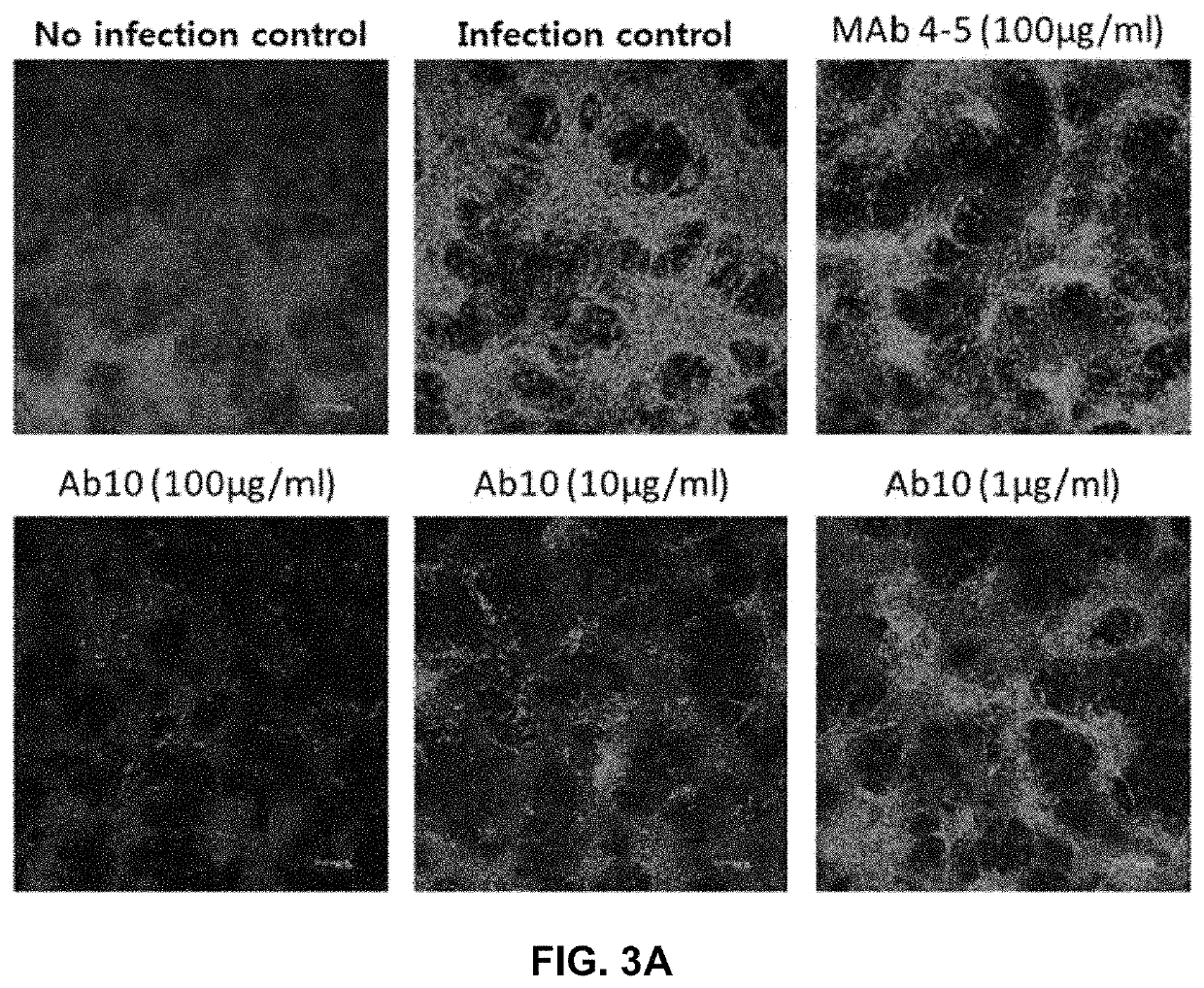
The invention discloses a severe fever with thrombocytopenia syndrome virus (SFTSV) inhibitor. The SFTSV inhibitor is a monoclonal antibody against SFTSV, wherein variable structural domain of heavy chain of the SFTSV inhibitor contains the sequence of CDR-H1 given in SEQ ID NO:1, the sequence of CDR-H2 given in SEQ ID NO:2 and the sequence of CDR-H3 given in SEQ ID NO:3; and moreover, variable structural domain of light chain of the SFTSV inhibitor contains the sequence of CDR-L1 given in SEQ ID NO:4, the sequence of CDR-L2 given in SEQ ID NO:5 and the sequence of CDR-L3 given in SEQ ID NO:6.The invention further extends to therapeutic use of the SFTSV inhibitor, compositions thereof, and a method for detecting SFTSV thereby.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE CONTROL & PREVENTION PUBLIC HEALTH RES INST OF JIANGSU PROVINCE
Primer probe set and detection method for detecting severe fever with thrombocytopeniasyndrome virus by real-time fluorescence RNA RT-RPA (reverse transcription-recombinase polymerase amplification)
InactiveCN112342316AReduce detectionEfficient detectionMicrobiological testing/measurementDNA/RNA fragmentationNucleotideField detection
The invention relates to the technical field of nucleic acid detection, in particular to a primer probe set and a detection method for detecting severe fever with thrombocytopeniasyndrome virus by real-time fluorescence RNA RT-RPA. The invention provides the primer probe set suitable for real-time fluorescence RNA RT-RPA for the severe fever with thrombocytopeniasyndrome virus, the nucleotide sequence of the primer probe set is shown as SEQ ID NO.2-4, and the primer probe set has high specificity and sensitivity. The real-time fluorescence RNA RT-RPA detection method provided by the inventioncan realize specific, sensitive, accurate and rapid detection of the severe fever with thrombocytopeniasyndrome virus, has the advantages of short detection time, simple operation, non-reliance on thermal cycle equipment and the like, can meet the requirements of field detection, and has a wide application prospect.
Owner:中国疾病预防控制中心病毒病预防控制所
Truncated Gn protein of severe fever with thrombocytopenia syndrome virus and application thereof
ActiveCN113061168AHigh titerImprove expression levelSsRNA viruses negative-sensePowder deliveryPlateletPharmaceutical drug
The invention relates to a truncated Gn protein of a severe fever with thrombocytopenia syndrome virus, the protein comprises a fusion protein comprising the truncated Gn protein, a nucleic acid molecule comprising a nucleotide sequence encoding the truncated Gn protein or the fusion protein, and a vector and a host cell comprising the nucleic acid molecule. In addition, the invention also relates to a pharmaceutical composition comprising the truncated Gn protein, the fusion protein, the nucleic acid molecule or the carrier.
Owner:BEIJING NORTHLAND BIOTECH
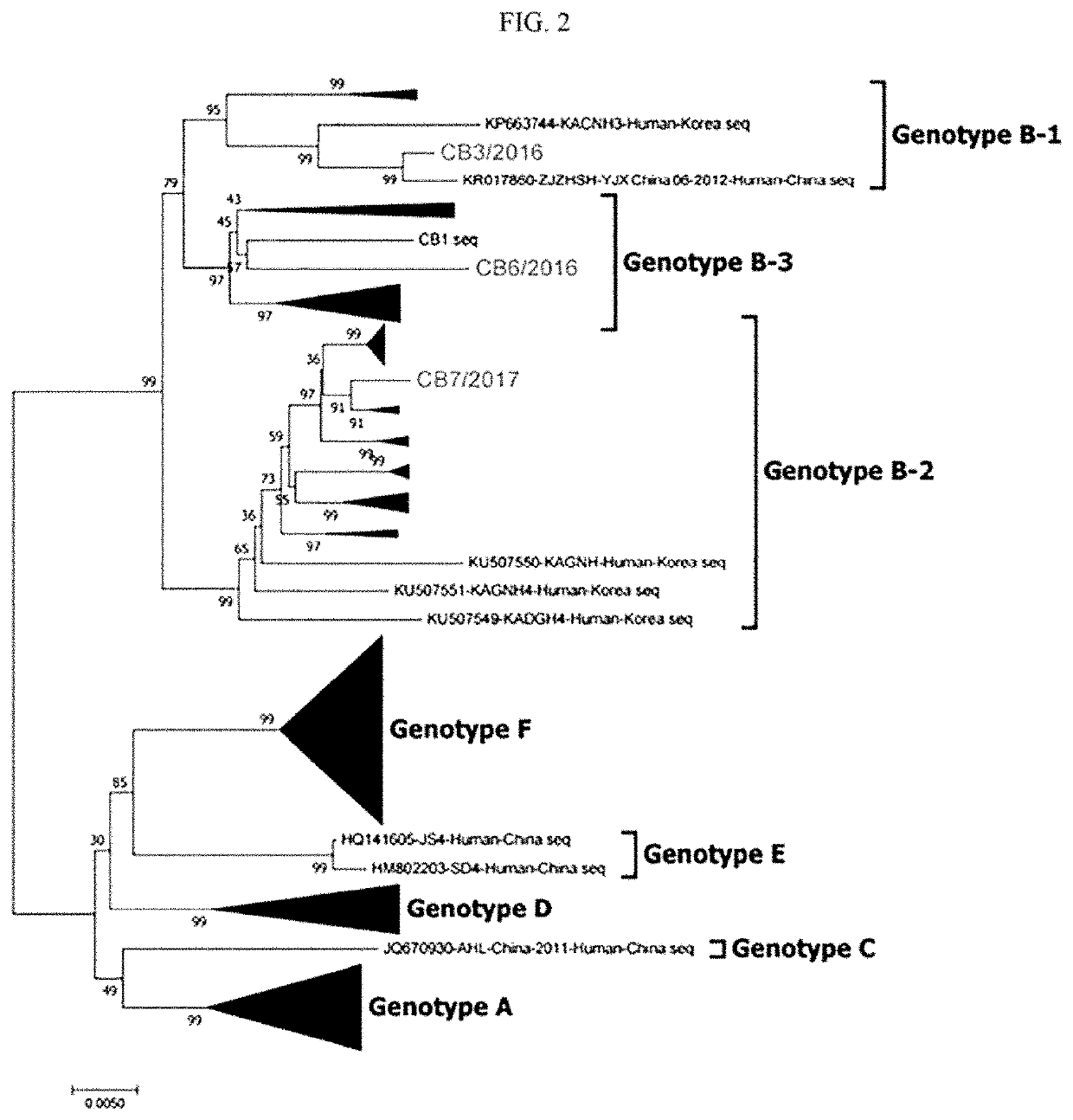
Novel severe fever with thrombocytopenia syndrome virus
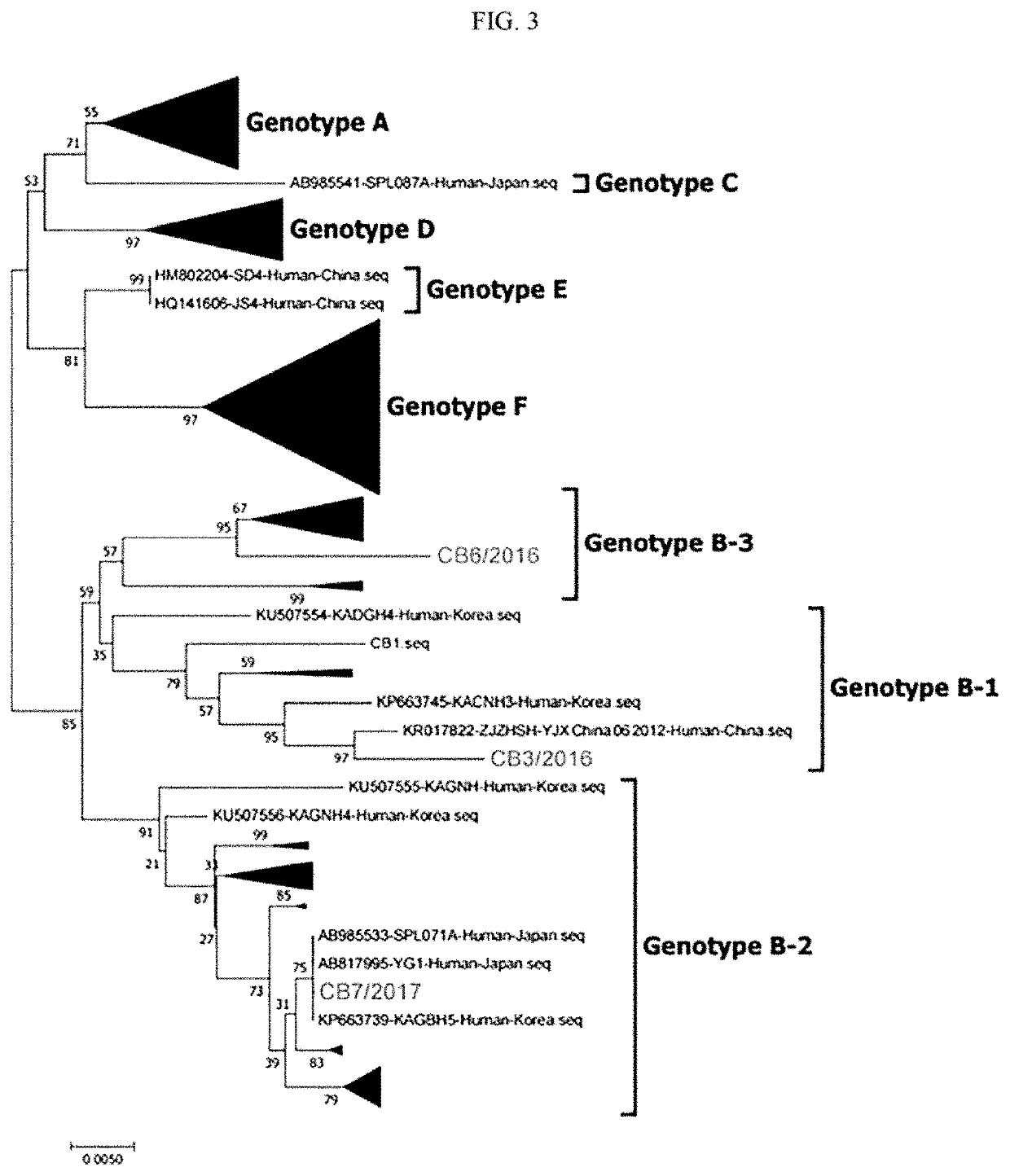
ActiveUS20210121557A1Excellent cross immunogenicityImprove protectionSsRNA viruses negative-senseViral antigen ingredientsPlateletGenotype
The present invention relates to a novel genotype of severe fever with thrombocytopenia syndrome viruses and use thereof as an immunogenic composition. The severe fever with thrombocytopenia syndrome viruses of the present invention are genetically different from conventional severe fever with thrombocytopenia syndrome viruses and are novel viruses taxonomically belonging to three sub-groups of genotype B. In view of the vaccine property that specific genotype viruses alone show only limited protective potential, the novel viruses of the present invention may be advantageously used as a vaccine having excellent cross-immunogenicity for SFTSV.
Owner:I D BIO
Immunofluorescence method for detecting fever with thrombocytopenia syndrome virus infection IgM antibody and detection kit thereof
PendingCN113341155AReduced characteristicsReduce sensitivityBiological testingFluorescence/phosphorescenceIgm antibodyCell
The invention belongs to the technical field of immunofluorescence method detection, particularly relates to an immunofluorescence method for detecting a fever with thrombocytopenia syndrome virus infected IgM antibody and a detection kit thereof, and solves the problems that in the prior art, the detection method has high requirements on experimental instruments, the operation is complicated, and the detection cost is high, and the prior art is not suitable for primary hospitals and on-site rapid detection due to the fact that products needs to be distinguished through electrophoresis, development and other steps after amplification is completed. The detection kit is composed of 12 holes, 2 microliters of cell suspension is dropwise added into each hole for hole laying and slide climbing, and after the cell suspension in the holes volatilizes, cold acetone is used for fixing; and serum of a patient is diluted with PBS and then added into antigen sheet holes, each antigen sheet is provided with a hole for blank control, and meanwhile positive control and negative control are set. The method has the advantages of relatively high specificity and sensitivity for detecting pathogens of the severe fever with thrombocytopenia syndrome, high detection accuracy, high detection efficiency and low detection cost.
Owner:李家斌
SFTSV (severe fever with thrombocytopenia syndrome virus) combinable polypeptide as well as nucleic acid coding sequence and application thereof
ActiveCN110713536AViral antigen ingredientsBiological material analysisComplementarity determining regionHumanized antibody
The invention relates to an SFTSV (severe fever with thrombocytopenia syndrome virus) combinable polypeptide which comprises three complementary determining regions CDR1-3, wherein the sequence of CDR1 is or comprises sequences shown in SEQ ID NO:1 in the description; the sequence of CDR2 is or comprises sequences shown in SEQ ID NO:2 in the description; and the sequence of CDR3 is or comprises one of sequences shown in SEQ ID NO:3 in the description. The invention aims to SFTS (severe fever with thrombocytopenia syndrome) which has a high fatality rate but the lack of effective vaccines or specific antiviral drugs and implements nano antibody drug development and research and development of diagnostic kits, through preparation of GN (glycoprotein N) protein, immune bactrian camels, a platform technology of displaying nano monoclonal antibodies through a phage library, and the like, a nano antibody VHH (variable heavy chain domain) which is specifically combined with GN is screened, CDR (complementary determining region) sequences of the nano antibody are identified, and a humanized antibody SNB is established; and meanwhile, the treatment effect of the SNB in treating SFTSV infection is assessed by using a humanized mouse model in vivo. By adopting the polypeptide, a novel potential nano antibody drug is provided for clinical treatment on SFTS, and meanwhile, a corresponding detection kit is provided for diagnosis on SFTS.
Owner:Y CLONE MEDICAL SCI CO LTD
A constant temperature amplification kit for detecting fever with thrombocytopenia syndrome virus and its application
ActiveCN105154585BReduced Pollution ChancesStrong specificityMicrobiological testing/measurementMicroorganism based processesBetaineReverse transcriptase
The invention relates to a reagent kit and application thereof, particularly relates to an isothermal amplification reagent kit for detecting fever and thrombocytopenia syndrome viruses and application of the isothermal amplification reagent kit, and belongs to the field of biotechnologies. The isothermal amplification reagent kit for detecting the fever and thrombocytopenia syndrome viruses contains oligonucleotide and comprises RNA (ribonucleic acid) extraction reagents (1), isothermal amplification reaction liquid (2), positive control templates (3) and negative control components (4). The isothermal amplification reaction liquid (1) comprises 1*Bst Buffer, betaine, MgSO<4>, dNTPs, RNase inhibitors, Bst DNA (deoxyribonucleic acid) polymerase, AMV reverse transcriptase, sterile deionized water, upstream and downstream strand displacement primers, upstream and downstream probes and a cross amplification primer; the positive control templates (3) are in-vitro transcription products of M gene segments of the fever and thrombocytopenia syndrome viruses; the negative control components (4) are sterile deionized water. The isothermal amplification reagent kit and the application have the advantages that the isothermal amplification reagent kit is high in detection sensitivity, specificity and repeatability, simple in operation step and short in reaction time, and has a broad application prospect, integral reaction procedures can be carried out at the same temperatures, the fever and thrombocytopenia syndrome viruses can be detected only by the aid of a single isothermal instrument, and the like.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL +1
Entire gene sequence of severe fever with thrombocytopenia syndrome virus (SFTSV) and application
ActiveCN102070704BStrong specificityPeptide/protein ingredientsMicrobiological testing/measurementStructural proteinTGE VACCINE
The invention relates to a severe fever with thrombocytopenia syndrome virus (SFTSV), an entire gene sequence represented by Hubei isolate HB29, amino acid sequences of coding proteins and application. The entire gene sequence of the virus is subjected to homology analysis. The virus belongs to bunyaviridae and comprises three gene segments, namely, L, M and S which represent polymerase and glycoprotein (Gn and Gc), nucleoprotein (NP) and non-structural proteins (NSs) of the virus respectively, and the three segments are all positioned on the branch of phlebovirus but farther from other viruses of phlebovirus. The entire gene sequence and the coding proteins of the virus can be used for developing drugs, vaccines or diagnostic reagents for preventing and treating the epidemic diseases caused by the SFTSV.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
RT-LAMP based primer set and kit for detecting SFTSV
InactiveCN108754029AEasy to useLow costMicrobiological testing/measurementDNA/RNA fragmentationMicrobiologyNucleotide sequencing
The invention discloses RT-LAMP based oligonucleotide and a kit for rapidly detecting severe fever with thrombocytopenia syndrome virus (SFTSV). The kit comprises outer primers (F3 and B3), inner primers (FIP and BIP), and loop primers (Loop F and Loop B). The nucleotide sequence of the outer primer F3 is represented by SEQ ID No.1; the nucleotide sequence of the outer primer B3 is represented bySEQ ID No.2; the nucleotide sequence of the inner primer FIP is represented by SEQ ID No.3; the nucleotide sequence of the inner primer BIP is represented by SEQ ID No.4; the nucleotide sequence of the loop primer Loop F is represented by SEQ ID No.5, and the nucleotide sequence of the loop primer Loop B is represented by SEQ ID No.6. The provided kit has the advantages of simple operation, high sensitivity, good specificity, low cost, rapid reaction, and easily observed results, and can rapidly detect SFTSV.
Owner:TAISHAN MEDICAL UNIV
sftsv inhibitor and its application
The invention discloses a severe fever with thrombocytopenia syndrome virus (SFTSV) inhibitor. The SFTSV inhibitor is a monoclonal antibody against SFTSV, wherein variable structural domain of heavy chain of the SFTSV inhibitor contains the sequence of CDR-H1 given in SEQ ID NO:1, the sequence of CDR-H2 given in SEQ ID NO:2 and the sequence of CDR-H3 given in SEQ ID NO:3; and moreover, variable structural domain of light chain of the SFTSV inhibitor contains the sequence of CDR-L1 given in SEQ ID NO:4, the sequence of CDR-L2 given in SEQ ID NO:5 and the sequence of CDR-L3 given in SEQ ID NO:6.The invention further extends to therapeutic use of the SFTSV inhibitor, compositions thereof, and a method for detecting SFTSV thereby.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE CONTROL & PREVENTION PUBLIC HEALTH RES INST OF JIANGSU PROVINCE
Application of Compound ps-341 in the Preparation of Bunyaviridae Phlebovirus Inhibitors
InactiveCN108210880BInhibitory activityInhibition of replicationDipeptide ingredientsAntiviralsSandflyPlatelet
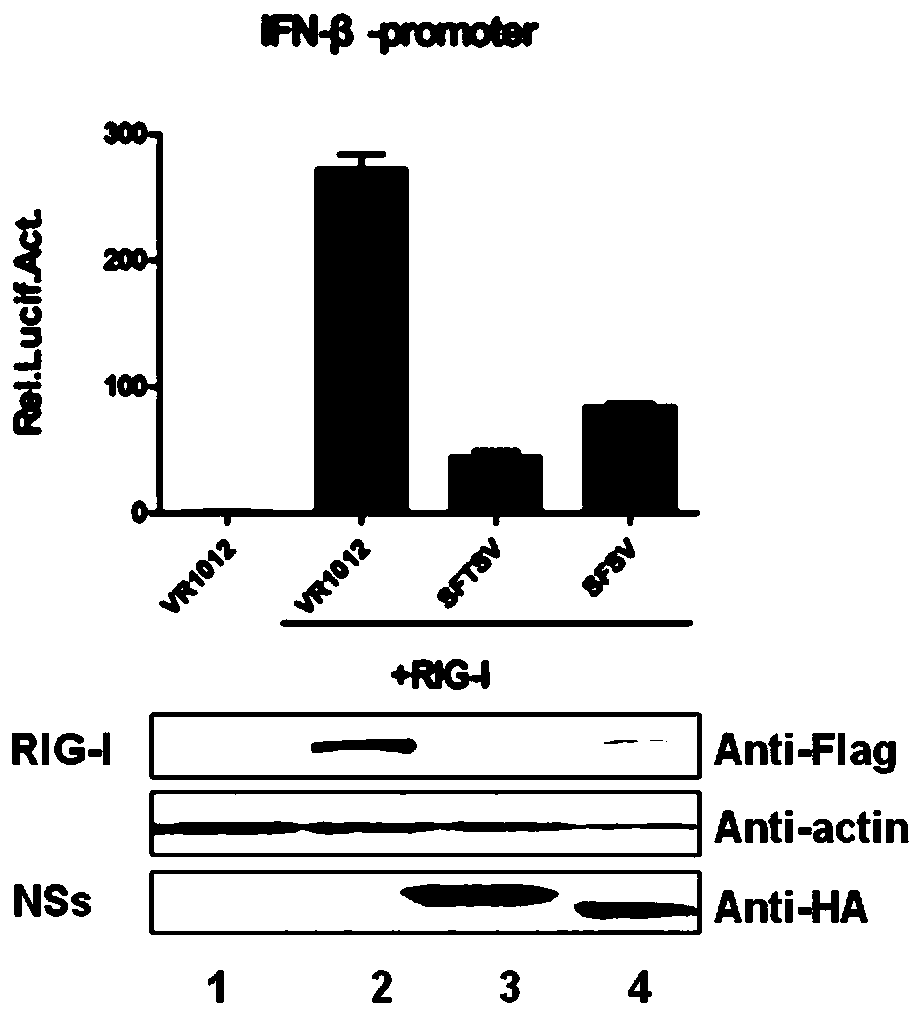
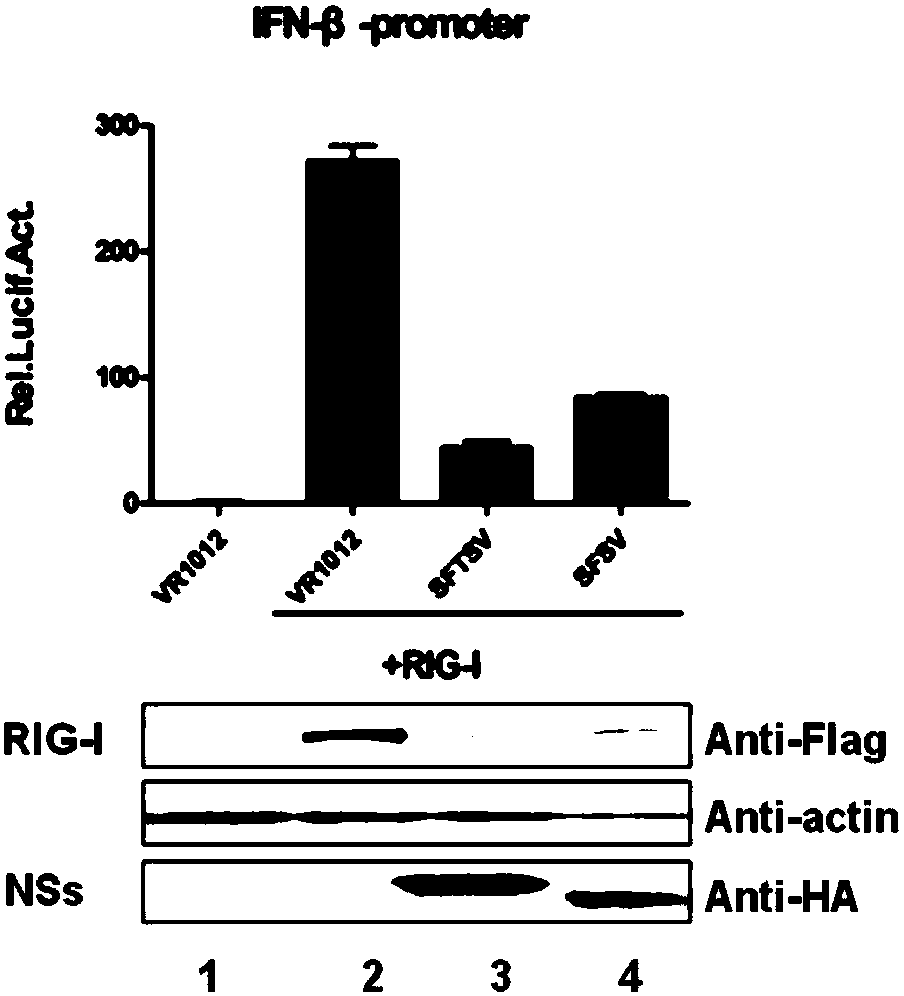
The invention discloses the application of compound PS-341 in the preparation of Bunyaviridae phlebovirus inhibitors, said compound PS-341 has a structure shown in formula (I): experiments have proved that compound PS-341 can inhibit Bunyaviridae Fever with thrombocytopenia syndrome virus, Ukuku virus, or Sicilian sandfly fever virus in the subviridae Phlebovirus genus. PS‑341 can inhibit the degradation of RIG‑I ubiquitination pathway mediated by SFTSV NSs, so that host cells can activate the interferon antiviral pathway during virus infection, and significantly inhibit virus replication and proliferation.
Owner:TIANJIN UNIV
Human monoclonal antibody specifically binding to envelope protein Gn of severe fever with thrombocytopenia syndrome virus and application thereof
ActiveCN114736291AImmunoglobulins against virusesAntiviralsPlateletSevere fever with thrombocytopenia syndrome virus
The invention relates to a human monoclonal antibody specifically combined with envelope protein Gn of severe fever with thrombocytopenia syndrome virus and application of the human monoclonal antibody. The antibody can specifically treat infection of severe fever with thrombocytopenia syndrome virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Application of Compound mln4924 in the Preparation of Bunyaviridae Phlebovirus Inhibitors
InactiveCN108210497BInhibitory activityInhibition of replicationOrganic active ingredientsAntiviralsFamily BunyaviridaeViral infection
The invention discloses an application of a compound MLN4924 in preparation of a bunyaviridae phlebovirus virus inhibitor. The compound MLN4924 has a structure shown in the formula (I) (shown in the specification), and experiments prove that the compound MLN4924 can inhibit severe fever with thrombocytopenia syndrome viruses or Sicilian phlebotomus fever viruses in bunyaviridae phlebovirus viruses. According to the invention, ubiquitination degradation of RIG-I mediated by SFTSV NSs is inhibited, so that a host cell activates an interferon antiviral pathway during virus infection, and replication and proliferation of viruses are obviously inhibited.
Owner:TIANJIN UNIV
Application of rs1800818 in the detection of febrile thrombocytopenia syndrome caused by neobunia virus
ActiveCN104789673BMicrobiological testing/measurementDNA/RNA fragmentationTreatment feverSevere fever with thrombocytopenia syndrome virus
The invention discloses application of rs1800818 to detection of severe fever with thrombocytopenia syndrome (FTLS) caused by severe fever with thrombocytopenia syndrome virus (FTLSV). The technical scheme to be protected is about application of substances for detecting the polymorphism or the genotype of rs1800818 in a personal genome to preparation of a product for screening FTLS and application of substances for detecting the polymorphism or the genotype of rs1800818 in a personal genome to preparation of a product for forecasting the condition of an FTLS patient. The substances for detecting the polymorphism or the genotype of rs1800818 can be combined with other substances (such as a substance for detecting other single nucleotide polymorphism or genotype related to FTLS) to prepare a product for screening FTLS patients or forecasting the conditions of FTLS patients.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Application of compound PS-341 in preparation of bunyaviridae phlebovirus virus inhibitor
InactiveCN108210880AInhibitory activityInhibition of replicationDipeptide ingredientsAntiviralsFamily BunyaviridaeViral infection
The invention discloses an application of a compound PS-341 in preparation of a bunyaviridae phlebovirus virus inhibitor. The compound PS-341 has a structure shown in the formula (I): (shown in the specification), and experiments prove that the compound PS-341 can inhibit severe fever with thrombocytopenia syndrome viruses, UUK viruses or Sicilian phlebotomus fever viruses in bunyaviridae phlebovirus viruses. By inhibiting ubiquitination way degradation of RIG-I mediated by SFTSV NSs, the PS-341 can enable a host cell to activate an interferon antiviral pathway during virus infection, therebyobviously inhibiting replication and proliferation of viruses.
Owner:TIANJIN UNIV
Preparation and application of an ELISA kit for detecting neobunia virus antigen
Owner:无锡鑫连鑫生物医药科技有限公司
Separation method of severe fever with thrombocytopenia syndrome virus strain
PendingCN113337477AHigh purityShort timeSsRNA viruses negative-senseSevere fever with thrombocytopenia syndrome virusBiomedical engineering
The invention belongs to the technical field of medical microbiology, particularly relates to a separation method of a severe fever with thrombocytopenia syndrome virus strain, and solves the problems that in the prior art, the severe fever with thrombocytopenia syndrome virus strain is difficult to separate, the strain obtained though separation is low in purity and is difficult to culture in cells, and no optimum scheme for separating the severe fever with thrombocytopenia syndrome virus strain exists in the prior art. The separation method of the severe fever with thrombocytopenia syndrome virus strain comprises the following steps of infecting Vero cells with the severe fever with thrombocytopenia syndrome virus, performing culturing, and collecting cell culture supernate; and performing filtration, adding a sucrose cushion layer, performing centrifugation, and taking a centrifugal precipitate to obtain a finished product. The separation method is simple, convenient and easy to operate, and consumes short time; and the severe fever with thrombocytopenia syndrome virus strain obtained through separation has high purity, can be used for subsequent research on pathogenic mechanisms and specific clinical manifestations of the severe fever with the thrombocytopenia syndrome, and has profound significance.
Owner:李家斌
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com