Patents
Literature
127 results about "Env Glycoproteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
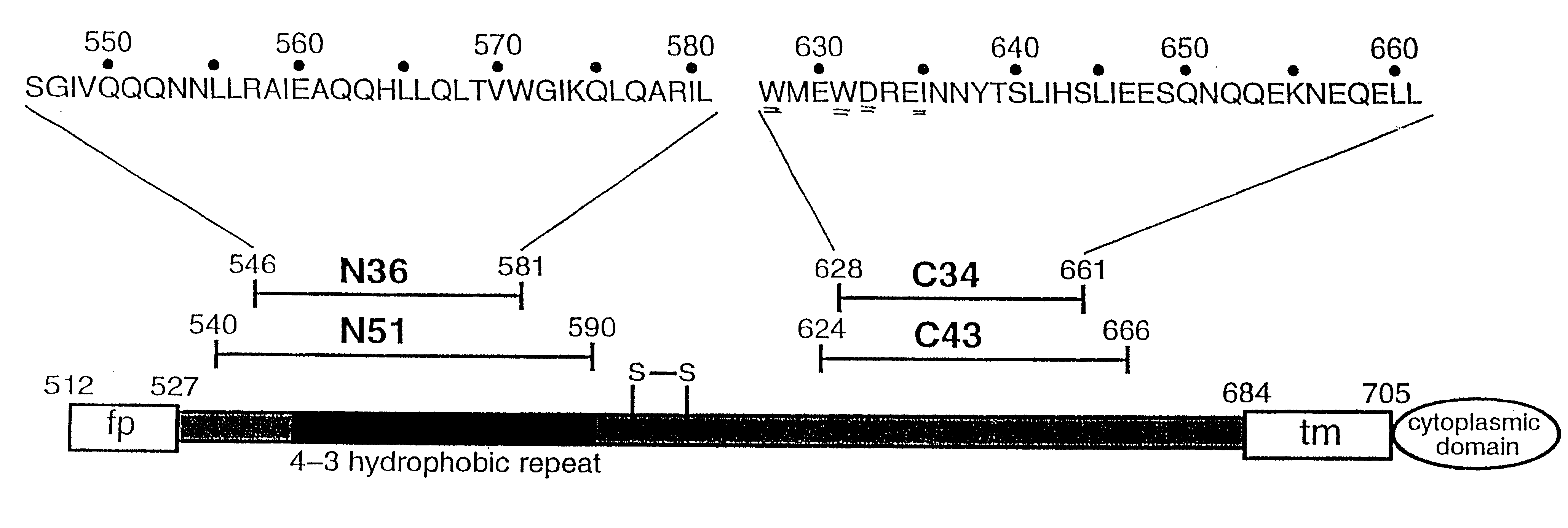
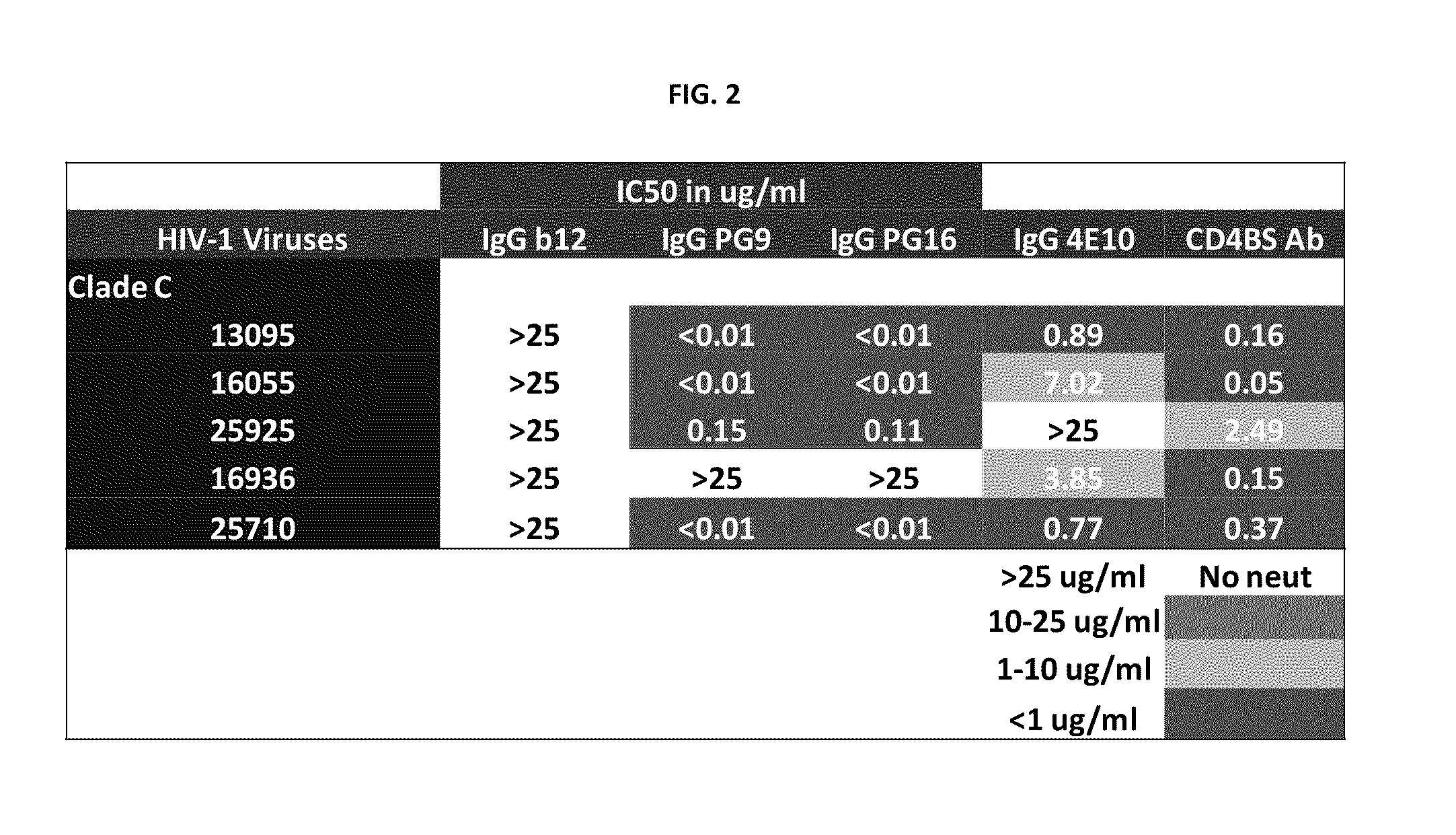
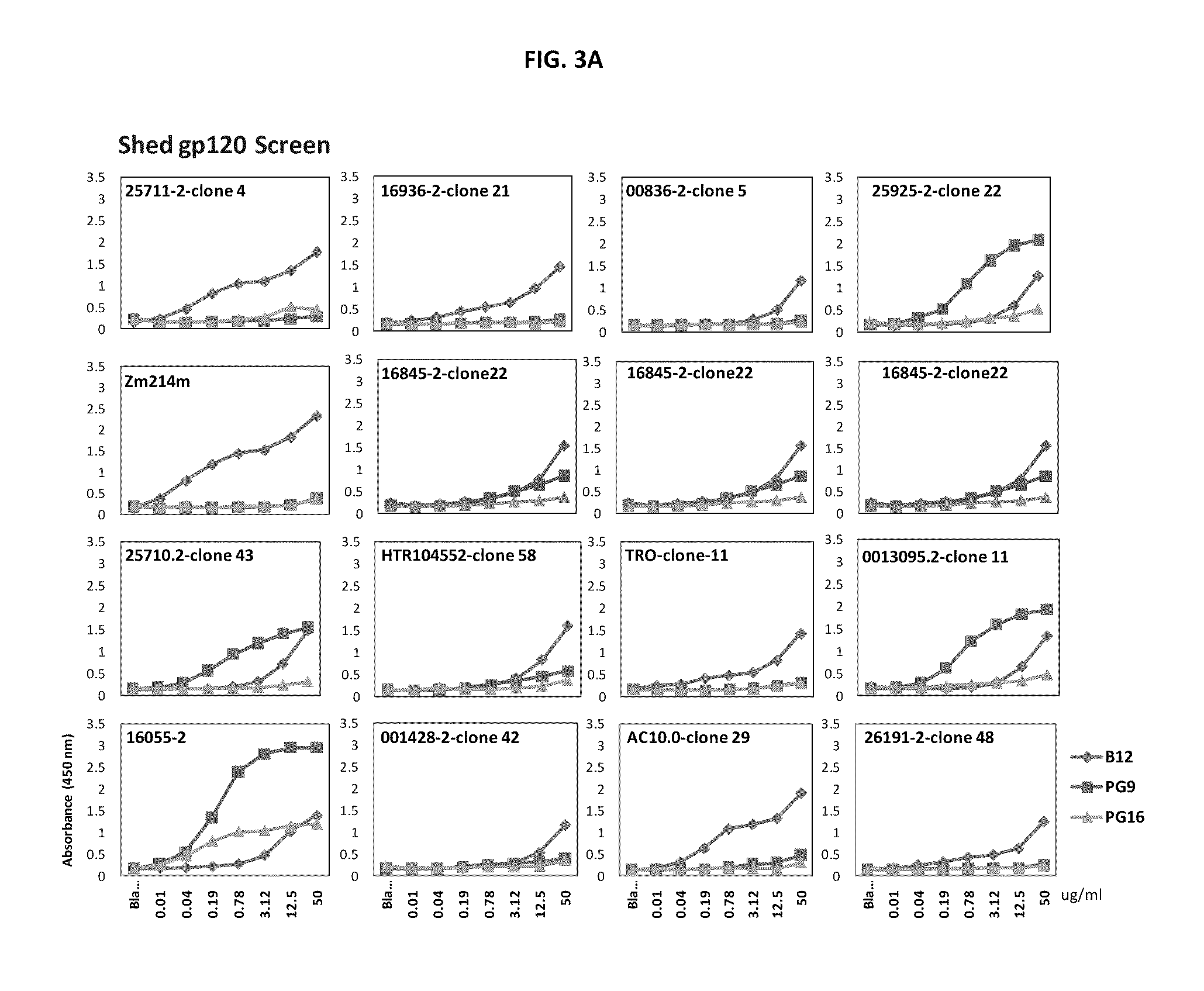
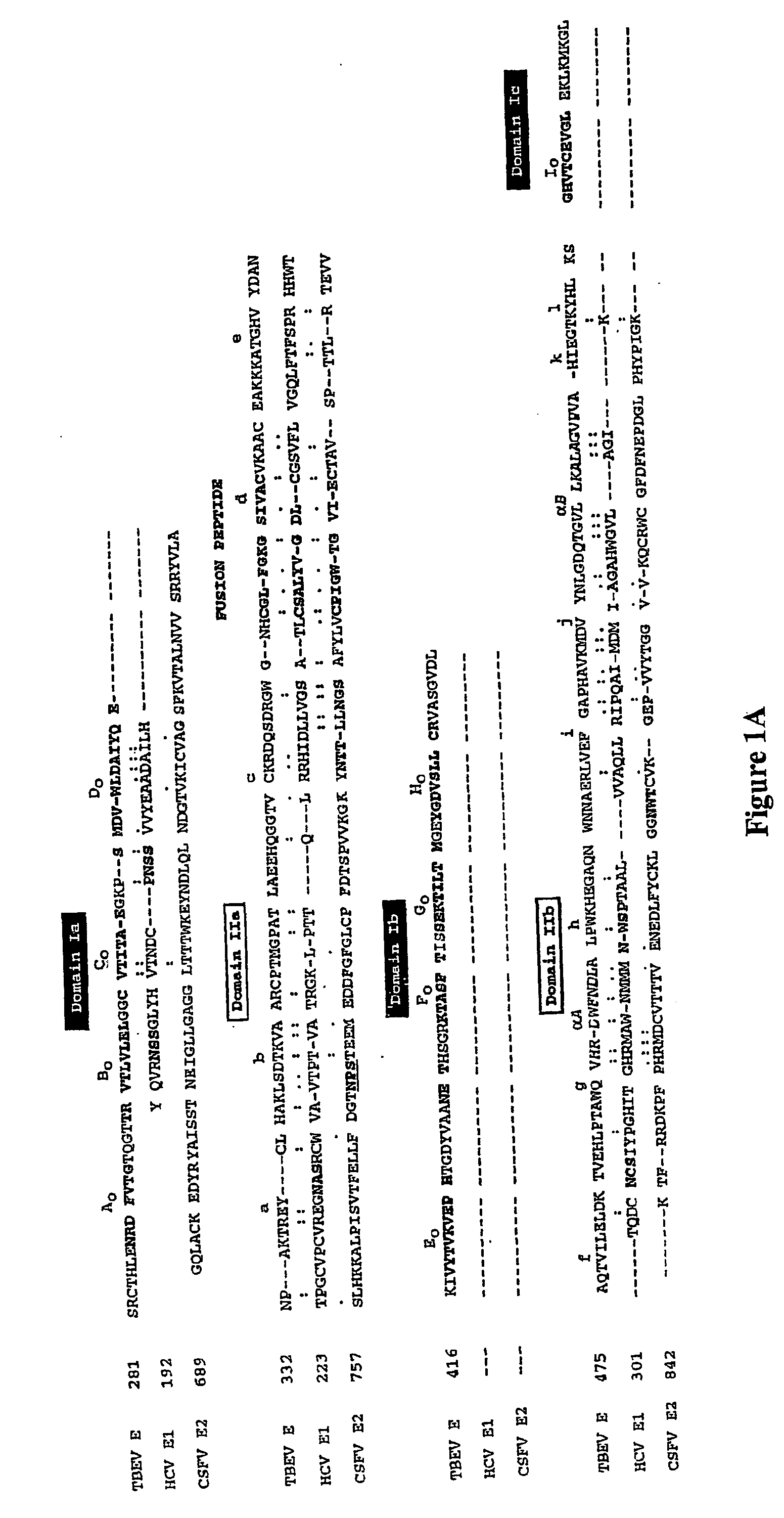
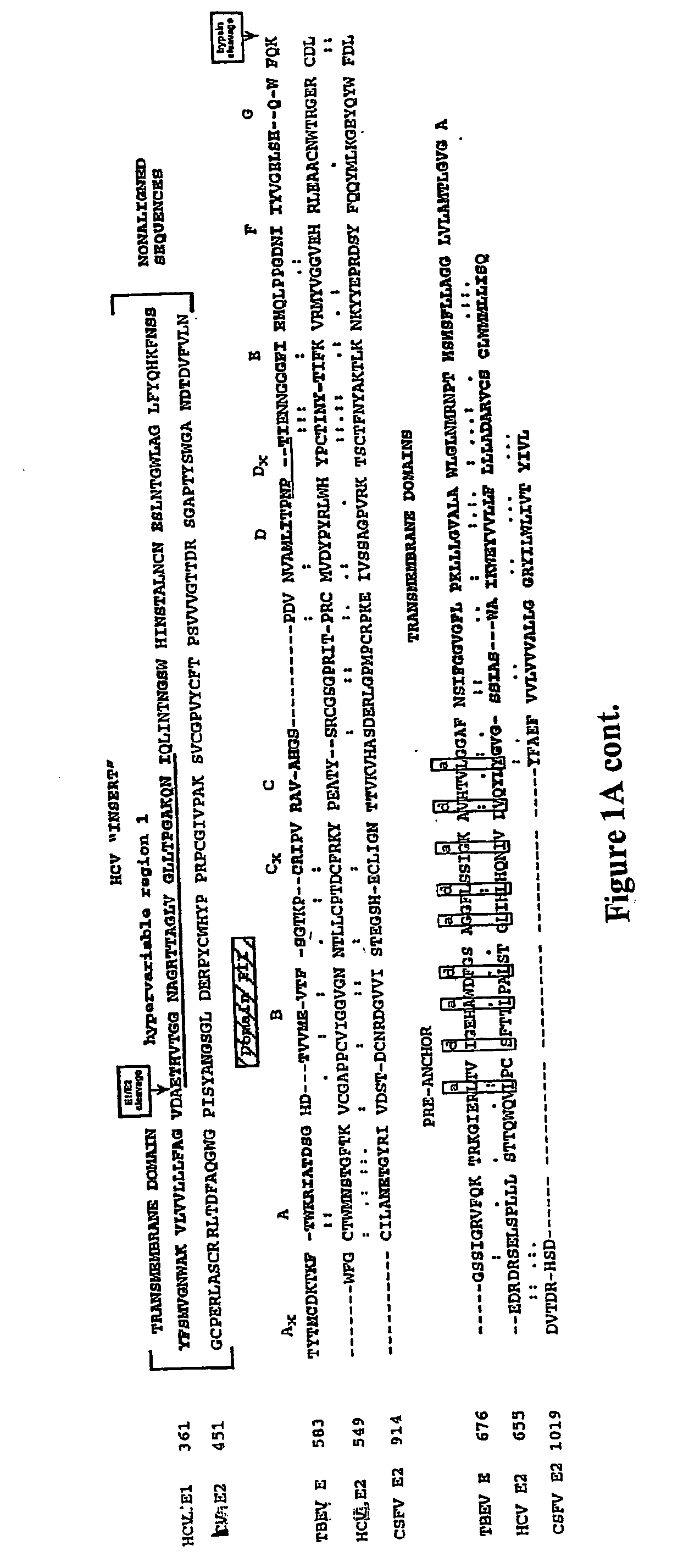
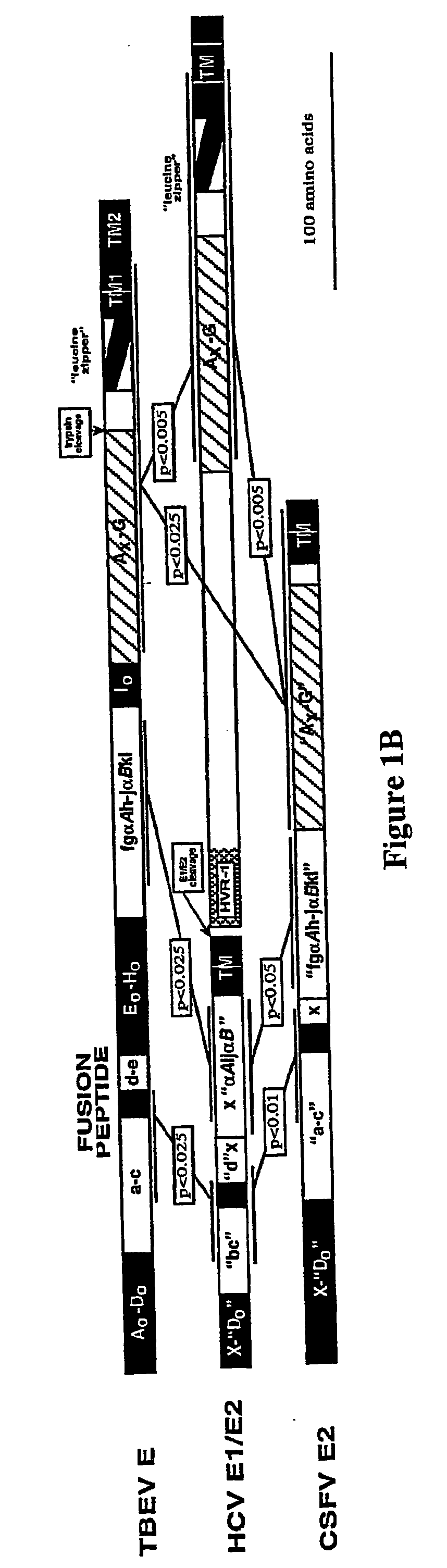
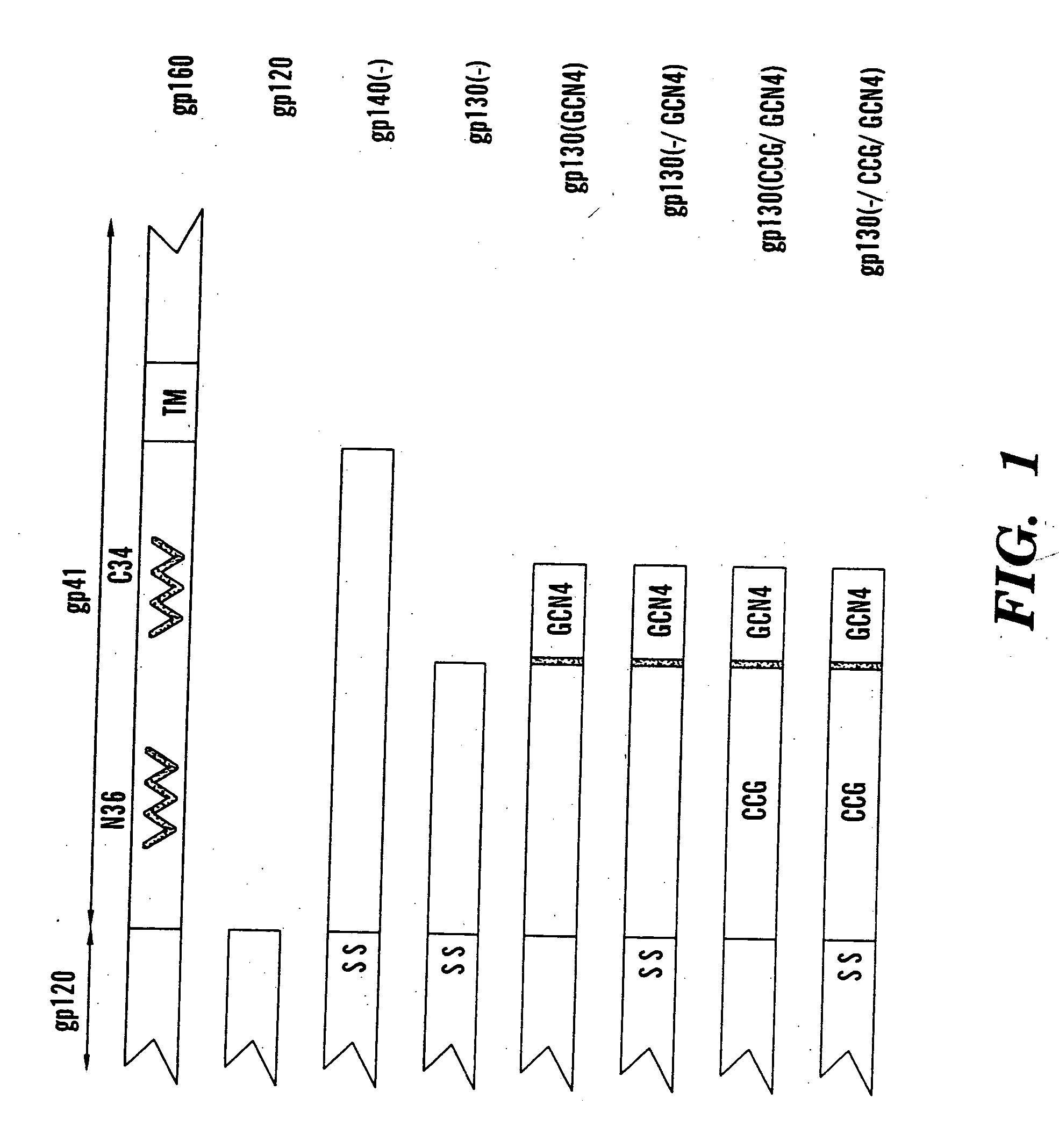
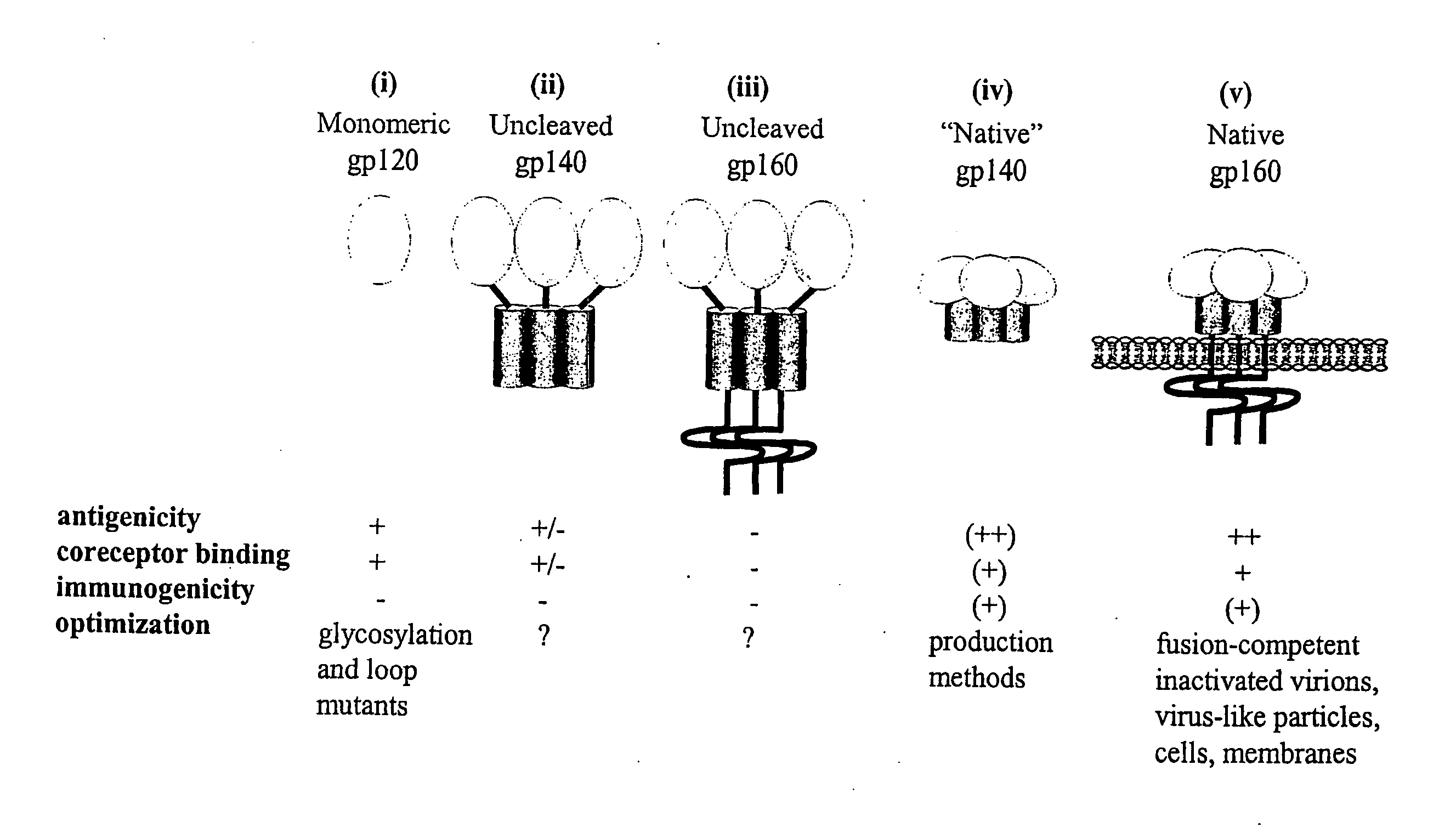
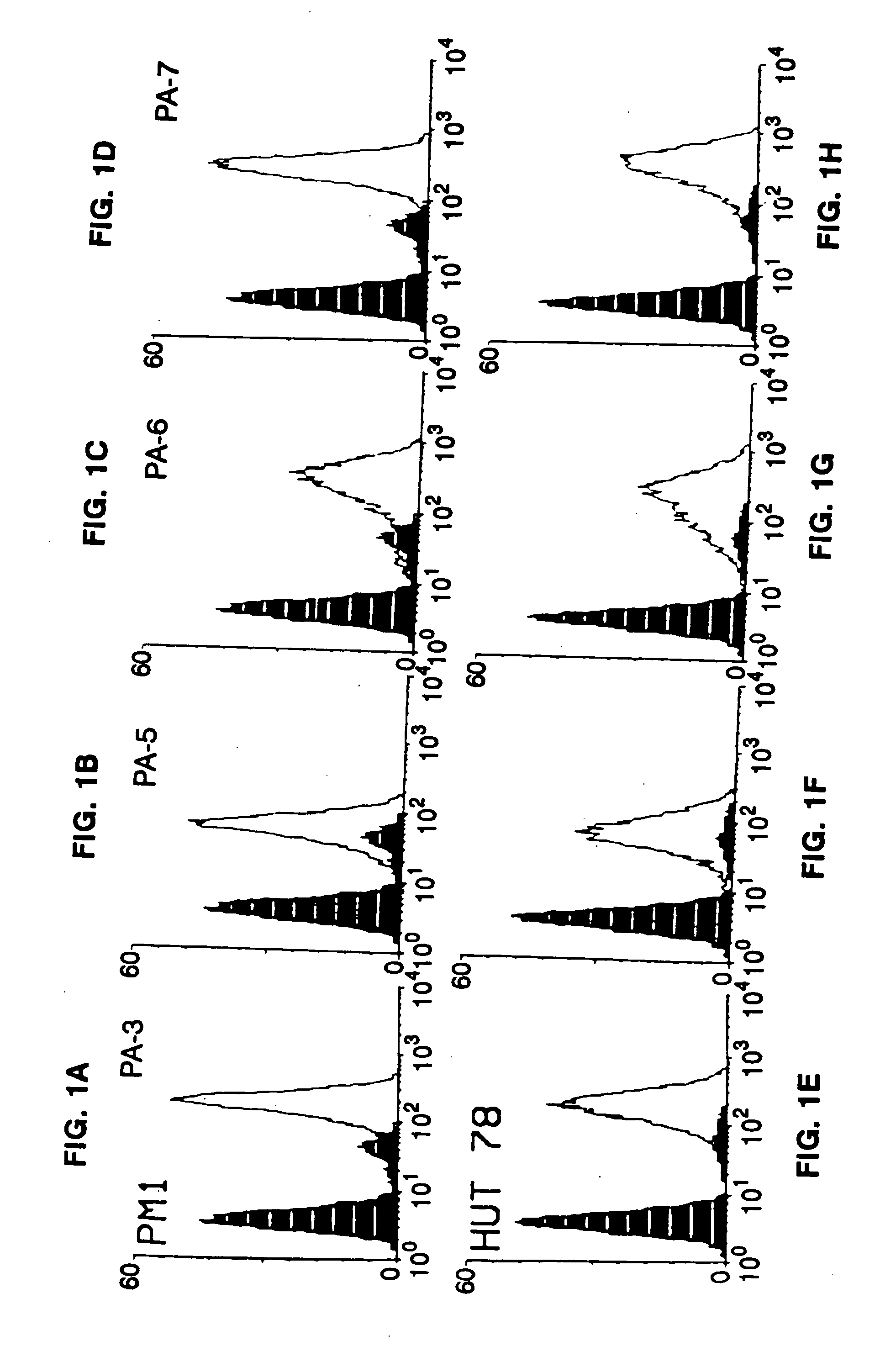
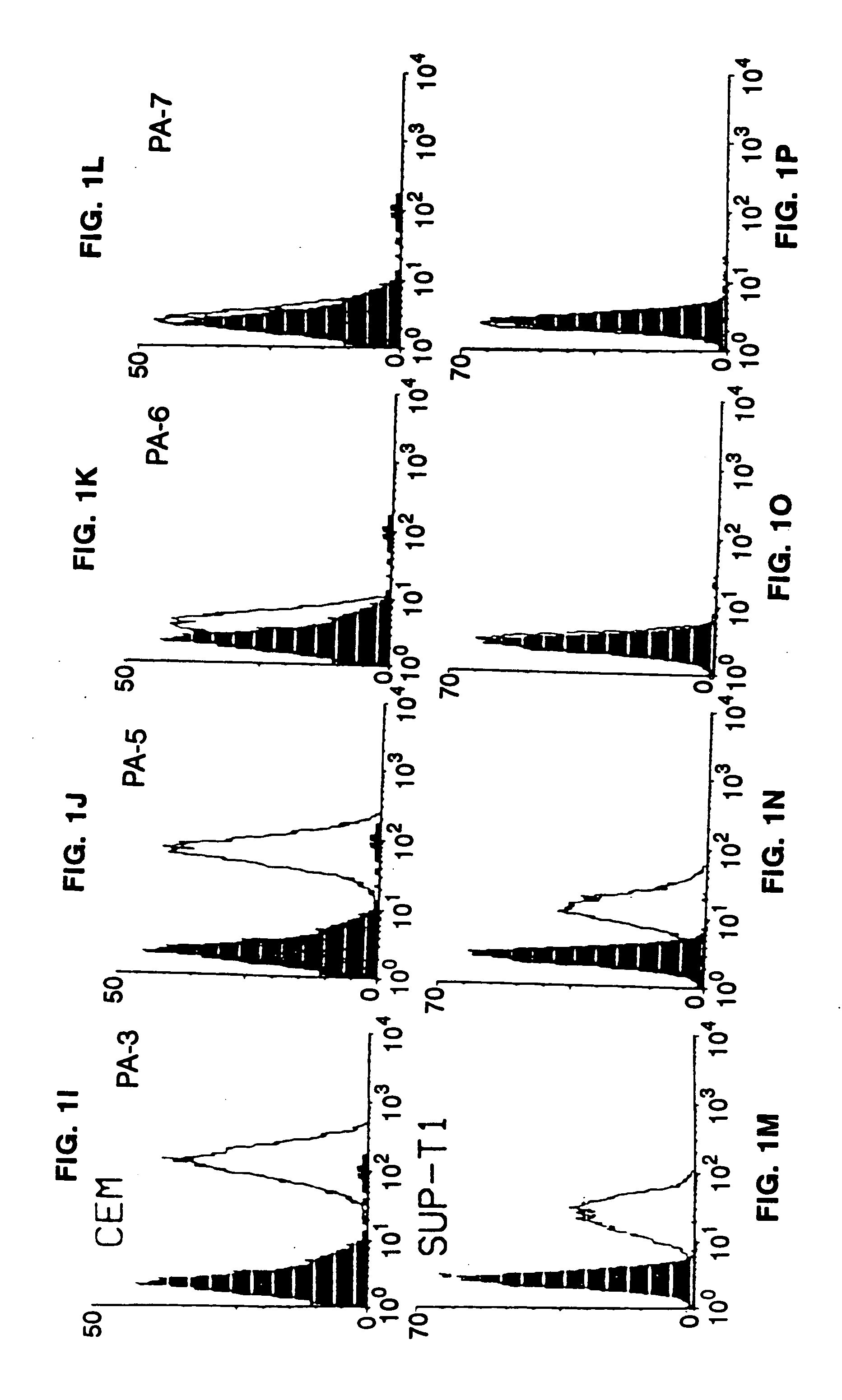
Core structure of gp41 from the HIV envelope glycoprotein
InactiveUS6506554B1Increase infectivityHigh activityPeptide/protein ingredientsMicrobiological testing/measurementHiv envelopeDesigning drug
Described are the crystal structure of the alpha-helical domain of the gp41 component of HIV-1 envelope glycoprotein which represents the core of fusion-active gp41, methods of identifying and designing drugs which inhibit gp41 function and drugs which do so.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
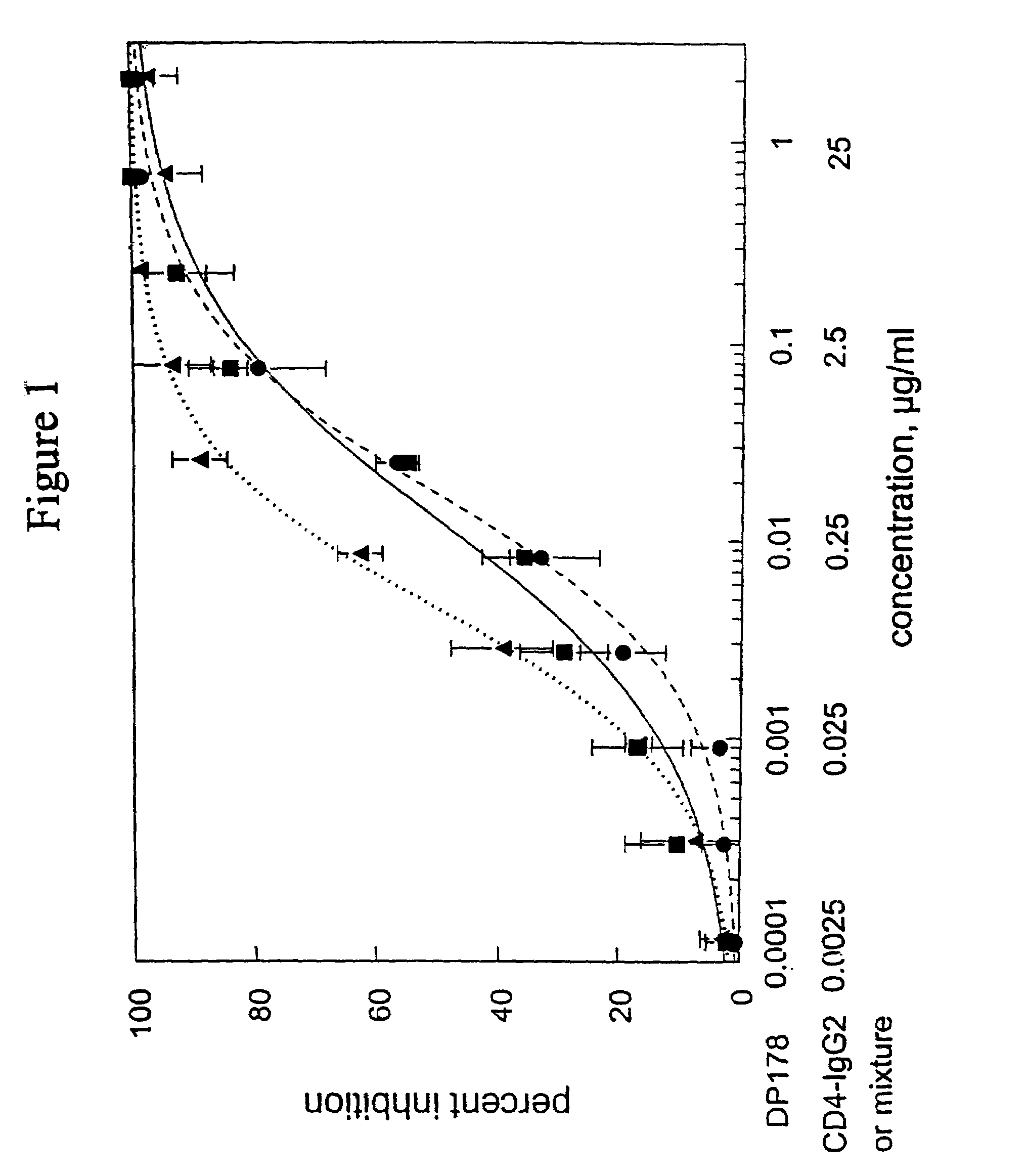
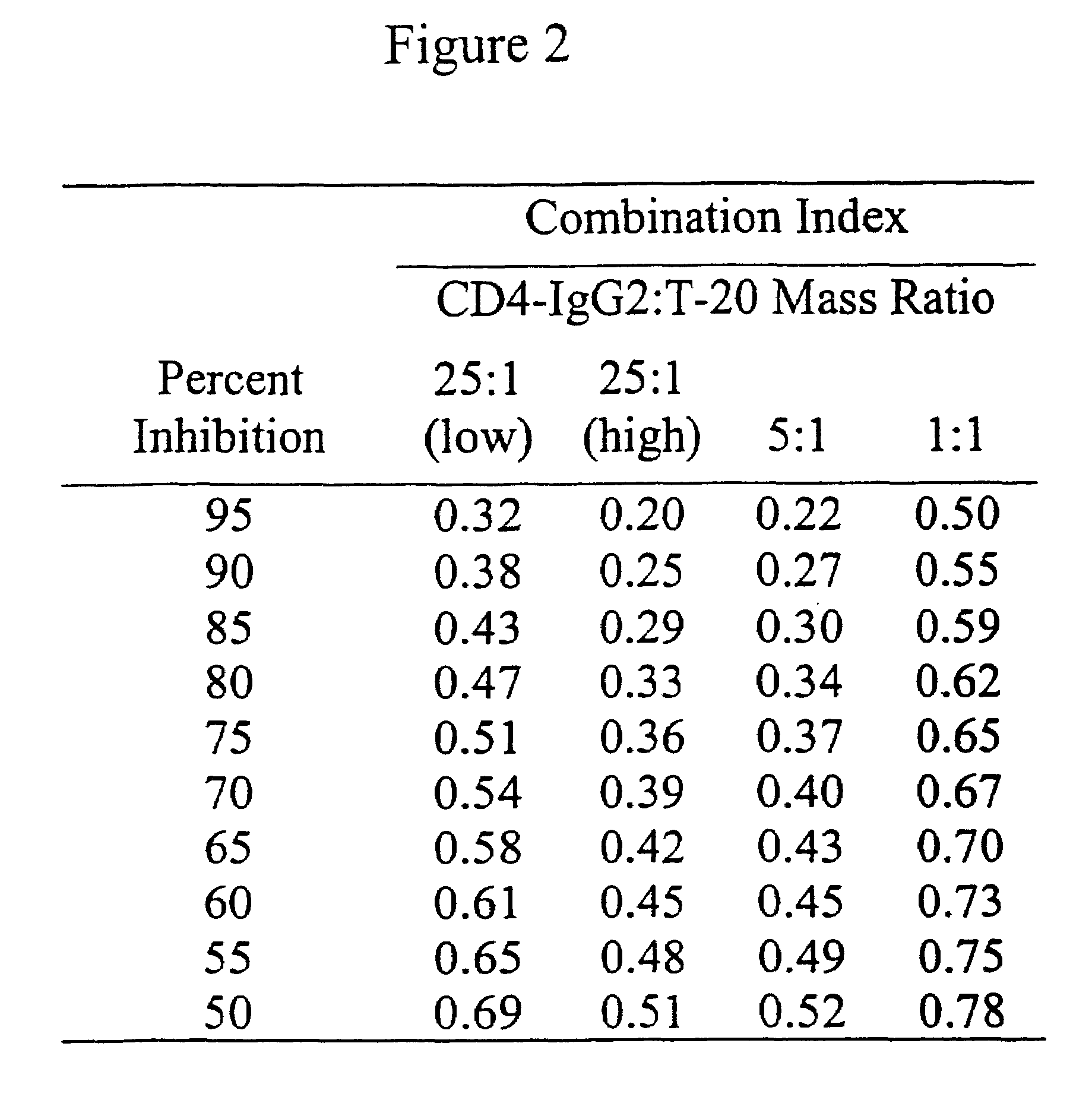
Methods for using resonance energy transfer-based assay of HIV-1 envelope glycoprotein-mediated membrane fusion, and kits for practicing same
This invention provides: agents determined to be capable of specifically inhibiting the fusion of a macrophage-tropic primary isolate of HIV-1 to a CD4+ cell, but not a T cell-tropic isolate of HIV-1 to a CD4+ cell; and agents determined to be capable of specifically inhibiting the fusion of a T cell-tropic isolate of HIV-1 to a CD4+ cell, but not a macrophage-tropic primary isolate of HIV-1 to a CD4+ cell. This invention also provides: agents capable of specifically inhibiting the fusion of a macrophage tropic primary isolate of HIV-1 with a CD+ cell susceptible to infection by a macrophage-tropic primary isolate of HIV-1; and agents capable of specifically inhibiting the fusion of a T cell-tropic isolate of HIV-1 with a CD4+ cell susceptible to infection by a T cell-tropic isolate of HIV-1. The agents include but are not limited to antibodies. This invention further provides: methods of inhibiting fusion of a macrophage-tropic primary isolate of HIV-1 with a CD+ cell susceptible to infection by a macrophage-tropic primary isolate of HIV-1 which comprises contacting the CD4+ cell with an amount of an agent capable of specifically inhibiting such fusion so as to thereby inhibit such fusion; and methods of inhibiting fusion of a T cell-tropic isolate of HIV-1 with a CD4+ cell susceptible to infection by a T cell-tropic isolate of HIV-1 which comprises contacting the CD4+ cell with an amount of an agent capable of specifically inhibiting such fusion so as to thereby inhibit such fusion.
Owner:CYTODYN
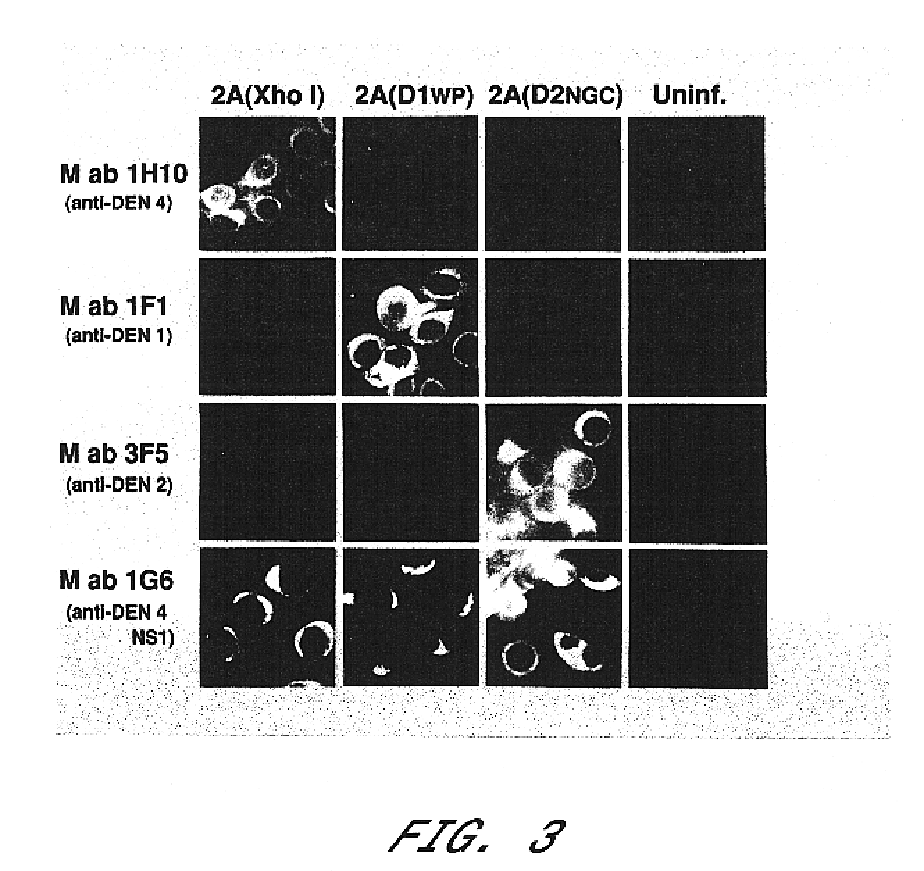
Chimeric and/or growth-restricted flaviviruses
InactiveUS6676936B1BiocideSsRNA viruses positive-senseVirulent characteristicsJapanese B Encephalitis Virus
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
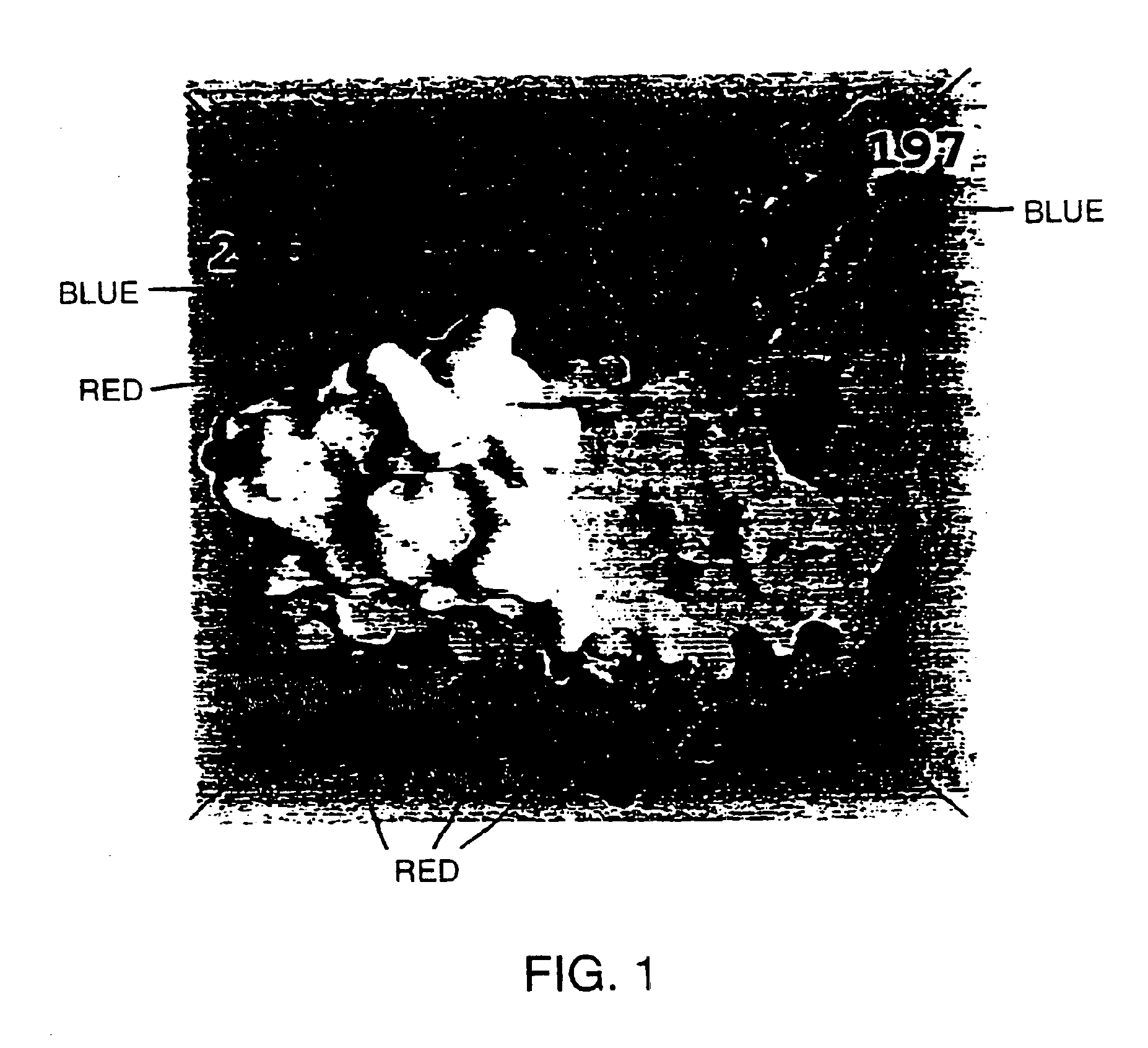
Glycosylated modified primate lentivirus envelope polypeptides
InactiveUS6908617B1Improving immunogenicityPeptide/protein ingredientsViral antigen ingredientsAdjuvantBinding site
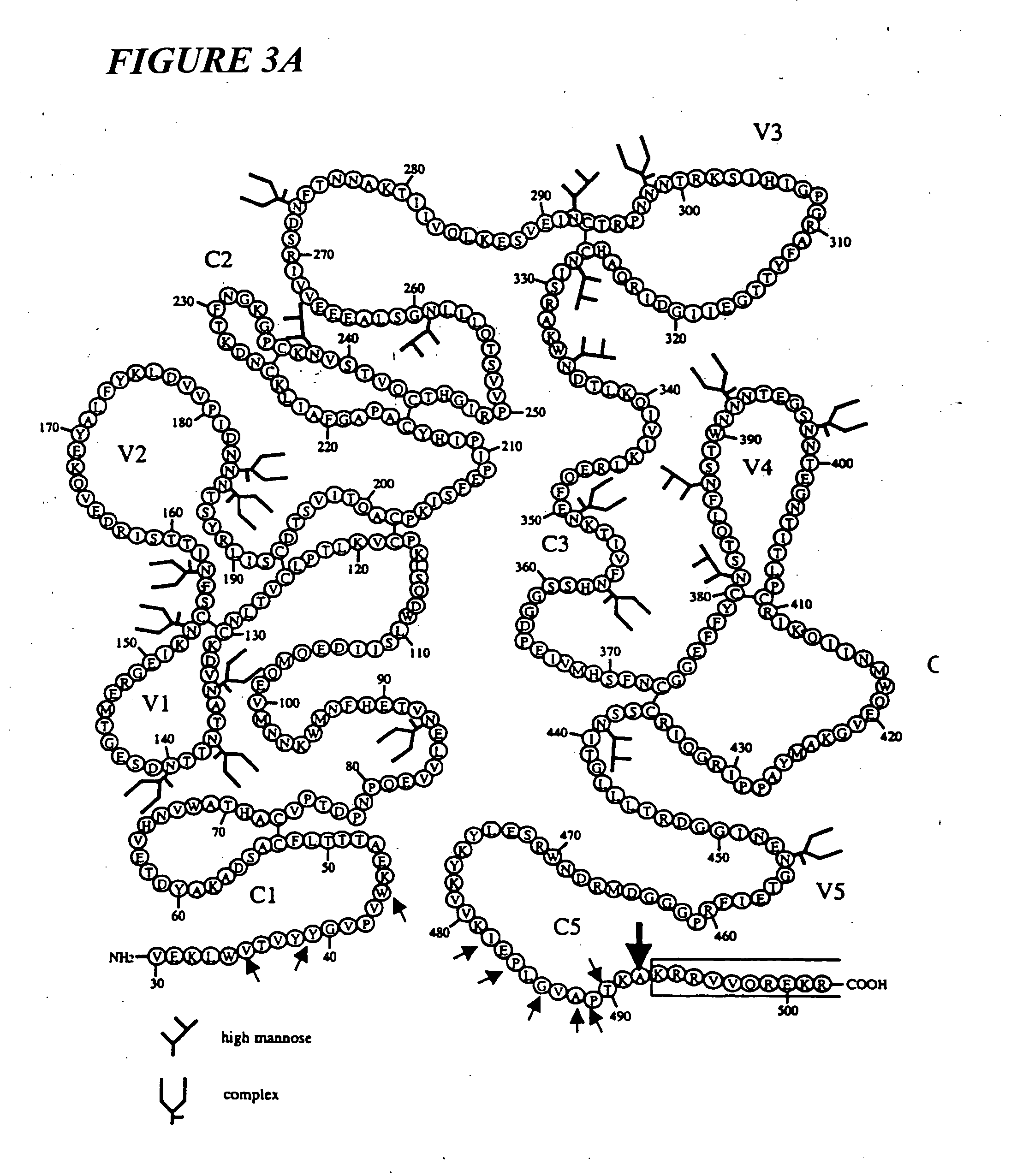
A modified polypeptide corresponding to an envelope glycoprotein of a primate lentivirus is described. The polypeptide has been modified from the wild-type structure so that it has at least two of the glycosylation sites proximal to the CD4 binding site or chemokine receptor site altered so that the alteration prevents glycosylation at that site or where glycosylation sites distal to these sites have been derivatized with a molecular adjuvant, while retaining the overall 3-dimensional structure of a discontinuous conserved epitope of the wild-type protein. Preferably, the polypeptide has both changes. Preferably, the primate lentivirus is HIV, and the protein is HIV-1 gp 120.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
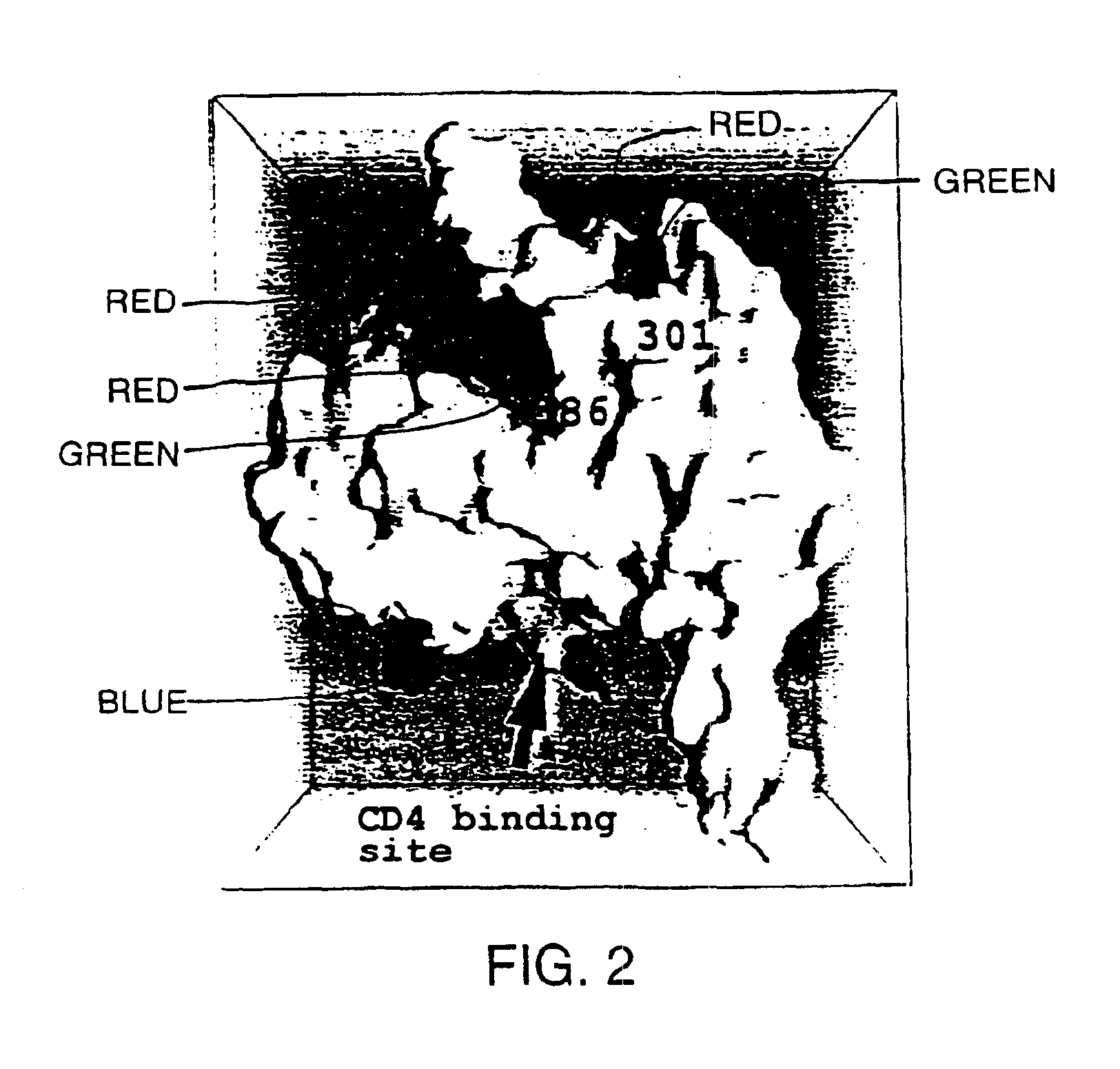
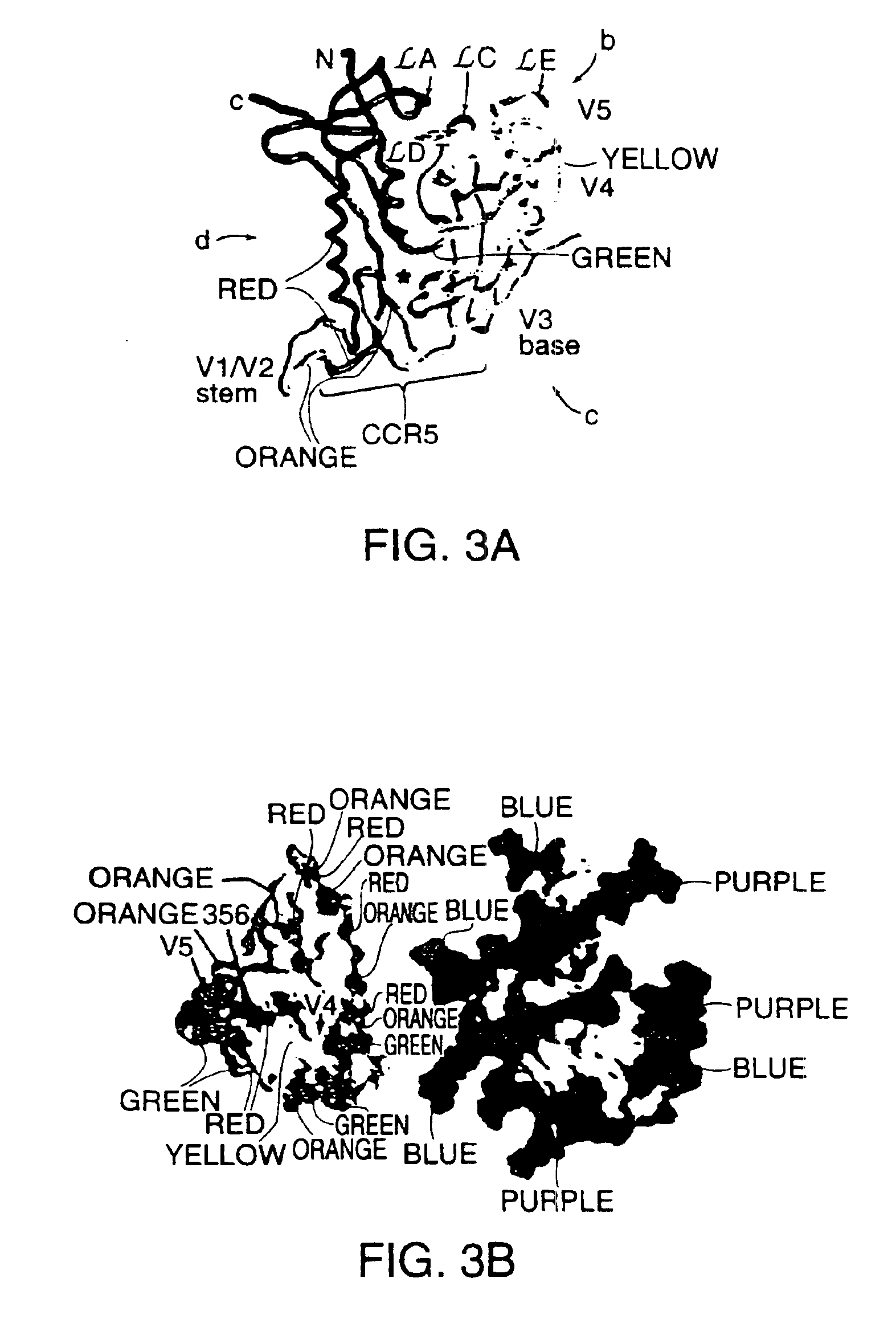
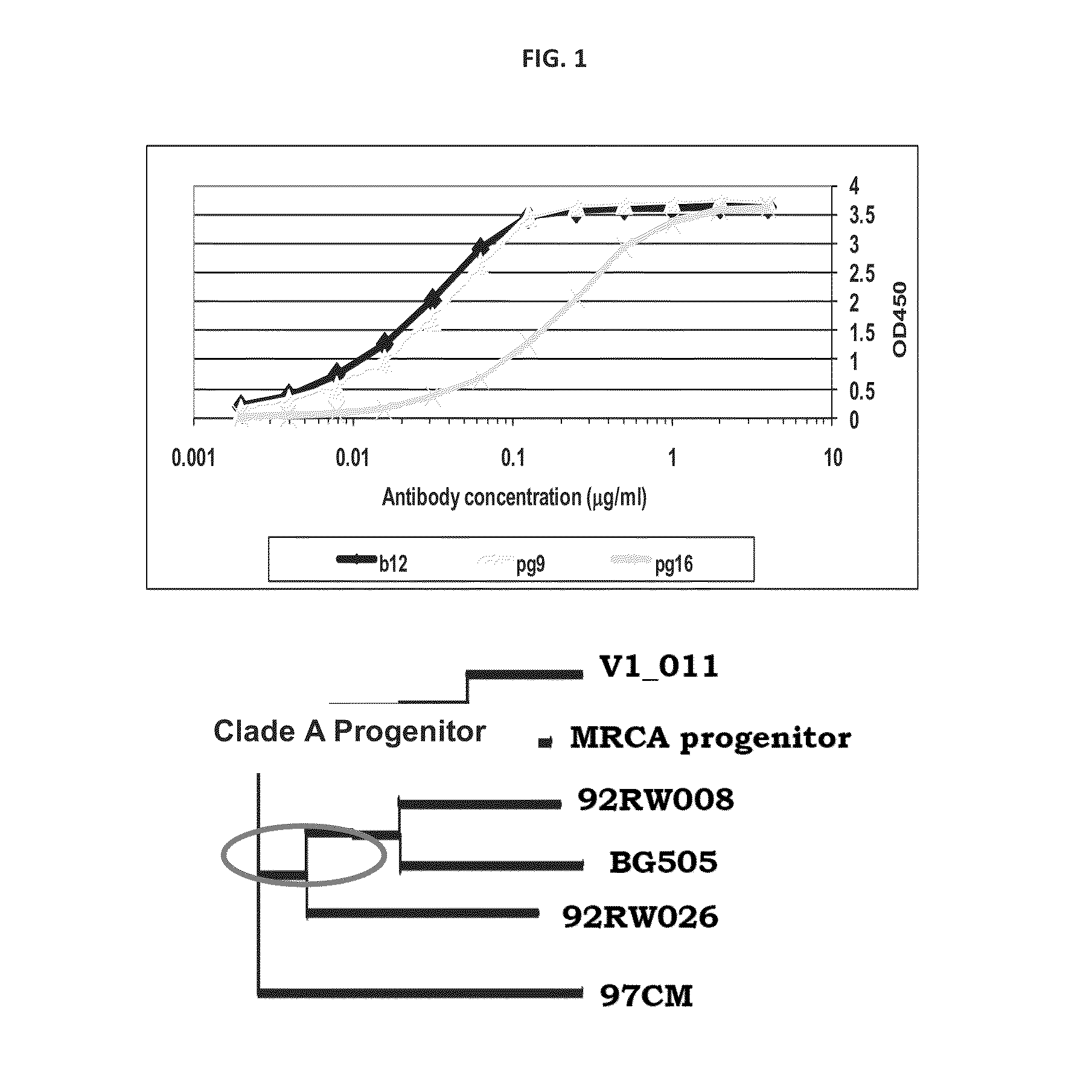
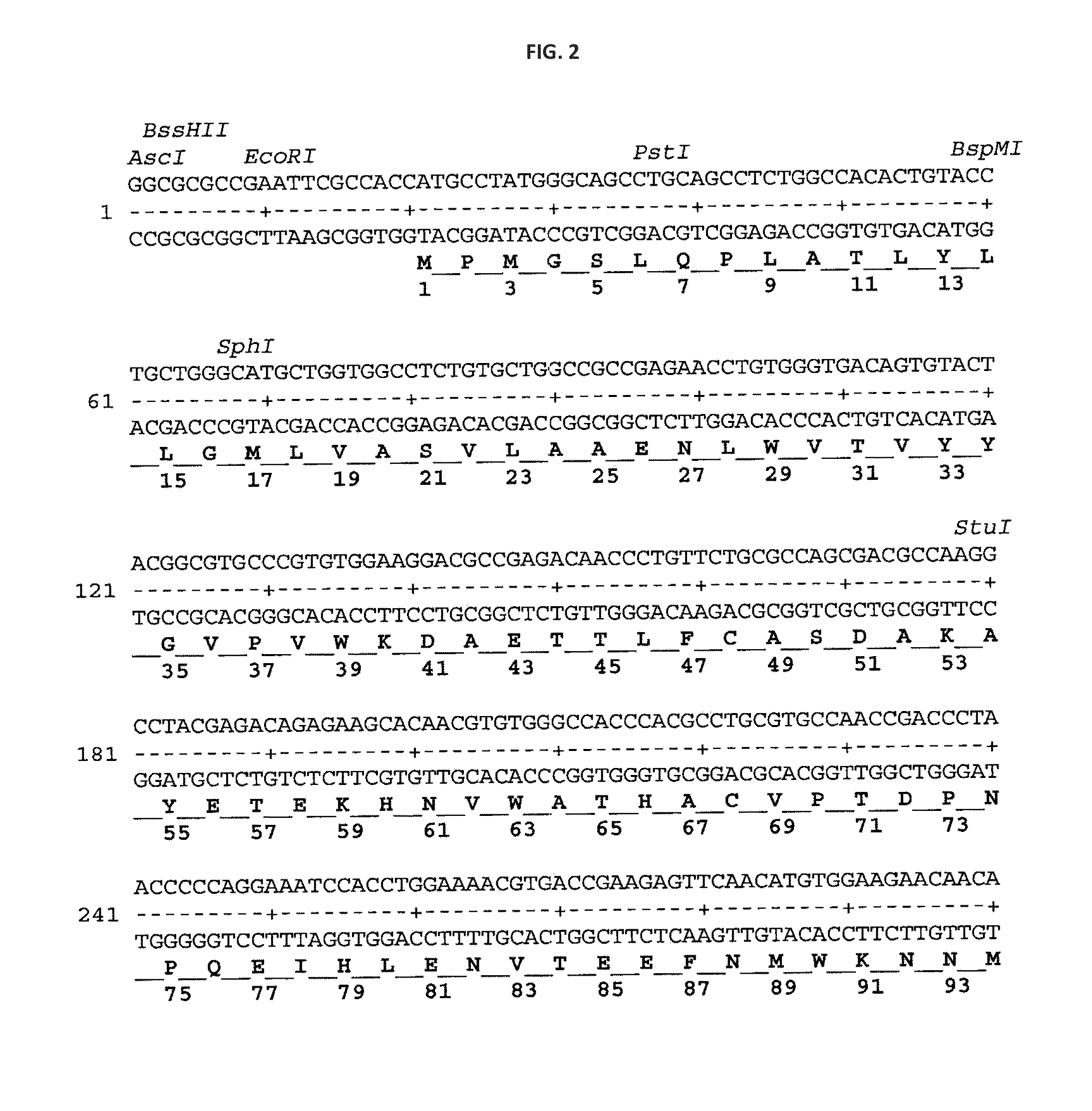
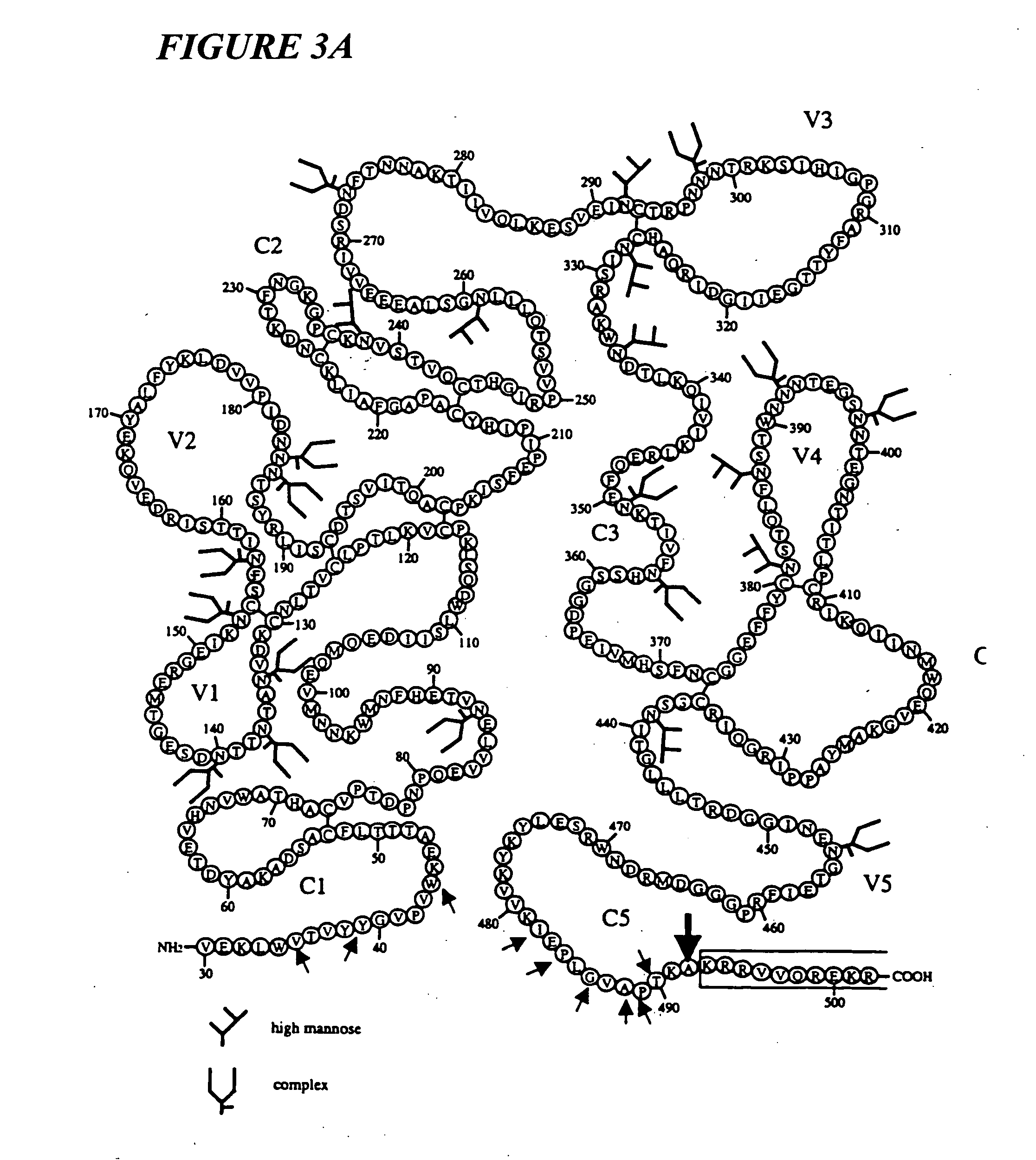
Novel HIV-1 envelope glycoprotein
The present application relates to novel HIV-1 envelope glycoproteins, which may be utilized as HIV-1 vaccine immunogens, and antigens for crystallization, electron micrsocopy and other biophysical, biochemical and immunological studies for the identification of broad neutralizing antibodies. The present invention encompasses the preparation and purification of immunogenic compositions, which are formulated into the vaccines of the present invention.
Owner:THE SCRIPPS RES INST +2
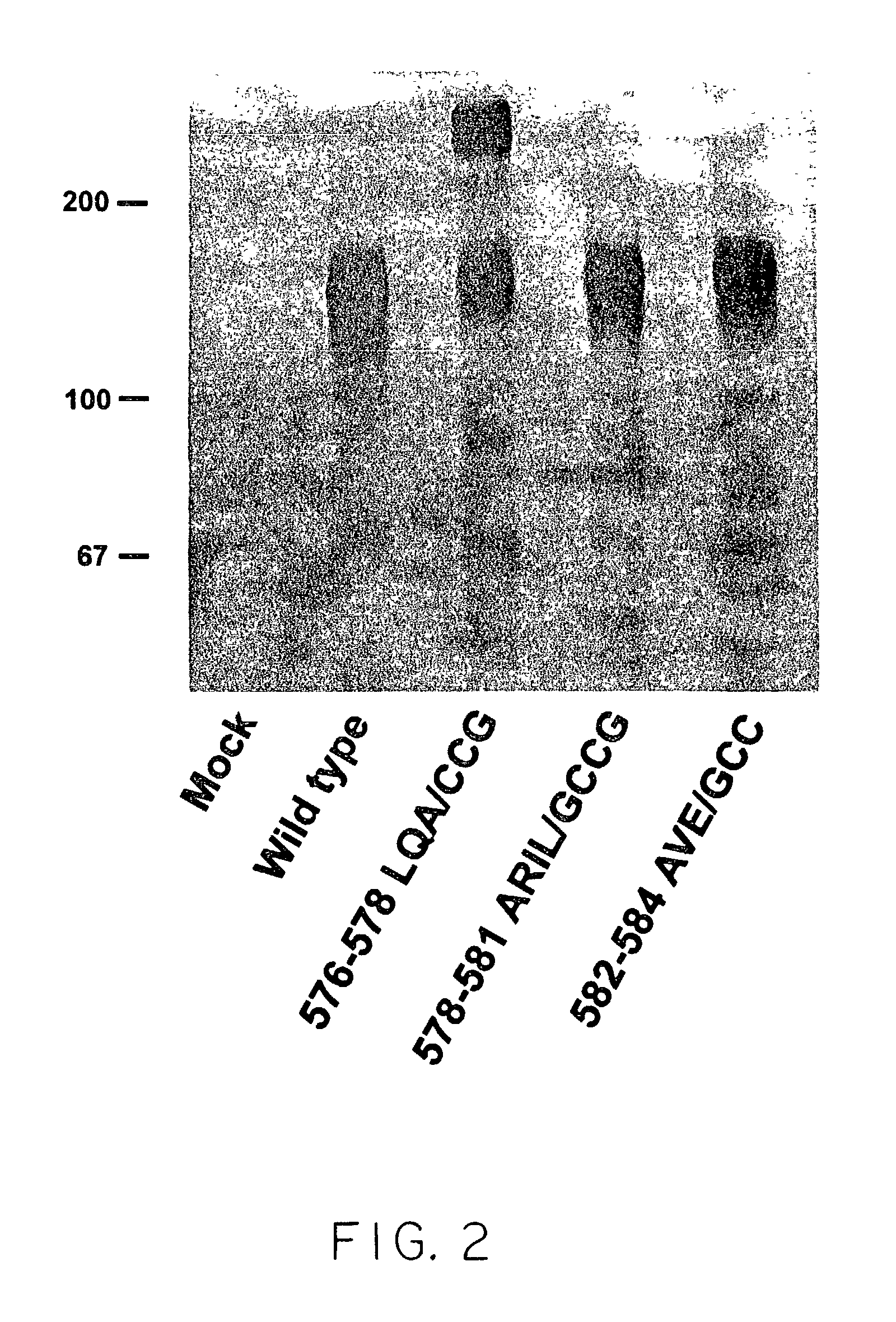
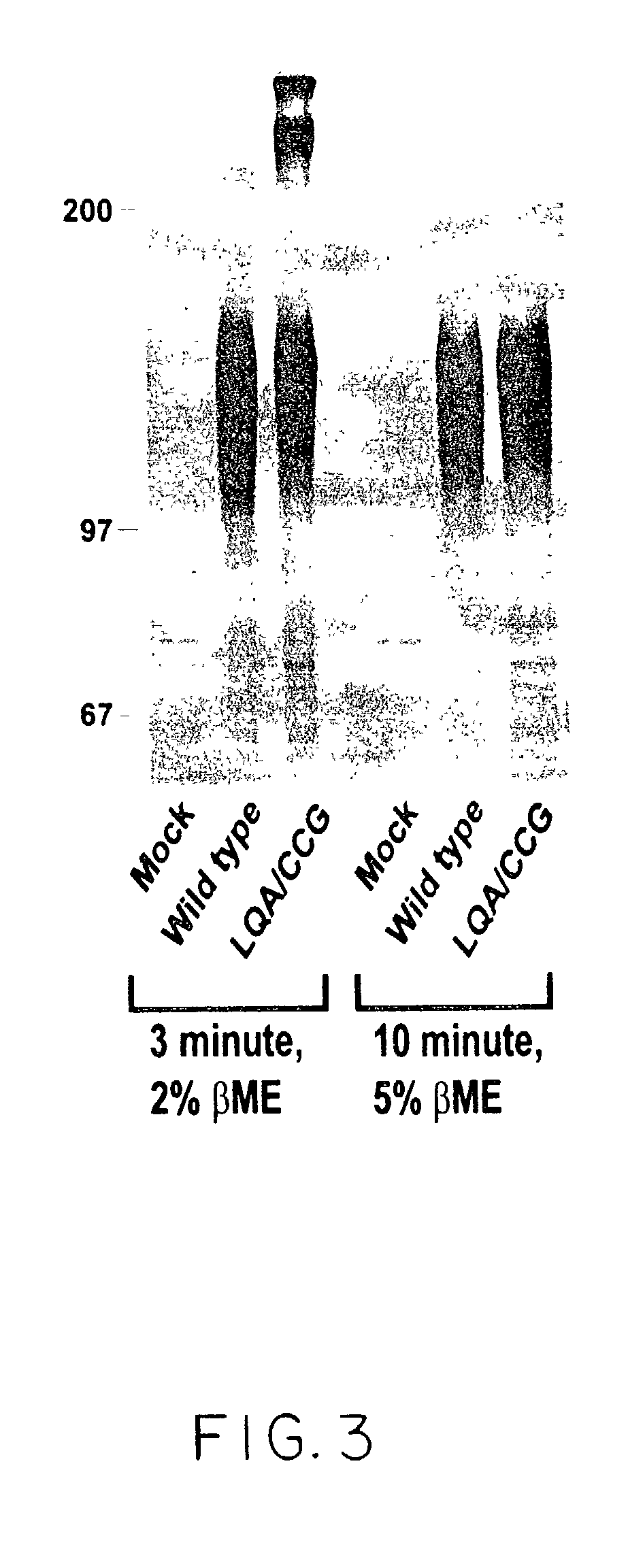
Human immunodeficiency virus envelope clycoprotein mutants and uses thereof
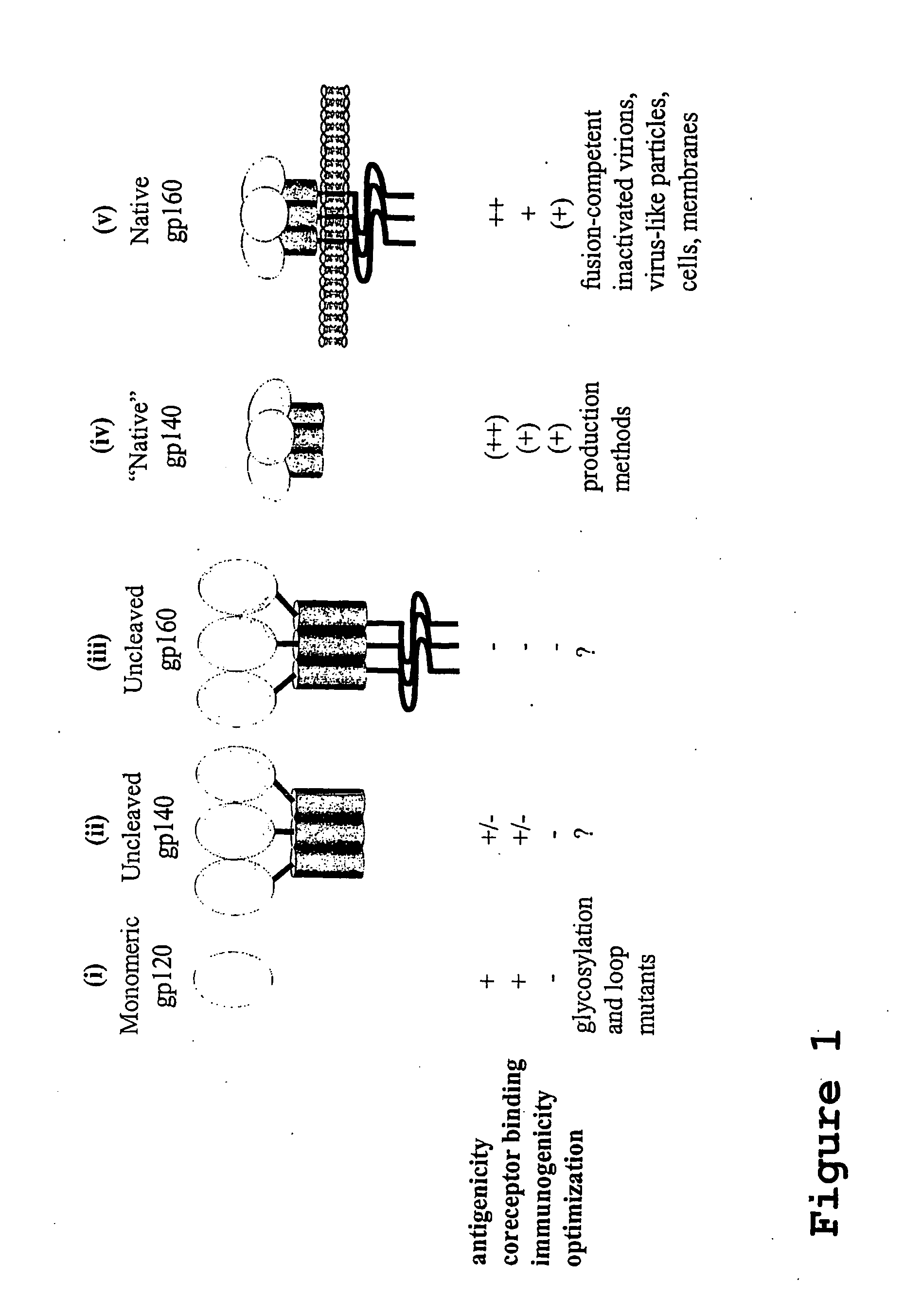
This invention provides stable HIV-1 pre-fusion envelope glycoprotein trimeric complexes. This invention also provides related polypeptides and compositions comprising pharmaceutically acceptable particles and the trimeric complexes operably affixed thereto. This invention further provides related nucleic acids, vectors, host cells, compositions, production methods, and prophylactic and therapeutic methods.
Owner:CORNELL RES FOUNDATION INC +1
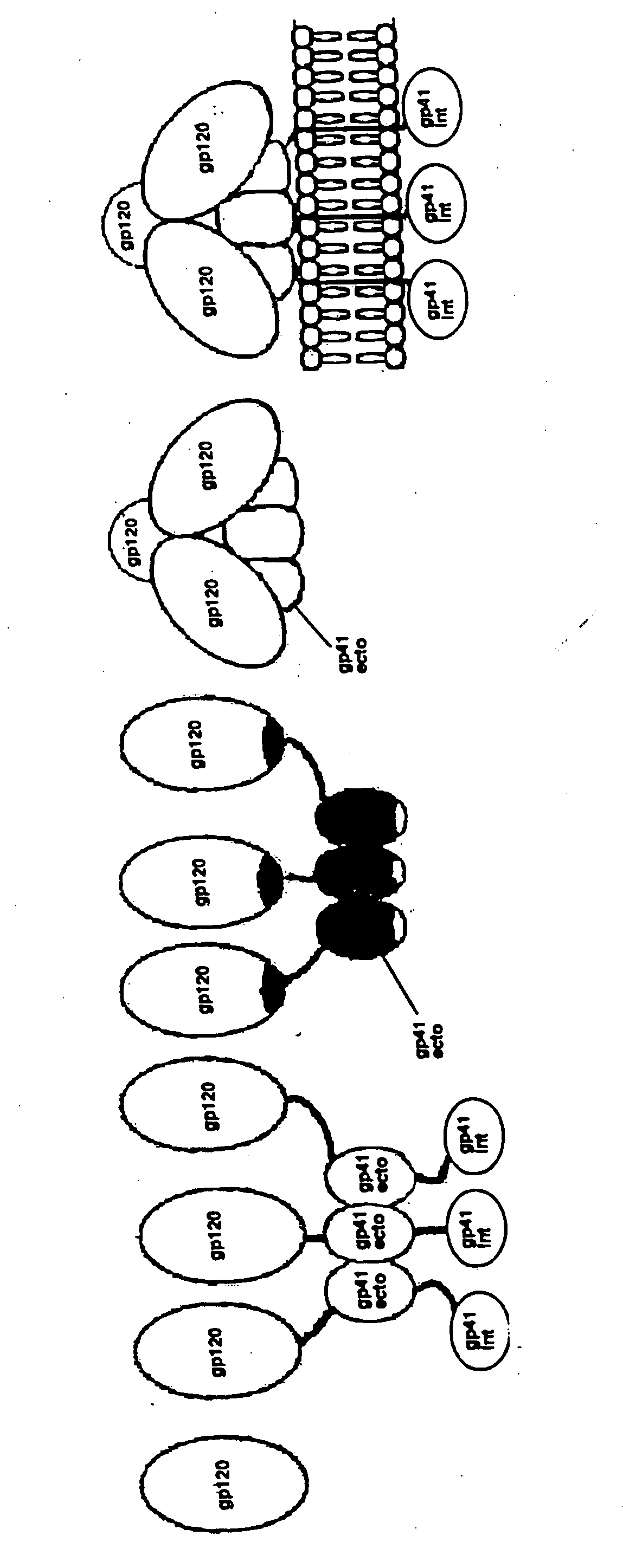
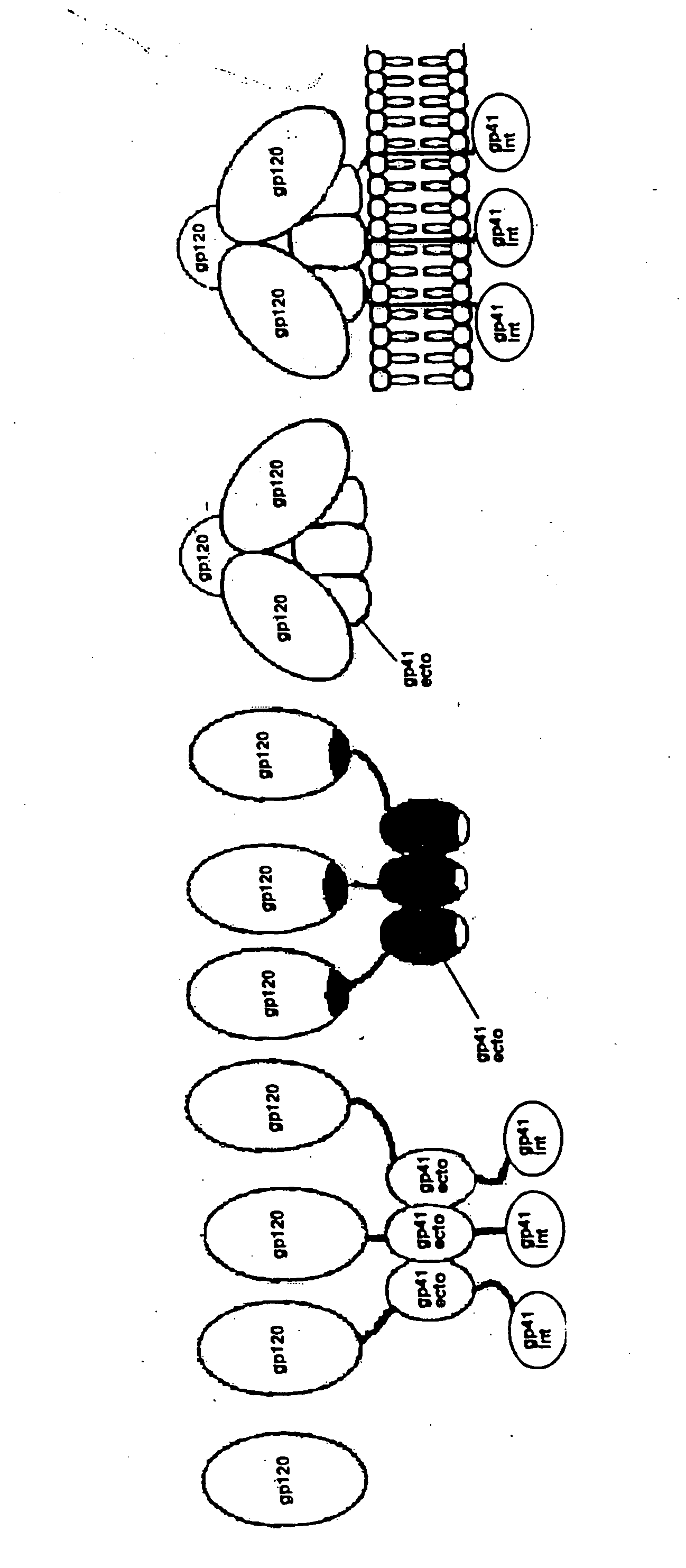
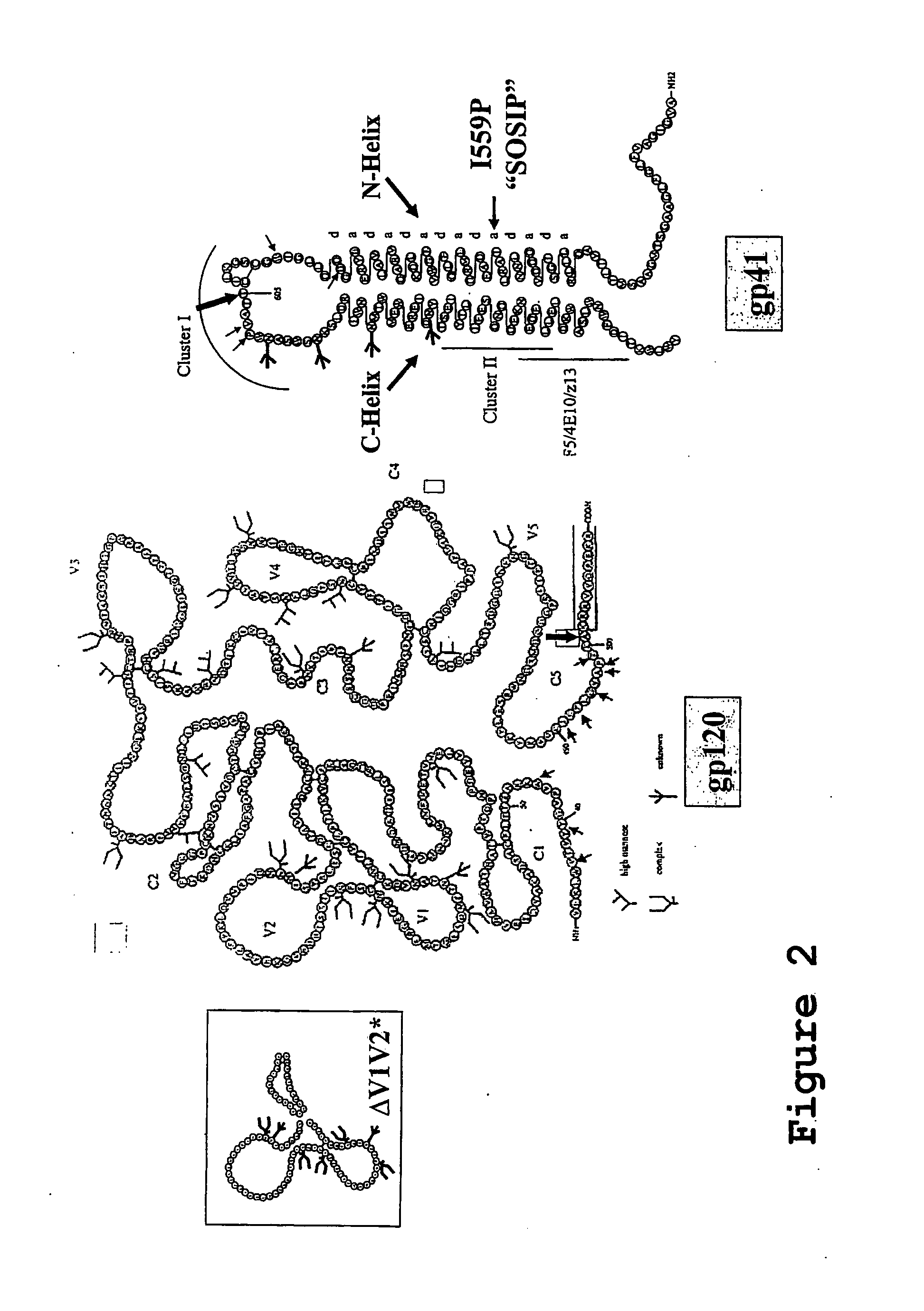
Stabilization of envelope glycoprotein trimers by disulfide bonds introduced into a gp41 glycoprotein ectodomain
The present application is directed to stabilized envelope glycoprotein trimers. The trimers are stabilized by introducing disulfide bonds at certain sites in the gp41 ectodomain. DNA molecules encoding such trimers can be used to generate an immunogenic reaction.
Owner:DANA FARBER CANCER INST INC
Particle-bound human immunodeficiency virus envelope glycoproteins and related compositions and methods
InactiveUS20060051373A1Inhibition of growth rateSlow growth rateBiocideOrganic active ingredientsParticulate antigenPharmaceutical medicine
This invention provides a first composition comprising a pharmaceutically acceptable particle and a stable HIV-1 pre-fusion envelope glycoprotein trimeric complex operably affixed thereto. This invention further provides a second composition comprising (a) a pharmaceutically acceptable particle, (b) an antigen, and (c) an agent which is operably affixed to the particle and is specifically bound to the antigen, whereby the antigen is operably bound to the particle. Finally, this invention provides related nucleic acids, vectors, cells, compositions, production methods, and prophylactic and therapeutic methods.
Owner:PROGENICS PHARMA INC
HIV vaccine
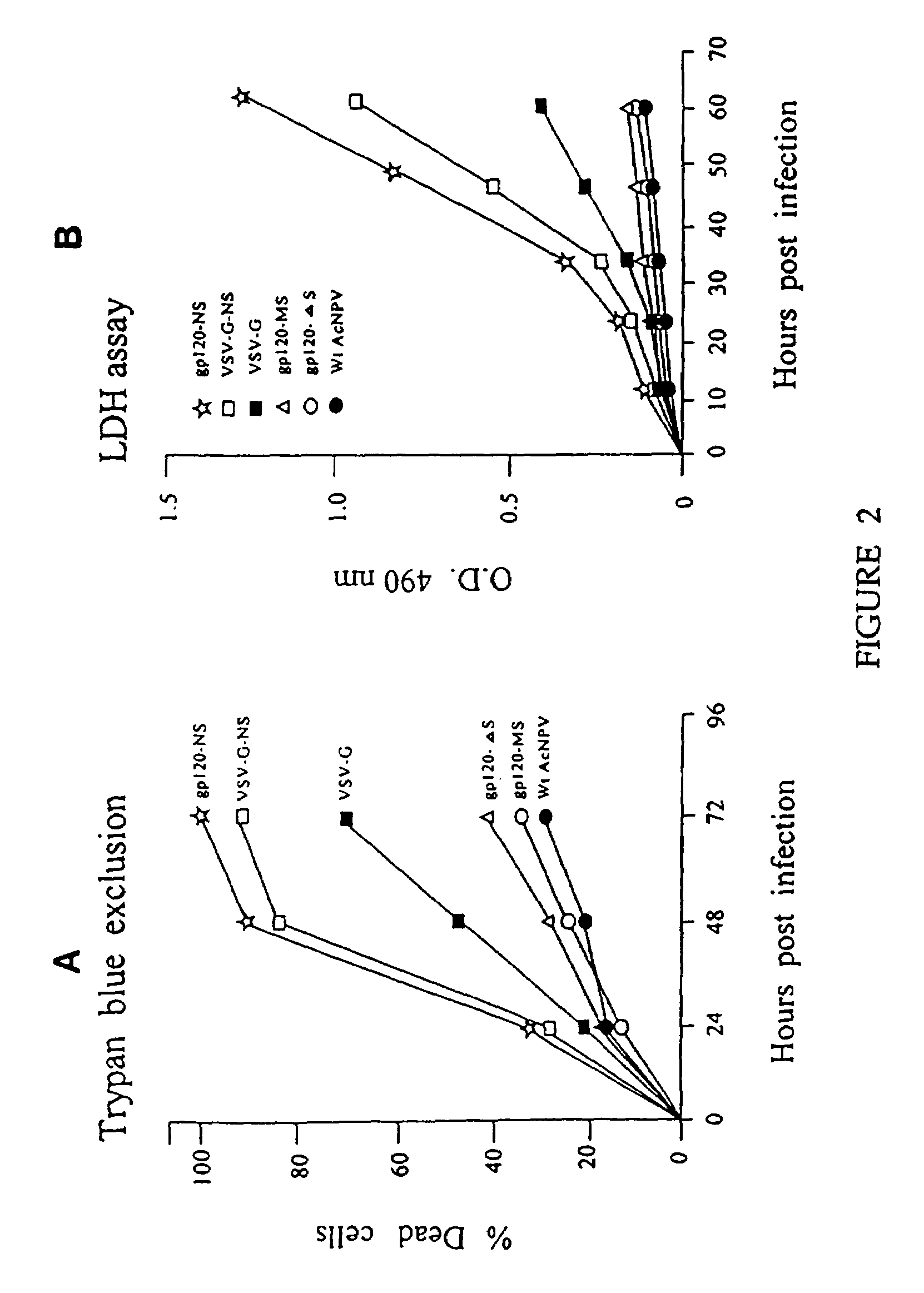
InactiveUS7067134B1Efficient replicationAvoid cell damageViral antigen ingredientsInactivation/attenuationHIV vaccineFhit gene
A novel HIV vaccine is provided. In particular, the vaccine comprises an avirulent and non-cytolytic recombinant HIV wherein the NSS of the virus' envelope glycoprotein is replaced with a non-cytolytic signal sequence and nef gene of the virus is deleted which renders the virus avirulent.
Owner:UNIV OF WESTERN ONTARIO
Stabilized primate lentivirus envelope glycoproteins
InactiveUS7048929B1Improve stabilityImproving immunogenicityAntibody mimetics/scaffoldsVirus peptidesEnv GlycoproteinsDisulfide bond
A modified polypeptide corresponding to an envelope glycoprotein of a primate lentivirus is described. The polypeptide has been modified from the wild-type structure so that it has cysteine amino acid residues introduced to create disulfide bonds, a cavity is filled with hydrophobic amino acids, a Proresidue is introduced at a defined turn structure of the protein, or the hydrophobicity is increased across the interface between different domains, while retaining the overall 3-dimensional structure of a discontinuous conserved epitope of the wild-type protein. Preferably, the polypeptide has more than one of those characteristics. Preferably, the primate lentivirus is HIV, and the protein is HIV-1 gp120.
Owner:DANA FARBER CANCER INST INC +1
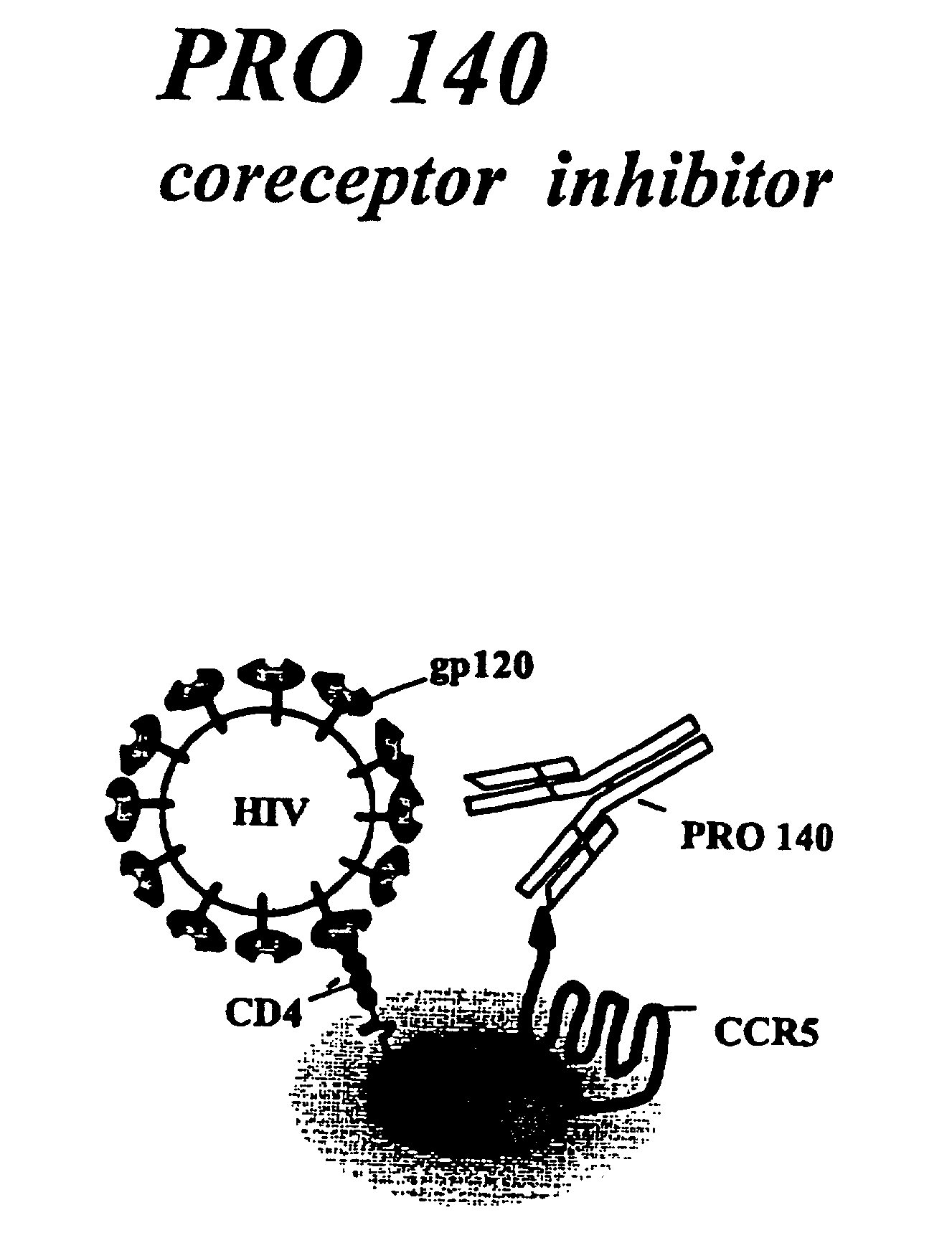
Compositions and methods for inhibition of HIV-1 infection
InactiveUS7138119B2Inhibit infectionAvoid infectionAnimal cellsPeptide/protein ingredientsMolecular biologyGlycophorin
Owner:CYTODYN
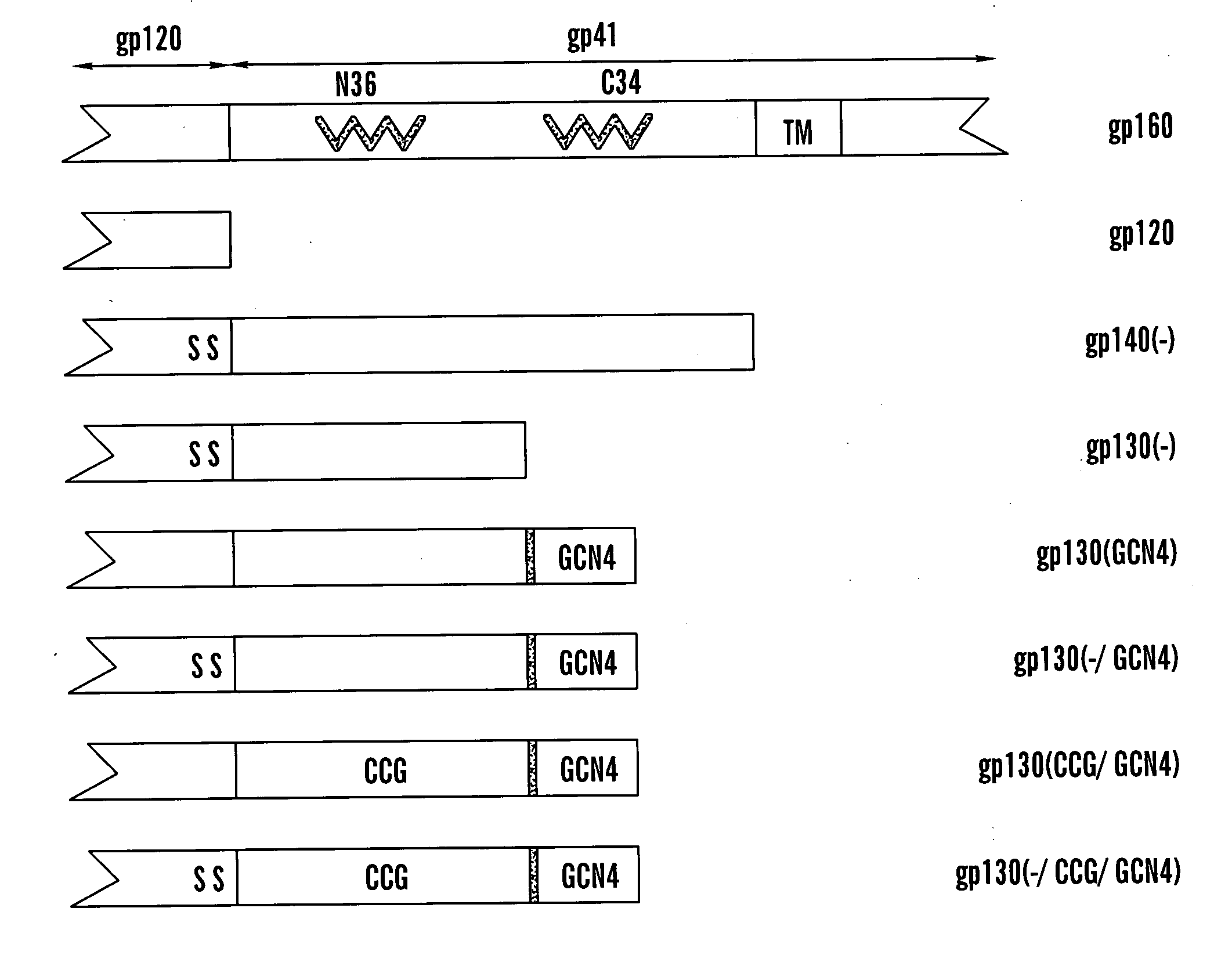
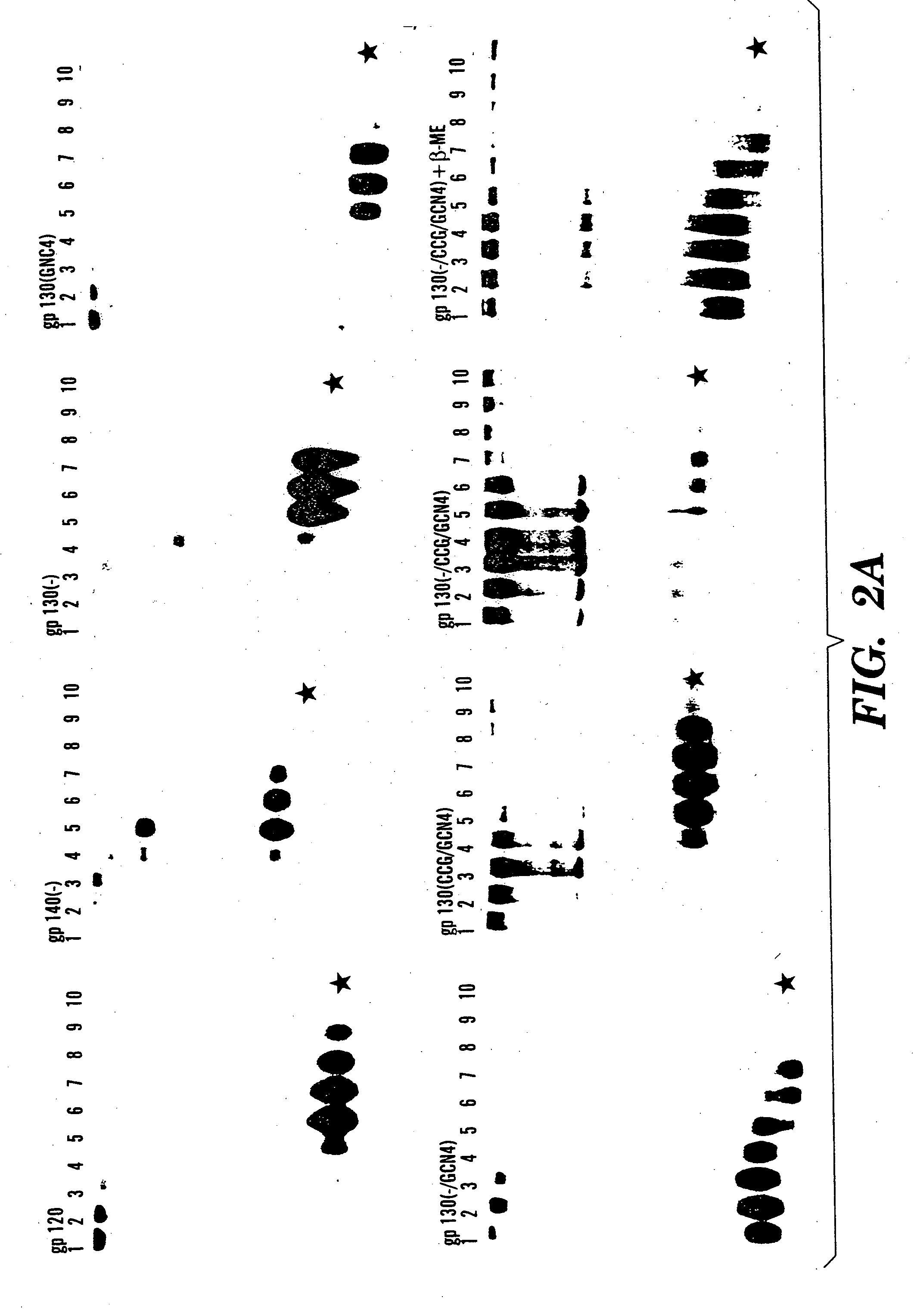
Stabilized soluble glycoprotein trimers
InactiveUS20050106177A1Stabilize trimerSimplifies isolationPeptide/protein ingredientsAntibody mimetics/scaffoldsHiv envelopeGp41
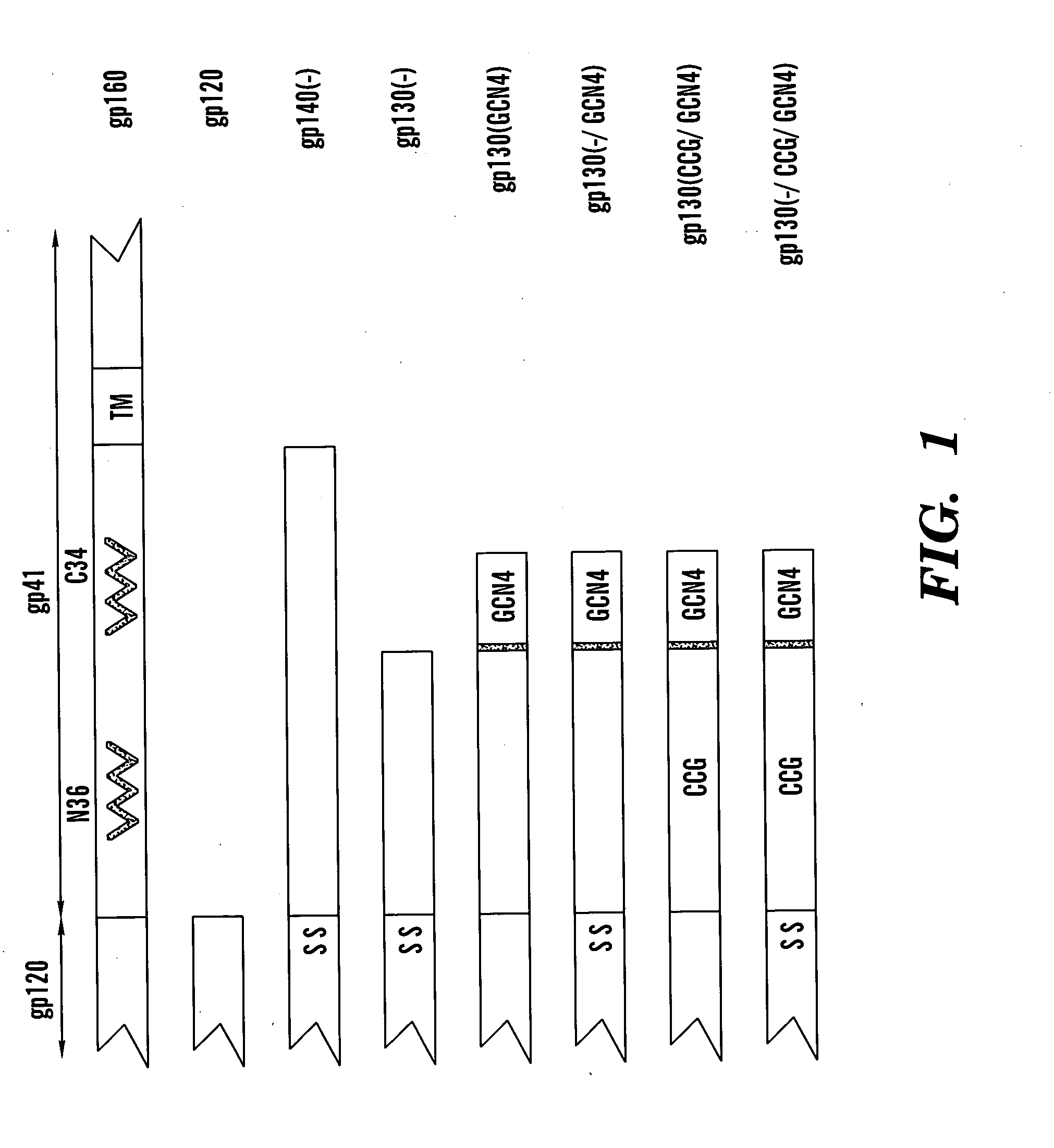
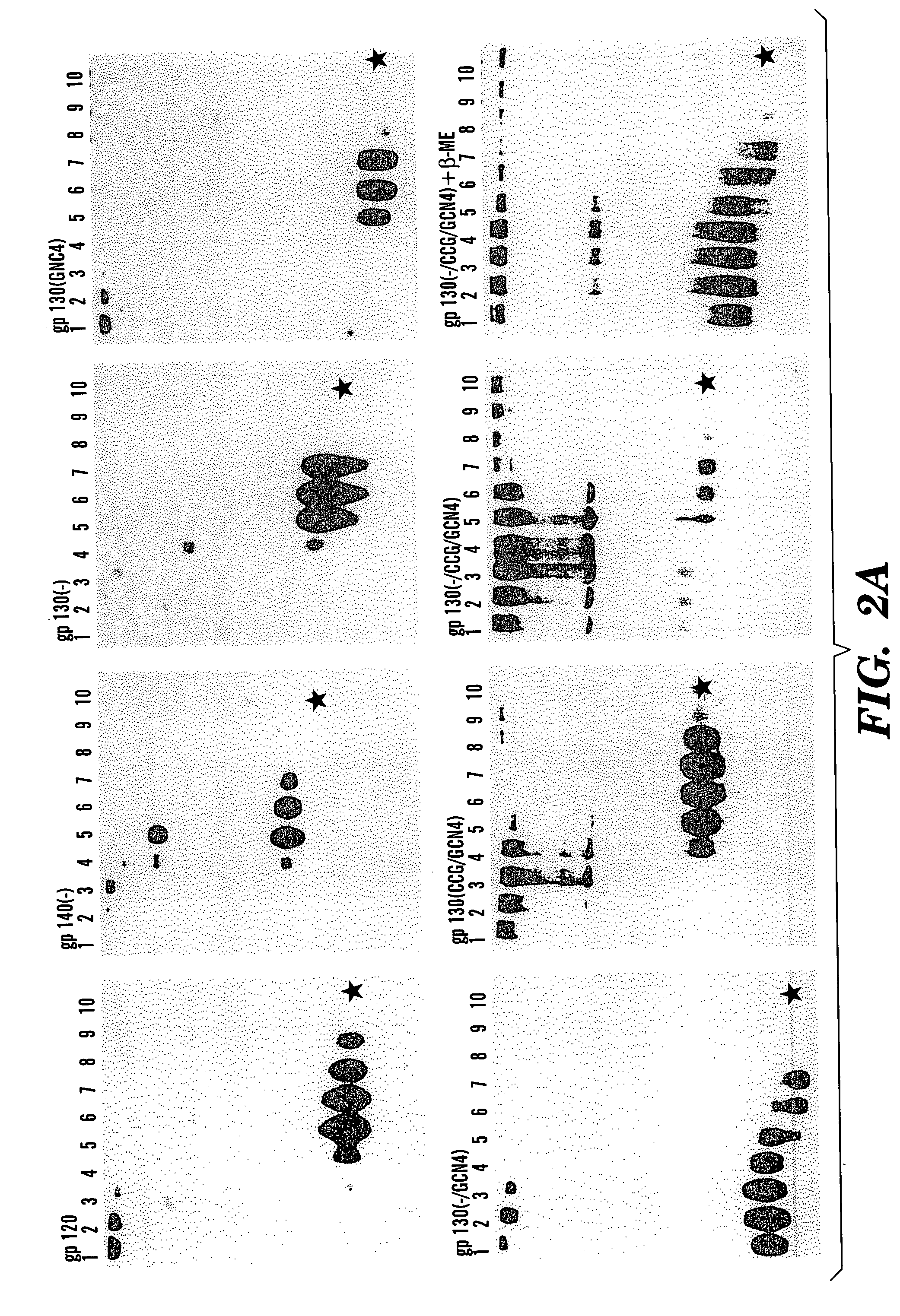
The present application is directed to stabilized HIV envelope glycoprotein trimers. The trimers are stabilized by introducing trimeric motifs, preferably the GCN4 coiled coil or the fibritin trimeric domain, at certain sites, for example in the gp41 ectodomain. These stabilized trimers or DNA molecules encoding such trimers can be used to generate an immunogenic reaction. The trimers can also be used in assays to screen for molecules that interact with them—and to identify molecules that interact with specific sites.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Nucleic acids encoding modified human immunodeficiency virus type 1 (HIV-1) group M consensus envelope glycoproteins
The present invention relates, in general, to an immunogen and, in particular, to an immunogen for inducing antibodies that neutralizes a wide spectrum of HIV primary isolates and / or to an immunogen that induces a T cell immune response. The invention also relates to a method of inducing anti-HIV antibodies, and / or to a method of inducing a T cell immune response, using such an immunogen. The invention further relates to nucleic acid sequences encoding the present immunogens.
Owner:UNIVERSITY OF ALABAMA +2
Methods for producing and using in vivo pseudotyped retroviruses
The present invention provides novel pseudotyped retroviral vectors that can transduce human and other cells. Vectors are provided that are packaged efficiently in packaging cells and cell lines to generate high titer recombinant virus stocks expressing novel envelope glycoproteins. The present invention further relates to compositions for gene therapy.
Owner:UNIV OF IOWA RES FOUND
Production of virus and purification of viral envelope proteins for vaccine use
Immunogenic envelope glycoproteins are produced from enveloped virus, such as of the paramyxoviridae family, particularly PIV-3 and RSV, by culturing the virus in the substantial absence of exogenous serum proteins, isolating the virus from the tissue culture, solubilizing the envelope glycoproteins and isolating the solubilized envelope glycoproteins by chromatography.
Owner:CONNAUGHT LAB
Methods for assaying inhibition of HIV-1 envelope glycoprotein-mediated membrane fusion
Owner:PROGENICS PHARMA INC
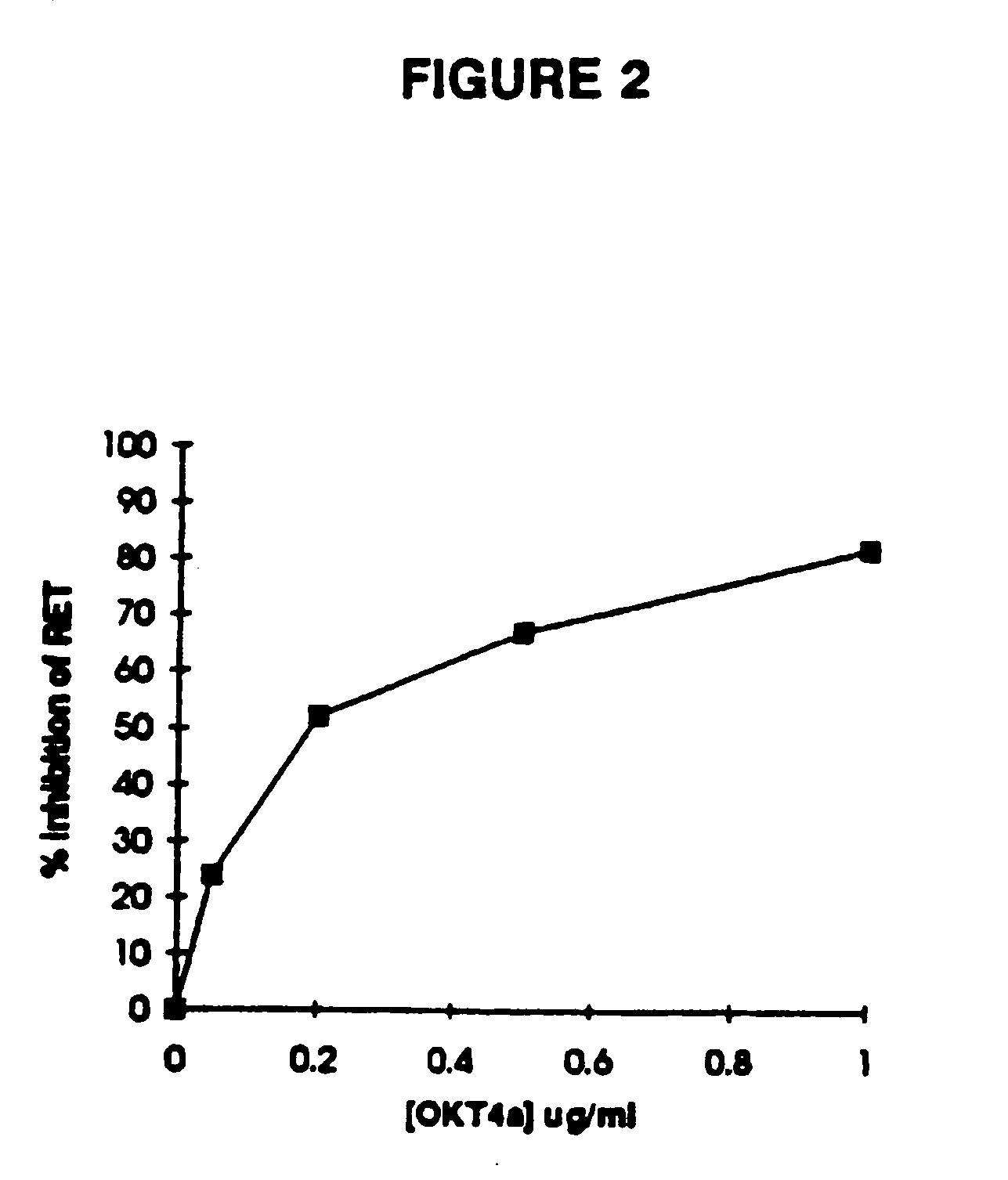
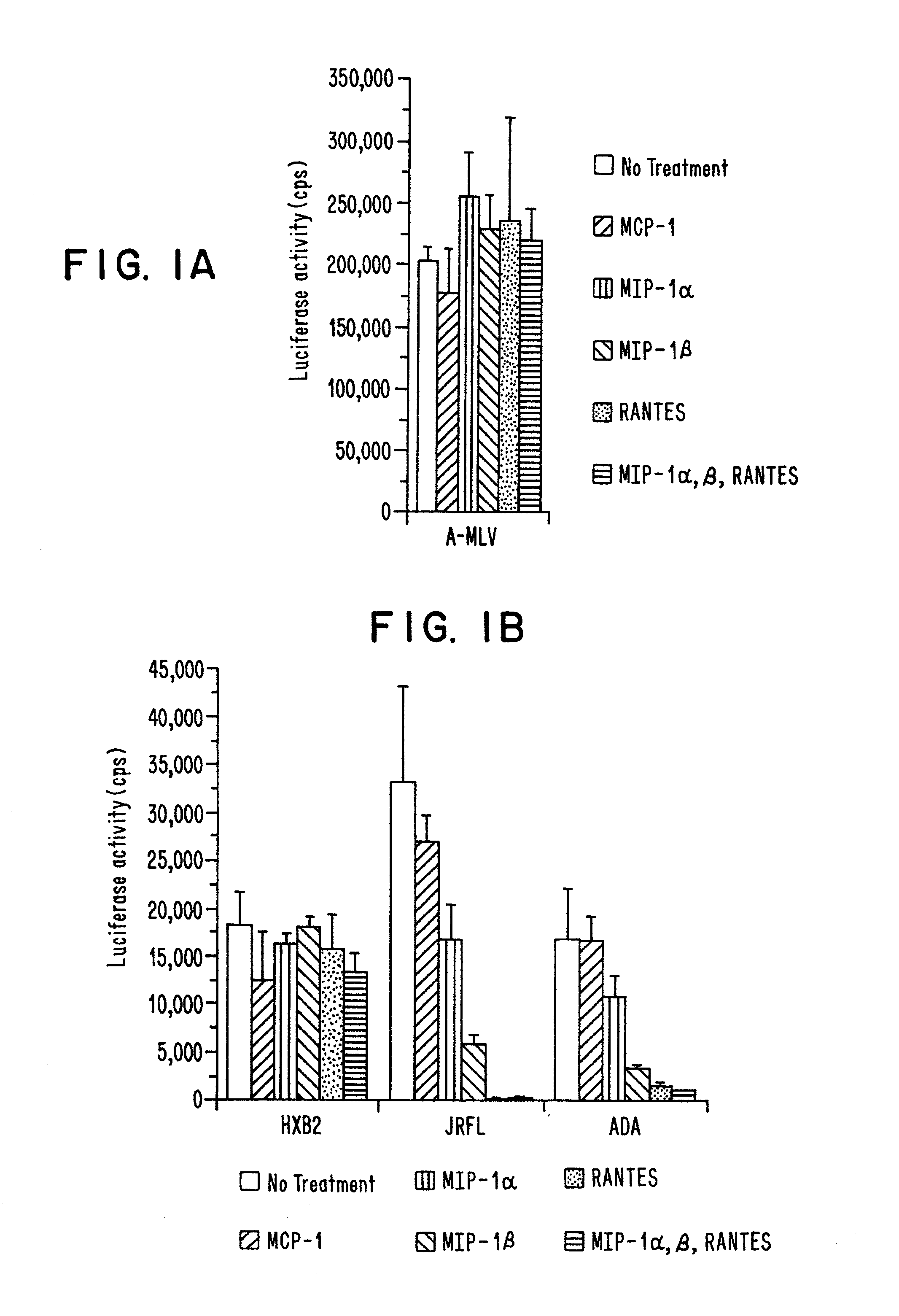
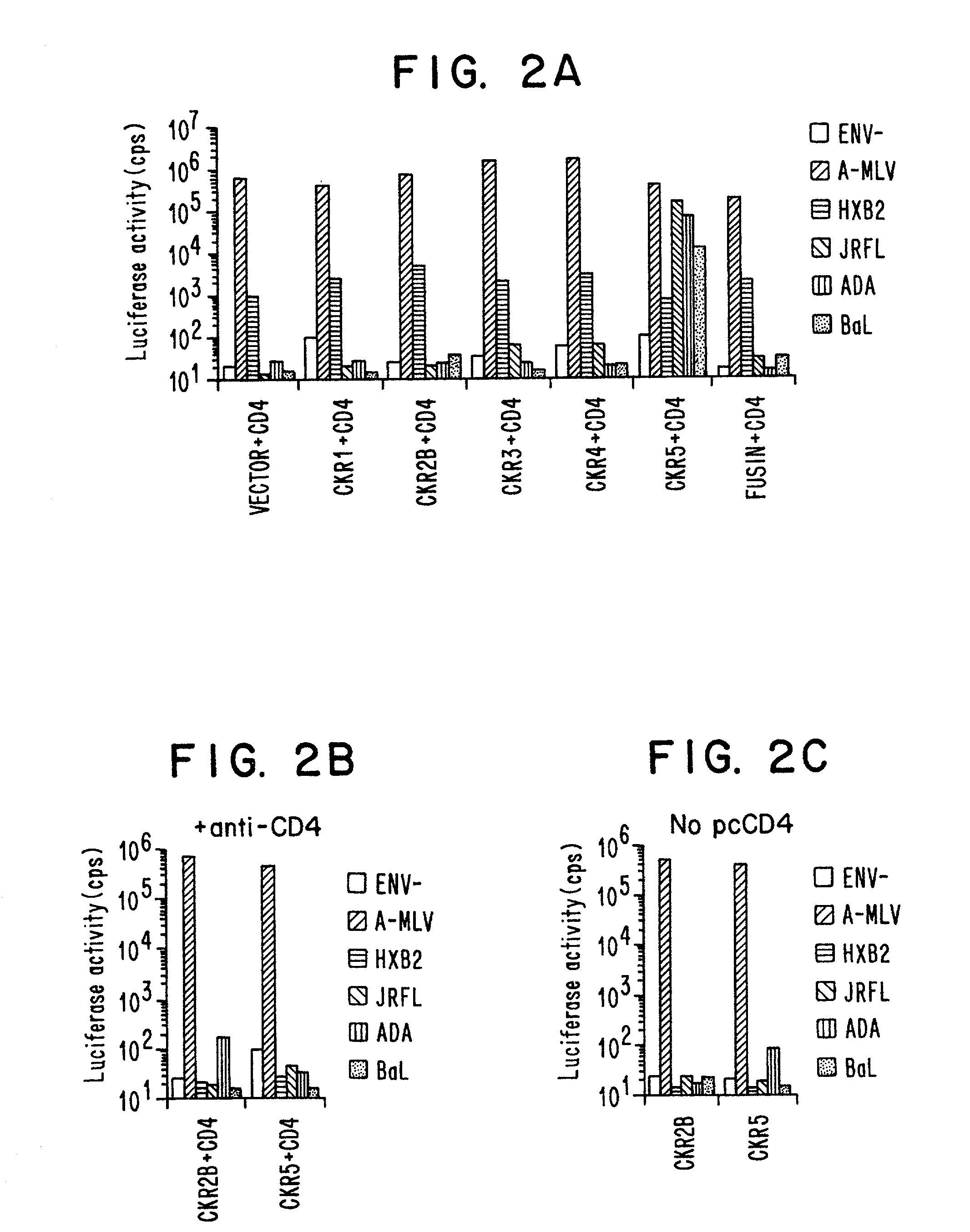
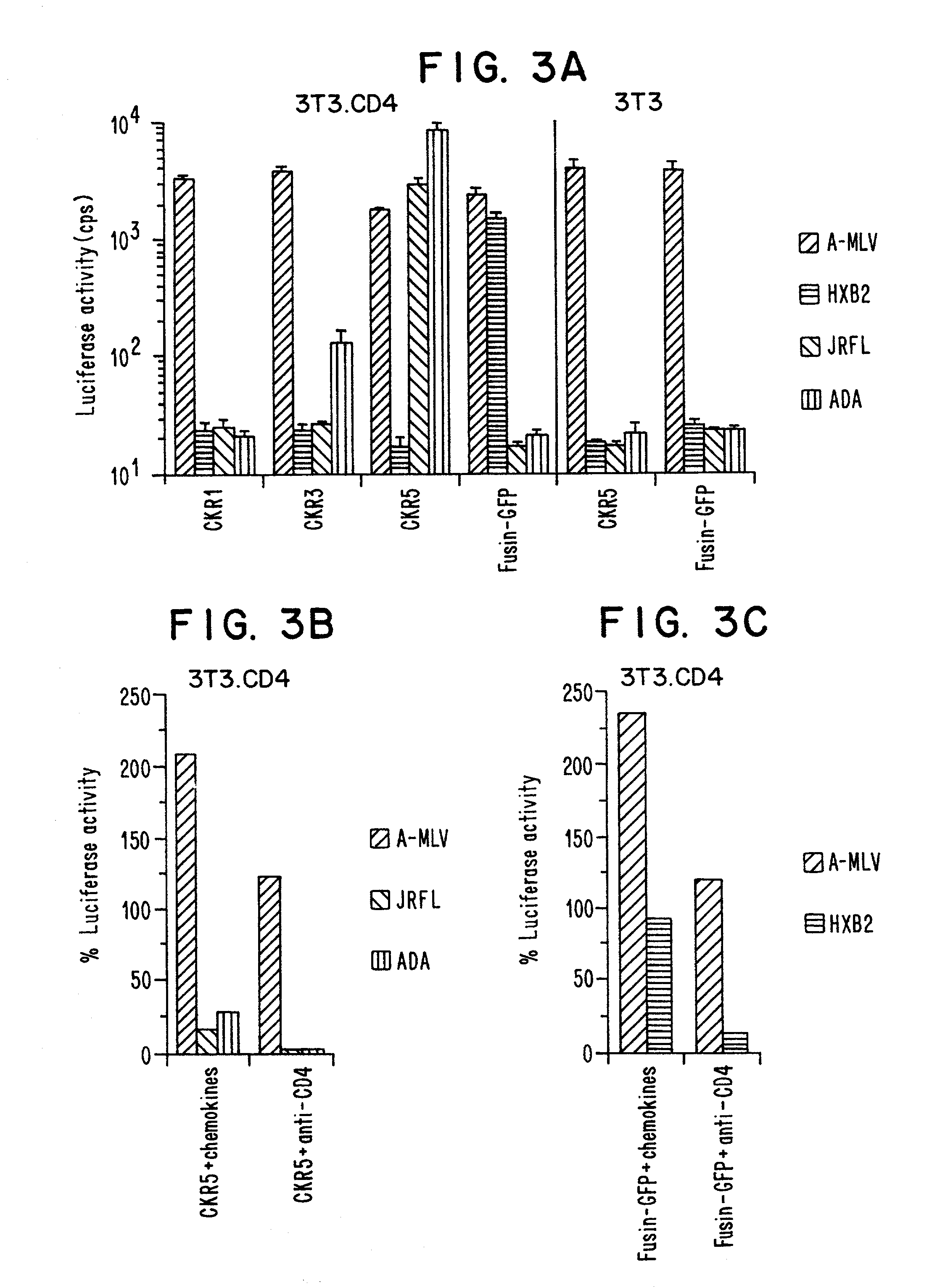
Methods of identifying g-couple receptors associated with macrophage-trophic HIV, and diagnostic and therapeutic uses thereof
InactiveUS7129055B2Effective activityHigh activityBiocideAntimycoticsTherapeutic Use StudyΒ chemokines
Entry of HIV-1 into target cells requires cell surface CD4 as well as additional host cell cofactors. A cofactor required for infection with virus adapted for growth in transformed T cell lines was recently identified and named fusin. Fusin, however, does not promote entry of macrophage-tropic viruses that are believed to be the key pathogenic strains in vivo. It has now been determined that the principal cofactor for entry mediated by the envelope glycoproteins of primary macrophage-tropic strains of HIV-1 is CC-CKR5, a receptor for the β-chemokines RANTES, MIP-1α, and MIP-1β.
Owner:NEW YORK UNIV
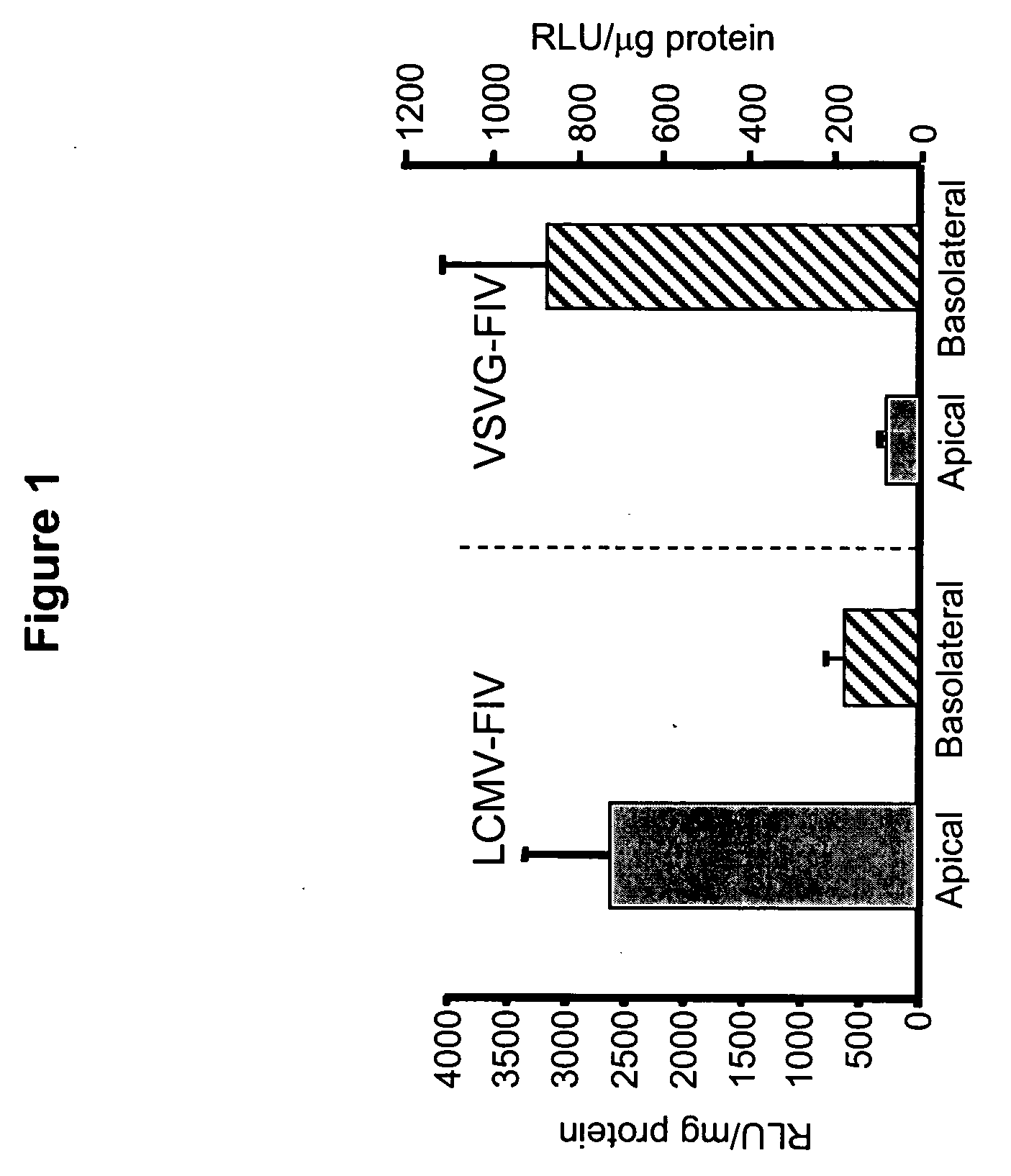
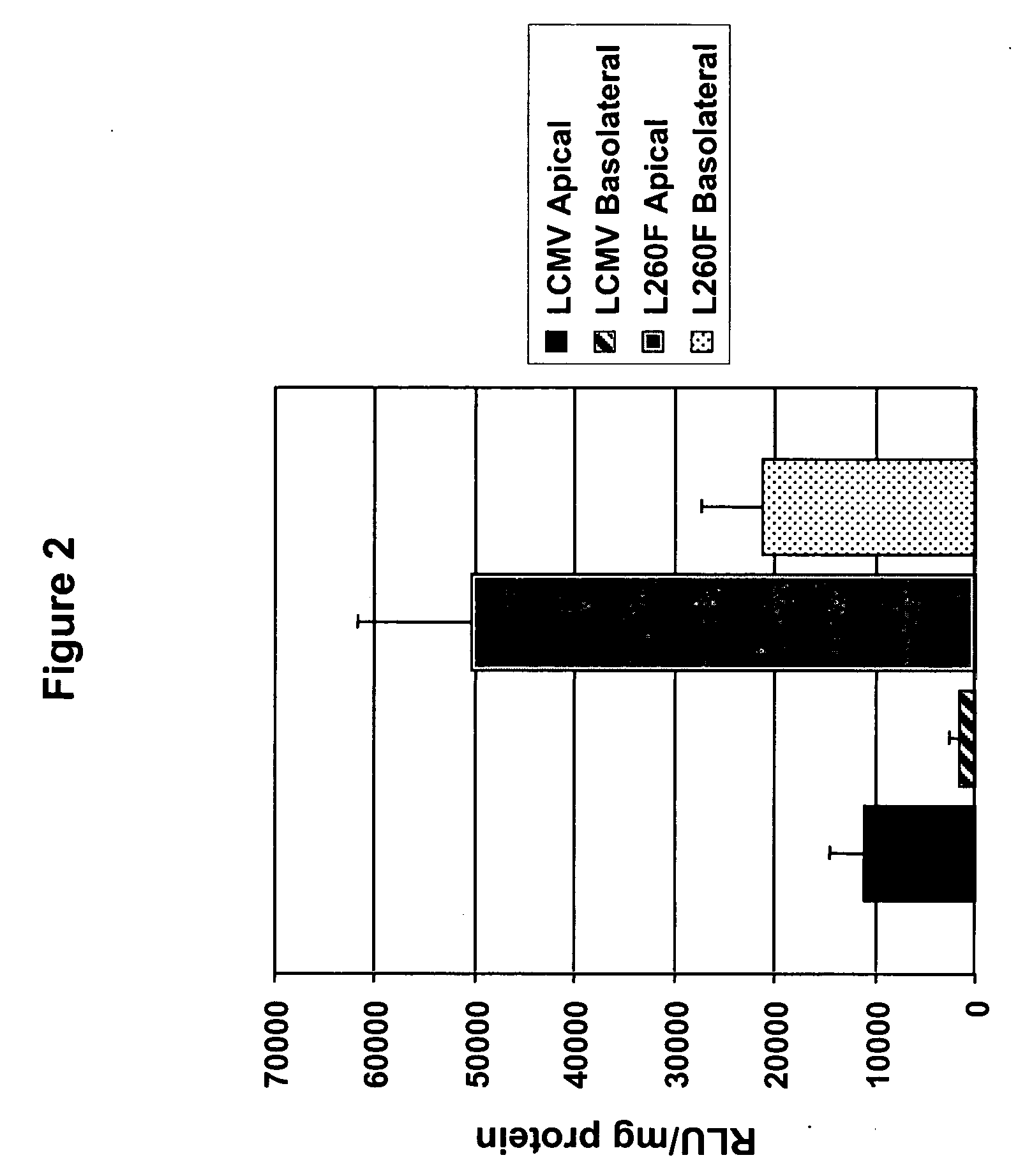
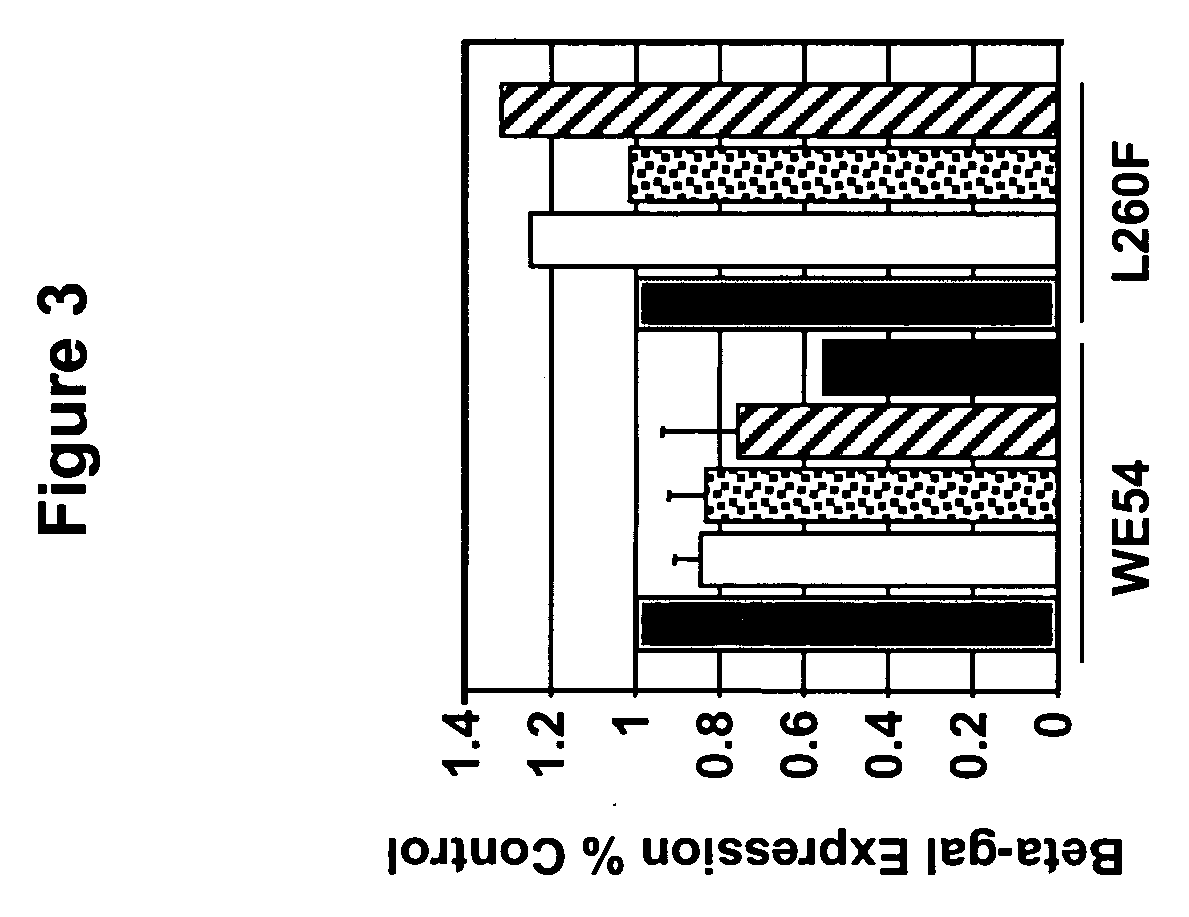
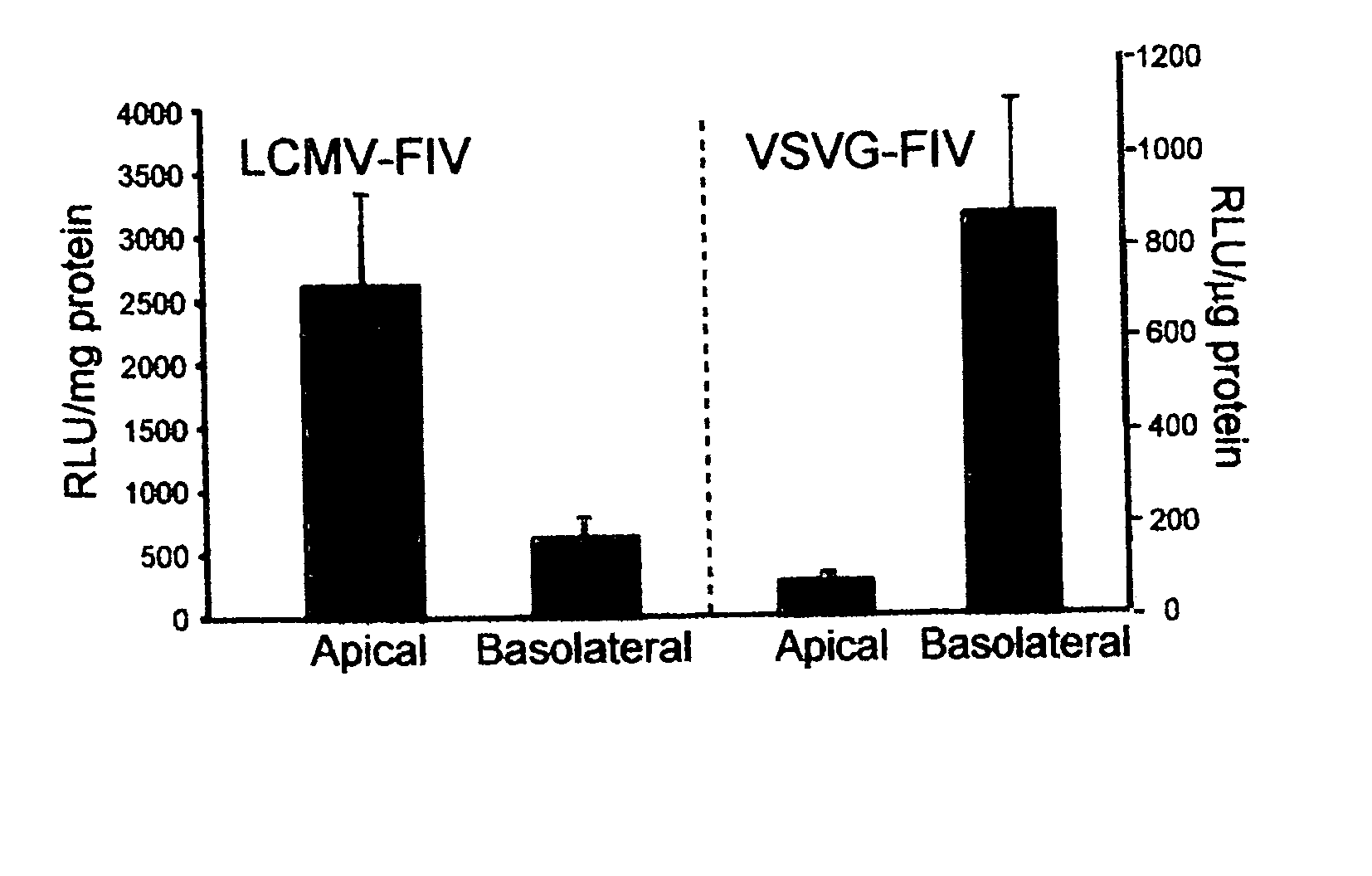
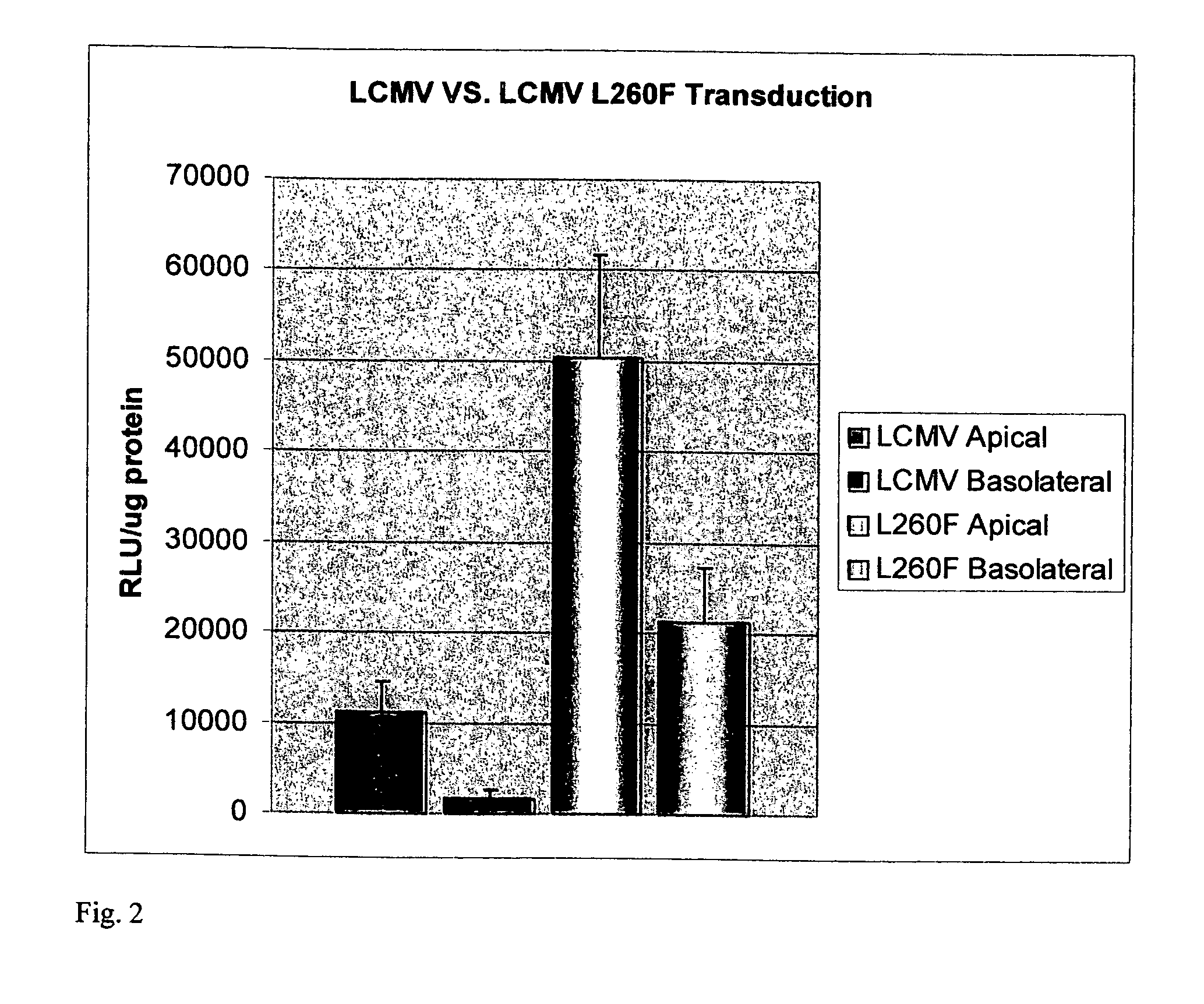
Methods for producing and using in vivo pseudotyped retroviruses using envelope glycoproteins from lymphocytic choriomeningitis virus (LCMV)
ActiveUS20050123517A1SsRNA viruses negative-senseBiocideLymphocytic choriomeningitis virus infectionIn vivo
The present invention provides novel pseudotyped retroviral vectors that can transduce human and other cells. Vectors are provided that are packaged efficiently in packaging cells and cell lines to generate high titer recombinant virus stocks expressing novel envelope glycoproteins. The present invention further relates to compositions for gene therapy.
Owner:IOWA RES FOUND UNIV OF
HIV-1 envelope glycoprotein
ActiveUS8586056B2Increase generationViral antigen ingredientsVirus peptidesNeutralising antibodyImmunogenicity
The present application relates to a novel HIV-1 envelope glycoprotein which may be utilized as an HIV-1 vaccine immunogen, antigens for crystallization and for the identification of broad neutralizing antibodies. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE
Flavivirus fusion inhibitors
InactiveUS20050271677A1Reduce HIV- loadImprove developmentSsRNA viruses positive-sensePeptide/protein ingredientsPestivirusVirosome
The present invention relates to peptides and methods of inhibiting fusion between the virion envelope of Flaviviruses and membranes of the target cell, the process that delivers the viral genome into the cell cytoplasm. The invention provides for methods which employ peptides or peptide derivatives to inhibit Flavivirus:cell fusion. The present invention is based in part on the discovery that E1 envelope glycoprotein of hepaciviruses and E2 envelope glycoprotein of pestivirus have previously undescribed structures, truncated class II fusion proteins. The present invention provides peptides and methods of treatment and prophylaxis of diseases induced by Flaviviruses.
Owner:TULANE EDUCATIONAL FUND +1
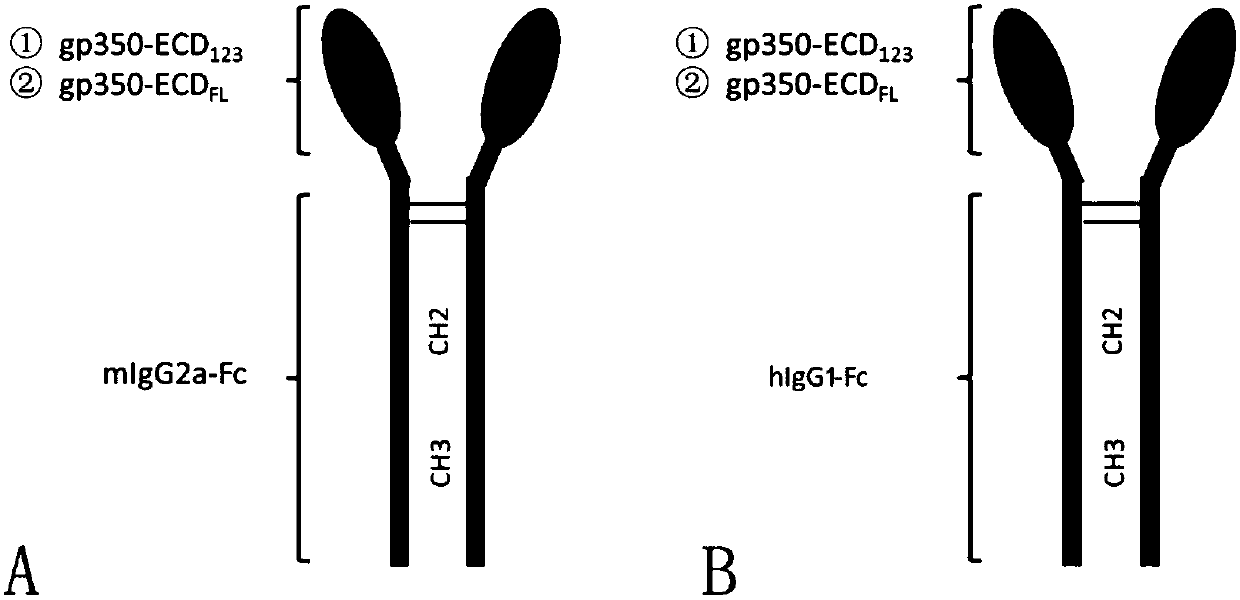
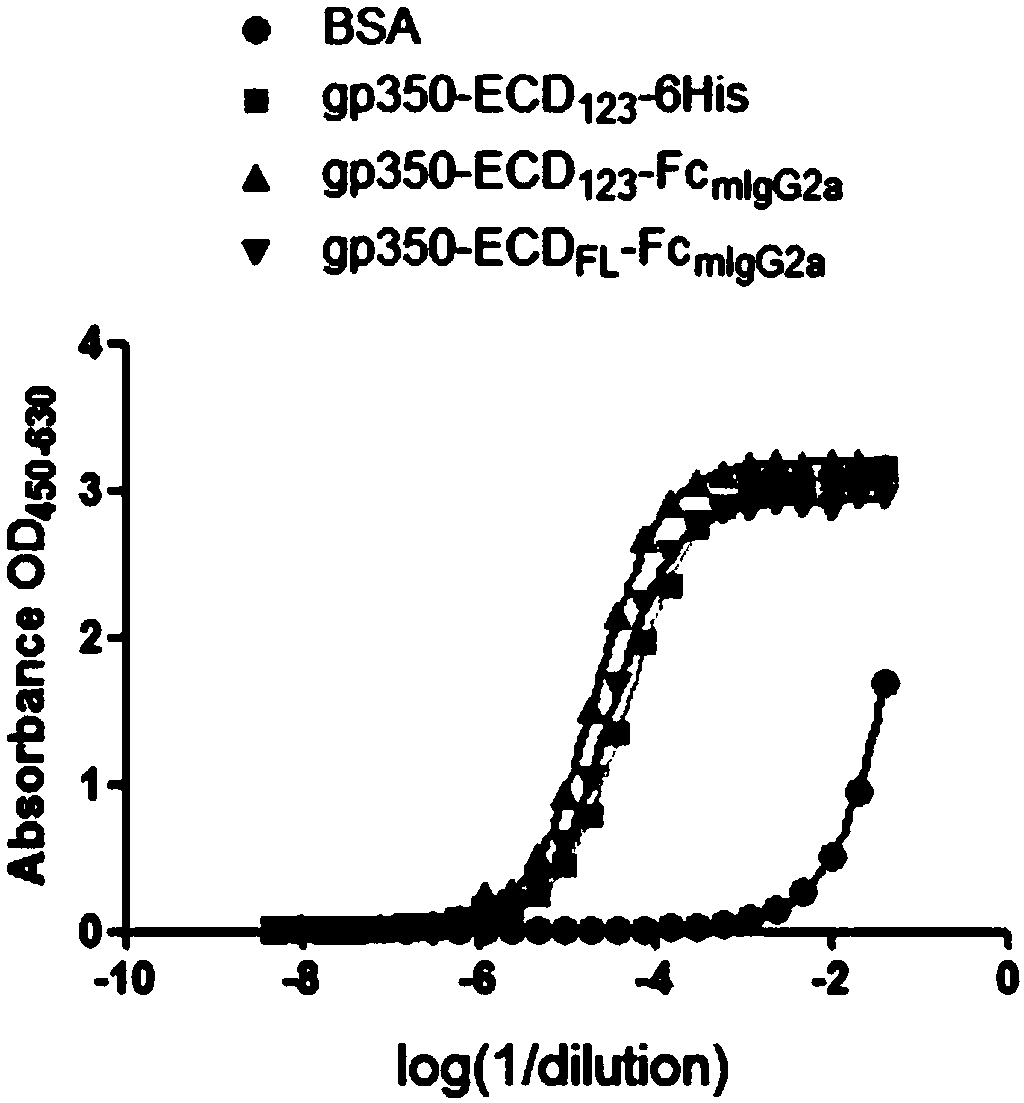
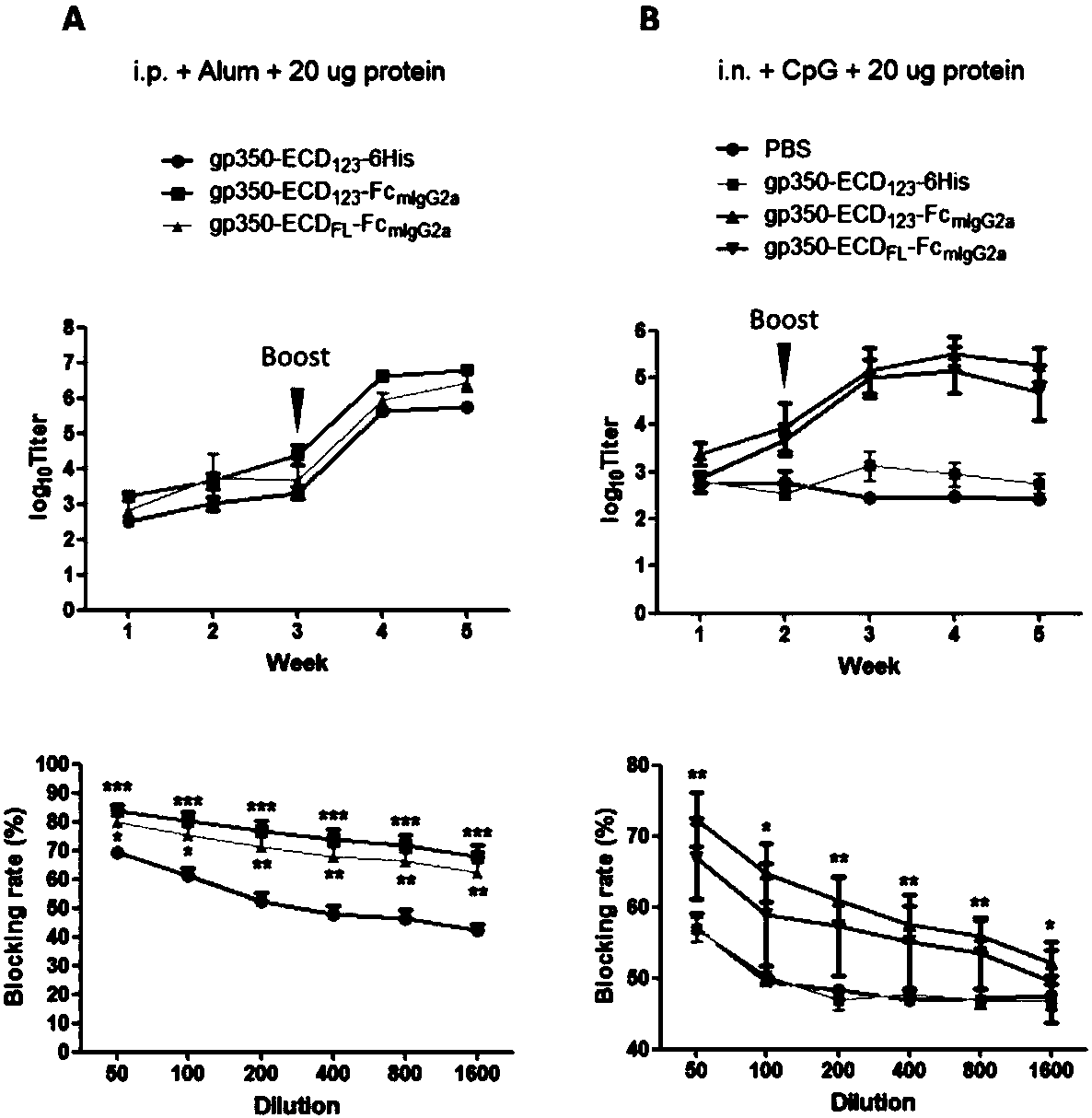
Fusion protein comprising Fc domain of IgG and extracellular domain of EB virus envelope glycoprotein
ActiveCN109824779AImproving immunogenicityHigh infection blocking efficiencyAntiviralsAntibody ingredientsDiseaseImmunogenicity
The invention discloses a fusion protein comprising an Fc domain of IgG and an extracellular domain of an EB virus envelope glycoprotein. The fusion protein is represented by the following formula: P-E-F, wherein P represents a secretion signal peptide, E represents an amino acid sequence of the extracellular domain of the EB virus envelope glycoprotein gp350, and F represents the amino acid sequence of the Fc domain of the IgG. It is found for the first time that after the fusion of the Fc domain of the immunoglobulin IgG with the envelope glycoprotein gp350 from the surface of the EB virus,the immunogenicity in vivo is significantly improved. After immunization with the fusion protein, the total serum titer an immunized animal, the serum specific neutralizing antibody titer and the serum in vitro viral infection blocking efficiency are significantly higher than that of a non-fused control protein. The fusion protein using the Fc domain of the immunoglobulin IgG and the EB virus membrane glycoprotein is adopted as an evidence for the efficacy of an EB virus vaccine and has important practical and theoretical significance and application prospects for prevention and treatment of EB virus-related diseases.
Owner:SUN YAT SEN UNIV +1
Stabilized soluble glycoprotein trimers
InactiveUS20050220817A1Stabilize trimerSimplifies isolationAntibody mimetics/scaffoldsVirus peptidesHiv envelopeGp41
The present application is directed to stabilized HIV envelope glycoprotein trimers. The trimers are stabilized by introducing trimeric motifs, preferably the GCN4 coiled coil or the fibritin trimeric domain, at certain sites, for example in the gp41 ectodomain. These stabilized trimers or DNA molecules encoding such trimers can be used to generate an immunogenic reaction. The trimers can also be used in assays to screen for molecules that interact with them—and to identify molecules that interact with specific sites.
Owner:DANA FARBER CANCER INST INC
Hiv-1 Neutralizing Antibodies Elicited By Trimeric Hiv-1 Envelope Glycoprotein Complex
InactiveUS20080274134A1Reduce likelihoodSlows rate of progressionViral antigen ingredientsVirus peptidesTherapeutic treatmentAntibody
This invention provides methods for eliciting an immune response in a subject comprising administering to the subject as part of a regimen (i) more than one dose of a nucleic acid prime, and (ii) more than one dose of a protein boost composition, wherein each dose of the nucleic acid prime is administered to the subject at a first predefined interval and each dose of the protein boost composition is administered to the subject at a second predefined interval, and wherein the protein boost composition comprises a prophylactically or therapeutically effective dose of (a) a trimeric HIV-1 gp140 protein complex, or (b) a superparamagnetic particle and a trimeric HIV-1 gp140 protein complex so affixed thereto as to permit recognition of the complex by a subject's immune system. The invention also provides methods for prophylactically or therapeutically treating HIV-1 infection in a subject. The invention further provides uses of a nucleic acid prime and a protein boost composition for the manufacture of separate coadministerable medicaments to be administered to a subject.
Owner:CORNELL RES FOUNDATION INC +1
Methods for producing and using in vivo pseudotyped retroviruses using envelope glycoproteins from lymphocytic choriomeningitis virus (LCMV)
ActiveUS20050112096A1SsRNA viruses negative-senseBiocideLymphocytic choriomeningitis virus infectionIn vivo
The present invention provides novel pseudotyped retroviral vectors that can transduce human and other cells. Vectors are provided that are packaged efficiently in packaging cells and cell lines to generate high titer recombinant virus stocks expressing novel envelope glycoproteins. The present invention further relates to compositions for gene therapy.
Owner:UNIV OF IOWA RES FOUND
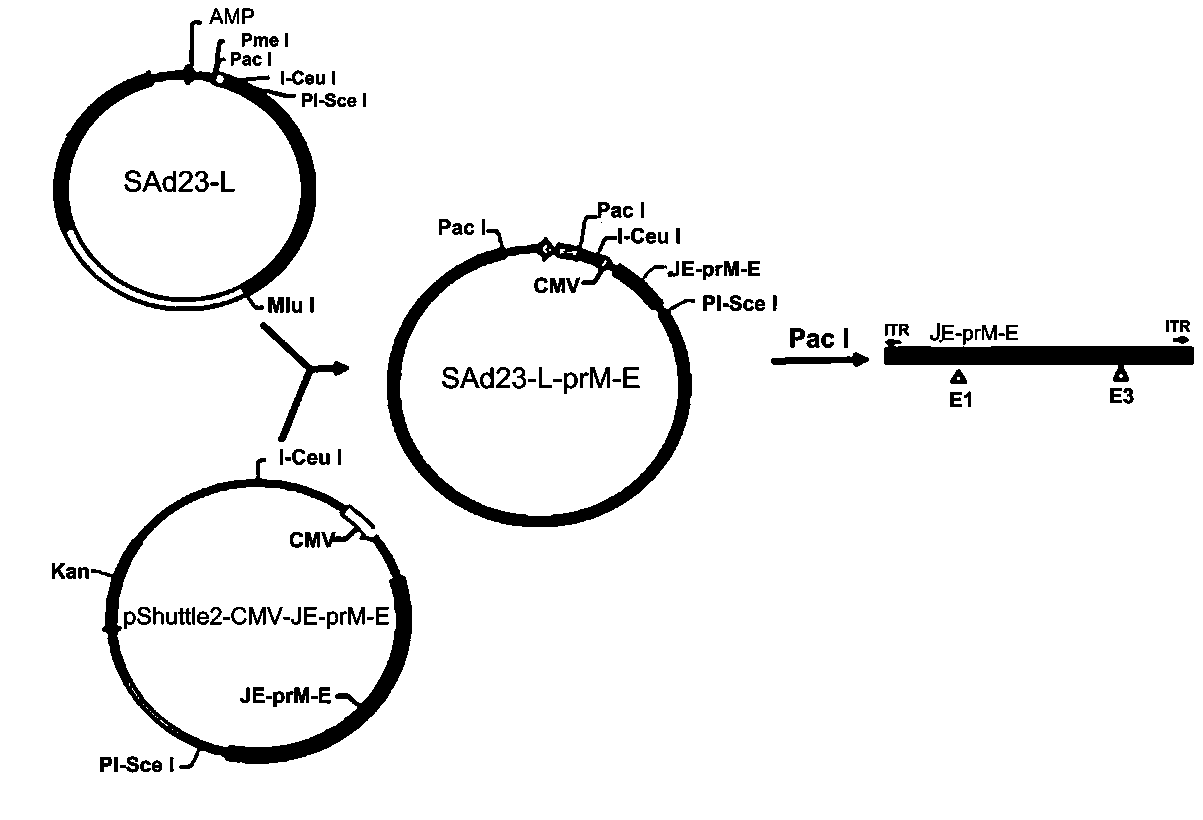
Zika virus vaccine based on replication-defective recombinant adenovirus vector
PendingCN111088271AImprove expression levelImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsNucleotideImmunogenicity
The invention discloses a Zika virus vaccine based on a replication-defective recombinant adenovirus vector. A nucleotide sequence of a JE signal peptide, and an optimized nucleotide sequence encodingZika virus membrane protein prM and envelope glycoprotein E are cloned into a shuttle plasmid vector pShutle2-CMV-Flag to obtain a recombinant shuttle plasmid, so that the expression of foreign proteins is significantly increased, and at the same time immunogenicity of an antigen is improved. Meanwhile, a recombinant adenovirus expression vector SAd23-L is used to escape pre-existing immune responses against common adenovirus vectors, and the humoral and cellular immunity with a higher level can be induced to generate in animals. After the recombinant adenovirus as a Zika virus vaccine is used to immunize animals, the humoral and cellular response to Zika virus is quickly induced to generate, especially a neutralizing antibody with a high level is induced to generate, and the specific cellular response to Zika virus antigen M and E protein is induced to generate in mice. Therefore, the Zika virus vaccine can be used to prevent large-scale outbreak and epidemic of Zika virus.
Owner:广州佰芮慷生物科技有限公司
Compositions and methods for inhibition of HIV-1 infection
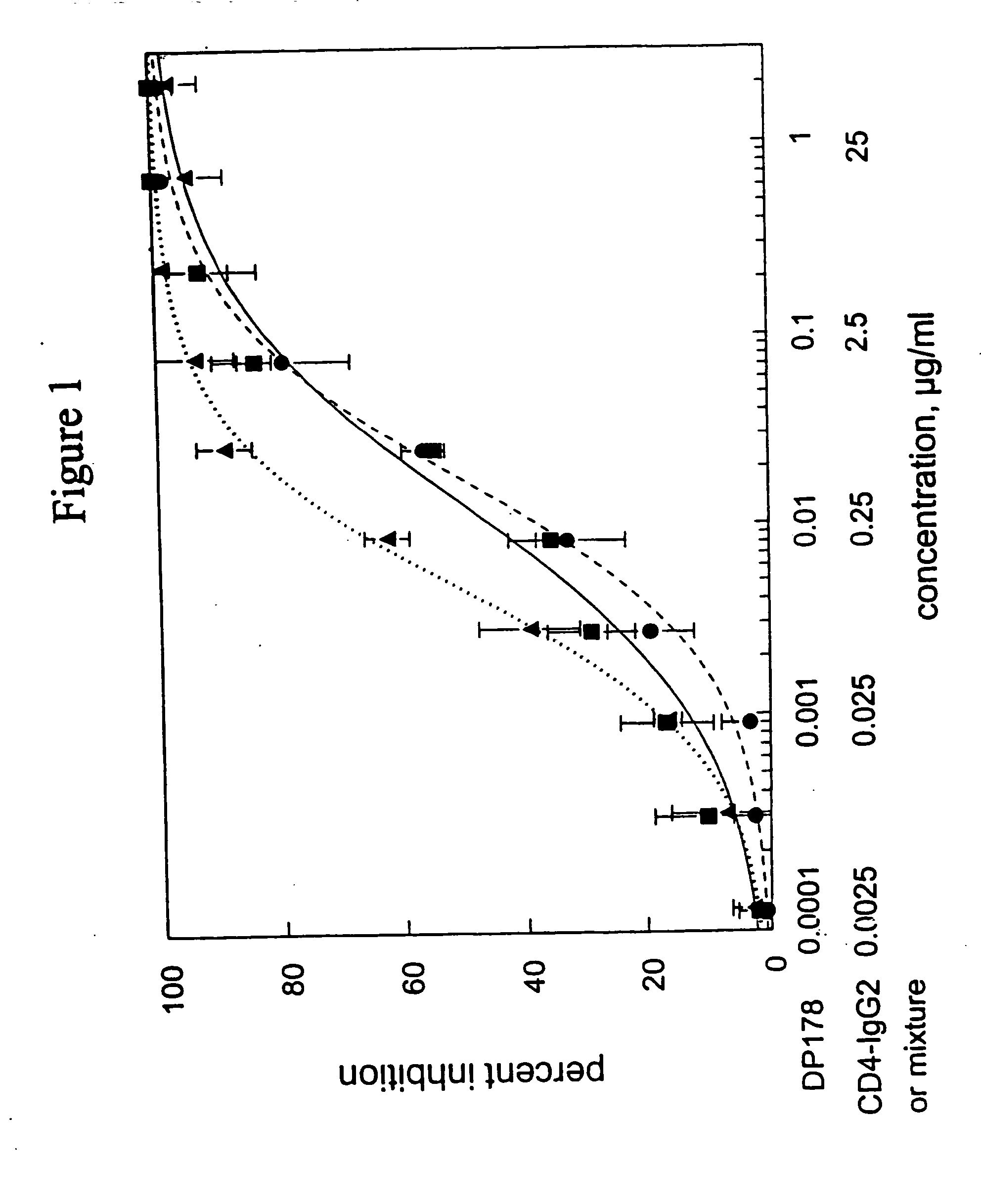
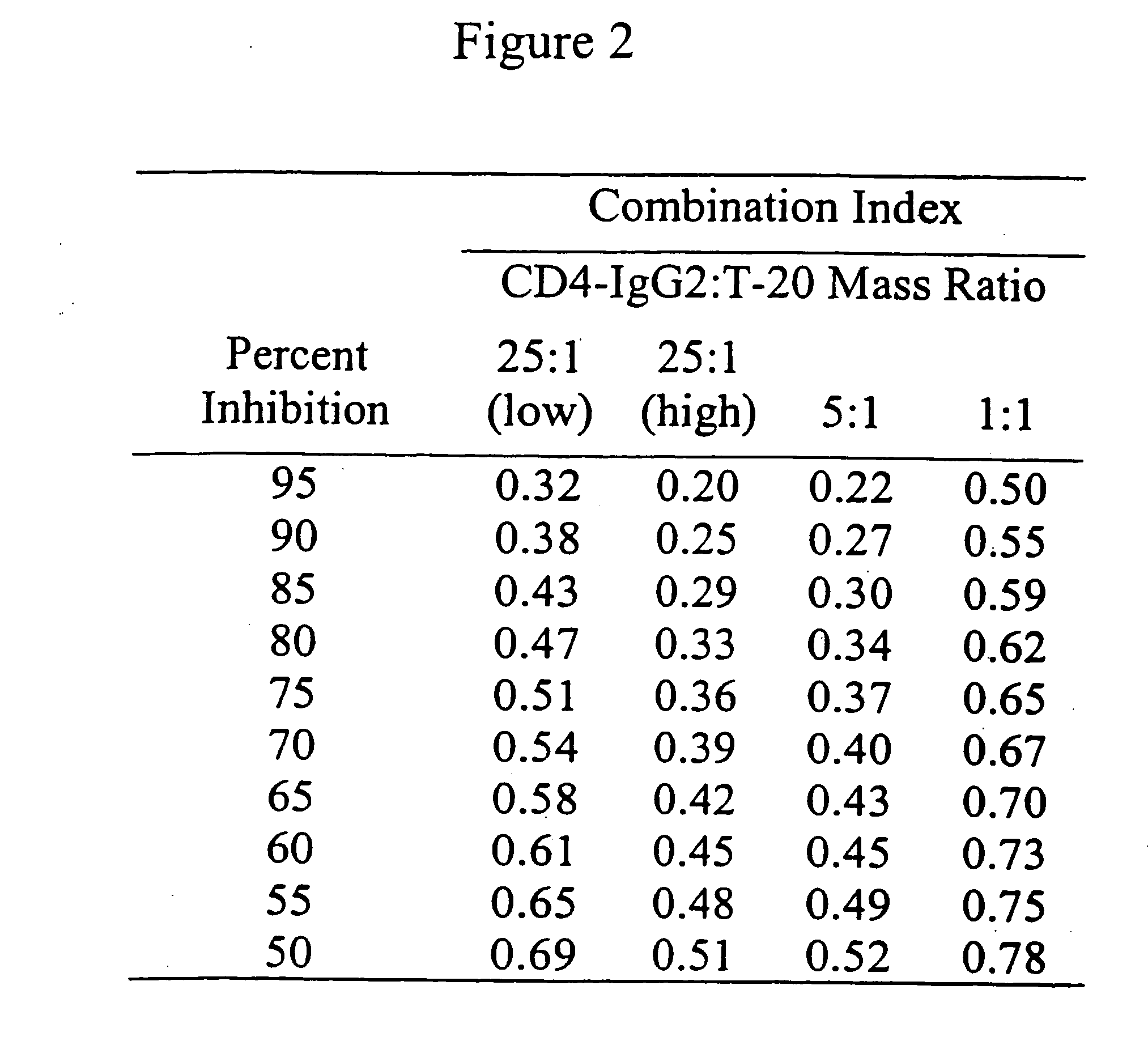
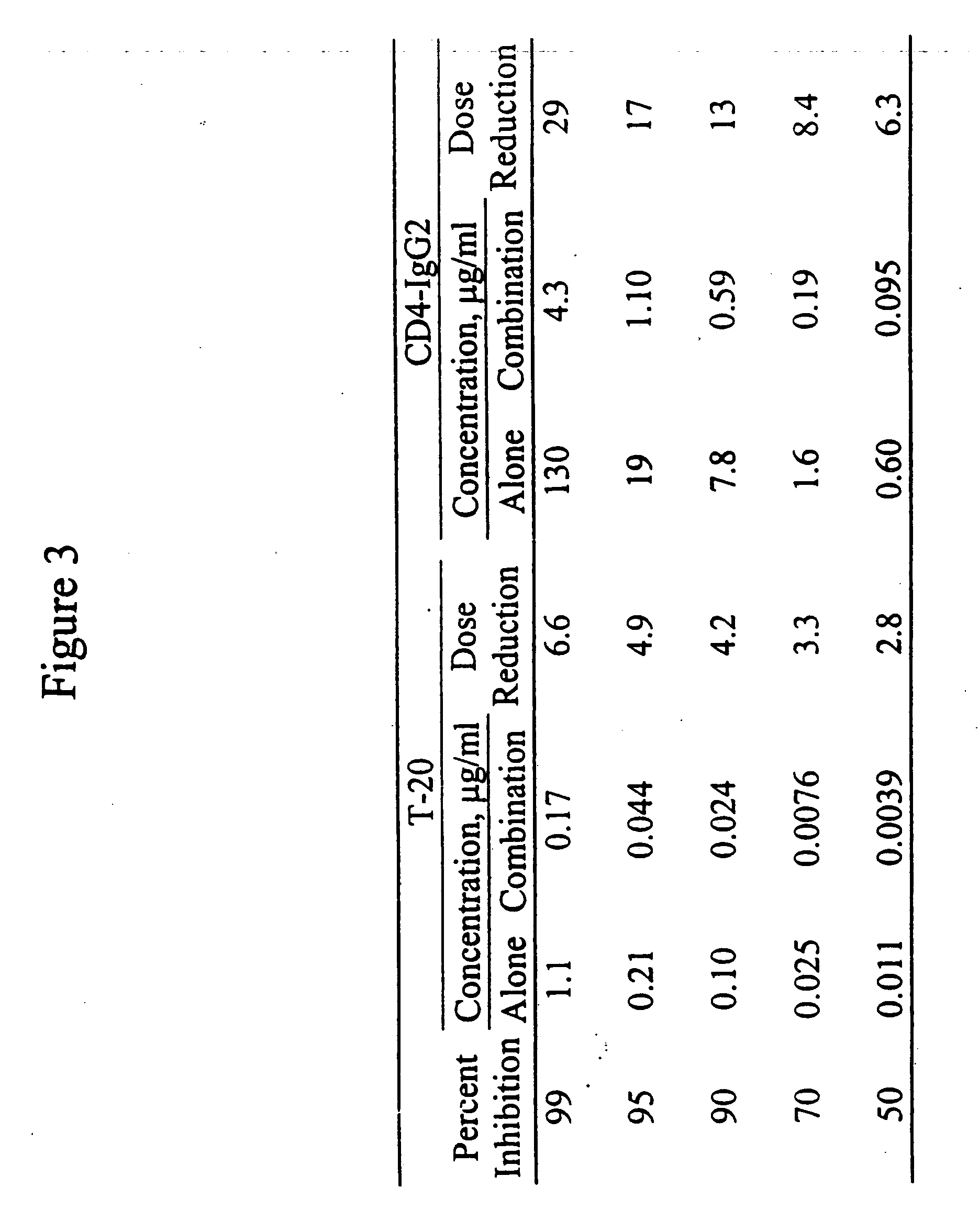
InactiveUS20070020280A1Avoid infectionPeptide/protein ingredientsVirus peptidesRelative massHIV receptor
This invention provides a composition which comprises an admixture of three compounds, wherein: (a) one compound is an antibody which binds to a CCR5 receptor; (b) one compound retards attachment of HIV-1 to a CD4+ cell by retarding binding of HIV-1 gp120 envelope glycoprotein to CD4 on the surface of the CD4+ cell; and (c) one compound retards gp41 from adopting a conformation capable of mediating fusion of HIV-1 to a CD4+ cell by binding noncovalently to an epitope on a gp41 fusion intermediate; wherein the relative mass ratio of any two of the compounds in the admixture ranges from about 100:1 to about 1:100, the composition being effective to inhibit HIV-1 infection of the CD4+ cell. This invention also provides a method of inhibiting HIV-1 infection of a CD4+ cell which comprises contacting the CD4+ cell with an amount of the composition of the subject invention effective to inhibit HIV-1 infection of the CD4+ cell so as to thereby inhibit HIV-1 infection of the CD4+ cell.
Owner:CYTODYN
Compounds capable of inhibiting HIV-1 infection
InactiveUS20070025983A1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsHiv 1 envelopeCD4 antigen
This invention provides an antibody capable of specifically inhibiting the fusion of an HIV-1 envelope glycoprotein+ cell with an appropriate CD4+ cell without cross reacting with the HIV-1 envelope glycoprotein or CD4 and capable of inhibiting infection by one or more strains of HIV-1. This antibody is then used to identify a molecule which is important for HIV infection. Different uses of the antibody and the molecule are described.
Owner:CYTODYN
Monoclonal antibodies specific to hemagglutinin and neuraminidase from influenza virus h5-subtype or n1-subtype and uses thereof
ActiveUS20130004497A1High sensitivityStrong specificitySsRNA viruses negative-senseMicrobiological testing/measurementHemagglutininNeuraminidase
Monoclonal antibodies and related binding proteins that bind specifically to the envelope glycoprotein of H5 subtypes or neuraminidase glycoprotein of N1 subtypes of avian influenza virus (AIV) are provided. The monoclonal antibodies and related binding proteins are useful for the detection of H5 and N1 subtypes of AIV, including H5N1 subtypes and provide means for the diagnosis, surveillance and treatment of dangerous viral infections.
Owner:TEMASEK LIFE SCIENCES LABORATORY
The DNA vaccine of an anti-infection of hepatitis C virus
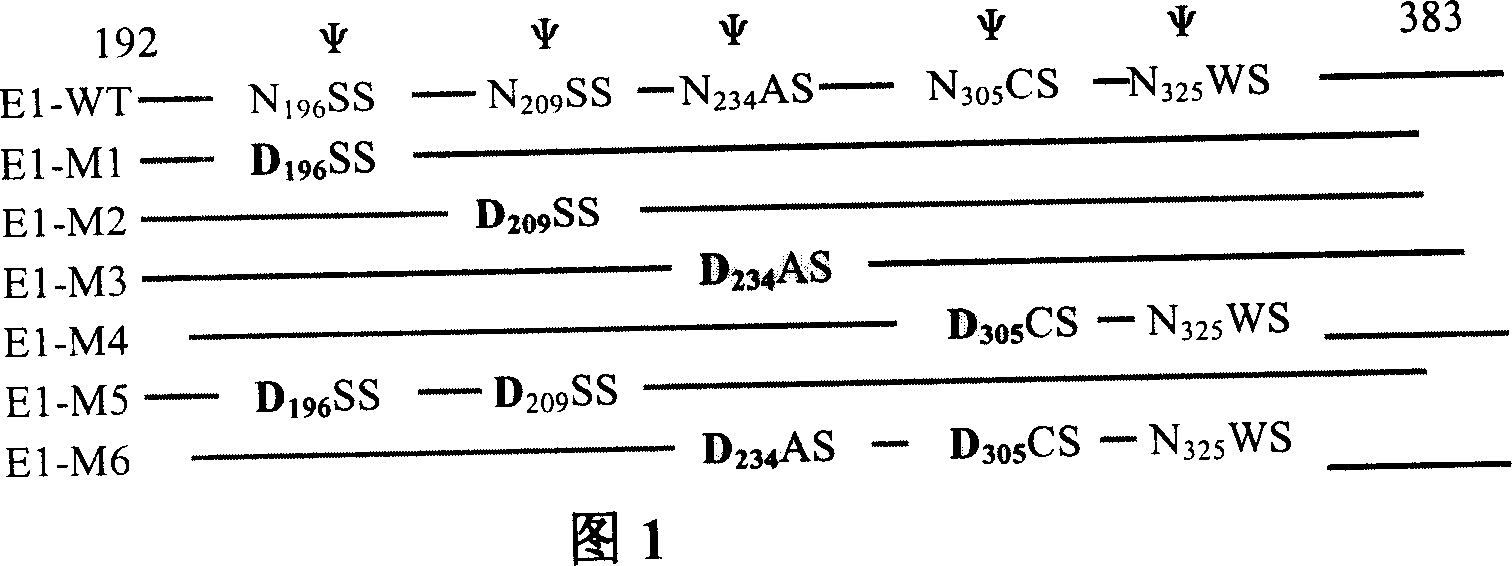
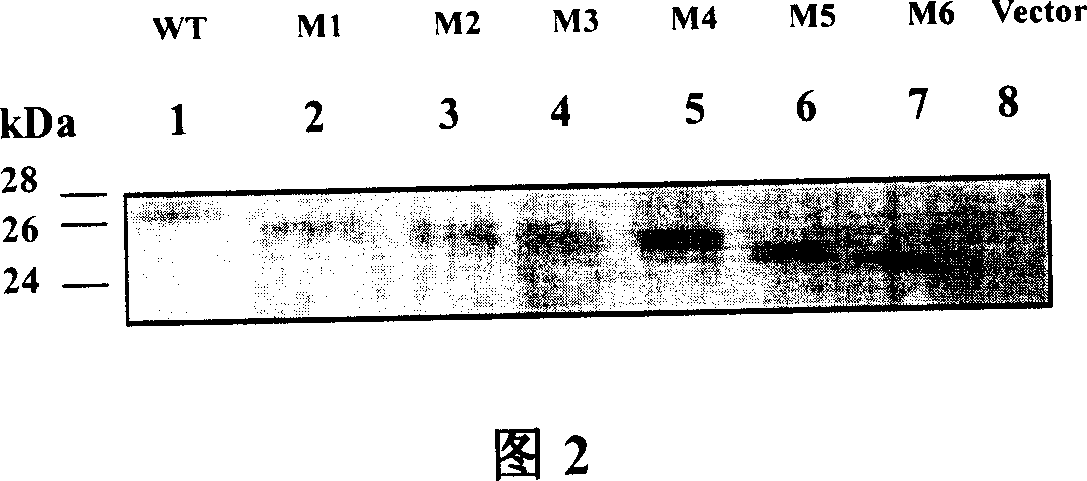
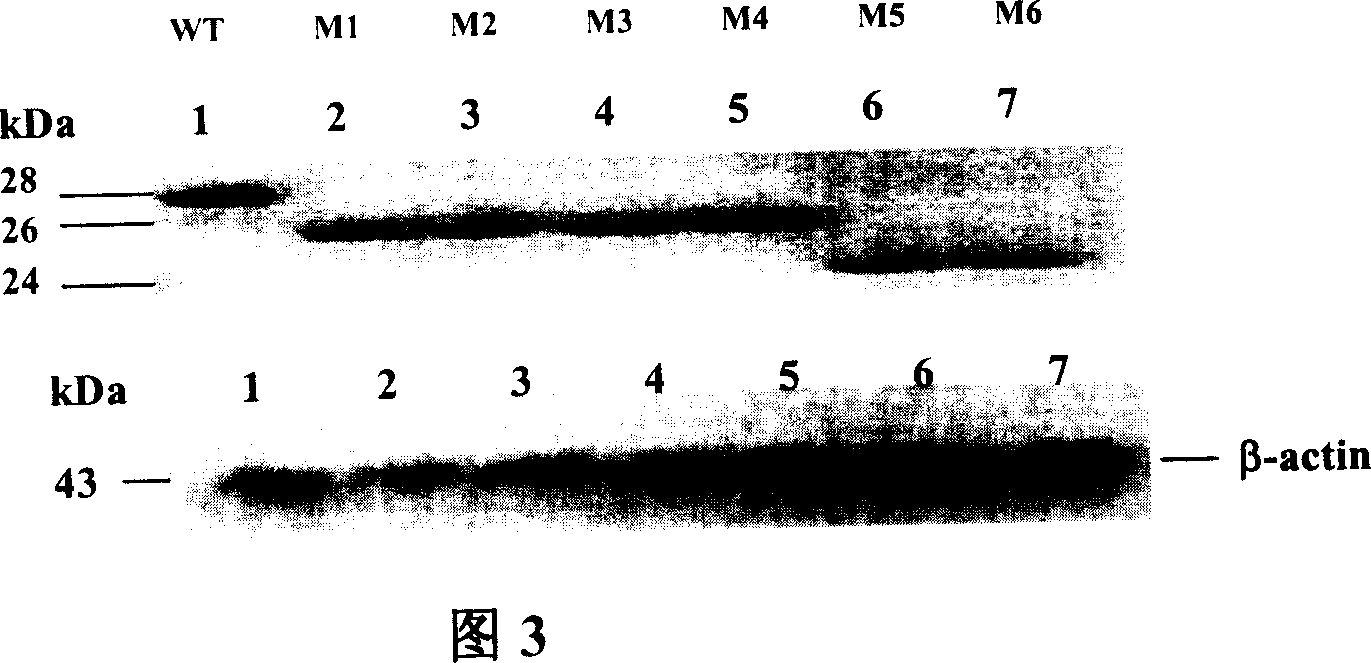
InactiveCN1943789AEasy to makeGood effectGenetic material ingredientsDigestive systemImmune recognitionMutant
The invention relates to preparation and application of DNA vaccine that can resist to the infection of hepatitis C virus. The invention constructed a recombinant DNA vaccine that can express HCV enveloped glycoprotein E1 with molecular cloning techniques, on this basis constructed six N-glycosylation mutants M1-M6 of the E1 and select N-glycosylation mutant M2 as DNA vaccine that resist to infection of hepatitis C virus and application in the preparation of DNA vaccine that prevent the HCV virus infection. The advantages of the invention are : experiments reveal that N-sugar chain of HCV E1 enveloped glycoprotein can limit the host's cellular immune responses, its glycosylation may shield the T and B cell epitope of virus, thereby enable the virus to evade immune recognition; with E1 glycosylate mutants M2 may be used as DNA vaccines that are HCV therapeutic and preventive and enhance the immunogenicity, besides preparation of mutants M2 E1 glycosylate is simple, convenient and effective, and has good application prospect.
Owner:WUHAN UNIV
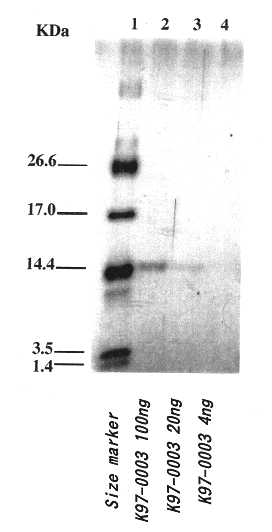
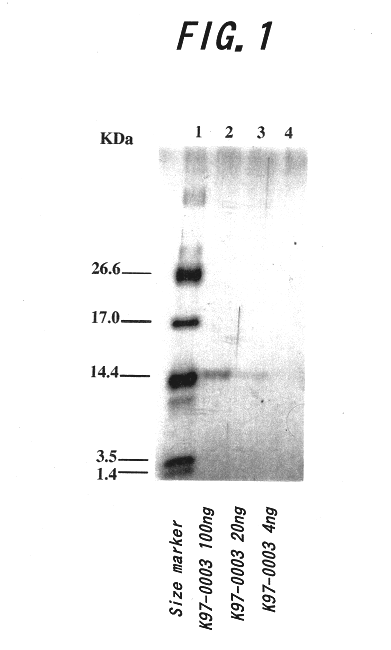
Polypeptide having human HIV inhibitory activity, a gene encoding the polypeptide, a method to produce the polypeptide
InactiveUS6482412B1Bacterial antigen ingredientsPeptide/protein ingredientsViral ReceptorHuman immunodeficiency
A novel compound, which is effective for treatment of AIDS and has inhibitory activity on human immunodeficiency viruses (HIV), was examined. The K97-0003 peptide, which has anti-HIV activity caused by inhibition of syncytium formation by fusion of envelope glycoprotein of HIV and the host cells expressing the receptor to said virus, was provided by the present invention. Furthermore, the base sequence of the gene coding for said polypeptide, and the method for preparing said polypeptide using strain K97-0003 were provided.
Owner:KIIM PHARM LAB +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com