Methods for producing and using in vivo pseudotyped retroviruses using envelope glycoproteins from lymphocytic choriomeningitis virus (LCMV)
a technology of lymphocytic choriomeningitis virus and envelope glycoprotein, which is applied in the direction of viruses, peptides, vectors, etc., can solve the problem that the application of viral vectors in vivo is often limited
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
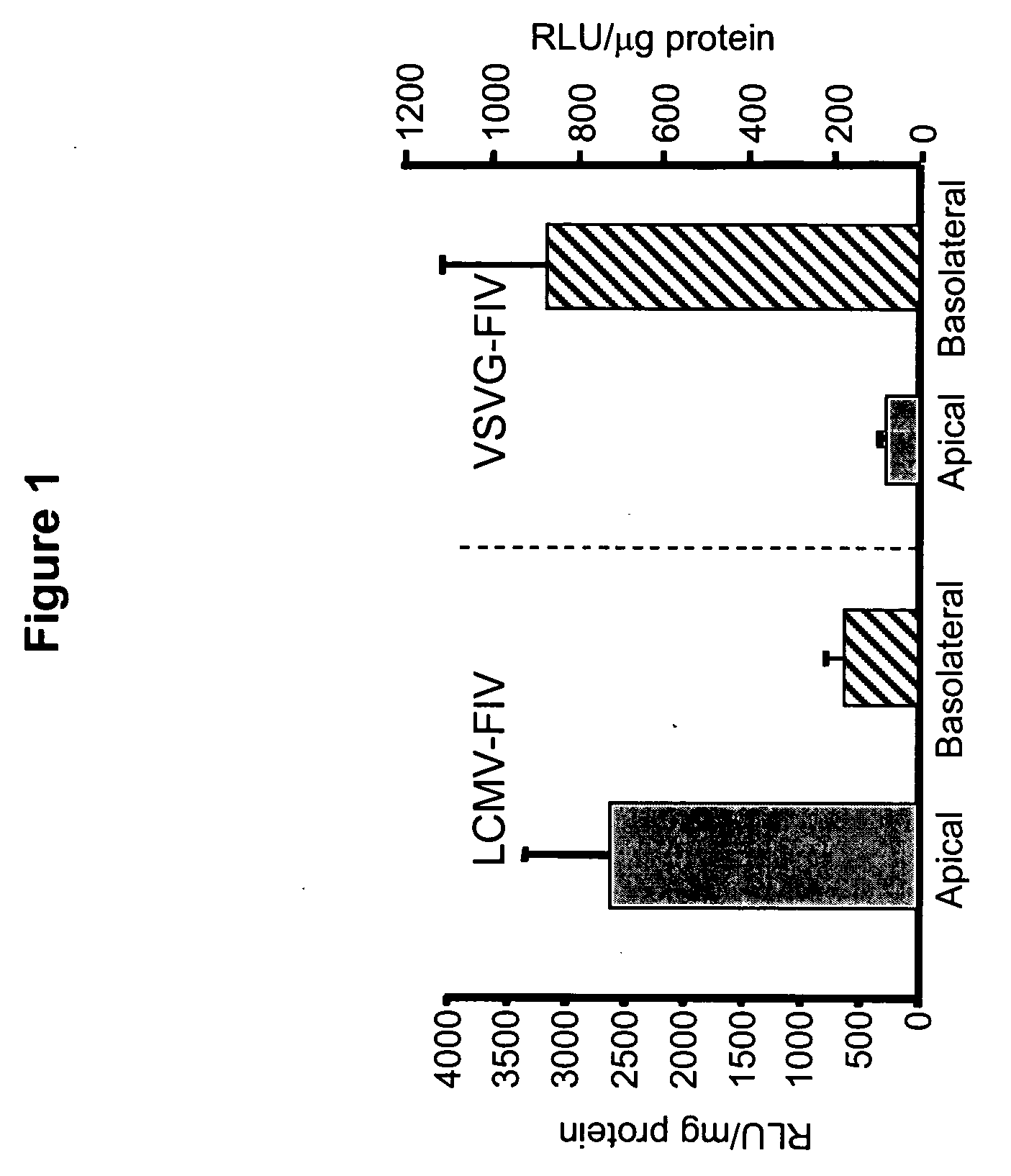
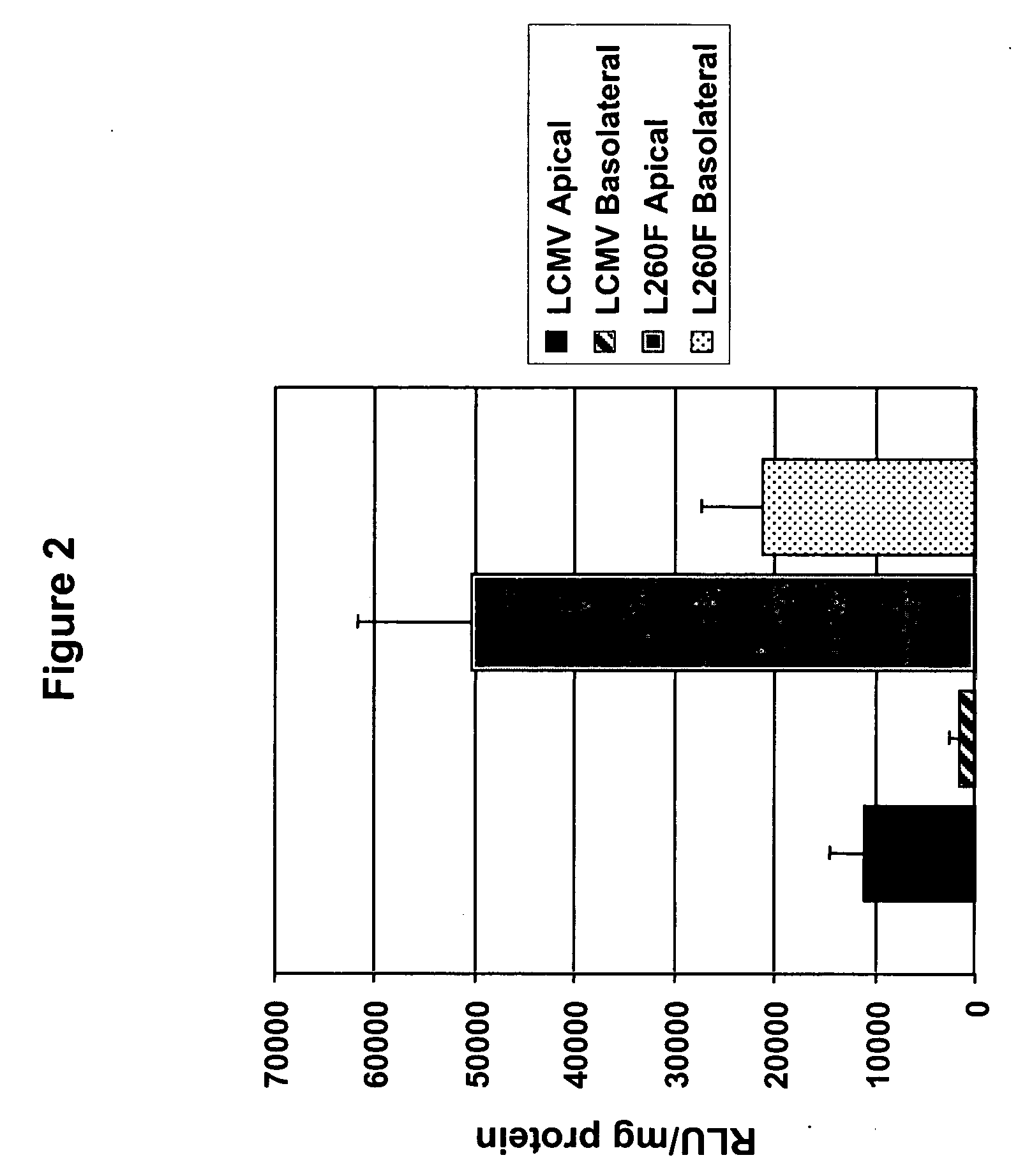
LCMV Pseudotyped FIV Targets the Apical Surface of Airway Epithelia for Entry
[0188] The inventors evaluated the ability of LCMV glycoproteins (GPs) to pseudotype the FIV vector. LCMV-GP is initially expressed as a precursor polypeptide, GP-C, which is post-translationally processed by a cellular protease into GP1 and GP2. GP1 meditates binding to the cellular receptor for LCMV, and GP2 contains the fusion peptide and the transmembrane region [Buchmeier et al., supra]. Recently Beyer and colleagues reported successful pseudotyping of HIV-based lentivirus vectors with glycoproteins from the LCMV WE54 strain [Beyer et al., J. Virol. 76:1488-1495 (2002)]. In contrast to amphotropic-MLV vector particles, LCMV pseudotypes could be efficiently concentrated by ultracentrifugation without loss of vector titer. The inventors obtained the LCMV strain WE54 GP from Dr. Beyer and evaluated its efficiency in pseudotyping FIV. Titers for the FIV pseudotyped with LCMV WE54 were ˜5×108 TU / ml, very s...
example 2
Receptors for LCMV
[0190] Alpha-dystroglycan (alpha-DG) was identified as a high affinity receptor for several arenaviruses including some strains of LCMV, Lassa virus, Oliveros virus, and Latino virus [Cao et al., Science, 282:2079-2081 (1998)]. Dystroglycan is a dystrophin-associated glycoprotein that connects the cytoskeleton with the extracellular matrix and is widely expressed in most tissues, including the lung [Henry and Campbell, Cell, 95:859-870 (1998); White et al., Am. J. Respir. Cell. Mol. Biol. 24:179-86 (2001)]. Dystroglycan is post-translationally cleaved into a highly glycosylated peripheral membrane protein alpha-DG, which is noncovalently associated with the membrane-spanning protein beta-dystroglycan [Henry and Campbell, Curr. Opin. Cell Biol., 11:602-607 (1999)]. Beta-dystroglycan is linked to the cytoskeleton. It is important to note that studies with wildtype LCMV WE54 strain demonstrate high affinity binding to alpha-DG (Spiropoulou et al., supra). However, so...
example 3
Gene Transfer to Mouse Liver Using FIV Pseudotyped with LCMV Envelope
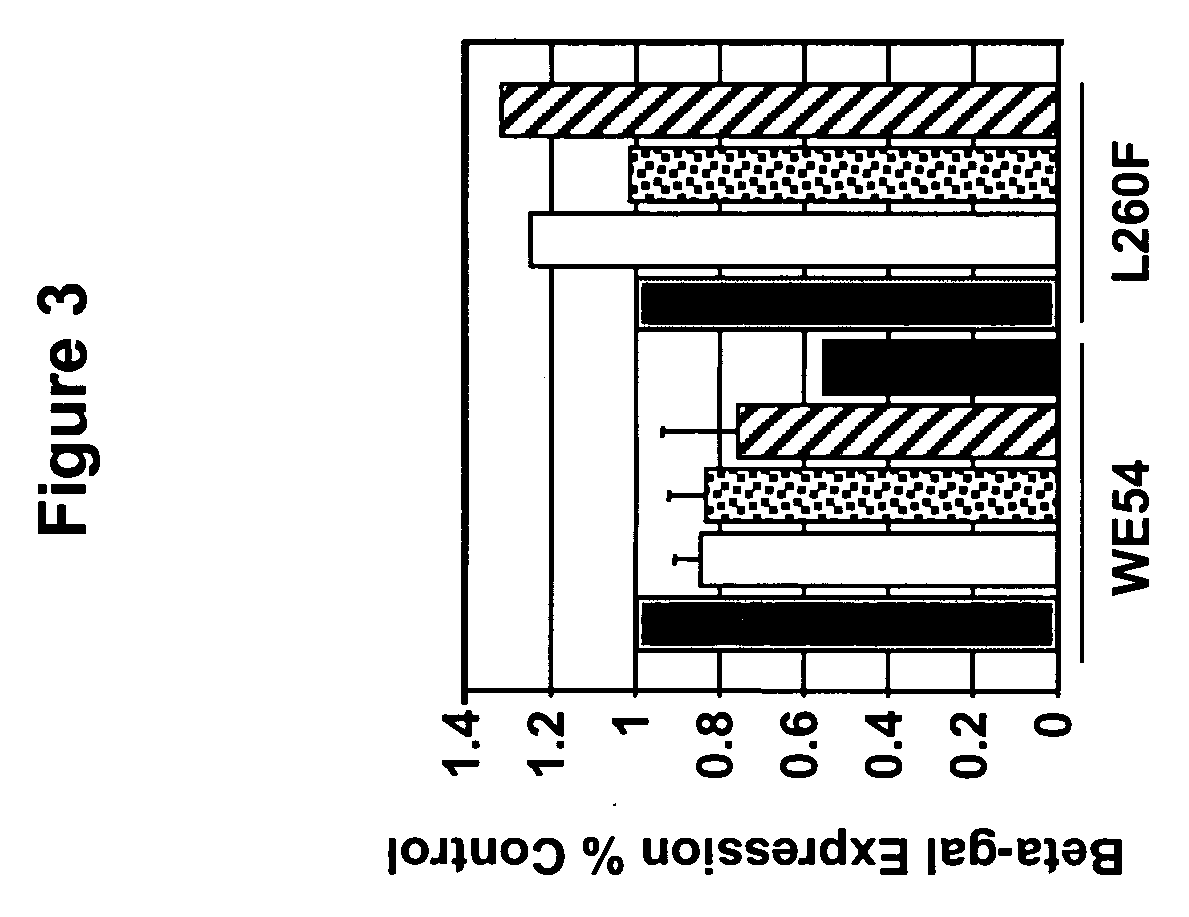
[0192] Gene transfer to the liver has potential clinical applications for many diseases. The inventors evaluated the liver transduction properties of the FIV pseudotyped with LCMV-L260F. For systemic vector delivery to the liver, C57BL / 6 mice received the LCMV pseudotyped FIV vector intravenously via the tail vein using methods as previously described [Stein et al., Mol. Ther., 3:850-856 (2001); Kang et al., J. Virol., 76:9378-9388 (2002)]. Three weeks later the animals were killed and the liver tissues examined for beta-galactosidase expression. Widespread expression was observed throughout the liver samples. This tropism for liver is useful for the production of secreted proteins or treatment of disorders primarily involving liver parenchyma, such as the mucopolysaccharidoses.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com