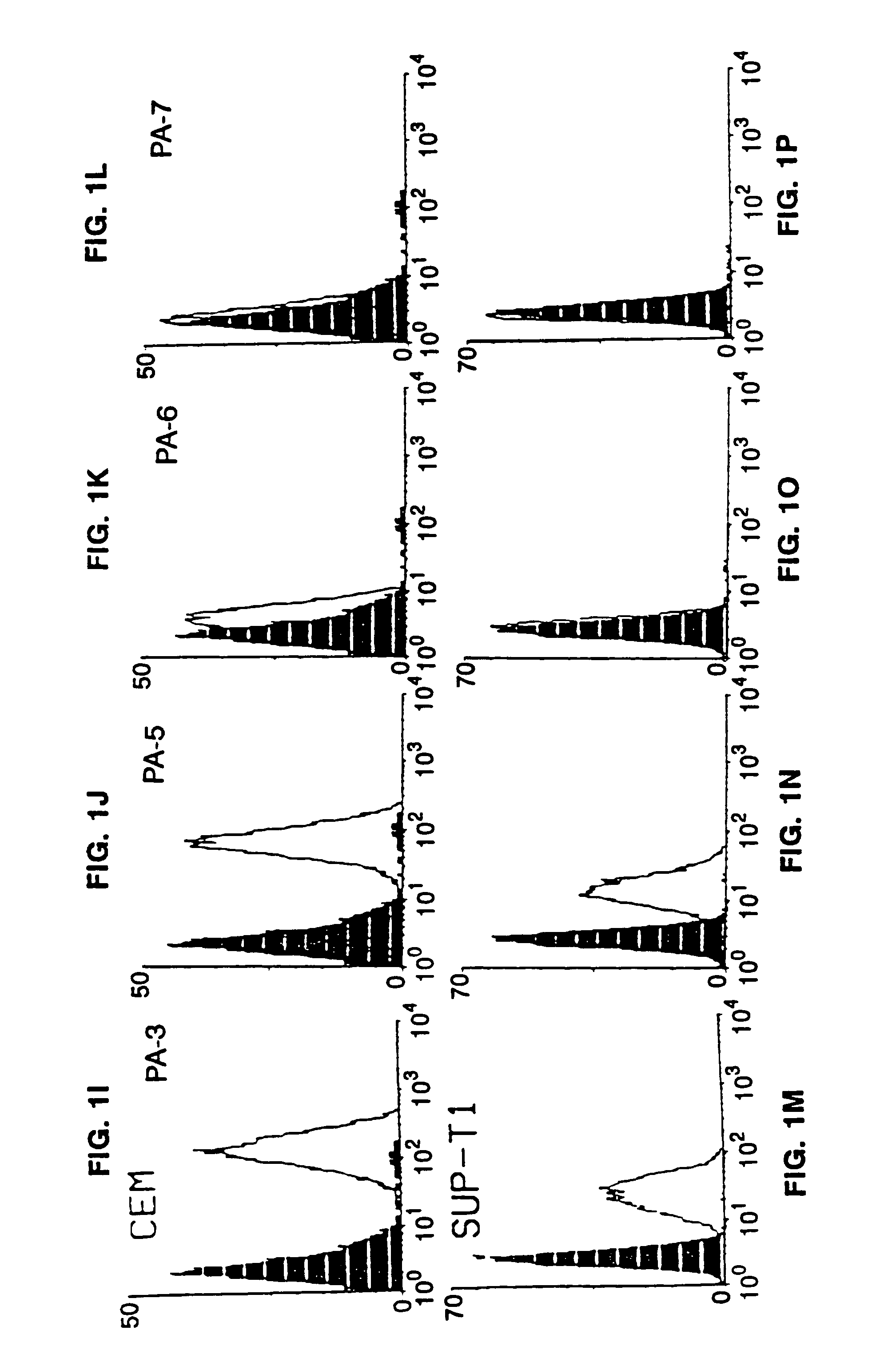
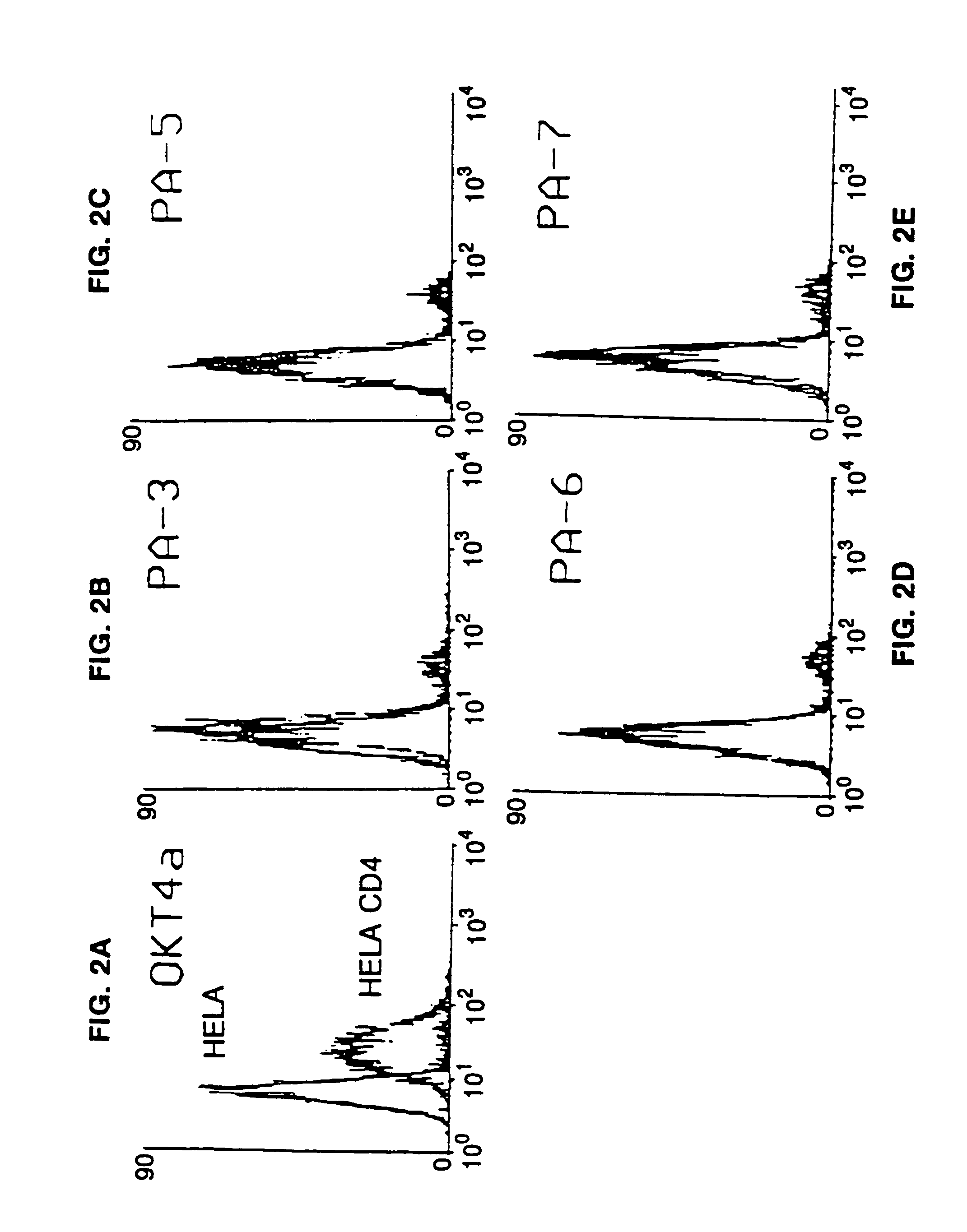
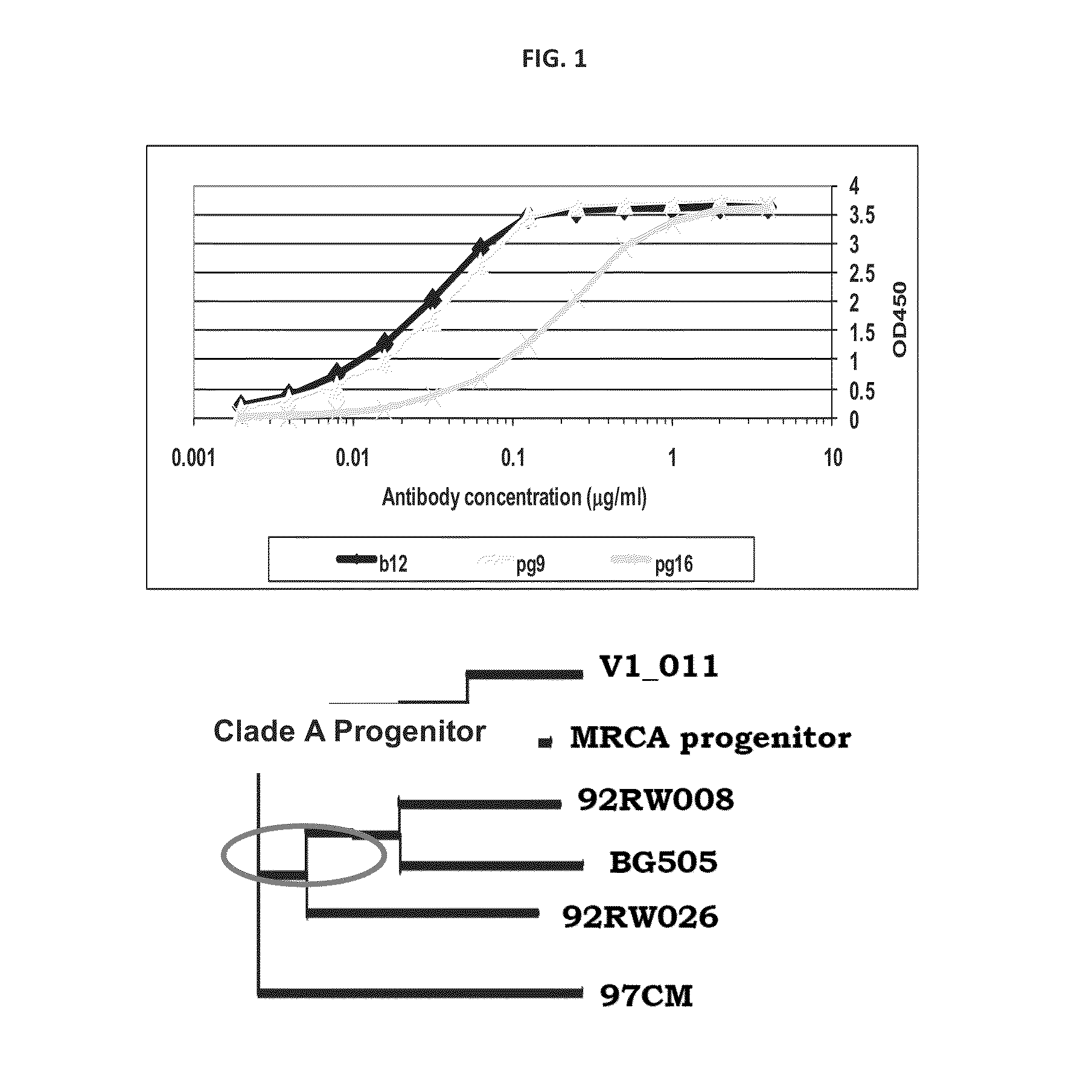
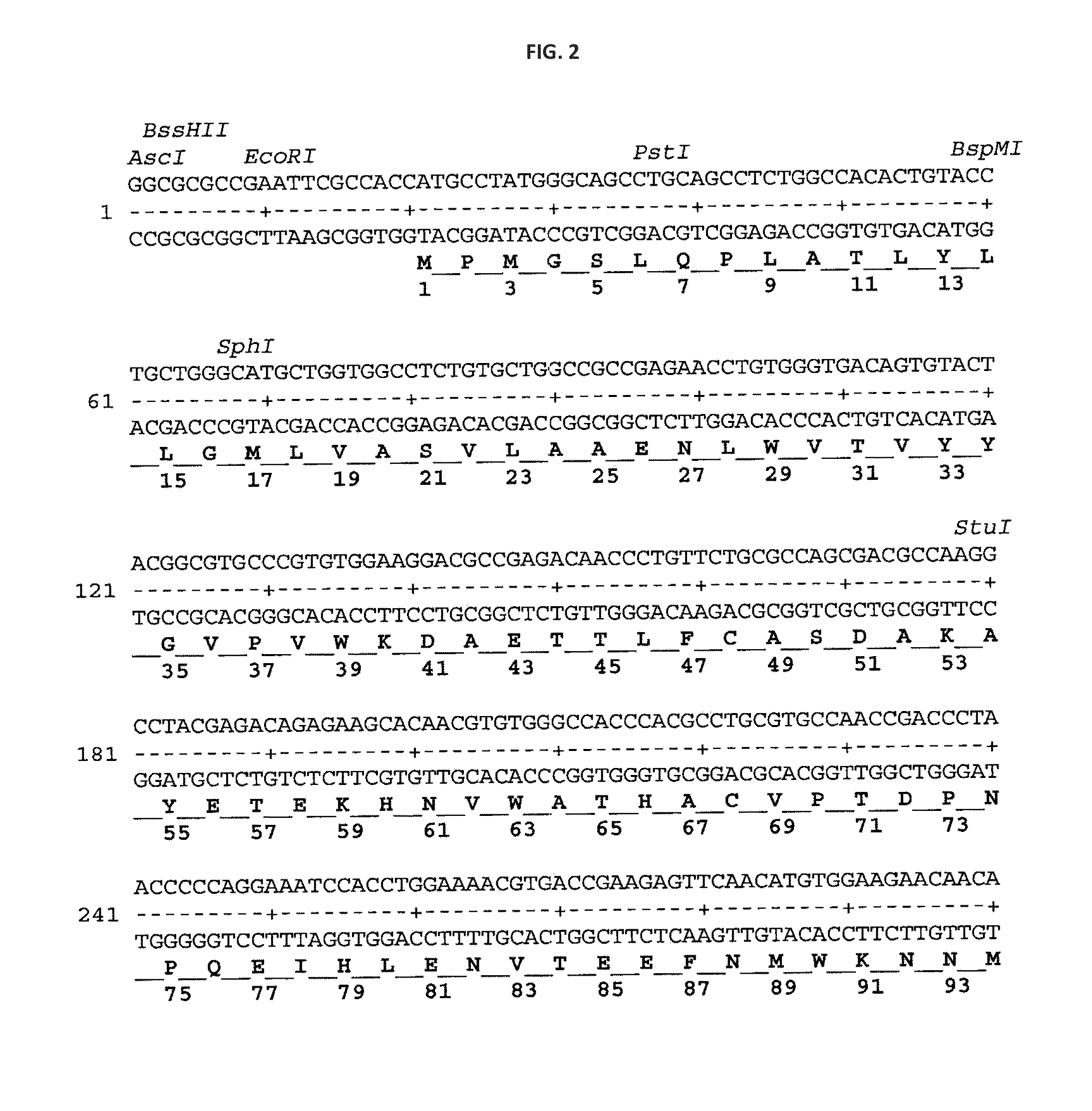
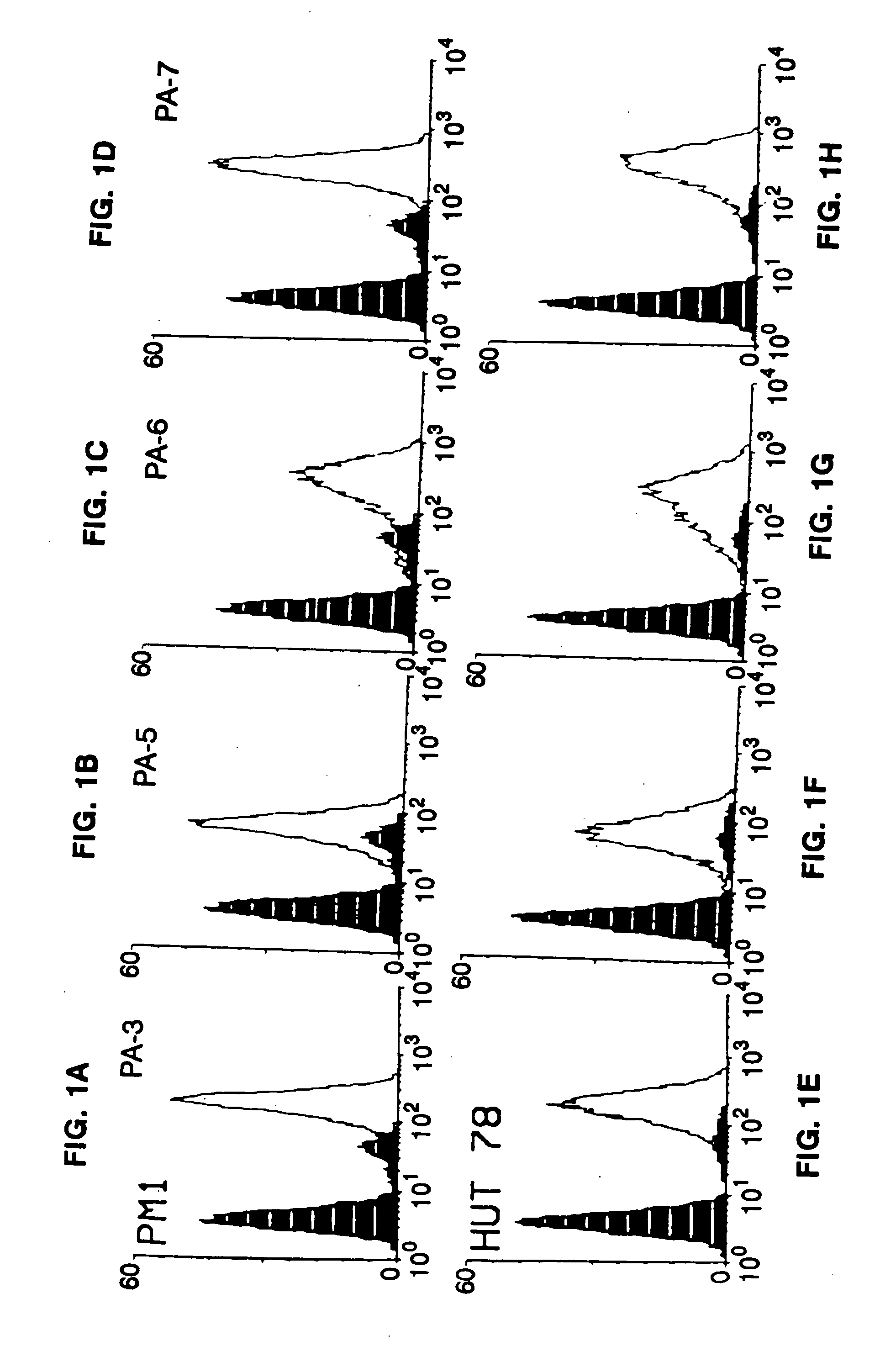
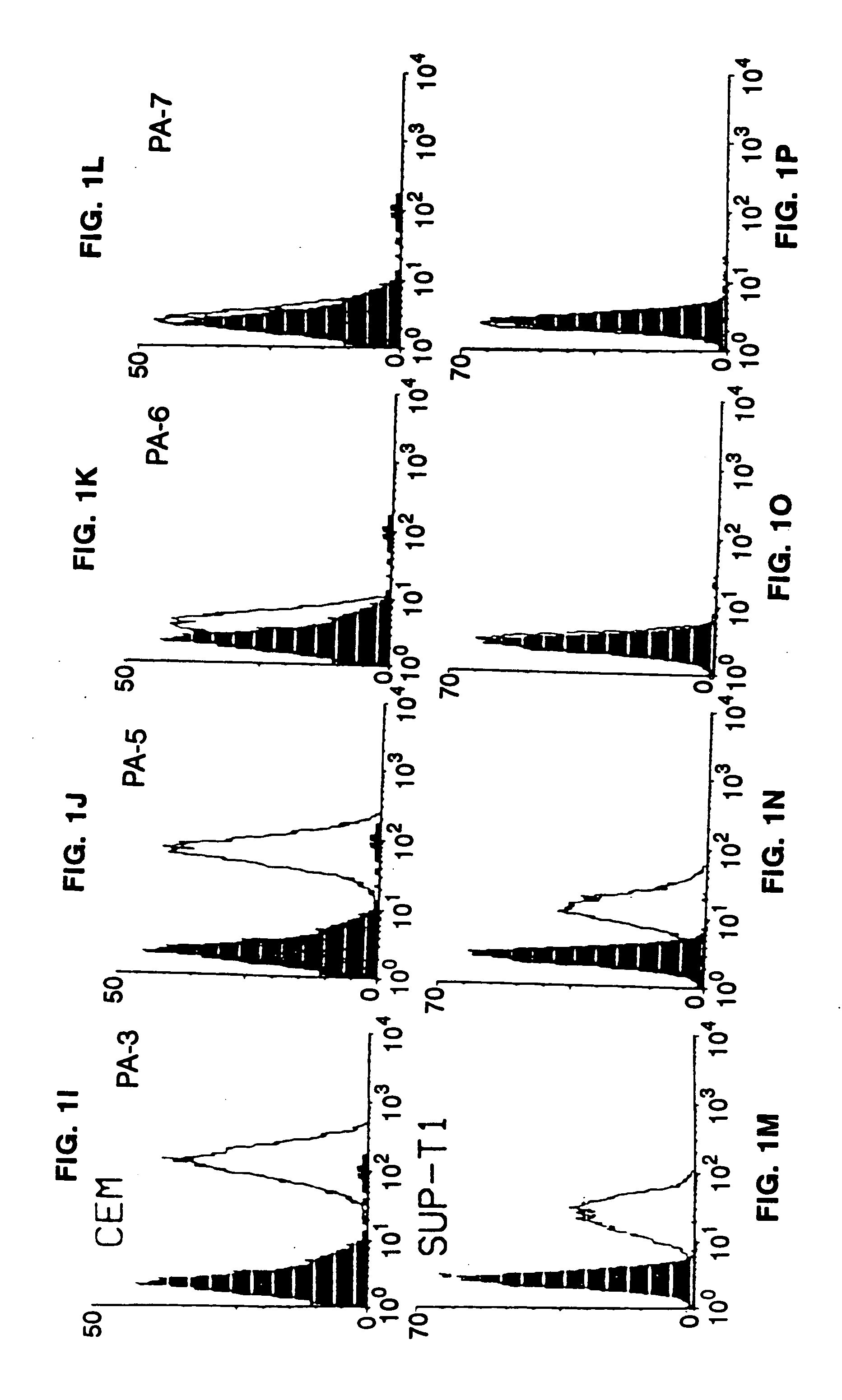
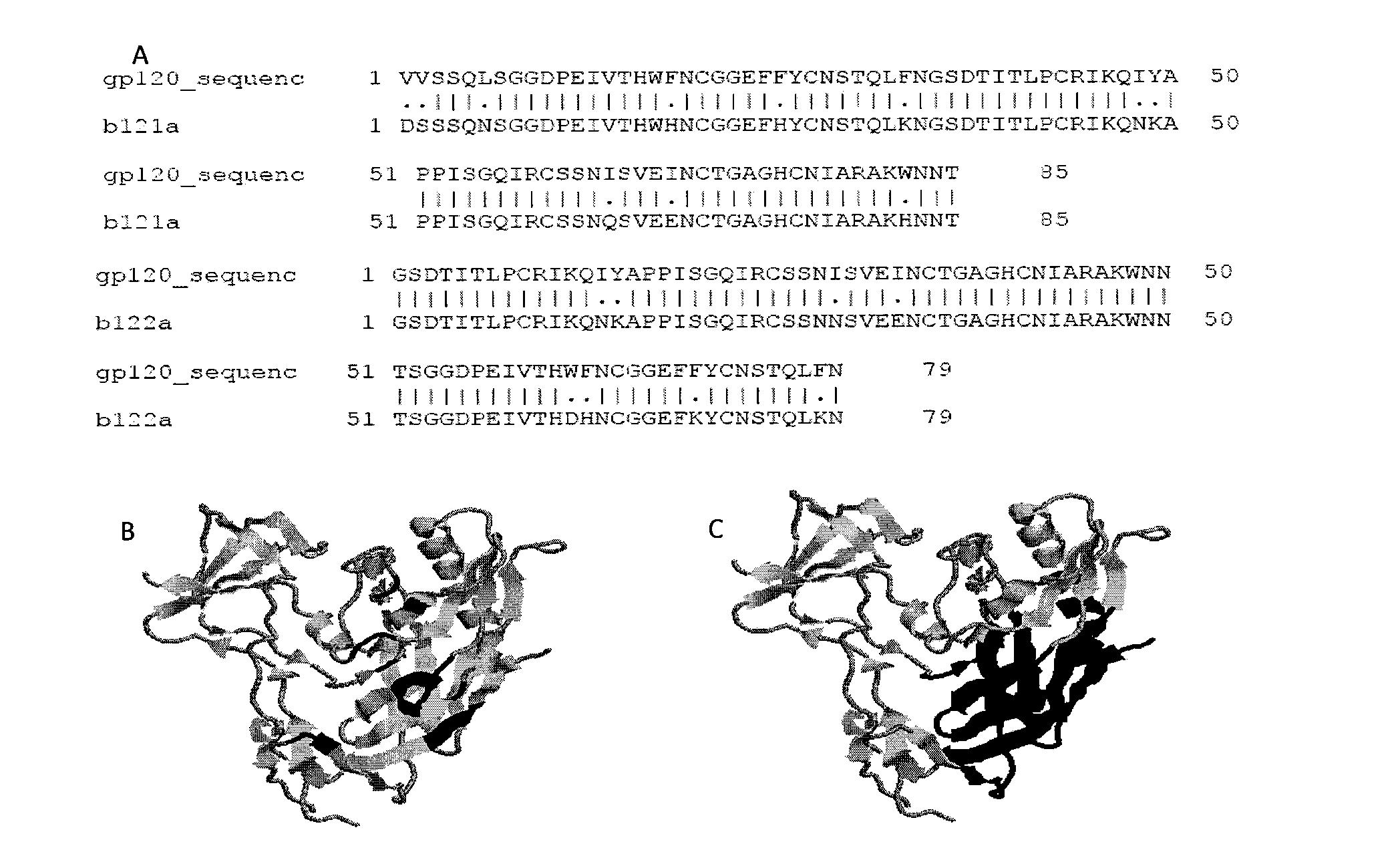
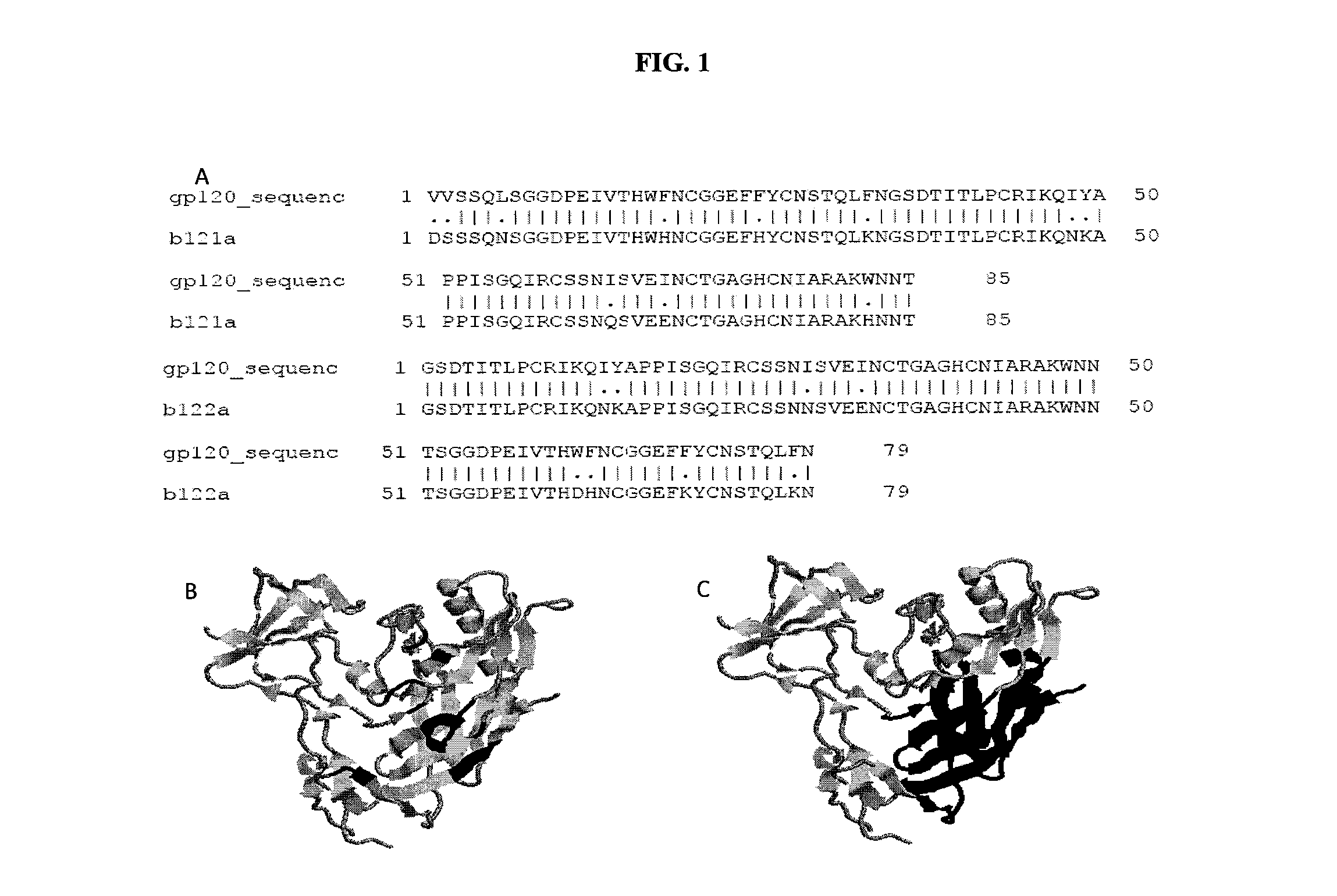
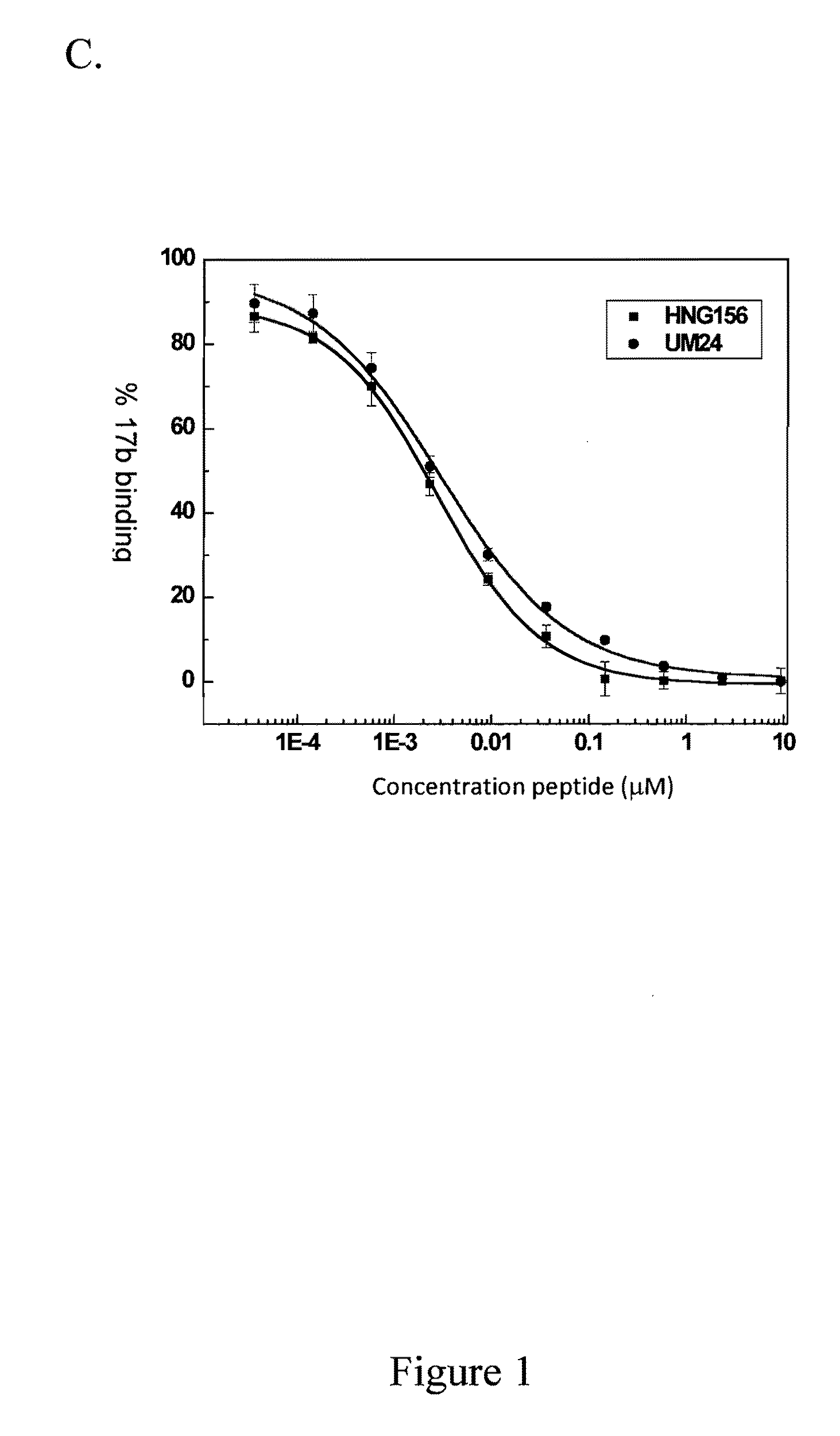
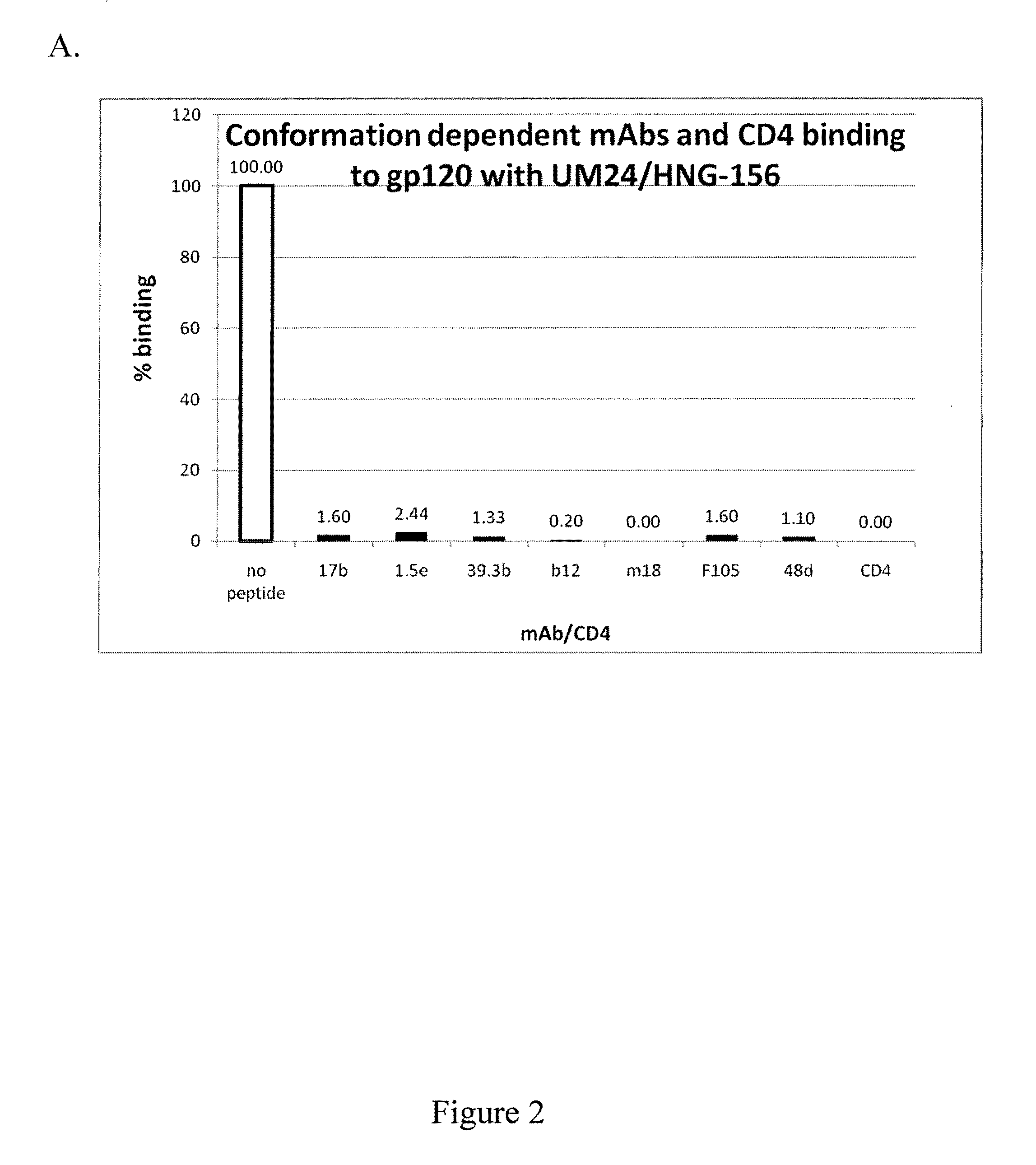
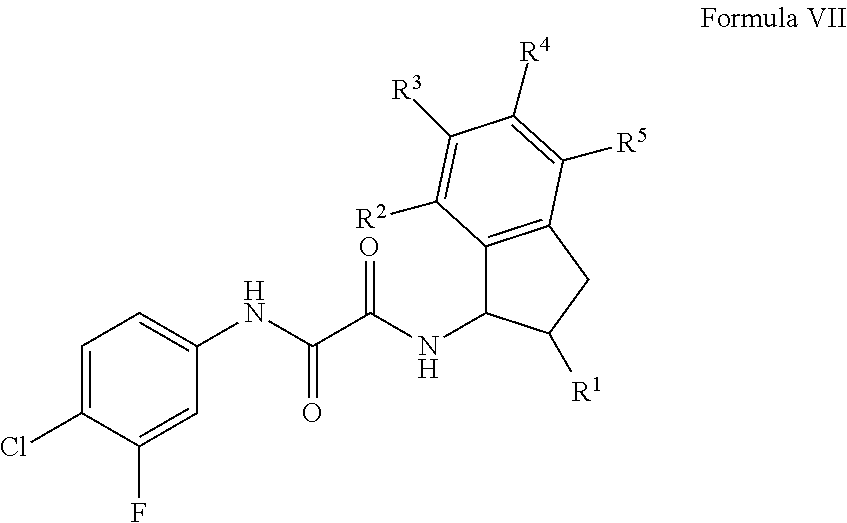
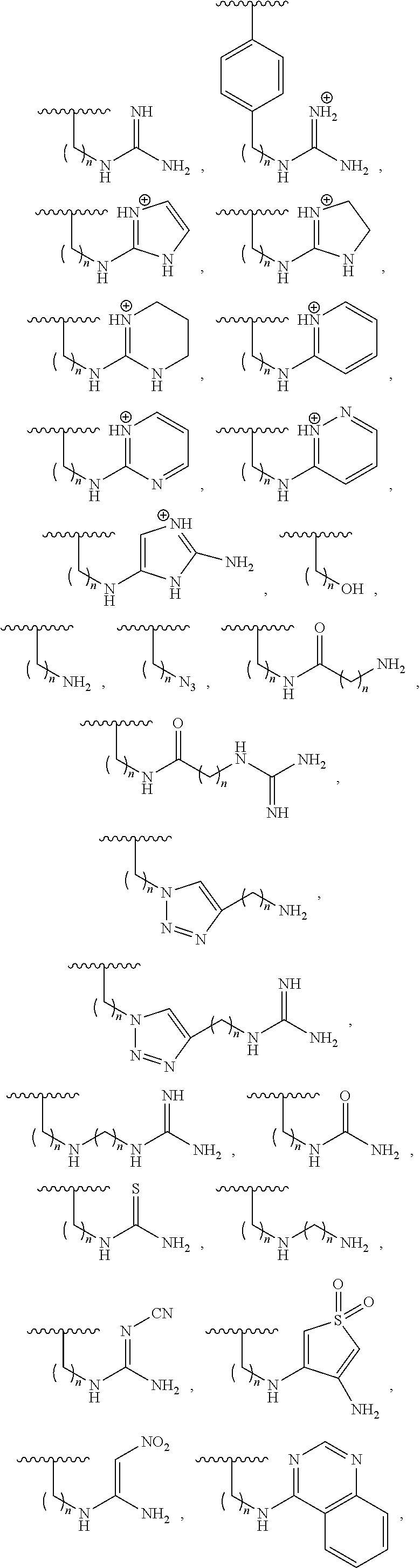
Patents
Literature
57 results about "Hiv 1 envelope" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
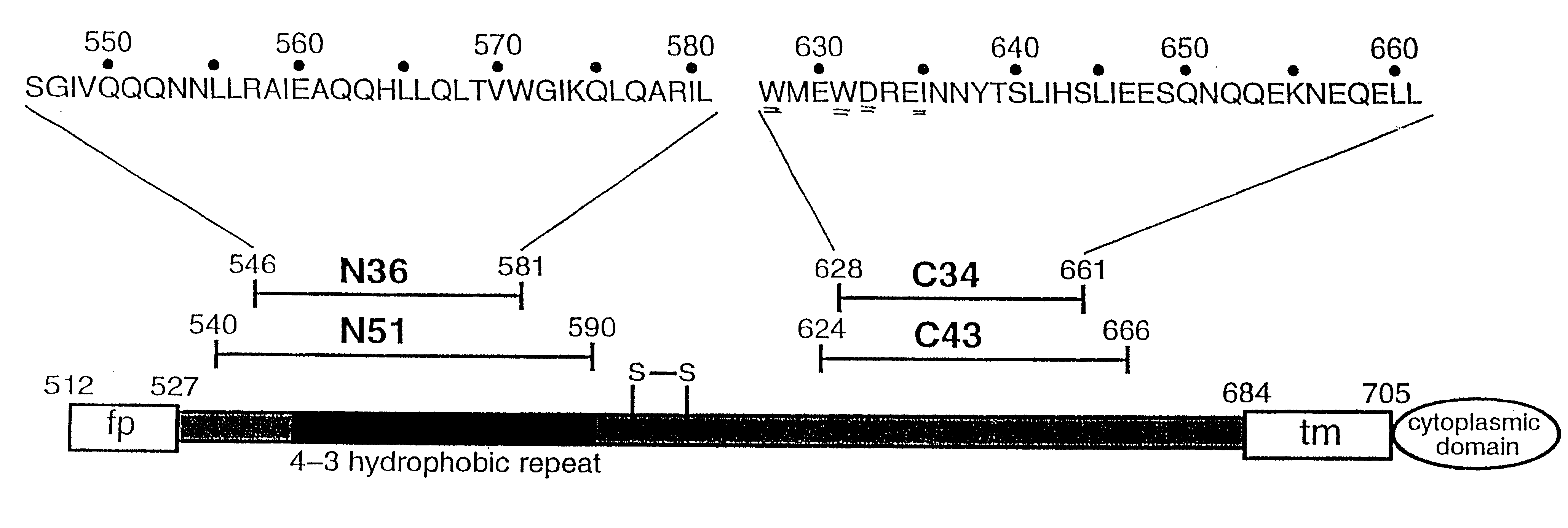
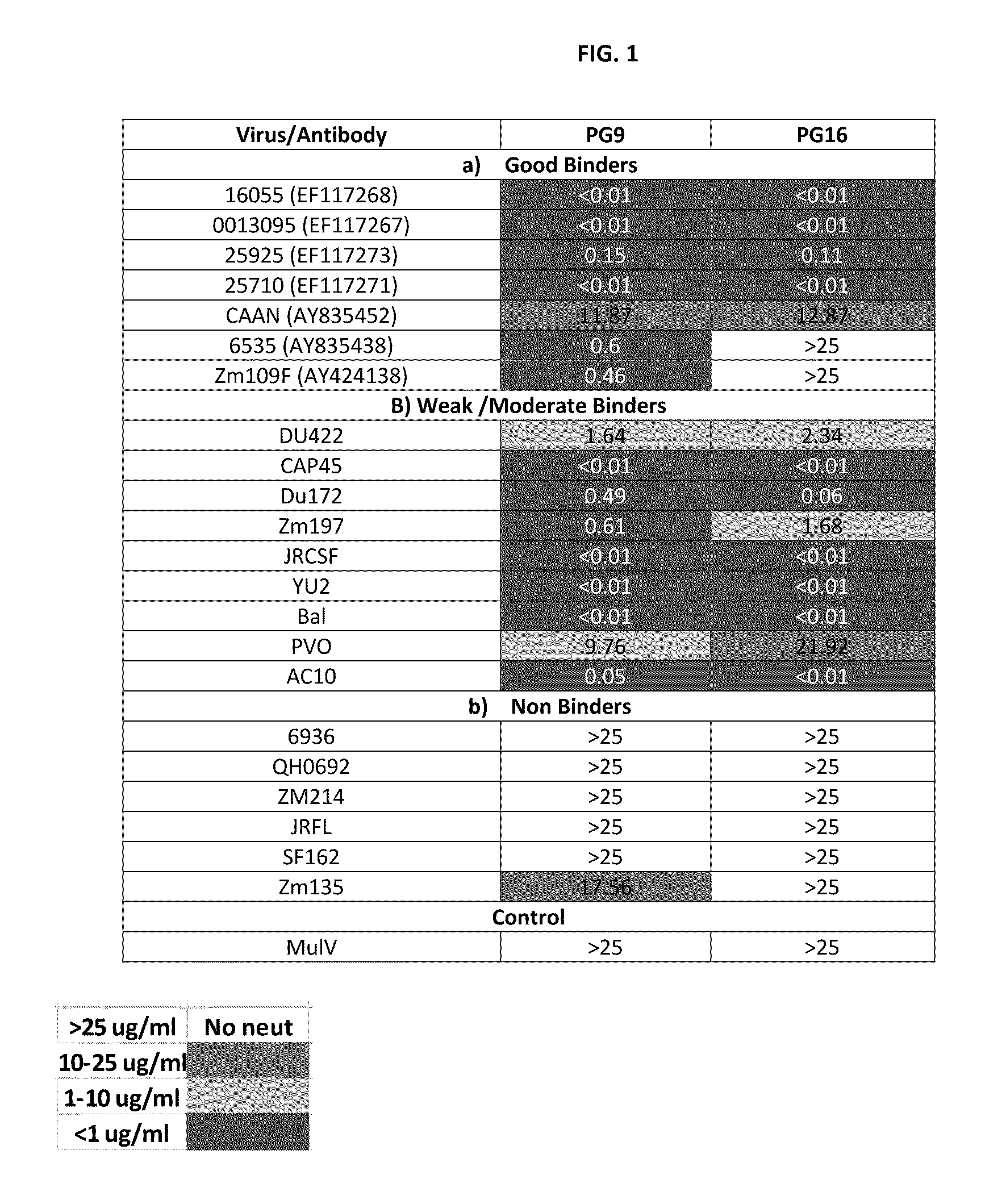
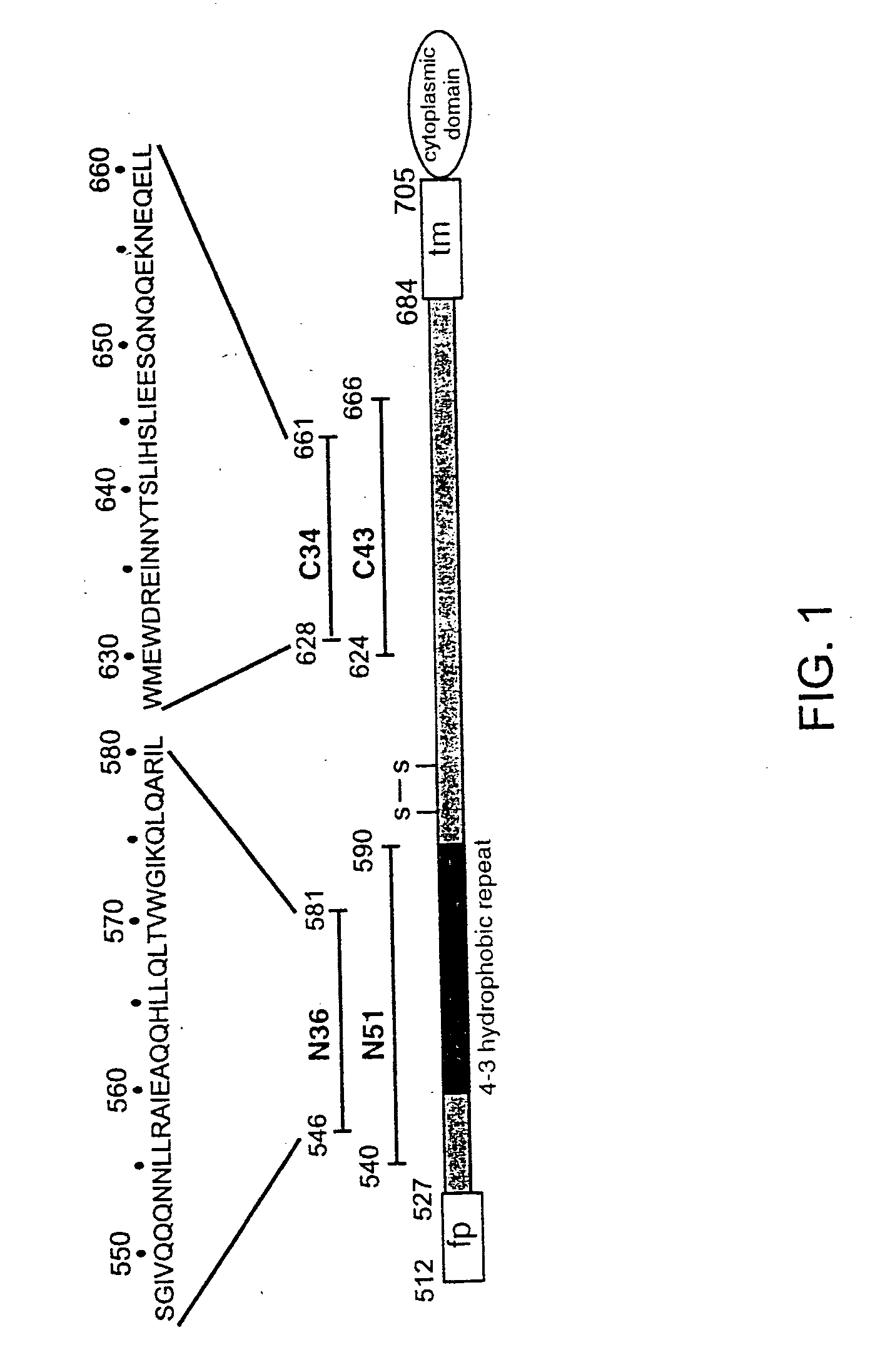
HIV-1 is a non-glycosylated, 233 amino acid polypeptide chain, having a molecular mass of 27275.88 dalton and pI=9.68. HIV-1 envelope protein spans the C-Terminus of gp120 and most of gp41.
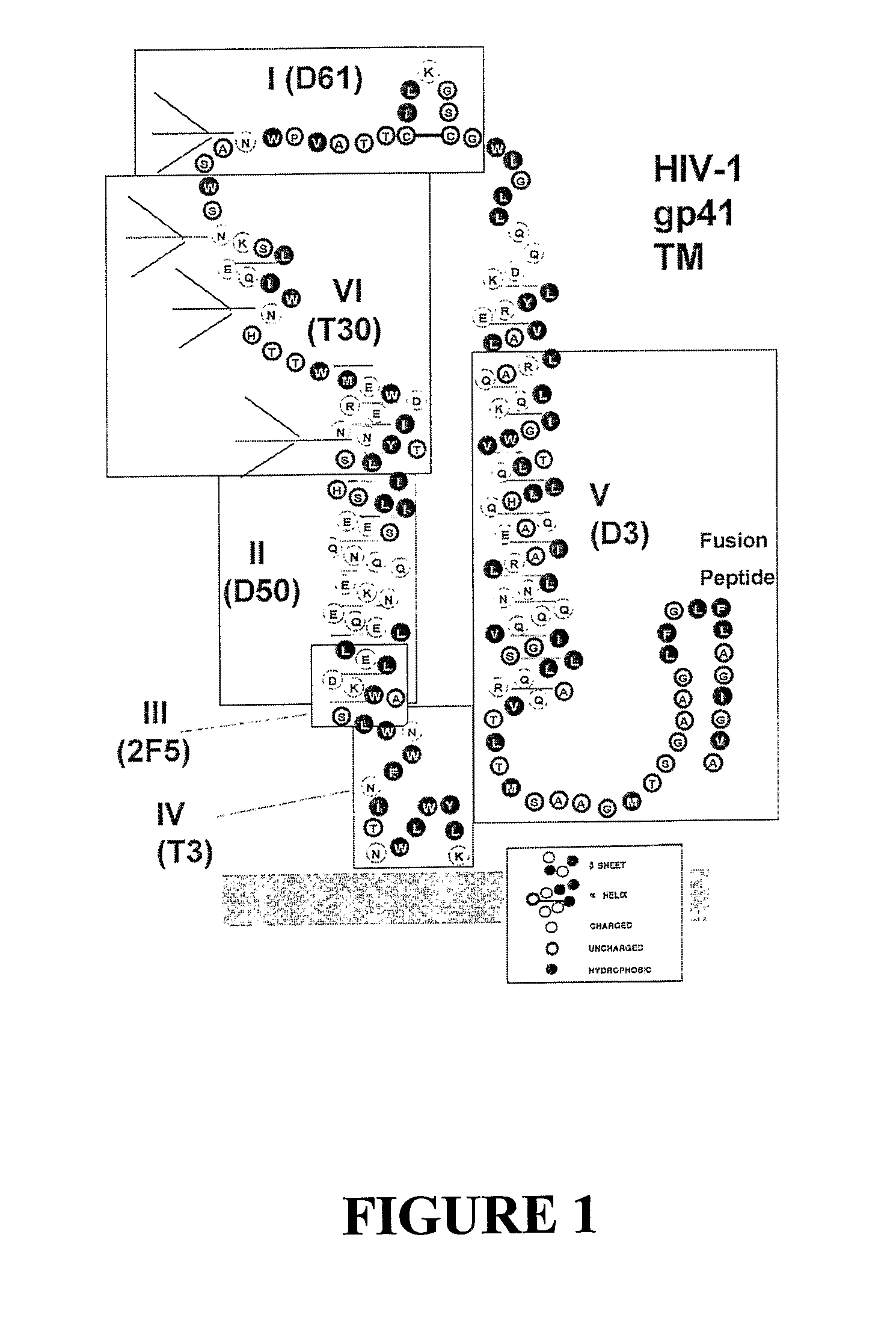
Core structure of gp41 from the HIV envelope glycoprotein
InactiveUS6506554B1Increase infectivityHigh activityPeptide/protein ingredientsMicrobiological testing/measurementHiv envelopeDesigning drug
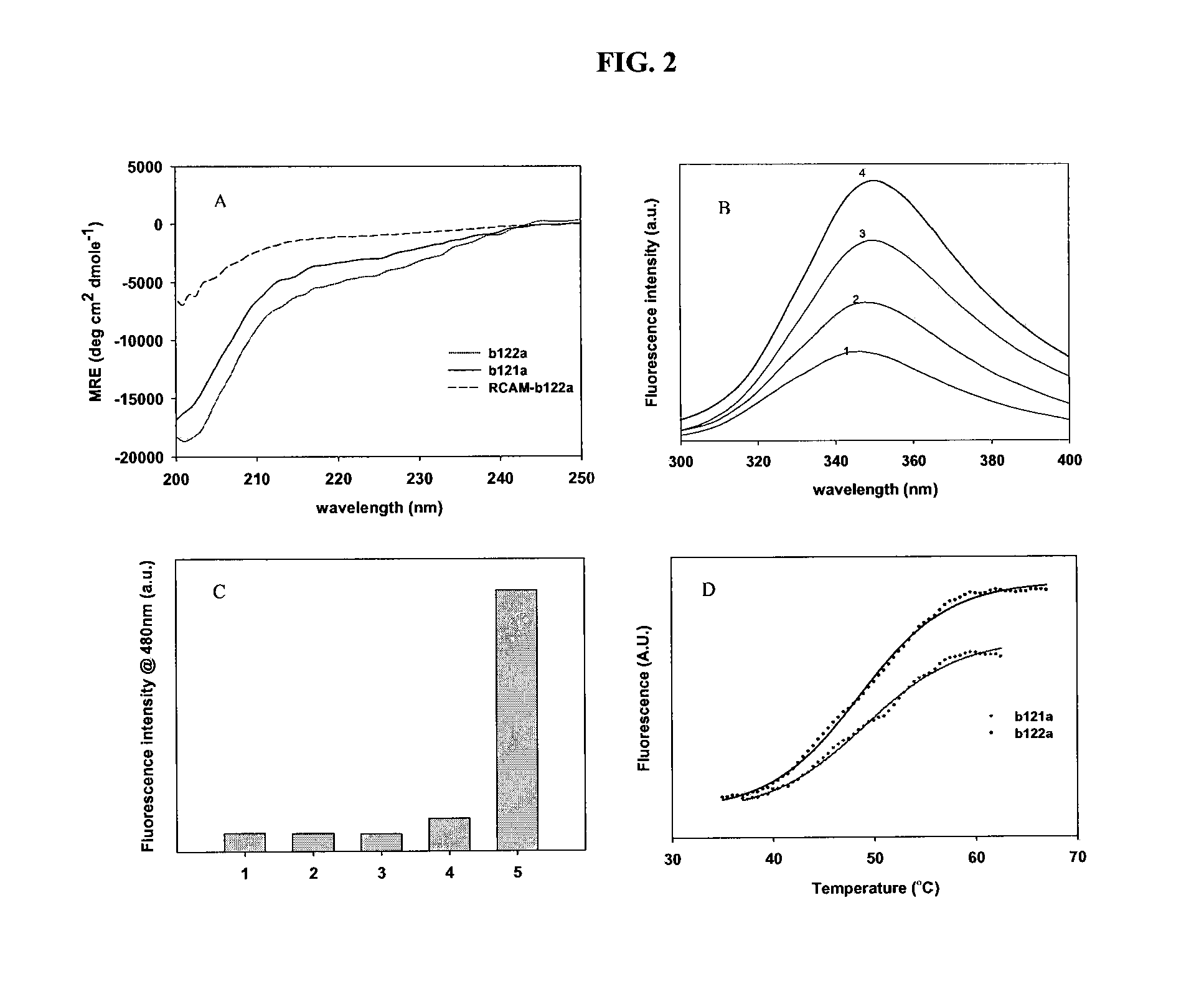
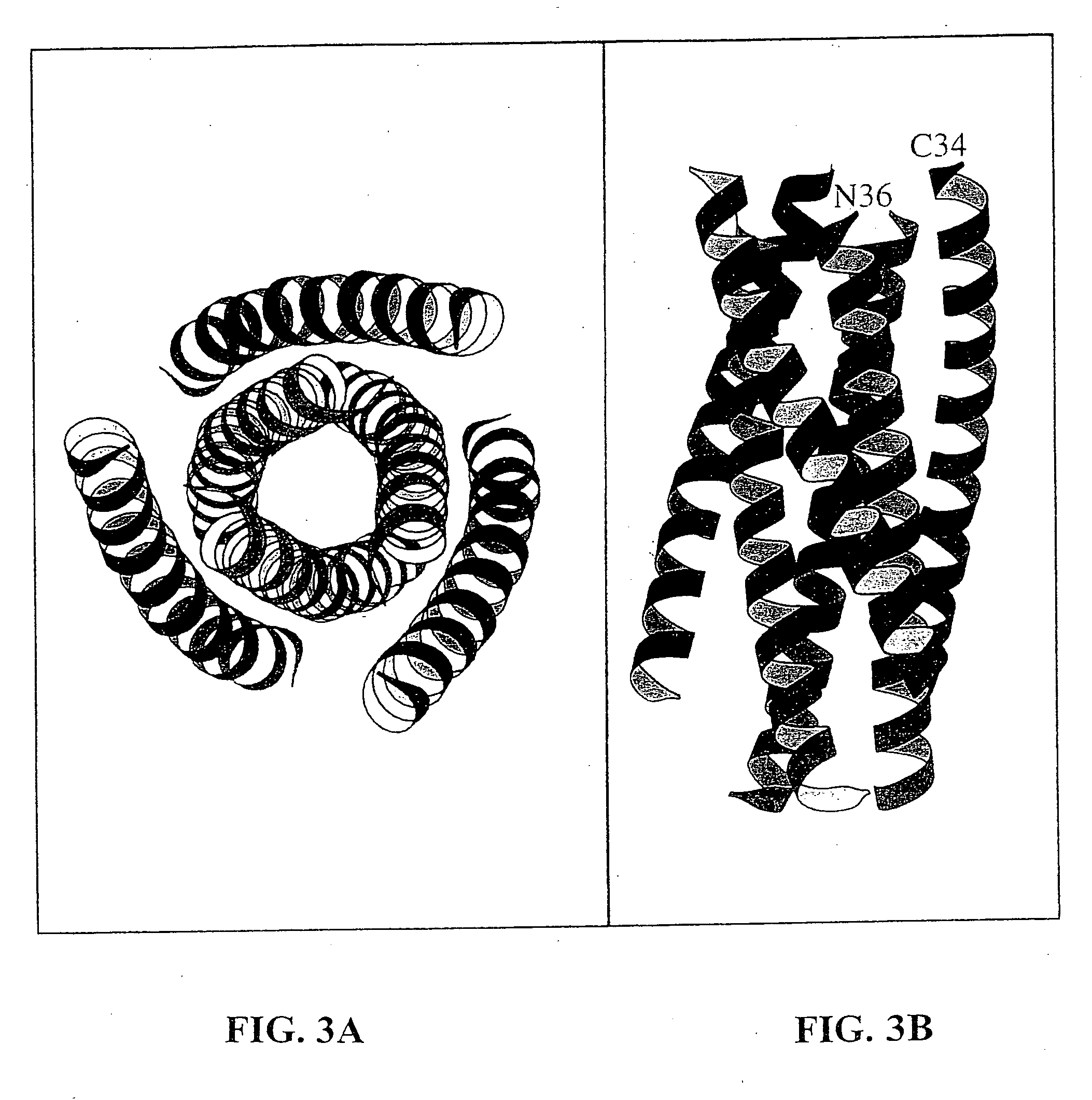
Described are the crystal structure of the alpha-helical domain of the gp41 component of HIV-1 envelope glycoprotein which represents the core of fusion-active gp41, methods of identifying and designing drugs which inhibit gp41 function and drugs which do so.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
Methods for inhibiting HIV-1 infection
This invention provides an antibody capable of specifically inhibiting the fusion of an HIV-1 envelope glycoprotein+ cell with an appropriate CD4+ cell without cross reacting with the HIV-1 envelope glycoprotein or CD4 and capable of inhibiting infection by one or more strains of HIV-1. This antibody is then used to identify a molecule which is important for HIV infection. Different uses of the antibody and the molecule are described.
Owner:CYTODYN
Novel HIV-1 envelope glycoprotein
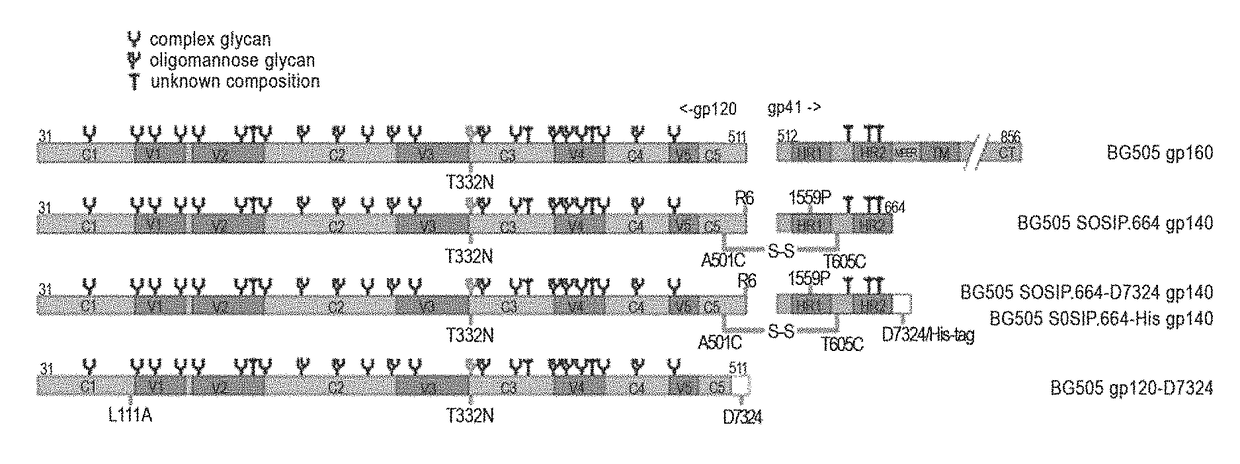
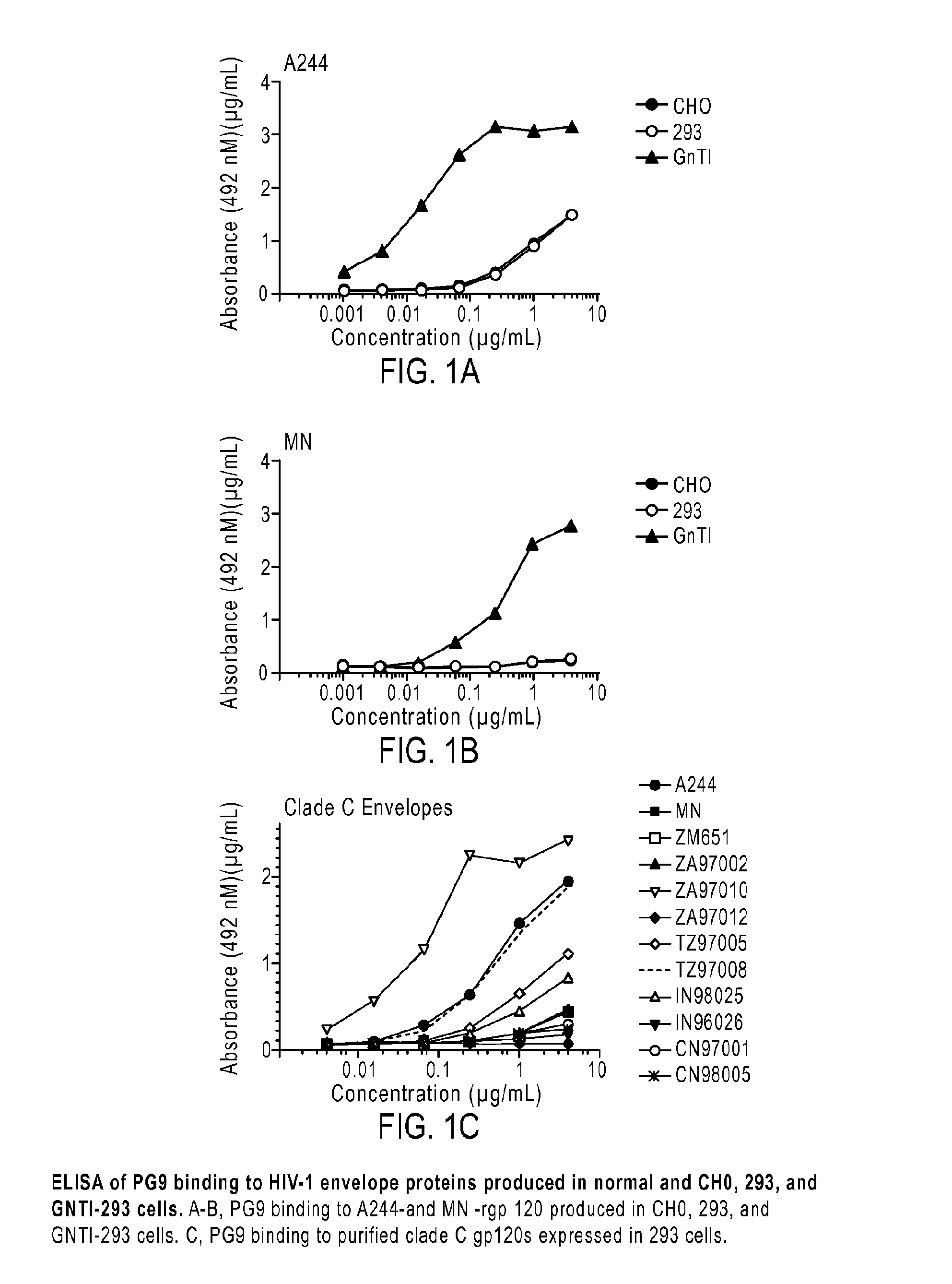
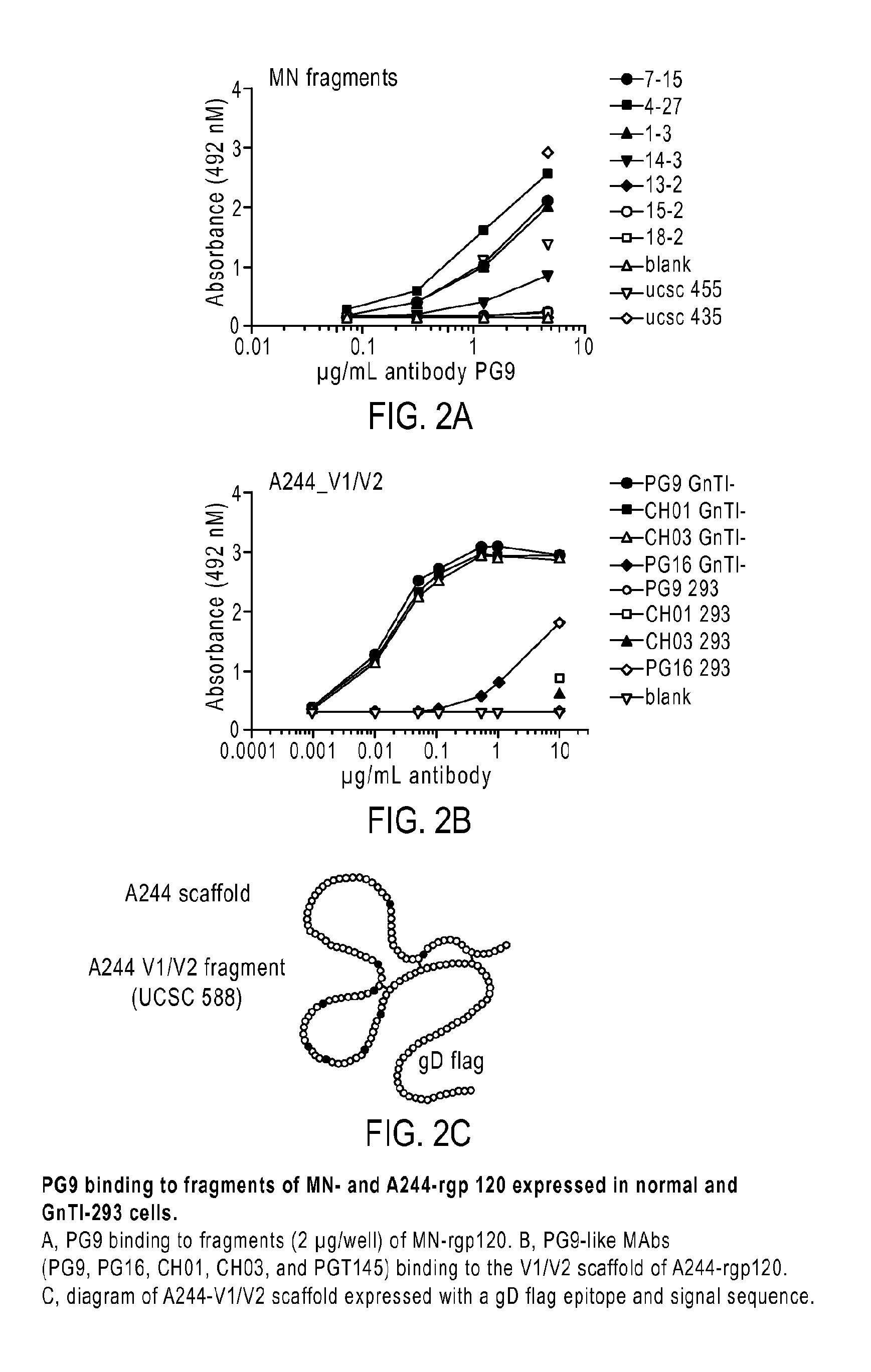
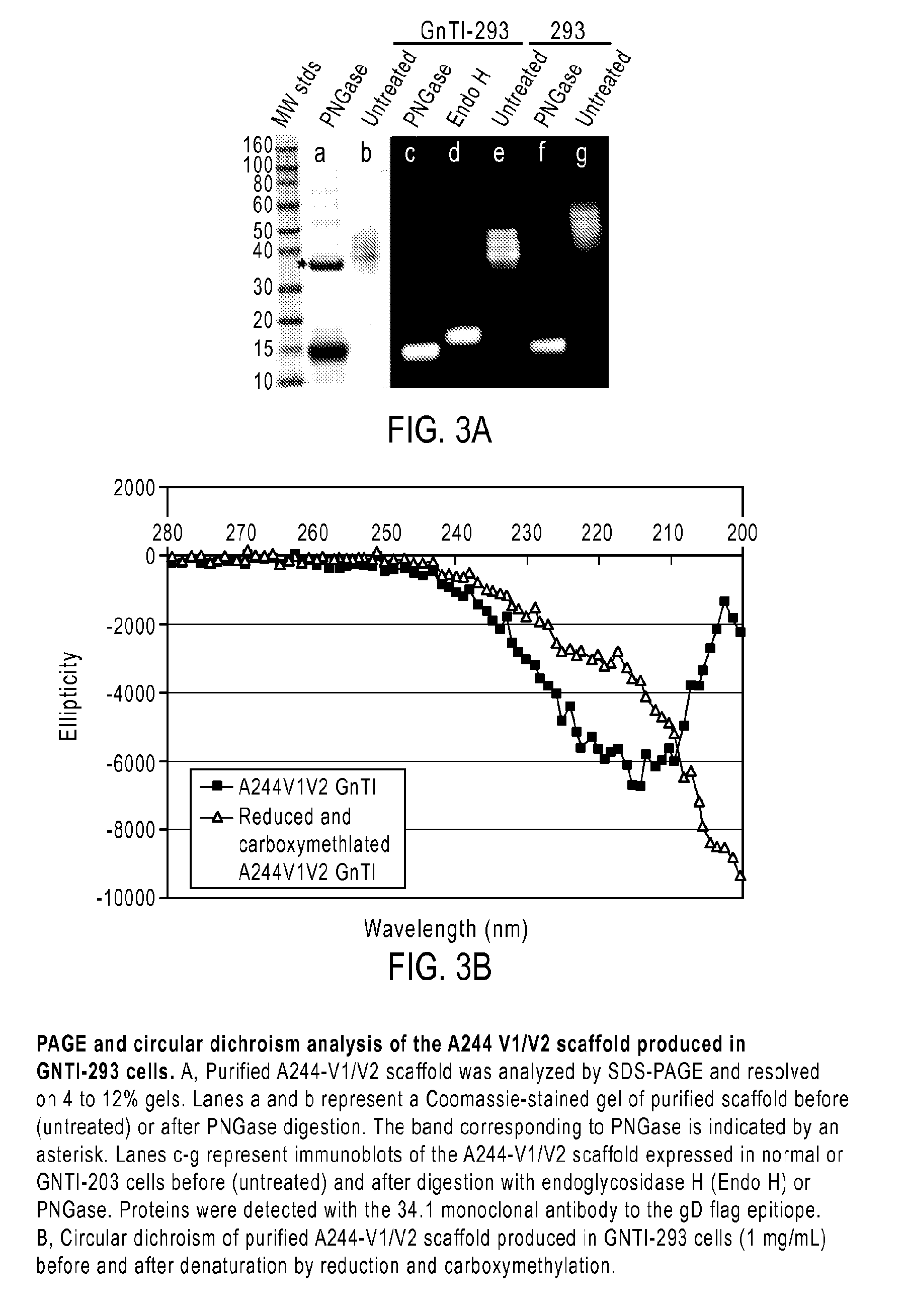
The present application relates to novel HIV-1 envelope glycoproteins, which may be utilized as HIV-1 vaccine immunogens, and antigens for crystallization, electron micrsocopy and other biophysical, biochemical and immunological studies for the identification of broad neutralizing antibodies. The present invention encompasses the preparation and purification of immunogenic compositions, which are formulated into the vaccines of the present invention.
Owner:THE SCRIPPS RES INST +2
Novel HIV-1 Envelope Glycoprotein
ActiveUS20110262488A1Increase generationViral antigen ingredientsVirus peptidesHiv 1 vaccineHiv 1 envelope
The present application relates to a novel HIV-1 envelope glycoprotein which may be utilized as an HIV-1 vaccine immunogen, antigens for crystallization and for the identification of broad neutralizing antibodies. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE
Potential preparation method for highly-efficient recombinant HIV-1 CRF07-BC gp140 immunogen
InactiveCN103992396AImprove uniformityHigh antigen reactivityVirus peptidesDepsipeptidesAntigenEndotoxin removal
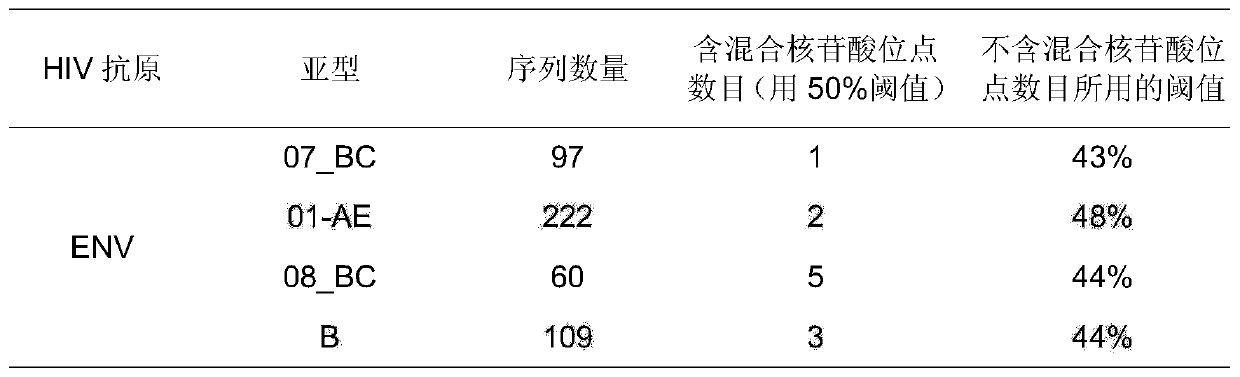
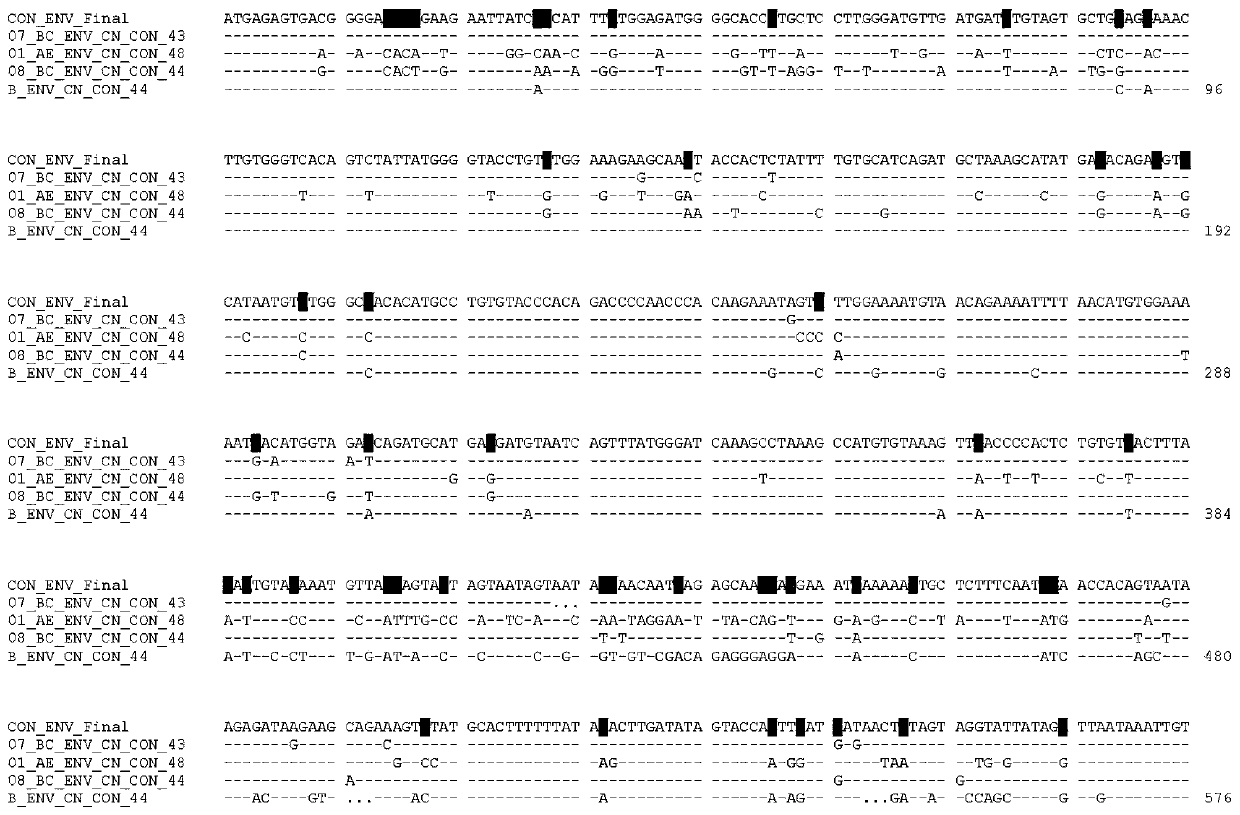
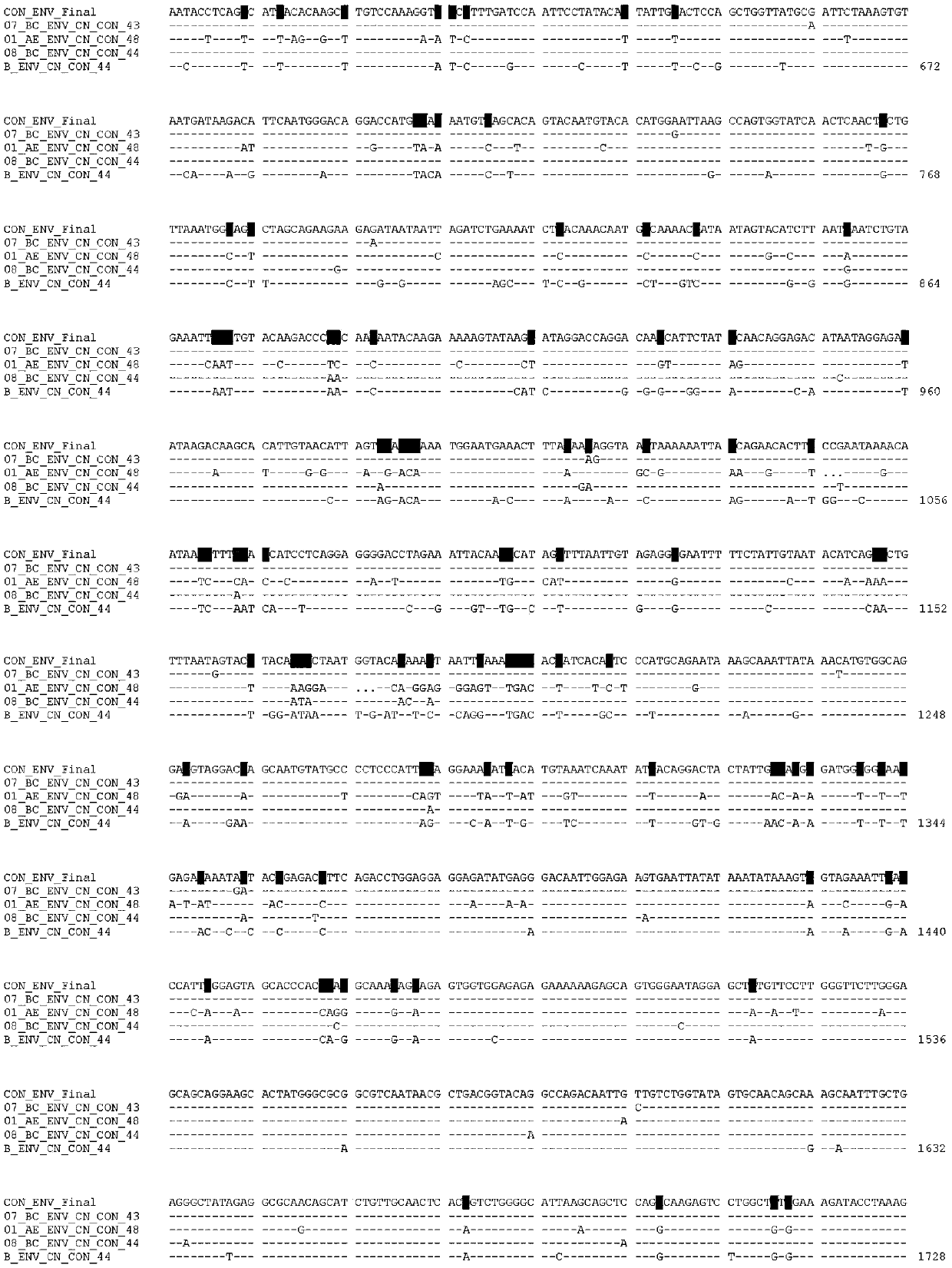
The invention discloses a potential preparation method for a highly-efficient recombinant HIV-1 CRF07-BC gp140 immunogen. The immunogen is designed based on the structure of HIV-1 envelope protein crystals which have been published internationally and obtained in our lab. The specific method uses an overlap extension PCR technology to obtain gp140 gene segments, and comprises the steps that target genes are cloned into an eukaryotic expression vector pMT, endotoxin is removed through extraction of a large amount of plasmids, the gp140 gene segments and resistance screening plasmids pCoBlast are co-transfected into drosophila melanogaster Schneider2 (S2) cells together, blasticidin (Blasticidin S) is used for positive clone screening, S2 cell lines for stably and efficiently secreting and expressing gp140 are screened, and after enlarged cultivation and through two steps of purification of nickel column affinity chromatography and gel filtration chromatography, the gp140 with high purity can be obtained. A series of biochemical and biophysical technologies indicate that the gp140 is uniform in polymeric states, and high in antigenic reactivity, and is quite fittingly used as the immunogen for the research and the development of AIDS subunit vaccines or multivalent combined vaccines.
Owner:NANKAI UNIV
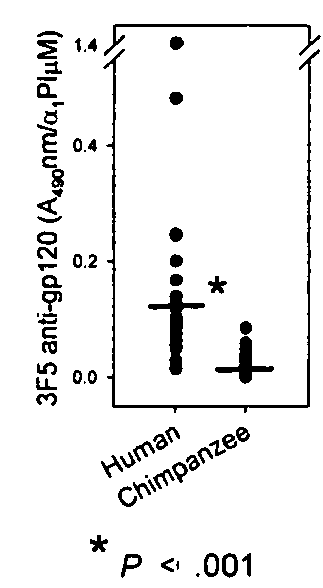
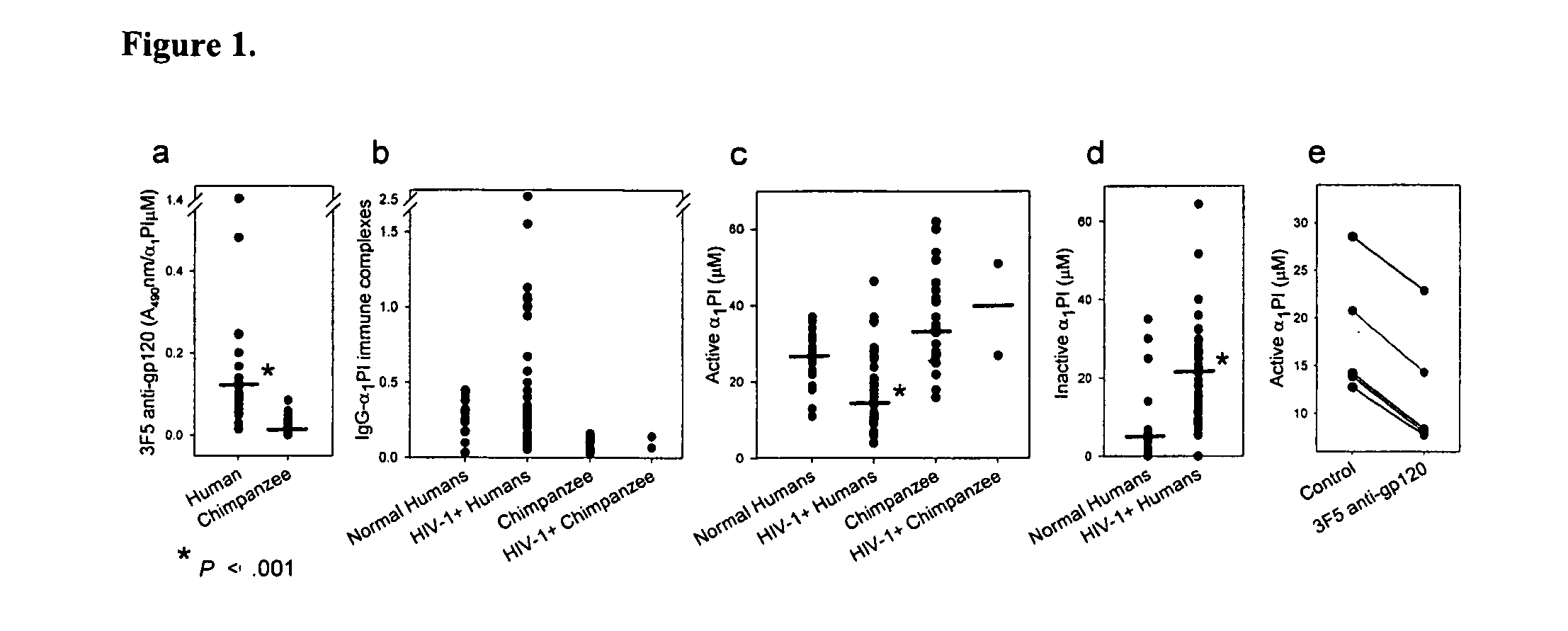
Alpha1 proteinase inhibitor peptides methods and use
InactiveUS20100029558A1Increasing CD lymphocyte renewalReturn to normal activitiesCompound screeningAnimal cellsDiseaseAlpha1-proteinase inhibitor
The invention is directed to the use of peptides that can bind and block the interaction of α1 proteinase inhibitor (α1PI) and one or more molecules, for example antibodies to HIV-1 envelope proteins. The invention features methods of activating α1PI in a cell, methods of treating or preventing a disease or disorder in a subject, for example HIV-1 or AIDS. The invention also features pharmaceutical compositions comprising one or more peptides that block the interaction of α1α1PI and one or more molecules. Also included in the invention are kits.
Owner:BRISTOW CYNTHIA L
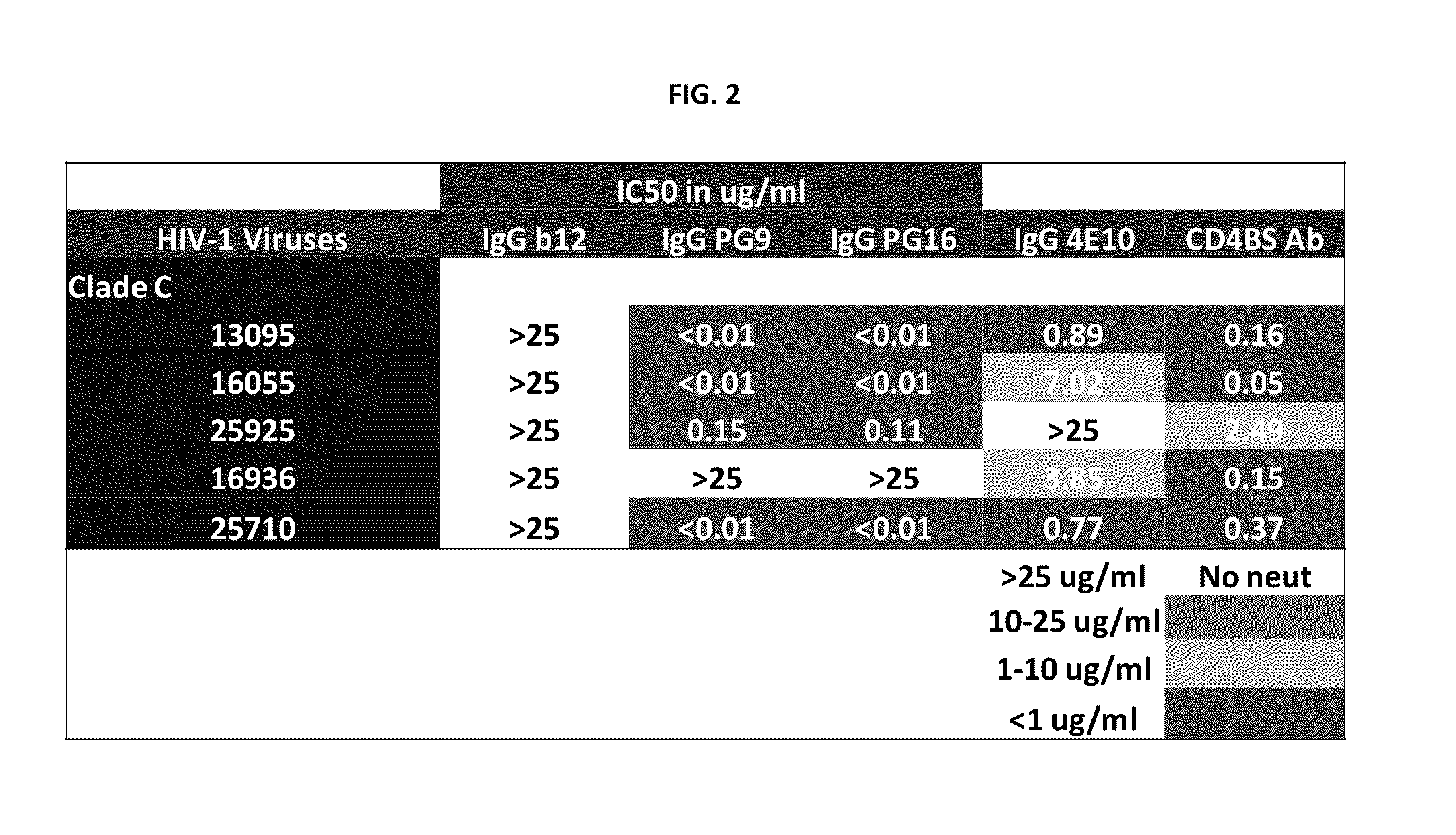
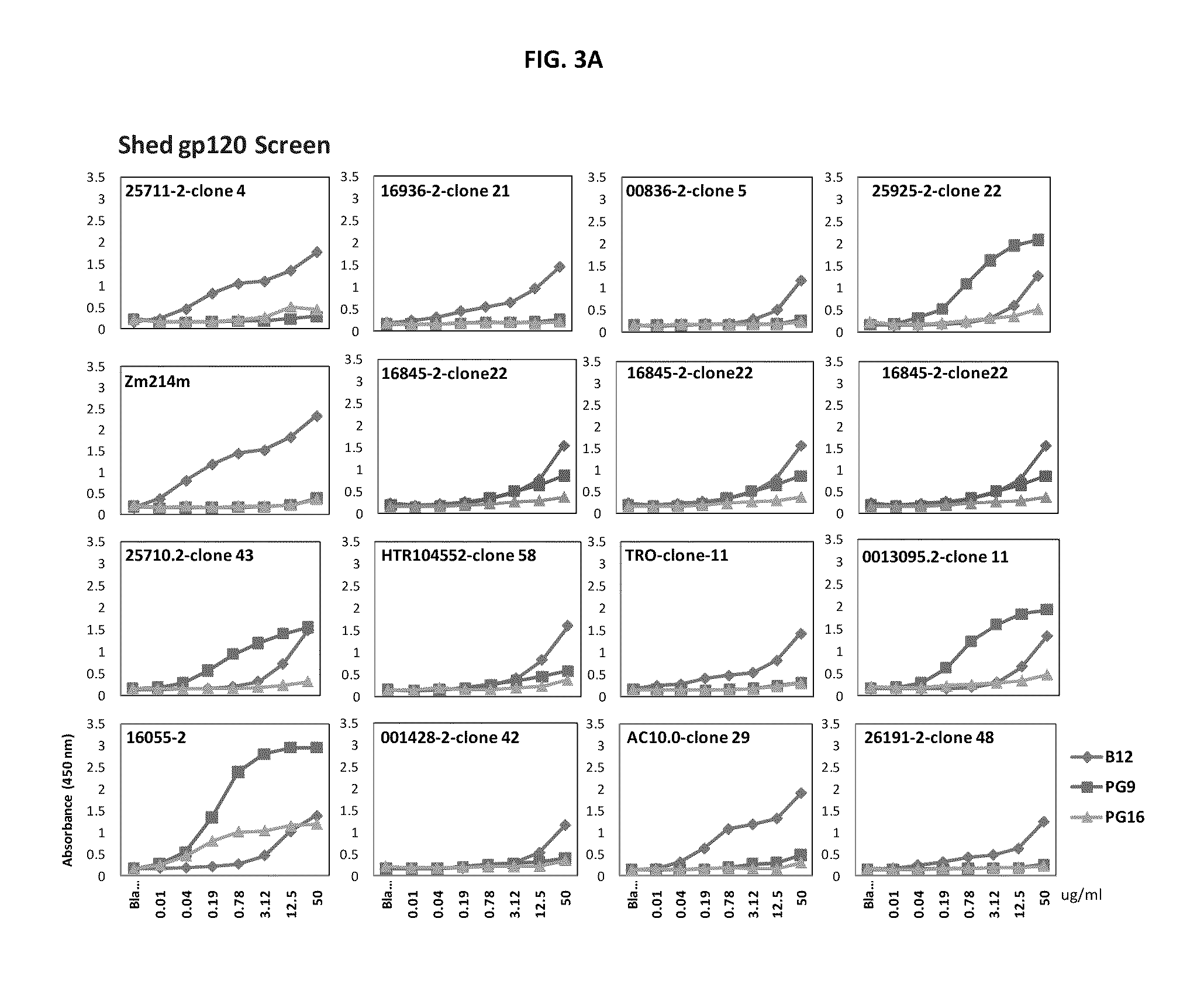
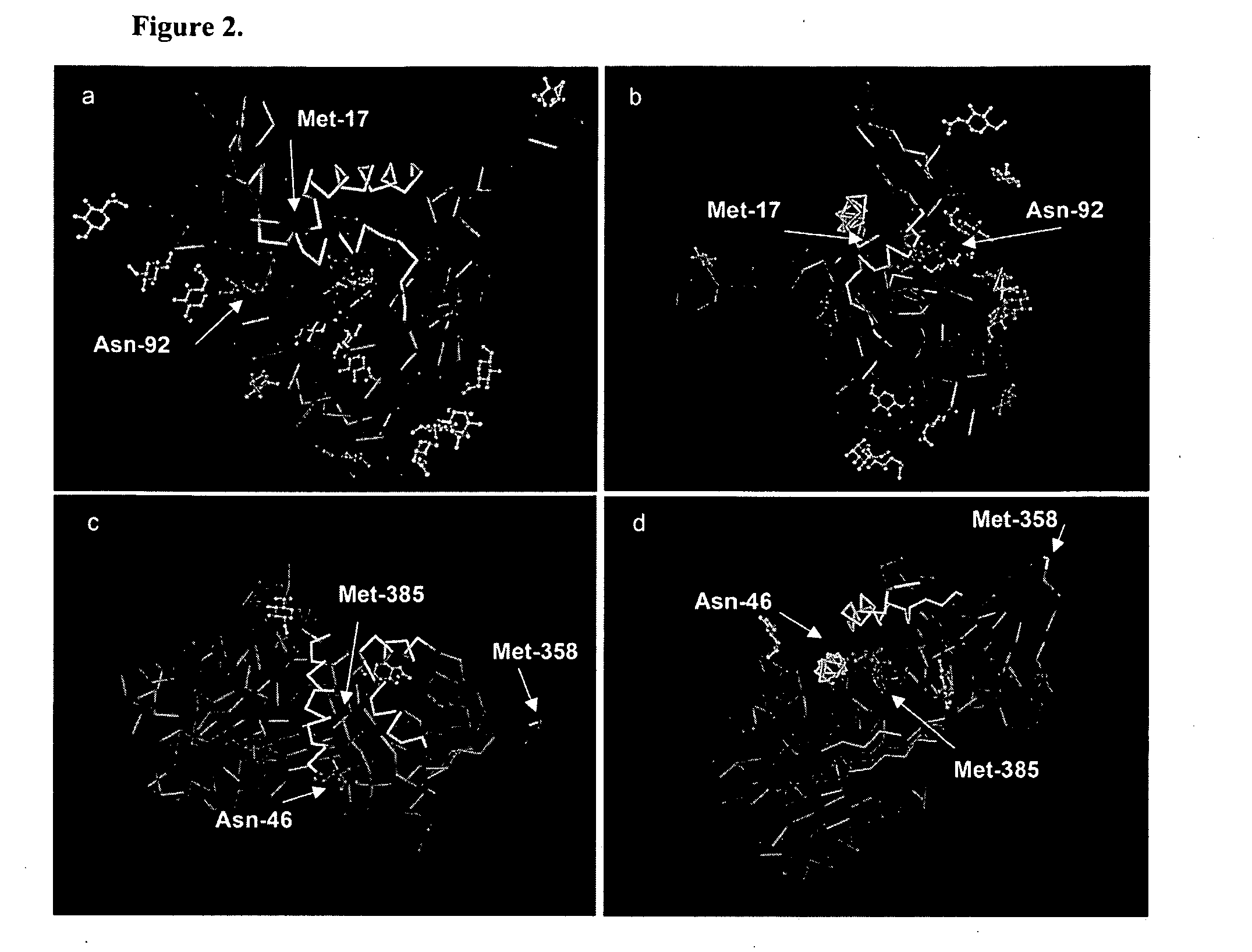
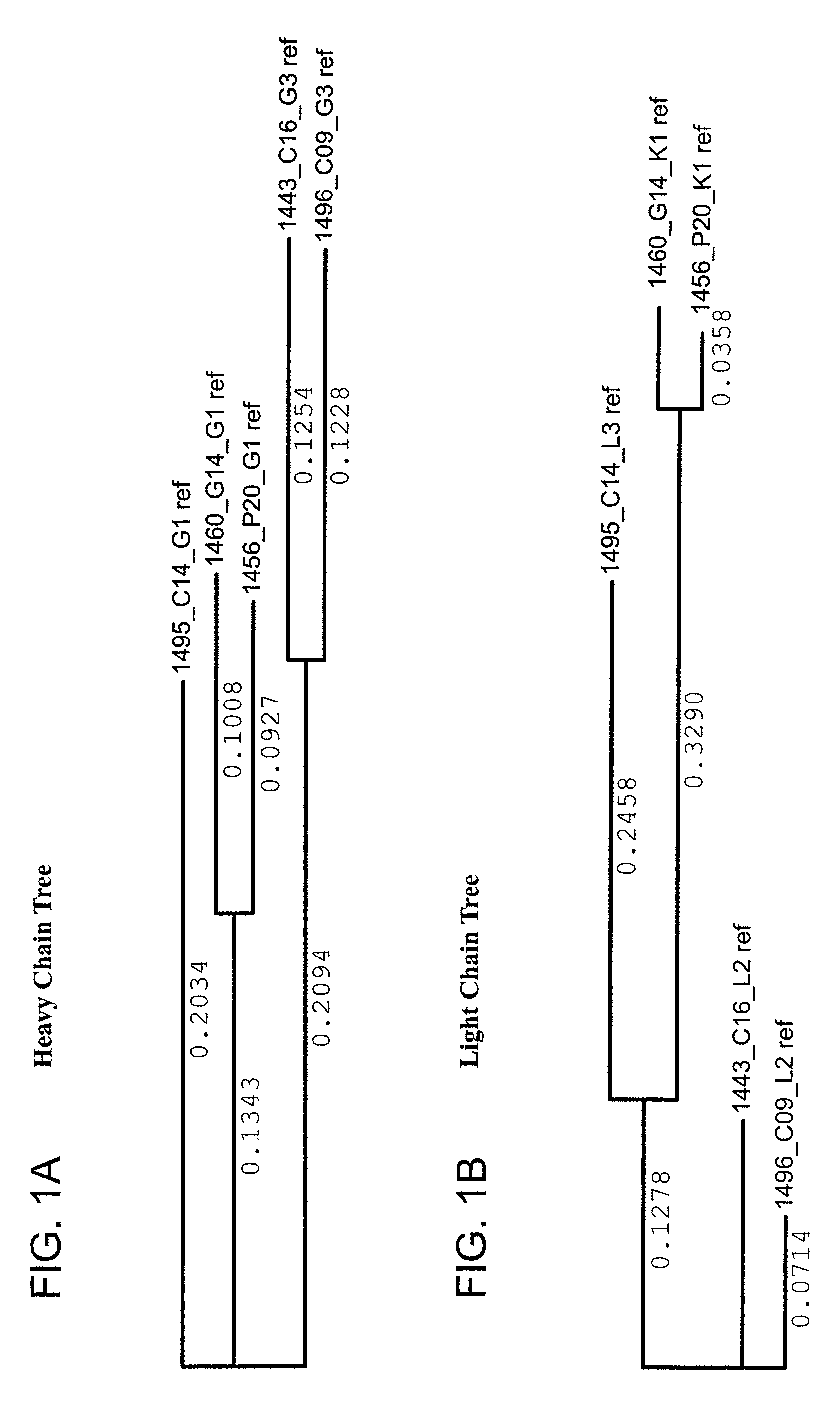
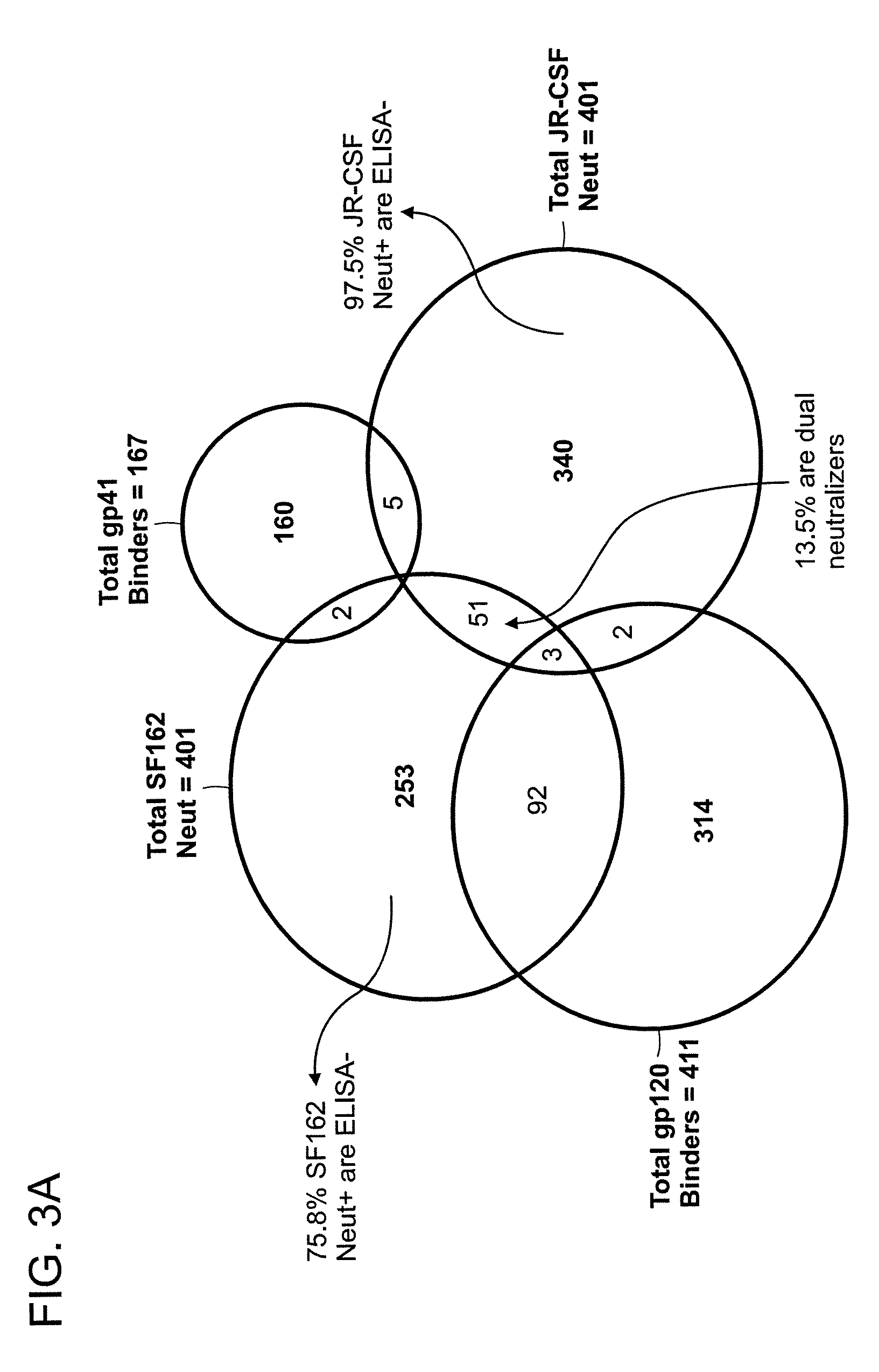
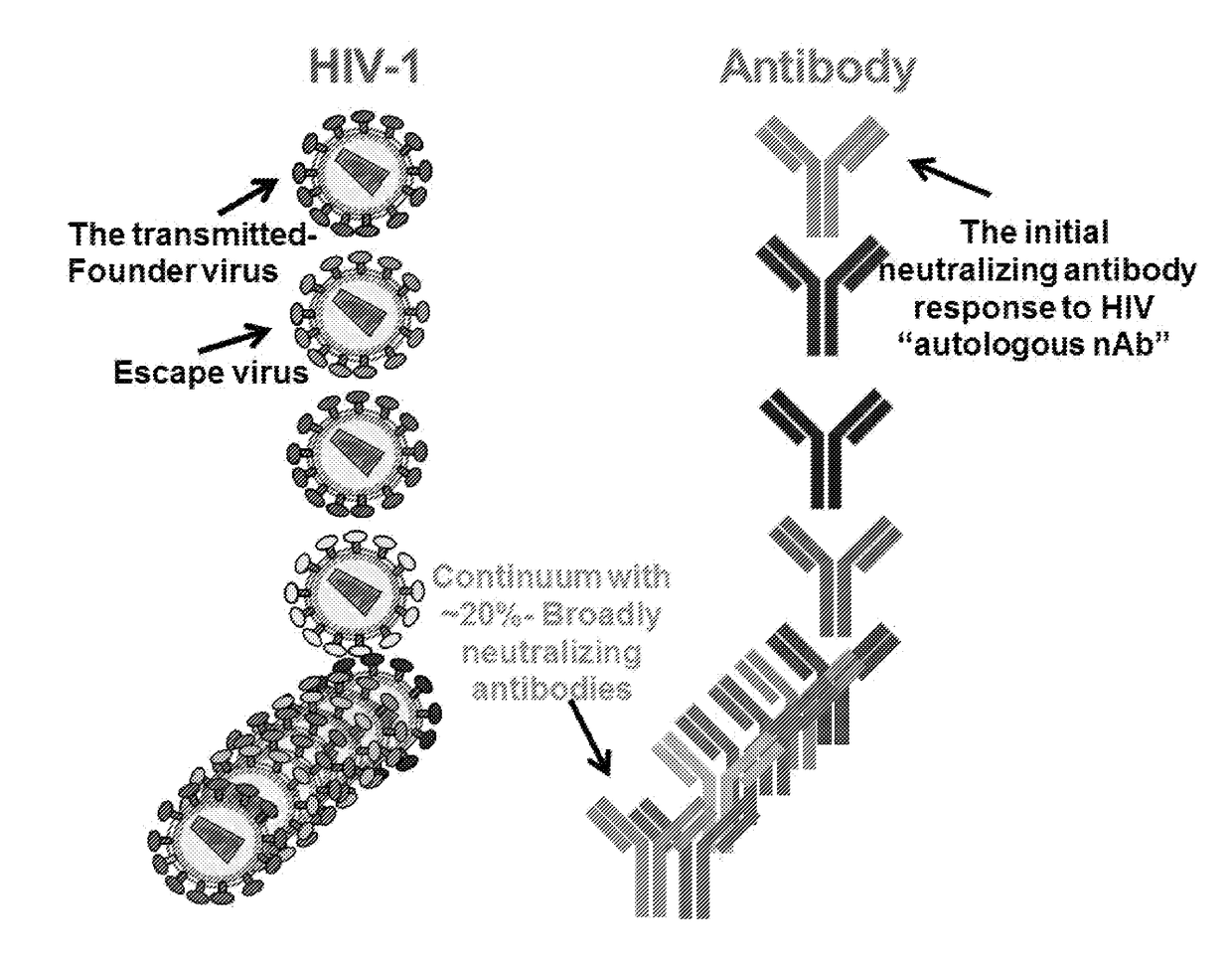
Monoclonal antibodies directed against trimeric forms of the HIV-1 envelope glycoprotein with broad and potent neutralizing activity
The invention provides a method for obtaining a broadly neutralizing antibody (bNab), including screening memory B cell cultures from a donor PBMC sample for neutralization activity against a plurality of HIV-1 species, cloning a memory B cell that exhibits broad neutralization activity; and rescuing a monoclonal antibody from that memory B cell culture. The resultant monoclonal antibodies are characterized by their ability to selectively bind epitopes from the Env proteins in native or monomeric form, as well as to inhibit infection of HIV-1 species from a plurality of clades. Compositions containing human monoclonal anti-HIV antibodies used for prophylaxis, diagnosis and treatment of HIV infection are provided. Methods for generating such antibodies by immunization using epitopes from conserved regions within the variable loops of gp120 are provided. Immunogens for generating anti-HIV1 bNAbs are also provided. Furthermore, methods for vaccination using suitable epitopes are provided.
Owner:THE SCRIPPS RES INST +2
Modified HIV-1 Envelope Proteins
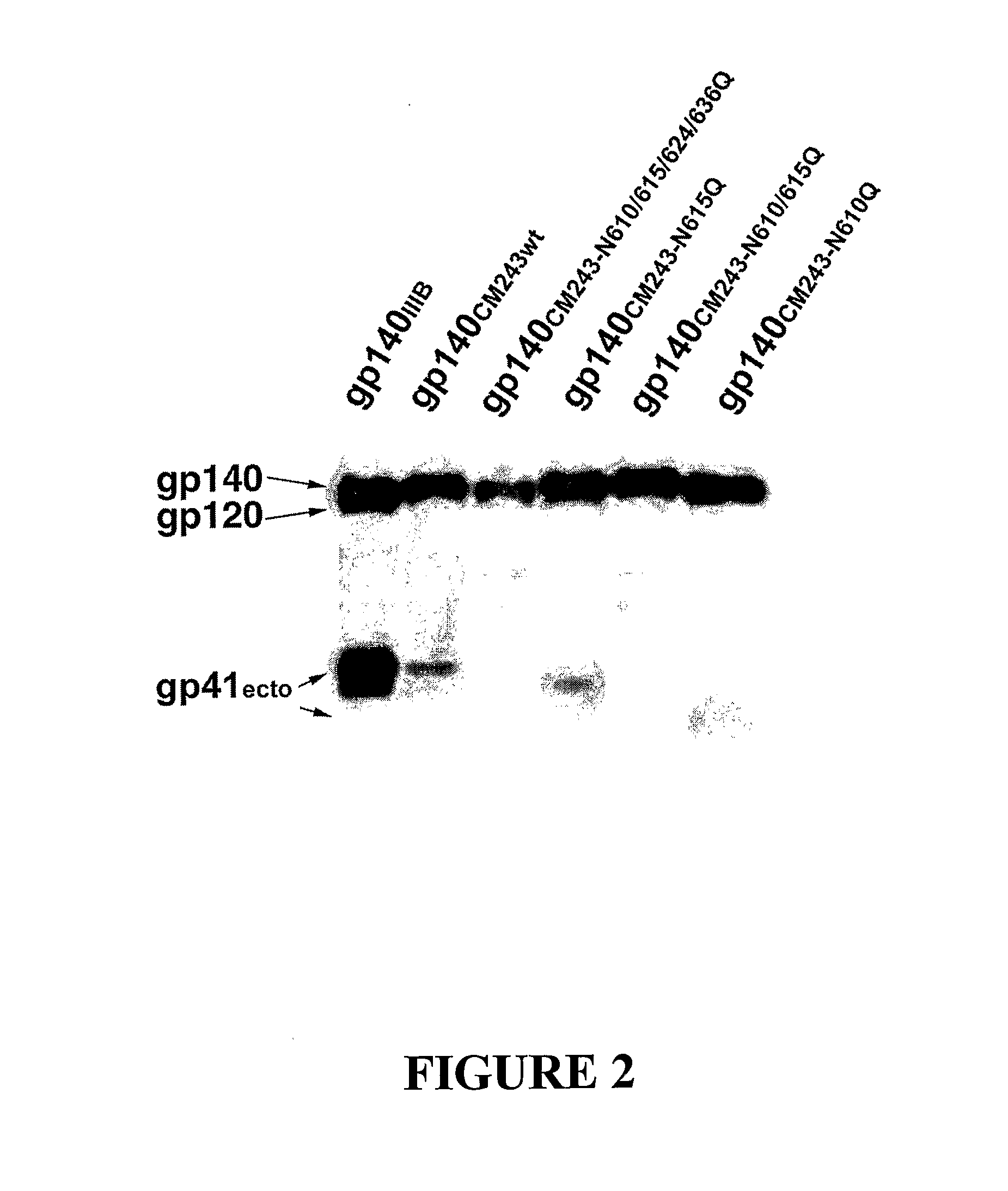
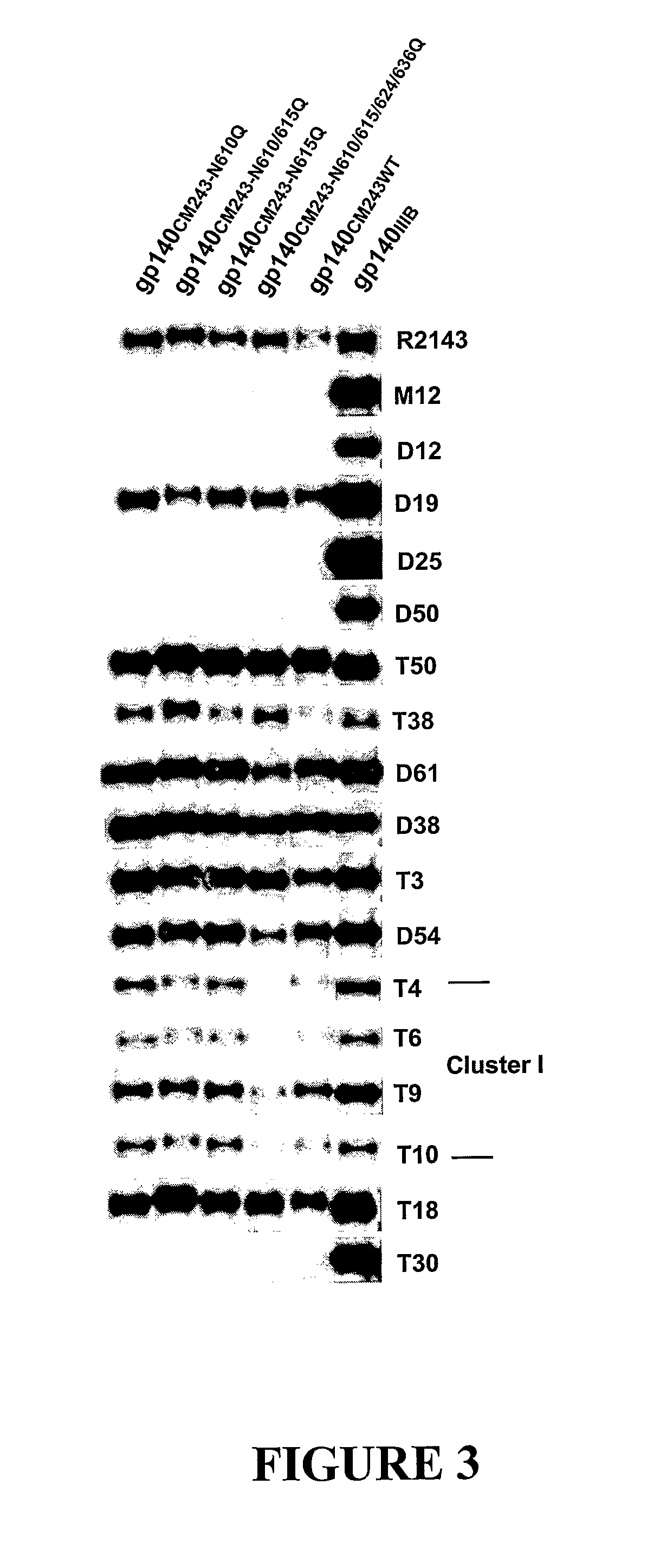
The present invention relates to modified HIV-1 envelope proteins where one or more N-glycosylation sites have been deleted or modified, which produce a broadly cross reactive neutralizing response, their methods of use and antibodies which bind to these proteins. The invention also provides for nucleic acids, vectors, antibodies and pharmaceutical compositions that comprise said modified HIV-1 envelope proteins.
Owner:HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF THE MILITARY MEDICINE INC
Compounds capable of inhibiting HIV-1 infection
InactiveUS20070025983A1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsHiv 1 envelopeCD4 antigen
This invention provides an antibody capable of specifically inhibiting the fusion of an HIV-1 envelope glycoprotein+ cell with an appropriate CD4+ cell without cross reacting with the HIV-1 envelope glycoprotein or CD4 and capable of inhibiting infection by one or more strains of HIV-1. This antibody is then used to identify a molecule which is important for HIV infection. Different uses of the antibody and the molecule are described.
Owner:CYTODYN
HIV-1 Envelope Based Fragments
InactiveUS20110217338A1Minimized in sizePeptide/protein ingredientsViral antigen ingredientsHiv 1 vaccineHiv 1 envelope
The present application relates to a novel HIV-1 envelope fragments containing the B12 epitope which may be utilized as an HIV-1 vaccine immunogen, in particular for eliciting broad neutralizing antibodies following a prime-boost immunization. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE +1
Core structure of gp41 from the HIV envelope glycoprotein
Described are the crystal structure of the α-helical domain of the gp41 component of HIV-1 envelope glycoprotein which represents the core of fusion-active gp41, methods of identifying and designing drugs which inhibit gp41 function and drugs which do so.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
HIV-1 envelope glycoprotein
The present application relates to novel HIV-1 envelope glycoproteins, which may be utilized as HIV-1 vaccine immunogens, and antigens for crystallization, electron microscopy and other biophysical, biochemical and immunological studies for the identification of broad neutralizing antibodies. The present invention encompasses the preparation and purification of immunogenic compositions, which are formulated into the vaccines of the present invention.
Owner:THE SCRIPPS RES INST +2
HIV-1 envelope proteins and fragments thereof that possess epitopes recognized by broadly neutralizing antibodies
ActiveUS20150246111A1Maximum efficiencyHigh strengthViral antigen ingredientsVirus peptidesHiv 1 envelopeVirology
Owner:RGT UNIV OF CALIFORNIA
HIV-1 vaccines and screening methods therefor
InactiveUS20020127238A1Organic active ingredientsPeptide/protein ingredientsHeterologousHiv 1 vaccine
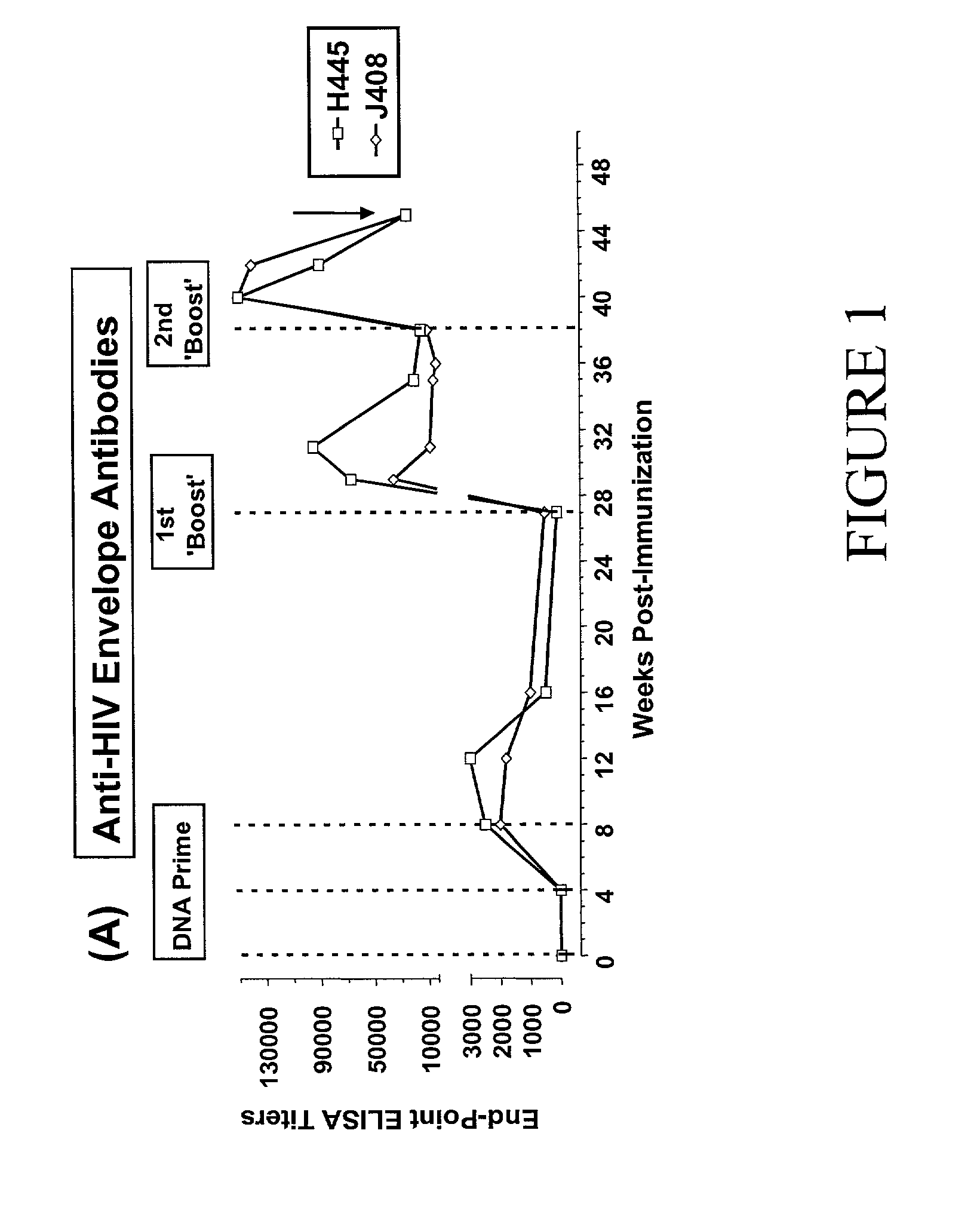
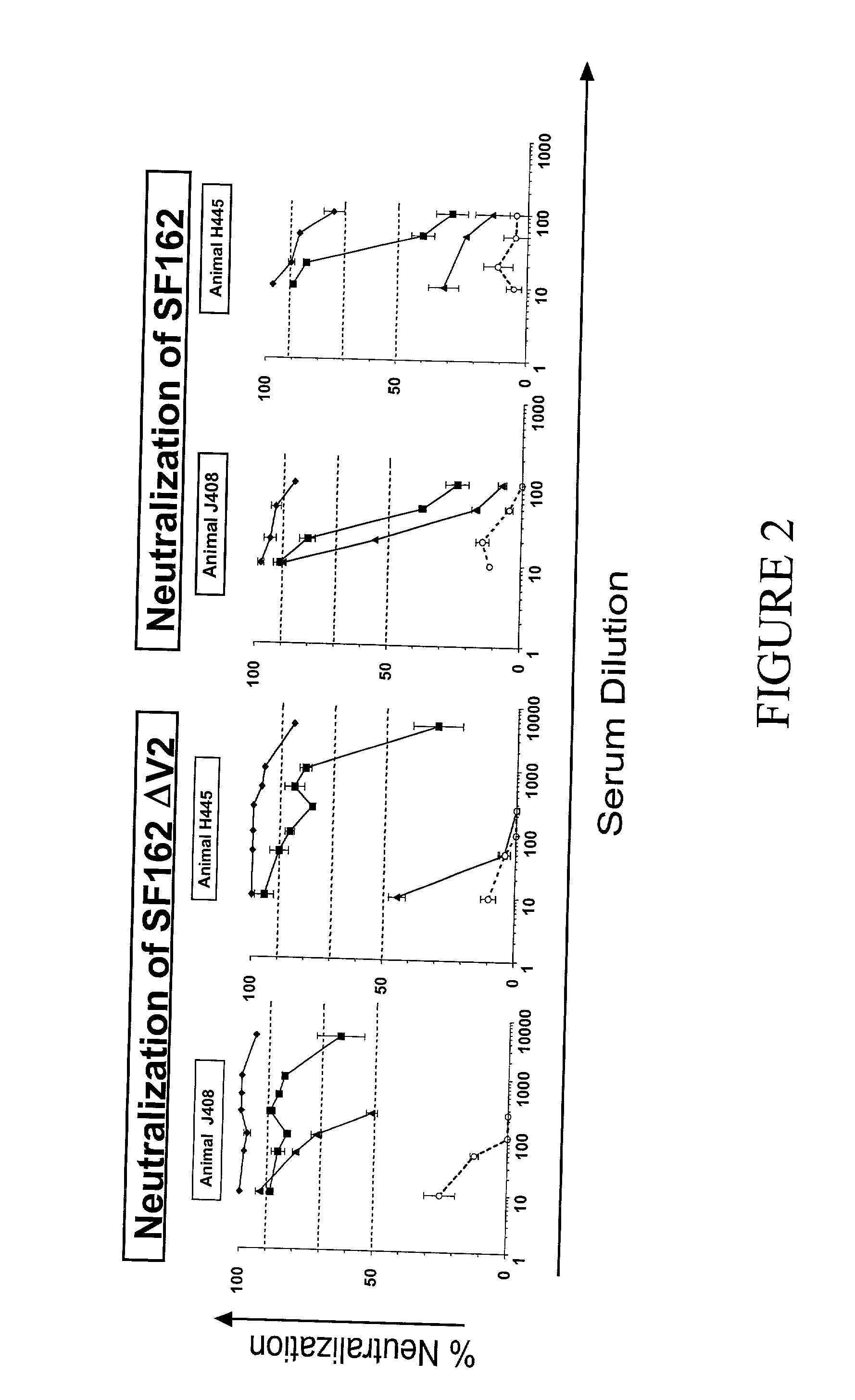
Methods for immunizing, and immunogen pharmaceutical compositions for eliciting a heterologous immune response to HIV-1 in an animal, preferably a human, are provided, utilizing a modified HIV-1 envelope protein or fragment or DNA encoding a modified HIV-1 envelope protein or fragment, the modified protein having a HIV-1 envelope protein V2 region deletion. A humoral response against heterologous HIV-1 strains is achieved.
Owner:CHIRON CORP
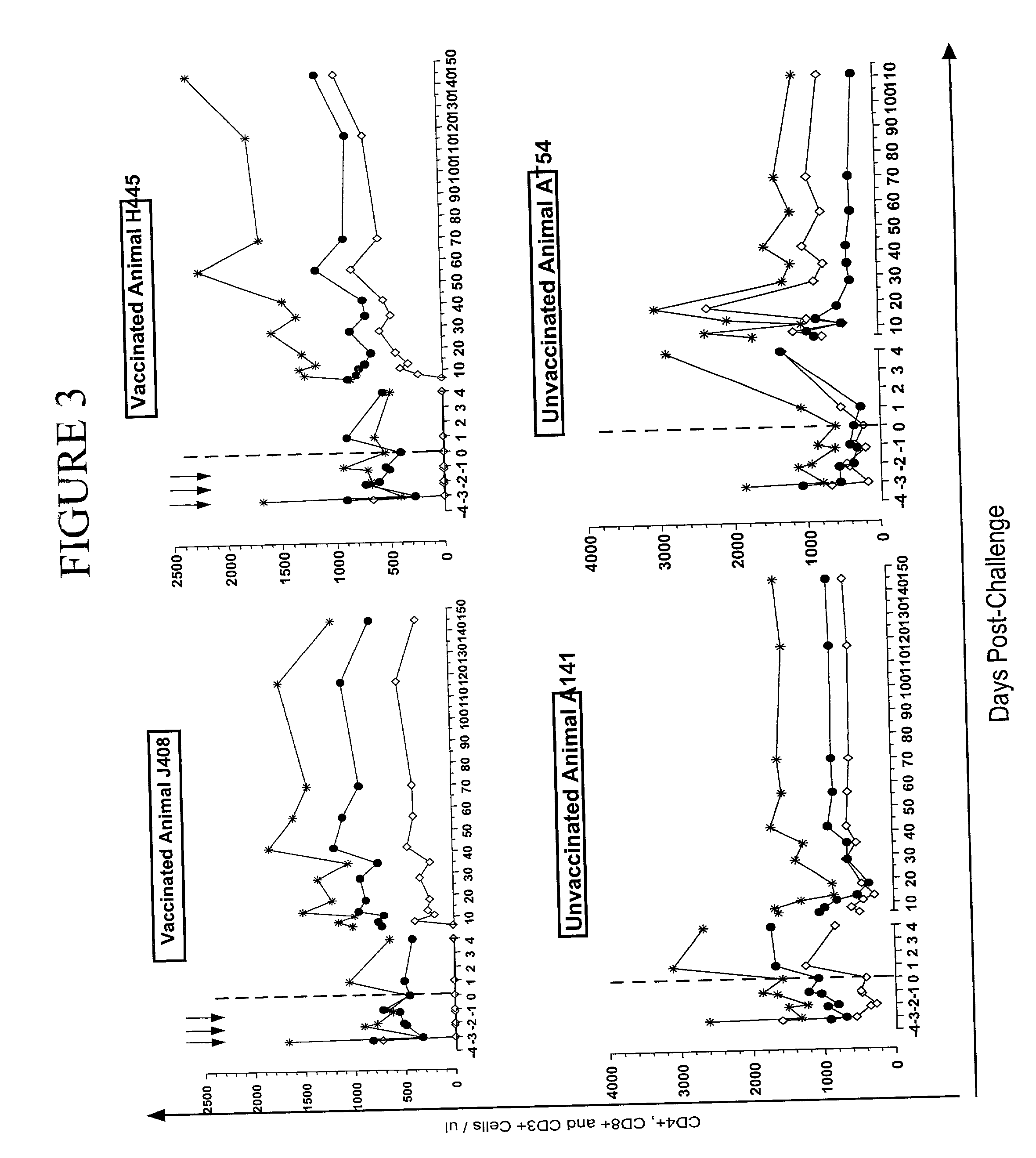
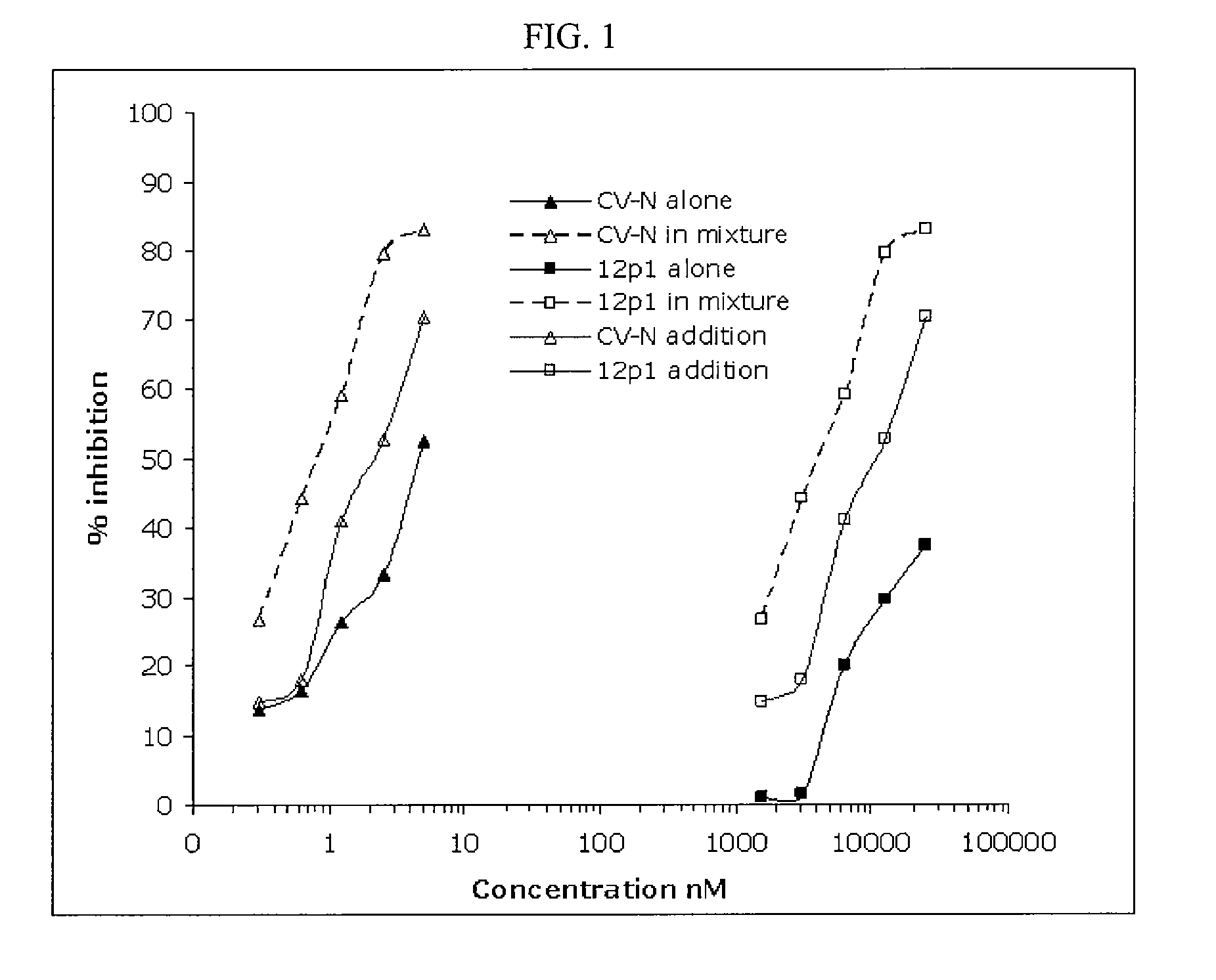
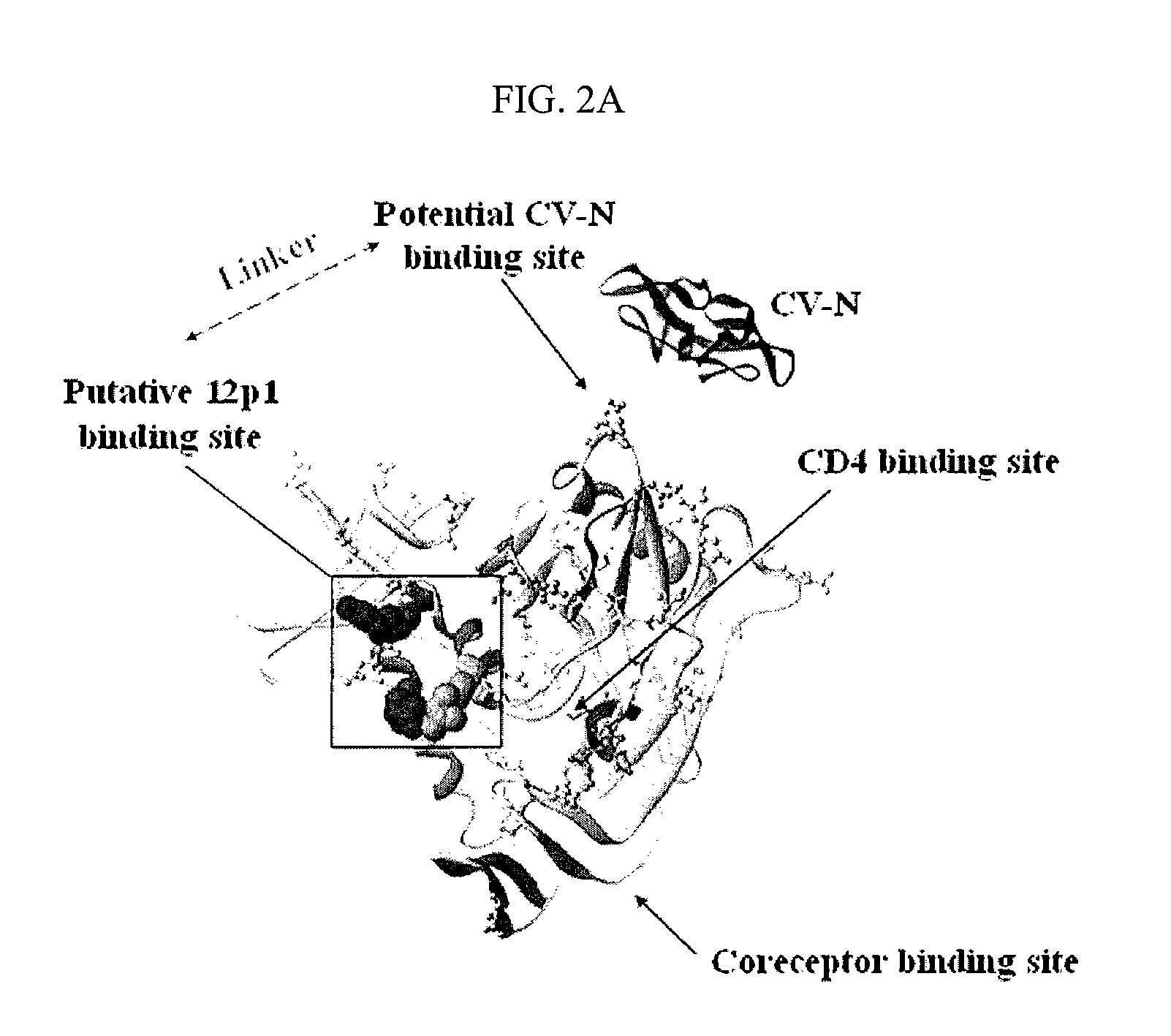
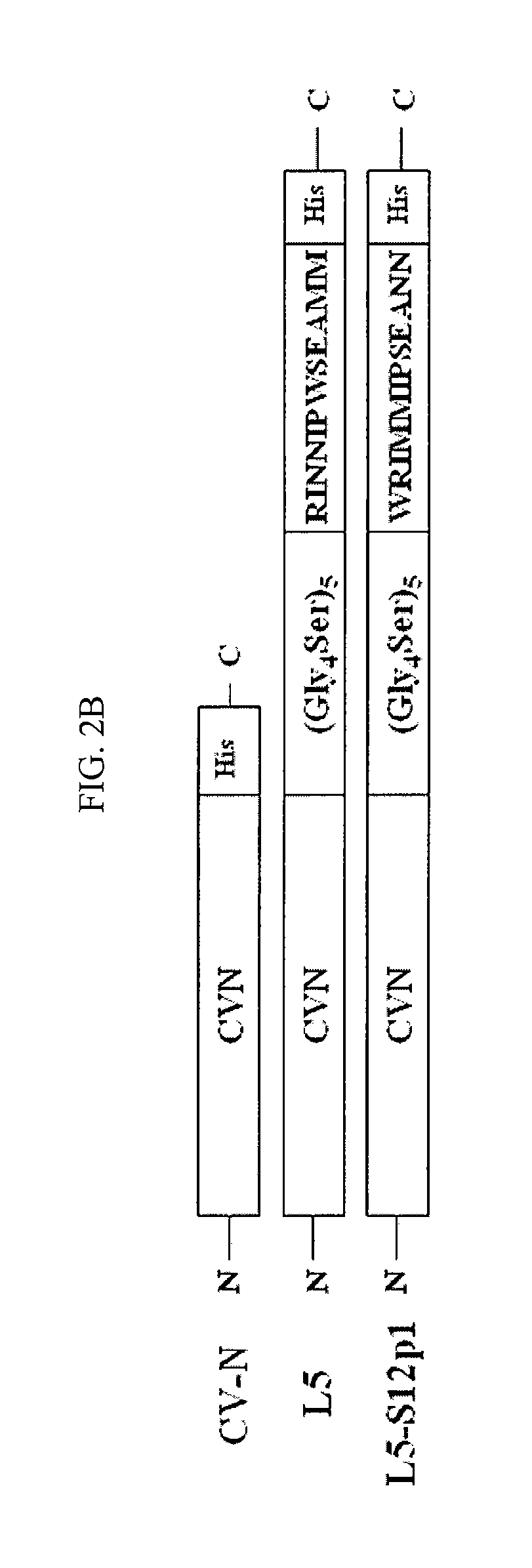
CVN-12p1: a recombinant allosteric lectin antagonist of HIV-1 envelope gp120 interactions
InactiveUS20090233850A1Protect against undesired immune responsesExtended half-lifeFungiBacteriaHiv 1 envelopeViral envelope
The invention provides a recombinant multi-functional chimera of CVN and 12p1. Chimeras of CVN and 12p1 present a model for targeting gp120 at two discrete sites, by two different modes of inhibition and with increasing potency versus either component alone. A chimera of the invention combines the high affinity suppression of viral activity by CVN with the allosteric suppression of viral envelope binding to both CD4 and co-receptor by 12p1.
Owner:DREXEL UNIV
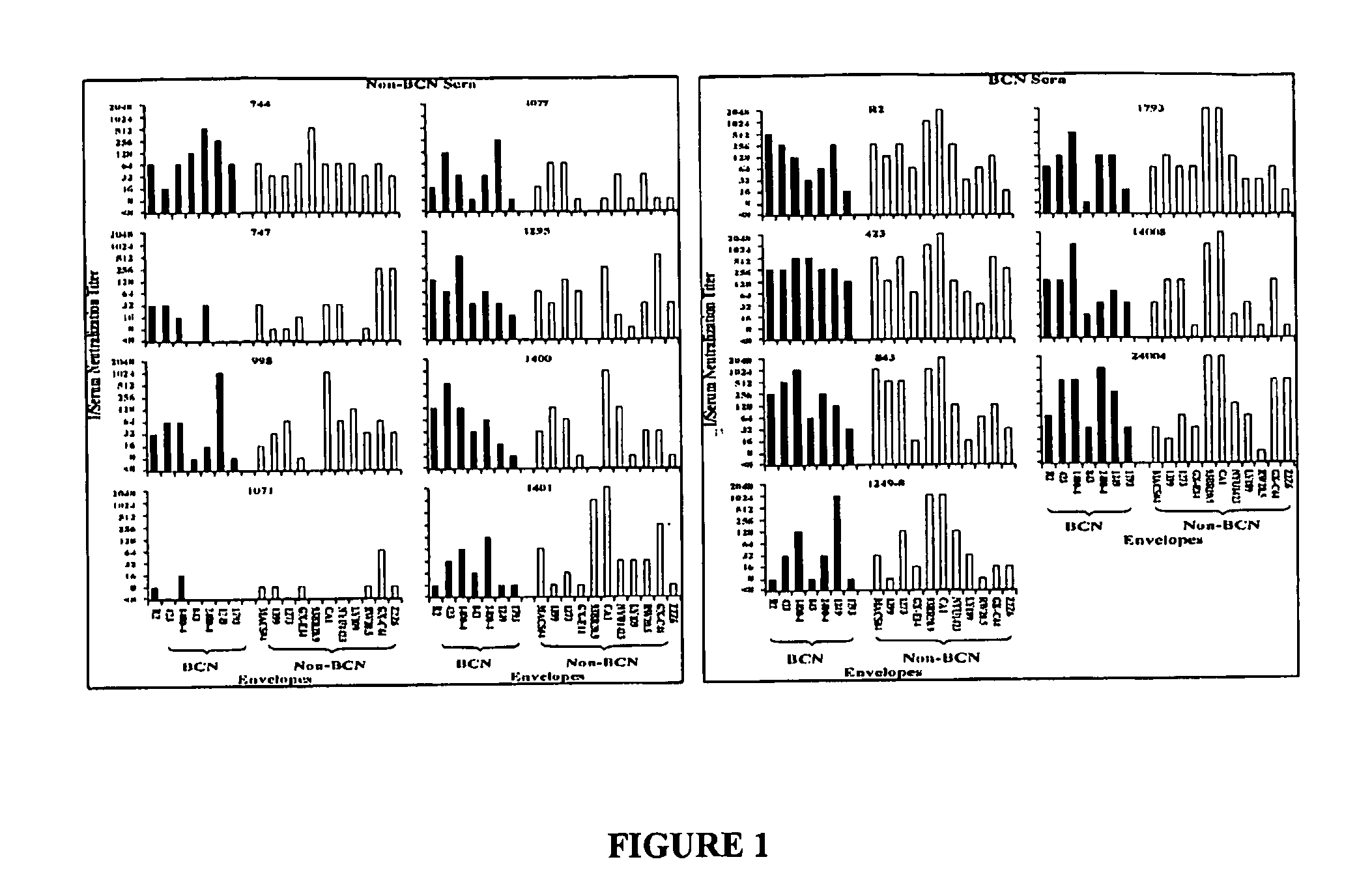
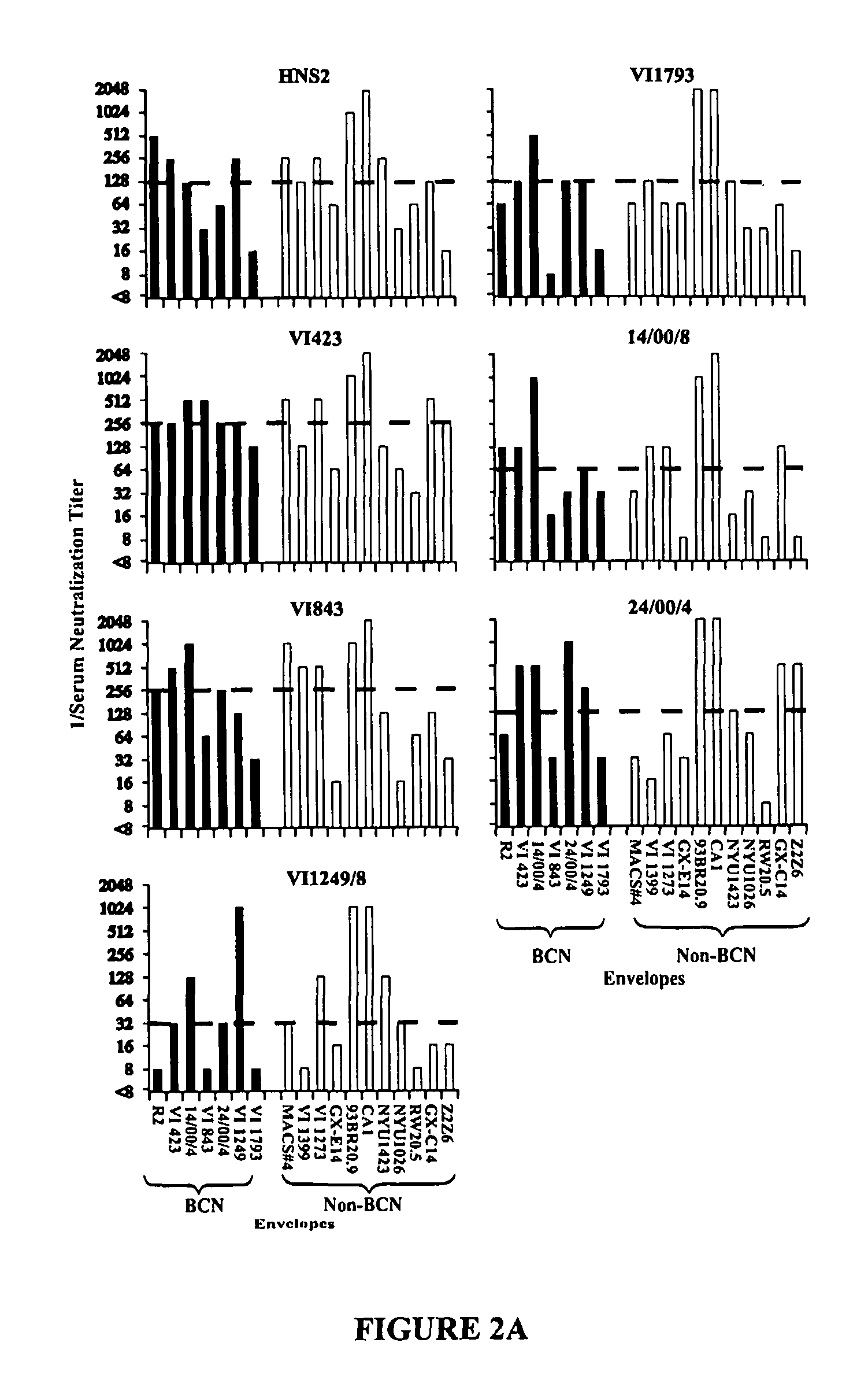
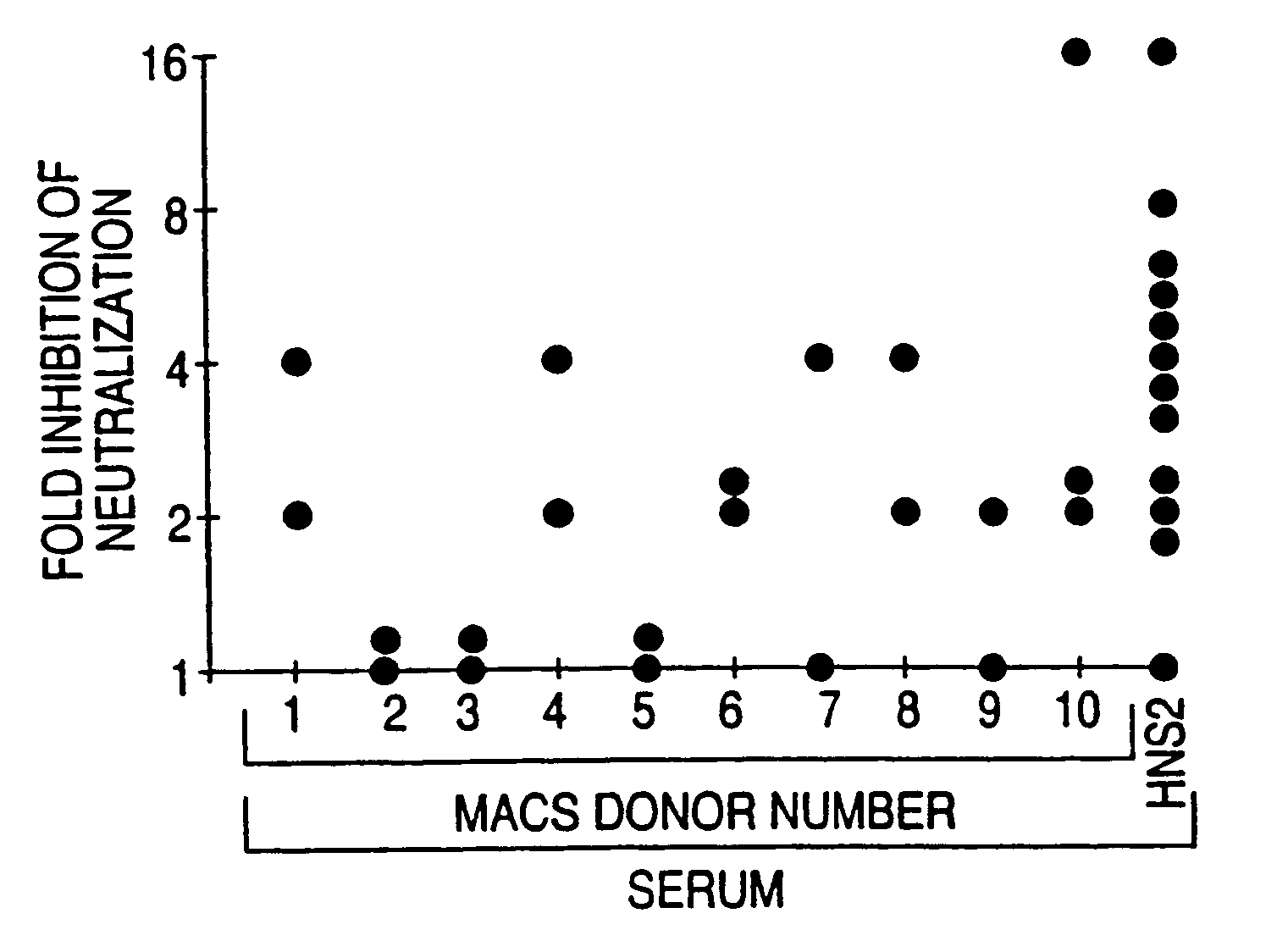
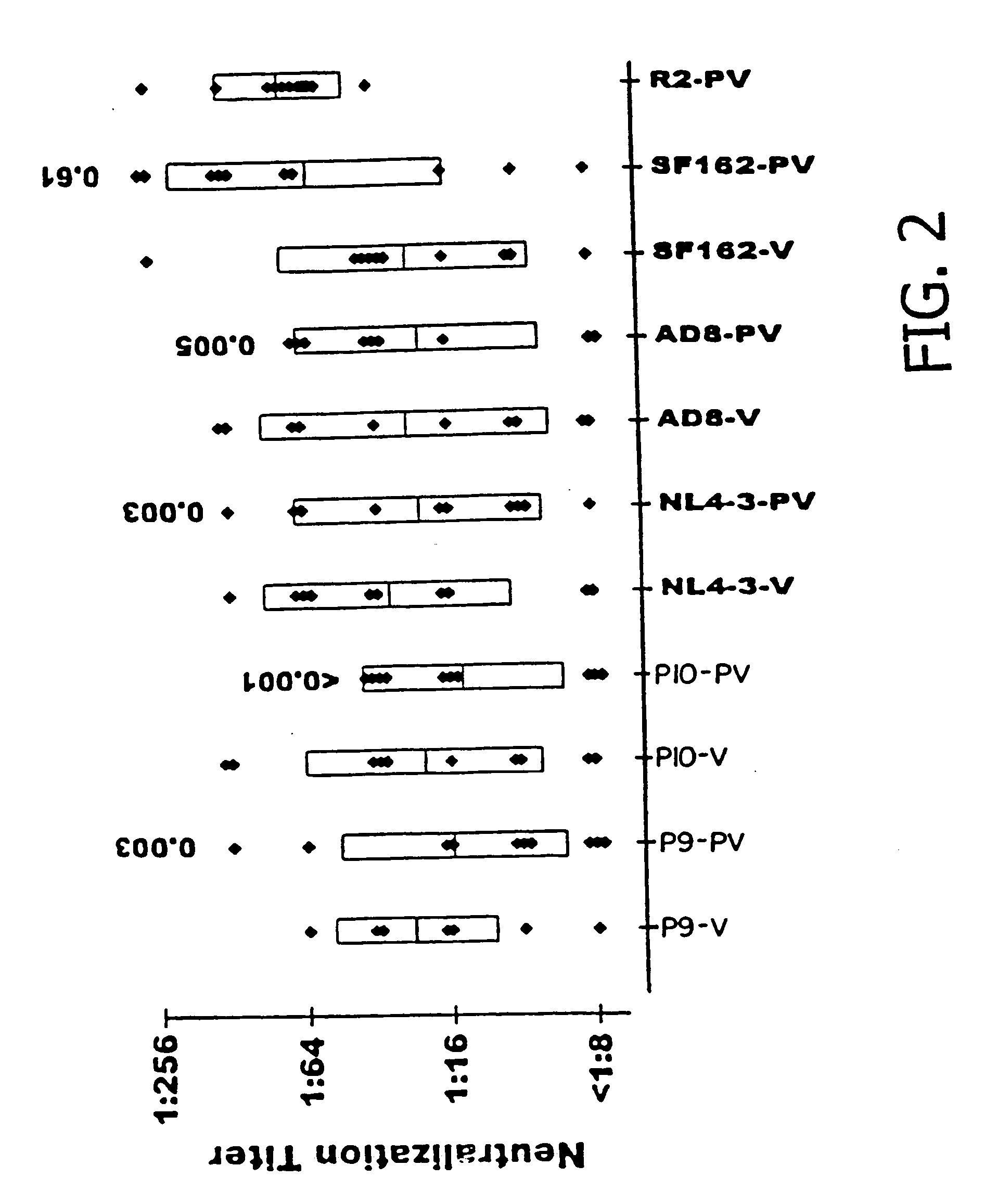
HIV-1 envelope protein associated with a broadly reactive neutralizing antibody response
The present invention relates to HIV-1 envelope proteins from a donor with non-progressive HIV-1 infection whose serum contains broadly cross-reactive, primary virus neutralizing antibody. The invention also relates to isolated or purified proteins and protein fragments that share certain amino acids at particular positions with the foregoing HIV-1 proteins.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
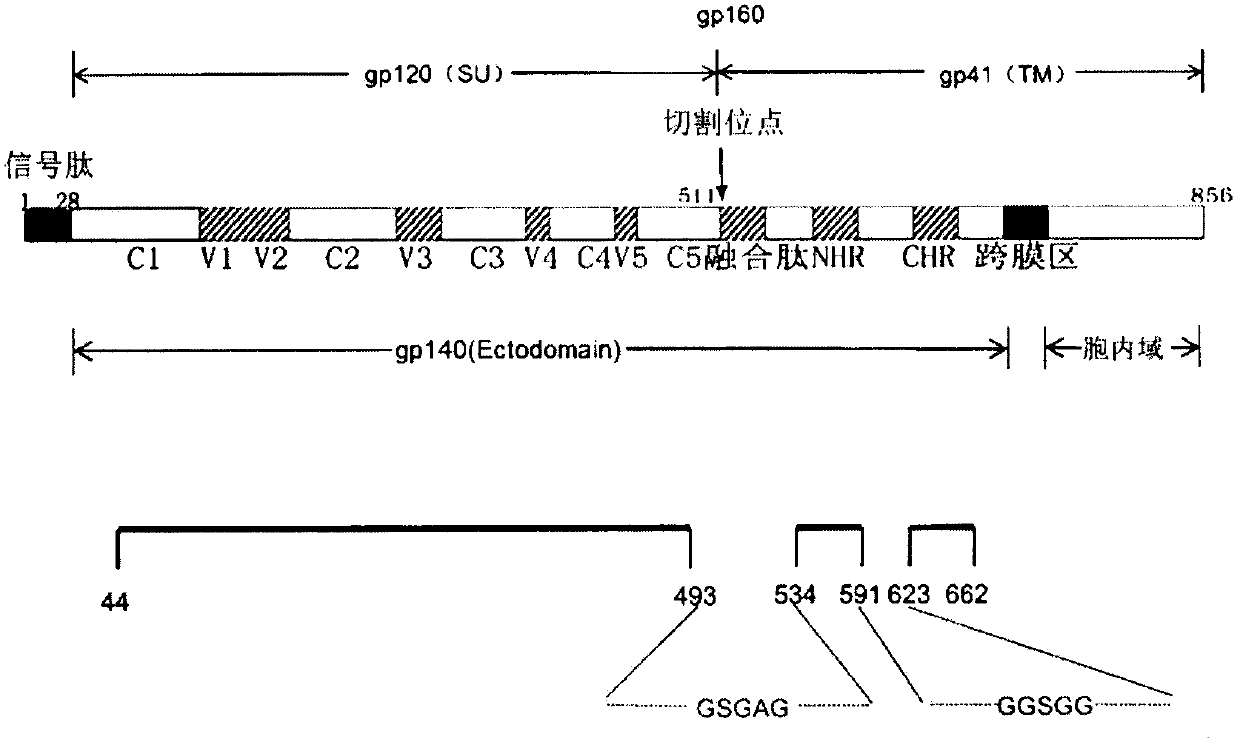
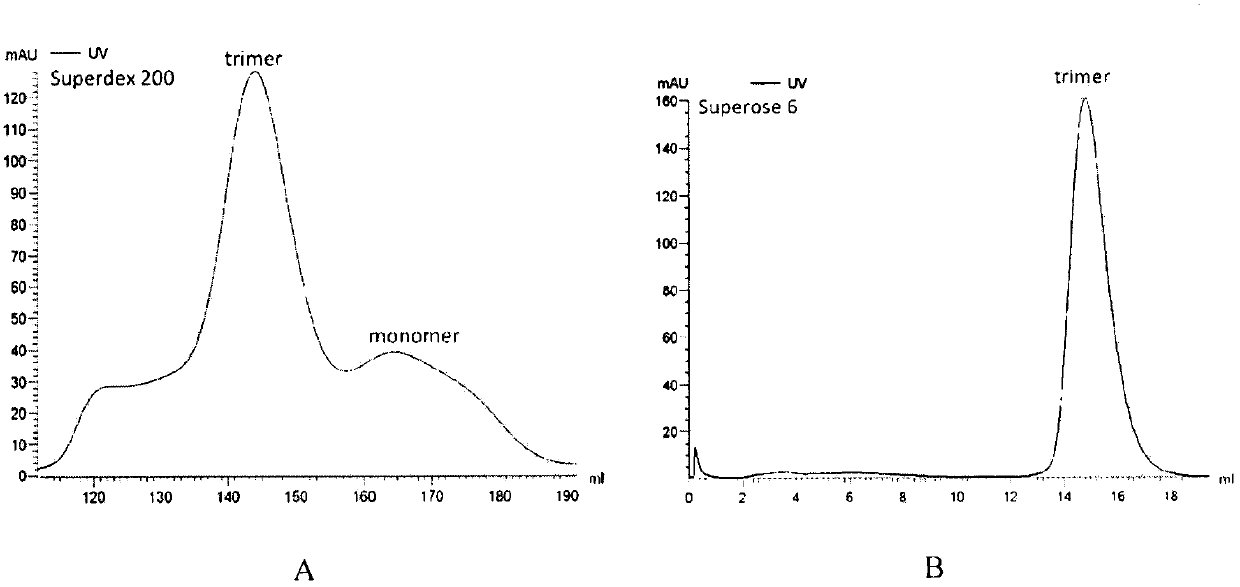
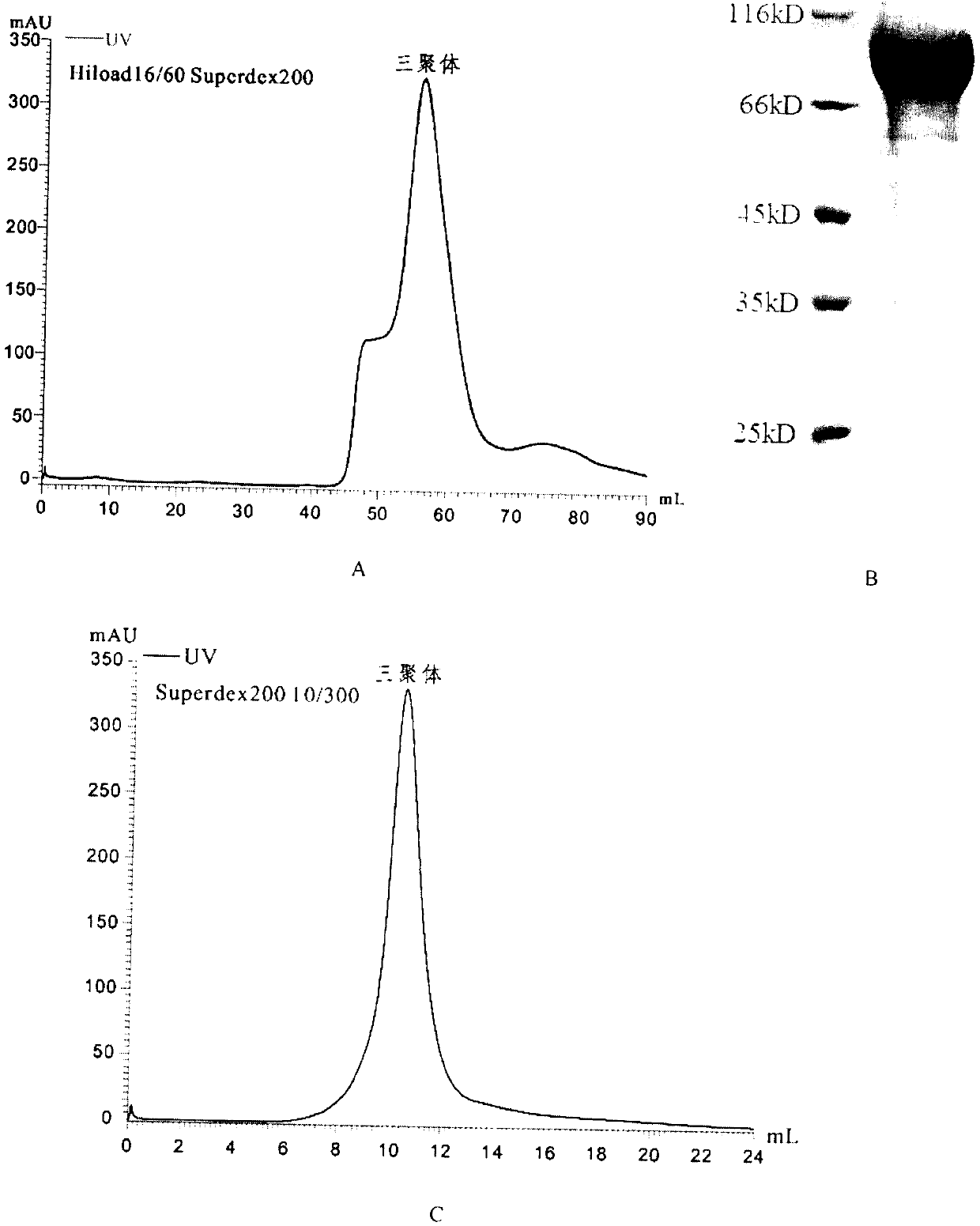
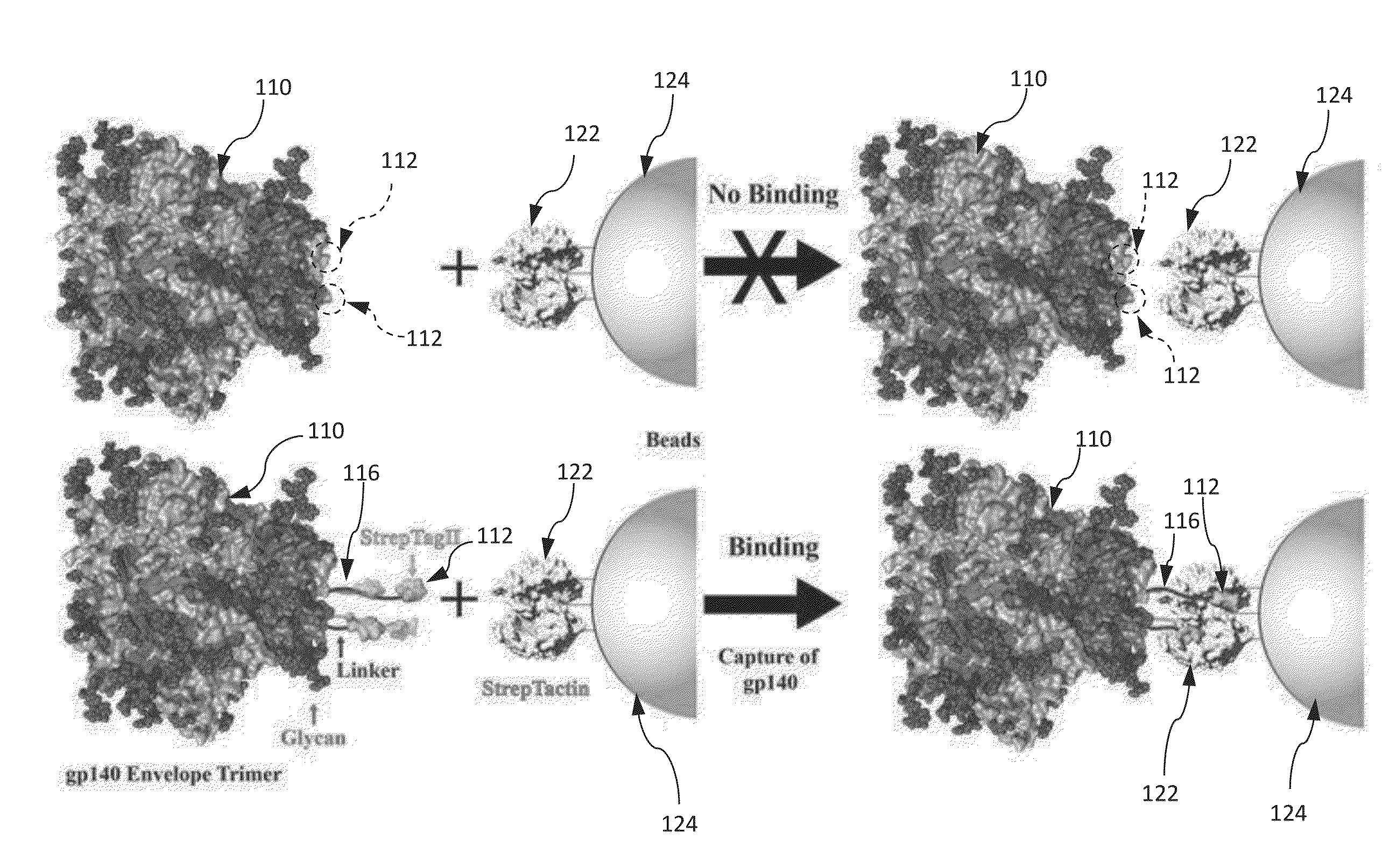
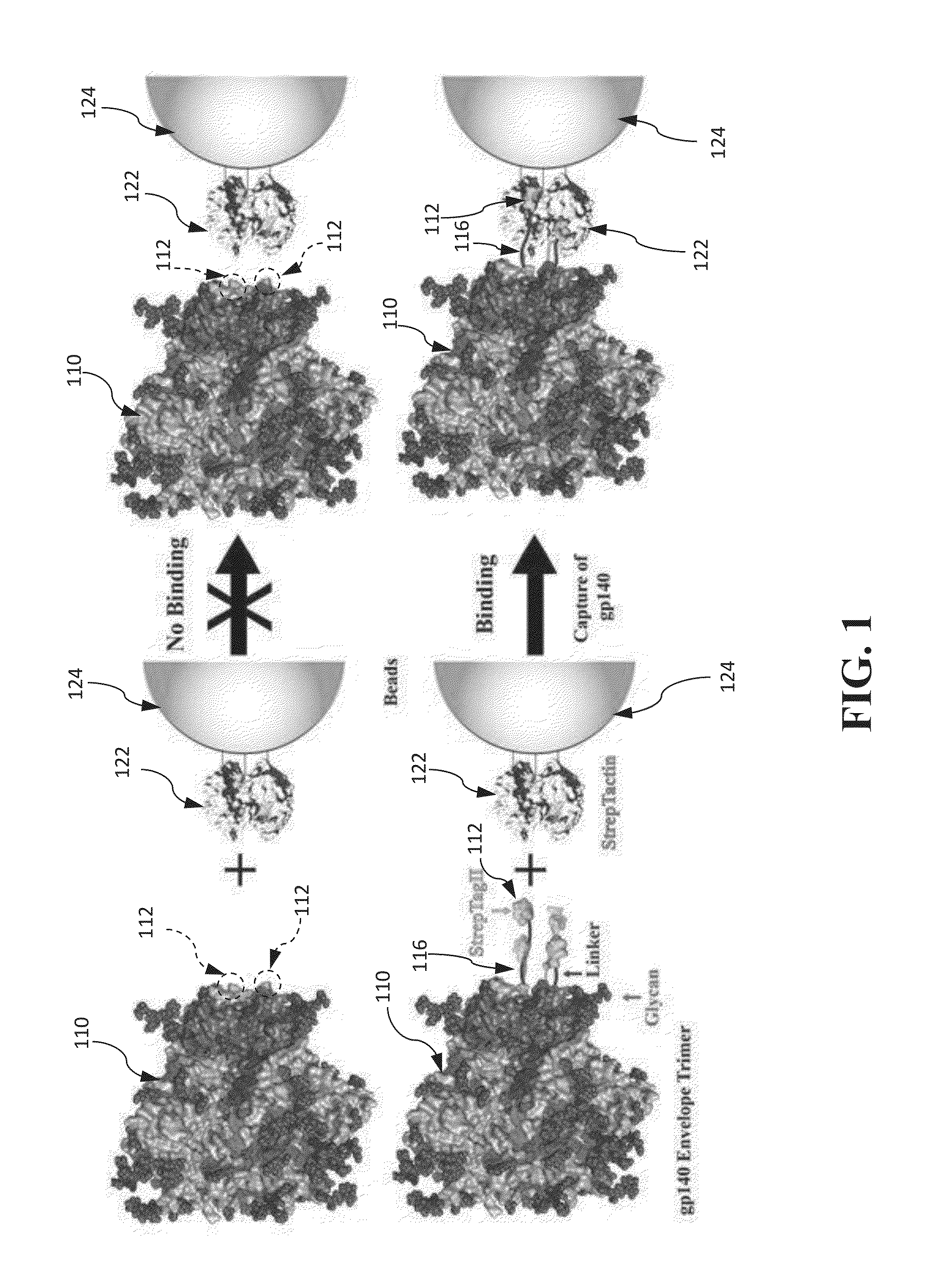
Approach to produce HIV-1 GP140 envelope protein trimers
Owner:CATHOLIC UNIV OF AMERICA
Modified HIV-1 envelope proteins
InactiveUS8017126B2Avoid infectionReduce the amount requiredSsRNA viruses positive-senseBacteriaHiv 1 envelopeVirology
Owner:INSTITUTE OF TROPICAL MEDICINE +1
Soluble HIV-1 envelope glycoprotein trimers
The present application relates to novel HIV-1 envelope glycoproteins which may be utilized as an HIV-1 vaccine immunogens, antigens for crystallization and for the identification of broad neutralizing antibodies. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE +1
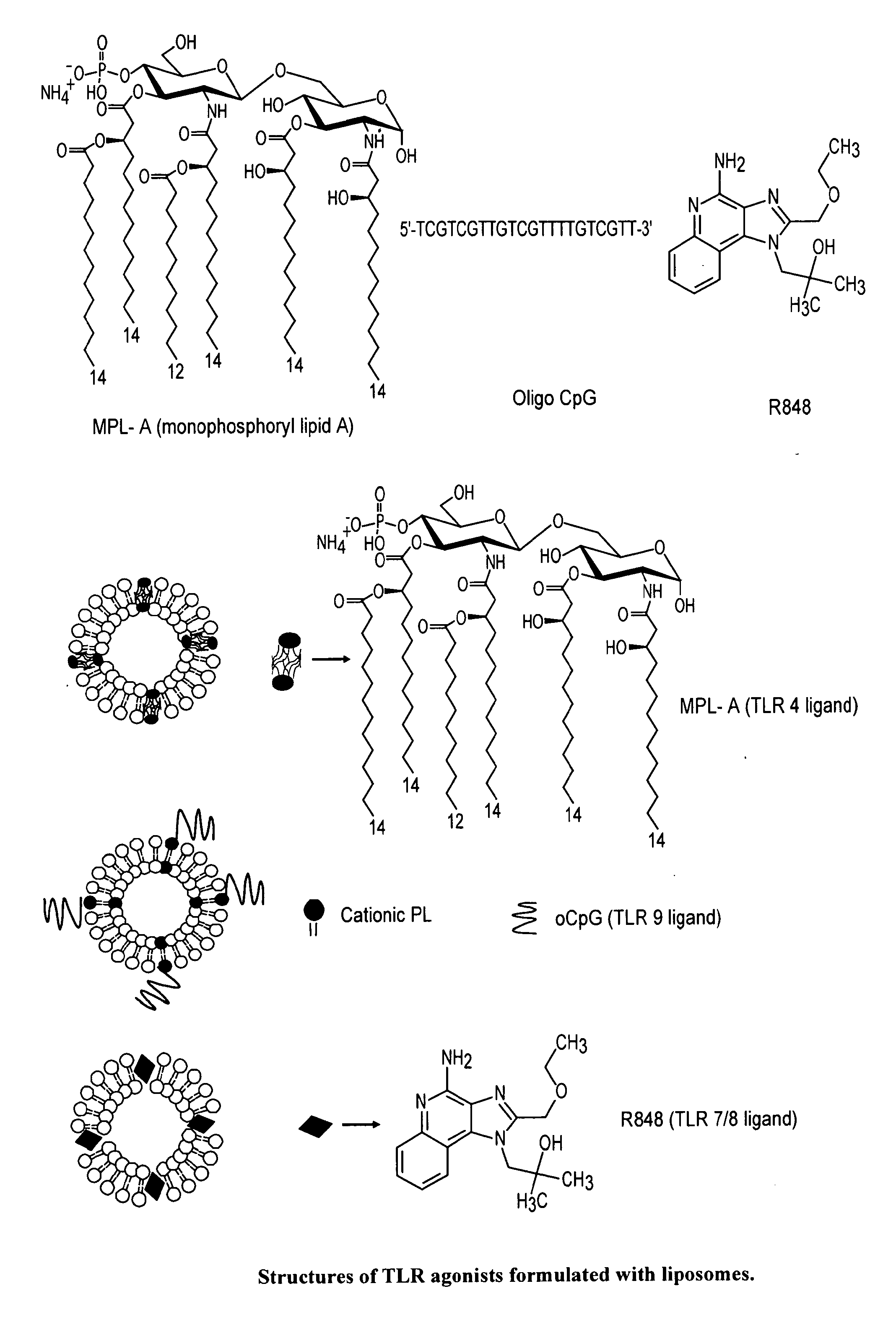
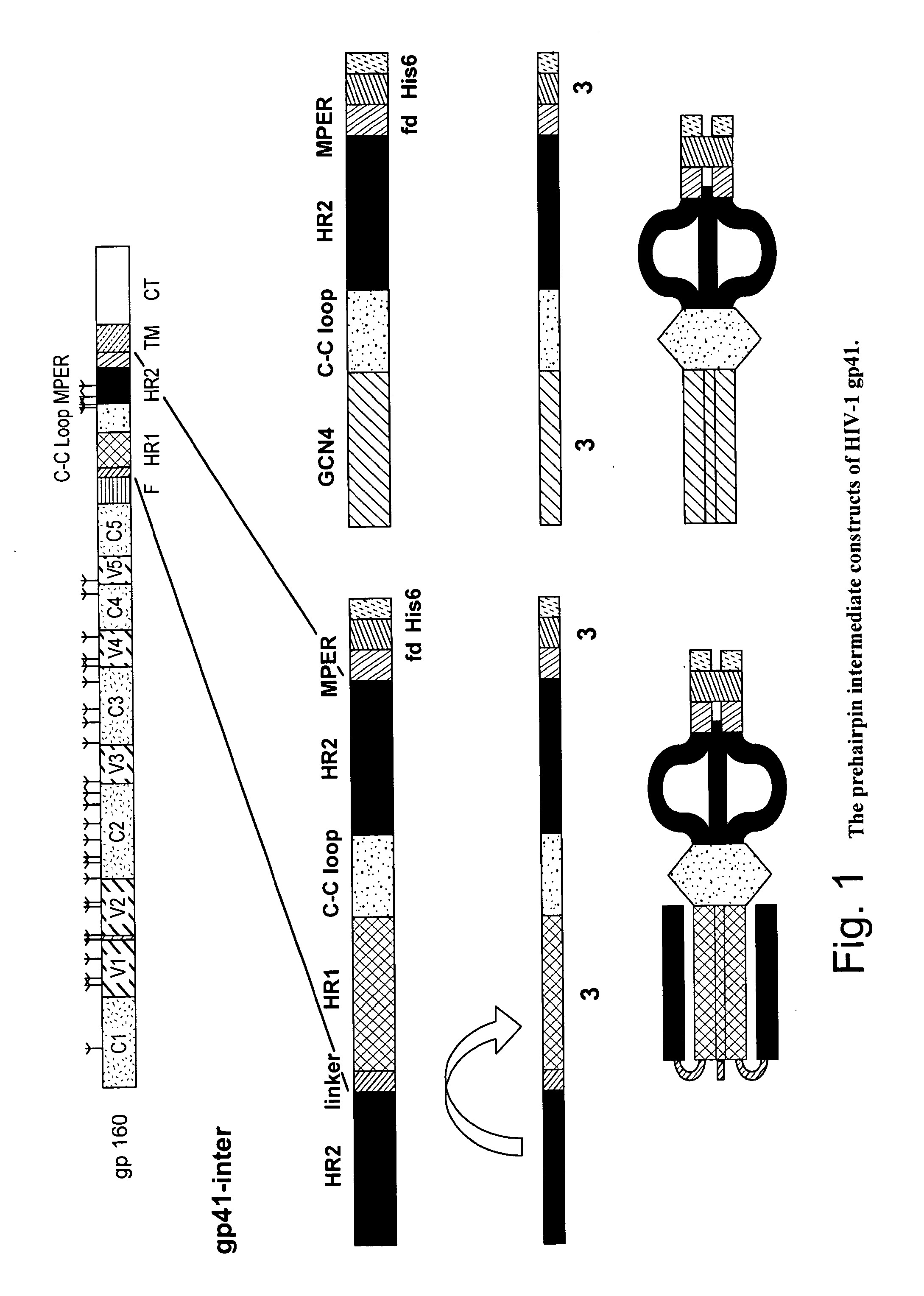
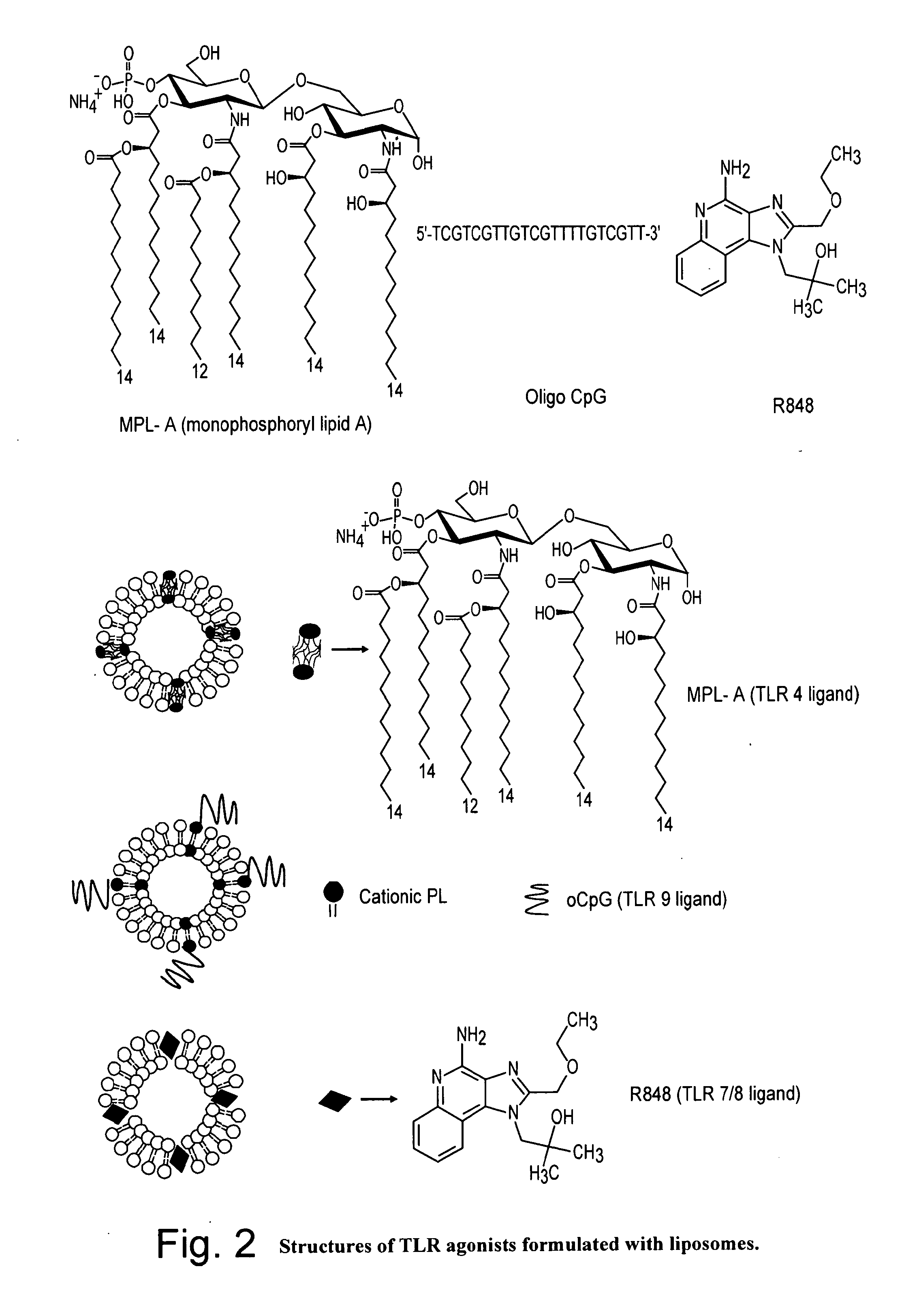
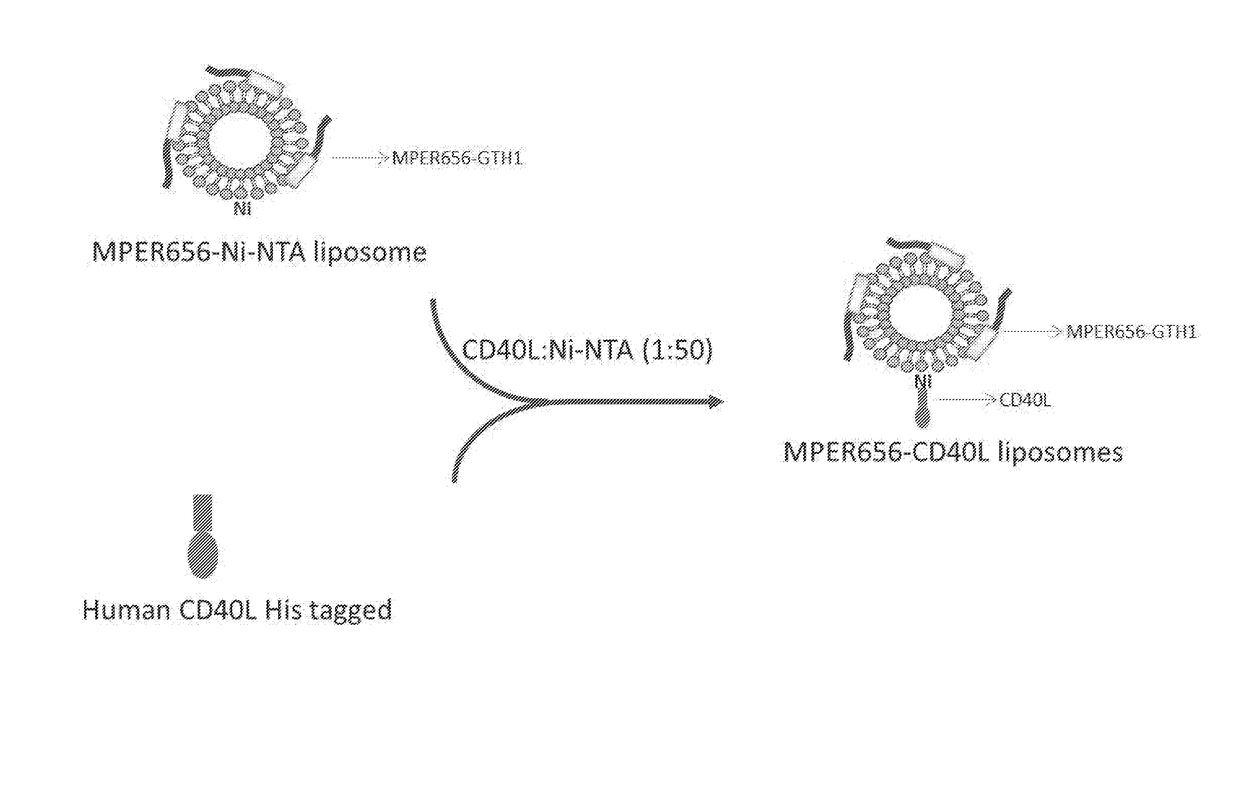
Formulation
The present invention relates, in general, to a formulation suitable for use in inducing anti-HIV-1 antibodies and, in particular, to a formulation comprising a prehairpin intermediate form of HIV-1 envelope gp41 linked to a liposome. The invention also relates to methods of inducing broadly neutralizing anti-HIV-1 antibodies using such a formulation.
Owner:DUKE UNIV +1
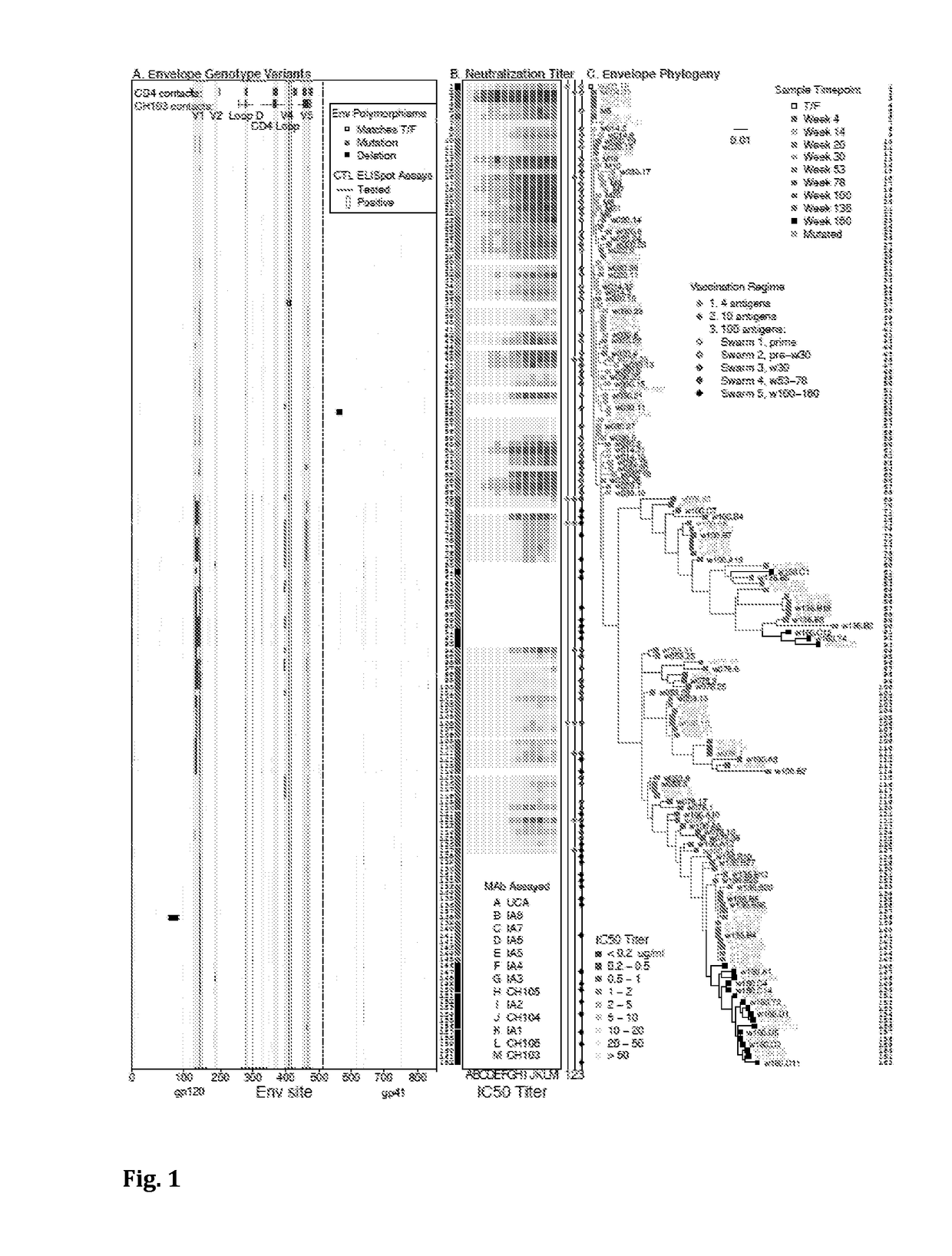
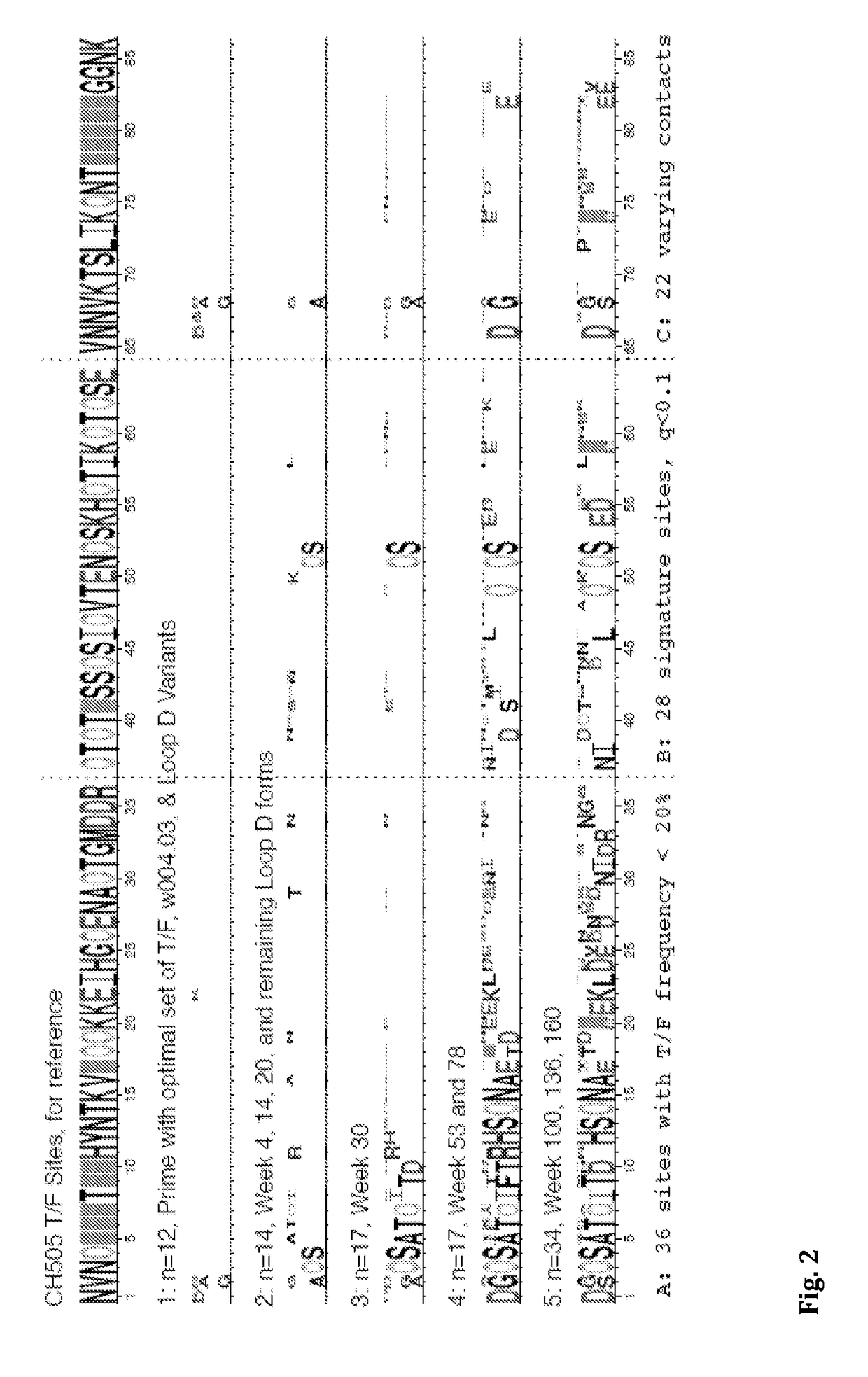
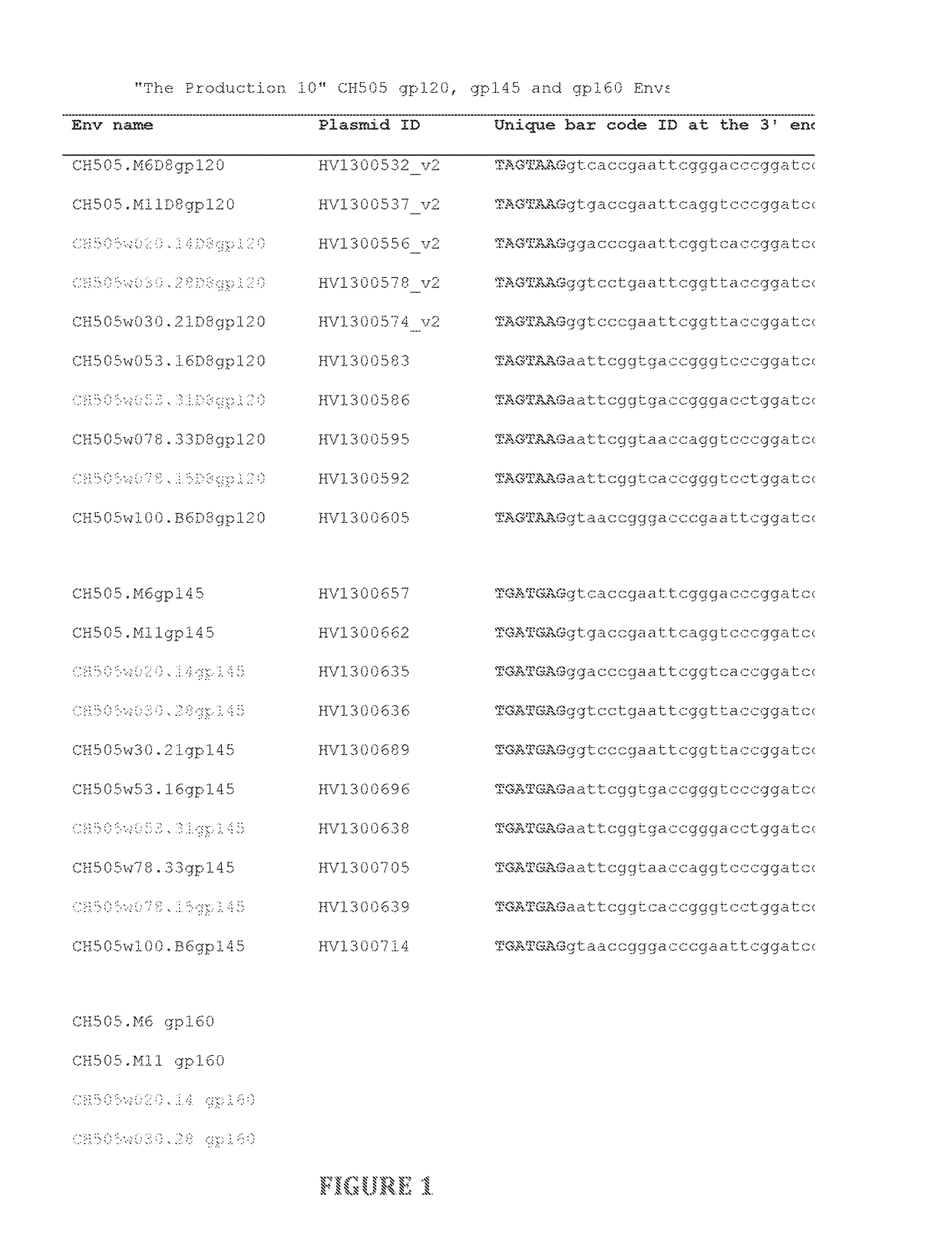
Swarm immunization with envelopes from ch505
Owner:DUKE UNIV +1
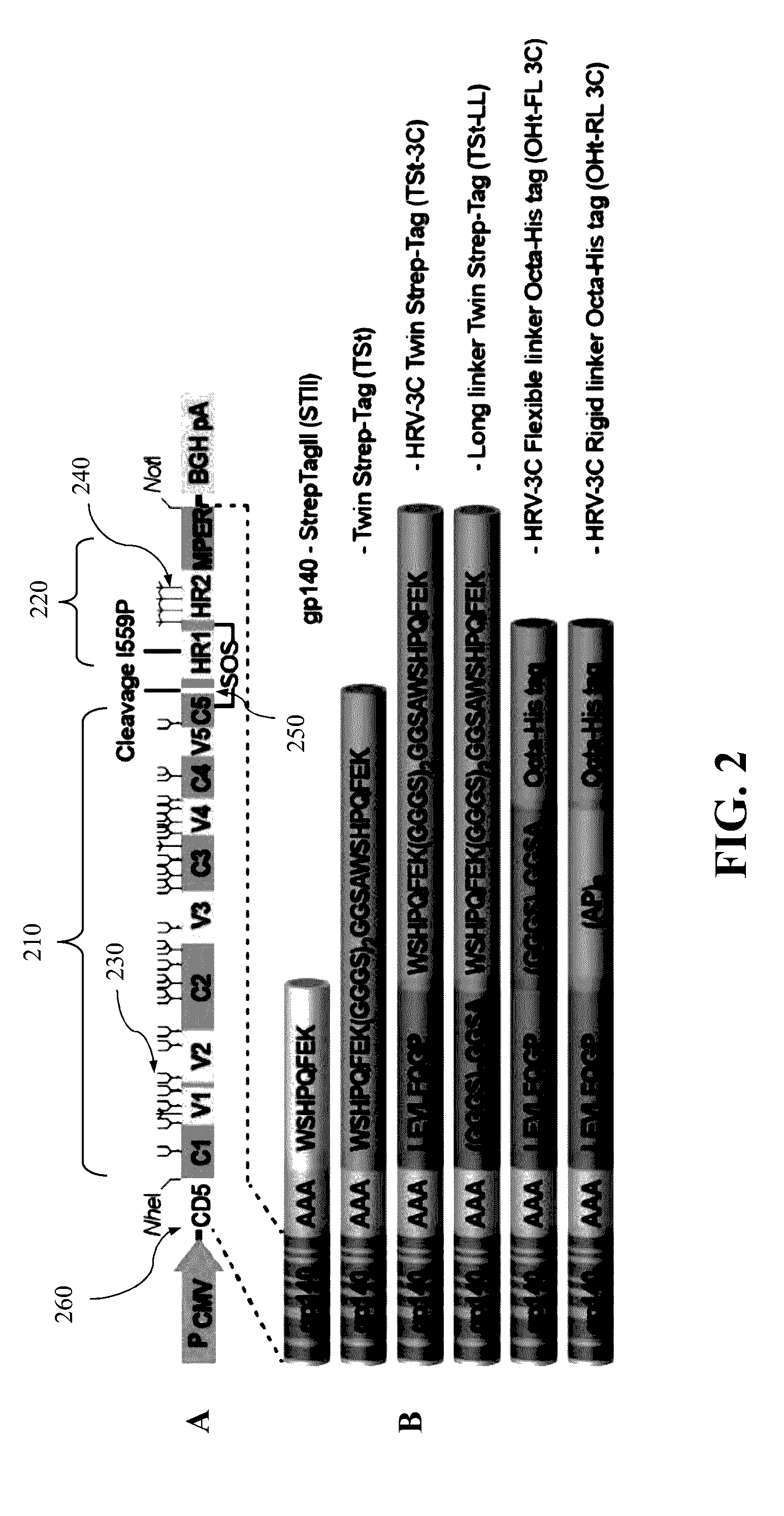
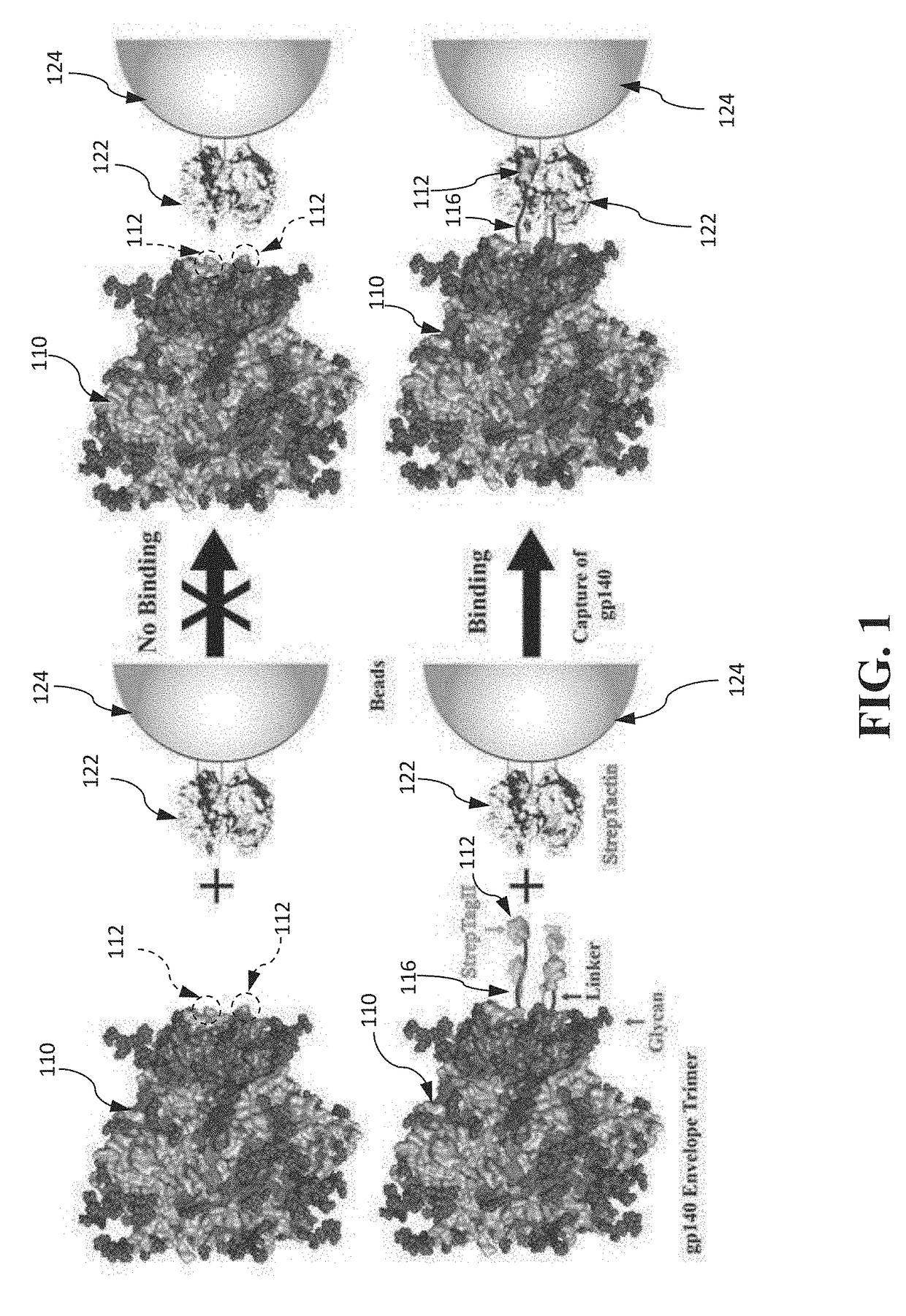
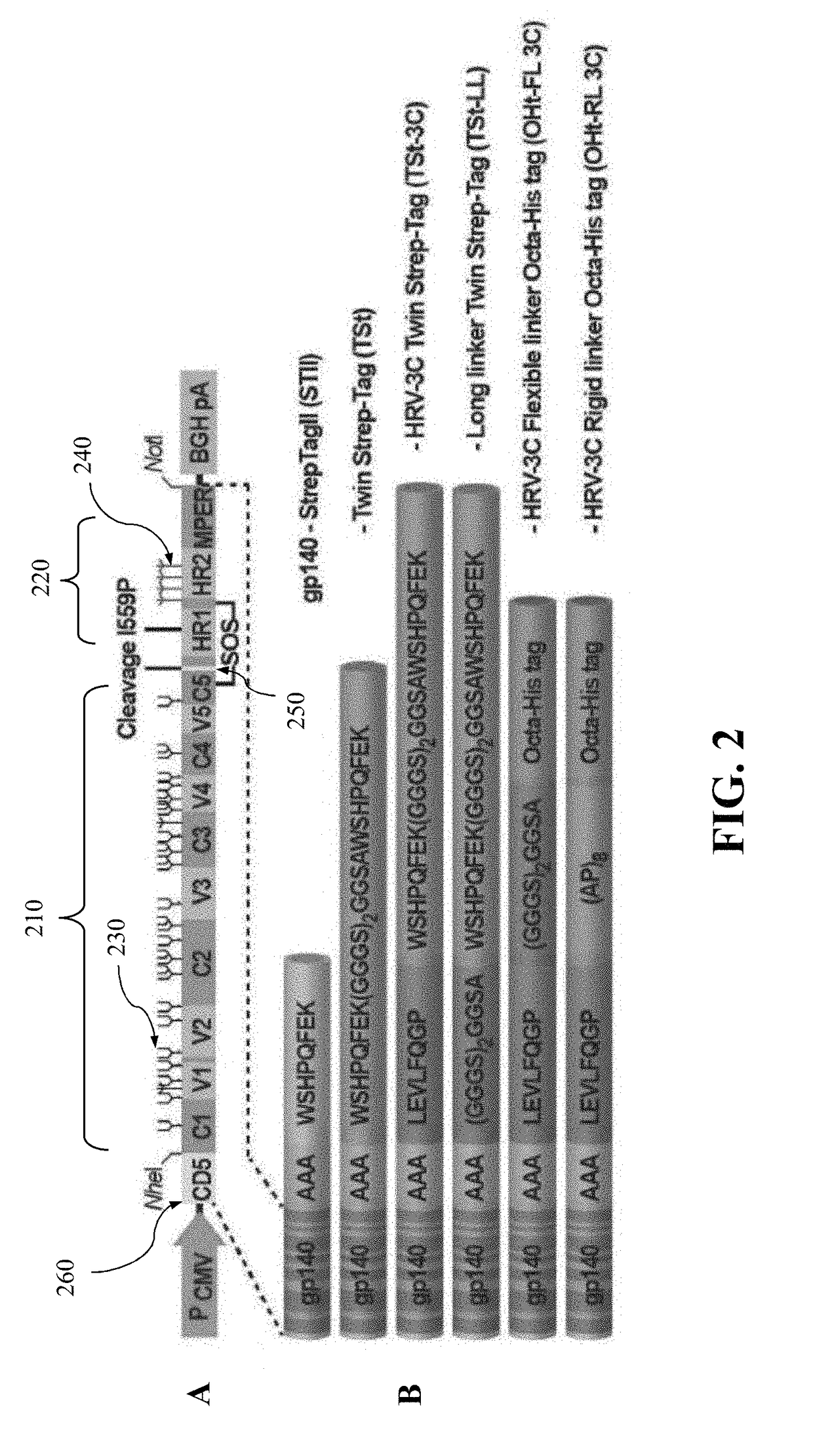
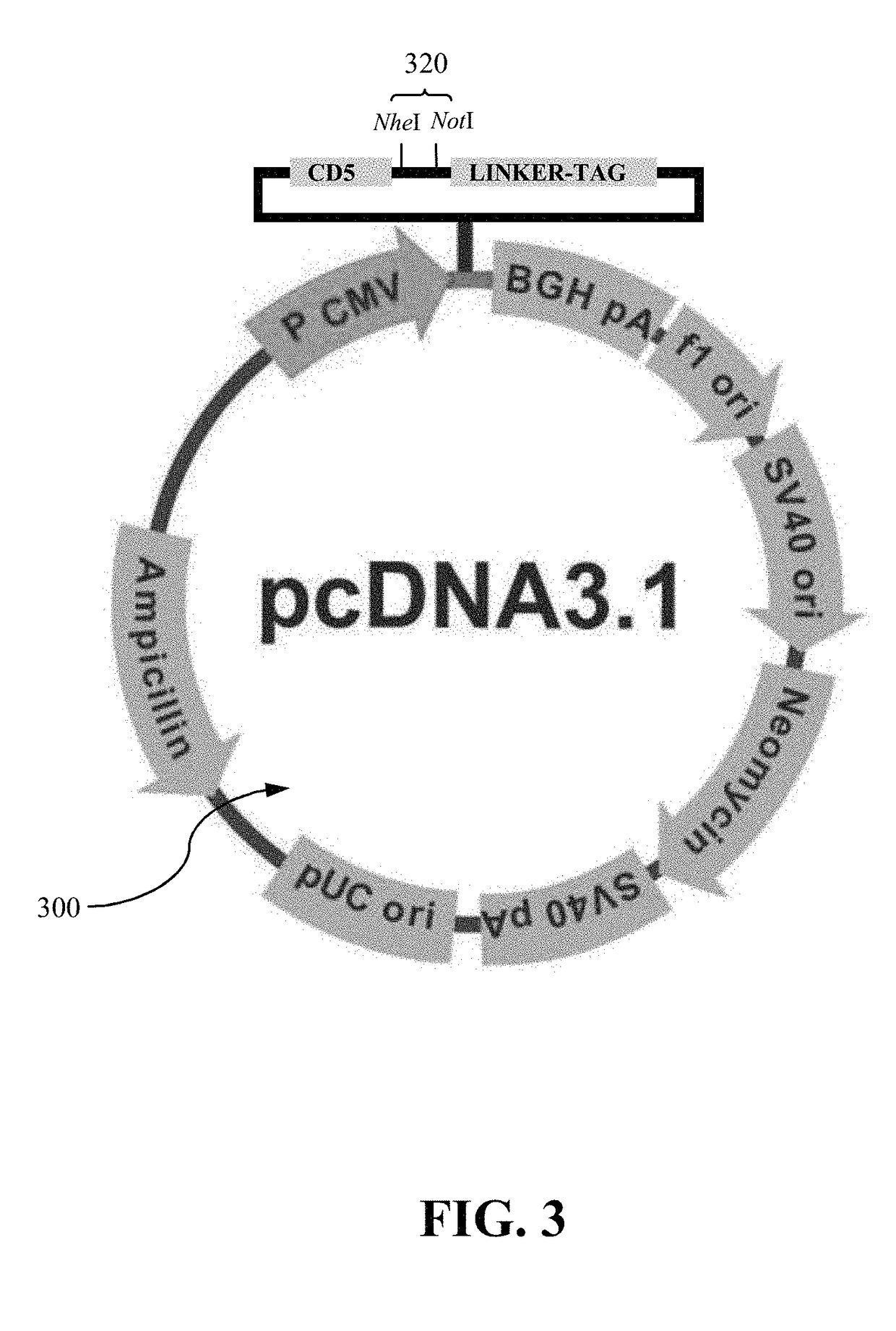
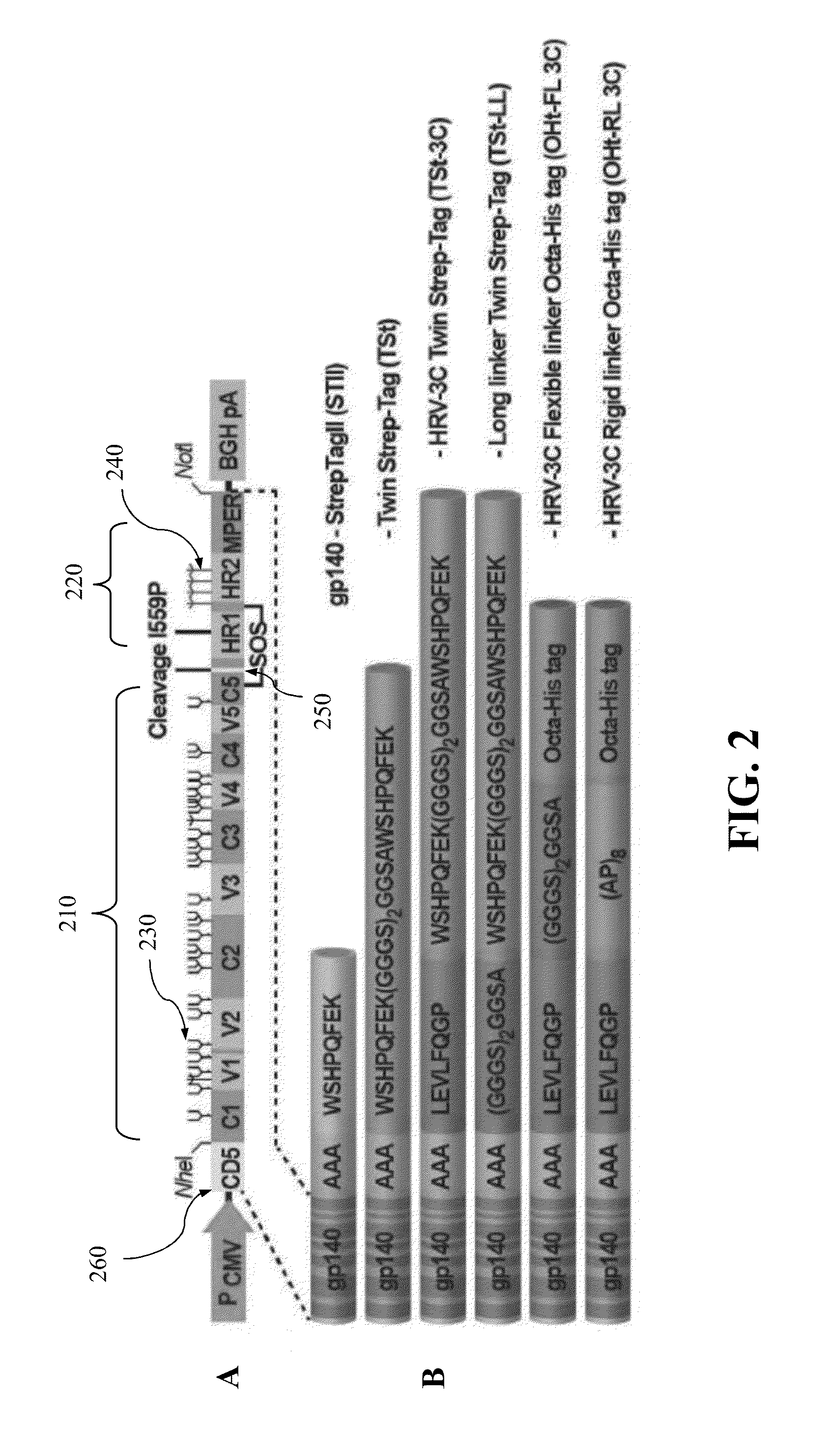
Authentic trimeric HIV-1 GP140 envelope glycoproteins comprising a long linker and tag
An approach of producing recombinant trimers that mimic native HIV-1 envelope trimers is developed. A recombinant protein forming the recombinant trimers encompasses a recombinant HIV-1 gp140 fused to a tag through a linker at C-terminus of the recombinant HIV-1 gp140. The linker is sufficiently long so that the tag is accessible for binding by a binding molecule bound on a solid matrix. After expressed in a cell, the recombinant protein is secreted into the culture medium and assembles into recombinant trimers therein. The recombinant trimers may be directly purified from the culture medium. Cleaved and uncleaved trimers from different clade viruses are produced.
Owner:CATHOLIC UNIV OF AMERICA
CD4-mimetic inhibitors of HIV-1 entry and methods of use thereof
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +2
Envelope protein trimer immunogen capable of inducing HIV-1 broad spectrum and neutralizing antibody and application thereof
ActiveCN110627911AGood immune resultsStrong broad spectrumAntibody mimetics/scaffoldsViral antigen ingredientsProtein trimerSequence design
The invention relates to the field of biomedicine, relates to an envelope protein trimer immunogen capable of inducing HIV-1 broad spectrum and neutralizing antibody and application thereof, in particular to an envelope protein Env common gene sequence designed based on HIV-1 Chinese epidemic strain and a stable envelope protein gp120 trimer immunogen obtained based on the sequence. The inventionfurther relates to the application of the HIV-1 envelope protein gp120 trimer immunogen in preparation of AIDS vaccines.
Owner:JILIN UNIV
Altered Immunogenic Landscape in HIV-1 Envelope Proteins
The present invention provides compositions and methods useful in the prevention and treatment of HIV-1 infection in a host subject. High affinity binding of an allosteric dual antagonist to HIV-1 gp120 induces a conformational change in the gp 120 protein that traps the gp 120 protein in a three-dimensional structure that suppresses its function and exposes novel antigenic epitopes to host immune surveillance.
Owner:PHILADELPHIA HEALTH & EDUCATION CORP
Cd4-mimetic inhibitors of hiv-1 entry and methods of use thereof
ActiveUS20140350113A1Avoid transmissionInhibit progressBiocideOrganic chemistryHiv 1 envelopeHIV receptor
Described herein are small-molecule mimics of CD4, which both enter the Phe43 cavity and target Asp368 of gp120, the HIV-1 envelope protein. Also described herein are methods of using these compounds to inhibit the transmission or progression of HIV infection. These compounds exhibit antiviral potency greater than that of a known antiviral, NBD-556, with 100% breadth against clade B and C viruses. Importantly, the compounds do not activate HIV infection of CD4-negative, CCR5-positive cells, in contrast to NBD-556.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +2
Approach to produce hiv-1 gp140 envelope protein trimers
ActiveUS20160271243A1Viral antigen ingredientsAntibody mimetics/scaffoldsProtein trimerHiv 1 envelope
Provided herein are HIV vaccines that encompasses recombinant trimers that mimic native HIV-1 envelope trimers. Also provided are methods of administering to a subject in need thereof an HIV vaccine provided herein to elicit antibodies against a recombinant trimer in the subject. A recombinant trimer is formed by a recombinant protein comprising a recombinant HIV-1 gp140 fused to a tag through a linker at C-terminus of the recombinant HIV-1 gp140, wherein the linker is sufficiently long so that the tag is accessible for binding by a binding molecule bound on a solid matrix during purification of the recombinant trimer.
Owner:CATHOLIC UNIV OF AMERICA
Compositions and methods for transient immune response modulation during vaccination
InactiveUS20170216426A1Facilitate cell uptakeHigh expressionOrganic active ingredientsPeptide/protein ingredientsVaccinationHiv 1 envelope
In certain aspects the invention provides a selection of HIV-1 envelopes suitable for use as immunogens, and methods of using these immunogens to induce neutralizing antibodies. In certain embodiments, the immunogens are designed to trimerize. In other embodiments, the immunogenic compositions and methods comprise at least one agent for transient immune response modulation during vaccination.
Owner:DUKE UNIV
HIV-1 envelope glycoprotein
ActiveUS20140037681A1Increase generationViral antigen ingredientsVirus peptidesHiv 1 vaccineHiv 1 envelope
The present application relates to novel HIV-1 envelope glycoproteins which may be utilized as an HIV-1 vaccine immunogens, antigens for crystallization and for the identification of broad neutralizing antibodies. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE
Expression and characterization of HIV-1 envelope protein associated with a broadly reactive neutralizing antibody response
The present invention relates to HIV-1 envelope proteins from a donor with non-progressive HIV-1 infection whose serum contains broadly cross-reactive, primary virus neutralizing antibody. The invention also relates to isolated or purified proteins and protein fragments that share certain amino acids at particular positions with the foregoing HIV-1 proteins.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com