Formulation
a formulation and peptide technology, applied in the field of formulation, can solve the problem that most induced antibodies are ineffective in preventing infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Description of gp41 MPER Peptide-gp41 Prehairpin Intermediate Conjugates
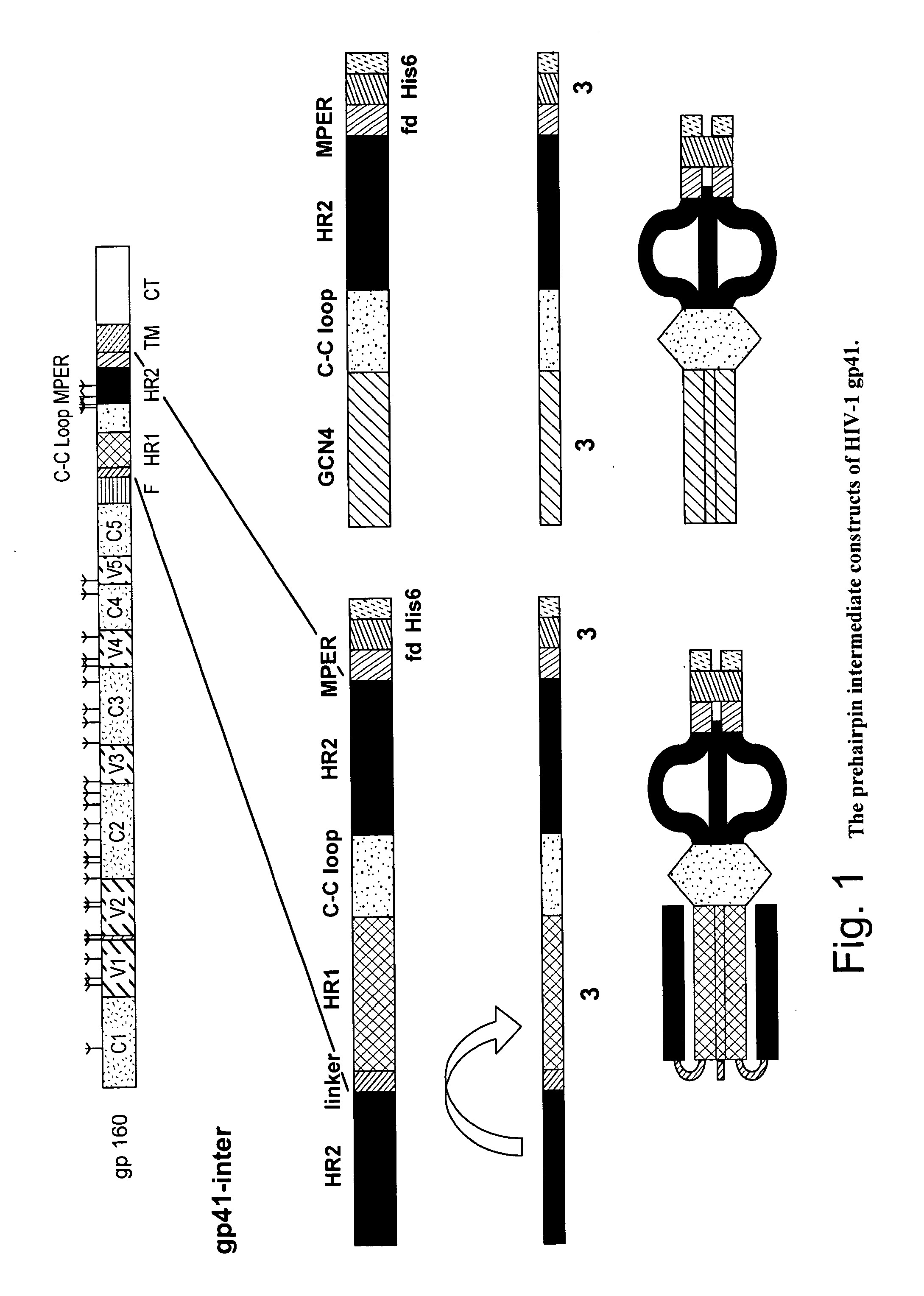
[0032]FIG. 1 shows the prehairpin intermediate forms of the HIV-1 gp41 MPER that can be conjugated to synthetic liposomes (Frey et al, Proc. Natl. Acad. Sci. 105:3739-3744 (2008)). To produce biochemically homogeneous forms of additional conformations, two constructs were made that were designed to capture gp41 in the extended, prehairpin intermediate conformation. As shown in FIG. 1, gp41-inter has the following sequence: (HR2)-linker-[HR1-CC loop-HR2-MPER]-(trimerization tag), where HR1 and HR2 are the first and second “heptad repeat” in gp41 (the segments that form helices in the postfusion trimer of hairpins) and the sequence in brackets is essentially the complete gp41 ectodomain, except for the fusion peptide. The “linker” is a short, flexible connector of serines and glycines. When gp41-inter chains trimerize, the N-terminal HR2 segments to form a six-helix bundle with the HR1 segments; the C-terminal HR2...
example 2
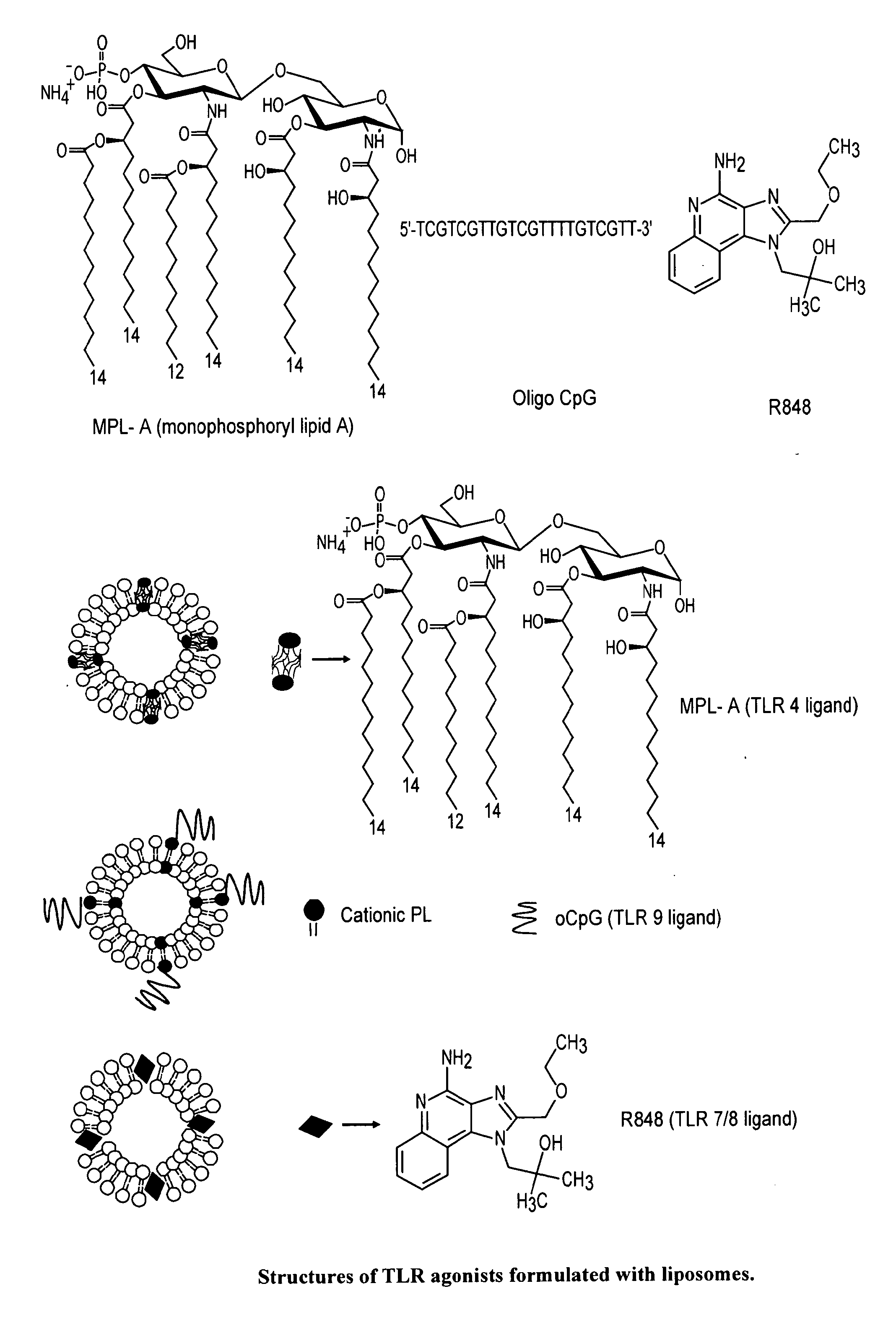
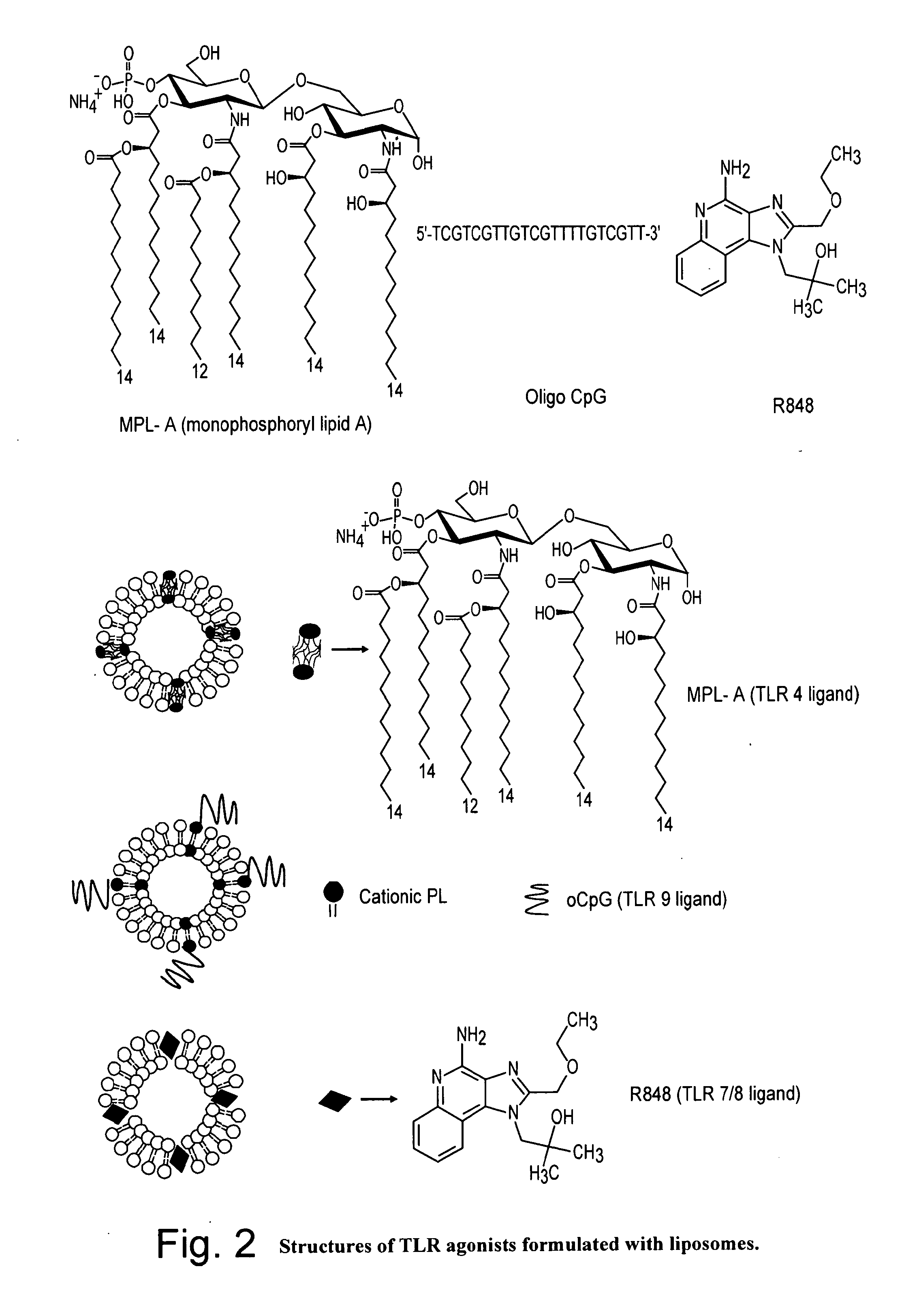
[0039]Autoreactive B cells can be activated by TLR ligands through a mechanism dependent on dual engagement of the BCR and TLR (Leadbetter et al, Nature 416:603 (2002); Marshak-Rothstein et al, Annu. Rev. Immunol. 25:419-41 (2007), Herlands et al, Immunity 29:249-260 (2008), Schlomchik, Immunity 28:18-28 (2008)). In this immunogen design, soluble IFN-α has been encapsulated into liposomes conjugated to either MPER656 or MPER656-L669S peptides. IFN-α has been reported to modulate and relax the selectivity for autoreactive B cells by lowering the BCR activation threshold (Uccellini et al, J. Immunol. 181:5875 (2008)). The design of these immunogens is also based on the observation that lipid reactivity of gp41 MPER antibodies is required for both binding to membrane bound MPER epitopes and in the neutralization of HIV-1.
[0040]The long CDR H3 loops of MPER neutralizing mAbs 4E10 and 2F5 have a hydrophobic face, postulated to interact with virion membrane lipids (Ofek et al, J. Virol. 7...
example 3
Experimental Details
[0044]Ni-NTA (N″,N″-bis[carboxymethyl]-L-lysine; nitriloacetic acid, NTA) liposomes were constructed from synthetic lipids POPC, POPE, DOGS (1,2 dioleoyl-sn- glycerol-3-succinyl-NTA-Ni) and cholesterol at mole fractions 45, 25, 5 and 25 respectively using methods described earlier (Alam et al., J. Immunol. 178:4424-4435 (2007)). Conjugation of His tagged gp41-inter to the Ni-NTA liposomes was verified by surface plasmon resonance experiment. The His tagged gp41-inter when injected over the immobilized liposomal surfaces bound selectively to the Ni-NTA liposomes when compared to the control liposomes that lacked Ni-NTA. The presentation of epitopes of MPER neutralizing antibodies in the liposome conjugated gp41-inter was examined by comparing the binding of 2F5 and 4E12 mAbs to the gp41-inter bearing Ni-NTA liposomes with that of unconjugated Ni-NTA liposomes. Both 2F5 and 4E10 mAbs bound selectively to the gp41-inter bearing Ni-NTA liposomes
Results
[0045]FIG. 10 s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| axial distance | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com