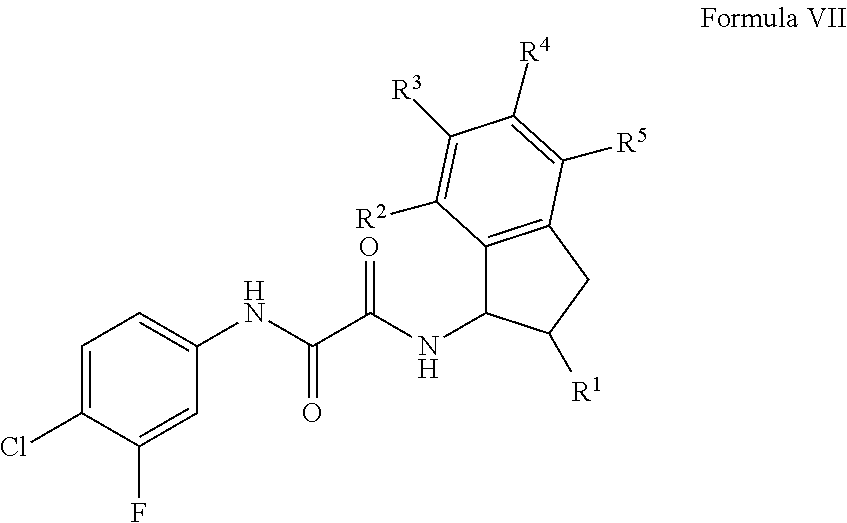
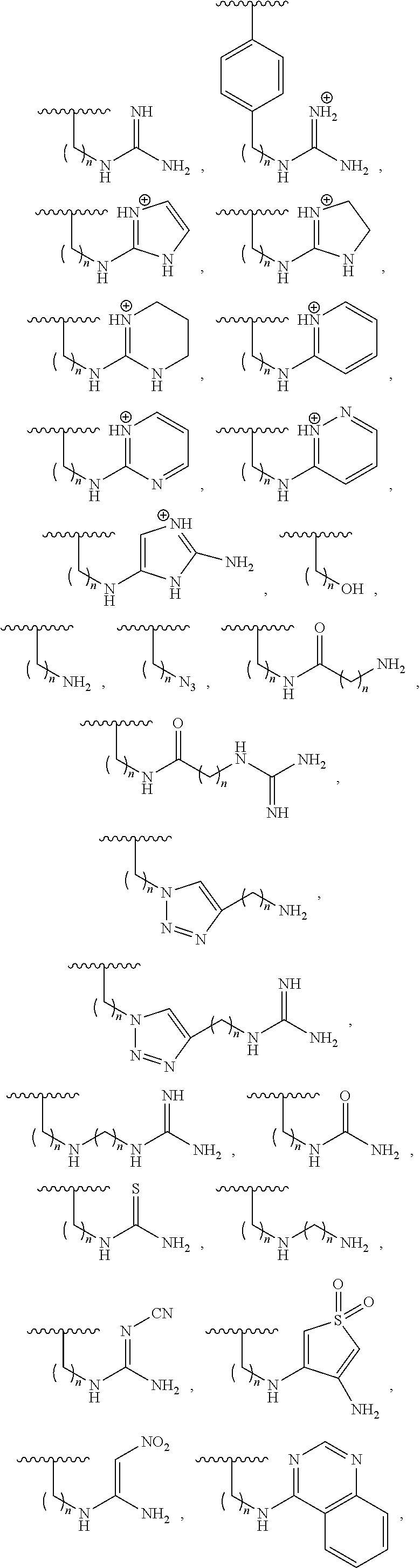
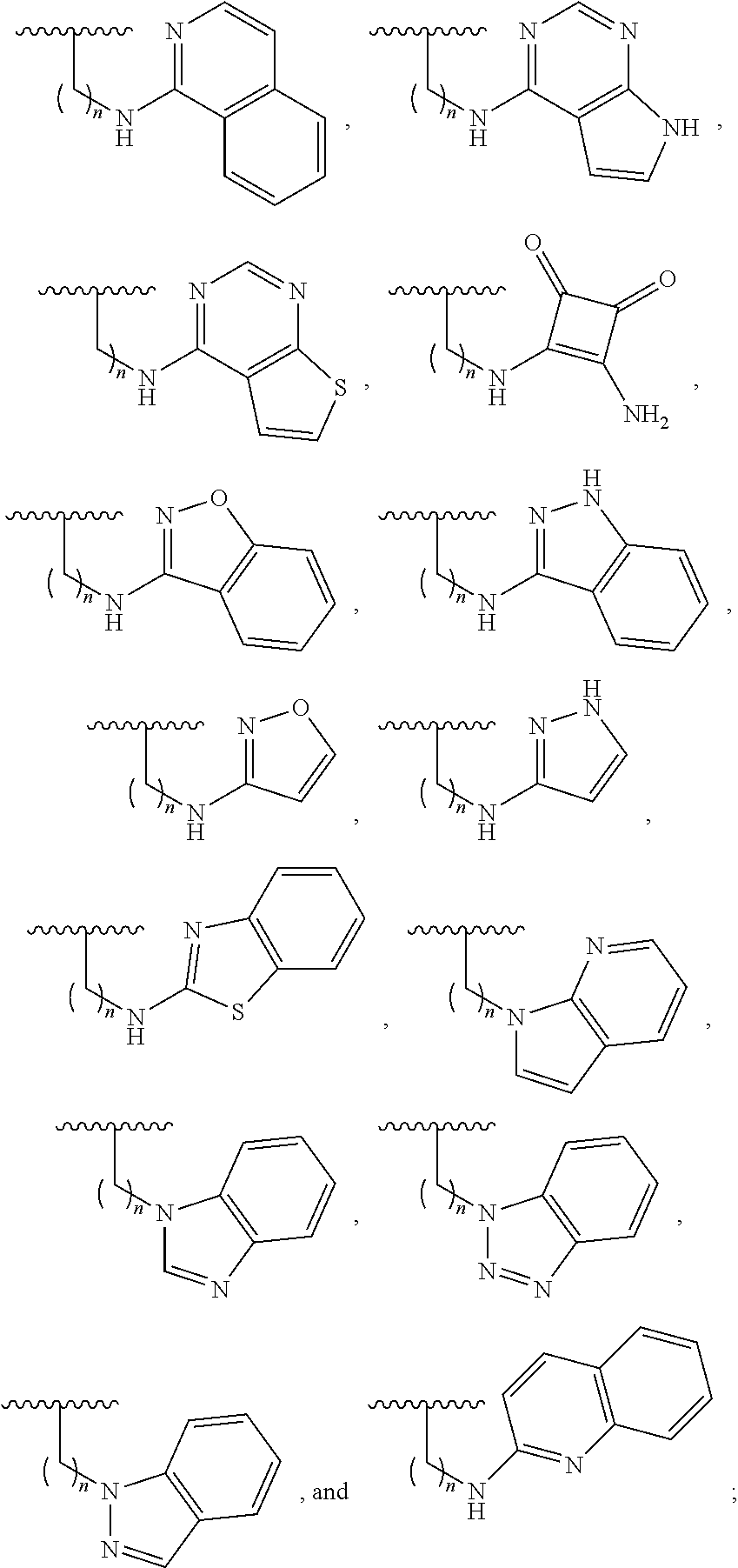
Cd4-mimetic inhibitors of hiv-1 entry and methods of use thereof
a technology of mimic inhibitors and inhibitors, applied in the direction of amide active ingredients, biocide, organic chemistry, etc., can solve the problems of refractory problems, difficult capture in small-molecule scaffolds, and inability to successfully integrate electrostatic interactions into nbd small-molecule design, etc., to inhibit transmission or progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Molecular Design and Synthesis
[0234]Analysis of the crystal structure of TS-II-224 (2) (FIG. 1) and NBD-556 bound to gp120 provided the opportunity to design small-molecule interactions with the Asp368 12o hotspot in the vestibule of the Phe43 cavity. Kwon, Y. D., et al. Submitted to PNAS (2011). The TS-II-224 crystal structure (FIG. 1 and FIG. 5d) indicated the close proximity of the C4 linker on the Region III tetramethylpiperidine with the carboxylate side-chain of Asp368gp120. Rather than performing systematic synthetic modifications of the tetramethylpiperidine moiety, a virtual screening strategy was chosen to identify a replacement moiety for Region III that would contain a basic amine oriented towards Asp368gp120. Hence, an analogue possessing a primary amine attached to C4 of the tetramethylpiperidine was constructed in silico. While the diamine (FIG. 1a) is not a chemically stable entity, it was used as an archetype to replicate desired interactions. The prototype was asse...
example 2
AWS-I-169 (9) and DMJ-I-228 (10) Inhibit Viral Infection and Compete with CD4
[0236]To evaluate anti-viral activity of the novel compounds, indanes analogues (3-10) and TS-II-224 (2) were first tested in mono-tropic (isolates that infect cells expressing CD4 / CCR5 or CD4 / CXCR4) and dual-tropic (isolates that infect cells expressing CD4 / CCR5 and CD4 / CXCR4) HIV-1 strains in single-round infection of recombinant HIV-1 encoding firefly luciferase. The recombinant viruses employed were pseudotyped with HIV-1 envelope glycoproteins derived from either an X4, laboratory-adapted HXBc2 isolate, or the R5, primary YU2 isolate. As a control for specificity, the viruses were pseudotyped with the envelope glycoproteins of the amphotropic murine leukemia virus (A-MLV), an unrelated retrovirus. Notably, in the case of both mono-tropic (HXBc2) and dual tropic (89.6 and KB9) viruses, AWS-I-169 (9) and DMJ-I-228 (10) inhibited entry on cells co-expressing CD4 and CXCR4 (FIG. 9 and FIG. 10) with the IC5...
example 3
DMJ-I-228 (10) Displays a Thermodynamic Signature Resembling Small Molecule Binding
[0238]Analogue binding to full-length gp120 from the YU2 strain was next characterized by isothermal titration calorimetry to assess the enthalpic and entropic contributions to binding affinity (FIG. 7 and FIG. 3c). Analogue TS-II-224 (2) binds to gp120 with a Kd of 0.33 μM at 25° C., FIG. 7. Although the 1,2-diaminoindane analogues [AWS-I-45 (7) and AWS-I-50 (8)] exhibit a three-fold loss in affinity, both AWS-I-169 (9) and DMJ-I-228 (10) bind with comparable affinity to TS-II-224 (2). As previously reported, the binding of CD4 to gp120 at 25° C. is associated with an enthalpy change of −34.5 kcal / mol that is partially compensated by a large unfavorable entropy change of −79 cal / (K×mol) and a change in heat capacity (ΔCp) of −1,800 cal / (K×mol). Schön, A. et al. Biochemistry 45, 10973-80 (2006). Such a binding event has the thermodynamic signature that resembles protein folding, rather than binding, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| dielectric constant | aaaaa | aaaaa |
| dielectric constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com