Patents
Literature
693 results about "CD4 antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
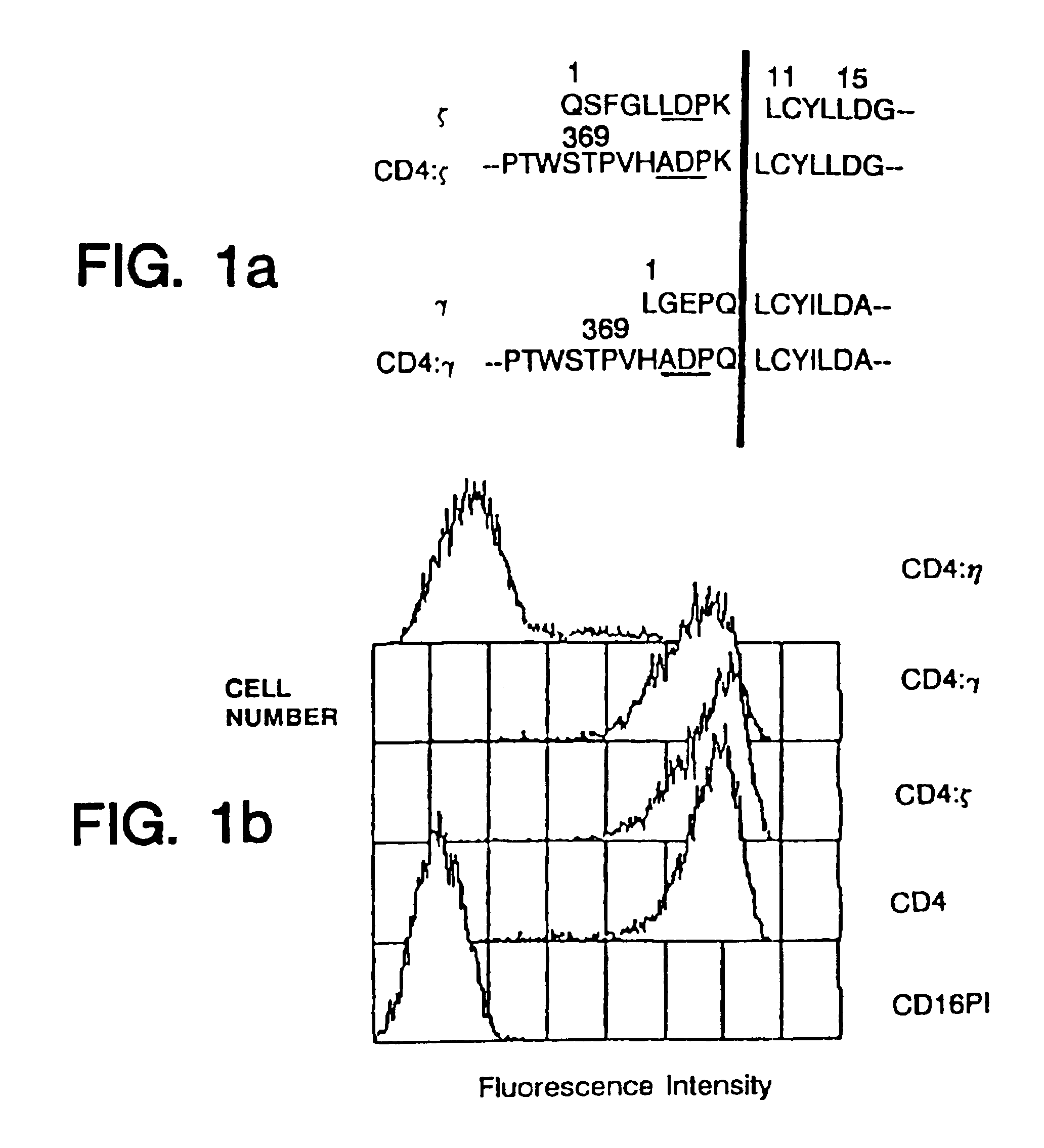
In molecular biology, CD4 (cluster of differentiation 4) is a glycoprotein found on the surface of immune cells such as T helper cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 (after the OKT4 monoclonal antibody that reacted with it) before being named CD4 in 1984. In humans, the CD4 protein is encoded by the CD4 gene.
CD19-specific chimeric T cell receptor
InactiveUS7446179B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingTransmembrane domain
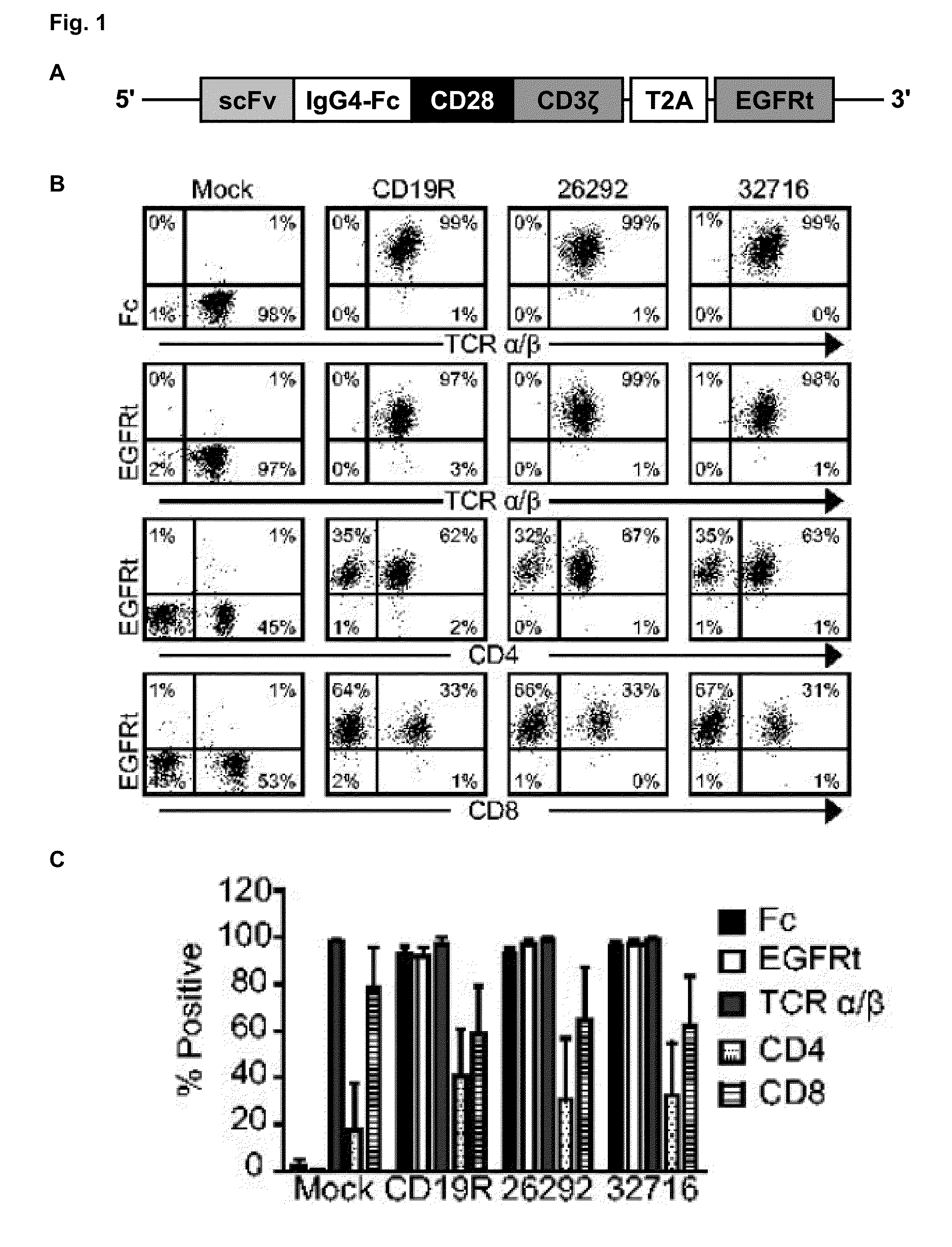
The present invention relates to a genetically engineered, CD19-specific chimeric T cell receptor and to immune cells expressing the chimeric receptor The present invention also relates to the use of such cells for cellular immunotherapy of CD9+ malignancies and for abrogating any untoward B cell function. The chimeric receptor is a single chain scFvFc:ζ receptor where scFvFc designates the extracellular domain, scFv designates the VH and VL chains of a single chain monoclonal antibody to CD19, Fc represents at least part of a constant region of an IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. The extracellular domain scFvFc and the intracellular domain ζ are linked by a transmembrane domain such as the transmembrane domain of CD4. In one aspect, the chimeric receptor comprises amino acids 23-634 of SEQ I DNO:2. The present invention further relates to a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor.
Owner:CITY OF HOPE
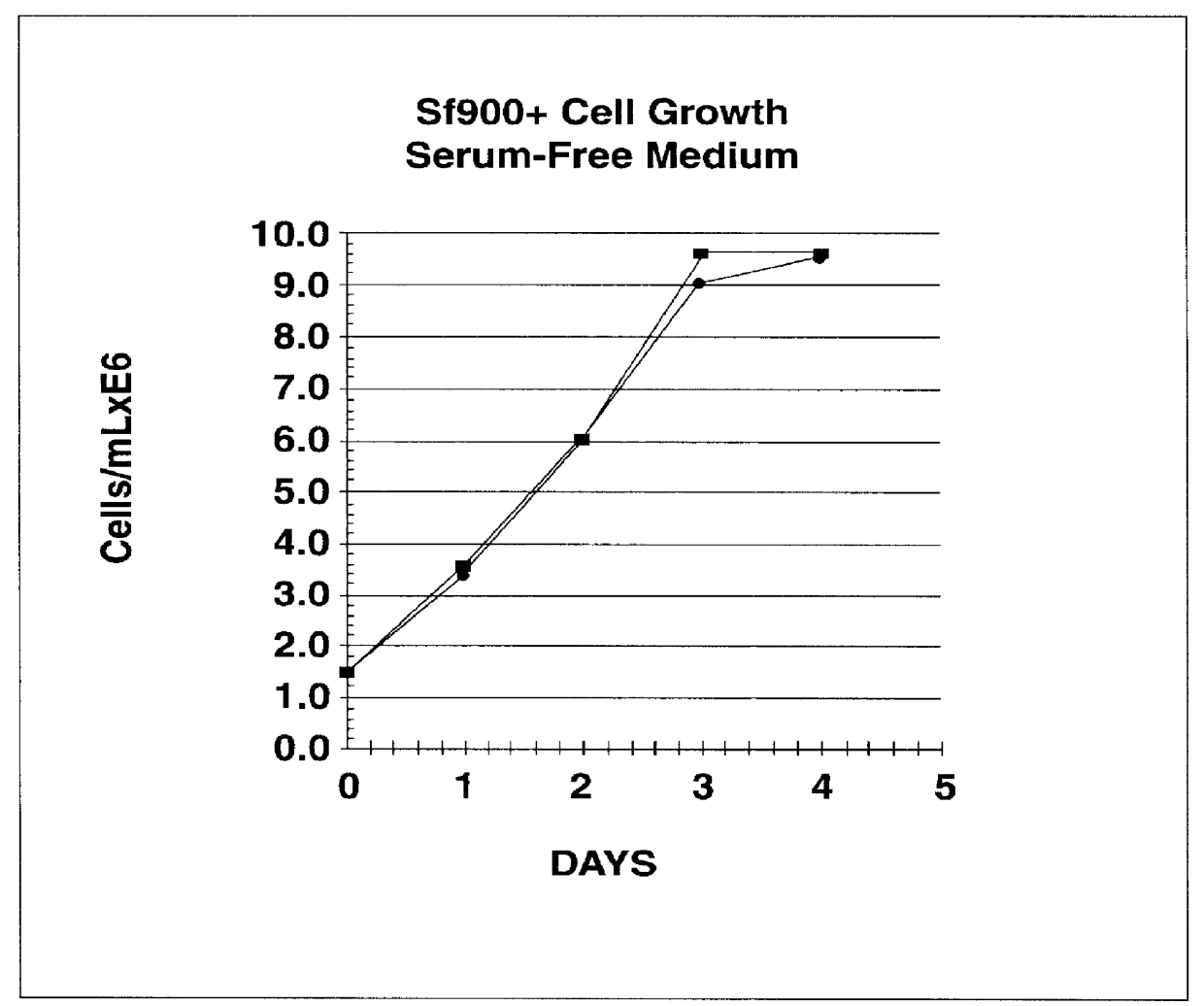
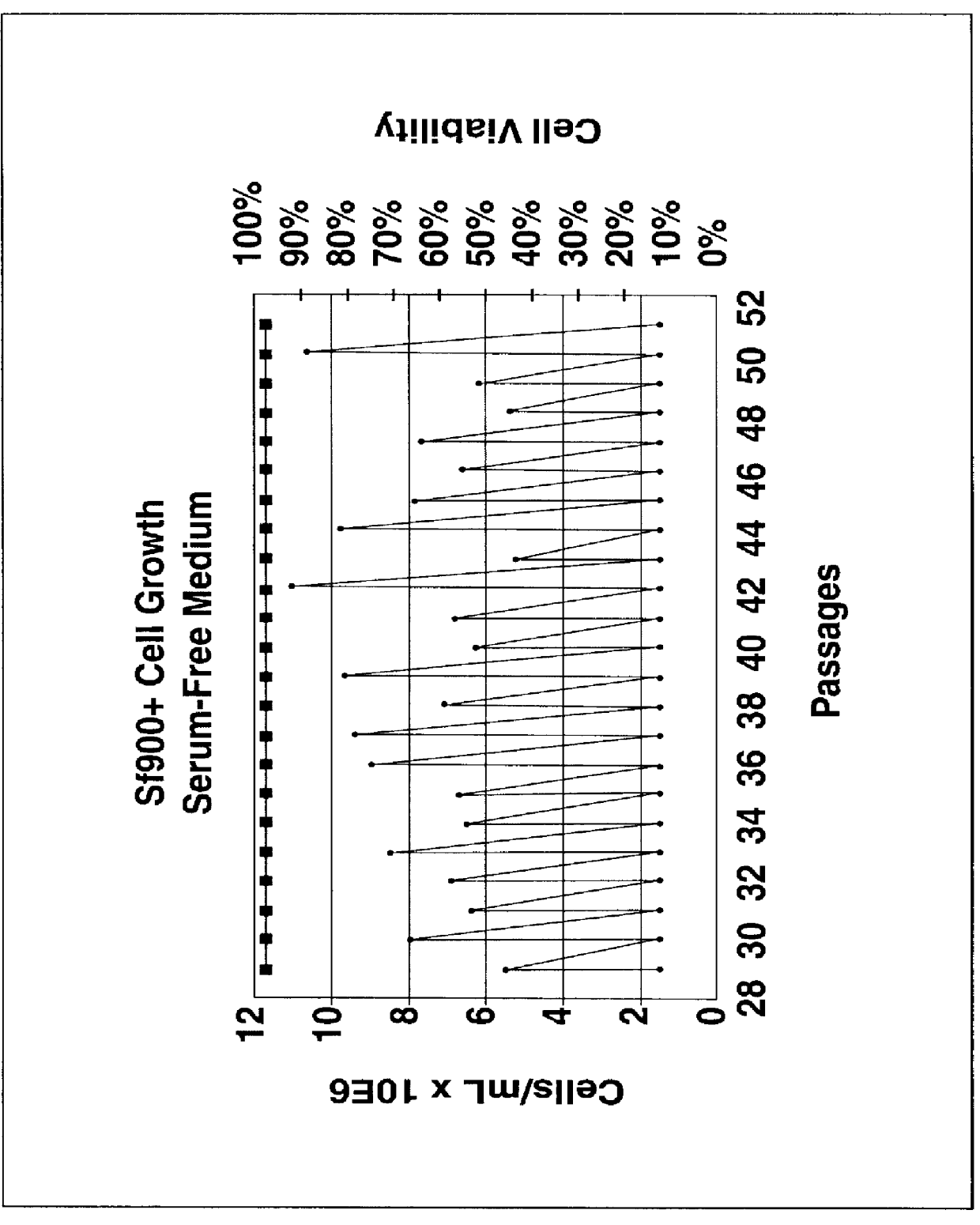
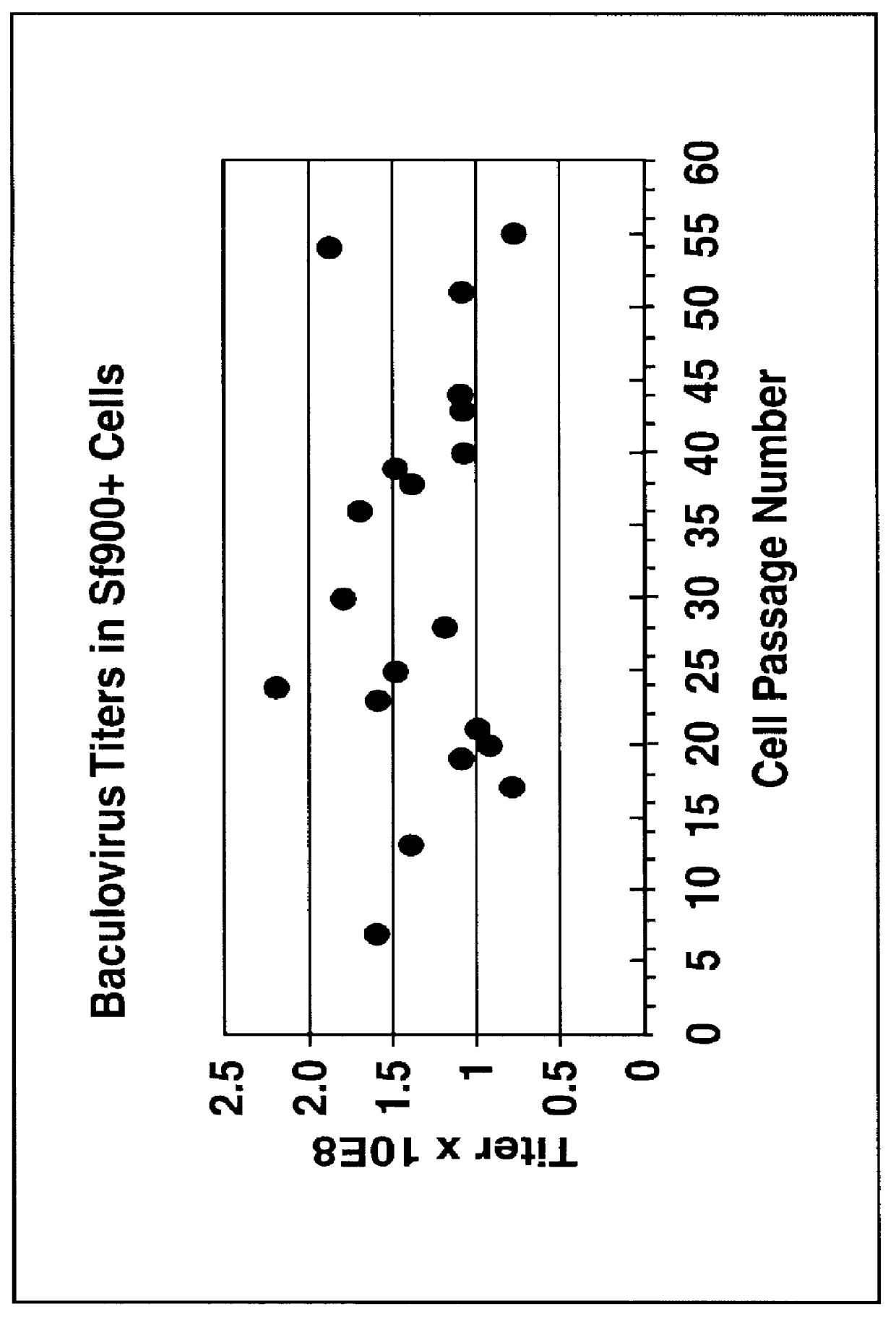
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
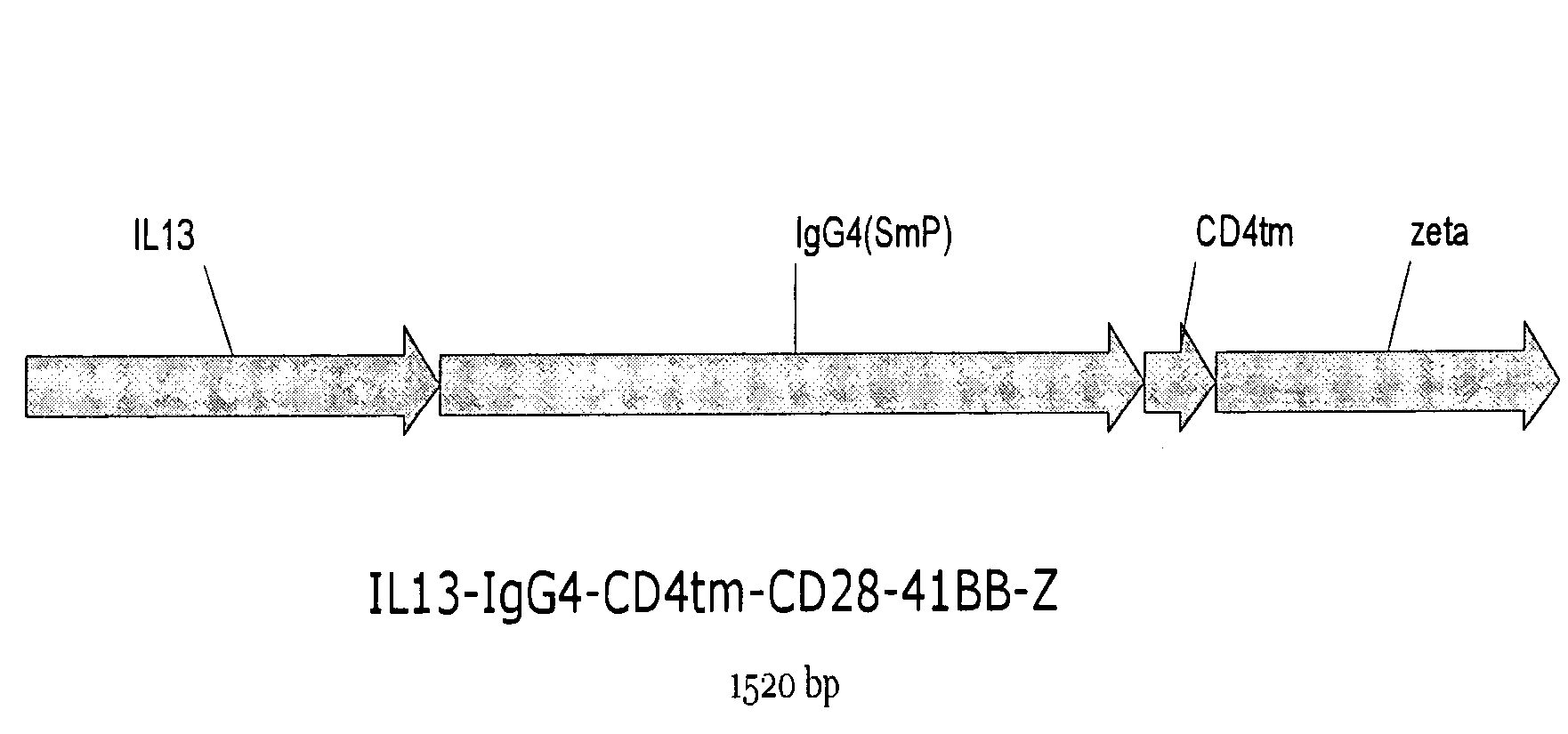
Chimeric immunoreceptor useful in treating human gliomas
InactiveUS7514537B2Negligible toxicityPotent and selectiveBiocideAntibody mimetics/scaffoldsIntracellular signallingHuman glioma
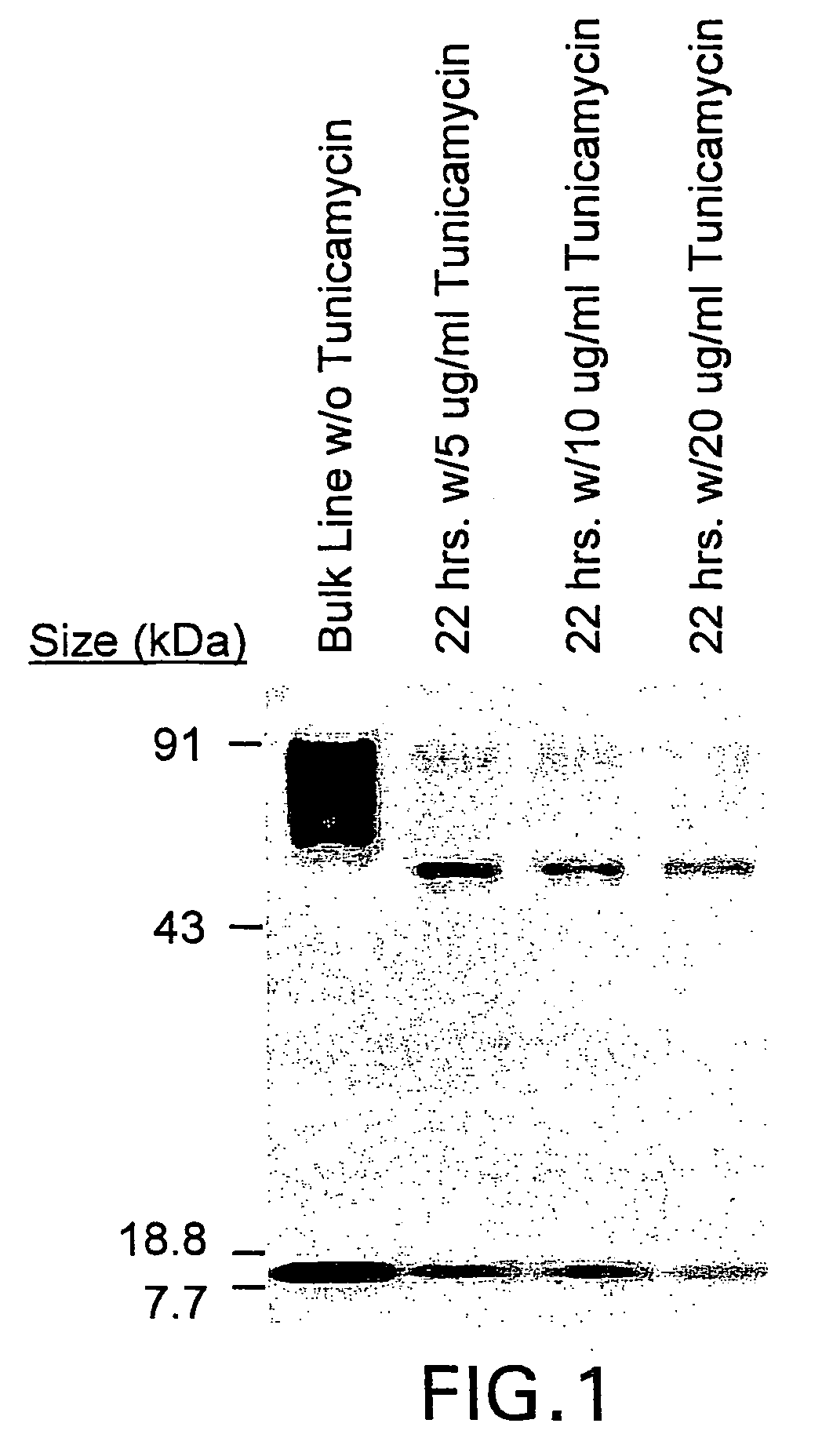
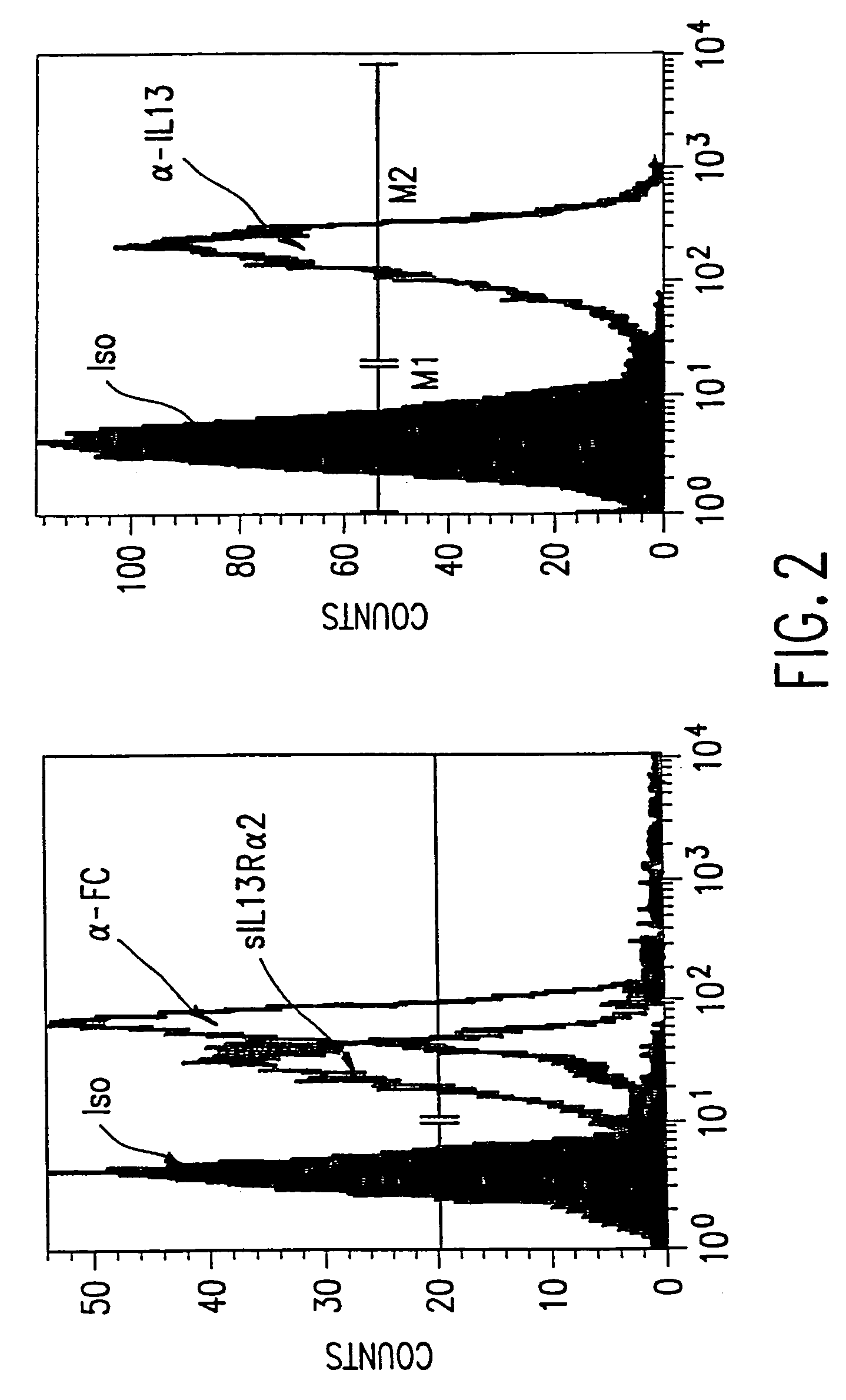
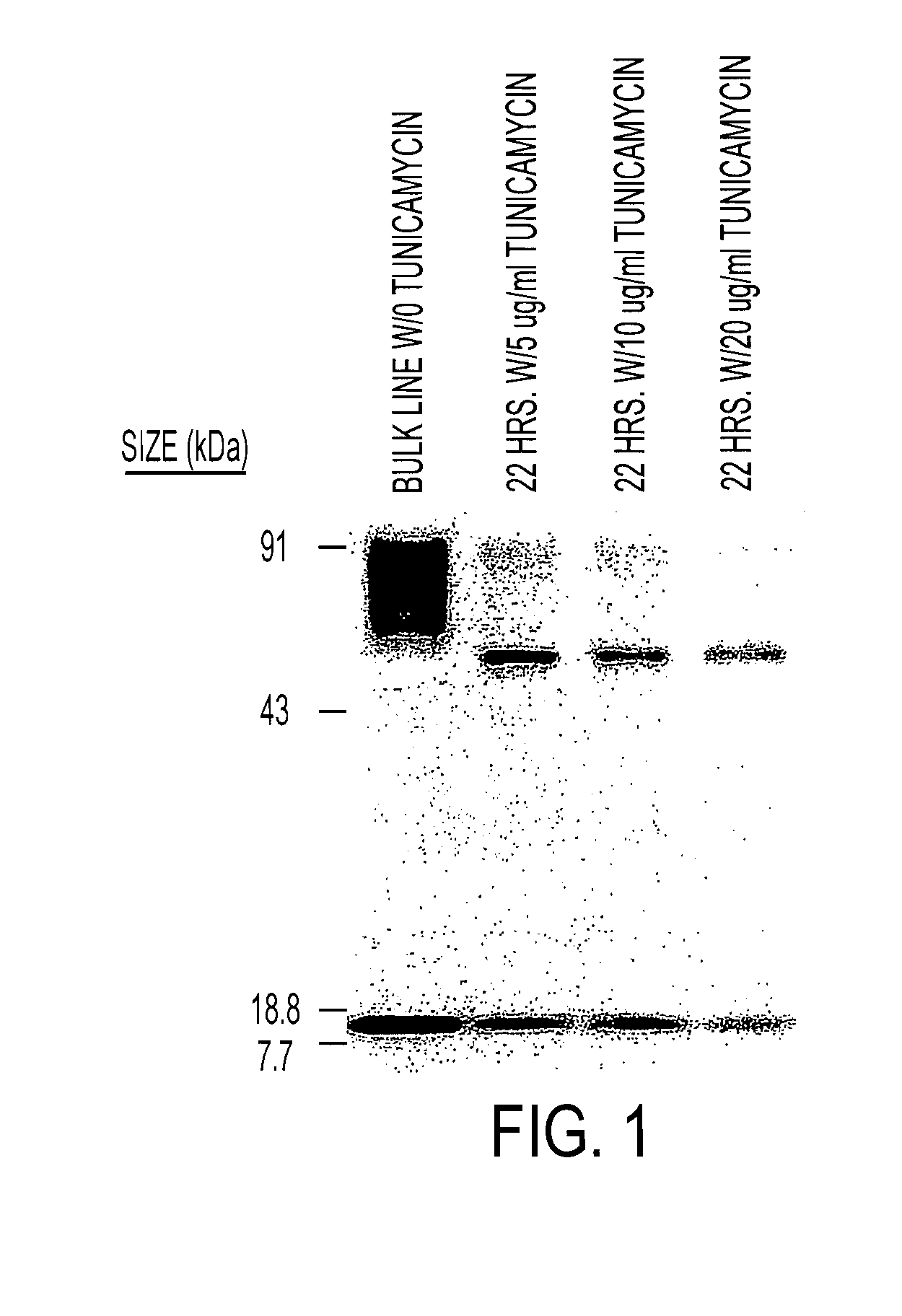
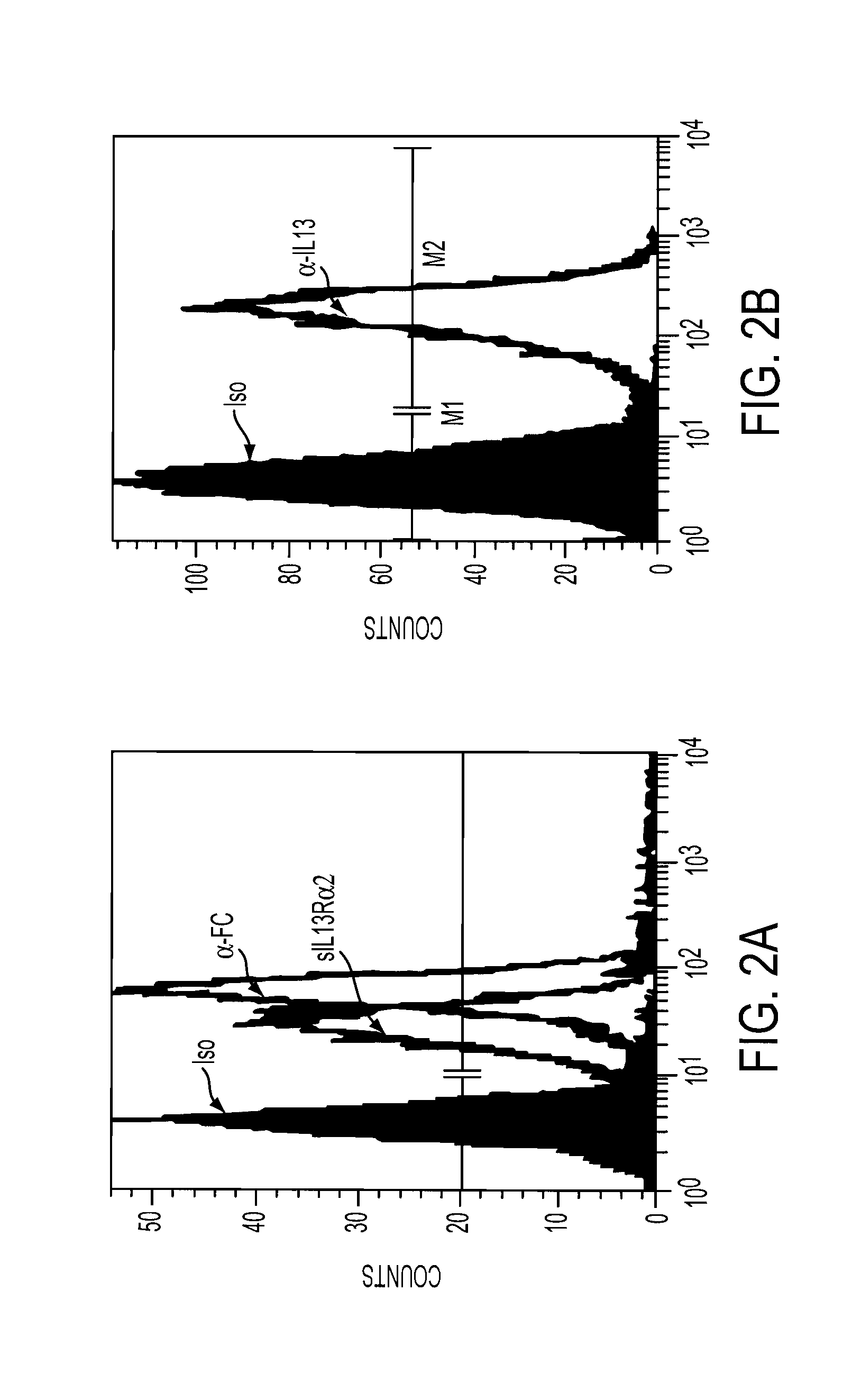
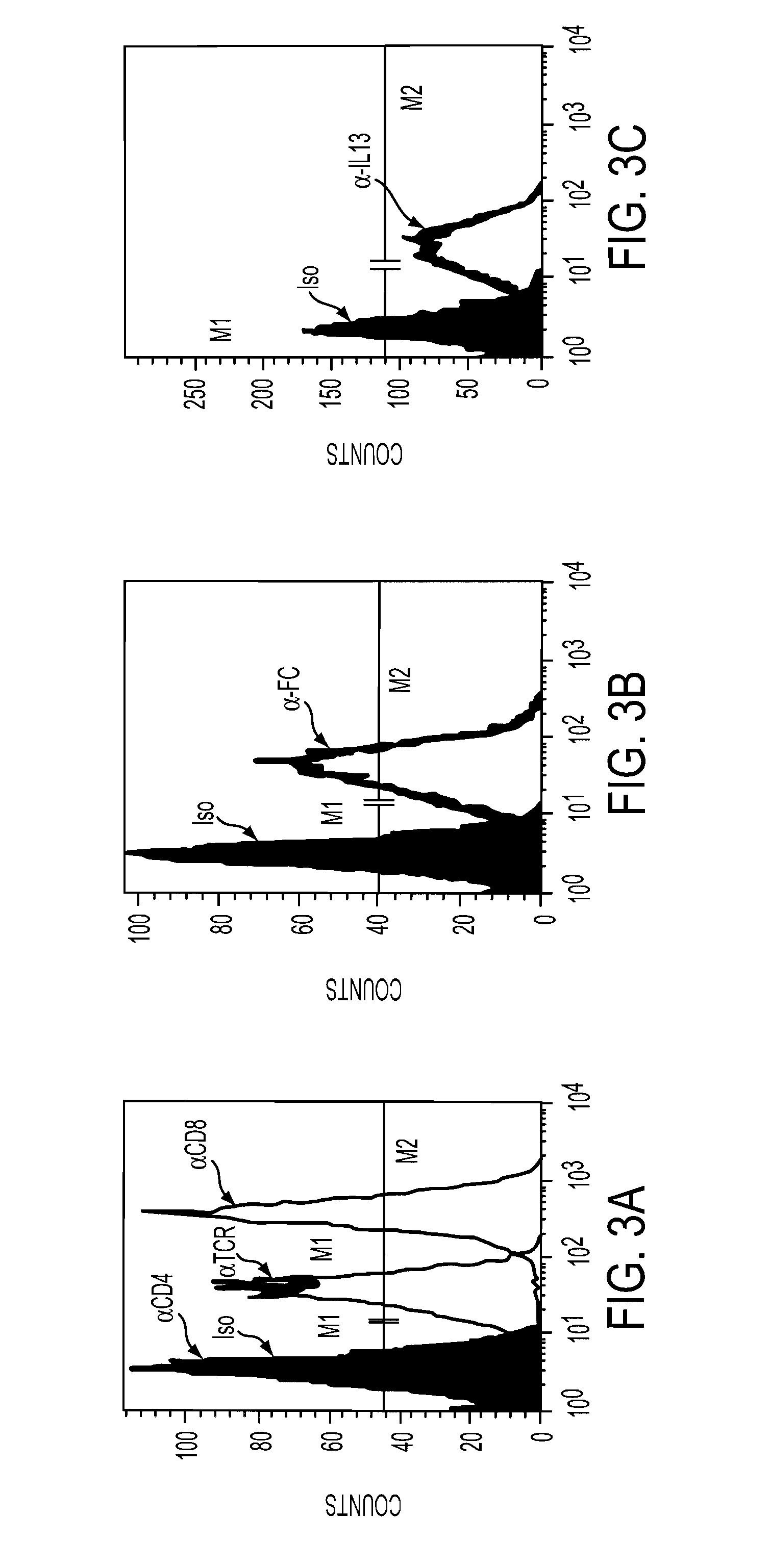
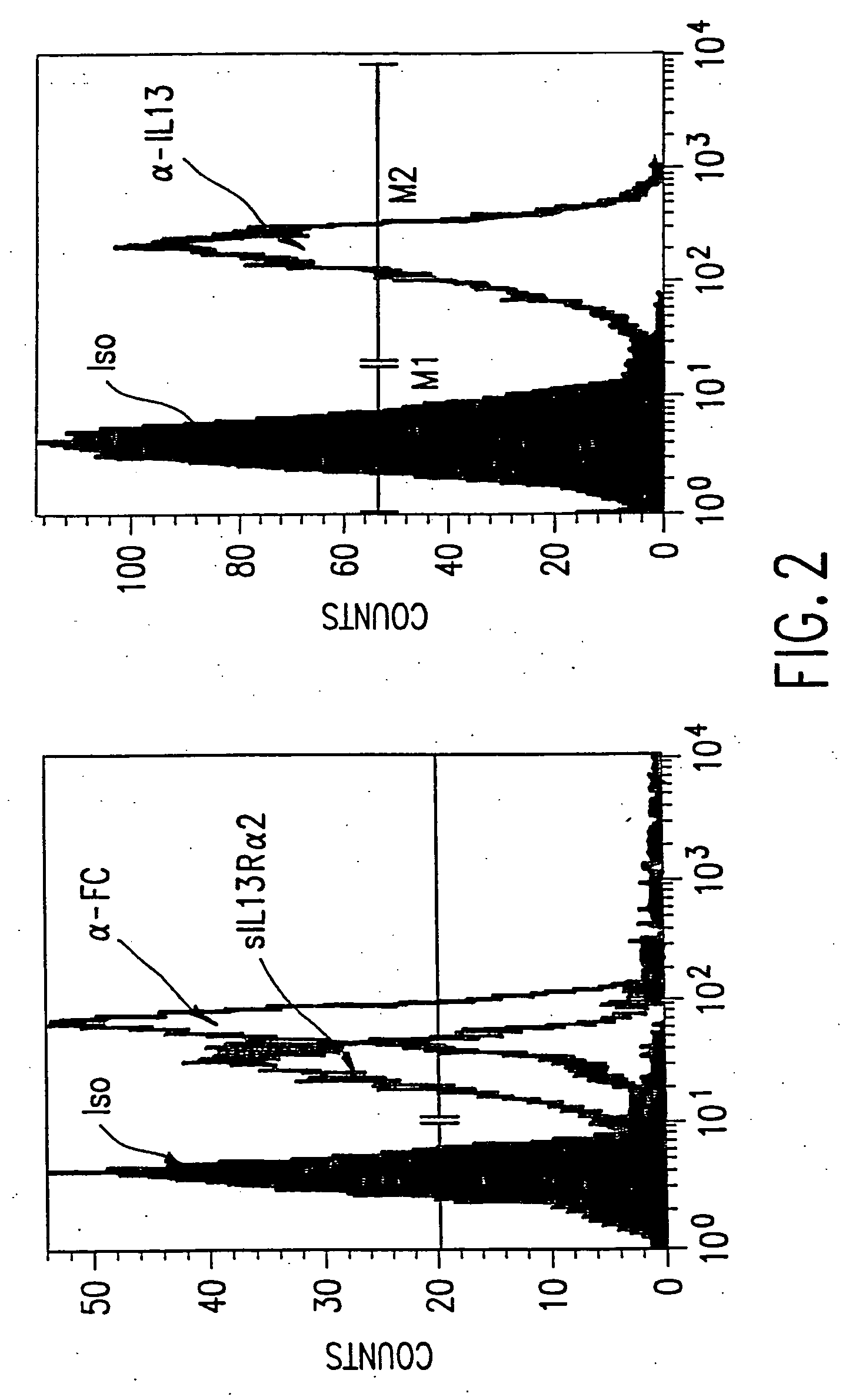
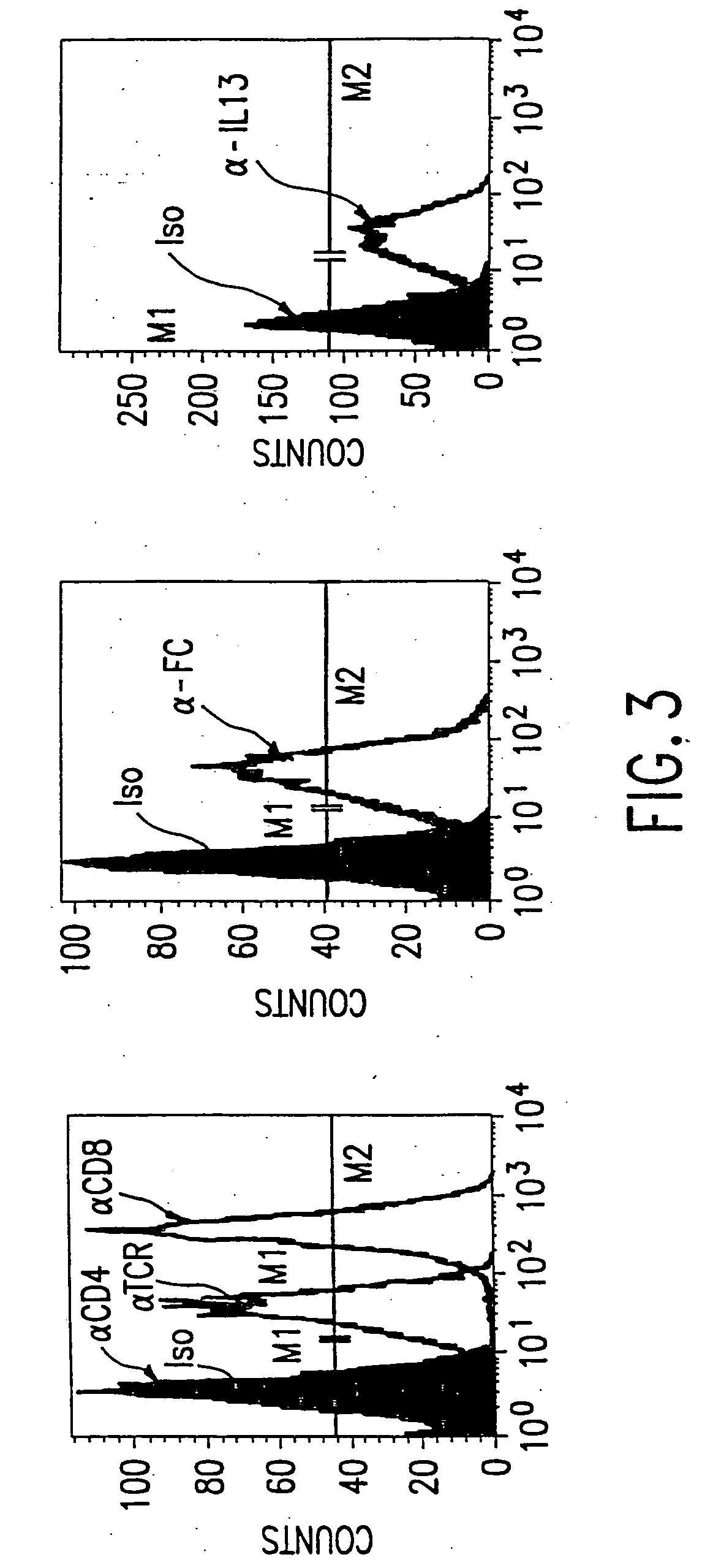
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signaling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human maligancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targeting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Methods and algorithms for cell enumeration in low-cost cytometer
InactiveUS20060024756A1Simple designReduce operating costsBioreactor/fermenter combinationsBiological substance pretreatmentsWhite blood cellCcd camera
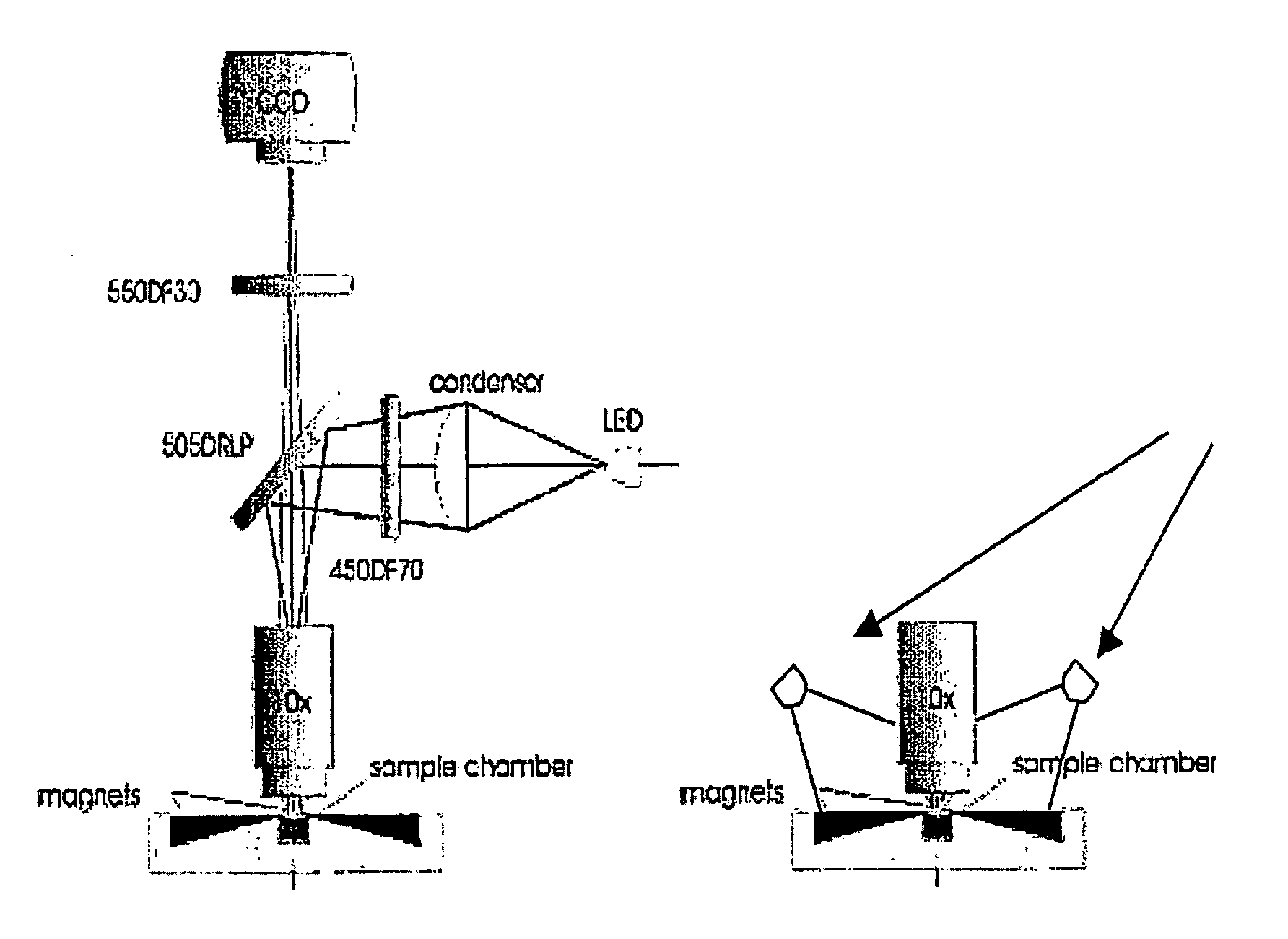
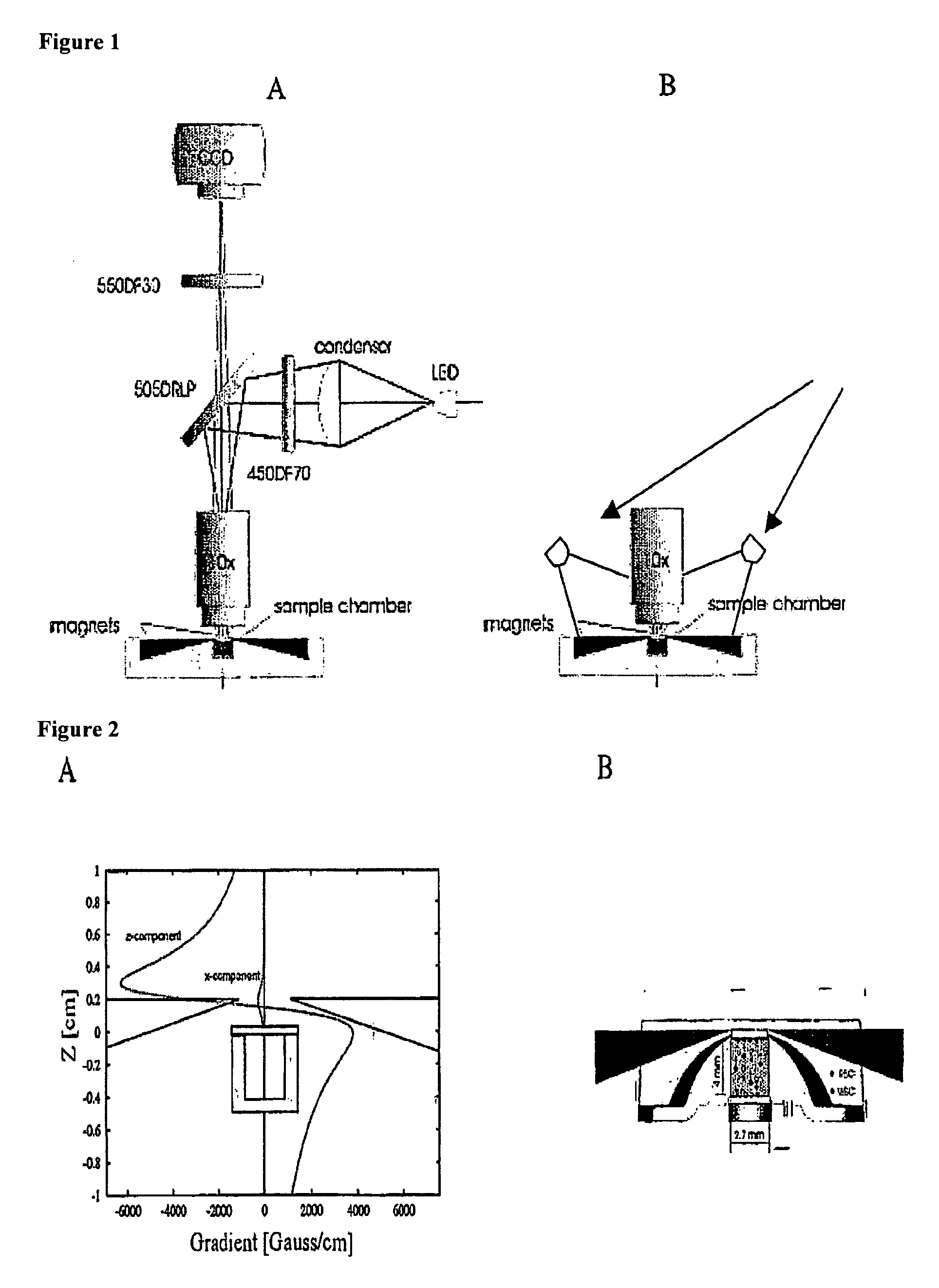
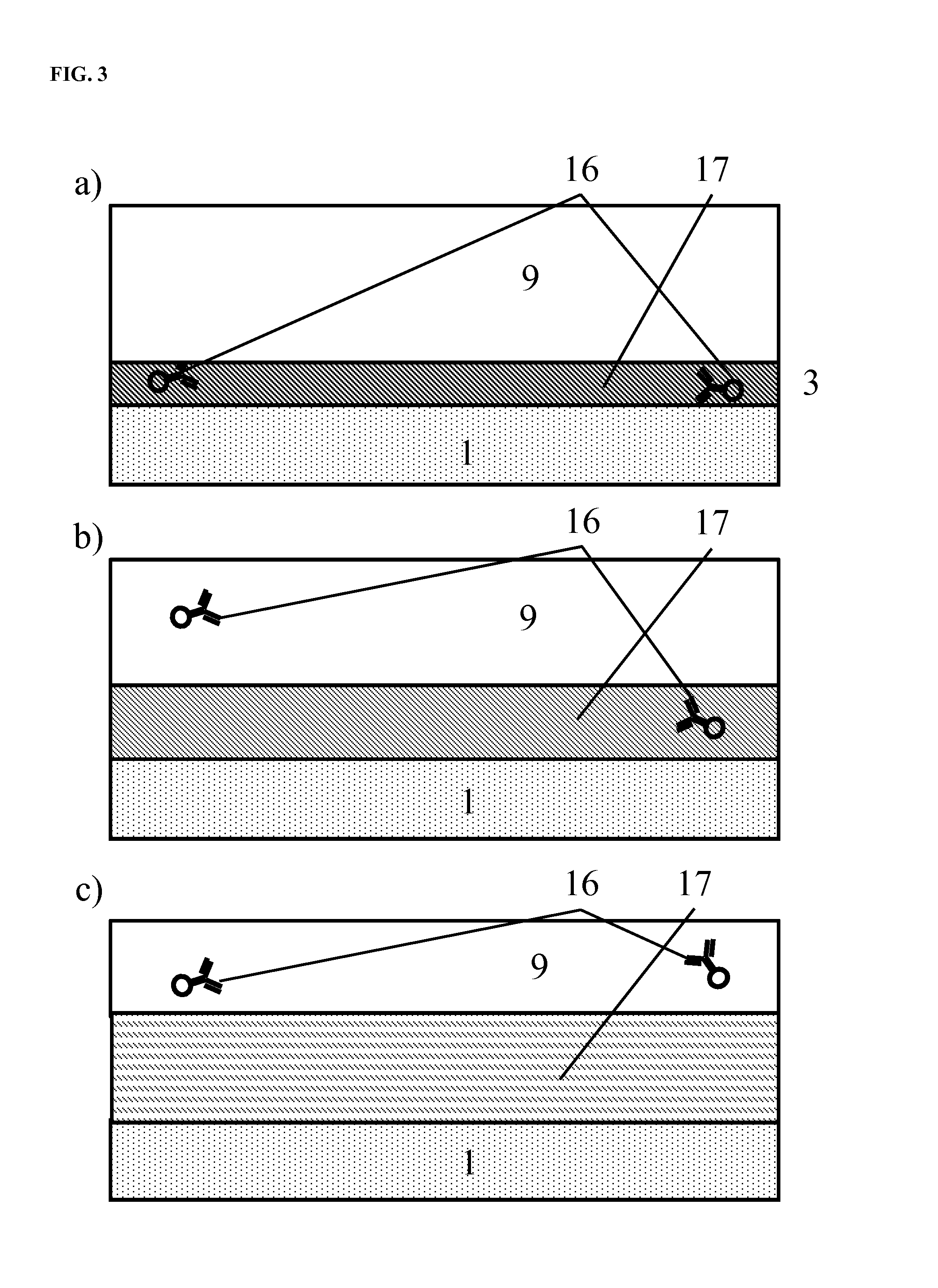
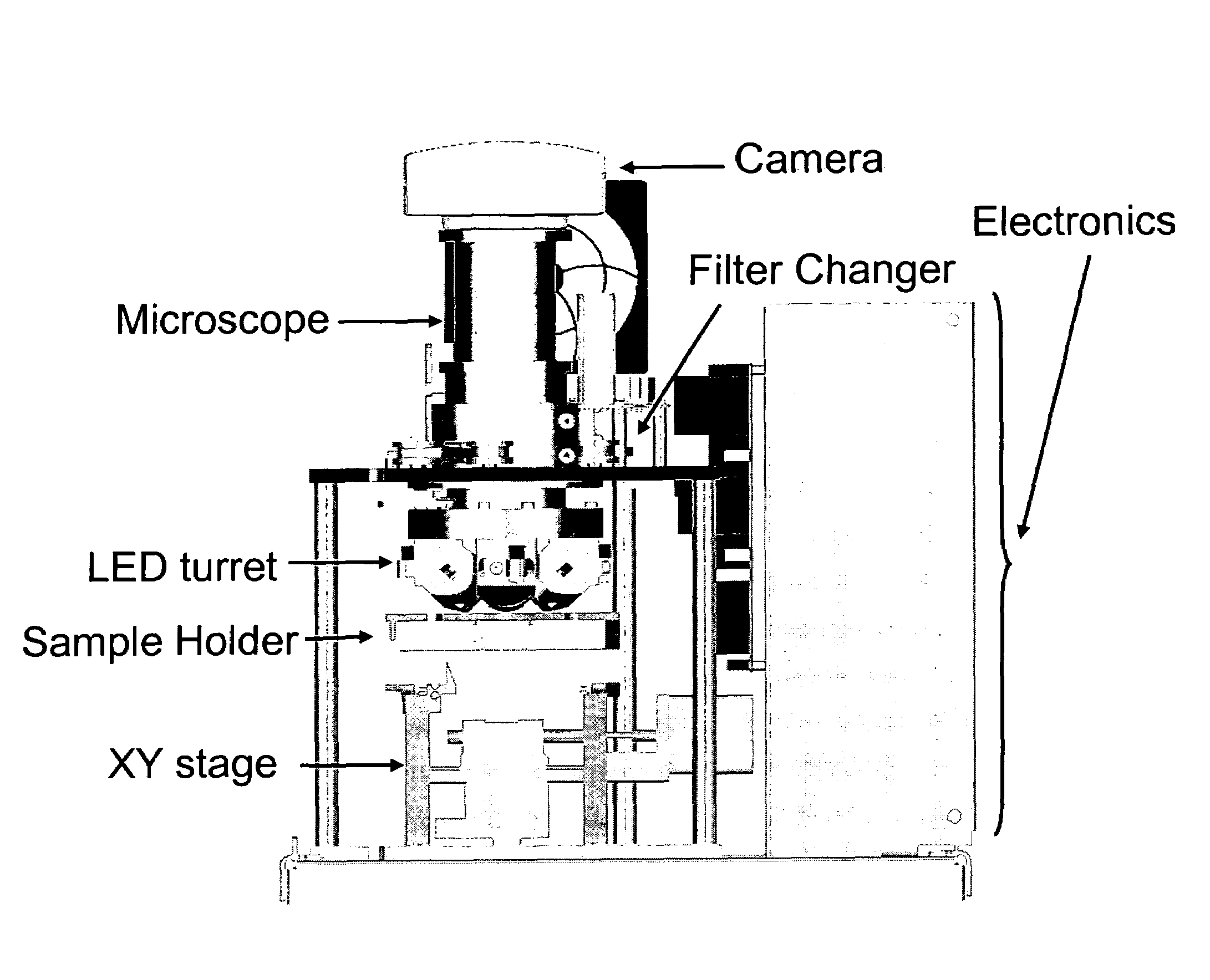
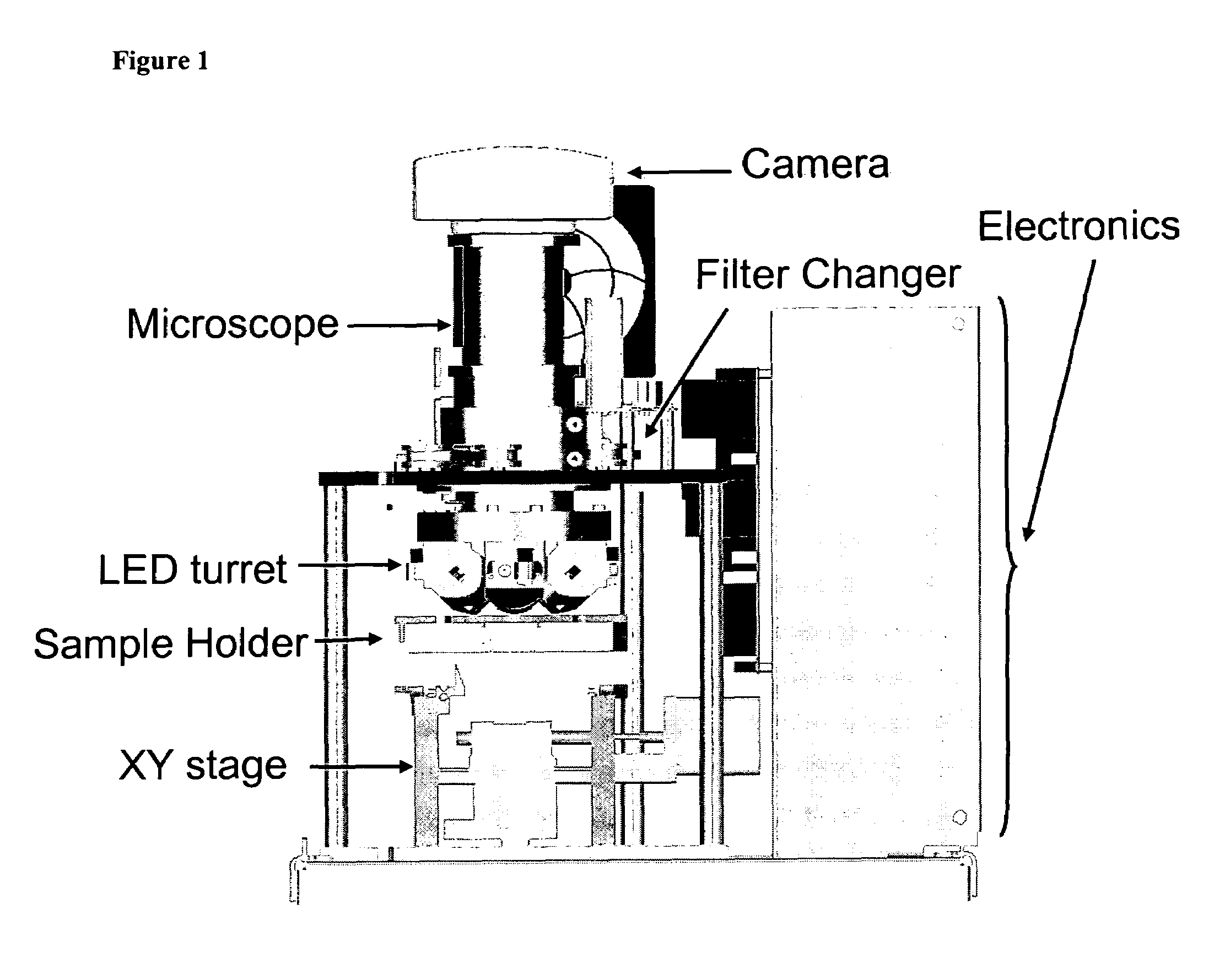
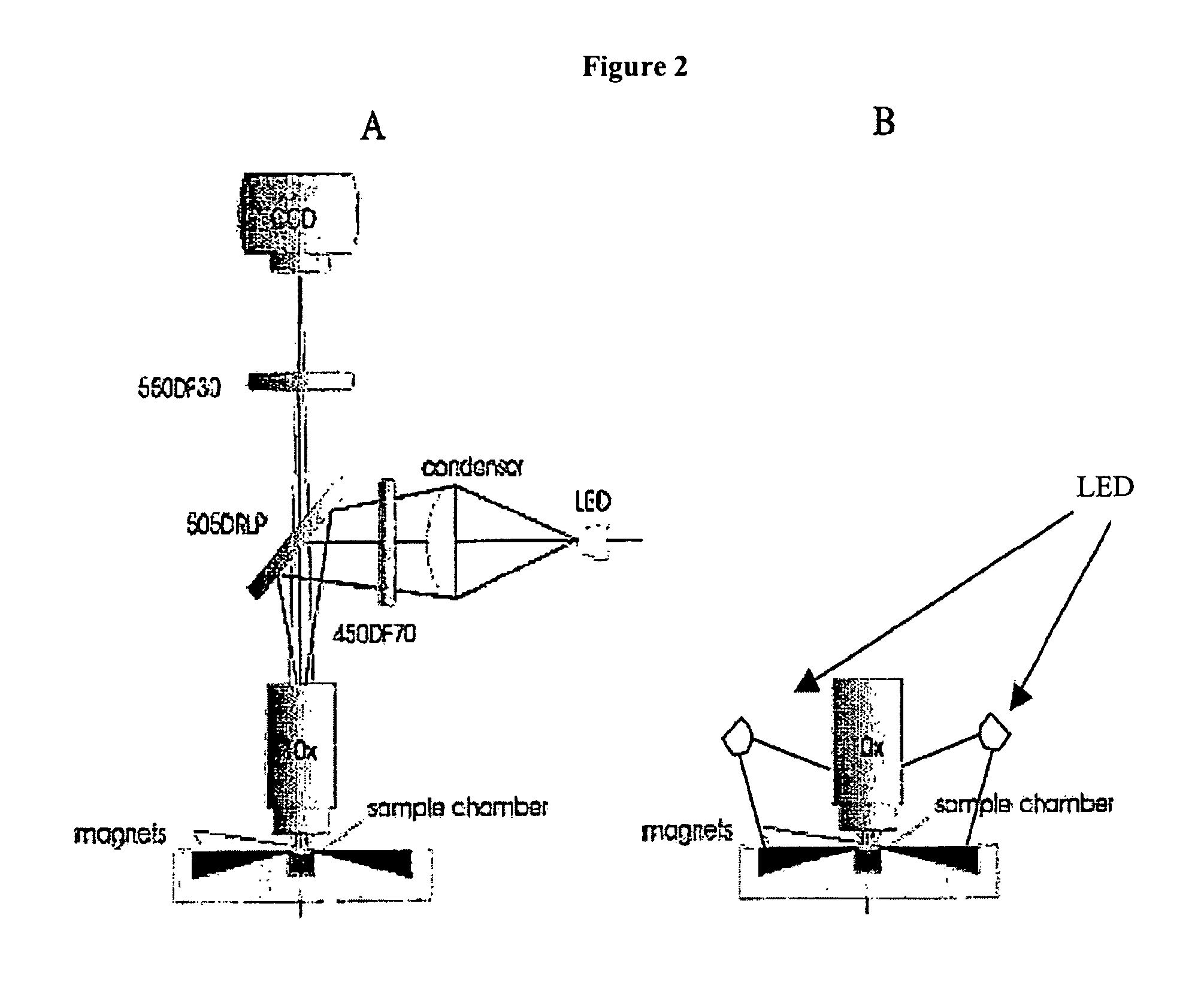
The enumeration of cells in fluids by flow cytometry is widely used across many disciplines such as assessment of leukocyte subsets in different bodily fluids or of bacterial contamination in environmental samples, food products and bodily fluids. For many applications the cost, size and complexity of the instruments prevents wider use, for example, CD4 analysis in HIV monitoring in resource-poor countries. The novel device, methods and algorithms disclosed herein largely overcome these limitations. Briefly, all cells in a biological sample are fluorescently labeled, but only the target cells are also magnetically labeled. The labeled sample, in a chamber or cuvet, is placed between two wedge-shaped magnets to selectively move the magnetically labeled cells to the observation surface of the cuvet. An LED illuminates the cells and a CCD camera captures the images of the fluorescent light emitted by the target cells. Image analysis performed with a novel algorithm provides a count of the cells on the surface that can be related to the target cell concentration of the original sample. The compact cytometer system provides a rugged, affordable and easy-to-use technique, which can be used in remote locations.
Owner:UNIVERSITY OF TWENTE
HIV env antibodies
InactiveUS7041293B1Reduce reverse transcriptase activityInhibit syncitia formationAnimal cellsMicrobiological testing/measurementReverse transcriptase activityCD4 antigen
The invention provides antibodies specific for HIV env, including monoclonal antibodies and related hybridomas. The antibodies block CD4 / g120 binding and reduce reverse transcriptase activity in vitro.
Owner:GENENTECH INC
Targeted cytolysis of HIV-infected cells by chimeric CD4 receptor-bearing cells
Disclosed is a method of directing a cellular immune response against an HIV-infected cell in a mammal involving administering to the mammal an effective amount of therapeutic cells which express a membrane-bound, proteinaceous chimeric receptor comprising (a) an extracellular portion which includes a fragment of CD4 which is capable of specifically recognizing and binding the HIV-infected cell but which does not mediate HIV infection and (b) an intracellular portion which is capable of signalling the therapeutic cell to destroy the receptor-bound HIV-infected cell. Also disclosed is a second method of treating HIV in a mammal involving administering to the mammal an effective amount of therapeutic cells expressing a membrane-bound, proteinaceous chimeric receptor comprising an extracellular portion which includes a fragment of CD4 which is capable of specifically recognizing and binding the HIV-infected cell but which does not mediate HIV infection. Also disclosed are cells which express the chimeric receptors and DNA and vectors encoding the chimeric receptors.
Owner:THE GENERAL HOSPITAL CORP
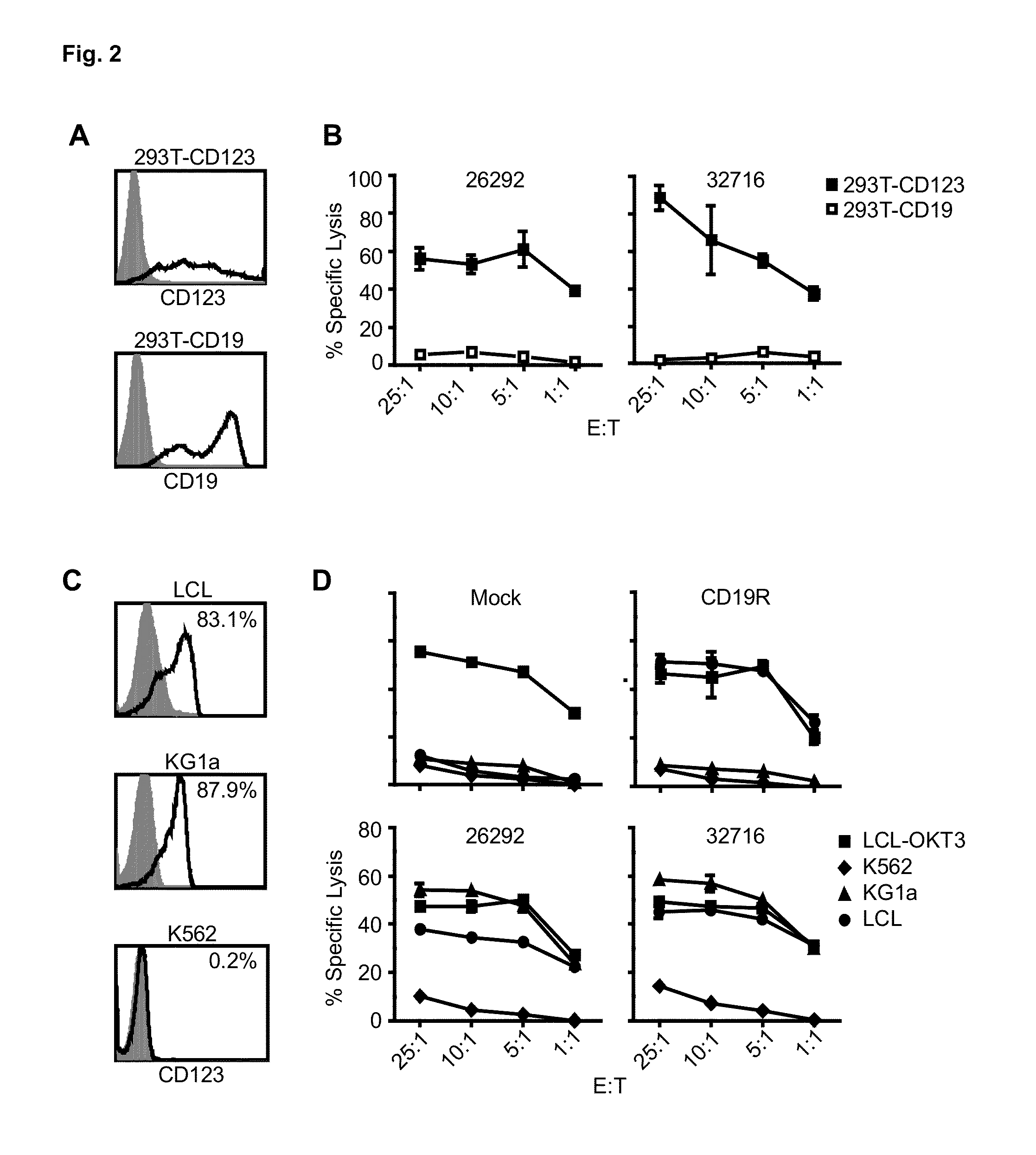
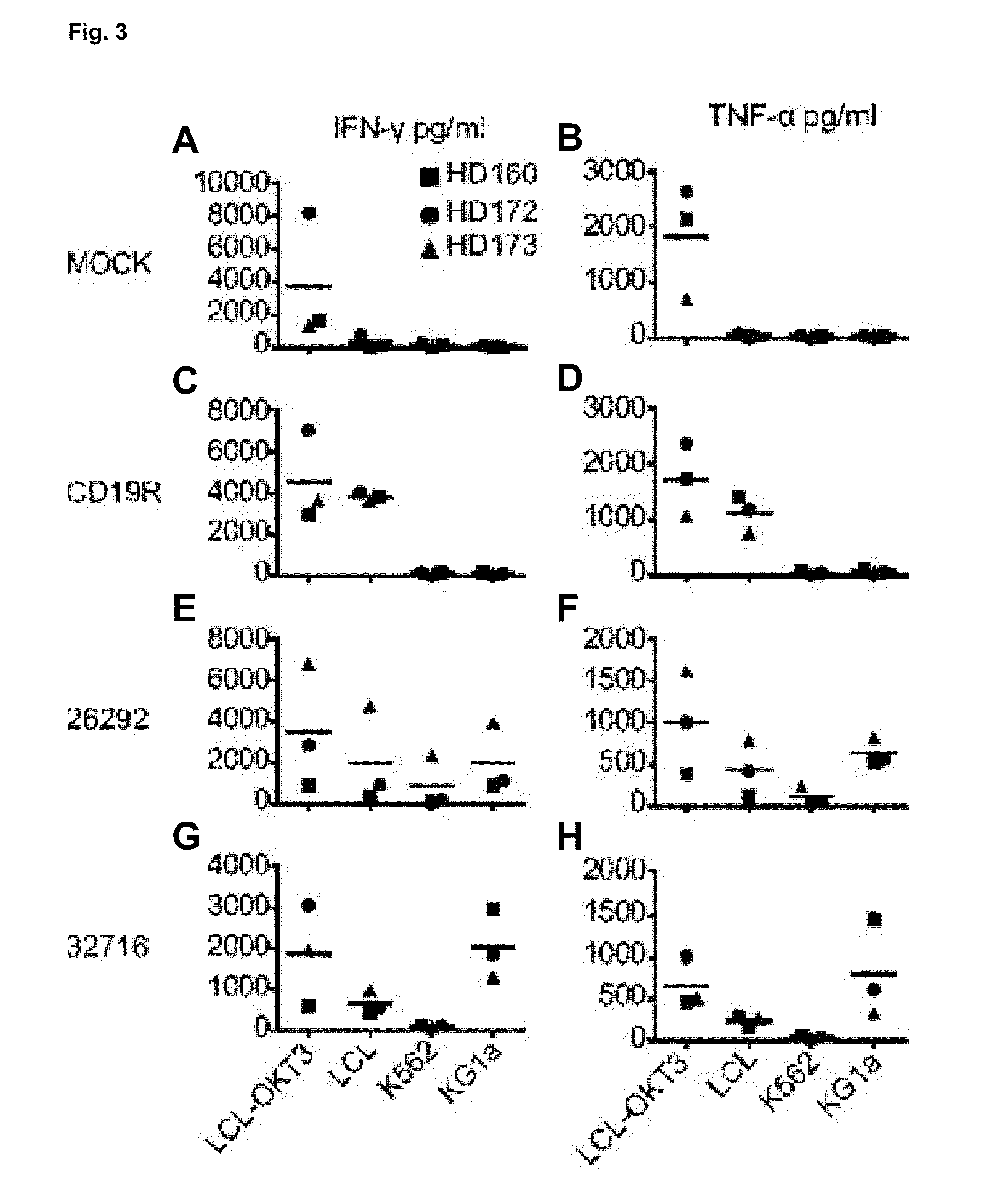
Cd123-specific chimeric antigen receptor redirected t cells and methods of their use
ActiveUS20140271582A1BiocideAntibody mimetics/scaffoldsCD19-specific chimeric antigen receptorT-Cell Specificity
A family of chimeric antigen receptors (CARs) containing a CD123 specific scFv was developed to target different epitopes on CD123. In some embodiments, such a CD123 chimeric antigen receptor (CD123CAR) gene includes an anti-CD123 scFv region fused in frame to a modified IgG4 hinge region comprising an S228P substitution, an L235E substitution, and optionally an N297Q substitution; a costimulatory signaling domain; and a T cell receptor (TCR) zeta chain signaling domain. When expressed in healthy donor T cells (CD4 / CD8), the CD123CARs redirect T cell specificity and mediated potent effector activity against CD123+ cell lines as well as primary AML patient samples. Further, T cells obtained from patients with active AML can be modified to express CD123CAR genes and are able to lyse autologous AML blasts in vitro. Finally, a single dose of 5.0×106 CAR123 T cells results in significantly delayed leukemic progression in mice. These results suggest that CD123CAR-transduced T cells may be used as an immunotherapy for the treatment of high risk AML.
Owner:CITY OF HOPE
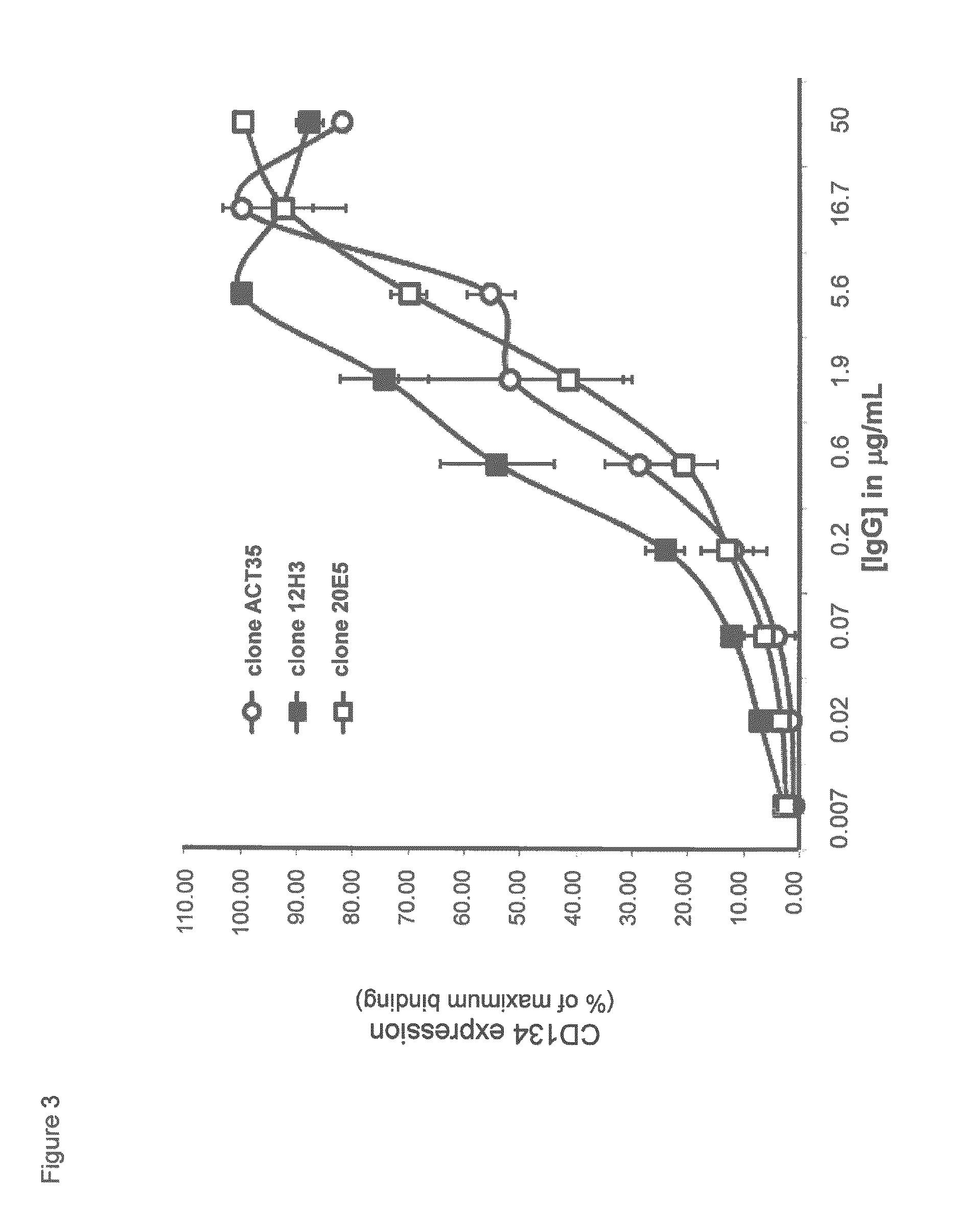
Humanized Anti-cd134 (OX40) antibodies and uses thereof
The invention provides antibodies that specifically bind to human CD134. Anti-human CD134 antibodies specifically bind to the extracellular domain of human CD134, including non-OX40 ligand (OX40L) binding domains on human CD134, which is expressed on e.g. activated human conventional effector CD4 and / or CD8 T lymphocytes (Teffs) and on activated human suppressive regulatory CD4 T lymphocytes (Tregs). Humanized anti-human CD134 antibodies are useful (e.g. to empower Teffs anti-cancer effector function and / or to inhibit Tregs suppressive function) for cancer treatment.
Owner:JANSSEN PHARMA INC
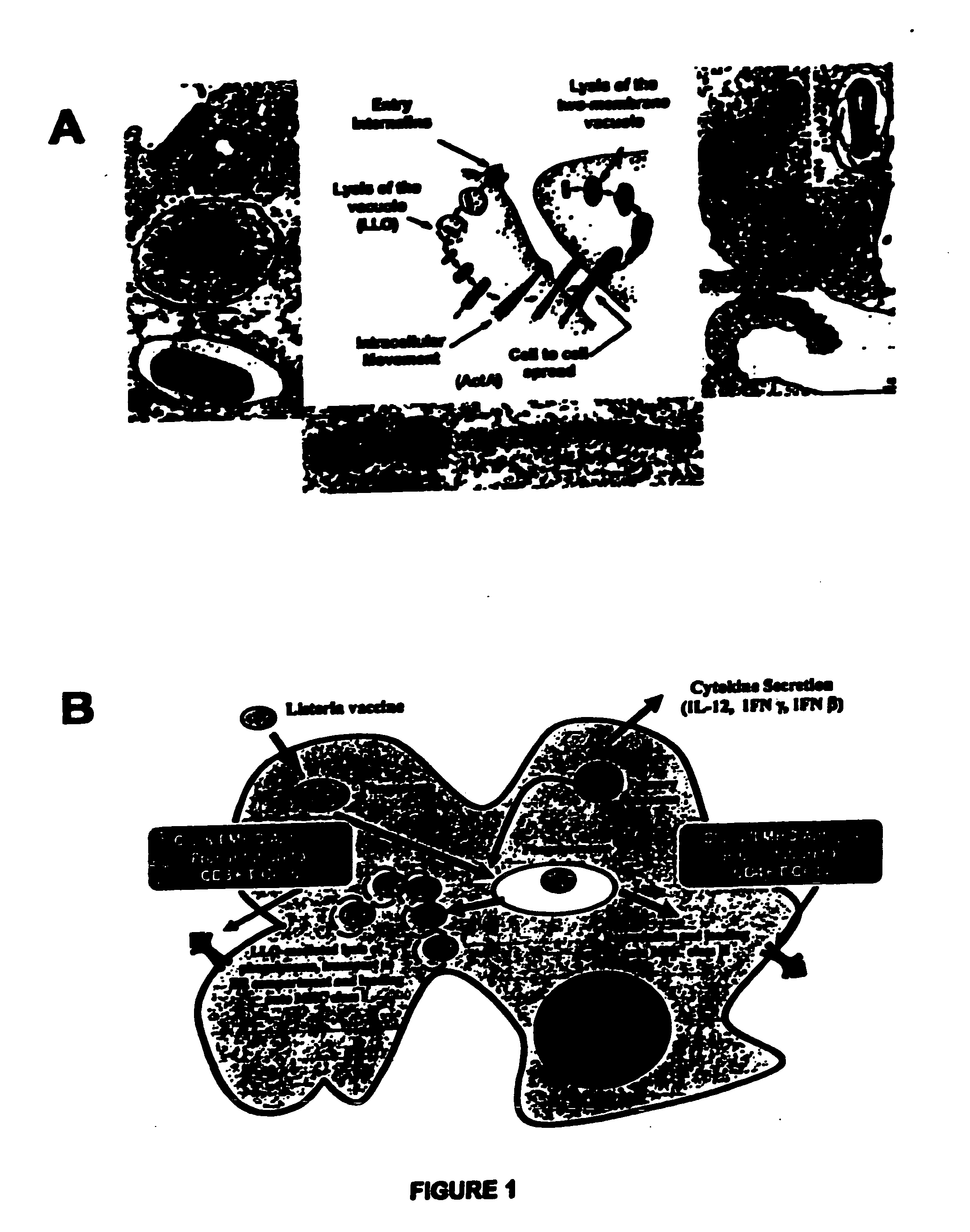
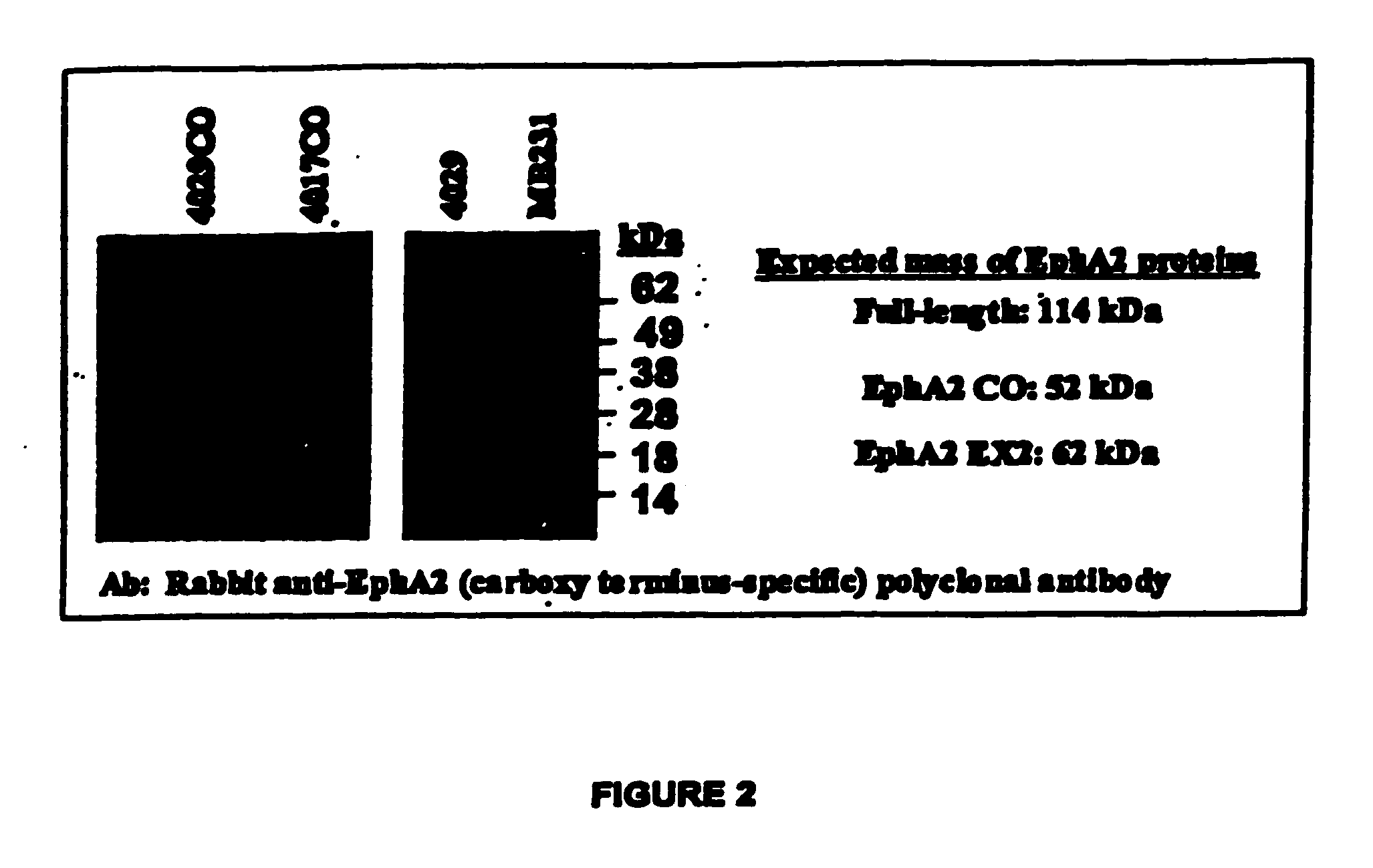
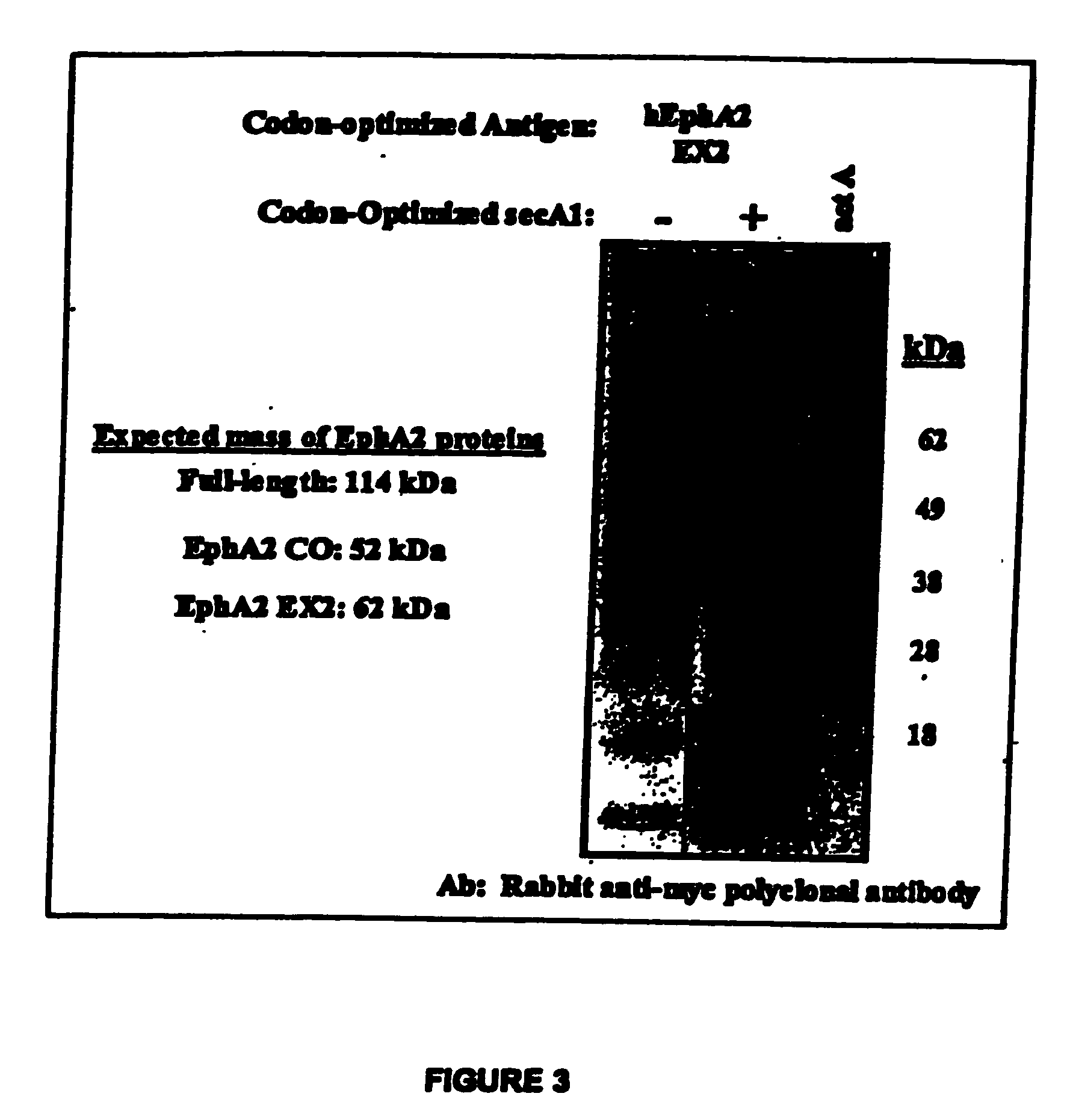
Listeria-based EphA2 vaccines
InactiveUS20050281783A1Beneficial therapeuticBeneficial prophylactic benefitBiocideSenses disorderCancer preventionCancer therapy
The present invention relates to methods and compositions designed for the treatment, management, or prevention of cancer, particularly metastatic cancer and cancers of T cell origin, and hyperproliferative diseases involving EphA2-expressing cells. The methods of the invention entail the use of a Listeria-based EphA2 vaccine. The invention also provides pharmaceutical compositions comprising one or more Listeria-based vaccines of the invention either alone or in combination with one or more other agents useful for cancer therapy. In certain aspects of the invention, the methods entail eliciting both CD4+ and CD8+ T-cell responses against EphA2 and / or EphA2-expressing cells.
Owner:CERUS CORP
High affinity human antibodies and human antibodies against human antigens
The invention relates to transgenic non-human animals capable of producing high affinity human sequence antibodies. The invention is also directed to human sequence antibodies specific for human antigens, such as, human CD4. The invention also is directed to methods for producing human sequence antibodies.
Owner:GENPHARM INT INC
Recombinant vector expressing multiple costimulatory molecules and uses thereof
InactiveUS7211432B2Enhance immune responseFacilitated DiffusionBiocideGenetic material ingredientsDendritic cellBiological activation
The present invention is a recombinant vector encoding and expressing at least three or more costimulatory molecules. The recombinant vector may additionally contain a gene encoding one or more target antigens or immunological epitope thereof. The synergistic effect of these costimulatory molecules on the enhanced activation of T cells is demonstrated. The degree of T-cell activation using recombinant vectors containing genes encoding three costimulatory molecules was far greater than the sum of recombinant vector constructs containing one costimulatory molecule and greater than the use of two costimulatory molecules. Results employing the triple costimulatory vectors were most dramatic under conditions of either low levels of first signal or low stimulator to T-cell ratios. This phenomenon was observed with both isolated CD4+ and CD8+ T cells. The recombinant vectors of the present invention are useful as immunogenes and vaccines against cancer and pathogenic micro-organisms, and in providing host cells, including dendritic cells and splenocytes with enhanced antigen-presenting functions.
Owner:UNITED STATES OF AMERICA
Anti-cd134 (OX40) antibodies and uses thereof
InactiveUS20150132288A1Enhance immune responseEnhancing immunostimulator/effector function of T-effectorLibrary screeningImmunoglobulins against cell receptors/antigens/surface-determinantsInhibitory modulationOX40 ligand
The invention provides antibodies that specifically bind to human CD134. Invention anti-human CD134 antibodies specifically bind to the extracellular domain of human CD134, including non-OX40 ligand (OX40L) binding domains on human CD134, which is expressed on e.g. activated human conventional effector CD4 and / or CD8 T lymphocytes (Teffs) and on activated human suppressive regulatory CD4 T lymphocytes (Tregs). Invention anti-human CD134 antibodies are useful (e.g. to empower Teffs anti-cancer effector function and / or to inhibit Tregs suppressive function) for cancer treatment.
Owner:JANSSEN PHARMA INC
Method and compositions for cellular immunotherapy
ActiveUS20140314795A1Maintain normalEnhance immune responsePeptide/protein ingredientsAntibody mimetics/scaffoldsPharmaceutical formulationWilms' tumor
The present invention provides methods and compositions to confer and / or augment immune responses mediated by cellular immunotherapy, such as by adoptively transferring genetically modified tumor specific CD8+ T cells in the presence of tumor-specific, subset specific genetically modified CD4+ T cells, wherein the CD4+ T cells confer and / or augment a CD8+ T cells ability to sustain anti-tumor reactivity and increase and / or maximize tumor-specific proliferation of the tumor-specific CD8+ T cells of interest. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:FRED HUTCHINSON CANCER CENT
Recombinant vector expressing multiple costimulatory molecules and uses thereof
InactiveUS6969609B1Enhance immune responseFacilitated DiffusionBiocideGenetic material ingredientsDendritic cellBiological activation
The present invention is a recombinant vector encoding and expressing at least three or more costimulatory molecules. The recombinant vector may additionally contain a gene encoding one or more target antigens or immunological epitope thereof. The synergistic effect of them costimulatory molecules on the enhanced activation of T cells is demonstrated. The degree of T-cell activation using recombinant vectors containing genes encoding three costimulatory molecules was far greater than the sum of recombinant vector constructs containing one costimulatory molecule and greater that the use of two costimulatory molecules. Results employing the triple costimulatory vectors were most dramatic under conditions of either low levels of first signal or low stimulator to T-cell ratios. This phenomenon was observed with both isolated CD4+and CD8+T cells. The recombinant vectors of the present invention are useful as immunogenes and vaccines against cancer and pathogenic micro-organisms, and in providing host cells, including dendritic cells and splenocytes with enhanced and antigen-presenting functions.
Owner:THE GOVERMENT OF THE UNITED STATES OF AMERICA REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES (SEE PF37)
Polyepitope carrier protein
InactiveUS6855321B1Facilitate subsequent purificationAccurate supervisionAntibacterial agentsVirusesCarrier proteinCD4 antigen
The invention relates to polyepitope carrier proteins that comprise at least five CD4+ T cell epitopes, for conjugation to capsular polysaccharides. The carrier proteins are use useful as components of vaccines that can elicit a T-cell dependent immune response. These vaccines arm particularly useful to confer protection against infection from encapsulated bacteria in infants between the ages of 3 months and about 2 years.
Owner:NOVARTIS AG
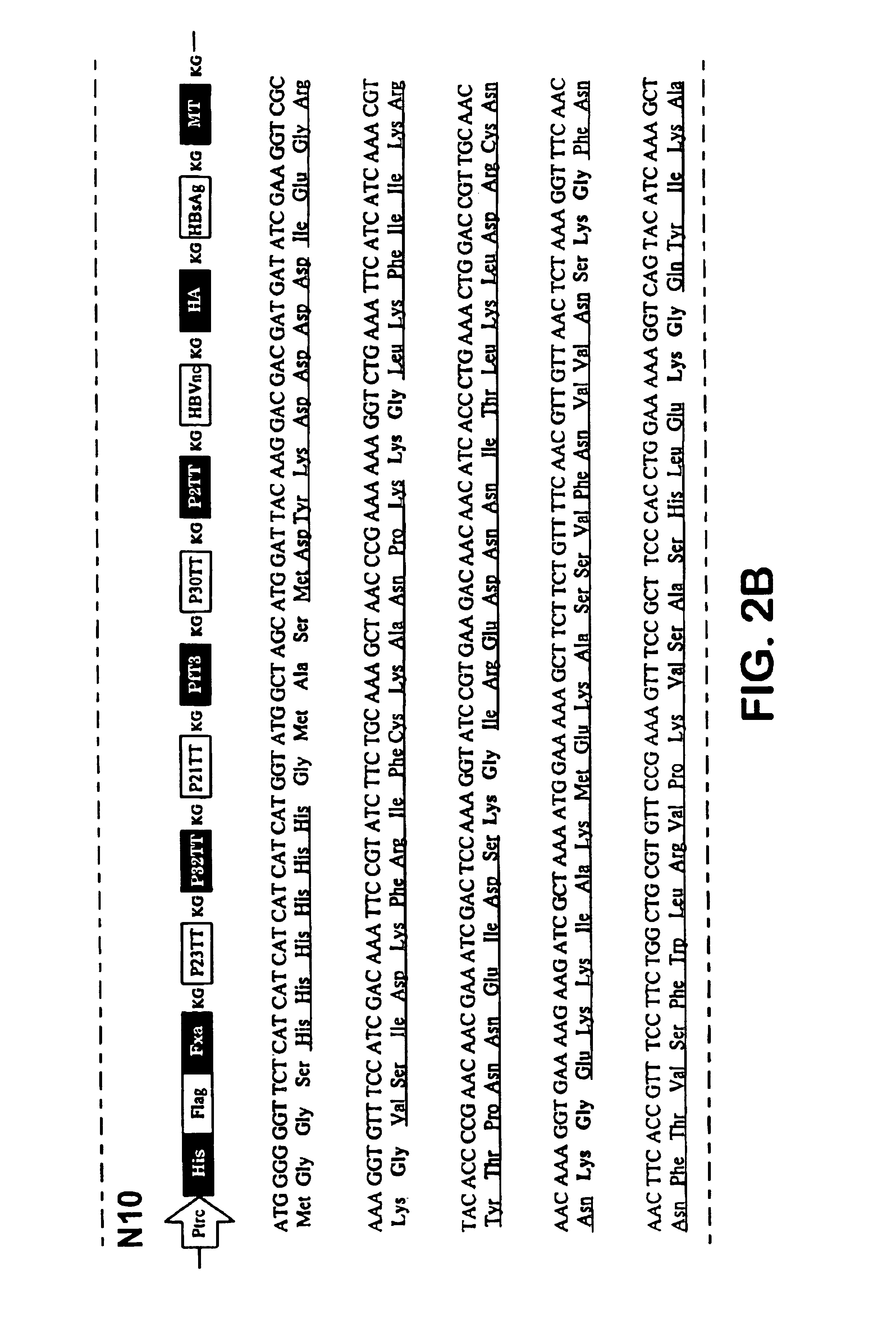
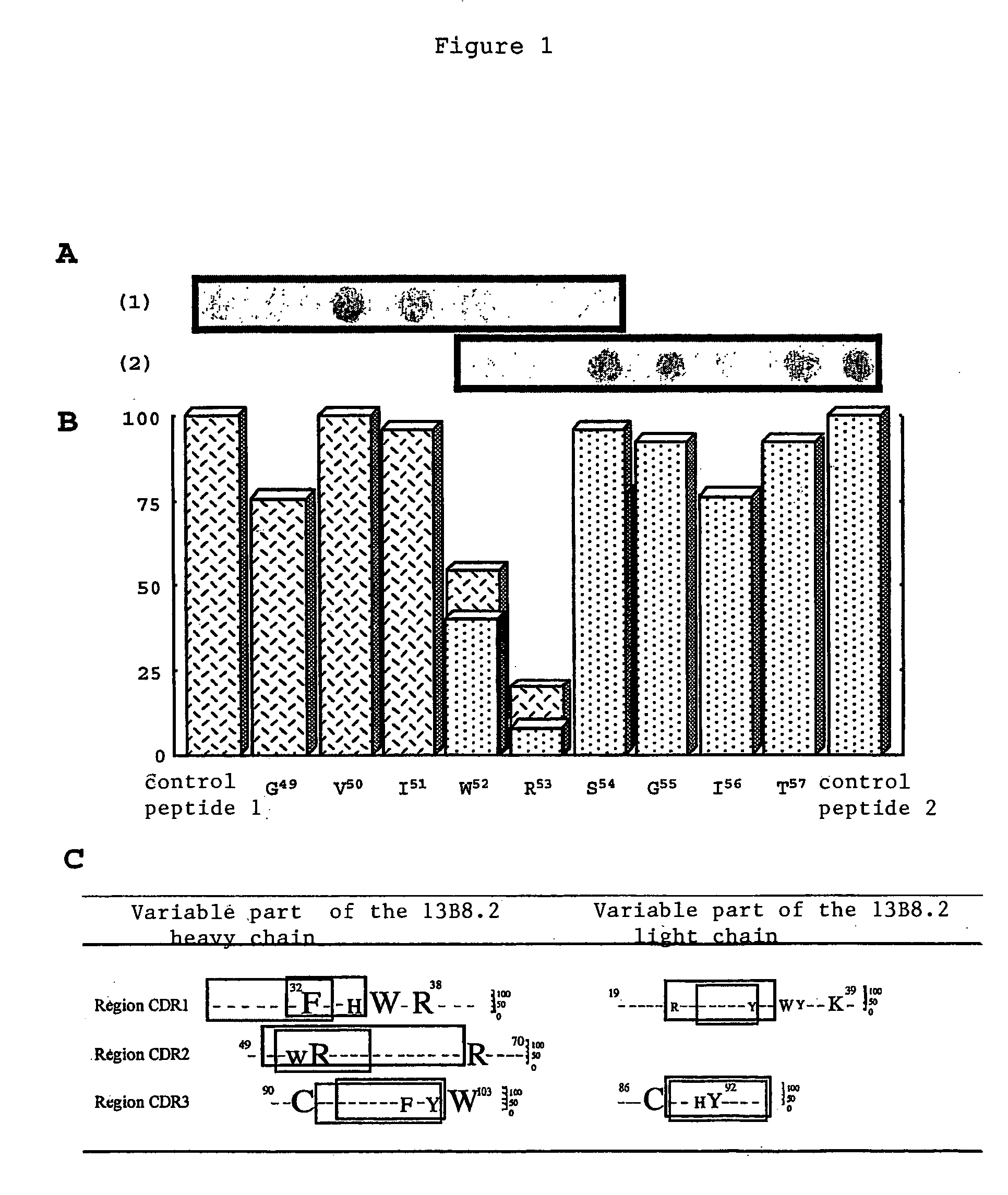
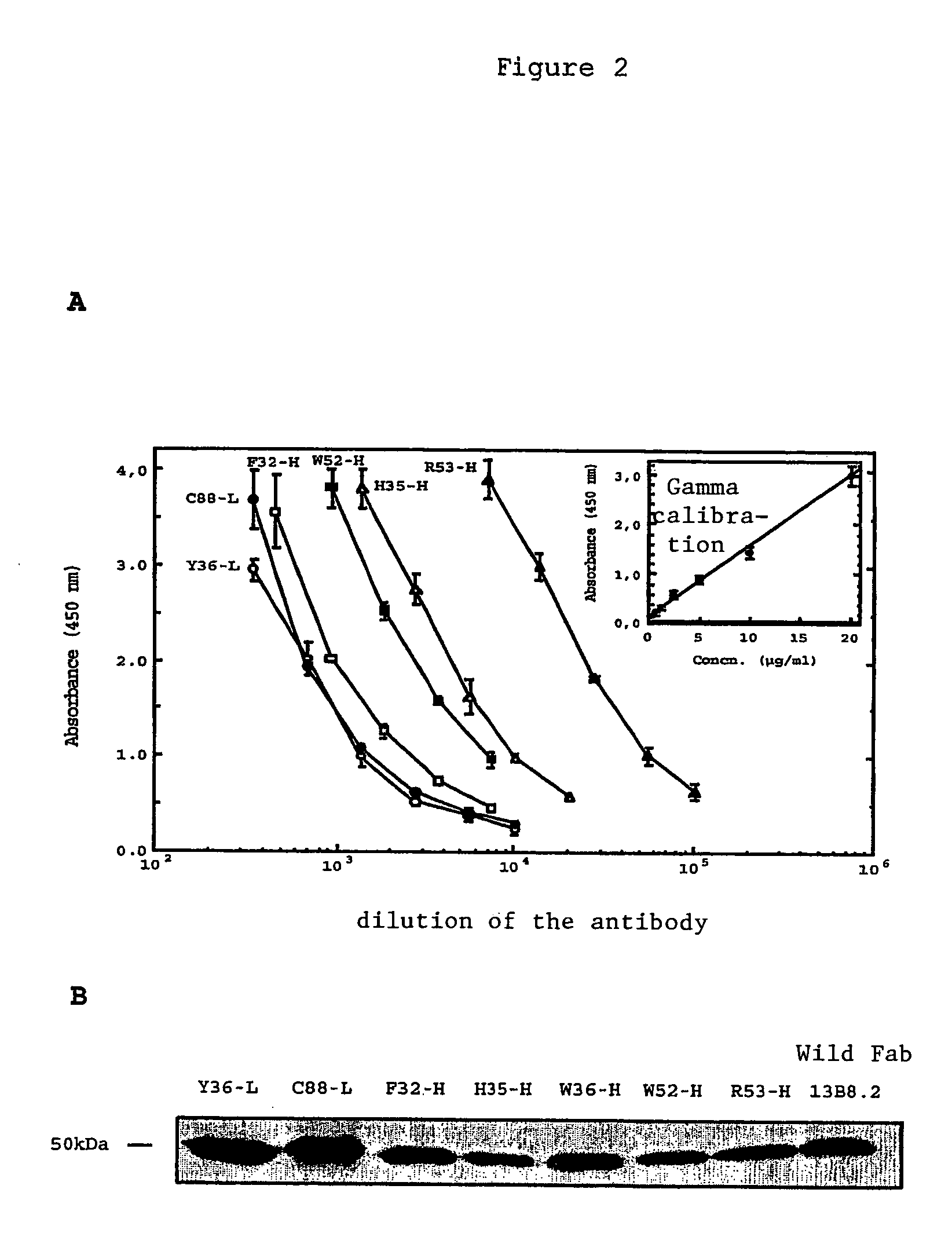
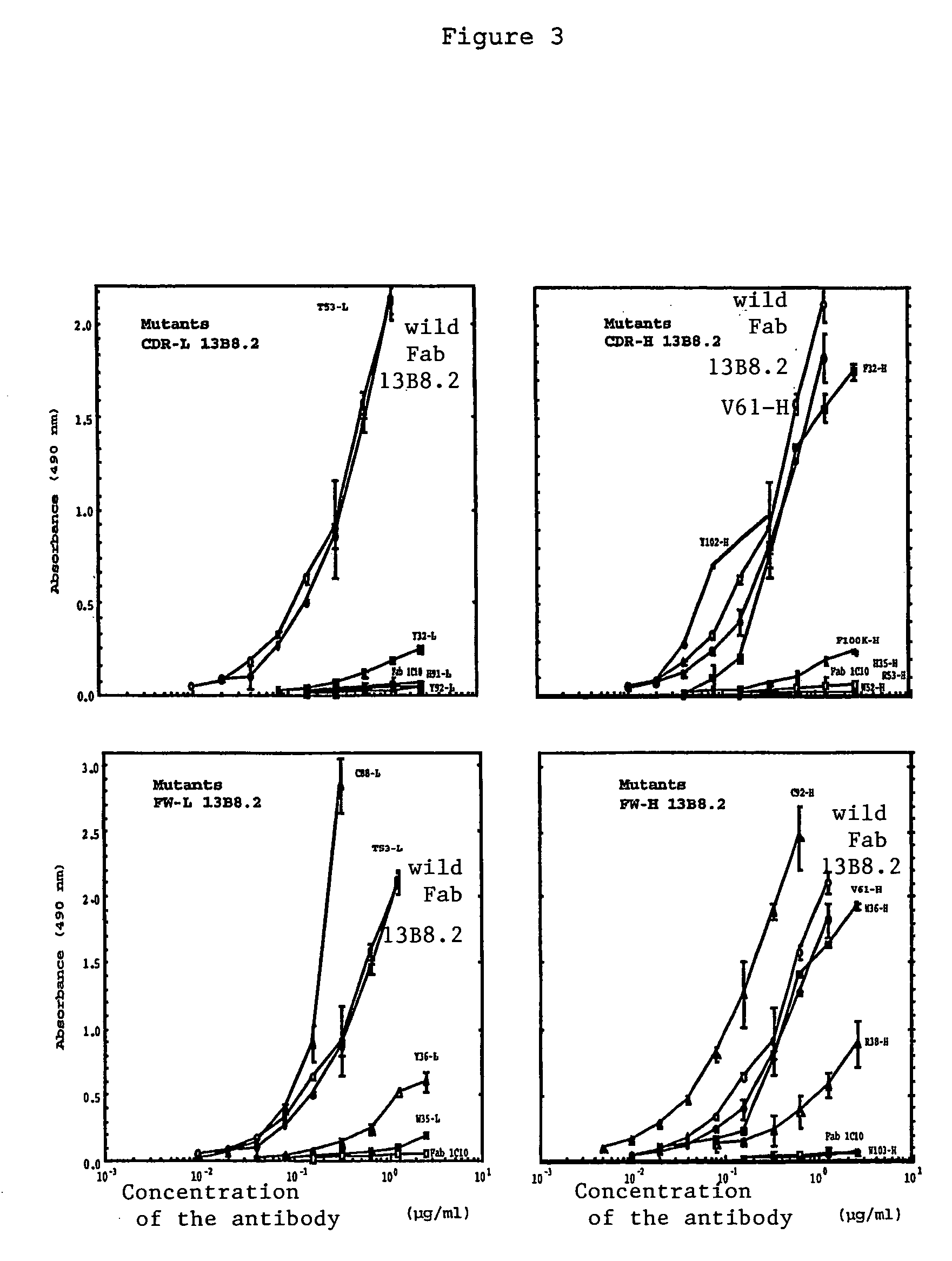
Mutant Fab fragments of the chimeric 13B8.2 anti-CD4 antibody and their applications
A mutant Fab fragment of the 13B8.2 anti-CD4 antibody that binds a CD4 molecule and includes a mutation of at least one residue in a position situated in the VH variable domain of the heavy chain and / or in a position situated in the Vκ variable domain of the light chain.
Owner:CENT NAT DE LA RECHERCHE SCI
Chimeric immunoreceptor useful in treating human cancers
InactiveUS20090257994A1Negligible toxicityPotent and selectiveBiocidePeptide/protein ingredientsIntracellular signallingMalignancy
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signalling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human malignancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targetting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Method and compositions for cellular immunotherapy
ActiveUS20150306141A1Enhanced cytokine productionIncreased proliferationPeptide/protein ingredientsAntibody mimetics/scaffoldsIn vivoTransmembrane domain
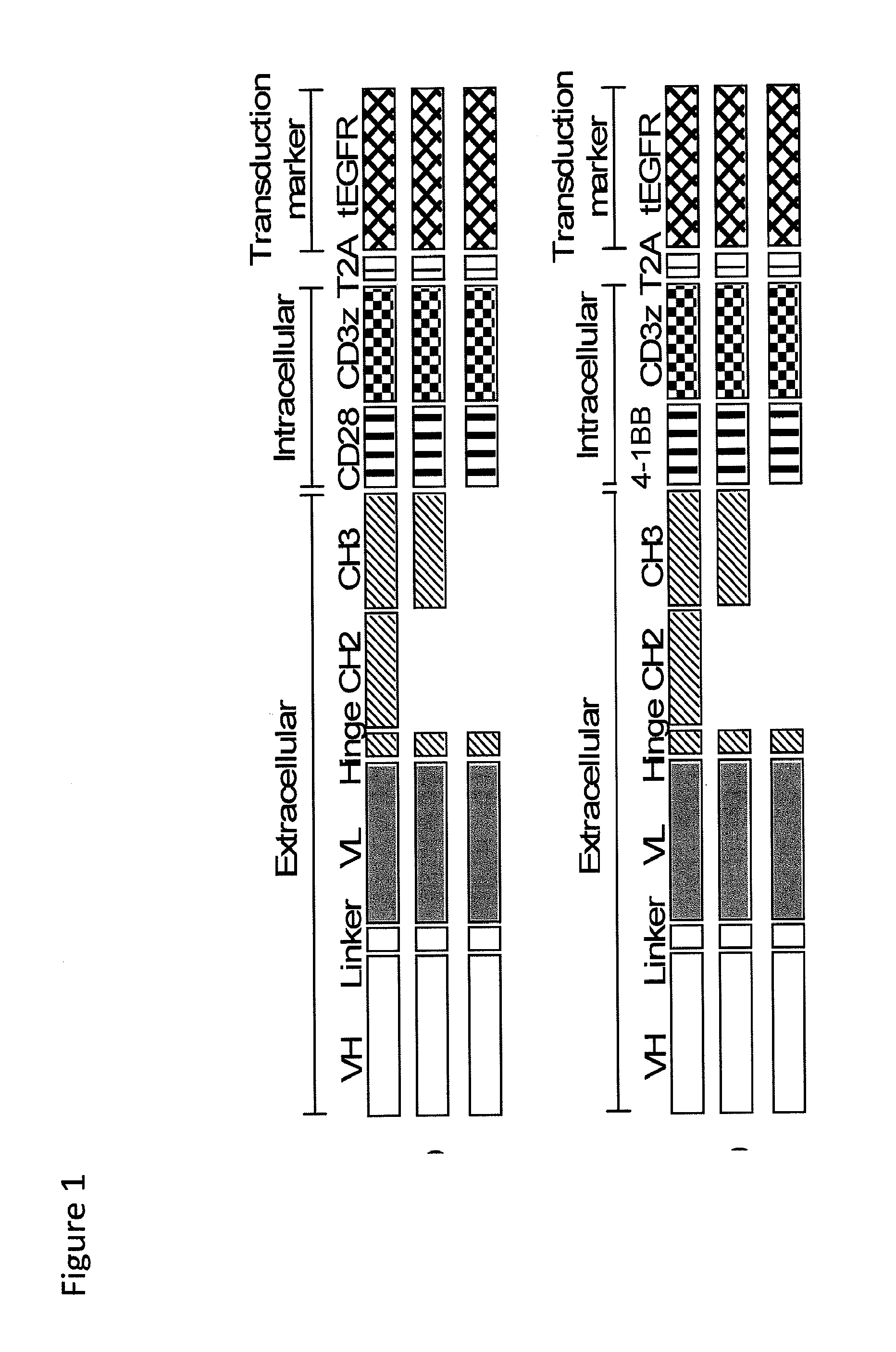
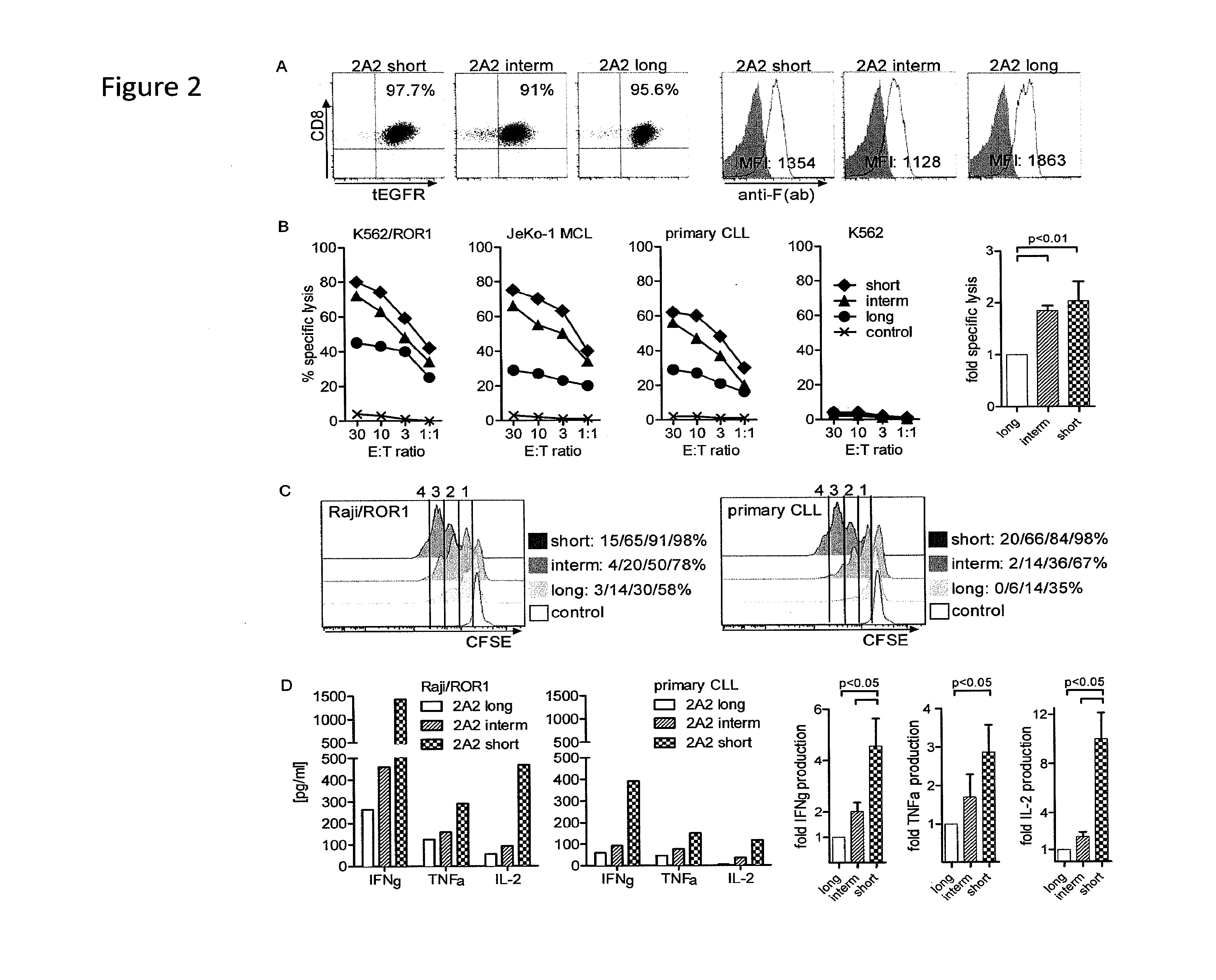
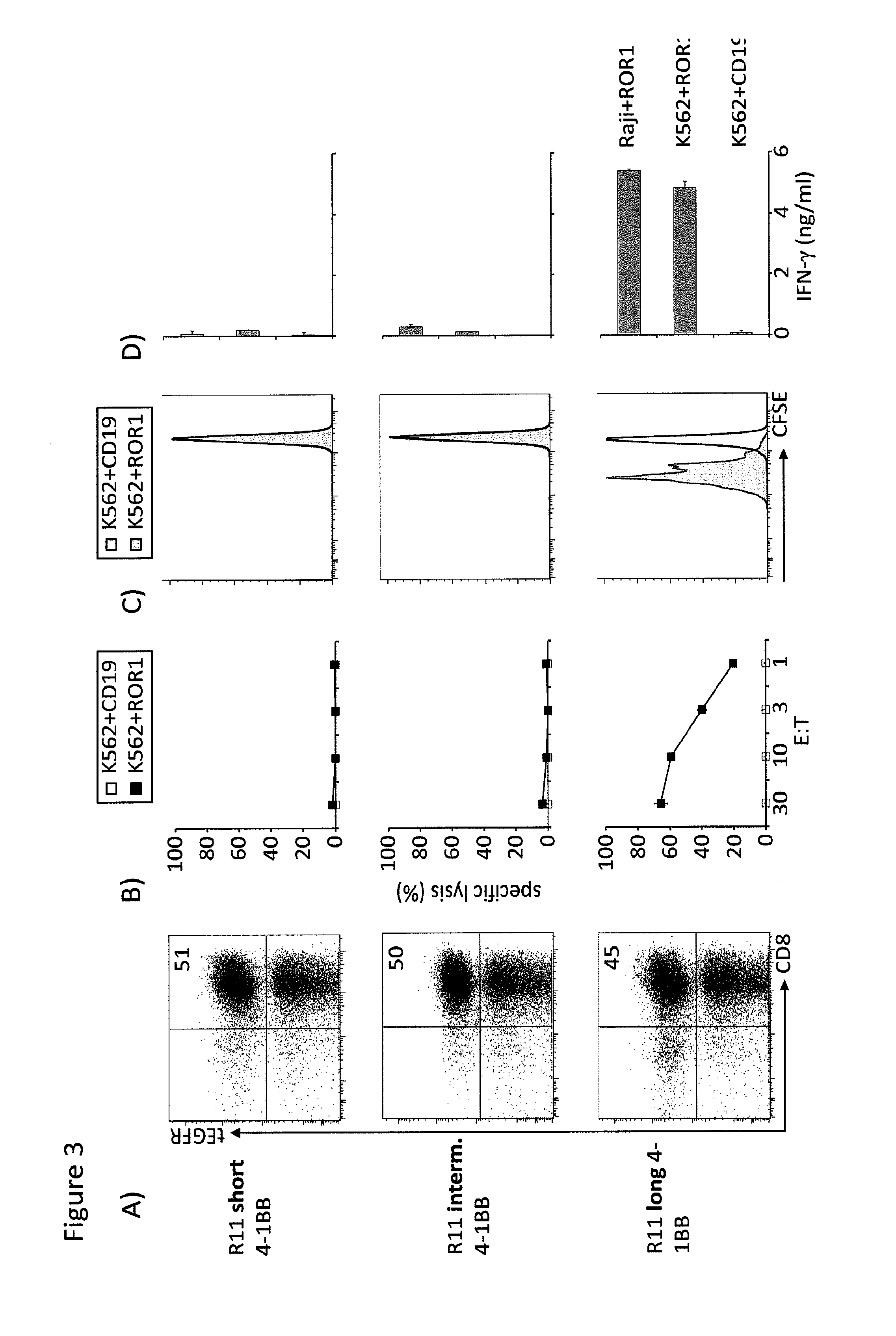
The present invention provides nucleic acids, vectors, host cells, methods and compositions to confer and / or augment immune responses mediated by cellular immunotherapy, such as by adoptively transferring CD8+ central memory T cells or combinations of central memory T cells with CD4+ T cells that are genetically modified to express a chimeric receptor. In embodiments the genetically modified host cell comprises a nucleic acid comprising a polynucleotide coding for a ligand binding domain, a polynucleotide comprising a customized spacer region, a polynucleotide comprising a transmembrane domain, and a polynucleotide comprising an intracellular signaling domain. It has been surprisingly found that the length of the spacer region can affects the ability of chimeric receptor modified T cells to recognize target cells in vitro and affects in vivo efficacy of the chimeric receptor modified T cells. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:SEATTLE CHILDRENS HOSPITAL +1
A simple and affordable method for immunophenotyping using a microfluidic chip sample preparation with image cytometry
ActiveUS20140038230A1Simple methodCheap componentBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careFluorescence
The enumeration of cells in fluids by flow cytometry is widely used across many disciplines such as assessment of leukocyte subsets in different bodily fluids or of bacterial contamination in environmental samples, food products and bodily fluids. For many applications the cost, size and complexity of the instruments prevents wider use, for example, CD4 analysis in HIV monitoring in resource-poor countries. The novel device, methods and system disclosed herein largely overcome these limitations. The system includes a simple system for CD4 and CD8 counting in point-of-care HIV staging within resource poor countries. Unlike previous approaches, no sample preparation is required with the sample added directly to a chip containing dried reagents by capillary flow. A large area image cytometer consisting of an LED module is used to excite the fluorochromes PerCP and APC labeled targets and a monochrome CCD camera with a combination of two macro lenses captures images of 40 mm2 of blood (approximately 1 microliter). CD4 and CD8-T-lymphocyte counts correlate well with those obtained by flow cytometry. The cytometer system described in the present invention provides an affordable and easy-to-use technique for use in remote locations.
Owner:UNIVERSITY OF TWENTE
Methods and algorithms for cell enumeration in a low-cost cytometer
InactiveUS7764821B2Low costFunction increaseBioreactor/fermenter combinationsBiological substance pretreatmentsWhite blood cellCcd camera
The enumeration of cells in fluids by flow cytometry is widely used across many disciplines such as assessment of leukocyte subsets in different bodily fluids or of bacterial contamination in environmental samples, food products and bodily fluids. For many applications the cost, size and complexity of the instruments prevents wider use, for example, CD4 analysis in HIV monitoring in resource-poor countries. The novel device, methods and algorithms disclosed herein largely overcome these limitations. Briefly, all cells in a biological sample are fluorescently labeled, but only the target cells are also magnetically labeled. In addition, non-magnetically labeled cells are imaged for viability in a modified slide configuration. The labeled sample, in a chamber or cuvet, is placed between two wedge-shaped magnets to selectively move the magnetically labeled cells to the observation surface of the cuvet. An LED illuminates the cells and a CCD camera captures the images of the fluorescent light emitted by the target cells. Image analysis performed with a novel algorithm provides a count of the cells on the surface that can be related to the target cell concentration of the original sample. The compact cytometer system provides a rugged, affordable and easy-to-use technique, which can be used in remote locations.
Owner:UNIVERSITY OF TWENTE
Antibodies directed against ICOS and uses thereof
ActiveUS9376493B2Effective conditioningShorten the progressAntibacterial agentsNervous disorderPlasmacytoid dendritic cellCD4 antigen
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
T cell regulation
InactiveUS20060240024A1Increases magnitudeSimple methodAntibacterial agentsNervous disorderRegulatory T cellAutoimmune responses
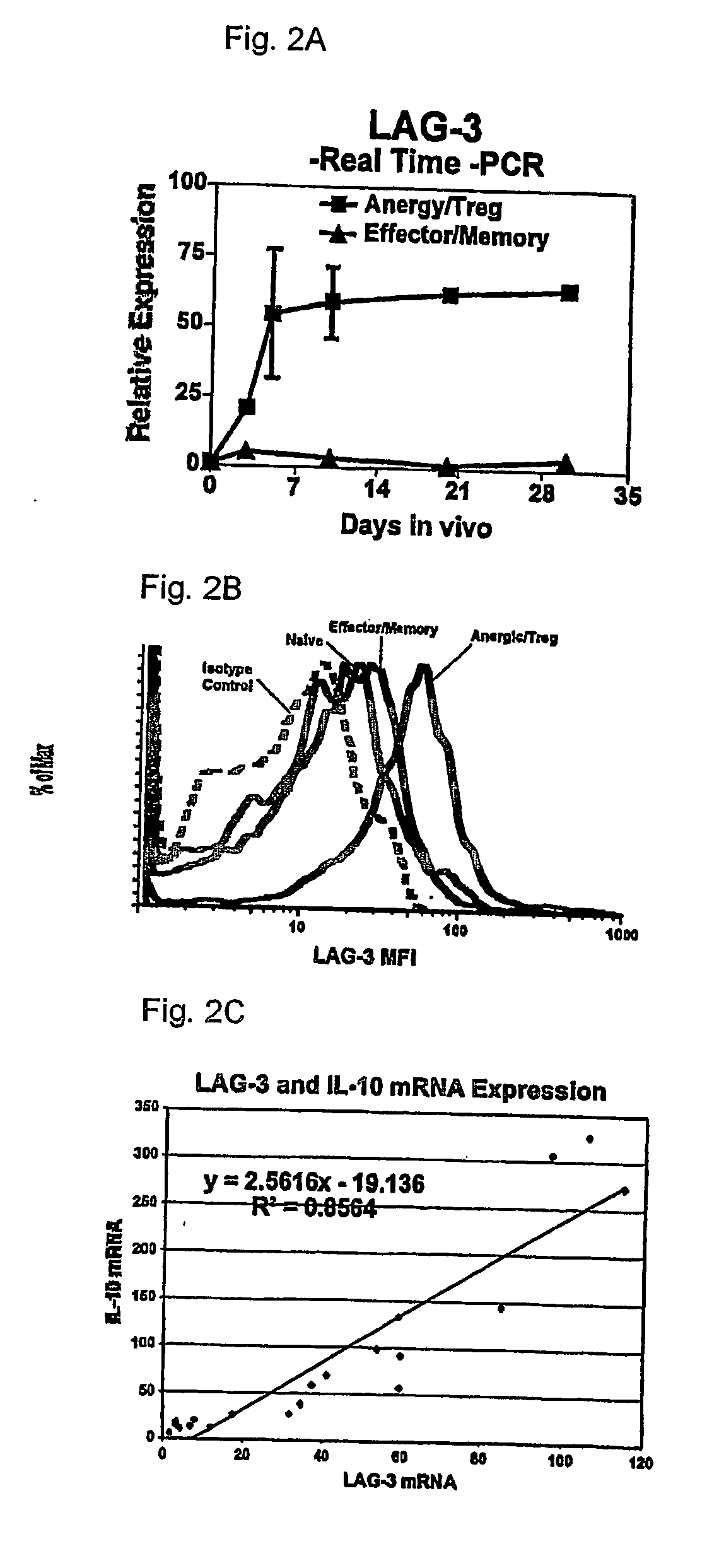
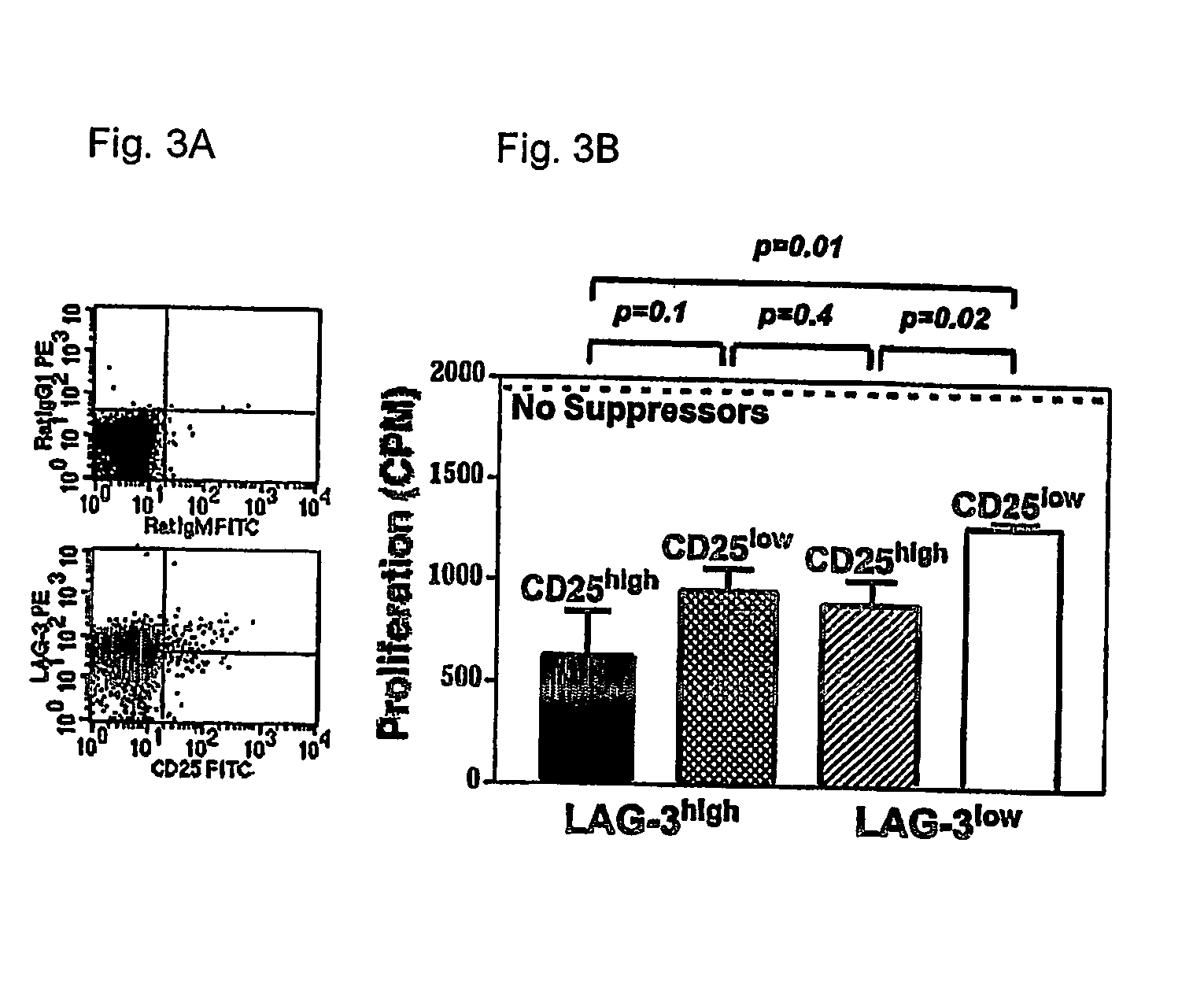
Regulatory T cells (Treg) limit autoimmunity but can also attenuate the magnitude of anti-pathogen and anti-tumor immunity. Understanding the mechanism of Treg function and therapeutic manipulation of Treg in vivo requires identification of Treg selective receptors. A comparative analysis of gene expression arrays from antigen specific CD4+ T cells differentiating to either an effector / memory or a regulatory phenotype revealed Treg selective expression of LAG-3 (CD223), a CD4-related molecule that binds MHC class II. LAG-3 expression on CD4+ T cells correlates with the cells' in vitro suppressor activity, and ectopic expression of LAG-3 on CD4 T cells confers suppressor activity on the T cells. Antibodies to LAG-3 inhibit suppression both in vitro and in vivo. LAG-3 marks regulatory T cell populations and contributes to their suppressor activity.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC +1
Dock-and-lock (DNL) constructs for human immunodeficiency virus (HIV) therapy
ActiveUS8481041B2Reduce eliminateReduce and eliminate focusOrganic active ingredientsImmunoglobulins against virusesAntigenAntibody fragments
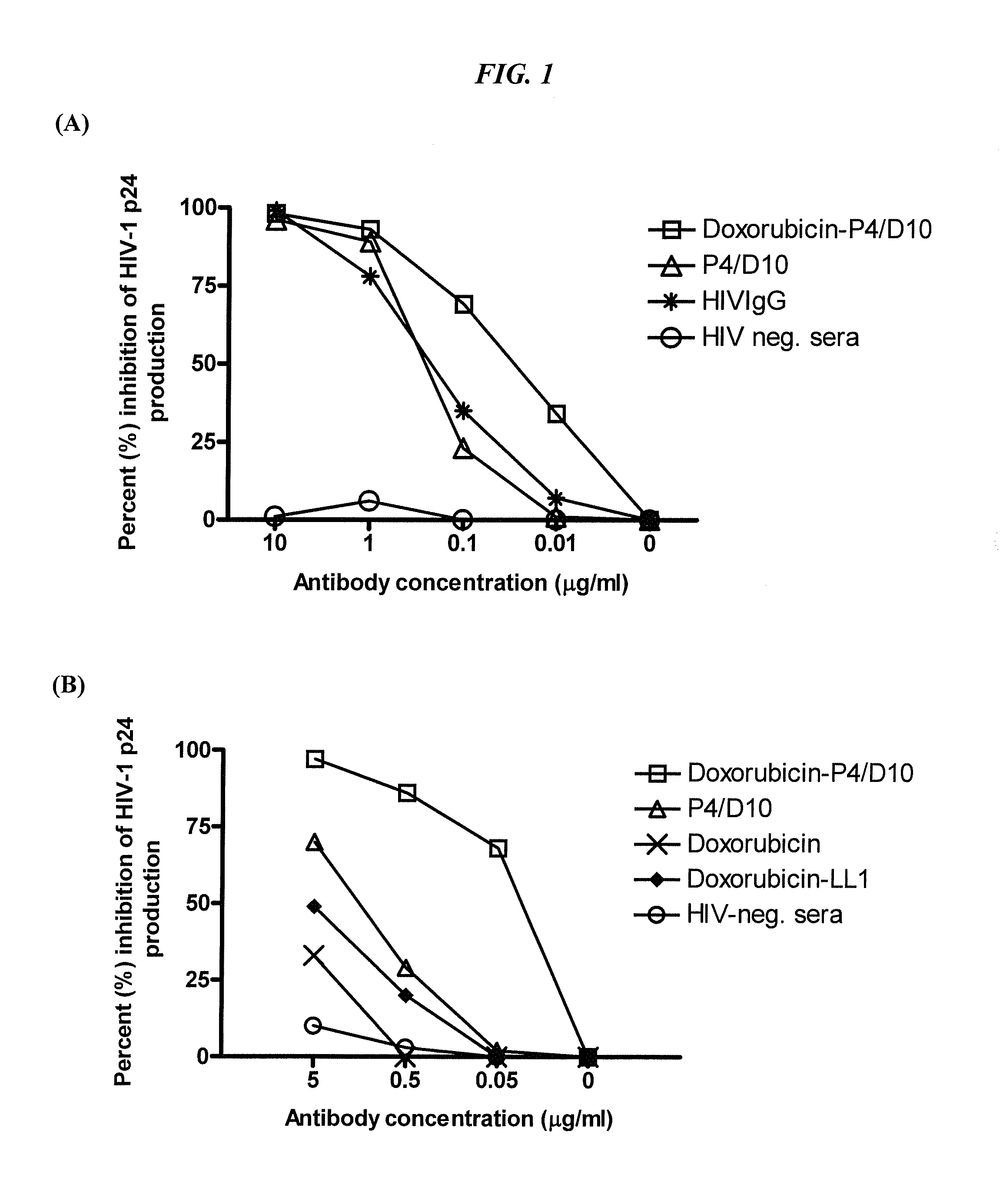
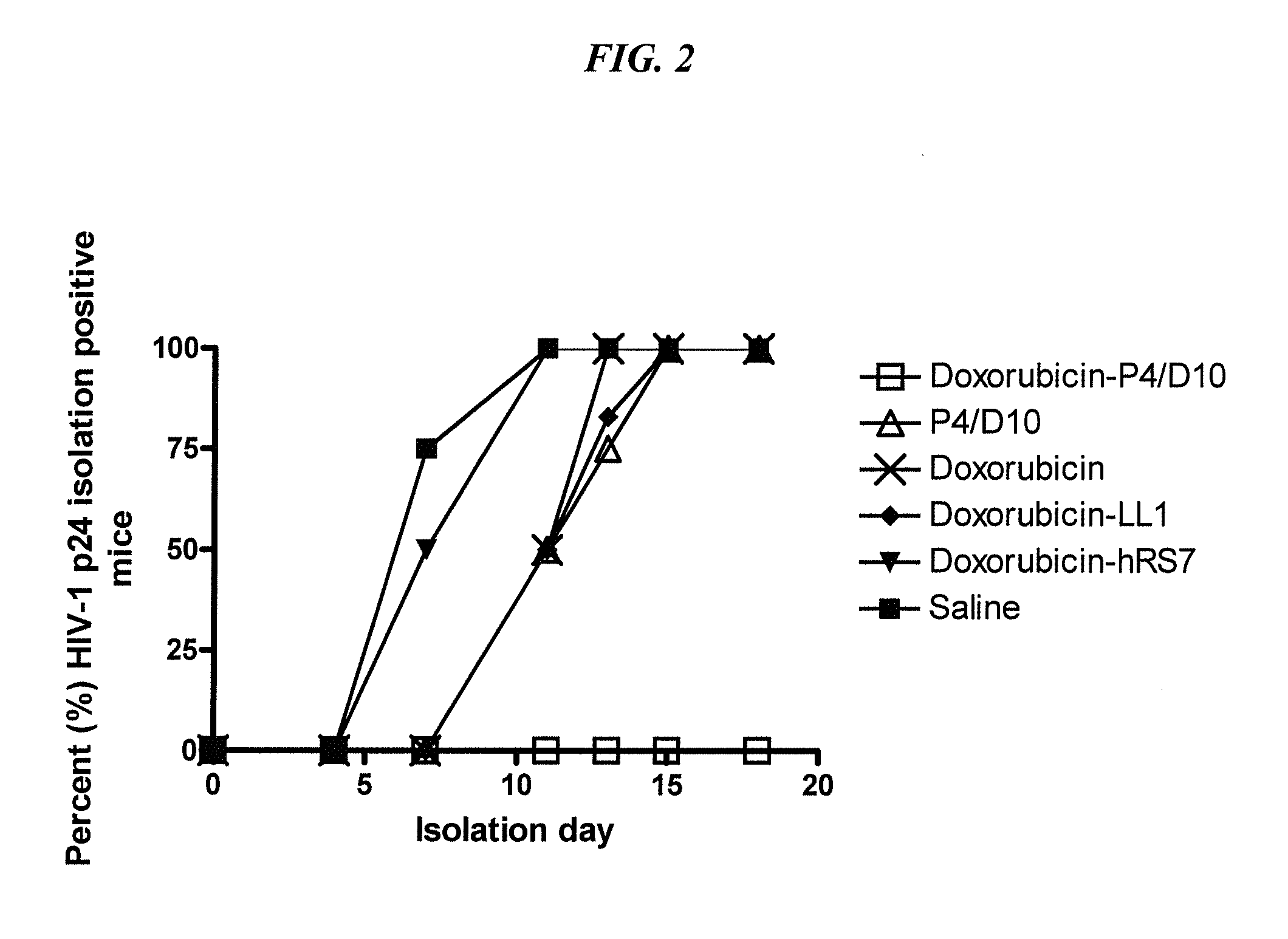
The present invention concerns methods and compositions for treatment of HIV infection in a subject, utilizing a DNL complex comprising at least one anti-HIV therapeutic agent, attached to an antibody, antibody fragment or PEG. In a preferred embodiment, the antibody or fragment binds to an antigen selected from gp120, gp41, CD4 and CCR5. In a more preferred embodiment the antibody is P4 / D10 or 2G12, although other anti-HIV antibodies are known and may be utilized. In a most preferred embodiment, the anti-HIV therapeutic agent is a fusion inhibitor, such as T20, T61, T651, T1249, T2635, CP32M or T-1444, although other anti-HIV therapeutic agents are known and may be utilized. The DNL complex may be administered alone or may be co-administered with one or more additional anti-HIV therapeutic agents.
Owner:IBC PHARMACEUTICALS INC
Humanized t cell co-receptor mice
The invention provides genetically modified non-human animals that express chimeric human / non-human T cell co-receptor polypeptides (e.g., CD4, CD8α, CD8β), as well as embryos, cells, and tissues comprising the same. Also provided are constructs for making said genetically modified animals and methods of making the same.
Owner:REGENERON PHARM INC
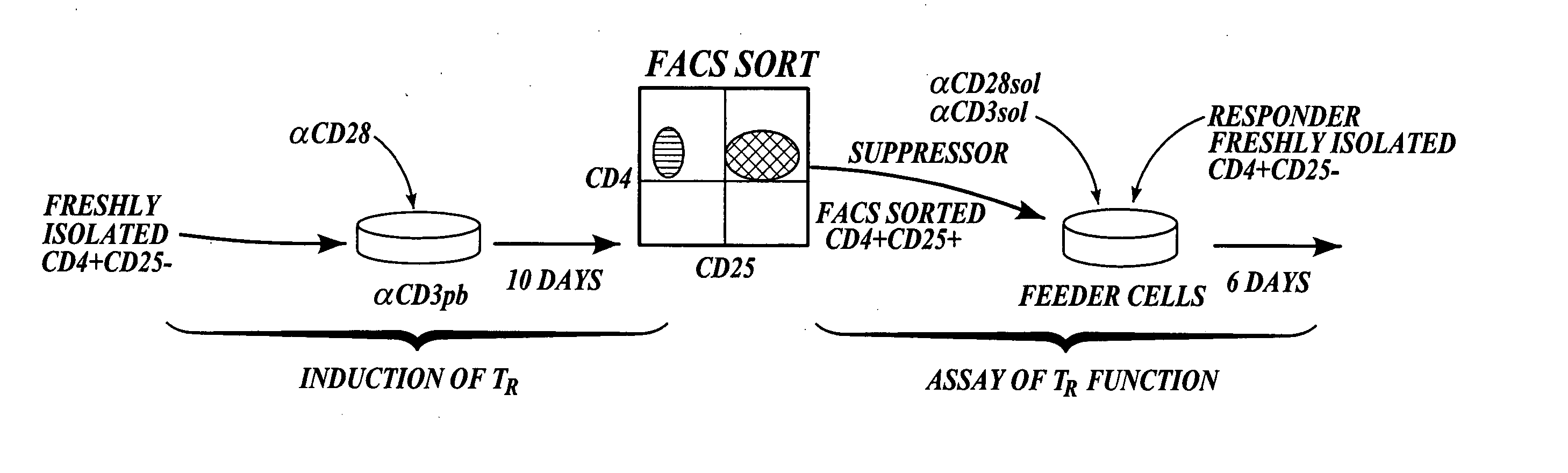
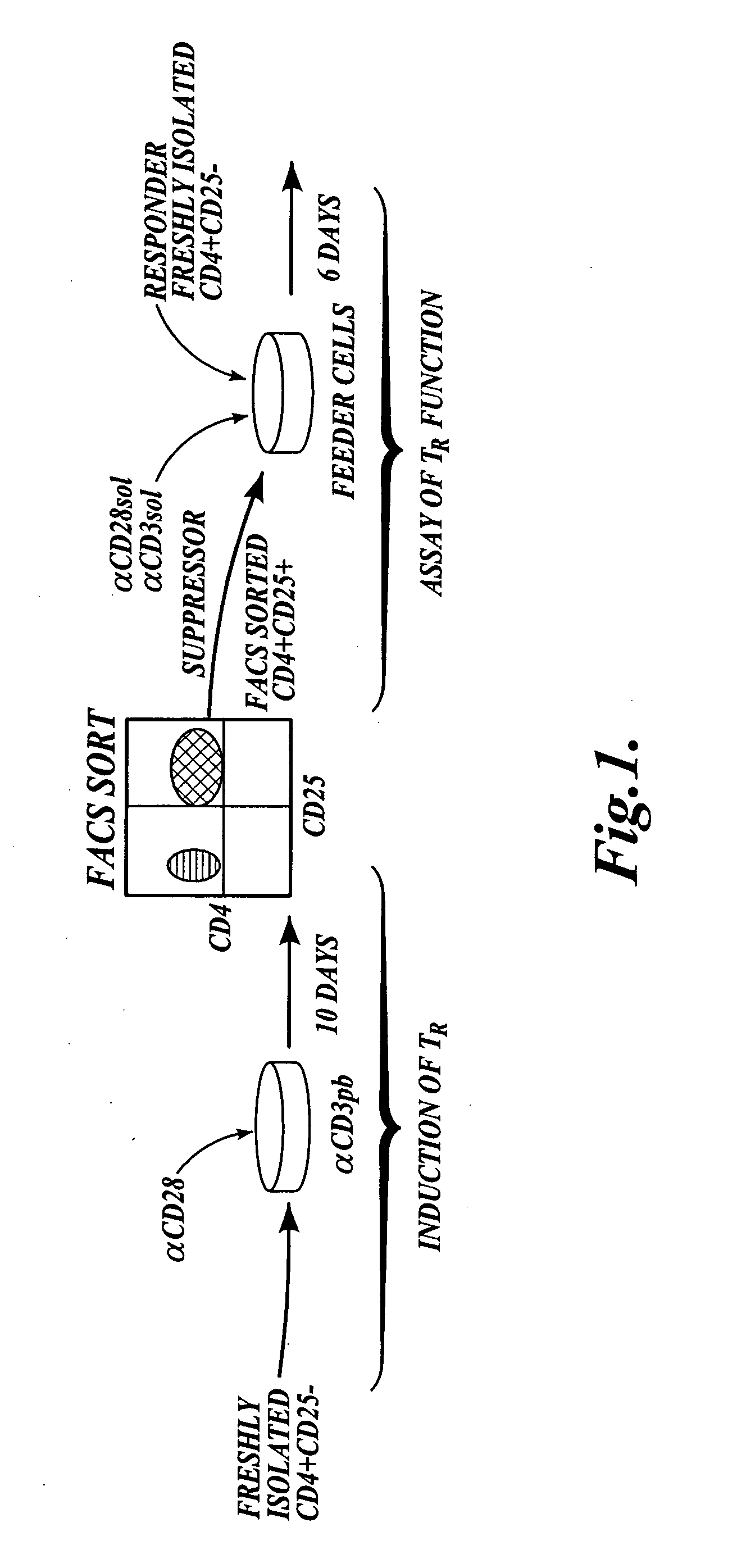
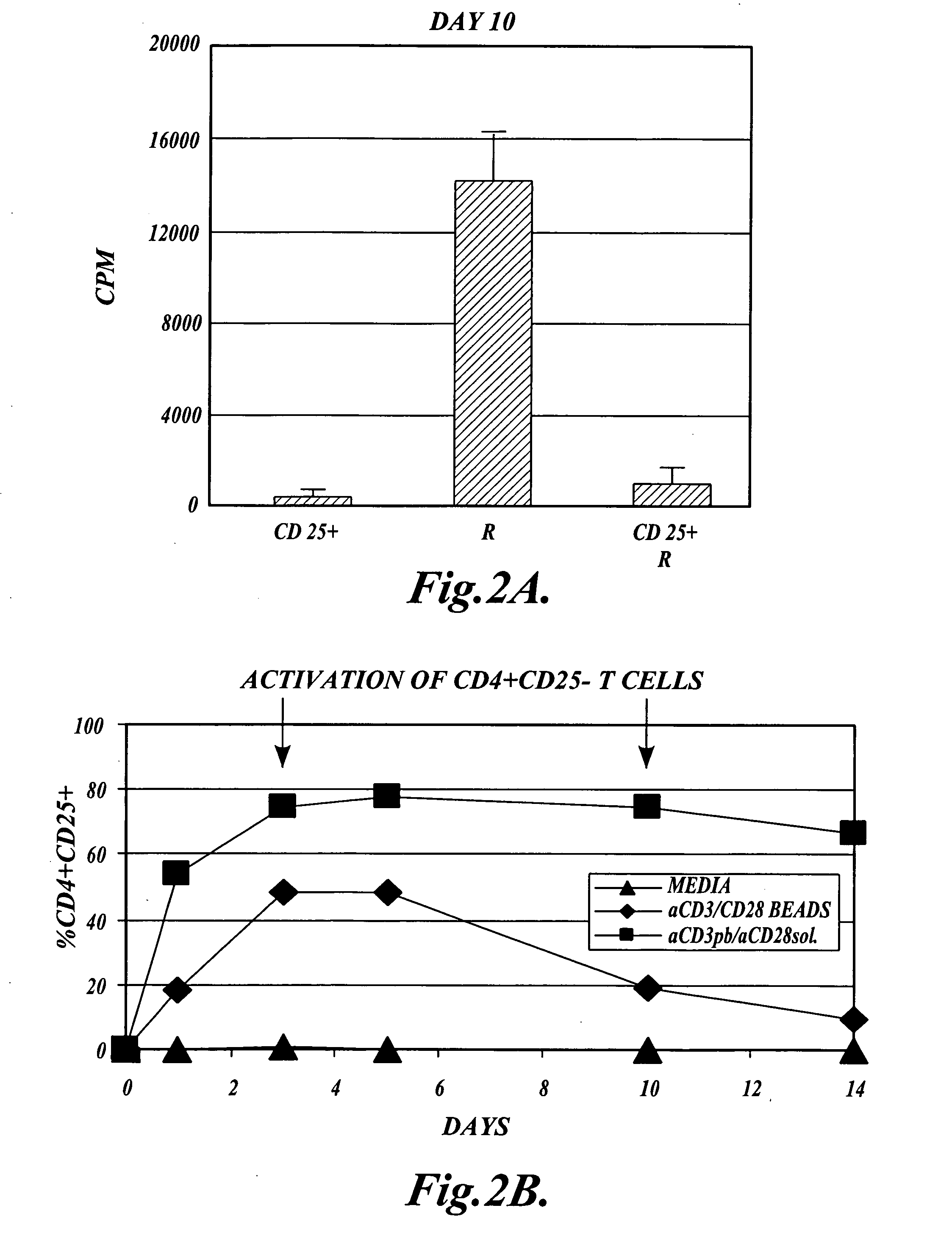
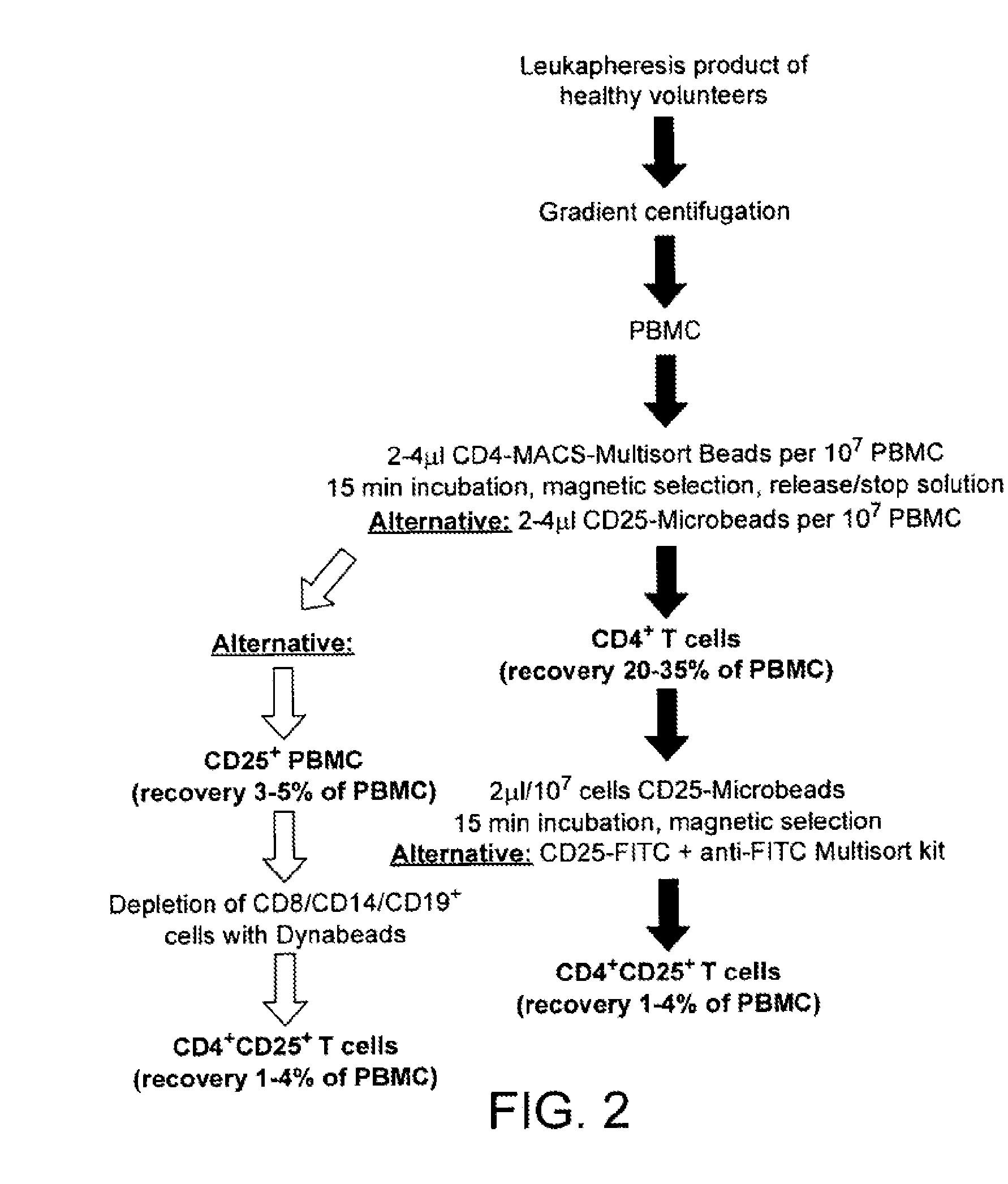
Methods of generating antigen-specific CD4+CD25+ regulatory T cells, compositions and methods of use
ActiveUS20060115899A1Reduce riskReduce severityArtificial cell constructsDead animal preservationRegulatory T cellSelect agent
The present invention provides methods for generating mammalian T cell populations comprising antigen-specific CD4+CD25+ regulatory T cells from freshly isolated CD4+CD25− T cells. The method comprises selecting CD4+CD25− T cells from a sample obtained from a mammalian subject; determining the MHC Class II type of the subject; inducing the generation of antigen-specific regulatory T cells by contacting the isolated CD4+CD25− T cells in a culture vessel with an induction agent for a time period sufficient to generate antigen-specific CD4+CD25+ regulatory T cells; and selecting the CD4+CD25+ antigen-specific regulatory T cells by sorting the cells in the induction culture with a selection agent comprising at least one artificial multimeric MHC Class II / peptide complex that corresponds to the MHC Class II type of the subject.
Owner:BENAROYA RES INST AT VIRGINIA MASON
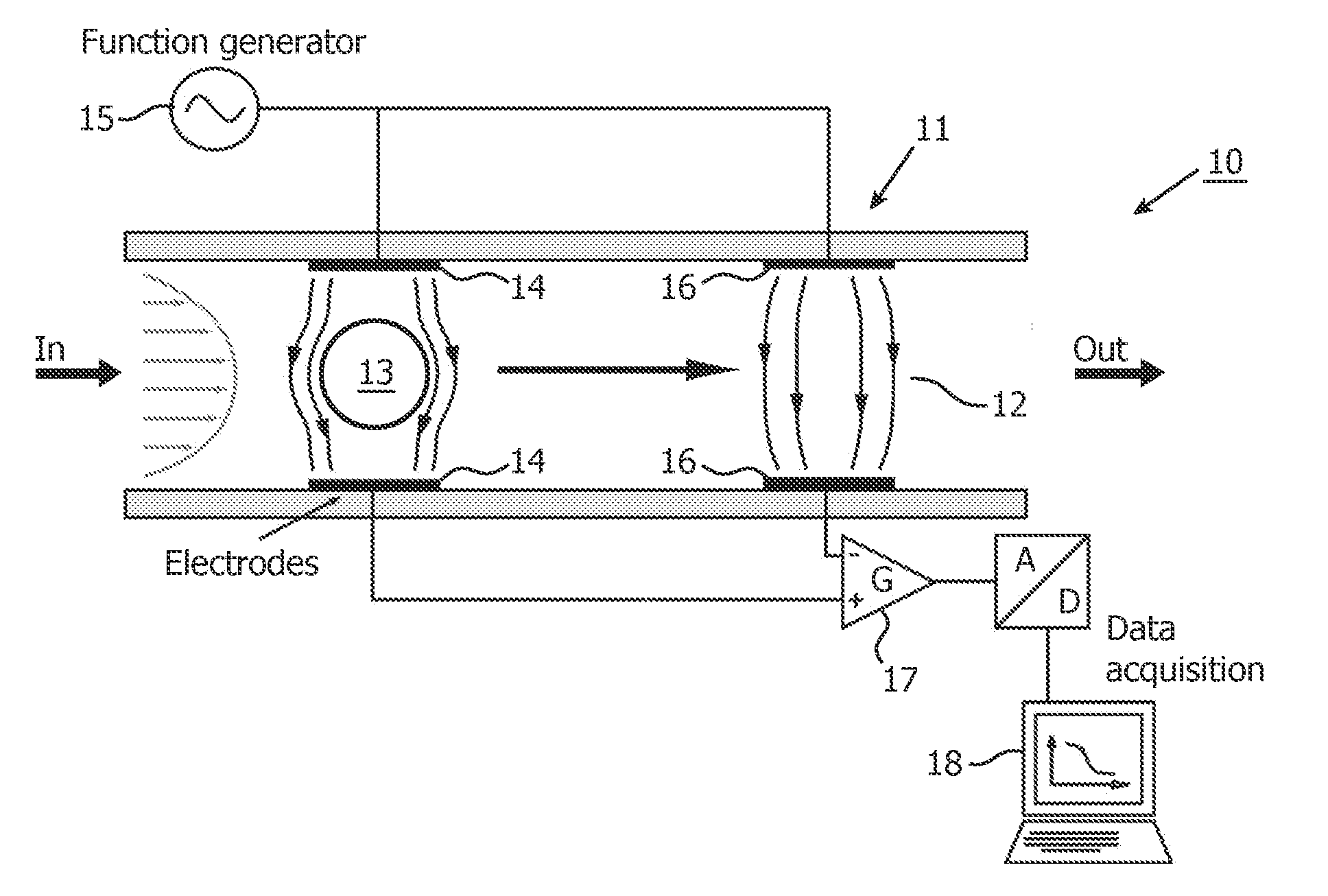
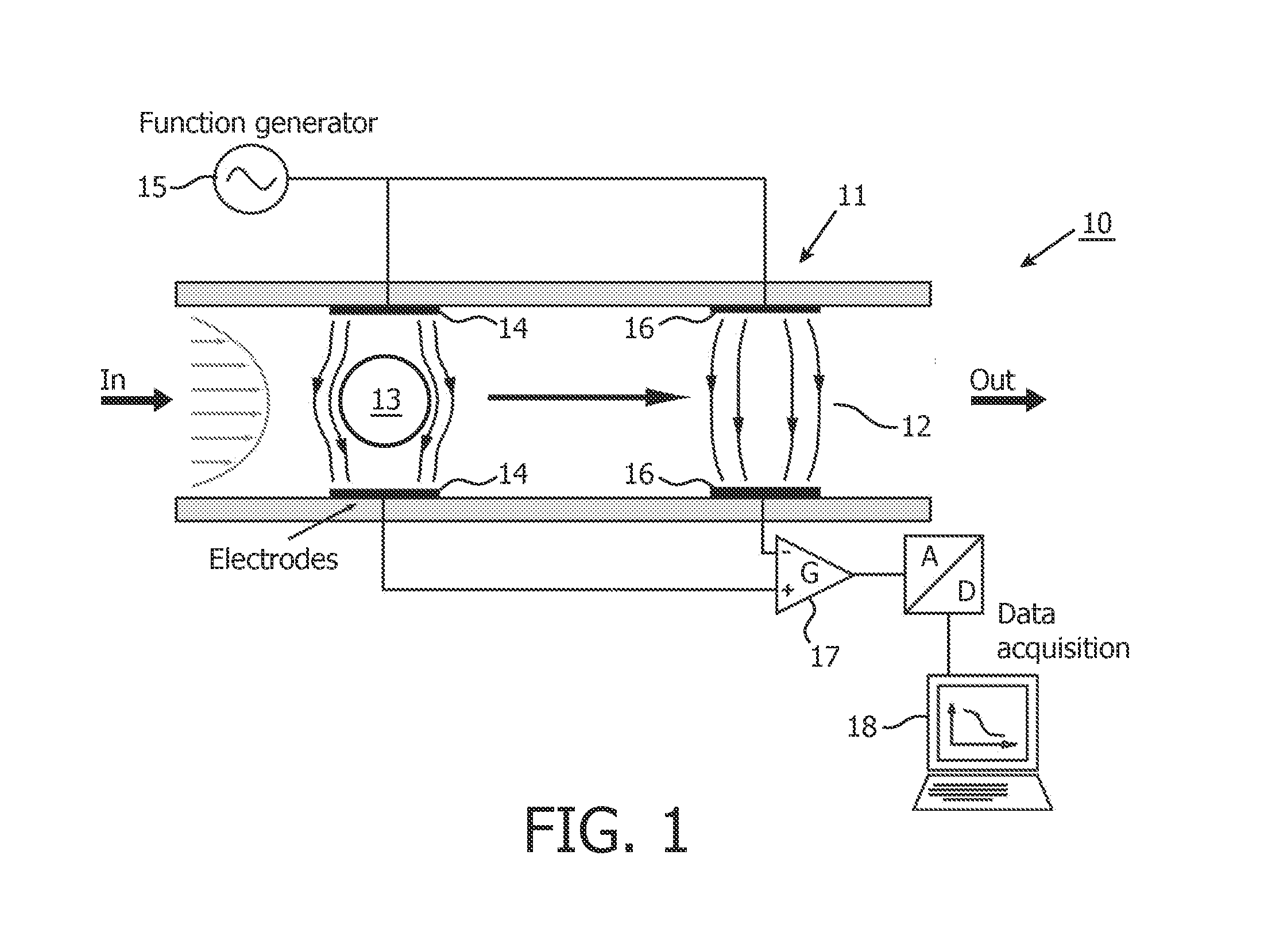
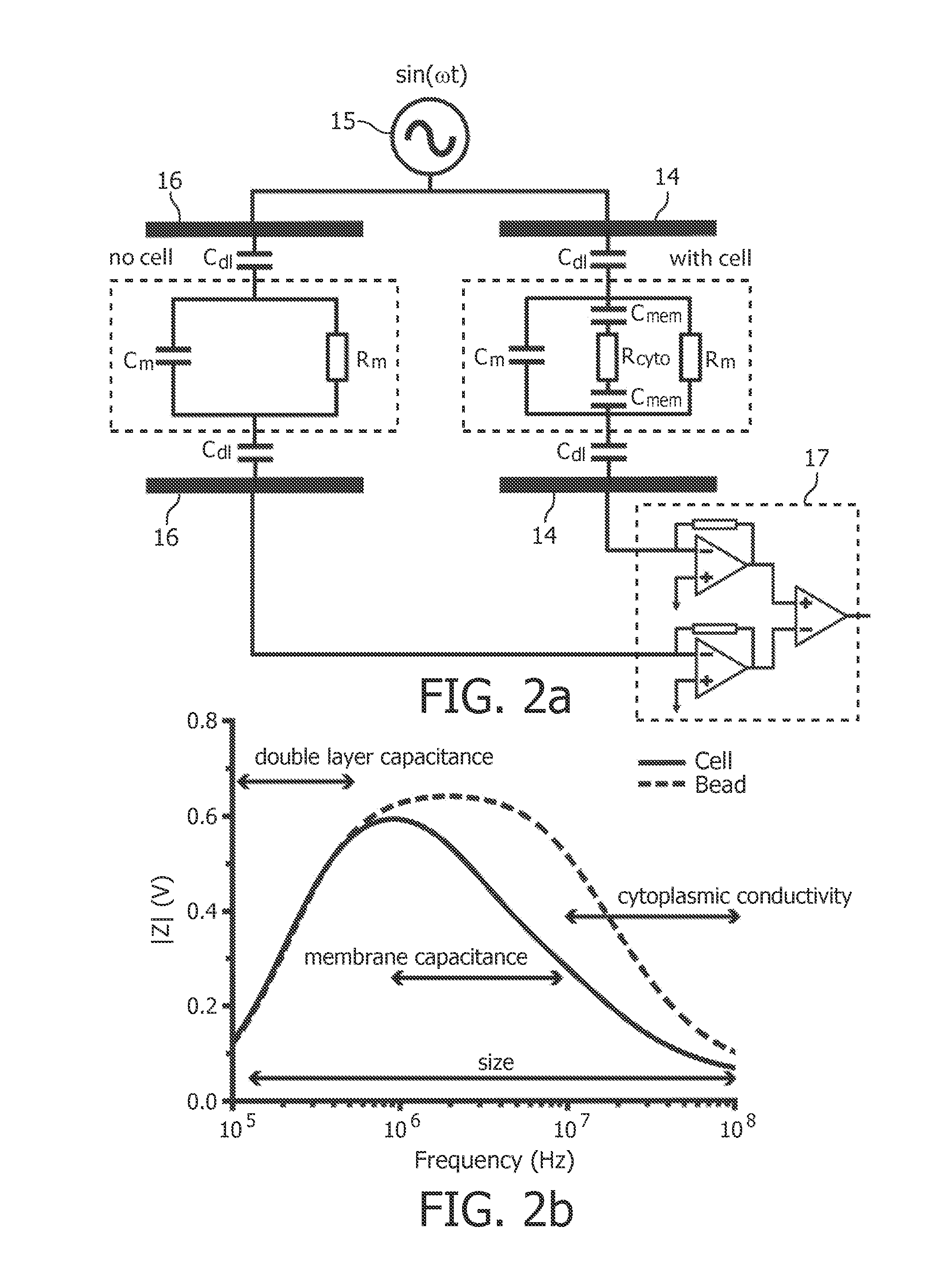
Multi-frequency impedance method and apparatus for discriminating and counting particles expressing a specific marker
InactiveUS20120142032A1Accurately determineFast and inexpensive and simple analysisBioreactor/fermenter combinationsBiological substance pretreatmentsDual frequencyLymphocyte
An analysis method for identifying and counting particles expressing a specific antigenic marker is proposed. One example (but not exclusive) is CD4+ T-lymphocyte analysis in blood. The method comprises obtaining a sample comprising particles in suspension, labeling the particles expressing the specific marker with an impedance label, and measuring the labeled sample using a dual frequency system to discriminate at least the labeled particles expressing the specific marker. The method allows an accurate count of particles e.g. the number CD4+ T-lymphocytes in a blood flow. A corresponding device is also provided.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Methods for inhibiting HIV-1 infection
This invention provides an antibody capable of specifically inhibiting the fusion of an HIV-1 envelope glycoprotein+ cell with an appropriate CD4+ cell without cross reacting with the HIV-1 envelope glycoprotein or CD4 and capable of inhibiting infection by one or more strains of HIV-1. This antibody is then used to identify a molecule which is important for HIV infection. Different uses of the antibody and the molecule are described.
Owner:CYTODYN
Product and process for T lymphocyte immunosuppression
InactiveUS6264950B1Peptide/protein ingredientsAntibody mimetics/scaffoldsMajor histocompatibilityActivation cells
The present invention relates to a product and process for suppressing an immune response using a T lymphocyte veto molecule capable of blocking cell surface molecules responsible for T cell activation. Disclosed is a CD4 or CD2 molecule, associated with an immunoglobulin molecule capable of binding to a major histocompatibility antigen. Also disclosed is a method to produce a T lymphocyte veto molecule, a therapeutic composition comprising a T lymphocyte veto molecule and methods to use T lymphocyte veto molecules in therapeutic processes requiring suppression of an immune response.
Owner:NAT JEWISH MEDICAL & RES CENT
Chimeric immunoreceptor useful in treating human cancers
InactiveUS20060067920A1Negligible toxicityPotent and selectiveBiocideAntibody mimetics/scaffoldsIntracellular signallingAutocrine paracrine
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signalling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human maligancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targetting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Agent for treating disease
InactiveUS20110059084A1Easy to manageReduce dosageNervous disorderAntipyreticAutoimmune diseaseCD4 antigen
The present invention provides a pharmaceutical composition for treating an autoimmune disease comprising a pharmaceutically acceptable carrier and an agent capable of activating CD4+CD25+ regulatory T cells, wherein the composition is to be administered to a subject in a dose of the agent from 0.2 mg to 30 mg.
Owner:BIOTEST SERUM INST GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com