T cell regulation
a technology of t cell and regulation, applied in the field of therapeutic and drug screening methods, can solve the problems of destroying t-cell activity, unable to meet the needs of patients, and unable to achieve optimal immunotherapy, so as to overcome the suppression of an immune response, increase the response of cancer patients, and increase the magnitude of anti-cancer response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Negative Regulation of T Cell Homeostasis by LAG-3 (CD223)
[0079] The following example shows that LAG-3 (CD223) negatively regulates CD4+ and CD8+ T cell homeostasis, supporting its identification as a novel therapeutic target for accelerating T cell engraftment following bone marrow transplantation.
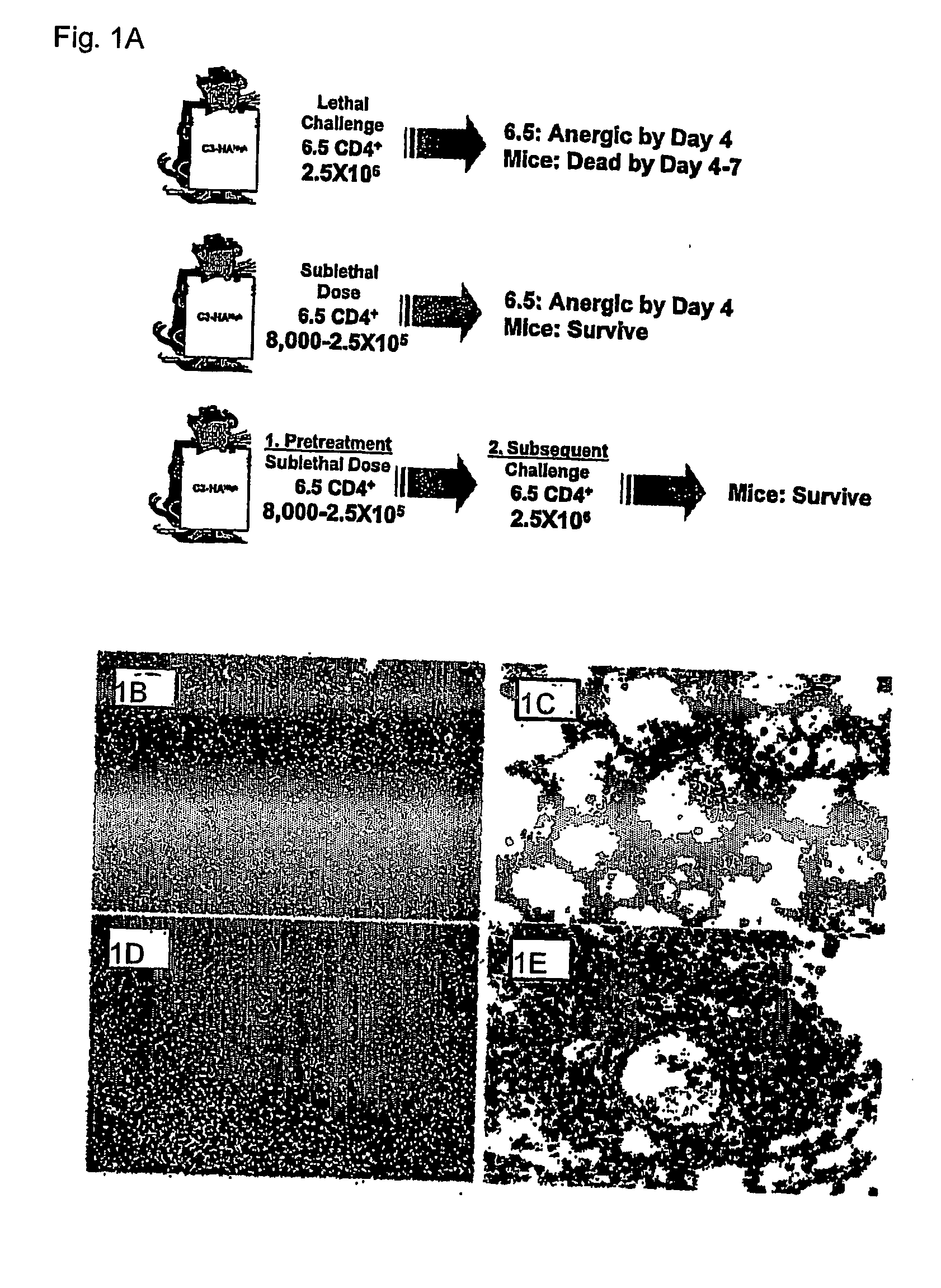
[0080] Wild type C57BL / 6 mice have a constant number of αβ+ cells from 4 to 52 weeks of age. As previously reported, young 4 week old LAG-3− / − mice have normal T cell numbers. Miyazaki, T. et al., Science 272: 405-408 (1996). In contrast, the number of αβ+ T cells in LAG-3− / − mice steadily increases from 3 months of age to numbers ˜2-fold higher than wild type mice. This difference is highly significant given the tight homeostatic regulation of αβ+ T cell number evidenced by the very low standard deviation. Both CD4+ and CD8+ cells were increased in LAG-3− / − mice but the CD4:CD8 ratio was unchanged. Similarly, LAG-3− / − mice transgenic for the OT-II TCR (ovalbumin 326-339-specific, H-2A...
example 2
Materials and Methods
[0088] This example provides the experimental methods and materials for example 1.
[0089] Mice: The following mice were used: LAG-3− / − [obtained from Yueh-Hsiu Chen, Stanford University, Palo Alto, Calif., with permission from Christophe Benoist and Diane Mathis, Joslin Diabetes Center, Boston, Mass.; Miyazaki, T. et al., Science 272: 405-408 (1996)]; C57BL / 6J [Jackson Labs, Bar Harbor, Me.]; B6.PL-Thy1a / Cy (Thy1.1 congenic) [Jackson Labs]; RAG-1− / − [Jackson Labs, Bar Harbor, Me.; Mombaerts, P. et al., Cell 68: 869-877 (1992)]; MHC class II− / − [provided by Peter Doherty, St. Jude Children's Research Hospital, Memphis, Term.; Grusby, M. J. et al., Science 253:1417-1420 (1991)]; MHC class I− / − / II− / − [Taconic, Germantown, N.Y.; Grusby, M. J. et al., Proc. Natl. Acad. Sci. U.S.A 90: 3913-3917 (1993)]; OT-II TCR transgenic mice [provided by Stephen Schoenberger, La Jolla Institute for Allergy and Immunology, La Jolla, Calif., with permission from William Heath, Walt...
example 3
Induced Treg Cells with Potent Regulatory Activity
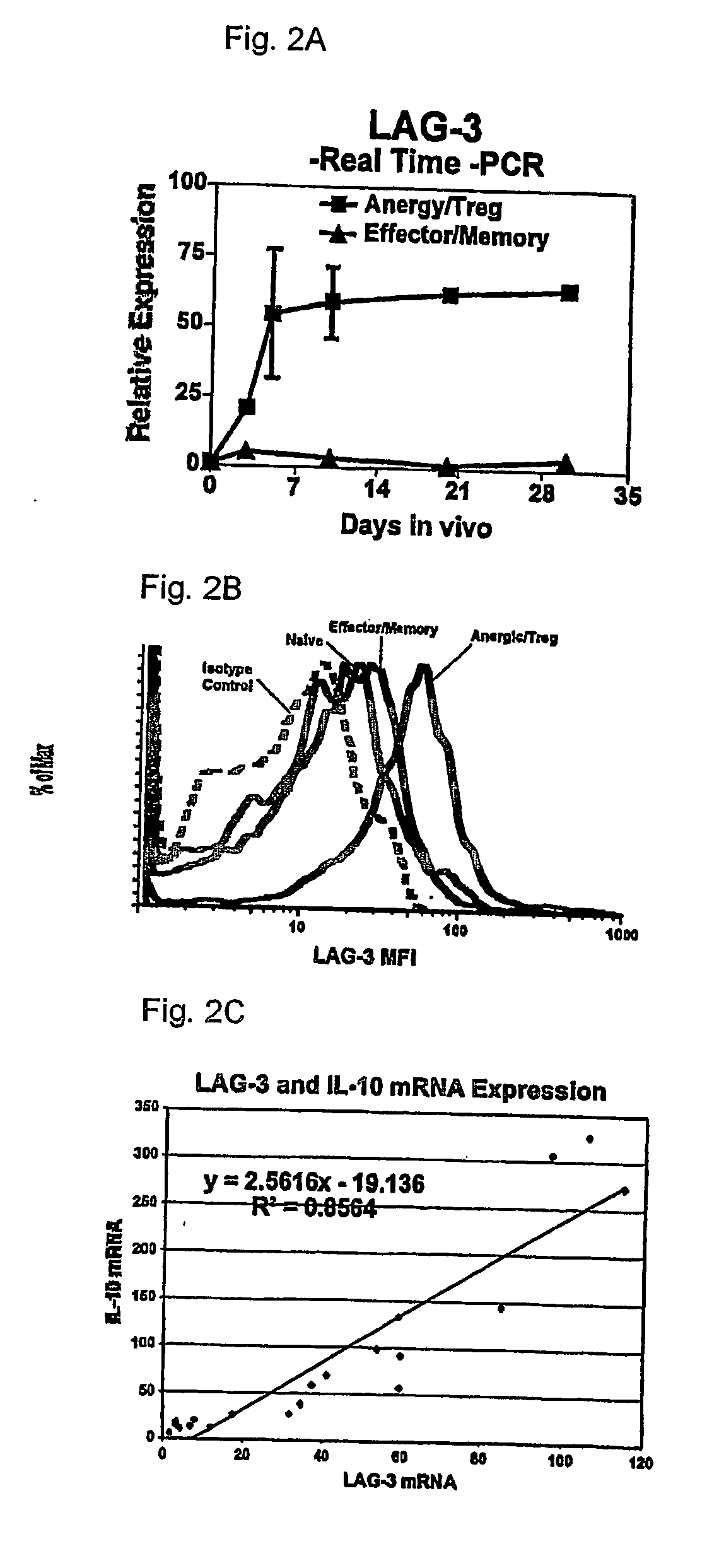
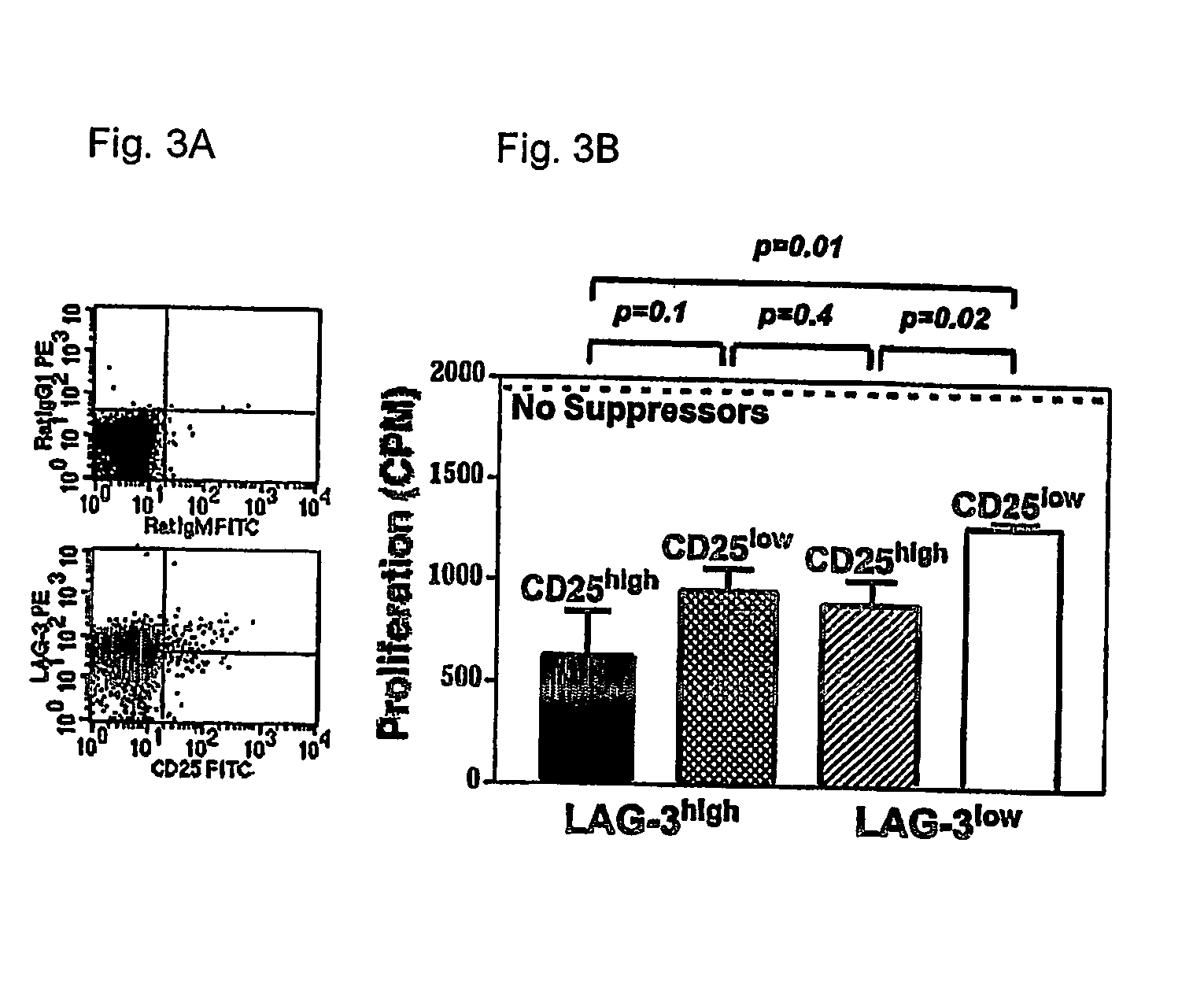
[0095] In order to identify Treg specific molecules, we performed a differential gene expression analysis of antigen-specific T cells differentiating to either effector / memory cells in response to viral infection or Treg cells upon encounter of cognate antigen as a self-antigen. This analysis revealed that the LAG-3 gene was selectively upregulated in Treg cells. The physiologic role of LAG-3, an MHC class II binding CD4 homologue, has not been clearly elucidated. Several in vitro studies have suggested that LAG-3 may have a negative regulatory function (Hannier et al., 1998; Huard et al., 1994; Workman et al., 2002a; Workman et al., 2002b). Here we show that membrane expression of LAG-3 selectively marks Treg cells independently of CD25 and that LAG-3 modulates both the in vitro and in vivo suppressive activity of Treg cells.
[0096] In order to study differences between T cell effector / memory and tolerance induction, we have utiliz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com