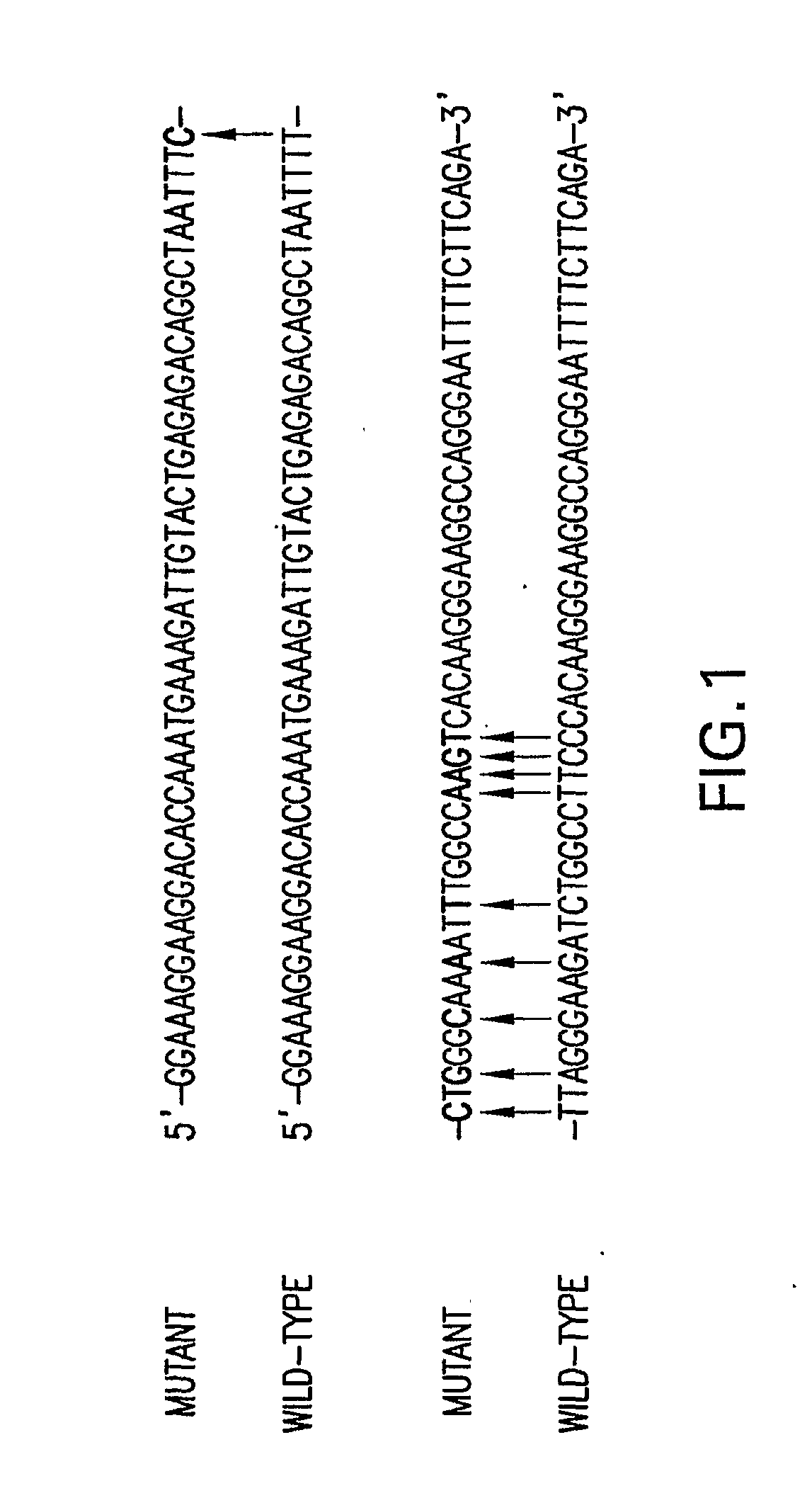
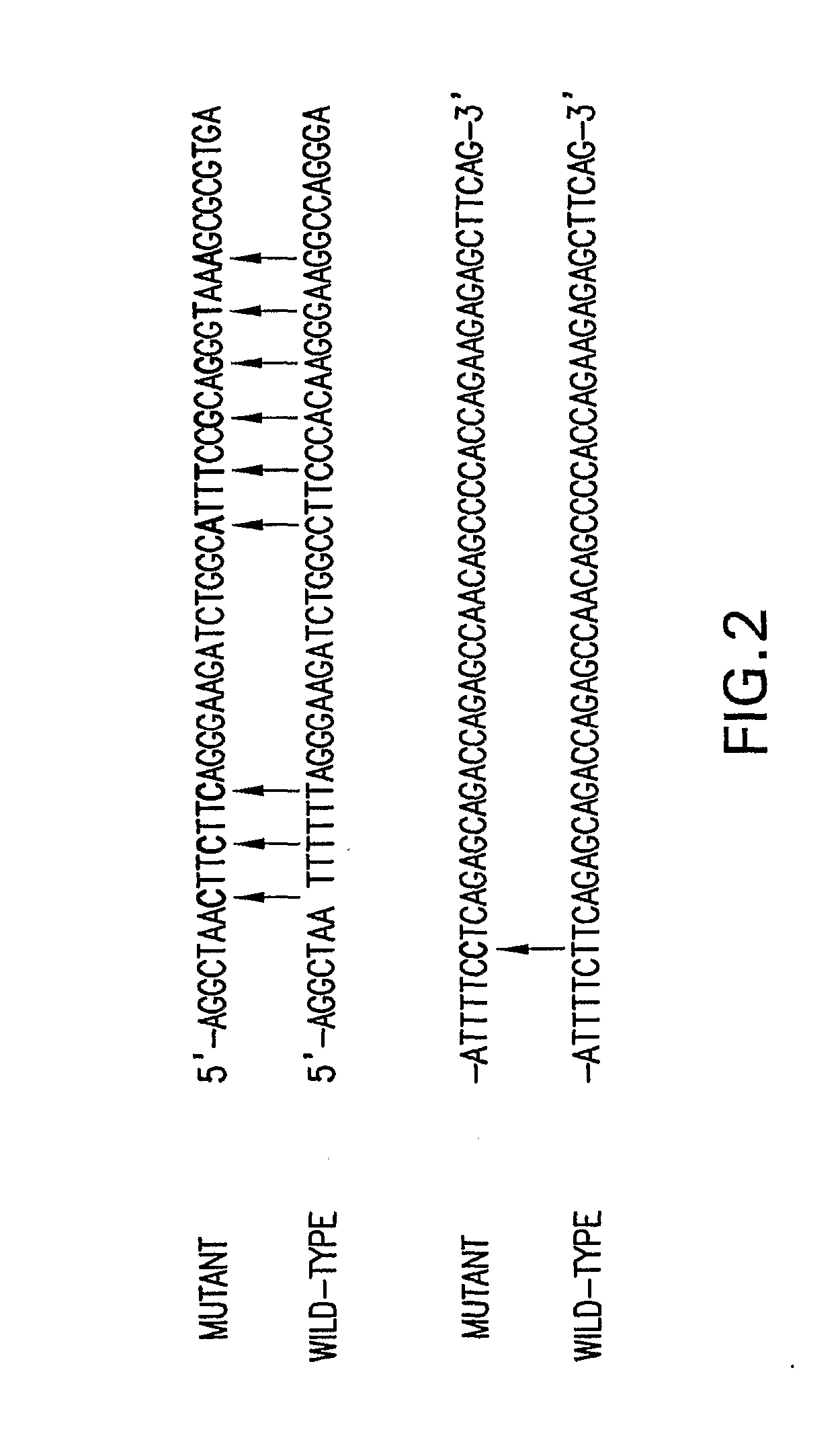
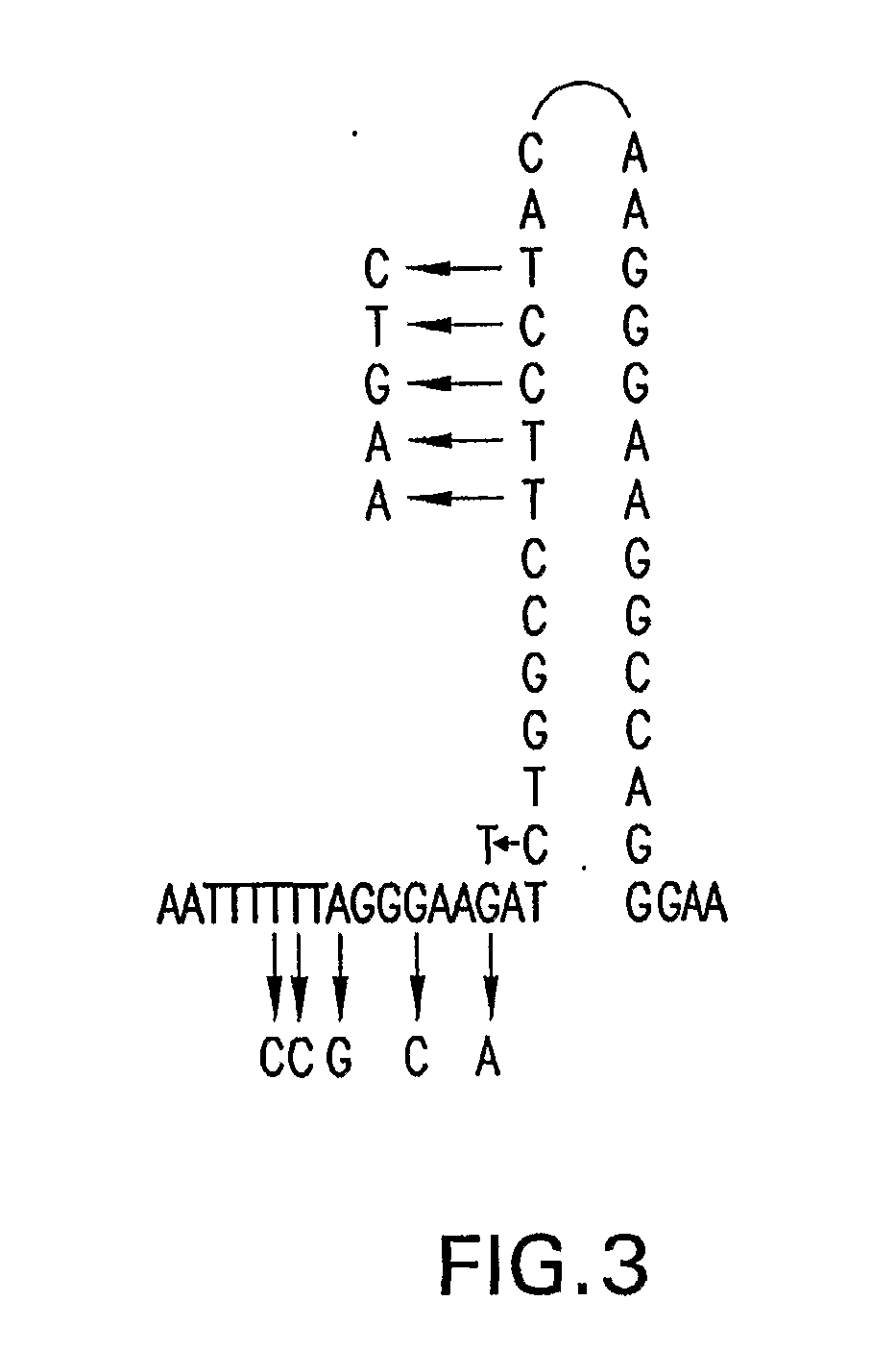
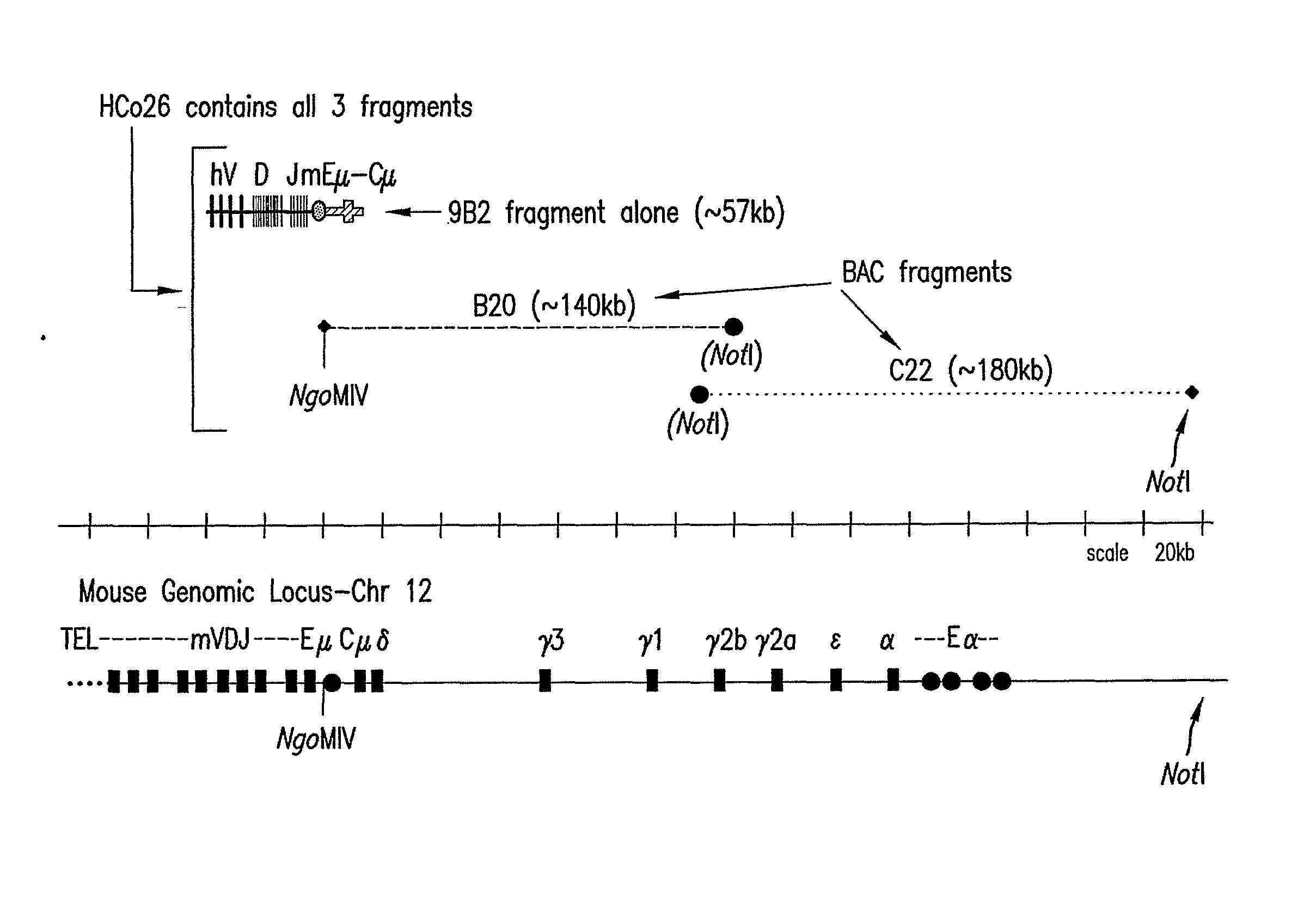
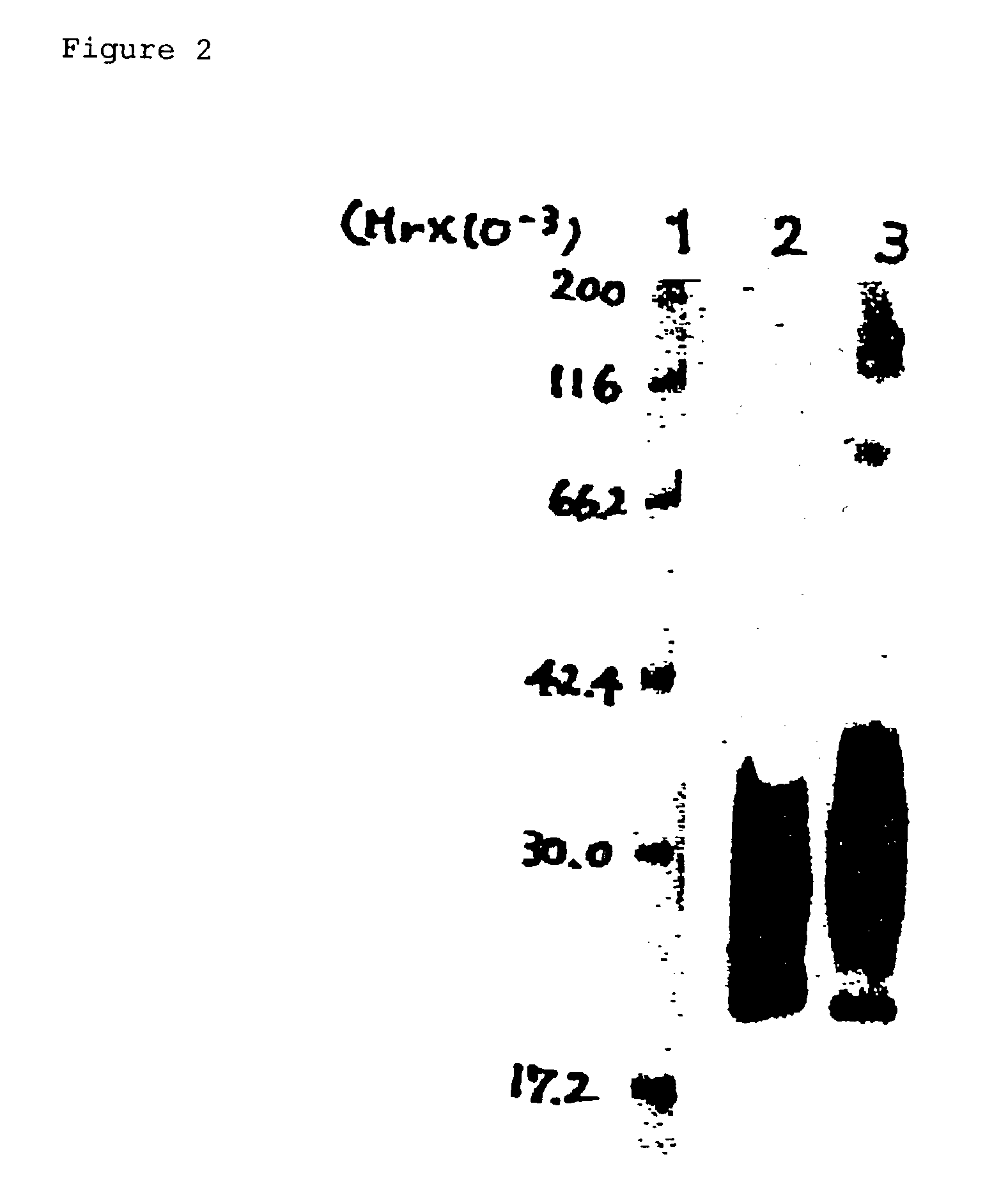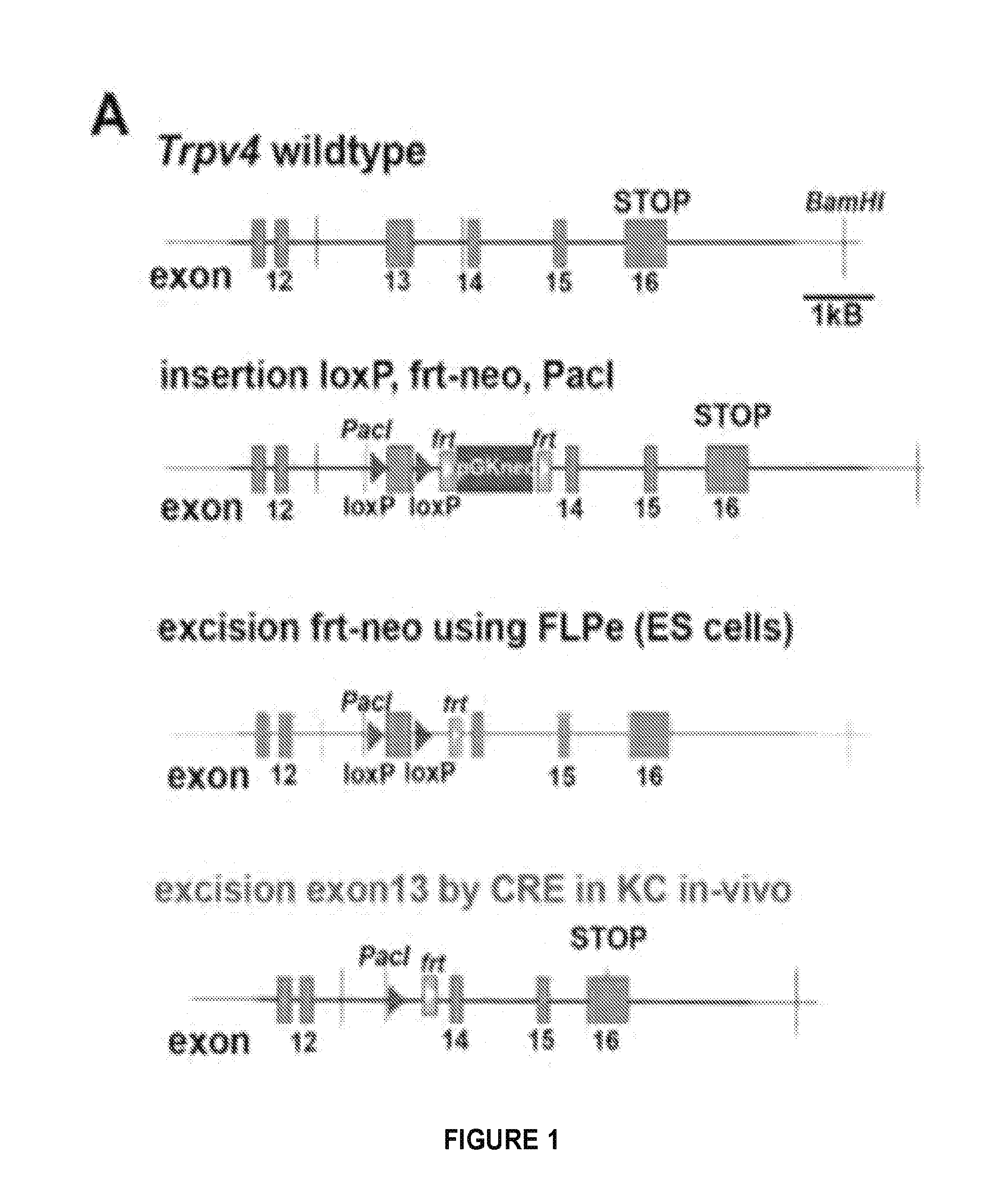Patents
Literature
218 results about "Mice transgenic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transgenic mice genetically engineered to develop cancer is a relatively new technology first realized during the 1980s. Since that time genetically modified mice have been used extensively in research as models for human disease. Knockout mice lacking tumor suppressing genes make for good models for human cancer.
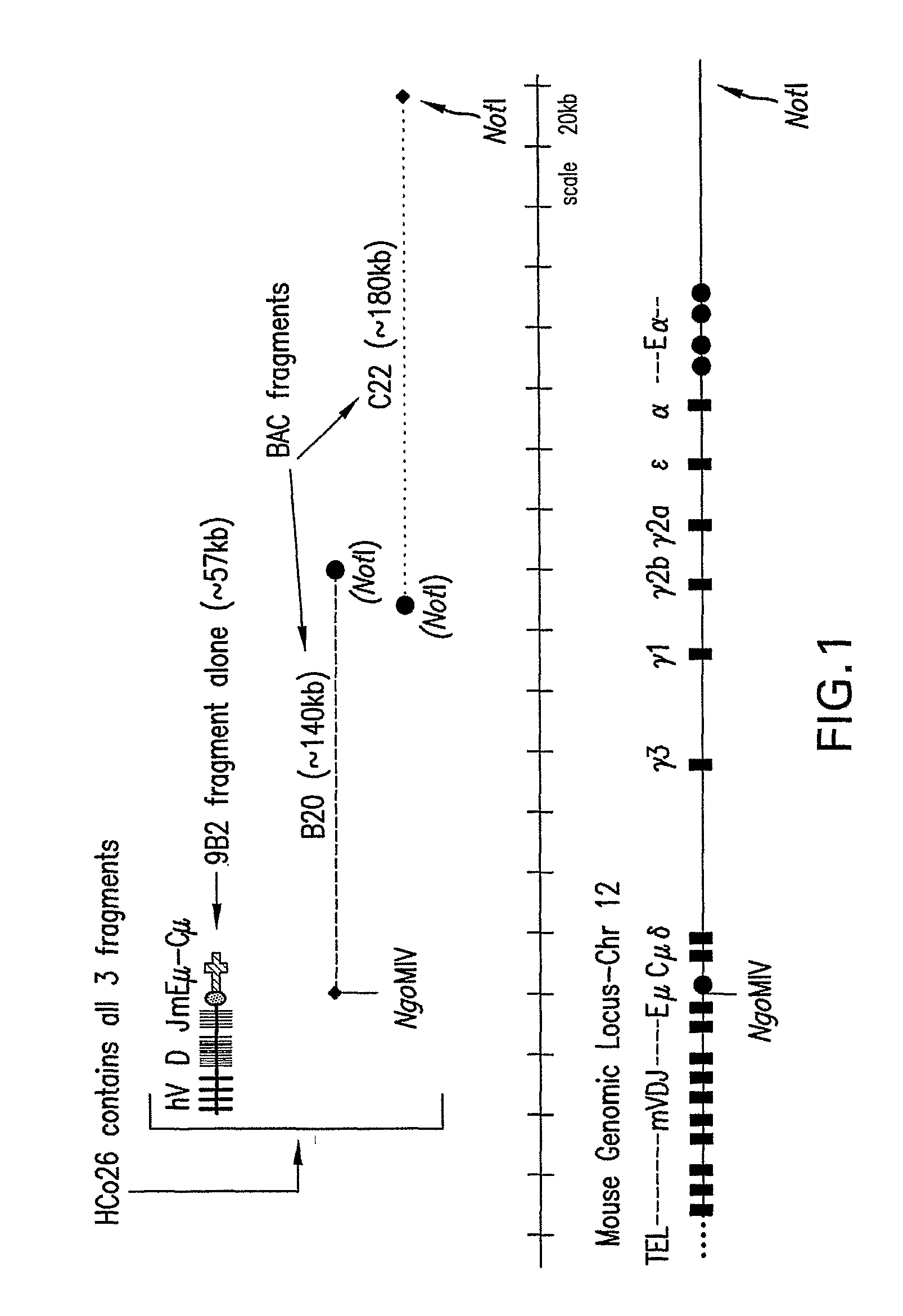
Transgenic animals expressing chimeric antibodies for use in preparing human antibodies
ActiveUS7910798B2Improved trafficking developmentStrengthen associationSugar derivativesImmunoglobulins against cytokines/lymphokines/interferonsTransgenesisIn vivo
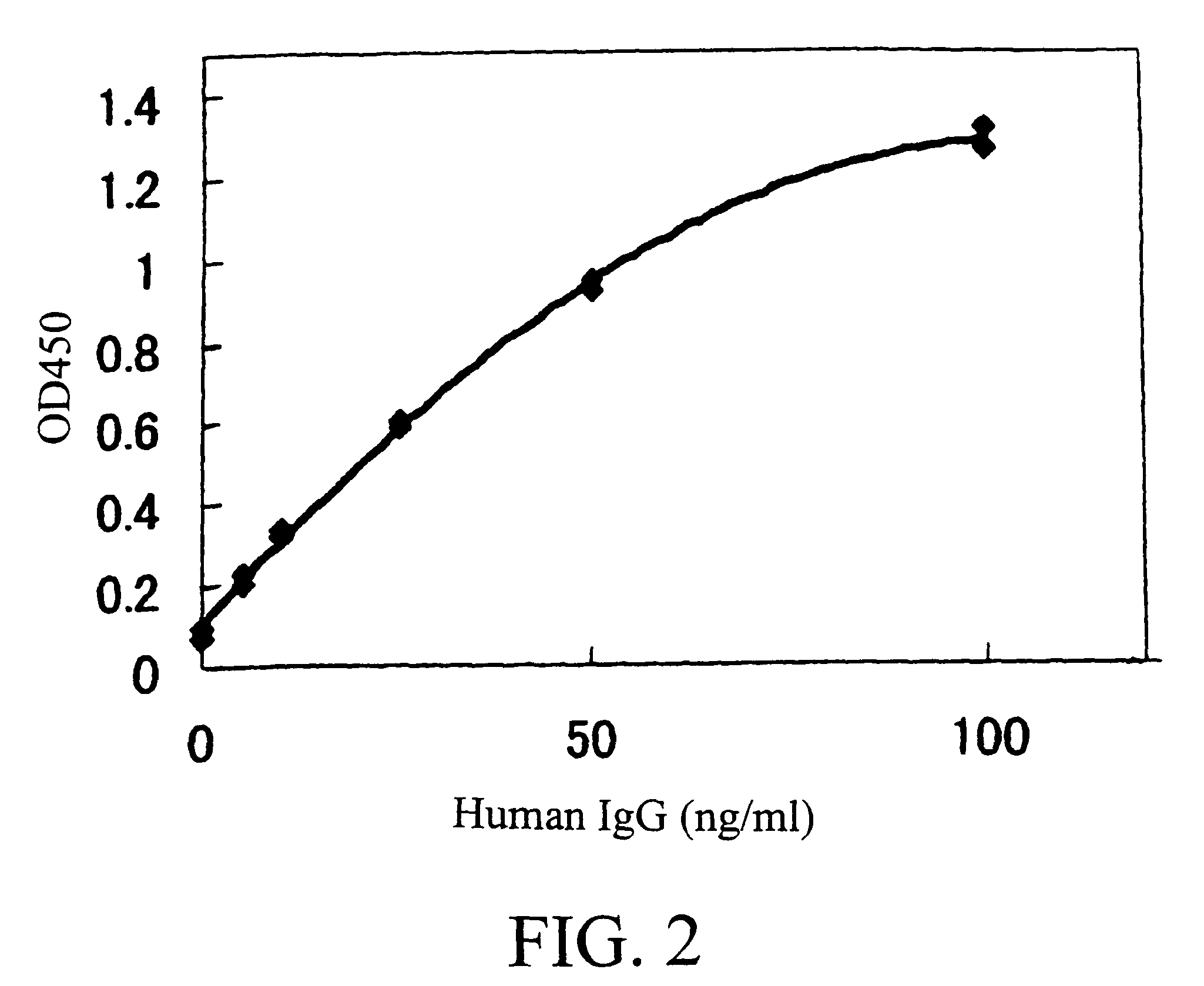
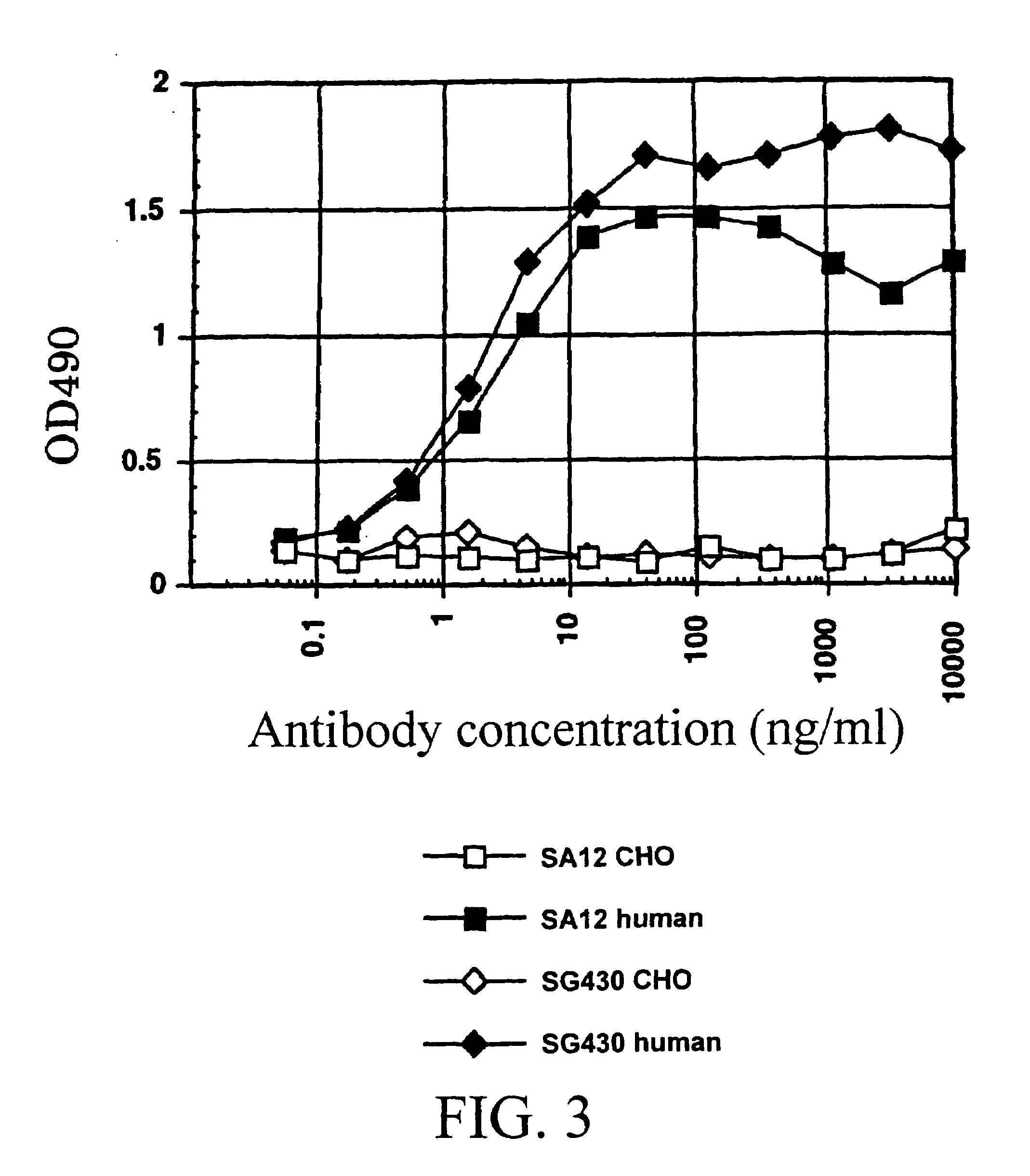
The invention provides transgene constructs for expressing chimeric antibodies, and transgenic non-human host animals carrying such constructs, wherein the chimeric antibodies comprise human variable regions and constant regions of the non-human transgenic host animal. The presence of immunoglobulin constant regions of the host animal allows for generation of improved antibodies in such transgenic host animals. Subsequently, the chimeric antibodies can be readily converted to fully human antibodies using recombinant DNA techniques. Thus, the invention provides compositions and methods for generating human antibodies in which chimeric antibodies raised in vivo in transgenic mice are used as intermediates and then converted to fully human antibodies in vitro.
Owner:ER SQUIBB & SONS INC
Purification of antigen-specific T cells
InactiveUS20060134125A1Snake antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMHC class IAbnormal tissue growth
A new method to capture, purify and expand antigen-specific T lymphocytes has been developed using magnetic beads coated with recombinant MHC class I molecules. This method was optimized using homogenous populations of naive T cells purified from mice transgenic for the 2C T cell receptor (TCR). These T cells were captured on beads coated with MHC class I molecules and the relevant antigenic peptides. MHC and peptide specificity was confirmed by the usage of irrelevant MHC peptide combinations. An enrichment of 800 to 1600 fold was measured, using 2C T cells mixed with irrelevant T cells, starting from a 2C T cell frequency of 1 / 3000. The same approach was used to purify antigen-specific CD8+ T cells from total CD8+ T cells from naive mice. The recovered cells could be expanded and specifically kill target cells in vitro; they had a significant effect in vivo as well. We expect this procedure to be suitable to purify and expand in vitro tumor- and virus-specific killer T cells for use in cell therapy.
Owner:ORTHO PHARMA
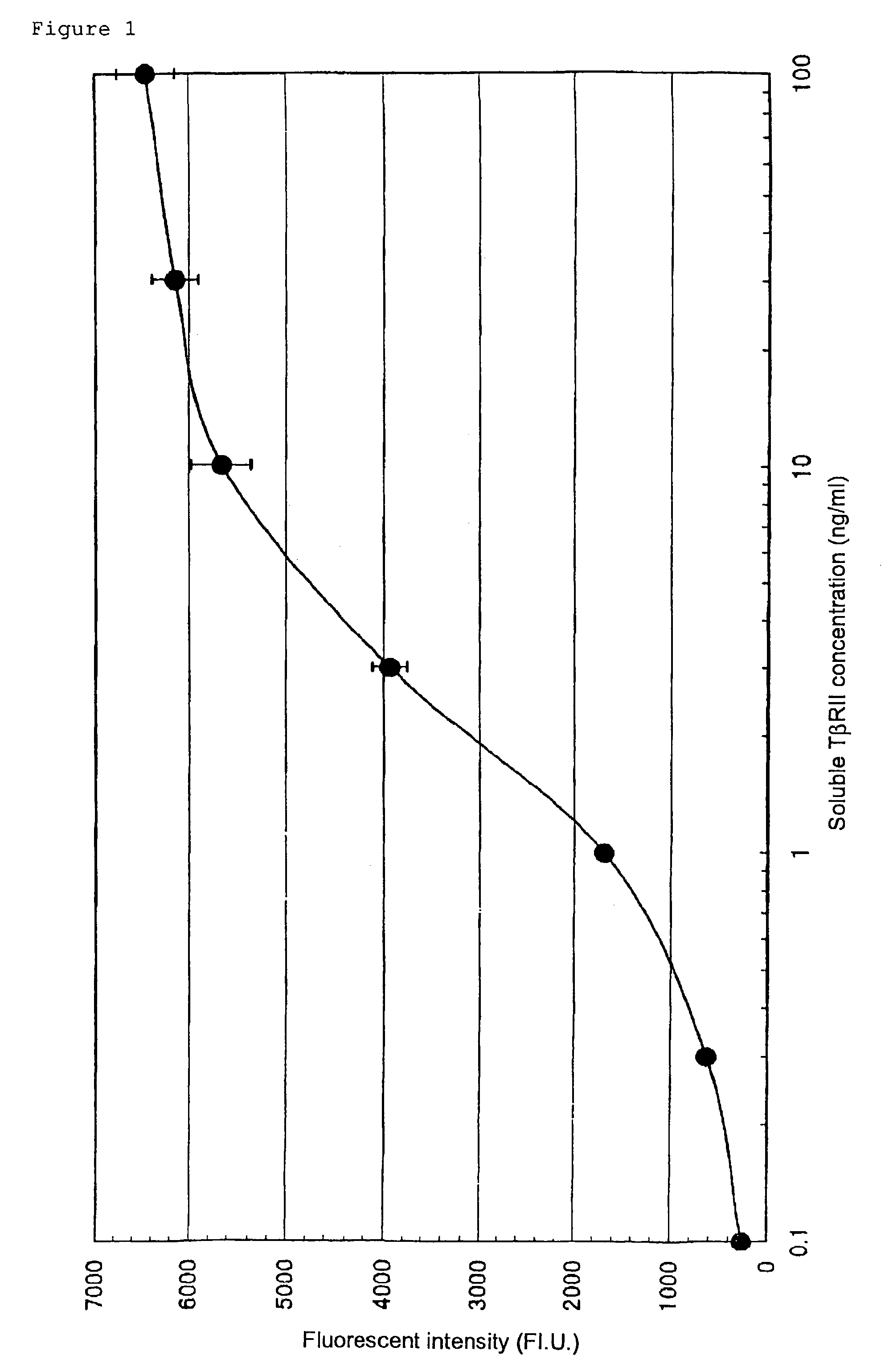
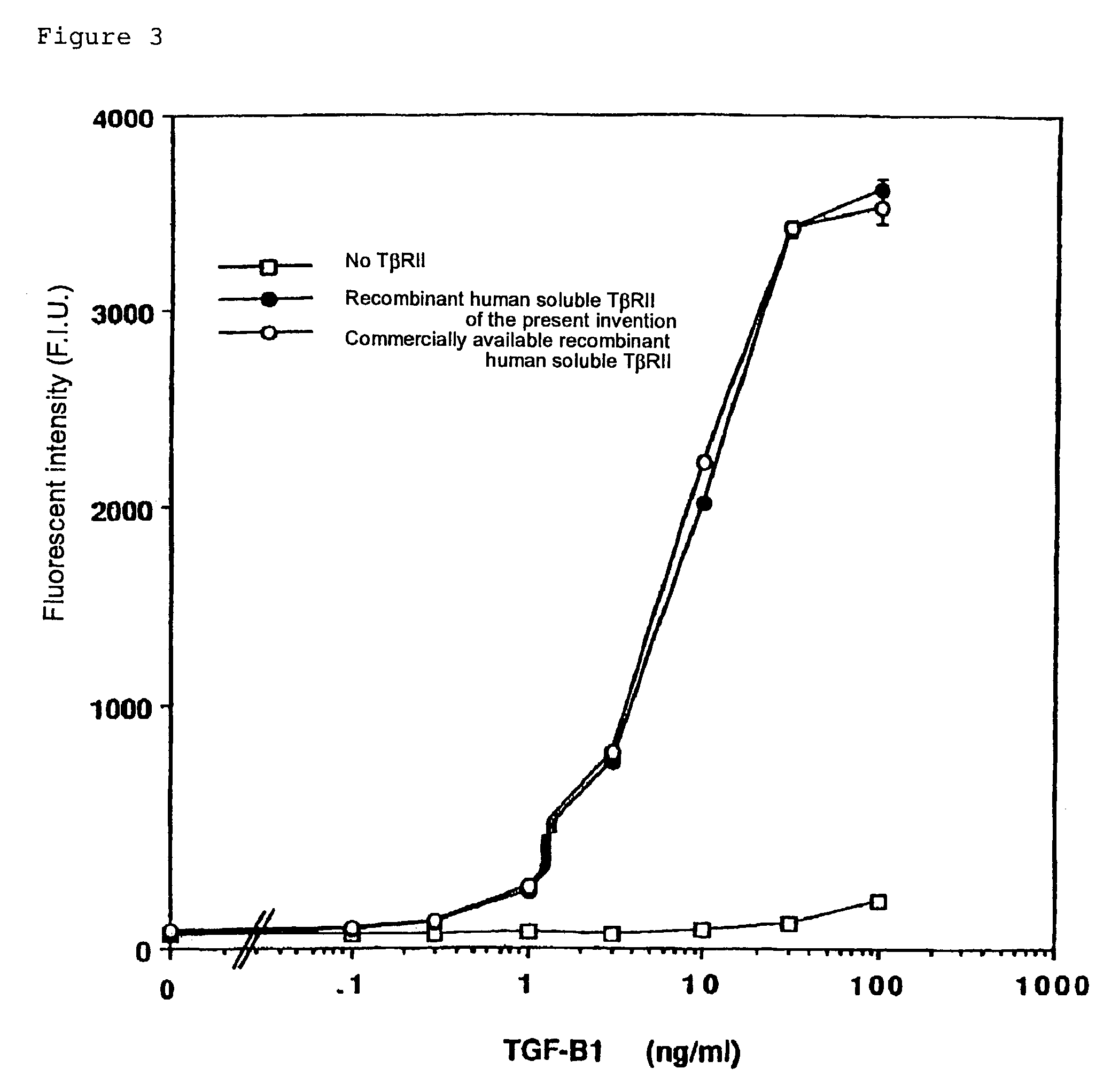
Human monoclonal antibody against TGF-beta type II receptor and medicinal use thereof
InactiveUS7579186B1Inhibits signal transductionIncrease valueAntipyreticAnalgesicsDiseaseMonoclonal antibody
Various human monoclonal antibodies that bind to human TGF-β type II receptor to inhibit the signal transduction of human TGF-β signal into cells are produced by immunizing human antibody-producing transgenic mice generated by genetic engineering techniques with the soluble human TGF-β type II receptor. Further, these human monoclonal antibodies were demonstrated to be effective for preventing and treating various diseases induced by human TGF-β in various organs (for example, tissue fibrosis).
Owner:AMGEN FREMONT INC
Methods of treating an inflammatory disorder and prohibiting proliferation, cytokine production, and signal transduction with antibody against costimulatory signal transduction molecule AILIM
InactiveUS7166283B2Increase valueEliminate the effects ofOrganic active ingredientsPeptide/protein ingredientsFhit geneGenetically modified mouse
Immunization of human antibody-producing transgenic mice, which have been created using genetic engineering techniques, with AILIM molecule as an antigen resulted in various human monoclonal antibodies capable of binding to AILIM and capable of controlling a variety of biological reactions (for example, cell proliferation, cytokine production, immune cytolysis, cell death, induction of ADCC, etc.) associated with AILIM-mediated costimulatory signal (secondary signal) transduction. Furthermore, it has been revealed that the human monoclonal antibody is effective to treat and prevent various diseases associated with AILIM-mediated costimulatory signal transduction, being capable of inhibiting the onset and / or advancement of the diseases.
Owner:JAPAN TOBACCO INC
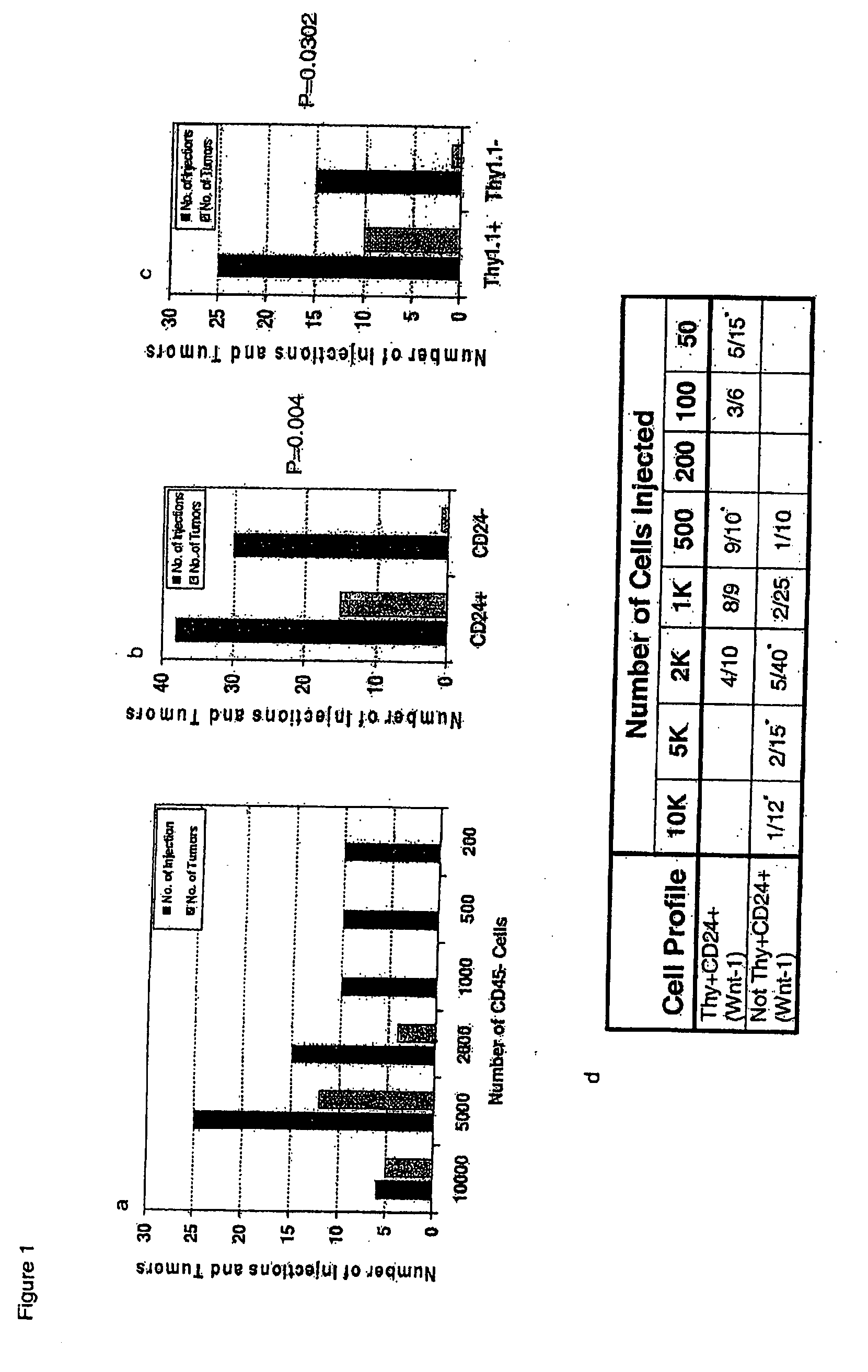
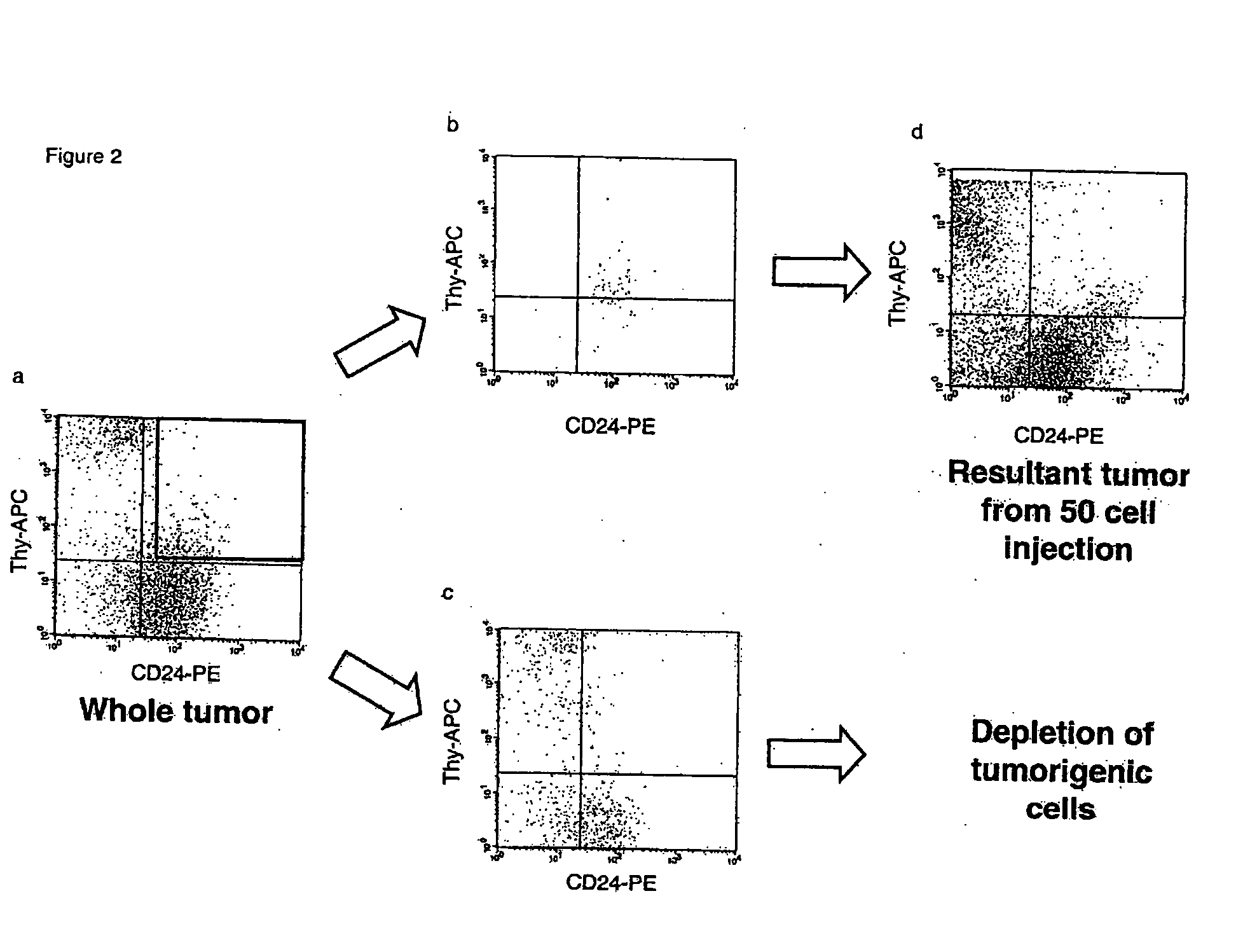
Genetic characterization and prognostic significance of cancer stem cells in cancer
InactiveUS20070220621A1Predictive of survivalReduce spreadNew breed animal cellsMicrobiological testing/measurementSolid tumorGene expression
The present invention is related to the identification of cancer stem cells using the MMTV-Wnt-1 transgenic mouse model. These cancer stem cells have a gene expression signature that allows them to be distinguished from their non-tumorigenic counterparts. Moreover, the gene expression pattern can also predict survival in a diverse group of solid tumors.
Owner:CLARKE MICHAEL F +2
Muir-Torre-like syndrome in Fhit deficient mice
InactiveUS20060075511A1Drug screeningNucleic acid vectorParanasal Sinus CarcinomaGastrointestinal cancer
The invention provides nonhuman transgenic animals with a disrupted FHIT gene. The invention further provides transgenic mice in which one or both Fhit alleles have been inactivated. Preferably, the Fhit-deficient mice develop multiple tumors of both visceral and sebaceous origin, similar to those of Muir-Torre familial cancer syndrome. The present invention further relates to the generation of these transgenic mice and their use as model systems to study the effects of carcinogenic agents in promoting clonal expansion of neoplastic cells in cancers, preferably gastrointestinal cancers of which Muir-Torre syndrome is a subset. The invention further relates to testing therapeutic agents for their efficacy in the prevention and treatment of cancer, preferably gastrointestinal cancer.
Owner:CROCE CARLO +1
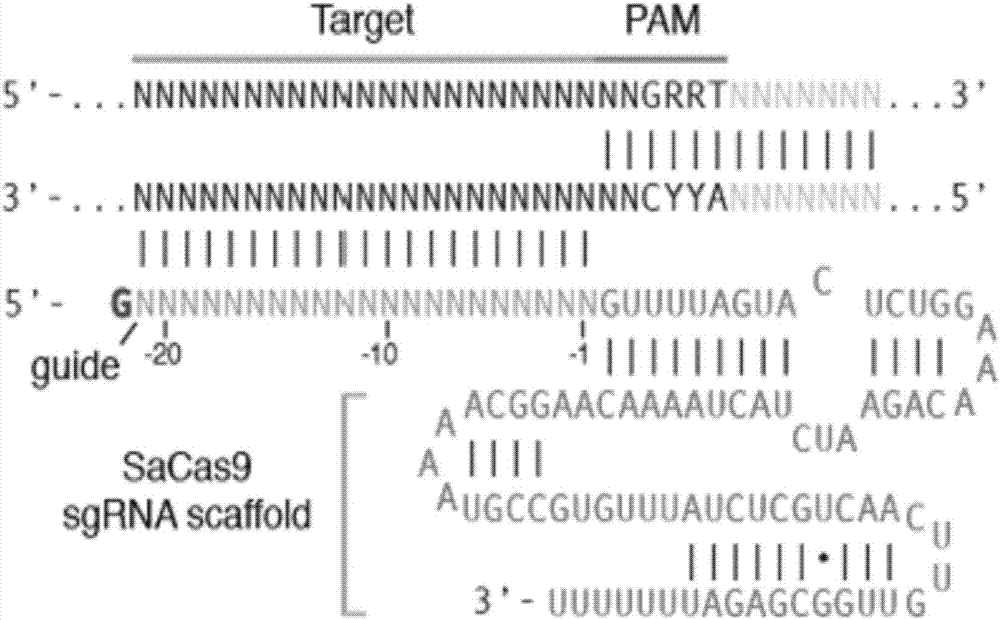
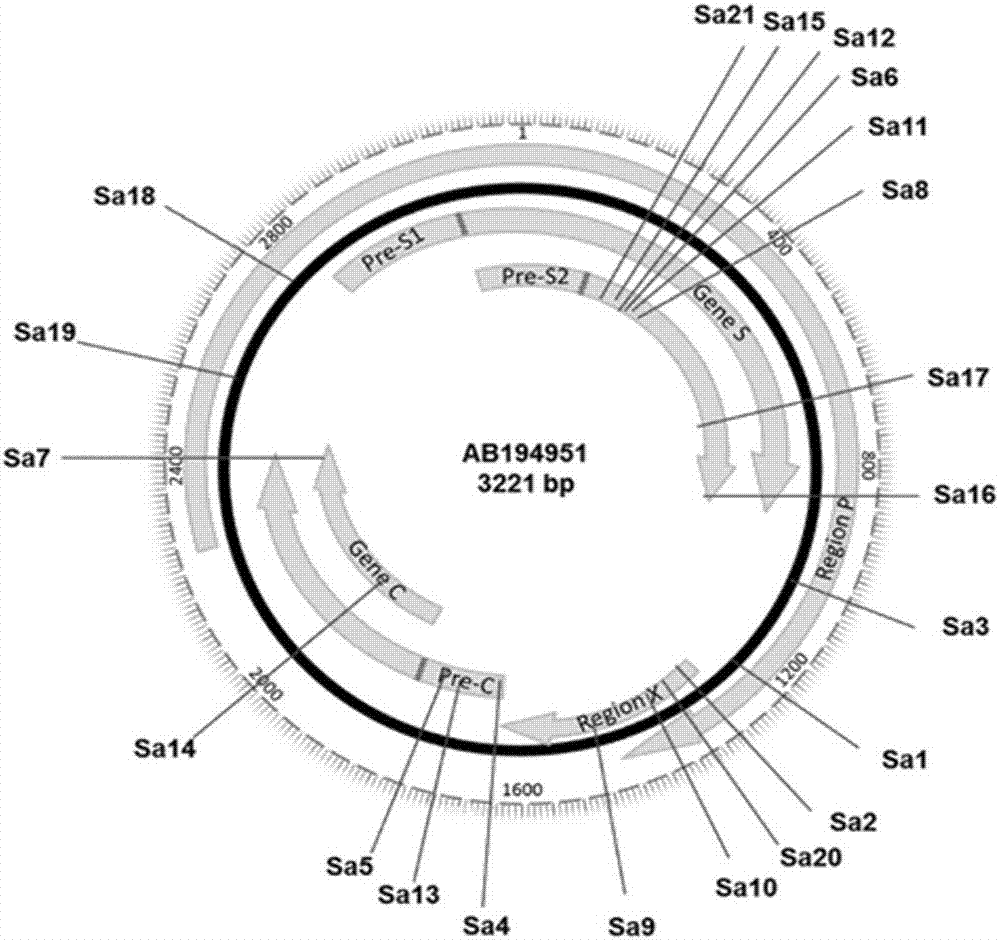
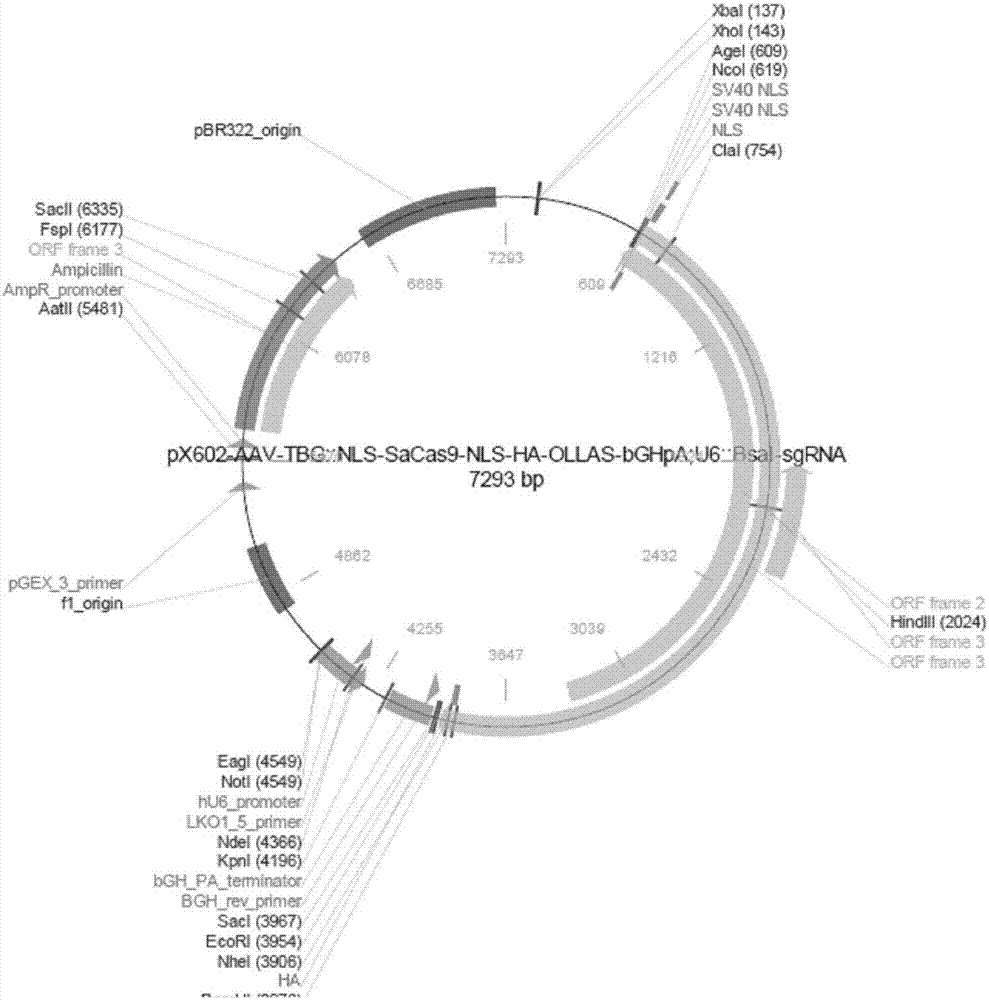
rAAV8-CRISPR-SaCas9 system and application of system in preparing hepatitis B therapeutics
The invention discloses a gRNA sequence. The sequence is capable of editing a DNA sequence in the manner of taking a hepatitis B viral genome specific locus as a target sequence. The invention also discloses a CRISPR-SaCas9 system containing the gRNA sequence and a recombinant adeno-associated virus packaged with the system. The system and the packaging virus show higher HBV scavenging activity in cells and in transgenic mice body. On the 38th day after twice continuous high dose injection, the contents of HBsAg and HBeAg in experimental group mice serum, compared with the control group, are respectively reduced by 62.96+ / -7.59% and 65.18+ / -3.08%; the HBV DNA content in the serum, compared with the control group, is reduced by 92.82+ / -3.67%; the liver and other visceral organs of the experimental mice are all free from any pathologic change, the off-target effect is also not detected and the application prospects of the gRNA sequence and the corresponding CRISPR-SaCas9 system provided by the invention in preparing the hepatitis B therapeutics are shown.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Non-invasive evaluation of physiological response in a transgenic mouse
Owner:XENOGEN CORP
Compositions and Methods Related to Controlled Gene Expression Using Viral Vectors
InactiveUS20090210952A1Significant advanceEfficient delivery and controlled expression of geneAntiviralsNucleic acid vectorViral vectorGenetically modified mouse
Provided herein are methods and compositions related to viral vectors. Also provided herein are methods and compositions for the efficient transfection of a host, for example through the highly efficient lentivector delivery system, and for the exquisite control of the timing and level of expression of the transferred sequence of interest by the simple administration of a modulator to the host harboring the transferred sequence of interest. Also disclosed are methods of making transgenic mice and transgenic mice made using compositions and methods relating to viral vectors.
Owner:UAB RES FOUND
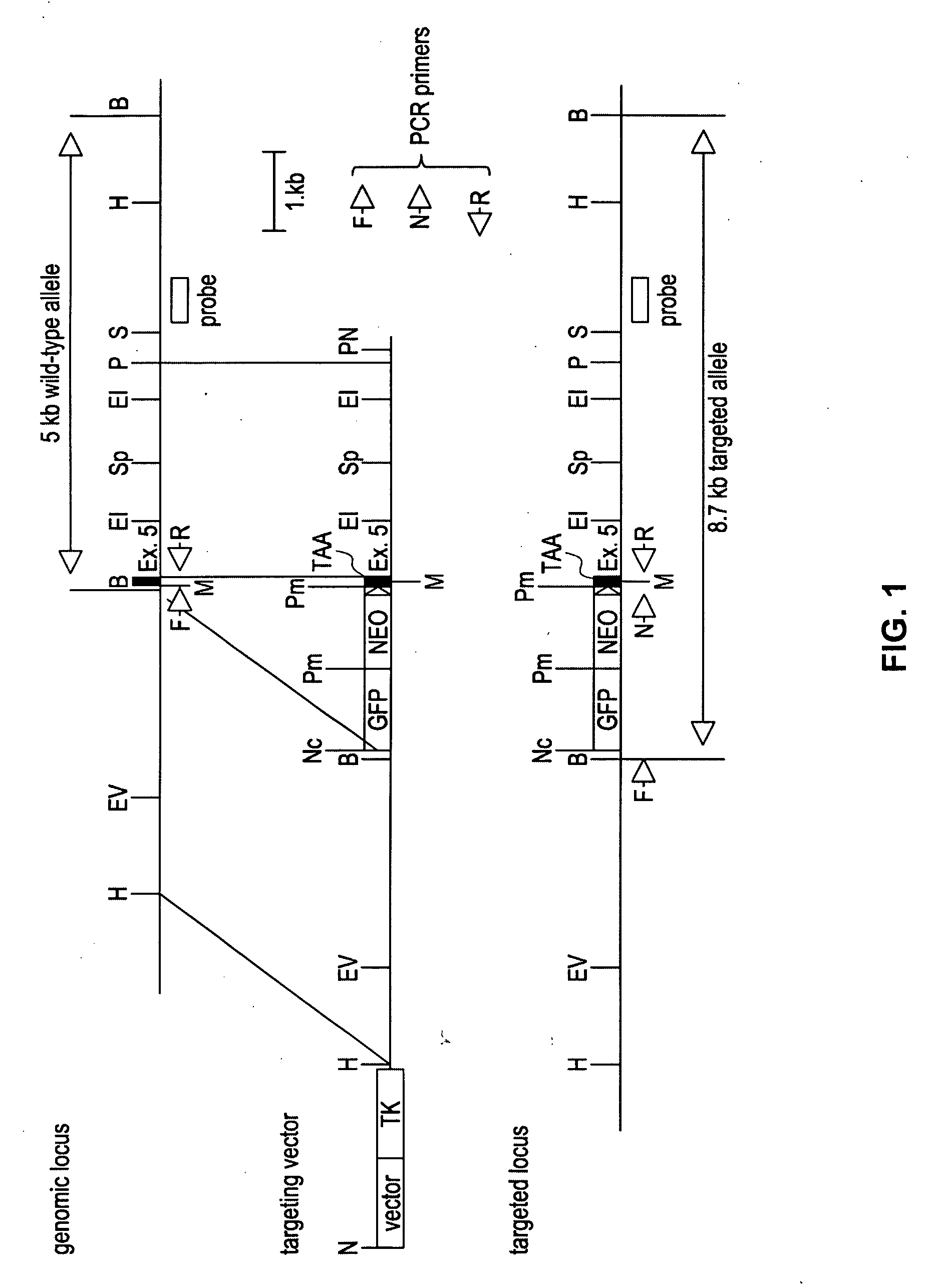
Transgenic mice expressing antibodies specific for genes of interest and uses thereof
The invention provides compositions and methods for the generation of novel non-human transgenic animals which contain an alteration in a gene of interest. These transgenic animals are capable of generating antibodies, e.g., human monoclonal antibodies, specific for the product of a gene of interest that has been functionally disrupted in the transgenic animal. Furthermore, the methods and compositions of the invention are suitable for use in the treatment, diagnosis, and imaging of disease.
Owner:ABBOTT GMBH & CO KG
Transgenic animals expressing chimeric antibodies for use in preparing human antibodies
ActiveUS20090260093A1Easily selectImprove traffic developmentSugar derivativesImmunoglobulins against cytokines/lymphokines/interferonsTransgeneAntibody
The invention provides transgene constructs for expressing chimeric antibodies, and transgenic non-human host animals carrying such constructs, wherein the chimeric antibodies comprise human variable regions and constant regions of the non-human transgenic host animal. The presence of immunoglobulin constant regions of the host animal allows for generation of improved antibodies in such transgenic host animals. Subsequently, the chimeric antibodies can be readily converted to fully human antibodies using recombinant DNA techniques. Thus, the invention provides compositions and methods for generating human antibodies in which chimeric antibodies raised in vivo in transgenic mice are used as intermediates and then converted to fully human antibodies in vitro.
Owner:MEDAREX LLC
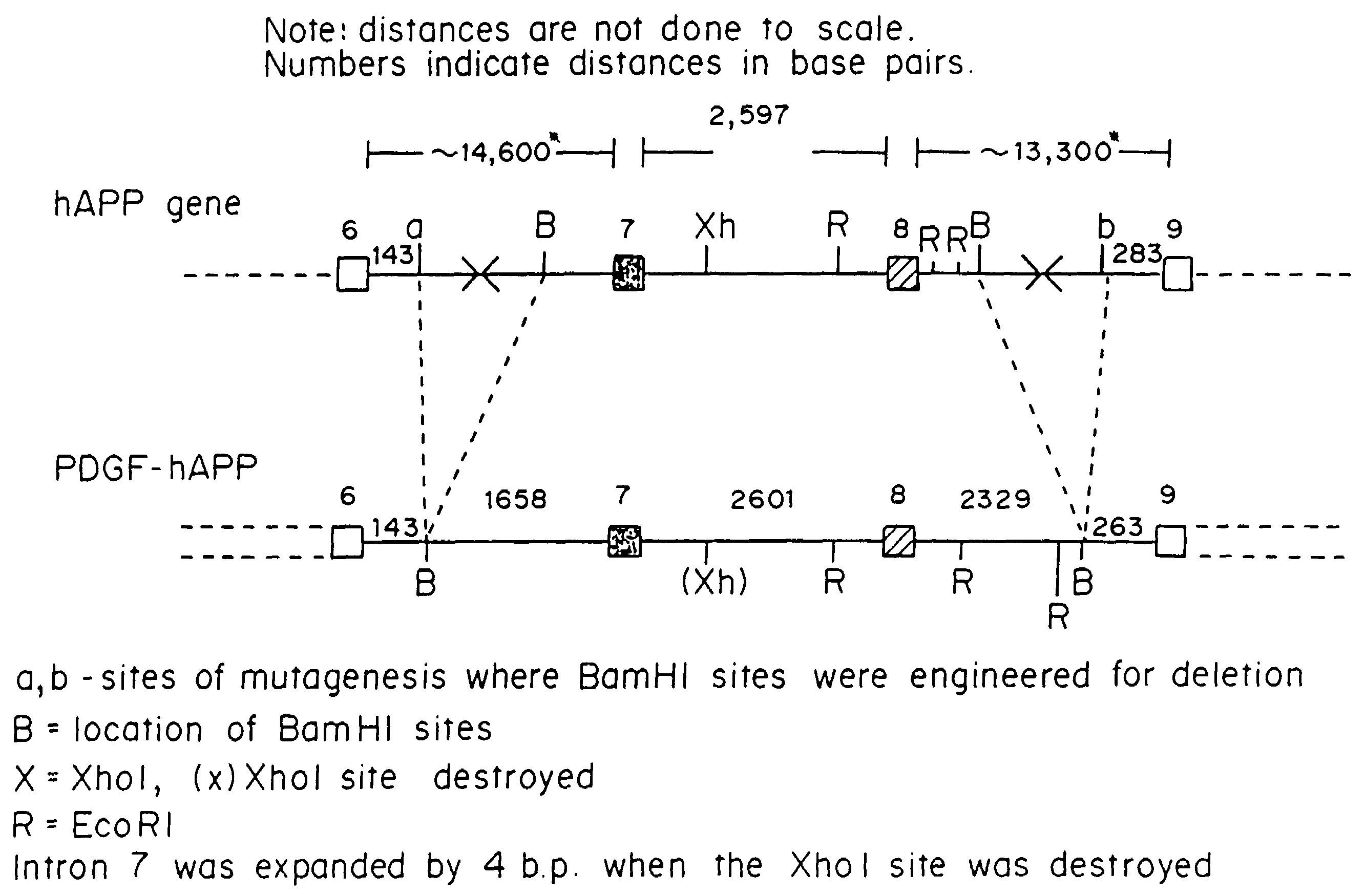
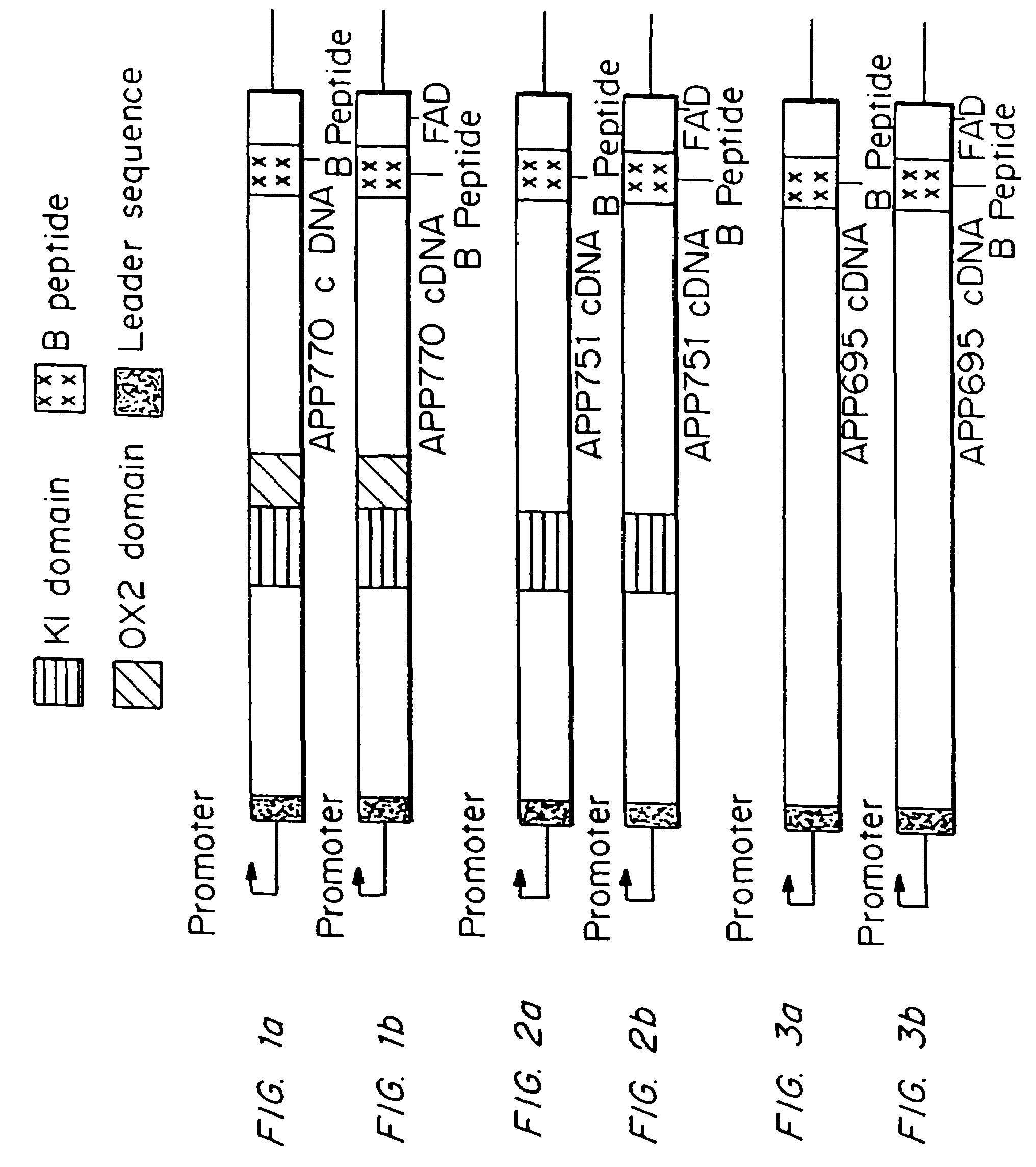
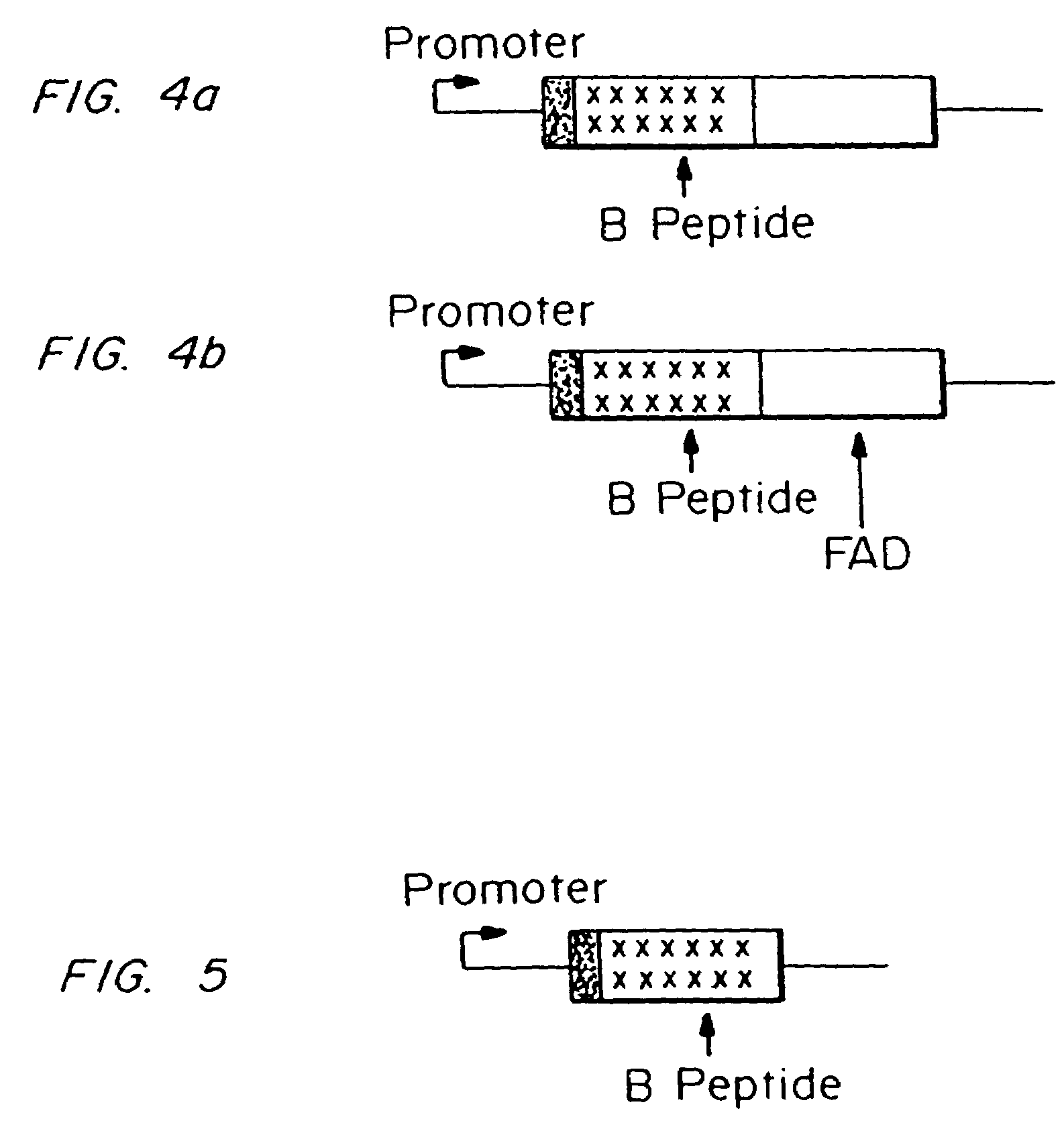
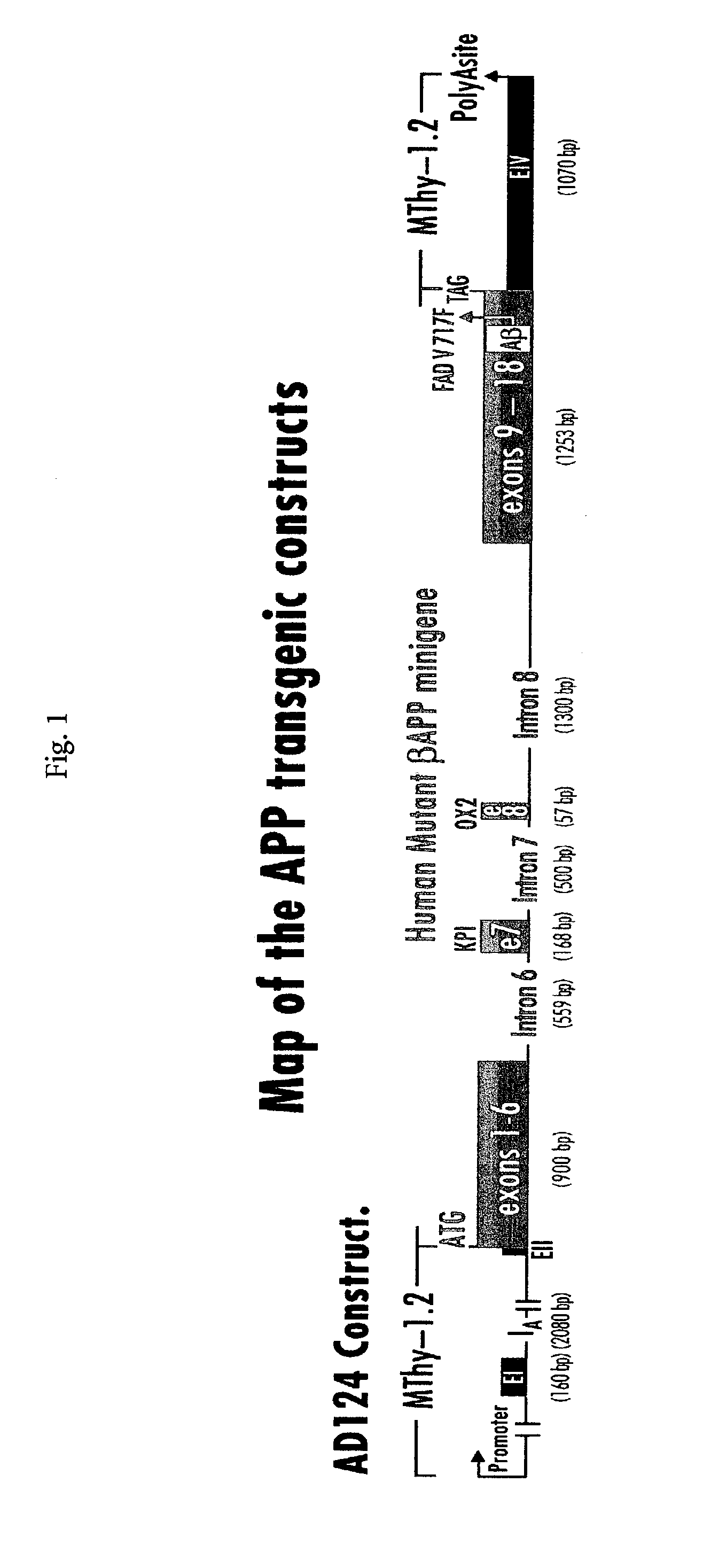
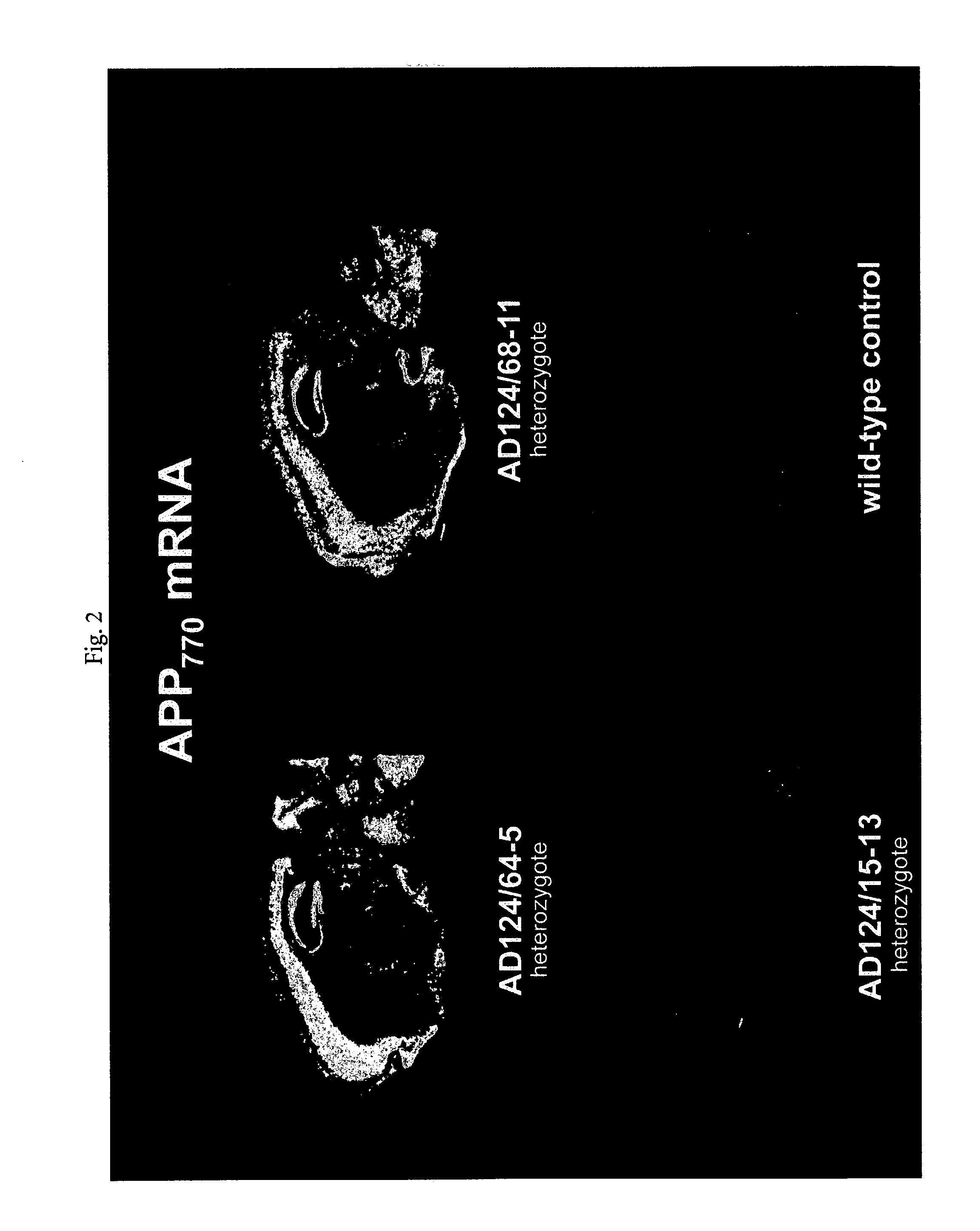
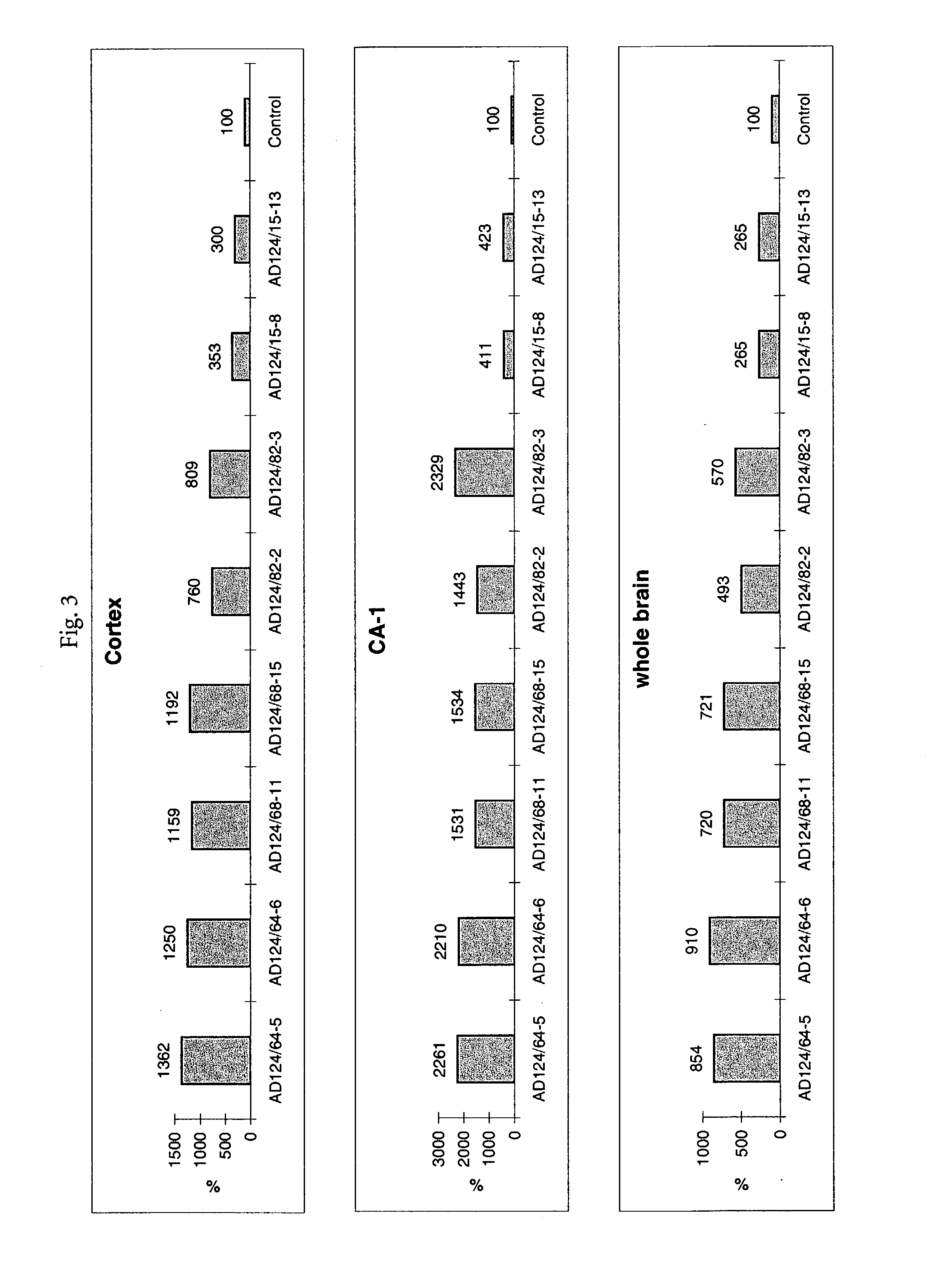
Testing compounds for effects on synaptophysin in transgenic mice expressing an Alzheimer's disease FAD DNA sequence
InactiveUS7186881B2Polypeptide with localisation/targeting motifAnimals/human peptidesSynaptophysinElectroporation
The construction of transgenic animal models of human Alzheimer's disease, and methods of using the models to screen potential Alzhe## disease therapeutics, are described. The models are characterized by pathologies similar to pathologies observed in Alzheimer's disease, based on expression of all three forms of the β-amyloid precursor protein (APP), APP695, APP751, and APP770, as well as various point mutations based on naturally occurring mutations, such as the London and Indiana familial Alzheimer's disease (FAD) mutations at amino acid 717, predicted mutations in the APP gene, and truncated forms of APP that contain the Aβ region. Animal cells can be isolated from the transgenic animals or prepared using the same constructs with standard techniques such as lipofection or electroporation. The transgenic animals, or animal cells, are used to screen for compounds altering the pathological course of Alzheimer's disease as measured by their effect on the amount of APP, β-amyloid peptide, and numerous other Alzheimer's disease markers in the animals, the neuropathology of the animals, as well as by behavioral alterations in the animals.
Owner:ELAN PHARM INC
Human Monoclonal Antibodies Against CD20
Isolated human monoclonal antibodies which bind to and inhibit human CD20, and related antibody-based compositions and molecules, are disclosed. The human antibodies can be produced by a transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB AS
Purification of antigen-specific T cells
InactiveUS7125964B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMHC class IMagnetic bead
Owner:ORTHO MCNEIL PHARM INC
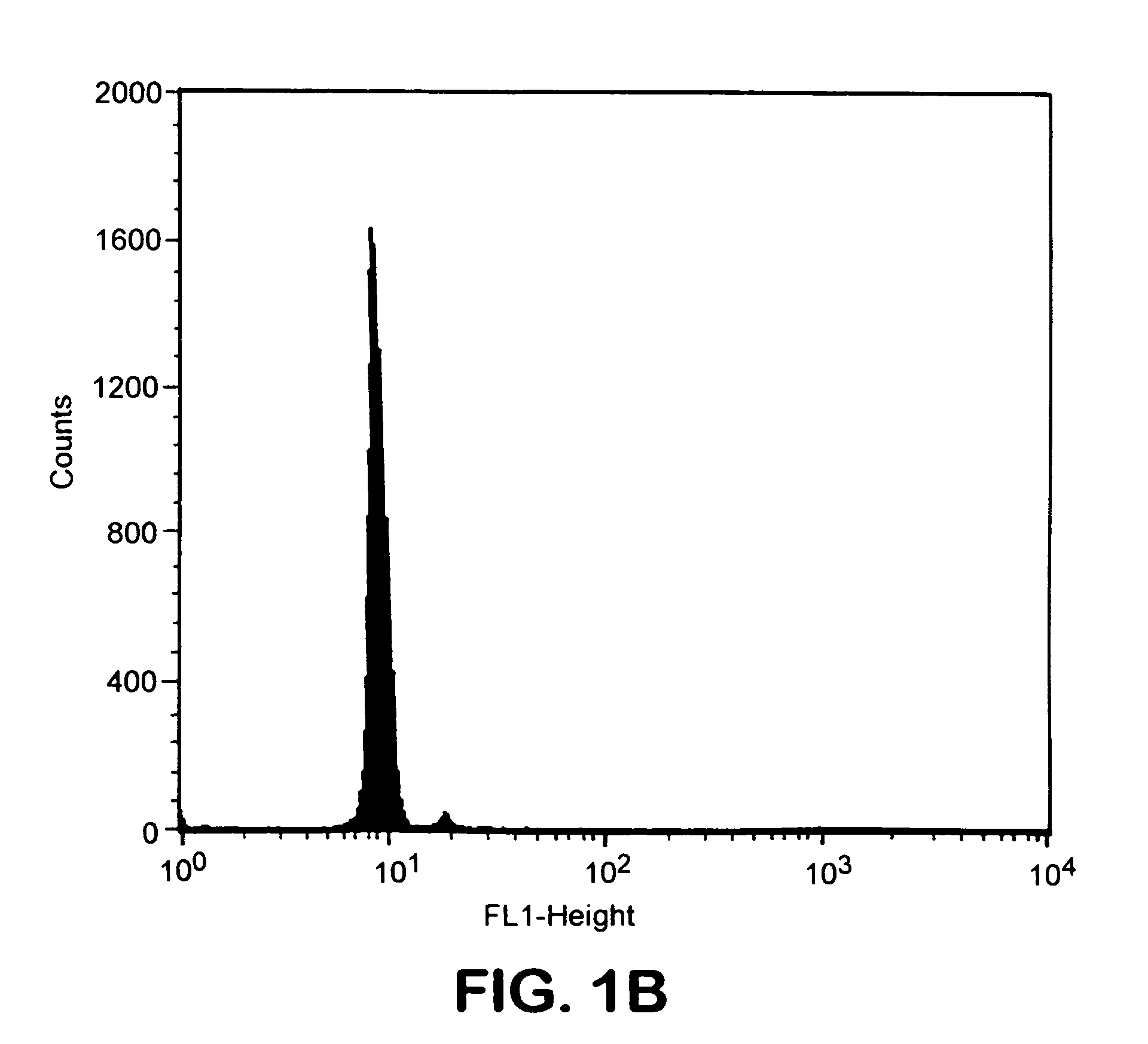
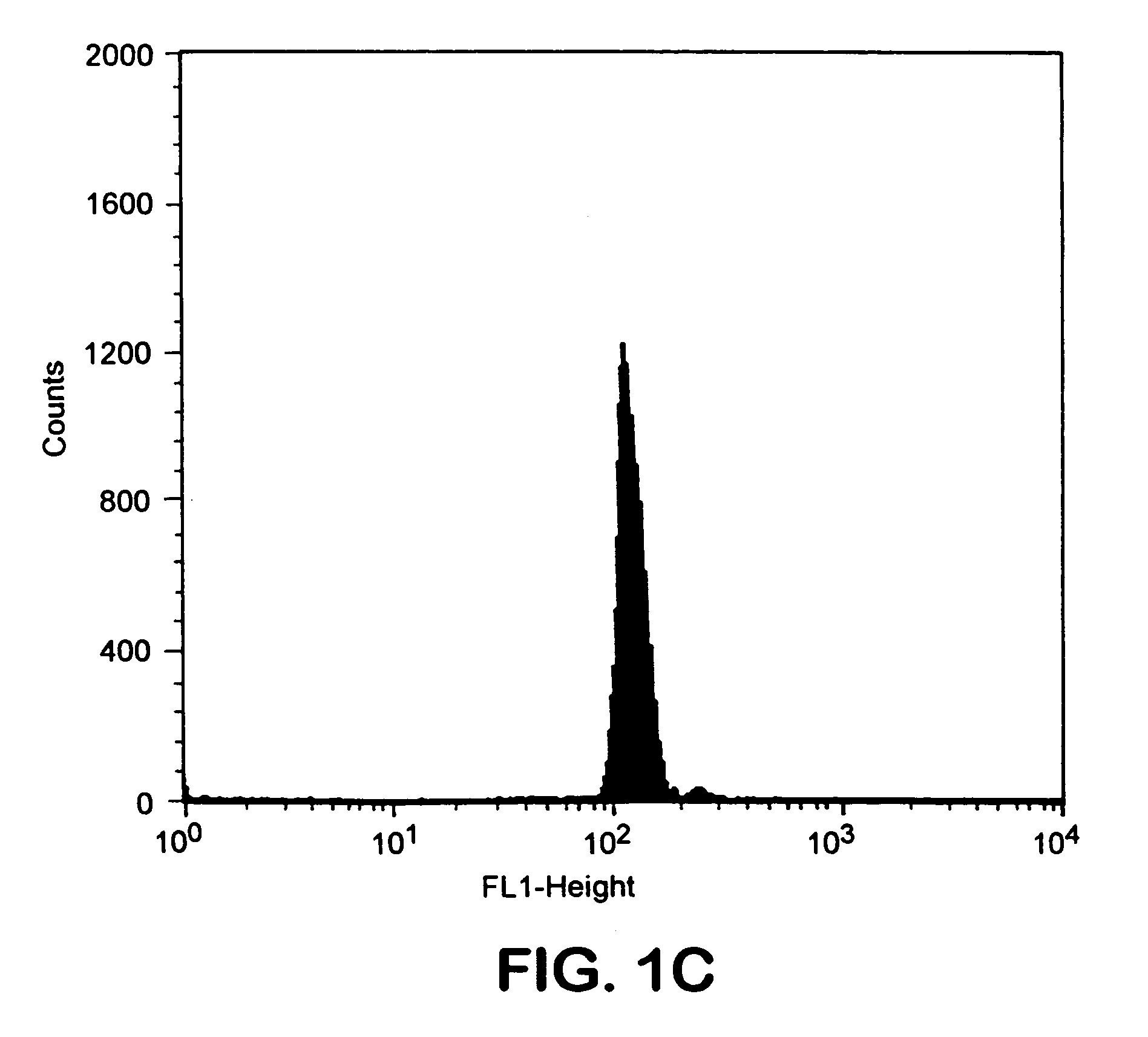
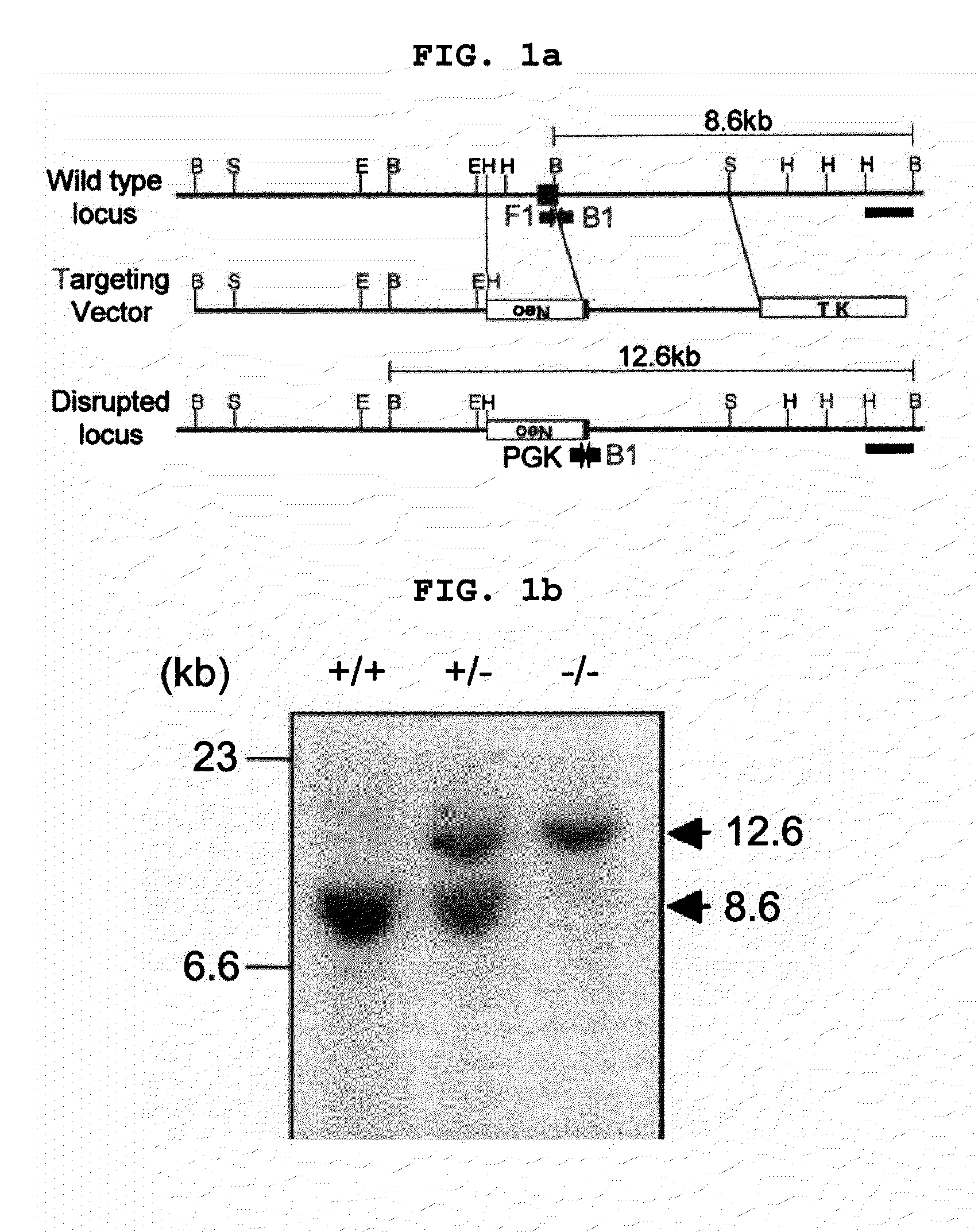
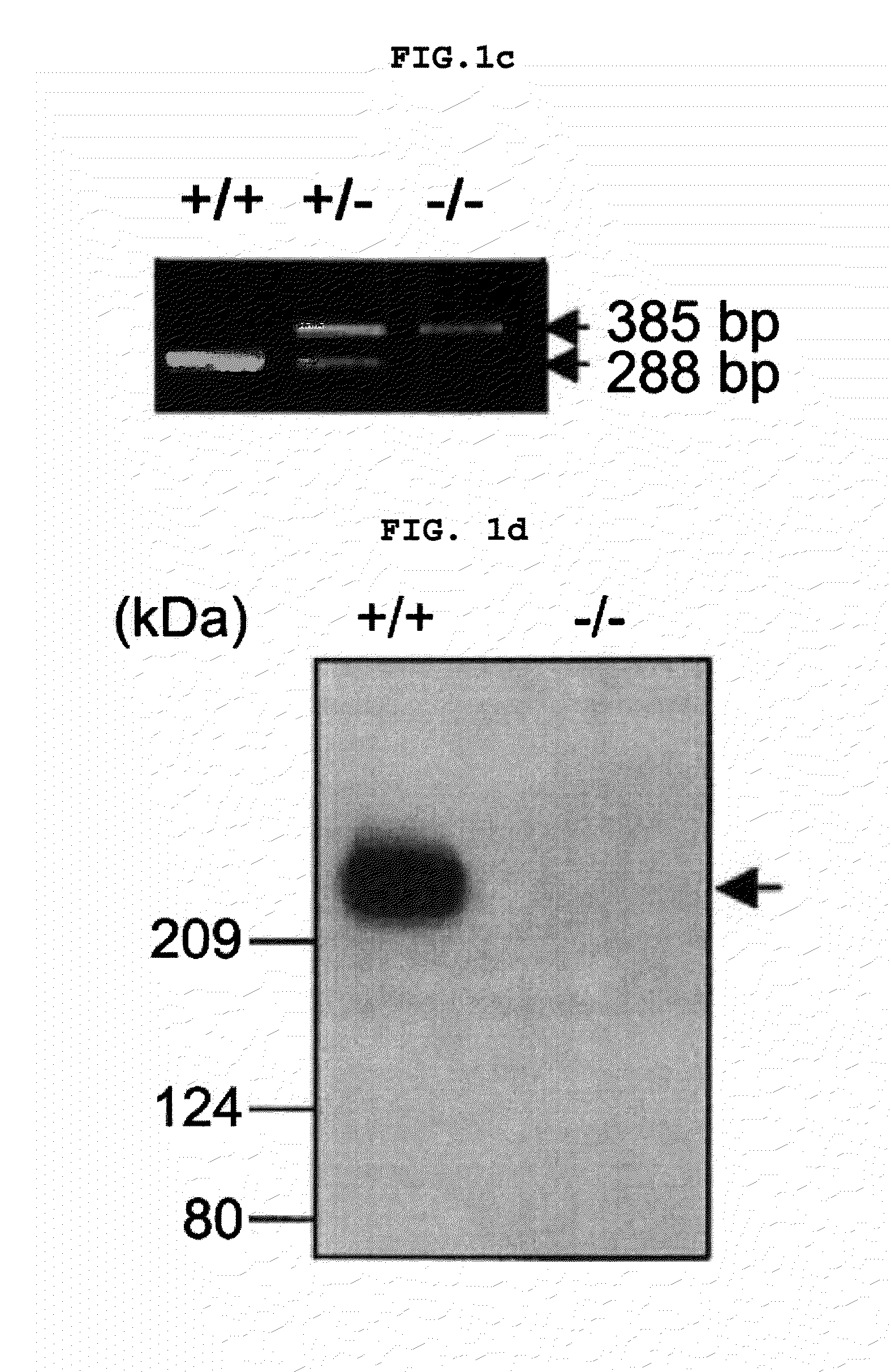
Transgenic mouse whose genome comprises a homozygous disruption of its α1G gene, a method of preparing the same and use thereof
InactiveUS7626076B2Compounds screening/testingCell receptors/surface-antigens/surface-determinantsNervous systemKnockout animal
The disclosure concerns a method for resistance of epilepsy by suppressing the function of alpha 1G protein of T-type calcium channels, use of suppressor of alpha 1G protein for prevention or treatment for epilepsy, knockout mice resisting epilepsy by disrupting alpha 1G subunit of T-type calcium channel, and preparation method thereof. The α1G-knockout transgenic mouse can be used for investigating the relationship between diseases particularly neuropathy or psychopathy and the function of α1G T-type calcium channel via various behavioral tests since α1G subunit is mainly expression in central nervous system (CNS) and pheripheral nervous system (PNS). Further, the α1G-knockout transgenic mouse can be used for screening antileptic agents.
Owner:ORIENTBIO
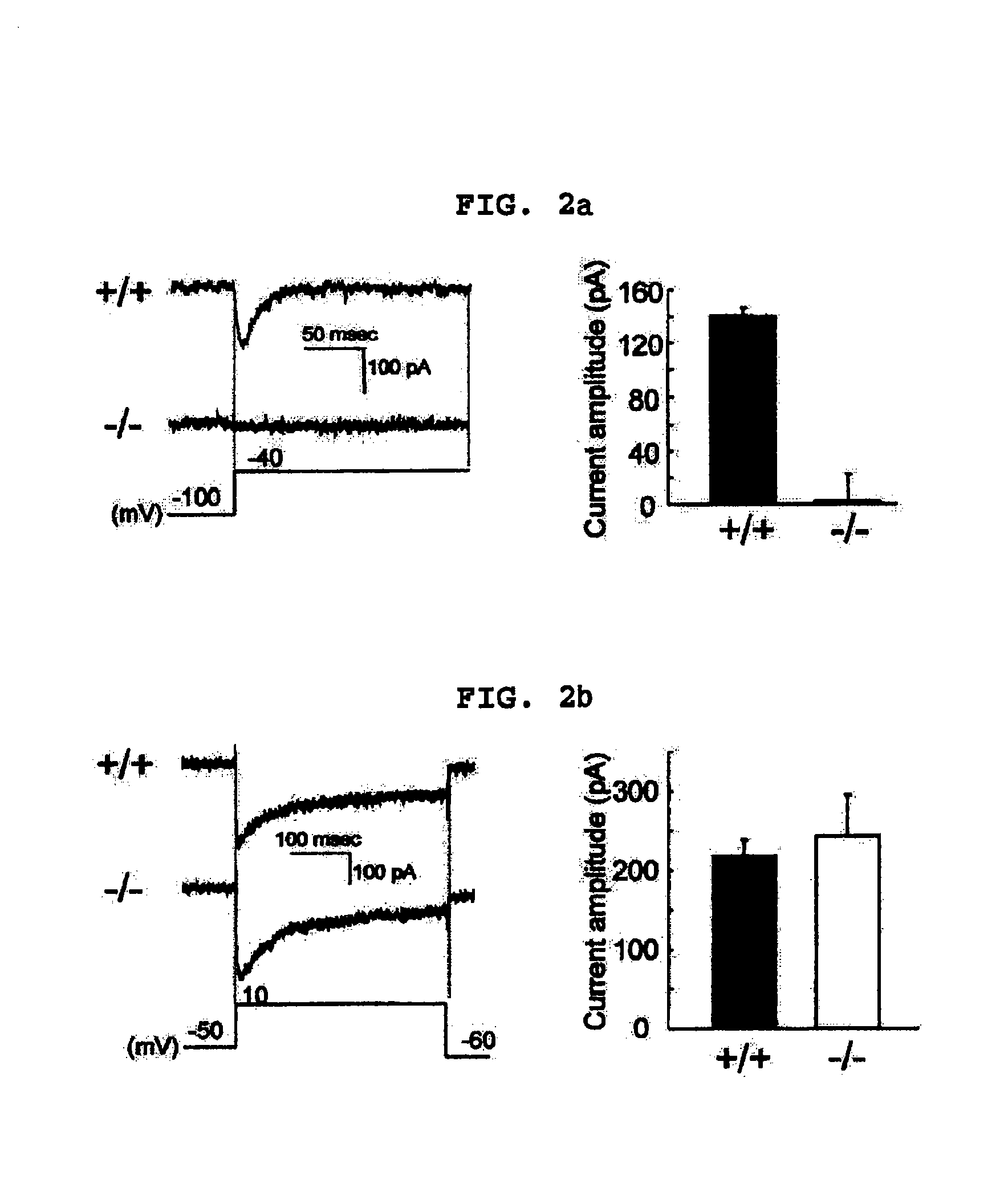
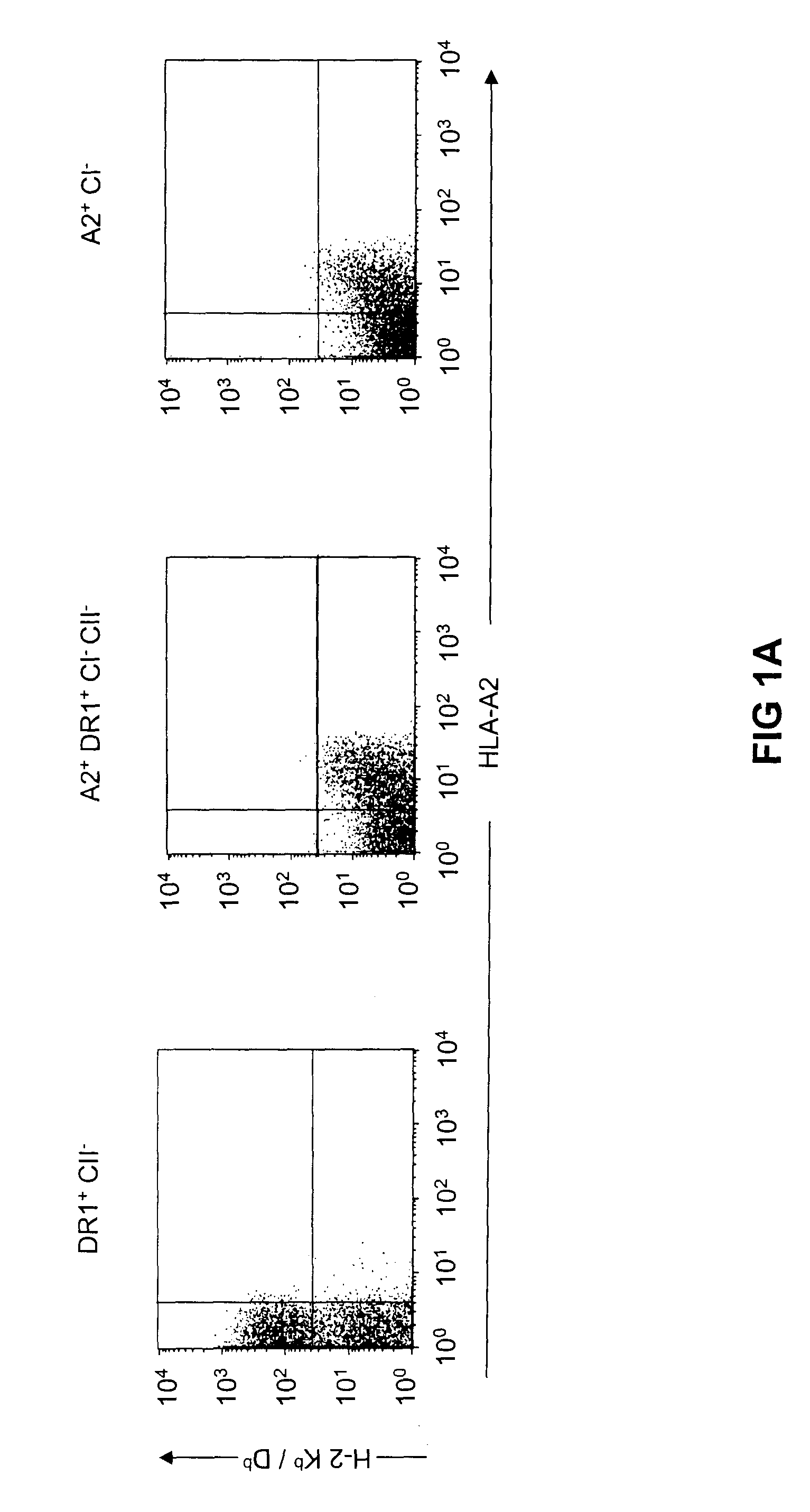
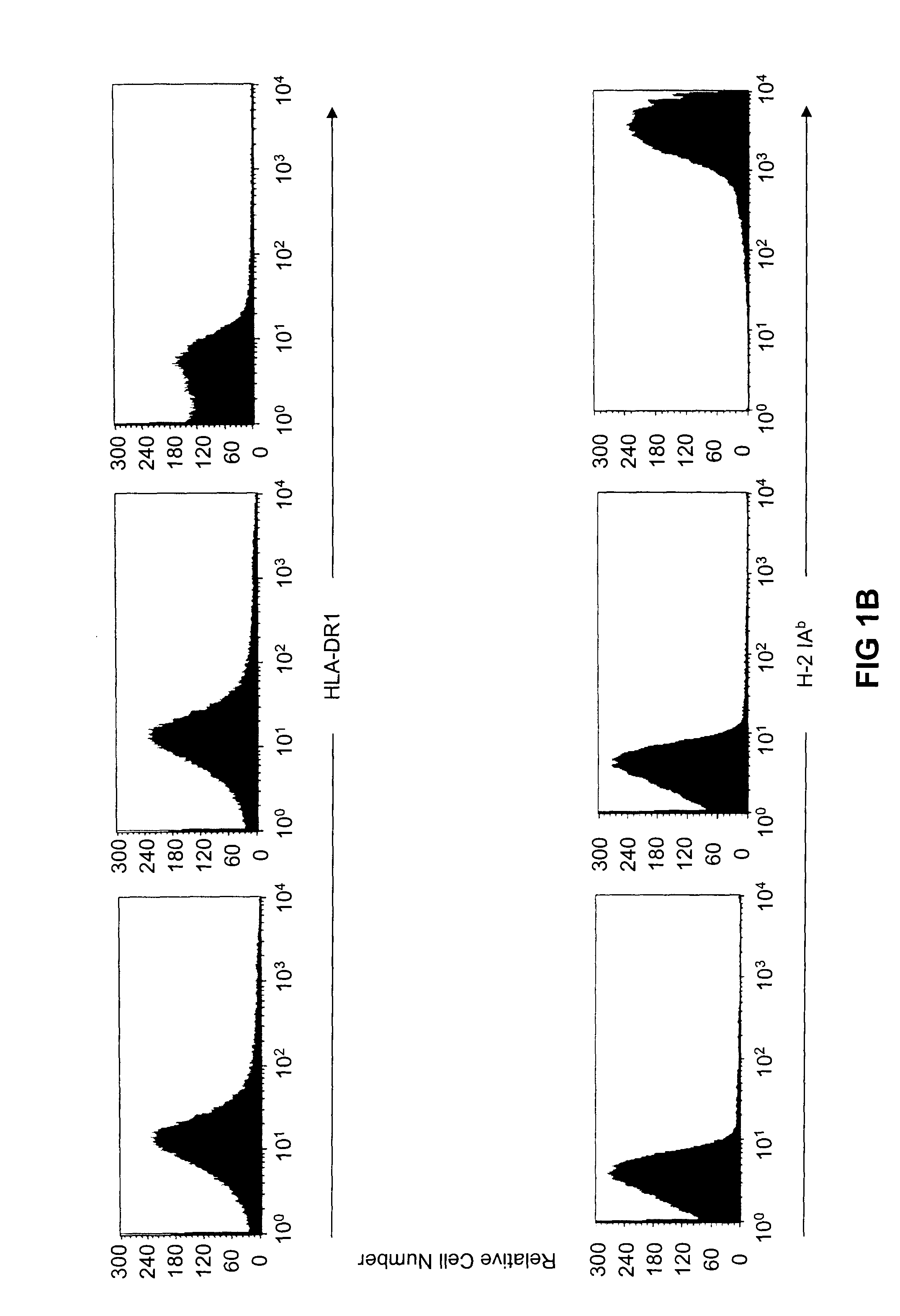
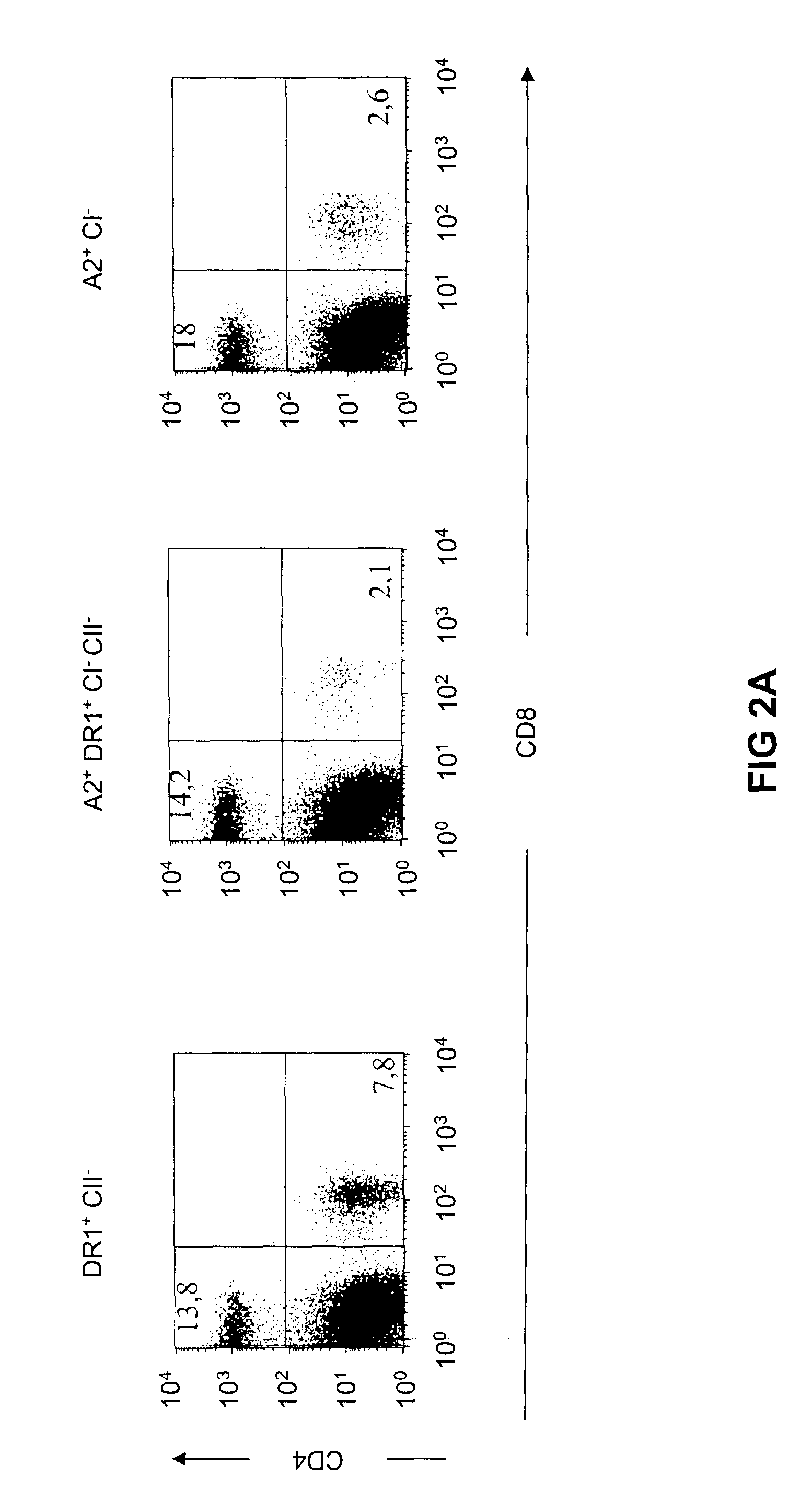
Transgenic mice having a human major histocompatibility complex (MHC) phenotype, experimental uses and applications
The present invention-relates to transgenic mice and isolated transgenic mouse cells, the mice and mouse cells comprising a disrupted H2 class I gene, a disrupted H2 class II gene, a functional HLA class I transgene, and a functional HLA class II transgene. In embodiments, the transgenic mouse or mouse cells are deficient for both H2 class I and class II molecules, wherein the transgenic mouse comprises a functional HLA class I transgene and a functional HLA class II transgene. In embodiments, the transgenic mouse or mouse cell has the genotype HLA-A2+HLA-DR1+β2m°IAβ°. The invention also relates to methods of using a transgenic mouse of the invention.
Owner:INST PASTEUR
Ubiquitin ligases as therapeutic targets
InactiveUS6720181B1Enhance ubiquitin ligase gene expressionOrganic active ingredientsFungiUbiquitin ligaseENCODE
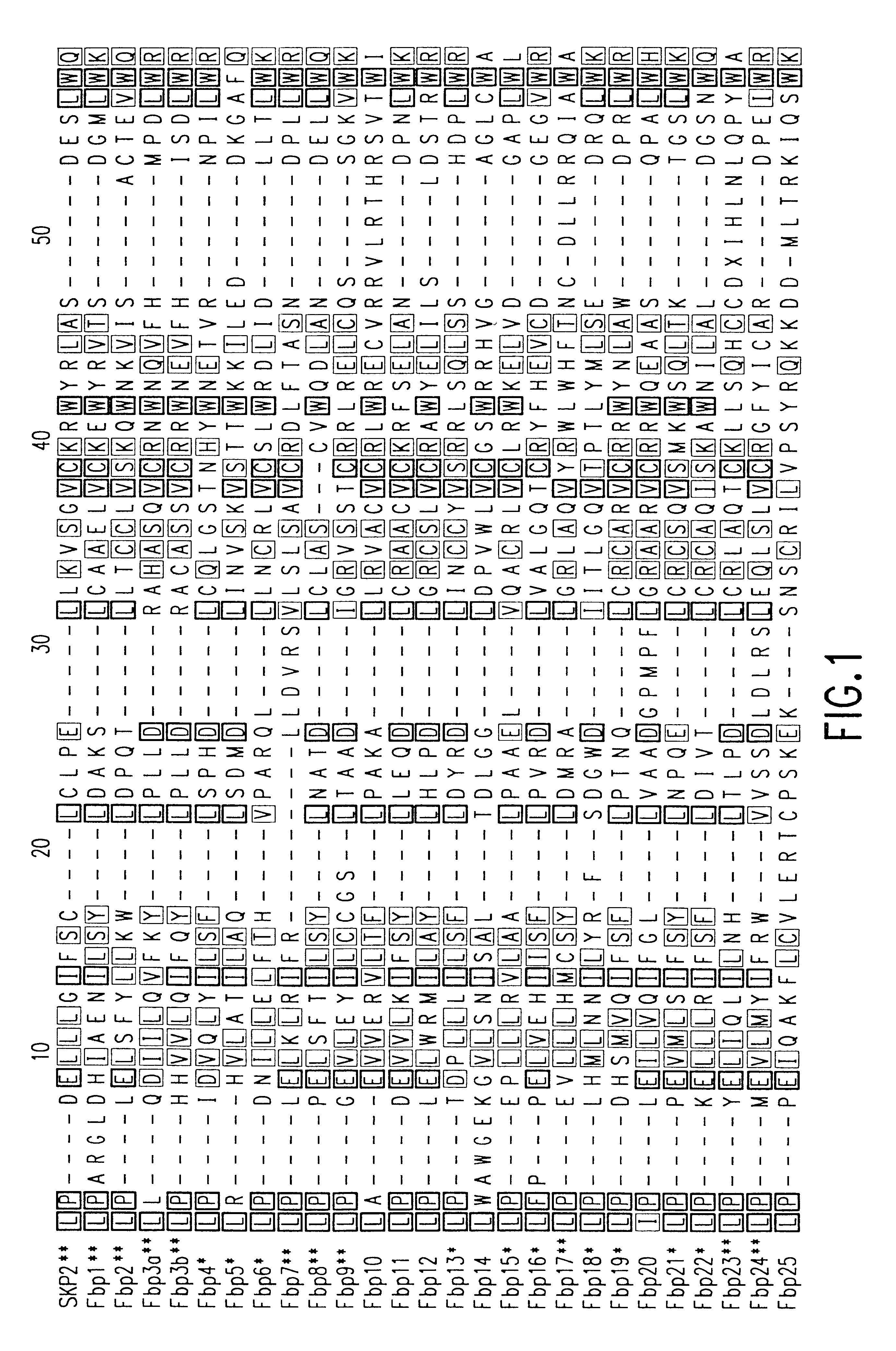
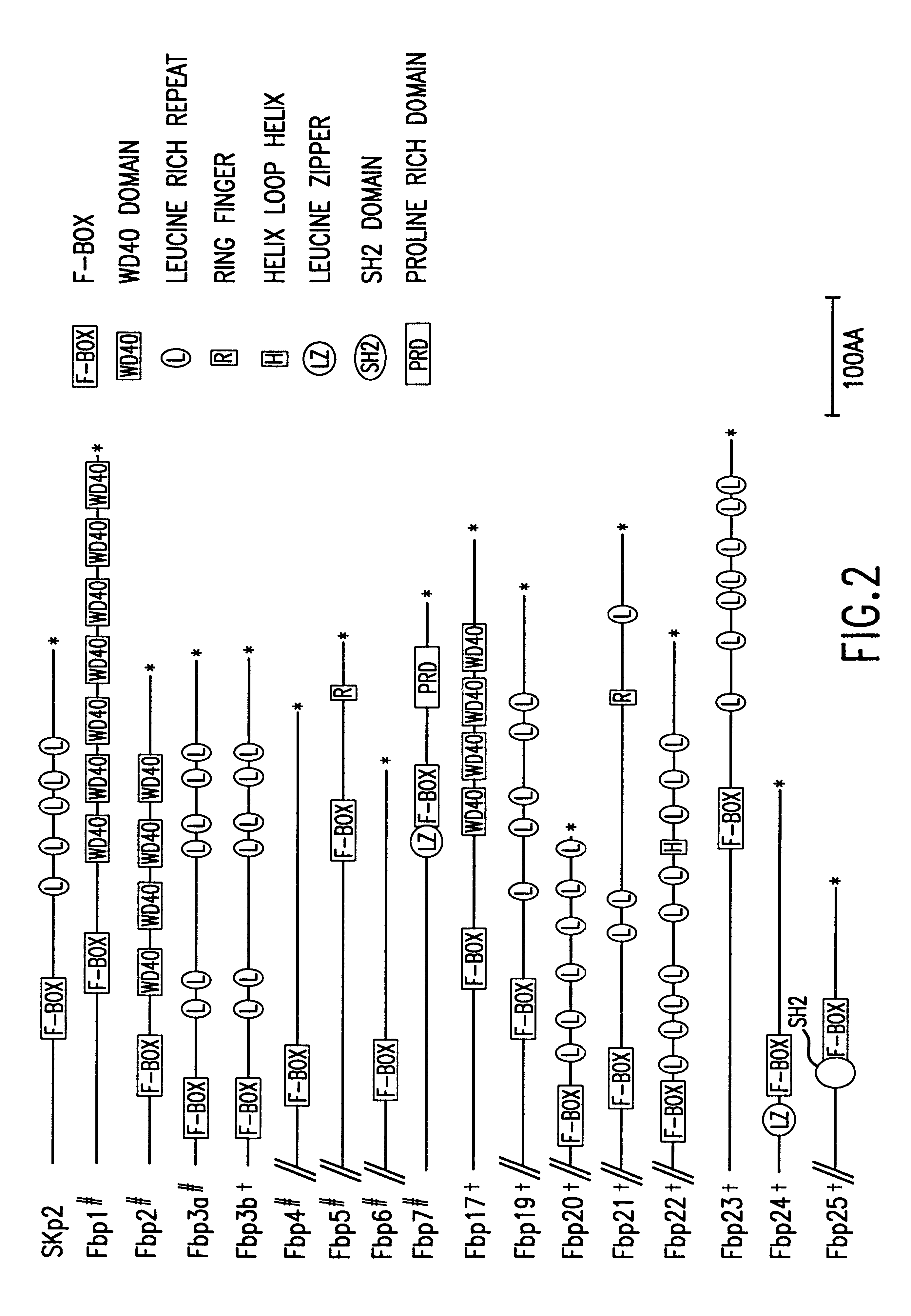
The present invention relates to the discovery, identification and characterization of nucleotides that encode novel substrate-targeting subunits of ubiquitin ligases. The invention encompasses nucleotides encoding novel substrate-targeting subunits of ubiquitin ligases: FBP1, FBP2, FBP3, FBP4, FBP5, FBP6, FBP7, FBP8, FBP9, FBP10, FBP11, FBP12, FBP13, FBP14, FBP15, FBP16, FBP17, FBP18, FBP19, FBP20, FBP21, FBP22, FBP23, FBP24, and FBP25, transgenic mice, knock-out mice, host cell expression systems and proteins encoded by the nucleotides of the present invention. The present invention relates to screening assays that use the novel substrate-targeting subunits to identify potential therapeutic agents such as small molecules, compounds or derivatives and analogues of the novel ubiquitin ligases which modulate activity of the novel ubiquitin ligases for the treatment of proliferative and differentiative disorders, such as cancer, major opportunistic infections, immune disorders, certain cardiovascular diseases, and inflammatory disorders. The invention further encompasses therapeutic protocols and pharmaceutical compositions designed to target ubiquitin ligases and their substrates for the treatment of proliferative disorders.
Owner:NEW YORK UNIV
Method for building special microRNA knock-down mouse model of heart
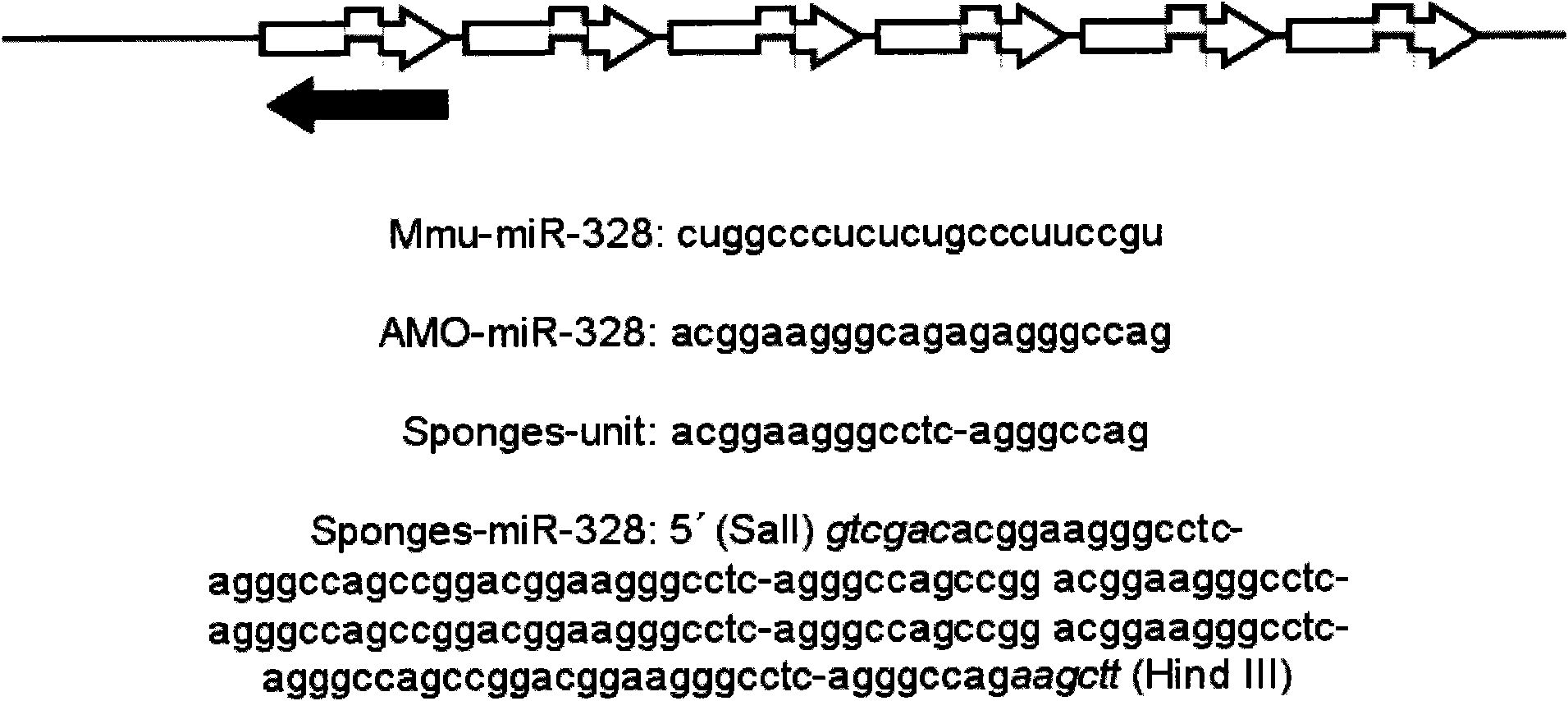
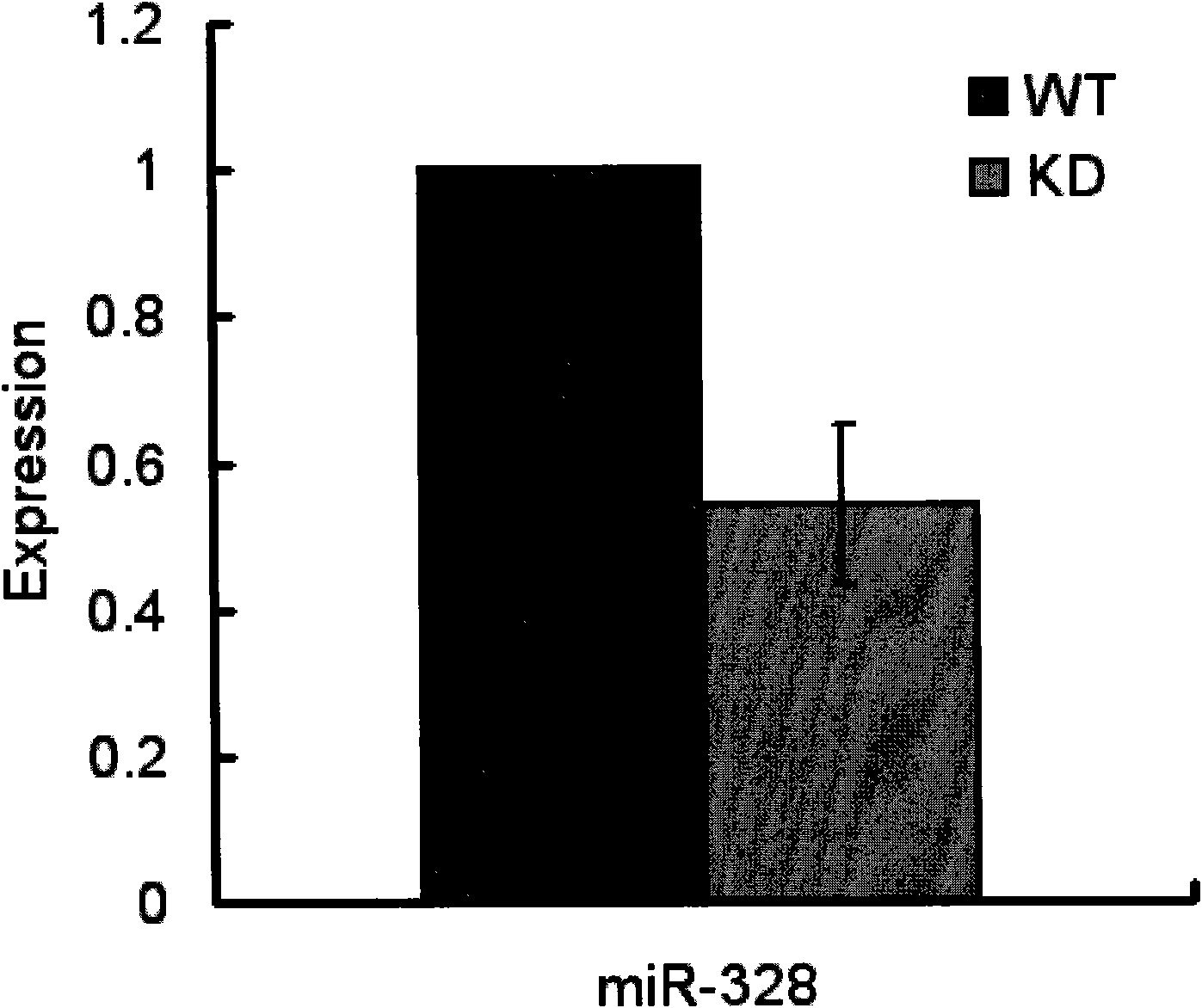
ActiveCN101985628AVector-based foreign material introductionAnimal husbandryGene ComponentCardiac muscle
The invention relates to the technical field of biotechnology, in particular to a method for building a special microRNA knock-down mouse model of a heart. The invention provides a transgenic mouse built by using a miRNA sponge technology. Exogenous gene components comprise a myocardium special promoter, a miR-328 sponge and a transcription termination signal. The myocardium miR-328 of the mouse is knocked down by almost 50%, and the mouse model is suitable for selection of antiarrhythmic drugs because the miR-328 is involved in the occurrence of arrhythmia. The method is based on the transgenic mouse, so the model can stably express the sponge to stably knock miRNA down and can inherit simultaneously. The mouse phenotype is stable, thus the mouse model is applicable for study of subsequent functions.
Owner:HARBIN MEDICAL UNIVERSITY
Highly safe intranasally administrable gene vaccines for treating alzheimer's disease
InactiveUS20090170798A1Large scaleLow costSsRNA viruses negative-senseOrganic active ingredientsDiseaseNose
An objective of the present invention is to provide a safe and effective vaccine therapy for Alzheimer's disease. A minus strand RNA viral vector carrying amyloid gene was constructed, and administered intranasally to 24- to 25-months-old APP transgenic mice. The level of serum anti-A 42 antibody was determined and showed to be markedly higher than the control. The results of histological investigation showed that the administration of a vector of the present invention markedly reduced senile plaques in all of the frontal lobe, parietal lobe, and hippocampus. The brain A level was also markedly reduced. Furthermore, the administration of a vector of the present invention did not result in lymphocyte infiltration in the central nervous system.
Owner:DNAVEC CORP +1
Activation of trpv4 ion channel by physical stimuli and critical role for trpv4 in organ-specific inflammation and itch
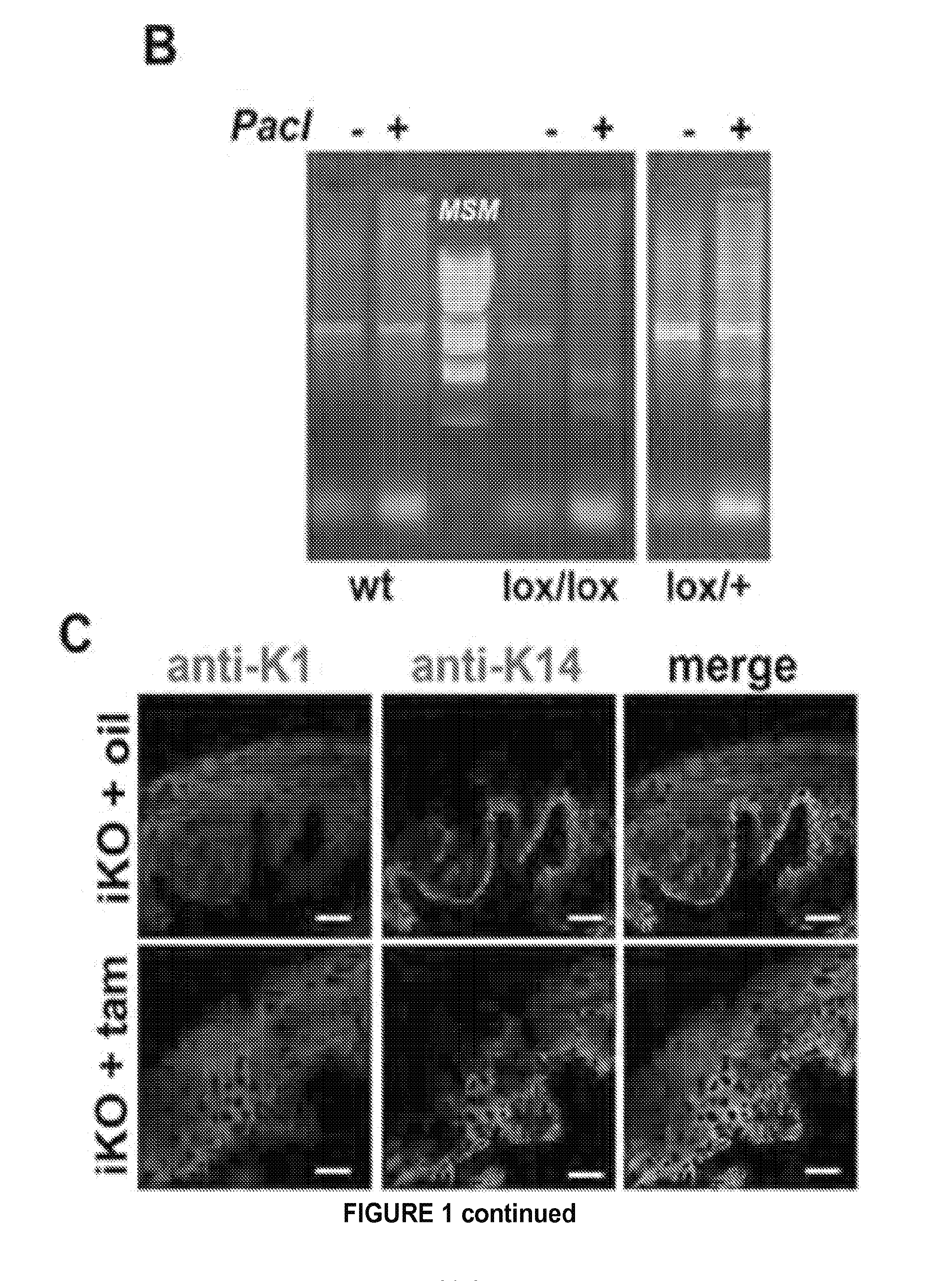
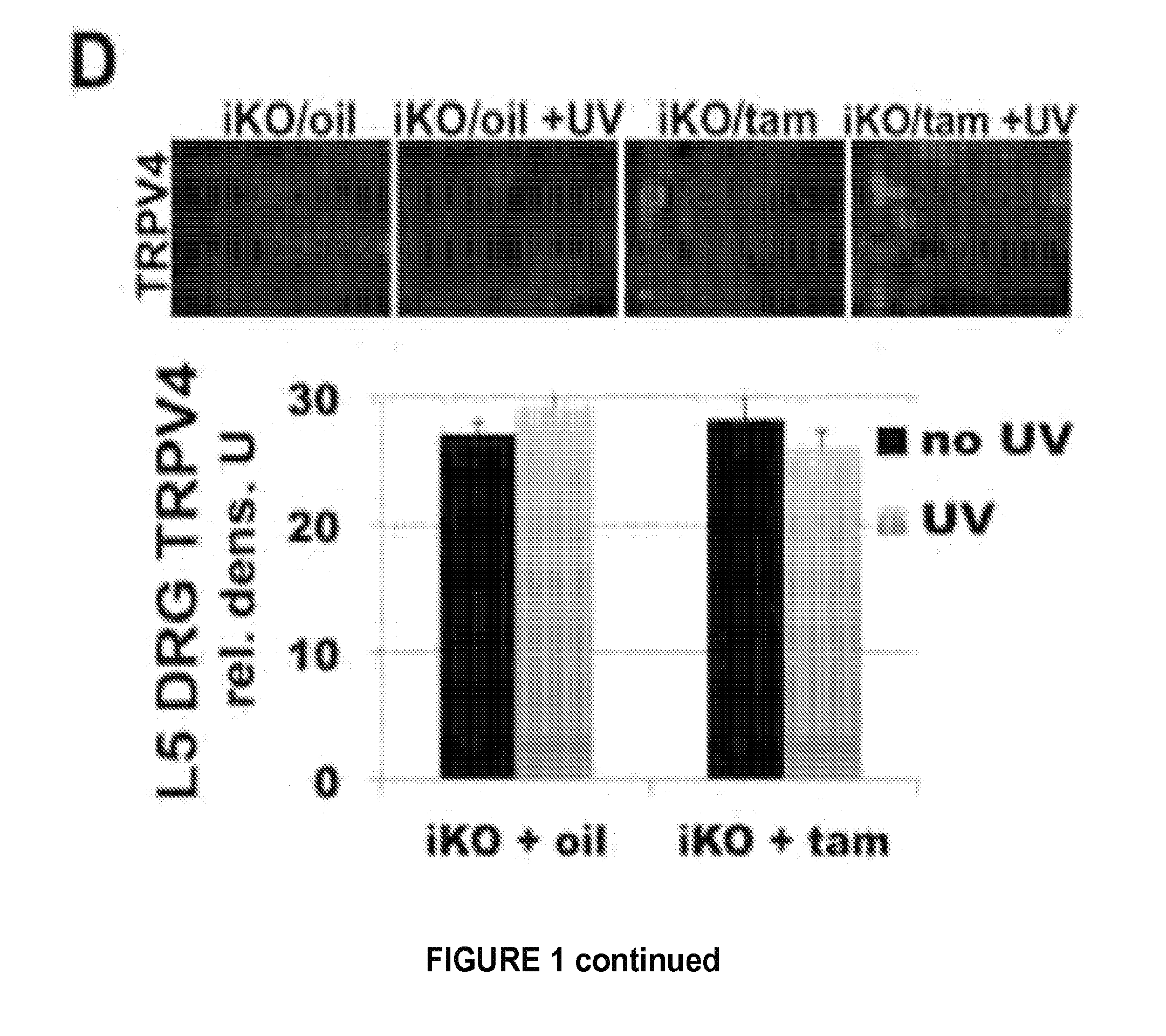
Provided are methods of treating and / or preventing dermatological disorders. Provided are methods of reducing skin inflammation, reducing pain, and / or reducing itch in a subject in need thereof. The methods may include administering to the subject an effective amount of a TRPV4 inhibitor. Further provided are compositions including a TRPV4 inhibitor compound in combination with a carrier, vehicle, or diluent that is suitable for topical application. Further provided is a transgenic mouse whose genome includes deletions of the Trpv4 gene in keratinocytes of the epidermis, wherein said transgenic mouse is a knockout for the Trpv4 gene in keratinocytes of the epidermis following keratinocyte-specific activation and expression of a site-specific recombination enzyme.
Owner:DUKE UNIV
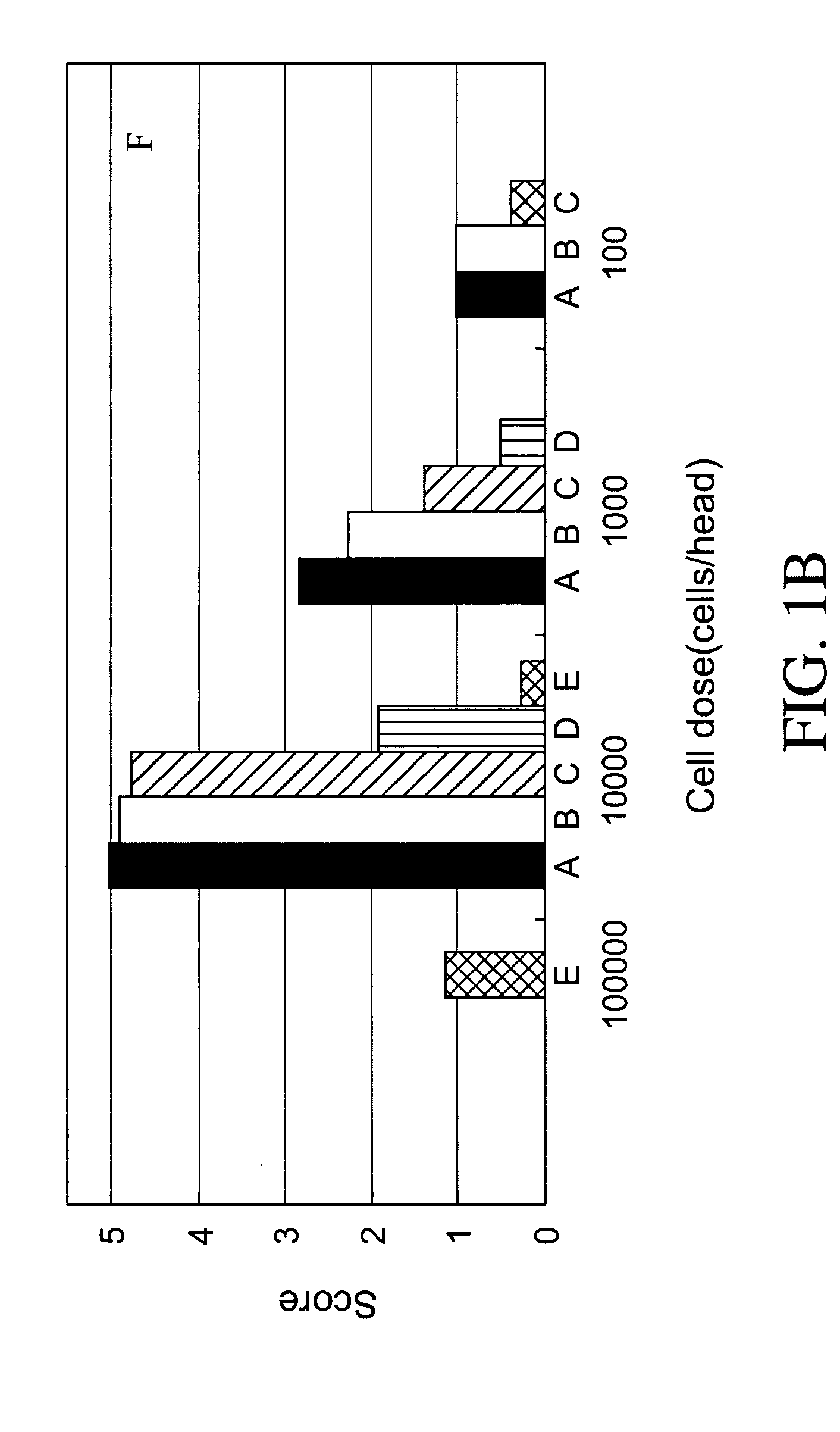
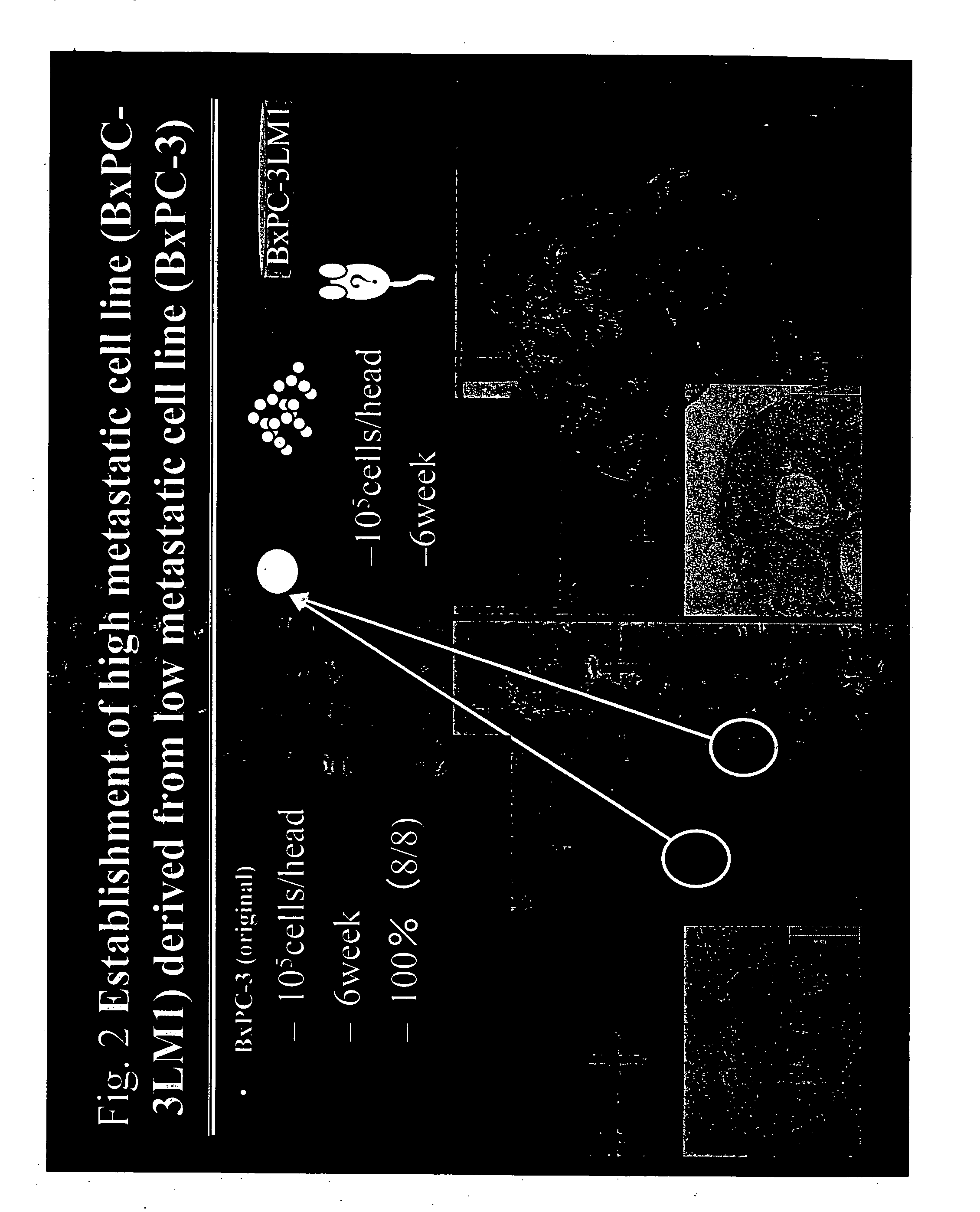
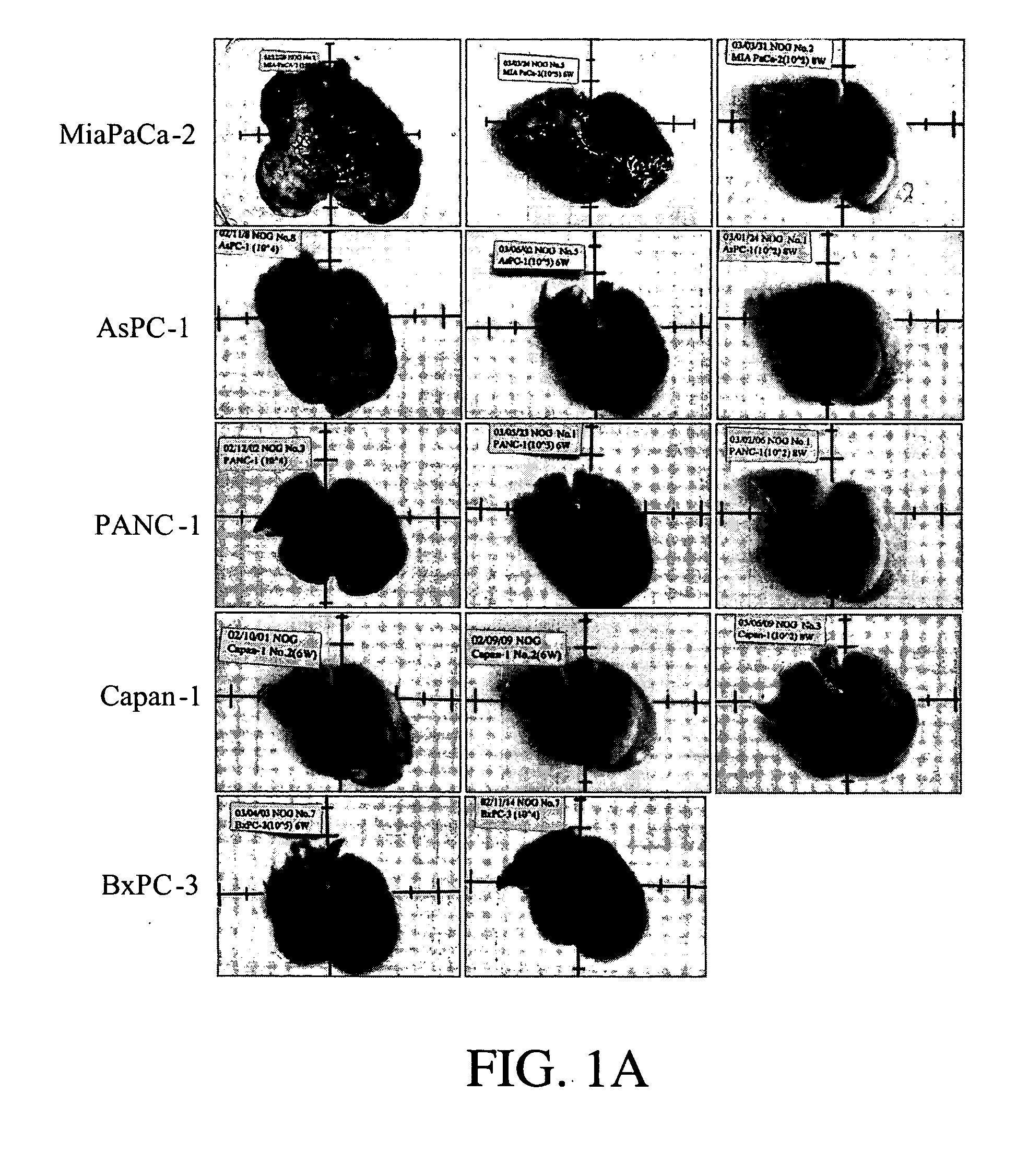
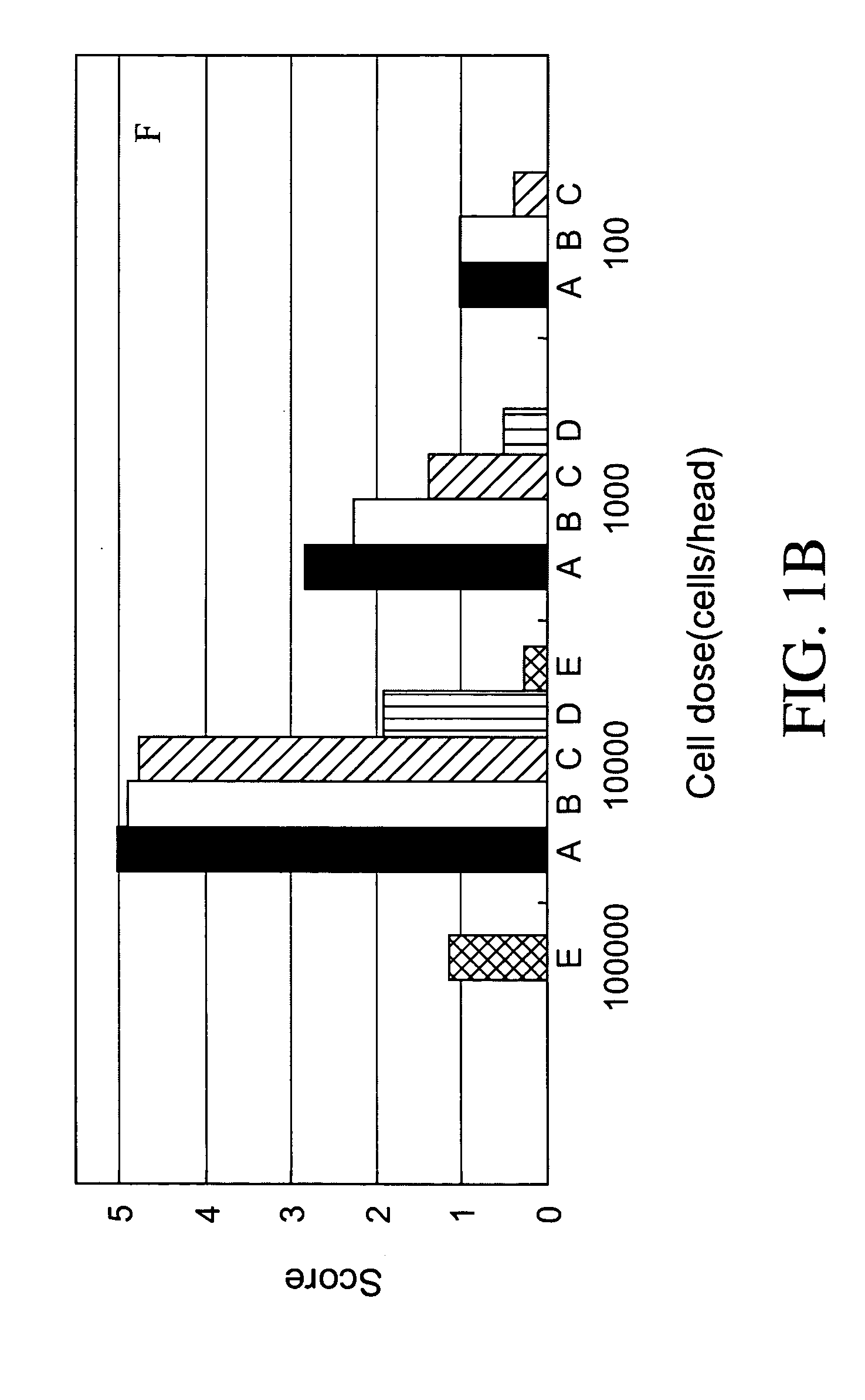
Establishment of human cancer cell lines with metastatic potential using NOD/SCID
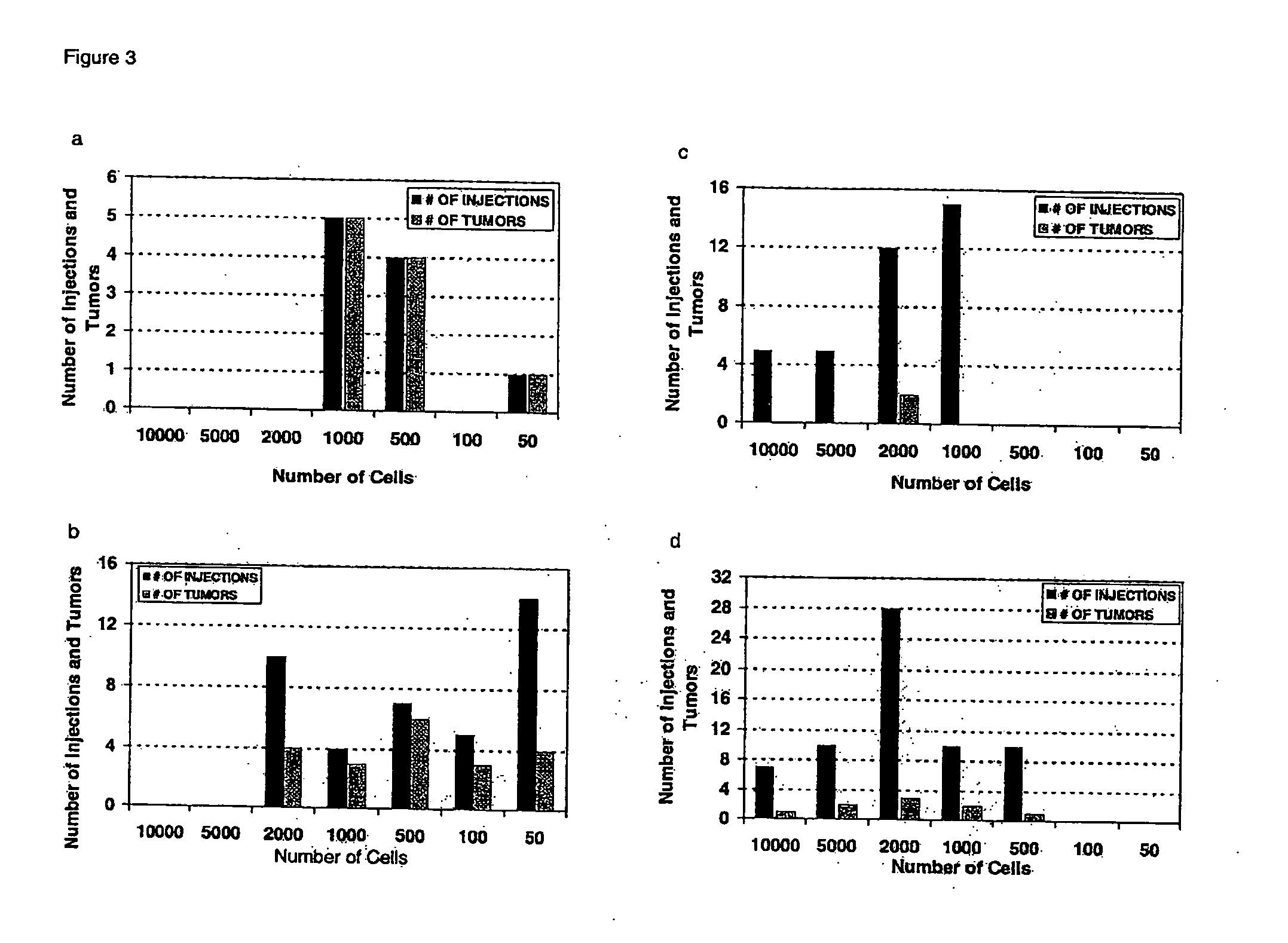
The invention provides a new reproducible transgenic mouse model for the study of tumor metastasis. In particular, the invention concerns the study of tumor metastasis in a NOD / SCID / γcnull transgenic mouse model.
Owner:CENT INST FOR EXPERIMENTAL ANIMALS +1
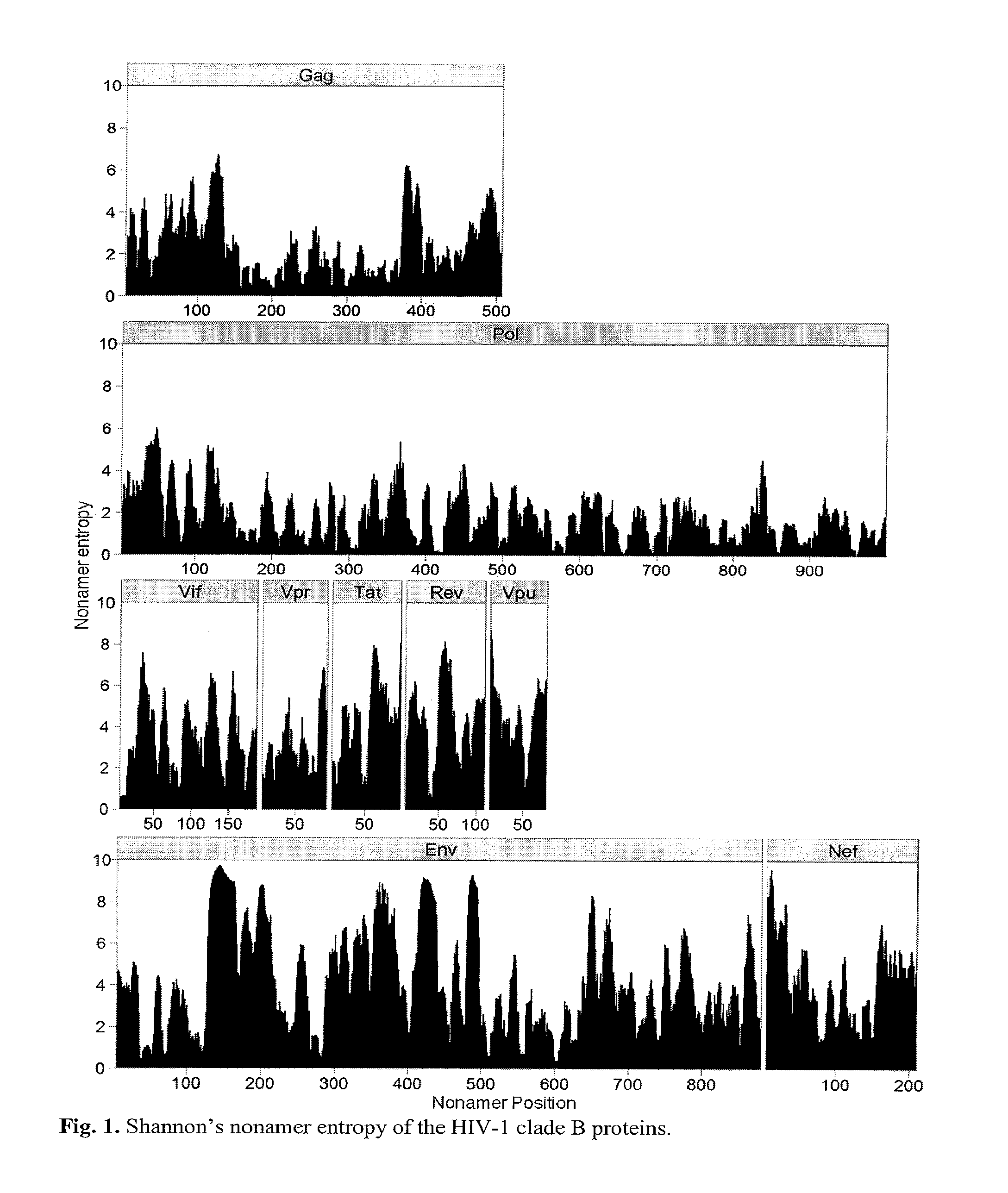
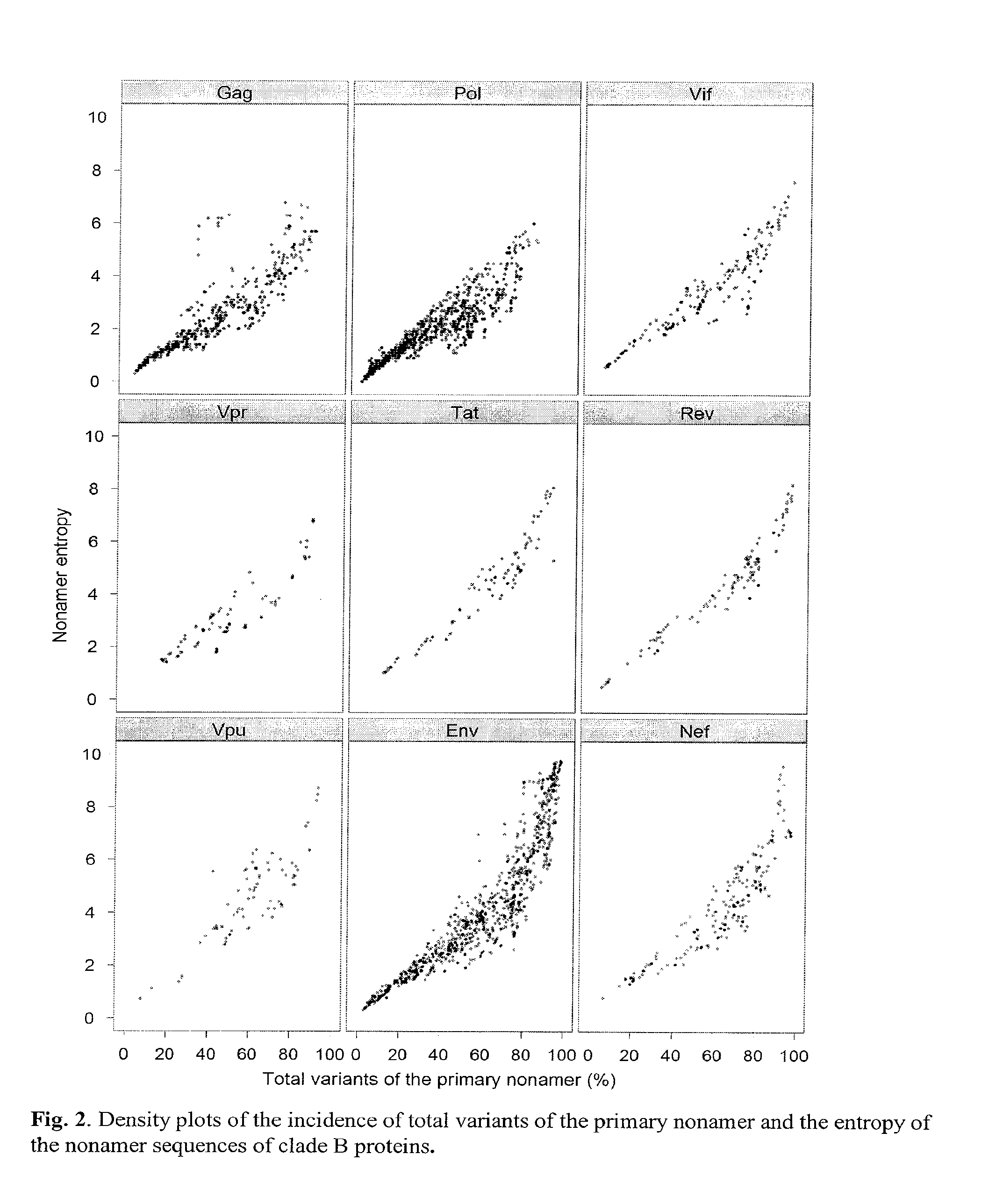
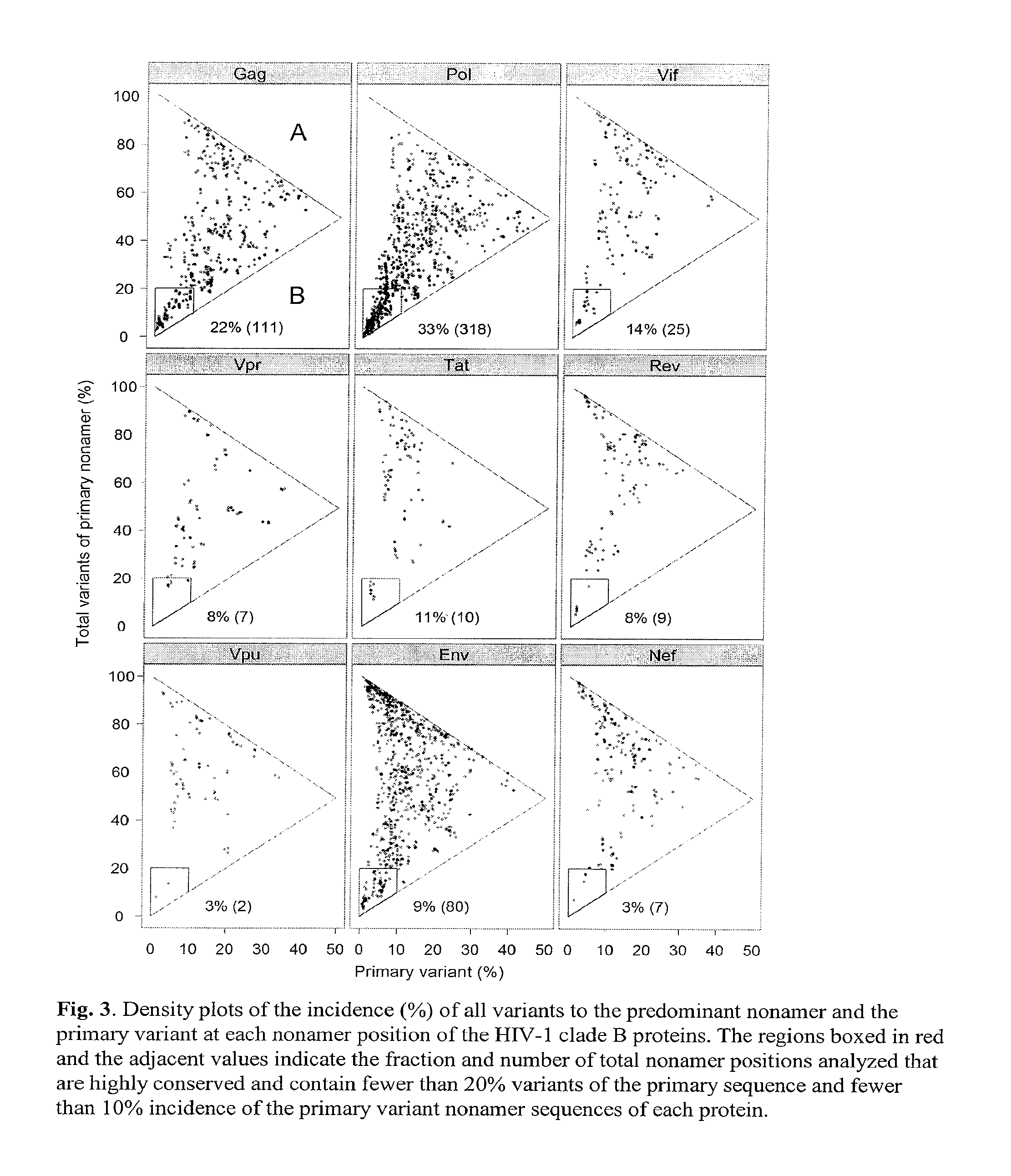
Human immunodeficiency virus (hiv-1) highly conserved and low variant sequences as targets for vaccine and diagnostic applications
InactiveUS20130195904A1Shorten the durationReduce riskPeptide/protein ingredientsVirus peptidesPrimate LentivirusesTGE VACCINE
We identified regions of the HIV-1 proteome with high conservation, and low variant incidence. Such highly conserved sequences have direct relevance to the development of new-generation vaccines and diagnostic applications. The immune relevance of these sequences was assessed by their correlation to previously reported human T-cell epitopes and to recently identified human HIV-1 T-cell epitopes (identified using HLA transgenic mice). We identified (a) sequences specific to HIV-1 with no shared identity to other viruses and organisms, and (b) sequences that are specific to primate lentivirus group, with multiclade HIV-1 conservation.
Owner:NAT UNIV OF SINGAPORE +1
Proliferator-Activated Receptor Disruptions, Compositions and Methods Relating Thereto
InactiveUS20070022486A1Increase kidney weight to body weight ratioDecrease latencyAnimal cellsNucleic acid vectorTransgeneDisease
The present disclosure relates to compositions, including transgenic and methods relating to the characterization of gene function. Specifically, the present disclosure provides transgenic mice comprising mutations in a PPAR gene. The present disclosure also provides methods for identifying agents that modulate PPAR expression and function, useful models, and potential treatments for various disease states and disease conditions.
Owner:DELTAGEN INC
Human monoclonal antibodies against bacillus anthracis protective antigen
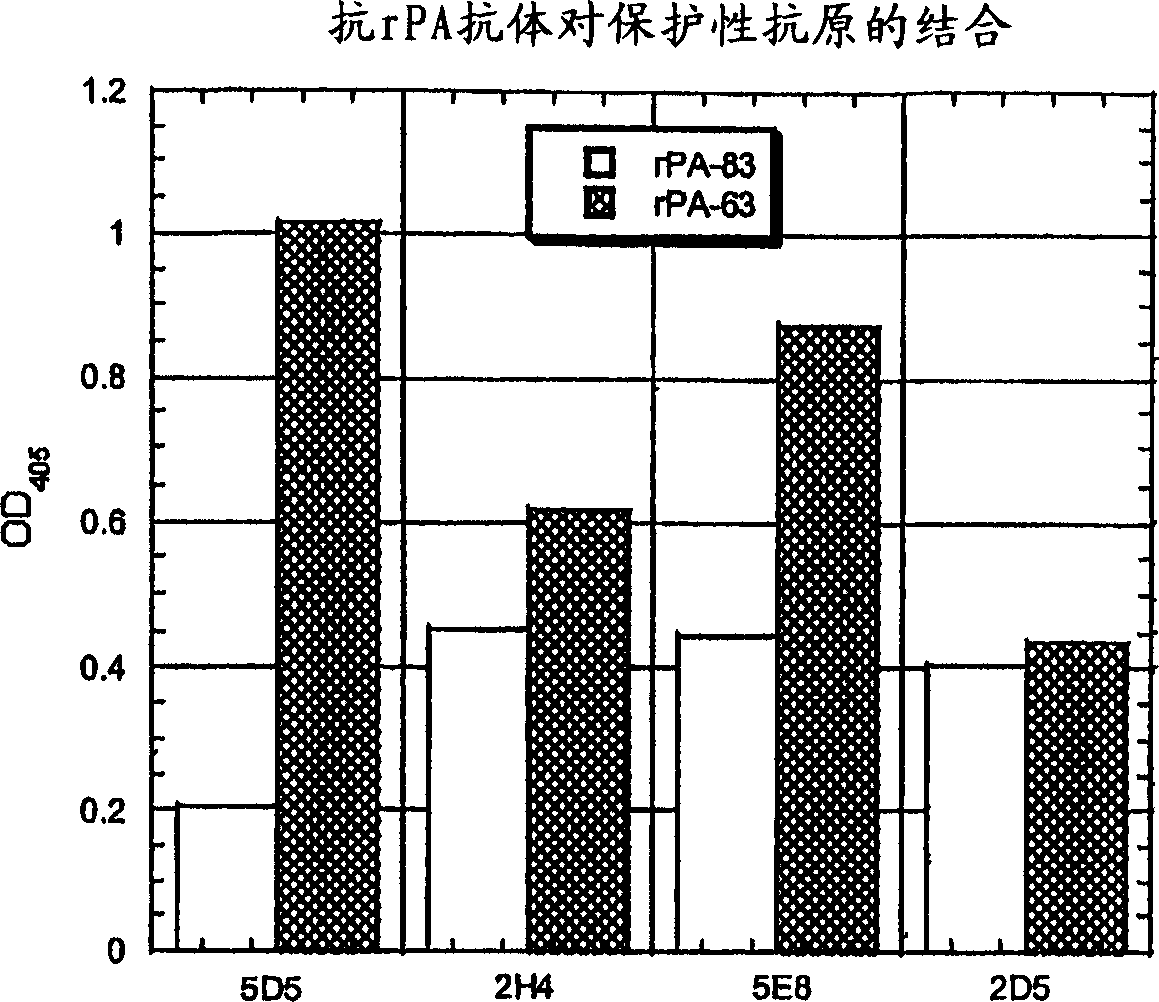
Isolated human monoclonal antibodies that bind anthrax protective antigens are disclosed. Human antibodies can be produced in non-human transgenic animals, such as transgenic mice, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are derivatives of human antibodies (e.g., bispecific antibodies and immunoconjugates), pharmaceutical compositions containing human antibodies, non-human transgenic animals and hybridomas producing human antibodies, and therapeutic and diagnostic uses of human antibodies method.
Owner:ER SQUIBB & SONS INC
Novel coronavirus SARS-CoV-2 mRNA vaccines and preparation method and application thereof
ActiveCN113151312AProlong half-lifeEasy to getSsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationSpecific igg
The invention provides novel coronavirus SARS-CoV-2mRNA vaccines and a preparation method and application thereof. The invention provides three mRNA vaccines, namely RBD, S1 and S vaccines. The RBD vaccine disclosed by the invention can induce a high-titer antigen-specific IgG antibody and a virus neutralization antibody after immunization with one dose, the high-titer neutralization antibody can be maintained for at least 26 weeks, and remarkable immune protection can be provided for human ACE2 transgenic mice in serum adoptive transfer protection experiments. The RBD and S vaccine disclosed by the invention can induce immune protection capable of completely resisting SARS-CoV-2 virus infection in the human ACE2 transgenic mice after immunization with two doses. A large number of experimental results show that the mRNA vaccine provided by the invention has good immunogenicity, forms powerful immune protection after immunizing an organism, and has a huge development potential.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Double transgenic mice overexpressing human beta secretase and human APP-London
The present invention relates to a novel animal model for amyloidopathies, especially Alzheimer' disease overexpressing human BACE and human APP London. This novel animal model exhibits several aspects of amyloidopathy and has utility in developping specific and general therapies for amyloidopathies. Moreover, this animal model can be used for screening methods to identify novel anti-amyloidogenic compounds and for testing their therapeutic effect. The present invention also relates to a method for producing the said double transgenic animals, to cells and cell lines derived from these animals and to a kit comprising these cells for screening compounds useful of amyloidopathies. Moreover, a method for the evaluation of the in vivo effects of beta-secretase activity on A-beta peptide generation, amyloidosis, neurodegeneration and AD pathology in these animals is provided.
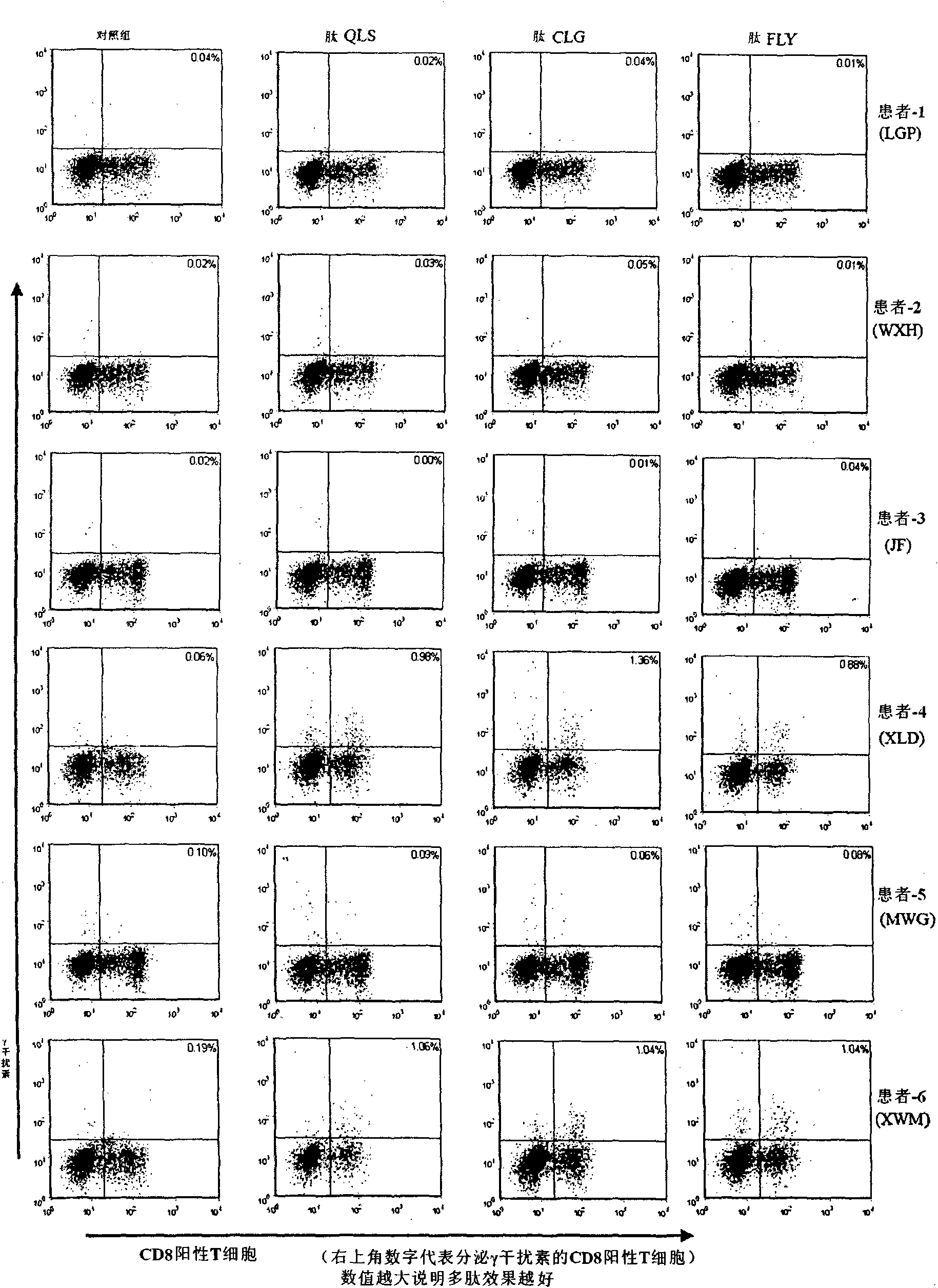
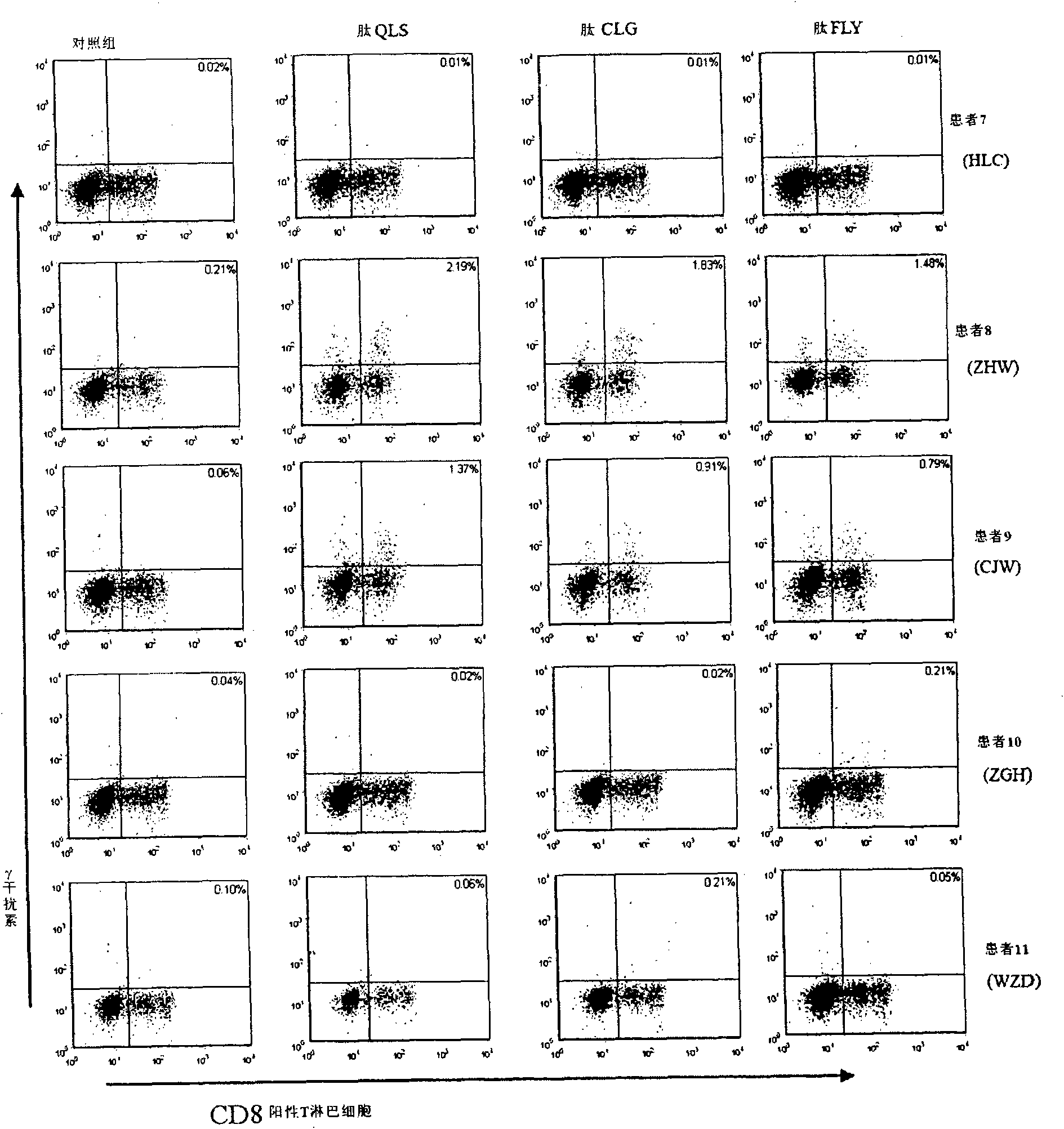
HLA-A2 restrictive epitope polypeptide of LMP2A protein source and purpose thereof
InactiveCN101643497AChemically stableEasy to manufacturePeptide/protein ingredientsPeptidesLMP2A-Specific Cytotoxic T-LymphocytesT lymphocyte
The invention discloses HLA-A2 restrictive epitope polypeptide of an LMP2A protein source, which is polypeptide LMP2A264 and comprises 9 amino acids in the positions of 264 to 272; the sequence is QLSPLLGAV. Mycobacteria heat shock protein 70 (HSP70) is expressed, identified and purified; a transgene animal model for evaluating EBV relevant polypeptide vaccine in vivo tests is established; the immune research on inducing EBV specific CTL inside and outside healthy volunteers and transgene rats for the new EBV epitope polypeptide LMP2A264 is systemically researched and the tumor prevention andthe tumor treating test of the polypeptide LMP2A264 on LMP2A positive solid tumors are evaluated, thereby proving that the polypeptide LMP2A264 can induce LMP2A specific cell toxic T lymphocyte reaction. Therefore, the polypeptide LMP2A264 can be applied to prepare medicaments for treating or preventing LMP2A positive solid tumors.
Owner:NANJING MEDICAL UNIV
Binding molecules
ActiveUS20100197897A1Antibacterial agentsAntibody mimetics/scaffoldsAntigen challengeAntigen binding
The present invention relates to the manufacture of a diverse repertoire of functional heavy chain-only antibodies that undergo affinity maturation, and uses thereof. The invention also relates to the manufacture and use of a diverse repertoire of class-specific heavy chain-only antibodies and to the manufacture and use of multivalent polypeptide complexes with antibody heavy chain functionality, preferably antibody heavy chain binding functionality, constant region effector activity and, optionally, additional effector functions.The present invention also relates to a method of generation of fully functional heavy chain-only antibodies in transgenic mice in response to antigen challenge. In particular, the present invention relates to a method for the generation of human antigen-specific, high affinity, heavy chain-only antibodies of any class, or mixture of classes and the isolation and expression of fully functional VH antigen-binding domains.
Owner:HARBOUR ANTIBODIES BV +1
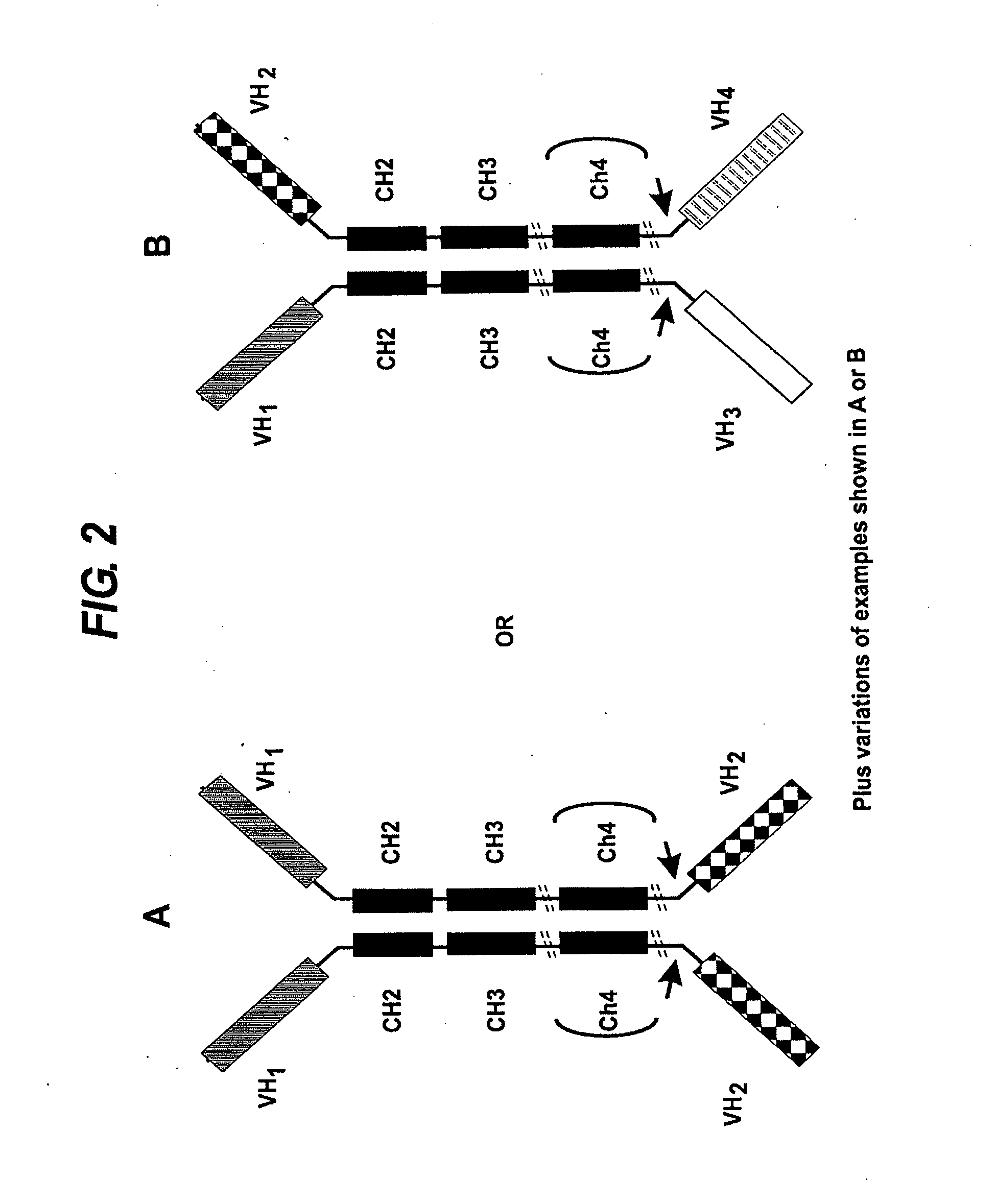
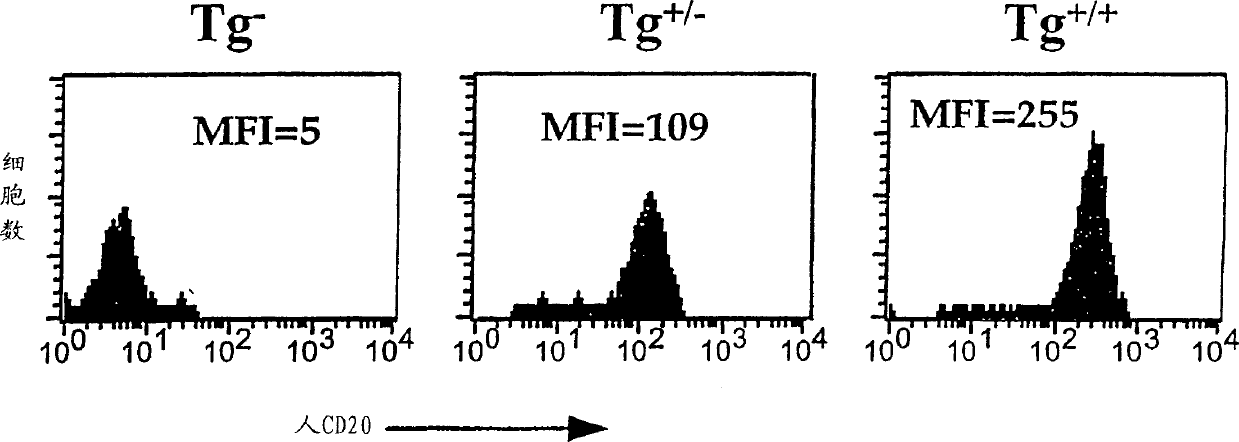
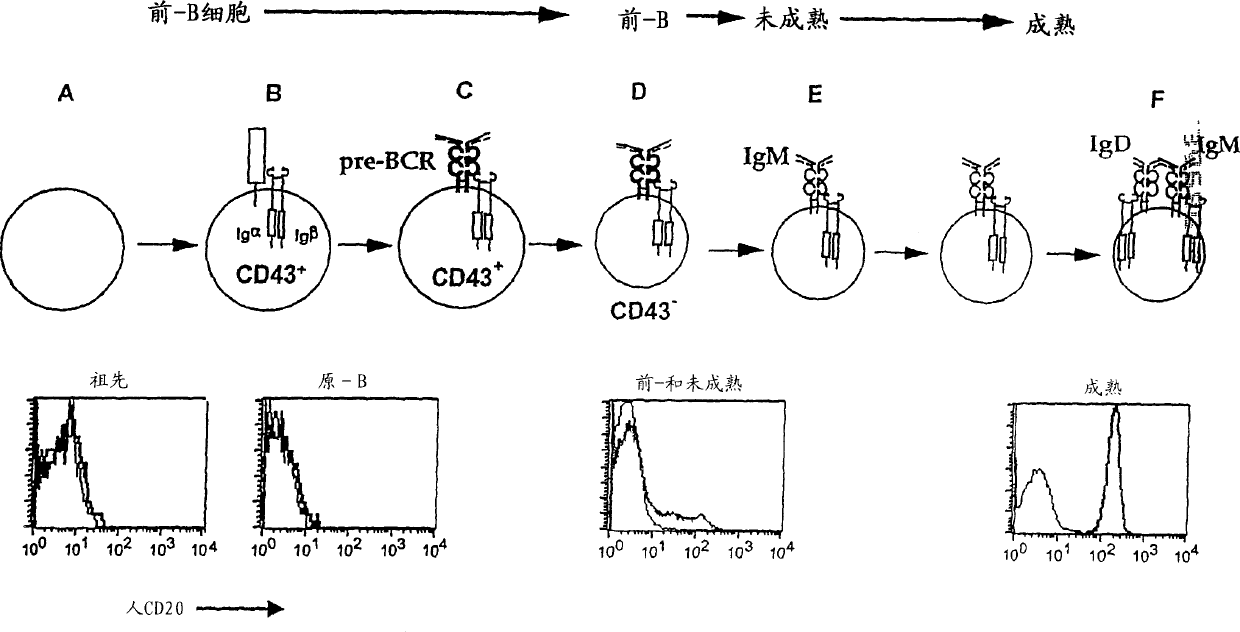
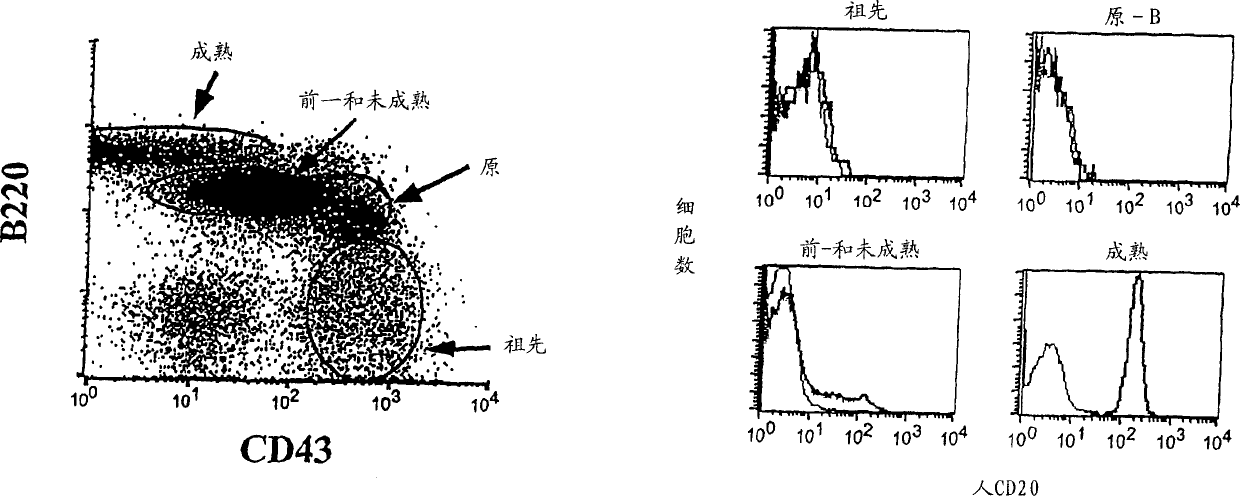
Transgenic mice expressing human cd20 and/or cd16
InactiveCN1748143ASugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthCD20
The present invention relates to immunoglobulin anamorphosis and uses thereof. The invenion provides a humanied and embeded aiti-CD20 body used for treating positive malignant tumor and auto-immune disease.
Owner:GENENTECH INC
Animal model for the analysis of tumor metastasis
The invention provides a new reproducible transgenic mouse model for the study of tumor metastasis. In particular, the invention concerns the study of tumor metastasis in a NOD / SCID / γcnull transgenic mouse model.
Owner:CENT INST FOR EXPERIMENTAL ANIMALS +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com