Transgenic mice expressing human cd20 and/or cd16
A transgenic animal and genome technology, applied in genetic engineering, plant genetic improvement, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problem of lack of co-expressed human marker animal model and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
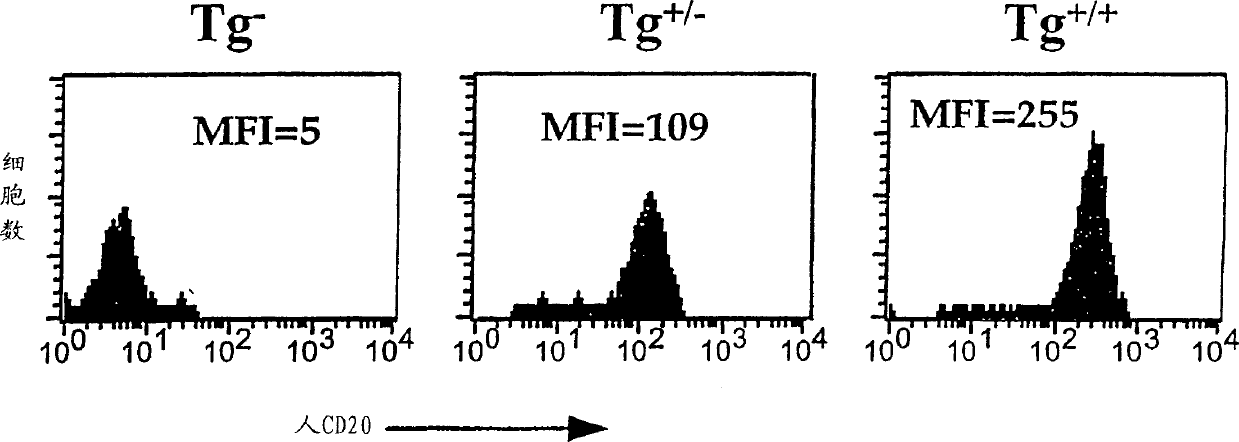
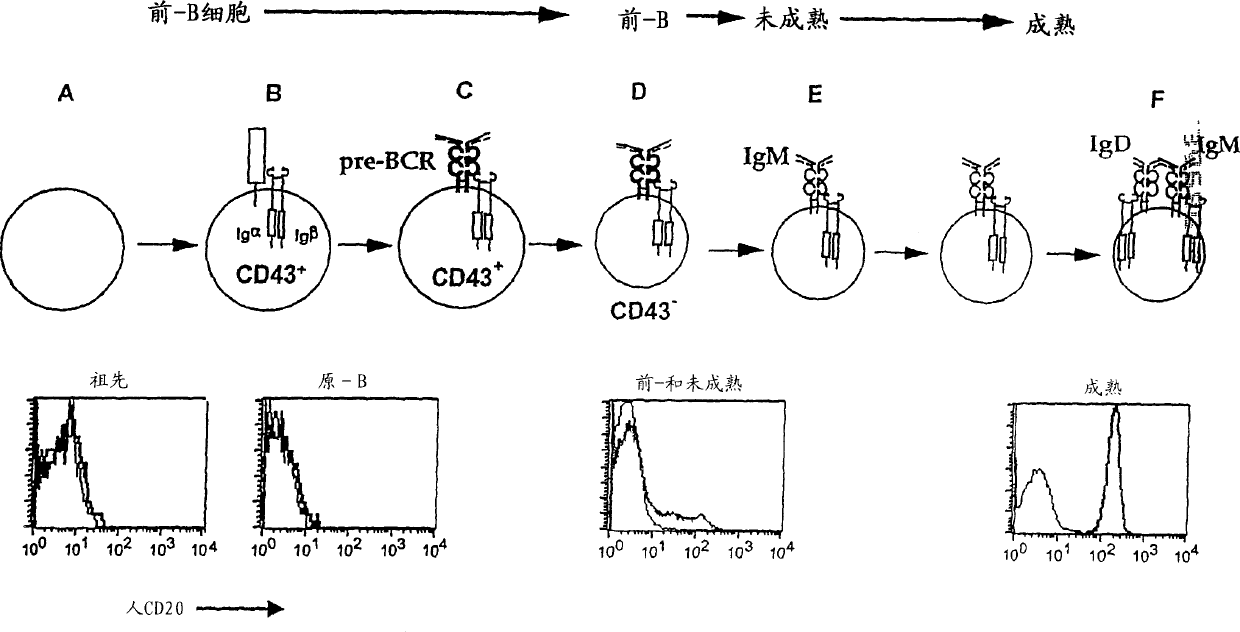
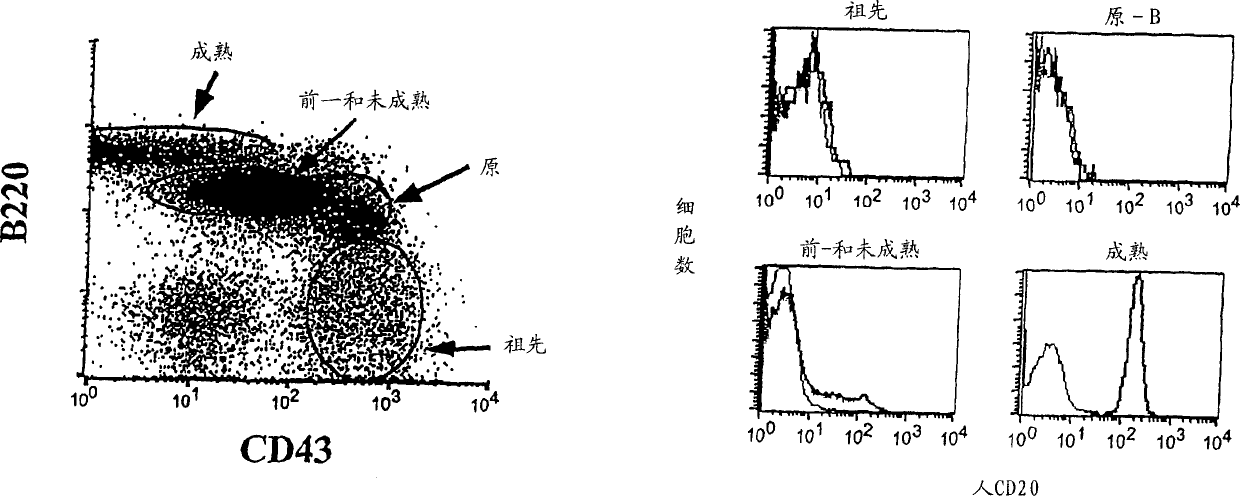
[0070] The present invention provides transgenic animals expressing heterologous CD20 markers, heterologous CD16 markers or both. In one embodiment, the human CD20 transgenic animal of the present invention expresses CD20 on the same type of B cells. Administration of anti-human CD20 antibodies to this currently described transgenic animal resulted in the depletion of lymphocytes expressing human CD20B. Due to the inability to incorporate effective transcription control regions into the transgene, previous attempts to prepare transgenic mice expressing human CD20 failed to achieve the expression of human CD20 in a suitable B cell subpopulation. In another embodiment, the transgenic mouse of the invention expresses human CD20 and human CD16.
[0071] The present invention provides transgenic animals that have cells that respond in a manner similar to human subjects. Administration of anti-human CD20 antibodies to this currently described transgenic animal resulted in the depletion ...
Embodiment 1
[0157] This example describes the generation of human CD20BAC transgenic (Tg+) mice, and studies the effect of anti-human CD20 antibody treatment in hCD20+ mice.
[0158] Human CD20 CITB human BAC-D-clone No. 117H19 from Invitrogen (Invitrogen, Carlsbad, CA) was used to produce human CD20 transgenic mice. DNA encoding human CD20 was isolated from human lymphocytes and sent to Invitrogen. Invitrogen tested the DNA with filter paper with clones from the human BAC library and identified clone 117H19. Previous attempts to produce transgenic mice expressing human CD20 were unsuccessful, perhaps partly due to the inability to incorporate sufficient transcription control regions into the transgenic construct. Transgenic mice were produced by microinjecting the human CD20BAC construct prepared by clone 117H19 into the fertilized eggs of the mouse FVB inbred line. The fertilized eggs are incubated for 1-7 days, and then transplanted into surrogate mice. Mice were screened based on FACS ana...
Embodiment 2
[0171] This example shows the synergy between anti-CD20 monoclonal antibody and BR3 antagonist treatment on B cell modulation / depletion. BR3-Fc is an immunoadhesin in which the extracellular domain of human BR3 is fused to the constant domain of an immunoglobulin sequence (in this case, human IgG1).
[0172] Transgenic mice expressing human CD20 (named hCD20 + Mouse) with anti-CD20 monoclonal antibody (100 μg single injection on day 9), BR3-Fc (from day 1 to day 12, 100 μg every other day), or anti-CD20 monoclonal antibody and BR3- The combination of Fc is treated by intraperitoneal injection. Each group consists of 4 mice. Two days after the last injection, kill the mice and analyze hCD20 + B cells. For B cell markers (CD21 + CD23 + ) FACS analysis of spleen, blood, lymph nodes and Peyer’s spots.
[0173] The results showed that anti-CD20 monoclonal antibody treatment reduced more than 99% of mature circulating B cells in the blood and lymph nodes, and BR3-Fc treatment reduced ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com