Purification of antigen-specific T cells
a technology of antigen-specific t cells and purification, which is applied in the field of derive antigen-specific t cell lines, can solve the problems of limited ex vivo immunotherapy for cancer or viral infections, and low precursor frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Attachment of Biotinylated MHC Class I Molecules on Avidin-Coated Magnetic Beads
[0027] Soluble MHC class I molecules Ld, Kb and Kbm3 were expressed in Drosophila melanogaster cells (Jackson et al, 1992) and purified as previously described (Sykulev et al, 1994a). Biotinylation was performed using biotin-BMCC (Pierce, Rockford, Ill.) according to the manufacturer instructions.
[0028] Dynabeads M500 (Dynal, Lake Success, N.Y.) were coated with neutravidin (Pierce, Rockford, Ill.), and subsequently incubated with biotinylated MHC class I molecules diluted in PBS containing 3% FCS for 2 hours at 4° C. under mild agitation. Beads were then washed 3 times in DMEM containing 10% FCS and incubated for 1 hour with 20 μM peptide. Beads were then used immediately for cell adsorption.
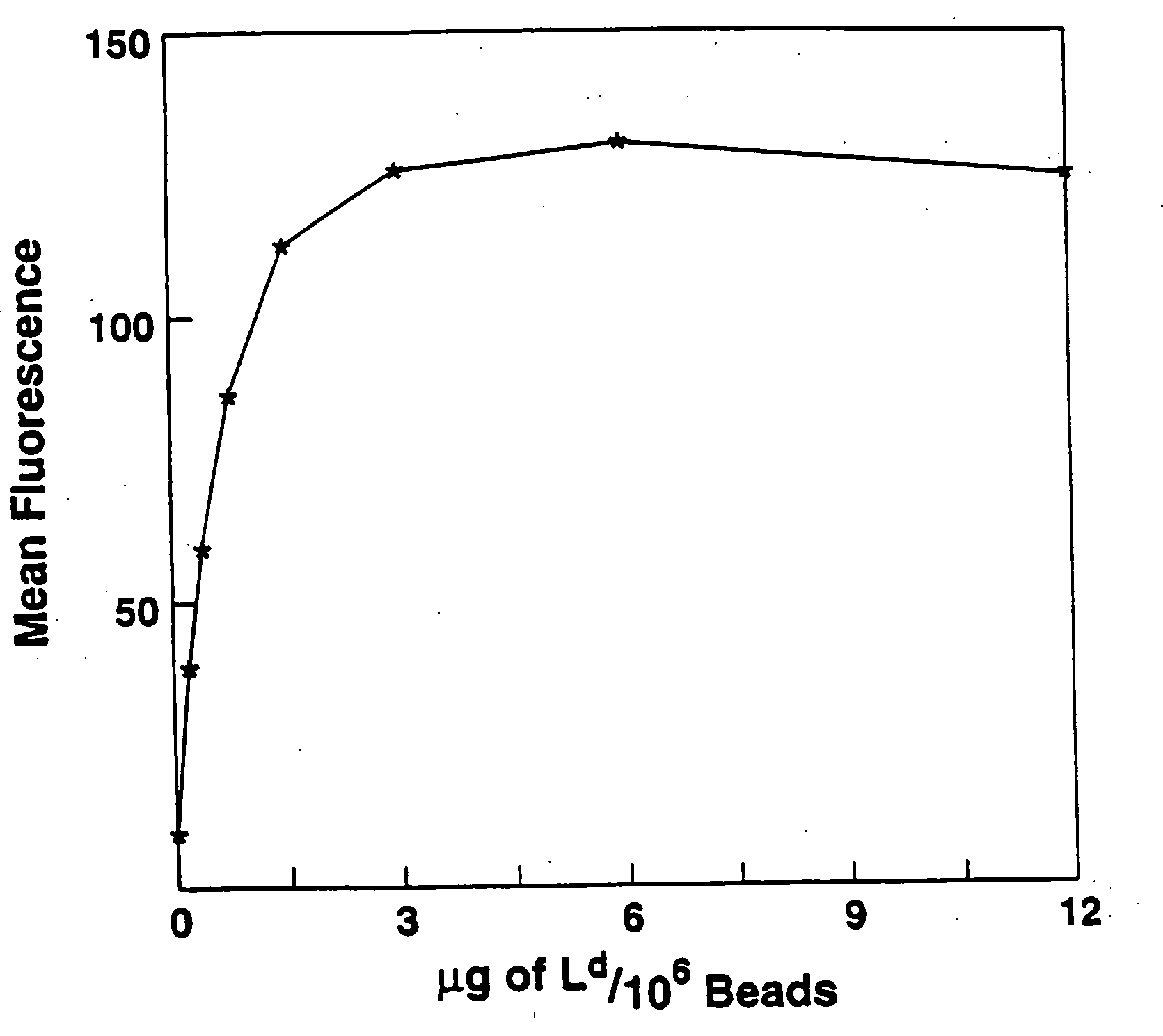
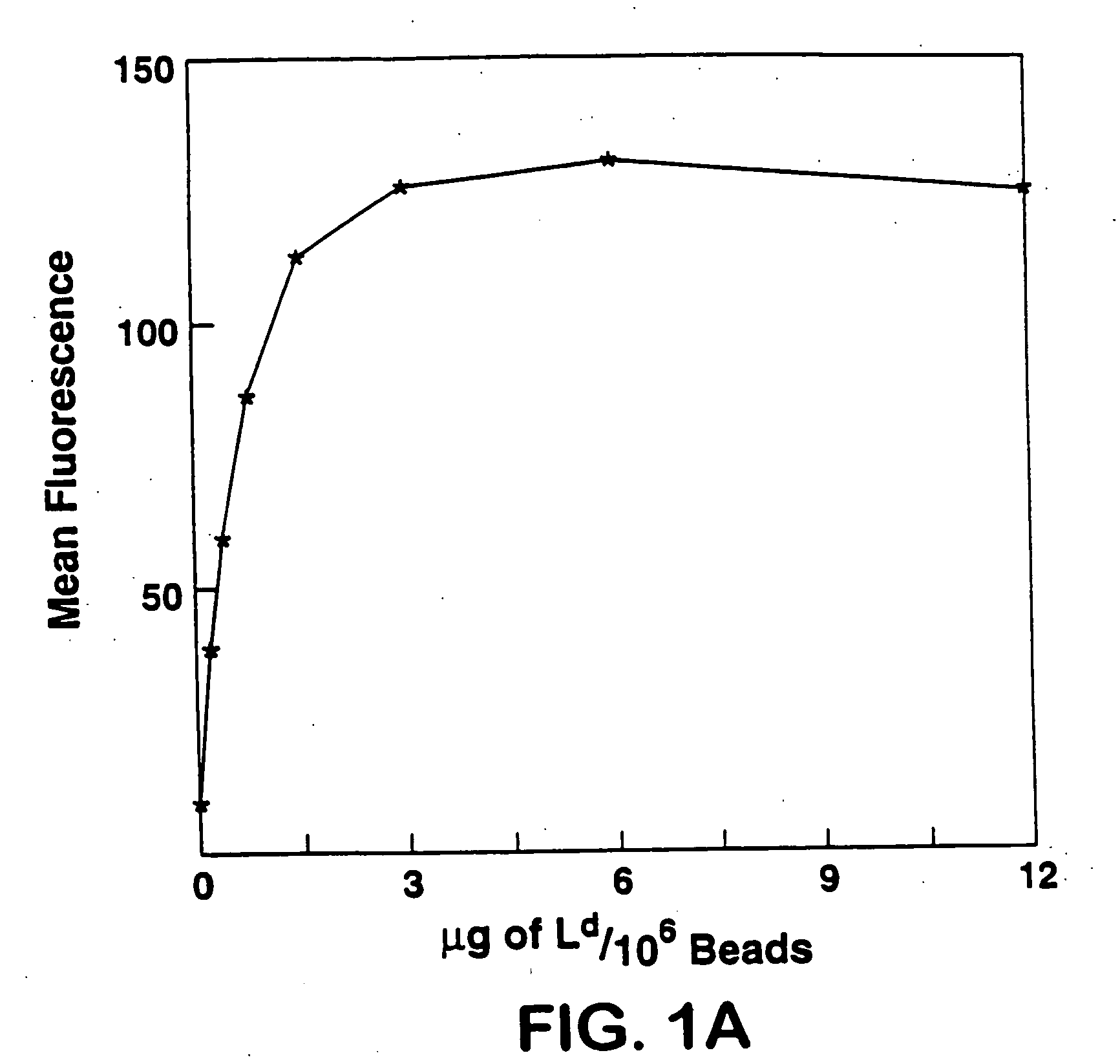
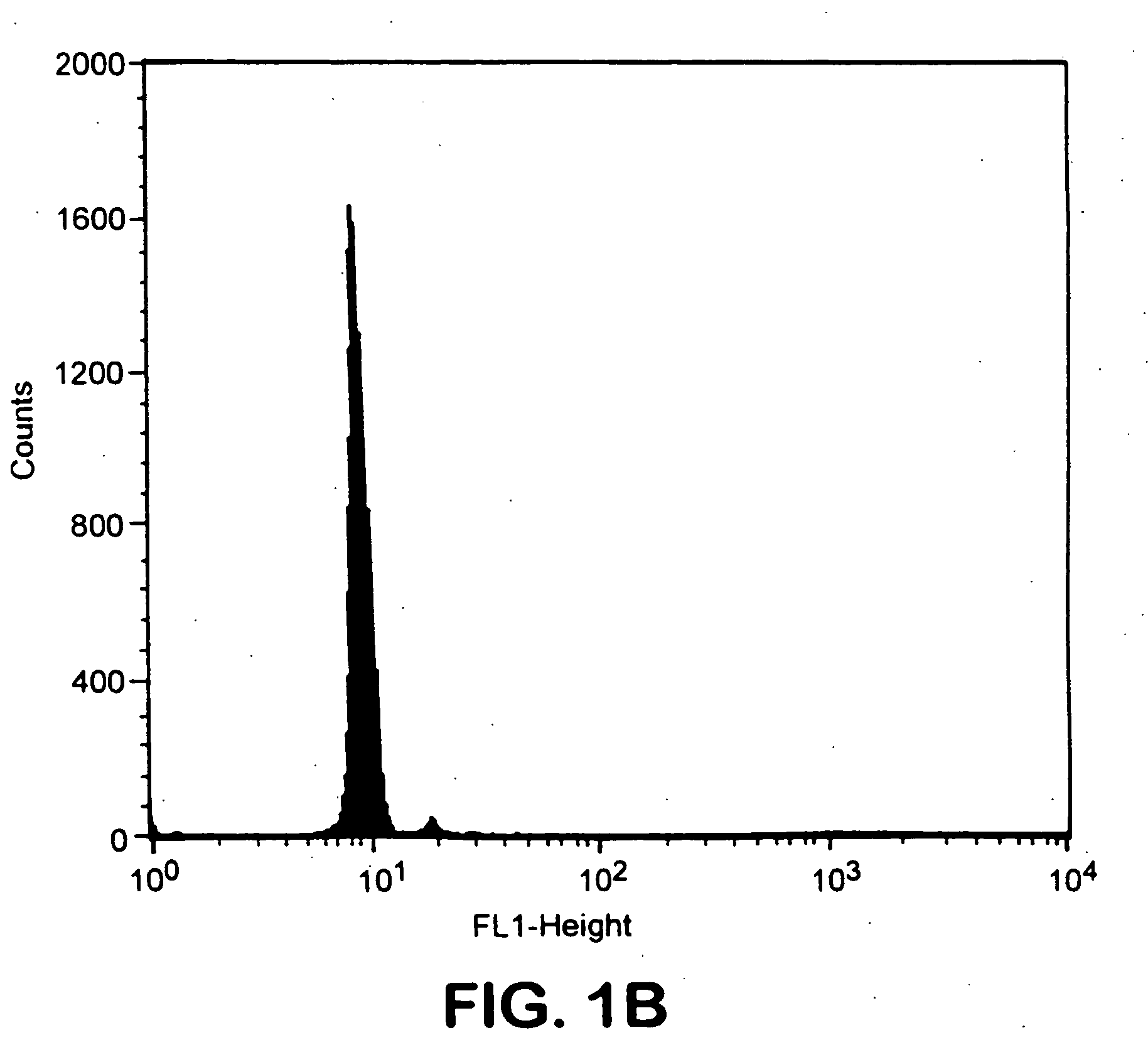
[0029] Biotinylated Ld was used as a test model to study attachment of biotinylated MHC class I to avidin-coated magnetic beads. Various amounts of this molecule were incubated with beads. Beads were then staine...
example 2
Capture of Antigen-Specific T Cells onto MHC Class 1-Coated Magnetic Beads in the Presence of Antigenic Peptides
[0031] Mice and cell lines. BALB / c (H-2d) and C57BL / 6 (H-2b) mice were from Harlan Sprague Dawley (San Diego, Calif.). 2C transgenic mice (Sha et al, 1988) were bred in R. W. Johnson P. R. I. vivarium. All mice were kept under specific pathogen free conditions. Ld-expressing RMA.S cells (Cai and Spent, 1996), and EL4 cells (H-2b, obtained from ATCC, Rockville, Md.) were used as target cells in CTL assays. The anti-clonotypic 1B2 hybridoma was previously described (Kranz et al., 1994).
Purification of CD8+ T Cells from Normal Mice
[0032] Purification was performed at 4° C. under sterile conditions. Mouse inguinal, axillary, cervical, iliac and mesenteric lymph nodes were dissected and separated into single cell suspension. Avidin-coated magnetic beads (Dynal, Lake Success, N.Y.) were coated with biotinylated goat anti-mouse Ig (Southern Biotechnology, Birmingham, Ala.), ...
example 3
Recovery of Antigen-Specific T Cells Mixed with Irrelevant T Cells
[0040] T cell precursor frequencies in a naive animal are typically low. Magnetic beads have been found suitable in other systems to enrich low frequency cell populations (Sawada et al., 1990; Kato and Radbruch, 1993). To assess whether MHC class I-coated magnetic beads could be used for T cell precursor enrichment, we mixed fluorescein-labeled 2C T cells with CD8+ T cells purified from naive C57BL / 6 mice. After incubation with MHC-coated magnetic beads in the presence of peptide, adsorbed cells were eluted and counted, and the percentage of 2C T cells was determined by flow cytofluorometry. In the experiment shown in FIG. 5, 2C T cells were undetectable at the initial frequency of 0.03%. Following adsorption using Kbm3-coated beads and dEV-8 peptide, a definite peak of green fluorescence was observed, displaying the same intensity as the original fluorescein-stained 2C T cell population. This peak represented 65.1%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass flow rate | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com