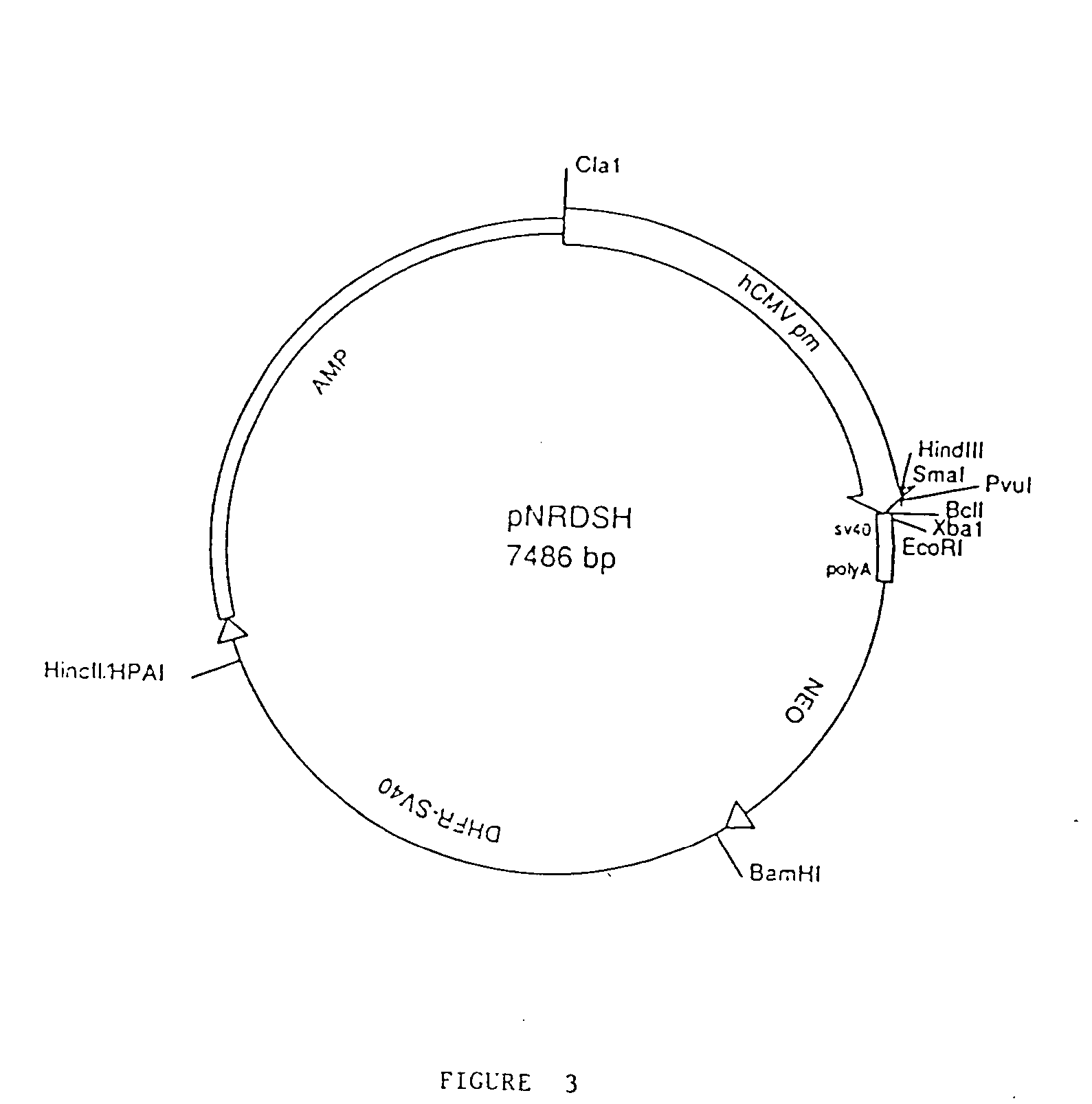
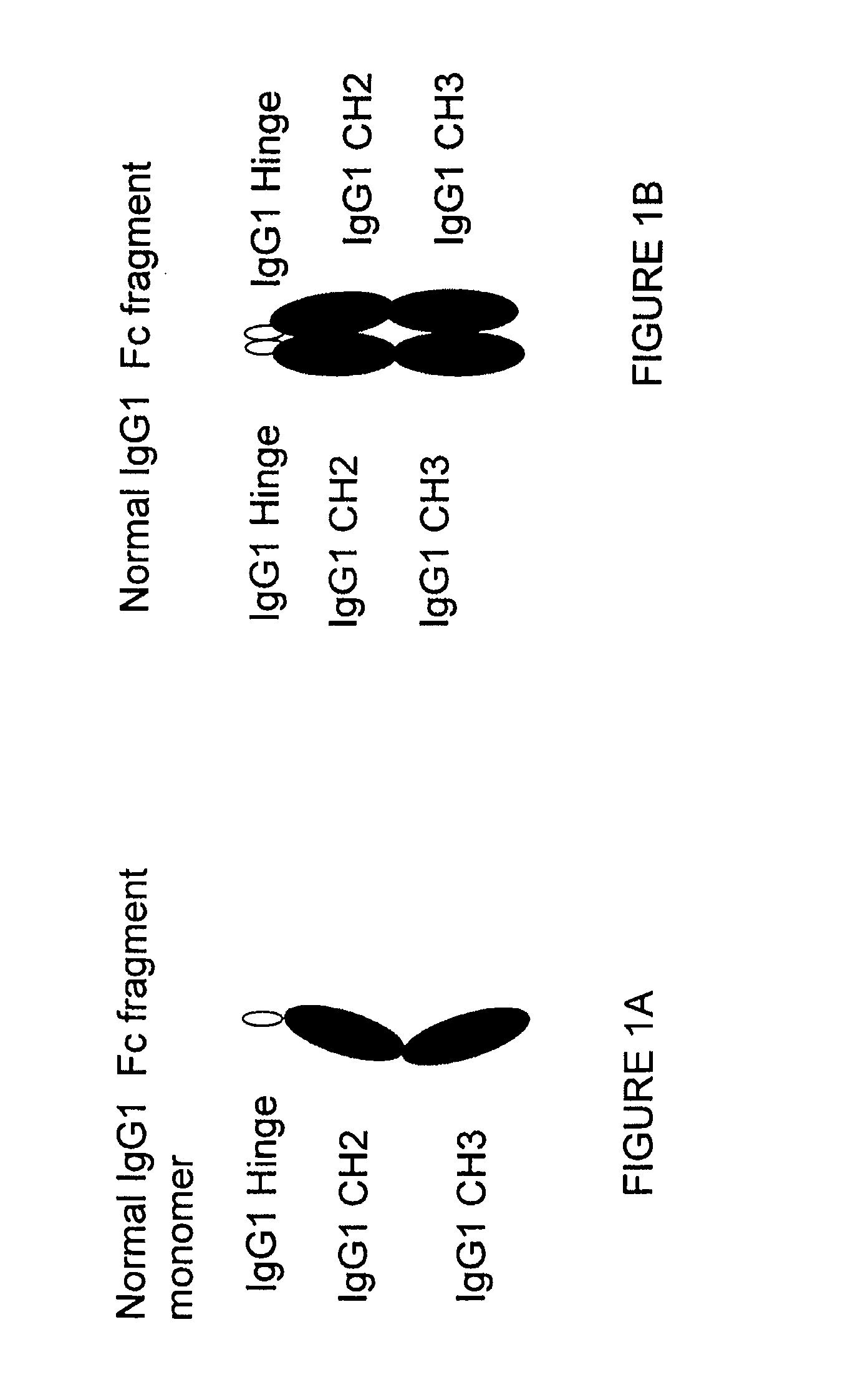
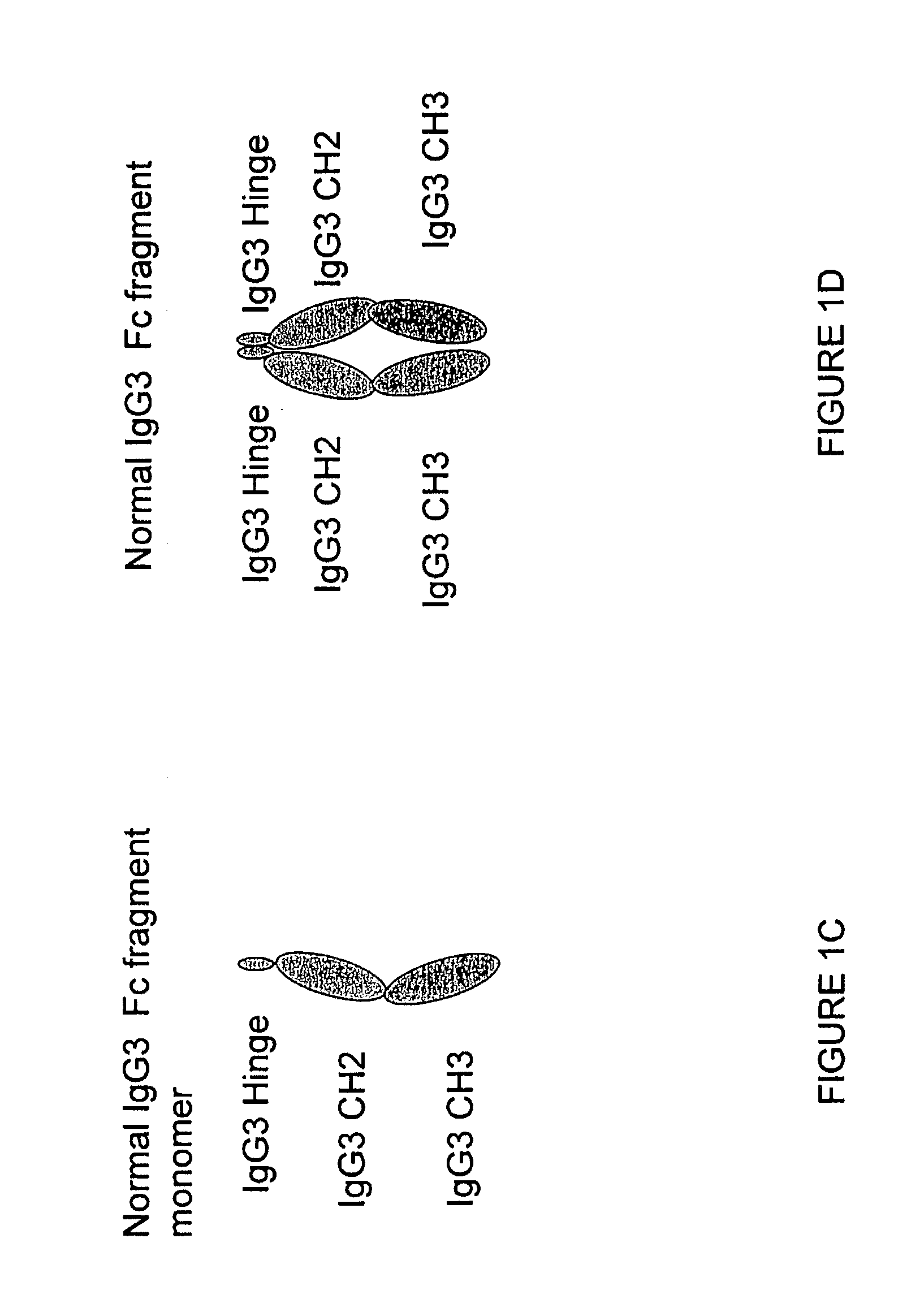
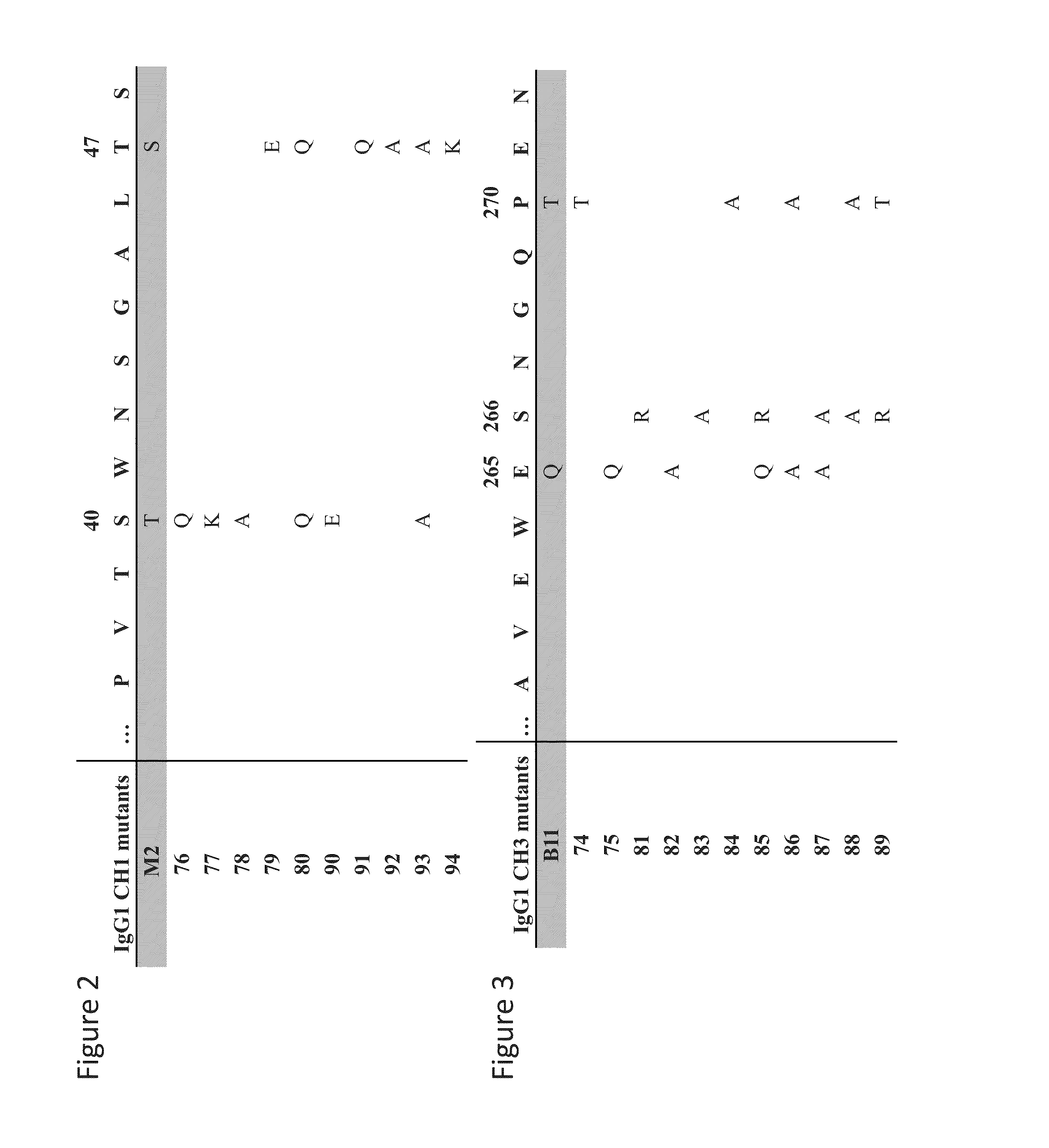
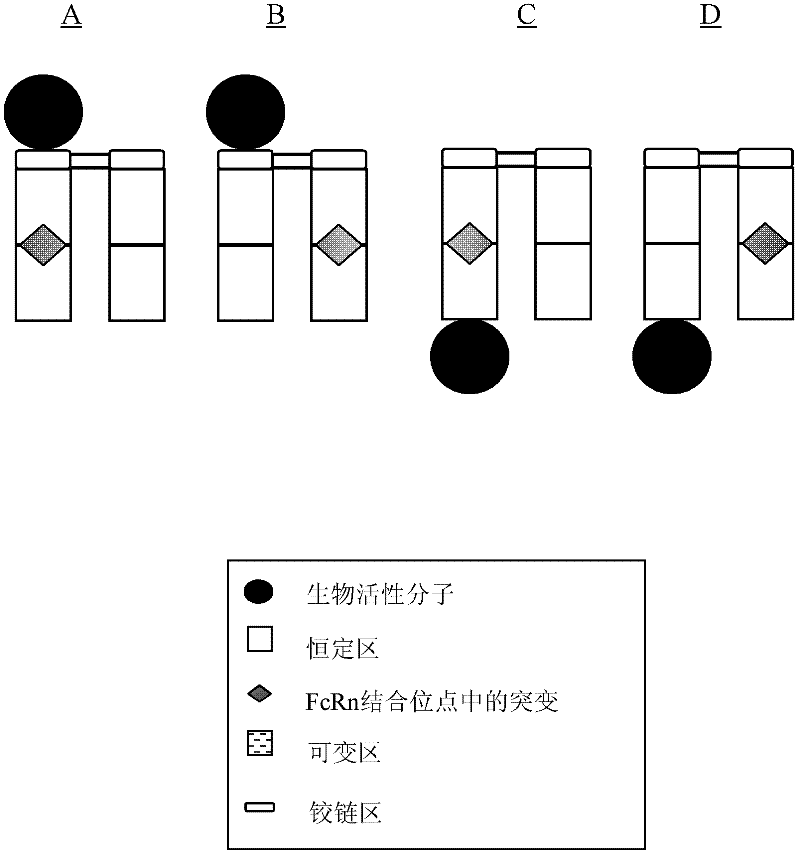
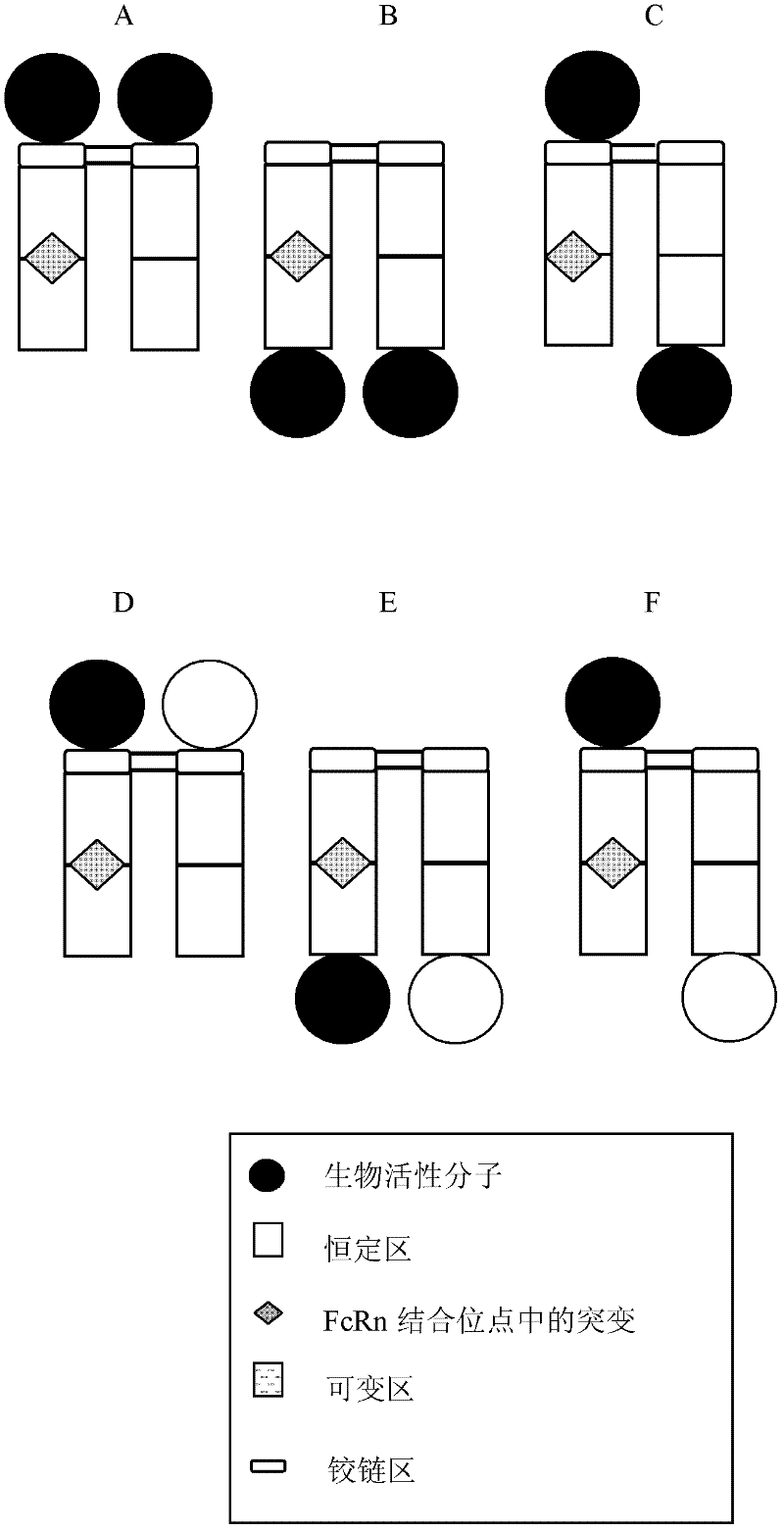
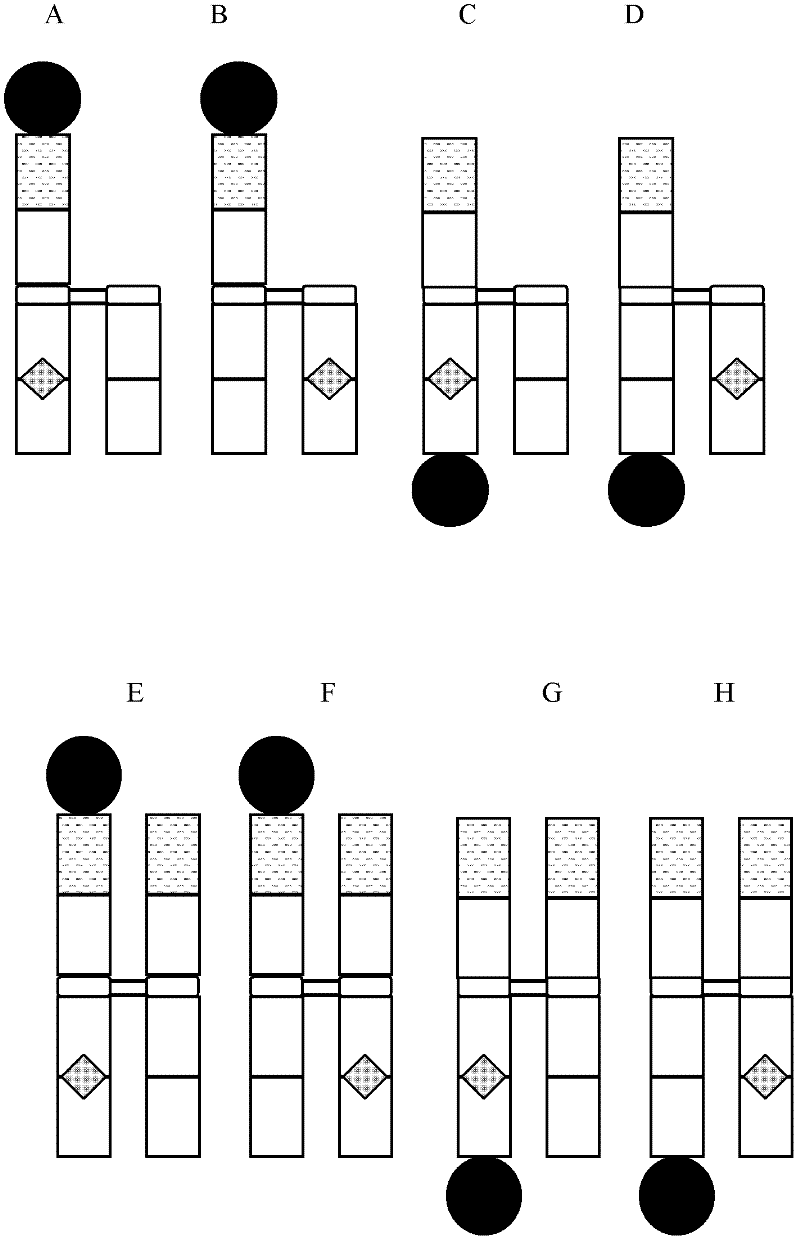
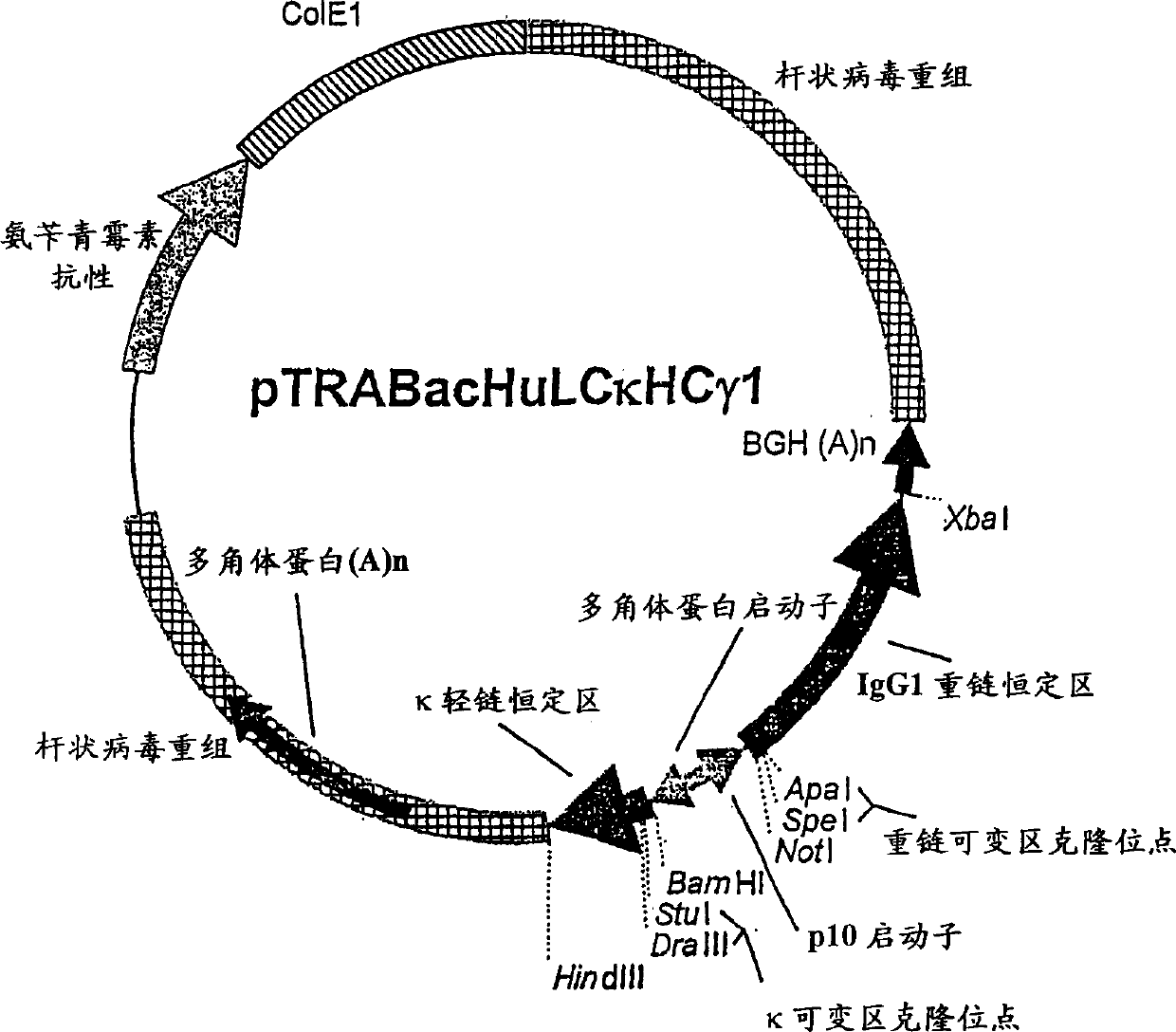
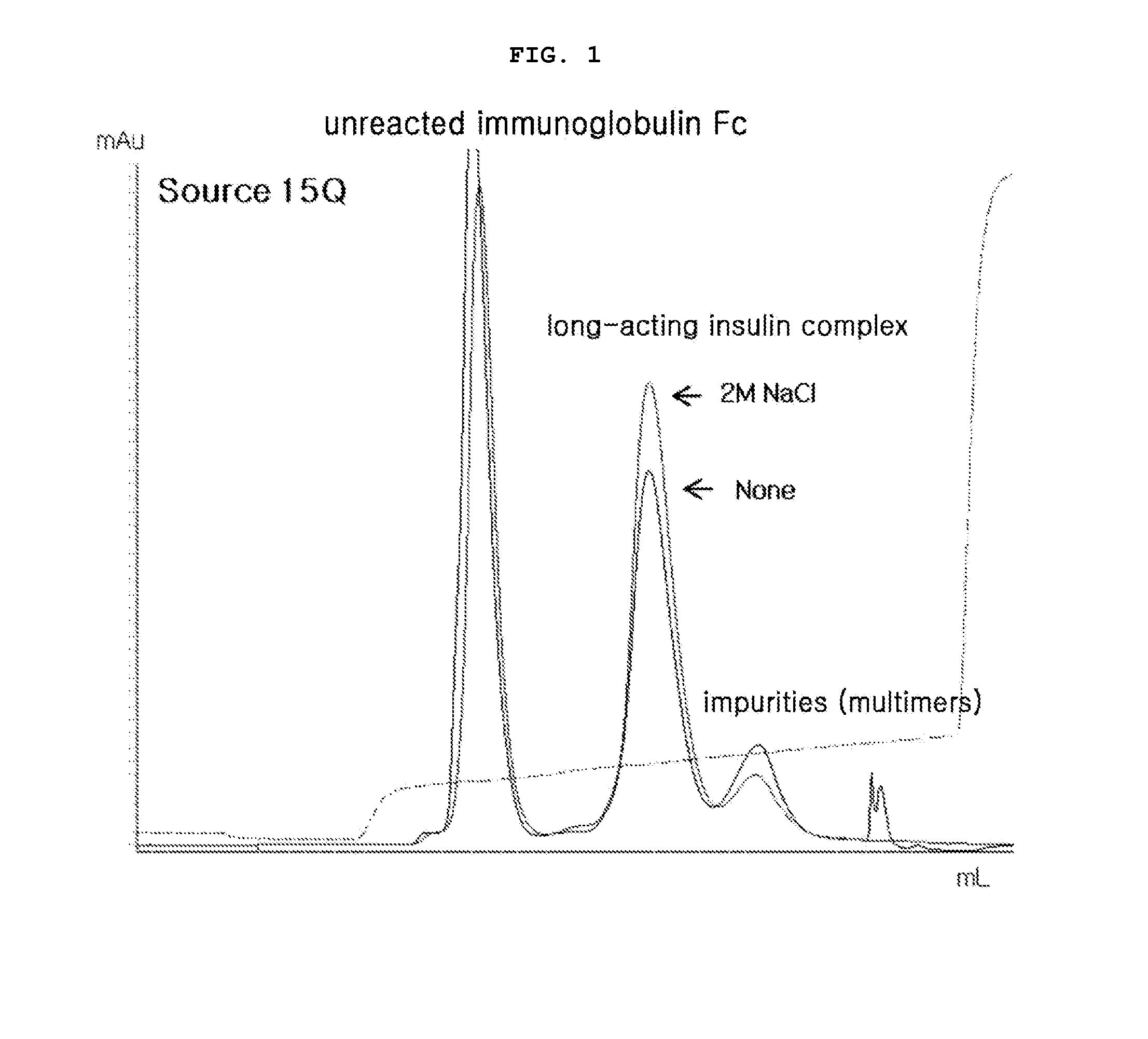
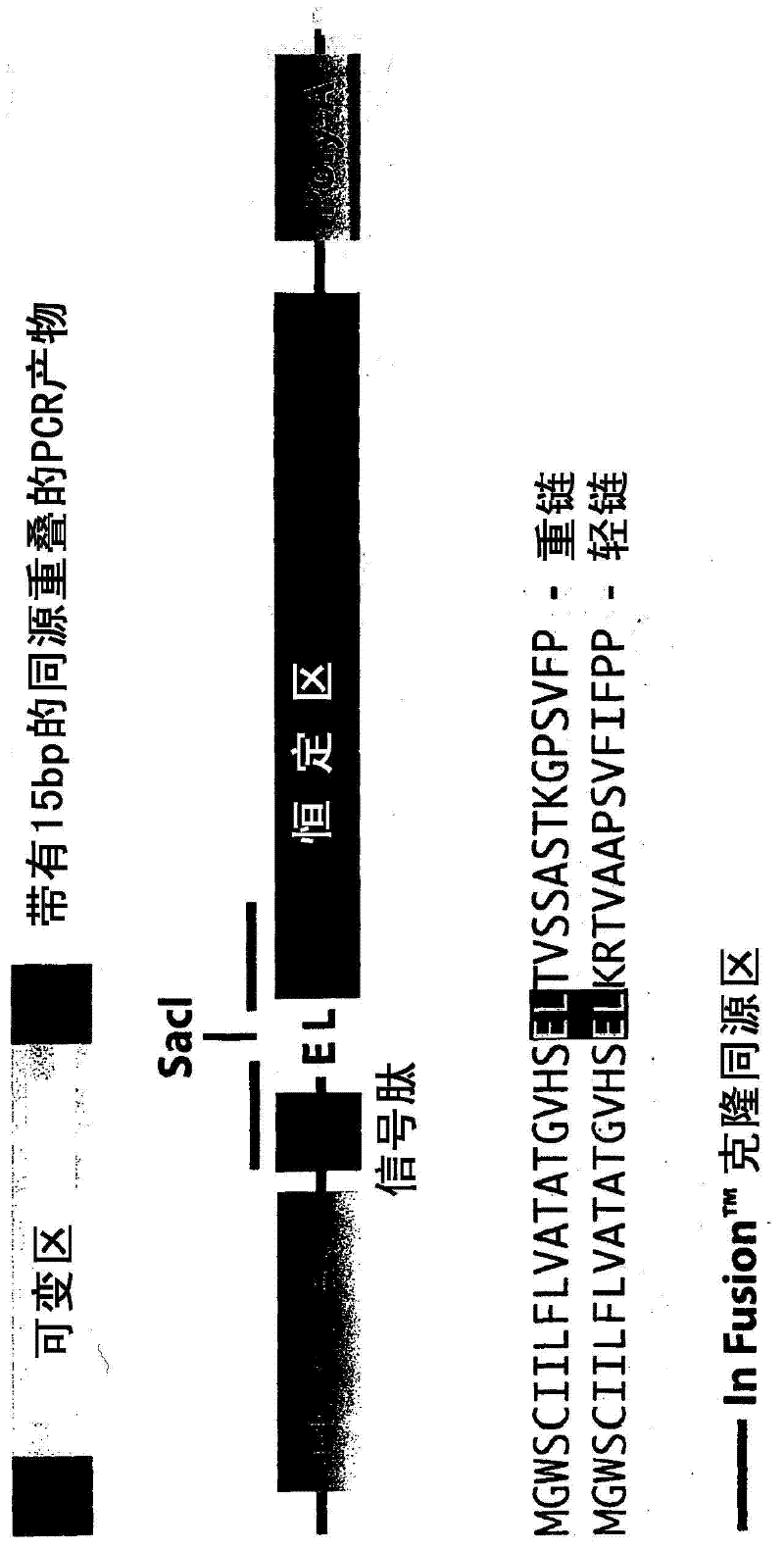
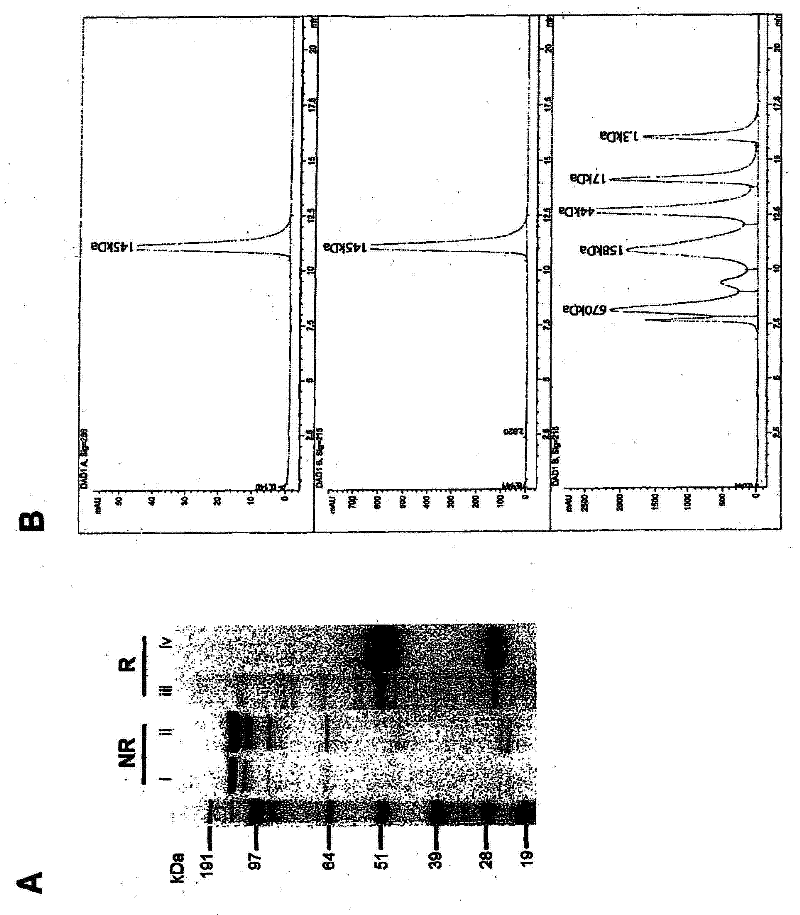
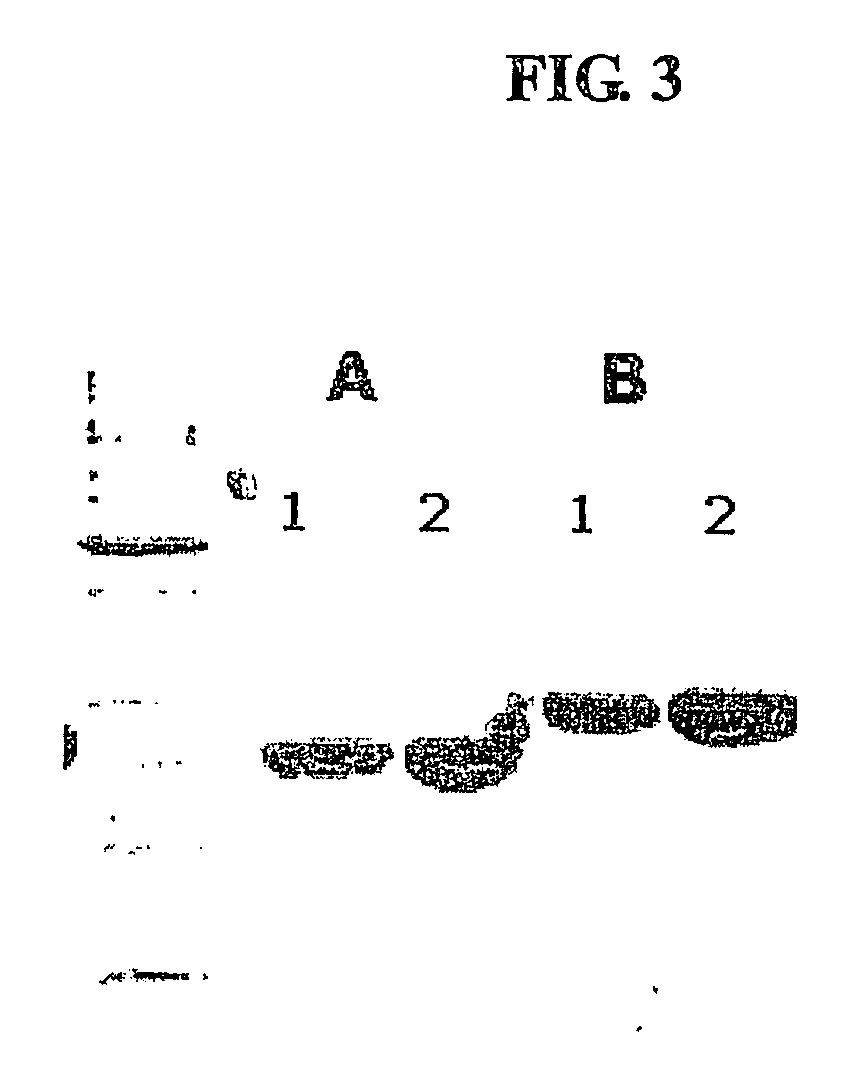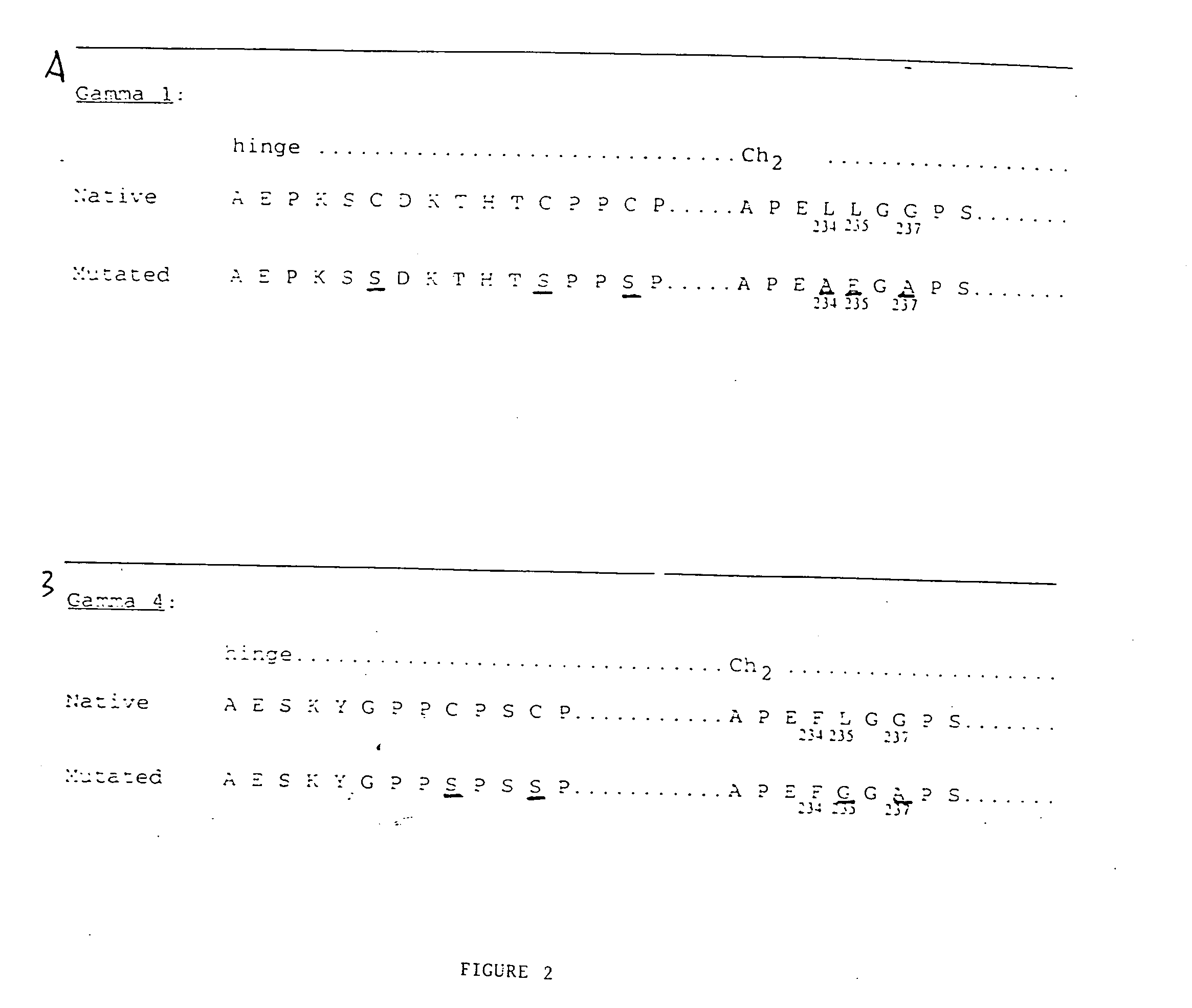Patents
Literature
39 results about "Immunoglobulin Constant Region" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
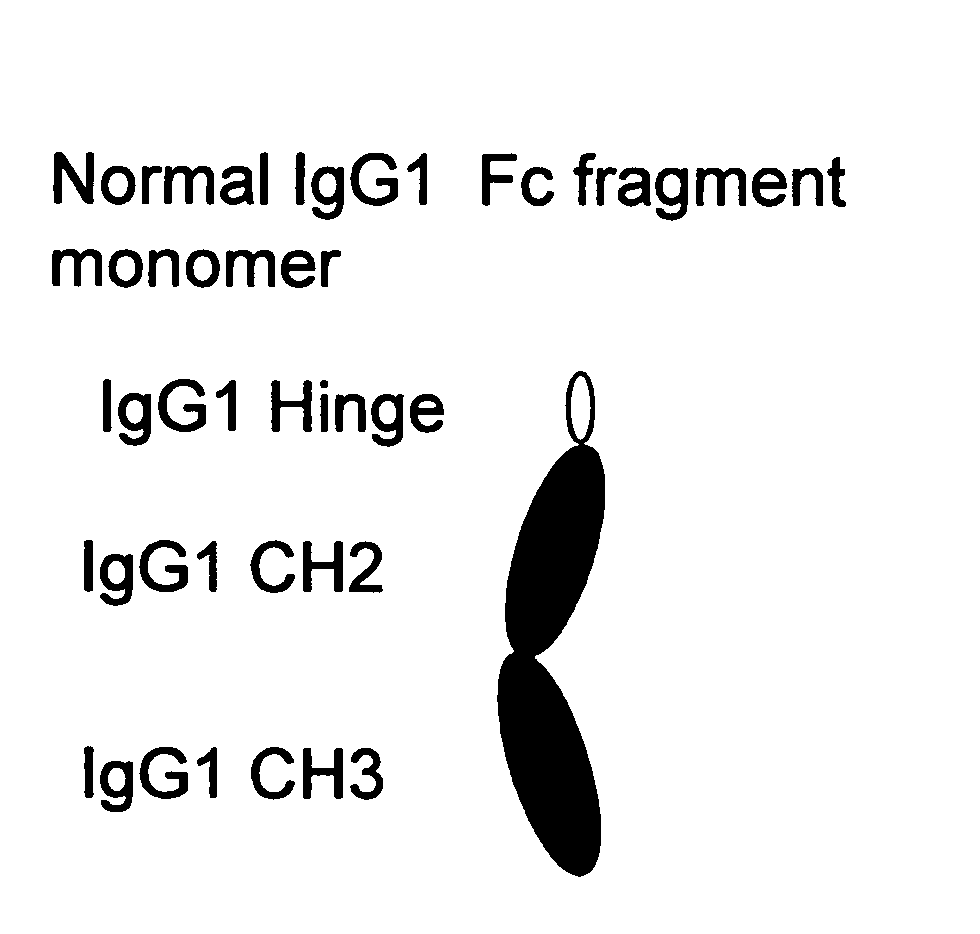
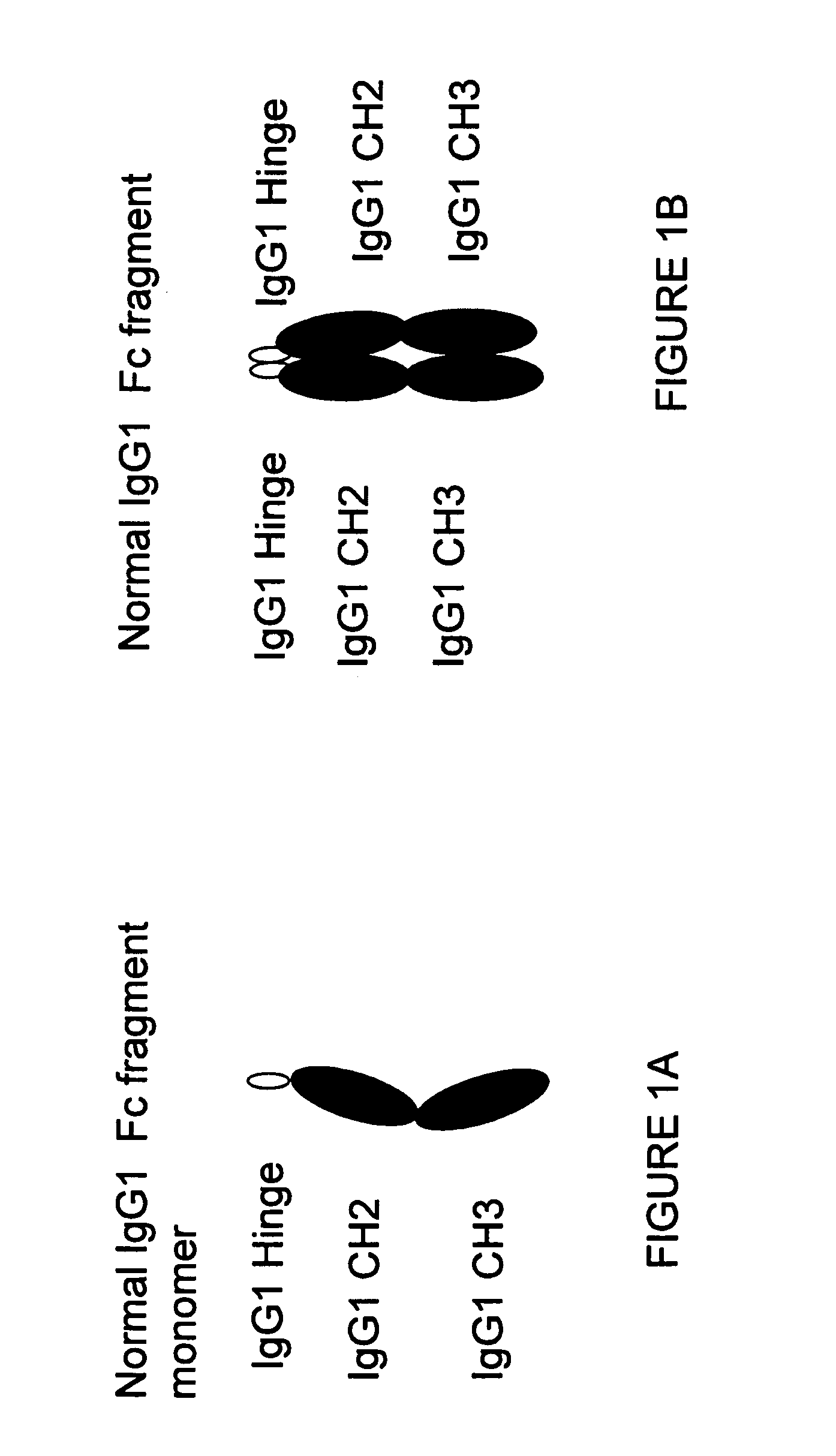
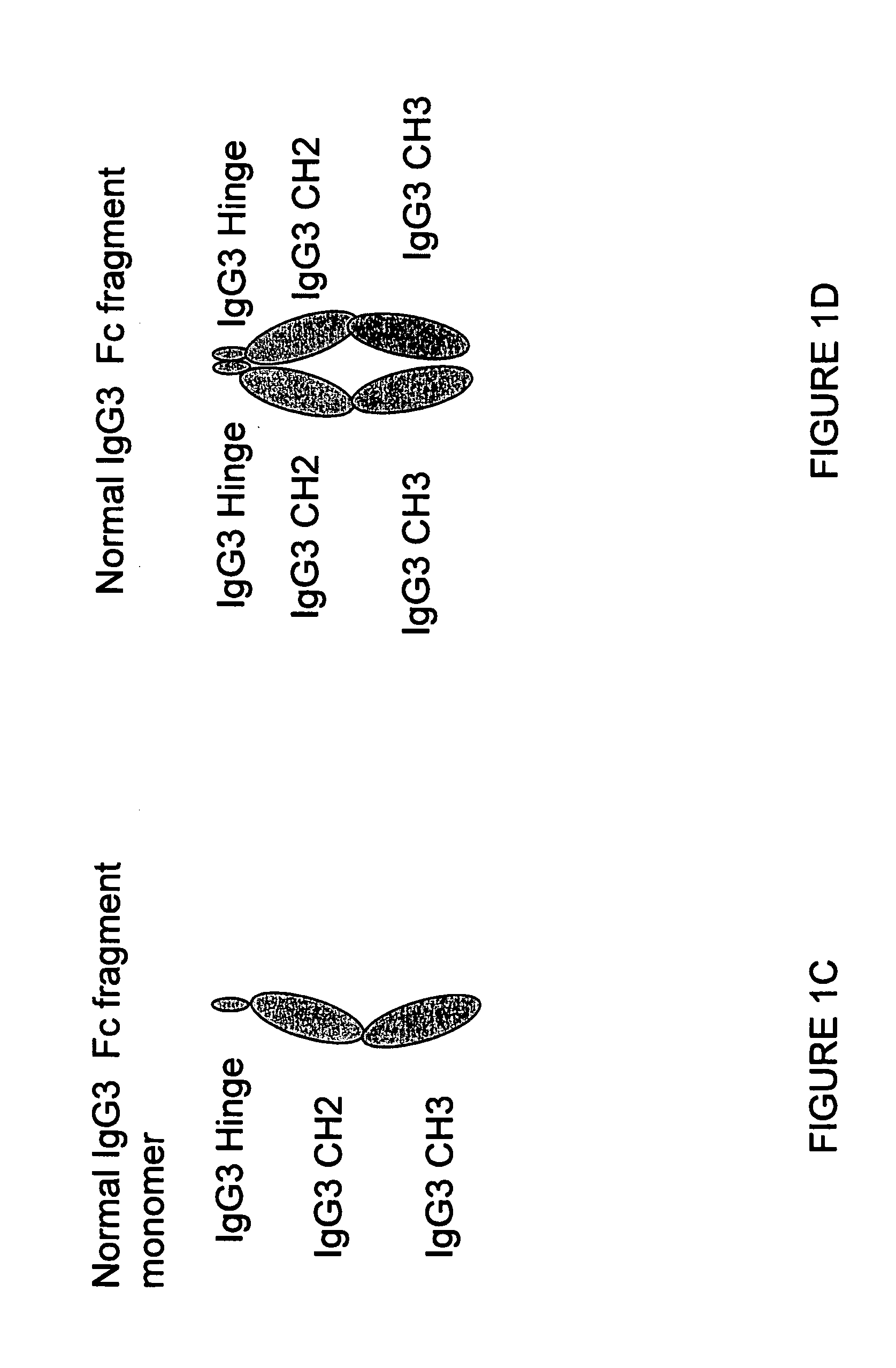
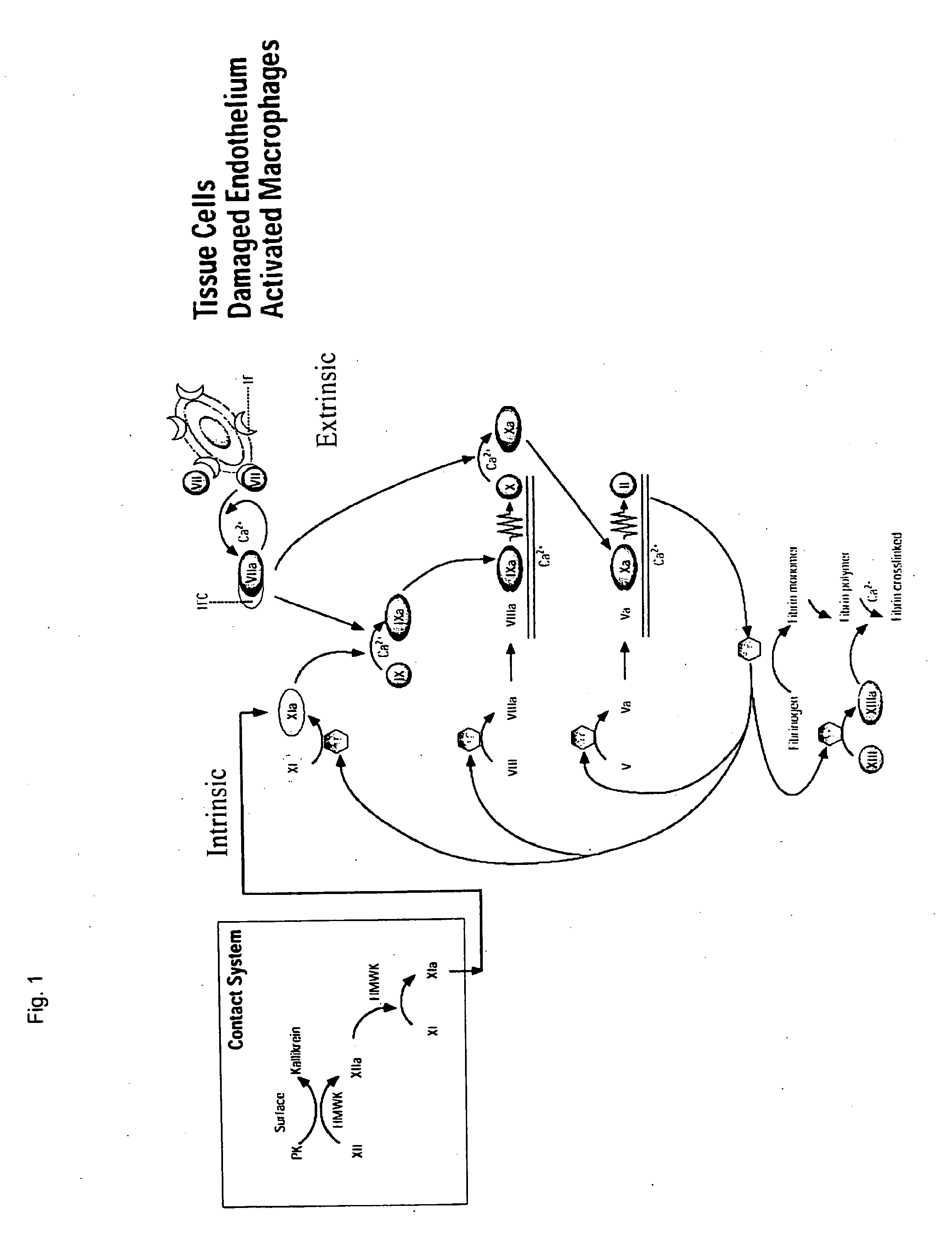
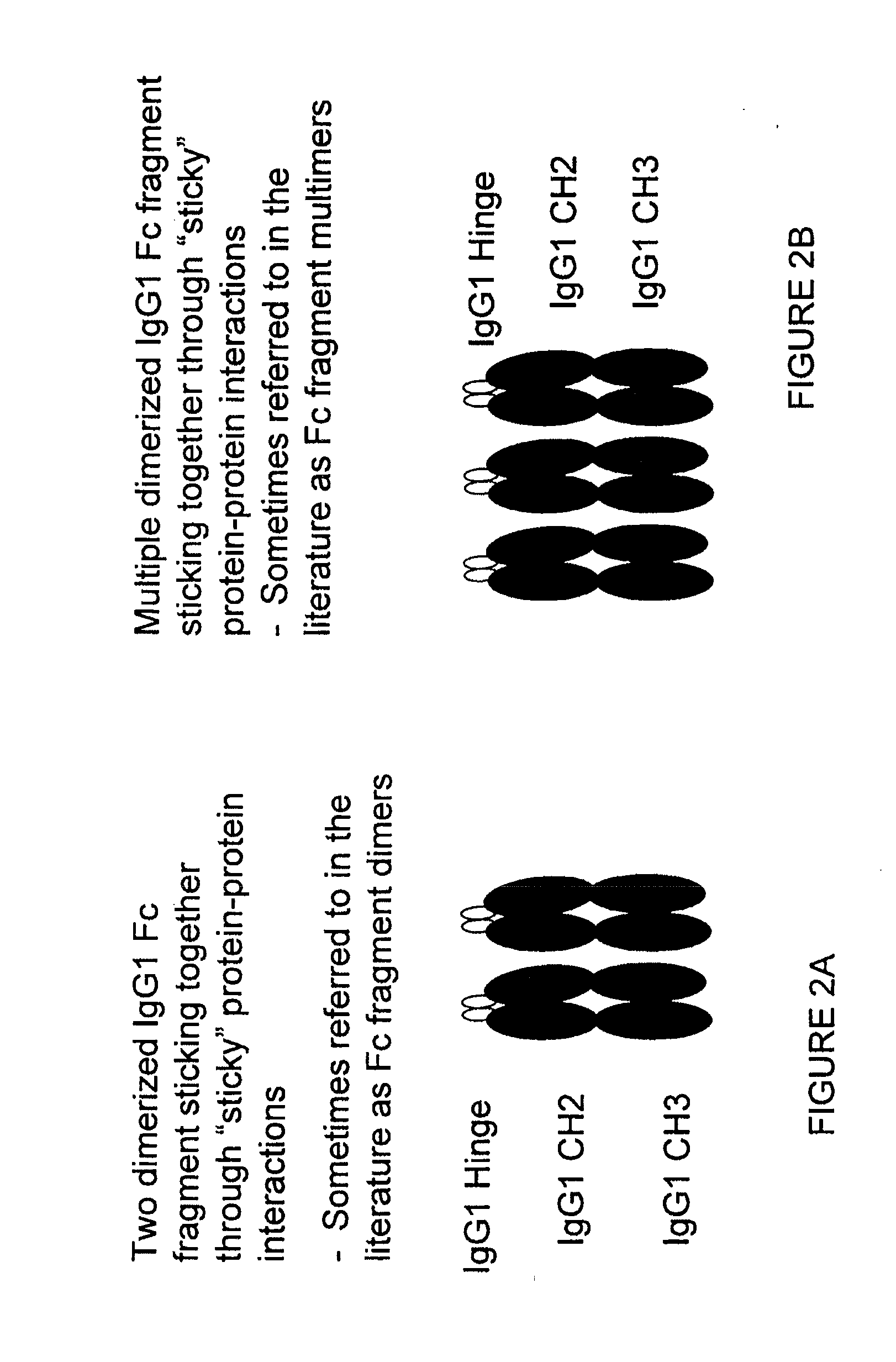
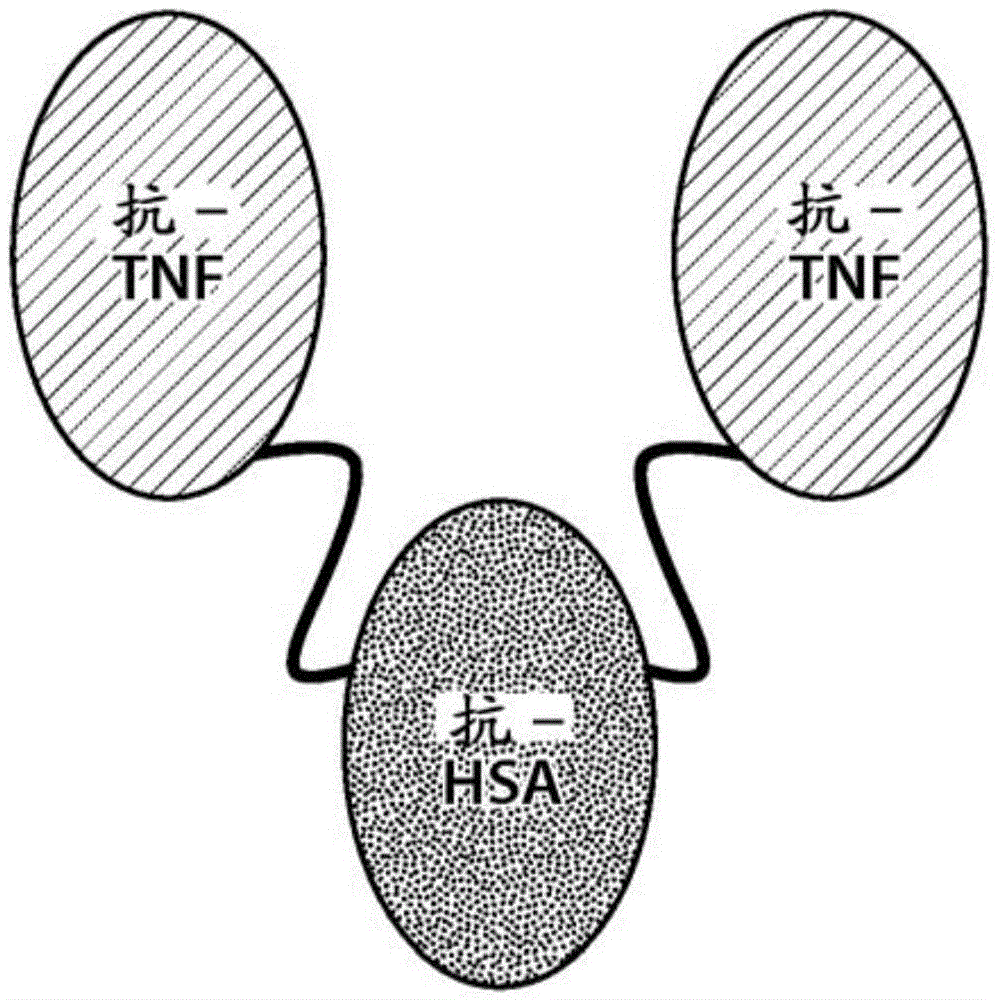
The portion of an antibody that is invariant within a class of immunoglobulins. It is comprised of the carboxyl terminal half of both light chains and at least three quarters of each heavy chain starting from the carboxyl terminus and excludes the variable regions of the heavy and light chains.
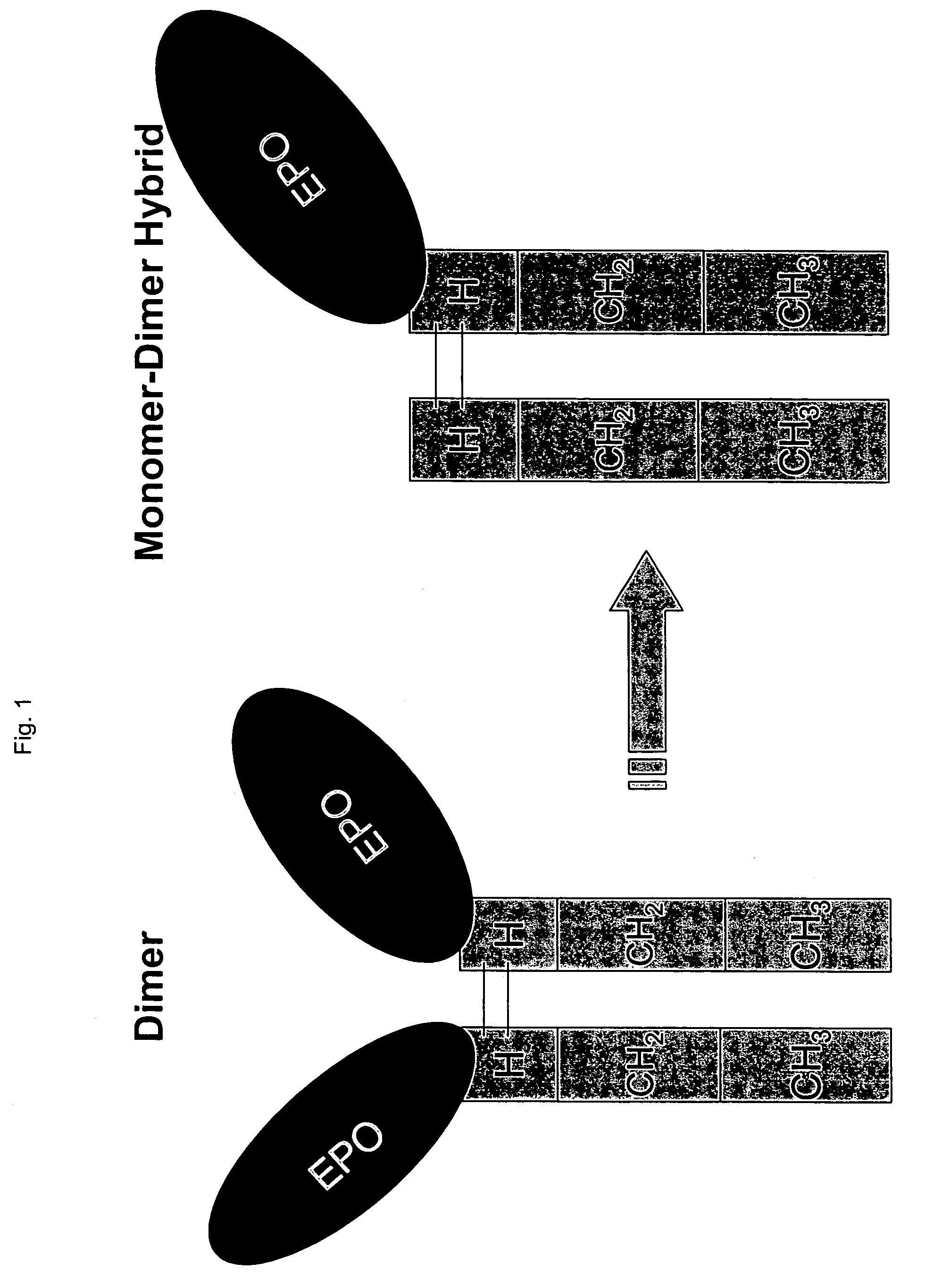
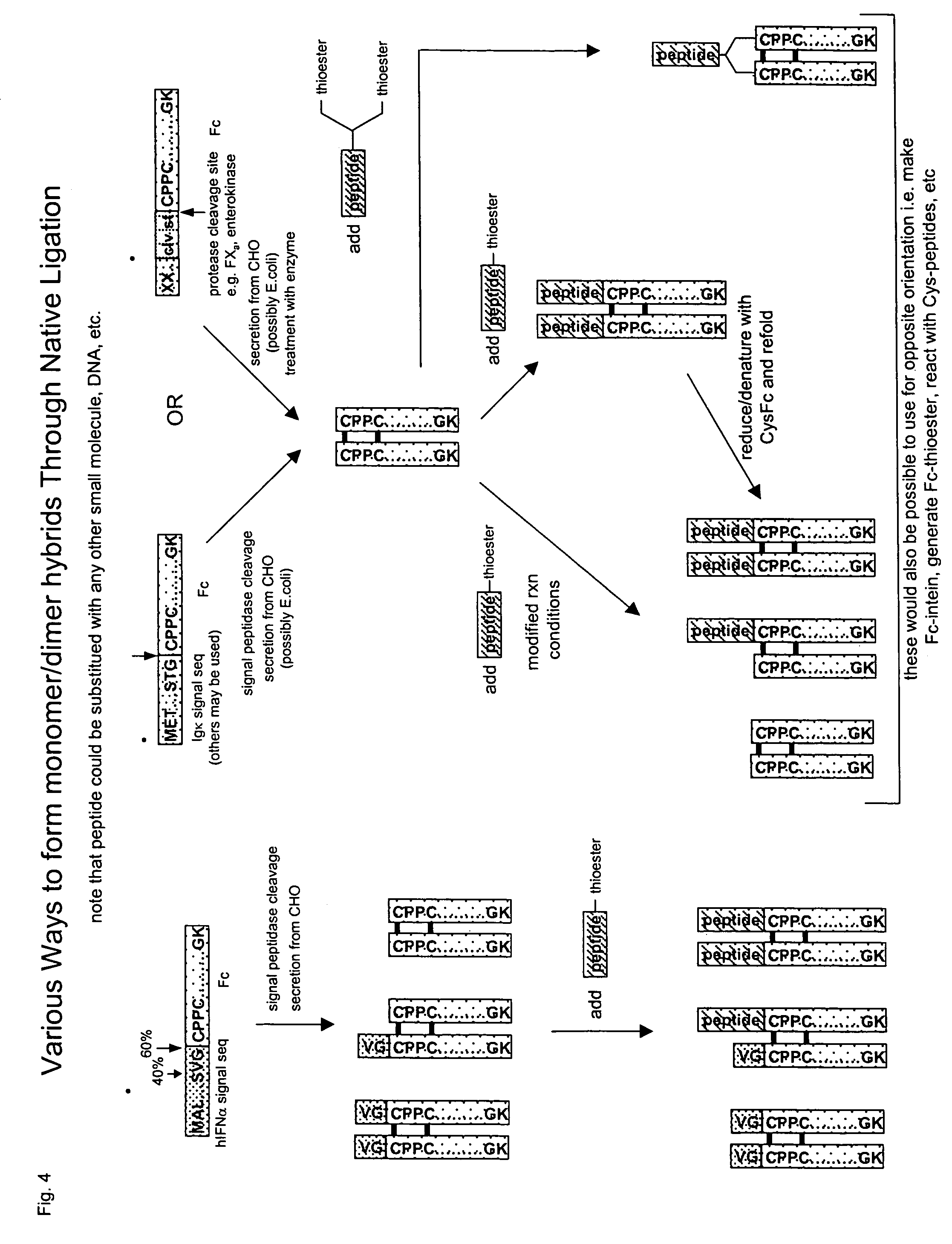
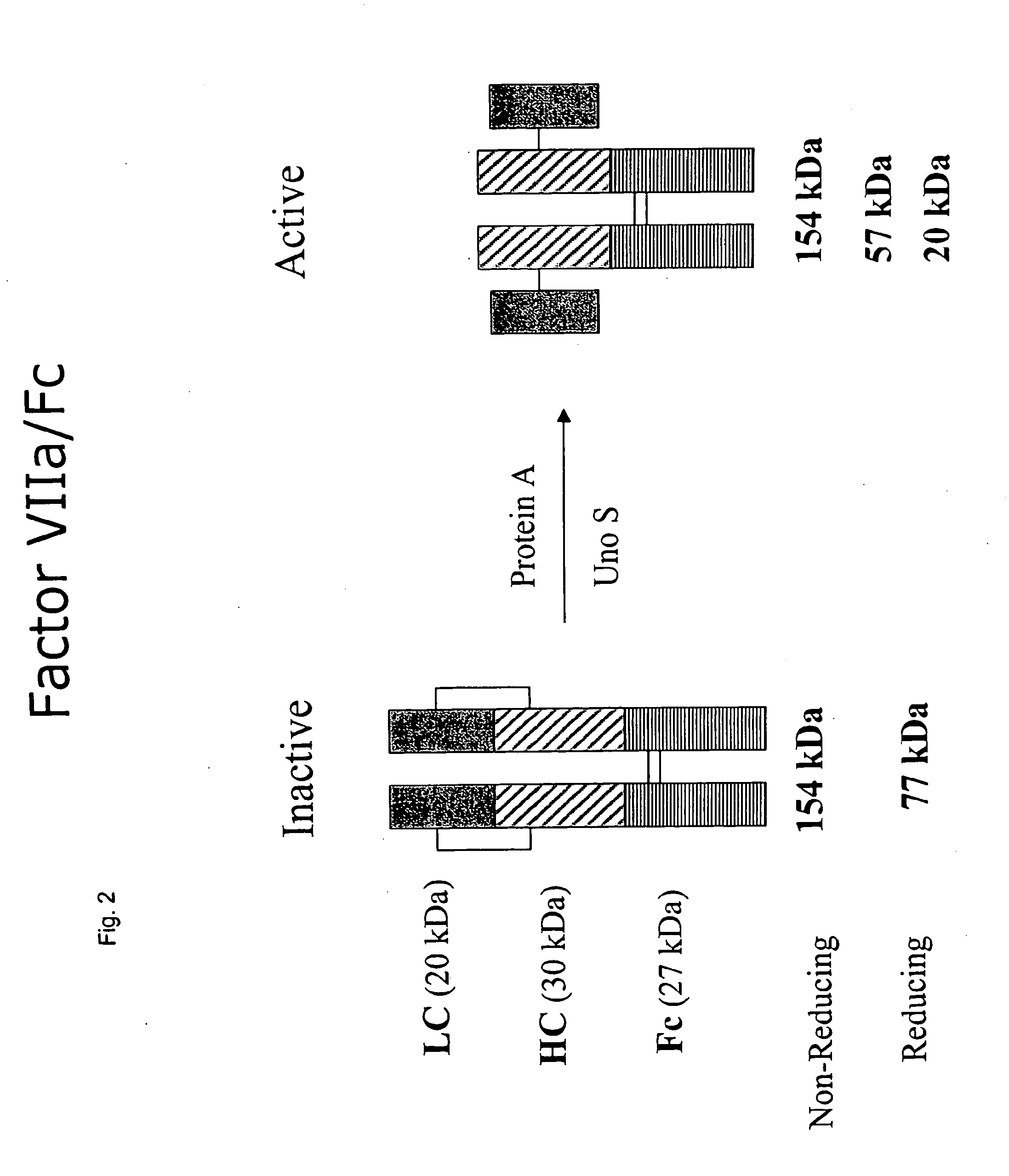
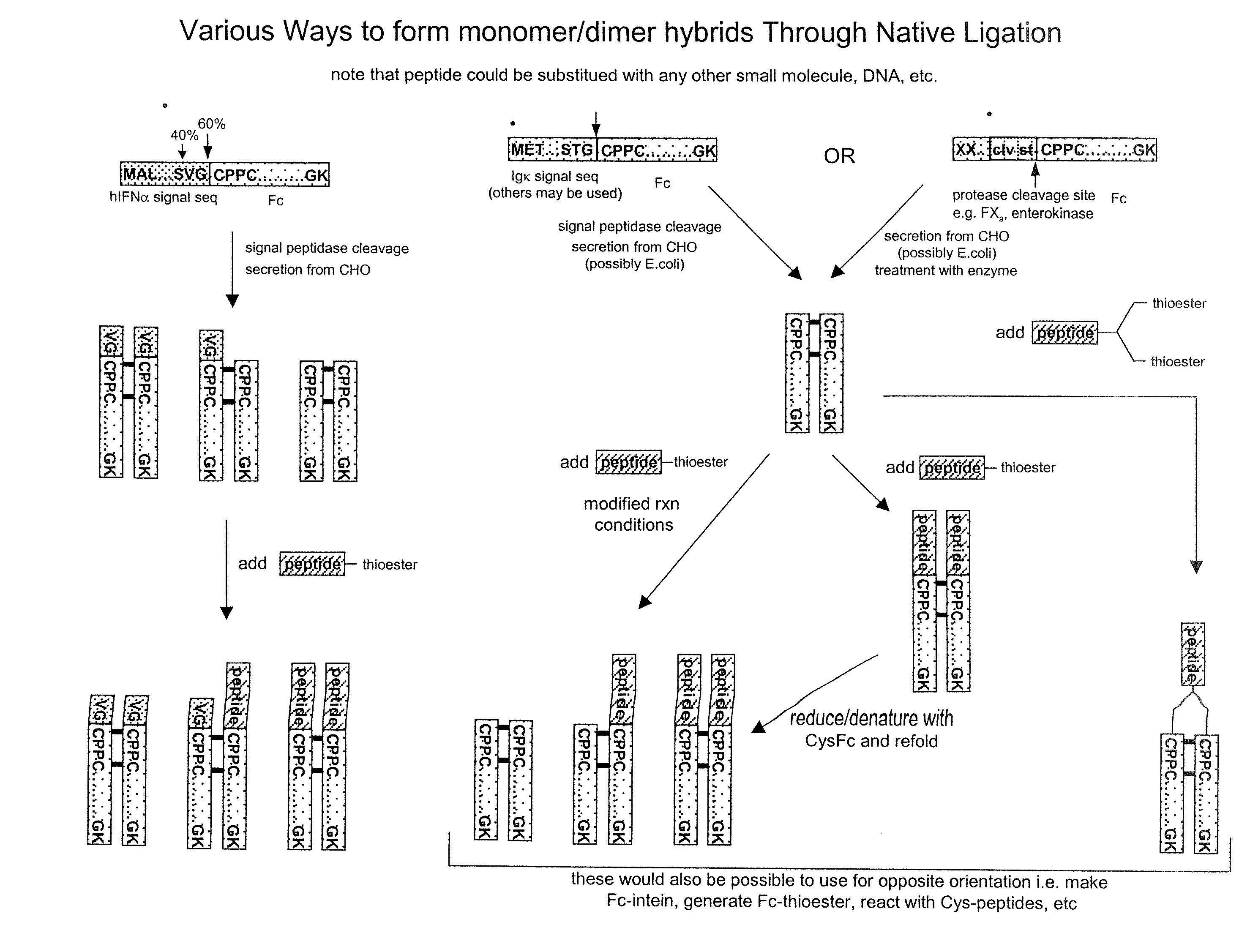
Methods for chemically synthesizing immunoglobulin chimeric proteins
ActiveUS7381408B2Prolong lifeEasy to purifyHydrolasesPeptide/protein ingredientsChimera ProteinBiochemistry
The invention provides methods of chemically synthesizing chimeric proteins comprising at least a portion of an immunoglobulin constant region and a biologically active molecule.
Owner:BIOVERATIV THERAPEUTICS INC
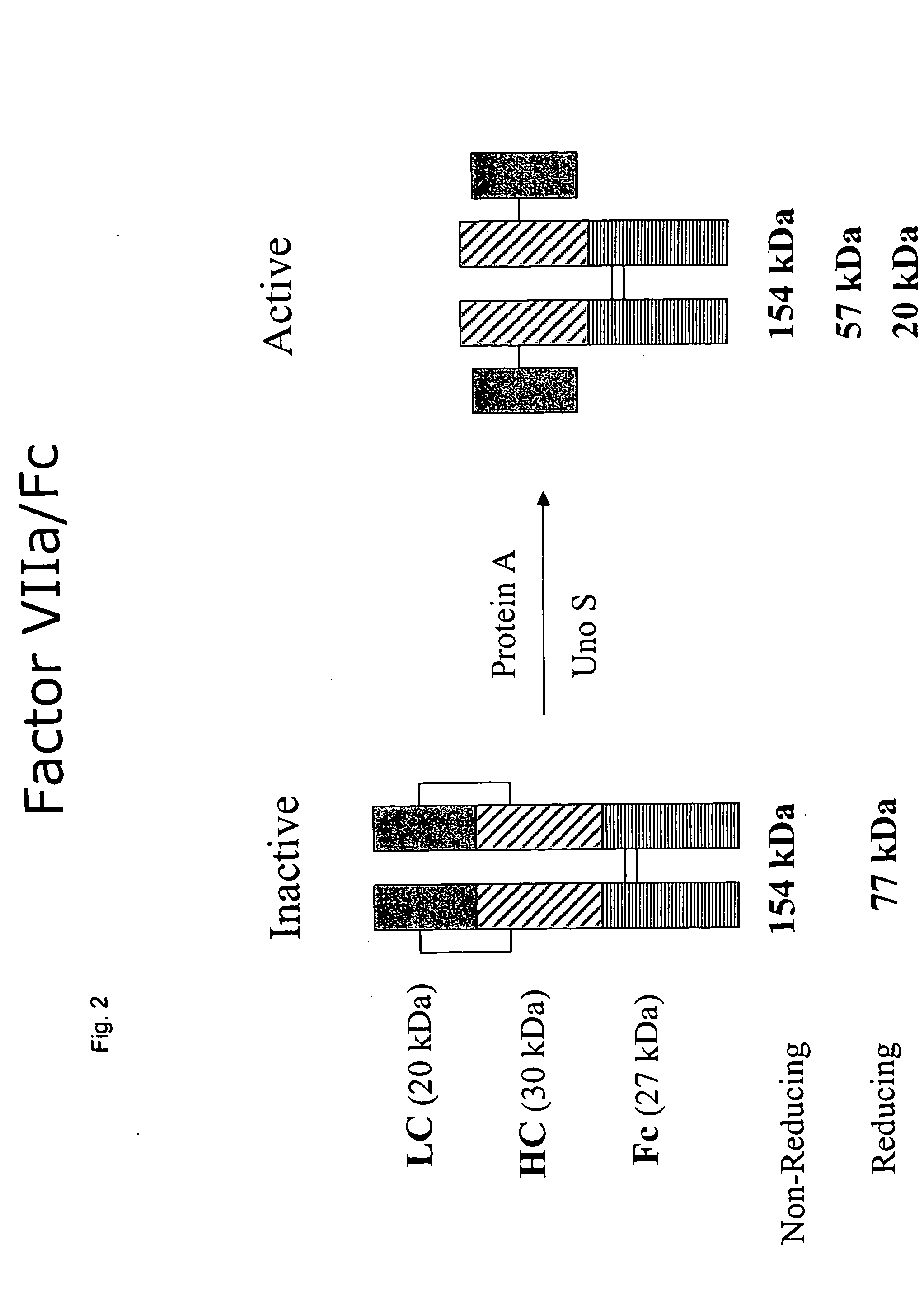
Clotting factor-Fc chimeric proteins to treat hemophilia
InactiveUS20050147618A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHemostatic DisordersChimera Protein
The invention relates to a chimeric protein comprising at least one clotting factor and at least a portion of an immunoglobulin constant region. The invention relates to a method of treating a hemostatic disorder comprising administering a therapeutically effective amount of a chimeric protein wherein the chimeric protein comprises at least one clotting factor and at least a portion of an immunoglobulin constant region.
Owner:BIOVERATIV THERAPEUTICS INC
Immunoglobulin constant region fc receptor binding agents
ActiveUS20100239633A1Wide applicationPathological conditionAntibacterial agentsAntibody mimetics/scaffoldsDiseaseFc(alpha) receptor
IVIG replacement compounds are derived from recombinant and / or biochemical creation of immunologically active biomimetic(s). These replacement compounds are then screened in vitro to assess each replacements compound's efficiency at modulating immune function. Particular replacement compounds are selected for further in vivo validation and dosage / administration optimization. Finally, the replacement compounds are used to treat a wide range of diseases, including inflammatory and autoimmune diseases.
Owner:GLIKNIK +1
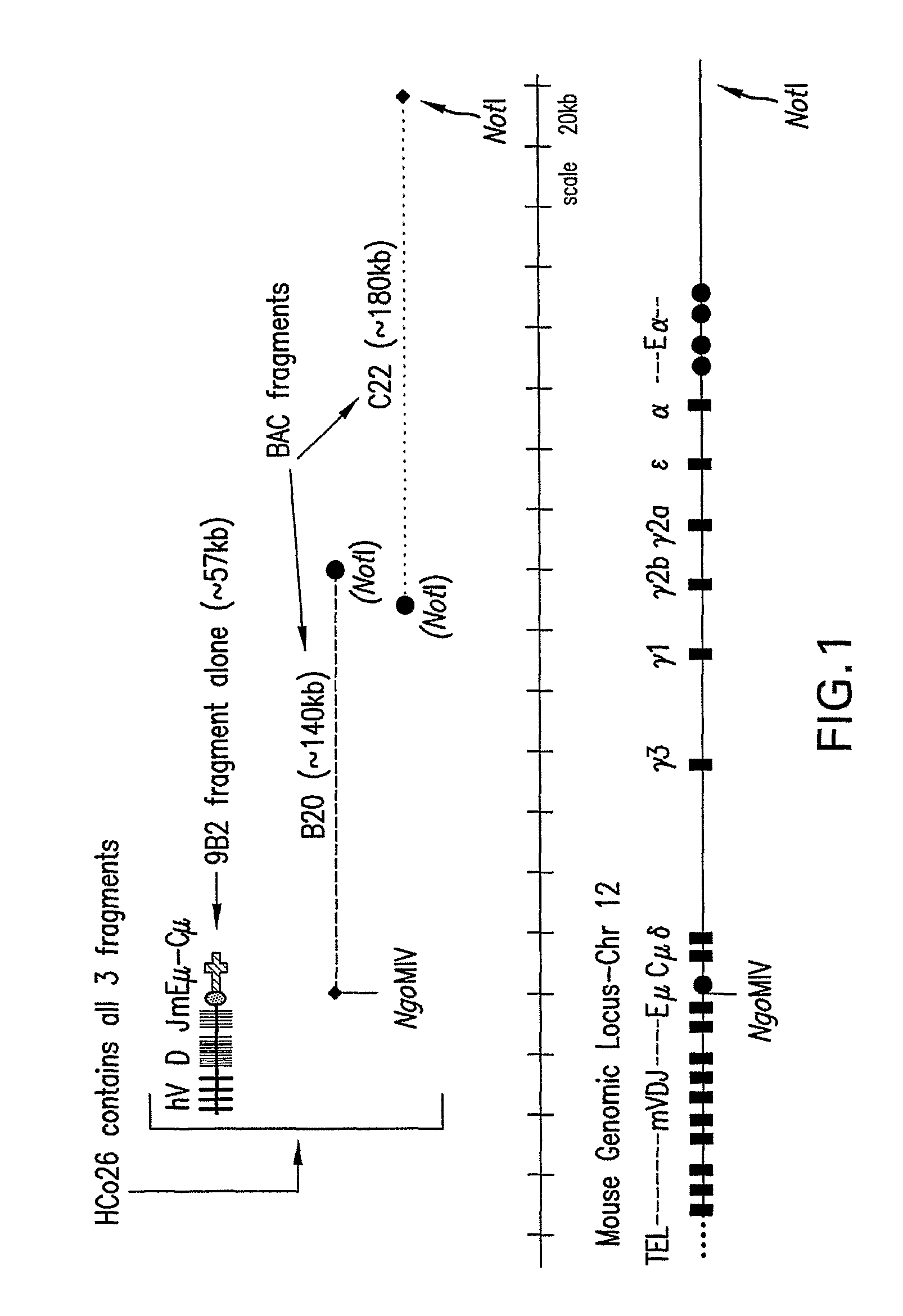
Transgenic animals expressing chimeric antibodies for use in preparing human antibodies
ActiveUS7910798B2Improved trafficking developmentStrengthen associationSugar derivativesImmunoglobulins against cytokines/lymphokines/interferonsTransgenesisIn vivo
The invention provides transgene constructs for expressing chimeric antibodies, and transgenic non-human host animals carrying such constructs, wherein the chimeric antibodies comprise human variable regions and constant regions of the non-human transgenic host animal. The presence of immunoglobulin constant regions of the host animal allows for generation of improved antibodies in such transgenic host animals. Subsequently, the chimeric antibodies can be readily converted to fully human antibodies using recombinant DNA techniques. Thus, the invention provides compositions and methods for generating human antibodies in which chimeric antibodies raised in vivo in transgenic mice are used as intermediates and then converted to fully human antibodies in vitro.
Owner:ER SQUIBB & SONS INC
Type II TGF-beta receptor/immunoglobulin constant region fusion proteins
InactiveUS20050203022A1Prevent overproductionPreventing postradiation fibrosisFungiSenses disorderDiseaseImmunoglobulin Constant Region
Owner:BIOGEN MA INC
CLOTTING FACTOR-Fc CHIMERIC PROTEINS TO TREAT HEMOPHILIA
ActiveUS20110182896A1Reduce riskEffective treatmentPeptide/protein ingredientsHydrolasesHemostatic DisordersImmunoglobulin IgE
Owner:BIOVERATIV THERAPEUTICS INC
Method for the mass production of immunoglobulin constant region
ActiveUS20060276633A1Good curative effectLow costBacteriaAntibody mimetics/scaffoldsEscherichia coliWater soluble
Disclosed are a recombinant expression vector comprising a nucleotide sequence encoding an E. coli-derived signal sequence and a nucleotide sequence encoding an immunoglobulin constant region, and a transformant transformed with the expression vector. Also, disclosed is a method of mass-producing an immunoglobulin constant region by culturing the transformant and expressing the immunoglobulin constant region in a water-soluble form.
Owner:HANMI SCI CO LTD
Fc chimeric proteins with anti-HIV drugs
InactiveUS20050281829A1Improve stabilityStable HIV chimeric proteinsPeptide/protein ingredientsAntibody mimetics/scaffoldsAntiviral drugImmunoglobulin Fc Fragments
The invention relates to anti viral agents comprised of viral fusion inhibitors and at least a portion of an immunoglobulin constant region. The invention further relates to anti viral agents comprised HIV viral fusion inhibitors and an Fc fragment of an immunoglobulin. The invention also relates to methods of treating a viral infection, including HIV infection.
Owner:HEHIR CRISTINA +5
Method and composition for altering a B cell mediated pathology
InactiveUS20050238645A1BiocidePeptide/protein ingredientsAntibody AffinitiesKeyhole-limpet haemocyanin
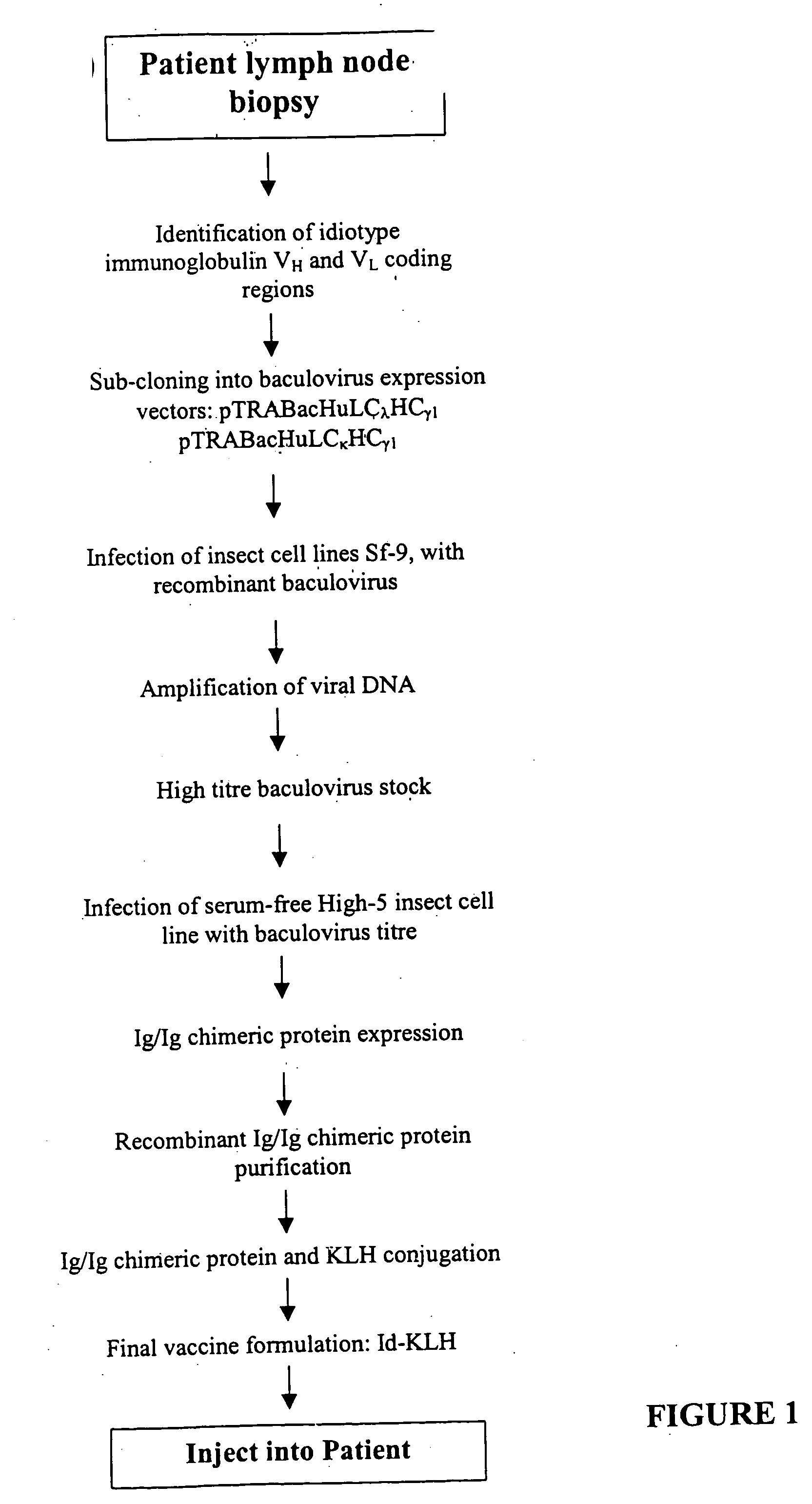
The present invention provides a method for altering a B cell mediated pathology in a patient. This method comprises administering a composition comprising at least one and / or two chimeric proteins. Each chimeric protein comprises at least a portion of either the VH or VL region of a immunoglobulin molecule from particular B cells from a patient having a B cell mediated pathology, and an immunoglobulin constant region. The genes encoding VH and / or VL regions and the genes encoding immunoglobulin constant regions are isolated and inserted into an expression vector. The chimeric proteins are produced by introducing the expression vectors into insect cell lines. The chimeric proteins are purified using antibody affinity columns, and then chemically conjugated to an immunogenic carrier, keyhole-limpet hemocyanin (KLH). Since the conjugates comprise chimeric proteins made specifically from particular B cells from a patient having B cell mediated pathology, when it is administered to such a patient, with or without a cytokine, such as granulocyte-macrophage-CSF, or a chemokine, it can induce immune responses to alter such a B cell mediated pathology.
Owner:MMRGLOBAL
CTLA4-Cy4 fusion proteins
CTLA4-immunoglobulin fusion proteins having modified immunoglobulin constant region-mediated effector functions, and nucleic acids encoding the fusion proteins, are described. The CTLA4-immunoglobulin fusion proteins comprise two components: a first peptide having a CTLA4 activity and a second peptide comprising an immunoglobulin constant region which is modified to reduce at least one constant region-mediated biological effector function relative to a CTLA4-IgG1 fusion protein. The nucleic acids of the invention can be integrated into various expression vectors, which in turn can direct the synthesis of the corresponding proteins in a variety of hosts, particularly eukaryotic cells. The CTLA4-immunoglobulin fusion proteins described herein can be administered to a subject to inhibit an interaction between a CTLA4 ligand (e.g., B7-1 and / or B7-2) on an antigen presenting cell and a receptor for the CTLA4 ligand (e.g., CD28 and / or CTLA4) on the surface of T cells to thereby suppress an immune response in the subject, for example to inhibit transplantation rejection, graft versus host disease or autoimmune responses.
Owner:REPLIGEN CORP
Immunoglobulin constant region fc receptor binding agents
InactiveUS20120309941A1Wide applicationPathological conditionAntibacterial agentsAntibody mimetics/scaffoldsFc(alpha) receptorAutoimmune condition
Owner:GLIKNIK +1
Methods for Chemically Synthesizing Immunoglobulin Chimeric Proteins
ActiveUS20080249288A1Longer in vivo halflifeEasy to purifyHydrolasesPeptide/protein ingredientsChemical synthesisImmunoglobulin Constant Region
The invention provides methods of chemically synthesizing chimeric proteins comprising at least a portion of an immunoglobulin constant region and a biologically active molecule.
Owner:BIOVERATIV THERAPEUTICS INC
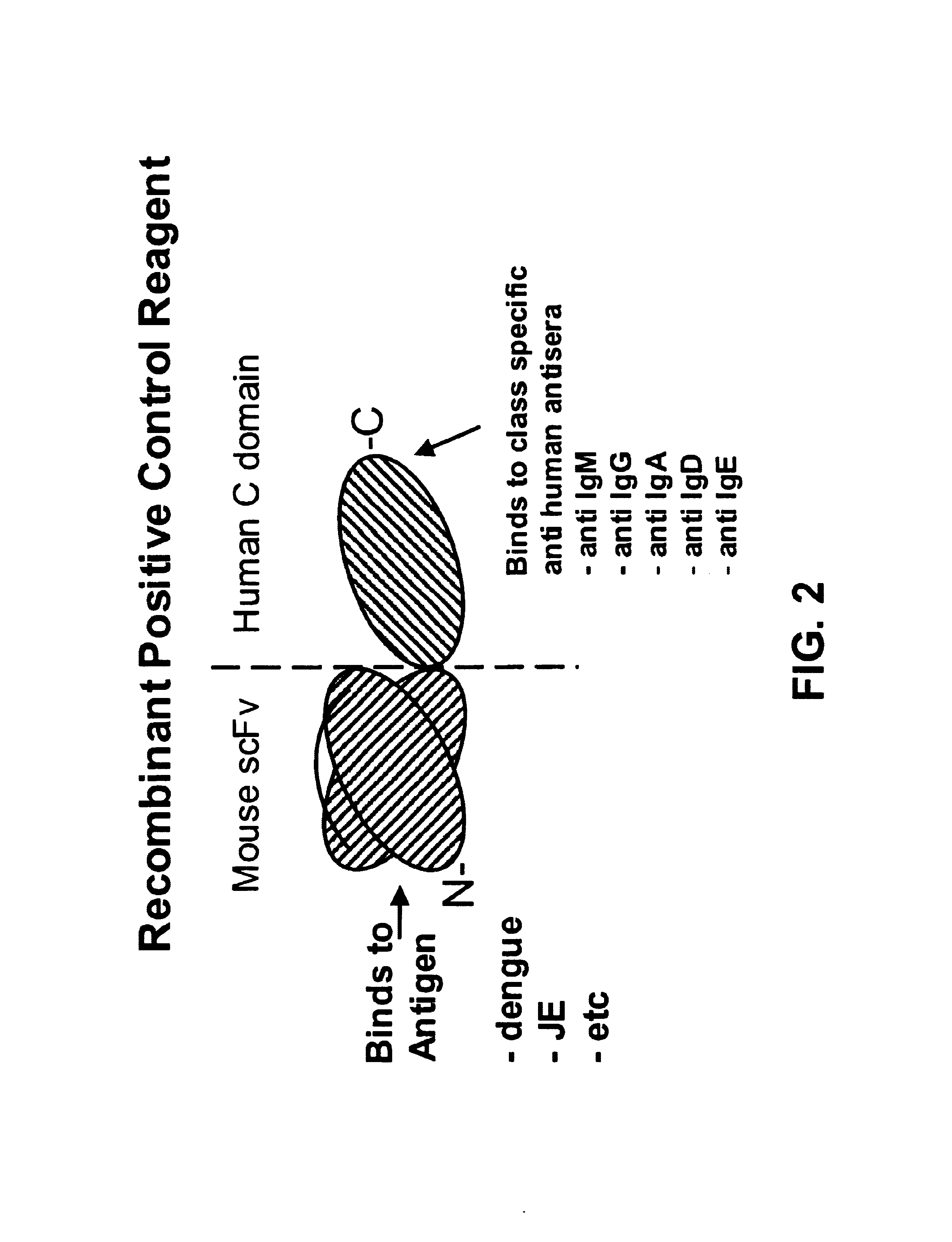
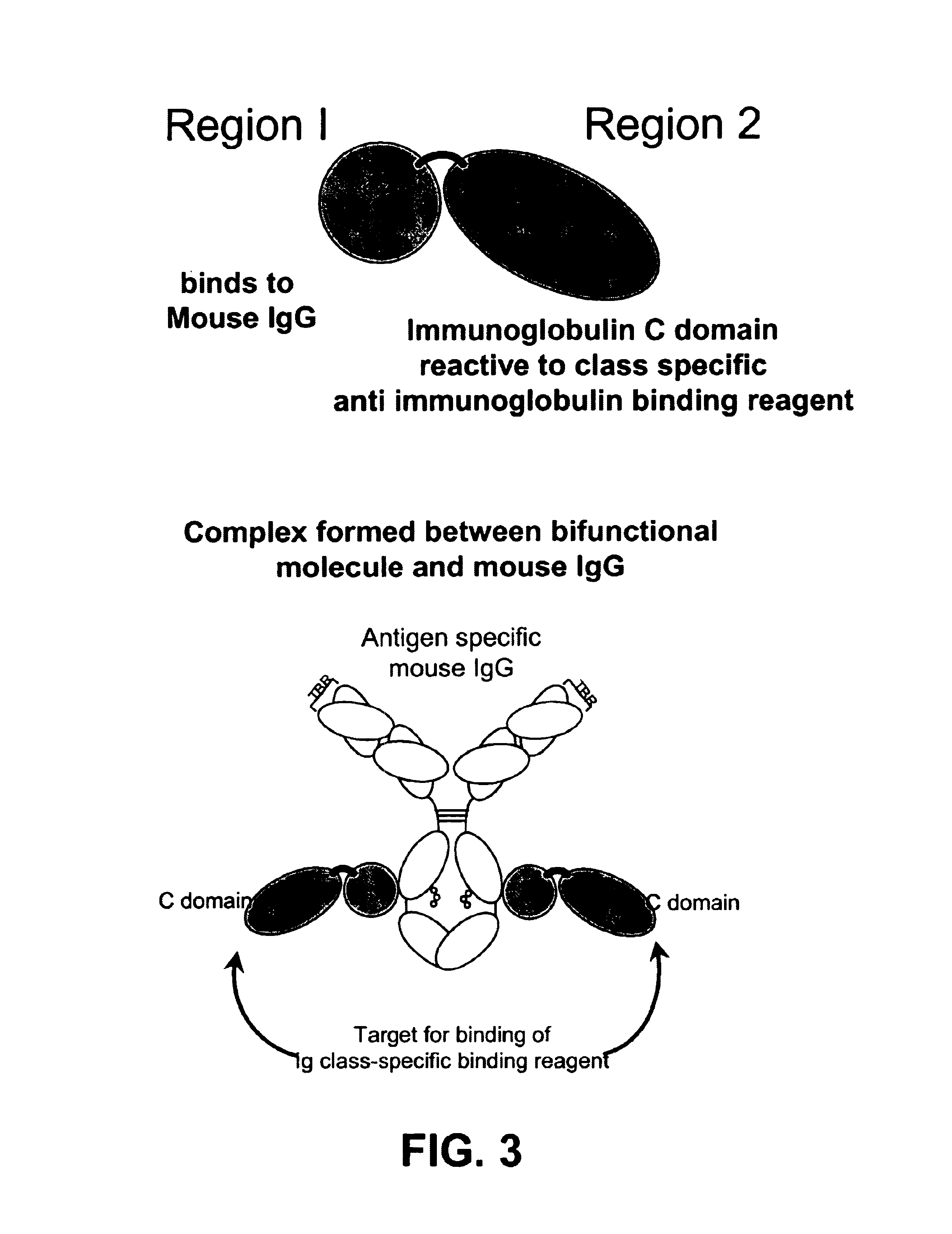
Bifunctional molecules
InactiveUS7026446B1Easy to produceAvoid inconvenienceAntibody mimetics/scaffoldsImmunoglobulins against bacteriaEpitopePositive control
The invention relates to a chimeric antibody conjugate comprising an antigen binding region of a non-human antibody and an immunoglobulin constant region which comprises at least one CH domain or epitope thereof, with the proviso that the constant region is not a naturally occurring FC fragment. A bifunctional molecule for use in labelling an antibody derived from a first species, the bifunctional molecule comprising a binding region which binds to the antibody of the first species or to one or more groups provided thereon, and a constant region derived from an antibody of a second species, the constant region comprising at least one CH domain or an epitope thereof. The present invention relates to bifunctional molecules and complexes which are useful as positive control reagents in antibody based diagnostic tests. The present invention also relates to polynucleotides encoding these bifunctional molecules, and to diagnostic assays involving the use of these molecules.
Owner:DIATECH PTY LTD
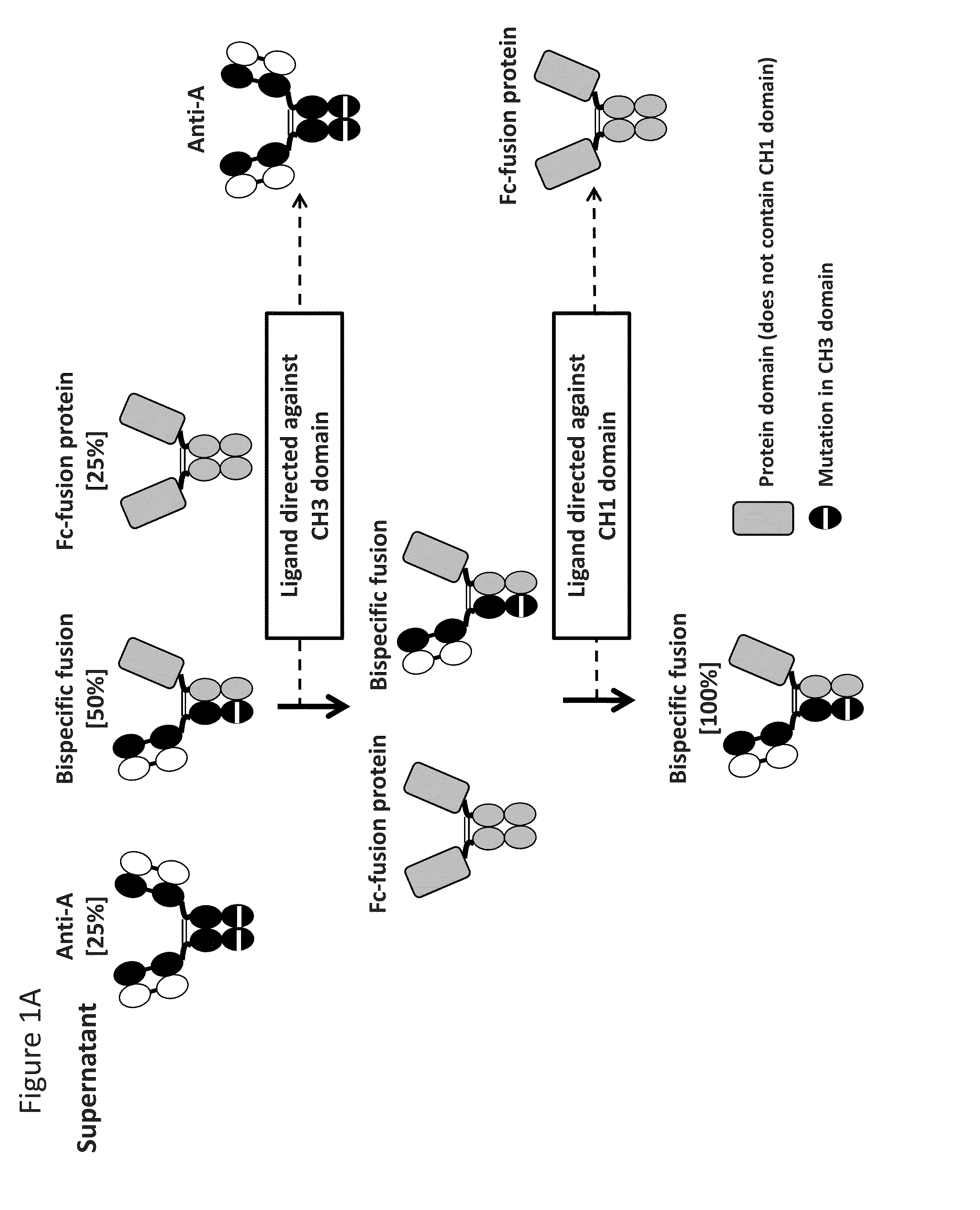
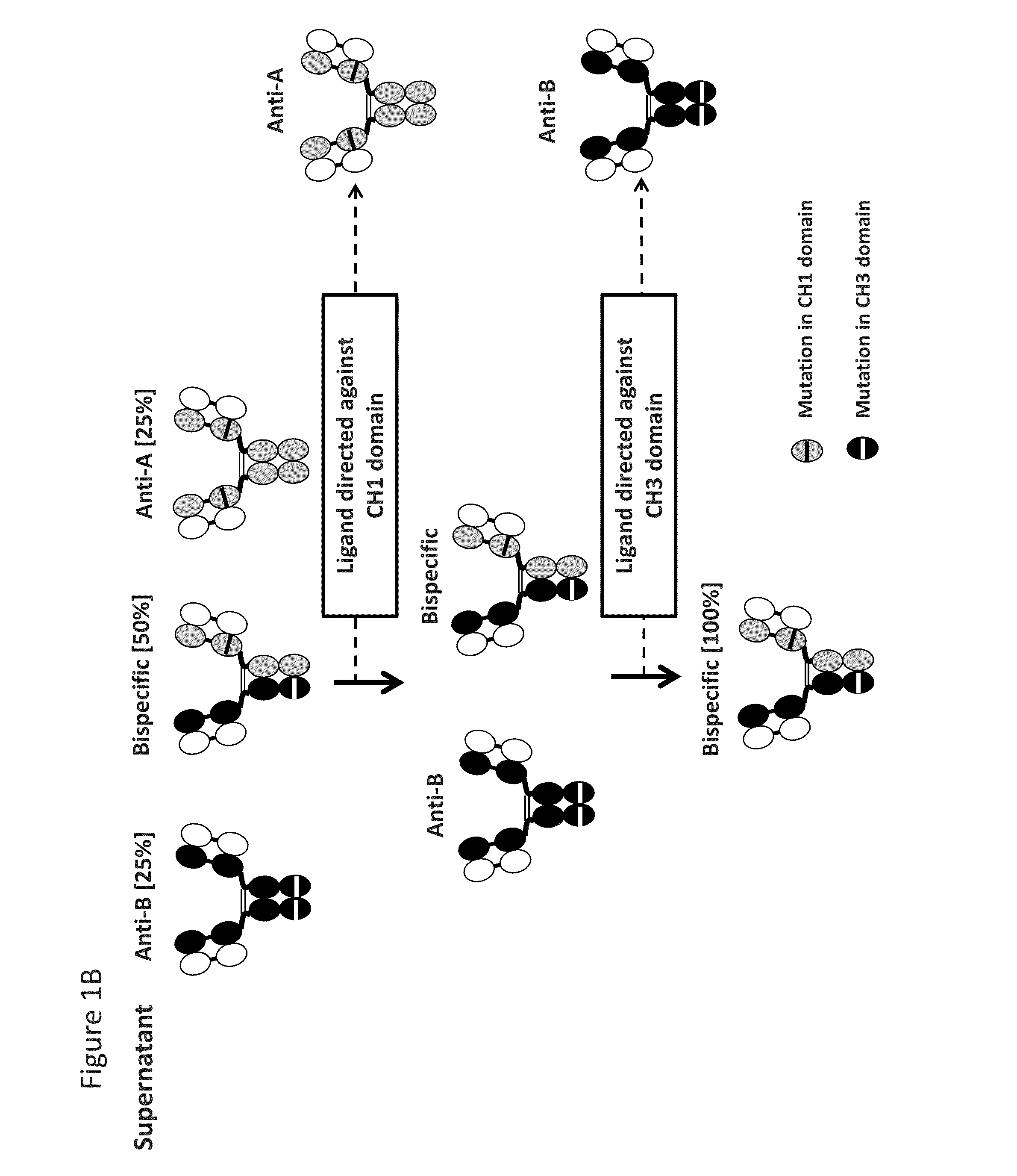
Readily Isolated Bispecific Binding Molecules with Native Format Having Mutated Constant Regions
ActiveUS20150094451A1Improve abilitiesHybrid immunoglobulinsDepsipeptidesBispecific antibodyAntigen binding
The invention provides heterodimer bispecific antigen-binding molecules that include a first polypeptide that does not include an IgG CH1 domain and a second polypeptide having an immunoglobulin constant region where there is at least one mutation in the IgG CH3 domain that abolishes the ability of the second polypeptide to bind CH3-specific affinity media such that the first and second polypeptides have different affinities with respect to CH1 and CH3 specific affinity reagents that allows rapid isolation by differential binding of the first and second polypeptides to these affinity reagents. The invention also provides bispecific antibodies that have IgG CH1 and CH3 regions with different affinities with respect to affinity reagents that allows rapid isolation by differential binding of the IgG regions to these affinity reagents. The invention also concerns bispecific antibodies which are heterodimers of heavy chains, i.e., two immunoglobulin heavy chains that differ by at least two amino acids that allow for the isolation of the bispecific antibody based on a differential affinity of one mutated immunoglobulin heavy chain and a second mutated immunoglobulin heavy chain toward two different affinity reagents.
Owner:NOVIMMUNE
Antibody fusion proteins with modified fcrn binding sites
Disclosed are antibody fusion proteins with a modified FcRn binding site and nucleic acid molecules encoding them. The antibody fusion protein include two polypeptide chains, wherein the first polypeptide chain includes a biologically, preferably therapeutically active molecule linked to at least a portion of an immunoglobulin constant region. The second polypeptide chain includes at least a portion of an immunoglobulin constant region. One of the polypeptide chains includes a mutation in the constant region that results in reduction or loss of FcRn binding but does not cause substantial reduction of serum half-life of said antibody fusion protein. In a preferred embodiment the invention relates to respectively constructed Fc-IFNss molecules. Also disclosed are methods of producing the fusion proteins and methods of using the fusion proteins for treating diseases and conditions alleviated by the administration of the fusion proteins.
Owner:MERCK PATENT GMBH
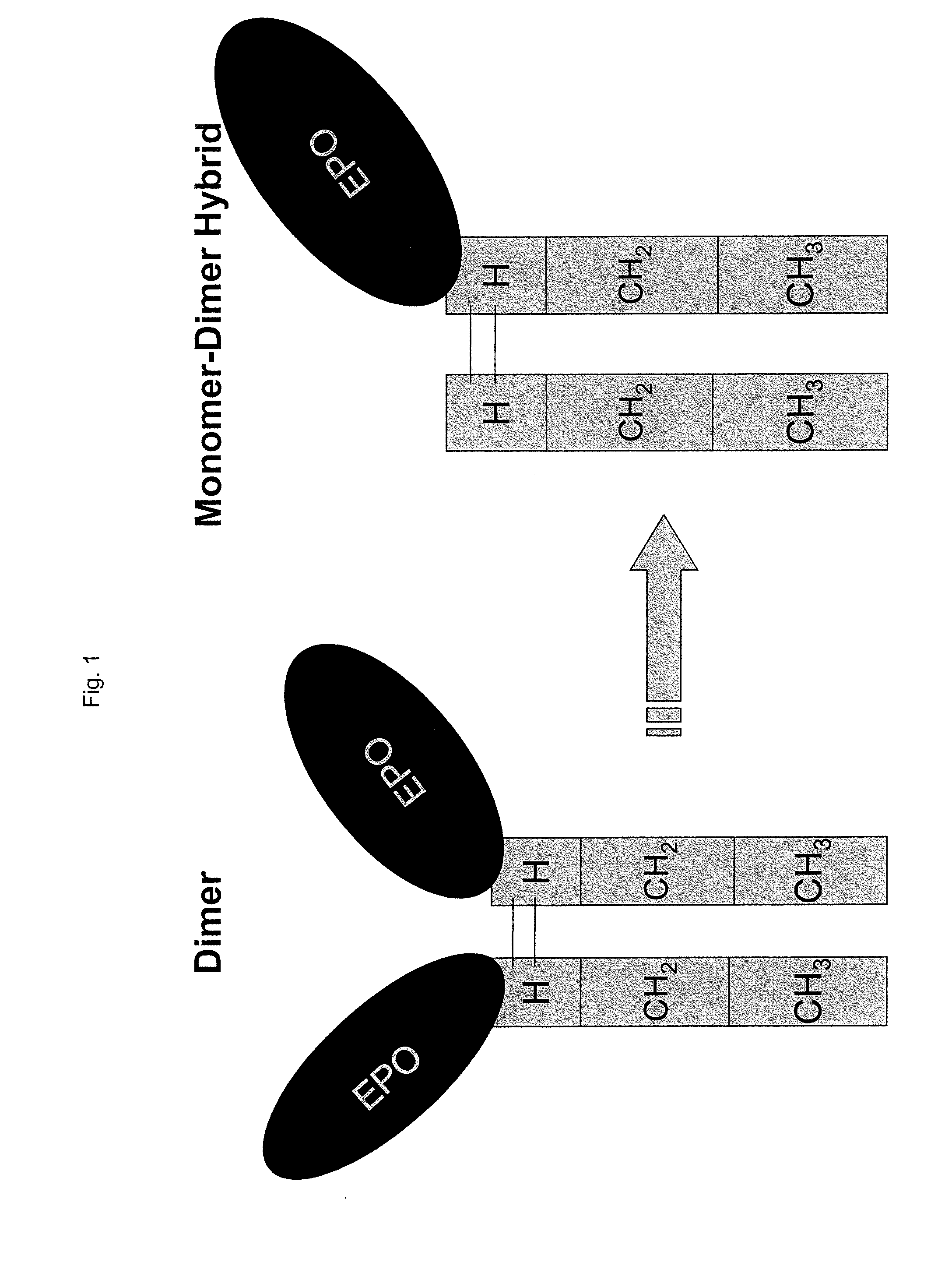
Dimer immunoadhesin, pharmaceutical composition and application thereof
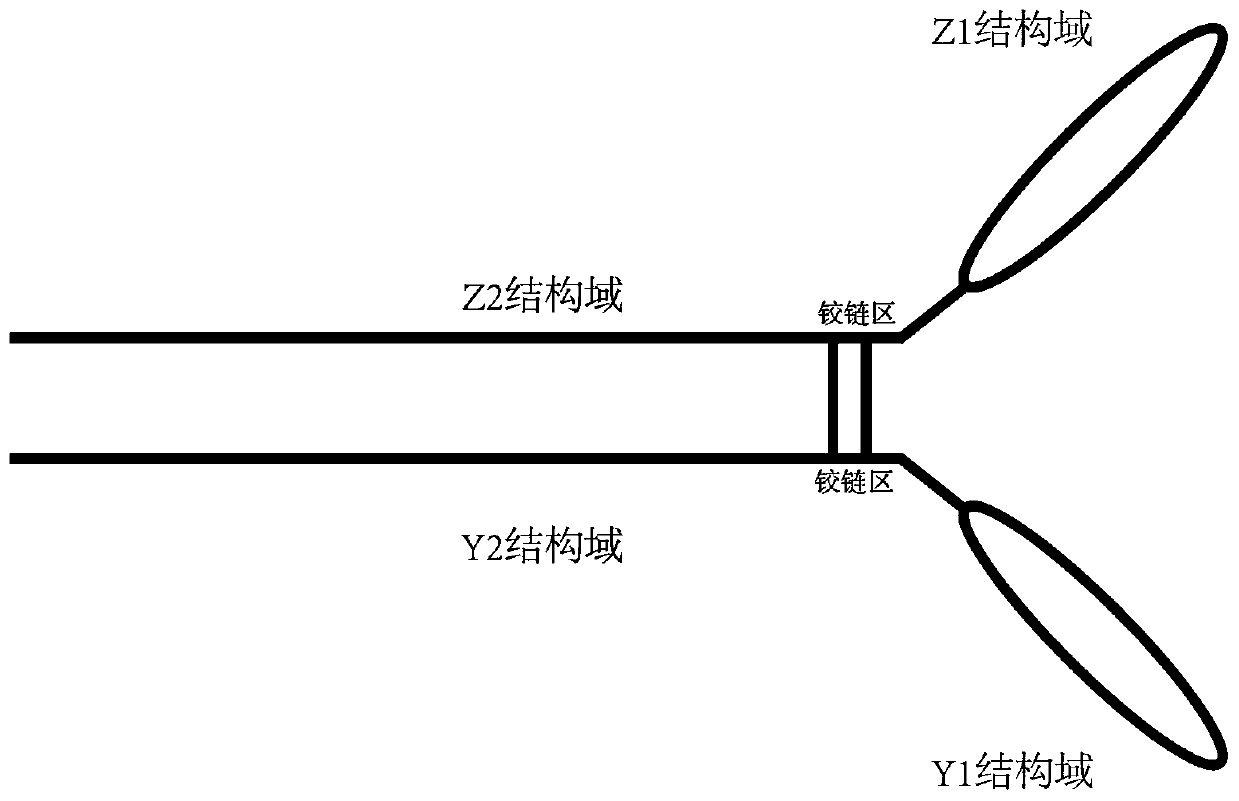
PendingCN110669139AEasy to buildHigh expressionImmunoglobulin superfamilyPeptide/protein ingredientsDiseaseDimer
The invention relates to the technical field of biomedical engineering, and provides a composition related to soluble dimeric immunoadherend and a method thereof. The dimeric immunoadhesin comprises afirst dimerized polypeptide chain and a second dimerized polypeptide chain. Wherein a structural general formula of the first polypeptide chain is Z1-Z2, and the structural general formula of the second polypeptide chain is Y1-Y2. Wherein Z1 is (i) an extracellular domain of a first cell surface receptor or a functional variant or fragment thereof, or (ii) a first cytokine or a functional variantor fragment thereof; Z2 is an immunoglobulin constant region dimerization domain or a functional variant or fragment thereof. Y1 is an extracellular domain or a functional variant or fragment thereofof a second cell surface receptor, or (ii) a second cytokine or a functional variant or fragment thereof. Y2 is an immunoglobulin constant region dimerization domain or the functional variant or fragment thereof. The dimer protein can be used for treating and preventing diseases related to infertility.
Owner:PHARCHOICE THERAPEUTICS INC
Preparation method for high-yield production of physiologically active polypeptide conjugate
ActiveUS10660940B2High purityHigh yieldPeptide/protein ingredientsAntibody mimetics/scaffoldsBiochemistryImmunoglobulin Constant Region
Owner:HANMI PHARMA
Single domain antigen combined molecule purifying method
The invention discloses a method of purifying or separating a single domain antigen combined molecule (SDAB) which includes one or more single combined domains (e.g., one or more nano antibody molecules) and basically does not include complementary antibody domain and immonoglubolin constant domain on the basis of affinity chromatography of protein A.
Owner:ABLYNX NV
Method and composition for altering a t cell mediated pathology
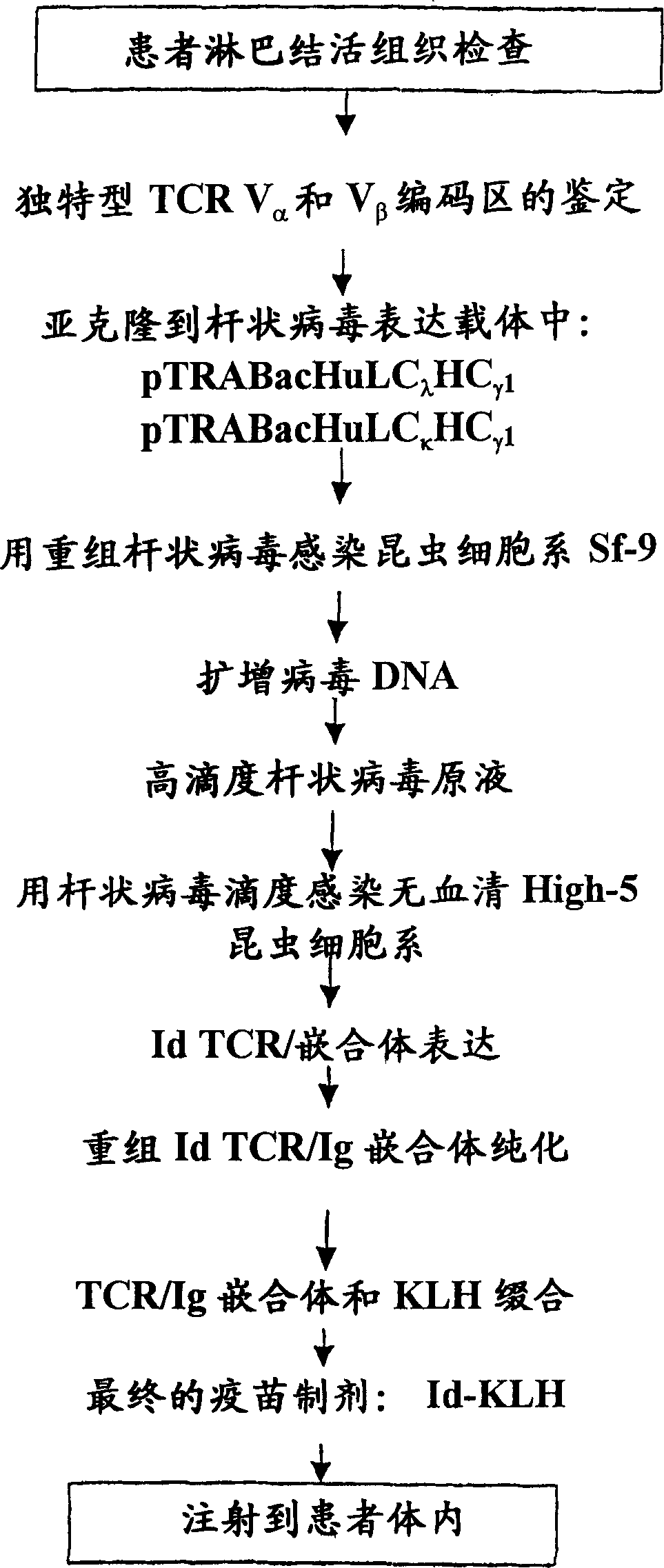
InactiveCN1468124AAntibody ingredientsCarrier-bound antigen/hapten ingredientsT-Cell SpecificityADAMTS Proteins
The present invention provides a method for altering a T cell mediated pathology in a patient. This method comprises administering a composition comprising at least one and / or two chimeric proteins. Each chimeric protein comprises at least a portion of either the Valpha or Vbeta region of a TCR from particular T cells from a patient having a T cell mediated pathology, and an immunoglobulin constant region. The genes encoding Valpha and / or Vbeta regions and the genes encoding immunoglobulin constant regions are isolated and inserted into an expression vector. The chimeric proteins are produced by introducing the expression vectors into insect cell lines. The chimeric proteins are purified using antibody affinity columns, and then chemically conjugated to an immunogenic carrier, keyhole-limpet hemocyanin (KLH). Since the conjugates comprises chimeric proteins made specifically from particular T cells from a patient having T cell mediated pathology, when it is administered to such a patient, with or without a cytokine, such as granulocyte-macrophage-CSF, or a chemokine, it can induce immune responses to alter such a T cell mediated pathology.
Owner:FAVRILLE
Improved preparation method for high-yield production of physiologically active polypeptide conjugate
ActiveUS20160008484A1High purityHigh yieldPeptide/protein ingredientsAntibody mimetics/scaffoldsCoupling reactionImmunoglobulin Constant Region
Provided is a method for preparing a conjugate by linking a physiologically active polypeptide, a non-peptidyl polymer linker, and an immunoglobulin constant region via a covalent bond. The method for efficiently preparing the physiologically active polypeptide conjugate uses a salt in a coupling reaction to solve the problem of low production yield during preparation of the physiologically active polypeptide conjugate. The conjugate of physiologically active polypeptide-non-peptidyl polymer-immunoglobulin constant region can be produced with high purity and yield by the subject method. Due to the method for preparing the physiologically active polypeptide conjugate, production costs can be reduced. Therefore, the method can be used to develop long-acting formulations of physiologically active polypeptides, which have improved industrial applicability and drug regulation compliance.
Owner:HANMI PHARMA
Recombinant antibody vector
A recombinant antibody vector for producing a single chain recombinant antibody comprises: (a) a contiguous nucleotide sequence: (i) that comprises a restriction endonuclease site that encodes an amino acid sequence of an immunoglobulin variable region; and (ii) that encodes an immunoglobulin constant region amino acid sequence in the same reading frame as (i), wherein another nucleotide sequence encoding (iii) an immunoglobulin variable region amino acid sequence, is insertable into the restriction endonuclease site in the same reading frame as (ii); and (b) one or more regulatory nucleotide sequences operably linked or connected to said nucleotide sequence, wherein the amino acid sequence in (i) comprises amino acids conserved in different immunoglobulin variable regions. The restriction endonuclease site may be a SacI site which encodes the conserved amino acids glutamate and leucine. In frame insertion of the nucleotide sequence of (iii) is facilitated by ligase independent cloning.
Owner:ACYTE BIOTEC
Methods for purification of single domain antigen-binding molecules
Processes and methods of purifying or separating Single Domain Antigen Binding (SDAB) molecules that include one or more single binding domains (e.g., one or more nanobody molecules), substantially devoid of a complementary antibody domain and an immunoglobulin constant region, using Protein A-based affinity chromatography, are disclosed.
Owner:ABLYNX NV
An improved process for preparation of physiologically active polypeptide complex
ActiveUS20150299247A1High purityHigh yieldPeptide/protein ingredientsMetabolism disorderDrug complianceImmunoglobulin Constant Region
Disclosed is a method for the preparation of a complex in which a physiologically active polypeptide is covalently bonded to an immunoglobulin constant region via a non-peptidyl linker. The method is characterized by the employment of a reducing agent, by which conventional problems of low production yield and modification of the polypeptide can be overcome. The physiologically active polypeptide-non-peptidyl polymer-immunoglobulin constant region complex can be produced with high purity and yield as well as at low cost. Thus, the method is industrially useful. Moreover, by exhibiting a prolonged action profile, the physiologically active polypeptide-non-peptidyl polymer-immunoglobulin constant region complex can be effectively used for developing long-acting formulations of physiologically active polypeptides which have improved drug compliance.
Owner:HANMI SCI CO LTD
Procoagulant fusion compound
ActiveUS10584147B2Peptide/protein ingredientsAntibody mimetics/scaffoldsCoagulation DisorderImmunoglobulin Constant Region
Owner:BIOVERATIV THERAPEUTICS INC
Process for preparation of physiologically active polypeptide complex
ActiveUS11168109B2High purityHigh yieldPeptide/protein ingredientsMetabolism disorderBiochemistryImmunoglobulin Constant Region
Disclosed is a method for the preparation of a complex in which a physiologically active polypeptide is covalently bonded to an immunoglobulin constant region via a non-peptidyl linker. The method is characterized by the employment of a reducing agent, by which conventional problems of low production yield and modification of the polypeptide can be overcome. The physiologically active polypeptide-non-peptidyl polymer-immunoglobulin constant region complex can be produced with high purity and yield as well as at low cost. Thus, the method is industrially useful. Moreover, by exhibiting a prolonged action profile, the physiologically active polypeptide-non-peptidyl polymer-immunoglobulin constant region complex can be effectively used for developing long-acting formulations of physiologically active polypeptides which have improved drug compliance.
Owner:HANMI SCI CO LTD
Method for preparing humanized immune globulin capable of antagonizing vessel endothelium cell growth factor and combined uses thereof
InactiveCN101134777AImmunoglobulins against animals/humansAntibody ingredientsVascular endotheliumOrganism
The present invention relates to biotechnology, belongs to the field of recombinant gene engineering, and is six kinds of humanized fused immunoglobulins prepared through recombinant gene engineering and cell culturing process and capable of recognizing and combining with vascular endothelial cell growth factor specifically and their application. These six kinds of fused immunoglobulins feature that each of them has immunoglobulin-like structure regions capable of being combined with VEGF in the N-end and human immunoglobulin constant region in the C-end. These fused immunoglobulins have the bioactivity of high combination with VEGF and the bioactivity of immunoglobulin constant region, and can neutralize the VEGF inside adsorbed body to antagonize and inhibit fission and proliferation of vascular endothelial cell. These fused immunoglobulins are applied as VEGF neutralizing adsorbent alone or in combination.
Owner:SUZHOU STAINWEI BIOTECH INC
Method and composition for altering a B cell mediated pathology
InactiveUS20050202004A1In-vivo radioactive preparationsPeptide/protein ingredientsAntibody AffinitiesKeyhole-limpet haemocyanin
Owner:MMRGLOBAL
Fusion protein of thymulin alpha1
InactiveCN105669871AImprove anti-tumor effectProlong half-life in vivoThymosin peptidesPeptide/protein ingredientsEscherichia coliHalf-life
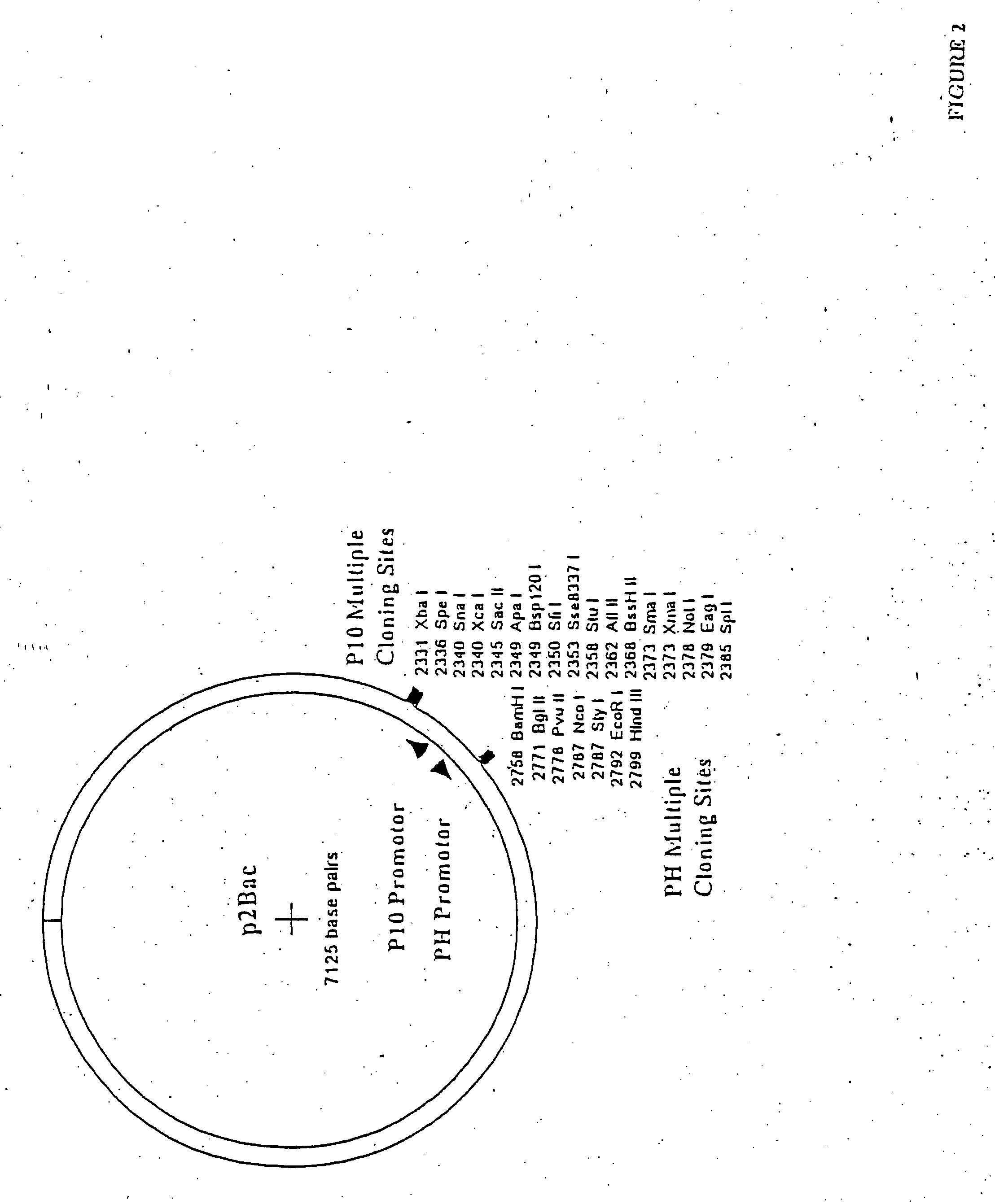
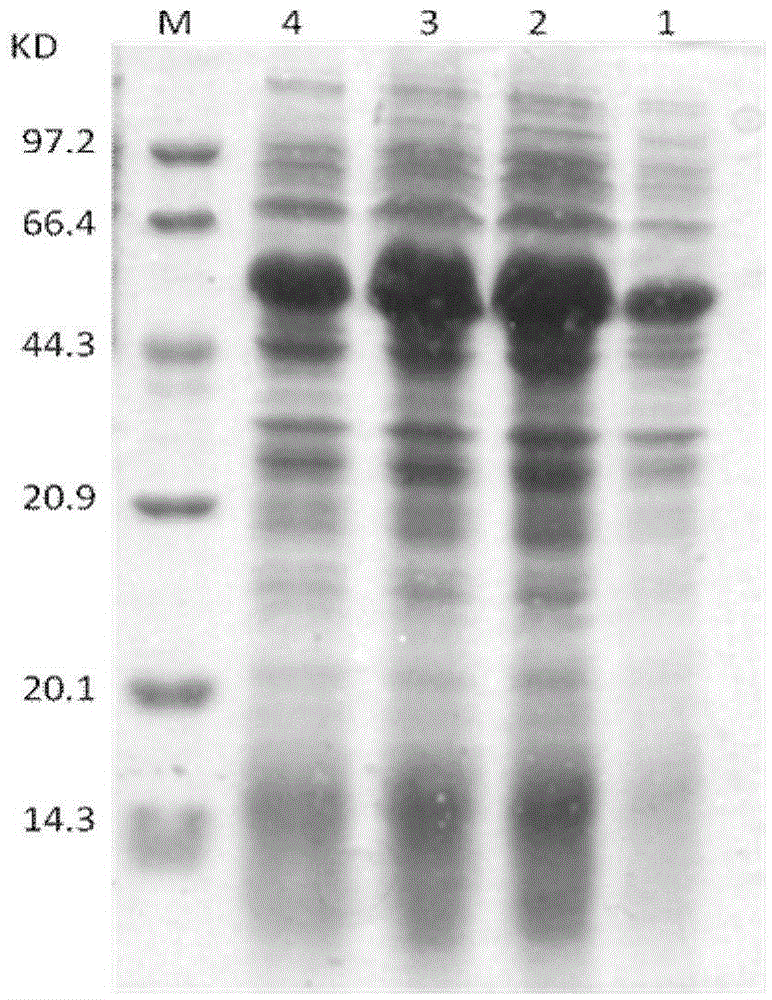
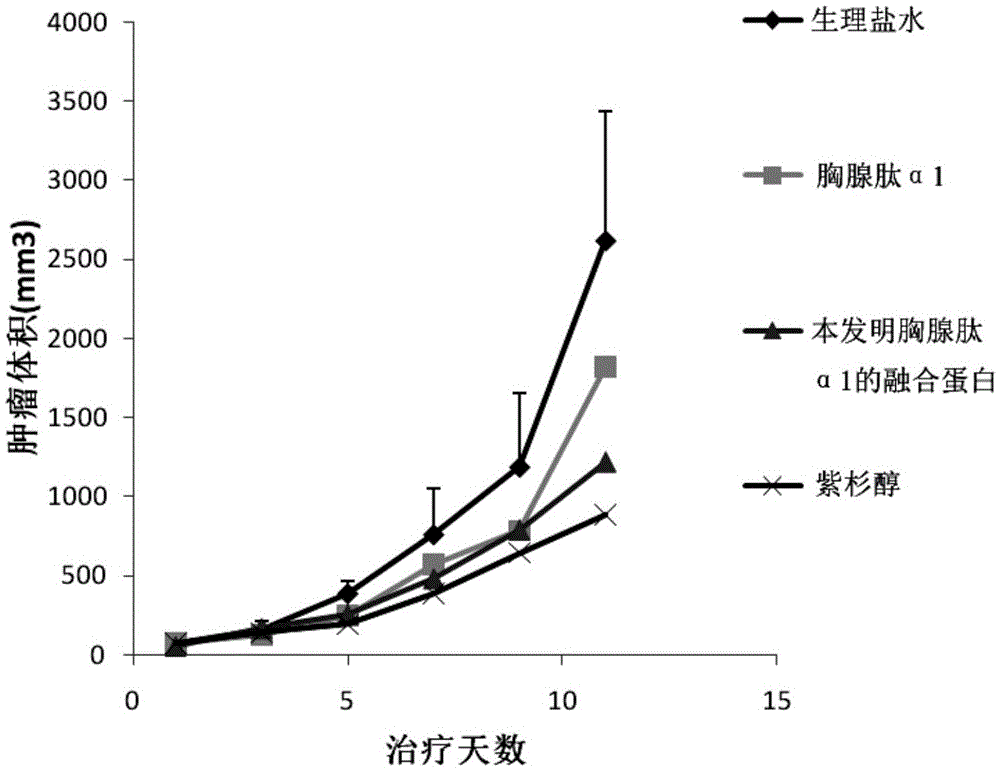
The invention discloses fusion protein of thymulin alpha1. The fusion protein is formed by connecting the thymulin alpha1 or active fragments of the thymulin alpha1with a constant region of immune globulin through connecting peptide or directly. The fusion protein can be expressed in eukaryotic cells and can also be expressed in prokaryotic cells. According to the fusion protein of the thymulin alpha1, a pronucleus carrier containing a target gene is prepared through the recombination DAN technology and expressed, separated and purified in escherichia coli, and compared with archetype thymulin alpha1, the obtained fusion protein of the thymulin alpha1 is longer in in-vivo half-life period and more efficient in anti-tumor effect.
Owner:CHINA PHARM UNIV
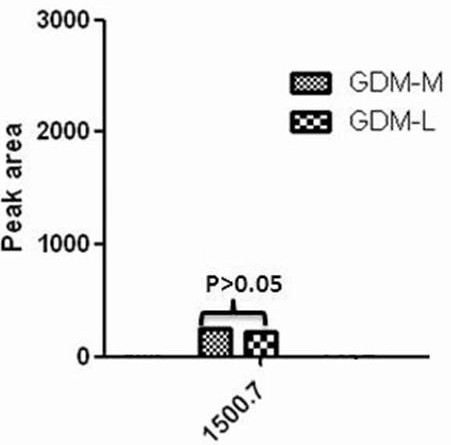
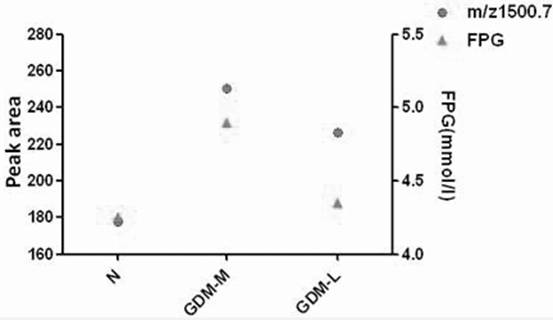
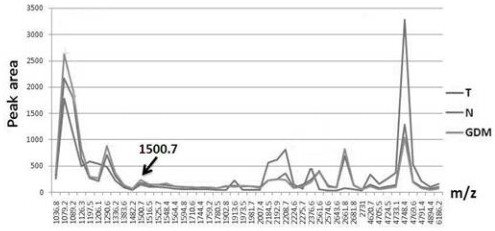
Application of urine IGKC protein and polypeptide fragment thereof in normal pregnancy or gestational diabetes
ActiveCN112904012AEasy to storeImmunoglobulins against animals/humansDisease diagnosisDiabetes mellitusDiabetes in pregnancy
The invention provides an application of a urine immunoglobulin kappa constant region (IGKC) and a polypeptide fragment of the IGKC, and particularly relates to an application of a urine IGKC protein and a polypeptide fragment of the IGKC protein in preparation of a preparation for monitoring normal pregnancy or gestational diabetes mellitus. Researches prove that compared with a normal childbearing age woman group (normal control), the expression of IGKC and polypeptide fragments thereof in normal pregnancy and gestational diabetes mellitus groups is increased, and the IGKC and polypeptide fragments thereof can be used for monitoring and auxiliary diagnosis of gestational state or gestational diabetes mellitus patients. According to the invention, the advantages of noninvasive acquisition, large-scale repeated sampling and convenient preservation of a urine sample are exerted, and the urine IGKC protein and the polypeptide fragment thereof are detected by using the urine sample.
Owner:张曼
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com