Method and composition for altering a t cell mediated pathology
A composition and cell technology, applied in the field of immunology and immunotherapy, can solve the problems of difficult production of TCR molecules and low production level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Long:
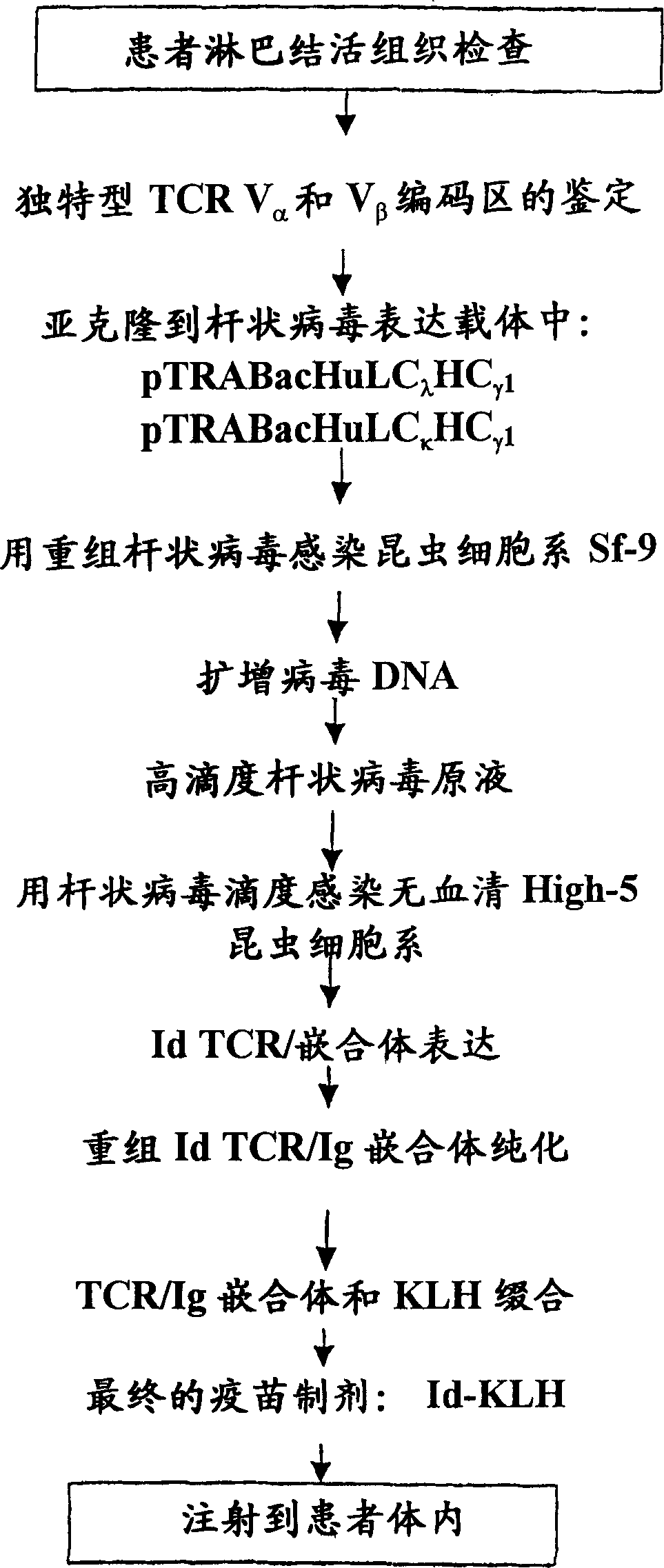
[0154] Tumor samples obtained from peripheral lymph nodes were biopsied under sterile conditions according to clinically prescribed methods, and the samples were used to produce patient idiotype-specific recombinant V α and V β - Ig chimeric protein. Store the remaining lymph node biopsy material in a tissue cell bank in liquid nitrogen for future use.
[0155] Cell isolation: Single cell suspensions of patient lymph node biopsy samples were obtained by forcing patient biopsy lymphoma tissue through a disposable 0.38 mm steel mesh and simultaneously immersed in sterile PBS. Dispersed cells were washed twice with PBS, then resuspended and counted. A 10% ratio of the cells was processed for total RNA extraction and the remaining cells were stored in liquid nitrogen after resuspension in RPMI 1640 tissue culture medium containing 30% fetal bovine serum and 10% DMSO. All clinical sample processing was performed in a biological safety cabinet.
[0156] Total...
Embodiment 2
[0158] Cloning and sequencing of PCR products: According to the manufacturer's recommendations, from multiple reactions, determined to contain V α and V β The PCR product of the tumor-specific sequence of the chain was cloned directly into the plasmid pCR2.1-TOPO, and then introduced into Top10 competent E. coli (E. coli) cells (Invitrogeh). Twenty-four miniprep DNA plasmids were prepared from carbenicillin-resistant colonies using the QIAPrep Spin Miniprep Kit (Qiagen) and quantified by spectrophotometry. 200 ng of each plasmid were sequenced using the Cy5 / Cy5.5 Dye Primer Cycle Sequencing Kit (Visible Genetics). After the sequencing reaction was completed, the samples were electrophoresed on the OpenGene DNA Automated Sequencing System (Automated DNASequencing System), and the data were processed with the GeneObjects software package (Visible Genetics). Additional analysis, including sequence alignment, was performed using SEQUENCHER Version 4.1.2 DNA analysis software (...
Embodiment 5
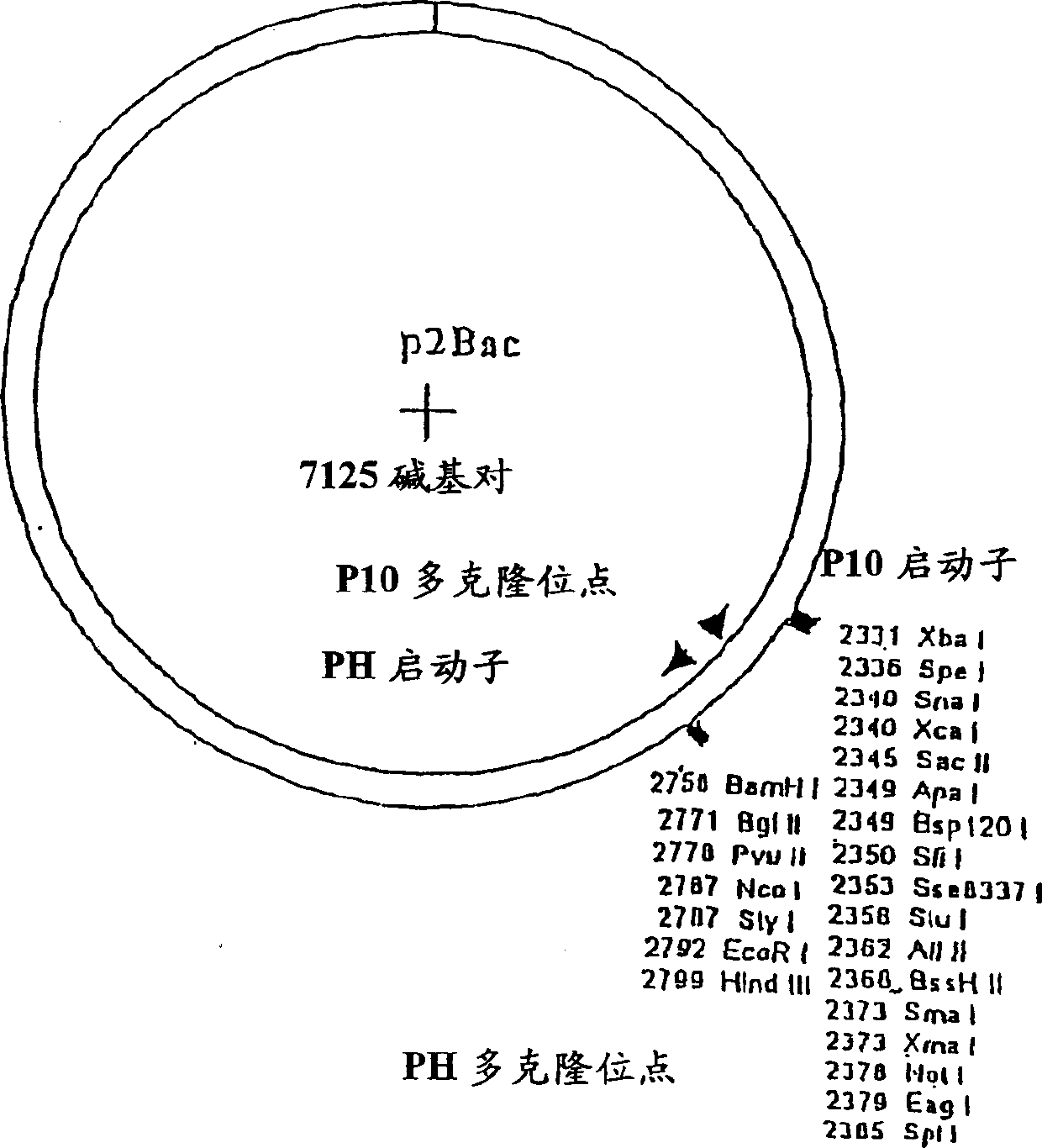
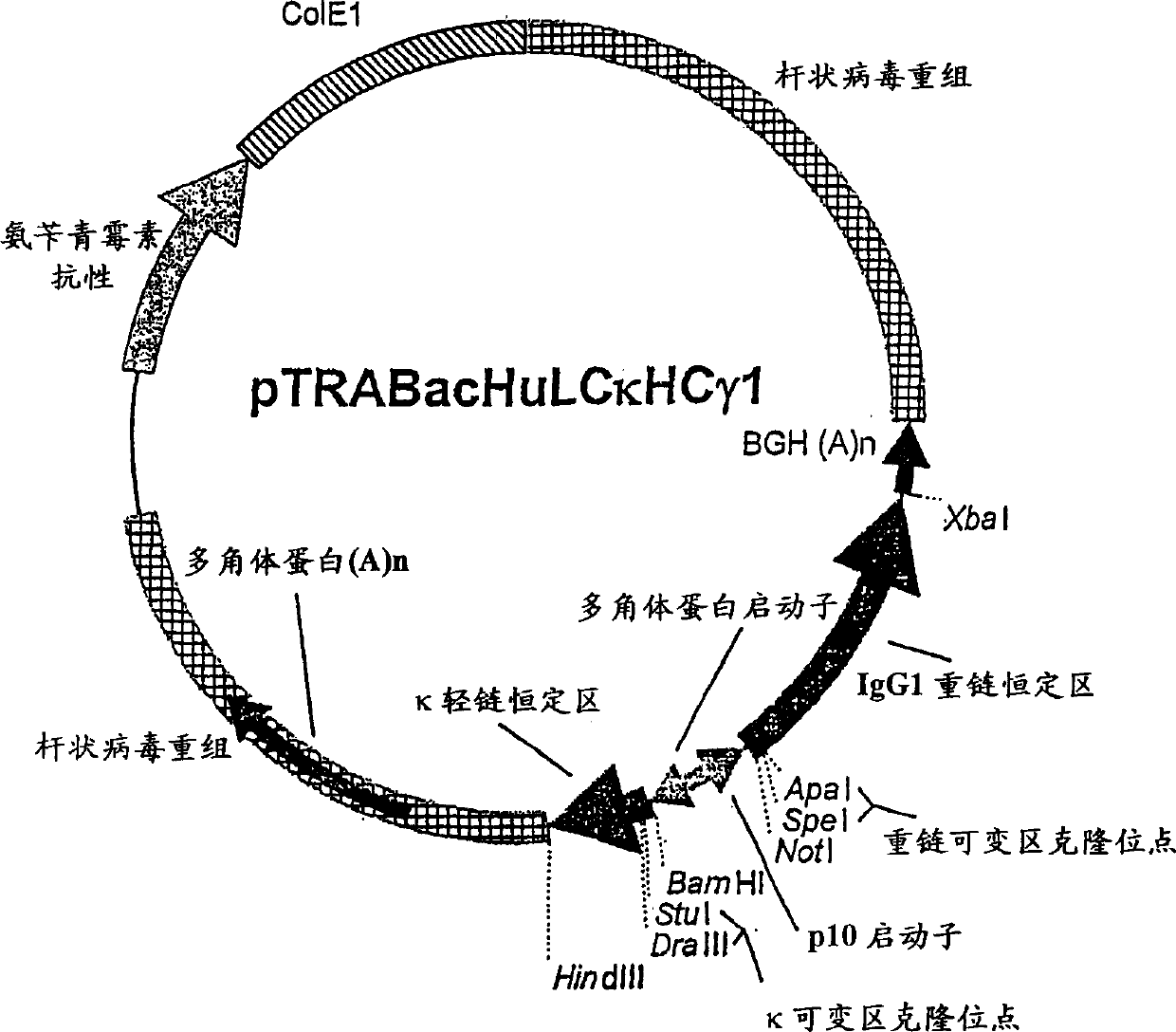
[0180] Production of TCR / Ig Chimeric Proteins in High-5 Insect Cells: High-5 insect cells (BTI-TN-5B1-4) secrete higher levels (2-20×) of recombinant immunoglobulins than Sf-9 cells and are selected for TCR / Ig chimeric protein production cell line. High-5 cells (1.0-2.0×10 6 cells / ml) at 5×10 5 Cells / ml were seeded in ESF921 medium (Expression Systems LLP) in 1 liter disposable culture flasks with vented closures. The culture bottle was shaken at 140-150 rpm at 28°C, and the volume of the culture medium in the culture bottle was adjusted within a specified time so that it did not exceed 500 ml. When the cell density reached 1.5-2.5 cells / ml in 500ml culture medium, the cells in the culture flask were infected with a high-titer recombinant baculovirus stock solution at a multiplicity of infection (MOI) of about 0.5:1 (pfn:cells). The flasks were then shaken at 140-150 rpm at 28°C. Cell viability was checked daily and cultures were harvested when viability did not drop to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com