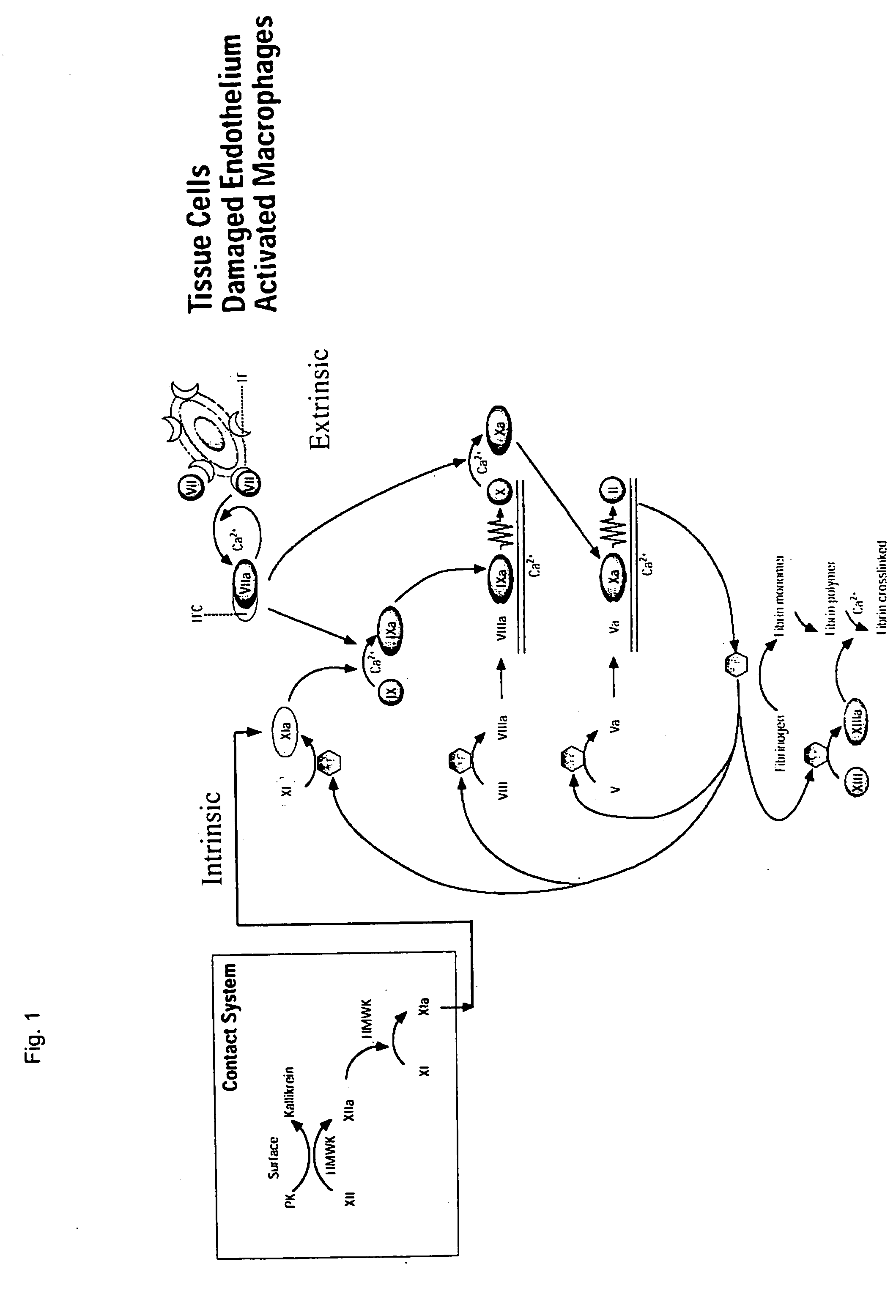
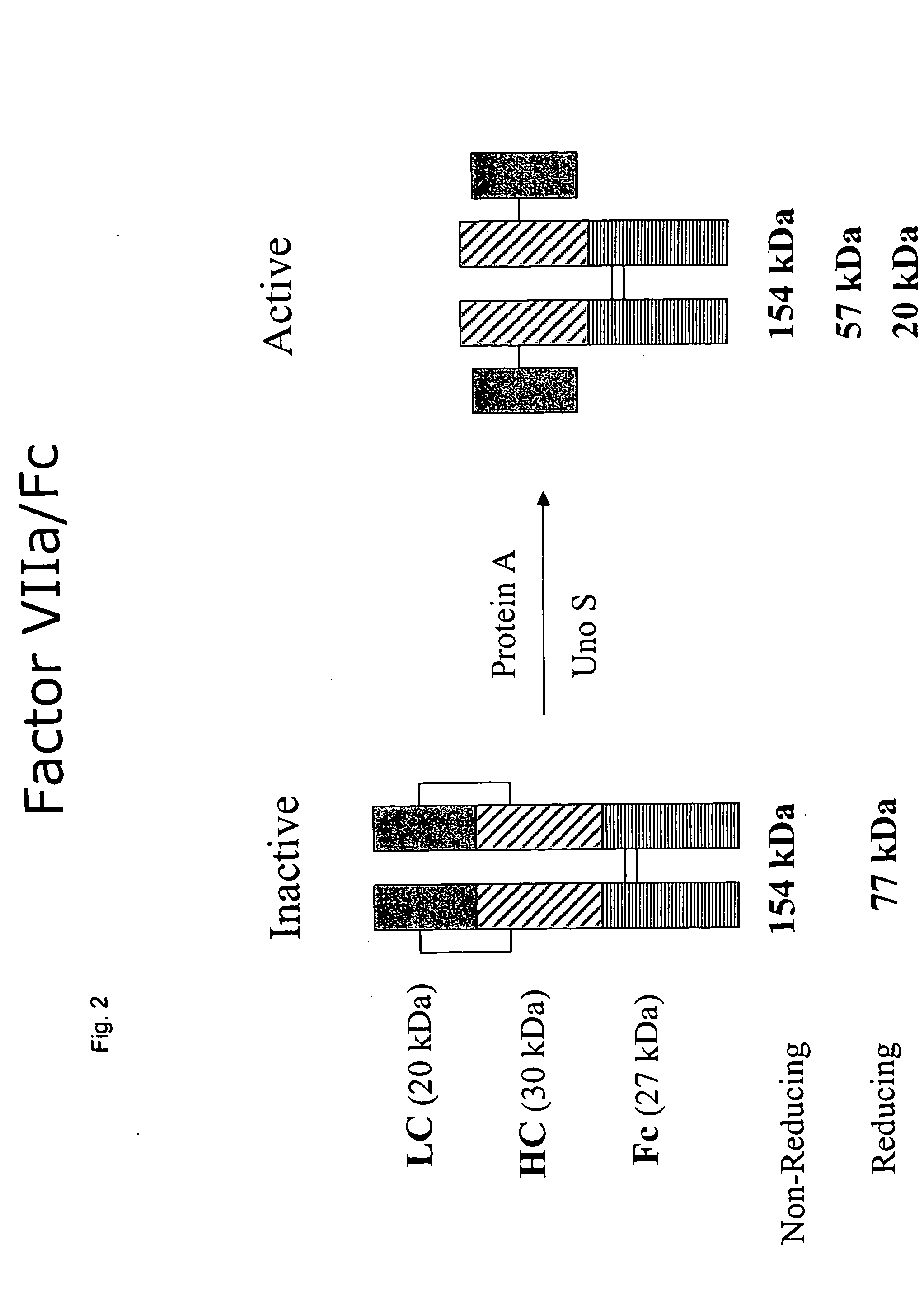
Clotting factor-Fc chimeric proteins to treat hemophilia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of pCDNA 3.1-Flag-Fc
[0129] The sequence for the FLAG peptide (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys)(SEQ ID NO: 23), a common affinity tag used to identify or purify proteins, was cloned into the pcDNA 3.1-Fc plasmid, which contains the mouse IgK signal sequence followed by the Fc fragment of human IgG1 (amino acids 221-447, EU numbering). The construct was created by overlapping PCR using the following primers:
FlagFc-F1:5′- GCTGGCTAGCCACCATGGA -3′(SEQ ID NO: 10)FlagFc-R1:5′- CTTGTCATCGTCGTCCTTGTAGTCGTCA(SEQ ID NO: 11)CCAGTGGAACCTGGAAC -3′FlagFc-F2:5′- GACTACAAGG ACGACGATGA(SEQ ID NO: 12)CAAGGACAAAACTCACACAT GCCCACCGTG CCCAGCTCCGGAACTCC -3′FlagFc-R2:5′- TAGTGGATCCTCATTTACCCG -3′(SEQ ID NO: 13)
[0130] The pcDNA 3.1-Fc template was then added to two separate PCR reactions containing 50 pmol each of the primer pairs FlagFc-F1 / R1 or FlagFc-F2 / R2 in a 50 μl reaction using Pfu Ultra DNA polymerase (Stratagene, Calif.) according to manufacturer's standard protocol in a MJ Thermocycler ...
example 2
Cloning of PACE Construct
[0131] The coding sequence for human PACE (paired basic amino acid cleaving enzyme), an endoprotease, was obtained by RT-PCR. The following primers were used:
(SEQ ID NO: 15)PACE-F1:5′- GGTAAGCTTGCCATGGAGCTGAGGCCCTGGTTGC -3′(SEQ ID NO: 16)PACE-R1:5′- GTTTTCAATCTCTAGGACCCACTCGCC -3′(SEQ ID NO: 17)PACE-F2:5′- GCCAGGCCACATGACTACTCCGC -3′(SEQ ID NO: 18)PACE-R2:5′- GGTGAATTCTCACTCAGGCAGGTGTGAGGGCAGC -3′
[0132] The PACE-F1 primer adds a HindIII site to the 5′ end of the PACE sequence beginning with 3 nucleotides before the start codon, while the PACE-R2 primer adds a stop codon after amino acid 715, which occurs at the end of the extracellular domain of PACE, as well as adding an EcoRI site to the 3′ end of the stop codon. The PACE-R1 and -F2 primers anneal on the 3′ and 5′ sides of an internal BamHI site, respectively. Two RT-PCR reactions were then set up using 25 pmol each of the primer pairs of PACE-F1 / R1 or PACE-F2 / R2 with 20 ng of adult human liver RNA (Clo...
example 3
Cloning of Fc-Factor VII construct
[0133] The coding sequence for Factor VII, was obtained by RT-PCR from human fetal liver RNA (Clontech, Palo Alto, Calif.). The cloned region is comprised of the cDNA sequence from bp 36 to bp 1430 terminating just before the stop codon. A SbfI site was introduced on the N-terminus. A BspEI site was introduced on the C-terminus. The construct was cloned by PCR using the primers:
(SEQ ID NO: 19)Down-5′ GCTACCTGCAGGCCACCATGGTCTCCCAGGCCCTCAGGstream:3′(SEQ ID NO: 20)Upstream:5′ CAGTTCCGGAGCTGGGCACGGCGGGCACGTGTGAGTTTTGTCGGGAAAT GG 3′
and the following conditions: 95° C. for 5 minutes followed by 30 cycles of 95° C. for 30 seconds, 55° C. for 30 seconds, 72° C. for 1 minute and 45 seconds, and a final extension cycle of 72° C. for 10 minutes.
[0134] The fragment was digested SbfI-BspE I and inserted into pED.dC-Fc a plasmid encoding for the Fc fragment of an IgG1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
| Disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com