Patents
Literature
223results about "Antigen carriers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
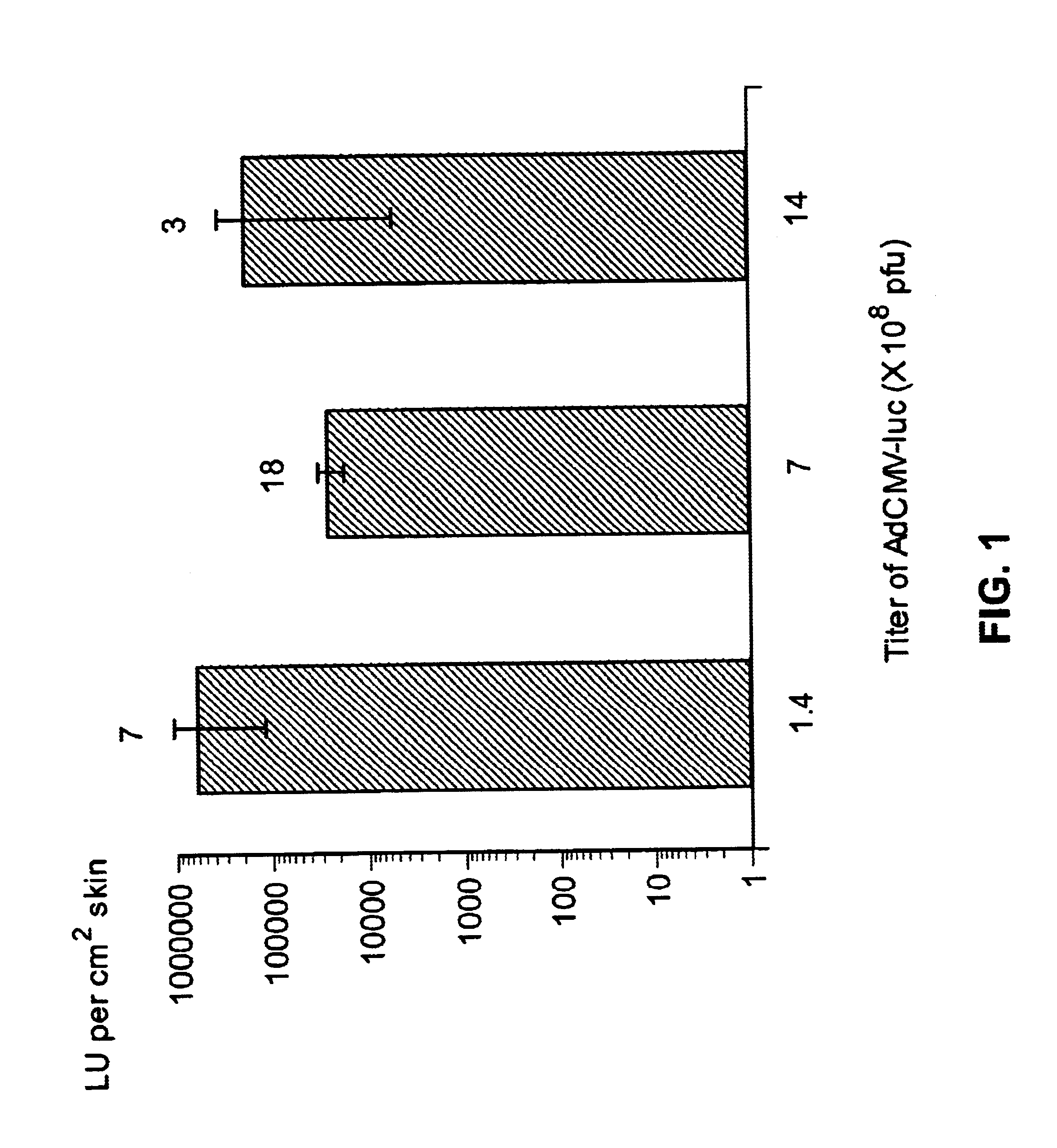
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
Vaccine Nanotechnology
ActiveUS20100233251A1Modulating immune systemEnhance and suppress and direct and immune responseNervous disorderAntipyreticDiseaseNanocarriers
Owner:MASSACHUSETTS INST OF TECH +4
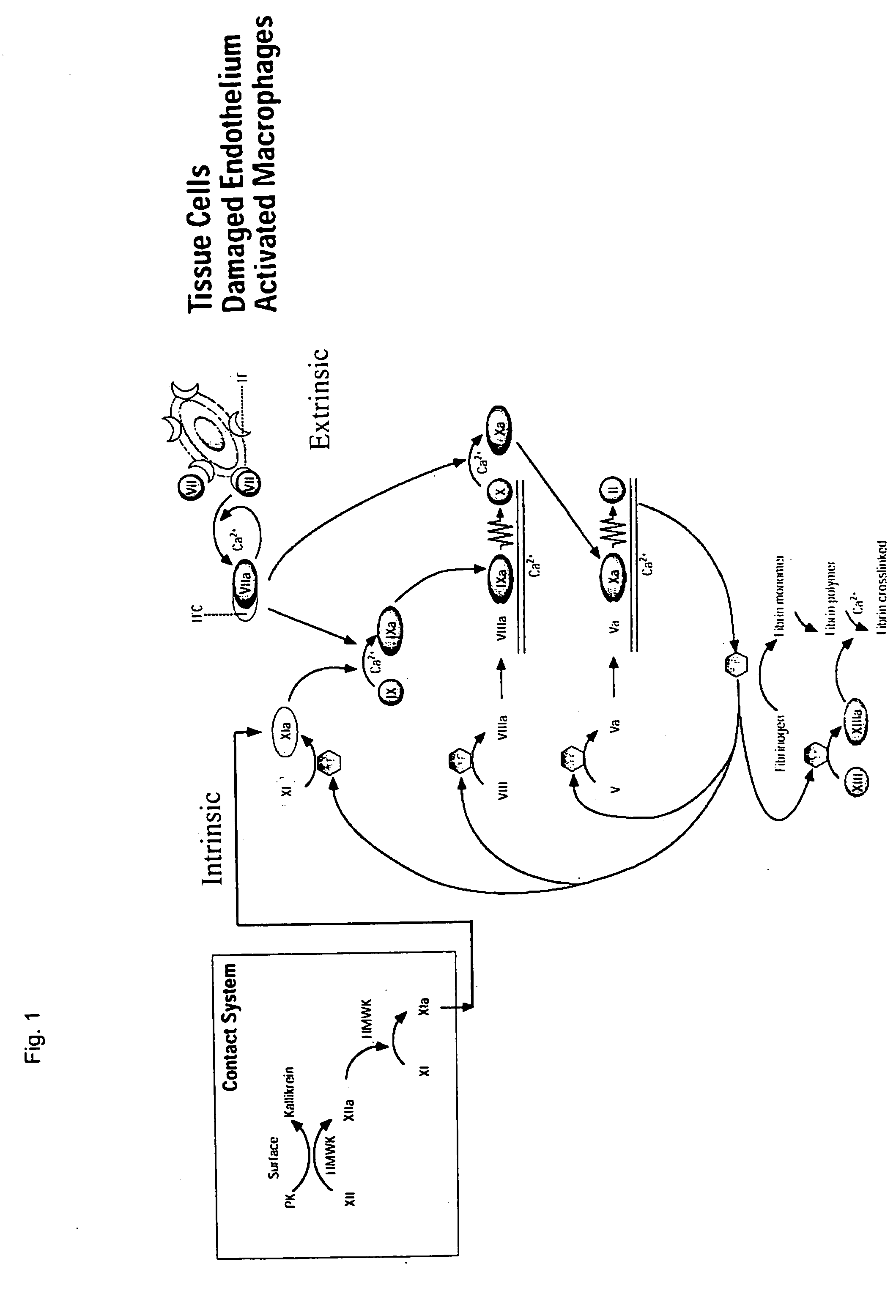
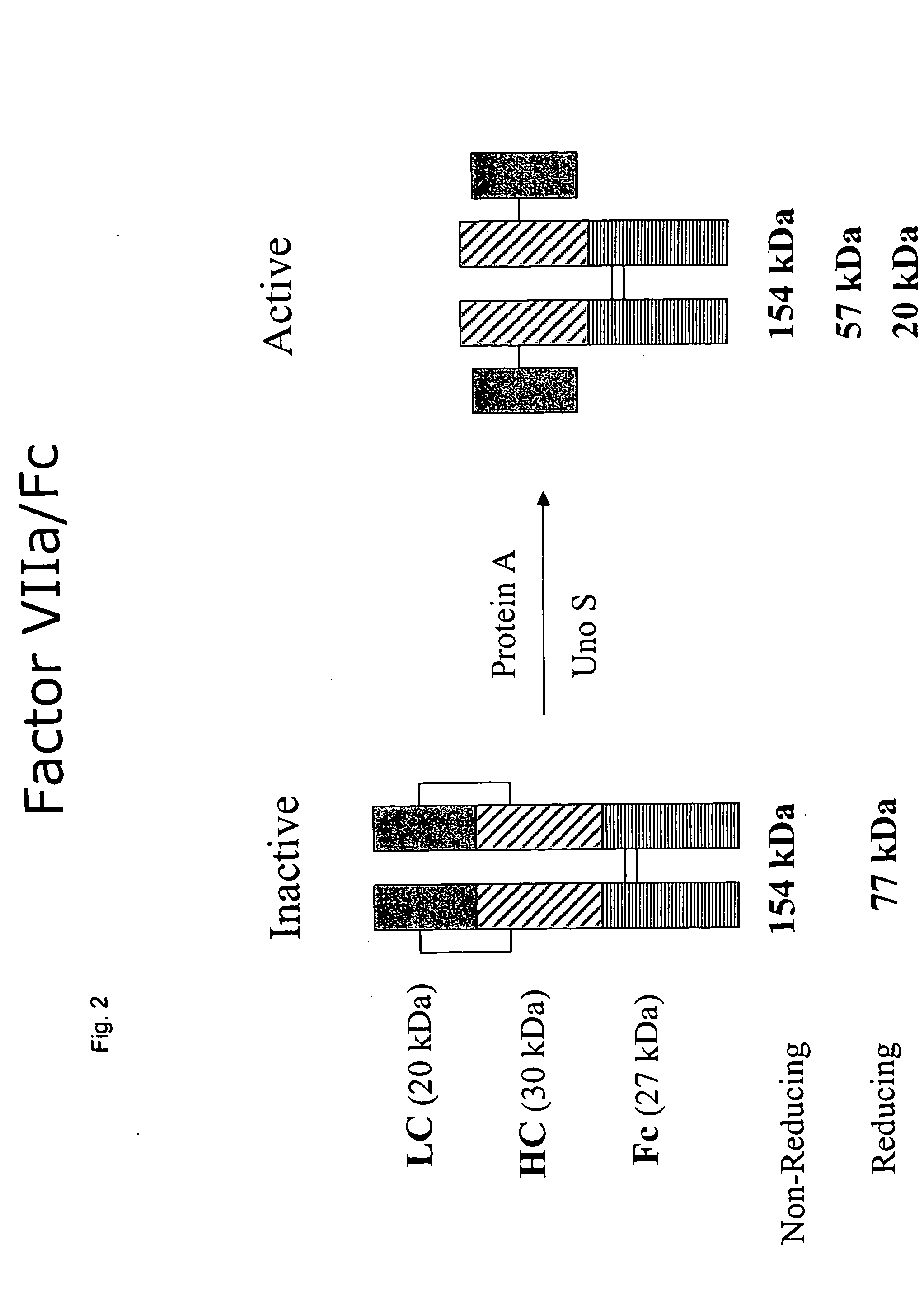
Clotting factor-Fc chimeric proteins to treat hemophilia
InactiveUS20050147618A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHemostatic DisordersChimera Protein
The invention relates to a chimeric protein comprising at least one clotting factor and at least a portion of an immunoglobulin constant region. The invention relates to a method of treating a hemostatic disorder comprising administering a therapeutically effective amount of a chimeric protein wherein the chimeric protein comprises at least one clotting factor and at least a portion of an immunoglobulin constant region.
Owner:BIOVERATIV THERAPEUTICS INC
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Vaccine Nanotechnology
ActiveUS20130236533A1Facilitate acquisitionModulating the immune systemNervous disorderAntipyreticDiseaseNanocarriers
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +3
Methods and compositions for increased priming of t-cells through cross-presentation of exogenous antigens
InactiveUS20080171059A1Easy to demonstrateEffective vaccineTissue cultureCancer antigen ingredientsDiseaseVaccination
Methods for eliciting in an animal in need thereof a cell-mediated immune response specific to an antigen, the method comprising providing an antigen preparation comprising particles on the surface of which the antigen is attached, and administering the antigen preparation to the animal, wherein the particles are taken up by antigen presenting cells (APC) of the animal via phagocytosis, forming a phagosome inside the APC, wherein the antigen is attached to the surface of the particle in such a way that the antigen is released in the phagosome before the phagosome fuses with a late endosome or a lysosome, and wherein the antigen is cross-presented on a Class I MHC molecule. Also provided are particulate antigen preparations or particulate vaccines that can be delivered to an animal in need thereof for vaccination against, for preventing or treating, a disease related to the antigen, such as cancer and a viral infection.
Owner:LUDWIG INST FOR CANCER RES +1
Vaccination by topical application of recombinant vectors
InactiveUS20030045492A1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseGenetic material ingredientsGene deliveryVaccination
The present invention relates to techniques of skin-targeted non-invasive gene delivery to elicit immune responses and uses thereof. The invention further relates to methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal following topical application of vectors, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal as well as such a method further including disposing the vector in and / or on the delivery device. The vector can be gram negative bacteria, preferably Salmonella and most preferably Salmonella typhimurium.
Owner:UAB RES FOUND
Saccharide Conjugate Vaccines
InactiveUS20080260773A1Improve bioavailabilityImprove efficacyAntibacterial agentsPeptide/protein ingredientsConjugate vaccineCarrier protein
The invention provides compositions comprising a combination of two or more monovalent conjugates, each of said two or more monovalent conjugates comprising a carrier protein comprising T cell epitopes from two or more pathogens conjugated to saccharide antigen. The invention also provides a multivalent conjugate comprising two or more antigenically distinct saccharide antigens conjugated to the same carrier protein molecule, wherein the carrier protein comprises T cell epitopes from two or more pathogens. Further compositions comprise one or more of said monovalent conjugates and one or more of said multivalent conjugates. The invention further provides methods for making said compositions and uses for said compositions.
Owner:NOVARTIS AG
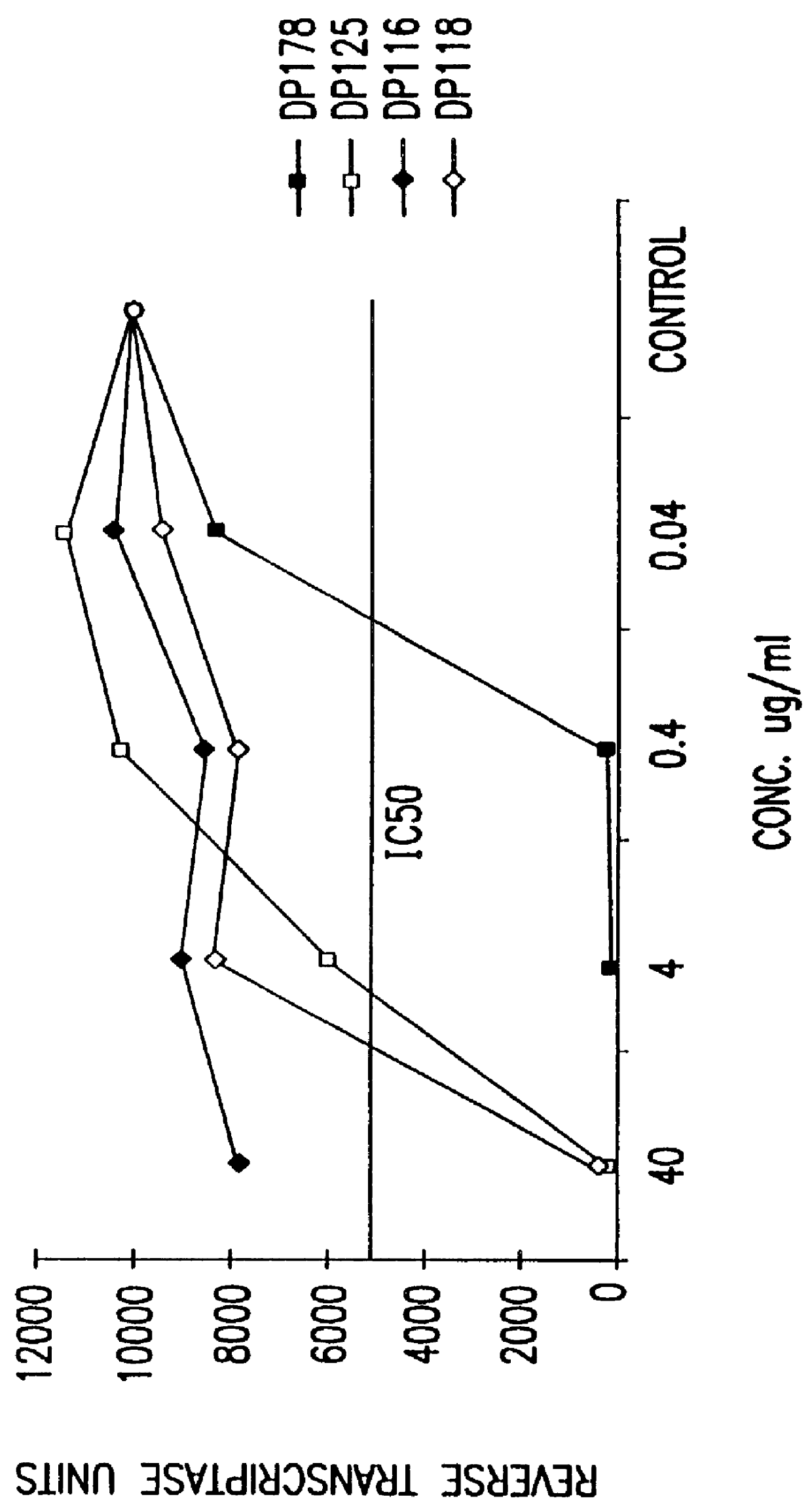
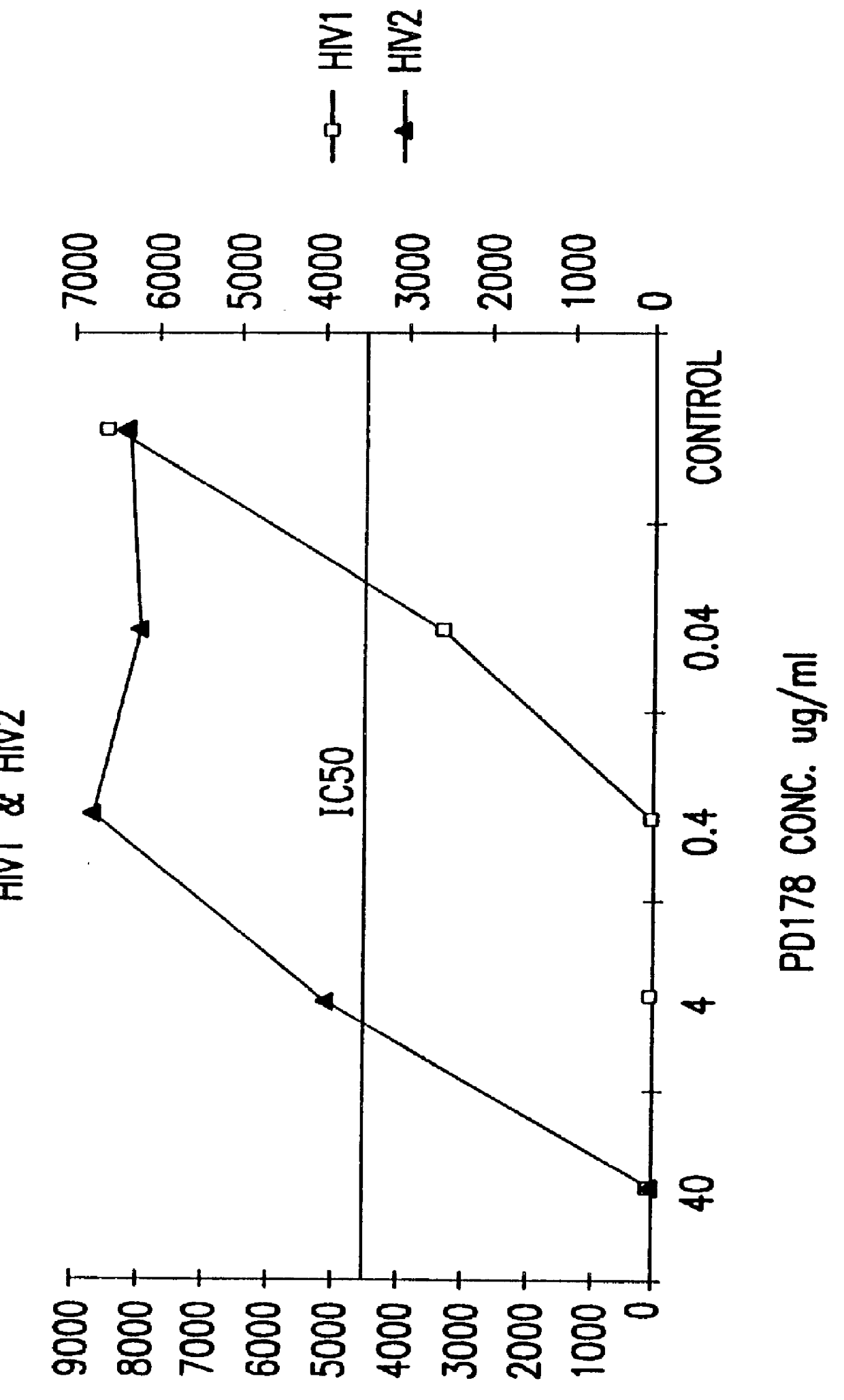
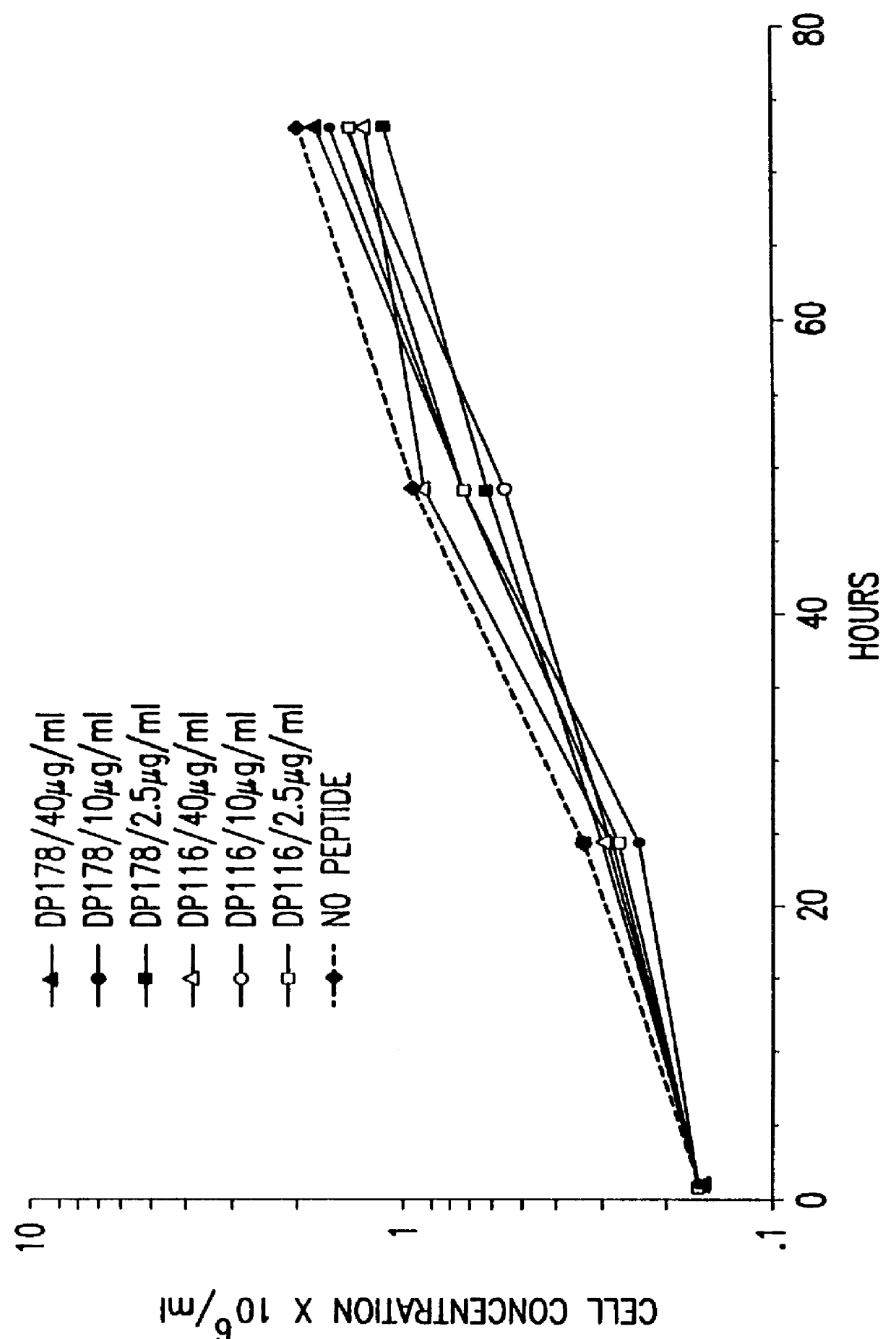
Screening assays for compounds that inhibit membrane fusion-associated events
The present invention relates to peptides which exhibit potent anti-retroviral activity. The peptides of the invention comprise DP178 (SEQ ID:1) peptide corresponding to amino acids 638 to 673 of the HIV-1LAI gp41 protein, and fragments, analogs and homologs of DP178. The invention further relates to the uses of such peptides as inhibitory of human and non-human retroviral, especially HIV, transmission to uninfected cells.
Owner:TRIMERIS
Methods and compositions for treatment with synthetic nanocarriers and immune checkpoint inhibitors
Disclosed are synthetic nanocarrier compositions and immune checkpoint inhibitor compositions and related methods for administration to a subject.
Owner:SELECTA BIOSCI
Vaccine Nanotechnology
ActiveUS20130287857A1Facilitate acquisitionModulating the immune systemNervous disorderAntipyreticDiseaseNanocarriers
The present invention provides compositions and systems for delivery of nanocarriers to cells of the immune system. The invention provides vaccine nanocarriers capable of stimulating an immune response in T cells and / or B cells, in some embodiments, comprising at least one immunomodulatory agent, and optionally comprising at last one targeting moiety and optionally at least one immunostimulatory agent. The invention provides pharmaceutical compositions comprising inventive vaccine nanocarriers. The present invention provides methods of designing, manufacturing, and using inventive vaccine nanocarriers and pharmaceutical compositions thereof. The invention provides methods of prophylaxis and / or treatment of diseases, disorders, and conditions comprising administering at least one inventive vaccine nanocarrier to a subject in need thereof.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +3
Methods and compositions for liposomal formulation of antigens and uses thereof
The present invention relates to liposomal vaccine compositions, methods for the manufacture thereof, and methods for the use thereof to stimulate an immune response in an animal. These compositions comprise dimyristoylphosphatidylcholine (“DMPC”); either dimyristoylphosphatidylglycerol (“DMPG”) or dimyristoyltrimethylammonium propane (“DMTAP”) or both DMPC and DMTAP; and at least one sterol derivative providing a covalent anchor for one or more immunogenic polypeptide(s) or carbohydrate(s).
Owner:RGT UNIV OF CALIFORNIA +1
Compounds and compositions for delivering active agents
InactiveUS6991798B1Increased and improved bioavailabilityOrganic active ingredientsDispersion deliveryActive agentMedicine
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
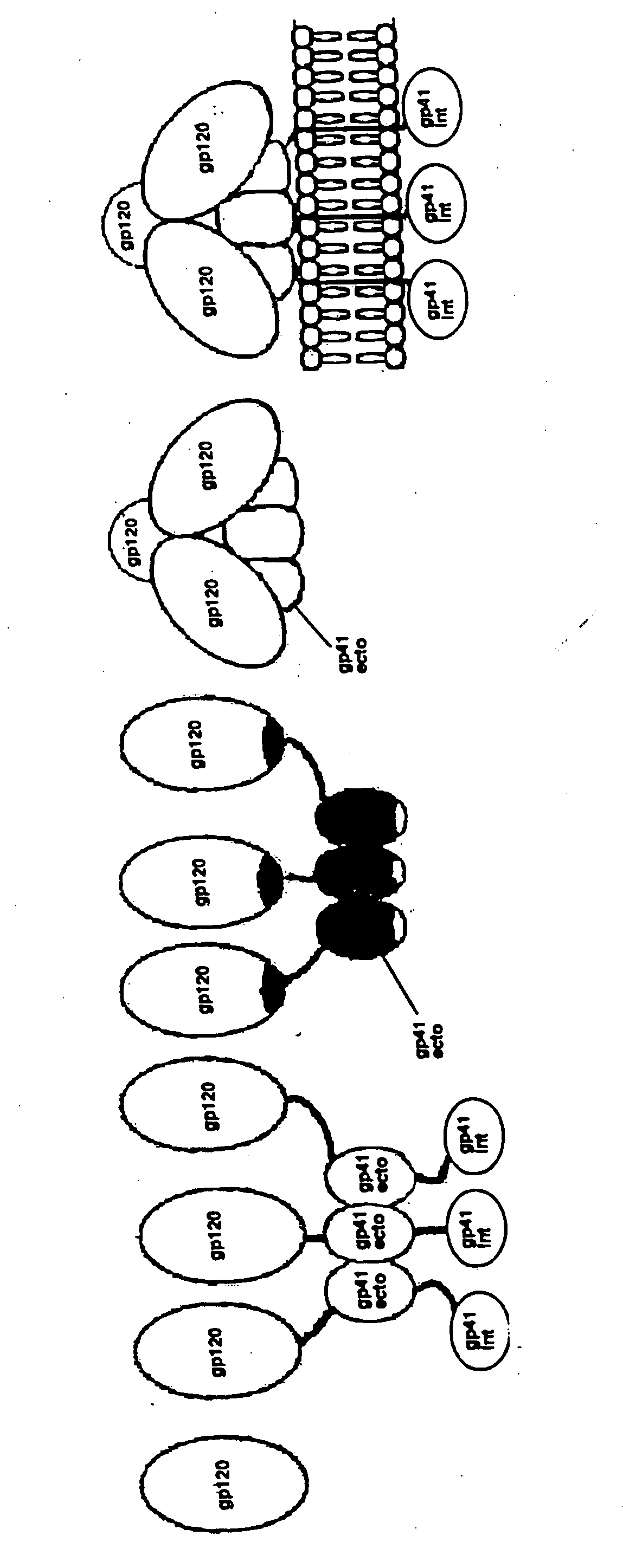
Human immunodeficiency virus envelope clycoprotein mutants and uses thereof
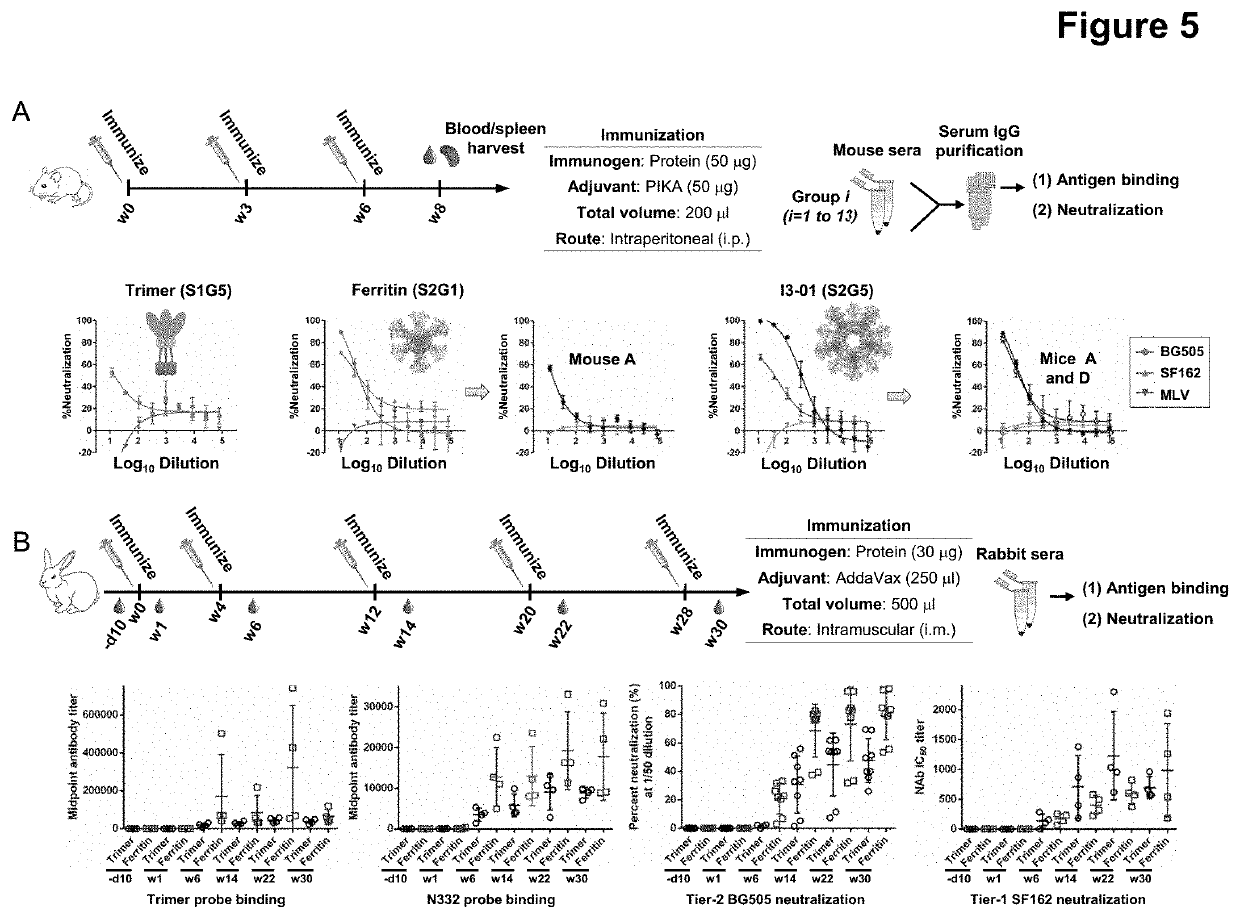
This invention provides stable HIV-1 pre-fusion envelope glycoprotein trimeric complexes. This invention also provides related polypeptides and compositions comprising pharmaceutically acceptable particles and the trimeric complexes operably affixed thereto. This invention further provides related nucleic acids, vectors, host cells, compositions, production methods, and prophylactic and therapeutic methods.
Owner:CORNELL RES FOUNDATION INC +1
Flagellin polypeptide vaccines
InactiveUS20090297552A1Facilitates cellular uptakeSsRNA viruses negative-senseAntibacterial agentsAntigenImmunomodulatory peptide
Vaccines that comprise or generate immunomodulatory flagellin polypeptides able to stimulate an innate immune response intracellularly and extracellularly employ viruses, bacteria or parasitic cells that contain expression systems for such polypeptides, as well as fusion proteins that contain antigens and / or cell penetrating peptides along with the immunomodulatory peptide.
Owner:INSTITUTE FOR SYSTEMS BIOLOGY
Immunoconjugates for programming or reprogramming of cells
InactiveUS20180117171A1Enhance Th immunityIncrease Th responseNervous disorderAntipyreticAutoimmune responsesReprogramming
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Tolerogenic synthetic nanocarriers to reduce immune responses to therapeutic proteins
InactiveUS20160220501A1Reduce in quantityReduce generationOrganic active ingredientsVertebrate antigen ingredientsAntigenNanocarriers
Disclosed are synthetic nanocarrier compositions, and related methods, comprising therapeutic protein APC presentable antigens and immunosuppressants that provide tolerogenic immune responses specific to therapeutic proteins.
Owner:SELECTA BIOSCI
Influenza antigen delivery vectors and constructs
ActiveUS20090191233A1Good curative effectImproving immunogenicitySsRNA viruses negative-sensePeptide/protein ingredientsAntigen deliveryImmunotherapeutic agent
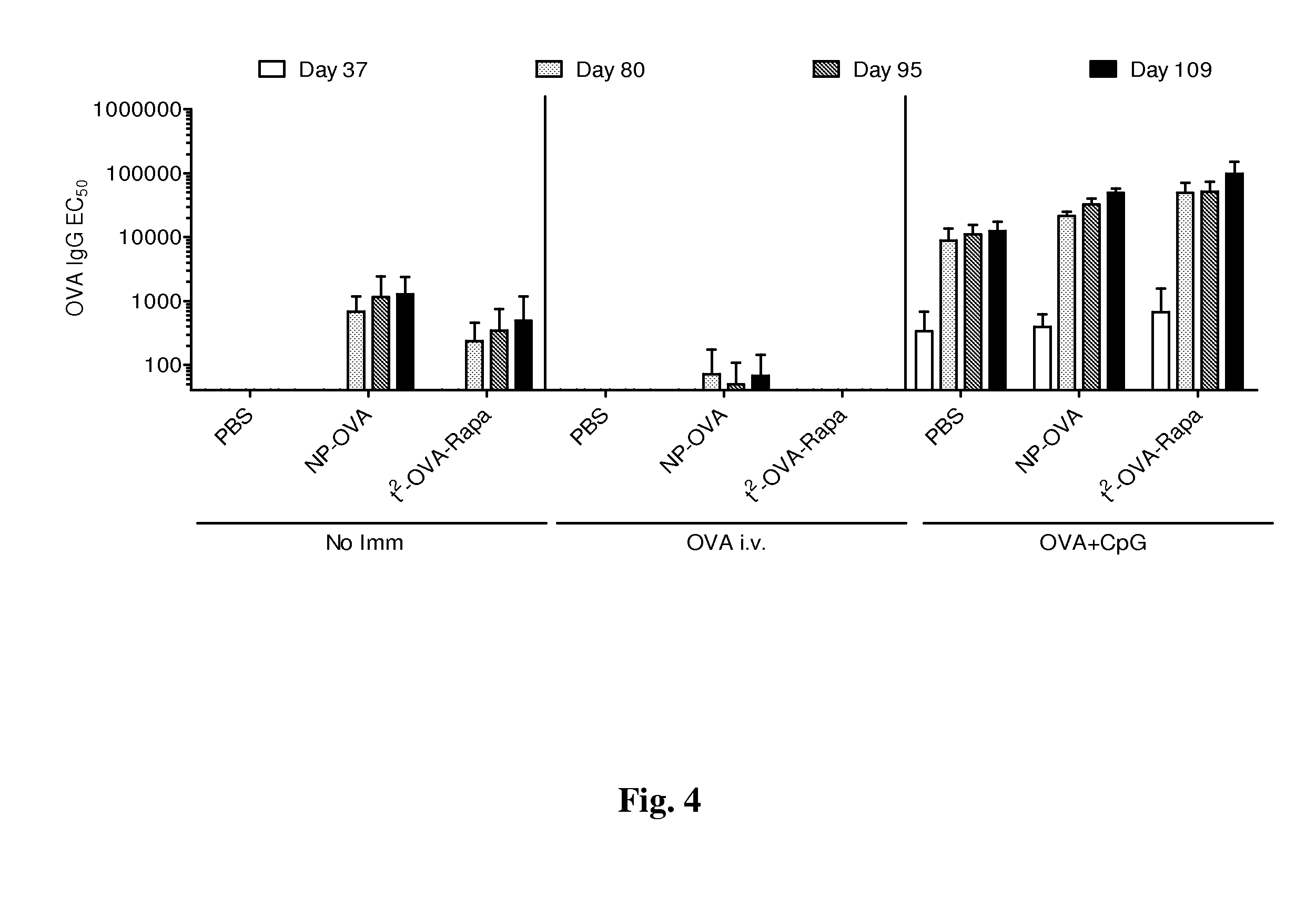
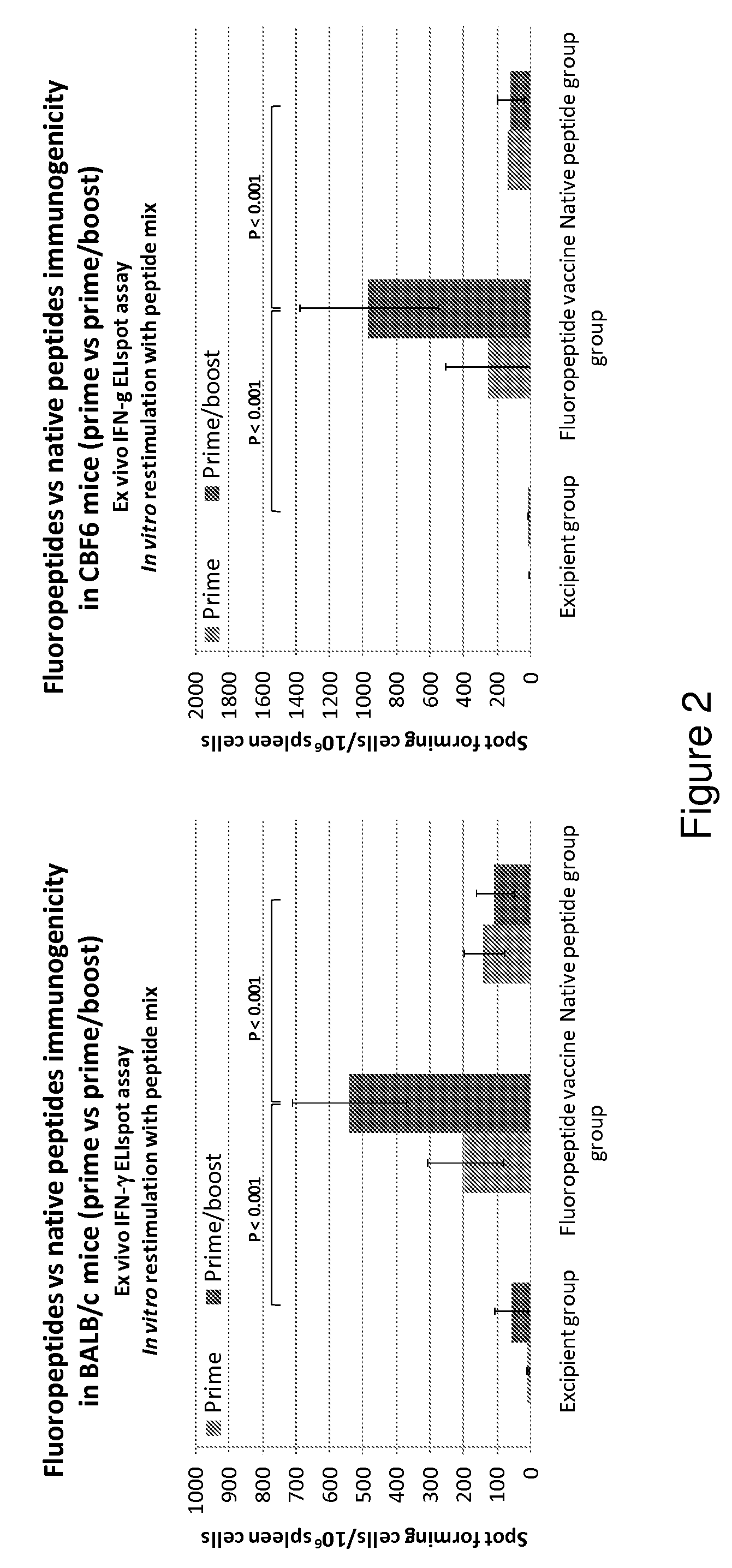
The present invention relates to fluorocarbon vectors for the delivery of influenza antigens to immunoresponsive target cells. It further relates to fluorocarbon vector-influenza antigen constructs and the use of such vectors associated with antigens as vaccines and immunotherapeutics in animals, including humans.
Owner:ALTIMMUNE UK LTD
Glycoconjugate vaccines containing peptidoglycan
InactiveUS20060134141A1Improve efficiencyImproving immunogenicityAntibacterial agentsOrganic active ingredientsMicrobiologyCarrier protein
The present invention relates to vaccines for treating bacterial infections, which vaccines comprise a glycoconjugate immunogen comprising at least one capsular polysaccharide conjugated to a carrier protein, such that the capsular polysaccharide contains an amount of peptidoglycan effective to improve the vaccine's properties.
Owner:NABI BIOPHARMLS
Vaccine compositions having improved stability and immunogenicity
ActiveUS20170202948A1MinimizeImprove stabilitySsRNA viruses negative-senseViral antigen ingredientsNanoparticleImmunogenicity
Disclosed herein are nanoparticles suitable for use in vaccines. The nanoparticles present antigens from pathogens surrounded to and associated with a detergent core resulting in enhanced stability and good immunogenicity. Dosages, formulations, and methods for preparing the vaccines and nanoparticles are also disclosed.
Owner:NOVAVAX
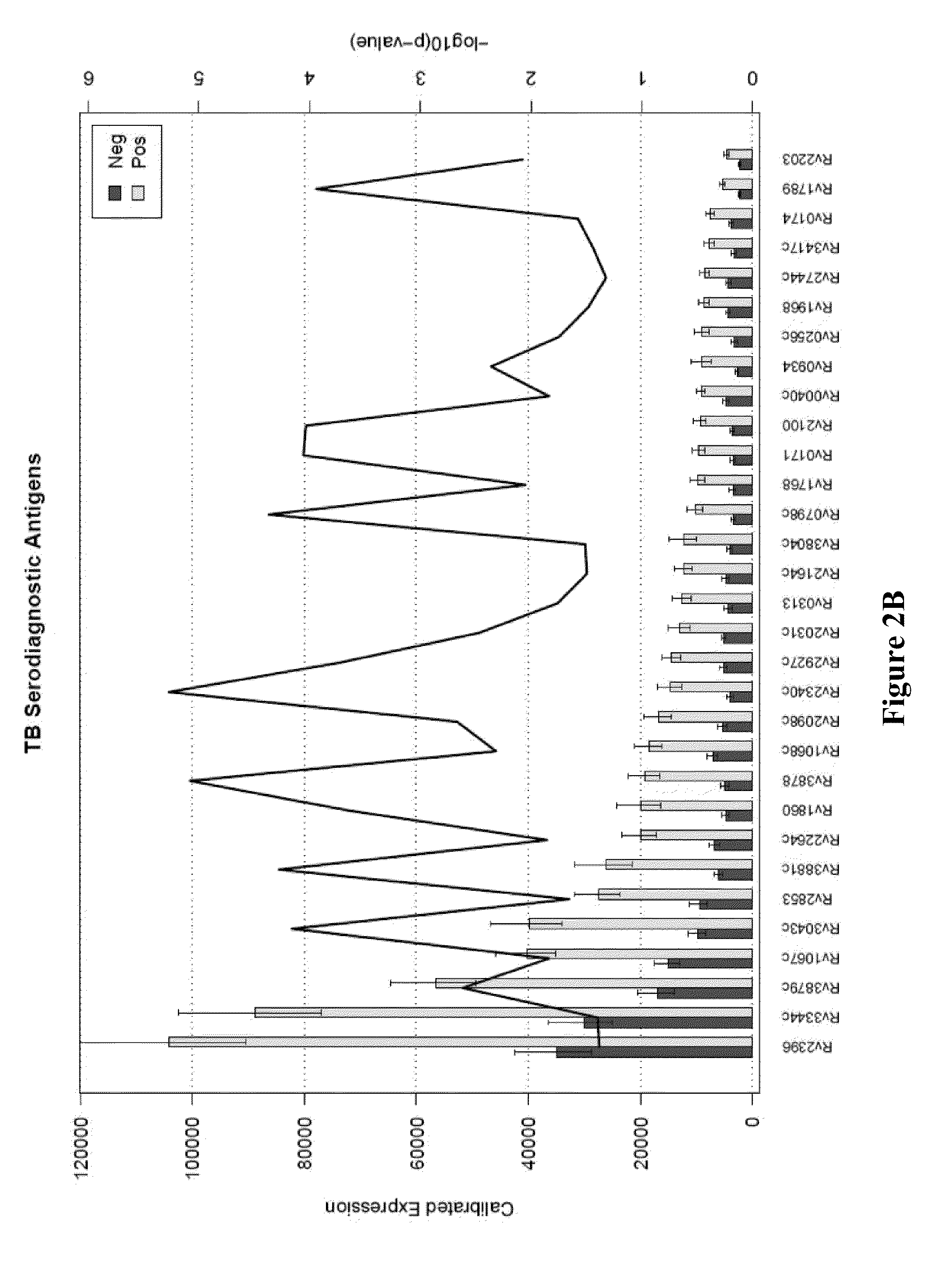
Compositions and methods for immunodominant antigens of Mycobacterium tuberculosis
Contemplated compositions, devices, and methods are drawn to various antigens from the pathogen M. tuberculosis and their use in vaccines, therapeutic agents, and various diagnostic tests. In particularly preferred aspects, the antigens are immunodominant and have quantified and known relative reactivities with respect to sera of a population infected with the pathogen, and / or have a known association with a disease parameter.
Owner:RUTGERS THE STATE UNIV +1
Nanoparticle vaccines with novel structural components
ActiveUS20200009244A1Treating and preventing HIV- infectionAvoid infectionSsRNA viruses negative-sensePowder deliveryEbola virusNanoparti cles
The present invention provides novel nanoparticle presented vaccine compositions that are stabilized with a locking domain. Various immunogens can be employed in the preparation of the vaccine compositions, including viral immunogens such as HIV-1 and Ebola viral immunogens, and non-viral immunogens such as immunogens derived from bacteria, parasites and mammalian species. The invention also provides methods of using such vaccine compositions in various therapeutic applications, e.g., for preventing or treating viral infections.
Owner:THE SCRIPPS RES INST
Conjugates for immunotherapy
ActiveUS20170202902A1Enhance immune responseControlling signalPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseMelanoma
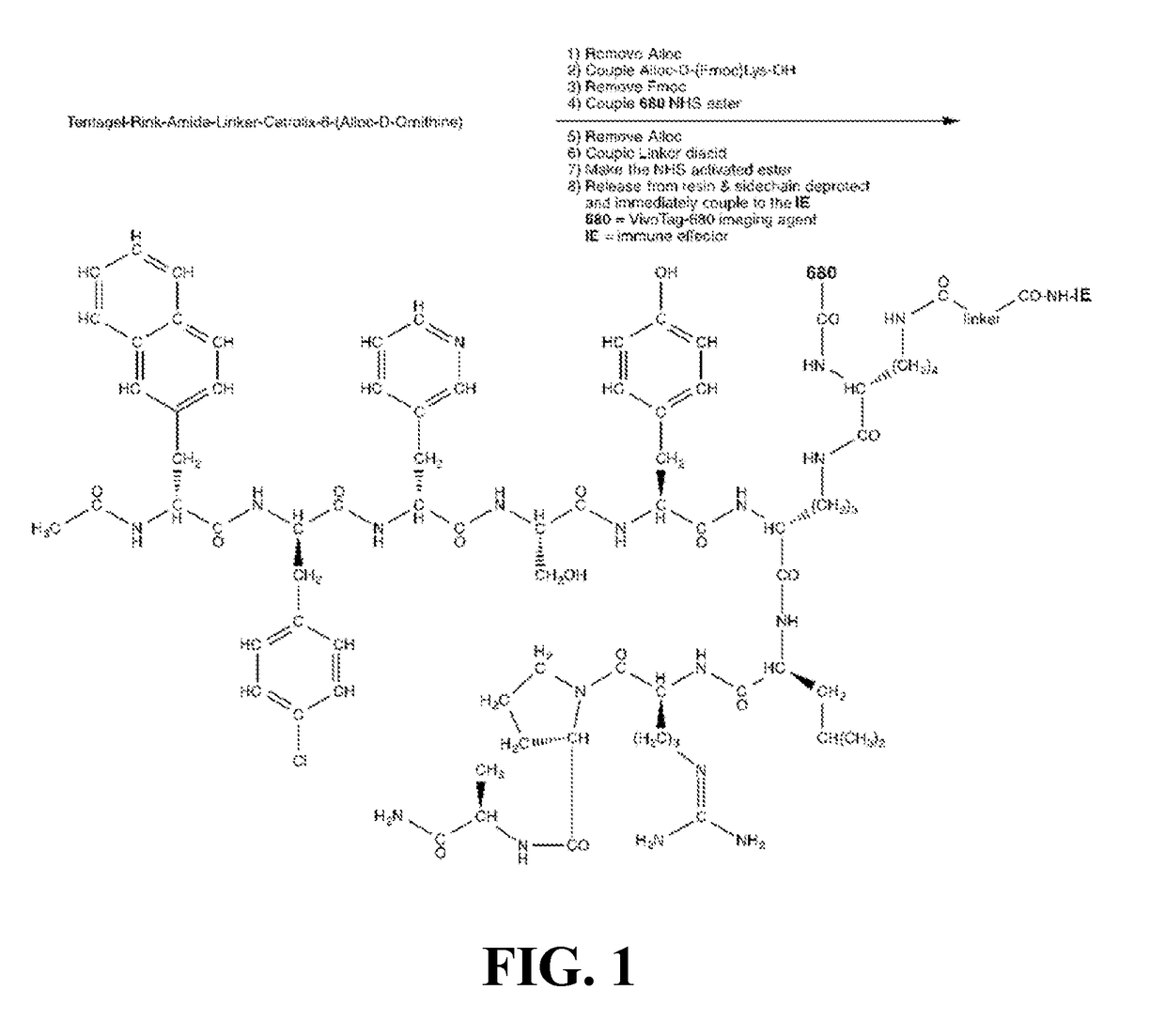
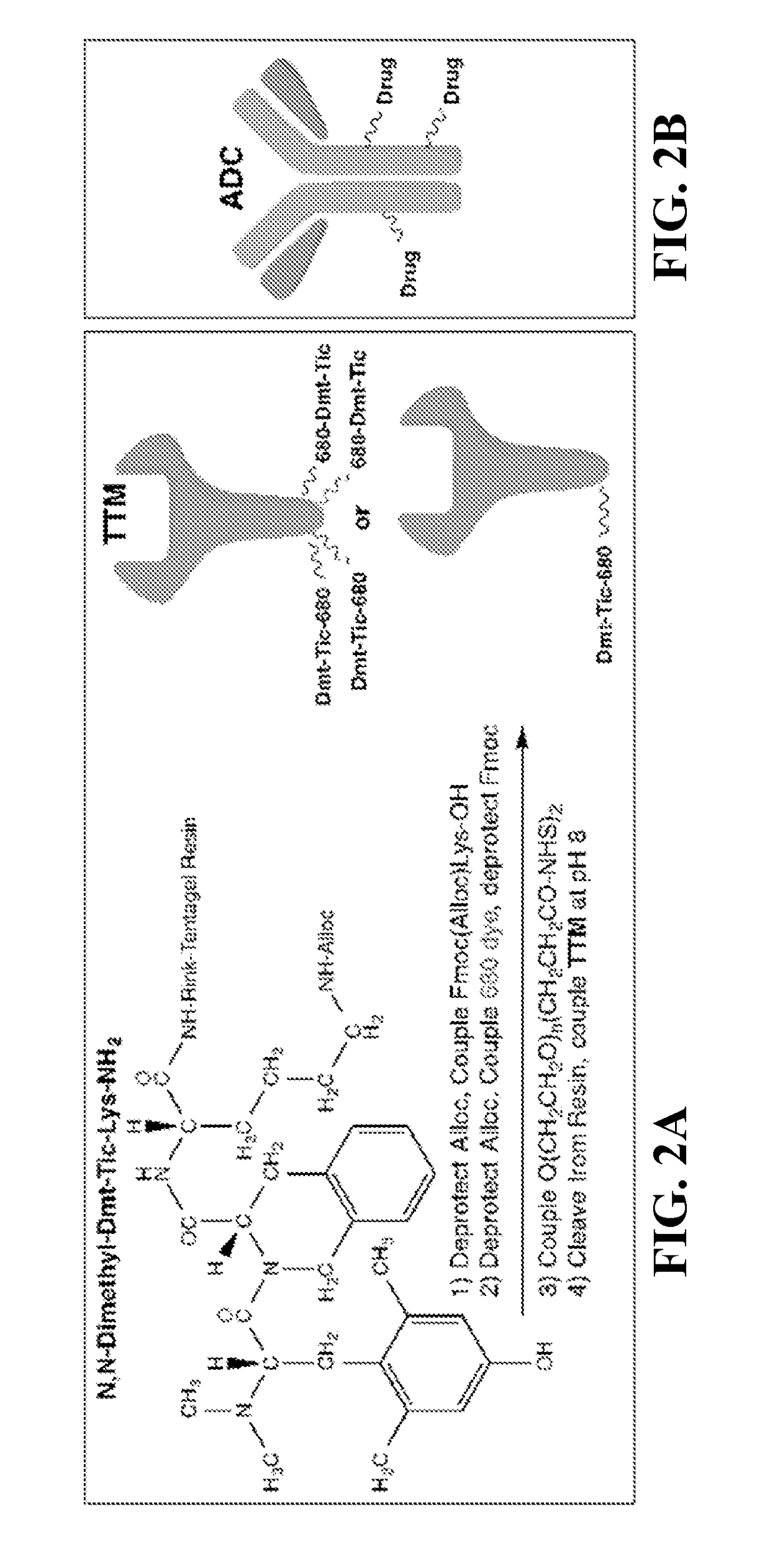
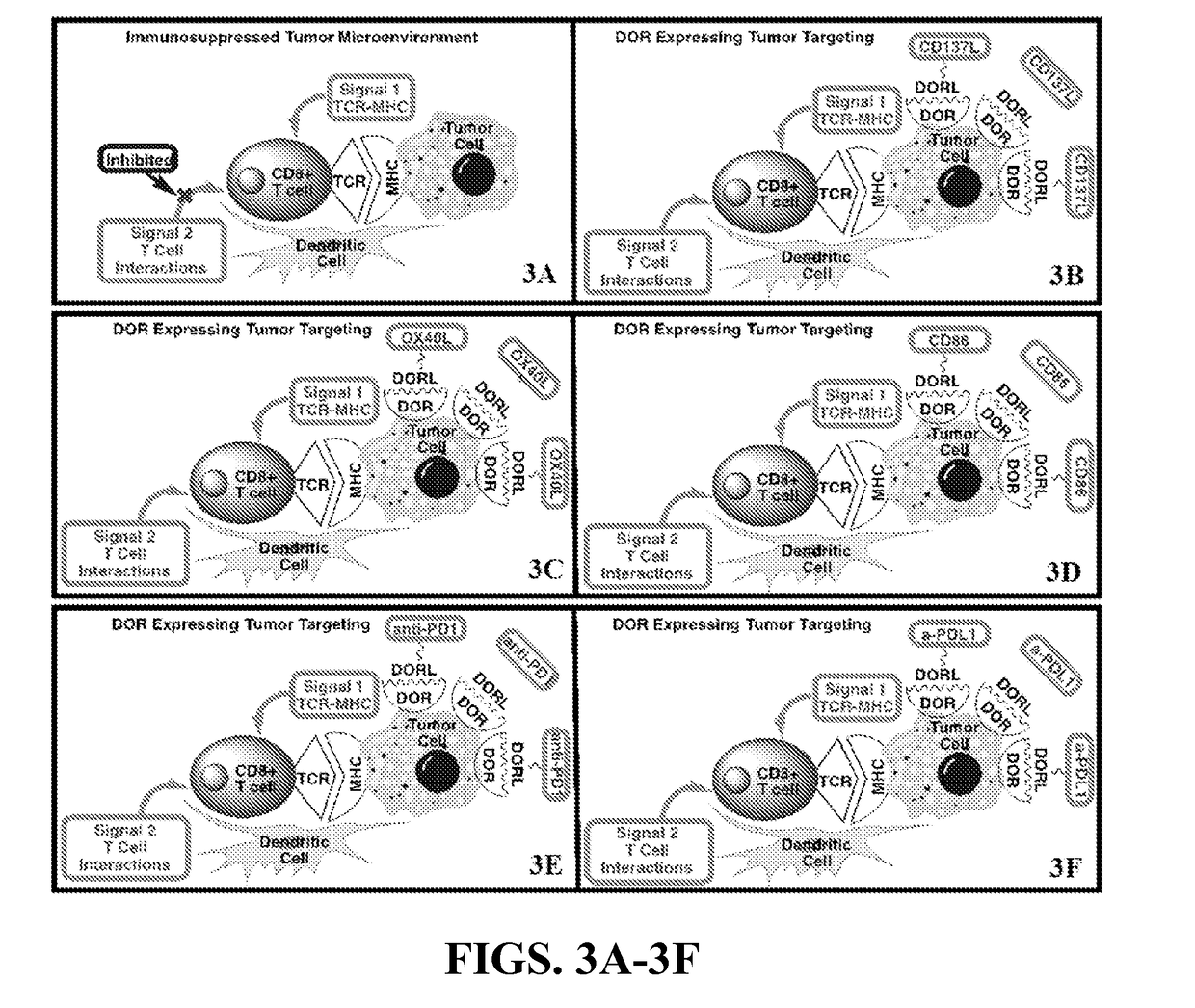
The current invention pertains to a molecular conjugate comprising an antagonist of a cell surface receptor specific to a target cell and an immune effector, such as a T cell modulator, conjugated to the antagonist. The target cell can be a cell responsible for development of a disease in a subject, for example, a cancer cell. In certain embodiments, the immune effector is an immune effector protein or an immune effector fragment thereof. The current invention also pertains to a method of treating a disease in a subject, the method comprising administering to the subject a pharmaceutically effective amount of the molecular conjugates of the current invention to the subject. The methods of the current invention can be used to treat cancer, such as breast cancer, ovarian cancer, prostate cancer, lung cancer, pancreatic cancer, or melanoma.
Owner:UNIV OF SOUTH FLORIDA +1
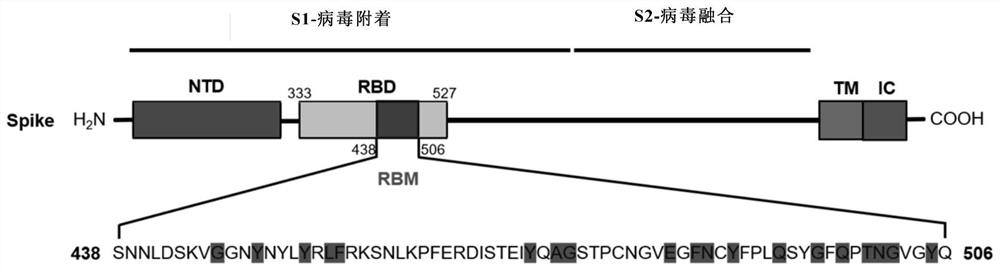
Novel coronavirus polypeptide vaccine coupled with TLR7 agonist and application of novel coronavirus polypeptide vaccine
ActiveCN111892648ASsRNA viruses positive-senseViral antigen ingredientsMolecular biologyImmune effects
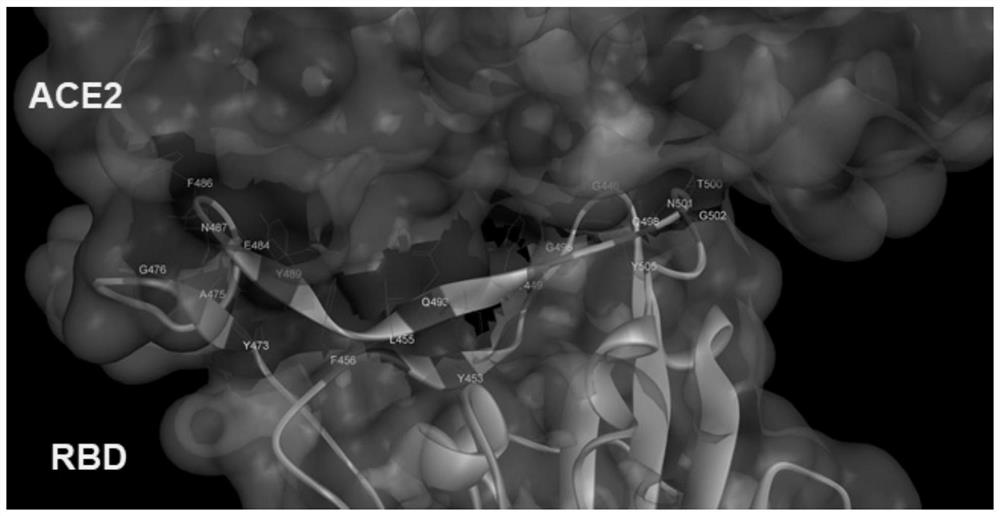
The invention discloses a novel coronavirus polypeptide vaccine coupled with a TLR7 agonist and application of the novel coronavirus polypeptide vaccine. Specifically, the invention provides a novel vaccine polypeptide for coronavirus pneumonia based on analysis and research of an RBD sequence and structural information of an S protein of SARS-CoV-2, the vaccine polypeptide has the following formula structure: Z-(J-U) n, and in the formula, Z, J, U, n and the like are as defined in the specification. The invention further provides a vaccine composition containing the vaccine polypeptide and application of the composition. Experiments show that the vaccine polypeptide can enable mice and cynomolgus monkeys to start strong cell and humoral immune effects, generates a neutralizing antibody for blocking combination of RBD and ACE2, and can be used for preventing and treating novel coronavirus pneumonia.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Compositions of vaccines and adjuvants and methods for the treatment of urinary tract infections
ActiveUS9415097B2Efficient and cost-effectiveLittle aggregationInorganic non-active ingredientsGranular deliveryEscherichia coliAdjuvant
This invention describes novel adjuvant compositions and formulations with excellent stability at refrigerated and room temperatures and up to and about 37° C. that can be produced at remarkably low costs. This invention describes novel vaccine compositions and formulations to treat and prevent urinary tract infections caused by gram-negative bacteria including Escherichia coli and multi-drug resistant E. coli. This invention also describes methods of administration of said novel vaccine compositions and formulations and methods of treatment to prevent and treat urinary tract infections caused by gram-negative bacteria including E. coli and multi-drug resistant E. coli.
Owner:SEQUOIA VACCINES INC
A preparing method of a recombinant fusion protein modified with bacterial polysaccharides and applications thereof
ActiveCN105695497AUniform polysaccharide binding siteQuality controllableAntibacterial agentsBacterial antigen ingredientsConjugate vaccineBacteroides
A preparing method of a recombinant fusion protein modified with bacterial polysaccharides and applications thereof are disclosed. The method includes co-expressing a recombinant fusion protein and neisseria meningitide O-oligosaccharyltransferase PglL in a bacterium with O-antigen ligase gene defect, and allowing polysaccharides of the bacterium or exogenous polysaccharides to be connected to the recombinant fusion protein through the neisseria meningitide O-oligosaccharyltransferase PglL to obtain the recombinant fusion protein modified with the bacterial polysaccharides. A specific antibody resisting bacterial polysaccharides can be prepared through immunizing mice with the prepared recombinant fusion protein modified with the bacterial polysaccharides. Through applying the recombinant fusion protein modified with the bacterial polysaccharides to preparation of bacterial polysaccharide protein conjugate vaccines, a plurality of problems of culturing of pathogenic bacteria can be avoided, homogeneity and a production efficiency of the vaccines can be increased and a vaccine preparing cost can be reduced.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Glycotargeting therapeutics
ActiveUS10046056B2Reduced responseRelieve symptomsPolypeptide with localisation/targeting motifPeptide/protein ingredientsAntigenAutoimmune condition
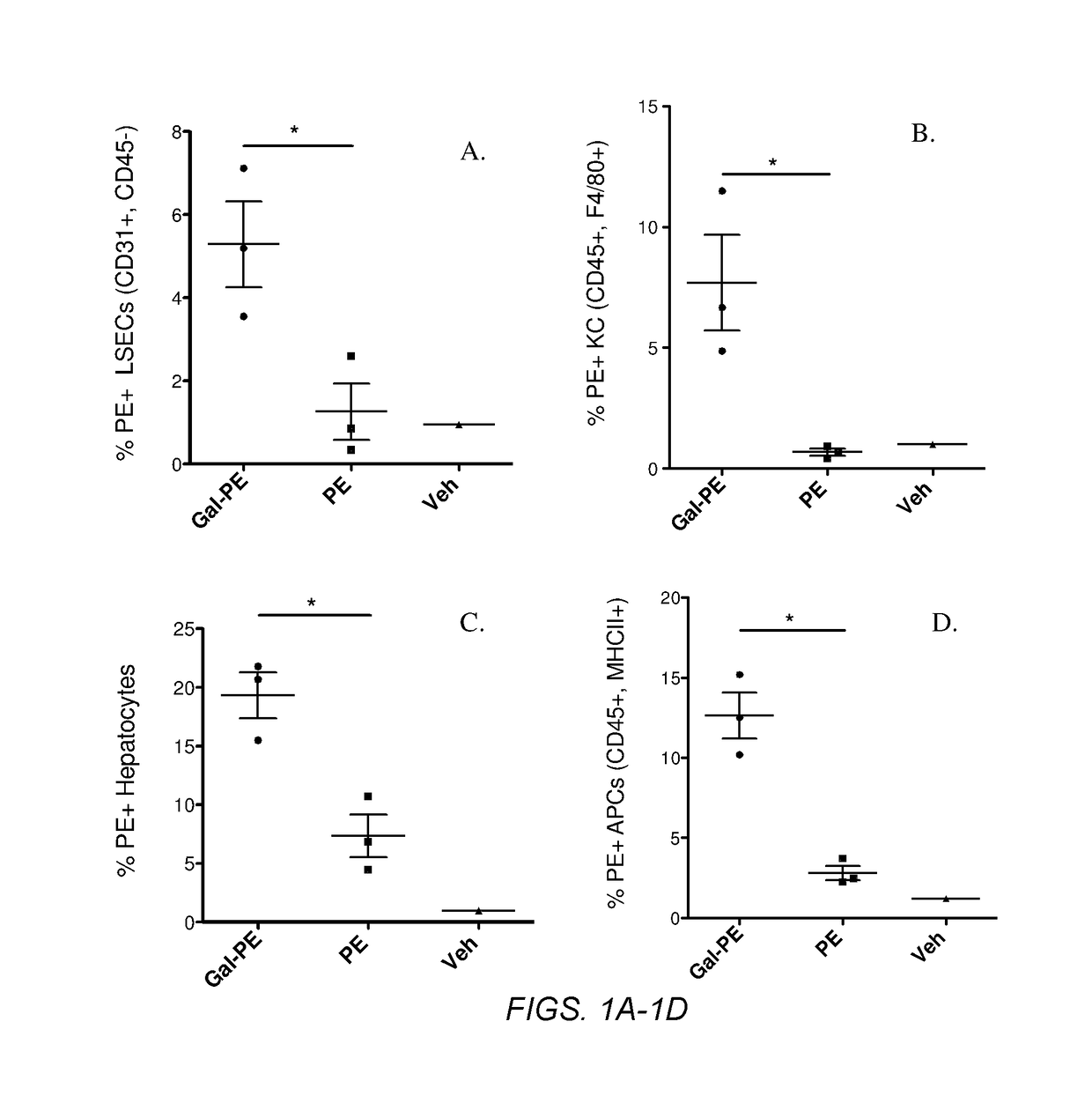
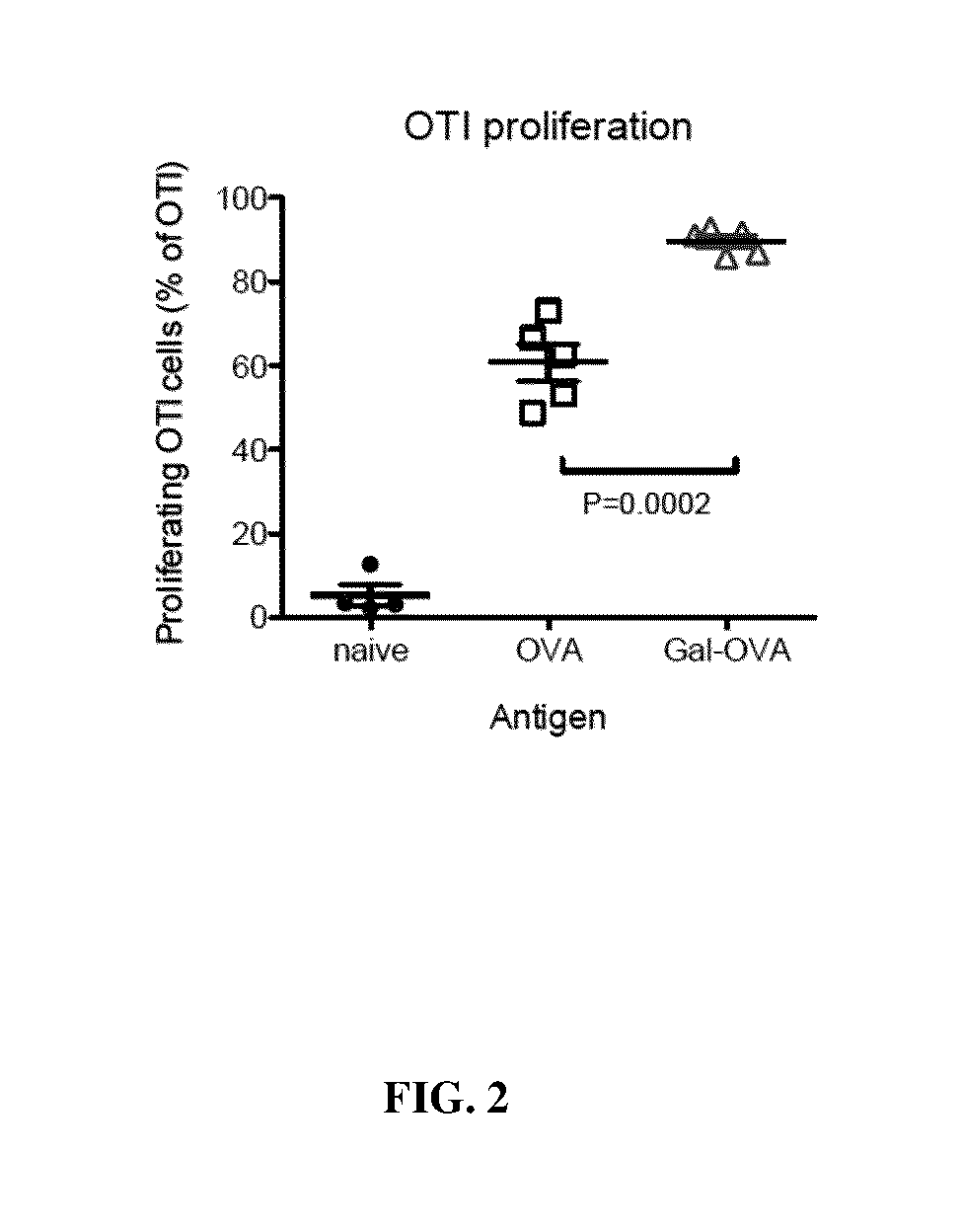
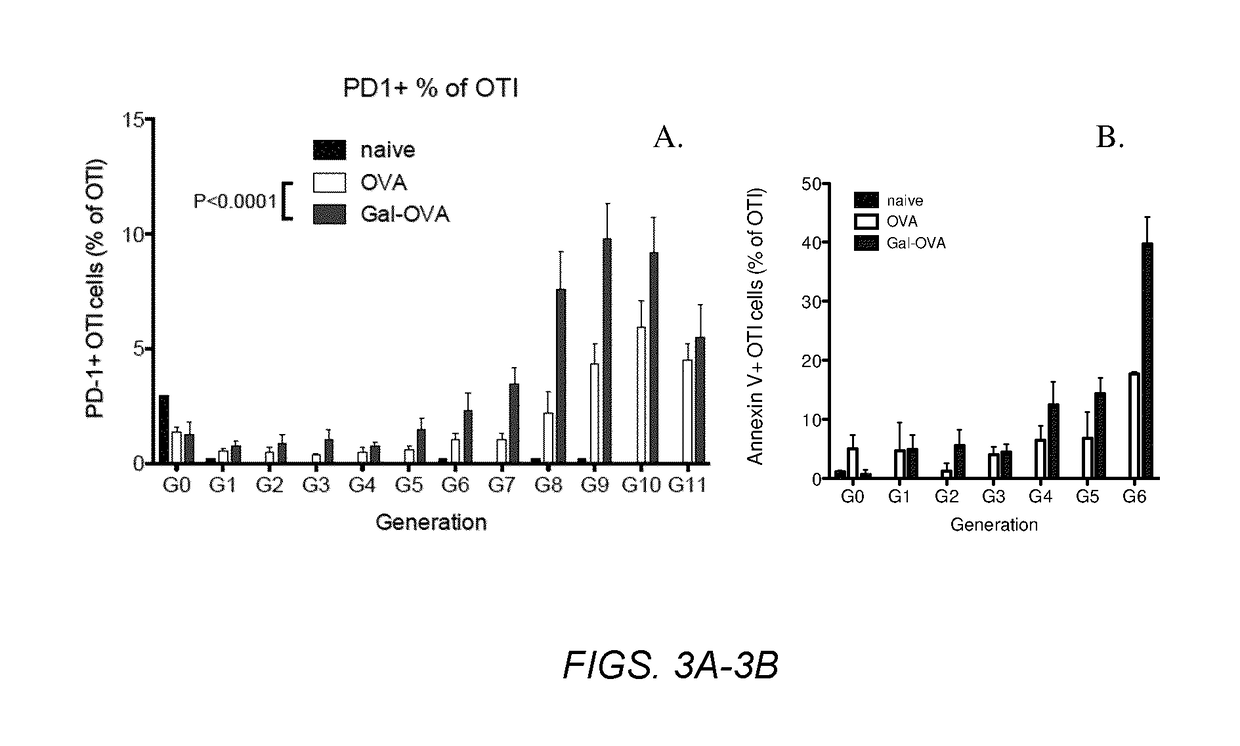
Several embodiments of the present disclosure relate to glycotargeting therapeutics that are useful in the treatment of transplant rejection, autoimmune disease, food allergy, and immune response against a therapeutic agent. In several embodiments, the compositions are configured to target the liver and deliver antigens to which tolerance is desired. Methods and uses of the compositions for induction of immune tolerance are also disclosed herein.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
Method of preparing an immunologically-active adjuvant-bound dried vaccine composition
ActiveUS8444991B2Reduce concentrationAntibacterial agentsPowder deliveryAdjuvantClostridial Neurotoxin
The disclosure provides a method of preparing an immunologically-active adjuvant-bound freeze dried vaccine composition. A specific embodiment provides a stable vaccine composition comprising an aluminum-salt adjuvant, a recombinant Clostridium botulinum neurotoxin protein and a glass-forming agent. These vaccine compositions are useful in the treatment of humans and other animals at risk of infection from Clostridium botulinum neurotoxin.
Owner:UNIV OF COLORADO THE REGENTS OF
Influenza antigen delivery vectors and constructs
ActiveUS8642531B2SsRNA viruses negative-senseHalogenated hydrocarbon active ingredientsAntigen deliveryImmunotherapeutic agent
The present invention relates to fluorocarbon vectors for the delivery of influenza antigens to immunoresponsive target cells. It further relates to fluorocarbon vector-influenza antigen constructs and the use of such vectors associated with antigens as vaccines and immunotherapeutics in animals, including humans.
Owner:ALTIMMUNE UK LTD
IL-1alpha IMMUNIZATION INDUCES AUTOANTIBODIES PROTECTIVE AGAINST ATHEROSCLEROSIS
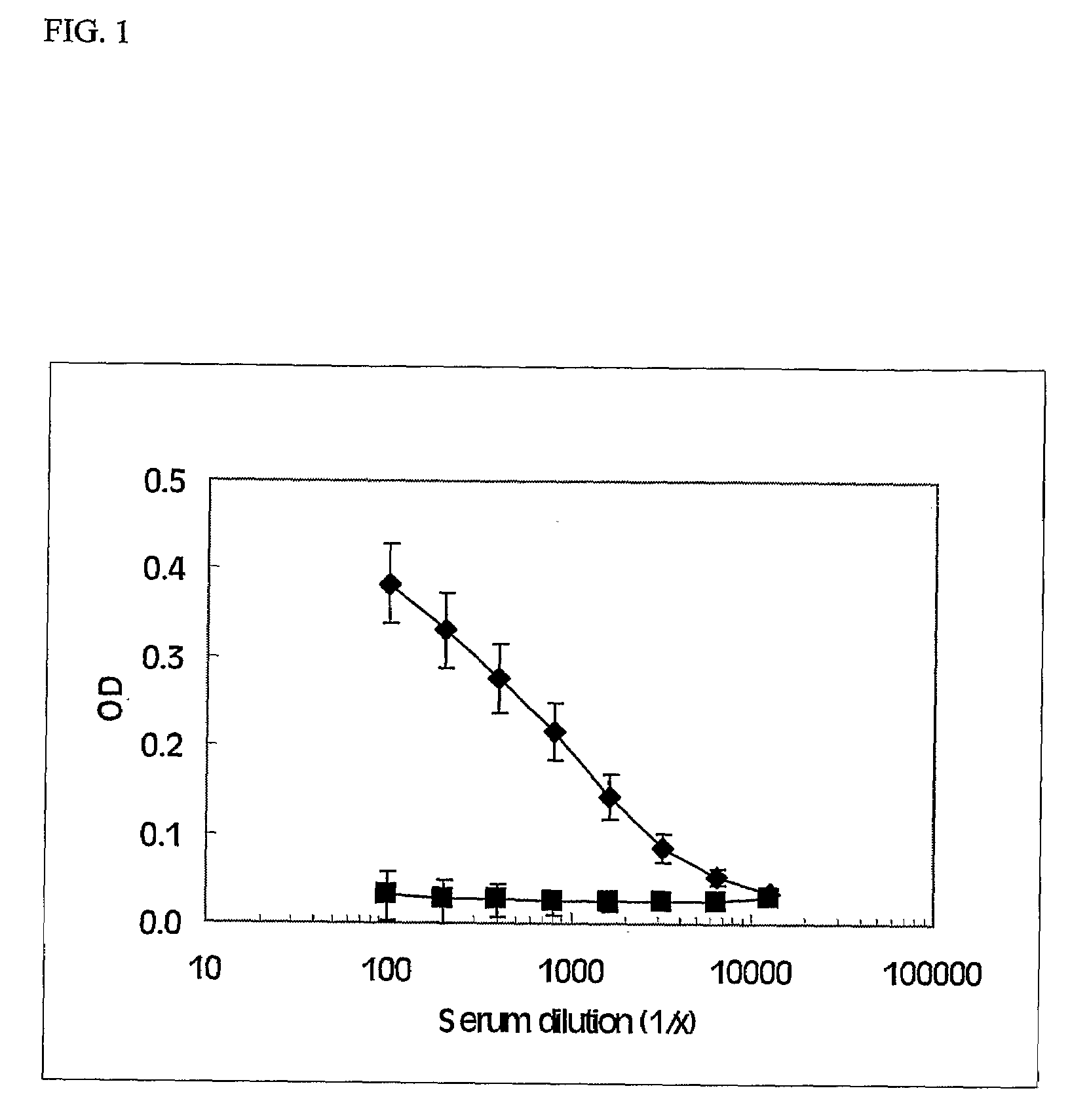
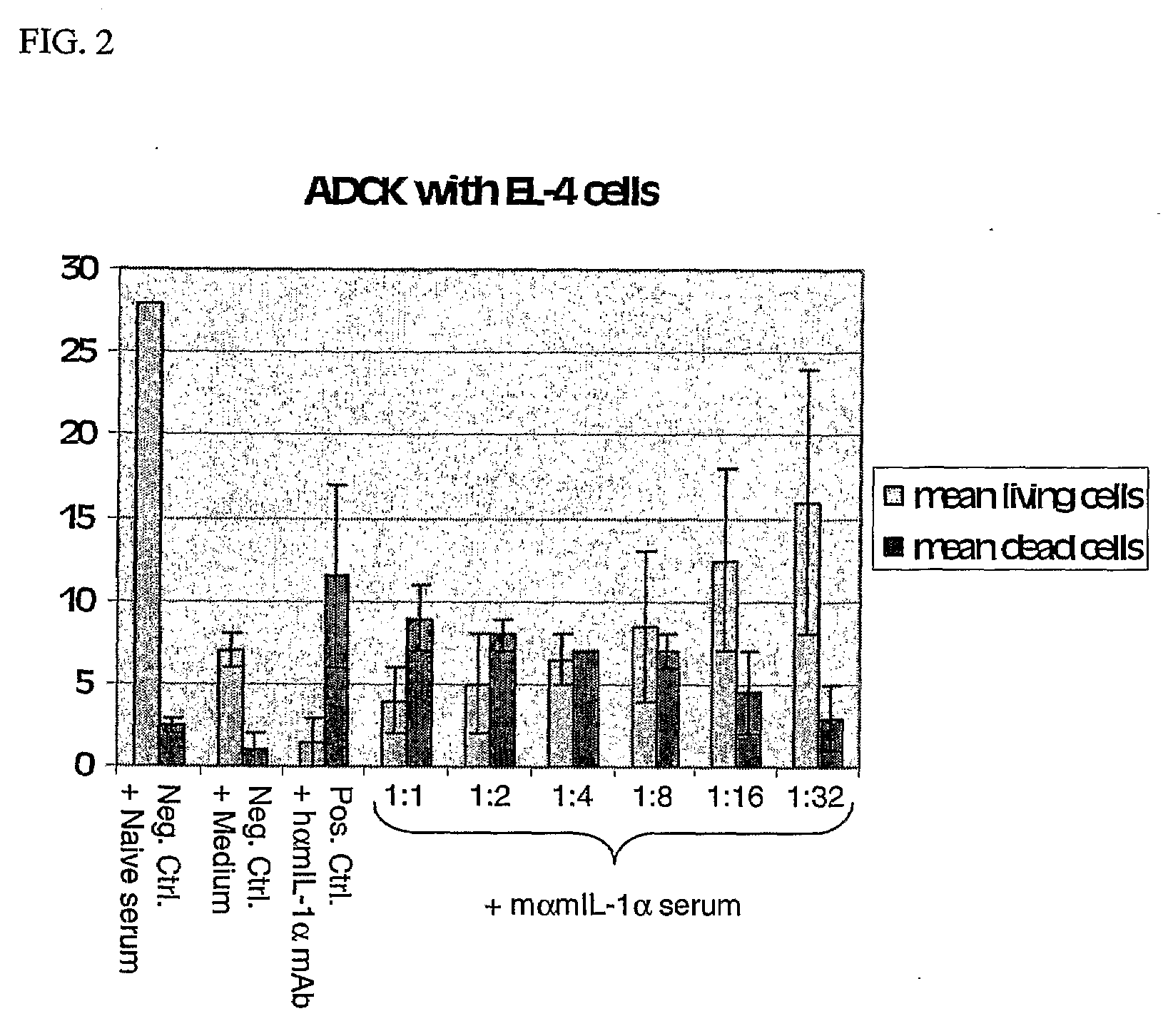
InactiveUS20090191149A1Lower Level RequirementsReduce morbidityPeptide/protein ingredientsViral antigen ingredientsCoronary artery diseaseCvd risk
Immunization of a mammal with IL-1α, which causes the mammal to generate IL-1α autoantibodies, can be used to reduce the risk and severity of, or to reduce progression of, an atherosclerosis-related disease in the mammal. Progression of atherosclerosis-related diseases such as peripheral ischemic heart disease, coronary artery disease, cerebrovascular disease, and peripheral arterial disease can be reduced using this treatment.
Owner:XBIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com