A preparing method of a recombinant fusion protein modified with bacterial polysaccharides and applications thereof
A fusion protein and bacterial polysaccharide technology, applied in the field of biomedicine, can solve problems such as poor product uniformity, difficulties in purification and quality control, and many steps in the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Example 1. Preparation of Shigella flexneri O-glycosylated recombinant fusion protein and its vaccine by one-step biological cross-linking method
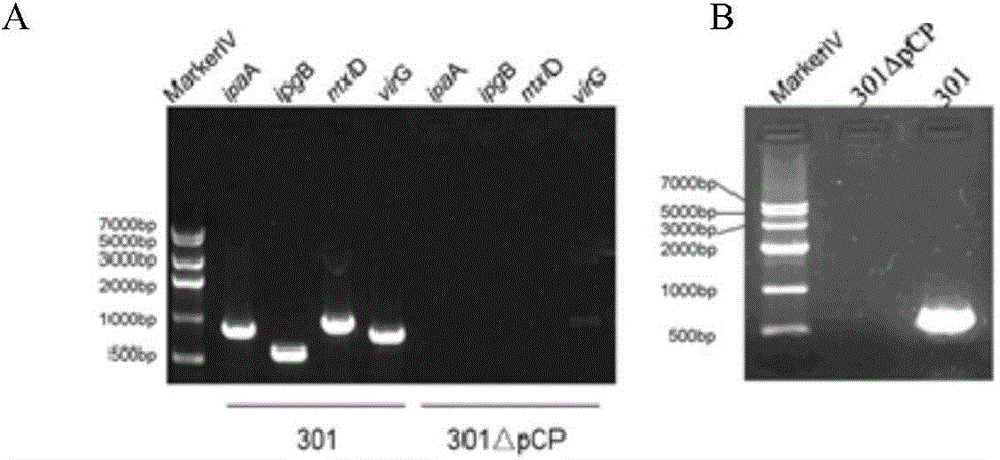
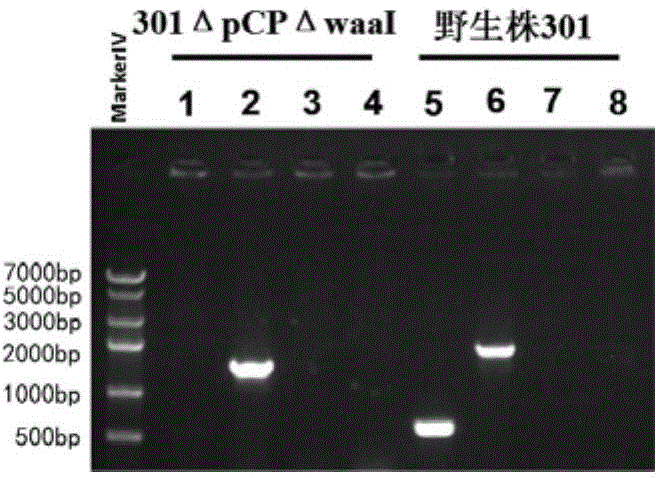
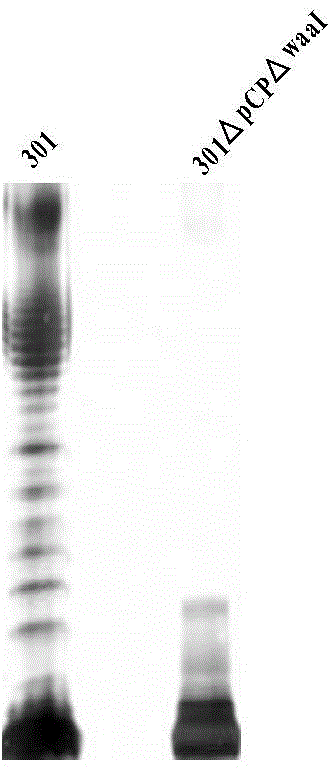
[0140] Shigella (Shigella spp.), commonly known as Shigella, is a highly contagious Gram-negative enteropathogenic bacterium, which mainly invades human colonic epithelial cells and finally locates in the large intestine, causing typical bacillary dysentery (fever , abdominal pain, tenesmus, fecal pus and blood), and the large virulence plasmid is the most important factor of its pathogenicity. The large virulence plasmid contains about 32 genes related to virulence, mainly including the type III secretion system Related virulence genes such as mxi-spa gene, ipaBCD and ipgC are closely related to bacterial invasion of epithelial cells. In order to develop it as a safe host bacterium, the large plasmid pCP encoding the virulence factor must be removed first, and on this basis, the O antigen ligase gene waaI is deficient, ther...
Embodiment 2
[0337] Example 2. Preparation of Salmonella paratyphi A O polysaccharide-recombinant CTB fusion protein by one-step biological cross-linking method
[0338] 1. Preparation of Salmonella paratyphi A deficient in O-antigen ligase gene waaL
[0339] (1) Preparation of linear targeting DNA fragments
[0340] 1. Design and synthesis of PCR primers
[0341] According to the waaL gene (GeneBank No. 3696236-3697450 of CP000026) listed in the whole genome sequence (NC_006511) of Salmonella paratyphi A 50973 strain (S.paratyphiCMCC50973) on NCBI and its upstream and downstream sequences, the upstream of the waaL gene (5' end) and downstream (3' end) respectively design a pair of primers, namely 73waaLu1 / 73waaLu2 and 73waaLd1 / 73waaLd2. For the convenience of operation, restriction enzyme cutting sites BamHI and SalI will be added to the end of the primer of the upstream homology arm up, and restriction enzyme cutting sites HindIII and XhoI will be added to the end of the primer of the ...
Embodiment 3
[0396] Example 3, Modification of Recombinant CTB Fusion Protein Using Exogenous O157 Escherichia coli O Polysaccharide
[0397] When exogenous polysaccharides are used to modify recombinant fusion proteins, bacteria that are double-deficient in O-antigen ligase gene and host O antigen synthesis should be used as the host. None of the polysaccharides can be used by the host LPS synthesis pathway, and the host's O antigen synthesis defect ensures that the glycosylation modification system only uses exogenous polysaccharides to modify the recombinant fusion protein, and does not appear to be modified by the host's own O antigen polysaccharides. pollution phenomenon. The host bacteria co-express the recombinant fusion protein gene, Neisseria meningitidis O-oligosaccharide transferase PglL gene, and exogenous polysaccharide synthesis gene cluster. Under the catalysis of oligosaccharide transferase PglL, the exogenous polysaccharide is transferred to the recombinant fusion protein ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com