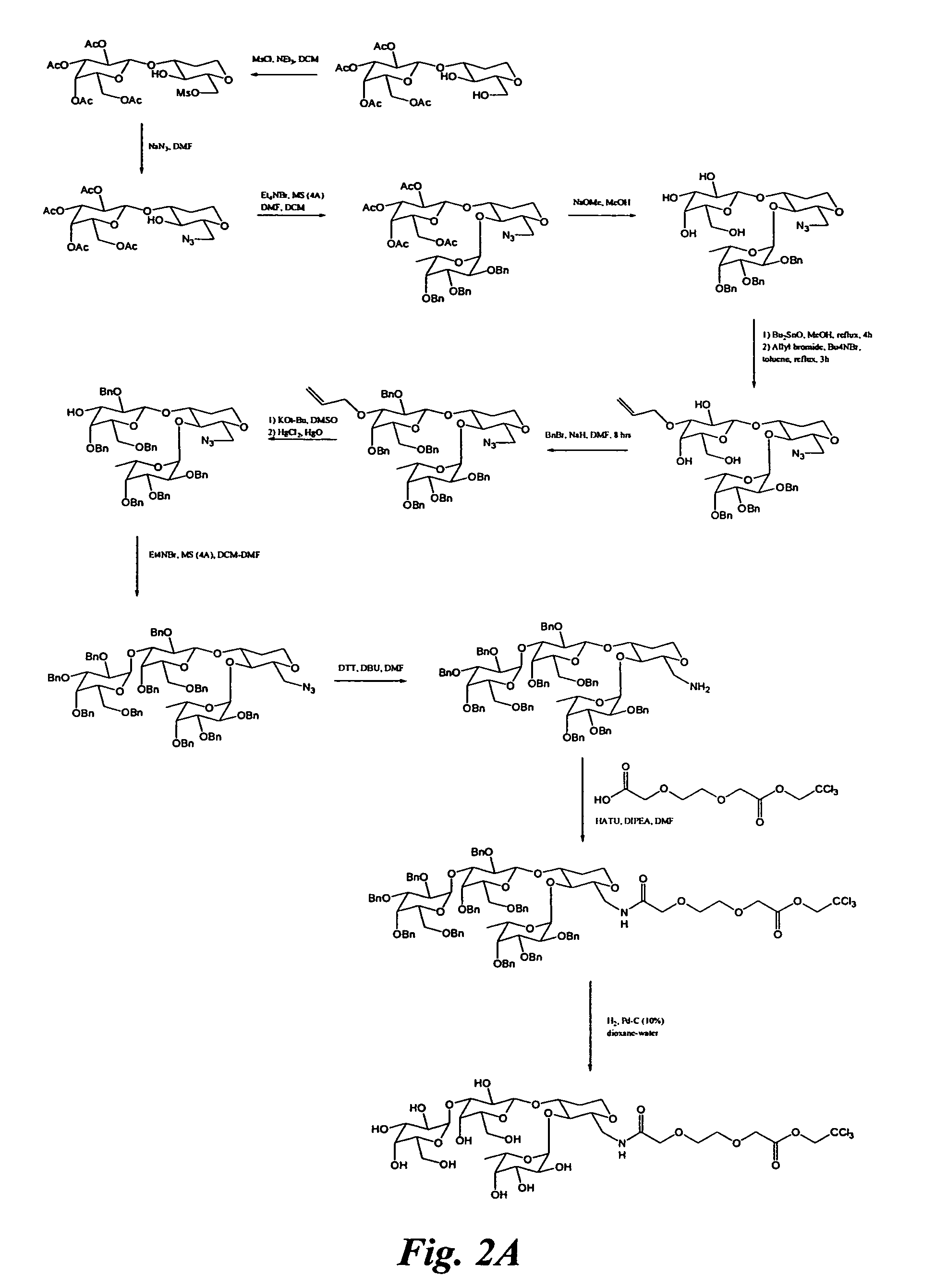
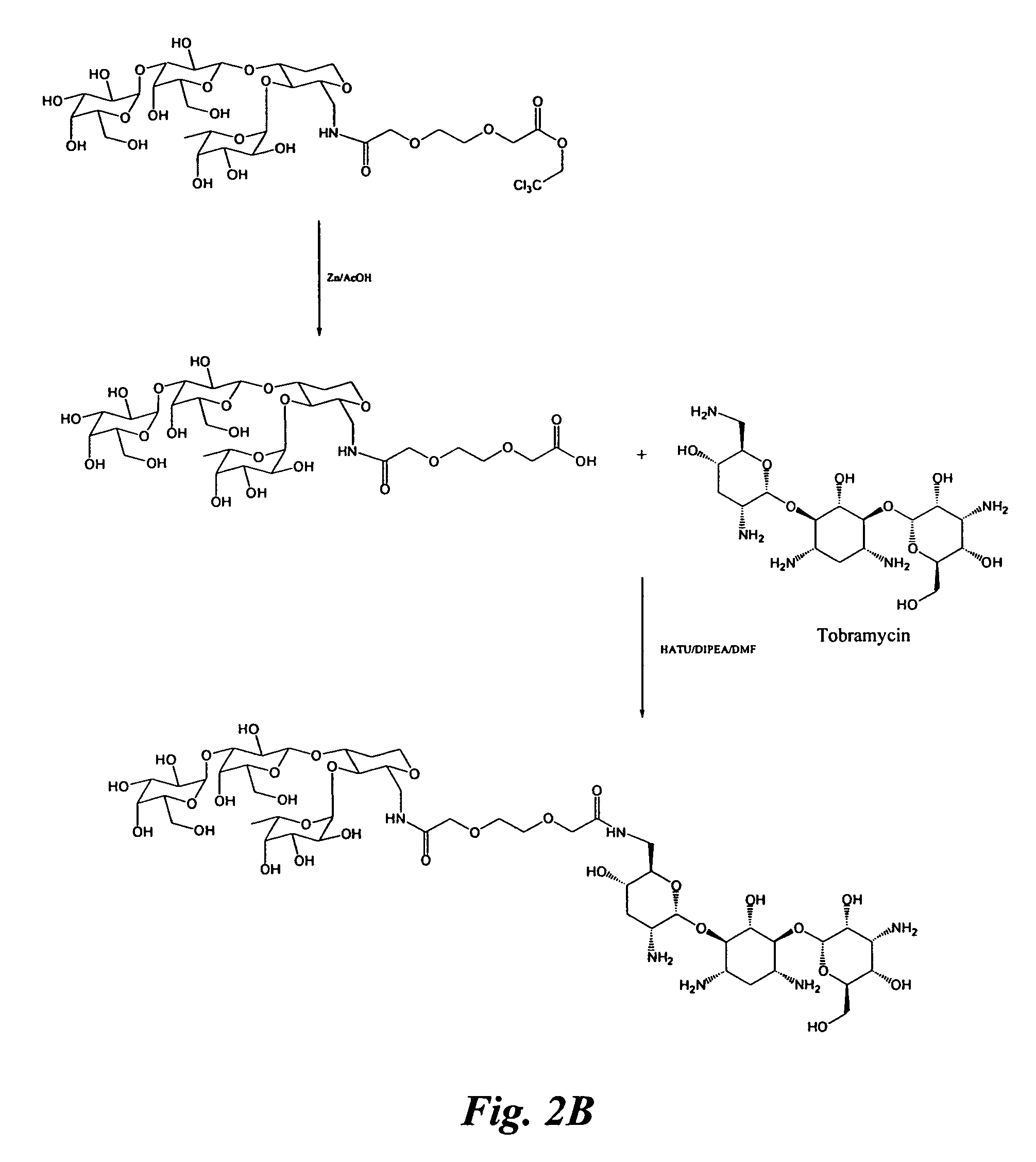
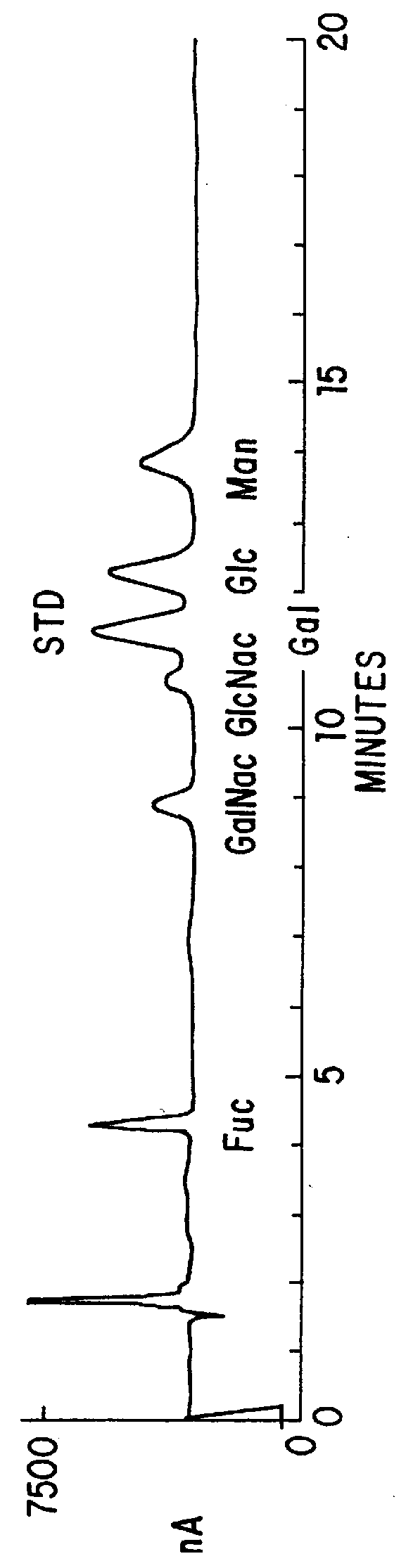
Patents
Literature
248 results about "Virulence factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
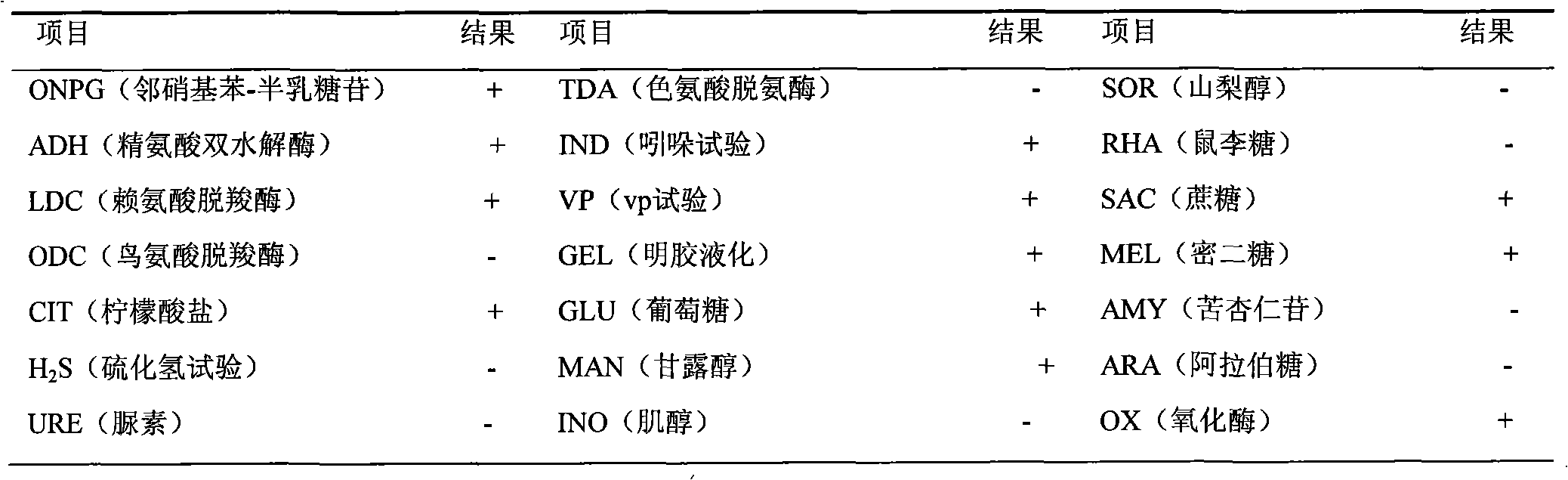
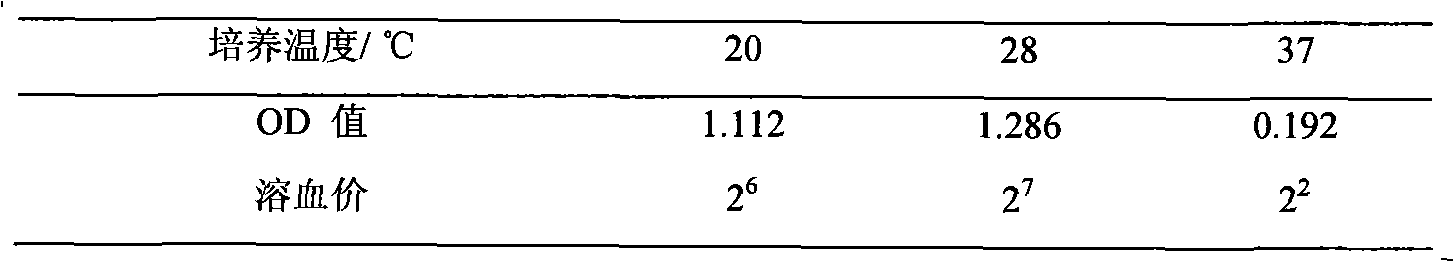
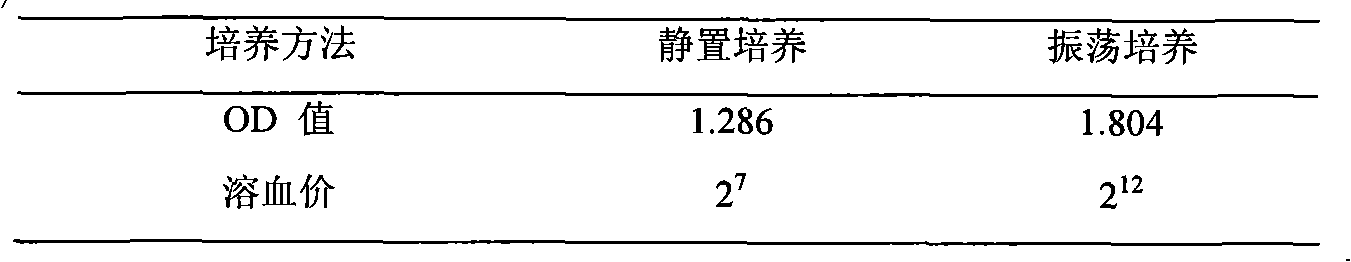
Virulence factors are molecules produced by bacteria, viruses, fungi, and protozoa that add to their effectiveness and enable them to achieve the following...
Pharmaceutical compositions and methods to vaccinate against candidiasis
InactiveUS20030124134A1Treat prevent alleviateToxic reductionPeptide/protein ingredientsSnake antigen ingredientsHospitalized patientsVirulent characteristics
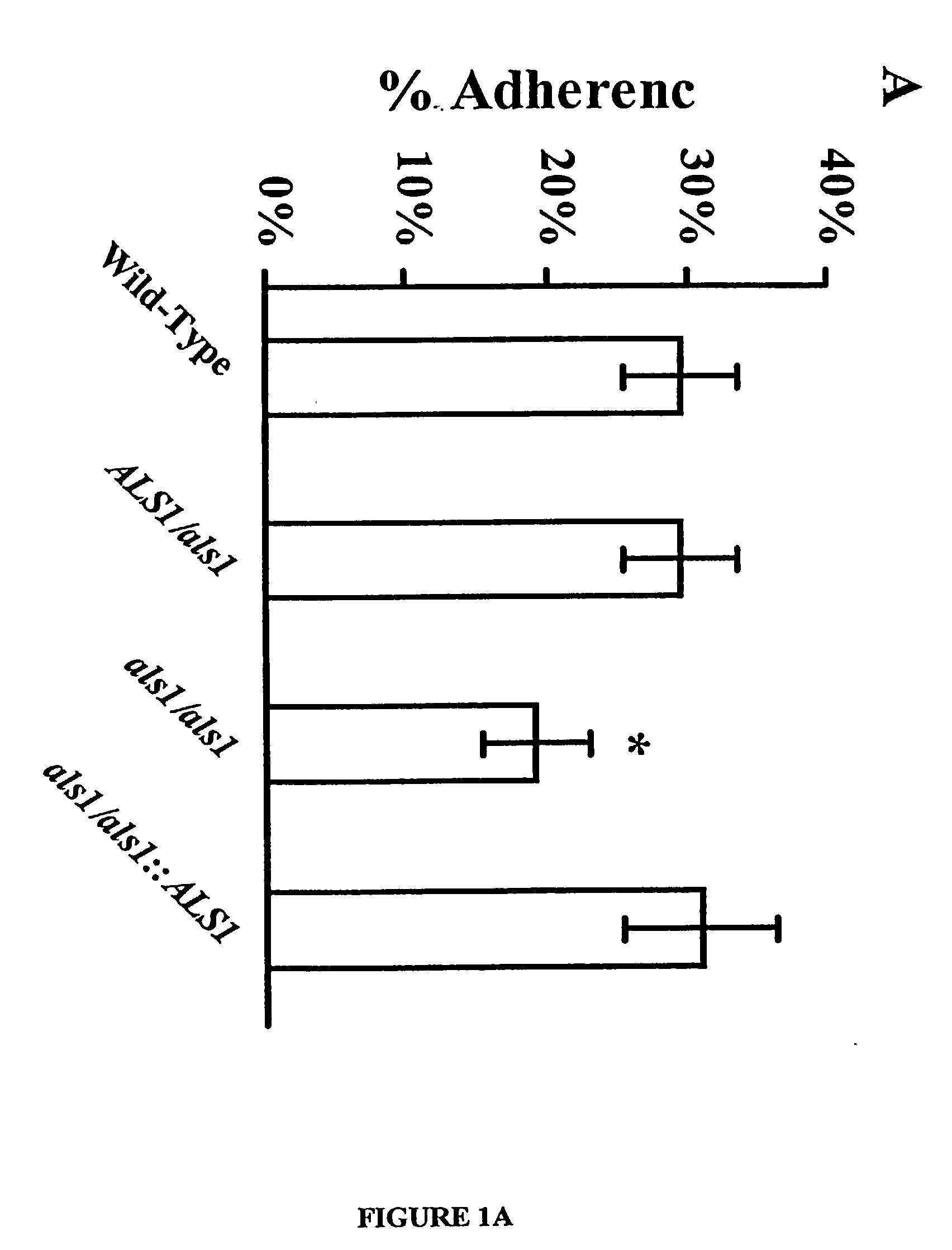
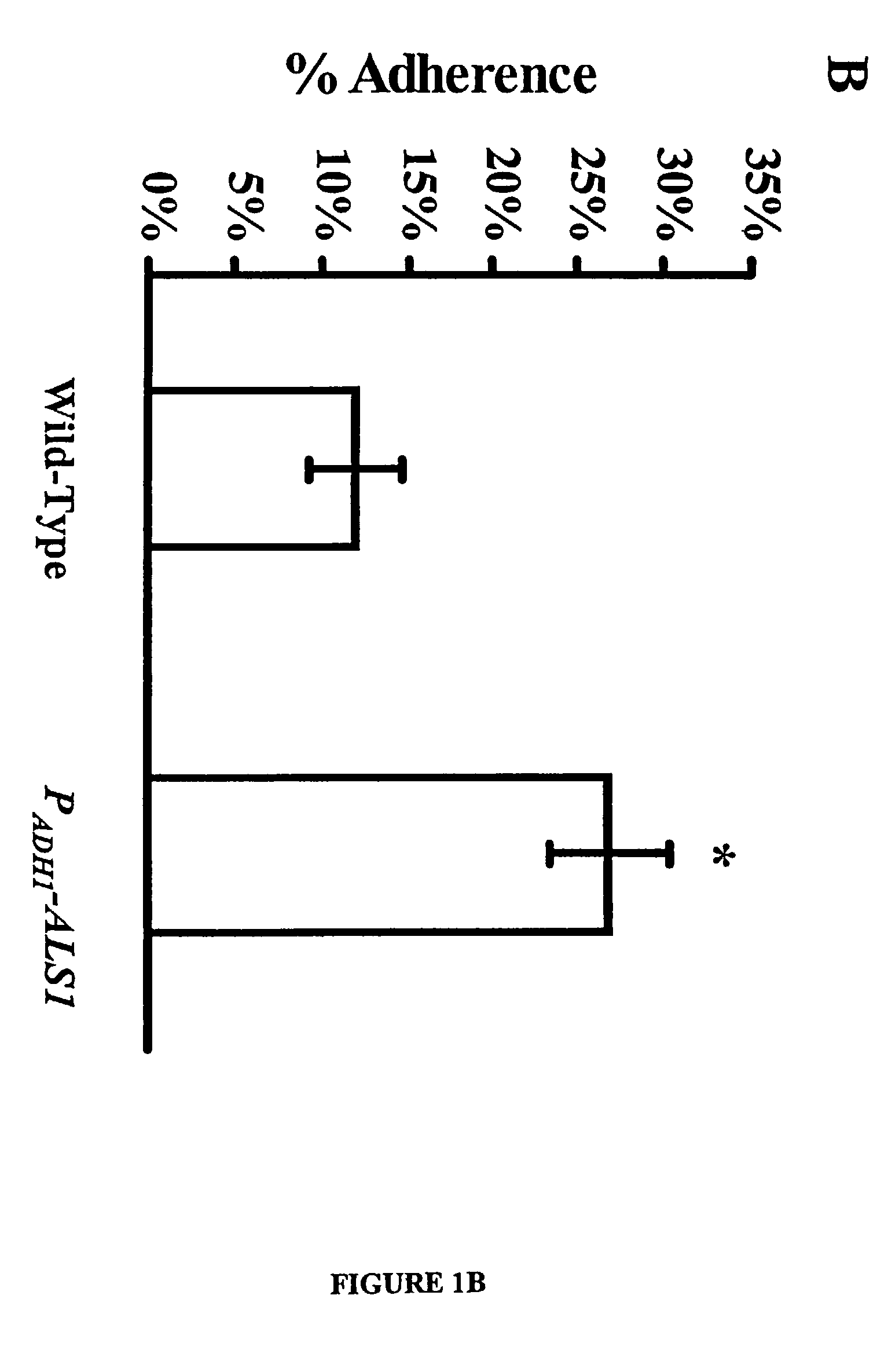
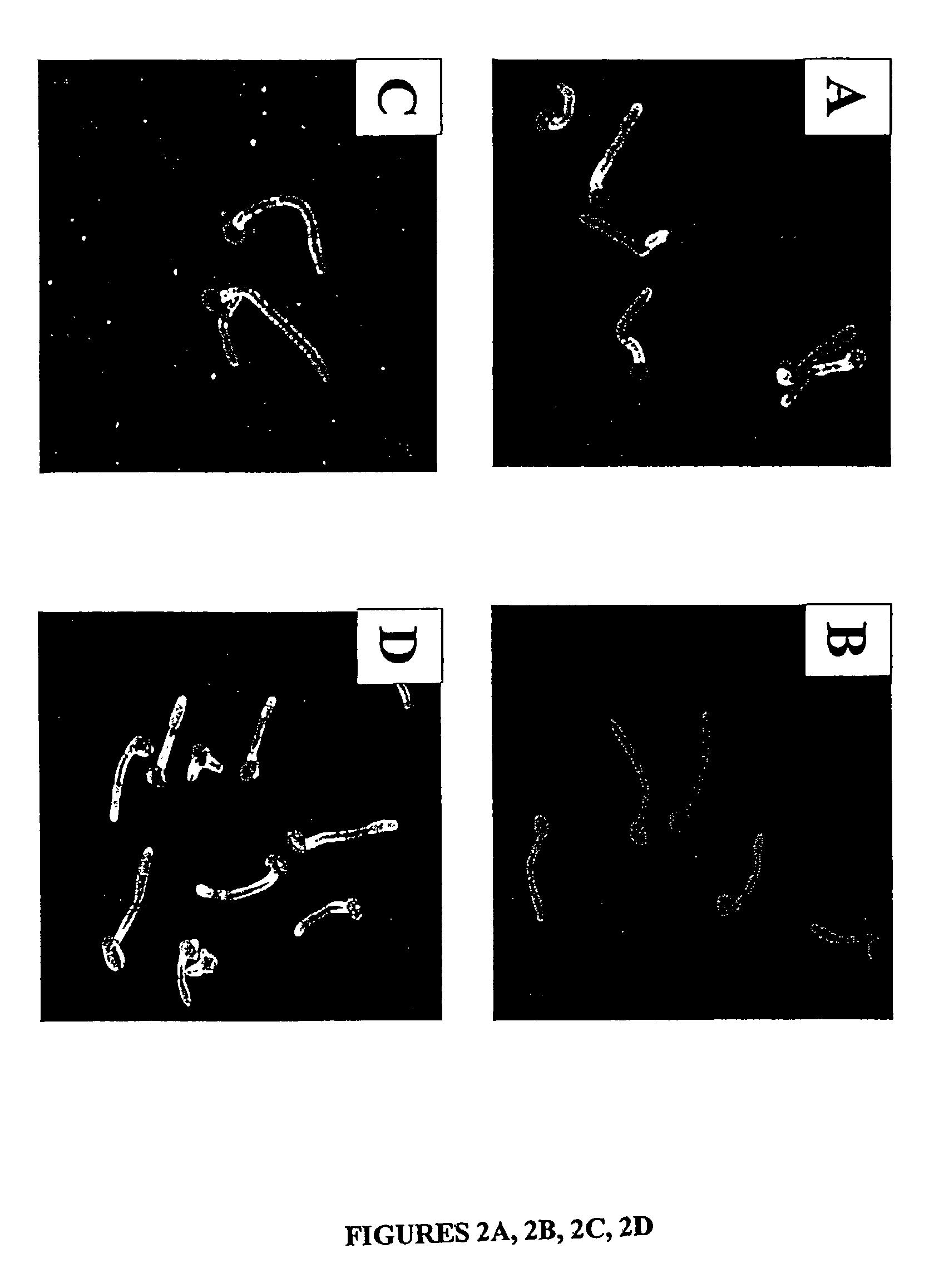
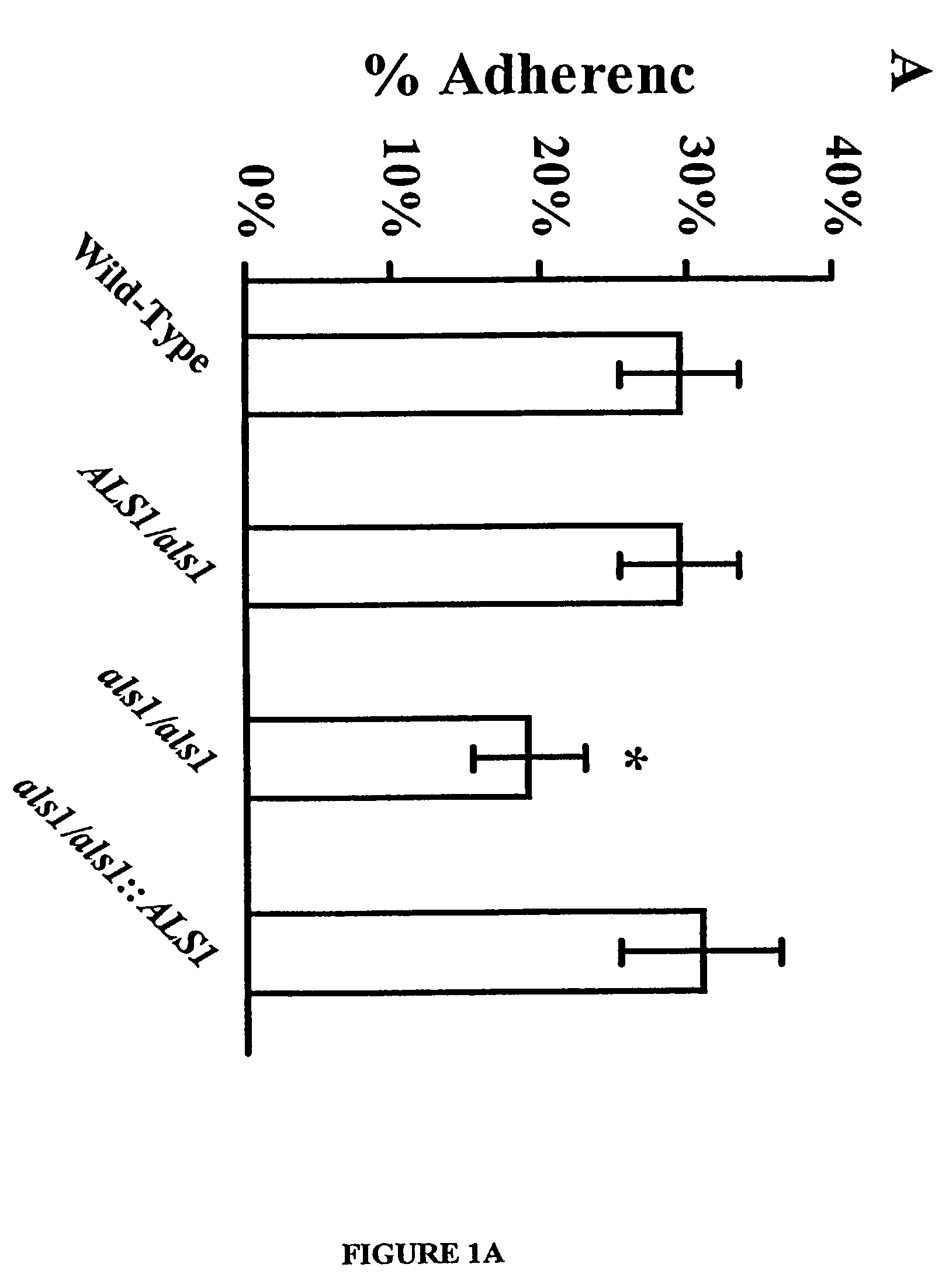
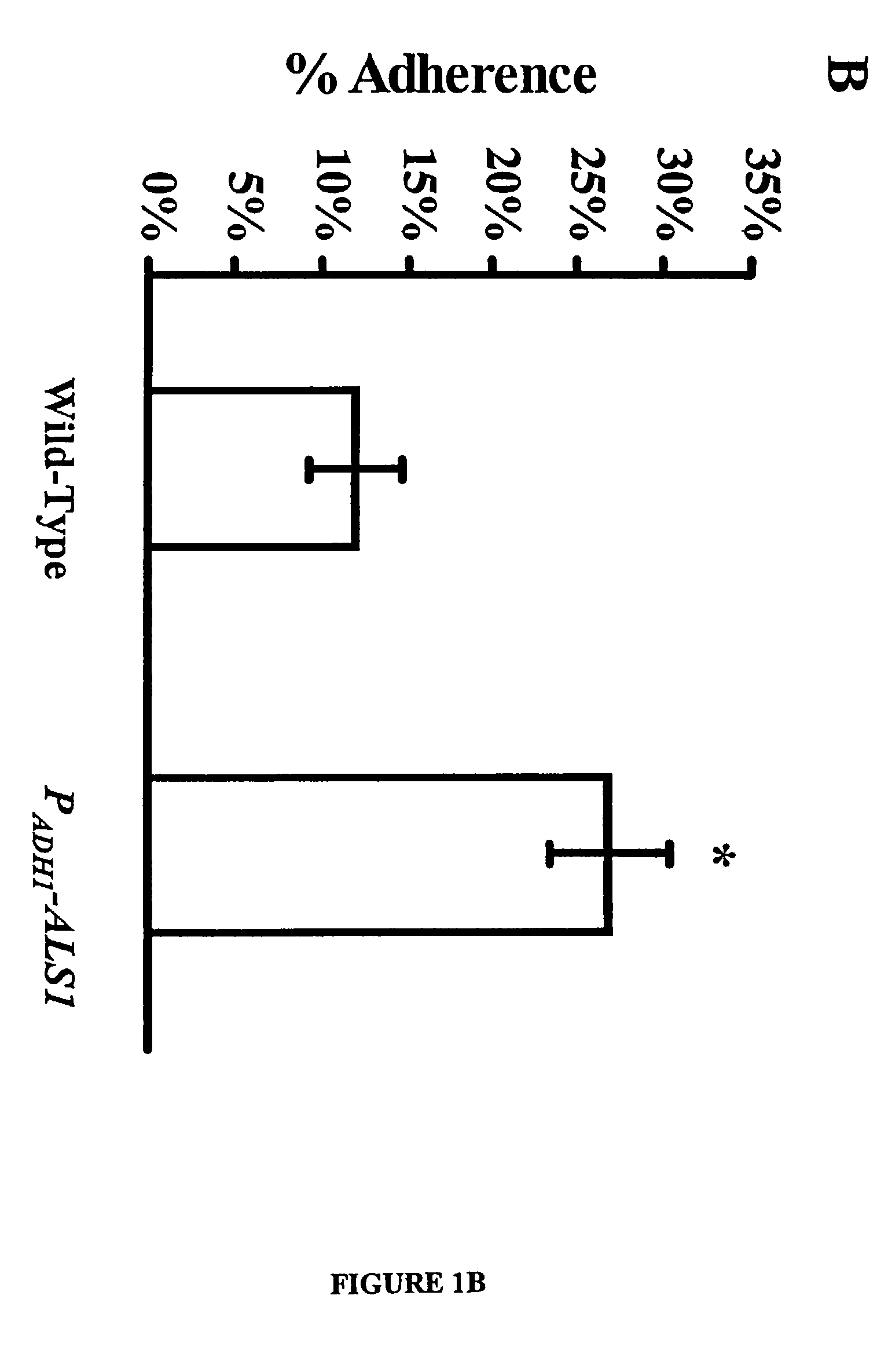
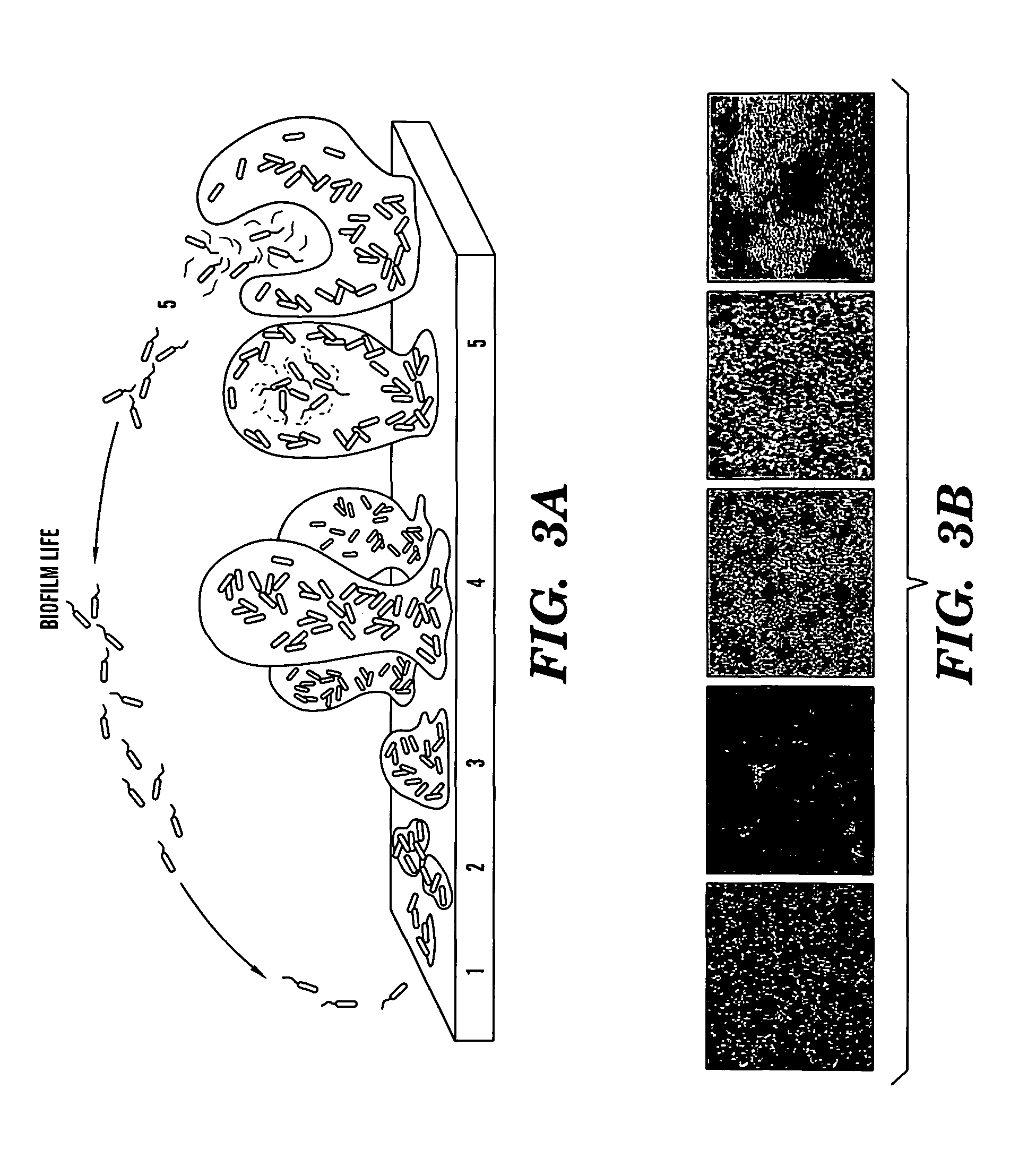
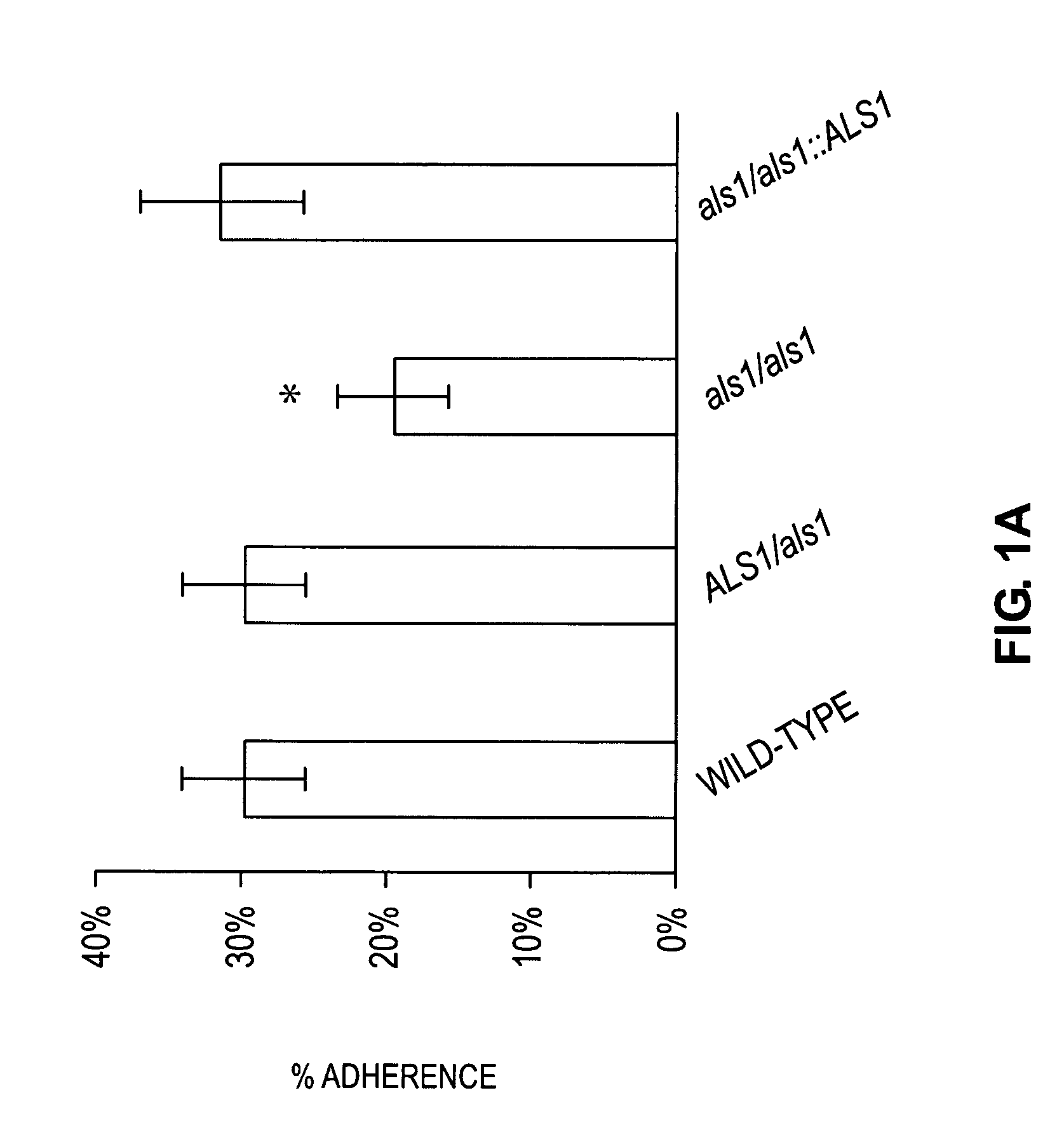
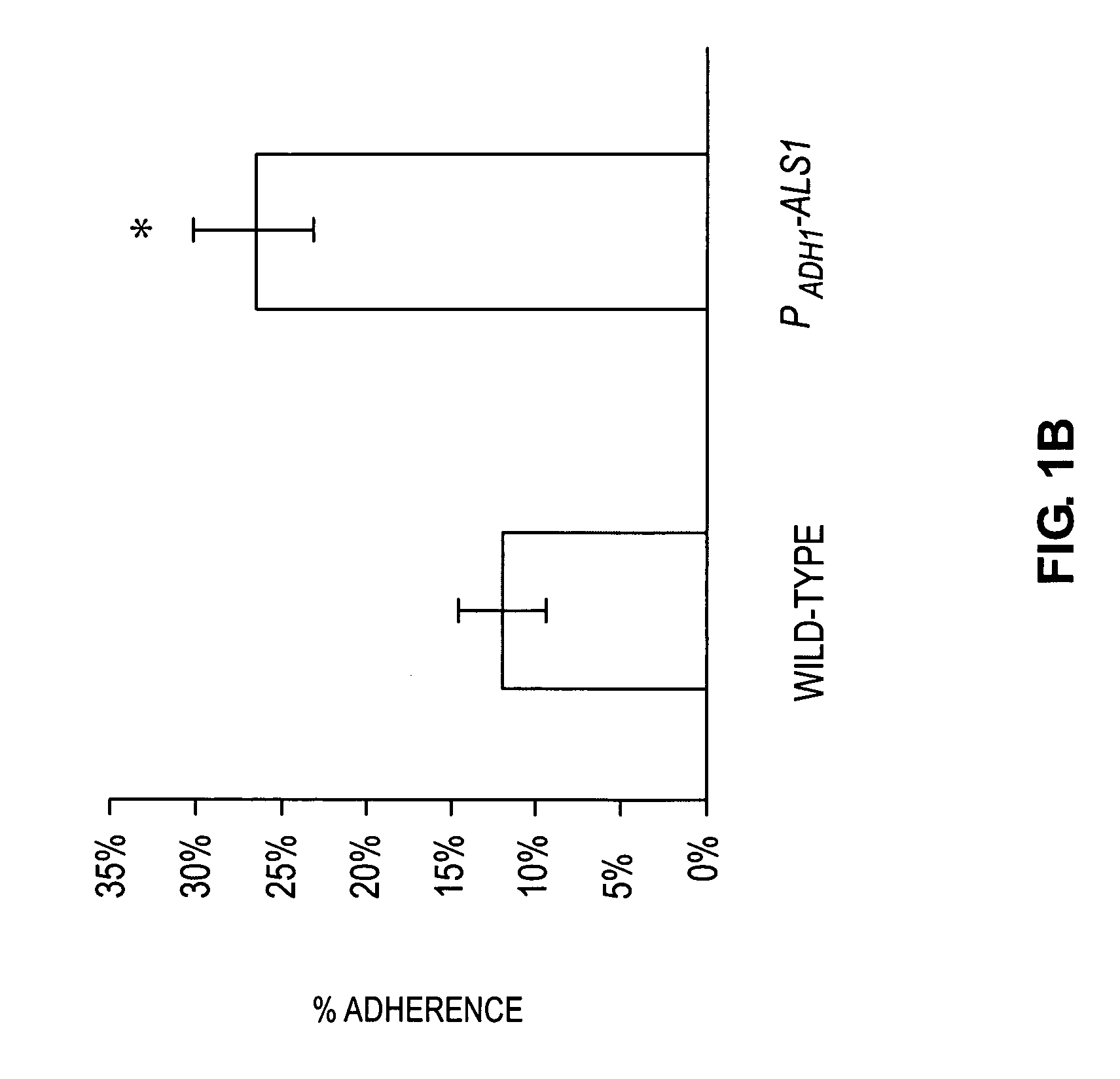
A Candida albicans bloodstream infections cause significant morbidity and mortality in hospitalized patients. Filament formation and adherence to host cells are critical virulence factors of C. albicans. Multiple filamentation regulatory pathways have been discovered, however the downstream effectors of these regulatory pathways remain unknown. The cell surface proteins in the ALS group are downstream effectors of the filamentation regulatory pathway. Particularly, Als1p mediates adherence to endothelial cells in vitro and is required for virulence. The blocking of adherence by the organism is described resulting from the use of a composition and method disclosed herein. Specifically, a pharmaceutical composition comprised of a gene, gene product, or specific antibody to the ALS gene family is administered as a vaccine to generate an immune response capable of blocking adherence of the organism.
Owner:LOS ANGELES BIOMEDICAL RES INST AT UCLA HARBOR MEDICAL CENT +1
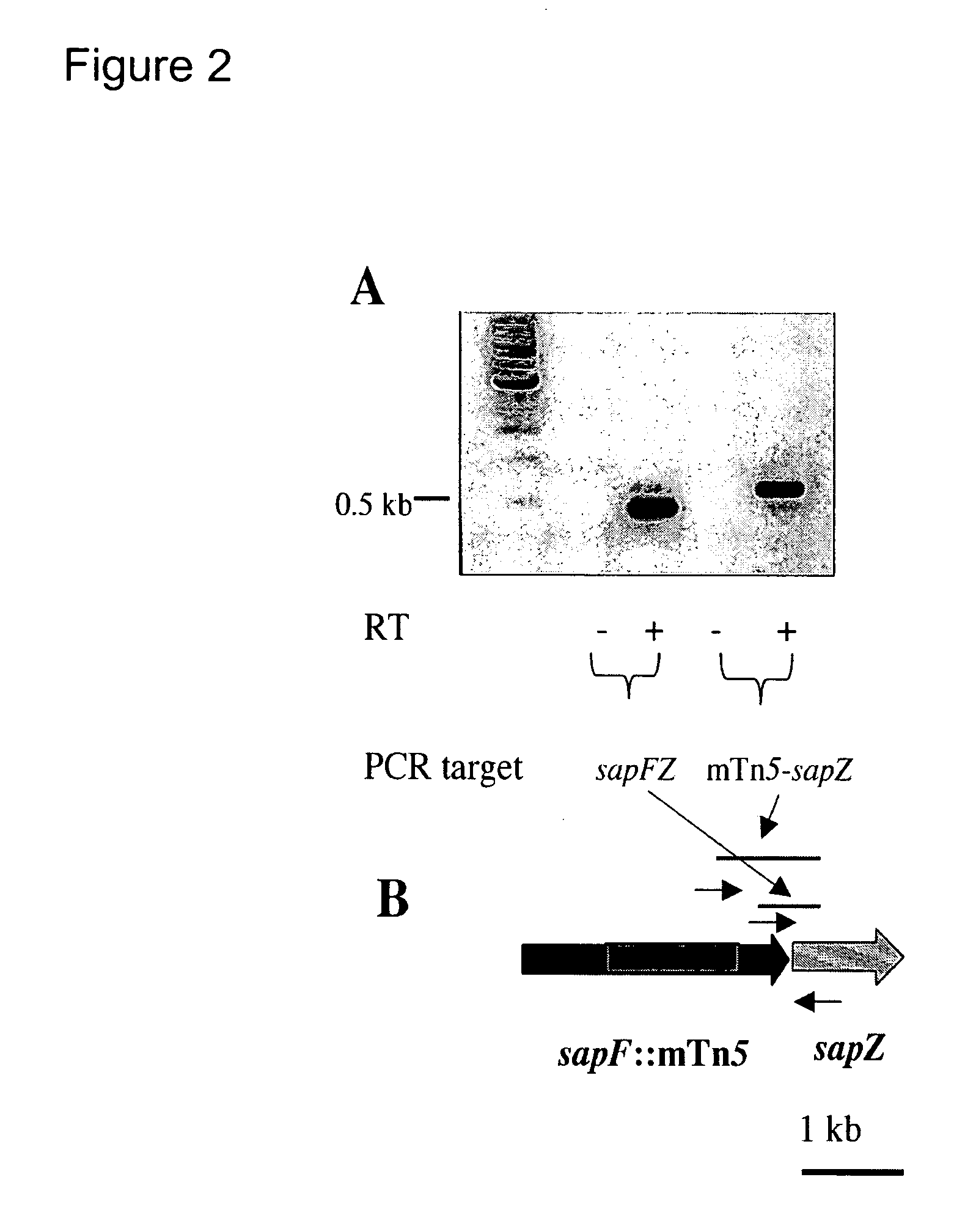
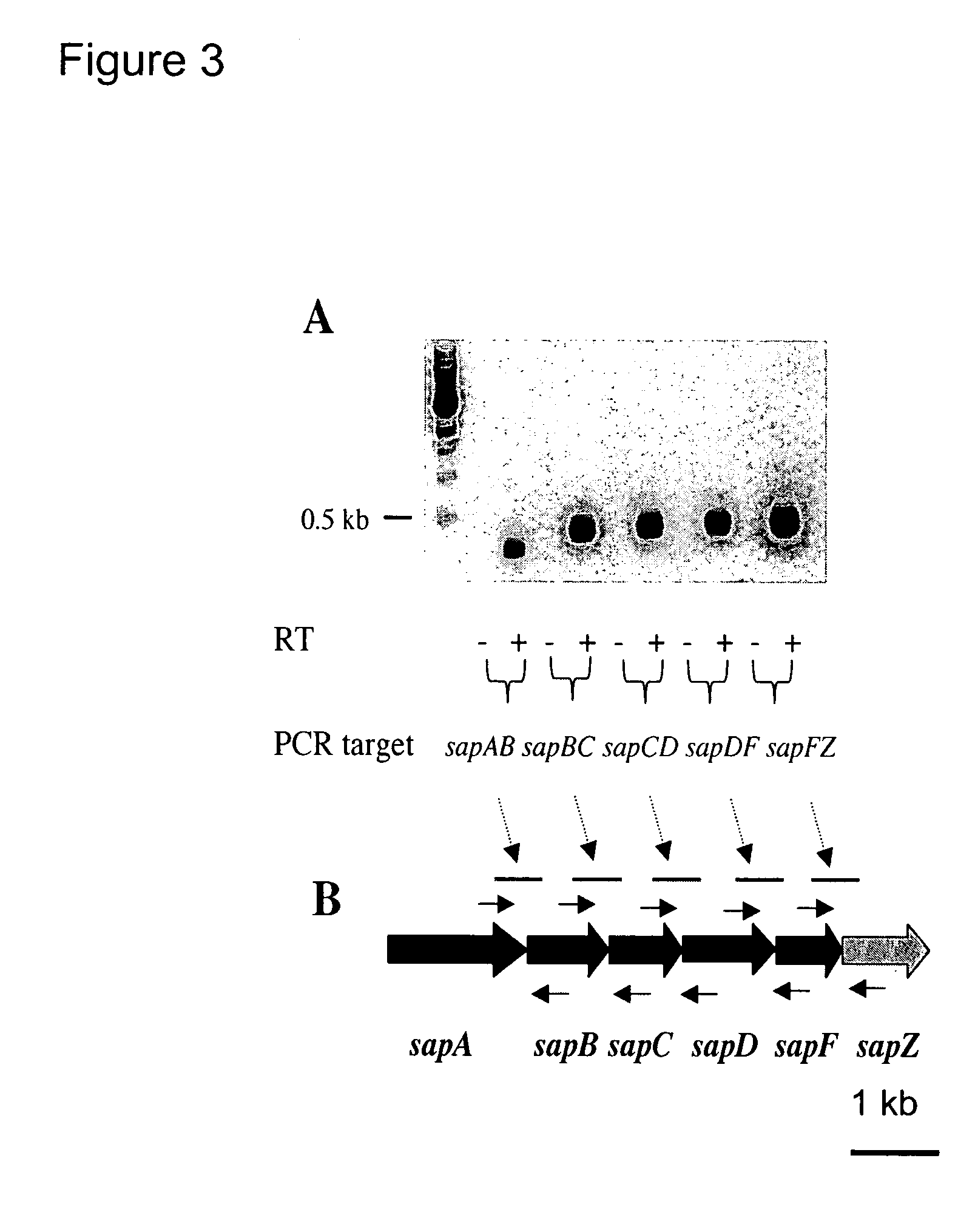
Nontypeable Haemophilus influenzae virulence factors
InactiveUS7306805B2Translation is prevented and reducedAvoid stickingAntibacterial agentsSenses disorderVirulent characteristicsOperon
The invention relates to a mutation within the sap operon of an avirulent clone of a nontypeable strain of Haemophilus influenzae (NTHi). The invention also relates to the NTHi sap operon genes and the polypeptides encoded by these polynucleotide sequences. The invention also relates to a novel 110 kDa NTHi outer membrane protein and the polynucleotide that encodes this outer membrane protein. Methods of screening for NTHi infection, and treating and preventing NTHi related disorders are also contemplated.
Owner:NATIONWIDE CHILDRENS HOSPITAL
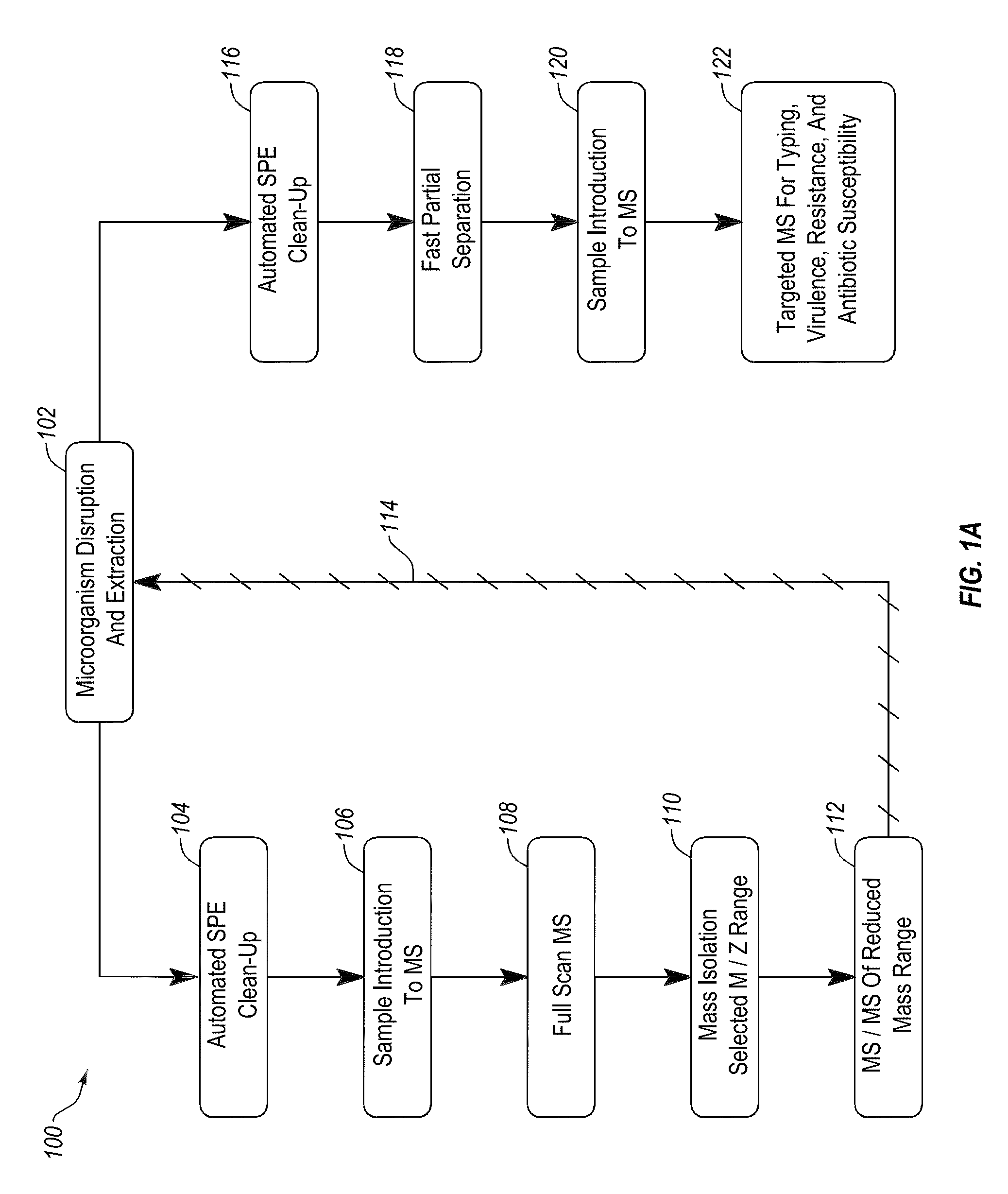
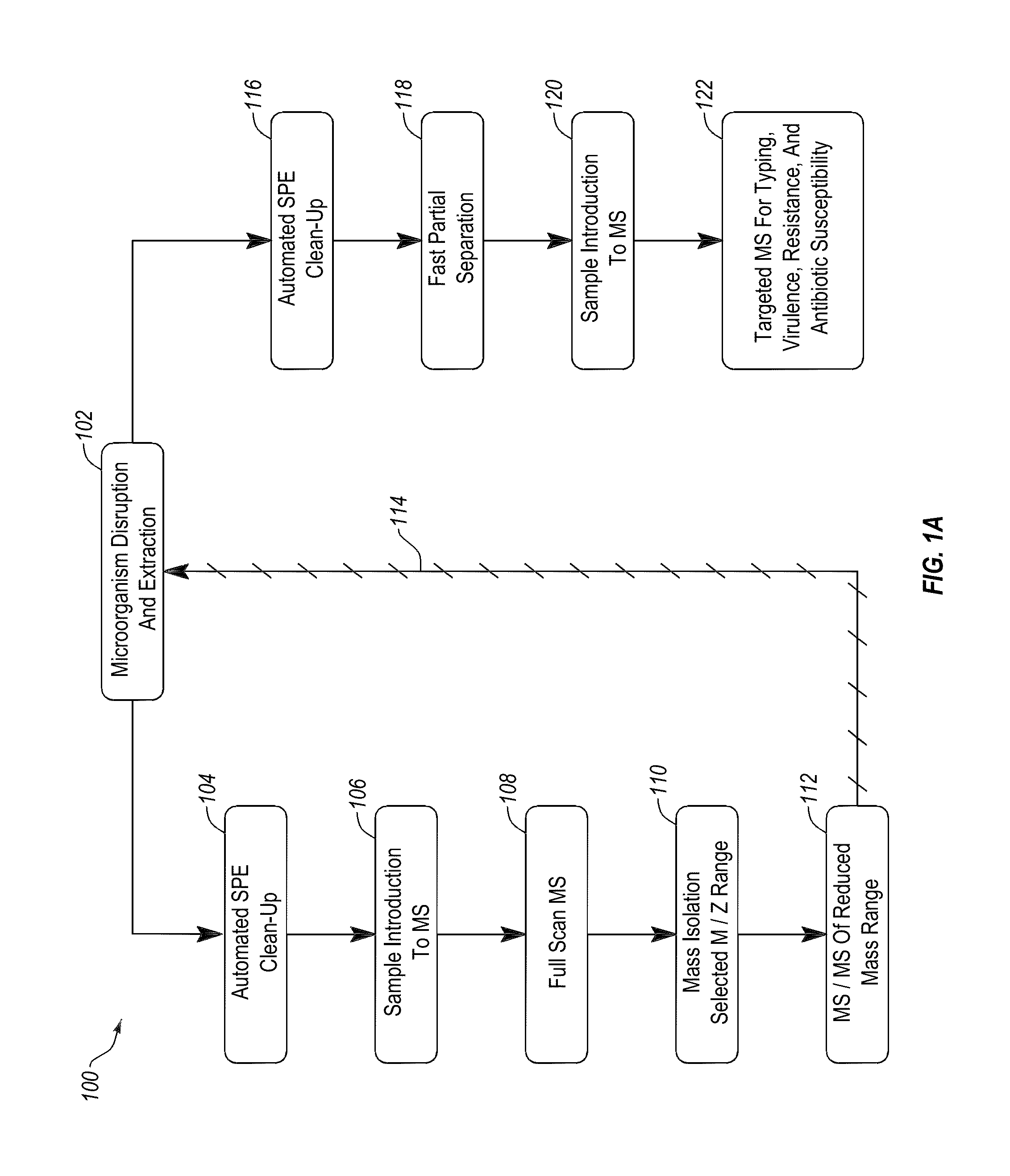
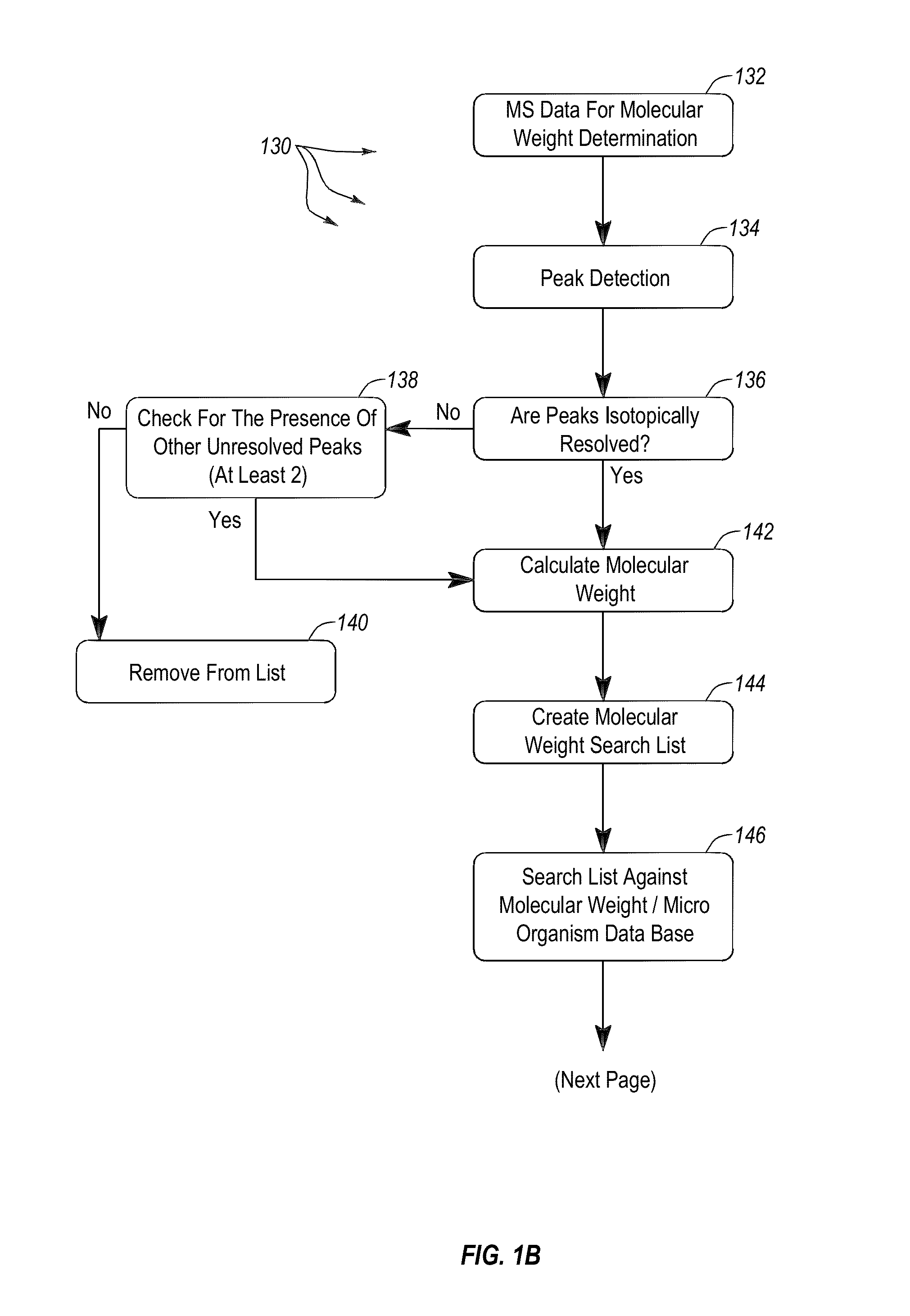
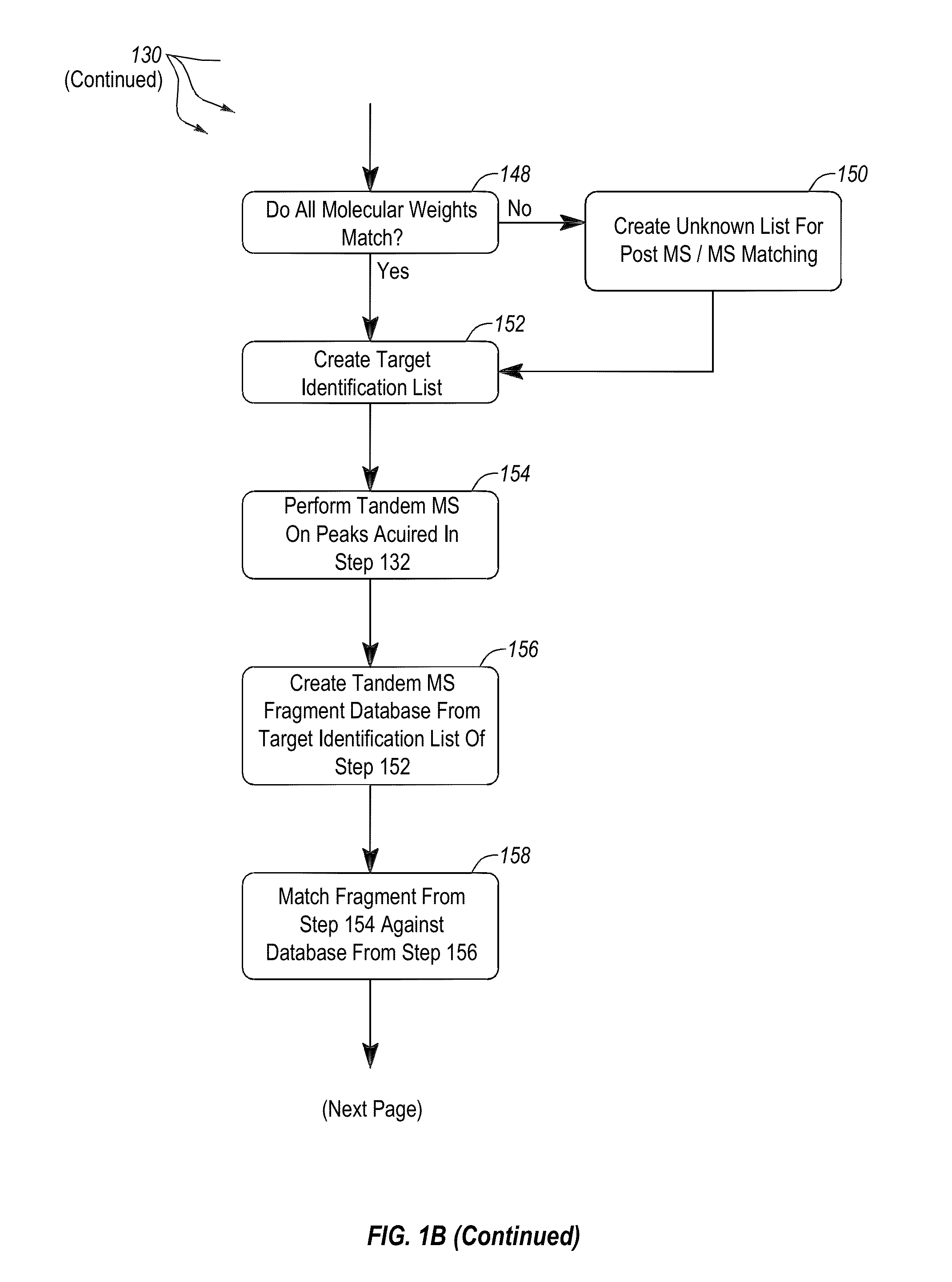
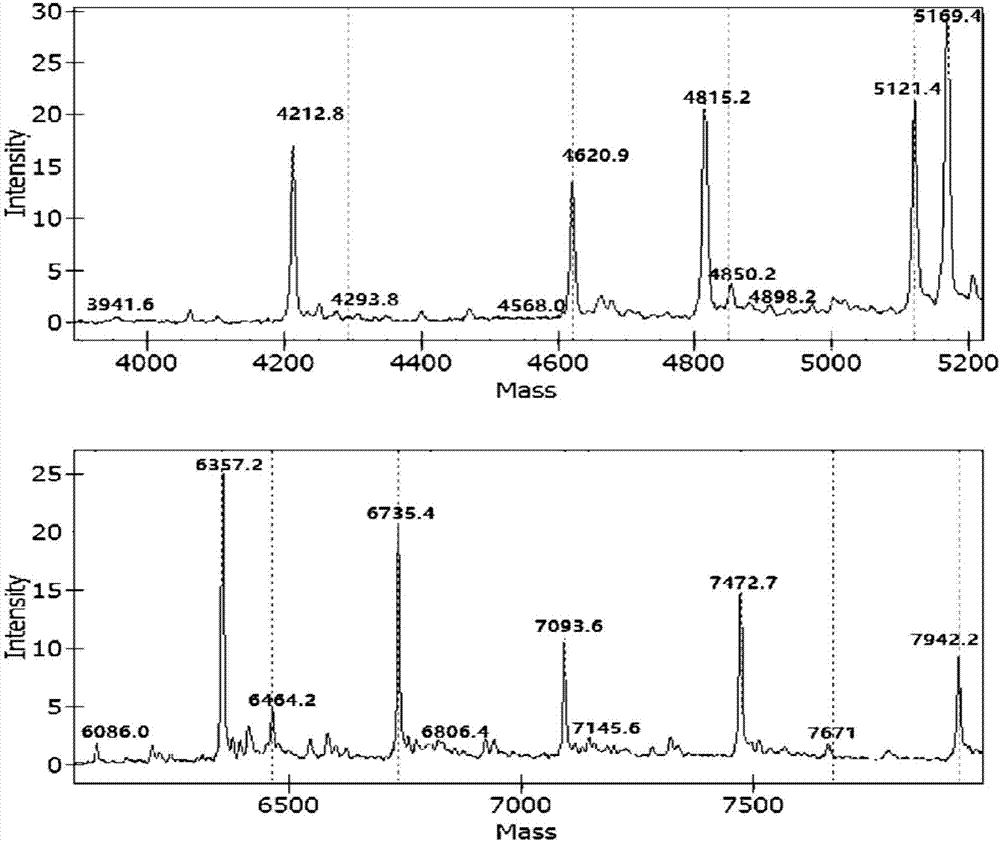
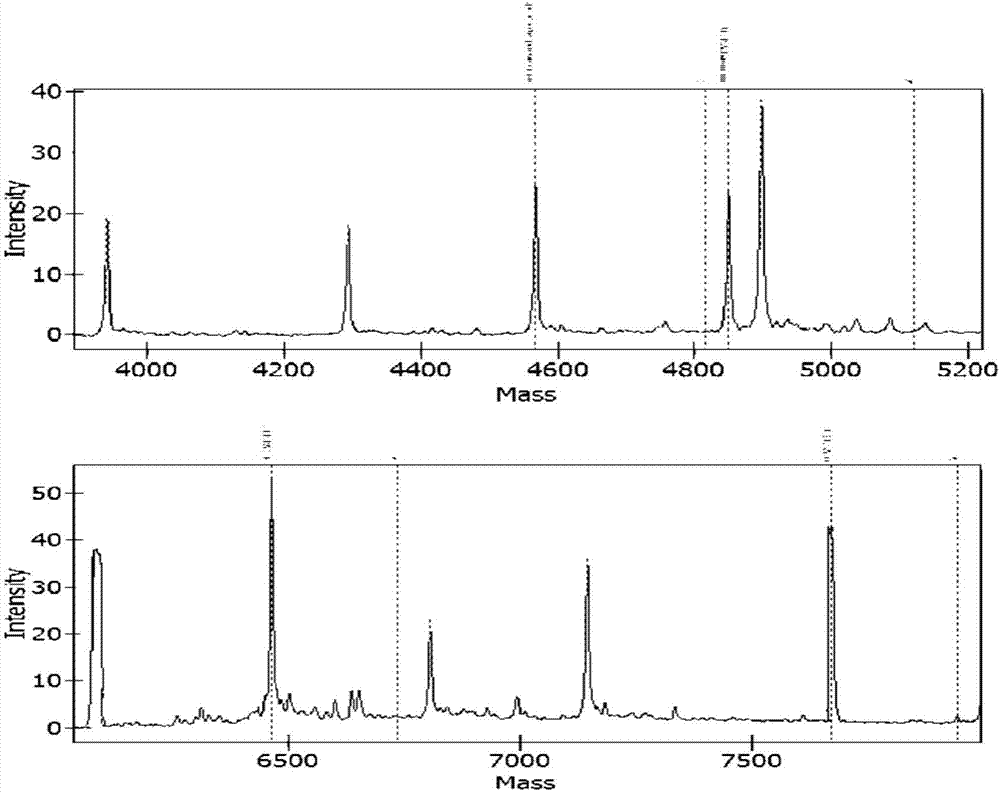
Apparatus and methods for microbial identification by mass spectrometry
ActiveUS9074236B2The process is simple and fastFacilitates epidemiological trackingBioreactor/fermenter combinationsBiological substance pretreatmentsMass spectrometry imagingMass spectrometric
Methods and systems for identification of microorganisms either after isolation from a culture or directly from a sample. The methods and systems are configured to identify microorganisms based on the characterization of proteins of the microorganisms via high-resolution / mass accuracy single-stage (MS) or multi-stage (MSn) mass spectrometry. Included herein are also discussion of targeted detection and evaluation of virulence factors, antibiotic resistance markers, antibiotic susceptibility markers, typing, or other characteristics using a method applicable to substantially all microorganisms and high-resolution / mass accuracy single-stage (MS) or multi-stage (MSn) mass spectrometry.
Owner:OXOID
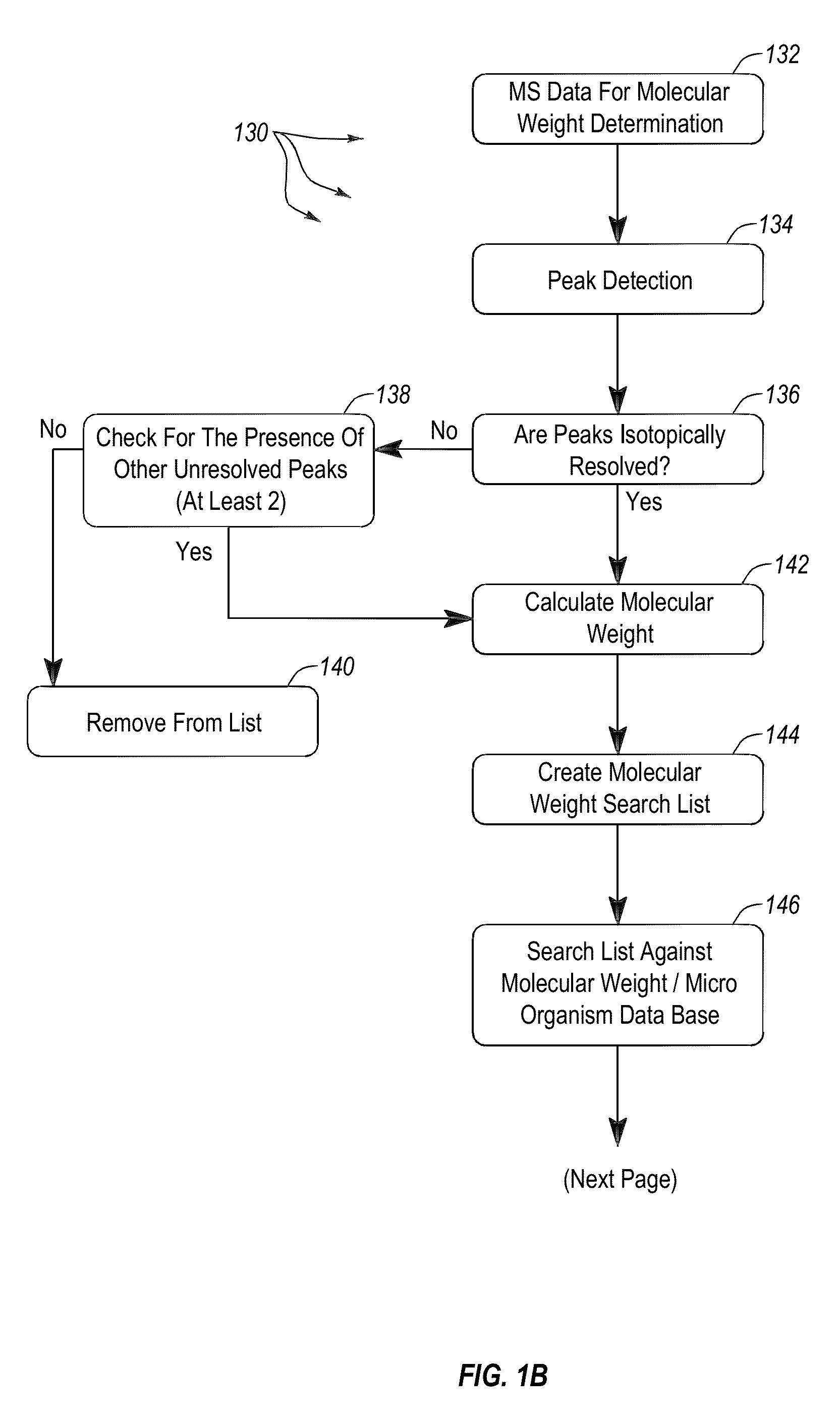
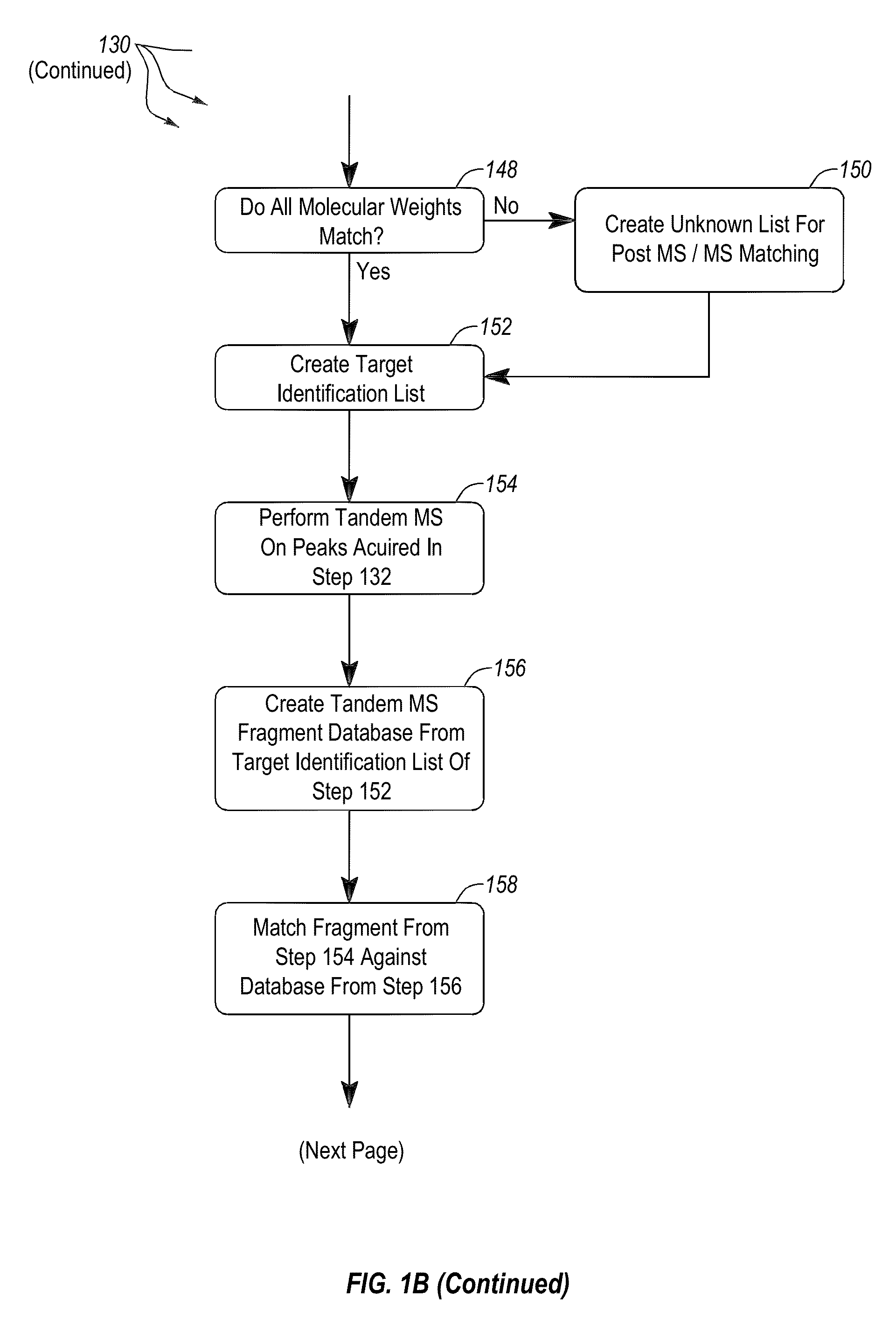
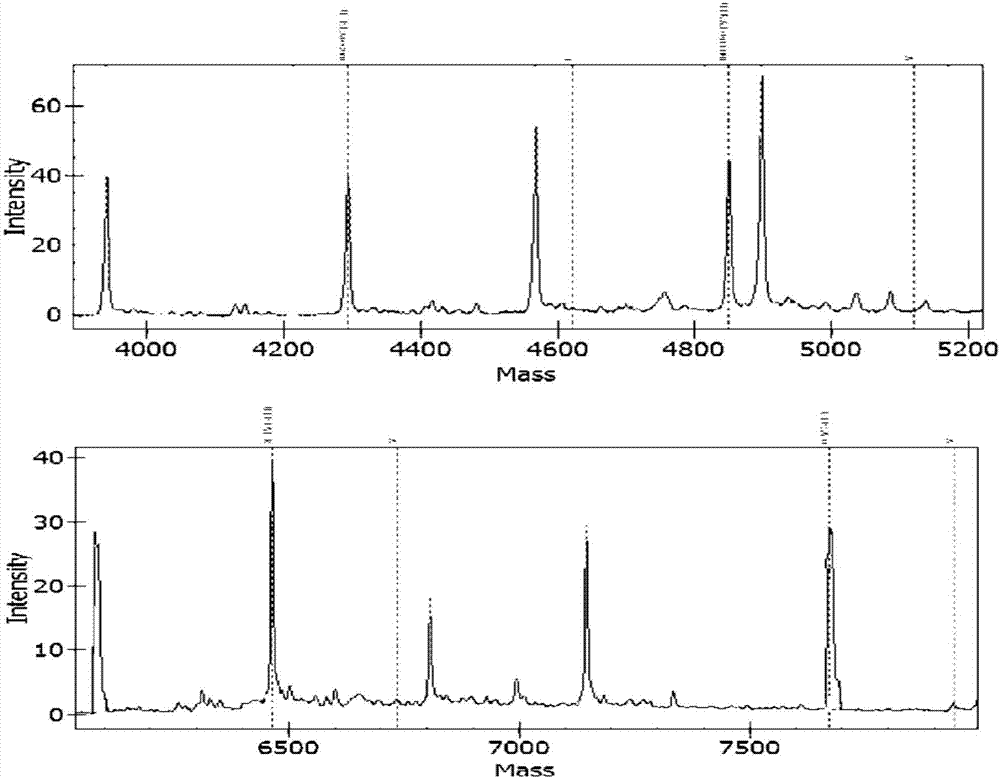
Apparatus and methods for microbiological analysis
ActiveUS20140051113A1Facilitates epidemiological trackingThe process is simple and fastBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismSingle stage
Methods and systems for identification of microorganisms either after isolation from a culture or directly from a sample. The methods and systems are configured to identify microorganisms based on the characterization of proteins of the microorganisms via high-resolution / mass accuracy single-stage (MS) or multi-stage (MSn) mass spectrometry. Included herein are also discussion of targeted detection and evaluation of virulence factors, antibiotic resistance markers, antibiotic susceptibility markers, typing, or other characteristics using a method applicable to substantially all microorganisms and high-resolution / mass accuracy single-stage (MS) or multi-stage (MSn) mass spectrometry.
Owner:OXOID
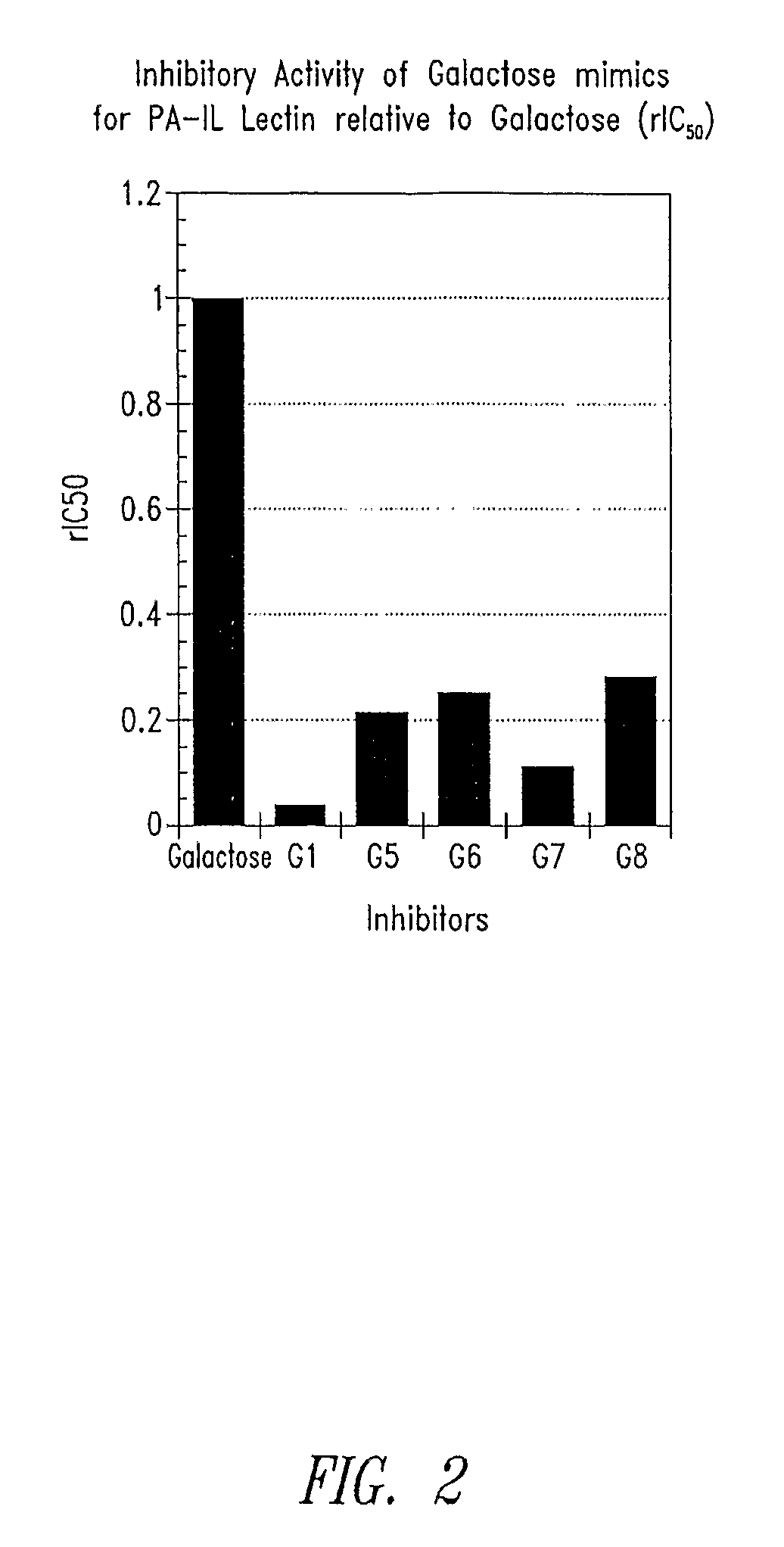
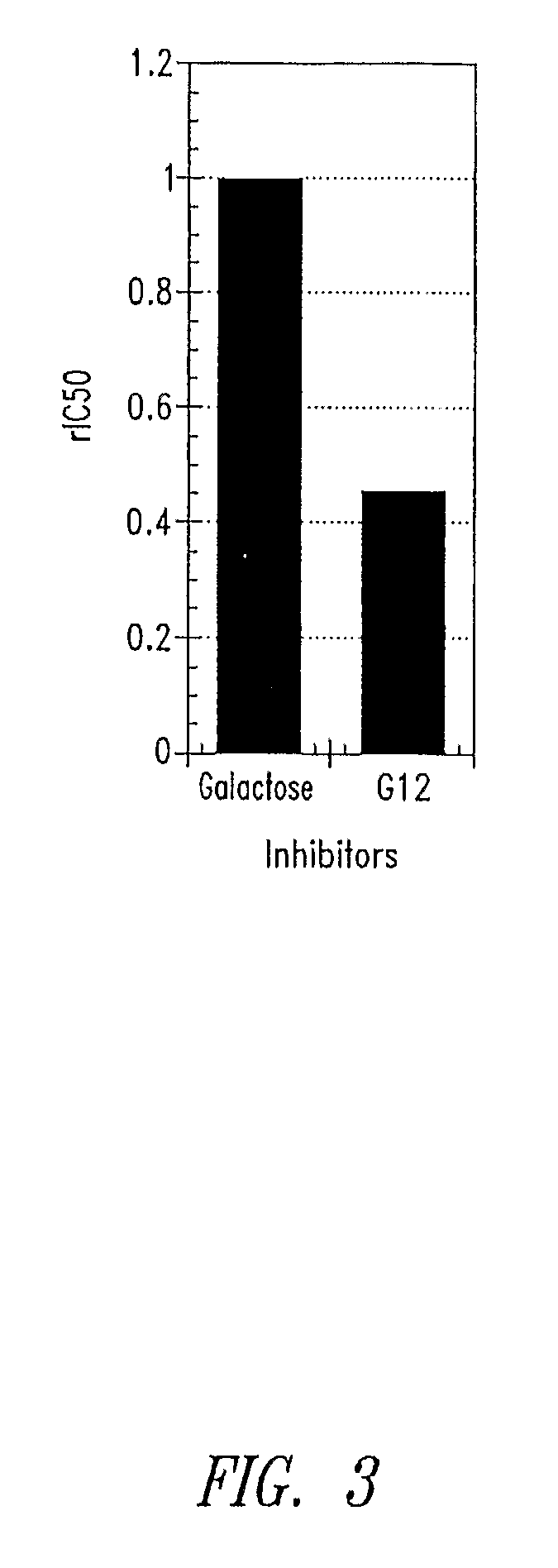
Glycomimetic inhibitors of the PA-IL lectin, PA-IIL lectin or both the lectins from Pseudomonas
Compositions and methods are provided related to Pseudomonas bacteria. The compositions and methods may be used for diagnosis and therapy of medical conditions involving infection with Pseudomonas bacteria. Such infections include Pseudomonas aeruginosa in the lungs of patients with cystic fibrosis. A compound useful in the present methods may be used in combination with a therapeutic agent or may be linked to a therapeutic agent. Pseudomonas bacteria may be inhibited by blocking colonization, inhibiting virulence factors, arresting growth or killing the bacteria.
Owner:GLYCOMIMETICS
Methods for producing enhanced antigenic Helicobacter sp.
InactiveUS6051416AEnhanced antigenic propertyIncrease proteinAntibacterial agentsBiocideBacteroidesVirulent characteristics
Methods using in vitro processes are disclosed for inducing or enhancing expression of enteric bacterial antigens or virulence factors. The methods, therefore, produce antigenically enhanced enteric bacteria. Also methods for using the antigenically enhanced bacteria are also disclosed, as well as vaccines containing the enteric bacteria. Specifically a whole enteric bacterium or components thereof are provided by Helicobacter species. Also there are other enteric bacteria which are useful for the disclosed invention; such as Campylobacter jejuni.
Owner:BIOPORT R&D
Nucleic acid mass spectrometry method for detecting 10 common pathogenic bacteria of clinical infection
InactiveCN107964565AHigh detection sensitivityOperational securityMicrobiological testing/measurementMicroorganism based processesRational useFluorescence
The invention discloses a primer system for detecting 10 common pathogenic bacteria of clinical infection. The detection system is used for detecting blood or body fluid (pleural fluid, ascitic fluid,drainage fluid, synovial fluid and cerebrospinal fluid) infected samples of patients infected by the pathogenic bacteria; detection results are combined with other clinical indicators, reference canbe provided for early diagnosis for clinicians and rational use of antibiotics, and treatment delaying and nonstandard use of the antibiotics are avoided. Virulence factor gene loci of 10 differentpathogenic bacteria can be simultaneously detected in two reaction systems; compared with sequencing, real-time fluorescence quantitative PCR and other technologies, the cost is lower, the operation is more convenient, and the accuracy and sensitivity are improved.
Owner:GENERAL HOSPITAL OF PLA
Attenuated, brightened and replication-controllable HSV recombinant virus, preparation method and applications thereof
InactiveCN107630009AEasy genetic manipulationEasy to insertViruses/bacteriophagesFermentationCell specificNervous system
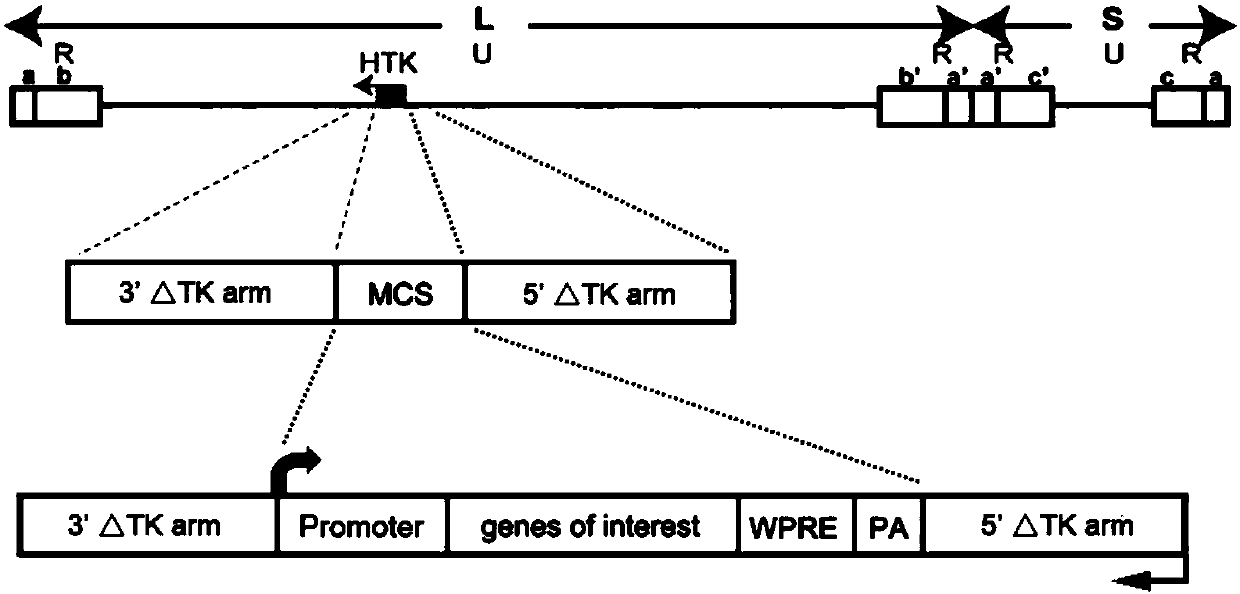
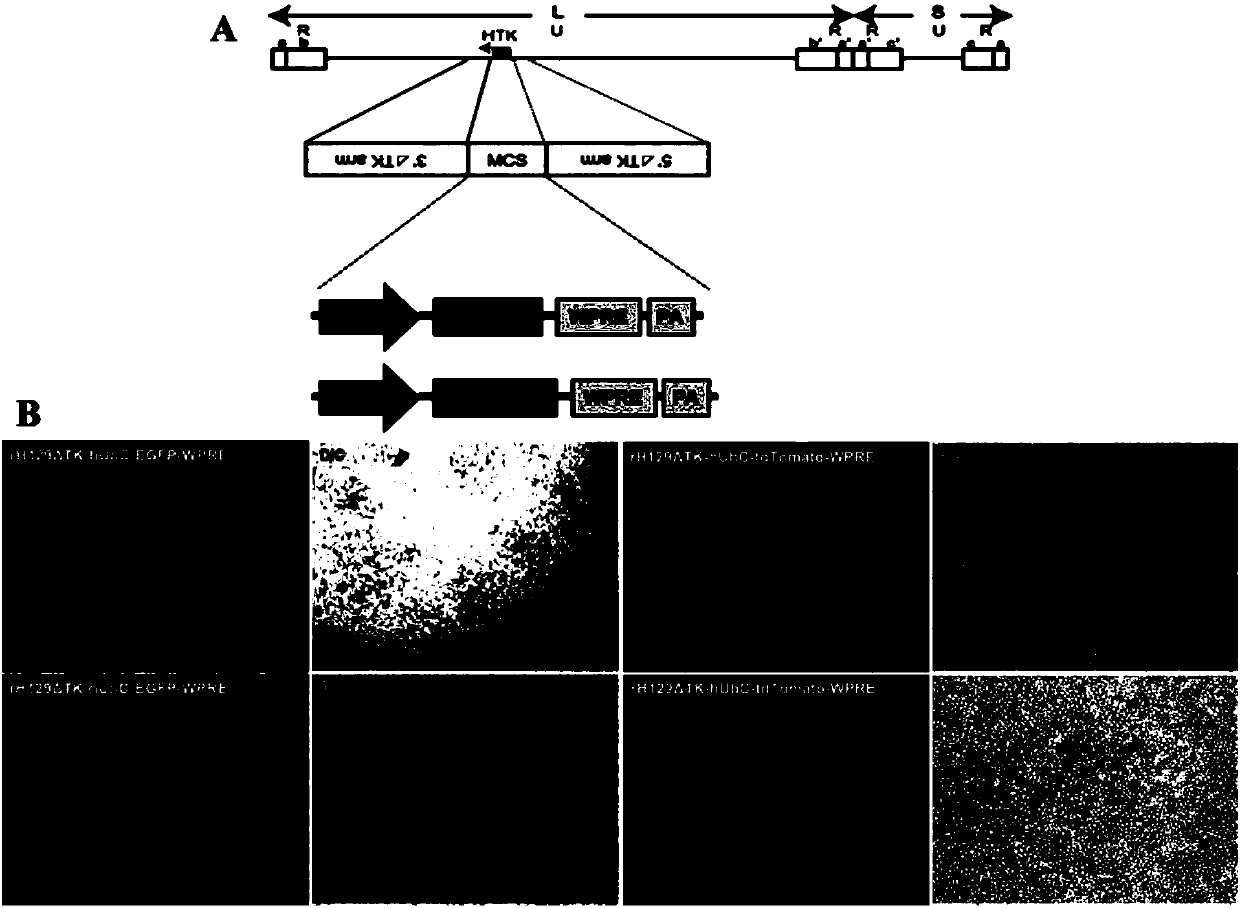
The invention discloses attenuated, brightened and replication-controllable HSV recombinant virus, a preparation method and applications thereof. According to the present invention, thymidine kinase (TK) gene essential for replicating viruses in neurons and being a main virulence factor is knocked out by using a homologous recombination method, and subsequently a red or green fluorescent gene enhancement expression cassette is recombined into the genome of the virus to construct a series of novel recombinants viruses, wherein the toxicity is markedly low, the states of infected mice are good,the fluorescence signal is strong, and the expression of the recombinant viruses is limited at the injection site after the recombinant viruses are used in in-vivo animal center labeling; by combiningwith Cre-dependent AAV helper viruses capable of expressing TK in a compensated manner, the cell-specific transmonosynaptic loop tracing is achieved; and the recombinant HSV has wide application value in nervous system targeted gene transduction, neural network transsynaptic tracing, tumor disintegration, viral replication and pathogenesis mechanism, antiviral drug screening and other fields.
Owner:WUHAN INST OF PHYSICS & MATHEMATICS CHINESE ACADEMY OF SCI
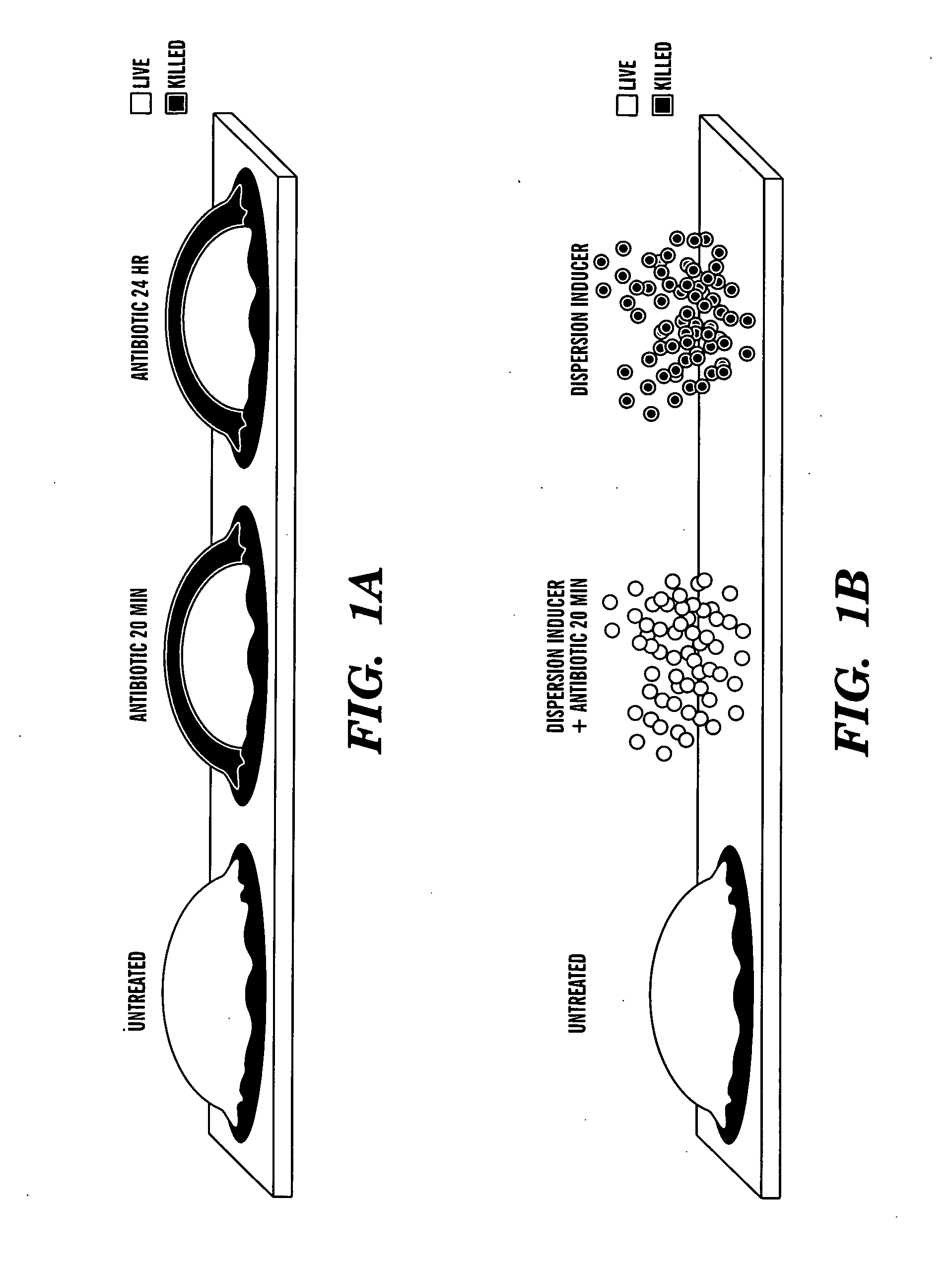
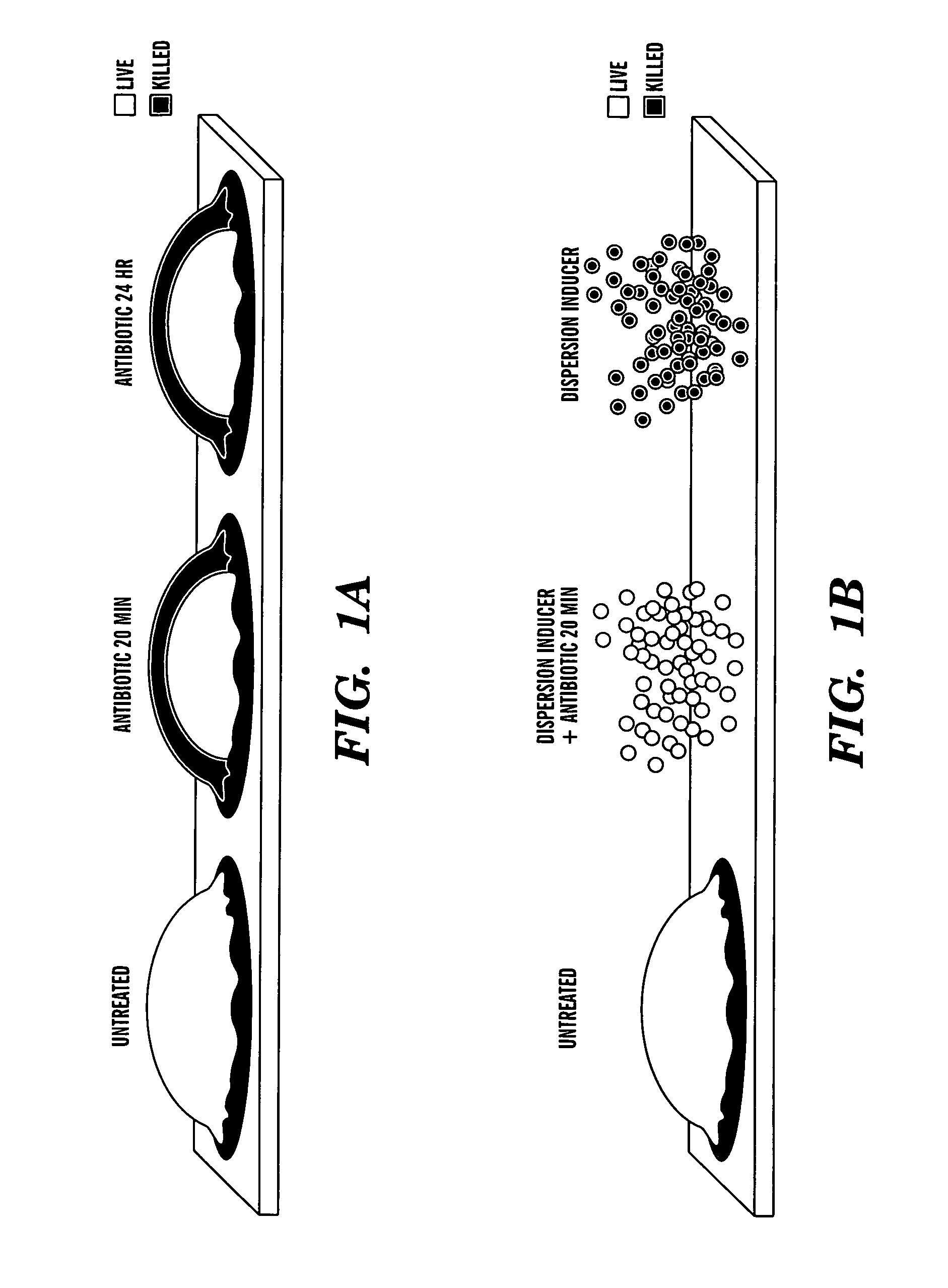
Induction of a physiological dispersion response in bacterial cells in a biofilm
ActiveUS20080317815A1Mild pHAntibacterial agentsOrganic active ingredientsDirect effectsCarbon–carbon bond
One aspect of the present invention is directed to a composition. The composition includes a dispersion inducer comprising:H3C—(CH2)n—CHmCHmR,where is a single or double carbon-carbon bond, m is 1 or 2, n is 2 to 15, and R is a carboxylic acid, a salt, an ester, or an amide, where the ester or amide is an isostere or biostere of the carboxylic acid. The composition additionally contains an additive component selected from one or more of the group consisting of biocides, surfactants, antibiotics, antiseptics, detergents, chelating agents, virulence factor inhibitors, gels, polymers, pastes, edible products, and chewable products. The composition is formulated so that when it is contacted with a biofilm produced by a microorganism, where the biofilm comprises a matrix and microorganism on a surface, the dispersion inducer selectively acts on the microorganism and has a suitable biological response without a required direct effect on the matrix to disperse the biofilm. The present invention is also directed to methods of using this compound.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Methods for identifying polypeptide targets and uses thereof for treating immunological diseases
InactiveUS20070264654A1Reduce development riskIncrease virulenceAntibacterial agentsAntipyreticDiseaseImmunological diseases
The present invention provides methods for identifying viral virulence factors and for identifying cellular polypeptides to which the viral polypeptides bind. The cellular polypeptide is useful as a therapeutic target or as a therapeutic agent for treating diseases and disorders, including immunological diseases or disorders.
Owner:VIRAL LOGIC SYST TECH CORP
Multiple PCR detection kit for virulence factors of streptococcus suis and detection method thereof
InactiveCN101812518AStrong specificityStable and reliableMicrobiological testing/measurementMicroorganism based processesSurvey researchVirulent characteristics
The invention discloses a multiple PCR detection kit for virulence factors of streptococcus suis and a detection method thereof, and belongs to the technical field of biology. The multiple PCR detection kit comprises nucleic acid shown by base sequences, such as SEQ ID NO:1 to SEQ ID NO:20. The detection method for the multiple PCR detection kit comprises the following steps of: 1, providing DNA of samples to be detected; 2, taking the kit, amplifying the DNA of the samples to be detected by adopting the conventional PCR method, detecting an amplified result by adopting an agarose gel electrophoresis method, and judging according to the result; and by using positive DNA of streptococcus suis 2 type as contrast, if the contrast proves that not all the strips are amplified, re-detecting, and if the contrast proves that all the strips are amplified, judging the result. The multiple PCR detection method established by the invention is specific and sensitive, and has the advantages of simpleness, convenience and quickness. The multiple PCR detection kit has reliable stability, and can be used for rapid detection of clinical samples and survey research on molecular epidemiology for the streptococcus suis.
Owner:SHANGHAI JIAO TONG UNIV
Pharmaceutical compositions and methods to vaccinate against candidiasis
InactiveUS8541008B2Toxic reductionReduce adhesionPeptide/protein ingredientsSnake antigen ingredientsHospitalized patientsVirulent characteristics
A Candida albicans bloodstream infections cause significant morbidity and mortality in hospitalized patients. Filament formation and adherence to host cells are critical virulence factors of C. albicans. Multiple filamentation regulatory pathways have been discovered, however the downstream effectors of these regulatory pathways remain unknown. The cell surface proteins in the ALS group are downstream effectors of the filamentation regulatory pathway. Particularly, Als1p mediates adherence to endothelial cells in vitro and is required for virulence. The blocking of adherence by the organism is described resulting from the use of a composition and method disclosed herein. Specifically, a pharmaceutical composition comprised of a gene, gene product, or specific antibody to the ALS gene family is administered as a vaccine to generate an immune response capable of blocking adherence of the organism.
Owner:LOS ANGELES BIOMEDICAL RES INST AT UCLA HARBOR MEDICAL CENT +1
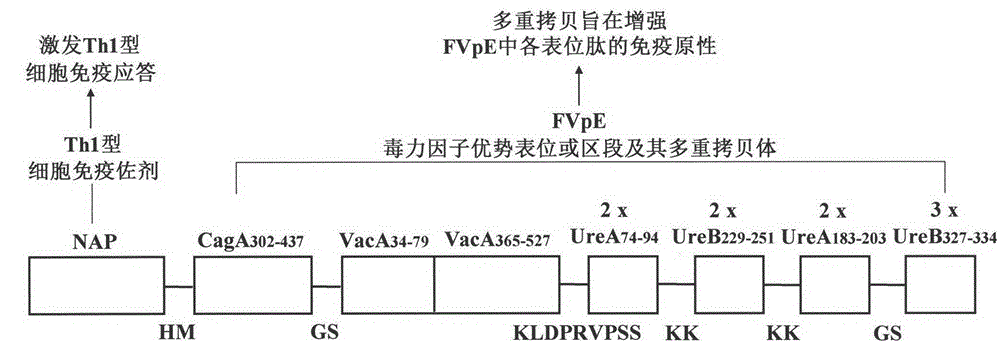
Helicobacter pylori tetravalent virulence factor multi-epitope vaccine and preparation method thereof
ActiveCN105106945ASmall molecular weightLow immunogenicityDigestive systemAntibody medical ingredientsVirulence factorELAV Proteins
The invention provides a helicobacter pylori tetravalent virulence factor multi-epitope vaccine and a preparation method thereof. The active constituent of the vaccine is polypeptide. The vaccine mainly comprises the dominant Th and B cell epitopes or sections of a urease A subunit, a urease B subunit, cytotoxin associated protein A and vacuolating cytotoxin associated protein A, and neutrophil activating protein. The gene synthesis and molecular cloning technology is used for building a fused gene containing the dominant Th and B cell epitopes or sections of ureases, the cytotoxin associated protein A and the vacuolating cytotoxin associated protein A, and the neutrophil activating protein. Escherichia coli is used for expressing the fused gene, and the tetravalent virulence factor multi-epitope vaccine is obtained after protein purification. The vaccine can stimulate a body to generate T cell immunity response and specific antibody humoral immunity response aiming at the ureases, the cytotoxin associated protein A, the vacuolating cytotoxin associated protein A and the neutrophil activating protein and can be used for preventing and treating diseases related to helicobacter pylori infection.
Owner:NINGXIA MEDICAL UNIV
Brucella molecule marking and virulence deletion attenuated vaccine and preparation method
InactiveCN101185756AWide application of practical valueAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsVaccine Immunogenicity
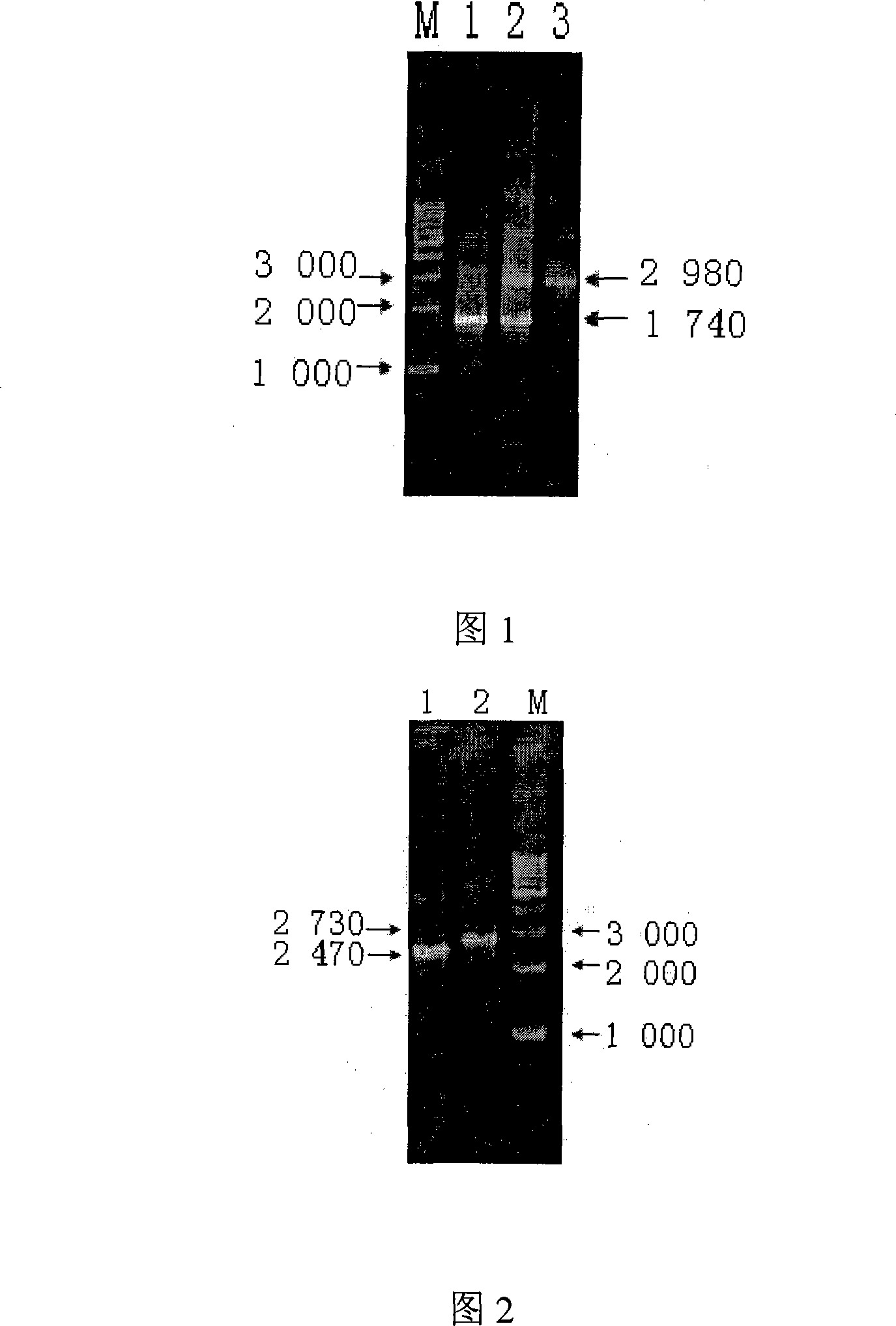
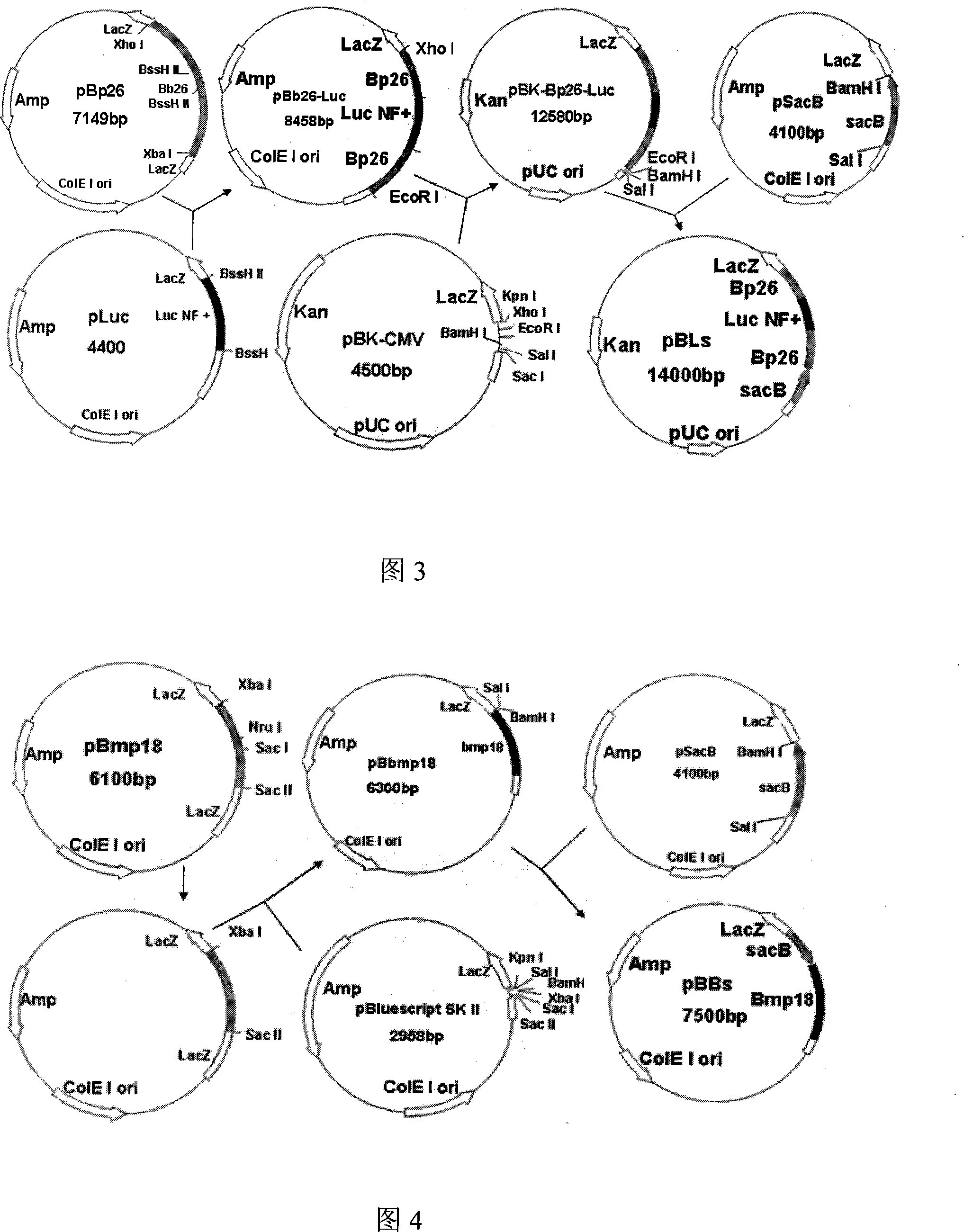
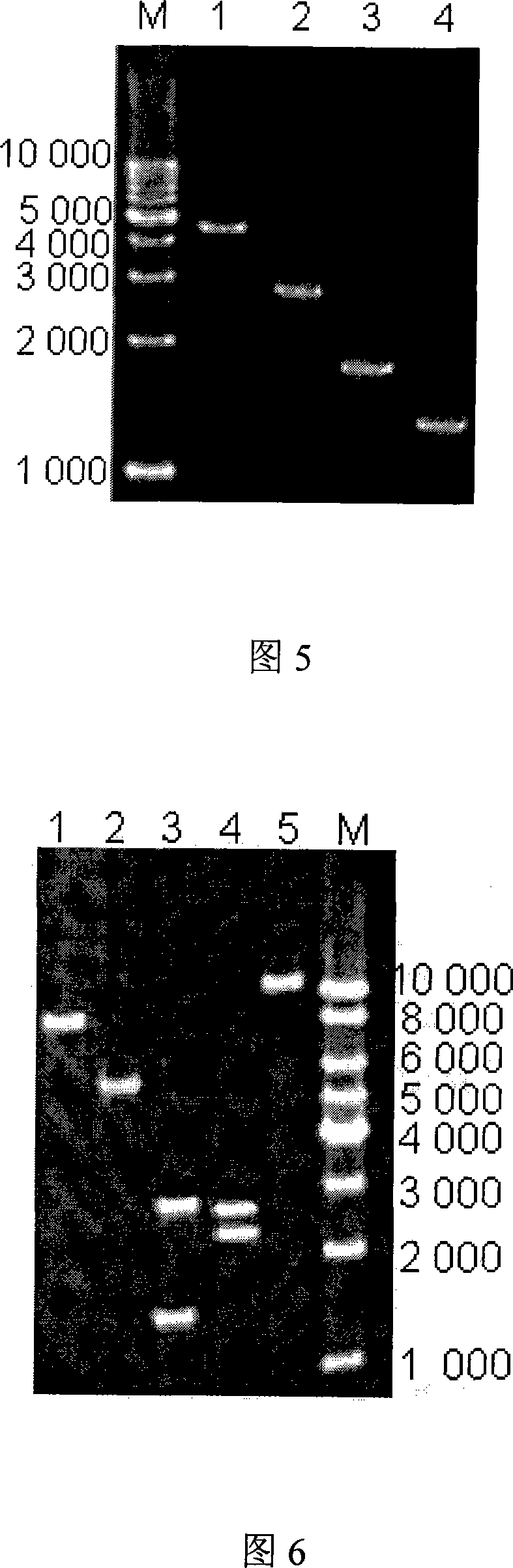
The invention relates to a Brucella vaccine, in particular to the molecular marker and virulence gene deletion of Brucella vaccine strain. The study uses luciferase modified gene (Luc NF plus) to replace the partial fragment of Bp26 gene of Brucella attenuated vaccine S19 strain by constructing suicide plasmid and adopting the method of targeted homologous recombination (gene targeting), so as to damage the expression of the immunogenicity protein BP26 and construct the gene deletion mutant strain Delta S19-1 of the Brucella Bp26. The BMP18 protein is one of the main virulence factors of Brucella. The invention adopts the same method to exclude the Bmp 18 gene of Delta S19-1, so as to lead the Delta S19-1 not to express the Bmp 18 protein and the Brucella virulence gene deletion mutant strain Delta S19-2 is constructed. The invention solves the problems that the conventional Brucella vaccine can not distinguish between the artificial immunization and the wild bacteria infection of people and animals, the virus is strong and the vaccine is easy to cause the illness of inoculated people and animals. The invention has important significance and practical application value of the monitoring, diagnosis, purification and all the controls of Brucella.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Galactosides and thiodigalactosides as inhibitors of pa-il lectin from pseudomonas
InactiveUS20090176717A1Good treatment effectGood curative effectBiocideSugar derivativesVirulent characteristicsPresent method
Compositions and methods are provided related to Pseudomonas bacteria. The compositions and methods may be used for diagnosis and therapy of medical conditions involving infection with Pseudomonas bacteria. Such infections include Pseudomonas aeruginosa in the lungs of patients with cystic fibrosis. A compound useful in the present methods may be used in combination with a therapeutic agent or may be linked to a therapeutic agent. Pseudomonas bacteria may be inhibited by blocking colonization, inhibiting virulence factors, arresting growth or killing the bacteria.
Owner:GLYCOMIMETICS
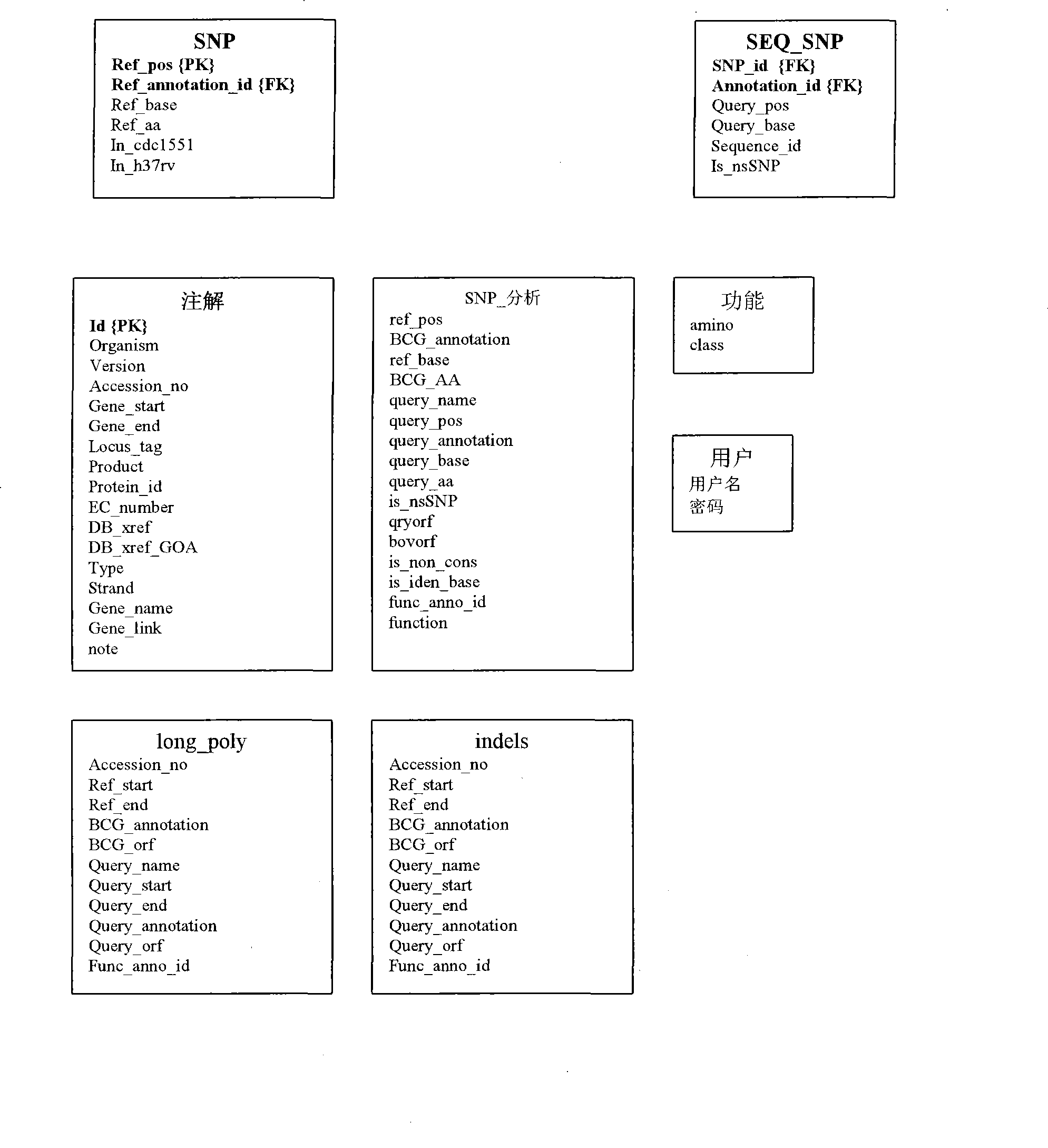
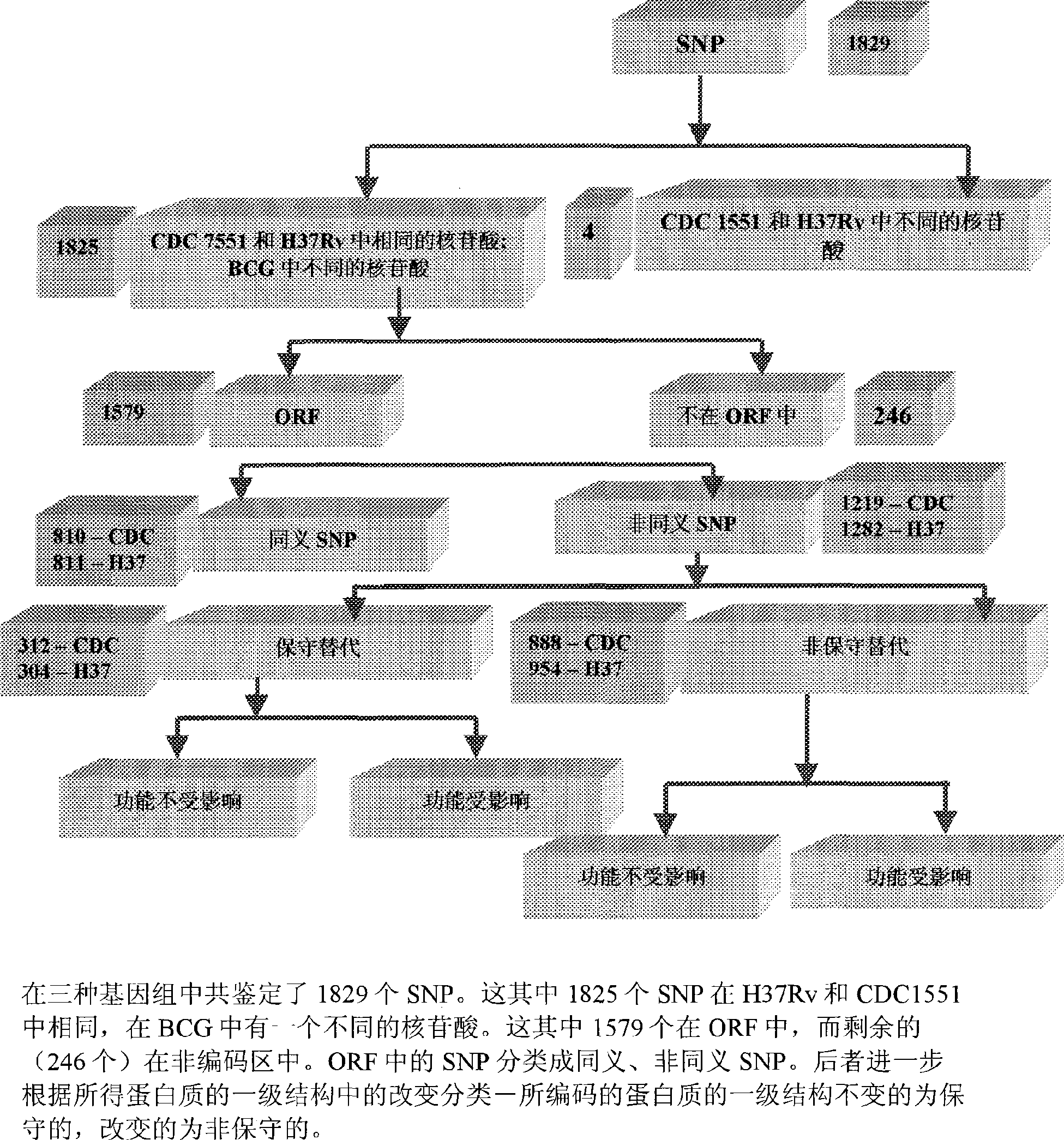
Construction of a comparative database and identification of virulence factors through comparison of polymorphic regions in clinical isolates of infectious organisms
InactiveCN101421415AReduce new targetsImprove protectionMicrobiological testing/measurementSpecial data processing applicationsVirulent characteristicsDrug target
The present invention is directed to novel nucleotide sequences to be used for diagnosis, identification of the strain, typing of the strain and giving orientation to its potential degree of virulence, infectivity and / or latency for all infectious diseases more particularly tuberculosis. The present invention also includes method for the identification and selection of polymorphisms associated with the virulence' and / or infectivity in infectious diseases more particularly in tuberculosis by a comparative genomic analysis of the sequences of different clinical isolates / strains of infectious organisms. The regions of polymorphisms, can also act as potential drug targets and vaccine targets.; More particularly, the invention also relates to identifying virulence factors of M. tuberculosis strains and other infectious organisms to be included in a diagnostic DNA chip allowing identification of the strain, typing of the strain and finally giving orientation to its potential degree of virulence. Although the present invention has been illustrated with specific reference to the polymorphic region in the Mycobacterium tuberculosis, the said invention is not to be understood and construed as being limited to Tuberculosis but is applicable to all infectious diseases.
Owner:阿维斯塔金格兰技术有限公司 +1
Bovine-derived single-chain antibody for resisting staphylococcus aureus LukD virulence factor and preparation and application thereof
PendingCN113493510AInhibition of pathogenic activityBacteriaImmunoglobulins against bacteriaSingle-Chain AntibodiesStaphylococcus aureus
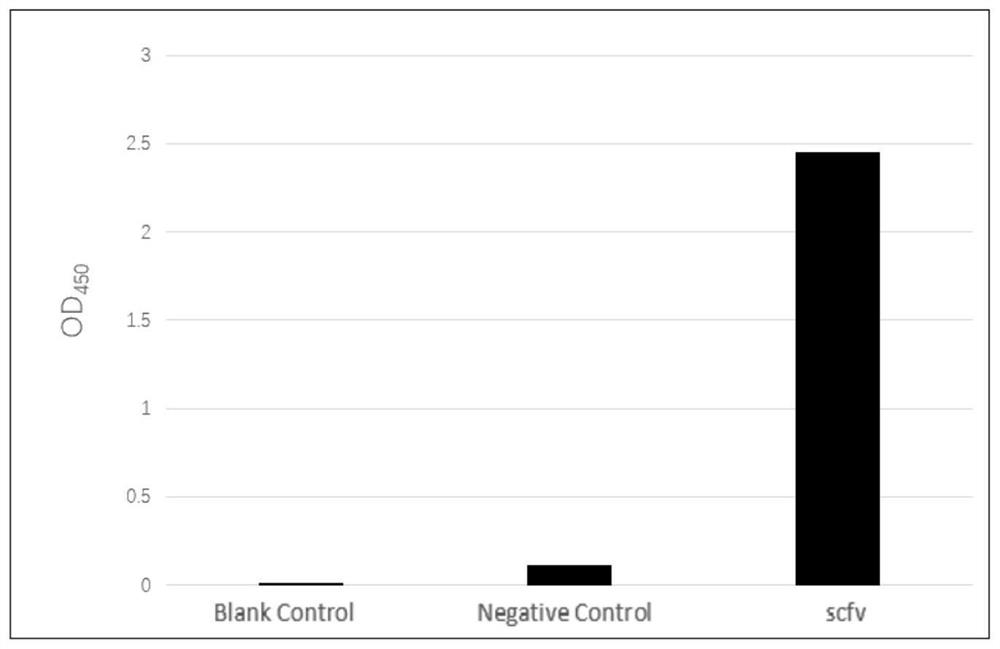
The invention relates to a bovine-derived single-chain antibody for resisting a staphylococcus aureus LukD virulence factor and preparation and application thereof, and specifically comprises the bovine-derived single-chain antibody for resisting the staphylococcus aureus LukD virulence factor, a carrier and a host cell for screening the single-chain antibody, a preparation method of the single-chain antibody and application of the single-chain antibody. The prokaryotic expression single-chain antibody at least comprises a light chain variable region with an amino acid sequence shown as SEQ ID No.1, a heavy chain variable region with an amino acid sequence shown as SEQ ID No.2 and an intermediate connecting peptide located between the light chain variable region and the heavy chain variable region. Compared with the prior art, the bovine-derived single-chain antibody for resisting the staphylococcus aureus LukD virulence factor can be specifically combined with staphylococcus aureus LukD protein and inhibit the membrane lysis effect of LukED on bovine mammary epithelial cells, so that the adhesion and damage of staphylococcus aureus to the bovine mammary epithelial cells are weakened, and the bovine-derived single-chain antibody has a certain function of inhibiting the damage of staphylococcus aureus to mammary gland.
Owner:SHANGHAI JIAO TONG UNIV
Methods for detecting Campylobacter bacteria or antibodies to Campylobacter bacteria with an immunoassay
This invention relates generally to in vitro methods for inducing or enhancing expression of enteric bacterial antigens and / or virulence factors thereby producing antigenically enhanced enteric bacteria, to methods for using antigenically enhanced enteric bacteria and to vaccines comprising antigenically enhanced enteric bacteria. A Campylobacter bacterium having enhanced antigenic property is disclosed. A culture medium useful for culturing the Campylobacteria comprises 0.05% to 3% bile or 0.025% to 0.6% of one or more bile acids or salts. In addition a divalent cation chelator can also be included in the culture medium. The bacteria culture is in a growth phase at early log phase and between early log phase and stationary phase. The enhanced antigenic property is a higher level of an immunogenic antigen when compared to the antigenic property of the same bacteria grown on brain heart infusion broth. Also, antibodies to Campylobacter bacteria or Campylobacteria in samples are detected by immunoassays.
Owner:BIOPORT R&D
Immunogenic compositions for use in vaccination against bordetella
InactiveUS20180071380A1Efficient combinationAntibacterial agentsBacterial antigen ingredientsVaccinationVirulent characteristics
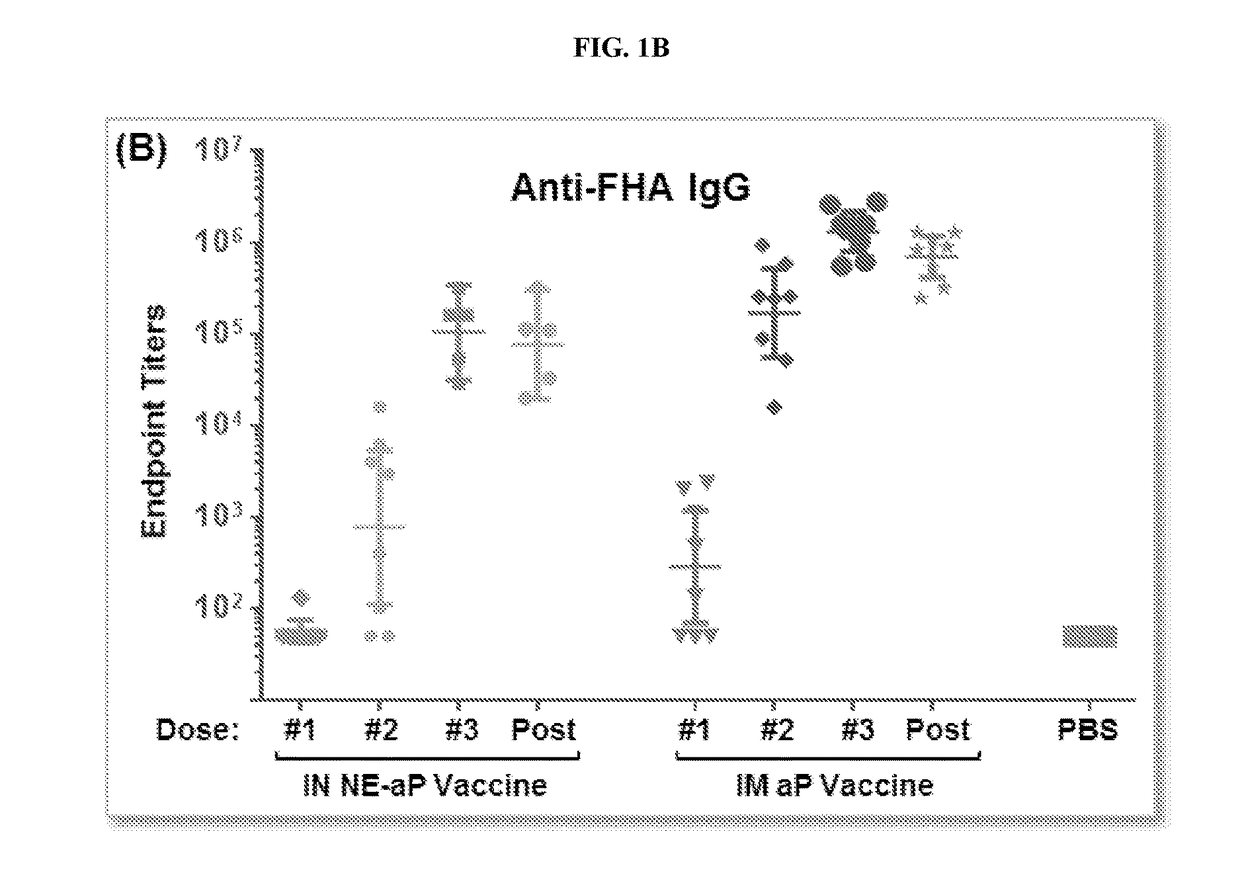
The present application relates to immunogenic compositions comprising a mixture of Bordetella (e.g., B. pertussis) antigens and an oil in water nanoemulsion. In particular, the invention provides immunogenic compositions comprising nanoemulsion and a combination of Bordetella (e.g., B. pertussis) antigens that have different functions, for example, combinations including B. pertussis adherence factors (adhesins), B. pertussis toxins or B. pertussis virulence factors. Vaccines, methods of treatment, uses of and processes to make a pertussis or whooping cough vaccine are also described. Compositions and methods of the present invention find use in, among other things, clinical (e.g. therapeutic and preventative medicine (e.g., vaccination)) and research applications.
Owner:NANOBIO CORP +1
Induction of a physiological dispersion response in bacterial cells in a biofilm
One aspect of the present invention is directed to a composition. The composition includes a dispersion inducer comprising:H3C—(CH2)n—CHmCHmR,where is a single or double carbon-carbon bond, m is 1 or 2, n is 2 to 15, and R is a carboxylic acid, a salt, an ester, or an amide, where the ester or amide is an isostere or biostere of the carboxylic acid. The composition additionally contains an additive component selected from one or more of the group consisting of biocides, surfactants, antibiotics, antiseptics, detergents, chelating agents, virulence factor inhibitors, gels, polymers, pastes, edible products, and chewable products. The composition is formulated so that when it is contacted with a biofilm produced by a microorganism, where the biofilm comprises a matrix and microorganism on a surface, the dispersion inducer selectively acts on the microorganism and has a suitable biological response without a required direct effect on the matrix to disperse the biofilm. The present invention is also directed to methods of using this compound.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Pharmaceutical compositions and methods to vaccinate against disseminated candidiasis
InactiveUS7067138B1Treat prevent alleviateRetard pathogenesisBiocideAntimycoticsHospitalized patientsVirulent characteristics
A Candida albicans bloodstream infections cause significant morbidity and mortality in hospitalized patients. Filament formation and adherence to host cells are critical virulence factors of C. albicans. Multiple filamentation regulatory pathways have been discovered, however the downstream effectors of these regulatory pathways remain unknown. The cell surface protein, Als1p, is a downstream effector of the filamentation regulatory pathway and is regulated by Efg1p. Als1p mediates adherence to endothelial cells in vitro and is required for virulence. The blocking of adherence by the organism is described resulting from the use of a composition and method disclosed herein. Specifically, a pharmaceutical composition comprised of a gene product from the ALS1 gene family is administered as a vaccine to generate an immune response capable of blocking adherence of the organism.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Aeromonas hydrophila inactivated vaccine and preparation thereof
InactiveCN101642567AOutbreak Prevention and ControlEpidemic prevention and controlAntibacterial agentsBacterial antigen ingredientsVirulent characteristicsAeromonas hydrophila
The invention relates to a fish vaccine and a preparation method thereof, in particular to an aeromonas hydrophila inactivated vaccine and the preparation thereof. The aeromonas hydrophila inactivatedvaccine is prepared by the following concrete steps: separating out a pathogen from a sick fish, determining the pathogen as an aeromonas hydrophila through morphology, virulence factors, biochemicalreaction and gene sequencing; rejuvenating the strain, selecting an optimum condition for cultivation; then selecting the optimum condition for inactivation to prepare the aeromonas hydrophila inactivated vaccine, and conforming the aeromonas hydrophila inactivated vaccine to be certificated through sterility test and security test. The inactivated vaccine is prepared from a pathogenic bacterialstrain separated from a sick fish body, has a simple operating method, strong pertinency, strong protective function, good immunological effect and low cost of the used material, and is suitable for mass production. The invention provides a practical method for preventing and curing the bacteremic septicemia of carps.
Owner:张秀军 +2
Muricauda olearia and application thereof
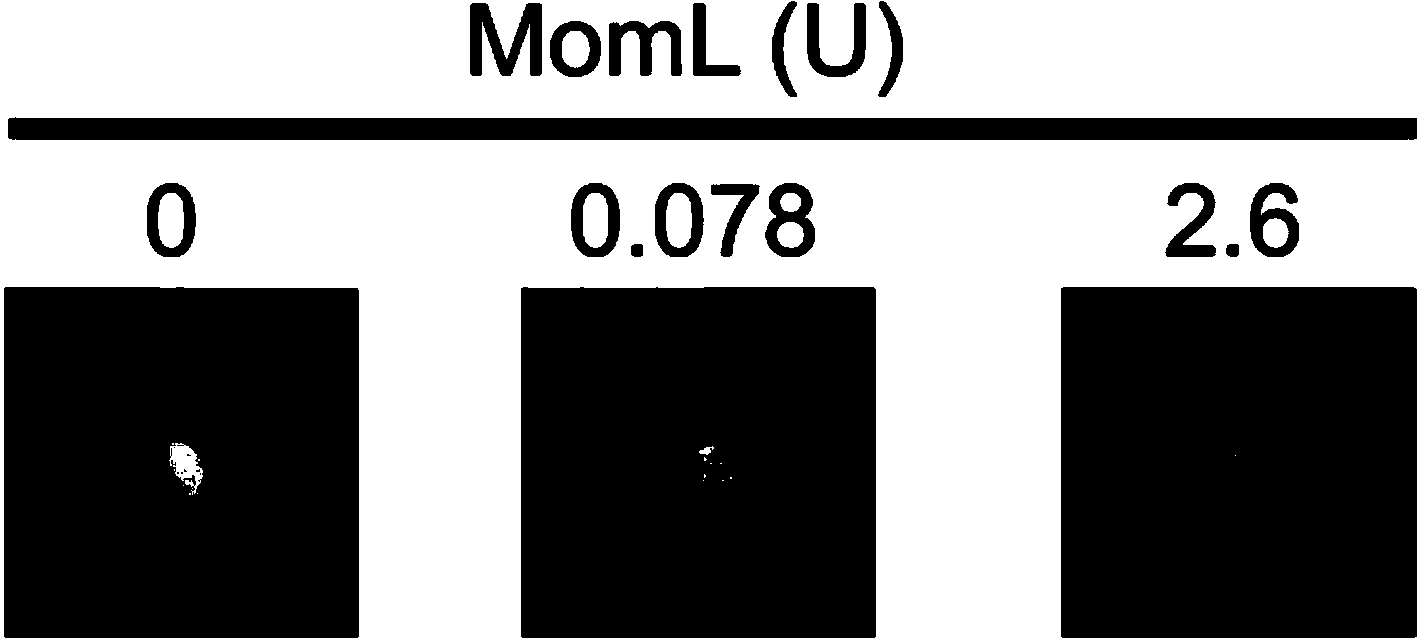
ActiveCN103981140AReduce secretionReduce synthesisBiocideBacteriaVirulent characteristicsVirulence factor
The invention provides Muricauda olearia. Muricauda oleariah is a quorum sensing quenching strain capable of inhibiting the secretion of pathogenic bacteria virulence factors, and the preservation number of Muricauda olearia LMM001 strain is CGMCC No. 8972. The invention also provides an AHL degrading enzyme MomL protein separated from the LMM001 strain, wherein the amino acid sequence of the MomL protein is SEQ ID NO: 1. The Muricauda olearia LMM001 strain disclosed by the invention can block the QS pathway of pathogenic bacteria by small molecule QS repressor, AHL lactonase MomL, AHL acyltransferase and other ways, and reduce the secretion of pathogenic bacteria virulence factors; the LMM001 strain has no toxic and side effects on zebrafish and can improve the resistance of zebrafish to the infection of Aeromonas hydrophila; AHL lactonase can greatly reduce the secretion of virulence factors such as Pseudomonas aeruginosa extracellular protease and pyocyanine and can reduce the synthesis of Chromobacterium violaceum and violacein.
Owner:OCEAN UNIV OF CHINA
Application of 1,3,4-thiadiazole compounds to prevention and treatment of rice xanthomonas oryzae
The invention discloses application of 1,3,4-thiadiazole compounds to prevention and treatment of rice xanthomonas oryzae. The structural general formula of the compounds is as shown in the formula I.The compounds with the general formula have remarkable inhibiting effects on the activity of an hpa1 promoter. Part of the compounds has a remarkable inhibiting effect on a key virulence factor T3SSunder the condition of not influencing the normal growth of the rice xanthomonas oryzae, so the 1,3,4-thiadiazole compounds can be applied to prevention and treatment of the rice xanthomonas oryzae well.
Owner:SOUTH CHINA AGRI UNIV
Application of luteolin in bacterial quorum sensing inhibition system
ActiveCN105147663AInhibition formationInhibit killAntibacterial agentsOrganic active ingredientsBacteroidesStaphylococcus cohnii
The invention relates to the technical field of medicine, and provides application of luteolin in a bacterial quorum sensing inhibition system, application of luteolin in preparing drug for lowering pathogenicity of staphylococcus aureus virulence factors and application of luteolin in preparing drug for inhibiting transcription of hla and agrA genes of staphylococcus aureus. Minimum effective concentration with application value is provided for the application of luteolin in the bacterial quorum sensing inhibition system, and a reference is provided for medically preparing specific bio-film forming inhibitors. The invention further provides a drug combination for inhibiting formation of staphylococcus aureus bio-films. The drug combination contains luteolin. According to the drug combination, formation of the bio-films of staphylococcus aureus can be inhibited by effectively inhibiting a quorum sensing system of staphylococcus aureus through tiny luteolin, prognosis risk, of staphylococcus aureus, for infection with high-drug-tolerance bio-films in anti-infection treatment is lowered, and a valuable reference is provided for medically preparing specific preparations for inhibiting the staphylococcus aureus bio-films.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Recombined cattle parainfluenza carrier for expressing protein VP1 of porcine O type foot-and-mouth disease virus
InactiveCN103773803ACapable of copyingVector-based foreign material introductionAntigen epitopeHeterologous
The invention relates to a recombined cattle parainfluenza carrier pcDNA-NM09-VP1 for expressing the protein VP1 of a porcine O type foot-and-mouth disease virus. The recombined cattle parainfluenza carrier is characterized in that an RNA (ribonucleic acid) extracted from a strain BPIV3NM09 is used as a template; a virus total-length gene group is segmentally amplified through RT-PCR (reverse transcription-polymerase chain reaction); the total-length cDNA of cattle parainfluenza is subjected to primary modification, namely AgeI is introduced between P and M, so that insertion and replacement of exogenous antigen gene fragments are facilitated; due to secondary modification, antigen epitope which is inserted into a heterologous virus in a modified manner is the protein VP1 of the O type foot-and-mouth disease virus; the amino acid sequence is SEQIDNO1, and the nucleotide sequence is SEQIDNO2. The foundation is laid for further development of a gene engineering recombined vaccine for preventing and controlling the porcine foot-and-mouth disease and research on the III-type toxicity factor and the molecular pathogenesis of the cattle parainfluenza; the recombined cattle parainfluenza carrier has the replication capacity.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS +1
Rhizobium sp. and application thereof
The invention discloses Rhizobium sp. and application thereof. The Rhizobium sp. is preserved in CGMCC (China General Microbiological Culture Collection Center) on March 11, 2016, and the preservation number of the Rhizobium sp. is CGMCC No. 12205. The Rhizobium sp. has the advantages that the Rhizobium sp. can efficiently inhibit the formation of a pathogen biofilm and inhibit pathogen virulence factors, the fermentation products or metabolites of the Rhizobium sp. can be effectively used as the active components of pathogen biofilm inhibitors, the Rhizobium sp. and the fermentation products or metabolites thereof can be used to effectively inhibit the formation of the pathogen biofilm and control the toxicity of pathogens, and accordingly the Rhizobium sp. can be effectively used for treating bacterial infection so as to enhance the susceptibility of the pathogens to common medicine and enhance a treatment effect.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Methods of screening compounds useful for prevention of infection or pathogenicity
InactiveUS6905670B2Improve responseImprove throughputCompounds screening/testingHydrolasesScreening proceduresVirulent characteristics
Screening procedures are disclosed for identifying compounds useful for inhibiting fungal infection or pathogenicity. Methods are also disclosed for identifying fungal pathogenic virulence factors.
Owner:THE GENERAL HOSPITAL CORP
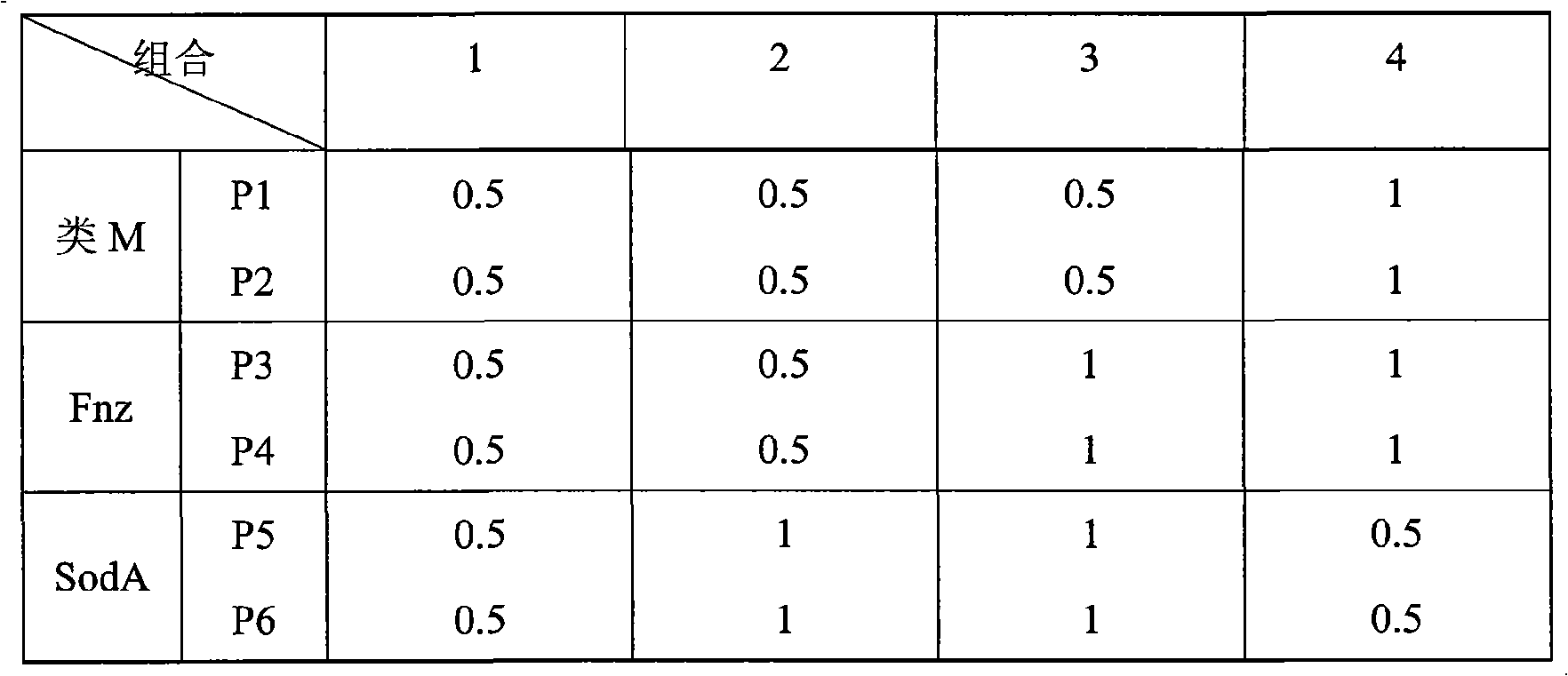
PCR detection method for streptococcus equi subsp zooepidemicus and kit used thereby
InactiveCN101597649AIncreased sensitivitySensitiveMicrobiological testing/measurementMicroorganism based processesBacteroidesVirulent characteristics
The invention belongs to the field of epidemiology and sanitation tests, and relates to a PCR quick detection kit for quickly detecting streptococcus equi subsp zooepidemicus and a PCR quick detection method. Particularly, the kit of the invention comprises three pairs of primers which are aimed at M-like proteins (SzP), fibronectin binding proteins (Fnz), superoxide dismutase A (SodA) coding gene respectively and specifically, so the kit can detect the three virulence factors of the streptococcus equi subsp zooepidemicus at the same time. The multiplex PCR detection method is quick and simple, can directly detect pathologic materials, avoids a normal complex process of bacteria isolation, cultivation and biochemical identification, reduces detection time and simplifies operation to allow for skillful use after simple training. The kit is high in sensitivity and can detect micro pathogen. And the kit is high in specificity and can distinguish streptococcus equi subsp zooepidemicus from other kinds of streptococcus without any cross reaction.
Owner:范红结 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com