Brucella molecule marking and virulence deletion attenuated vaccine and preparation method
A technology of Brucella and attenuated vaccine, applied in the direction of bacterial antigen components, bacteria, antibacterial drugs, etc., can solve the problems of disease, adverse reactions between humans and animals, strong virus, etc., and achieve the effect of wide application and practical value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Construction of homologous recombination vector (suicide plasmid)
[0068] 1 Materials and methods
[0069] 1.1 Bacterial strains, plasmids, vectors Brucella S19 strain, DH5 a, pBluescript SK + Preserved in our laboratory; pBK-CMV was purchased from Stratagene; pSP-Luc NF + Purchased from Promega Company; pIB279 was donated by Dr. Jiang Jiandong of Nanjing Agricultural University; pMD18-T simple vector was purchased from TakaRa Company.
[0070] 1.2 Reagents Restriction endonuclease, T4 DNA ligase, DNA polymerase I (Klenow large fragment), Escherichia coli DNA polymerase I, LA-Taq DNA polymerase, 1kbp DNA Ladder Marker, plasmid DNA extraction kit, DNA gel The gel recovery kits were all products of TaKaRa Company; the luciferase detection system was purchased from Promega Company.
[0071] 1.3 PCR amplification
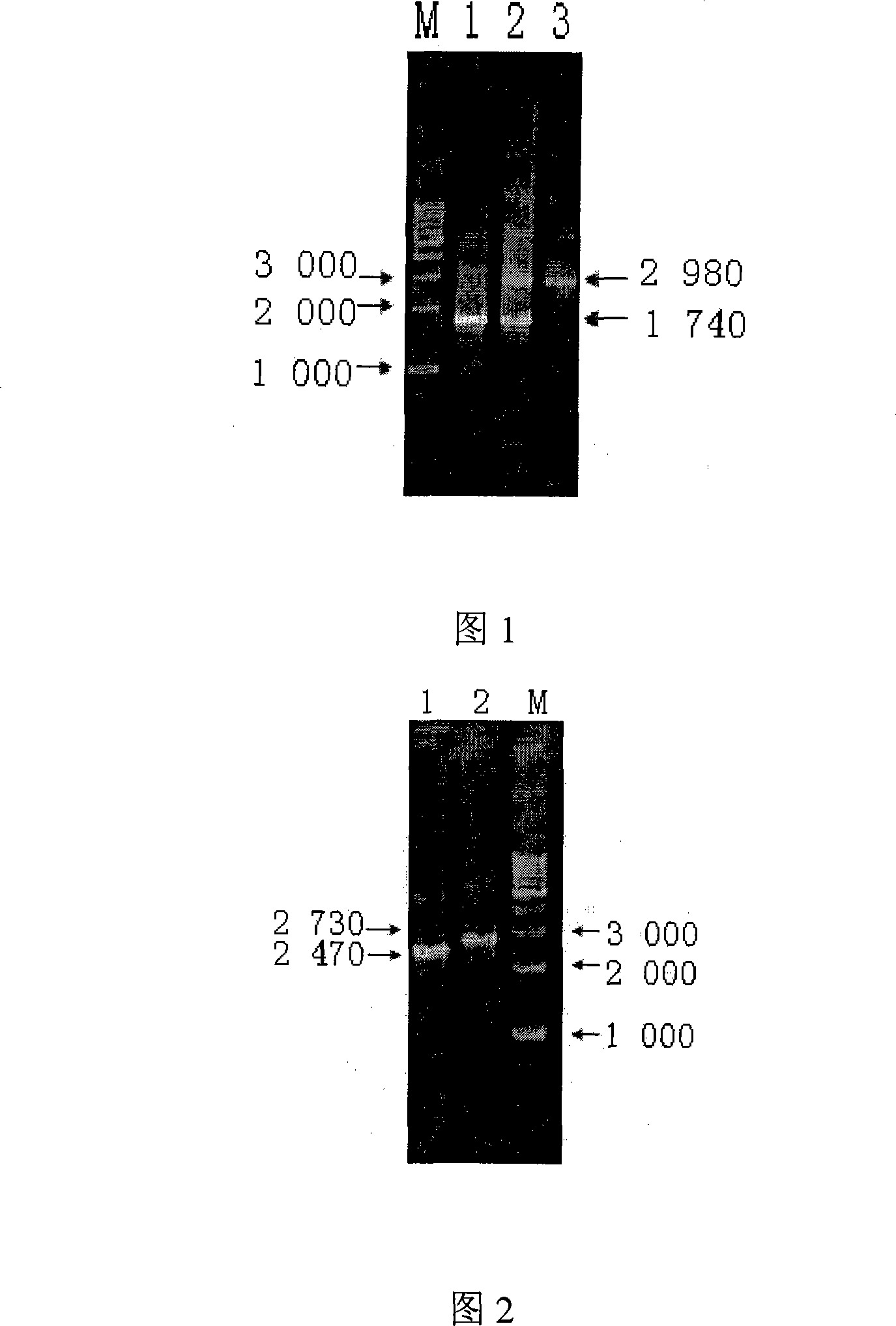
[0072] 1.3.1 Amplification containing the Bp26 gene homologous recombination guide sequence According to the sequence of the Bp26 gene and the characteristi...
Embodiment 2
[0083] Construction of Brucella Molecular Marker and Virulence Lost Vaccine Strain ΔS19-2
[0084] 1.1 Reagents The luciferase detection system was purchased from Promega, and other reagents were the same as in Example 1.
[0085] 1.2 Liver soup medium Take fresh beef liver, remove fat and fascia, mince it, weigh 500g, add 1000mL of tap water to mix with it, boil in a pot for 1h, filter, add water to make up the original amount, add NaCl5g, peptone 10g. Liver soup agar preparation: Take 1000 mL of the above liver soup medium, add 20 g of agar, and sterilize for later use
[0086] 1.3 Electroconversion
[0087] 1.3.1 Preparation of Competent State Take 240mL pre-logarithmic growth mid-phase (OD 600 =0.15) Put the Brucella liquid culture into a pre-cooled 250mL centrifuge tube, quickly place the culture in the ice-water mixture for 15-30min, shake slowly from time to time to ensure that the contents are fully cooled. Then, centrifuge at 1000×g for 15 minutes at 4° C. to recov...
Embodiment 3
[0101] Identification of ΔS19-2 virulence
[0102] In Example 2 of the present invention, we deleted Bmp18, one of the main virulence genes of S19, and constructed a mutant strain of ΔS19-2. Theoretically, ΔS19-2 is less virulent than S19. In the present invention, mice are used as experimental animals to detect the bacteria content of ΔS19-2 and S19 in the spleen of mice inoculated at different times, and to inoculate mice with different high concentrations of ΔS19-2 and S19, and to observe the clinical symptoms and the minimum lethal number of mice , and compared their virulence.
[0103] 1 Materials and methods
[0104] 1.1 Materials
[0105] 1.1.1 Strains
[0106] See Example 2
[0107] 1.2 Method
[0108] 1.2.1 Bacteria count method
[0109] (1) Wash the 48-hour Brucella culture with sterile saline, and make a 1 billion / mL bacterial suspension using standard turbidimetry.
[0110] (2) Then make 10-fold serial dilutions per mL: 100 million, 10 million, 1 million, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com